 Open Access Article
Open Access ArticleA review of interface optimization strategies for solid electrolytes and anode materials
Dandan
Wang
ab,
Xinyang
Wu
ab,
Yongpeng
Ren
 *abc,
Yaru
Li
*ab,
Xiaolin
Xie
c,
Xiqiang
Ma
c,
Ihar
Razanau
d,
Xuemin
Chen
ab,
Junhao
Lu
ab and
Kunming
Pan
abc
*abc,
Yaru
Li
*ab,
Xiaolin
Xie
c,
Xiqiang
Ma
c,
Ihar
Razanau
d,
Xuemin
Chen
ab,
Junhao
Lu
ab and
Kunming
Pan
abc
aSchool of Materials Science and Engineering, Henan University of Science and Technology, Luoyang 471000, China
bHenan Key Laboratory of High-temperature Structural and Functional Materials, Henan University of Science and Technology, Luoyang 471003, China
cLongmen Laboratory, Luoyang 471000, China
dLaboratory of Physical-Chemical Technologies, Scientific-Practical Materials Research Centre of National Academy of Sciences of Belarus, Minsk 220072, Belarus
First published on 9th May 2025
Abstract
With the increasing demand for high-performance power batteries in electric vehicles, low-altitude economy, military applications, and other fields, existing liquid electrolyte-based battery technologies are gradually becoming incapable of meeting the energy density and safety requirements. New battery systems based on solid electrolytes are the main candidate materials for future power batteries owing to their high safety and energy density. Thus far, researchers have conducted extensive studies on the ionic/electronic transfer mechanisms of solid electrolytes and electrode materials, as well as the cooperative effects and interface issues between them. Although much progress has been made, the practical application of solid-state batteries is still severely limited by the high interface impedance between the solid electrolyte and the anode. This impedance stems from incompatible physical and chemical properties and dynamic interface evolution. This paper focuses on the latest progress in the interface engineering strategies of solid electrolytes and anodes and systematically analyzes the cooperative coupling effect between charge transfer dynamics and mechanical stability at the interface. This review provides insights into the future research in this field, aiming to offer a new perspective to enhance our understanding of solid-state lithium batteries, thereby facilitating their more optimal design and promoting their practical applications.
1. Introduction
Driven by the rapid development of new energy vehicles, the installation of power batteries in China has shown a continuous upward trend. However, the explosive growth in sales of new energy vehicles has highlighted energy density and safety issues in traditional high-performance lithium batteries. Flammable organic liquid electrolytes (LEs) can easily heat up, decompose, and swell under abnormal conditions such as overcharging and short circuits, leading to thermal runaway. Additionally, liquid lithium-ion batteries are limited by their system, with a maximum energy density of only 300 Wh kg−1, which struggles to meet the increasing performance demands of power batteries.1–4 To simplify battery design while pursuing more efficient and safer batteries, solid-state batteries came into being.Solid-state electrolytes (SSEs) are the core component of solid-state batteries, replacing flammable traditional organic LEs. SSEs possess mechanical strength that effectively mitigates the safety risks associated with lithium dendrites, fundamentally reducing the risk of battery fires and explosions. Most SSEs maintain good ion transport capabilities in both low- and high-temperature environments, ensuring stable operation of solid-state batteries across a wide temperature range, which further enhances battery cycle stability.4,5 Additionally, SSEs are compatible with more reactive anode materials, such as lithium, and have a lighter weight, which can further enhance battery energy density. Although LEs exhibit good interfacial wettability and low interfacial impedance, the solid–solid interface between SSEs and electrodes suffers from high interfacial impedance, which significantly hinders Li+ transport across the interface.6 Therefore, the electrolyte–electrode interface issue is a critical challenge that urgently needs to be addressed in the field of solid-state batteries. In contrast to the cathode interface, the electrolyte–anode interface faces a range of challenges, including the formation of lithium dendrites, substantial volume changes, and various side reactions. These factors lead to unstable interface structures and limited ion transport, posing a greater challenge to optimizing solid-state battery performance, especially in high energy density anode material systems.7,8
Although lithium metal and silicon based anode materials are considered ideal anode materials for solid-state batteries due to their high specific capacity and low reduction potential, their inherent properties exacerbate the instability of the electrolyte–anode interface. For example, the high theoretical capacity of lithium metal anodes leads to continuous dissolution and deposition of active lithium, while the significant volume expansion of silicon anodes during lithiation leads to a vicious cycle of mechanical stress accumulation and interfacial chemical instability. Problems such as side reactions at the solid-state electrolyte–anode interface, instability of the anode solid electrolyte interphase (SEI) layer, and restricted ion transport at the interface severely impact the electrochemical performance and safety of solid-state batteries.9–11 Therefore, it is very important to study and solve the problem of the electrolyte–anode interface for the construction of a high-performance solid-state battery system. In response to this, this paper offers a comprehensive review of the research progress on the solid-state electrolyte–anode interface in lithium batteries. It systematically examines the challenges and underlying mechanisms encountered by solid-state electrolytes, anode materials, and their interfaces. The goal is to elucidate design strategies and methods for optimizing electrolytes, anodes, and interfaces for next-generation high-performance solid-state batteries, ultimately facilitating their large-scale deployment.
2. Solid-state electrolytes (SSEs)
SSEs have the advantages of low volatility, high temperature resistance, corrosion resistance, chemical stability and excellent mechanical properties, and higher safety compared with LEs. They are also compatible with high-energy-density systems, with streamlined battery construction and enhanced energy density.12–14 Currently, SSEs are primarily categorized into inorganic solid electrolytes (ISEs), solid polymer electrolytes (SPEs), and composite solid polymer electrolytes (CSPEs) (Fig. 1).152.1 Inorganic solid electrolytes (ISEs)
Inorganic solid electrolytes (ISEs) exhibit high ionic conductivity, thermal stability, Young's modulus, and safety. However, they face challenges such as the high interface impedance of the electrodes and the difficulty of handling thin ISE layers, which often require pressure to be applied during testing. Based on the choice of electrolyte materials, ISEs can be categorized into three types: oxides, sulfides, and halides.In addition, strategies such as enhancing the flexibility of the electrolyte,21 reducing the surface roughness,22 and coating or modifying the electrolyte surface23 can improve the interface contact with the anode and promote the application of oxide solid electrolytes in solid-state batteries. Future research will continue to address the core challenges of interface contact, using strategies such as element doping, grain boundary modification, and the construction of composite electrolytes to reduce interface impedance.
2.2 Solid polymer electrolytes (SPEs)
SPEs primarily consist of polymer matrices (such as PEO) and lithium salts (like LiTFSI, LiDFOB, and LiPF6). Flexible polymer chains have the advantages of good flexibility and adaptability. These polymer chains can adjust to various electrode interfaces through the movement and entanglement of molecular segments, ensuring a close fit to the electrode surface while maintaining efficient ionic transport pathways.33,34Single-phase SPEs cannot meet the needs of high-performance solid-state batteries. The emergence of organic–inorganic composite solid-state polymer electrolytes may combine the advantages of both types, potentially breaking existing limitations and serving as a key to advancing solid-state battery development (Fig. 3 and Table 1).
 | ||
| Fig. 3 (a) Schematic of ion diffusion in highly crystalline PEO.36 (b) Schematic of the PEO-based electrolyte interface and performance enhancement.43 (c) Schematic representation of Li+ transport in the PVDF-based electrolyte.44 (d) Diffusion of [Li(DMF)x]+ in P-NCM cathodes.45 (e) Transport mechanism of [Li(DMF)x]+ in residual DMF solvent within the PVDF-based electrolyte.41 | ||
| Classification | Advantages | Disadvantages | Matching issue with anode (such as lithium metal) |
|---|---|---|---|
| Oxide | High-pressure resistance (4–5 V) | High rigidity and poor interface contact | Poor infiltration with lithium metal |
| Good stability and low cost | Low ionic conductivity (10−4 to 10−3 S cm−1) | Interface side reaction may result in the formation of a high-resistance layer | |
| Sulfide | High ionic conductivity (10−3 to 10−2 S cm−1) | Sensitive to air and generates H2S when in contact with water | Poor interface stability with lithium metal, prone to form lithium dendrites |
| Good extensibility, easy to be processed into films | Low oxidation potential (easily reacts with the positive electrode at high voltage) | ||
| Halide | High ionic conductivity (10−3 S cm−1) and superior air stability compared with sulfides | High cost of raw materials | Poor infiltration of lithium metal |
| Combining the advantages of sulfides and oxides | Low reduction potential, poor compatibility with lithium metal | Interface side effect | |
| Polymer | Good flexibility | Low ionic conductivity (10−7 to 10−5 S cm−1) | Insufficient mechanical strength, lithium dendrites are prone to penetrate |
| Good interface contact | Poor ability to withstand high pressure (<4 V) |
2.3 Organic–inorganic composite solid polymer electrolytes (CSPEs)
CSPEs are composed of polymers, lithium salts and fillers, combining the benefits of SPEs and ISEs. However, they also face serious interface problems.46 The physical and chemical properties of organic phase and inorganic phase are different, so that the two are prone to phase separation, and some inorganic materials have high chemical activity on the surface, prone to side reactions, with the side reaction products covering the surface of the electrode or filling the interface between the electrolyte and the electrode, which will lead to increased interface impedance.47–50 Jin et al. used the “multi-affinity” of the 12C4-TFSI supramolecular nanomodification layer to connect PVDF, LLZTO and LiTFSI, and realized the interface optimization among the components in CSPEs.51 Wu et al. used PAA with a small LUMO–HOMO gap to remove alkaline impurities such as Li2CO3, reduce the degree of dehydrofluorination of PVDF, and improve the compatibility with polymer matrix.52In addition, a layer of material with good compatibility and ionic conductivity is inserted at the interface as the interface buffer layer, which can improve the interface contact condition and effectively avoid the interface side reaction. Wang et al. coated nano-porous KANF on the LATP surface and constructed a SE@KANF protective layer by in situ polymerization, which could improve the interface stability of the effective lithium anode.53 Wang et al. improved the mechanical properties of the electrolyte by in situ polymerization of low molecular weight polyethylene glycol dimethyl ether (PEGDME) and polymethyl methacrylate (PMMA). Lithium difluoro (oxalate) borate (LiDFOB) forms a fluorine-rich SEI film at the lithium anode interface, effectively restraining the side reaction and improving the interface stability.54
In several mainstream solid electrolytes, the movement of polymer chain segments in polymer solid electrolytes is relatively limited under RT, resulting in a slow Li+ migration rate.55 The oxide-based solid electrolyte has a relatively stable crystal structure framework and a suitable Li+ transmission channel. Some electrolytes can achieve ionic conductivity of about 10−4 S cm−1 under RT. However, issues related to interface compatibility remain difficult to resolve.56 Sulfide-based solid electrolytes achieve ionic conductivities of 10−3 S cm−1 or higher at RT due to their relatively low ion migration energy barriers and loose structures. However, significant side reactions present urgent challenges. The composite electrolyte has high ionic conductivity, good thermal stability and mechanical properties, which can promote the rapid development of solid-state battery applications. Solving problems such as oxide interface impedance and sulfide side reactions will promote the medium-and long-term application of solid-state batteries (Fig. 4).
 | ||
| Fig. 4 (a) Schematic of Li+ transport and charge distribution at the electrolyte–electrode interface in LLZTO and (b) PDL@LLZTO effect of electrolyte on 2D/3D lithium anodes.57 (c) Diagram of the PEGDME@PMMA electrolyte and internal Li+ transport.54 Digital photos and SEM of lithium deposition before cycling and after 10 hours for (d) PEGDME/LiTFSI, (e) PEGDME/LiTFSI/LiDFOB, and (f) PEGDME/LiDFOB.54 SEM of cross-sections after cycling of (g) Li|SE-LATP and (h) Li|SE@KANF-LATP.53 (i) Performance of Li|SE@KANF-LATP|LFP and Li|SE-LATP|LFP.53 | ||
3. Anode of solid-state batteries
Anode materials in solid-state batteries are critical for energy storage and release, significantly influencing energy density, stability, and compatibility with solid electrolytes. It is essential for anode materials to maintain a stable interface with solid electrolytes to ensure battery efficiency and longevity.58 Traditional graphite anodes have struggled to meet the needs of high energy density solid-state batteries, leading to a shift towards high-performance anode materials such as silicon, lithium, and even anode-free designs. Lithium metal and silicon-based anodes have extremely high specific capacities and are becoming prime candidates for next-generation solid-state batteries. It is expected that silicon-based anodes will first be applied to polymer and polymer oxide solid-state batteries, followed by the integration of silicon and lithium in pure oxide or sulfide systems, with the ultimate goal of high-performance solid-state batteries with lithium anodes or no anodes at all. The lithium metal anode provides the lithium source directly to the battery, significantly increasing the energy density and reducing the charging time. However, they still face challenges such as lithium dendrite formation, unstable and uneven interface contact, unstable solid electrolyte interphase (SEI) films, and low critical current density (CCD).59 Silicon has the advantages of large theoretical capacity and abundant availability. The risk of forming lithium dendrites is low due to good alloy reaction kinetics and high lithium deimpingement potential. However, significant volume expansion, irreversible side reactions at the interface, and poor intrinsic conductivity severely hinder its practical application.60 Achieving dense contact between the solid electrolyte and anode materials not only reduces interfacial resistance and ensures smooth Li+ transport between the anode and electrolyte but also effectively suppresses lithium dendrite formation, thereby enhancing the cycling stability and safety of the battery.61,62 However, most solid electrolytes are difficult to wet and permeate porous electrodes, with side reactions further exacerbating interface problems (Fig. 5). | ||
| Fig. 5 Comparison of silicon anodes and lithium metal anodes in all-solid-state lithium batteries.63 | ||
In solid-state batteries, anode materials store and release energy through repeated Li+ insertion and deinsertion. The solid electrolyte isolates the anode from the cathode to prevent short circuits, while providing high ionic conductivity and good chemical stability, ensuring efficient Li+ transfer between the anode and cathode. During the charge and discharge process, Li+ migrates through the solid electrolyte, and a redox reaction occurs to promote the storage and release of energy. The close contact between the solid electrolyte and the negative electrode material reduces the interface resistance, ensures the smooth transfer of Li+, effectively inhibits the growth of lithium dendrites, and improves the stability and safety of the cycle.61,62,64 But, most SSEs are difficult to wet and permeate porous electrodes, resulting in poor electrolyte–electrode interface contact. However, because of their minimal interfacial side reactions, they are compatible with lithium metal and silicon-based anodes, offering the potential to overcome the energy density limitations of commercial lithium batteries.
3.1 Interface issues of silicon-based anodes
Silicon has a theoretical capacity of about 3600 mA h g−1 (nine times that of graphite), is abundant, environmentally friendly, and has a suitable electrode potential (0.4 V vs. Li/Li+), which effectively prevents lithium deposition and dendrite growth, solving key problems related to the anode.65 However, due to the volumetric expansion effect, silicon-based anode materials often experience particle breakage and continuous solid–electrolyte interface (SEI) formation, leading to lithium loss and rapid capacity decline. Unlike liquid batteries, the mechanical rigidity and external stacking pressure of the solid electrolyte in solid-state batteries reduce or alter SEI formation and particle breakage in silicon-based anodes, potentially improving cycle stability.66,67 In all-solid-state batteries, the low mobility of silicon SEI films makes inorganic solid-state silicon anodes an effective method to reduce the side reactions at the electrode–electrolyte interface. Although the formation of a less mobile SEI in solid-state silicon anodes can slow down the repeated growth of interfacial phases and enhance cycling life, it may also lead to a lower initial coulombic efficiency (ICE) (Table 2).68,69| Anode | SE | Ionic conductivity/S cm−1 | Current density | Capacity/mAh g−1 | Ref. |
|---|---|---|---|---|---|
| Si–N-MXene | PEO@LATP | 3.4 × 10−4/50 °C | 0.4 A g−1/50 °C | First: 2305 | 70 |
| 10th: 1362 | |||||
| Si@SiO2@LPO@C | PEO/LATP | 2.86 × 10−5/50 °C | 0.2 A g−1/50 °C | First: 2482 | 71 |
| 0.5 A g−1/50 °C | First: 2279 | ||||
| 20th: 1001 | |||||
| Si@MOF | PVDF/PEO/LLZTO | 8.1 × 10−5/25 °C | 200 mA g−1/60 °C | First: 1416 | 72 |
| Si | LLZAO | 4 × 10−4/RT | 0.66 mA h cm−2 | First: 2685 | 73 |
| Si@Li6PS5Cl | Li6PS5Cl | — | 0.1 mA cm−2 | First: 2412 | 74 |
| Si@LPSCl | Li5.4PS4.4Cl1.6 | ∼8 × 10−3/RT | 0.2–0.5 mA cm−2/RT | First: 2412 | 75 |
| 50th: 1136 | |||||
| Si/CNTs/C/Li6PS5Cl | Li6PS5Cl | 2.13 × 10−3/RT | 50 mA g−1 | 50th: 1226 | 76 |
3.2 Interface issues of lithium metal anodes
Lithium metal anode has a high theoretical specific capacity (3860 mA h g−1), low electrode potential and low density, and is considered to be the ideal anode material for high-performance solid-state batteries. However, issues such as lithium dendrite formation, poor physical contact, and poor interfacial compatibility significantly hinder their application.77,78SSEs will inevitably be spontaneously reduced by the lithium metal, resulting in the formation of unstable SEI on the surface of the lithium metal anode. In addition, the rigid contact between the electrolyte and the anode causes ions and electrons to be unevenly distributed at the interface, hindering the transfer of ions and electrons.79 In addition, the volume change of lithium metal during the cycle causes the electrolyte–anode interface to lose contact, reducing ion transport pathways. Moreover, the high reactivity of lithium metal will produce side reactions when in contact with solid electrolyte, which will not only consume active lithium, increase interface resistance, hinder the transmission of lithium ions, but also lead to uneven Li+ flux and local high current density which promotes the growth of lithium dendrites, thereby penetrating the electrolyte layer and causing short circuit.80,81 In recent years, the RT ionic conductivity of solid-state batteries has been significantly improved, transforming the performance bottleneck of solid-state batteries from the problem of low ionic conductivity to the problem of electrolyte–electrode interface compatibility (Fig. 6).
 | ||
| Fig. 6 (a) and (b) Characteristics of lithium anodes;82 (c) strategies for improving interfacial issues.83 | ||
4. Interface mechanism issues between solid-state electrolytes and anodes
The ideal SSEs-anode interface should exhibit low interfacial resistance, close contact, and excellent electrochemical/mechanical stability. However, due to the complex interaction between the SSE and anode, as well as their mismatch in physical, chemical, and electrochemical properties, the solid–solid contact interface has poor contact, compatibility issues, and unstable ion transport, which seriously hinders the performance improvement and commercialization of solid-state batteries.62,84 The interfacial instability between SSEs and anodes is a primary cause of performance degradation in solid-state batteries. Although interfacial electrochemical reactions and dendrite growth both contribute to the transport of lithium ions, the mechanisms for the formation of gaps at the interface of lithium metal and silicon-based anodes are fundamentally different. Therefore, it is necessary to study each of these mechanisms and their effects in depth (Fig. 7).85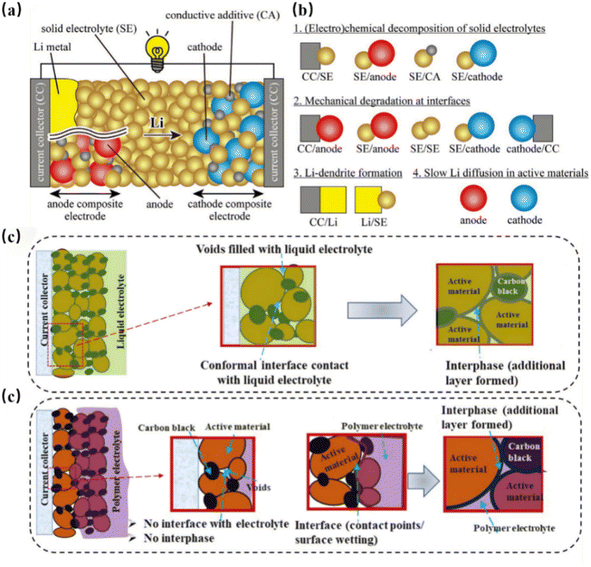 | ||
| Fig. 7 (a) Configuration of ASSBs and (b) key issues at the solid electrolyte–electrode interface.86 Schematic of the electrolyte–electrode interface in (c) liquid and (d) solid batteries.82 | ||
4.1 Growth of lithium dendrites
Lithium dendrites pose a serious challenge to lithium metal anodes. Although SSEs have many advantages over LEs, they have difficulty in uniformly conducting Li+, resulting in local concentrations of Li+ and promoting dendrite growth in specific areas.87,88 Nagao et al. characterized the growth of lithium along grain boundaries using in situ scanning electron microscopy and found that dendrite growth induced local stress and electron accumulation, leading to the formation of cracks. This leads to the continuous accumulation and growth of dendrites at grain boundaries and cracks, which eventually punctures the electrolyte film, causing short circuits and thermal runaway.89 The growth of lithium dendrites is a complex electrochemical process influenced by many factors such as ionic diffusion, overpotential, concentration gradients, and mechanical stress.90 Harry et al. found that the random distribution of irregular impurities, such as Li2O or LiOH, leads to uneven lithium deposition and dendrite growth.90 Current density is also a crucial factor affecting dendrite growth. Due to the low Li+ mobility in some SSEs, the high current density creates a cation concentration gradient on the surface of the lithium metal anode, further exacerbating dendrite growth.91 Near the SSS-lithium anode interface, the uneven distribution of local ion transport paths, crystal defects, ion concentration, current, overpotential and stress are important factors that trigger and aggravate the formation of lithium dendrites. Future systematic studies should focus on these factors within specific solid electrolyte systems to clarify the underlying mechanisms and guide the design of electrolyte–anode interfaces (Fig. 8).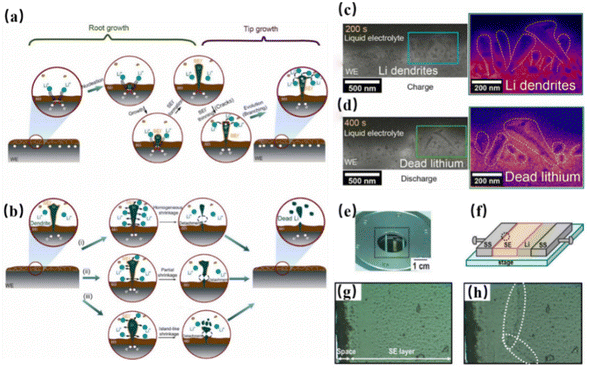 | ||
| Fig. 8 Schematics of lithium dendrite growth and dissolution dynamics during (a) charging and (b) discharging;92 ADF-STEM images showing dendrite growth at the interface (c) after 200 s and (d) 400 s of cycling.87 (e) Photo of in situ optical microscope viewing device and (f) measurement schematic, highlighting morphological changes at the dashed circle. (g) Optical microscopy images of the SSE-SS interface before testing and (h) after lithium deposition for 730 s at 2 mA cm−2.93 | ||
4.2 Volume effect exacerbates interfacial impedance
On the one hand, the solid–solid interface between the solid electrolyte and anode features a small contact area, poor physical contact, and inadequate interface stability (e.g., due to byproducts from interfacial side reactions), which can increase charge transfer resistance and hinder effective Li+ transport. In solid-state batteries, the combined effect of anode volume and lithium dendrite growth significantly changes the interface structure, further disrupting the existing ion conduction pathway and increasing the interface resistance. On the other hand, high-performance anode materials like silicon and lithium typically experience significant volume expansion and contraction during cycling. The rigid contact interface between the SSE and anode is particularly sensitive to these volume changes, resulting in interface contact degradation, potential crack formation, and the creation of large stresses that can directly break the interface. In addition, the interfacial side reaction alters the stress distribution at the electrolyte–anode interface, leading to continuous degradation and hindering ion transport across the interface.94 Liu et al. used a multi-physics simulation method to monitor the change of stress distribution at the interface between Li1.3Al0.3Ge1.7(PO4)3 (LAGP) and lithium metal anode. Their results confirm that lithium metal electrodeposition causes interfacial compression and stress concentration, disrupting the interfacial balance and leading to a rapid decline in battery performance.95 Cao et al. constructed a continuous, crack-free MgF2 nanoscale film on the surface of garnet-type solid electrolyte Li6.5La3Zr1.5Ta0.5O12 (LLZTO). This resulted in a significant reduction in the LLZTO–lithium anode interfacial impedance from 1190 Ω cm2 to 6 Ω cm2, effectively suppressing lithium dendrite formation and ensuring stable battery cycling.96 Zhang et al. introduced polystyrene sulfonate lithium (PLSS) to the surface of LLZTO and used the –SO3Li group to bind to metal elements on LLZTO. This effectively reduces the Li+ migration activation energy, increases the Li+ diffusion coefficient, inhibits the growth of lithium dendrites, and achieves a stable cycle, so that the interface impedance of Li/LLZTO–PLSS/Li is significantly reduced to 9 Ω cm2, and the critical current density is increased to 1.1 mA cm−2.974.3 Stress–strain and electronic structure affect interfacial impedance
In addition, due to the differences in surface roughness and crystal structure between solid electrolyte and anode material, it is difficult to achieve tight and uniform contact when the two are assembled into batteries, hindering effective ion transport at the interface. With the generation and gradual accumulation of structural stress at the interface during charge and discharge cycle, interface superlattice, stress strain and structural defects occur. This further hinders ion transport, resulting in a continuous increase in interface impedance.95 The energy level corresponding to the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) between the electrolyte and the anode plays a decisive role in the chemical stability of the interface. For example, the anodic reduction of Li0.33La0.56TiO3 (LLTO) and Li1.3Al0.3Ti1.7(PO4)3 (LATP) occurs at 1.7 V and 2.4 V, respectively. The reduction of Ti4+ to Ti3+, which is unstable to lithium metal, limits the electrochemical stability window, forms cracks between adjacent ion channels and increases grain boundary resistance (Fig. 9).98,99 | ||
| Fig. 9 Schematic of the chemical and structural evolution of (a) Si-SE-C, (b) Si-SE, and (c) Si anodes in all-solid-state batteries;100 SEM of (d) LLZTO/Li and (e) LLZTO–PLSS/Li;101 EIS for (f) Li/LLZTO/Li and (g) Li/LLZTO–PLSS/Li.101 | ||
4.4 Side reactions at the interface reduce energy density and increase impedance
Under the dual drive of electrochemical and chemical processes, side reactions occur at the electrolyte–anode interface and form by-products, resulting in increased interface impedance, which is a major key problem of solid-state batteries. On the one hand, the electronic conductivity of amorphous mixed ionic intermediates in these side reactions perpetuates irreversible reactions, leading to continuous decomposition of the electrolyte and worsening of lithium metal's non-uniform growth. This further intensifies interfacial degradation, leading to irreversible loss of the by-product Li+, ultimately reducing the coulombic efficiency and energy density of the battery.102 On the other hand, the poor ionic conductivity of the byproducts from interfacial side reactions can hinder ionic transport at the interface, increasing interfacial impedance and reducing the battery's power density, capacity, and lifespan. Due to the high electrochemical reactivity of lithium metal anodes, almost all solid electrolytes are thermodynamically unstable to them, except for common by-products such as LiF, Li3N, and Li2O.8,103,104 By-products often have a negative impact on battery performance. For example, the side reaction between lithium and the PEO electrolyte produces Li2O, C2H4 and H2, which not only breaks the close contact between the electrolyte and anode interface, but also hinders the transmission of Li+ due to the low ionic conductivity of Li2O.105 The accumulation of gas generated by side reactions at the internal interface of the battery cannot be discharged, resulting in increased internal stress and battery expansion, compromising structural integrity and increasing the risk of safety accidents.5. Effective measures to mitigate interface issues
5.1 First-principles and artificial intelligence computing guide the design of materials and charge–discharge processes
First-principles and artificial intelligence calculations are powerful tools for predicting the (electro)chemical stability of electrolyte–electrode interfaces. Huang et al. investigated the thermodynamic stability between silicon and sulfide-SSEs, revealing that the reactions are primarily driven by interactions between Si and P, resulting in the formation of SiP2, SiP, and Li5SiP3. The electrode potential of SiP2, SiP (1.5 to 0.97 V vs. Li+/Li) and Li5SiP3 (0.96 to 0.76 V vs. Li+/Li) is significantly higher than that of silicon, resulting in a lack of reversibility in the operating voltage range of silicon, which increases the anode potential, internal resistance, and reduces the coulombic efficiency.106 In the field of solid-state battery research, effectively screening and designing materials suitable for battery interfaces remains a critical challenge for scientists. However, traditional material screening methods often rely on extensive experimentation and high computational costs, resulting in lengthy development cycles and low efficiency. Li et al. proposed a material screening framework based on transfer learning that integrates crystallogram convolutional neural networks to train highly predictive models using limited data. The method successfully identified 12 promising SEI materials, breaking the traditional reliance on large data sets and providing a viable way to rapidly screen materials in solid-state batteries, demonstrating the great potential of artificial intelligence in guiding material design.107 Xu et al. developed a systematic Ge atom substitution screening process to solve the interface problem of Li10GeP2S12. This process led to the identification of a new solid electrolyte, Li10SrP2S12. The results show that the LSrPS–Li interface has a larger Schottky barrier (0.13 eV), smaller electron transfer region (3.10 Å), and a stronger ability to block excess electrons. Replacing Ge with Sr improves the stability of the battery interface without affecting the lithium-ion conductivity of the LGPS.1085.2 Optimizing the electrochemical window
The interaction and compatibility between solid electrolytes and anode materials are crucial for the performance of solid-state batteries. Poor interface compatibility can lead to issues such as internal cracking, hindered ionic transport, and increased battery impedance. Enhanced interface compatibility optimizes the SSE-anode interaction, thereby improving the stability and efficiency of the battery.The electrochemical stability window of an electrolyte reflects its ability to resist undesirable electron transfer and is a key parameter for evaluating the compatibility of solid electrolyte–anode interface. Both CV tests and DFT calculations can determine the electrochemical window, but their results often show significant differences. For example, the calculated electrochemical window of Li6PS5Cl is only 1.71–2.01 V, while its measured value can reach 7 V.109,110 Due to the high reactivity of lithium metal anodes, most electrolytes are unstable in their presence. The formation of an interface phase with high ion conductivity and low electron transport capacity can act as a passivation layer, hindering the continuous decomposition of the electrolyte–lithium metal anode interface, thus expanding the electrochemical window.
5.3 Suppressing lithium dendrite growth
The growth of lithium dendrites is influenced by various factors, including the intrinsic characteristics of the electrolyte, temperature, lithium deposition rate, and electrode surface conditions. Incomplete dissolution of dendrites during cycling can lead to the formation of dead lithium, causing loss of electrical contact with the current collector upon stripping. Furthermore, dendrite growth may penetrate solid electrolytes, resulting in internal short circuits, capacity degradation, and thermal runaway risks. Therefore, suppressing lithium dendrite growth is crucial for addressing interfacial issues between solid electrolytes and electrodes. Liu et al. utilized magnetron sputtering to construct an electromechanical buffering layer on the LATP surface through the spontaneous reaction between metallic lithium and Ti–LiF films. This approach facilitated uniform and effective Li+ migration across the interface while dissipating interfacial stress generated during lithium growth.111 Sun et al. inhibited the growth of lithium dendrites by introducing an interfacial layer doped fluorocarbon phosphate (FA). The inherent electrostatic shielding and self-healing properties of Cs reduce the nucleation potential of lithium ions and the binding energy of LiTFSI, promote the uniform deposition of Li+, and enhance the stability of the lithium metal surface.112 Xie et al. used Sn(Oct)2 to initiate in situ ring-opening polymerization of ε-caprolactone (ε-CL), forming LiSn mesophase layer at the anode interface and inhibiting the growth of lithium dendrites. At a current density of 0.05 mA h cm−2, the assembled symmetric battery can be stably cycled for 900 h, while the non-in situ polyelectrolyte battery shorted out after only 200 h.113 Wang et al. constructed a Li/LLZO interface in situ that could be observed from the cross-sectional direction in TEM. Through phase field simulation and molecular dynamics calculations, they clarified the mechanism of the interface pores causing local electric field concentration and the difference in lithium atom diffusion rate, providing theoretical guidance for interface optimization.1145.4 Controlling volume effects in anode materials
Controlling the volume effect of the anode material is essential to maintain the stability of the electrolyte–anode interface and ensure electrochemical performance. For example, active materials such as lithium metal and silicon-based anodes experience significant volume fluctuations during cycling, leading to interface contact failure and electrolyte structure destruction, resulting in increased interface impedance and blocked Li+ transport pathways. Ci et al. introduced a LiAlO2 coating with high ionic conductivity and mechanical strength on the Si surface, which not only facilitates Li+ transfer at the interface but also mitigates silicon's volume expansion, thereby preserving the electrode structure and the electrolyte–silicon anode interface.115 Zhang et al. developed a Si@MOF anode by embedding nano-silicon into MOF-derived carbon matrix, which effectively alleviated the volume expansion of silicon. The assembled LFP/PVDF-PEO-LLZTO/Si@MOF full battery has an initial capacity of 135 mA h g−1 and a capacity retention rate of 73.1% after 500 cycles at 0.5C and 60 °C.1165.5 Enhancing interfacial wettability
The effective contact area between the electrolyte and anode in solid-state batteries primarily depends on their interfacial wettability. For example, LLZO exhibits high contact angles, resulting in elevated apparent interface resistance. However, rapid acid treatment and in situ shielding can produce Li2CO3-free lithium wettable LLZO, which reduces interfacial resistance.117,118 The side reaction between electrolyte and electrode can change the interface properties, thus affecting the wettability. Certain composite coatings can simultaneously provide rapid Li+ diffusion pathways, high modulus, and shape consistency. For example, films composed of PVDF-HFP mixed with rigid LiF particles can enhance interfacial ion conductivity and physical/chemical stability when used as an artificial SEI on lithium metal anodes.119 Cao et al. assembled a high-voltage bipolar stacked all-solid-state battery using NCM811, Li6PS5Cl, and Si, employing ethyl cellulose as a binder due to its amphiphilic nature and strong adhesion. This approach achieved good compatibility with sulfide solid electrolytes and high thermal stability.74 Yan et al. synthesized a stable lithium–silicon alloy anode with hard carbon, consisting of a deformable lithium-rich phase (Li15Si4 and LiC6) to form a three-dimensional ion/electron conducting network. This structure increases the active surface area, reduces stress concentration, and optimizes interface contact.120In situ synthesis technology makes the contact between the solid electrolyte and the anode material uniform and dense, improves the mechanical strength and stability of the interface, and realizes the good compatibility between the two components.5.6 Suppressing of interfacial side reactions
Interface side reactions can lead to increased internal resistance, reduced capacity, shortened life, and even safety issues. Therefore, inhibiting these side reactions is crucial to improving battery performance. Utilizing materials such as PPF40,121 lithium polymer in F diluents,122 ionic liquids,123 Cu3P,124 graphene,125 Li3OCl,126 aluminosilicate,127 Mo6S8/C@Li,128 and clay/cross-linked network polymers129 as interfacial buffer coatings can significantly improve the contact interface between the electrolyte and electrode, thereby reducing interfacial side reactions. By using molecular layer deposition technology, Sun et al. prepared an inorganic–organic composite coating at the interface of silicon anode and sulfide solid electrolyte, which significantly reduced the side reactions at the interface.130 Hyeon et al. effectively suppressed interfacial side reactions and promoted ion migration and uniform reactions by coating Li6PS5Cl solid electrolyte on two-dimensional conductive graphene-like carbon (GLC@LPSCl), resulting in approximately 90% capacity retention after 200 cycles.131 Kaskel et al. constructed a cylindrical silicon anode structure-Li6PS5Cl 2D transverse SEI, which combined with mechanical stability under external pressure reduced the surface area of the side reaction, and the copper dendrites on the fluid collection ensured good electrical conductivity and adhesion of active substances along the direction of the column.68 In addition, electrolyte additives can form a stable protective film at the interface, promote Li+ migration, inhibit interface side reactions, and enhance the chemical stability of the interface.132–134In summary, in the optimization of the interface between solid-state electrolytes and anode materials, multiple strategies exhibit complementarity and limitations. Computational-guided design accelerates material development through atomic-level mechanism prediction and high-throughput screening, but is limited by computational resource consumption and the accuracy of dynamic process modeling (such as the multi-scale evolution of lithium dendrite growth). In terms of electrolyte modification, doping technology can enhance the electrochemical window of sulfides, but is limited by its inherent voltage tolerance and requires combined composite material design to balance its performance and process feasibility. In interface engineering, high mechanical strength electrolytes can inhibit lithium dendrite growth, but rigid interfaces are prone to contact failure; ionic liquids or polymer coatings can improve wettability, but may introduce thermal stability risks. In negative electrode optimization, although nanoscale or pre-lithiation treatment of silicon-carbon anodes can alleviate volume effects, it sacrifices energy density and cycle life. The control of side reactions relies on the construction of stable SEI membranes, but the dynamic evolution mechanism still lacks precise control means. Currently, semi-solid batteries have achieved preliminary commercialization through compromise solutions, while the all-solid-state system still needs to break through interface impedance and process bottlenecks. In the future, it is necessary to integrate collaborative strategies such as AI simulation-guided material design, in situ characterization dynamic monitoring, and multi-dimensional interface regulation to promote the industrialization process.
6. Summary and outlook
This paper reviews the recent progress of solid electrolyte–anode interface problems and optimization strategies to promote the development of high-performance sensors. At present, the solid electrolyte–anode interface faces the following challenges: (a) uneven lithium deposition leads to the growth of dendrites, which can penetrate the electrolyte, leading to short circuits and thermal runaway; (b) bad contact leads to high interface impedance, and the volume effect and stress changes during charge and discharge lead to interface degradation, which limits ion transport and reduces capacity; (c) serious interfacial side reactions lead to lithium loss, deterioration of interfacial contact, limited ion transport and gas production expansion, resulting in decreased energy density and damage to the overall structure of the battery.In response to these interface problems, researchers have tried a series of strategies to alleviate the interface problems, but the side reactions and interface contact impedance problems have not been completely solved. In the future, the following research ideas may be used to promote the application of all-solid-state lithium batteries: (a) employing advanced in situ characterization techniques and simulation calculations to gain a deeper understanding of the electrochemical behavior at the solid electrolyte–anode interface; (b) investigating the effects of charge–discharge parameters on the evolution of the electrolyte–anode interface; (c) improving interfacial stability through the construction of interfacial transition layers, modification of electrolyte and anode materials, and optimizing interfacial fabrication processes.
Solid electrolyte–anode interface with good physical contact, high ionic conductivity, stable (electrical) chemical stability and excellent self-healing ability is crucial to improve the high energy density and high safety of all-solid-state lithium batteries. The solution of this problem will strongly promote the large-scale practical application of high-performance all-solid-state lithium batteries and greatly accelerate the arrival of the era of comprehensive electrification in the field of energy transportation.
Data availability
Data will be made available upon request.Author contributions
Dandan Wang: conceptualization, methodology, and writing-original draft. Xinyang Wu: investigation and methodology. Yongpeng Ren: conceptualization, funding acquisition, and writing-review & editing. Yaru Li: conceptualization, funding acquisition, and writing-review & editing. Xiaolin Xie: investigation, formal analysis, and funding acquisition. Xiqiang Ma: investigation, formal analysis, and funding acquisition. Xuemin Chen: investigation. Junhao Lu: investigation. Kunming Pan: funding acquisition, project administration, and supervision.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationship that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by Frontier exploration Projects of Longmen Laboratory (LMQYTSKT012 and LMQYTSKT037), Major Scientific and Technological Innovation Project in Henan Province (231100220100), Youth Project of Henan Natural Science Foundation (222300420134), the Key Research and Development Project of Henan Province (No. 241111231600), Major Science and Technology Projects of Longmen Laboratory (ZDYF-001), the Longmen Laboratory “Trendy Industry Projects” (LMFKCY2023002), and Longmen laboratory concept verification project (GNYZ-202501).References
- D. Razzaghi, M. Babazadeh-Mamaqani, A. Babaie, F. Esmati, H. Roghani-Mamaqani, M. Rezaei, K. De Clerck and R. Hoogenboom, ACS Appl. Mater. Interfaces, 2025, 17, 4247–4289 CrossRef PubMed.
- R. Götz, R. Streng, J. Sterzinger, T. Steeger, M. M. Kaye, M. Vitort and A. S. Bandarenka, InfoMat, 2024, 6, e12627 CrossRef.
- J. Wu, M. Zheng, T. Liu, Y. Wang, Y. Liu, J. Nai, L. Zhang, S. Zhang and X. Tao, Energy Storage Mater., 2023, 54, 120–134 CrossRef.
- B. B. Gicha, L. T. Tufa, N. Nwaji, X. Hu and J. Lee, Nano-Micro Lett., 2024, 16, 172 CrossRef.
- T. Schmaltz, F. Hartmann, T. Wicke, L. Weymann, C. Neef and J. Janek, Adv. Energy Mater., 2023, 13, 2301886 CrossRef.
- M. Wan, S. Kang, L. Wang, H. W. Lee, G. W. Zheng, Y. Cui and Y. Sun, Nat. Commun., 2020, 11, 829 CrossRef PubMed.
- M. Liu, A. Song, X. Zhang, J. Wang, Y. Fan, G. Wang, H. Tian, Z. Ma and G. Shao, Nano Energy, 2025, 136, 110749 CrossRef.
- Y. Zheng, S. Zhang, J. Ma, F. Sun, M. Osenberg, A. Hilger, H. Marktter, F. Wilde, I. Manke and Z. Hu, Sci. Bull., 2023, 68, 13 Search PubMed.
- J. Kim, G. Yoon, S. Kim, S. Sugata, N. Yashiro, S. Suzuki, M. Lee, R. Kim, M. Badding, Z. Song, J. Chang and D. Im, Nat. Commun., 2023, 14, 782 CrossRef.
- K. Shen, W. Shi, H. Song, C. Zheng, Y. Yan, X. Hong, X. Liu, Y. An, Y. Li, F. Ye, M. He, G. Ye, C. Ma, L. Zheng, P. Gao and Q. Pang, Adv. Mater., 2025, 37, 2417171 CrossRef.
- E. P. Alsaç, D. L. Nelson, S. G. Yoon, K. A. Cavallaro, C. Wang, S. E. Sandoval, U. D. Eze, W. J. Jeong and M. T. McDowell, Chem. Rev., 2025, 125, 2009–2119 CrossRef PubMed.
- X. Wang, D. Guan, X. Ma, X. Yuan, Q. Zhu, H. Deng, H. Wang and J. Xu, Angew. Chem., Int. Ed., 2025, 64, e202415727 CrossRef PubMed.
- X. Zhan, M. Li, S. Li, X. Pang, F. Mao, H. Wang, Z. Sun, X. Han, B. Jiang, Y. He, M. Li, Q. Zhang and L. Zhang, Energy Storage Mater., 2023, 61, 102875 CrossRef.
- X. Li, J. Liang, X. Cao, S. Zhu, Y. Bai, J. Sun, H. Luo and J. Kong, Rare Met., 2025, 44, 2871–2899 CrossRef.
- A. G. Nguyen and C. J. Park, J. Membr. Sci., 2023, 675, 121552 CrossRef.
- L. Fan, S. Wei, S. Li, Q. Li and Y. Lu, Adv. Energy Mater., 2018, 8, 1702657 CrossRef.
- W. Li, M. Li, S. Wang, P. H. Chien, J. Luo, J. Fu, X. Lin, G. King, R. Feng, J. Wang, J. Zhou, R. Li, J. Liu, Y. Mo, T. K. Sham and X. Sun, Nat. Nanotechnol., 2025, 20, 265–275 CrossRef.
- R. Murugan, V. Thangadurai and W. Weppner, Angew. Chem., Int. Ed., 2007, 46, 7778–7781 CrossRef PubMed.
- R. Dubey, J. Sastre, C. Cancellieri, F. Okur, A. Forster, L. Pompizii, A. Priebe, Y. E. Romanyuk, L. P. H. Jeurgens, M. V. Kovalenko and K. V. Kravchyk, Adv. Energy Mater., 2021, 11, 2102086 CrossRef.
- Y. Chen, J. Qian, X. Hu, Y. Ma, Y. Li, T. Xue, T. Yu, L. Li, F. Wu and R. Chen, Adv. Mater., 2023, 35, 2212096 CrossRef.
- J. Li, J. Chen, X. Xu, J. Sun, B. Huang and T. Zhao, Energy Environ. Sci., 2024, 17, 5521–5531 RSC.
- J. Zhang, C. Wang, J. Fu, M. Ye, H. Zhai, J. Li, G. Tan, X. Tang and X. Sun, Adv. Funct. Mater., 2024, 35, 2416229 CrossRef.
- D. Dai, P. Yan, X. Zhou, H. Li, Z. Zhang, L. Wang, M. Han, X. Lai, Y. Qiao, M. Jia, B. Li and D. Liu, Green Carbon, 2024, 2, 310–315 CrossRef.
- H. Li, Q. Lin, J. Wang, L. Hu, F. Chen, Z. Zhang and C. Ma, Angew. Chem., 2024, 136, e202407892 CrossRef.
- M. S. Nafis, Z. Liang, S. Lee and C. Ban, Nano Energy, 2025, 133, 110447 CrossRef.
- L. Hu, Y. Ren, C. Wang, J. Li, Z. Wang, F. Sun, J. Ju, J. Ma, P. Han, S. Dong and G. Cui, Adv. Mater., 2024, 36, 2401909 CrossRef.
- J. E. Trevey, J. Wang, C. M. DeLuca, K. K. Maute, M. L. Dunn, S. H. Lee and V. M. Bright, Sens. Actuators, A, 2011, 167, 139–145 CrossRef.
- B. Zhang, R. Tan, L. Yang, J. Zheng, K. Zhang, S. Mo, Z. Lin and F. Pan, Energy Storage Mater., 2018, 10, 139–159 CrossRef.
- Z. Gao, H. Sun, L. Fu, F. Ye, Y. Zhang, W. Luo and Y. Huang, Adv. Mater., 2018, 30, 1705702 CrossRef PubMed.
- Y. Huang, J. Zeng, S. Li, C. Dai, J. Liu, C. Liu and Y. He, Adv. Energy Mater., 2023, 13, 2203888 CrossRef.
- G. Wang, H. Wu, M. Zheng, C. Zhao, J. Liang, L. Zhou, J. Yue, X. Zhu, Y. Xu, N. Zhang, T. Pang, J. Fu, W. Li, Y. Xia, W. Yin, X. Sun and X. Li, Adv. Mater., 2024, 37, 2410402 CrossRef.
- W. Ji, D. Zheng, X. Zhang, T. Ding and D. Qu, J. Mater. Chem. A, 2021, 9, 15012–15018 RSC.
- X. Shi, Z. Jia, D. Wang, B. Jiang, Y. Liao, G. Zhang, Q. Wang, D. He and Y. Huang, Adv. Mater., 2024, 36, 2405097 CrossRef.
- P. Zhai, N. Ahmad, S. Qu, L. Feng and W. Yang, Adv. Funct. Mater., 2024, 34, 2316561 CrossRef.
- A. Du, H. Lu, S. Liu, S. Chen, Z. Chen, W. Li, J. Song, Q. Yang and C. Yang, Adv. Energy Mater., 2024, 14, 2400808 CrossRef.
- S. Xu, K. Zhang, R. Xu, P. Tang, H. Cheng, Z. Sun and F. Li, Energy Storage Mater., 2025, 74, 103941 CrossRef.
- H. An, M. Li, Q. Liu, Y. Song, J. Liu, Z. Yu, X. Liu, B. Deng and J. Wang, Nat. Commun., 2024, 15, 9150 CrossRef.
- Y. Jiang, C. Xu, K. Xu, S. Li, J. Ni, Y. Wang, Y. Liu, J. Cai and C. Lai, Chem. Eng. J., 2022, 442, 136245 CrossRef.
- Z. Jia, M. Jia, Q. Sun, N. Wang, Z. Bi and X. Guo, Energy Storage Mater., 2024, 68, 103325 CrossRef.
- M. Lemaalem and P. Carbonniere, Solid State Ionics, 2023, 399, 116304 CrossRef.
- Q. Liu, G. Yang, X. Li, S. Zhang, R. Chen, X. Wang, Y. Gao, Z. Wang and L. Chen, Energy Storage Mater., 2022, 51, 443–452 CrossRef.
- Y. Zhang, J. Li, M. Ge, Y. Huang and H. Xu, Rare Met., 2024, 43, 5625–5636 CrossRef CAS.
- X. Su, X. Xu, Z. Ji, J. Wu, F. Ma and L. Fan, Electrochem. Energy Rev., 2024, 7, 1–38 CrossRef.
- J. Liu, Z. Wu, F. J. Stadler and Y. Huang, Angew. Chem., Int. Ed., 2023, 62, e202300243 CrossRef CAS.
- Y. Liu, X. An, K. Yang, J. Ma, J. Mi, D. Zhang, X. Cheng, Y. Li, Y. Ma, M. Liu, F. Kang and Y. He, Energy Environ. Sci., 2024, 17, 344–353 RSC.
- S. Guo, Y. Su, K. Yan, C. Zhao, Y. Lu, H. Wang, J. Dong, N. Li, Y. Liu, Y. Guan, F. Wu and L. Chen, Adv. Sci., 2024, 11, 2404307 CrossRef CAS PubMed.
- X. Zhang, S. Cheng, C. Fu, G. Yin, L. Wang, Y. Wu and H. Huo, Nano-Micro Lett., 2025, 17, 2 CrossRef CAS.
- Z. Zhang, J. Guo, K. Cui, X. Zhang, Y. Yao, S. Wang and H. Wang, Nano-Micro Lett., 2024, 16, 181 CrossRef CAS PubMed.
- J. Hou, W. Sun, Q. Yuan, L. Ding, Y. Wan, Z. Xiao, T. Zhu, X. Lei, J. Lin, R. Cheacharoen, Y. Zhou, S. Wang, F. Manshaii, J. Xie, W. Li and J. Zhao, Angew. Chem., Int. Ed., 2025, 64, e202421427 CrossRef CAS.
- G. Polizos, M. Goswami, J. K. Keum, L. He, C. J. Jafta, J. Sharma, Y. Wang, L. T. Kearney, R. Tao and J. Li, ACS Nano, 2024, 18, 2750–2762 CrossRef CAS.
- C. Liu, S. Wang, Z. Lu, J. Zhao, Y. Wu, C. Ren, R. Yang and C. Jin, Nano Res., 2024, 18, 94907087 CrossRef.
- B. Luo, J. Wu, M. Zhang, Z. Zhang, X. Zhang, Z. Fang, Z. Xu and M. Wu, Chem. Sci., 2023, 14, 13067–13079 RSC.
- W. Kong, Z. Jiang, Y. Liu, Q. Han, L. Ding, S. Wang and H. Wang, Adv. Funct. Mater., 2023, 33, 2306748 CrossRef CAS.
- R. Tong, Y. Huang, C. Feng, Y. Dong and C. Wang, Adv. Funct. Mater., 2024, 34, 2315777 CrossRef CAS.
- P. Zhao, Y. Wang, Q. Huang, Z. Jin and C. Li, Angew. Chem., Int. Ed., 2025, 64, e202416897 CrossRef CAS PubMed.
- K. Li, J. Huang, X. Qu, G. Fu, X. Chen, W. Shen and Y. Lin, ACS Appl. Mater. Interfaces, 2025, 17, 3146–3162 CrossRef CAS.
- C. Shen, W. Feng, Y. Yu, H. Wang, Y. Cheng, C. Dong, J. Gu, A. Zheng, X. Liao, X. Xu and L. Mai, Adv. Energy Mater., 2024, 14, 2304511 CrossRef CAS.
- M. Cui, Y. Li, B. Jin, S. Sun and Q. Jiang, Chem. Eng. J., 2024, 500, 156748 CrossRef CAS.
- X. Wang, Z. Chen, X. Xue, J. Wang, Y. Wang, D. Bresser, X. Liu, M. Chen and S. Passerini, Nano Energy, 2025, 133, 110439 CrossRef CAS.
- T. Wang, Z. Wang, H. Li, L. Cheng, Y. Wu, X. Liu, L. Meng, Y. Zhang and S. Jiang, Carbon, 2024, 230, 119615 CrossRef CAS.
- Z. Wang, C. Zhao, N. Yao, Y. Lu, Z. Xue, X. Huang, P. Xu, W. Huang, Z. Wang, J. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2024, 64, e202414524 CrossRef.
- X. Zhang, S. Cheng, C. Fu, G. Yin, P. Zuo, L. Wang and H. Huo, Adv. Energy Mater., 2024, 14, 2401802 CrossRef CAS.
- D. Cao, X. Sun, Y. Li, A. Anderson, W. Lu and H. Zhu, Adv. Mater., 2022, 34, 2200401 CrossRef CAS.
- M. Li, S. Yang and B. Li, Interdiscip. Mater., 2024, 3, 805–834 CAS.
- B. Chen, D. Xu, Z. Chang and A. Pan, Adv. Sustainable Syst., 2024, 8, 2400220 CrossRef CAS.
- D. L. Nelson, S. E. Sandoval, J. Pyo, D. Bistri, T. A. Thomas, K. A. Cavallaro, J. A. Lewis, A. S. Iyer, P. Shevchenko, C. V. Di Leo and M. T. McDowell, ACS Energy Lett., 2024, 9, 6085–6095 CrossRef CAS.
- F. Frie, H. Ditler, S. Klick, G. Stahl, C. Rahe, T. Ghaddar and D. U. Sauer, ChemElectroChem, 2024, 11, e202400020 CrossRef CAS.
- S. Cangaz, F. Hippauf, F. S. Reuter, S. Doerfler, T. Abendroth, H. Althues and S. Kaskel, Adv. Energy Mater., 2020, 10, 2001320 CrossRef CAS.
- Y. Wang, T. Li, X. Yang, Q. Yin, S. Wang, H. Zhang and X. Li, Adv. Energy Mater., 2023, 14, 2303189 CrossRef.
- X. Han, W. Zhou, M. Chen, J. Chen, G. Wang, B. Liu, L. Luo, S. Chen, Q. Zhang, S. Shi and C. Wong, J. Energy Chem., 2022, 67, 727–735 CrossRef CAS.
- L. Gu, J. Han, M. Chen, W. Zhou, X. Wang, M. Xu, H. Lin, H. Liu, H. Chen, J. Chen, Q. Zhang and X. Han, Energy Storage Mater., 2022, 52, 547–561 CrossRef.
- L. Zhang, Y. Lin, X. Peng, M. Wu and T. Zhao, ACS Appl. Mater. Interfaces, 2022, 14, 24798–24805 CrossRef CAS.
- W. Ping, C. Yang, Y. Bao, C. Wang, H. Xie, E. Hitz, J. Cheng, T. Li and L. Hu, Energy Storage Mater., 2019, 21, 246–252 CrossRef.
- D. Cao, X. Sun, Y. Wang and H. Zhu, Energy Storage Mater., 2022, 48, 458–465 CrossRef.
- D. Cao, T. Ji, A. Singh, S. Bak, Y. Du, X. Xiao, H. Xu, J. Zhu and H. Zhu, Adv. Energy Mater., 2024, 13, 2203969 CrossRef.
- L. Hu, X. Yan, Z. Fu, J. Zhang, Y. Xia, W. Zhang, Y. Gan, X. He and H. Huang, ACS Appl. Energy Mater., 2022, 5, 14353–14360 CrossRef CAS.
- X. Xu, P. V. Evdokimov, V. S. Volkov, S. Xiong, X. Jiao, O. O. Kapitanova and Y. Liu, Energy Storage Mater., 2023, 57, 421–428 CrossRef.
- J. Ma, S. Zhang, Y. Zheng, T. Huang, F. Sun, S. Dong and G. Cui, Adv. Mater., 2023, 35, 2301892 CrossRef CAS PubMed.
- Q. Liu, D. Zhou, D. Shanmukaraj, P. Li, F. Kang, B. Li, M. Armand and G. Wang, ACS Energy Lett., 2020, 5, 1456–1464 CrossRef CAS.
- S. Guo, T. Wu, Y. Sun, S. Zhang, B. Li, H. Zhang, M. Qi, X. Liu, A. Cao and L. Wan, Adv. Funct. Mater., 2022, 32, 2201498 CrossRef CAS.
- O. B. Chae and B. L. Lucht, Adv. Energy Mater., 2023, 13, 2203791 CrossRef CAS.
- Q. Liu and L. Wang, Adv. Energy Mater., 2023, 13, 2301742 CrossRef CAS.
- G. Lu, J. Nai, D. Luan, X. Tao and X. W. D. Lou, Sci. Adv., 2023, 9, 14 Search PubMed.
- Q. Xia, S. Yuan, Q. Zhang, C. Huang, J. Liu and H. Jin, Adv. Sci., 2024, 11, 2401453 CrossRef CAS.
- Z. Deng, S. Chen, K. Yang, Y. Song, S. Xue, X. Yao, L. Yang and F. Pan, Adv. Mater., 2024, 36, 2407923 CrossRef CAS.
- Y. Nomura and K. Yamamoto, Adv. Energy Mater., 2023, 13, 2203883 CrossRef CAS.
- W. Dachraoui, R. S. Kühnel, C. Battaglia and R. Erni, Nano Energy, 2024, 130, 110086 CrossRef CAS.
- H. Liu, Y. Chen, P. H. Chien, G. Amouzandeh, D. Hou, E. Truong, I. P. Oyekunle, J. Bhagu, S. W. Holder, H. Xiong, P. L. Gor’kov, J. T. Rosenberg, S. C. Grant and Y. Hu, Nat. Mater., 2025, 24, 581–588 CrossRef CAS PubMed.
- M. Nagao, A. Hayashi, M. Tatsumisago, T. Kanetsuku, T. Tsudacd and S. Kuwabata, Phys. Chem. Chem. Phys., 2013, 15, 18600–18606 RSC.
- P. Hartmann, T. Leichtweiss, M. R. Busche, M. Schneider, M. Reich, J. Sann, P. Adelhelm and J. Janek, J. Phys. Chem. C, 2013, 117, 21064–21074 CrossRef CAS.
- R. Raj and J. Wolfenstine, J. Power Sources, 2017, 343, 119–126 CrossRef CAS.
- W. Dachraoui, R. S. Kühnel, C. Battaglia and R. Erni, Nano Energy, 2024, 130, 110086 CrossRef CAS.
- M. Nagao, A. Hayashi, M. Tatsumisago, T. Kanetsuku, T. Tsudacd and S. Kuwabata, Phys. Chem. Chem. Phys., 2013, 15, 18600–18606 RSC.
- X. Gao, Z. Xing, M. Wang, C. Nie, Z. Shang, Z. Bai, S. Dou and N. Wang, Energy Storage Mater., 2023, 60, 102821 CrossRef.
- Y. Liu, X. Xu, X. Jiao, O. O. Kapitanova, Z. Song and S. Xiong, Adv. Mater., 2023, 35, 2301152 CrossRef CAS PubMed.
- M. Jia, T. Wu, S. Zhang, S. Guo, Y. Fu and A. Cao, Adv. Funct. Mater., 2024, 35, 2415542 CrossRef.
- G. Yang, X. Bai, Y. Zhang, Z. Guo, C. Zhao, L. Fan and N. Zhang, Adv. Funct. Mater., 2023, 33, 2211387 CrossRef CAS.
- L. Zhu, Y. Wang, Y. Wu, W. Feng, Z. Liu, W. Tang, X. Wang and Y. Xia, Adv. Funct. Mater., 2022, 32, 2201136 CrossRef CAS.
- K. Lee, E. Kazyak, M. J. Wang, N. P. Dasgupta and J. Sakamoto, Joule, 2022, 6, 2547–2565 CrossRef CAS.
- D. Cao, T. Ji, A. Singh, S. Bak, Y. Du, X. Xiao, H. Xu, J. Zhu and H. Zhu, Adv. Energy Mater., 2023, 13, 2203969 CrossRef CAS.
- G. Yang, X. Bai, Y. Zhang, Z. Guo, C. Zhao, L. Fan and N. Zhang, Adv. Funct. Mater., 2022, 33, 2211387 CrossRef.
- O. Sheng, H. Hu, T. Liu, Z. Ju, G. Lu, Y. Liu, J. Nai, Y. Wang, W. Zhang and X. Tao, Adv. Funct. Mater., 2022, 32, 2111026 CrossRef CAS.
- A. Banerjee, X. Wang, C. Fang, E. A. Wu and Y. S. Meng, Chem. Rev., 2020, 120, 6878–6933 CrossRef CAS.
- F. Sun, C. Wang, M. Osenberg, K. Dong, S. Zhang, C. Yang, Y. Wang, A. Hilger, J. Zhang, S. Dong, H. Markötter, I. Manke and G. Cui, Adv. Energy Mater., 2022, 12, 2103714 CrossRef CAS.
- H. Adenusi, G. A. Chass, S. Passerini, K. V. Tian and G. Chen, Adv. Energy Mater., 2023, 13, 2203307 CrossRef CAS.
- Y. Huang, B. Shao, Y. Wang and F. Han, Energy Environ. Sci., 2023, 16, 1569–1580 RSC.
- K. Tao, W. He, A. Chen, Y. Han and J. Li, Energy Storage Mater., 2025, 75, 104034 CrossRef.
- Z. Wan, X. Chen, Z. Zhou, X. Zhong, X. Luo and D. Xu, J. Energy Chem., 2024, 88, 28–38 CrossRef CAS.
- Y. He, C. Lu, S. Liu, W. Zheng and J. Luo, Adv. Energy Mater., 2019, 9, 1901810 CrossRef.
- F. Sun, L. Duchêne, M. Osenberg, S. Risse, C. Yang, L. Chen, N. Chen, Y. Huang, A. Hilger, K. Dong, T. Arlt, C. Battaglia, A. Remhof, I. Manke and R. Chen, Nano Energy, 2021, 82, 105762 CrossRef CAS.
- X. Wang, W. Hou, Y. Chen, Y. Yang, Y. Wang, Z. Guo, Z. Song and Y. Liu, Adv. Energy Mater., 2024, 15, 2402731 CrossRef.
- Y. Mao, F. Mi, T. Wang and C. Sun, Chem. Eng. J., 2024, 496, 153823 CrossRef CAS.
- M. Sun, Z. Zeng, L. Peng, Z. Han, C. Yu, S. Cheng and J. Xie, Mater. Today Energy, 2021, 21, 100785 CrossRef CAS.
- H. Gao, C. Lin, Y. Liu, J. Shi, B. Zhang, Z. Sun, Z. Li, Y. Wang, M. Yang and Y. Cheng, Sci. Adv., 2025, 11, eadt4666 CrossRef CAS.
- X. Xu, Q. Sun, Y. Li, F. Ji, J. Cheng, H. Zhang, Z. Zeng, Y. Rao, H. Liu, D. Li and L. Ci, Small, 2023, 19, 2302934 CrossRef CAS PubMed.
- L. Zhang, Y. Lin, X. Peng, M. Wu and T. Zhao, ACS Appl. Mater. Interfaces, 2022, 14, 24798–24805 CrossRef CAS.
- H. Huo, Y. Chen, N. Zhao, X. Lin, J. Luo, X. Yang, Y. Liu, X. Guo and X. Sun, Nano Energy, 2019, 61, 119–125 CrossRef CAS.
- J. Wu, B. Pu, D. Wang, S. Shi, N. Zhao, X. Guo and X. Guo, ACS Appl. Mater. Interfaces, 2018, 11, 898–905 CrossRef PubMed.
- R. Xu, X. Zhang, X. Cheng, H. Peng, C. Zhao, C. Yan and J. Huang, Adv. Funct. Mater., 2018, 28, 1705838 CrossRef.
- W. Yan, Z. Mu, Z. Wang, Y. Huang, D. Wu, P. Lu, J. Lu, J. Xu, Y. Wu, T. Ma, M. Yang, X. Zhu, Y. Xia, S. Shi, L. Chen, H. Li and F. Wu, Nat. Energy, 2023, 8, 800–813 CrossRef CAS.
- C. Zheng, Y. Lu, Q. Chang, Z. Song, T. Xiu, J. Jin, M. E. Badding and Z. Wen, Adv. Funct. Mater., 2023, 33, 2302729 CrossRef CAS.
- W. Zhang, V. Koverga, S. Liu, J. Zhou, J. Wang, P. Bai, S. Tan, N. K. Dandu, Z. Wang, F. Chen, J. Xia, H. Wan, X. Zhang, H. Yang, B. L. Lucht, A. M. Li, X. Yang, E. Hu, S. R. Raghavan, A. T. Ngo and C. Wang, Nat. Energy, 2024, 9, 386–400 CrossRef CAS.
- S. Xiong, Y. Liu, P. Jankowski, Q. Liu, F. Nitze, K. Xie, J. Song and A. Matic, Adv. Funct. Mater., 2020, 30, 2001444 CrossRef CAS.
- C. Sun, A. Lin, W. Li, J. Jin, Y. Sun, J. Yang and Z. Wen, Adv. Energy Mater., 2020, 10, 1902989 CrossRef CAS.
- Y. Ma, P. Qi, J. Ma, L. Wei, L. Zhao, J. Cheng, Y. Su, Y. Gu, Y. Lian, Y. Peng, Y. Shen, L. Chen, Z. Deng and Z. Liu, Adv. Sci., 2021, 8, 2100488 CrossRef CAS.
- B. Han, D. Feng, S. Li, Z. Zhang, Y. Zou, M. Gu, H. Meng, C. Wang, K. Xu, Y. Zhao, Ho. Zeng, C. Wang and Y. Deng, Nano Lett., 2020, 20, 4029–4037 CrossRef CAS PubMed.
- Z. Ju, C. Jin, H. Yuan, T. Yang, O. Sheng, T. Liu, Y. Liu, Y. Wang, F. Ma, W. Zhang, J. Nai and X. Tao, Chem. Eng. J., 2021, 408, 128016 CrossRef CAS.
- K. Lu, S. Gao, R. J. Dick, Z. Sattara and Y. Cheng, J. Mater. Chem. A, 2019, 7, 6038–6044 RSC.
- H. Liu, R. Tao, C. Guo, W. Zhang, X. Liu, P. Guo, T. Zhang and J. Liang, Chem. Eng. J., 2022, 429, 132239 CrossRef CAS.
- C. Wang, Y. Zhao, Q. Sun, X. Li, Y. Liu, J. Liang, X. Li, X. Lin, R. Li, K. R. Adair, L. Zhang, R. Yang, S. Lu and X. Sun, Nano Energy, 2018, 53, 168–174 CrossRef CAS.
- H. J. Shin, J. T. Kim, D. Han, H. S. Kim, K. Y. Chung, J. Mun, J. Kim, K. W. Nam and H. G. Jung, Adv. Energy Mater., 2024, 15, 2403247 CrossRef.
- Y. Yang, J. Wang, Z. Li, Z. Yang, B. Wang and H. Zhao, ACS Nano, 2024, 18, 7666–7676 CrossRef CAS.
- F. Wu, Z. Wen, Z. Zhao, J. Bi, Y. Shang, Y. Liang, L. Li, N. Chen, Y. Li and R. Chen, ACS Appl. Mater. Interfaces, 2022, 14, 38807–38814 CrossRef PubMed.
- X. Zhuang, S. Zhang, Z. Cui, B. Xie, T. Gong, X. Zhang, J. Li, R. Wu, S. Wang, L. Qiao, T. Liu, S. Dong, G. Xu, L. Huang and G. Cui, Angew. Chem., Int. Ed., 2023, 136, e202315710 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |


