 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tailored synthesis and morphological analysis of Mo2CTx and Ti3C2Tx MXenes: a study on multilayered and delaminated architectures
Vasanth
Magesh†
a,
Raji
Atchudan†
b,
Sandeep
Arya
 c,
Surendra H.
Mahadevegowda
c,
Surendra H.
Mahadevegowda
 d and
Ashok K.
Sundramoorthy
d and
Ashok K.
Sundramoorthy
 *a
*a
aDepartment of Prosthodontics and Materials Science, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Chennai, 600077, Tamil Nadu, India. E-mail: ashok.sundramoorthy@gmail.com
bSchool of Chemical Engineering, Yeungnam University, Gyeongsan 38541, Republic of Korea
cDepartment of Physics, University of Jammu, Jammu 180006, Jammu and Kashmir, India
dDepartment of Chemistry, School of Sciences, National Institute of Technology Andhra Pradesh, Tadepalligudem 534101, Andhra Pradesh, India
First published on 3rd November 2025
Abstract
MXenes, a family of two-dimensional transition metal carbides, nitrides, and carbonitrides, display high conductivity, large surface area, hydrophilicity, and biocompatibility. This work demonstrates a comparative scalable synthesis of multilayer (m-MXene) and delaminated (d-MXene) Mo2CTx and Ti3C2Tx from their MAX phases (Mo2Ga2C and Ti3AlC2) using optimized HCl/HF/DI water etchants (ratios of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for Mo2Ga2C and 6
1 for Mo2Ga2C and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for Ti3AlC2) via an LiCl-assisted delamination strategy. Significant interlayer expansion is confirmed by XRD (002) peak shifts (from 9.81° to 8.03° for Mo2CTx and from 9.51° to 8.80° for Ti3C2Tx), while their lateral sizes reached 2–5 µm for Ti3C2Tx and 0.5–1 µm for Mo2CTx. The UV-Vis spectra showed characteristic absorption peaks at 209, 230, 295, and 568 nm for Mo2CTx and at 263, 325, and 798 nm for Ti3C2Tx, confirming their delamination and distinctive electronic structure. Thorough structural and compositional characterizations (UV-Vis spectroscopy, XRD, HR-SEM, EDS, Raman spectroscopy, and FTIR spectroscopy) verified the successful synthesis of MXenes. This study provides the first direct systematic comparison of Mo2CTx and Ti3C2Tx MXenes, establishing benchmarks for the scalable production and applications of MXenes.
3 for Ti3AlC2) via an LiCl-assisted delamination strategy. Significant interlayer expansion is confirmed by XRD (002) peak shifts (from 9.81° to 8.03° for Mo2CTx and from 9.51° to 8.80° for Ti3C2Tx), while their lateral sizes reached 2–5 µm for Ti3C2Tx and 0.5–1 µm for Mo2CTx. The UV-Vis spectra showed characteristic absorption peaks at 209, 230, 295, and 568 nm for Mo2CTx and at 263, 325, and 798 nm for Ti3C2Tx, confirming their delamination and distinctive electronic structure. Thorough structural and compositional characterizations (UV-Vis spectroscopy, XRD, HR-SEM, EDS, Raman spectroscopy, and FTIR spectroscopy) verified the successful synthesis of MXenes. This study provides the first direct systematic comparison of Mo2CTx and Ti3C2Tx MXenes, establishing benchmarks for the scalable production and applications of MXenes.
1 Introduction
MXenes are two-dimensional (2-D) transition metal carbides, nitrides, and carbonitrides with the general formula of Mn+1XnTx. In this formula, “M” represents an early transition metal (such as Ti, Mo, V, Sc, Nb, and Cr), “X” is carbon and/or nitrogen, “n” is an integer from 1 to 4 indicating the layer thickness, and “Tx” represents surface functional groups (–F, –O, and –OH) introduced during the wet chemical etching process, where “A” is typically a group 13 or 14 element, except boron (B) and carbon (C).1,2 MXenes have gained global research interest for their potential in energy storage, photovoltaics, nanoelectronics, and optoelectronics,3,4 motivating fundamental studies on their synthesis, structure, and surface chemistry. Both stacked multilayer and single-layer delaminated MXenes have attracted tremendous attention in many fields including supercapacitors, sensors, EMI shielding, actuators, field-effect transistors, photothermal therapy, and cancer treatment.5–12 Their unique properties, including high electrical conductivity,13 metallic nature,14 hydrophilicity,15 biocompatibility,16,17 and large surface area, make MXenes highly valuable across these research fields.18 Recent publications have highlighted Ti3C2Tx, Mo2CTx, and other related MXenes and their applications in adsorption,19 antimicrobial activity,20 environmental remediation,21 water treatment and pollution control.21,22 To date, over 62 distinct MXenes have been synthesized experimentally, and many others have been theoretically examined.23–25 However, the majority of research to date has focused on Ti3C2Tx.26,27Various synthesis methods have been developed to etch the MAX phase for producing multilayer MXenes (m-MXenes) and to utilize intercalating agents for delaminating m-MXenes into few-layer or monolayer MXenes (d-MXenes).28 The etching and delamination processes significantly influence the shape, size, morphology, and surface chemistry of MXenes, which in turn affect their processing and performance.14,29–31 Wet chemical etching is the most commonly used method for preparing MXenes. In this approach, immersing MAX phases in concentrated hydrofluoric acid (HF) leads to the separation of layers and the introduction of –OH and –F terminal groups.32 During this wet etching, ‘A’-layer is gradually removed to produce m-MXenes, while organic agents (e.g., TBAOH and DMSO) or inorganic compounds (e.g., cationic surfactants) are used to delaminate m-MXenes into d-MXenes.28 However, HF-etched MXenes often exhibit small lateral sizes and structural defects.33,34 To address these issues, a minimally intensive layer delamination (MILD) method was adopted to produce high-quality MXenes, which was based on in situ HF generated using LiF, HCl and bifluoride-based etchants (NH4HF2, NaHF2, and KHF2).35–39 These methods have their own strengths and limitations. MILD etching requires extended durations at room temperature (RT) or shorter times at elevated temperatures and typically involves organic intercalation or ultrasonication for successful delamination. In contrast, the in situ HF method produces clay-like aggregated m-MXenes, and delamination into d-MXenes can be achieved using deionized (DI) water by a simple hand-shaking liquid-assisted process (no need for organic intercalation or ultrasonication).28,40 However, the in situ formation of HF leads to a slower etching rate and sometimes incomplete removal of the A-layer, which may leave residual A-site elements in the MXene structure due to its less aggressive nature compared to concentrated HF. Therefore, longer etching durations are required, which can affect the overall quality and yield of the resulting MXene.41 Additionally, the etching process can generate unwanted byproducts such as salts (e.g., LiCl and GaF3) or gel-like residues during the washing process, which are difficult to remove completely and may also interfere with the delamination process and the final application of MXenes.42–44 Bifluoride-based etching methods require additional steps to remove the residual inorganic cations after the etching process, especially when intended for specific applications.45,46 Furthermore, Mathis et al. emphasized that the production of high-quality m-MXenes and d-MXenes depends not only on optimized etching methods but also on the quality of the starting MAX phase.47 They had demonstrated that synthesizing Ti3AlC2 with an excess of aluminum enhances the structural integrity and morphology of MAX phase grains. This approach resulted in high-quality Al–Ti3C2Tx MXene nanosheets with superior chemical stability, allowing storage for up to 10 months without significant oxidation or degradation.47 This study highlights the importance of optimizing both the MAX phase synthesis protocols and etching processes to achieve consistent and reliable MXene production.
Despite notable progress in MXene synthesis methodologies, significant research gaps persist, particularly in conducting systematic comparative studies among different MXene types and optimizing synthesis protocols for less-explored families such as Mo2CTx. Most existing studies focus on individual MXenes, which restricts our understanding of how synthesis parameters should be customized for distinct MAX phase precursors with diverse chemical compositions. Moreover, the correlation between precursor properties and the resulting d-MXene morphology remains poorly elucidated.
To bridge these gaps, the present study reports the first comprehensive comparative synthesis and characterization of Mo2CTx and Ti3C2Tx MXenes under identical experimental conditions. The novelty of this work is fourfold: (1) optimization of etchant composition (HCl : HF : DI water), determining ideal ratios (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for Mo2Ga2C and 6
1 for Mo2Ga2C and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for Ti3AlC2) based on the intrinsic chemical differences between Mo–Ga and Ti–Al bonding in their respective MAX phases; (2) detailed morphological and structural comparison of Mo- and Ti-based MXenes throughout the synthesis pathway (MAX → m-MXene → d-MXene); (3) successful fabrication of d-MXenes via LiCl intercalation alone, removing the need for organic intercalants, ultrasonication, or other aggressive delamination treatments; and (4) systematic evaluation of precursor influence on final d-MXene lateral dimensions, revealing that Ti3C2Tx MXenes exhibit 2–5 µm lateral sizes, whereas Mo2CTx MXenes achieve 0.5–1 µm when subjected to optimized HCl/HF/DI water etching followed by LiCl-assisted delamination at room temperature. This integrated approach effectively promotes interlayer expansion and enables direct side-by-side assessment of Mo- and Ti-based MXenes, offering valuable insights into how etching and delamination conditions affect MXene structural integrity.
3 for Ti3AlC2) based on the intrinsic chemical differences between Mo–Ga and Ti–Al bonding in their respective MAX phases; (2) detailed morphological and structural comparison of Mo- and Ti-based MXenes throughout the synthesis pathway (MAX → m-MXene → d-MXene); (3) successful fabrication of d-MXenes via LiCl intercalation alone, removing the need for organic intercalants, ultrasonication, or other aggressive delamination treatments; and (4) systematic evaluation of precursor influence on final d-MXene lateral dimensions, revealing that Ti3C2Tx MXenes exhibit 2–5 µm lateral sizes, whereas Mo2CTx MXenes achieve 0.5–1 µm when subjected to optimized HCl/HF/DI water etching followed by LiCl-assisted delamination at room temperature. This integrated approach effectively promotes interlayer expansion and enables direct side-by-side assessment of Mo- and Ti-based MXenes, offering valuable insights into how etching and delamination conditions affect MXene structural integrity.
Overall, this study establishes the first unified comparative framework that links synthesis parameters, structural evolution, and morphological characteristics across different MXene families. The findings provide critical benchmarks for optimizing MXene synthesis and deepen the understanding of how MAX phase chemistry dictates the properties of derived 2D MXenes. This comparative methodology is anticipated to advance the rational design of synthesis strategies for emerging MXene compositions, facilitating more predictable control over their structure and performance for diverse applications.
2 Experimental
2.1 Chemicals and reagents
Titanium aluminum carbide (Ti3AlC2) and molybdenum gallium carbide (Mo2Ga2C) MAX phase powders were purchased from Aritech Chemazone, India. Hydrochloric acid (HCl, 35%) and lithium chloride (LiCl, 99%) were obtained from Sigma-Aldrich, India. Hydrofluoric acid (HF, 48%) was sourced from NICE Chemicals, India. All chemicals were used as received, without further purification. Common laboratory-grade reagents were employed as per standard protocols. Distilled water (DI water) with a resistivity of 18.2 MΩ·cm was supplied using a Millipore system.2.2 Preparation of Mo2CTx m-MXenes and d-MXenes
To prepare Mo2CTx m-MXenes, the Mo2Ga2C MAX phase powder was pretreated to remove impurities (unreacted gallium (Ga), molybdenum (Mo), and carbon (C) during MAX phase synthesis), and then wet etching was performed. For the removal of impurities, 1 g of Mo2Ga2C MAX phase powder was pretreated with 30 mL of 9 M HCl for 24 h.47 During this process, the solution turned yellow after 10 h and became completely golden yellow within 24 h (Fig. 1 and S1A). The treated powder was vacuum-filtered, thoroughly rinsed with DI water, and dried at 80 °C for 24 h in a vacuum oven under −25 inHg pressure, resulting in a greenish-gray powder with a flour-like texture (Fig. S2A). Next, 0.5 g of the dried powder was etched in 30 mL of a solution comprising 12 M HCl, 48% HF, and DI water in a ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.47 The etching process was conducted at 70 °C ± 3 °C in an oil bath with continuous stirring at 400 rpm for 120 h. The resulting etched mixture was washed multiple times by centrifugation at 3500 rpm for 5 min per cycle using 30 mL of DI water. Washing process was continued until the supernatant achieved a pH between 6 and 7, which was typically reached after the fifth wash.
1.47 The etching process was conducted at 70 °C ± 3 °C in an oil bath with continuous stirring at 400 rpm for 120 h. The resulting etched mixture was washed multiple times by centrifugation at 3500 rpm for 5 min per cycle using 30 mL of DI water. Washing process was continued until the supernatant achieved a pH between 6 and 7, which was typically reached after the fifth wash.
For Mo2CTx d-MXene preparation, the centrifuged precipitate (m-Mo2CTx) was added to 50 mL of 0.5 M LiCl and stirred at 400 rpm at RT for 24 h.47 The mixture was washed by centrifugation at 3500 rpm for 5 min to remove unreacted LiCl. By the fourth wash, the supernatant became slightly transparent with a brown tint, indicating the successful removal of excess LiCl. After the fourth wash, N2-purged DI water was added to the precipitate, hand-shaken for 2 min and centrifuged at 3500 rpm for 30 min. The resulting supernatant appeared as a transparent brownish-green solution (Fig. S3A), confirming the delamination of Mo2CTx MXenes. This process was repeated three times, and the collected transparent brownish-green supernatant containing the d-MXene was stored separately. After three repetitions, the remaining precipitate was re-dispersed and stored in tightly sealed PTFE bottles at 4 °C.
The product yields at each synthesis stage are summarized as follows: For Mo2CTx, starting from 500 mg of Mo2Ga2C MAX precursor, approximately 305 mg of multilayer MXenes were obtained after acid etching, corresponding to a yield of ∼61%. Subsequent delamination produced about 120 mg of delaminated MXenes, equivalent to ∼24% of the initial MAX precursor. Some material loss is expected during repeated washing steps following etching and LiCl treatment. Only the delaminated monolayer and few-layer MXene sheets remain suspended in the supernatant after sedimentation, representing the actual yield of the delaminated product. These variations in yield are attributed to the intrinsic structure of the MAX phase and the efficiency of the etching process.
2.3 Preparation of Ti3C2Tx m-MXenes and d-MXenes
The Ti3C2Tx MXene was synthesized by following a similar procedure to that used for Mo2CTx with slight changes. First, 1 g of Ti3AlC2 powder was immersed in 30 mL of 9 M HCl for 24 h at RT without stirring to remove impurities (unreacted titanium (Ti), aluminum (Al), and C during MAX phase synthesis).47 In the beginning, (first 2 min), the air bubbles began to emerge from the powder in the HCl solution (likely hydrogen gas [2Al(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2(g)]) (Fig. 2). After 10 h, the solution's color shifted to a pinkish–purple hue, and later it turned completely pinkish–purple after 24 h (Fig. S1B).28 The treated powder was then filtered using a vacuum filtration system and rinsed several times with DI water to remove residual HCl. Subsequently, the powder was dried at 80 °C for 24 h in a vacuum oven under a pressure of −25 inHg, resulting in a blackish-gray powder with a texture resembling that of fine black-sand (Fig. S2B). Next, 0.5 g of the dried powder was etched in 30 mL of an etching solution composed of 12 M HCl, 48% HF, and DI water in a ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.47 The etching process was conducted with stirring at 400 rpm for 24 h at a controlled temperature of 35 ± 2 °C using an oil bath. The resulting mixture was washed repeatedly by centrifugation at 3500 rpm for 5 min per cycle with 30 mL of DI water until the supernatant achieved a pH of 6 or higher. In this procedure, the pH of the supernatant was stabilized between 6 and 7 after the sixth wash.
3.47 The etching process was conducted with stirring at 400 rpm for 24 h at a controlled temperature of 35 ± 2 °C using an oil bath. The resulting mixture was washed repeatedly by centrifugation at 3500 rpm for 5 min per cycle with 30 mL of DI water until the supernatant achieved a pH of 6 or higher. In this procedure, the pH of the supernatant was stabilized between 6 and 7 after the sixth wash.
For Ti3C2Tx d-MXene preparation, the centrifuged precipitate of m-MXenes was added to 50 mL of 0.5 M LiCl solution and stirred at 400 rpm at RT for 24 h.47 The mixture was then washed with DI water by hand-shaking for 1 min, followed by centrifugation at 3500 rpm for 5 min to remove unreacted LiCl. After the third wash, the supernatant became slightly transparent with a greenish tint, indicating the successful removal of unreacted LiCl. Thereafter, N2-purged DI water was added to the precipitate, hand-shaken for 2 min, and centrifuged at 3500 rpm for 30 min. Finally, the supernatant appeared as a transparent green solution (Fig. S3B), confirming the delamination of Ti3C2Tx MXenes. This process was repeated three times, and the collected transparent green supernatant containing the d-MXene was stored separately. After three repetitions, the remaining precipitate was re-dispersed and stored in tightly sealed PTFE bottles at 4 °C.
The mass yields at each synthesis stage are summarized as follows: for Ti3C2Tx, acid etching of 500 mg of Ti3AlC2 MAX precursor produced approximately 360 mg of multilayer MXenes, corresponding to a yield of ∼72%. Subsequent delamination yielded around 155 mg of delaminated MXenes, equivalent to ∼31% of the initial MAX material. Minor material loss occurs during the repeated washing steps following etching and LiCl treatment. The true yield of the delaminated product corresponds to the monolayer and few-layer MXene sheets that remain suspended in the supernatant after sedimentation. These yield variations are influenced by the intrinsic MAX phase structure and the efficiency of the etching process.
2.4 Instrumentation
The absorption spectra of the Ti3C2Tx and Mo2CTx d-MXenes were examined by UV-Visible spectroscopy (UV-Vis, LAMBDA 365+, PerkinElmer, USA). A D8-Advance XRD (BRUKER, Billerica, MA, USA) was used for recording the X-ray diffraction (XRD) data. The surface structure and morphology of the Ti3C2Tx and Mo2CTx d-MXenes were investigated using a high-resolution scanning electron microscope (HR-SEM, Quanta FEG 200F, FEI, USA). Energy-dispersive X-ray spectrum (EDS) data were obtained using an XPLORE-30 (Oxford, UK). Raman spectra were acquired using an XPLORA plus (HORIBA, Kyoto, Japan) with 532 nm laser excitation. Spectrum-II (PerkinElmer, USA) equipment was used to record the Fourier transform infrared (FTIR) spectra. All characterizations (except UV-Vis) were performed using vacuum-dried powder samples of the MAX phases, m-MXenes, and d-MXenes.3 Results and discussion
3.1 UV-visible spectra of Mo2CTx and Ti3C2Tx d-MXenes
The UV-Vis spectra of Mo2CTx and Ti3C2Tx d-MXenes reveal distinct absorbance peaks for each material. Mo2CTx exhibits peaks at 209 nm, 230 nm, 295 nm, and 568 nm (Fig. 3A), whereas Ti3C2Tx shows peaks at 263 nm, 325 nm, and 798 nm (Fig. 3B).48 The absorption peaks at 209 nm and 263 nm are attributed to π → π* transitions within the Mo–C and Ti–C framework, reflecting strong electronic interactions in their carbon structures.49 The absorption peak at 230 nm is also attributed to the π → π* transitions in the Mo–C framework.The dual peaks observed at 209 nm and 230 nm for Mo2CTx d-MXenes with the same transition are probably due to differences in bonding environments and the influence of surface terminations such as –OH, –F, or ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. The peaks at 295 nm (Mo2CTx) and 325 nm (Ti3C2Tx) are probably attributed to n → π* transitions, involving lone pairs of electrons on oxygen or other surface groups, interacting with the carbon π-system. These transitions may also be influenced by the surface terminations of MXenes.49 In the visible region, a broad peak at 568 nm (for Mo2CTx) suggests d–d electronic transitions characteristic of molybdenum atoms. Similarly, the absorbance at 798 nm in the near-infrared region for Ti3C2Tx indicates d–d transitions typical of titanium atoms.28,50 The absorption peaks observed in the visible and near-infrared regions reflect significant electronic interactions within the Mo2CTx and Ti3C2Tx d-MXene structures. These observations indicated the charge transfer between metal centers and ligands, influenced by surface terminations.51,52 Additionally, the broad absorbance features may arise from plasmonic-like collective oscillations of delocalized electrons, as suggested in earlier MXene studies.53,54
O groups. The peaks at 295 nm (Mo2CTx) and 325 nm (Ti3C2Tx) are probably attributed to n → π* transitions, involving lone pairs of electrons on oxygen or other surface groups, interacting with the carbon π-system. These transitions may also be influenced by the surface terminations of MXenes.49 In the visible region, a broad peak at 568 nm (for Mo2CTx) suggests d–d electronic transitions characteristic of molybdenum atoms. Similarly, the absorbance at 798 nm in the near-infrared region for Ti3C2Tx indicates d–d transitions typical of titanium atoms.28,50 The absorption peaks observed in the visible and near-infrared regions reflect significant electronic interactions within the Mo2CTx and Ti3C2Tx d-MXene structures. These observations indicated the charge transfer between metal centers and ligands, influenced by surface terminations.51,52 Additionally, the broad absorbance features may arise from plasmonic-like collective oscillations of delocalized electrons, as suggested in earlier MXene studies.53,54
UV-Vis spectroscopy validated the optical properties and colloidal stability of both Mo2CTx and Ti3C2Tx MXenes. The observed electronic transitions in their UV-Vis spectra are consistent with the characteristic features reported by Alhabeb et al. (2017), Mathis et al. (2021) and Sunderiya et al. (2024).28,47,55 These results confirm that the optical transitions and dispersion achieved in this study are in line with other earlier studies, affirming successful synthesis and delamination of both Mo- and Ti-based MXenes for advanced applications. To further support these results, the oxidation stability of delaminated Mo2CTx and Ti3C2Tx MXene dispersions was evaluated by monitoring their colloidal stability in DI water over 32 days under ambient conditions (Fig. S4). Both dispersions maintained a stable, highly dispersed state with no visible sedimentation or color change throughout this period. The minimal sedimentation observed after 32 days indicated excellent resistance to oxidation and prolonged colloidal stability for both MXene types. While these observations demonstrated good stability over 32 days, the longer-term oxidation stability was not assessed. Moreover, the 32-day data suggested that the as-prepared MXenes have strong resistance to oxidation and sustained dispersion quality, supporting the retention of electronic and optical transitions, as reflected by the UV-Vis spectra.
3.2 XRD analysis of Mo2Ga2C and Ti3AlC2 systems
The XRD results of the Mo2Ga2C and Ti3AlC2 MAX phases, along with their corresponding m-MXene and d-MXene forms, are presented in Fig. 4. The XRD pattern of the pure Mo2Ga2C MAX phase (Fig. 4A(i), red curve) exhibits diffraction peaks at 9.81° (002), 19.68° (004), 34.16° (100), 37.35° (103), 39.91° (008), 42.56° (105), 45.86° (106), 49.48° (107), 53.51° (108), and 61.06° (110).56,57 After etching Ga from Mo2Ga2C, significant changes in crystallinity and structure were observed, as shown in the m-MXene XRD pattern (Fig. 4A(ii), blue curve). The (002) peak at 9.81° shifted slightly to a lower angle of 8.86°, indicating an increased d-spacing due to the expanded interlayer gap. A new peak appeared at 25.77°, corresponding to the (006) reflection. This new peak is a characteristic feature of Mo2CTx MXenes, signifying successful etching and the formation of the layered MXene structure.56,58 In the Mo2Ga2C MAX phase, the compact structure and the presence of Ga layers suppress the periodicity required for the (006) reflection. After Ga is eliminated, the Mo2C layers undergo rearrangement, establishing a new periodicity along the c-axis and resulting in the emergence of the (006) peak.59 The disappearance of the (103) and (008) peaks at 37.35° and 39.94°, respectively, further confirms the successful removal of Ga. Additionally, the (100) peak, originally located at 34.16°, shifted slightly to a higher angle of 34.38° after Ga removal. This shift can be attributed to a slight contraction in the lattice structure within the ab-plane (a and b axes).60 The removal of Ga reduces interlayer interactions, leading to a decrease in lattice parameters and causing the diffraction angle of the (100) plane to increase, as explained by Bragg's law.61 These structural changes reflect the successful etching of Ga and the subsequent formation of the Mo2CTx MXene. The XRD pattern of the Mo2CTx d-MXene (Fig. 4A(iii), green curve) reveals a reduction in the intensity of most peaks, except for the (002) peak. Notably, the (002) peak is further shifted to a lower angle of 8.03°. This shift is attributed to the intercalation of Li+ ions during the delamination process, which increases the interlayer spacing between the sheets.The XRD pattern of the pure Ti3AlC2 MAX phase (Fig. 4B(i), red curve, JCPDS No. 52-0875) exhibits diffraction peaks at 9.51° (002), 19.14° (004), 33.98° (101), 36.70° (103), 38.85° (104), 41.71° (105), 44.82° (106), 48.38° (107), 52.25° (108), 56.31° (109), and 60.17° (110).62–64 After etching aluminum (Al) from Ti3AlC2, the crystallinity and structure underwent significant changes, as evident from the m-MXene XRD pattern (Fig. 4B(ii), blue curve). The (002) and (004) peaks of Ti3AlC2, originally found at 9.51° and 19.14°, were shifted to lower angles of 8.92° and 18.48°, respectively.
This indicated an increased d-spacing due to the expanded interlayer gap. A new peak at 27.86°, corresponding to the (006) reflection, emerged as a characteristic feature of Ti3C2Tx MXene, signifying successful etching and the formation of the layered MXene structure.65–67 In the Ti3AlC2 MAX phase, the compact structure and presence of Al layers inhibit the periodicity required for the (006) reflection. Upon Al removal, the Ti3C2 layers rearrange and create a new periodicity along the c-axis, which gives rise to the (006) peak.68 Additionally, the absence of the (104) peak at 38.85°, originally present in the MAX phase, further corroborates the successful removal of Al.69 The Ti3C2Tx d-MXene XRD pattern (Fig. 4B(iii), green curve) reveals a reduction in the intensity of the (002), (004), and (006) peaks, which have slightly shifted to lower angles of 8.80°, 18.25°, and 27.32°, respectively. These shifts result from the intercalation of Li+ ions, which increase the d-spacing between the sheets during the delamination process.70,71 Additionally, delaminated MXene layers align preferentially with their basal planes parallel to the substrate or solvent interface, altering the XRD patterns. In Mo2CTx d-MXenes, this alignment enhances basal reflections ((002) and (006)) while reducing the intensity of the (100) peak, confirming successful delamination. In the Ti3C2Tx d-MXene, the opposite trend is observed, with basal plane reflections being reduced and the (103) peak diminished or disappeared, indicating successful delamination. The combined effects of peak shifts, reduced peak intensities, and the disappearance of specific peaks demonstrated the successful exfoliation of both Mo2CTx and Ti3C2Tx MXenes into monolayer or few-layer delaminated sheets. The observed (002) diffraction peak shift for Ti3C2Tx (from 9.51° in the MAX phase to 8.80° in the d-MXene) is consistent with the values reported by Naguib et al. and Ghidiu et al. (2014), confirming a significant increase in interlayer spacing upon delamination and Li+ intercalation.40,62 Similarly, for Mo2CTx, the (002) peak shift aligns with the findings of Gupta et al. (2023) and Pazniak et al. (2021), which also attribute to the expansion in d-spacing to Li+ insertion during the delamination process.56,57 These comparable results validated the efficiency of the tailored synthesis protocol employed in this study for achieving effective intercalation and exfoliation.
To quantify the structural changes, we calculated the (002) interlayer spacing from Bragg's law (eqn (1) and (2)) using Cu Kα radiation (λ = 1.5406 Å) (Table 1). The Mo-system shows an increase in d (002) from 9.01 Å (Mo2Ga2C MAX) to 9.97 Å (m-Mo2CTx) and to 11.00 Å after delamination (d-Mo2CTx), corresponding to +10.70% and +22.12% increases, respectively. The Ti-system increases from 9.29 Å (Ti3AlC2 MAX) to 9.91 Å (m-Ti3C2Tx) and to 10.04 Å (d-Ti3C2Tx), i.e., +6.60% and +8.06%, respectively. These quantitative changes support the interpretation that Li+ intercalation and delamination substantially increase the interlayer separation, and that the Mo-system undergoes a larger relative expansion than the Ti-system under our etching/delamination conditions.
nλ = 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (1) |
 | (2) |
| System | Phase | 2θ (°) (current work) | d (002) (Å) | % Change vs. MAX |
|---|---|---|---|---|
| Mo2Ga2C → Mo2CTx | MAX (002) | 9.81 | 9.009 Å | — |
| m-MXene (002) | 8.86 | 9.973 Å | 10.70% | |
| d-MXene (002) | 8.03 | 11.002 Å | 22.12% | |
| Ti3AlC2 → Ti3C2Tx | MAX (002) | 9.51 | 9.292 Å | — |
| m-MXene (002) | 8.92 | 9.906 Å | 6.60% | |
| d-MXene (002) | 8.8 | 10.041 Å | 8.06% |
3.3 HR-SEM and EDS analysis of Mo2Ga2C and Ti3AlC2 systems
The surface morphology and elemental composition of Mo2Ga2C and Ti3AlC2 MAX phases, m-MXenes, and d-MXenes were analyzed by HR-SEM imaging and EDS, as illustrated in Fig. 5 and 6. The molecular structures of the Mo2Ga2C and Ti3AlC2 MAX phases, along with the etched m-MXenes and d-MXenes, are depicted in Fig. 5A and 6A. The HR-SEM images of the Mo2Ga2C MAX phase revealed a white-stone-like morphology characteristic of Mo-MAX phases with well-defined stacked grains (Fig. 5B(i)). After the etching process to produce Mo2CTx m-MXenes, the HR-SEM images revealed significant exfoliation of Ga layers, resulting in a loosely packed layered morphology (Fig. 5B(ii)). In the proposed etching method, the Mo2Ga2C MAX phase did not undergo vigorous etching as it would with highly concentrated HF. The slow removal of the A-layer and gradual release of H2 during etching led to the formation of this type of structure. The d-Mo2CTx MXene demonstrated further delamination, with monolayer sheets, confirming the successful delamination of Mo2CTxMXenes (Fig. 5B(iii)). The delaminated MXene sheets exhibited slightly bent edges and a smooth surface morphology.Similarly, the HR-SEM images of the Ti3AlC2 MAX phase display its characteristic rock-like layered morphology, with individual particles measuring approximately 10–15 µm and exhibiting distinctly stacked grains (Fig. 6B(i)). Upon etching to form the multilayer Ti3C2Tx (m-MXene), the morphology transforms into a layered structure (Fig. 6B(ii)), where the stacked layers, oriented perpendicular to the imaging plane, clearly reveal the material's multi-layered nature.72,73 Subsequent delamination leads to the formation of Ti3C2Tx d-MXenes, consisting of completely separated, monolayer MXene sheets (Fig. 6B(iii)).
The d-MXene sheets of Ti3C2Tx exhibited sharp edges and a smooth surface, in contrast to the bent edges observed in Mo2CTx d-MXenes. This difference can be attributed to the structural composition of the MXenes: Ti3C2Tx has three layers of titanium and two layers of carbon in each sheet, whereas Mo2CTx comprises two layers of molybdenum and one layer of carbon, making the Mo2CTx sheets more prone to bending and uneven edges compared to the sharper edges of Ti3C2Tx MXenes. The HR-SEM images showed larger lateral sizes for the Ti3AlC2 MAX phase (5–10 µm) compared to the Mo2Ga2C MAX phase (1–2 µm). After delamination, the lateral size of the Ti3C2Tx MXene observed in this study (2–5 µm) is slightly larger but broadly consistent with the atomic-scale characterization reported by Pazniak et al. (2019), who produced Ti3C2Tx flakes with an average lateral size close to 1–2 µm with uniform morphology using self-propagation high-temperature synthesis (SHS) and grinding to produce Ti3AlC2 MAX phases.74 For Mo2CTx MXenes, the observed lateral sizes (0.5–1 µm) are comparable to those reported by Guo et al.(2020),75 who found it difficult to obtain Mo2CTx flakes larger than 1 µm regardless of etching technique, where most flakes being sub-micrometer in size. The lateral sheet size distributions of the exfoliated Mo2CTx and Ti3C2Tx MXenes were quantified using the ImageJ software from the HR-SEM images. The size measurements were plotted as histograms and fitted with a normal (Gaussian) distribution using the Origin software (shown in Fig. S5). This analysis revealed a notable difference in mean lateral sheet sizes: approximately 620 nm for Mo2CTx and 3.7 µm for Ti3C2Tx. These findings confirmed that both of the synthesis routes and the quality of the precursor MAX phase play crucial roles in determining MXene sheet morphology and size.
The EDS spectra were used to confirm the formation of Mo2CTx by analyzing the atomic weight percentages of elemental compositions in the MAX phase, m-MXene, and d-MXene. The MAX phase (Mo2Ga2C) showed the presence of Mo (35.80%), Ga (34.71%), C (15.39%), and O (14.10%) (Fig. 5C(i)). For Mo2CTx m-MXenes, the EDS spectrum confirmed the presence of Mo (19.49%), C (47.01%), and O (29.30%) (Fig. 5C(ii)). Moreover, fluorine (F, 2.09%) and chlorine (Cl, 2.11%) were detected in the EDS spectrum of the m-MXene, originating from the etchant used. In the Mo2CTx d-MXene, the EDS analysis confirmed the presence of Mo (16.19%), C (50.94%), and O (28.85%) (Fig. 5C(iii)).
Similarly, the EDS spectrum of the Ti3AlC2 MAX phase (Fig. 6C(i)) confirmed its elemental composition, with Ti (37.93%), Al (11.49%), C (37.11%), and O (13.47%). In the m-MXene (Ti3C2Tx) spectrum (Fig. 6C(ii)), the removal of Al is evident, along with increased ‘O’ and ‘F’ contents, attributed to surface terminations. The elemental composition of m-MXenes includes Ti (29.39%), C (33.23%), O (22.30%), F (14.69%), and Cl (0.39%). For d-MXenes (Fig. 6C(iii)), the spectrum highlighted the presence of Ti (27.89%), C (34.17%), O (23.56%), F (14.09%), and Cl (0.29%), with a significantly reduced Al content, further confirming successful etching and delamination processes. The EDS analysis of both Mo2CTx and Ti3C2Tx d-MXenes showed elements with weight percentages, which are well consistent with the values reported by Guo et al. (2020), Wei et al. (2023), Yan et al. (2019) and Feng et al. (2019), confirming preservation of the Mo–C and Ti–C framework.75–78 Both MXenes exhibited strong F and O signals, reflecting surface terminations introduced during HF etching and subsequent ambient oxidation, in line with Alhabeb et al. (2017).28 The higher carbon content is attributed to the direct adherence of both titanium and molybdenum MAX phase, m-MXene, and d-MXene powders to the carbon tape during SEM and EDS analyses. The slightly elevated O and F contents, compared to some previous reports, can be ascribed to the optimized etching ratios employed, which promote enhanced termination coverage and surface uniformity. These findings not only align with earlier studies but also set new benchmarks, highlighting how the etching chemistry critically governs the surface termination density and stability of MXenes.
3.4 Raman analysis of Mo2Ga2C and Ti3AlC2 systems
The Raman spectra of Mo2Ga2C and Ti3AlC2 systems of MAX phase, m-MXene, and d-MXene are presented in Fig. 7. The Raman peaks were observed at different stages of the material transformation, highlighting structural and vibrational changes during etching and delamination. The Raman spectrum of the Mo2Ga2C MAX phase (Fig. 7A(i), red curve) exhibited distinct peaks at 201.5, 331.8, 419.8, 633.1, and 748.4 cm−1. The Raman bands at 201.5, 331.8, and 419.8 cm−1 labeled as S1, S2, and S3 correspond to the characteristic shear (E2g) and longitudinal (A1g) vibrational modes of Mo and Ga atoms along the c-axis in the layered MAX phase structure.79,80 Specifically, the S1 peak is associated with the vibrations of Ga atoms.81 The peaks at 633.1 and 748.4 cm−1 are attributed to Mo–Ga and Mo2C vibrations within an octahedral coordination. Upon etching to form Mo2CTx m-MXenes (Fig. 7A(ii), blue curve), significant changes were observed in the Raman spectrum. A sharp, high-intensity peak appeared at 827.7 cm−1, while the peaks at 201.5 and 633.1 cm−1 corresponding to the vibrations of Ga and Mo–Ga in the MAX phase were significantly reduced, indicating the successful removal of Ga layers and the formation of Mo2CTx MXene. The peaks at 827.7 and 998.1 cm−1 are characteristic of the E2g and A1g vibrations of the Mo2C layer in the MXene structure.82–84 Low-intensity peaks at 289.3, 350.2, and 432.5 cm−1 (labeled as S2, S3, and S4 in Fig. 7A(ii)) correspond to out-of-plane Mo and C vibrations influenced by surface terminations (–F, –O, or –OH groups) introduced during the etching process.85 For delaminated Mo2CTx MXenes (Fig. 7A(iii), green curve), further spectral changes confirmed successful delamination. The increased intensity and sharpness of the peak at 207.2 cm−1 (labeled as S2 in Fig. 7A(iii)) are attributed to the formation of surface oxide on the Mo2CTx MXene surface.86 Additional peaks at 298.5 and 348.4 cm−1 (labeled as S3 and S4 in Fig. 7A(iii)) were attributed to Mo–C vibrations influenced by intercalated Li+ ions or structural rearrangements.80 New peaks at 574.6 and 1110.2 cm−1 were identified as artifacts from the glass microscope slide (marked with “+” in Fig. 7A(iii)) used for MXene sample preparation as the substrate.87,88 The Raman peaks at 207.2, 298.5, and 348.4 cm−1 exhibited slight shifts compared to the corresponding peaks at 289.3, 350.2, and 432.5 cm−1 of m-MXenes, probably due to interference from the glass substrate peaks (574.6 and 1110.2 cm−1). Similarly, the characteristic peaks of Mo2CTx MXenes at 827.7 and 998.1 cm−1 shifted slightly to 729.4 and 912.6 cm−1, which also attributed to the glass substrate. The peaks at 729.4 and 912.6 cm−1 represent characteristic Mo2C vibrations, with their reduced intensity further confirming the successful delamination of the MXene.The Raman spectrum of the Ti3AlC2 MAX phase exhibits distinct peaks at 156.3, 270.7, 403.4, 617.2, 1346.1, and 1569.3 cm−1 (Fig. 7B(i), red curve). The characteristic peaks of the layered MAX structure at 270.7, 403.4, and 617.2 cm−1 are labeled as ψ1, ψ2, and ψ3, respectively, and correspond to the E2g and A1g vibrational modes of Ti and Al atoms.89–92 The ψ1 peak is specifically associated with the vibrations of Al atoms. The peak at 156.3 cm−1 marked with “*” is attributed to the out-of-plane vibrations of Ti and C atoms.93 Raman bands at 1346.1 and 1569.3 cm−1 are associated with sp2-hybridized carbon, indicating the presence of carbon-based vibrations in the structure.94 Upon etching to produce m-MXenes, significant changes were observed in the Raman spectrum (Fig. 7B(ii), blue curve). The disappearance of the ψ1 peak in the MXene spectrum correlates with the successful removal of Al layers and the formation of the MXene structure. Low-intensity peaks at 408.8 and 624.2 cm−1 correspond to Ti–C vibrational modes influenced by surface terminations such as –F, –O, or –OH groups.86,93,95,96 A sharp high-intensity peak at 163.9 cm−1 and a low-intensity peak at 214.7 cm−1, marked with “*”, are attributed to the formation of oxide on the Ti3C2 surface86,97,98 and the out-of-plane vibrations of Ti–C atoms, respectively.99,100 The D and G bands, located at 1337.4 and 1578.5 cm−1, respectively, indicate the presence of disordered and graphitic carbon within the material.101,102 Further changes in the Raman spectrum of delaminated Ti3C2Tx MXenes confirmed the successful delamination. The d-MXene spectrum displayed peaks at 163.9, 216.5, 408.1, 578.2, 638.4, and 1102.1 cm−1 (Fig. 7B(iii), green curve). The peaks at 163.9 and 216.5 cm−1 are similar to those observed in m-MXenes correspond to the surface-oxidized Ti3C2 peak and out-of-plane vibrations of Ti–C atoms, respectively (marked with “*”). However, the intensity of the 163.9 cm−1 peak was reduced in the delaminated spectrum. The peaks at 408.1 and 638.4 cm−1 are associated with Ti–C vibrations, with their increased intensity probably influenced by interactions with intercalated ions or functional groups.103 Conversely, the peaks at 578.2 and 1102.1 cm−1 are attributed to artifacts from the glass microscope slide (marked with “+”) used for Raman sample preparation.87,88 The slight shift observed in the 638.4 cm−1 peak compared to m-MXenes is probably due to Li+ ion intercalation and also the interference from the glass substrate. The D and G bands at 1346.8 and 1569.4 cm−1 remain prominent in the delaminated MXene spectrum, indicating that carbon-based vibrations are retained after delamination. The reduced intensity of the surface-oxidized peak at 163.9 cm−1 and the increased intensity of the characteristics peak of Ti3C2 vibration and G band in the d-MXene spectrum further confirm the successful delamination of Ti3C2Tx MXene. The ID/IG ratio decreased for d-MXene (0.82) compared to the m-MXene (0.98) and the MAX phase (1.15), indicating the successful formation of the Ti3C2Tx d-MXene with a reduced structural disorder.
The Raman spectra distinctly captured the transformation from MAX phases to m-MXenes and d-MXenes, reflecting the structural and chemical modifications induced during the etching and delamination processes. In Mo2CTx, the disappearance of Ga-related peaks and the emergence of Mo–C vibrational modes at 729 and 912 cm−1, along with additional weak bands between 207 and 432 cm−1 associated with surface terminations, confirm the successful conversion. These observations align well with those reported by Bayhan et al. (2023) and Gupta et al. (2023), who identified similar Mo–C bands and termination-related features, indicating effective Ga removal and preservation of the carbide framework.57,104 Likewise, for Ti3C2Tx, the spectra exhibit characteristic Ti–C modes at 163–217 and 408–638 cm−1, together with pronounced D (∼1340 cm−1) and G (∼1570 cm−1) bands, indicating sp2 carbon ordering. This spectral profile is consistent with earlier findings by Sarycheva and Gogotsi (2020), Ferrari and Robertson (2000), and Rajendran et al. (2022), who reported comparable Raman features in delaminated Ti-based MXenes.92,95,99,101 Overall, the Raman signatures of both MXenes substantiate the phase purity, structural integrity, and low defect density achieved through the present synthesis approach.
3.5 FTIR analysis of Mo2Ga2C and Ti3AlC2 systems
FTIR and Raman spectroscopies are complementary techniques widely used to study vibrational modes in materials. FTIR spectroscopy is particularly effective in detecting Raman-inactive vibrational modes, such as Eu and A2u, making it a valuable tool for characterizing surface terminations, such as chemical bonds, MXene-based organic hybrids, and hydrogen bonding.105 The FTIR spectra of Mo2Ga2C and Ti3AlC2 MAX phases, m-MXenes, and d-MXenes exhibit distinct features across two main regions. The surface functional group region (4000–1500 cm−1) reveals peaks associated with hydroxyl (–OH), oxygen (–O) and carbon bonds (C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), while the fingerprint region (1500–400 cm−1) contains unique absorption peaks that identify molecular vibrations characteristic of each material's structure.
O), while the fingerprint region (1500–400 cm−1) contains unique absorption peaks that identify molecular vibrations characteristic of each material's structure.
For the Mo2Ga2C MAX phase (as shown in Fig. 8A(i), red curve), the Ga–C stretching peak at ∼1023 cm−1 is specific to gallium carbide bonds,106 Mo–O stretching at ∼762 cm−1 is linked to partial surface oxidation of molybdenum while Mo–C stretching and bending vibrations at ∼2322 cm−1 and ∼580 cm−1 are characteristic of molybdenum carbide in the MAX phase.107,108 The spectrum of the Mo2CTx m-MXene (as shown in Fig. 8A(ii), blue curve) reveals O–H stretching vibrations between ∼3600 and ∼3200 cm−1, confirming the presence of hydroxyl surface terminations introduced during etching, and O–H bending at ∼1411 cm−1 further supports this functionalization.99 The C–H stretching at ∼2890 cm−1 reflects aliphatic hydrocarbon vibrations. The spectrum also shows C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching at ∼1621 cm−1 reflecting carbonyl groups.109,110 Additionally, Mo–F stretching at ∼852 cm−1 indicates fluorine terminations from HF etching, Mo–O stretching at ∼733 cm−1 suggests molybdenum oxide species, the Mo–C stretching and bending at ∼2343 cm−1 and ∼610 cm−1 confirmed the presence of molybdenum carbide bonds.109,110 The spectrum of the Mo2CTx d-MXene is similar to that of the m-MXene, with slight shifts in peaks due to delamination (Fig. 8A(iii), green curve). These include O–H bending at ∼1410 cm−1 and C–H stretching at ∼2901 cm−1. Additionally, Mo–F stretching at ∼883 cm−1 suggests increased fluorine terminations, while Mo–C stretching and bending at ∼2326 cm−1 and ∼619 cm−1 indicated the carbide framework's retention.
O stretching at ∼1621 cm−1 reflecting carbonyl groups.109,110 Additionally, Mo–F stretching at ∼852 cm−1 indicates fluorine terminations from HF etching, Mo–O stretching at ∼733 cm−1 suggests molybdenum oxide species, the Mo–C stretching and bending at ∼2343 cm−1 and ∼610 cm−1 confirmed the presence of molybdenum carbide bonds.109,110 The spectrum of the Mo2CTx d-MXene is similar to that of the m-MXene, with slight shifts in peaks due to delamination (Fig. 8A(iii), green curve). These include O–H bending at ∼1410 cm−1 and C–H stretching at ∼2901 cm−1. Additionally, Mo–F stretching at ∼883 cm−1 suggests increased fluorine terminations, while Mo–C stretching and bending at ∼2326 cm−1 and ∼619 cm−1 indicated the carbide framework's retention.
Similarly, the FTIR spectrum of the Ti3AlC2 MAX phase (Fig. 8B(i), red curve) confirms its structural integrity through distinct vibrational signatures. The C–H stretching at ∼2331 cm−1 indicates hydrocarbon interactions, while the C–O stretching at ∼1251 cm−1 suggests surface oxides or possible impurities. The Al–C stretching at ∼799 cm−1 underscores aluminum–carbon bonds, essential for the MAX phase structure.111 The Ti–O stretching at ∼599 cm−1 reveals partial surface oxidation of titanium, possibly due to environmental exposure, while the Ti–C stretching and bending at ∼2322 cm−1 and ∼559 cm−1 confirmed the presence of titanium carbide bonds, a defining feature of the MAX phase.109,112 Additionally, the C–C bending vibration at ∼445 cm−1 highlights in-plane deformation of carbon–carbon bonds, characteristic of the layered structure.99,113
The FTIR spectrum of the etched Ti3C2Tx m-MXene (Fig. 8B(ii), blue curve) shows significant functionalization and new surface terminations after etching process. The broad O–H stretching (3600–3200 cm−1) and O–H bending (∼1394 cm−1) confirmed the addition of –OH groups.99 The C–H stretching at ∼2892 cm−1 reflected hydrocarbon functionalities. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching at ∼1802 cm−1 indicated a carbonyl group.109,110 The C–F stretching at ∼1248 cm−1 and Ti–F stretching at ∼764 cm−1 confirmed fluorination from the etching solution, while Ti–O stretching at ∼654 cm−1 and Ti–C stretching and bending at ∼2323 cm−1 and ∼584 cm−1 demonstrated retention of titanium oxide and carbide bonds.109,112 The Ti3C2Tx d-MXene spectrum (Fig. 8B(iii), green curve) resembles that of the m-MXene, with peak shifts due to delamination. These include O–H bending at ∼1410 cm−1 and C–H stretching at ∼2901 cm−1, reflecting altered chemical environments. Enhanced C–F stretching at ∼1252 cm−1 and Ti–F stretching at ∼773 cm−1 highlighted fluorination, while Ti–O stretching at ∼652 cm−1 and Ti–C stretching and bending at ∼2327 cm−1 and ∼598 cm−1 confirmed structural stability. A C–C bending vibration in the range of 550−450 cm−1 is observed in the Ti3AlC2 MAX phase, m-MXene, and d-MXene, within the fingerprint region.99,113 However, this vibration corresponds to a weak A2u (C–C) IR mode, making it less significant for FTIR-based identification of Ti3AlC2. The FTIR analysis revealed the detailed surface chemistry of both Mo2CTx and Ti3C2Tx MXenes following etching and delamination processes.105
O stretching at ∼1802 cm−1 indicated a carbonyl group.109,110 The C–F stretching at ∼1248 cm−1 and Ti–F stretching at ∼764 cm−1 confirmed fluorination from the etching solution, while Ti–O stretching at ∼654 cm−1 and Ti–C stretching and bending at ∼2323 cm−1 and ∼584 cm−1 demonstrated retention of titanium oxide and carbide bonds.109,112 The Ti3C2Tx d-MXene spectrum (Fig. 8B(iii), green curve) resembles that of the m-MXene, with peak shifts due to delamination. These include O–H bending at ∼1410 cm−1 and C–H stretching at ∼2901 cm−1, reflecting altered chemical environments. Enhanced C–F stretching at ∼1252 cm−1 and Ti–F stretching at ∼773 cm−1 highlighted fluorination, while Ti–O stretching at ∼652 cm−1 and Ti–C stretching and bending at ∼2327 cm−1 and ∼598 cm−1 confirmed structural stability. A C–C bending vibration in the range of 550−450 cm−1 is observed in the Ti3AlC2 MAX phase, m-MXene, and d-MXene, within the fingerprint region.99,113 However, this vibration corresponds to a weak A2u (C–C) IR mode, making it less significant for FTIR-based identification of Ti3AlC2. The FTIR analysis revealed the detailed surface chemistry of both Mo2CTx and Ti3C2Tx MXenes following etching and delamination processes.105
This is evidenced by the appearance of broad –OH peaks, which are absent in the MAX phase but become prominent in the m-MXene and d-MXene, indicating surface functionalization. For Mo2CTx, distinct O–H, C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and Mo–F absorption bands confirmed the presence of hydroxyl, carboxyl, and fluoride terminations, while the preserved Mo–C vibrations indicate retention of the carbide framework. These features are consistent with DFT predictions and spectral assignments reported by Parker et al. (2024), reflecting the complex and overlapping contributions of mixed surface terminations.105 Similarly, Ti3C2Tx exhibits characteristic O–H, C
O, and Mo–F absorption bands confirmed the presence of hydroxyl, carboxyl, and fluoride terminations, while the preserved Mo–C vibrations indicate retention of the carbide framework. These features are consistent with DFT predictions and spectral assignments reported by Parker et al. (2024), reflecting the complex and overlapping contributions of mixed surface terminations.105 Similarly, Ti3C2Tx exhibits characteristic O–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, Ti–F, and backbone Ti–C vibrational modes, in agreement with both theoretical and experimental analyses by Hu et al. (2015) and Parker et al. (2024).99,105 Collectively, these studies establish a robust framework for interpreting MXene FTIR signatures. The strong correspondence between experimental spectra and DFT-predicted vibrational modes (Table 2) confirms effective delamination, functionalization, and structural integrity across Mo- and Ti-based MXenes, thereby validating the surface chemistry and phase preservation crucial for advanced MXene applications. Moreover, the FTIR vibrational modes of Mo2CTx and Ti3C2Tx (Table 2) demonstrate strong alignment between empirically observed peaks and DFT-predicted modes for surface terminations such as –F2 and –O2, further supporting the presence of these functional groups on the MXene surfaces.
O, Ti–F, and backbone Ti–C vibrational modes, in agreement with both theoretical and experimental analyses by Hu et al. (2015) and Parker et al. (2024).99,105 Collectively, these studies establish a robust framework for interpreting MXene FTIR signatures. The strong correspondence between experimental spectra and DFT-predicted vibrational modes (Table 2) confirms effective delamination, functionalization, and structural integrity across Mo- and Ti-based MXenes, thereby validating the surface chemistry and phase preservation crucial for advanced MXene applications. Moreover, the FTIR vibrational modes of Mo2CTx and Ti3C2Tx (Table 2) demonstrate strong alignment between empirically observed peaks and DFT-predicted modes for surface terminations such as –F2 and –O2, further supporting the presence of these functional groups on the MXene surfaces.
| MXene type | MXene vibration modes | |||
|---|---|---|---|---|
| M–C ∼350−450 cm−1 | C–C ∼400−500 cm−1 | M–O ∼500−650 cm−1 | M–F ∼700−750 cm−1 | |
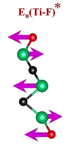
a Footnotes: in the table, empirical terminations are presented in parentheses, while DFT-predicted terminations with –F are marked with “*”, and terminations with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O remain unmarked. Discrepancies between DFT predictions and empirical data arise from the use of a single-termination model and the inherently broad range of empirical FTIR data. All structural visualizations were created using the VESTA software. O remain unmarked. Discrepancies between DFT predictions and empirical data arise from the use of a single-termination model and the inherently broad range of empirical FTIR data. All structural visualizations were created using the VESTA software.
|
||||
| Mo2CTx | (Mo–C)99,115,116 | (C–C)114,117–119 | (Mo–O)99,115,116 | (Mo–F)116,120 |

|

|

|
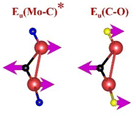
|
|
| Ti3C2Tx | (Ti–C)109,110 | (C–C)114,117–119 | (Ti–O)109,110 | (Ti–F)118,120 |
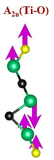
|
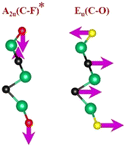
|
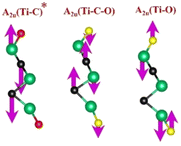
|
|
|
4 Limitations of the study
While this study provides the first systematic comparative synthesis of Mo2CTx and Ti3C2Tx MXenes under identical processing conditions, several limitations should be noted. First, the investigation was restricted to only two MXene systems (Mo- and Ti-based), and the conclusions may not directly extend to other emerging MXene families. Second, the structural and morphological characterizations were comprehensive. However, quantitative surface chemistry (e.g., XPS depth profiling) and computational modeling (e.g., DFT-based electronic structure calculations) were not included, which would have further validated the interpretations of the UV-Vis, FTIR, and Raman features. Third, only short-term oxidation stability (up to 32 days) was observed, despite the importance of long-term stability for practical applications. Finally, the study was limited to laboratory-scale synthesis; thus, the scalability of the optimized etching protocols to industrial levels remains yet to be demonstrated. These limitations define the scope of our work while also pointing to clear directions for future research aimed at extending the comparative framework to other MXene families, integrating advanced surface/electronic characterizations, and assessing long-term performance in device-relevant environments.5 Conclusion
This study demonstrates a comparative scalable synthesis strategy of both multilayer and delaminated Mo2CTx and Ti3C2Tx MXenes, by tailored acid etching and LiCl-based delamination without organic intercalants or ultrasonication. This unified protocol contrasts with conventional in situ HF and concentrated HF approaches by yielding enhanced flake quality, scalable yields, and reproducible control of MXene morphology and surface chemistry. Key findings including significant interlayer expansion (XRD shifts: 9.81° → 8.03° for Mo2CTx, 9.51° → 8.80° for Ti3C2Tx), lateral sheet sizes of 0.5–1 µm (Mo2CTx) and 2–5 µm (Ti3C2Tx), and distinct UV-Vis absorption features confirmed the strong electronic transitions and high-quality delamination. HR-SEM and EDS analyses confirmed robust morphology and elemental composition, while spectroscopic analysis confirms delamination and structural integrity. This work offers a direct, rigorous comparison of Mo- and Ti-based MXenes under identical conditions, showcasing a facile, safe, scalable route to high-quality MXenes with well-preserved carbide frameworks and surface terminations (–OH, –F, and![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). These results provide critical comparative data and clearly demonstrated how etchant formulation and delamination strategies influence the MXene properties. Although no application-specific measurements are included, the detailed structural benchmarks established here serve as a foundation for future research into energy, sensing, and filtration technologies utilizing MXenes.
O). These results provide critical comparative data and clearly demonstrated how etchant formulation and delamination strategies influence the MXene properties. Although no application-specific measurements are included, the detailed structural benchmarks established here serve as a foundation for future research into energy, sensing, and filtration technologies utilizing MXenes.
Author contributions
Conceptualization: V. M. and A. K. S.; methodology, validation, formal analysis, data curation, software, writing – original draft preparation: V. M.; resources, review and editing: R. A., S. A., and S. H. M.; resources, supervision, project administration, funding acquisition, writing – review & editing, validation: A. K. S. all authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Data availability
We have provided all the obtained data in the manuscript.Supplementary information: includes photographs of MAXphase impurity removal, delaminated MXene dispersion, and oxidation stability of d-MXene, along with histogram plots of the lateral sheet size distribution for Mo2CTx and Ti3C2Tx MXenes. See DOI: https://doi.org/10.1039/d5na00669d.
Acknowledgements
We appreciate the Anusandhan National Research Foundation (ANRF), India, for funding through CRG/2021/001517. We thank the Indian Institute of Science (IISc)-Centre for Nano Science and Engineering (CeNSE) department for providing Micro Nano Characterization Facility (MNCF) for the characterization of the MAX phase and MXene materials.References
- K. R. G. Lim, M. Shekhirev, B. C. Wyatt, B. Anasori, Y. Gogotsi and Z. W. Seh, Nat. Synth., 2022, 1, 601–614 CrossRef CAS.
- D. N. Ampong, E. Agyekum, F. O. Agyemang, K. Mensah-Darkwa, A. Andrews, A. Kumar and R. K. Gupta, Discover Nano, 2023, 18, 3 CrossRef CAS PubMed.
- P. Kamakshi, J. Chandrappan, S. Chella and G. K. Krishnamoorthy, Eur. Phys. J. Appl. Phys., 2024, 99, 23 CrossRef CAS.
- J. Liew, S. Bashir, K. Ramesh and S. Ramesh, Mater. Sci. Eng., B, 2025, 314, 118076 CrossRef CAS.
- L. Damptey, B. N. Jaato, C. S. Ribeiro, S. Varagnolo, N. P. Power, V. Selvaraj, D. Dodoo-Arhin, R. V. Kumar, S. P. Sreenilayam, D. Brabazon, V. Kumar Thakur and S. Krishnamurthy, Global Chall., 2022, 6, 2100120 CrossRef PubMed.
- X. Li, Z. Huang, C. E. Shuck, G. Liang, Y. Gogotsi and C. Zhi, Nat. Rev. Chem., 2022, 6, 389–404 CrossRef PubMed.
- M. Yadav, M. Kumar and A. Sharma, ACS Appl. Nano Mater., 2024, 7, 9847–9867 CrossRef CAS.
- C. K. Raj, R. Siranjeevi, S. S. Shabnum, P. Nivetha, K. Benazir, A. Saravanan and A. S. Vickram, Environ. Qual. Manag., 2024, 34, e22360 CrossRef.
- S. Balasamy, D. Ganapathy, R. Atchudan, S. Arya and A. K. Sundramoorthy, Curr. Cancer Ther. Rev., 2025, 21, 1016–1029 CrossRef CAS.
- P. Iravani, S. Iravani and R. S. Varma, Micromachines, 2022, 13, 1383 CrossRef PubMed.
- B. Zhu, J. Shi, C. Liu, J. Li and S. Cao, Ceram. Int., 2021, 47, 24252–24261 CrossRef CAS.
- A. Liu, Y. Liu, G. Liu, A. Zhang, Y. Cheng, Y. Li, L. Zhang, L. Wang, H. Zhou, J. Liu and H. Wang, Chem. Eng. J., 2022, 448, 137691 CrossRef CAS.
- A. Lipatov, A. Goad, M. J. Loes, N. S. Vorobeva, J. Abourahma, Y. Gogotsi and A. Sinitskii, Matter, 2021, 4, 1413–1427 CrossRef CAS.
- R. M. Ronchi, J. T. Arantes and S. F. Santos, Ceram. Int., 2019, 45, 18167–18188 CrossRef CAS.
- L. Pandey, W. Liang, A. VahidMohammadi, T. Zhang, Y. Gogotsi and M. Wanunu, RSC Adv., 2024, 14, 21635–21643 RSC.
- H. Li, R. Fan, B. Zou, J. Yan, Q. Shi and G. Guo, J. Nanobiotechnol., 2023, 21, 73 CrossRef PubMed.
- S. Balasamy, P. Sakthivelan, V. Magesh and A. K. Sundramoorthy, Nanomedicine, 2025, 20, 1537–1547 CrossRef CAS PubMed.
- R. Akhter and S. S. Maktedar, J. Mater., 2023, 9, 1196–1241 Search PubMed.
- A. M. Amani, E. Vafa, M. Mirzae, M. Abbasi, A. Vaez, A. Najdian, A. Jahanbin, S. R. Kasaei, S. Mosleh-Shirazi, H. Kamyab, S. Chelliapan, S. Rajendran and L. P. Guamán, J. Ind. Eng. Chem., 2025, 149, 275–312 CrossRef CAS.
- A. M. Amani, A. Rahbar, E. Vafa, L. Tayebi, M. Abbasi, H. Kamyab, S. Chelliapan, S. R. Kasaee, A. Vaez and S. Mosleh-Shirazi, Mater. Today Commun., 2024, 41, 110774 CrossRef CAS.
- A. M. Amani, M. Abbasi, A. Najdian, F. Mohamadpour, S. R. Kasaee, H. Kamyab, S. Chelliapan, M. Shafiee, L. Tayebi, A. Vaez, A. Najafian, E. Vafa and S. Mosleh-Shirazi, Ecotoxicol. Environ. Saf., 2025, 291, 117817 CrossRef CAS PubMed.
- A. M. Amani, M. Abbasi, A. Najdian, F. Mohamadpour, S. R. Kasaee, H. Kamyab, S. Chelliapan, H. Ardeshiri, L. Tayebi, E. Vafa, S. Mosleh-Shirazi, A. Jahanbin, S. Rajendran and D. Simancas-Racines, Ecotoxicol. Environ. Saf., 2025, 297, 118222 CrossRef CAS PubMed.
- B. Anasori and Y. Gogotsi, 2D Metal Carbides and Nitrides (MXenes), Springer Nature, Cham, Switzerland, 1st edn, 2020 Search PubMed.
- M. Han, C. E. Shuck, R. Rakhmanov, D. Parchment, B. Anasori, C. M. Koo, G. Friedman and Y. Gogotsi, ACS Nano, 2020, 14, 5008–5016 CrossRef CAS PubMed.
- Z. Otgonbayar and W.-C. Oh, FlatChem, 2023, 40, 100524 CrossRef CAS.
- P. A. Rasheed, R. P. Pandey, F. Banat and S. W. Hasan, Matter, 2022, 5, 546–572 CrossRef CAS.
- W. Cao, J. Nie, Y. Cao, C. Gao, M. Wang, W. Wang, X. Lu, X. Ma and P. Zhong, Chem. Eng. J., 2024, 496, 154097 CrossRef CAS.
- M. Alhabeb, K. Maleski, B. Anasori, P. Lelyukh, L. Clark, S. Sin and Y. Gogotsi, Chem. Mater., 2017, 29, 7633–7644 CrossRef CAS.
- X. Sang, Y. Xie, M.-W. Lin, M. Alhabeb, K. L. Van Aken, Y. Gogotsi, P. R. C. Kent, K. Xiao and R. R. Unocic, ACS Nano, 2016, 10, 9193–9200 CrossRef CAS PubMed.
- M. A. Hope, A. C. Forse, K. J. Griffith, M. R. Lukatskaya, M. Ghidiu, Y. Gogotsi and C. P. Grey, Phys. Chem. Chem. Phys., 2016, 18, 5099–5102 RSC.
- D. Xiong, X. Li, Z. Bai and S. Lu, Small, 2018, 14, e1703419 CrossRef PubMed.
- S. Abdolhosseinzadeh, X. Jiang, H. Zhang, J. Qiu and C. (john) Zhang, Mater. Today, 2021, 48, 214–240 CrossRef CAS.
- R. Verma, A. Sharma, V. Dutta, A. Chauhan, D. Pathak and S. Ghotekar, Emergent Mater., 2024, 7, 35–62 CrossRef CAS.
- S. Lai, J. Jeon, S. K. Jang, J. Xu, Y. J. Choi, J.-H. Park, E. Hwang and S. Lee, Nanoscale, 2015, 7, 19390–19396 RSC.
- N. Tyagi, M. K. Singh, S. Moka and M. Khanuja, Hybrid Adv., 2024, 7, 100285 CrossRef.
- A. Feng, Y. Yu, F. Jiang, Y. Wang, L. Mi, Y. Yu and L. Song, Ceram. Int., 2017, 43, 6322–6328 CrossRef CAS.
- A. Lipatov, M. Alhabeb, M. R. Lukatskaya, A. Boson, Y. Gogotsi and A. Sinitskii, Adv. Electron. Mater., 2016, 2, 1600255 CrossRef.
- C. E. Shuck, M. Han, K. Maleski, K. Hantanasirisakul, S. J. Kim, J. Choi, W. E. B. Reil and Y. Gogotsi, ACS Appl. Nano Mater., 2019, 2, 3368–3376 CrossRef CAS.
- Y. Tian, W. Que, Y. Luo, C. Yang, X. Yin and L. B. Kong, J. Mater. Chem. A, 2019, 7, 5416–5425 RSC.
- M. Ghidiu, M. R. Lukatskaya, M.-Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CrossRef CAS PubMed.
- X. Zhang, W. Zhang and H. Zhao, Mater. Today Commun., 2022, 33, 104384 CrossRef CAS.
- B. Gurzęda, N. Boulanger, A. Nordenström, C. Dejoie and A. V. Talyzin, Adv. Sci., 2024, 11, e2408448 CrossRef PubMed.
- J. A. Jaffri, M. Ihsan, S. Bashir, J. Selvaraj, S. Ramesh, B. Vengadaesvaran, N. A. A. Samad, I. Thiyahuddin, T. Husaini, G. Srinivasan, T. Prasankumar and K. Ramesh, Sustain. Mater. Technol., 2025, e01502 CAS.
- A. Shayesteh Zeraati, S. A. Mirkhani, P. Sun, M. Naguib, P. V. Braun and U. Sundararaj, Nanoscale, 2021, 13, 3572–3580 RSC.
- S. Biswas and P. S. Alegaonkar, Surfaces, 2021, 5, 1–34 CrossRef.
- P. Wang, B. Wang and R. Wang, Materials, 2023, 16, 6816 CrossRef CAS PubMed.
- T. S. Mathis, K. Maleski, A. Goad, A. Sarycheva, M. Anayee, A. C. Foucher, K. Hantanasirisakul, C. E. Shuck, E. A. Stach and Y. Gogotsi, ACS Nano, 2021, 15, 6420–6429 CrossRef CAS PubMed.
- V. Magesh, D. Ganapathy and A. K. Sundramoorthy, Nanosci. Nanotechnol.-Asia, 2025, 15, e22106812391332 CrossRef.
- B. Kartick, S. K. Srivastava and A. I. Srivastava, J. Nanosci. Nanotechnol., 2013, 13, 4320–4324 CrossRef CAS PubMed.
- D. R. Everett, Chem. Educ., 1998, 3, 1–2 CrossRef.
- N. Sinha and O. S. Wenger, J. Am. Chem. Soc., 2023, 145, 4903–4920 CrossRef CAS.
- R. Otero, A. L. Vázquez de Parga and J. M. Gallego, Surf. Sci. Rep., 2017, 72, 105–145 CrossRef CAS.
- B. Zhang, Y. Wang, Z. Wang, G. Tan, T. Liu, S. Feng, Y. Tan, W. Liu, Q. Yang, Y. Liu, A. Xia, H. Ren and Y. Wu, Appl. Catal., B, 2023, 339, 123132 CrossRef CAS.
- M. Rahman and M. S. Al Mamun, Nanoscale Adv., 2024, 6, 367–385 RSC.
- S. Sunderiya, S. Suragtkhuu, S. Purevdorj, T. Ochirkhuyag, M. Bat-Erdene, P. Myagmarsereejid, A. D. Slattery, A. S. R. Bati, J. G. Shapter, D. Odkhuu, S. Davaasambuu and M. Batmunkh, J. Energy Chem., 2024, 88, 437–445 CrossRef CAS.
- H. Pazniak, A. S. Varezhnikov, D. A. Kolosov, I. A. Plugin, A. D. Vito, O. E. Glukhova, P. M. Sheverdyaeva, M. Spasova, I. Kaikov, E. A. Kolesnikov, P. Moras, A. M. Bainyashev, M. A. Solomatin, I. Kiselev, U. Wiedwald and V. V. Sysoev, Adv. Mater., 2021, 33, 2104878 CrossRef CAS PubMed.
- M. Gupta, A. Verma, P. Chaudhary and B. C. Yadav, Mater. Adv., 2023, 4, 3989–4010 RSC.
- C. Wang, H. Shou, S. Chen, S. Wei, Y. Lin, P. Zhang, Z. Liu, K. Zhu, X. Guo, X. Wu, P. M. Ajayan and L. Song, Adv. Mater., 2021, 33, e2101015 CrossRef PubMed.
- M. A. Shehzad, P. M. Das, A. C. Tyner, M. Cheng, Y.-S. Lee, P. Goswami, R. Dos Reis, X. Chen and V. P. Dravid, 2D Mater., 2022, 9, 015039 CrossRef CAS.
- U. M. Kuruppu, A. J. Magdaleno, A. S. Kshirsagar, B. Donnadieu, F. Prins and M. K. Gangishetty, Chem. Commun., 2024, 60, 14960–14963 RSC.
- G. Kostorz, in Physical Metallurgy, Elsevier, 1996, pp. 1115–1199 Search PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- P. Yan, R. Zhang, J. Jia, C. Wu, A. Zhou, J. Xu and X. Zhang, J. Power Sources, 2015, 284, 38–43 CrossRef CAS.
- S. M. M. Raj, A. K. Sundramoorthy, R. Atchudan, D. Ganapathy and A. Khosla, J. Electrochem. Soc., 2022, 169, 077501 CrossRef CAS.
- H. Fang, Y. Pan, M. Yin and C. Pan, J. Mater. Sci.: Mater. Electron., 2019, 30, 14954–14966 CrossRef CAS.
- Q. T. H. Ta, N. M. Tran and J.-S. Noh, Catalysts, 2020, 10, 1140 CrossRef CAS.
- H. Wang, Y. Wu, J. Zhang, G. Li, H. Huang, X. Zhang and Q. Jiang, Mater. Lett., 2015, 160, 537–540 CrossRef CAS.
- T. B. Sobyra, K. Matthews, T. S. Mathis, Y. Gogotsi and P. Fenter, ACS Energy Lett., 2022, 7, 3612–3617 CrossRef CAS.
- S. Munir, A. Rasheed, T. Rasheed, I. Ayman, S. Ajmal, A. Rehman, I. Shakir, P. O. Agboola and M. F. Warsi, ACS Omega, 2020, 5, 26845–26854 CrossRef CAS PubMed.
- O. Mashtalir, M. Naguib, V. N. Mochalin, Y. Dall'Agnese, M. Heon, M. W. Barsoum and Y. Gogotsi, Nat. Commun., 2013, 4, 1716 CrossRef PubMed.
- Y. Long, Y. Tao, T. Shang, H. Yang, Z. Sun, W. Chen and Q.-H. Yang, Adv. Sci., 2022, 9, e2200296 CrossRef PubMed.
- M. Bandpey and D. P. J. Barz, Nanoscale, 2024, 16, 15078–15093 RSC.
- X. Li, F. Ran, F. Yang, J. Long and L. Shao, Trans. Tianjin Univ., 2021, 27, 217–247 CrossRef CAS.
- A. Pazniak, P. Bazhin, N. Shplis, E. Kolesnikov, I. Shchetinin, A. Komissarov, J. Polcak, A. Stolin and D. Kuznetsov, Mater. Des., 2019, 183, 108143 CrossRef CAS.
- Y. Guo, S. Jin, L. Wang, P. He, Q. Hu, L.-Z. Fan and A. Zhou, Ceram. Int., 2020, 46, 19550–19556 CrossRef CAS.
- L. Wei, K. Zhou, Q. Rao, H.-Q. Li and P. Yang, Environ. Eng. Sci., 2023, 40, 657–666 CrossRef CAS.
- S. Yan, C. Cao, J. He, L. He and Z. Qu, J. Mater. Sci.: Mater. Electron., 2019, 30, 6537–6543 CrossRef CAS.
- W. Feng, H. Luo, Y. Wang, S. Zeng, Y. Tan, L. Deng, X. Zhou, H. Zhang and S. Peng, Sci. Rep., 2019, 9, 3957 CrossRef PubMed.
- O. Chaix-Pluchery, A. Thore, S. Kota, J. Halim, C. Hu, J. Rosen, T. Ouisse and M. W. Barsoum, J. Raman Spectrosc., 2017, 48, 631–638 CrossRef CAS.
- W. Guo, S. G. Surya, V. Babar, F. Ming, S. Sharma, H. N. Alshareef, U. Schwingenschlögl and K. N. Salama, ACS Appl. Mater. Interfaces, 2020, 12, 57218–57227 CrossRef CAS.
- J. A. Creighton and R. Withnall, Chem. Phys. Lett., 2000, 326, 311–313 CrossRef CAS.
- S. Hussain, S. A. Zaidi, D. Vikraman, H.-S. Kim and J. Jung, Biosens. Bioelectron., 2019, 140, 111330 CrossRef CAS PubMed.
- S. Hussain, D. Vikraman, A. Feroze, W. Song, K.-S. An, H.-S. Kim, S.-H. Chun and J. Jung, Front. Chem., 2019, 7, 716 CrossRef CAS.
- Department of Physics, SFR College for Women, Sivakasi, TamilNadu, India, R. S. Periathai and K. Rajagopal, IOSR J. Appl. Phys., 2014, 6, 09–12 CrossRef.
- Y. Zhou, L. Liang, C. Wang, F. Sun, L. Zheng, H. Qi, B. Wang, X. Wang, C.-T. Au, J. Wang, L. Jiang and H. Hosono, J. Am. Chem. Soc., 2024, 146, 23054–23066 CrossRef CAS PubMed.
- Y. Cao, Q. Deng, Z. Liu, D. Shen, T. Wang, Q. Huang, S. Du, N. Jiang, C.-T. Lin and J. Yu, RSC Adv., 2017, 7, 20494–20501 RSC.
- D. Tuschel, Spectroscopy, 2017, 32, 26–33 CAS.
- A. W. Zia, I. Anestopoulos, L. Bowen, M. I. Panayiotidis and M. Birkett, Surf. Coat. Technol., 2024, 493, 131281 CrossRef CAS.
- C. Zhao, Q. Wang, H. Zhang, S. Passerini and X. Qian, ACS Appl. Mater. Interfaces, 2016, 8, 15661–15667 CrossRef CAS.
- L. Wang, H. Zhang, B. Wang, C. Shen, C. Zhang, Q. Hu, A. Zhou and B. Liu, Electron. Mater. Lett., 2016, 12, 702–710 CrossRef CAS.
- J. E. Spanier, S. Gupta, M. Amer and M. W. Barsoum, Phys. Rev. B:Condens. Matter Mater. Phys., 2005, 71, 012103 CrossRef.
- A. Sarycheva and Y. Gogotsi, Chem. Mater., 2020, 32, 3480–3488 CrossRef CAS.
- A. Iqbal and N. M. Hamdan, Materials, 2021, 14, 6292 CrossRef CAS PubMed.
- F. Liu, A. Zhou, J. Chen, H. Zhang, J. Cao, L. Wang and Q. Hu, Adsorption, 2016, 22, 915–922 CrossRef CAS.
- J. Rajendran, A. K. Sundramoorthy, D. Ganapathy, R. Atchudan, M. A. Habila and D. Nallaswamy, J. Hazard. Mater., 2022, 440, 129705 CrossRef CAS PubMed.
- E. Saita, M. Iwata, Y. Shibata, Y. Matsunaga, R. Suizu, K. Awaga, J. Hirotani and H. Omachi, Front. Chem., 2022, 10, 841313 CrossRef CAS PubMed.
- O. Kaipoldayev, Y. Mukhametkarimov, R. Nemkaeva, G. Baigarinova, M. Aitzhanov, A. Muradov and N. Guseinov, Eurasian Chem.-Technol. J., 2017, 19, 181 Search PubMed.
- S. S. El-Deen, A. M. Hashem, A. E. Abdel Ghany, S. Indris, H. Ehrenberg, A. Mauger and C. M. Julien, Ionics, 2018, 24, 2925–2934 CrossRef CAS.
- T. Hu, J. Wang, H. Zhang, Z. Li, M. Hu and X. Wang, Phys. Chem. Chem. Phys., 2015, 17, 9997–10003 RSC.
- G. P. Lim, C. F. Soon, M. Morsin, M. K. Ahmad, N. Nayan and K. S. Tee, Ceram. Int., 2020, 46, 20306–20312 CrossRef CAS.
- A. C. Ferrari and J. Robertson, Phys. Rev. B:Condens. Matter Mater. Phys., 2000, 61, 14095–14107 CrossRef CAS.
- M. Han, X. Yin, H. Wu, Z. Hou, C. Song, X. Li, L. Zhang and L. Cheng, ACS Appl. Mater. Interfaces, 2016, 8, 21011–21019 CrossRef CAS.
- M. Amer, M. W. Barsoum, T. El-Raghy, I. Weiss, S. Leclair and D. Liptak, J. Appl. Phys., 1998, 84, 5817–5819 CrossRef CAS.
- Z. Bayhan, J. K. El-Demellawi, J. Yin, Y. Khan, Y. Lei, E. Alhajji, Q. Wang, M. N. Hedhili and H. N. Alshareef, Small, 2023, 19, e2208253 CrossRef PubMed.
- T. Parker, D. Zhang, D. Bugallo, K. Shevchuk, M. Downes, G. Valurouthu, A. Inman, B. Chacon, T. Zhang, C. E. Shuck, Y.-J. Hu and Y. Gogotsi, Chem. Mater., 2024, 36, 8437–8446 CrossRef CAS PubMed.
- K. Parveen, U. Rafique, I. Jamil and A. Ashraf, Environ. Monit. Assess., 2023, 195, 1106 CrossRef CAS PubMed.
- Y. Qi, W. Chen, L. Mai, Q. Zhu and A. Jin, Int. J. Electrochem. Sci., 2006, 1, 317–323 CrossRef CAS.
- N. Maheswari and G. Muralidharan, Appl. Surf. Sci., 2017, 416, 461–469 CrossRef CAS.
- D. Liu, T. Li, W. Sun, W. Zhou and G. Zhang, ACS Omega, 2022, 7, 31945–31953 CrossRef CAS PubMed.
- T. Cygan, J. Wozniak, M. Petrus, A. Lachowski, W. Pawlak, B. Adamczyk-Cieślak, A. Jastrzębska, A. Rozmysłowska-Wojciechowska, T. Wojciechowski, W. Ziemkowska and A. Olszyna, Materials, 2021, 14, 829 CrossRef CAS PubMed.
- L. Yate, J. C. Caicedo, A. H. Macias, F. J. Espinoza-Beltrán, G. Zambrano, J. Muñoz-Saldaña and P. Prieto, Surf. Coat. Technol., 2009, 203, 1904–1907 CrossRef CAS.
- C. Liu, K. Shih, Y. Gao, F. Li and L. Wei, J. Soils Sediments, 2012, 12, 724–733 CrossRef CAS.
- J. W. Robinson and E. M. Skelly Frame, Undergraduate Instrumental Analysis, Sixth Edition, CRC Press, Boca Raton, 2004 Search PubMed.
- IR Spectrum Table & Chart - Sigma-Aldrich, https://www.scribd.com/document/432355477/IR-Spectrum-Table-Chart-Sigma-Aldrich, accessed December 23, 2024 Search PubMed.
- R. A. A. Khan, A. Zulfqar, M. Mateen, M. Hussain, R. T. Rasool, G. A. Ashraf and G. Xianlong, Ceram. Int., 2023, 49, 26322–26330 CrossRef CAS.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- Infrared spectroscopy absorption table, https://chem.libretexts.org/Ancillary_Materials/Reference/Reference_Tables/Spectroscopic_Reference_Tables/Infrared_Spectroscopy_Absorption_Table, accessed December 23, 2024 Search PubMed.
- H.-E. Lai, R. M. S. Yoo, A. Djire and P. B. Balbuena, J. Phys. Chem. C, 2024, 128, 3327–3342 CrossRef CAS.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, Wiley-Interscience, Newy York, 6th edn, 2009 Search PubMed.
- N. M. Laptash and J. Fluor, Chem, 2000, 105, 59–64 CAS.
Footnote |
| † These authors equally contributed. |
| This journal is © The Royal Society of Chemistry 2025 |








