 Open Access Article
Open Access ArticleNano-enhanced Fenton/Fenton-like chemistry: integrating peroxidase nanozymes, MOFs, and MXenes for next-generation colorimetric biosensors
Hanh An
Nguyen†
 a,
Nguyen Tran Truc
Phuong†
a,
Nguyen Tran Truc
Phuong†
 bc,
Nguyen Bao
Tran
de,
Thi Ngoc Diep
Trinh
bc,
Nguyen Bao
Tran
de,
Thi Ngoc Diep
Trinh
 f,
Ngoc Xuan Dat
Mai
eg,
Ngoc Quang
Tran
f,
Ngoc Xuan Dat
Mai
eg,
Ngoc Quang
Tran
 eg,
Nhu Hoa Thi
Tran
eg,
Nhu Hoa Thi
Tran
 *de and
Kieu The Loan
Trinh
*de and
Kieu The Loan
Trinh
 *eg
*eg
aDepartment of Molecular Biology, Institute of Food and Biotechnology, Can Tho University, Can Tho City, Vietnam
bNguyen Tat Thanh University Center for Hi-Tech Development, Saigon Hi-Tech Park, Ho Chi Minh City, Vietnam
cNTT Hi-Tech Institute, Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam
dFaculty of Materials Science and Technology, University of Science, Ho Chi Minh City 70000, Vietnam. E-mail: ttnhoa@hcmus.edu.vn
eVietnam National University, Ho Chi Minh City 70000, Vietnam
fBioTechnology Institute, Tra Vinh University, Tra Vinh City 87000, Vietnam
gCenter for Innovative Materials and Architectures, Ho Chi Minh City 70000, Vietnam. E-mail: tktloan@inomar.edu.vn
First published on 30th June 2025
Abstract
Colorimetric biosensors exhibit promising potential toward molecular analysis with a wide range of sample types such as clinical, environmental, animal, and plant samples due to their high portability, sensitivity, specificity, and accuracy. Colorimetric biosensors rely on chromogenic reactions to transduce biochemical signals into visible color changes. Among the various signal transduction mechanisms, the Fenton/Fenton-like reaction is an outstanding reaction that can be used to detect a broad spectrum of analytes under diverse conditions. The ability to detect a wide range of analytes expands the application of the Fenton/Fenton-like reaction; this comes at the cost of specificity. This review highlights how integrating nanomaterials, such as peroxidase nanozymes, metal–organic frameworks (MOFs), and MXenes, can improve conventional Fenton/Fenton-like reactions, significantly enhancing specificity, selectivity, and catalytic efficiency. Besides, we demonstrated that the terms “Fenton/Fenton-like reaction” and “peroxidase-mimic nanozyme activity” fundamentally describe the same catalytic process, providing a unified perspective for researchers in the field. Moreover, the incorporation of MOFs and MXenes offers abundant active sites and enhanced electron transfer, resulting in improved sensitivity and selectivity in Fenton-based colorimetric assays.
1. Introduction
Colorimetric biosensors hold significant promise for molecular analysis and are widely utilized in clinical diagnostics, environmental monitoring, and quality control in the food industry. Colorimetric biosensors meet the urgent needs of on-field target monitoring due to the ability to operate outside the laboratory, simple fabrication, fast detection, and equipment-free result readout. The outstanding performance of colorimetric biosensors has been demonstrated in terms of high sensitivity, specificity, and portability. Their detection principle is based on chromogenic reactions, where the presence of target analytes triggers conformational changes in chromogenic substrates or influences chromogenic reactions, leading to distinct color shifts in the solution.1–3Most well-known chemical reactions for colorimetric detection include the Fenton/Fenton-like reaction, Schiff's base reaction, Lowry reaction, Bradford reaction, and plasmonic effects.4–9 Among them, the Fenton/Fenton-like reaction stands out as a particularly promising approach for several reasons. First, the Fenton/Fenton-like reaction can be used to detect a wide range of analytes such as metal ions, proteins, nucleic acids, and microorganisms, whereas the Schiff's base reaction, Lowry reaction, and Bradford reaction only detect aldehydes or proteins. Second, in contrast to plasmonic effects, which rely on limited types of materials, the Fenton/Fenton-like reaction can be initiated by a wide range of reagents, making it highly adaptable for different sensing applications. Given these advantages, this reaction holds great potential for the development of colorimetric biosensors. However, the Fenton/Fenton-like reaction still has some limitations that need to be overcome. For instance, the ability to respond to broad analytes expands its application; nevertheless, this comes at the cost of reduced specificity.10–13
The latest advancements in nanotechnology have created a fresh window of opportunity to improve the limitations of the Fenton/Fenton-like reaction. By leveraging nanotechnology, the analytical performance of the Fenton/Fenton-like reaction can be impressively enhanced through decreasing the size of Fenton/Fenton-like reagents down to the nanoscale, where unique quantum effects emerge, thereby increasing their catalytic activity. Unlike the traditional Fenton/Fenton-like reaction, which depends on strongly acidic conditions to trigger the reaction and has poor molecular accessibility, the nanoscale Fenton/Fenton-like reaction has mild reactive conditions and high molecular accessibility. For example, Meng et al. synthesized a ferric hydroxide nanocage (Fe(OH)3-NC) to trigger the Fenton/Fenton-like reaction, which was applied for the detection of CA 19-9, a model analyte.14 Fe(OH)3-NC the triggered Fenton/Fenton-like reaction under neutral pH conditions and provided more accessible catalytic sites, resulting in increasing analytical performance. Liang et al. used a Cu-Fenton system, mimicking peroxidase activity for colorimetric detection of creatinine.13 This research demonstrated that the distinction between the ‘Fenton/Fenton-like reaction’ and ‘peroxidase activity’ can be conceptually merged, especially within the framework of colorimetric biosensing. This conceptual merging enables the application of recent advantages from both systems to enhance the design and performance of colorimetric biosensors. Furthermore, along with significant advances in nanotechnology, tailorable nanomaterials can be rationally designed to enable the Fenton/Fenton-like reaction in response to specific analytes. Metal–organic frameworks (MOFs), for example, offer tunable porosity, allowing precise control over pore sizes and geometries to selectively bind target analytes. This not only enhances selectivity but also minimizes interference from non-specific molecules.15,16 Moreover, the stability of the Fenton/Fenton-like reaction can be improved when Fenton/Fenton-like reagents are anchored onto nanomaterials. MXenes, with a unique accordion structure, large surface area, and abundant terminal groups, serve as excellent nanomaterials for anchoring and stabilizing Fenton/Fenton-like reagents.17,18
In this review, the mechanisms of the Fenton/Fenton-like reaction for catalyzing colorimetric detection are first described. Then, the role of nanotechnology in improving the Fenton/Fenton-like reaction for colorimetric biosensor applications is discussed.
2. Principle of the Fenton/Fenton-like reaction
Initially, in 1876, Henry John Horstman Fenton discovered the special feature of ferrous ions (Fe2+) allowing them to oxidize tartaric acid (C4H6O6) with enhanced activity in the presence of hydrogen peroxide (H2O2). This catalytic oxidation system was then named after him as the Fenton reagent. Afterwards, finding the mechanism of the reagent he discovered became his career goal. Unfortunately, Fenton died in 1929 without knowing the mechanism fully explaining the Fenton reaction.19,20 Following Fenton's work, Fritz Haber and Joseph Weiss explained the mechanism of the Fenton reaction involving the generation of hydroxyl radicals (˙OH) in H2O2 reduction by Fe2+.21 They proposed that the Fe2+ ion donates an electron to an H2O2 molecule resulting in the generation of ˙OH through cleaving the O–O bridge of H2O2. The ˙OH radical then reacts with another H2O2 molecule to generate superoxide (O2˙−) and eventually form oxygen. The reaction chain is summarized below.| HOOH + M+x → HO˙ + M(+x+1) + OH− | (1) |
 | (2) |
 | (3) |
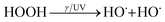 | (4) |
| HO˙ + RH → R˙ + H2O | (5) |
| HO˙ + R˙ → R–OH | (6) |
| R˙ + R˙ → R–R | (7) |
The hydroxyl radicals, which are generated in the Fenton reaction, have an extremely strong oxidation activity with oxidation potential varying between ∼2.0 and 2.8 E° (V). Thus, hydroxyl radicals are placed between ozone and fluorine among common oxidants.22 With the strong oxidation feature, the Fenton reaction has been widely used to degrade organic pollutants such as phenols, pesticides, pharmaceuticals, organic solvents, organic dyes, etc. Furthermore, the Fenton reaction has attracted great interest due to the rapid reaction, cheap and easy-to-find chemicals, and the ability to operate at ambient pressure and temperature. Recently, the application of the Fenton reaction has been extended to colorimetric assays for target analysis through employing the ability to rapidly react with organic dyes. In colorimetric assays, the Fenton reaction is commonly used to oxidize 3,3′,5,5′-tetramethylbenzidine (TMB)—a chromogenic substrate. The mechanism of this reaction involves a reaction between the surface Fe2+ (oxidation) and H2O2 (reduction) to generate ˙OH and Fe3+. Then, the resultant ˙OH participates in the oxidation of TMB, which causes a perceptible change from colorless to blue (Fig. 1).23 The presence of targets interrupts or enhances the effect of the Fenton reaction on TMB, resulting in altering the original color-changing trend. Guo et al. reported that Fe3O4 first forms an intermediate with H2O by capturing an O atom from a H2O2 molecule.24 Then, TMB binds to the surface of Fe3O4–O* and donates an electron to the exposed O atom to form an Fe3O4–O–TMB complex. In this step, TMB is oxidized, while Fe3O4–O* is reduced to form an Fe3O4–OH–oxTMB complex. oxTMB then leaves this complex resulting in Fe3O4–OH formation. Another TMB molecule approaches the Fe3O4–OH structure to form an Fe3O4–OH–TMB complex, which in turn creates another oxTMB and water molecule, while Fe3O4 is regenerated. In short, a cycle of this catalysis consumes one H2O2 molecule to oxidize two TMB molecules and form two H2O. Although the reaction of surface Fe2+ is the key process accounting for generating ˙OH, internal atomic changes and their contribution to the catalytic reaction should not be underestimated. Recently, Dong et al. proved that the electrons can be transferred from the internal Fe2+ within Fe3O4 to the surface through the Fe2+–O–Fe3+ chain, resulting in the regeneration of Fe2+.25 Since the Fenton reaction can occur with a variety of chromogenic substrates, Fenton reaction-based colorimetric assays can be expanded by using other chromogenic substrates such as o-phenylenediamine (OPD),26 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS),27 3,3′-diamino benzidine (DAB),28etc. These substrates can produce colored products during target analysis.
 | ||
| Fig. 1 Schematic diagram showing the color shift effect via the Fenton reaction.23 | ||
Modifications and improvements to the traditional Fenton reaction have been investigated aiming to increase reaction kinetics and enhance catalysis reactivity. Measures to achieve the improvement include the usage of alternative reagents to replace iron ions. Such reagents are commonly multivalent metals such as Cu, Mn, Co, Ce, Ag, Cr, Ru, Mo, V, W, Ti, etc.29,30 Like Fe ions, these metal ions can react with H2O2 to generate ˙OH. The reactions of these alternative reagents with H2O2 are referred to as Fenton-like reactions. Lower valent metals (Mn+) possess reduction properties and are converted to higher valent metals (M(n+x)+) with oxidation properties. Meanwhile, H2O2 possesses both reduction and oxidation properties. Although higher valent metals can also react with H2O2 to generate ˙OH, the reaction rate is quite low as compared to that of lower valent metals. Thus, the regeneration of lower valent metals from higher valent metals is essentially important. Other oxidants such as peroxymonosulfate (PMS)31 and peroxydisulfate (PDS)32 are alternative options for replacing H2O2 in the Fenton/Fenton-like reaction. The positively charged catalytic metal center can form complexes with the electronegative oxygen atoms in the peroxyl O–O bond of the peroxide family (H2O2, PMS, PDS, etc.). Due to their multivalent nature and lower electronegativity (XTM = 1.36–2.54) compared to O (XO = 3.44),33 transition metals can activate the O–O bond. Subsequently, electrons migrate from metal sites to peroxide, resulting in the breaking of the O–O bond and ˙OH generation. The decomposition of H2O2 (HO–OH) by the Fenton/Fenton-like reaction generates ˙OH, while the decomposition of PMS (HO–O–SO3–) and PDS (–O3S–O–O–SO3–) generates ˙OH and/or SO4˙−.34
3. Fenton/Fenton-like reaction coupling with nanotechnology for colorimetric biosensors
3.1. Fenton/Fenton-like reaction and peroxidase-mimic nanozyme: the two different terminologies describing the same fundamental catalytic process
The Fenton/Fenton-like reaction shares a fundamental mechanism with peroxidase-mimic enzymes, as they both generate reactive oxygen species (ROS) as the major products that play a vital role in colorimetric biosensors. Both systems rely on the reaction between transition metals and H2O2 to generate ROS such as ˙OH and O2˙− stimulating oxidation of chromogenic substrates. Peroxidase-mimic nanozymes describe a context in nanotechnology, which is involved in nanomaterials that exhibit similar catalytic activity to natural peroxidase. Meanwhile, the traditional Fenton/Fenton-like reaction is a term relating to homogeneous or heterogeneous catalysis, where the oxidation states of metal ions are cycled to degrade H2O2 into ˙OH. Although peroxidase-mimic nanozymes are described using different terminology, the underlying chemistry remains the same. That is related to the generation of ˙OH and other types of ROS through the decomposition of H2O2 driven by transition metals, which subsequently oxidizes chromogenic substrates in colorimetric biosensors.35The distinction between the Fenton/Fenton-like reaction and peroxidase-mimic nanozymes primarily arises from differences in research fields rather than fundamental chemistry. The term “peroxidase-mimic nanozymes” is used to describe a concept in nanotechnology that emphasizes the enzyme-like catalytic activity of nanomaterials, making them more convenient than natural peroxidase. Nevertheless, at its core, the working principle underlying peroxidase-mimic nanozymes is essentially a nanoscale adaptation of the Fenton/Fenton-like reaction. Moreover, both the Fenton/Fenton-like reaction and peroxidase-mimic nanozymes share one more similar reaction pathway regarding O2˙− generation as the secondary product, further reinforcing their mechanistic overlap.36 Therefore, rather than separating the Fenton/Fenton-like reaction and peroxidase-mimic nanozymes as distinct phenomena, they should be considered as the different terminologies describing the same fundamental catalytic process, one from the chemical viewpoint and the other from the nanomaterial engineering perspective. By doing this, the advantages and recent developments in both systems can be considered to improve colorimetric biosensors.
As a class of nanomaterials, nanozymes possessing intrinsic enzyme-mimic properties have been booming over the last decade due to their potential to overcome the limitations of traditional enzyme-based colorimetric assays. Along with the development of nanotechnology, nanozymes have superior properties over traditional enzymes regarding the capability to be rationally designed at the active centers and the surface. To date, nanozymes can mimic peroxidase, catalase, oxidase, superoxide dismutase, uricase, haloperoxidase, glutathione peroxidase, etc. However, only peroxidase-mimic nanozymes employ the Fenton/Fenton-like reaction. Therefore, this section mainly discusses peroxidase-mimic nanozymes.
In the conventional Fenton/Fenton-like reaction, metal ions and H2O2 need to be stored separately to prevent premature reactions because they react immediately upon contact with each other. This issue increases costs and complicates assay preparation. Recently, Lin et al. provided a feasible method to synthesize a copper peroxide (CP) nanodot through coordinating H2O2 to Cu2O nanoparticles with the aid of polyvinylpyrrolidone (PVP) and NaOH.56 NaOH plays a key role in deprotonating H2O2, facilitating the coordination of H2O2 with Cu2+. The CP nanodot integrates both H2O2 and Cu2+ into a single nanostructure, thus addressing the issue of separate reagent storage. The CP nanodot can release H2O2 and Cu2+ to trigger Fenton-like reactions upon adding acid to neutralize NaOH. Although this technique has not been applied in colorimetric assays, the CP nanodot can become a great strategy for developing more feasible, more cost-effective, and simpler Fenton-like reaction-based colorimetric assays.
Colorimetric assays applying a Fenton-based peroxidase can be used to detect a wide range of targets including nucleic acids, proteins, biomarkers, insecticides, antibiotics, organic compounds, metal ions, etc. The large variants of Fenton-like reactions depending on changing their nanocomposite enable Fenton-based colorimetric detection with a large number of target types. Liang et al. used a MoO3–Cu2+ system, in which MoO3 acted as a co-catalyst for enhancing peroxidase activity in creatinine assay.13 The MoO3–Cu2+ complex facilitated the oxidation of TMB and had high selectivity for creatinine because its chemical and physical properties facilitated a specific binding effect toward creatinine. Each nanocomposite seems to have specific affinity for its respective substrate. Ray et al. demonstrated that gold nanoparticles confined in the wall of mesoporous silica (AuMS) had high affinity toward dopamine.57 Among various biomolecules in the human body such as glucose, tryptophan, phenylalanine, tyrosine, ascorbic acid, and uric acid, only dopamine inhibits the Fenton-based oxidation of TMB, resulting in quenching of the blue color. To further increase the specificity of the Fenton/Fenton-like reaction, the surface of metal nanostructures can be conjugated with biorecognition elements such as antibodies, aptamers, peptides, and molecular imprinted polymers (MIPs). This ensures that the Fenton/Fenton-like reaction occurs preferentially in the presence of specific target molecules, enhancing the specificity of the colorimetric biosensors. For example, Ali et al. conjugated an aptamer to Au@Fe3O4 nanoparticles.58 The specific conformation of the aptamer endowed the Au@Fe3O4 nanoparticles with high affinity to E. coli O157:H7. Upon E. coli O157:H7 binding, Au@Fe3O4 nanoparticles cannot stimulate the Fenton/Fenton-like reaction, resulting in a significant decrease in absorbance. The specificity of aptamer-conjugated Au@Fe3O4 nanoparticles toward E. coli O157:H7 was demonstrated by evaluating their binding affinity against other pathogenic strains, including S. aureus, K. pneumoniae, P. aeruginosa, L. monocytogenes, S. typhimurium, C. albicans, and various E. coli serotypes (O78:H11, O1, O2, O6, O26, O33, O78, and O111). As a result, only E. coli O157:H7 inhibited the Fenton/Fenton-like reaction of Au@Fe3O4 nanoparticles.
In nanocomposites, the synergistic effect of multi-metal complexes is commonly used for enhancing the catalytic performance. Multimetallic nanozymes generally possessed greater catalytic activity than that obtained from monometallic or bimetallic nanozymes. For example, Au@PtRu nanorods exhibited much higher peroxidase-like activity than Au and Au@Pt nanorods.60 PtPdCu trimetallic nanoalloys had enhanced peroxidase activity as compared to PtPd bimetallic nanoalloys and commercial Pt/C.61 The insertion of Cu into the PtPd structure not only provides more active sites but also causes a change in the electronic structure, which is beneficial for improving the catalytic performance. InCex nanoparticles possessed great peroxidase-like activity compared to pure In2O3 and CeO2.62 Su et al. demonstrated that NiCo2O4 mesoporous spheres enabled a lower limit of detection than the method using Co3O4 and NiO nanoparticles individually, highlighting the advantage of the bimetallic oxide over its single-metal counterparts.63 Zhi et al. inserted Cu into three noble metals including Pt, Rh, and Ru to form a quaternary alloy nanozyme (PtRhRuCu), exhibiting peroxidase activity for a specific glucose assay (Fig. 2A).59 The morphology of PtRhRuCu nanoparticles is shown in Fig. 2B. This nanocomposite possessed unique electronic structures and the modulation of the electronic states due to the multielement effect. The Cu ions were responsible for selective activation of Fenton-like reactions in the presence of H2O2 and regulation of the d-band center, which could obviously boost peroxidase activity. The PtRhRuCu nanoparticles show good selectivity for glucose detection, with minimal interference from other sugars (D-fructose, sucrose, D-galactose, and maltobiose), as shown in Fig. 2C. Moreover, the PtRhRuCu nanoparticles can detect glucose in the concentration range of 0 to 1 mM (Fig. 2D). The colorimetric assay applying the PtRhRuCu quaternary nanozyme can detect glucose as low as 0.98 μM, which is significantly lower than the limit of detection when using the PtRhRu ternary alloy nanozyme (29.59 μM).
 | ||
| Fig. 2 (A) Mechanisms of enhanced peroxidase-like activity induced by the presence of Cu in a quaternary nanozyme. (B) TEM images of PtRhRuCu nanoparticles, containing size distribution. (C) Results showing the specificity test of glucose detection via PtRhRuCu-based colorimetric analysis. (D) Calibration curves of various glucose concentrations (0–1 mM; y = −77.47557x + 113.18419, R2 = 0.99893).59 | ||
One of the biggest disadvantages of Fenton and Fenton-like reactions is that metal ions are not stable in liquid solution and their catalytic activities are reduced over time, affecting the efficiency of colorimetric detection. For example, in a copper-based Fenton-like reaction, to initiate the generation of ˙OH, Cu2+ needs to be reduced to Cu+; then, Cu+ mainly contributes to the catalytic reaction. However, Cu+ is unstable and easily oxidized to Cu2+ due to the low redox potential of Cu2+/Cu+. Moreover, the slow conversion of Cu2+ to Cu+ limits the reaction rate. To solve this problem, Hong et al. synthesized a catalyst that enables Cu to be in the reduced state (Cu+), namely the 4-(dimethylamino) cinnamaldehyde oxalyl dihydrazone Cu(I) complex.64 However, this complex only provides Cu+, which does not assist the Cu2+/Cu+ cycle. Later, Li et al. used fullerenes (C60)—a carbon-based nanomaterial—for dual purposes including stabilizing Cu+ and accelerating the Cu2+/Cu+ cycle.65 C60 can transfer electrons to Cu2+ and serve as a single electron acceptor of Cu0, making C60 beneficial for stabilizing Cu+.66 The C60-doped Cu+ exhibited impressive peroxidase activity resulting in excellent sensitivity with a detection limit of 115 nM bleomycin. The combination between metal ions and supports not only enhances the Fenton/Fenton-like reaction but also endows the catalysts with new features regarding the electrochemical signal output. For example, Song et al. used Cu2+-doped polypyrrole nanotubes for dual-mode biosensors (colorimetric and electrochemical modes).67 The polypyrrole nanotubes with low dimensionality and a distinctive π-conjugated chain structure of the polymer provided high conductivity, while Cu2+ facilitated a Fenton-like reaction. As a result, this Cu2+-doped polypyrrole nanotube biosensor had dual-signal outcomes including colorimetric and electrochemical signals.
3.2. MOF-assisted Fenton/Fenton-like reaction
Metal–organic frameworks (MOFs) are porous crystal coordination polymers with various pore sizes and functions. MOFs are self-assembled from metal ions and organic ligands via coordination action, endowing them with both inorganic and organic characteristics. MOF-based materials have been widely used in biosensors and catalysis due to their large specific surface area, abundant active exposure sites, favorable access of reactants to active sites, tailorable properties, etc. The ability for regular arrangement of metal nodes in MOFs endows MOF-based materials with peroxidase-like activity via catalyzing the Fenton/Fenton-like reaction.68–71 Some MOF-based nanomaterials are constantly emerging in Fenton-based colorimetric biosensors, such as MILs,72–74 PCNs,75–77 ZIFs,78–80etc. Ren et al. used a MIL-53(Cu) support for immobilizing nFe2O3 nanoparticles (nFe2O3/MIL-53(Cu)).81 nFe2O3/MIL-53(Cu) exhibited supreme catalytic activity due to better dispersion of nFe2O3, small size of particles, and the iron–copper synergistic effect. The nFe2O3/MIL-53(Cu)/H2O2 system reached a pseudo-first-order rate constant of 0.0123 min−1 for bisphenol A degradation, while MIL-53(Cu)/H2O2 and nFe2O3/H2O2 systems reached only 0.0026 and 0.0040 min−1, respectively. This result revealed that MOFs could enhance the Fenton reaction.One of the most useful strategies for enhancing the Fenton-based catalytic performance of MOFs is employing synergistic effects when multiple metals are used together. For example, Deng et al. employed synergistic effects by doping Mn and Fe ions along with Pd nanoparticles into Fe-MOF (Mn/Fe-MOF@Pd1.0) for colorimetric detection of hydroquinone (Fig. 3A).82 The morphology of Mn/Fe-MOF@Pd1.0 is illustrated in Fig. 3B. In this system, Mn and Fe ions provide different redox potentials and work synergistically to activate H2O2 for producing active radicals, while Pd nanoparticles improve the structural stability and tolerance toward acidic conditions. Mn/Fe-MOF@Pd1.0 oxidizes colorless TMB in the presence of H2O2 to form blue oxidized TMB (Ox-TMB). However, the presence of hydroquinone interferes with this chromogenic reaction, effectively maintaining TMB in its colorless form. Fig. 3C demonstrates that Mn/Fe-MOF@Pd1.0 has significantly enhanced catalytic performance toward TMB oxidation compared to Fe-MOF alone. Moreover, the Mn/Fe-MOF@Pd1.0 nanocomposite shows high selectivity for hydroquinone, with minimal interference from other structurally similar phenolic compounds (Fig. 3D). The Mn/Fe-MOF@Pd1.0-based colorimetric biosensor showed a good linear relationship with hydroquinone concentrations ranging from 0.3 to 30 μM (R2 = 0.998), with a detection limit of 0.09 μM (Fig. 3E). Similarly, bimetallic Fe/Co-MIL-88(NH2) can convert colorless TMB to colored TMB at a higher reaction rate than the monometallic Fe-MIL-88(NH2), resulting in the production of a 2.1 times higher signal.83 In this system, Co(II) probably represents the reason for the enormous catalytic performance of Fe/Co-MIL-88(NH2). Besides, the bimetallic MOF has higher affinities for both H2O2 and TMB as compared to the monometallic Fe-MIL-88(NH2). The stronger affinity of the bimetallic MOF could be due to the highly porous nature of the MOF and the large number of active sites due to the presence of dual metals that strongly facilitate the binding and reaction with H2O2. For another example of multi-metallic MOFs, MoCu-2MI (2-methylimidazole) synthesized by Li et al. exhibited stronger peroxidase-like activity than pure Cu-2MI.16 Mo served as a co-catalyst to accelerate the electron transfer and facilitated the Cu2+/Cu+ cycle in the Fenton-like reaction. As a result, MoCu-2MI produced large levels of ˙OH in the presence of H2O2 and finally improved the catalytic activity.
 | ||
| Fig. 3 (A) Solvothermal synthesis and synergistic Fenton/Fenton-like catalytic performance of the Mn/Fe-MOF@Pd1.0 nanocomposite for the colorimetric detection of hydroquinone. (B) SEM image of Mn/Fe-MOF@Pd1.0. (C) UV-vis absorption of different reaction systems demonstrating the enhanced catalytic activity of the Mn/Fe-MOF@Pd nanocomposite for the oxidation of TMB. (D) Selectivity of the Mn/Fe-MOF@Pd1.0-based colorimetric biosensor for hydroquinone detection in the presence of various phenolic substances as interfering substances. (E) Linear relationship between absorption and hydroquinone concentrations.82 | ||
One of the most distinctive features of MOFs is their highly porous structure and the sizes of these pores can be easily controlled, which enables specific binding of targets. This feature not only enhances the specificity of colorimetric assays but also endows them with another function that is target separation and extraction. During the process of target separation, target molecules are drawn toward the MOF surface by diffusion from the bulk solution to the active pores of the MOF. This process occurs due to two types of intermolecular forces of attraction: (1) chemisorption (such as ionic interactions) and (2) physisorption (such as hydrogen bonding, van der Waals, and π–π interactions).84,85 Some MOF-based materials that have been commonly used for target separation are MIL-101(Cr) for penicillin,86 ZIF-8 for fluoroquinolones,87 MOF-199 for benzene homologs,88 MIL-53(Al) for PAHs,89 CoFe2O4/MIL-101(Fe) for N-nitrosamines,90etc. Li et al. used Fe-NH2-MIL-88B for both extraction and colorimetric detection of tetracycline.15 The porous nature of Fe-NH2-MIL-88B facilitated size-selective separation, while –NH2 could react with –OH from tetracycline. More interestingly, the hydrogen-bonding interaction between –NH2 and –OH caused electronic interactions that enhanced the Fenton reaction of Fe-NH2-MIL-88B.
Some MOFs can contribute to the generation of fluorescence signals, making them valuable components in the design of dual-mode biosensors.91,92 Combining fluorescence and colorimetric properties, MOFs serve as a promising approach for fabricating dual-mode biosensors. MIL-101(Fe)-NH2 is a representative example, which can be synthesized by coordinating 2-aminoterephthalic acid (NH2-BDC) as an organic ligand with FeCl3·6H2O as a metal node. NH2-BDC incorporates a fluorescence emission peak at 455 nm when excited at 380 nm, while the mixed-valence metal node of Fe3+/Fe2+ contributes to the Fenton reaction. This dual functionality enables MIL-101(Fe)-NH2 to simultaneously generate both fluorescence and colorimetric signals. He et al. employed the bifunctionality of MIL-101(Fe)-NH2@MIP for ratiometric fluorescent/colorimetric detection of chloramphenicol (CAP) (Fig. 4).93 MIL-101(Fe)-NH2@MIP catalyzed the oxidation of colorless o-phenylenediamine (OPD) to form a yellow solution of 2,3-diaminophenazine (DAP) in the presence of H2O2. In the meantime, the fluorescence intensity of MIL-101(Fe)-NH2@MIP at 455 nm decreased, while the fluorescence of DAP at 560 nm increased under excitation at 380 nm. In the presence of CAP as a target molecule, the molecularly imprinted polymer (MIP) cavities on MIL-101(Fe)-NH2@MIP capture CAP. This binding prevented H2O2 from entering the pores of MIL-101(Fe)-NH2@MIP, thereby suppressing the Fenton reaction and reducing hydroxyl radical generation. As a result, catalyzed OPD chromogenic reactions are hindered, maintaining colorless OPD. Simultaneously, the fluorescence signal at 560 nm from DAP is suppressed, while the peak fluorescence signal of MIL-101(Fe)-NH2@MIP at 455 nm is restored. Alternatively, Liu et al. reported a dual-mode biosensor for the early diagnosis of acute myocardial infarction (AMI) (Fig. 4B). Using cardiac troponin I (cTnI) as a diagnosis biomarker for AMI, they employed an ultrathin Fe-MOF-74 nanosheet, which exhibited both peroxidase-mimicking activity and fluorescence, to construct a biosensor capable of dual-mode detection of cTnI.94 Upon cTnI binding, a cascade of reactions is triggered. First, glucose oxidase (GOx) is activated to generate hydrogen peroxide. Subsequently, ROS is produced through a Fenton-like reaction catalyzed by the generated hydrogen peroxide and Fe-MOF-74 nanosheet. ROS further changes colorless TMB into blue oxTMB. For the fluorescence signal, the Fe-MOF-74 nanosheet contains 2,5-dihydroxy-1,4-benzenedicarboxylic acid (DOBDC) ligands, which act as fluorophores with an excitation wavelength of 355 nm and an emission peak at 552 nm. The presence of cTnI increases the level of oxTMB, resulting in quenching fluorescence of the Fe-MOF-74 nanosheet. As a result, the fluorescence intensity decreases and is inversely proportional to the cTnI concentration. The biosensor showed good linearity with cTnI concentrations in the range of 10–2000 pg mL−1 in both fluorescence and colorimetric modes with the detection limits of 6.4 pg mL−1 and 8.4 pg mL−1, respectively.
 | ||
| Fig. 4 (A) Schematic representation of colorimetric/ratiometric fluorescent dual-mode platform determination of chloramphenicol (CAP) via MIL-101 (Fe)-NH2@MIP.93 (B) Colorimetric/fluorescent detection of cardiac troponin I induced by Fe-MOF-74 NS-1.94 | ||
For target analysis, MOFs have become promising nanomaterials for assisting the Fenton/Fenton-like reaction to improve colorimetric biosensors due to the following advantages. (1) Tailorable porosity: this feature offers the capability to control specific pore sizes and geometries for specific binding of target analytes. It results in enhanced selectivity and reduces interference from non-specific molecules. (2) High specific surface area: this feature provides a large interface for target binding, resulting in enhanced sensitivity of biosensors. (3) Tunable surface functional groups: various ligands, metal ions, aldehyde groups, carboxylic groups, and other functional groups can be selected to regulate appropriate interaction and affinity between MOFs and target analytes. (4) Great biocompatibility and biodegradability: the low toxicity of MOFs enables the development of biosensors for in vivo monitoring and target detection.
3.3. MXene-assisted Fenton/Fenton-like reaction
MXene is a new type of two-dimensional (2D) nanomaterial, which was first discovered when 2D titanium-carbide (Ti3C2) layers were exfoliated from bulk three-dimensional (3D) titanium aluminum carbide (Ti3AlC2) using hydrofluoric acid.95 MXenes have the chemical formula Mn+1XnTx, where M implies transition metals (Cr, Mn, Ce, Ti, Mo, etc.); X implies either nitrogen or carbon; n = 1, 2, or 3; and T represents the functional groups (–O, –OH, and –F).96 MXene has the suffix “ene” to indicate that it has graphene-like structures and shares the beneficial properties of graphene such as 2D architecture, great metallic conductivity, excellent adherence to substrates, large surface area, and fluorescence quenching ability. However, unlike graphene, MXenes show quick dispersion in aqueous media without the need for any additives. MXenes also contain more diverse functional groups resulting from the etching process. With these excellent properties, MXenes have become prospective materials for sensing applications. Unfortunately, MXenes alone cannot be used for colorimetric detection because pristine MXenes do not exhibit peroxidase-like activity and cannot trigger the Fenton/Fenton-like reaction. Consequently, MXenes are not commonly able to change the color of chromogenic substrates. Therefore, MXenes are mostly used as supports for increasing the catalytic performance, stability, recyclability, pH, thermal resistance, etc. of the Fenton/Fenton-like reaction.The ability of MXenes to improve the catalytic performance of the Fenton/Fenton-like reaction relies on the low valence transition metals surrounding the surface of MXenes. These low valence transition metals are considered as efficient reducing agents and can reduce Fe3+, resulting in the facilitation of the Fe3+/Fe2+ cycle. For example, the surface of Ti3C2 is filled with low valence titanium, which can trigger Fe3+/Fe2+ redox cycling to activate H2O2 due to the ability to effectively reduce Fe3+ through Ti–C bond breakage.96 Facure et al. demonstrated that the presence of Ti3C2 in ZnO–Co3O4 nanofibers led to a nanocomposite with superior catalytic activity.18 The novel architecture formed when combining multiple types of nanomaterials probably promoted the synergistic effect. The van der Waals forces between Ti3C2 and ZnO–Co3O4 nanofibers can lead to more suitable conformations, which led to a nanocomposite with a higher specific surface area and a superior peroxidase-like activity. The Ti3C2 matrix has high electronic density and high electron mobility, which enhance rapid charge transfer from TMB to H2O2. The 2D morphology of the Ti3C2 matrix provides a large surface area and great absorption ability, resulting in a boosted Fenton/Fenton-like reaction (Fig. 5A and B). Wang et al. used a Ti3C2@Fe3O4 nanocomposite to detect H2O2 with a detection limit of 0.4 μM.97 The Ti3C2@Fe3O4 nanocomposite possessed a higher peroxidase-like activity than its individual components. The enhanced peroxidase-like activity of the Ti3C2@Fe3O4 nanocomposite was ascribed to the large ion-accessible interface, quick electron transfer channels in the MXene architecture, and the synergistic effect of Ti3C2 and Fe3O4.
 | ||
| Fig. 5 (A) Synthesis and (B) application of a MXene-based nanocomposite for colorimetric detection.18 | ||
Apart from Ti3C2, vanadium-based MXenes are other representative MXenes that can trigger the Fenton/Fenton-like reaction. V2C MXene does not require the incorporation of other metals or metal oxides to promote the Fenton/Fenton-like reaction as Ti3C2. However, V2C requires proper oxidation to be transformed into VOxC before it can be used to trigger the Fenton/Fenton-like reaction.98 In the chemistry of the VOxC complex, vanadium has three adjacently mixed valence states: V3+, V4+, and V5+ in aqueous solution. Among these valence states, V4+ is dominant. This mixed valence state, dominated by V4+, possesses abundant electron transport behaviors.99
Taking advantage of nanotechnology, transition metals have been controlled at the nanoscale level providing more active sites resulting in a more efficient Fenton/Fenton-like reaction. However, nano-sized metals are prone to aggregation due to the exponential increase of surface energy. The aggregation of nanoscale metals in aqueous solution significantly reduces the efficiency of the Fenton/Fenton-like reaction because it can block active sites resulting in instability and poor recyclability. In this regard, MXenes with layered structures serve as ideal substrates for anchoring catalytically active sites, thus increasing the stability of Fenton catalysts such as metals and metal oxides. The key that makes MXenes highly suitable for anchoring Fenton catalysts is the unique synthesis process of MXenes. Taking the most studied MXene as an example, Ti3C2 MXene is synthesized by selectively exfoliating the Al atom layers from the Ti3AlC2 precursor, followed by intercalation with dimethyl sulfoxide (DMSO). This process generates a unique accordion structure, which provides MXenes with more space for immobilization of Fenton/Fenton-like catalysts.100 Some common metals and metal oxides coupled with MXene to form nanocomposites include Fe3O4/Ti3C2,100 Cu2O/Ti3C2,101 Co3O4/Ti3C2,102 Cu2O/TiO2/Ti3C2,103 ZnO/Nb2C, and ZnO/V2C.104 Fe3O4 anchored onto Ti3C2 MXene showed high recyclability over four cycles with high stability.105 Meanwhile, TiO1.47@C synthesized by direct oxidation of Ti3C2 MXene showed little change in the efficiency of Fenton-like reactions after five reactions, which demonstrates its high recyclability. The shelf life of TiO1.47@C was highly desirable as it demonstrated good structural stability.17 Another benefit of the etching process is that it could occasionally strip away transition metal atoms in MXenes leaving single vacancies or vacancy cluster defects that are highly oxophilic. These defect sites can easily form oxide clusters through abstraction of oxygen or hydrolysis. As a result, the compositing transition metals of MXenes such as V, Ti, and Nb can become source materials for the in situ formation of transition metal oxides. These multivalent metal oxides efficiently promote the Fenton/Fenton-like reaction in the presence of H2O2.
Moreover, MXenes not only enhance the efficiency of the Fenton/Fenton-like reaction for colorimetric detection, but also contribute to fluorescence-based detection due to their excellent fluorescence quenching properties. The dual properties regarding Fenton/Fenton-like catalysis and fluorescence quenching have made MXene nanocomposites become promising materials to fabricate dual-mode biosensors giving both colorimetric and fluorescent outputs. For example, Kong et al. used a Ti3C2 MXene nanozyme for the colorimetric and fluorescence dual-mode detection of aflatoxin B1 (Fig. 6). The morphology and nanostructure of the synthesized Ti3C2 MXene were characterized using SEM and TEM, as shown in Fig. 6A–E. Together, the SEM and TEM analyses confirm the typical layered morphology with an accordion-like structure of MXene. For fluorescence-based mode, the surface of Ti3C2 MXene was functionalized with a fluorescein (FAM) conjugated ssDNA aptamer (Fig. 6F). Ti3C2 MXene can absorb the emission energy of the FAM probe, resulting in the fluorescence quenching effect. However, the presence of the target aflatoxin B1 separates ssDNA aptamer-FAM due to the strong affinity between aflatoxin B1 and the ssDNA aptamer. Once the ssDNA aptamer-FAM structure left Ti3C2 MXene, its fluorescence emission was recovered. Meanwhile, colorimetric mode relied on the Ti vacancies, which were generated from the etching process in MXene synthesis. These Ti vacancies have high proton affinity and nucleophilicity, facilitating electron transfer from the surface of MXene to nearby H2O2 and further promoting the electron transfer from TMB to Ti3C2. When TMB loses electrons, the color of the solution changes from colorless to blue. The presence of the ssDNA aptamer effectively enhanced the activity of Ti3C2 MXene, while the dissociation of the aptamer due to the presence of aflatoxin B1 reduced the activity of Ti3C2 MXene. This dual-mode biosensor had detection limits of 0.09 ng mL−1 and 0.61 ng mL−1 for fluorescence and colorimetric modes, respectively. To further expand the application and increase the practicability of this biosensing strategy, a smartphone-based RGB analysis system was incorporated for quantitative analysis (Fig. 6G–I). After the colorimetric reaction catalyzed by Ti3C2 MXene through a Fenton-like mechanism, the results were captured using a smartphone under controlled lighting conditions for color intensity analysis. The Ti3C2 MXenes played a central role in this process, not only acting as peroxidase mimics but also facilitating the Fenton-like reaction. These MXene-driven Fenton-like reactions produced dual and reliable colorimetric outputs that varied with AFB1 concentrations. The MXene-based Fenton-like reaction combining a smartphone-based system showed a good linear relationship between RGB values and aflatoxin B1 concentrations in the range of 1–800 ng mL−1 (Fig. 6J).106 Similarly, Nb2C MXene coupled with aptamer-FAM was used to fabricate dual-mode (fluorescence and colorimetric) biosensors for the detection of aflatoxin B1.107 This Nb2C MXene-based dual-mode biosensor achieved a detection line as low as 0.0950 ng mL−1 under optimal conditions.
 | ||
| Fig. 6 (A) The SEM image of Ti3C2 MXene. (B–E) The TEM images of Ti3C2 MXene. (F) Preparation and application of Ti3C2@ssDNA for a multimode biosensor (colorimetric, fluorescence, and smartphone-based modes). (G and H) Design of a smartphone-based system for quantitative analysis. (I) Analysis of aflatoxin B1 concentration using the three channels of RGB. (J) Calibration curve showing the relationship between color intensity and aflatoxin B1 concentration in RGB mode.106 | ||
MXenes, with an accordion-like layered structure, provide a large surface specific area and abundant channels for electron transport. These unique properties make MXenes good candidates for fabricating dual-mode biosensors with both colorimetric and electrochemical outputs, especially when integrated with Fenton/Fenton-like reactions. This approach was effectively demonstrated in the work of Liu and co-worker (Fig. 7).108 In their work, Ti3C2Tx MXene nanoribbons were loaded with Au to form a Ti3C2Tx MNR@Au nanohybrid via a simple self-reduction process and then used as a nanozyme to detect Hg2+. The Ti3C2Tx MNR@Au nanohybrid is extremely limited in triggering Fenton-like reactions. However, the presence of Hg2+ significantly enhances Fenton-like reactions of the Ti3C2Tx MNR@Au nanohybrid, which can efficiently catalyze the oxidation of o-phenylenediamine (OPD) to form colored products (2,3-diaminophenazine, DAP) (Fig. 7A). Simultaneously, DAP produced a distinct reduction peak at −0.4 V, serving as an electrochemical signal. Based on this dual-mode approach, a highly sensitive biosensor was fabricated for the colorimetric and electrochemical detection of Hg2+. The colorimetric mode enables convenient and naked-eye detection, while the electrochemical mode provides high sensitivity, with a detection limit as low as 17 pM and a wide linear range of 0.4 nM to 2 μM. The Ti3C2Tx MNR@Au nanohybrid was demonstrated for high selectivity toward Hg2+ detection (Fig. 7B and C). This remarkable selectivity originates from the formation of an Au–Hg amalgam on the surface of the Ti3C2Tx MNR@Au nanohybrid. The presence of Hg2+ significantly (Table 1) enhances the Fenton/Fenton-like reaction of the hybrid material, resulting in signal amplification that surpasses the response induced by other tested metal ions, including Na+, K+, Ca2+, NH4+, Cu2+, Zn2+, Ni2+, Fe2+, Co2+, Mg2+, Mn2+, Pb2+, Cd2+, Fe3+, and As3+.
 | ||
| Fig. 7 (A) Mechanism underlying dual-mode (electrochemical/colorimetric mode) sensing of Hg2+via the Ti3C2Tx MNR@Au nanohybrid. (B) Differential pulse voltammetry (DVP) current response of different metal ions and Hg2+ added into the biosensor including Na+, K+, Ca2+, NH4+, Cu2+, Zn2+, Ni2+, Fe2+, Co2+, Mg2+, Mn2+, Pb2+, Cd2+, Fe3+, and As3+. (C) The corresponding image to the color change of different metal ions and Hg2+.108 | ||
| Fenton reagent and dye | Type of nanomaterial | LOD | Target | Type of sample | Application |
|---|---|---|---|---|---|
| Fenton reagent: Fe3O4–Fe0/Fe3C, H2O2 dye: TMB39 | Peroxidase nanozyme | 67.1 pM | H2O2 | Milk, live HepG2 cells | Food monitoring, cell monitoring |
| Fenton reagent: Fe3O4/nitrogen-doped porous carbon nanocomposite (Fe3O4/N-PCNC), H2O2 dye: TMB41 | Peroxidase nanozyme | 0.1 μM (H2O2) 2.6 μM (glucose) | H2O2, glucose | Various drinks | Food monitoring |
| Fenton reagent: Fe3O4@Pt nanoparticles, H2O2 dye: TMB45 | Peroxidase nanozyme | 0.36 μM (H2O2) 1.27 μM (glucose) | H2O2, glucose | Human serum samples, urine samples | Health monitoring |
| Fenton reagent: Ag–Fe3O4, H2O2 dye: TMB46 | Peroxidase nanozyme | 0.4 μM | Sulfur ions | Water | Environmental monitoring |
| Fenton reagent: PtRhRuCu quaternary alloy nanozymes, H2O2 dye: TMB59 | Peroxidase nanozyme | 0.021 mM | Glucose | Amino acid drink, probiotic drink, vitamin drink | Beverage monitoring |
| Fenton reagent: gold nanoparticles mesoporous silica (AuMS), H2O2 dye: TMB57 | Peroxidase nanozyme | 1.28 nM | Dopamine | Dopamine hydrochloride injection | Drug monitoring |
| Fenton reagent: Pt/Fe-MOF, H2O2 dye: TMB109 | MOF | 2.3 μM | Glucose | Human serum | Health monitoring |
| Fenton reagent: Fe/Co-MIL-88(NH2) MOF, H2O2 dye: TMB83 | MOF | 7.8 × 104 particles per mL | Extracellular vesicles | Extracellular vesicles secreted by HeLa cells, sera samples | Health monitoring |
| Fenton reagent: Mn/Fe-MOF@Pd1.0, H2O2 dye: TMB82 | MOF | 0.09 μM | Hydroquinone | Whitening cream and actual water samples | Environmental and cosmetic monitoring |
| Fenton reagent: MXene@Fe3O4, H2O2 dye: TMB97 | MXene | 0.4 μM (H2O2) 0.5 μM (glutathione) | H2O2, glutathione | Human blood samples | Health monitoring |
| Fenton reagent: VOxC, H2O2 dye: TMB99 | MXene | 2.5 μM (dopamine) 0.36 μM (glutathione) | Dopamine, glutathione | Spiked solution | Health monitoring |
| Fenton reagent: Ti3C2, H2O2 dye: TMB106 | MXene | 0.09 ng mL−1 (fluorescent mode) 0.61 ng mL−1 (colorimetric mode) 0.96 ng mL−1 (smartphone mode) | Aflatoxin B1 | Peanut sample | Food monitoring |
| Fenton reagent: ZnO–Co3O4 NFs/Ti3C2Tx, H2O2 dye: TMB18 | MXene | 0.58 μmol L−1 | Ascorbic acid | Orange juice | Beverage monitoring |
4. Conclusion and future perspectives
This review summarized current reports discussing the mechanisms as well as current progress in the development and application of the Fenton/Fenton-like reaction for colorimetric biosensors. Along with significant advances in nanotechnology, the catalytic performance, stability, and recyclability of the Fenton/Fenton-like reaction have been significantly improved. First, from the perspective that the Fenton/Fenton-like reaction and peroxidase-mimic nanozymes are the two terminologies describing the same fundamental catalytic process, the advancements of nanozymes also increase the catalytic performance of the Fenton/Fenton-like reaction, especially for biosensor applications. For example, recent advances in nanozyme synthesis allow rational design of the active centers and the surface, which can control the catalytic performance of the Fenton/Fenton-like reaction. Second, MOFs assist the Fenton/Fenton-like reaction exhibiting supreme catalytic activity due to the ability to increase the dispersion of metals and metal oxides, resulting in smaller particles and more active sites. Besides, MOFs have a unique morphology with high porosity and the pore size can be easily adjusted. This feature enables MOFs to specifically capture analytes. Thus, by using a MOF-assisted Fenton/Fenton-like reaction, both sensitivity and selectivity of the colorimetric biosensors can be increased. Third, the MXene-assisted Fenton/Fenton-like reaction enables better stability and catalytic performance. MXenes with a layered structure serve as ideal substrates for anchoring catalytically active sites, thus increasing the stability of Fenton catalysts. The unique accordion structure of MXenes provides quick electron transfer channels and a large ion-accessible interface in the MXene architecture, which eventually enhances the catalytic performance of the Fenton/Fenton-like reaction. Besides, MXenes have excellent fluorescence quenching properties, enabling the fabrication of dual-mode biosensors, which give both colorimetric and fluorescence outputs. However, some limitations remain to be overcome for further improvement.First, although MOFs have been used for enhancing the selectivity of the Fenton/Fenton-like reaction through controlling pore sizes, the selectivity is still relatively low. Besides, optimization is required to find the pore size that offers the highest affinity toward the analytes which can cause extra cost and effort. Thus, future research needs to focus on improving selectivity as well as simplifying the optimization process. First, for enhancing selectivity, some current emerging technologies can be used such as molecular imprinting, aptamer conjugating, antibody/antigen coating, etc. Second, leveraging computer science can offer key benefits such as rational design for specific properties, a simple optimization process, discovery of Fenton/Fenton-like reaction pathways, etc. via simulation and computational modeling.
Second, the Fenton/Fenton-like reaction possesses multiple key features such as fast electron transfer cycles, strong catalytic performance, abundant sources of Fenton agents, etc. With these features, the Fenton/Fenton-like reaction can participate in several signal releasing pathways such as electrochemical, colorimetric, and fluorescent signals. Leveraging multiple signal releasing pathways, the Fenton/Fenton-like reaction possesses great potential for fabricating multiple-mode biosensors. There are several publications demonstrating the application of the Fenton/Fenton-like reaction for dual-mode biosensors. However, trio- and quad-mode biosensors have not been studied much yet. Future research should investigate more Fenton/Fenton-like nanocomposites that can release more types of signals for the fabrication of multiple-mode biosensors.
Third, the Fenton/Fenton-like reaction can produce hydroxyl radicals. These highly active species have strong antimicrobial activity, which can be employed for killing harmful microorganisms. The antimicrobial properties of the Fenton/Fenton-like reaction should be investigated and used together with biosensors to decrease the infectious risk from patients.
Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Vietnam National University, Ho Chi Minh City under grant number TX2025-50-01. The authors show their sincere gratitude to Tra Vinh University for generous support in this work. Also, the authors would like to gratefully acknowledge the Nguyen Tat Thanh University for support in this work.References
- L. Tessaro, A. Aquino, P. Panzenhagen, N. Joshi and C. A. Conte-Junior, J. Pharm. Biomed. Anal., 2023, 222, 115087 CrossRef CAS PubMed.
- A. Márquez, S. Santiago, M. V. Dos Santos, S. D. Aznar-Cervantes, C. Domínguez, F. G. Omenetto, G. Guirado and X. Muñoz-Berbel, ACS Appl. Bio Mater., 2024, 7, 853–862 CrossRef.
- M. Adampourezare, B. Nikzad, S. Sajedi-Amin and E. Rahimpour, BMC Chem., 2024, 18, 80 CrossRef CAS.
- M. A. Redmile-Gordon, E. Armenise, R. P. White, P. R. Hirsch and K. W. T. Goulding, Soil Biol. Biochem., 2013, 67, 166–173 CrossRef CAS.
- S. Warakaulle, H. Mohamed, M. Ranasinghe, I. Shah, X. Yanyang, G. Chen, M. M. Ayyash, D. Vincent and A. Kamal-Eldin, J. Food Compos. Anal., 2024, 126, 105854 CrossRef CAS.
- G. M. Abu-Taweel, H. M. Al-Saidi, M. Alshareef, M. A. M. Alhamami, J. S. Algethami and S. S. Alharthi, J. Fluoresc., 2025, 35, 221–236 CrossRef.
- Z.-P. Deng, B. Zhao, L.-L. Gao, B.-T. Ji, J.-G. Li, Y.-X. Sun, J. Li and Y. Sun, J. Mol. Struct., 2024, 1299, 137203 CrossRef CAS.
- C. Chen, Q. Fan, Z. Li, Z. Cai, Z. Ye and Y. Yin, Nano Lett., 2024, 24, 3737–3743 CrossRef CAS.
- C. Wenck, D. Leopoldt, M. Habib, J. Hegermann, M. Stiesch, K. Doll-Nikutta, A. Heisterkamp and M. L. Torres-Mapa, Nanoscale Adv., 2024, 6, 1447–1459 RSC.
- J. Xiao, S. Guo, D. Wang and Q. An, Chem.–Eur. J., 2024, 30, e202304337 CrossRef CAS.
- A. A. Babu Christus, P. Panneerselvam and A. Ravikumar, Anal. Methods, 2018, 10, 4378–4386 RSC.
- H. A. Nguyen, H. Choi and N. Y. Lee, Biosensors, 2022, 12, 488 CrossRef CAS PubMed.
- L. Liang, Y. Xiong, Y. Duan, W. Zuo, L. Liu, F. Ye and S. Zhao, Biosens. Bioelectron., 2022, 206, 114121 CrossRef CAS.
- X. Meng, Y. Xu, N. Zhang, B. Ma, Z. Ma and H. Han, Sens. Actuators, B, 2021, 338, 129840 CrossRef CAS.
- Z. Li, F. Meng, R. Li, Y. Fang, Y. Cui, Y. Qin and M. Zhang, Biosens. Bioelectron., 2023, 234, 115294 CrossRef CAS PubMed.
- S. Li, L. Liang, L. Tian, J. Wu, Y. Zhu, Y. Qin, S. Zhao and F. Ye, J. Mater. Chem. B, 2023, 11, 7913–7919 RSC.
- Y. Jiang, D. Baimanov, S. Jin, J. Cheuk-Fung Law, P. Zhao, J. Tang, J. Peng, L. Wang, K. S.-Y. Leung, W. Sheng and S. Lin, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2210211120 CrossRef CAS PubMed.
- M. H. M. Facure, L. A. Mercante, Y. Gogotsi and D. S. Correa, ACS Appl. Nano Mater., 2025, 8(9), 4291–4299 CrossRef CAS.
- R. E. Coleman, R. B. Boulton and A. A. Stuchebrukhov, J. Phys. Chem. B, 2023, 127, 4300–4308 CrossRef CAS.
- R. Ovalle, in Biochemistry, ed, R. Ahmad, IntechOpen, 2022, vol. 28 Search PubMed.
- F. Haber and W. Joseph, Proc. R. Soc. London, A, 1934, 147, 332–351 CAS.
- S. Navalon, M. Alvaro and H. Garcia, Appl. Catal., B, 2010, 99, 1–26 CrossRef CAS.
- B. Yuan, H.-L. Chou and Y.-K. Peng, ACS Appl. Mater. Interfaces, 2022, 14, 22728–22736 CrossRef CAS.
- S. Guo and L. Guo, J. Phys. Chem. C, 2019, 123(50), 30318–30334 CrossRef CAS.
- H. Dong, W. Du, J. Dong, R. Che, F. Kong, W. Cheng, M. Ma, N. Gu and Y. Zhang, Nat. Commun., 2022, 13, 5365 CrossRef CAS.
- Q. Ye, S. Ren, H. Huang, G. Duan, K. Liu and J.-B. Liu, ACS Omega, 2020, 5, 20698–20706 CrossRef CAS PubMed.
- B. Bekdeşer and R. Apak, ACS Omega, 2024, 9, 11738–11746 CrossRef.
- R. Ai and Y. He, Sens. Actuators, B, 2020, 304, 127372 CrossRef CAS.
- W. Cao, M. Jin, K. Yang, B. Chen, M. Xiong, X. Li and G. Cao, J. Nanobiotechnol., 2021, 19, 325 CrossRef CAS PubMed.
- Y. Liu and J. Wang, Chem. Eng. J., 2023, 466, 143147 CrossRef CAS.
- Y. Zhao, X. Deng, Y. Yang, Y. Zhang, H. Wang and B. Xin, J. Environ. Chem. Eng., 2023, 11, 110220 CrossRef CAS.
- S. Tang, H. Liu, E. Zhu, T. Zhao, Z. Wang, T. Jiao, Q. Zhang and D. Yuan, Sep. Purif. Technol., 2022, 301, 122056 CrossRef CAS.
- A. Ugartemendia, I. Casademont-Reig, L. Zhao, Z. Zhang, G. Frenking, J. M. Ugalde, A. Garcia-Lekue and E. Jimenez-Izal, Chem. Sci., 2024, 15, 6151–6159 RSC.
- J. Guo, B. Gao, Q. Li, S. Wang, Y. Shang, X. Duan and X. Xu, Adv. Mater., 2024, 2403965 CrossRef CAS PubMed.
- B. Das, J. L. Franco, N. Logan, P. Balasubramanian, M. I. Kim and C. Cao, Nano-Micro Lett., 2021, 13, 193 CrossRef CAS.
- C. Abe, T. Miyazawa and T. Miyazawa, Molecules, 2022, 27, 5451 CrossRef CAS PubMed.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nature Nanotech., 2007, 2, 577–583 CrossRef CAS PubMed.
- N. N. Ulusu, J. Mol. Evol., 2015, 80, 251–257 CrossRef CAS PubMed.
- A. F. Baye, H. Thi Nguyen and H. Kim, Sens. Actuators, B, 2023, 377, 133097 CrossRef CAS.
- A. Jonidi Jafari, B. Kakavandi, N. Jaafarzadeh, R. Rezaei Kalantary, M. Ahmadi and A. Akbar Babaei, J. Ind. Eng. Chem., 2017, 45, 323–333 CrossRef CAS.
- A. Nsabimana, S. A. Kitte, F. Wu, L. Qi, Z. Liu, M. N. Zafar, R. Luque and G. Xu, Appl. Surf. Sci., 2019, 467–468, 89–97 CrossRef CAS.
- V. Cao, P. A. Cao, D. L. Han, M. T. Ngo, T. X. Vuong and H. N. Manh, Nat. Env. Poll. Tech, 2024, 23, 255–263 CrossRef CAS.
- Z. Duan, W. Zhang, M. Lu, Z. Shao, W. Huang, J. Li, Y. Li, J. Mo, Y. Li and C. Chen, Carbon, 2020, 167, 351–363 CrossRef CAS.
- R. Zeng, Y. Li, X. Hu, W. Wang, Y. Li, H. Gong, J. Xu, L. Huang, L. Lu, Y. Zhang, D. Tang and J. Song, Nano Lett., 2023, 23, 6073–6080 CrossRef CAS.
- Y. He, P. Wang, X. Chen, Y. Li, J. Wei, G. Cai, K. Aoyagi and W. Wang, R. Soc. Open Sci., 2022, 9, 220484 CrossRef CAS PubMed.
- Y. Wang, Y. Ding, Y. Tan, X. Liu, L. Fu and W. Qing, J. Environ. Chem. Eng., 2023, 11, 109150 CrossRef CAS.
- B. Han, H. Guan, B. Peng, Y. Zhang and Y. Liu, Anal. Methods, 2022, 14, 4832–4841 RSC.
- J. Zhu and Z. Nan, J. Phys. Chem. C, 2017, 121, 9612–9620 CrossRef CAS.
- X. Huang, C. Xu, J. Ma and F. Chen, Adv. Powder Technol., 2018, 29, 796–803 CrossRef CAS.
- J. Xiao, J. Lai, R. Li, X. Fang, D. Zhang, P. Tsiakaras and Y. Wang, Ind. Eng. Chem. Res., 2020, 59, 12431–12440 CrossRef CAS.
- T. T. N. Le, H. B. Truong, L. Thi Hoa, H. S. Le, T. T. T. Tran, T. D. Manh, V. T. Le, Q. K. Dinh and X. C. Nguyen, Heliyon, 2023, 9, e20466 CrossRef CAS PubMed.
- Y. Wang, H. Li, L. Guo, Q. Jiang and F. Liu, RSC Adv., 2019, 9, 18815–18822 RSC.
- Y. J. Jang, V. K. H. Bui, P. T. Nguyen, Y.-C. Lee and M. I. Kim, Chemosensors, 2021, 9, 219 CrossRef CAS.
- Y. Zhong, L. Yu, Z.-F. Chen, H. He, F. Ye, G. Cheng and Q. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 29203–29212 CrossRef CAS.
- Y. Huang, H. Zhong, C. Jiang, J. Yang, J. Zhang, F. Zhao and C. Liu, Particuology, 2024, 84, 126–135 CrossRef CAS.
- L.-S. Lin, T. Huang, J. Song, X.-Y. Ou, Z. Wang, H. Deng, R. Tian, Y. Liu, J.-F. Wang, Y. Liu, G. Yu, Z. Zhou, S. Wang, G. Niu, H.-H. Yang and X. Chen, J. Am. Chem. Soc., 2019, 141, 9937–9945 CrossRef CAS PubMed.
- S. Ray, R. Biswas, R. Banerjee and P. Biswas, Nanoscale Adv., 2020, 2, 734–745 RSC.
- R. Ali, A. Alattar, R. Alshaman, A. Ghabban, S. Alanazi, H. Al-Brahimi, M. Alatwi, A. Jlawi, A. Albalawi, A. Moutair Awad Alatawi, B. Al Balawi, A. Al-Marwani and M. M. El-Wekil, Food Chem., 2024, 443, 138564 CrossRef CAS.
- X. Zhi, Q. Yang, X. Zhang, H. Zhang, Y. Gao, L. Zhang, Y. Tong and W. He, Food Chem., 2024, 445, 138788 CrossRef CAS PubMed.
- F. Lv, Y. Gong, Y. Cao, Y. Deng, S. Liang, X. Tian, H. Gu and J.-J. Yin, Nanoscale Adv., 2020, 2, 1583–1589 RSC.
- Y. Mao, F. Jia, T. Jing, T. Li, H. Jia and W. He, ACS Sustainable Chem. Eng., 2021, 9, 569–579 CrossRef CAS.
- B. Liu, H. Ruan, C. Li, J. Yao, B. Wei, L. Wang, S. Ban and J. Xie, New J. Chem., 2023, 47, 18476–18484 RSC.
- L. Su, W. Dong, C. Wu, Y. Gong, Y. Zhang, L. Li, G. Mao and S. Feng, Anal. Chim. Acta, 2017, 951, 124–132 CrossRef CAS PubMed.
- Z. Hong, J. Zhong, D. Ding, S. Gong, L. Zhang, S. Zhao, X.-C. Shen, H. Liang and F.-P. Huang, Dalton Trans., 2023, 52, 6187–6193 RSC.
- S. Li, Y. Pan, M. Li, S.-H. Li, S. Zhao and F. Ye, Anal. Bioanal. Chem., 2024, 416, 6021–6031 CrossRef CAS PubMed.
- J. Zheng, L. Huang, C.-H. Cui, Z.-C. Chen, X.-F. Liu, X. Duan, X.-Y. Cao, T.-Z. Yang, H. Zhu, K. Shi, P. Du, S.-W. Ying, C.-F. Zhu, Y.-G. Yao, G.-C. Guo, Y. Yuan, S.-Y. Xie and L.-S. Zheng, Science, 2022, 376, 288–292 CrossRef CAS PubMed.
- N. Song, S. Chen, D. Tian, Y. Li, C. Wang and X. Lu, Mater. Today Chem., 2020, 18, 100374 CrossRef CAS.
- P. Wu, F. Gong, X. Feng, Y. Xia, L. Xia, T. Kai and P. Ding, J. Nanobiotechnol., 2023, 21, 185 CrossRef CAS.
- C. Gao, S. Chen, X. Quan, H. Yu and Y. Zhang, J. Catal., 2017, 356, 125–132 CrossRef CAS.
- S. Lu, L. Liu, H. Demissie, G. An and D. Wang, Environ. Int., 2021, 146, 106273 CrossRef CAS PubMed.
- H. Sohrabi, S. Ghasemzadeh, S. Shakib, M. R. Majidi, A. Razmjou, Y. Yoon and A. Khataee, Ind. Eng. Chem. Res., 2023, 62, 4611–4627 CrossRef CAS.
- L. Liu, J. Wang, J. Wang, J. Wu, S. Wu and L. Xie, ChemistrySelect, 2021, 6, 7143–7149 CrossRef CAS.
- P. Huang, Q. Chang, G. Jiang, X. Wang, H. Zhu and Q. Liu, Spectrochim. Acta, Part A, 2023, 285, 121943 CrossRef CAS.
- Y. Lu, T. Wang, C. Tang, Q. Niu and T. You, New J. Chem., 2023, 47, 21505–21512 RSC.
- M. Xia, T. Liu and Y. Zhang, Anal. Sci., 2021, 37, 1023–1027 CrossRef CAS PubMed.
- J. Li, X. Yang, Q. Li, D. Yang, Q. Hu, Z. Zhong and Y. Yang, Food Biosci., 2024, 59, 103910 CrossRef CAS.
- Y. Wu, C. Ke, Z. Song, H. Zhu, H. Guo, H. Sun and M. Liu, Analyst, 2024, 149, 935–946 RSC.
- Z. Zhao, T. Lin, W. Liu, L. Hou, F. Ye and S. Zhao, Spectrochim. Acta, Part A, 2019, 219, 240–247 CrossRef CAS.
- K. Yang, G. Chen, L. Wang, M. Guo, J. Xu, Y. Ma, Z. Luo, A. Zeng and Q. Fu, New J. Chem., 2023, 47, 4103–4112 RSC.
- S. Wang, D. Xu, L. Ma, J. Qiu, X. Wang, Q. Dong, Q. Zhang, J. Pan and Q. Liu, Anal. Bioanal. Chem., 2018, 410, 7145–7152 CrossRef CAS.
- Y. Ren, M. Shi, W. Zhang, D. D. Dionysiou, J. Lu, C. Shan, Y. Zhang, L. Lv and B. Pan, Environ. Sci. Technol., 2020, 54, 5258–5267 CrossRef CAS.
- D. Deng, Y. Wang, S. Wen, Y. Kang, X. Cui, R. Tang and X. Yang, Anal. Chim. Acta, 2023, 1279, 341797 CrossRef CAS.
- Q. Jiang, Y. Xiao, A. N. Hong, Z. Gao, Y. Shen, Q. Fan, P. Feng and W. Zhong, ACS Appl. Mater. Interfaces, 2022, 14, 41800–41808 CrossRef CAS.
- M. Safari, in Recent Trends in the Application of Metal–Organic Frameworks, IntechOpen, 2024 Search PubMed.
- Z. Hasan and S. H. Jhung, J. Hazard. Mater., 2015, 283, 329–339 CrossRef CAS.
- C. Lin, S. Lirio, Y. Chen, C. Lin and H. Huang, Chem.–Eur. J., 2014, 20, 3317–3321 CrossRef CAS PubMed.
- J. Pang, Y. Liao, X. Huang, Z. Ye and D. Yuan, Talanta, 2019, 199, 499–506 CrossRef CAS PubMed.
- X.-Y. Cui, Z.-Y. Gu, D.-Q. Jiang, Y. Li, H.-F. Wang and X.-P. Yan, Anal. Chem., 2009, 81, 9771–9777 CrossRef CAS PubMed.
- X.-F. Chen, H. Zang, X. Wang, J.-G. Cheng, R.-S. Zhao, C.-G. Cheng and X.-Q. Lu, Analyst, 2012, 137, 5411 RSC.
- P. Miralles, I. Van Gemert, A. Chisvert and A. Salvador, J. Chromatogr. A., 2019, 1604, 460465 CrossRef CAS PubMed.
- C. Lou, F. Yang, L. Zhu, Q. Sun, Y. Yang and J. Guo, Colloids Surf., A, 2023, 677, 132398 CrossRef CAS.
- S. Zhang, H. Li, D. Yang and Y. Yang, Talanta, 2024, 280, 126785 CrossRef CAS PubMed.
- X.-Y. He, Y. Wang, Q. Xue, W.-F. Qian, G.-L. Li and Q. Li, Food Chem.: X, 2025, 26, 102322 CAS.
- J. Liu, Y. Wang, W. Peng, B. Qiu, K. Wong and S. Hu, Anal. Chim. Acta, 2025, 1350, 343800 CrossRef CAS.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- S. Xu, C. Liu, X. Jiang, X. Wang, S. Zhang, Y. Zhang, Q. Wang, W. Xiong and J. Zhang, J. Hazard. Mater., 2023, 444, 130450 CrossRef CAS PubMed.
- J. Wang, W. Xu, L. Zhou, T. Zhang, N. Yang, M. Wang, X. Luo, L. Jin, H. Zhu and W. Ge, Microchim. Acta, 2022, 189, 452 CrossRef CAS.
- H. Geng, X. Li, X. J. Gao, Y. Cong, Q. Liu, J. Li, Y. Guan, L. Wang and W. He, Nano Today, 2023, 52, 101989 CrossRef CAS.
- H. Jia, Q. Liu, J. Si, Y. Chen, G. Zhou, H. Lan and W. He, Nanoscale Adv., 2023, 5, 5799–5809 RSC.
- Y. Cui, D. Zhang, K. Shen, S. Nie, M. Liu, H. Huang, F. Deng, N. Zhou, X. Zhang and Y. Wei, J. Environ. Chem. Eng., 2020, 8, 104369 CrossRef CAS.
- H. Xia, B. Li, Y. Ye, S. Wang, A. Chen and R. K. Kankala, Adv. Healthcare Materials, 2024, 13, 2303582 CrossRef CAS PubMed.
- Y. Liu, R. Luo, Y. Li, J. Qi, C. Wang, J. Li, X. Sun and L. Wang, Chem. Eng. J., 2018, 347, 731–740 CrossRef CAS.
- J. Yin, B. Ge, T. Jiao, Z. Qin, M. Yu, L. Zhang, Q. Zhang and Q. Peng, Langmuir, 2021, 37, 1267–1278 CrossRef CAS.
- W. Zhou, B. Yu, J. Zhu, K. Li and S. Tian, Appl. Surf. Sci., 2022, 590, 153095 CrossRef CAS.
- M. Rethinasabapathy, G. Bhaskaran, B. Park, J.-Y. Shin, W.-S. Kim, J. Ryu and Y. S. Huh, Chemosphere, 2022, 286, 131679 CrossRef CAS.
- Y. Kong, Y. Zhu, J. Song, Q. Liu, L. Song, X. Fei and X. Li, Food Chem., 2023, 426, 136645 CrossRef CAS.
- Y. Kong, Z. Li, L. Zhang, J. Song, Q. Liu, Y. Zhu, N. Li, L. Song and X. Li, Biosens. Bioelectron., 2023, 242, 115725 CrossRef CAS PubMed.
- T. Liu, R. Zhou, K. Wu and G. Zhu, Anal. Chim. Acta, 2023, 1250, 340975 CrossRef CAS PubMed.
- J. Li, J. Zhao, S. Li, Y. Chen, W. Lv, J. Zhang, L. Zhang, Z. Zhang and X. Lu, Nano Res., 2021, 14, 4689–4695 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |








