Open Access Article
Open Access ArticleSequential treatment of cyanide and phenolic mixtures using CMC-PVP-nZVI/Pd and Rhodococcus pyridinivorans strain PDB9T N1
Ankita Priyadarshinia,
Naresh Kumar Sahoo *a,
Soumya Mishraa,
Prasant Kumar Sahoob,
Ranjan Kumar Bhuyanc,
Prangya Ranjan Routd and
Bankim Chandra Tripathye
*a,
Soumya Mishraa,
Prasant Kumar Sahoob,
Ranjan Kumar Bhuyanc,
Prangya Ranjan Routd and
Bankim Chandra Tripathye
aDepartment of Chemistry, Environmental Science and Technology Program, Faculty of Engineering and Technology (ITER), Siksha 'O' Anusandhan (Deemed to be University), Bhubaneswar 751030, Odisha, India. E-mail: nareshsahoo@soa.ac.in; nareshks2010@gmail.com
bEnvironmental Hydrology Division, National Institute of Hydrology, Jalvigyan Bhawan, Roorkee 247667, India
cP. G. Department of Physics, Government Autonomous College Angul Odisha, India
dDepartment of BioTechnology, Dr B. R Ambedkar National Institute of Technology Jalandhar, India
eFaculty of Chemical Sciences (AcSIR), Department of Hydro and Electrometallurgy, Institute of Minerals and Materials Technology, Bhubaneswar 751 013, India
First published on 19th December 2025
Abstract
Cyanide and phenol are considered the major toxic pollutants in coke-oven wastewater. Cyanide, at a pH less than 10, is converted to HCN gas (pKa = 9.2). Hence, cyanide treatment requires strongly alkaline conditions, i.e., a pH of more than 10; however, at such a high pH, the viability of microbes is not feasible. Therefore, to overcome this incompatibility, a sequential nano-bio treatment system was developed, integrating a novel carboxymethyl cellulose-polyvinylpyrrolidone-stabilized nanoscale zero-valent iron doped with palladium (CMC-PVP-nZVI/Pd) nanocomposite, followed by bio-treatment using R. pyridinivorans strain PDB9T N-1. The first nanostage was operated at pH 12 to stabilize cyanide (CN−) and initiate its removal, while the subsequent biostage was operated at a pH of 7.4 to achieve the complete removal of phenol. To prevent the atmospheric oxidation of nZVI and to improve its reusability and electron mobility, it was doped with palladium and conjugated with CMC and PVP. The synthesised nanomaterials were characterized using XRD, FTIR spectroscopy, FESEM, EDX, and XPS analyses. Results revealed that about 99% of cyanide was removed with an initial dose of 100 mg L−1 at 30 °C using the nanocomposite, followed by the complete biodegradation of the remaining phenol (300 mg L−1). The cyanide removal efficiency of the nanocomposite was 1.8-fold higher than that of the bare nZVI. Overall, the cyanide removal process followed a pseudo-2nd-order kinetics model, revealing a chemisorption nature with a superior sorption capacity of 93.37 mg g−1. The intraparticle diffusion model showed that exterior mass transfer primarily governed the cyanide removal. Additionally, the nanocomposite exhibited strong reusability, demonstrating the efficacy of the proposed sequential nano-bio system.
1. Introduction
Steel plant industries generate vast amounts of wastewater primarily from coke oven plants, varying in the range of 0.3 to 4 m3 per ton of coke production.1,2 In general, coke-oven wastewater contains high levels of phenol (ranging from 150 to 2000 mg L−1) and cyanide (ranging from 0.1 to 0.6 kilograms per ton of coke), significantly surpassing the standard limits for phenol at 1 mg L−1 and cyanide at 0.2 mg L−1.3,4 Cyanide inhibits the cytochrome oxidase enzyme system and, subsequently, the mitochondrial electron transport chain and adenosine triphosphate (ATP) formation, thus causing serious human health hazards.5,6 Similarly, phenolic compounds cause significant human health and environmental damages due to their acute toxicity and carcinogenic nature.7,8 Thus, cyanide and phenolic compounds are listed as priority pollutants by the United States Environmental Protection Agency (USEPA).6,9Conventional treatment methods such as chlorination, photocatalysis, electrolysis, and membrane filtration are often not cost-effective and typically result in only the partial degradation of pollutants, leading to the formation of harmful secondary intermediates. Among biological treatment methods, reactors based on the activated sludge process are most frequently utilized for this wastewater.7,10 In general, several studies have reported cyanide biodegradation, with most experiments conducted at neutral or sub-alkaline pH (<10). However, at pH values below 10, cyanide (CN−) is converted to hydrogen cyanide (HCN) gas,11 and the authors of these studies often did not verify whether cyanide removal occurred through microbial degradation or volatilization as HCN.11,12 Therefore, industrial wastewater containing cyanide must be maintained at an alkaline pH above 10 (pKa = 9.2) to prevent HCN gas formation.13 Nonetheless, at pH levels higher than 10, microbial viability and effective cyanide biodegradation are limited.14 To address these challenges, the present study adopted a two-stage sequential nano-bio treatment strategy. In the first stage, a nanocomposite was applied under alkaline conditions for rapid cyanide removal. This was followed by pH adjustment and aeration, after which a bacterial strain was grown at a pH of 7.4 to completely degrade phenol and remaining intermediates. Such combined approaches are now increasingly recognized for their ability to merge redox catalysis and biological oxidation, resulting a safer and more comprehensive remediation.15
Nanoscale zero-valent iron (nZVI) has emerged as a promising candidate for on-site remediation of toxic heavy metals,16 halogenated hydrocarbons,17,18 and other pollutants.19 Its appeal lies in its nontoxicity, high reactivity, rapid kinetics, and large specific surface area, which collectively enable efficient contaminant removal from water.20–23 However, several challenges limit its effectiveness. In particular, nZVI particles readily agglomerate due to their nanoscale size and intrinsic magnetic properties,24,25 leading to a transition from the nanoscale to the microscale. This agglomeration diminishes their specific surface area, mobility, and overall reactivity.26,27 Secondly, nZVI is highly reactive and can readily interact with water and oxygen, which reduces its long-term effectiveness. To address these challenges, several strategies have been developed. A common approach to prevent particle aggregation involves using ecofriendly biopolymers such as chitosan and carboxymethyl cellulose (CMC). CMC, in particular, is a nontoxic, biodegradable polymer that forms a protective coating around nZVI particles. This coating generates electrostatic and steric repulsion forces, which keep the particles apart, thereby enhancing their stability and dispersion in water.28,29 The CMC stabilization process enables nZVI to remain suspended for longer durations, thereby increasing its contact time with environmental contaminants and enhancing remediation efficiency. Similarly, polyvinylpyrrolidone (PVP), a conductive polymer, has attracted attention as a modifying agent because of its distinct properties, including low scattering loss and sufficient charge storage capacity. The pyrrole group of PVP allows it to interact with other molecules, form complexes with salts, and act as a stabilizer, which improves particle dispersion and may also contribute to redox reactions involving nZVI. Consequently, PVP was employed in this study to enhance both the efficiency and stability of nZVI. However, due to the high reactivity of nZVI, oxidation readily occurs upon exposure to air or water, producing a core–shell structure with metallic iron at the core and iron oxides on the surface. CMC and PVP together facilitate a well-stabilized hybrid network that enhances nanoscale reactivity and extends the catalyst lifetime.30 Another strategy for enhancing nZVI's performance is the deposition of secondary metals such as Pd, Pt, or Ni onto its surface. Recent advances indicate that modified or doped nZVI systems exhibit superior degradation efficiency via the synergistic activation of oxidants and electron transfer.31,32 This modification increases the availability of electrons for contaminant reduction, thereby improving nZVI's reactivity. Among these, Pd has been widely recognized as the most effective catalyst due to its excellent chemical stability and superior catalytic activity.33 When Pd4+ ions are introduced, they impede the oxidation of iron particles by accepting electrons to reduce to Pd2+, thereby protecting nZVI from oxidation and facilitating the formation of Fe–Pd bimetallic nanoparticles.34 According to recent reports, oxygen-promoted redox pathways and partial denitrification systems can further increase the effectiveness of oxidative transformation.35,36 In certain cases, this modification also improves nanoparticle stability under aerobic conditions by further inhibiting surface oxidation.23
Based on these principles, a carboxymethyl cellulose-polyvinylpyrrolidone-stabilized nanoscale zero-valent iron/palladium (CMC-PVP-nZVI/Pd) nanocomposite was synthesised, characterised, and used for cyanide removal from its phenolic mixture. The cyanide-treated effluent was subsequently subjected to biological treatment using the R. pyridinivorans strain PDB9T N-1 for the degradation of phenolic compounds. The overall efficiency of this sequential nano-bio process was assessed through several kinetic and thermodynamic models. The mechanism of pollutant removal was thoroughly discussed as well, and the reusability capacity was systematically investigated.
2. Materials and methods
2.1. Chemicals
In this research, all chemicals and reagents used were of analytical reagent grade (AR). Chemicals such as ferric chloride tetrahydrate (FeCl3·4H2O), sodium borohydride (NaBH4), and potassium hexachloropalladate (K2PdCl6) were sourced from Merck, India. Polyvinylpyrrolidone (PVP), carboxymethyl cellulose (CMC), and phenol crystals (C6H5OH) were sourced from SRL India.2.2. Methods
| 4Fe3+ + 3BH4− + 9H2O → 4Fe0 + 3H2BO3− + 12H+ + 6H2 | (1) |
The procedure was carried out until the formation of a black nZVI precipitate. Then, the solution was centrifuged for 10 minutes at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The obtained nZVI NMs were washed three times with 100% ethanol, dried at 100 °C, and preserved in an airtight container to prevent further oxidation.
000 rpm. The obtained nZVI NMs were washed three times with 100% ethanol, dried at 100 °C, and preserved in an airtight container to prevent further oxidation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The CMC-PVP-nZVI NPs were washed three times with 100% ethanol, dried at 100 °C and preserved in an airtight container.
000 rpm. The CMC-PVP-nZVI NPs were washed three times with 100% ethanol, dried at 100 °C and preserved in an airtight container.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and dried at 60 °C. The prepared NMs were kept under vacuum overnight and then stored in a sealed container.
000 rpm for 10 min and dried at 60 °C. The prepared NMs were kept under vacuum overnight and then stored in a sealed container.2.3. Experimental procedure
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, and the supernatant was collected to determine the remaining cyanide concentration using the colorimetric method at 578 nm, as outlined in the literature.39 The efficiency of cyanide removal was calculated as follows:
000 rpm, and the supernatant was collected to determine the remaining cyanide concentration using the colorimetric method at 578 nm, as outlined in the literature.39 The efficiency of cyanide removal was calculated as follows:
 | (2) |
2.4. Analytical method
The cyanide and phenol concentrations were assessed using the PBA method (pyridine-barbituric acid) and 4-amino antipyrine method, respectively, as outlined in standard methods.38 The developed colours of cyanide and phenol were analysed calorimetrically at 578 nm and 510 nm, respectively, using a UV-Vis spectrophotometer (Evolution 220, Thermo Fisher Scientific, USA). The size distribution, shape, and structure of different synthesized NMs were analyzed by X-ray diffraction (XRD). XRD analysis was conducted using Rigaku Ultima (Japan) by varying the 2θ value between 20° and 90° with X'Pert High Score software. Fourier transform infrared (FTIR) spectroscopy was used to detect the functional groups present on the surface of the NMs using KBr pellets (Thermo Fisher Nicolet 10 spectrometer, USA). FTIR measurements were performed within the 400–4000 cm−1 wavenumber range. To examine the structural and morphological configurations of the NMs, the FE-SEM method was utilized (Zeiss Gemini Sigma 300, UK). Using the same Zeiss Gemini Sigma 300 instrument, energy-dispersive X-ray spectroscopy (EDX) was performed to evaluate the compositional purity. X-ray photoelectron spectroscopy (XPS) was used to analyze the surface composition of the nanocomposite using an imaging photoelectron spectrometer (Thermo Escalab, USA), model no. PHI 5000 Versa Probe III.2.5. Regeneration experiments
After the CN− removal reaction, the NMs were centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes and dried at 60 ⸰C. Then, the dried NMs were dispersed in a solution of hydrochloric acid (0.1 M). The mixtures were agitated for 120 minutes. To determine the reusability of the NMs, the regeneration experiments of the composites were performed 3 times with HCl; then, the sorbent was cleaned with distilled water till the supernatant had a neutral pH, confirming that there was no acid content on the NMs, which were utilized for the next regeneration cycle.
000 rpm for 10 minutes and dried at 60 ⸰C. Then, the dried NMs were dispersed in a solution of hydrochloric acid (0.1 M). The mixtures were agitated for 120 minutes. To determine the reusability of the NMs, the regeneration experiments of the composites were performed 3 times with HCl; then, the sorbent was cleaned with distilled water till the supernatant had a neutral pH, confirming that there was no acid content on the NMs, which were utilized for the next regeneration cycle.
3. Results and discussion
3.1. Characterization
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching vibration, suggesting cyanide sorption on the surface of nZVI.50
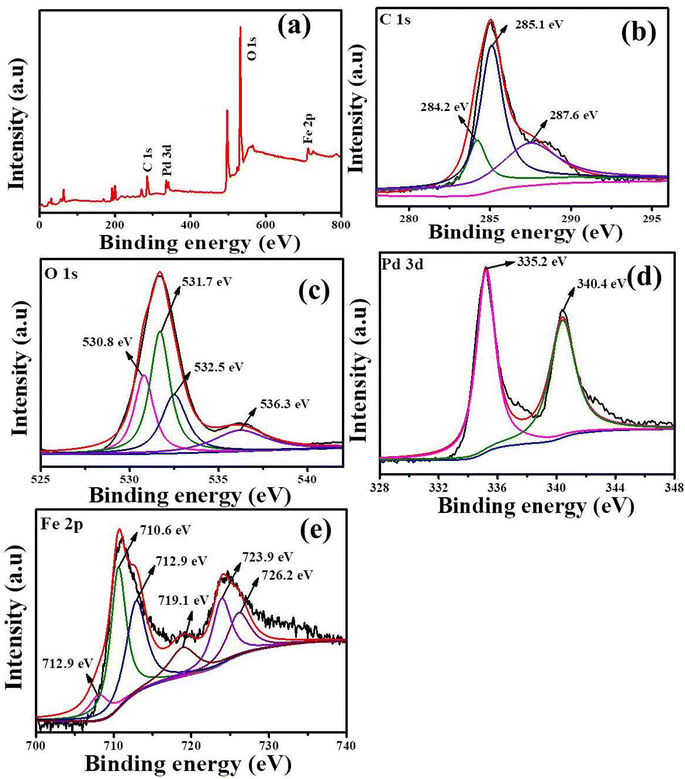
N stretching vibration, suggesting cyanide sorption on the surface of nZVI.50![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/O–C–O bonds, respectively, as shown in Fig. 3(b).58 These signals generally arise from the hydroxyl, carboxyl, and pyrrolidone groups of CMC and PVP, which indicate that the polymers are chemically bound to the Fe surface through Fe–O–C and Fe–N linkages. This coordination not only stabilizes the nanoparticles but also facilitates charge delocalization between the Fe and Pd sites, thereby improving electron transfer during redox reactions.42,59,60 As depicted in Fig. 3(c), the high-resolution O 1s spectra can be fitted with three characteristic peaks located at approximately 530.8 eV, 531.7 eV, and 532.5 eV, corresponding to C
O/O–C–O bonds, respectively, as shown in Fig. 3(b).58 These signals generally arise from the hydroxyl, carboxyl, and pyrrolidone groups of CMC and PVP, which indicate that the polymers are chemically bound to the Fe surface through Fe–O–C and Fe–N linkages. This coordination not only stabilizes the nanoparticles but also facilitates charge delocalization between the Fe and Pd sites, thereby improving electron transfer during redox reactions.42,59,60 As depicted in Fig. 3(c), the high-resolution O 1s spectra can be fitted with three characteristic peaks located at approximately 530.8 eV, 531.7 eV, and 532.5 eV, corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH/C–O, and C–OO−/O–C–O functional groups, respectively.61 The additional shoulder at 536.3 eV can be attributed to adsorbed oxygen from air contaminants and moisture. These oxygenated species confirm the coexistence of polymeric oxygen groups and surface iron oxides or hydroxides, forming a thin passivation layer that protects the Fe0 core and provides reactive sites for pollutant reduction. As shown in Fig. 3(d), the deconvoluted XPS spectrum of Pd exhibits two distinct peaks at 335.2 eV and 340.4 eV, corresponding to the Pd 3d5/2 and Pd 3d3/2 orbitals, respectively. These characteristic binding energies confirm that Pd is present in the metallic zero-valent state (Pd0).62 Further, a slight negative shift in the Pd binding energy relative to the standard Pd0 (335.6 eV) suggests electron donation from Fe0 to Pd, implying a synergistic Fe–Pd coupling that enhances catalytic activity. The Fe 2p profile (Fig. 3(e)) shows two peaks centered at binding energies of 710.6 eV and 723.9 eV, corresponding to Fe(II), while the peaks at 712.9 eV and 726.2 eV are attributed to Fe(III).63 The presence of Fe(II) and Fe(III) oxides may result from oxidation processes occurring during sample storage or testing. Additionally, the doublet peaks observed at binding energies of 718.9 eV and 707.8 eV correspond to Fe(0) 2p1/2 and Fe(0) 2p3/2, respectively, confirming that the metallic Fe core remains intact. The simultaneous presence of Fe(0), Fe(II), and Fe(III) signals supports the formation of a core–shell Fe0/Fe-oxide structure, where the polymer coating and Pd nanoparticles collectively improve structural stability, electron conductivity, and the accessibility of reactive sites within the nanocomposite, demonstrating the effective formation of the CMC-PVP-nZVI/Pd nanocomposite.
O, C–OH/C–O, and C–OO−/O–C–O functional groups, respectively.61 The additional shoulder at 536.3 eV can be attributed to adsorbed oxygen from air contaminants and moisture. These oxygenated species confirm the coexistence of polymeric oxygen groups and surface iron oxides or hydroxides, forming a thin passivation layer that protects the Fe0 core and provides reactive sites for pollutant reduction. As shown in Fig. 3(d), the deconvoluted XPS spectrum of Pd exhibits two distinct peaks at 335.2 eV and 340.4 eV, corresponding to the Pd 3d5/2 and Pd 3d3/2 orbitals, respectively. These characteristic binding energies confirm that Pd is present in the metallic zero-valent state (Pd0).62 Further, a slight negative shift in the Pd binding energy relative to the standard Pd0 (335.6 eV) suggests electron donation from Fe0 to Pd, implying a synergistic Fe–Pd coupling that enhances catalytic activity. The Fe 2p profile (Fig. 3(e)) shows two peaks centered at binding energies of 710.6 eV and 723.9 eV, corresponding to Fe(II), while the peaks at 712.9 eV and 726.2 eV are attributed to Fe(III).63 The presence of Fe(II) and Fe(III) oxides may result from oxidation processes occurring during sample storage or testing. Additionally, the doublet peaks observed at binding energies of 718.9 eV and 707.8 eV correspond to Fe(0) 2p1/2 and Fe(0) 2p3/2, respectively, confirming that the metallic Fe core remains intact. The simultaneous presence of Fe(0), Fe(II), and Fe(III) signals supports the formation of a core–shell Fe0/Fe-oxide structure, where the polymer coating and Pd nanoparticles collectively improve structural stability, electron conductivity, and the accessibility of reactive sites within the nanocomposite, demonstrating the effective formation of the CMC-PVP-nZVI/Pd nanocomposite.
3.2. Cyanide removal performance of the synthesized NMs
The cyanide reduction performance of the synthesized NMs is presented in Fig. 4(a). As shown, bare nZVI achieved only 49.65% cyanide removal at an initial dose of 100 mg L−1 within 2 h. The incorporation of CMC into nZVI significantly improved the performance, increasing cyanide removal to 76.42%. A further enhancement was observed with PVP-nZVI, which achieved 87.43% removal. The highest efficiency was obtained using the PVP-Pd-nZVI nanocomposite, reaching 99.82% removal. Interestingly, when CMC was introduced into the PVP-Pd-nZVI system (CMC-PVP-nZVI/Pd), the removal efficiency slightly decreased to 89.44%. This reduction can be attributed to the CMC biopolymer coating, which acts as a protective barrier that shields nZVI, thereby slowing its oxidation and deactivation and also partially limiting its reactivity. CMC creates electrostatic and steric forces that push individual nZVI particles apart, thereby maintaining their stability and improving their dispersion in water.28,29 It also provides the sustained release of nZVI over time, extending its longevity.53 However, despite these advantages, CMC did not enhance cyanide removal. This reduced efficiency may be attributed to the blocking of nZVI reactive sites by CMC molecules, which serve as stabilizing and coating agents, thus hindering cyanide adsorption and subsequent reduction. In addition, the reductive sorption of cyanide by CMC-PVP-nZVI/Pd was suppressed due to electrostatic repulsion between the negatively charged COO− groups of CMC and the negatively charged CN− ions.20,64 In contrast, PVP significantly improved cyanide reduction by minimizing particle aggregation, increasing the available surface area, and enhancing the electron transfer rate. Further, PVP polymers contain a pyrrole group, which can interact with other molecules, enhancing dispersion and potentially participating in the redox reactions of nZVI.The incorporation of Pd-doped PVP into nZVI markedly increased cyanide removal efficiency. This improvement is attributed to the ability of Pd4+ ions to prevent the further oxidation of iron particles by accepting electrons and being reduced to Pd2+. This process inhibits nZVI oxidation and facilitates the formation of Fe–Pd bimetallic nanoparticles.34,65 These results demonstrate that PVP-Pd is an effective polymer for enhancing cyanide removal by nZVI. Subsequently, the cyanide-treated sample was subjected to phenol degradation (300 mg L−1) using the R. pyridinivorans strain PDB9T, achieving 99.88% phenol removal within 16 h, as shown in Fig. 4(b).
3.3. Phenol degradation performance by Rhodococcus pyridinivorans PDB9T N-1
The phenol degradation efficiency of the obtained effluent after nanotreatment was evaluated at an initial phenol concentration of 300 mg L−1 over a reaction period of 24 h at pH 7.4. The phenol concentration decreased gradually with time, as shown in Fig. 4(b), reaching 8.32% removal at 2 hours, 46.6% at 10 hours, and 99.88% total degradation by 16 hours. The biological stage was initiated only after complete cyanide removal (<0.1 mg L−1), ensuring that Rhodococcus pyridinivorans PDB9T N-1 was not exposed to inhibitory cyanide levels. The consistent increase in the degradation efficiency and the near-complete removal within 16 hours demonstrate the high catalytic activity, adaptability, and robustness of R. pyridinivorans in the postdetoxified effluent.3.4. Effect of the initial cyanide concentration on its removal
The study evaluated the performance of the CMC-PVP-nZVI/Pd nanocomposite at different initial cyanide concentrations ranging from 20 to 200 mg L−1 while keeping other parameters constant. Almost complete cyanide removal was observed when the initial concentration was below 40 mg L−1. However, as the cyanide concentration increased, the removal efficiency gradually declined. For example, the efficiency decreased from 100% to 58.6% as the initial dose increased from 40 to 200 mg L−1, as shown in Fig. 4(c). This reduction in efficiency at higher cyanide concentrations can be attributed to the limited availability of active binding sites on the CMC-PVP-nZVI/Pd nanocomposite.12,663.5. Effect of the temperature and thermodynamics of cyanide removal
Experiments were conducted to examine the effect of the temperature on cyanide removal under fixed conditions (initial cyanide concentration = 100 mg L−1, pH = 12, contact time = 2 h) at four different temperatures (293, 298, 303, and 313 K), as presented in Fig. 4(d). The results indicated that the cyanide removal efficiency increased with rising temperature. This enhancement can be attributed to the disruption of internal bonds within the adsorbent's active sites, which improves the availability of reactive sites for cyanide binding. Moreover, the higher temperature lowers the solution's viscosity, thereby facilitating cyanide sorption onto nZVI.12,63 Remarkably, almost complete removal (>99%) was achieved at 303 K. The impact of the temperature on the sorption dynamics of cyanide can be analysed using thermodynamic principles, where ΔG°, ΔH°, and ΔS° denote the Gibbs free energy, enthalpy, and entropy, respectively. The values of these thermodynamic parameters were computed using the following equations:
ΔG° = − RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kd kd
| (3) |
 | (4) |
| ΔG° = ΔH° − TΔS° | (5) |
The van't Hoff equation is obtained by combining eqn (4) and (5), as shown below:
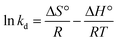 | (6) |
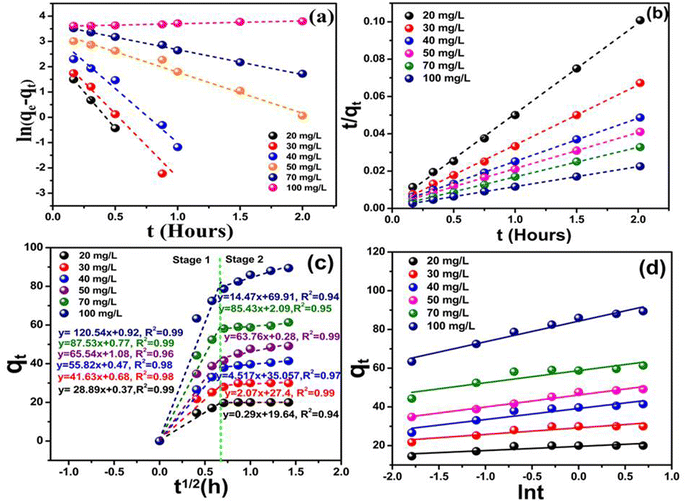
The slope of the thermodynamic plot of 1/T vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kd yielded a positive enthalpy change (ΔH°) of 99.80 kJ mol−1. Fig. 4(e) reveals that the cyanide removal process is endothermic. However, when the temperature was raised to 313 K (Fig. 4(d)), more water molecules desorbed, and the increased molecular energy weakened the attractive forces between them, which subsequently decreased the sorption rate.67 The Gibbs free energy changes (ΔG°) at different temperatures were determined to be −105.86, −107.67, and −109.78 kJ mol−1, as presented in Table 1. The negative ΔG° value indicates that cyanide removal is a spontaneous process, with chemisorption likely being the dominant mechanism. The positive entropy change (ΔS°) suggests an increased degree of disorder at the solid–liquid interface, which may result from structural reorganization as cyanide ions bind to the active sites of the nanocomposite.
kd yielded a positive enthalpy change (ΔH°) of 99.80 kJ mol−1. Fig. 4(e) reveals that the cyanide removal process is endothermic. However, when the temperature was raised to 313 K (Fig. 4(d)), more water molecules desorbed, and the increased molecular energy weakened the attractive forces between them, which subsequently decreased the sorption rate.67 The Gibbs free energy changes (ΔG°) at different temperatures were determined to be −105.86, −107.67, and −109.78 kJ mol−1, as presented in Table 1. The negative ΔG° value indicates that cyanide removal is a spontaneous process, with chemisorption likely being the dominant mechanism. The positive entropy change (ΔS°) suggests an increased degree of disorder at the solid–liquid interface, which may result from structural reorganization as cyanide ions bind to the active sites of the nanocomposite.
| Temperature (K) | Kd | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) |
|---|---|---|---|---|
| 293 | 15.125 | −105.86 | 99.8 | 361.659 |
| 298 | 24.5 | −107.67 | ||
| 303 | 49.5 | −109.78 |
3.6. Regeneration and recycling of the nanocomposite
Reducing the production cost of adsorption materials is crucial, and regeneration methods play an important role in this regard. Acid washing is a widely used technique to restore NMs by removing their passivation layer.68 After four regeneration cycles, the CMC-PVP-nZVI/Pd nanocomposite maintained a high cyanide removal efficiency of 81.25% (Fig. 4(f)), likely due to the protective CMC coating on nZVI. This demonstrates that CMC-PVP-nZVI/Pd is a cost-effective material for treating cyanide-contaminated water. In contrast, the regeneration efficiency of PVP-nZVI/Pd was only 60.6%, lower than that of the complete CMC-PVP-nZVI/Pd composite, confirming that the CMC coating enhances the stability and longevity of nZVI.Although the PVP-nZVI/Pd composite exhibited the highest cyanide removal efficiency (99.82%), its catalytic activity rapidly declined upon reuse due to aggregation and oxidation. The addition of CMC led to a slight decrease in performance (89.44%). This decline is mainly due to the partial coverage of reactive Fe0 sites by CMC molecules, which act as stabilizing agents and restrict the direct contact between Fe0 and cyanide ions. Moreover, the negatively charged carboxyl (–COO−) groups of CMC may repel cyanide anions (CN−), further limiting adsorption and reduction. Despite this reduction in the initial efficiency, the incorporation of CMC provided substantial benefits in terms of stability and recyclability. The CMC coating prevented oxidation, minimized particle aggregation, and maintained catalytic activity over several reuse cycles. Hence, a clear balance emerged. While PVP-nZVI/Pd was more efficient in a single treatment, the CMC-PVP-nZVI/Pd composite offered greater durability and operational reliability, making it more suitable for long-term and cost-effective wastewater treatment.
3.7. Kinetics of cyanide removal using the CMC-PVP-nZVI/Pd nanocomposite
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (qe − qt) = ln (qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − K1t qe − K1t
| (7) |
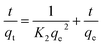 | (8) |
| qt = K3t1/2 + C1 | (9) |
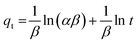 | (10) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t graph. The slope of the line represents
t graph. The slope of the line represents 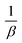 , and the intercept corresponds to
, and the intercept corresponds to 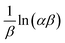 ; the calculated β value is 0.0939, as presented in Table 1. The lower β value observed in this study suggests that the chemisorption process has high feasibility. Similarly, the α value of the adsorption rate is calculated to be 29
; the calculated β value is 0.0939, as presented in Table 1. The lower β value observed in this study suggests that the chemisorption process has high feasibility. Similarly, the α value of the adsorption rate is calculated to be 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319.26 mg g−1 h−1 with an R2 of 0.96. This suggests that the reductive sorption of cyanide on the CMC-PVP-nZVI/Pd nanocomposite is primarily driven by chemisorption rather than physisorption, which is further confirmed by the rapid cyanide reductive sorption process.74 Hence, it can be concluded that cyanide removal on the CMC-PVP-nZVI/Pd nanocomposite probably occurs according to surface exchange reactions until the available sites on the surface are saturated.
319.26 mg g−1 h−1 with an R2 of 0.96. This suggests that the reductive sorption of cyanide on the CMC-PVP-nZVI/Pd nanocomposite is primarily driven by chemisorption rather than physisorption, which is further confirmed by the rapid cyanide reductive sorption process.74 Hence, it can be concluded that cyanide removal on the CMC-PVP-nZVI/Pd nanocomposite probably occurs according to surface exchange reactions until the available sites on the surface are saturated.3.8 Stabilization mechanisms
To prevent the aggregation of nZVI particles and enhance their catalytic activity and conductivity, various stabilization strategies have been developed, with carboxymethyl cellulose (CMC) being one of the most effective. The stabilization process involves several critical stages. The overall mechanism of sequential cyanide and phenolic removal by the CMC-PVP-nZVI/Pd nanocomposite and R. pyridinivorans strain PDB9T is illustrated in Fig. 6. In the initial stage, CMC, a water-soluble polymer derived from cellulose, adsorbs onto the nZVI surface, forming a protective coating. This coating acts as a physical and electrostatic barrier, preventing particle aggregation and shielding nZVI from the rapid oxidation and deactivation caused by direct exposure to the surrounding environment. The incorporation of CMC also helps reduce the dissolution rate of nZVI. By forming a protective coating, CMC not only enhances the reduction capacity of nZVI but also enables the sustained release of reducing agents over an extended period, thereby improving the longevity of nZVI.75 Similarly, PVP is employed as a conductive polymer due to its superior charge storage capacity and low scattering loss49. The PVP coating significantly enhances the stability and reactivity of nZVI particles, resulting in improved pollutant removal efficiency.76 Furthermore, the unique structure of PVP, characterized by rigid pyrrole groups, promotes the formation of complexes with various inorganic compounds, underscoring its suitability for polymeric nanomaterial synthesis, as illustrated in Fig. 6.77 In this study, PVP is employed as both a highly conductive material and a stabiliser to synthesise stable and efficient nZVI. The introduced Pd4+ ions prevent further oxidation of iron particles by accepting electrons and reducing to Pd2+, thereby protecting nZVI from oxidation and facilitating the formation of Fe–Pd bimetallic nanoparticles.29 Palladium not only enhances the reactivity of nZVI but also inhibits atmospheric oxidation on its surface.18,41 | ||
| Fig. 6 Detailed mechanism of sequential cyanide and phenol removal by the CMC-PVP-nZVI/Pd nanocomposite and R. pyridinivorans strain PDB9T. | ||
The overall mechanism of cyanide removal by the CMC-PVP-nZVI/Pd nanocomposite involves Pd-assisted reductive chemisorption. Initially, the XRD peak at 35.41° (440 plane) indicates that CN− ions are chemisorbed onto Fe0 active sites, forming transient Fe–CN surface complexes (Fe4[Fe(CN)6]3).12
| Fe0 + CN− → Fe − CN (surface complexation/chemisorption) | (11) |
The FTIR spectra further support this mechanism. After cyanide uptake, nZVI exhibits an N–H stretching band at 3431 cm−1, confirming the formation of amine groups,12 and a distinct C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching band at 1384 cm−1, confirming cyanide sorption and partial reduction.50 The N–H signal reveals that CN− undergoes reductive transformation to less-toxic amine species, facilitated by Pd catalysis. During synthesis, K2PdCl6 (Pd4+) serves as the palladium precursor and is spontaneously reduced by Fe0 through a two-step reduction process:78–80
N stretching band at 1384 cm−1, confirming cyanide sorption and partial reduction.50 The N–H signal reveals that CN− undergoes reductive transformation to less-toxic amine species, facilitated by Pd catalysis. During synthesis, K2PdCl6 (Pd4+) serves as the palladium precursor and is spontaneously reduced by Fe0 through a two-step reduction process:78–80
| Fe0 + Pd4+ → Fe2+ + Pd2+ | (12) |
| Fe0 + Pd2+ → Fe2+ + Pd0 | (13) |
This reduction leads to the deposition of bimetallic Pd0 nanoparticles on the Fe0 surface, forming a bimetallic Fe–Pd0 structure.81 This is well agreement with the XPS analysis, wherein the deconvoluted XPS spectrum of Pd exhibits two distinct peaks at 335.2 eV and 340.4 eV, corresponding to the Pd 3d5/2 and Pd 3d3/2 orbitals, respectively. These characteristic binding energies confirm that Pd is present in the metallic zero-valent state (Pd0).62
Consequently, the CMC-PVP-nZVI/Pd nanocomposite exhibits the highest cyanide removal of 99% at an initial dose of 100 mg L−1. Following cyanide removal, the filtered sample is subsequently utilized for the next phase of phenol degradation. The degradation of phenol is caused by the phenol monooxygenase enzyme of Rhodococcus pyridinivorans N-1, which converts phenol into hydroquinone. The hydroquinone is then subjected to an oxidation reaction to generate protocatechuic acid by hydroquinone carboxylase. Later, the protocatechuate is converted to beta-carboxy cis–cis muconate by a ring-fission reaction catalysed by the protocatechuate dioxygenase enzyme system. This is followed by several decarboxylation and oxidation reactions to generate TCA cycle intermediates, ultimately leading to complete mineralization into CO2 and H2O.7 This enzymatic sequence transforms phenol into less-toxic intermediates and finally into harmless end products.
4. Conclusion
In this study, the synthesized nanomaterials (NMs) were characterized and evaluated for cyanide removal. The FTIR and XPS analyses confirmed that the functional groups, such as hydroxyl, carbonyl, and pyrrolidone groups, of CMC and PVP chemically interacted with the Fe/Pd surface through Fe–O–C and Fe–N linkages. This coordination improved electron delocalization between Fe0 and Pd, increased the accessibility of active sites, and enhanced the overall redox reactivity of the nanocomposite. The coating of polymers also provided both steric and electrostatic stabilization that successfully inhibited nZVI aggregation, as confirmed by FE-SEM analysis. The stabilized CMC-PVP-nZVI/Pd nanocomposite achieved more than 99% cyanide removal at an initial concentration of 100 mg L−1 at 30 °C, demonstrating 1.8-fold higher efficiency than bare nZVI. Kinetic studies revealed that cyanide removal by CMC-PVP-nZVI/Pd followed a pseudo-second-order model, indicating a chemisorption mechanism with a high sorption capacity of 93.37 mg g−1. While PVP-nZVI/Pd exhibited the maximum cyanide removal efficiency, the CMC-PVP-nZVI/Pd nanocomposite showed superior regeneration performance, highlighting the role of CMC in enhancing the stability and longevity of nZVI. This balance between the initial efficiency and durability underscores its practical value, providing a sustainable and reusable solution for continuous industrial wastewater treatment. Thermodynamic analysis further confirmed the spontaneity of the sorption process (negative ΔG°) and its endothermic nature (positive ΔH°). In parallel, the isolated strain Rhodococcus pyridinivorans PDB9T demonstrated effective phenol removal from partially treated wastewater. Together, this sequential nano-bio treatment system presents a promising, economical, and environmentally sustainable strategy for treating hazardous industrial effluents.Author contributions
Ankita Priyadarshini: conduct experiments, investigation, methodology, formal analysis, validation, data curation, writing – original draft. Naresh Kumar Sahoo: investigation, conceptualization, supervision, analyzed sample and data, writing draft manuscript, writing – review and editing, funding acquisition, project administration, funding resources. Soumya Mishra: conduct experiments, investigation, methodology, formal analysis, validation, data curation, and writing – original draft. Prasanta Kumar Sahoo: conceptualization, analyzed sample and data, writing draft manuscript, formal analysis, review & editing. Ranjan Kumar Bhuyan: conceptualization, supervision, visualization, data curation, formal analysis, review, and editing. Prangya Ranjan Rout: conceptualization, supervision, visualization, data curation, formal analysis, review, and editing. Bankim Chandra Tripathy: conceptualization, supervision, visualization, data curation, formal analysis, review, and editing.Conflicts of interest
There are no conflicts to declare.Data availability
Data will be made available on request.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5na00920k.
Acknowledgements
The authors acknowledge the financial support received from the Siksha ‘O’Anusandhan (Deemed to be University), Bhubaneswar 751030, Odisha, India, for conducting this research work.References
- N. K. Sharma and L. Philip, Combined biological and photocatalytic treatment of real coke oven wastewater, Chem. Eng. J., 2016, 295, 20–28 CrossRef CAS.
- X. Xiang, M. Zhang, Q. Huang, Y. Mao, J. Jia, X. Zeng, Y. Dong, J. Liao, X. Chen and X. Yao, et.al, Construction of S-scheme CuInS2/ZnIn2S4 heterostructures for enhanced photocatalytic activity towards Cr (VI) removal and antibiotics degradation, Chemosphere, 2024, 352, 141351 CrossRef CAS PubMed.
- N. Panigrahy, M. Barik and N. K. Sahoo, Kinetics of phenol biodegradation by an indigenous Pseudomonas citronellolis NS1 isolated from coke oven wastewater, J. Hazard., Toxic Radioact. Waste, 2020, 24, 4020019 CrossRef CAS.
- J. Mishra, D. S. Pattanayak, A. A. Das, D. K. Mishra, D. Rath and N. K. Sahoo, Enhanced photocatalytic degradation of cyanide employing Fe-porphyrin sensitizer with hydroxyapatite palladium doped TiO2 nano-composite system, J. Mol. Liq., 2019, 287, 110821 CrossRef CAS.
- D. S. Pattanayak, J. Mishra, J. Nanda, P. K. Sahoo, R. Kumar and N. K. Sahoo, Photocatalytic degradation of cyanide using polyurethane foam immobilized Fe-TCPP-S-TiO2-rGO nano-composite, J. Environ. Manage., 2021, 297, 113312 CrossRef CAS PubMed.
- S. Bhattacharya, A. A. Das, G. C. Dhal, P. K. Sahoo, A. Tripathi and N. K. Sahoo, Evaluation of N doped rGO-ZnO-CoPc (COOH)8 nanocomposite in cyanide degradation and its bactericidal activities, J. Environ. Manage., 2022, 302, 114022 CrossRef CAS PubMed.
- M. Barik, C. P. Das, A. K. Verma, S. Sahoo and N. K. Sahoo, Metabolic profiling of phenol biodegradation by an indigenous Rhodococcus pyridinivorans strain PDB9T N-1 isolated from paper pulp wastewater, Int. Biodeterior. Biodegrad., 2021, 158, 105168 CrossRef CAS.
- A. K. Bakthavatsalam, et.al, A comparative study on growth and degradation behavior of C. pyrenoidosa on synthetic phenol and phenolic wastewater of a coal gasification plant, J. Environ. Chem. Eng., 2019, 7, 103079 CrossRef.
- S. K. Sahoo, A. A. Das, D. Deka, B. Naik and N. K. Sahoo, Organic-inorganic hybrid hydroquinone bridged V-CdS/HAP/Pd-TCPP: A novel visible light active photocatalyst for phenol degradation, J. Mol. Liq., 2021, 339, 116721 CrossRef CAS.
- T. Felföldi, Z. Nagymáté, A. J. Székely, L. Jurecska and K. Márialigeti, Biological treatment of coke plant effluents: from a microbiological perspective, Biol. Futura, 2020, 71, 359–370 CrossRef PubMed.
- S. C. Curry and M. B. Spyres, Cyanide: hydrogen cyanide, inorganic cyanide salts, and nitriles, in Critical Care Toxicology, Springer, 2015, pp. 1–21 Search PubMed.
- M. Tyagi, A. Rana, S. Kumari and S. Jagadevan, Adsorptive removal of cyanide from coke oven wastewater onto zero-valent iron: Optimization through response surface methodology, isotherm and kinetic studies, J. Clean. Prod., 2018, 178, 398–407 CrossRef CAS.
- A. Alvillo-Rivera, S. Garrido-Hoyos, G. Buitrón, P. Thangarasu-Sarasvathi and G. Rosano-Ortega, Biological treatment for the degradation of cyanide: A review, J. Mater. Res. Technol., 2021, 12, 1418–1433 CrossRef CAS.
- U. Singh, N. K. Arora and P. Sachan, Simultaneous biodegradation of phenol and cyanide present in coke-oven effluent using immobilized Pseudomonas putida and Pseudomonas stutzeri, Braz. J. Microbiol., 2018, 49, 38–44 CrossRef CAS PubMed.
- J. Chen, H. Luo, D. Luo, Y. Chen, J. Tang, H. Ma and S. Pu, New insights into the degradation of nitrobenzene by activated persulfate with sulfidated nanoscale zero-valent iron: Synergistic effects of reduction and reactive oxygen species oxidation, Sep. Purif. Technol., 2023, 322, 124252 CrossRef CAS.
- Y. Zou, X. Wang, A. Khan, P. Wang, Y. Liu, A. Alsaedi, T. Hayat and X. Wang, Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: a review, Environ. Sci. Technol., 2016, 50, 7290–7304 CrossRef CAS PubMed.
- D. O'Carroll, B. Sleep, M. Krol, H. Boparai and C. Kocur, Nanoscale zero valent iron and bimetallic particles for contaminated site remediation, Adv. Water Resour., 2013, 51, 104–122 CrossRef.
- Y. Gao, F. Wang, Y. Wu, R. Naidu and Z. Chen, Comparison of degradation mechanisms of microcystin-LR using nanoscale zero-valent iron (nZVI) and bimetallic Fe/Ni and Fe/Pd nanoparticles, Chem. Eng. J., 2016, 285, 459–466 CrossRef CAS.
- M. Fu, Q. Jiao and Y. Zhao, One-step vapor diffusion synthesis of uniform CdS quantum dots/reduced graphene oxide composites as efficient visible-light photocatalysts, RSC Adv., 2014, 4, 23242–23250 RSC.
- R. Eljamal, O. Eljamal, I. Maamoun, G. Yilmaz and Y. Sugihara, Enhancing the characteristics and reactivity of nZVI: Polymers effect and mechanisms, J. Mol. Liq., 2020, 315, 113714 CrossRef CAS.
- J. Shi, J. Wang, W. Wang, W. Teng and W. Zhang, Stabilization of nanoscale zero-valent iron in water with mesoporous carbon (nZVI@ MC), J. Environ. Sci., 2019, 81, 28–33 CrossRef CAS PubMed.
- J. Deng, H. Dong, C. Zhang, Z. Jiang, Y. Cheng, K. Hou, L. Zhang and C. Fan, Nanoscale zero-valent iron/biochar composite as an activator for Fenton-like removal of sulfamethazine, Sep. Purif. Technol., 2018, 202, 130–137 CrossRef CAS.
- C. Wang, H. Luo, Z. Zhang, Y. Wu, J. Zhang and S. Chen, Removal of As (III) and As (V) from aqueous solutions using nanoscale zero valent iron-reduced graphite oxide modified composites, J. Hazard. Mater., 2014, 268, 124–131 CrossRef CAS PubMed.
- E. Lefevre, N. Bossa, M. R. Wiesner and C. K. Gunsch, A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): behavior, transport and impacts on microbial communities, Sci. Total Environ., 2016, 565, 889–901 CrossRef CAS PubMed.
- H. Dong, F. Zhao, G. Zeng, L. Tang, C. Fan, L. Zhang, Y. Zeng, Q. He, Y. Xie and Y. Wu, Aging study on carboxymethyl cellulose-coated zero-valent iron nanoparticles in water: Chemical transformation and structural evolution, J. Hazard. Mater., 2016, 312, 234–242 CrossRef CAS PubMed.
- T. Phenrat, N. Saleh, K. Sirk, H.-J. Kim, R. D. Tilton and G. V Lowry, Stabilization of aqueous nanoscale zerovalent iron dispersions by anionic polyelectrolytes: adsorbed anionic polyelectrolyte layer properties and their effect on aggregation and sedimentation, J. Nanoparticle Res., 2008, 10, 795–814 CrossRef CAS.
- T. Phenrat, N. Saleh, K. Sirk, R. D. Tilton and G. V Lowry, Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions, Environ. Sci. Technol., 2007, 41, 284–290 CrossRef CAS PubMed.
- Y. Xie, Y. Yi, Y. Qin, L. Wang, G. Liu, Y. Wu, Z. Diao, T. Zhou and M. Xu, Perchlorate degradation in aqueous solution using chitosan-stabilized zero-valent iron nanoparticles, Sep. Purif. Technol., 2016, 171, 164–173 CrossRef CAS.
- F. He and D. Zhao, Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers, Environ. Sci. Technol., 2007, 41, 6216–6221 CrossRef CAS PubMed.
- H. Wang, C. Jin, X. Li, J.-X. Ma, Y.-F. Ye, L.-X. Tang, J. Si and B.-K. Cui, A green biocatalyst fabricated by fungal laccase immobilized onto Fe3O4@ polyaniline-chitosan nanofibrous composites for the removal of phenolic compounds, Chem. Eng. J., 2025, 507, 160486 CrossRef CAS.
- J. Y. Lu, Z. Q. Bu, Y. Q. Lei, D. Wang, B. He, J. Wang and W. T. Huang, Facile microwave-assisted synthesis of Sb2O3-CuO nanocomposites for catalytic degradation of p-nitrophenol, J. Mol. Liq., 2024, 409, 125503 CrossRef CAS.
- Y. Li, J. Bu, Y. Sun, Z. Huang, X. Zhu, S. Li, P. Chen, Y. Tang, G. He and S. Zhong, Efficient degradation of norfloxacin by synergistic activation of PMS with a three-dimensional electrocatalytic system based on Cu-MOF, Sep. Purif. Technol., 2025, 356, 129945 CrossRef CAS.
- J. Xie, C. Lei, W. Chen and B. Huang, Conductive-polymer-supported palladium-iron bimetallic nanocatalyst for simultaneous 4-chlorophenol and Cr (VI) removal: Enhanced interfacial electron transfer and mechanism, J. Hazard. Mater., 2022, 424, 127748 CrossRef CAS PubMed.
- F. He, D. Zhao, J. Liu and C. B. Roberts, Stabilization of Fe- Pd nanoparticles with sodium carboxymethyl cellulose for enhanced transport and dechlorination of trichloroethylene in soil and groundwater, Ind. Eng. Chem. Res., 2007, 46, 29–34 CrossRef CAS.
- Z.-B. Wang, J. Zhang, Q. Miao, H.-Y. Cao, F. Xiong, T. Lee, A. El-Baz, L. Xie and S.-Q. Ni, Achieving stable partial denitrification by selective inhibition of nitrite reductase with the biosafe aprotic solvent DMSO, Environ. Sci. Technol., 2024, 58, 21242–21250 CrossRef CAS PubMed.
- Z. Qi, S. Wen, Z. Liu and D. Jiang, Oxygen-promoted 6-endo-trig cyclization of $β$, $γ$-unsaturated hydrazones/ketoximes with diazonium tetrafluoroborates for pyridazin-4 (1 H)-ones/oxazin-4 (1 H)-ones, Org. Lett., 2023, 25, 6110–6115 CrossRef CAS PubMed.
- W. Shen, X. Wang, F. Jia, Z. Tong, H. Sun, X. Wang, F. Song, Z. Ai, L. Zhang and B. Chai, Amorphization enables highly efficient anaerobic thiamphenicol reduction by zero-valent iron, Appl. Catal., B, 2020, 264, 118550 CrossRef CAS.
- A. Priyadarshini, S. Mishra, N. K. Sahoo, S. Raut, A. Daverey and B. C. Tripathy, Biodegradation of phenol using the indigenous Rhodococcus pyridinivorans Strain PDB9T NS-1 immobilized in calcium alginate beads, Appl. Biochem. Biotechnol., 2024, 196, 2798–2818 CrossRef CAS PubMed.
- S. Mishra, N. K. Sahoo, P. K. Sahoo, S. Sahoo, L. Nayak and P. R. Rout, Construction of a novel ternary synergistic CuFe2O4-SnO2-rGO heterojunction for efficient removal of cyanide from contaminated water, RSC Adv., 2024, 14, 13850–13861 RSC.
- S. Mandal, S. Pu, L. Shangguan, S. Liu, H. Ma, S. Adhikari and D. Hou, Synergistic construction of green tea biochar supported nZVI for immobilization of lead in soil: A mechanistic investigation, Environ. Int., 2020, 135, 105374 CrossRef CAS PubMed.
- C. H. Nguyen, M. L. Tran, T. T. Van Tran and R.-S. Juang, Efficient removal of antibiotic oxytetracycline from water by Fenton-like reactions using reduced graphene oxide-supported bimetallic Pd/nZVI nanocomposites, J. Taiwan Inst. Chem. Eng., 2021, 119, 80–89 CrossRef CAS.
- C. He, Y. Ding, C. Li, W. Yan, A. Mao, S. Wei and M. Li, Cost-effective core@ shell structured zero-valent iron nanoparticles@ magnetic (nZVI@ Fe3O4) for Cr (VI) removal from aqueous solutions: preparation by disproportionation of Fe (ii), RSC Adv., 2023, 13, 26983–26994 RSC.
- M. Liu, Y. Ye, L. Xu, T. Gao, A. Zhong and Z. Song, Recent advances in nanoscale zero-valent iron (nZVI)-based advanced oxidation processes (AOPs): Applications, mechanisms, and future prospects, Nanomaterials, 2023, 13, 2830 CrossRef CAS PubMed.
- F. Jiao, J.-C. Jumas, M. Womes, A. V Chadwick, A. Harrison and P. G. Bruce, Synthesis of ordered mesoporous Fe3O4 and $γ$-Fe2O3 with crystalline walls using post-template reduction/oxidation, J. Am. Chem. Soc., 2006, 128, 12905–12909 CrossRef CAS PubMed.
- R. Singh, V. Misra and R. P. Singh, Synthesis, characterization and role of zero-valent iron nanoparticle in removal of hexavalent chromium from chromium-spiked soil, J. Nanoparticle Res., 2011, 13, 4063–4073 CrossRef CAS.
- L. Qian, S. Liu, W. Zhang, Y. Chen, D. Ouyang, L. Han, J. Yan and M. Chen, Enhanced reduction and adsorption of hexavalent chromium by palladium and silicon rich biochar supported nanoscale zero-valent iron, J. Colloid Interface Sci., 2019, 533, 428–436 CrossRef CAS PubMed.
- M. Sarmah, A. B. Neog, P. K. Boruah, M. R. Das, P. Bharali and U. Bora, Effect of substrates on catalytic activity of biogenic palladium nanoparticles in C--C cross-coupling reactions, ACS Omega, 2019, 4, 3329–3340 CrossRef CAS PubMed.
- I. A. Safo, M. Werheid, C. Dosche and M. Oezaslan, The role of polyvinylpyrrolidone (PVP) as a capping and structure-directing agent in the formation of Pt nanocubes, Nanoscale Adv., 2019, 1, 3095–3106 RSC.
- L. Chen, T. Yuan, R. Ni, Q. Yue and B. Gao, Multivariate optimization of ciprofloxacin removal by polyvinylpyrrolidone stabilized NZVI/Cu bimetallic particles, Chem. Eng. J., 2019, 365, 183–192 CrossRef CAS.
- H. Uppal, S. S. Tripathy, S. Chawla, B. Sharma, M. K. Dalai, S. P. Singh, S. Singh and N. Singh, Study of cyanide removal from contaminated water using zinc peroxide nanomaterial, J. Environ. Sci., 2017, 55, 76–85 CrossRef CAS PubMed.
- H. Khorsandi, A. Azarnioush, A.-A. Aghapour, S. Nemati, S. Karimzadeh and H.-R. Khalkhali, An analysis of boron removal from water using modified zero-valent iron nanoparticles, Desalin. Water Treat., 2017, 70, 284–289 CrossRef CAS.
- Y.-H. Shih, C.-P. Tso and L.-Y. Tung, Rapid degradation of methyl orange with nanoscale zerovalent iron particles, Nanotechnology, 2010, 7, 7 Search PubMed.
- H. K. Boparai, M. Joseph and D. M. O'Carroll, Cadmium (Cd 2+) removal by nano zerovalent iron: surface analysis, effects of solution chemistry and surface complexation modeling, Environ. Sci. Pollut. Res., 2013, 20, 6210–6221 CrossRef CAS PubMed.
- A. Saberinasr, M. Rezaei, M. Nakhaei and S. M. Hosseini, Transport of CMC-stabilized nZVI in saturated sand column: The effect of particle concentration and soil grain size, Water Air Soil Pollut., 2016, 227, 1–16 CrossRef CAS.
- D. Edikresnha, I. Sriyanti, M. M. Munir, et.al, in IOP Conference Series: Materials Science and Engineering, 2017, vol. 202, p. 12043 Search PubMed.
- H. Zhou, M. Ma, Y. Zhao, S. A. Baig, S. Hu, M. Ye and J. Wang, Integrated green complexing agent and biochar modified nano zero-valent iron for hexavalent chromium removal: A characterisation and performance study, Sci. Total Environ., 2022, 834, 155080 CrossRef CAS PubMed.
- W. Guo, J. Duan, Z. Shi, X. Yu and Z. Shao, Biodegradation of PET by the membrane-anchored PET esterase from the marine bacterium Rhodococcus pyridinivorans P23, Commun. Biol., 2023, 6, 1090 CrossRef CAS PubMed.
- J.-L. Zhu, M.-L. Wang, S.-C. Shi, J.-X. Ren, H.-D. Huang, W. Lin and Z.-M. Li, In-situ constructing robust cellulose nanocomposite hydrogel network with well-dispersed dual catalysts for the efficient, stable and recyclable photo-Fenton degradation, Cellulose, 2022, 29, 1929–1942 CrossRef CAS.
- R. Shamshirgaran, R. Malakooti, A. Akbarpour and A. Z. Moghaddam, Fabrication of Polyvinylpyrrolidone-Stabilized Nano Zero-Valent Iron Supported by Hydrophilic Biochar for Efficient Cr (VI) Removal from Groundwater, ChemistrySelect, 2022, 7, e202202927 CrossRef CAS.
- B. Zhang, J. Zhan, J. Fan, B. Zhu, W. Shen, S. Zhang, W. Li, Z. Li and F. Zeng, Application of Carboxymethyl Cellulose (CMC)-Coated Nanoscale Zero-Valent Iron in Chromium-Containing Soil Remediation, Clean Technol., 2024, 6, 1610–1624 CrossRef.
- Y. O. Al-Ghamdi, M. Jabli, M. H. Alhalafi, A. Khan and K. A. Alamry, Hybridized sulfated-carboxymethyl cellulose/MWNT nanocomposite as highly selective electrochemical probe for trace detection of arsenic in real environmental samples, RSC Adv., 2023, 13, 18382–18395 RSC.
- Z. Zhu, S. Liang, H. Sun, W. Zhang, J. Yang and Z. Gao, Magnetic Pd--Fe nanoparticles for sustainable Suzuki--Miyaura cross-coupling reactions, Catal. Sci. Technol., 2024, 14, 3176–3183 RSC.
- A. Jena, B. Mahanty, D. Deka, P. K. Sahoo, S. Pradhan, P. R. Rout, S. Mishra and N. K. Sahoo, Green synthesis of a potential magnetic and mesoporous EG-nZVI/CA-MCM41 nanocomposite for reductive sorption of europium, Environ. Sci. Nano, 2024, 11, 855–869 RSC.
- L. Gong, S. Shi, N. Lv, W. Xu, Z. Ye, B. Gao, D. M. O’carroll and F. He, Sulfidation enhances stability and mobility of carboxymethyl cellulose stabilized nanoscale zero-valent iron in saturated porous media, Sci. Total Environ., 2020, 718, 137427 CrossRef CAS PubMed.
- G.-H. Han, S.-H. Lee, M. Seo and K.-Y. Lee, Effect of polyvinylpyrrolidone (PVP) on palladium catalysts for direct synthesis of hydrogen peroxide from hydrogen and oxygen, RSC Adv., 2020, 10, 19952–19960 RSC.
- B. Agarwal, C. Balomajumder and P. K. Thakur, Simultaneous co-adsorptive removal of phenol and cyanide from binary solution using granular activated carbon, Chem. Eng. J., 2013, 228, 655–664 CrossRef CAS.
- J. E. Asuquo, I. S. Udegbunam and E. E. Etim, Effect of temperature on the adsorption of metallic soaps of castor seed oil onto haematite, Int. J. Adv. Res. Chem. Sci, 2017, 4, 345–353 Search PubMed.
- J. Yang, L. Zhou, F. Ma, H. Zhao, F. Deng, S. Pi, A. Tang and A. Li, Magnetic nanocomposite microbial extracellular polymeric substances@ Fe3O4 supported nZVI for Sb (V) reduction and adsorption under aerobic and anaerobic conditions, Environ. Res., 2020, 189, 109950 CrossRef CAS PubMed.
- A. Jena, N. K. Sahoo, P. K. Sahoo, S. Mishra, P. R. Rout and Y. Zhong, Design of a novel green synthesized ZVS/S-rGO-Deinococcus radiodurans R1 chitosan hydrogel beads for enhanced recovery of europium, J. Clean. Prod., 2025, 145367 CrossRef CAS.
- F. Liu, W. Huang, S. Wang and B. Hu, Investigation of adsorption properties and mechanism of uranium (VI) and europium (III) on magnetic amidoxime-functionalized MCM-41, Appl. Surf. Sci., 2022, 594, 153376 CrossRef CAS.
- N. Singh and C. Balomajumder, Phytoremediation potential of water hyacinth (Eichhornia crassipes) for phenol and cyanide elimination from synthetic/simulated wastewater, Appl. Water Sci., 2021, 11, 144 CrossRef CAS.
- Y. Huang, X. Lee, M. Grattieri, F. C. Macazo, R. Cai and S. D. Minteer, A sustainable adsorbent for phosphate removal: modifying multi-walled carbon nanotubes with chitosan, J. Mater. Sci., 2018, 53, 12641–12649 CrossRef CAS.
- K. Riahi, S. Chaabane and B. Ben Thayer, A kinetic modeling study of phosphate adsorption onto Phoenix dactylifera L. date palm fibers in batch mode, J. Saudi Chem. Soc., 2017, 21, S143–S152 CrossRef CAS.
- P. Zong, M. Shao, D. Cao, X. Xu, S. Wang and H. Zhang, Synthesis of potential Ca--Mg--Al layered double hydroxides coated graphene oxide composites for simultaneous uptake of europium and fulvic acid from wastewater systems, Environ. Res., 2021, 196, 110375 CrossRef CAS PubMed.
- X. Kong, L. Xuan, Y. Fu, F. Yuan and C. Qin, Effect of the modification sequence on the reactivity, electron selectivity, and mobility of sulfidated and CMC-stabilized nanoscale zerovalent iron, Sci. Total Environ., 2021, 793, 148487 CrossRef CAS PubMed.
- J. Li, M. Fan, M. Li and X. Liu, Cr (VI) removal from groundwater using double surfactant-modified nanoscale zero-valent iron (nZVI): Effects of materials in different status, Sci. Total Environ., 2020, 717, 137112 CrossRef CAS PubMed.
- S. Bubel, N. Mechau and R. Schmechel, Electronic properties of polyvinylpyrrolidone at the zinc oxide nanoparticle surface: PVP in ZnO dispersions and nanoparticulate ZnO thin films for thin film transistors, J. Mater. Sci., 2011, 46, 7776–7783 CrossRef CAS.
- S. Park, J. E. Zenobio and L. S. Lee, Perfluorooctane sulfonate (PFOS) removal with Pd0/nFe0 nanoparticles: adsorption or aqueous Fe-complexation, not transformation?, J. Hazard. Mater., 2018, 342, 20–28 CrossRef CAS PubMed.
- H.-L. Lien and W. Zhang, Nanoscale iron particles for complete reduction of chlorinated ethenes, Colloids Surf., A, 2001, 191, 97–105 CrossRef CAS.
- C. Noubactep, On the operating mode of bimetallic systems for environmental remediation, J. Hazard. Mater., 2009, 164, 394–395 CrossRef CAS PubMed.
- X. Wang, C. Chen, Y. Chang and H. Liu, Dechlorination of chlorinated methanes by Pd/Fe bimetallic nanoparticles, J. Hazard. Mater., 2009, 161, 815–823 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |