 Open Access Article
Open Access ArticleA comprehensive review on carbonylation reactions: catalysis by magnetic nanoparticle-supported transition metals
Irfan
Ahmad
a,
Munthar
Kedhim
bcd,
Yashwantsinh
Jadeja
e,
Gargi
Sangwan
f,
Kavitha
V.
g,
Aditya
Kashyap
h,
Shirin
Shomurotova
i,
Mosstafa
Kazemi
 *j and
Ramin
Javahershenas
*j and
Ramin
Javahershenas
 *k
*k
aDepartment of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia. E-mail: irfancsmmu@gmail.com
bCollege of Pharmacy, The Islamic University, Najaf, Iraq. E-mail: muntherabosoda@iunajaf.edu.iq
cDepartment of Medical Analysis, Medical Laboratory Technique College, The Islamic University of Al Diwaniyah, Al Diwaniyah, Iraq
dDepartment of Medical Analysis, Medical Laboratory Technique College, The Islamic University of Babylon, Babylon, Iraq
eMarwadi University Research Center, Department of Chemistry, Faculty of Science, Marwadi University, Rajkot 360003, Gujarat, India. E-mail: yashwantsinh.jadeja@marwadieducation.edu.in
fChitkara Centre for Research and Development, Chitkara University, Baddi, Himachal Pradesh 174103, India. E-mail: gargi24@outlook.in
gDepartment of Chemistry, Sathyabama Institute of Science and Technology, Chennai, Tamil Nadu, India. E-mail: kavitha.chemistry@sathyabama.ac.in
hCentre for Research Impact & Outcome, Chitkara University Institute of Engineering and Technology, Chitkara University, Rajpura, 140401, Punjab, India. E-mail: aditya_kashyap2024@outlook.com
iDepartment of Chemistry Teaching Methods, Tashkent State Pedagogical University named after Nizami, Bunyodkor Street 27, Tashkent, Uzbekistan. E-mail: author.uzb@mail.ru
jYoung Researchers and Elite Club, Islamic Azad University, Tehran Branch, Tehran, Iran. E-mail: mosstafakazemi@gmail.com
kOrganic Development, Chemistry Faculty, Urmia University, Urmia, Iran. E-mail: jshbco@gmail.com
First published on 14th March 2025
Abstract
Magnetic catalysts have become a crucial innovation in carbonylation reactions, providing a sustainable and highly efficient means of synthesizing compounds that contain carbonyl groups. This review article explores the diverse and significant role of magnetic catalysts in various carbonylation processes, emphasizing their essential contributions to improving reaction rates, selectivity, and recyclability of catalysts. The distinctive magnetic properties of these catalysts enable straightforward separation and recovery, a feature that significantly mitigates waste and reduces environmental impact. As a result, magnetic catalysts' environmental and economic advantages position them as key players in contemporary synthetic chemistry, driving the evolution of green chemistry practices. Particularly noteworthy is the combination of magnetic nanoparticles with transition metals, resulting in the development of robust catalytic systems that exploit the complementary effects of magnetism and catalysis. Recent advances have showcased the adaptability of magnetic nanoparticles supported by transition metal catalysts in various carbonylation reactions, including carbonylative coupling, alkoxy carbonylation, thio carbonylation, and amino carbonylation. This review meticulously examines the mechanistic aspects of how magnetic fields influenced catalytic performance between 2014 and the end of 2024.
1 Introduction
Catalysts play a crucial role in chemical reactions, primarily by increasing the reaction rate without being consumed.1 They function by providing an alternative pathway for the reaction with a lower activation energy, which is the minimum energy required for a reaction to occur.2 This not only speeds up reactions that would otherwise be too slow for practical use but also allows for less extreme conditions in terms of temperature and pressure.3,4 In industrial applications, catalysts are vital for efficiently producing many chemicals and materials. They enable more sustainable processes by reducing energy consumption and minimizing the production of unwanted byproducts through selectivity.5,6 In biological systems, enzymes, natural catalysts, are essential for life, as they regulate the speed of the biochemical reactions necessary for cellular function. Therefore, the study and use of catalysts are fundamental to industrial chemistry and biological sciences. Catalysts also play a significant role in research and development.7,8 They are used to synthesize new materials and chemicals, which can lead to the development of new drugs, materials, and technologies.9,10 The study of catalysis is a dynamic field that focuses on improving existing processes and discovering new ways to catalyze reactions, which can lead to groundbreaking advancements in science and technology.111.1. Preparation and characterization of magnetic catalysts
Transition metals possess unique properties, making them highly effective catalysts for chemical reactions (Fig. 1).12 These metals, including iron, copper, and nickel, are characterized by their ability to exist in multiple oxidation states and their capacity to form complex compounds with various ligands.13–17 This versatility is crucial for catalysis, as it allows transition metals to facilitate a wide range of chemical transformations by providing an alternative reaction pathway with lower activation energy.18–21 The advantages of using transition metals as catalysts are manifold.They often enable reactions to occur at lower temperatures and pressures, reducing energy consumption and the environmental impact of chemical manufacturing.22–24 Additionally, transition metals can catalyze reactions that would otherwise be slow or impossible, opening up new pathways for synthesizing complex molecules.25,26
Transition metals are effective catalysts due to their electronic configuration, which allows them to accept and donate electrons easily. They can form complexes with ligands, stabilizing reaction intermediates, and their surface properties are important in heterogeneous catalysis. Their thermal stability makes them suitable for high-temperature reactions, and their variable oxidation states enable participation in redox reactions. These characteristics contribute to their high catalytic activity, allowing reactions to occur faster and under milder conditions.25–34
Despite their widespread use, transition metal catalysts face challenges, such as deactivation by poisoning and the need for precise control over their environment to maintain activity.35,36 However, ongoing research and development continue to enhance the efficiency and sustainability of these catalysts, ensuring their pivotal role in chemistry and industry.37,38 With their remarkable catalytic properties, transition metals remain at the forefront of scientific innovation, driving advancements in everything from pharmaceuticals to renewable energy technologies.39,40 Magnetic nanoparticles (MNPs) are an innovative catalyst substrate due to their unique properties and advantages in various chemical processes.41,42 The application of MNPs as catalyst substrates is extensive, including their use in organic synthesis, environmental remediation, and pharmaceutical production.43,44 One of the key benefits of magnetic nanoparticles (MNPs) is their ability to respond to magnetic fields, which facilitates their quick and efficient separation from reaction mixtures. When exposed to an external magnetic field, these nanoparticles can be easily pulled out of the solution, allowing for simple recovery and reuse. This characteristic not only streamlines the purification process but also significantly minimizes waste, resulting in reduced overall costs associated with catalyst recycling.45–47
Additionally, MNPs exhibit a high surface area-to-volume ratio, enhancing the catalytic activity by providing more active sites for reactions.48 This characteristic also contributes to the efficiency and speed of catalytic processes.49–51 Furthermore, MNPs can be engineered with a core–shell structure, where the core provides magnetic properties, and the shell can be tailored with specific functional groups to support various catalytic activities.52 This versatility enables the design of highly selective and targeted catalysts.53,54 MNPs also promote greener chemistry practices by minimizing the need for harmful chemicals and solvents in the separation process.55–57
In summary, magnetic nanoparticles utilized as catalyst substrates provide a unique blend of enhanced catalytic efficiency, environmental advantages, and cost-effectiveness. This combination not only boosts the performance of catalytic processes but also aligns with sustainable practices, making magnetic nanoparticles a significant asset in modern catalysis. Their magnetic properties facilitate easy separation and recovery after reactions, contributing to environmentally friendly and economically viable solutions (Fig. 2).58–60
Magnetic nanoparticles (MNPs) are fascinating little particles that can be constructed through various innovative methods. Each approach is designed to fine-tune its size, shape, and unique properties, allowing for various applications. The versatility of their synthesis opens the door to endless possibilities in fields like medicine, electronics, and environmental science.61–67 Here are several popular and effective preparation methods (Fig. 3).
When preparing magnetic nanoparticles, it is important to recognize that each method has distinct advantages and limitations affecting particle size, uniformity, reproducibility, and scalability. Selecting a preparation technique should align with the intended application, considering desired magnetic properties, shape, and size factors. Operational conditions like temperature and reaction time also play a crucial role in determining the final characteristics. Ultimately, a careful evaluation of purity and cost-effectiveness is essential to ensure that the produced nanoparticles meet the performance criteria for practical applications. Determining the magnetic structure of catalysts is a complex process that employs various sophisticated techniques. These methods are essential for unraveling the intricate magnetic properties and behaviors of different catalysts.68,69
Below are some of the most frequently utilized approaches in this field (Fig. 4):70,71
Transition metals immobilized on magnetic nanoparticles (MNPs) serve as innovative catalysts in chemical reactions, offering a multifaceted approach to catalysis with distinct advantages over conventional systems.72–74 These catalysts are characterized by their unique ability to combine homogeneous catalysts' high activity and selectivity with the ease of separation and recyclability of heterogeneous catalysts.75,76 The application of these MNPs spans various chemical reactions, including but not limited to chemical reactions, where they have been shown to enhance reaction rates and improve yield and selectivity.66,77 They provide a versatile and efficient alternative to traditional catalysts, with the added benefits of enhanced activity, selectivity, and sustainability.78,79 As research continues to evolve, these MNPs promise to revolutionize the field of catalysis, offering solutions that are more effective and environmentally responsible.80,81
Transition metals are key catalysts in chemical reactions due to their electronic properties, coordination complex formation, and variable oxidation states. Transition metals exhibit distinct and interconnected properties as catalysts, impacting their applications (Table 1). The choice of metal depends on the reaction type, economic factors, and desired performance under specific conditions.60–75
| Differences | Connections | Application scopes |
|---|---|---|
| Electronic configuration | Reactivity trends | Palladium (Pd) cross-coupling and carbonylation reactions |
| Oxidation states | Ligand effects | Nickel (Ni) a cost-effective alternative in cross-coupling and hydrogenation |
| Catalytic mechanism | Shared mechanistic pathways | Platinum (Pt) primarily for hydrogenation and fuel cell applications |
| Stability and reactivity | Alloying effects | Ruthenium (Ru) utilized in metathesis and oxidation processes |
| Coordination chemistry | Iron (Fe) sustainable catalysis in various organic processes | |
| Cobalt (Co) Fischer–Tropsch synthesis and hydrogenation |
MNPs–TM catalysts offer several advantages: they are easily separated from reaction mixtures using a magnetic field, can be reused with minimal activity loss, and have a high surface area due to their small size. Immobilizing transition metals on MNPs enhances their thermal and chemical stability, and surface functionalization can improve metal dispersion and catalytic activity. The size and shape of MNPs can be controlled to optimize catalytic performance, with some being biocompatible for biomedical applications. These catalysts exhibit high catalytic performance, and are cost-effective, environmentally friendly, and effective in green chemistry by allowing reactions in benign solvents under mild conditions, aligning with sustainable chemistry principles.82–89
1.2. Advantages of magnetic catalysts in carbonylation reactions
Carbonylation reactions, vital for producing a wide array of industrial chemicals like pharmaceuticals and polymers, often rely on catalysts to ensure efficient progress. The need for employing magnetic catalysts in these reactions stems from their ease of separation and recyclability, addressing a significant drawback of traditional homogeneous catalysts: the difficulty in separating them from the reaction mixture. This reduces waste, lowers production costs, and aligns with the growing demand for sustainable and environmentally friendly chemical processes. Magnetic catalysts, therefore, represent a significant advancement, offering a pathway to cleaner and more economical carbonylation chemistry.90Carbonylation reactions are a cornerstone of modern synthetic chemistry, pivotal in creating various chemical products.90 Central to these reactions is the introduction of a carbonyl group into an organic molecule, a transformation facilitated by using carbon monoxide as a reagent.91 This simple yet powerful process is harnessed in producing various carbonyl compounds integral to pharmaceuticals, agrochemicals, and materials science.92 The versatility of carbonylation is further demonstrated by its use in synthesizing acetic acid, a chemical of immense industrial importance, produced on a massive scale for use in food, plastics, and pharmaceuticals.93 The significance of carbonylation reactions extends beyond their industrial applications; they are also a fundamental tool in organic synthesis.94 Through the strategic use of metal catalysts, these reactions enable the efficient and selective formation of complex molecules from simpler substrates.95 For instance, the hydroformylation process, a type of carbonylation, is instrumental in converting alkenes into aldehydes, which can then be used as building blocks for more intricate compounds.96,97 Similarly, alkoxy-carbonylation and Pauson–Khand reactions are other notable examples where carbonylation steps are key to achieving the desired molecular architecture.98
Carbonylation reactions are essential in various fields:
• Pharmaceuticals: they introduce carbonyl groups into molecules, crucial for synthesizing biologically active compounds and complex structures with high selectivity and yield while also aligning with green chemistry principles.99–104
• Agrochemicals: carbonylation, especially palladium-catalyzed reactions, streamlines the synthesis of complex natural products, developing new agrochemicals and pharmaceuticals while converting carbon dioxide into valuable chemicals.105–112
• Materials science: these reactions efficiently introduce carbonyl groups, creating polymers and complex molecules with high precision and versatility, essential for producing pharmaceuticals, solvents, and plastics.113–115
• Industrial chemistry: carbonylation is used to synthesize acetic acid and other carboxylic acids, which are vital for solvents, plastics, and pharmaceuticals. Processes like hydroformylation and the Monsanto process highlight their significance.116–120
• Catalysis: catalyzing carbonylation introduces carbonyl groups efficiently, synthesizing pharmaceuticals, agrochemicals, and polymers. It maximizes atom economy and operates under milder conditions, advancing sustainable chemistry.121–130
Carbonylation reactions are a class of chemical processes where carbon monoxide (CO) is introduced into a molecule to form a carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).126 Metal catalysts facilitate these reactions and are fundamental in creating a wide range of organic compounds. Typically, the process involves the reaction of CO with an organic substrate in the presence of a metal catalyst, such as palladium or rhodium, and often a co-catalyst or ligand to enhance the reaction's efficiency and selectivity.131,132 The materials required for a carbonylation reaction include the substrate (often an alkene or alcohol), carbon monoxide, a metal catalyst, and sometimes additional solvents or reagents, depending on the specific type of carbonylation.127,133
O).126 Metal catalysts facilitate these reactions and are fundamental in creating a wide range of organic compounds. Typically, the process involves the reaction of CO with an organic substrate in the presence of a metal catalyst, such as palladium or rhodium, and often a co-catalyst or ligand to enhance the reaction's efficiency and selectivity.131,132 The materials required for a carbonylation reaction include the substrate (often an alkene or alcohol), carbon monoxide, a metal catalyst, and sometimes additional solvents or reagents, depending on the specific type of carbonylation.127,133
Magnetic catalysts offer a modern approach to carbonylation reactions, providing several advantages over traditional catalysts.127 They are designed with magnetic properties enabling easy separation from the reaction mixture using an external magnetic field, which is impossible with conventional catalysts. This magnetic separation process is less time-consuming and more environmentally friendly, reducing the need for additional solvents or energy-intensive methods typically required for catalyst recovery.127,134 Moreover, magnetic catalysts can be reused multiple times without significant activity loss, contributing to cost-effectiveness and sustainability in chemical processes.127 Traditional catalysts, often precious-metal-based, engage in substrate carbonylation through two-electron pathways and can be challenging to recover and reuse, leading to higher costs and potential environmental concerns due to metal residues.127,135 Magnetic catalysts, particularly those integrating magnetic nanoparticles with transition metals, have been shown to create synergistic effects that enhance both magnetism and catalytic activity. This has opened new avenues for carbonyl compound synthesis, with improved reaction rates and selectivity.127 Moreover, the stabilization of magnetic catalysts can be managed more effectively, resulting in improved reproducibility and consistency in reactions compared to traditional catalysts, which tend to be more variable, less uniform, and less reliable.127
Magnetic catalysts enhance carbonylation reactions due to their high surface area, which provides more active sites and the ability to be functionalized, improving selectivity and activity. They are quickly recovered and reused via magnetic separation, reducing waste and aligning with green chemistry. These catalysts are compatible with green solvents and operate efficiently under mild conditions, reducing energy consumption and enhancing process safety. Conventional catalysts remain vital for many industrial applications due to their proven performance and stability.
1.3. Application of magnetic catalysts in carbonylation reactions
Integrating magnetic nanoparticles with transition metals has been particularly noteworthy, yielding robust catalytic systems that leverage the synergistic effects of magnetism and catalytic activity.136 Recent advancements have demonstrated the versatility of magnetic nanoparticle-supported transition metal catalysts in various carbonylation reactions, including carbonylative coupling, alkoxycarbonylation, thiocarbonylation, and aminocarbonylation reactions (Fig. 5). This review comprehensively examines the mechanistic insights into how magnetic fields influence catalytic performance, the design and functionalization of magnetic catalysts for specific reactions, and the practical implications of these catalysts in industrial applications. Through critical analysis of current literature and case studies, the article presents a forward-looking perspective on the potential of magnetic catalysts to revolutionize carbonylation reactions, paving the way for more sustainable and innovative approaches in organic synthesis.2 Synthesis of ketones via carbonylation reactions
The synthesis of ketones is a fundamental aspect of organic chemistry due to their versatility and central role in various chemical processes.137 Ketones serve as key intermediates in synthesizing complex organic compounds, often acting as pivotal building blocks in constructing larger molecules.138 Their unique chemical properties enable various reactions, making them indispensable for creating pharmaceuticals, natural products, and other specialized chemicals.139 The ability to synthesize ketones through methods such as oxidation of secondary alcohols, ozonolysis of alkenes, and hydration or hydroboration of alkynes provides chemists with powerful tools to manipulate molecular structures and develop new compounds with desired properties.140 Moreover, ketones are used in various industries as solvents and are involved in essential biological processes, such as the formation of ketone bodies during fat metabolism in living organisms.141 This multifaceted utility underscores the importance of ketone synthesis in advancing both chemical research and industrial applications.142 The synthesis of ketones through carbonylation reactions is a crucial process in organic chemistry due to its atom efficiency and the ability to produce a wide range of valuable compounds.143 Carbonylation reactions introduce a carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) into an organic substrate. When applied to the synthesis of ketones, it transforms readily available starting materials into complex ketone structures.144 These ketones are essential in various fields, including pharmaceuticals, which serve as building blocks for drug design and materials science, where they contribute to developing new materials with specific properties.115 The importance of this method lies in its versatility and efficiency, enabling chemists to create a diverse array of ketone compounds in a relatively straightforward manner, which can be further used to synthesize more complex molecules.115
O) into an organic substrate. When applied to the synthesis of ketones, it transforms readily available starting materials into complex ketone structures.144 These ketones are essential in various fields, including pharmaceuticals, which serve as building blocks for drug design and materials science, where they contribute to developing new materials with specific properties.115 The importance of this method lies in its versatility and efficiency, enabling chemists to create a diverse array of ketone compounds in a relatively straightforward manner, which can be further used to synthesize more complex molecules.115
In 2022, the Hajipour research group reported that palladium immobilized on methionine-modified iron nanoparticles [ImmPd(0)–MNPs] [MRC-1] (Fig. 6) in the presence of KOH can serve as an excellent catalytic system for the preparation of diaryl ketone derivatives through a one-pot three-component carbonylative Suzuki–Miyaura coupling reaction of aryl iodides with aryl boronic acid and Mo(CO)6 in DMF under mild conditions.122 Fe3O4 nanoparticles were modified with methionine; then, the palladium complex was stabilized in the presence of ethanol and acetone on its surface to synthesize a magnetic palladium catalyst. SEM and TEM analyses showed that the fabricated particles are well within the nanometer range, and XRD analysis confirmed the structure and phase of the ImmPd(0)–MNPs catalyst. After conducting optimization experiments, the authors synthesized different derivatives of diaryl ketones in 4–12 hours with good to high yields through a one-pot three-component carbonylative Suzuki–Miyaura coupling reaction of aryl iodides with aryl boronic acid and Mo(CO)6. According to the mechanism proposed in Scheme 1 by the authors, the presence of palladium and base was essential to carry out the reaction. According to the recycling tests, the ImmPd(0)–MNPs catalyst was separated through magnetic decantation and could be used 8 times without reducing its catalytic efficiency.
 | ||
| Scheme 1 Scope of the ImmPd(0)–MNPs catalyst [MRC-1] for a one-pot three-component carbonylative Suzuki–Miyaura coupling reaction of aryl iodides with aryl boronic acid and Mo(CO)6. | ||
Li's research team reported the fabrication of palladium immobilized on Fe3O4@SiO2 functionalized with mercaptopropyl [Fe3O4@SiO2–SH–PdII] for the preparation of diaryl ketone derivatives via carbonylative Suzuki coupling reactions.110 The Fe3O4@SiO2–SH–PdII catalyst [MRC-2] (Fig. 6) was synthesized using a simple method from available materials. Several identification analyses confirmed the successful preparation of the Fe3O4@SiO2–SH–PdII catalyst. The VSM technique confirmed the magnetic properties of the Fe3O4@SiO2–SH–PdII catalyst [MRC-2], and TGA analysis showed that the catalyst has acceptable thermal stability. Various tests were performed to obtain optimal conditions for preparing diaryl ketone derivatives. Under optimal conditions, the one-pot, three-component carbonylative Suzuki coupling reactions of aryl halides, aryl boronic acids, and carbon monoxide were successfully catalyzed by the Fe3O4@SiO2–SH–PdII nanocomposite to synthesize various products of diaryl ketones with high acceptable yields (Scheme 2). According to the recycling tests, the Fe3O4@SiO2–SH–PdII catalyst was separated through magnetic decantation and could be used 5 times without reducing its catalytic efficiency.
 | ||
| Scheme 2 Scope of the Fe3O4@SiO2–SH–PdII catalyst [MRC-2] for the one-pot, three-component carbonylative Suzuki coupling reactions of aryl iodides, aryl boronic acids, and carbon monoxide. | ||
In a similar method, Li's research group reported the use of a new magnetic palladium catalyst [Fe3O4/PPy–Pd(II)] to prepare diaryl ketones via one-pot three-component carbonylative reactions of aryl iodides, aryl boronic acids and carbon monoxide.114 Several spectroscopic techniques were used to determine the structure of the Fe3O4/PPy–Pd(II) catalyst [MRC-3] (Fig. 4); XRD analysis confirmed that the structure of the catalyst is consistent with that of the reported samples. Under the standardized conditions shown in Scheme 3, the reactions of aryl halides with arylboronic acids and carbon monoxide were well catalyzed by the Fe3O4/PPy–Pd(II) nanomaterial, and the diaryl ketone products were synthesized with moderate to good yields. According to the recycling tests, the Fe3O4/PPy–Pd(II) catalyst was separated through magnetic decantation and could be used 5 times without reducing its catalytic efficiency.
 | ||
| Scheme 3 Scope of the Fe3O4/PPy–Pd(II) catalyst [MRC-3] for one-pot, three-component carbonylative Suzuki coupling reactions of aryl iodides, aryl boronic acids, and carbon monoxide. | ||
In an exciting method, Shafiq's group prepared a magnetic ligand by immobilizing 2-picolylamine (pyridin-2-ylmethanamine) and forming an imine bond on the surface of Fe3O4 nanoparticles and immobilized palladium on its surface to form a magnetic palladium catalyst (Fe3O4–bis(Py-imine)–PdCl2) [MRC-4].144 The catalytic application of this magnetic palladium nanocomposite was studied in the preparation of diaryl ketone derivatives, and the results of these experiments are summarized in Scheme 4. As shown in Scheme 4, a broad range of derivatives of diaryl ketones (24 examples) were synthesized with high yields from several derivatives of aryl iodides or aryl boronic acids with different functional groups (electron donating and withdrawing groups) under eco-friendly conditions. According to the designed mechanism, first the Fe3O4–bis(Py-imine)–PdCl2 catalyst [MRC-4] (Fig. 4) reacts with aryl iodide, and then a carbonylated intermediate is formed due to the carbonyl group entering the reaction. Then, the desired diaryl ketone products were synthesized by adding aryl boronic acid to the reaction in the presence of a base. According to the recycling tests, the Fe3O4/PPy–Pd(II) catalyst [MRC-4] was separated through magnetic decantation and could be used 6 times without reducing its catalytic efficiency.
 | ||
| Scheme 4 Scope of the Fe3O4–bis(Py-imine)–PdCl2 catalyst [MRC-4] for one-pot, three-component carbonylative Suzuki coupling reactions of aryl iodides, aryl boronic acids, and Mo(CO)6. | ||
A palladium complex stabilized on triphenylphosphine attached to Fe3O4@SiO2 nanoparticles as a recoverable catalyst [MRC-5] (Fig. 6) was developed by Cai's group research, and it was used in the preparation of diaryl ketone derivatives.112 FT-IR analysis confirmed the presence of a phosphine functional group and the formation of Fe–O and Fe3O4@SiO2 bonds. In order to enhance the reaction conditions for the synthesis of diaryl ketones, a series of meticulous experiments were conducted. These experiments explored various factors, including the selection and quantity of the catalyst, the choice of base, and the type of solvent used in the model reaction. Each variable was systematically tested to determine its impact on the overall efficiency and yield of the desired products. Under the optimal conditions shown in Scheme 5, various derivatives of diaryl ketones were synthesized with poor to high yields through the carbonylation reactions of aryl boronic acids, aryl iodides, and formic acid as the carbonyl source. One of the important features of this work is the use of formic acid as both a solvent supplement and a carbonyl agent. According to recycling tests, the Fe3O4@SiO2–2P–PdCl2 catalyst [MRC-5] was separated through magnetic decantation and could be used 6 times without a decease in its catalytic efficiency.
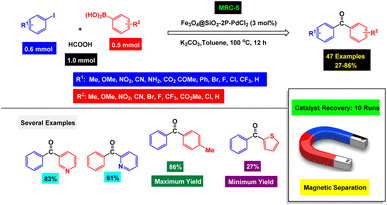 | ||
| Scheme 5 Scope of the Fe3O4@SiO2–2P–PdCl2 catalyst [MRC-5] for one-pot, three-component carbonylative Suzuki coupling reactions of aryl iodides, aryl boronic acids, and HCCOH as the carbonyl source. | ||
In another method used to prepare diaryl ketones, Ichie's research group reported that arginine immobilized on Fe3O4 nanoparticles can act as an excellent ligand to catalyze the carbonylation reactions by palladium metal. BET, VSM, and ICP-OES confirmed the Fe3O4@arginine–Pd(0) catalyst's structure.134 Experimental tests showed that the amount of the Fe3O4@arginine–Pd(0) catalyst [MRC-6] (Fig. 7), along with the use of the base, is a very crucial component in optimizing the reaction conditions. As seen in Scheme 6, the carbonylation reaction of aryl iodides with aryl boronic acids and Cr(CO)6 as the carbonyl source in the presence of KOH base in DMF solvent was efficiently catalyzed by the Fe3O4@arginine–Pd(0) nanocomposite [MRC-6] and ten derivatives of diaryl ketones were obtained with high yields in less than 5 h. According to the recycling tests, the Fe3O4@arginine–Pd(0) catalyst [MRC-6] was separated through magnetic decantation and could be used six times without reducing its catalytic efficiency.
In a similar method, So-Ho's research group also showed that immobilization of dopamine on Fe3O4 nanoparticles and its reaction with pyridine 2-carbaldehyde leads to the formation of an imine bond and the production of an excellent magnetic ligand to stabilize palladium metal [Fe3O4@DopPy–Pd(0) catalyst] [MRC-7] (Fig. 5).145,146 VSM analysis showed that the Fe3O4@DopPy–Pd(0) catalyst has strong magnetic properties. The optimized palladium catalyst demonstrated remarkable effectiveness in synthesizing diaryl ketone derivatives through an innovative one-pot, three-component reaction mechanism. This process involves the interaction between aryl iodides and aryl boronic acids, with Mo(CO)6 serving as the carbonyl source, leading to the efficient formation of the desired ketone products (Scheme 7). The streamlined approach facilitates a direct and efficient route to these complex compounds, underscoring the catalyst's high catalytic efficiency and versatility in organic synthesis. The carbonylation reactions were carried out in the presence of sodium hydroxide base in DMSO solution under thermal conditions for 6 hours, and 10 examples of diaryl ketone derivatives were prepared with acceptable yields. According to the recycling tests, the Fe3O4@DopPy–Pd(0) catalyst [MRC-7] was separated through magnetic decantation and could be utilized six times without diminishing its catalytic effectiveness.
 | ||
| Scheme 7 Scope of the Fe3O4@DopPy–Pd(0) catalyst [MRC-7] for one-pot, three-component carbonylative Suzuki coupling reactions of aryl iodides, aryl boronic acids, and Mo(CO)6 as the carbonyl source. | ||
Jin's research group prepared a magnetic ligand through the reaction of Fe3O4 nanoparticles coated with 3,4-dihydroxybenzaldehyde with 1,10-phenanthroline-5,6-diamine; and by immobilizing the palladium(0) complex on the surface of the Fe3O4@DH/Ph–ImH–Phen ligand, they constructed a palladium magnetic catalyst. In this publication, the authors showed that the palladium magnetic catalyst [Fe3O4@DH/Ph–ImH–Phen–Pd(0)][MRC-8] (Fig. 5) in the presence of KHCO3 in PEG can act as a green and efficient catalytic system for the preparation of diaryl ketone derivatives through carbonylation reactions.134 By optimizing the conditions by studying the amount of the Fe3O4@DH/Ph–ImH–Phen–Pd(0) catalyst and the effect of base and solvent, the authors studied the effect of different substrates, including aryl and heteroaryl iodides and boronic acids, and the desired diaryl ketone products were synthesized with high yields (Scheme 8). According to the recycling tests, the Fe3O4@DH/Ph–ImH–Phen–Pd(0) catalyst [MRC-8] was separated through magnetic decantation and could be used 8 times without reducing its catalytic efficiency. The reaction proceeded through the formation of carbonyl–palladium intermediate and then by adding aryl boronic acids in the presence of KHCO3, the reaction cycle was completed, and the desired diaryl ketone products were synthesized with satisfactory yields.
2.1. Synthesis of flavones via carbonylation/cyclization reactions
Flavone derivatives are a significant class of natural compounds with a broad spectrum of biological and pharmaceutical applications.107,147 Biologically, flavones are known for their antioxidant, anti-inflammatory, and antimicrobial properties, which contribute to their role in defining mechanisms and potential health benefits in human nutrition and pharmacology.148–150 From a pharmaceutical perspective, flavones and their derivatives have been extensively studied for their therapeutic potential, including anticancer, neuroprotective, and cardioprotective effects.151,152 The flavones can be synthesized through various methods, including the carbonylation reaction, a palladium-catalyzed process that introduces a carbonyl group into organic compounds. Such synthetic versatility is crucial for developing new compounds with improved biological properties for further pharmaceutical applications.To prepare flavone derivatives via carbonylation reaction, Wang's research group reported the use of a palladium magnetic catalyst [Fe3O4@AMBA–BiPy–Pd(0)]. The Fe3O4@AMBA–BiPy–Pd(0) catalyst [MRC-9] (Fig. 7) was prepared by modifying the surface of Fe3O4 NPs with 4-aminobenzoic acid and reacting it with [2,2′-bipyridine]-6,6′-dicarbaldehyde and stabilizing palladium metal on its surface. Various analyses were used to confirm the successful preparation of the Fe3O4@AMBA–BiPy–Pd(0) catalyst; SEM and TEM analyses, in line with XRD analysis, showed that the particles are entirely spherical and synthesized in the nanometer range.153 Also, the magnetic properties and thermal stability of the Fe3O4@AMBA–BiPy–Pd(0) catalyst [MRC-9] were confirmed by VSM and TGA analyses, respectively. Experiments to optimize the model reaction showed that the presence of a base and a catalyst is vital for carrying out the reaction, and in their absence, the reaction will not be carried out. Under the optimized conditions shown in Scheme 9, 24 examples of flavones were synthesized with satisfactory yields through three-component carbonylation reactions of 2-iodophenol derivatives, terminal alkynes, and Cr(CO)6 in the presence of DABCO in PEG for 6 h. The reaction first proceeded through the interaction of 2-iodophenol with the Fe3O4@AMBA–BiPy–Pd(0) catalyst in the presence of Cr(CO)6 (carbonyl source) to prepare the carbonylated intermediate; subsequently, with the introduction of terminal alkyne into the reaction cycle in the presence of DABCO base and the Fe3O4@AMBA–BiPy–Pd(0) catalyst, flavone products were synthesized through several intramolecular reactions. According to the recycling tests, the Fe3O4@AMBA–BiPy–Pd(0) catalyst [MRC-9] was separated through magnetic decantation and could be used six times without reducing its catalytic efficiency.
 | ||
| Scheme 9 Scope of the Fe3O4@AMBA–BiPy–Pd(0) catalyst [MRC-9] for one-pot, three-component carbonylative annulation reactions of 2-iodophenol derivatives, terminal alkynes, and Cr(CO)6. | ||
More recently, Martin reported that multicomponent reactions of 2-halide phenols (halide: I, Br) with terminal alkynes and Mn(CO)3 (as the carbonyl source) can be successfully catalyzed by the Fe3O4@AFPA–Pd(0) nanocomposite [MRC-10] (Fig. 7).154 The structure of the Fe3O4@AFPA–Pd(0) catalyst was evaluated by FT-IR, TGA, and SEM analysis; the high thermal stability of the Fe3O4@AFPA–Pd(0) catalyst was proved by TGA. By performing optimization reactions, and testing various bases and solvents, 15 mol% catalyst in the presence of DABCO in DMF under thermal conditions for 8 hours was considered as the standardized condition for the preparation of flavone derivatives. In this publication, as shown in Scheme 10, the scope of the catalytic system was tested by studying several aryl and heteroaryl alkynes, and 8 examples of flavone derivatives were synthesized with high yields. According to the recycling tests, the Fe3O4@AFPA–Pd(0) catalyst [MRC-10] was separated through magnetic decantation and could be used six times without reducing its catalytic efficiency. By reacting the Fe3O4@AFPA–Pd(0) catalyst with 2-halide phenols and then introducing Mn(CO)3 into the reaction cycle, first the carbonylated palladium intermediate was formed; then this process was accompanied by the reaction with activated alkynes (through the reaction with DABCO) to synthesize the flavone products.
 | ||
| Scheme 10 Scope of the Fe3O4@AFPA–Pd(0) catalyst [MRC-10] for one-pot, three-component carbonylative annulation reactions of 2-iodophenol derivatives, terminal alkynes, and Mn(CO)3. | ||
The synthesis of quinolines is critically significant due to their extensive applications across various fields, including pharmaceuticals, agrochemicals, and material sciences. Quinolines are key structural components in many biologically active compounds, prominently featuring in anti-malarial medications like chloroquine and in agents prescribed for cancer treatment, inflammation reduction, and microbial infections. Their wide-ranging pharmacological properties underscore their importance in drug discovery and the ongoing research within medicinal chemistry. Beyond their medicinal uses, quinoline derivatives also play vital roles in developing vibrant dyes and innovative organic light-emitting diodes (OLEDs) and serve as essential ligands in coordination chemistry. The quest for efficient and sustainable methodologies for synthesizing quinolines has led to the emergence of catalytic approaches, including one-pot, three-component carbonylative reactions. These strategies not only enhance the accessibility of quinolines but also streamline the overall synthesis process, reducing the number of reaction steps involved. Such advancements align with principles of green chemistry, making quinolines increasingly valuable for industrial applications and academic research endeavors.155–158
Kazemi and his research group introduced a novel and highly effective methodology for synthesizing various derivatives of quinoline-4(1H)-ones, as shown in Scheme 11.159 This innovative approach employs a palladium nanocomposite catalyst, specifically [Fe3O4@SiO2–Diol–Phen–Pd(0)], which is not only magnetic but also reusable, making it attractive in green chemistry [MRC-13] (Fig. 7). The procedure involves three-component reactions, where a diverse range of heteroaryl alkynes react seamlessly with nitrobenzene. The catalytic role of the Fe3O4@SiO2–Diol–Phen–Pd(0) nanocomposite is notably pronounced, particularly when paired with Cr(CO)6 acting as a carbon monoxide source. The reaction takes place in an essential aqueous environment using potassium acetate (KOAc) in a mixture of water and polyethylene glycol (PEG), and it yields an impressive array of quinoline-4(1H)-one derivatives with remarkable efficiency. Water plays an important role in this reaction, facilitating the conversion of the nitro functional group into an amine, significantly enhancing the overall efficiency of the process. Scheme 10 beautifully illustrates the versatility of the Fe3O4@SiO2–Diol–Phen–Pd(0) catalyst (referred to as MRC-4) in this one-pot, three-component carbonylative transformation. The reaction involves explicitly a bromo-nitro-substituted aromatic compound, an alkyne, and hexacarbonylchromium [Cr(CO)6] as a source of carbon monoxide, all occurring under basic aqueous conditions at a temperature of 100 °C for two hours. The results are striking, showcasing 24 examples achieved with high yields ranging from 87% to an impressive 99%. The substrate scope is comprehensive, accommodating various functional groups—including heteroaryl, methoxy (OMe), nitro (NO2), bromo (Br), chloro (Cl), and hydrogen (H)—as well as a wide variety of aryl and heteroaryl alkynes, including phenyl, naphthyl, thiophene, furan, and pyridine. One of the key advantages of this process is the ability to easily separate the magnetic catalyst, allowing for its recyclability up to eight reaction cycles. The catalytic performance is exemplary, with the highest yield recorded at 99% and the lowest at 84%, underscoring both the effectiveness of the catalyst and its broad substrate tolerance.
 | ||
| Scheme 11 Scope of the Fe3O4@SiO2–Diol–Phen–Pd(0) catalyst [MRC-13] for the synthesis of quinoline-4(1H)-one derivatives via one-pot, three-component carbonylative reactions. | ||
2.2. Synthesis of esters and thioesters via alkoxy and thio carbonylation reactions
Esters and thioesters play pivotal roles in both biological systems and pharmaceutical applications.160 Biologically, esters are essential for forming fats and oils, serving as energy storage molecules, while thioesters are crucial in metabolic pathways. For instance, acetyl coenzyme A, a central metabolite, is a thioester that transports acyl groups in the citric acid cycle, a fundamental energy-releasing pathway.161 In pharmaceuticals, esters are often employed as prodrugs, where the ester moiety is metabolized to release the active drug, enhancing its absorption and distribution.162–165 Aspirin, one of the most widely used medications, is an ester of salicylic acid and is a prime example of this application.166,167 The preparation of esters and thioesters typically involves the reaction of an acid derivative with an alcohol or thiol, respectively.168–170 Carbonylation reactions introduce a carbonyl group into a compound and are a standard method for synthesizing these derivatives.171 Recent advancements have enabled the synthesis of thioesters via carbonylation reactions that are more atom-efficient and environmentally friendly.172 These methods often involve transition-metal-free conditions, broadening the substrate scope and improving functional group tolerance, which is particularly advantageous in synthesizing complex molecules for pharmaceutical applications. The ability to perform these reactions under mild conditions is also beneficial for preserving the integrity of sensitive molecules.To synthesize ester and thioester derivatives, Wei's research groups prepared a nickel magnetic catalyst [Fe3O4@BA/Pyrim–carboxamide–NiCl2]. They studied its catalytic activity in three-component reactions of aryl iodides, aryl or benzyl phenols or thiols, and carbonyl source (Cr(CO)6), as shown in Scheme 12.168 In order to prepare a magnetic nickel catalyst, Fe3O4 NPs were first surface modified with 4-(aminomethyl)benzoic acid. The aminolysis via the amine reaction with pyrimidine-2-carbonyl chloride led to the production of the magnetic Fe3O4@BA/Pyrim–carboxamide ligand. With the addition of nickel metal, the Fe3O4@BA/Pyrim–carboxamide–NiCl2 catalyst [MRC-11] (Fig. 8) was successfully synthesized. VSM analysis clearly showed that the Fe3O4@BA/Pyrim–carboxamide–NiCl2 catalyst has high magnetic potential. The structure and functional groups in the Fe3O4@BA/Pyrim–carboxamide–NiCl2 catalyst [MRC-11] were confirmed by XRD and FT-IR analyses. The authors conducted various experiments to optimize the conditions for preparing esters and thioesters. The base type and the catalyst amount were crucial in the optimization process. Also, by testing different solvents, the authors found that the ChCl–urea solvent is more efficient than other solvents for preparing ester and thioester derivatives. Three-component reactions of aryl iodides with aryl or benzyl phenols or thiols and Cr(CO)6 (carbonyl source) were catalyzed by the Fe3O4@BA/Pyrim–carboxamide–NiCl2 nanocomposite in the presence of KOAc and various derivatives of esters and thioesters were synthesized with high yields in ChCl–urea under air and mild conditions. According to the recycling tests, the Fe3O4@BA/Pyrim–carboxamide–NiCl2 catalyst [MRC-11] was separated through magnetic decantation and could be used eight times without reducing its catalytic efficiency.
Al-Shakarji and Kamel researched the synthesis of thioesters, demonstrating the effective use of a nanocomposite consisting of Fe3O4@benzo[d]imidazole–Zr, as shown in Scheme 13. This innovative material was employed in thiocarbonylation reactions involving aryl iodides.173 Notably, they utilized Cr(CO)6 as a solid source, showcasing a novel approach for facilitating these reactions. The plausible reaction mechanism for the synthesis of thioesters from iodobenzene catalyzed by Fe3O4@benzo[d]imidazole–Zr follows a typical catalytic cycle involving oxidative addition, carbonyl insertion, and nucleophilic substitution (Scheme 13). Initially, the zirconium catalyst (Zr(0)) undergoes oxidative addition with iodobenzene, forming an aryl–Zr(II) complex. This intermediate reacts with carbon monoxide (CO) to generate an acyl–Zr(II) species [MRC-12] (Fig. 7). Subsequently, the acyl complex undergoes nucleophilic attack by a thiol (Ar–SH) in the presence of a base (K2CO3), forming the thioester–Zr(II) complex. Finally, protonation releases the thioester product, regenerating the active Zr catalyst for the next cycle. The Fe3O4 support enhances catalyst stability and reusability, making the system efficient for thioester synthesis. The condensation reaction exhibited a faster rate for aryl halides and thiols that contained electron-withdrawing substituents, such as nitro groups, in comparison to those with electron-donating substituents, like alkyl, alkoxy, and hydroxy groups. This observation aligns with the expected predictions regarding the influence of substituent types on the reactivity of these compounds. After conducting a thorough series of recycling tests, we noted that the catalyst maintained its integrity throughout the process, showing no significant decline in its quantity or catalytic activity. This remarkable stability allowed for the successful recovery and reuse of the catalyst in four consecutive cycles. The results highlighted the catalyst's durability and effectiveness, underscoring its potential for sustainable applications in various catalytic processes.174
 | ||
| Scheme 13 Scope of the Fe3O4@benzo[d]imidazole–Zr catalyst [MRC-12] for the synthesis of thioester derivatives via one-pot, three-component thiocarbonylation reactions. | ||
In order to prepare thioester derivatives, Khalili's research group reported an interesting and valuable method for the reaction of methylene derivatives with aryl or alkyl thiols in the presence of t-BuOOH based on the use of rGO/Fe3O4–CuO nanocomposite as an efficient and reusable catalyst.174 The rGO/Fe3O4–CuO catalyst [MRC-15] (Fig. 8) was prepared in a multi-step process by coating graphene with Fe3O4 NPs and then fixing copper oxide on its surface. The structure and morphology of the rGO/Fe3O4–CuO catalyst were well confirmed by XRD and TEM analyses. The effect of the amount of catalyst and solvent was studied to optimize the reaction. As seen in Scheme 14, 10 derivatives of thioesters were synthesized with relatively good yields in an aqueous medium under mild conditions. A carbonyl radical was generated from the reaction of toluene derivatives with t-BuOOH in the presence of the rGO/Fe3O4–CuO. A sulfide–copper intermediate was formed by the reaction of aryl and alkyl thiols in the presence of the rGO/Fe3O4–CuO catalyst. The desired thioester products were synthesized with good yields when this intermediate entered the reaction cycle. According to the recycling tests, the rGO/Fe3O4–CuO catalyst [MRC-15] was separated through magnetic decantation and could be used six times without reducing its catalytic efficiency.
 | ||
| Scheme 14 Scope of the rGO/Fe3O4–CuO catalyst [MRC-15] for one-pot, three-component thioesterification annulation reactions. | ||
In a fascinating and convenient research method, Prasad's research group reported that the magnetic palladium catalyst [Pd/Fe3O4] in the presence of K3PO4 base in DMF can act as an effective catalytic system for the preparation of ester derivatives through alkoxy carboxylation reactions of iodo benzenes with aryl, benzyl or alkyl alcohols and carbon monoxide.175 TEM images showed that the catalyst was successfully constructed in the nanometre range. The conditions for carrying out alkoxy carboxylation reactions were optimized by examining the effect of base and solvent. The results showed that this catalytic system has relatively good efficiency for synthesizing esters and is a general and suitable method for carrying out alkoxy carboxylation reactions of aryl iodides. The scope of the catalytic system was studied by using iodo benzenes with electron-withdrawing and electron-donating groups, and under standardized conditions, the ester products were synthesized with different yields, which shows that the effect of substitution and substrate on the catalyst is practical (Scheme 15). The reaction cycle proceeded through the reaction of iodo benzenes with the Pd/Fe3O4 catalyst [MRC-16] and carbon monoxide to form the carbonyl–palladium intermediate. The reaction of alcohol and K3PO4 leads to the production of an alkoxy nucleophile, which then enters the cycle and reacts with the carbonyl–palladium intermediate, completing the process.176 The alkoxy carbonylation reaction is completed, and the ester products are synthesized with acceptable yields. According to the recycling tests, the Pd/Fe3O4 catalyst [MRC-16] was separated through magnetic decantation and could be used five times without reducing its catalytic efficiency.
 | ||
| Scheme 15 Scope of the Pd/Fe3O4 catalyst [MRC-16] for one-pot, three-component esterification annulation reactions. | ||
Zhang and Zheyuan introduced a highly effective methodology for synthesizing thioester derivatives through the interaction of tertiary thioamides and alcohols, utilizing a nanocatalyst known as MNPs–Acac@Cu, combined with PEG as a solvent (Scheme 16). To achieve optimal results, the researchers meticulously adjusted various reaction parameters, such as temperature, solvent choice, and the amount of catalyst, at the outset of their investigation. The morphology and structural characteristics of the nanocatalyst were assessed through TEM, which also provided insights into the average particle size. The TEM images revealed that copper is effectively immobilized on the Fe3O4 substrate, forming the MNPs–Acac@Cu nanocatalyst at the nanoscale level. Under the specified conditions, the method yielded thioesters with impressive efficiencies, achieving yields ranging from 81% to 90% in a remarkably short reaction time. The proposed mechanism, illustrated in Scheme 3, begins with the MNPs–MA@Cu catalyst protonating the alcohol, followed by a nucleophilic attack from the thioamide after removing water and forming a carbocation. This process leads to S-alkylation and the subsequent creation of thioformamidinium salt. Finally, the hydrolysis reaction, facilitated by water, results in the formation of desired thioester products. Following a series of comprehensive recycling tests, it was observed that there was no significant reduction in either the quantity or the activity of the catalyst during the process. As a result, the catalyst was successfully recovered and reused four consecutive times, demonstrating its durability and effectiveness.
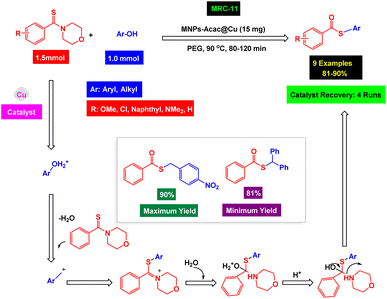 | ||
| Scheme 16 Scope of the MNPs–Acac@Cu catalyst [MRC-11] for the synthesis of thioester derivatives via one-pot, three-component thiocarbonylation reactions. | ||
2.3. Synthesis of amides via amino carbonylation reactions
Amide derivatives are pivotal in various scientific fields due to their structural significance and functional versatility.177 Biologically, amides form the backbone of peptides and proteins, essential for life's myriad functions.178 The amide bond is a fundamental component of biomolecules, playing a crucial role in the structure and function of living organisms.98 In the pharmaceutical industry, amides are integral, with a significant portion of drugs containing at least one amide bond due to their stability and ability to engage in hydrogen bonding, which is vital for drug–target interactions. Medically, amides are found in various therapeutic agents, highlighting their importance in treatment and disease management.179 Chemically, amides are synthetically versatile and capable of undergoing a range of transformations, making them valuable in creating complex molecules for various applications.180 The preparation of amides can be achieved through several methods, with carbonylation reactions being a prominent technique.181 This process involves introducing a carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) into a molecule. Specifically, amino carbonylation is a method where a nitrogen-containing group is introduced into the molecule along with the carbonyl group, forming an amide.182 This reaction typically employs a palladium catalyst and involves carbon monoxide, offering a route to synthesize amides from aryl halides and amines. The carbonylation reaction is important for the synthesis of amides and exemplifies the advancement of green chemistry practices, as it often allows for the creation of amides in a more environmentally friendly manner compared to traditional methods. Developing new, efficient, and sustainable synthetic routes for amide formation is an ongoing area of research, reflecting the compound's significance across multiple disciplines.
O) into a molecule. Specifically, amino carbonylation is a method where a nitrogen-containing group is introduced into the molecule along with the carbonyl group, forming an amide.182 This reaction typically employs a palladium catalyst and involves carbon monoxide, offering a route to synthesize amides from aryl halides and amines. The carbonylation reaction is important for the synthesis of amides and exemplifies the advancement of green chemistry practices, as it often allows for the creation of amides in a more environmentally friendly manner compared to traditional methods. Developing new, efficient, and sustainable synthetic routes for amide formation is an ongoing area of research, reflecting the compound's significance across multiple disciplines.
In order to prepare derivatives of amides and esters, the Abu-Reziq research group used a magnetic palladium catalyst [MNP–Im–NH2–Pd] [MRC-16] (Fig. 8) (made by immobilizing palladium on the surface of functionalized magnetic nanoparticles with imidazole–amine) to carry out alkoxy and amino carbonylation reactions.183 TEM analyses, in line with the XRD analysis, confirmed that the particles of the MNP–Im–NH2–Pd catalyst were in the nanometer range. A series of studies were conducted on optimization reactions to obtain standard conditions for preparing amide and ester derivatives. As can be seen in Scheme 17, three-component reactions of aryl and heteroaryl halides with various derivatives of amines and alcohols in the presence of carbon monoxide were successfully catalyzed by the MNP–Im–NH2–Pd nanocomposite [MRC-16] and Et3N in toluene for 24 h, and the desired ester and amide products were synthesized with good to high yields. According to the recycling tests, the MNP–Im–NH2–Pd catalyst [MRC-16] was separated through magnetic decantation and could be used five times without reducing its catalytic efficiency.
 | ||
| Scheme 17 Scope of the MNP–Im–NH2–Pd catalyst [MRC-16] for one-pot, three-component esterification and amino carbonylation annulation reactions. | ||
In a similar method, Jain's research group developed a palladium magnetic catalyst [Fe3O4@APPA–Pd] [MRC-17] (Fig. 9) by immobilizing palladium on a magnetic ligand (made from the reaction of magnetic Fe3O4 nanoparticles with amino acid). The catalytic activity was investigated in alkoxy and amino carbonylation reactions.96 TGA analysis showed that the synthesized Fe3O4@APPA–Pd catalyst [MRC-17] has high thermal stability, and the presence of functional groups in the catalyst was confirmed by FT-IR analysis. By studying different amounts of the Fe3O4@APPA–Pd catalyst [MRC-17] in the presence of several bases in different solvents on the model reaction, the optimal conditions for preparing amide and ester derivatives were optimized. In alkoxy carbonylation reactions, various derivatives of aryl and heteroaryl iodides reacted readily with phenol or benzyl alcohols in the presence of carbon monoxide under optimal conditions, and the desired ester products were synthesized with acceptable yields (Scheme 18). Also, by reacting anilines or pyridine amines with a carbonyl-containing palladium intermediate (made from the reaction of a palladium catalyst with aryl halides with a carbonyl source), amide products were synthesized with relatively high yields (Scheme 19). According to the recycling tests, the Fe3O4@APPA–Pd catalyst [MRC-18] was separated through magnetic decantation and could be used five times without reducing its catalytic efficiency.
 | ||
| Scheme 18 Scope of the Fe3O4@APPA–Pd catalyst [MRC-17] for one-pot, three-component esterification annulation reactions. | ||
 | ||
| Scheme 19 Scope of the Fe3O4@APPA–Pd catalyst [MRC-17] for one-pot, three-component amino carbonylation annulation reactions. | ||
In order to pepare a palladium magnetic catalyst [MNPs–dopamine–BiPy–Pd(0)], Jain's research group first fixed dopamine on magnetic Fe3O4 nanoparticles and then reacted with [2,2′-bipyridine]-6,6′-dicarbaldehyde to synthesize the desired magnetic ligand by forming an imine bond between dopamine and bipyridine. By immobilizing palladium metal in the presence of NaBH4 on the prepared MNPs–dopamine–BiPy magnetic ligand, the MNPs–dopamine–BiPy–Pd(0) magnetic catalyst [MRC-18] (Fig. 9) was prepared. Its catalytic activity was evaluated in the preparation of amide derivatives through amino carbonylation reactions of iodobenzenes with Mo(CO)6 and sodium azide in PEG solvent under mild conditions. To confirm the successful production of the MNPs–dopamine–BiPy–Pd(0) catalyst [MRC-18], several analyses such as FT-IR, SEM, TEM, TGA, VSM, EDX, ICP-OES, XRD, and elemental mapping analysis were used.97 The structure of the catalyst was confirmed by XRD and FT-IR analysis, and EDX and elemental mapping analysis also confirmed the elements present in the catalyst structure. The scope and efficiency of the catalytic system were evaluated by using several derivatives of iodobenzenes with electron-donating and electron-withdrawing groups, and the desired amide products were synthesized with high yields (Scheme 20). According to the mechanism shown in Scheme 20, the cyclization of amides proceeded through the reaction of iodobenzenes with the MNPs–dopamine–BiPy–Pd(0) catalyst [MRC-18] in the presence of the carbonyl source (Mo(CO)6) to form a carbonyl–palladium intermediate. By reacting palladium–iodobenzenes intermediates with sodium azide, benzene azide was formed, and when this compound entered the mechanism cycle, amine–palladium–carbonyl intermediates were formed, leading to the synthesis of amide products. According to the recycling tests, the MNPs–dopamine–BiPy–Pd(0) catalyst [MRC-18] separated through magnetic decantation and could be used eight times without reducing its catalytic efficiency.
 | ||
| Scheme 20 Scope of the MNPs–dopamine–BiPy–Pd(0) catalyst [MRC-16] for one-pot, three-component amino carbonylation annulation reactions. | ||
Recently, Jain's research group presented a desirable, general, and efficient method in which magnetic palladium catalyst [Fe3O4@SiO2–Phen–Pd(0)] (made by immobilizing palladium on a magnetic nanoparticle substrate modified with phenanthroline dicarboxylic acid) in the presence of potassium acetate to prepare benzothiazole amide derivatives were used.184 SEM and TEM analyses showed that the catalyst particles were synthesized in the nanometer range, and VSM analysis confirmed the high magnetic properties of the Fe3O4@SiO2–Phen–Pd(0) catalyst [MRC-19] (Fig. 9). By conducting studies on the model reaction and optimization experiments, it was found that the presence of the Fe3O4@SiO2–Phen–Pd(0) catalyst [MRC-19] and a base is essential to carry out the amino carbonylation reactions. As can be seen in Scheme 21, numerous derivatives (19 examples) of the benzothiazole amides were synthesized with very high yields through Fe3O4@SiO2–Phen–Pd(0) nanocomposite-catalyzed amino carbonylation reactions of aryl or heteroaryl iodides with Mo(CO)6 and 2-aminobenzothiazoles using KOAc in glycerol. FT-IR and XRD analyses showed that the structure of the Fe3O4@SiO2–Phen–Pd(0) catalyst [MRC-19] t did not change even after recovery, which indicates the high stability of the Fe3O4@SiO2–Phen–Pd(0) catalyst. According to the reported mechanism, the reaction cycle is completed with the introduction of the nucleophile 2-amino benzothiazole (obtained from the reaction in the presence of KOAc) to the palladium carbonylated intermediates (obtained from the reaction of iodobenzene with the palladium catalyst and the carbonyl source) and benzothiazole amide products were synthesized with high yields. According to the recycling tests, the Fe3O4@SiO2–Phen–Pd(0) catalyst [MRC-19] separated through magnetic decantation and could be used eight times without reducing its catalytic efficiency.
 | ||
| Scheme 21 Scope of the Fe3O4@SiO2–Phen–Pd(0) catalyst [MRC-19] for one-pot, three-component amino carbonylation annulation reactions of 2-aminobenzothiazoles. | ||
3 Summary and outlook
In conclusion, exploring magnetic catalysts in carbonylation reactions significantly advances organic synthesis. The unique properties of these catalysts, including their enhanced reactivity, selectivity, and ease of separation via magnetic decantation, have opened new avenues for developing more efficient and sustainable chemical processes. Integrating magnetic catalysts has improved the yield and purity of carbonylation products and contributed to reducing environmental impact by minimizing waste and simplifying purification steps. As highlighted in this review, the versatility and robustness of magnetic catalysts suggest a promising future for their application across various domains of chemical manufacturing. The ongoing research and development in this area are expected to refine these catalysts further, potentially leading to groundbreaking discoveries that could revolutionize the industry. Therefore, continued investigation into the use and importance of magnetic catalysts in carbonylation reactions is not only of academic interest but also of substantial practical value, holding the potential to contribute significantly to the advancement of green chemistry and process optimization.While offering numerous advantages, using magnetic catalysts in carbonylation reactions also presents challenges that must be addressed to harness their potential fully. One of the primary concerns is the stability of these catalysts under reaction conditions. The harsh environment can compromise the catalytic activity, leading to deactivation and a decrease in efficiency over time. Additionally, the magnetic properties of these catalysts, which are crucial for their separation and recovery, can be affected by the reaction milieu, potentially leading to difficulties in catalyst retrieval and reuse. Another challenge lies in the specificity of the catalysts; ensuring that they selectively facilitate the desired reaction without unwanted side reactions is a complex task that requires precise control over the catalyst's structure and the reaction conditions.
Furthermore, the scalability of processes using magnetic catalysts is often questioned, as reactions that are successful on a laboratory scale may encounter obstacles when translated to industrial-scale production, such as maintaining consistent magnetic separation or dealing with the increased mass transfer limitations. Lastly, the synthesis of magnetic catalysts themselves can be intricate and costly, necessitating the development of more economical and straightforward methods for their production. Despite these challenges, the continuous advancements in the field are promising, and ongoing research is dedicated to overcoming these hurdles, paving the way for broader application of magnetic catalysts in carbonylation reactions and beyond.
Magnetic catalysts have emerged as a promising alternative to traditional carbonylation reactions due to their unique properties and operational advantages.50–80 Below is a comparison between the two types of catalysts (Tables 2–6).
| Aspect | Magnetic catalysts | Traditional catalysts |
|---|---|---|
| Composition | Nanomaterials (e.g., Fe3O4) are often combined with active metals or ligands | Homogeneous (e.g., metal complexes) or heterogeneous (e.g., supported catalysts) |
| Surface area | The high surface area is due to nanoscale particles | Variable surface area; traditional supports like silica or alumina may limit dispersion |
| Functionalization | Easy functionalization of magnetic nanoparticles to improve activity | Functionalization can be more complex for homogeneous or bulk catalysts |
| Aspect | Magnetic catalysts | Traditional catalysts |
|---|---|---|
| Catalytic activity | Comparable or higher activity due to nanoscale effects | Well-established activity for various systems |
| Selectivity | High selectivity achieved via tunable surface chemistry | Depends on the catalyst structure and reaction conditions |
| Aspect | Magnetic catalysts | Traditional catalysts |
|---|---|---|
| Separation | Easily separated using an external magnetic field, reducing processing steps and solvent use | Separation often requires filtration, centrifugation, or phase separation |
| Reusability | High reusability with minimal activity loss | Reusability can be challenging, particularly for homogeneous catalysts |
| Waste reduction | Reduces waste by avoiding additional separation agents | May generate more waste due to additional recovery steps |
| Aspect | Magnetic catalysts | Traditional catalysts |
|---|---|---|
| Eco-friendliness | Lower environmental footprint due to easy recyclability and reduced use of harmful solvents | Potentially higher environmental impact, especially for heavy metal catalysts |
| Toxicity | Magnetic cores (e.g., iron oxide) are generally less toxic | Toxicity depends on the catalyst composition (e.g., Pd, Rh, and Co) |
| Aspect | Magnetic catalysts | Traditional catalysts |
|---|---|---|
| Scalability | Challenges in synthesizing magnetic catalysts at scale | Established production methods |
| Stability | Susceptible to aggregation or oxidation if not properly stabilized | Typically more stable under reaction conditions |
| Cost | Initial synthesis can be expensive | Costs depend on the material and preparation method |
Magnetic catalysts offer significant advantages over traditional catalysts, especially regarding reusability, separation ease, and environmental impact. However, scalability and long-term stability must be addressed to realize their full potential in industrial applications.
4 Conclusion
In conclusion, the continued development of magnetic catalysts will likely focus on enhancing their selectivity and stability, which could lead to breakthroughs in carbonylation methods. This would further solidify their role as a cornerstone in the pursuit of green chemistry, where the goal is to minimize environmental impact while maximizing chemical productivity. As research progresses, we anticipate a surge in the application of magnetic catalysts across various domains of chemical synthesis, particularly carbonylative reactions, potentially transforming the landscape of industrial and pharmaceutical chemistry.5 Perspective
The field of magnetic catalysts in carbonylation reactions presents both opportunities and challenges. While magnetic catalysts offer simplified separation and recyclability, future development should enhance their catalytic activity and stability to match or exceed homogeneous counterparts. The key directions are exploring novel magnetic nanomaterials, optimizing catalyst immobilization techniques, and designing magnetically recoverable ligands. Challenges include preventing metal leaching, maintaining a high dispersion of active sites, and ensuring compatibility with diverse reaction conditions. Overcoming these hurdles will unlock the full potential of magnetic catalysts, paving the way for greener and more sustainable carbonylation processes in academia and industry. Further research into understanding the structure–activity relationships and developing scalable synthesis methods is crucial for widespread adoption.Building on the potential of magnetic catalysts in carbonylation reactions, the future landscape is set for transformative advancements deeply rooted in green chemistry principles. We anticipate breakthroughs in magnetic nanoparticle design and functionalization, boosting catalytic performance, selectivity, and longevity. This evolution will unlock new realms of carbonylation reactions conducted under gentle, eco-friendly conditions. Integrating machine learning and high-throughput experimentation can revolutionize catalyst design by predicting optimal surface modifications and reaction parameters, accelerating efficiency gains. Flow chemistry systems offer another exciting avenue, enabling continuous, scalable carbonylation with minimal waste through magnetic catalyst integration. Further exploration of synergistic combinations of magnetic catalysts with photocatalytic or electrocatalytic systems could pave the way for innovative methodologies that harness sustainable energy sources. By proactively tackling challenges like catalyst deactivation and expanding the synthesis scope, magnetic catalysts are poised to redefine carbonylation reactions, aligning seamlessly with the eco-conscious objectives of the industrial and pharmaceutical chemistry sectors. The journey forward necessitates collaborative efforts, merging expertise from materials science, chemical engineering, and catalysis to fully realize the potential of magnetic catalysts in shaping a sustainable future for carbonylation chemistry.
Conflicts of interest
The authors declare no conflict of interest.References
- S. Sultana, G. Borah and P. K. Gogoi, Appl. Organomet. Chem., 2019, 33, e4595 CrossRef.
- Y. Zhao, ACS Appl. Nano Mater., 2020, 3, 4917 Search PubMed.
- Z. Li, L. Wang, L. Qin, C. Lai, Z. Wang, M. Zhou, L. Xiao, S. Liu and M. Zhang, Chemosphere, 2021, 285, 131432 Search PubMed.
- M. Ghobadi, P. P. Qhazvini and M. Kazemi, Synth. Commun., 2020, 50, 3717 Search PubMed.
- S. Kanithan, N. A. Vignesh, K. M. Katubi, P. S. Subudhi, E. Yanmaz, J. A. Dhanraj, N. S. Alsaiari, K. M. Abualnaja, M. Sukumar, M. Sundararajan, S. Baskar, S. Sahu and C. S. Dash, J. Mol. Struct., 2022, 1265, 133289 Search PubMed.
- R. J. Albadr, W. M. Taher, M. Alwan, M. J. Jawad, H. Mushtaq, B. M. Saadi, A. Smerat, M. Kazemi and R. Javahershenas, Copper Catalyzed Formation of Carbon–Silicon Bond: A Review, J. Inorg. Organomet. Polym. Mater., 2025 DOI:10.1007/s10904-024-03558-7.
- R. Wang, C. Jia, N. Zheng, S. Liu, Z. Qi, R. Wang, L. Zhang, Y. Niu and S. Pan, Photodiagn. Photodyn. Ther., 2022, 103141 Search PubMed.
- M. K. Razi, R. Javahershenas, M. Adelzadeh, M. Ghobadi and M. Kazemi, Synth. Commun., 2020, 50, 3739 Search PubMed.
- F. F. Sead, V. Jain, R. Roopashree, A. Kashyap, S. Saini, G. C. Sharma, P. N. Bhakuni, M. Kazemi and R. Javahershenas, Front. Chem., 2025, 13, 1545252 CrossRef CAS PubMed.
- F. F. Sead, V. Jain, A. Kumar, R. M. M., M. Kundlas, S. Gupta, M. Kumari, M. Kazemi and R. Javahershenas, RSC Adv., 2025, 15, 3928–3953 RSC.
- H. He, Q.-Q. Zhu, Y. Yan, H.-W. Zhang, Z.-Y. Han, H. Sun, J. Chen, C.-P. Li, Z. Zhang and M. Du, Appl. Catal., B, 2022, 302, 120840 Search PubMed.
- M. I. Obaid and W. A. Jaafar, Chem. Methodol., 2022, 6, 457 CAS.
- R. Tamatam, S.-H. Kim and D. Shin, Front. Chem., 2023, 1140562 CrossRef CAS PubMed.
- S. M. Reda and A. A. S. Al-Hamdani, Chem. Methodol., 2022, 6, 475 Search PubMed.
- E. P. Beaumier, A. J. Pearce, X. Y. See and I. A. Tonks, Nat. Rev. Chem, 2018, 3, 15 Search PubMed.
- L. Chen, A. N. Fajer, Z. Yessimbekov, M. Kazemi and M. Mohammadi, J. Sulfur Chem., 2019, 40, 451 CrossRef CAS.
- H. Tavakoli, S. Hashemi, R. Amirkhani and M. Gholampour, J. Synth. Chem., 2023, 1, 137 Search PubMed.
- Q. Pu, M. Kazemi and M. Mohammadi, Mini-Rev. Org. Chem., 2020, 17, 423 Search PubMed.
- H. S. Lihumis, Z. A. Al Talebi and A. K. Khaleel, J. Med. Chem. Sci., 2022, 5, 596 CAS.
- N. Kaplaneris and L. Ackermann, Beilstein J. Org. Chem., 2022, 18, 86 CrossRef CAS PubMed.
- M. Ghobadi, J. Synth. Chem., 2022, 1, 84 Search PubMed.
- F. F. Sead, V. Jain, S. Ballal, A. Singh, A. Devi, G. C. Sharma, K. K. Joshi, M. Kazemi and R. Javahershenas, RSC Adv., 2025, 15, 2334–2346 RSC.
- H. Lin, L. Wu and M. Kazemi, Synth. Commun., 2021, 51, 1609–1635 CrossRef CAS.
- A. M. Mustafa and A. Younes, Nanomater. Chem., 2024, 1, 120 Search PubMed.
- M. S. Yusubov and V. V. Zhdankin, Resour.-Effic. Technol., 2015, 1, 49 Search PubMed.
- D. Bhattacherjee, M. Rahman, S. Ghosh, A. K. Bagdi, G. V. Zyryanov, O. N. Chupakhin, P. Das and A. Hajra, Adv. Synth. Catal., 2021, 363, 1597 CrossRef CAS.
- Y. Sun, W. Jin and C. Liu, Molecules, 2019, 24, 3838 CrossRef CAS PubMed.
- F. V. Singh, S. E. Shetgaonkar, M. Krishnan and T. Wirth, Chem. Soc. Rev., 2022, 51, 8102 RSC.
- X. Yin, Q. Zhang and Q. Zeng, Organics, 2023, 4, 173 CrossRef CAS.
- E. Doustkhah, S. Rostamnia, M. Imura, Y. Ide, S. Mohammadi, C. J. T. Hyland, J. You, N. Tsunoji, B. Zeynizadeh and Y. Yamauchi, RSC Adv., 2017, 7, 56306 RSC.
- M. Nasrollahzadeh, F. Ghorbannezhad and S. M. Sajadi, Appl. Organomet. Chem., 2018, e4698 Search PubMed.
- L. Shiri, A. Ghorbani-Choghamarani and M. Kazemi, Aust. J. Chem., 2016, 69, 585 CrossRef CAS.
- L. Zuo, S. Yu, R. Zhang, H. Li, Y. Wu, R. Abiev, Z. Sun and Z. Sun, J. Cleaner Prod., 2023, 410, 137212 CrossRef CAS.
- Q. Hu, X. Liu, L. Tang, D. Min, T. Shi and W. Zhang, RSC Adv., 2017, 7, 7964 RSC.
- B. Baghernejad and L. Nazari, Eurasian Chem. Commun., 2021, 3, 319 CAS.
- N. Chen, W. Fu, J. Zhou, L. Mei, J. Yang, Y. Tian, Q. Wang and W. Yin, Chin. Chem. Lett., 2021, 32, 2405 CrossRef CAS.
- G. Xia, Y. Zheng, Z. Sun, S. Xia, Z. Ni and J. Yao, Environ. Sci. Pollut. Res., 2022, 29, 39441 CrossRef CAS PubMed.
- H. Li, Y. Wang, F. Jiang, M. Li and Z. Xu, Dalton Trans., 2023, 52, 3846 RSC.
- R. Eisavi and A. Karimi, RSC Adv., 2019, 9, 29873 RSC.
- M. M. K. Siuki, M. Bakavoli and H. Eshghi, Appl. Organomet. Chem., 2019, 33, e4774 CrossRef.
- M. P. Conte, J. K. Sahoo, Y. M. Abul-Haija, K. H. A. Lau and R. V. Ulijn, ACS Appl. Mater. Interfaces, 2018, 10, 3069 CrossRef CAS PubMed.
- M. Kazemi, Synth. Commun., 2020, 50, 2095 CrossRef CAS.
- D. Wang, C. Deraedt, J. Ruiz and D. Astruc, Acc. Chem. Res., 2015, 48, 1871 CrossRef CAS PubMed.
- M. R. Abdi, Biol. Mol. Chem., 2023, 1, 1 Search PubMed.
- Y. Yan, S. Liang, X. Wang and Z. Lin, Proc. Natl. Acad. Sci. U.S.A., 2021, 118, e2110036118 CrossRef CAS PubMed.
- A. M. K. Pasha, S. Raoufi, M. Ghobadi and M. Kazemi, Synth. Commun., 2020, 50, 3685 CrossRef.
- R. Ghorbani and A. Ramezani, Biol. Mol. Chem., 2023, 1, 71 Search PubMed.
- H. R. Saadati-Moshtaghin and F. M. Zonoz, Res. Chem. Intermed., 2018, 44, 2195 CrossRef CAS.
- K. Fukushima and Y. Hidaka, Phys. Rev. Lett., 2013, 110, 031601 CrossRef PubMed.
- M. Kazemi, Synth. Commun., 2020, 50, 2095 CrossRef CAS.
- T. Mandal, J. Synth. Chem., 2023, 2, 54 Search PubMed.
- L. Tang, X. Meng, D. Deng and X. Bao, Adv. Mater., 2019, 31, 1901996 CrossRef CAS PubMed.
- (a) M. Kazemi, R. Javahershenas and K. D. Klika, Mini-Rev. Org. Chem., 2024 DOI:10.2174/0118756298339198241015055314; (b) M. Kazemi, M. Ghobadi and A. Mirzaie, Nanotechnol. Rev., 2018, 7, 43 CrossRef CAS.
- I. Khan, K. Saeed and I. Khan, Arabian J. Chem., 2019, 12, 908 CrossRef CAS.
- M. Kazemi, Synth. Commun., 2020, 50, 2095 CrossRef CAS.
- A. Naderi, Biol. Mol. Chem., 2023, 1, 61 Search PubMed.
- L. Tang, H. Peng, J. Kang, H. Chen, M. Zhang, Y. Liu, D. H. Kim, Y. Liu and Z. Lin, Chem. Soc. Rev., 2024, 53, 4877 RSC.
- W. Li, J. Yan, W. Xu and L. Y. Zhang, RSC Adv., 2023, 13, 28964 RSC.
- M. Ghobadi, P. P. Qhazvini, M. Eslami and M. Kazemi, Synth. Commun., 2021, 51, 325 CrossRef CAS.
- E. T. Talgatov, A. S. Auyezkhanova, K. S. Seitkalieva, N. Z. Tumabayev, S. N. Akhmetova and A. K. Zharmagambetova, J. Porous Mater., 2020, 27, 919 CrossRef CAS.
- N. M. Basith, R. A. Raj, M. S. AlSalhi, S. Devanesan, S. J. A. Ali, S. Rajasekar, R. Sundaram and C. Ragupathi, J. Supercond. Novel Magn., 2016, 29, 2053 CrossRef CAS.
- M. Su, C. He and K. Shih, Ceram. Int., 2016, 42, 14793 Search PubMed.
- A. Makarem, M. Gheibi, R. Mirsafaei and M. Eftekhari, J. Mol. Liq., 2023, 388, 122743 CrossRef CAS.
- Z. Ghorannevis, T. Kato, T. Kaneko and R. Hatakeyama, Jpn. J. Appl. Phys., 2010, 49, 02BA01 CrossRef.
- Z. Yingzhe, H. Yuxing, Q. Qingdong, W. Fuchun, W. Wankun and L. Yongmei, Superlattices Microstruct., 2018, 118, 123 Search PubMed.
- M. A. M. Gijs, F. Lacharme and U. Lehmann, Chem. Rev., 2010, 110, 1518 CrossRef CAS PubMed.
- E. Karaoğlu, A. Baykal, M. Şenel, H. Sözeri and M. S. Toprak, Mater. Res. Bull., 2012, 47, 2480 Search PubMed.
- B. Jaleh, A. Khalilipour, S. Habibi, M. Niyaifar and M. Nasrollahzadeh, J. Mater. Sci.:Mater. Electron., 2017, 28, 4974 CrossRef CAS.
- Z. Chen, Y. Zhao, J. Ma, C. Liu and Y. Ma, Ceram. Int., 2017, 43, 16763 CrossRef CAS.
- Z. Moghadasi, A. N. Fajer, A. M. H. Abudken and H. Ali Al-Bahrani, Nanomater. Chem., 2023, 1, 24 Search PubMed.
- A. M. Abu-Dief and S. M. Abdel-Fatah, Beni-Suef Univ. J. Basic Appl. Sci., 2018, 7, 55 Search PubMed.
- N. Fajer, H. A. Al-Bahrani, A. A. H. Kadhum and M. Kazemi, J. Mol. Struct., 2024, 1296, 136800 CrossRef.
- A. Shakarji and B. al-Rabi'i, Nanomater. Chem., 2023, 1, 81 Search PubMed.
- M. Sun, W. Liu, W. Wu, Q. Li and L. Shen, RSC Adv., 2023, 13, 20351 RSC.
- A. N. Fajer, H. K. Aboud, H. A. Al-Bahrani and M. Kazemi, Polycyclic Aromat. Compd., 2023, 1, 1–47 Search PubMed.
- A. Arabmarkadeh, R. Javahershenas and M. Kazemi, Synth. Commun., 2021, 51, 880 CAS.
- Y. Riadi, M. M. Kadhim, S. J. Shoja, M. H. Ali, Y. F. Mustafa and A. Sajjadi, Synth. Commun., 2022, 52, 875 CrossRef CAS.
- M. Ghobadi, M. K. Razi, R. Javahershenas and M. Kazemi, Synth. Commun., 2021, 51, 647 CrossRef CAS.
- R. Eisavi and F. Ahmadi, Sci. Rep., 2022, 12, 11939 CrossRef CAS PubMed.
- P. Hou and M. Kazemi, Res. Chem. Intermed., 2024, 50, 1713 CrossRef.
- S. Sheikh, M. A. Nasseri, A. Allahresani and R. S. Varma, Sci. Rep., 2022, 12, 17986 CrossRef CAS PubMed.
- Z. Fekri, M. Nikpassand, S. Shariati, B. Aghazadeh, R. Zarkeshvari and N. N. Pour, J. Organomet. Chem., 2018, 871, 60 CrossRef.
- C. Sappino, L. Primitivo, M. De Angelis, M. O. Domenici, A. Mastrodonato, I. B. Romdan, C. Tatangelo, L. Suber, L. Pilloni, A. Ricelli and G. Righi, ACS Omega, 2019, 4, 21809 CrossRef CAS PubMed.
- M. Ma, P. Hou, P. Zhang, J. Cao, H. Liu, H. Yue, G. Tian and S. Feng, Appl. Catal., A, 2020, 602, 117709 CrossRef CAS.
- S. Jiang, Mol. Diversity, 2024, 3859 CrossRef CAS PubMed.
- J. Zhao, X. Luo, X. Li and L.-Y. Chang, Res. Chem. Intermed., 2024, 50, 2131 CrossRef CAS.
- P. Rai and D. Gupta, Synth. Commun., 2021, 51, 3059 Search PubMed.
- R. Hudson, Y. Feng, R. S. Varma and A. Moores, Green Chem., 2014, 16, 4493 RSC.
- W. Zhang, C. Ai, K. Wang, J. Guo and B. Zhao, Asian J. Org. Chem., 2023, e202300413 CrossRef CAS.
- S. T. Gadge and B. M. Bhanage, RSC Adv., 2014, 4, 10367 RSC.
- d. Albuquerque, W. Teixeira, S. Narayanaperumal and R. Schwab, J. Braz. Chem. Soc., 2022, 33, 637–663 Search PubMed.
- Z.-P. Bao and X.-F. Wu, Ind. Chem. Mater., 2024, 2, 276 RSC.
- V. Botla, A. Voronov, E. Motti, C. Carfagna, R. Mancuso, B. Gabriele and N. Della Ca', Catalysts, 2021, 11, 918 CrossRef CAS.
- S. Layek, B. Agrahari, R. Ganguly, P. Das and D. D. Pathak, Appl. Organomet. Chem., 2020, 34, e5414 CrossRef CAS.
- M. Beigi and S. Fathizadeh, J. Synth. Chem., 2024, 3, 91 Search PubMed.
- Y. Zheng, M. R. Abukhadra, M. Tlija and L. Y. Zhang, J. Inorg. Organomet. Polym. Mater., 2024 DOI:10.1007/s10904-024-03380-1.
- H. Li, K. Dong, H. Neumann and M. Beller, Angew. Chem., 2015, 127, 10377 CrossRef.
- Z.-J. Wang, X.-Y. Wang, X. Wang, Z.-W. Liang and X. Xu, Catal. Commun., 2017, 101, 10 CrossRef CAS.
- A. W. Augustyniak, W. Zawartka, J. A. R. Navarro and A. M. Trzeciak, Dalton Trans., 2016, 45, 13525 RSC.
- J. Zhang and X.-F. Wu, Org. Lett., 2023, 25, 2162 CrossRef CAS PubMed.
- M. Genelot, A. Bendjeriou, V. Dufaud and L. Djakovitch, Appl. Catal., A, 2009, 369, 125 CrossRef CAS.
- F. Wu, J. Peng, X. Qi and X. Wu, ChemCatChem, 2018, 10, 173 CrossRef CAS.
- G. Zheng, P. Wang and M. Cai, Chin. J. Chem., 2009, 27, 1420 Search PubMed.
- W. Zawartka, P. Pośpiech, M. Cypryk and A. M. Trzeciak, J. Mol. Catal. A:Chem., 2016, 417, 76 CrossRef CAS.
- H. Yin and T. Skrydstrup, J. Org. Chem., 2017, 82, 6474 Search PubMed.
- J. Liu, M. Liu, Y. Yue, N. Zhang, Y. Zhang and K. Zhuo, Tetrahedron Lett., 2013, 54, 1802 CrossRef CAS.
- M. Cai, G. Zheng, L. Zha and J. Peng, Eur. J. Org. Chem., 2009, 2009, 1585 CrossRef.
- L. Åkerbladh, P. Nordeman, M. Wejdemar, L. R. Odell and M. Larhed, J. Org. Chem., 2015, 80, 1464 CrossRef PubMed.
- M. Niakan, Z. Asadi and M. Emami, Catal. Lett., 2020, 150, 404 CrossRef CAS.
- J. Niu, X. Huo, F. Zhang, H. Wang, P. Zhao, W. Hu, J. Ma and R. Li, ChemCatChem, 2013, 5, 349 CrossRef CAS.
- P. Mehara, P. Sharma, A. K. Sharma, Shaifali and P. Das, Mol. Catal., 2023, 550, 113546 CrossRef CAS.
- S. You, C. Yan, R. Zhang and M. Cai, Appl. Organomet. Chem., 2019, 33, e4650 CrossRef.
- J. Niu, M. Xie, X. Zhu, Y. Long, P. Wang, R. Li and J. Ma, J. Mol. Catal. A:Chem., 2014, 392, 247 CrossRef CAS.
- Z. Zhu, Z. Wang, Y. Jian, H. Sun, G. Zhang, J. M. Lynam, C. R. McElroy, T. J. Burden, R. L. Inight, I. J. S. Fairlamb, W. Zhang and Z. Gao, Green Chem., 2021, 23, 920 RSC.
- M. Cai, J. Peng, W. Hao and G. Ding, Green Chem., 2011, 13, 190 RSC.
- J. Ying, J. Wang, L. Yao, W. Lu and X. Wu, Chem. - Eur. J., 2020, 26, 14565 CrossRef CAS PubMed.
- G. Xie, J. Zhan, M. Cai and B. Huang, Synthesis, 2023, 55, 647 CrossRef CAS.
- B. Akhlaghinia and A. Makarem, J. Sulfur Chem., 2011, 32, 575–581 CrossRef CAS.
- T. Ketike, V. R. K. Velpula, V. R. Madduluri, S. R. R. Kamaraju and D. R. Burri, ChemistrySelect, 2018, 3, 7164 CrossRef CAS.
- Z. Zhou, B. Huang and M. Cai, Synth. Commun., 2021, 51, 3150 CrossRef CAS.
- N. Jiao, Z. Li, Y. Wang, J. Liu and C. Xia, RSC Adv., 2015, 5, 26913 RSC.
- A. R. Hajipour and Z. Tavangar-Rizi, ChemistrySelect, 2017, 2, 8990 CrossRef CAS.
- X. Wu, J. Schranck, H. Neumann and M. Beller, Chem.–Eur. J., 2011, 17, 12246 CrossRef CAS PubMed.
- P. Sharma, S. Rohilla and N. Jain, J. Org. Chem., 2017, 82, 1105 CrossRef CAS PubMed.
- J.-B. Peng, F.-P. Wu and X.-F. Wu, Chem. Rev., 2019, 119, 2090 CrossRef CAS.
- P. Hu and M. Kazemi, J. Coord. Chem., 2024, 77, 1324–1348 CrossRef CAS.
- E. Etemadi-Davan and N. Iranpoor, ChemistrySelect, 2016, 1, 4300 CrossRef CAS.
- H.-Q. Geng, Y.-H. Zhao, P. Yang and X.-F. Wu, Chem. Sci., 2024, 15, 3996 RSC.
- P. Gautam, M. Dhiman, V. Polshettiwar and B. M. Bhanage, Green Chem., 2016, 18, 5890 RSC.
- Y. Cui, X. Guo, Y. Wang and X. Guo, Chin. J. Catal., 2015, 36, 322 CrossRef CAS.
- T. T. Dang, A. Chen and A. M. Seayad, RSC Adv., 2014, 4, 30019 RSC.
- S. Guo, J. Zhai, F. Wang and X. Fan, Org. Biomol. Chem., 2017, 15, 3674 RSC.
- K. A. Dahlous, S. Mohammad and X. Liu, J. Inorg. Organomet. Polym. Mater., 2024, 34, 4813–4827 CrossRef CAS.
- S. Ichie and R. Bracho, J. Synth. Chem., 2023, 2, 156 Search PubMed.
- A. M. Mustafa and A. Younes, Nanomater. Chem., 2023, 1, 12 Search PubMed.
- A. Liu, Z. Liang, A. Jialingbieke, J. Gao and D. Du, Org. Lett., 2023, 25, 2657 CrossRef PubMed.
- Z. Wang, H. Zeng and C.-J. Li, Org. Lett., 2019, 21, 2302 Search PubMed.
- Q. G. Karwi, D. Biswas, T. Pulinilkunnil and G. D. Lopaschuk, J. Card. Failure, 2020, 26, 998 CrossRef PubMed.
- N. Chalotra, S. Sultan and B. A. Shah, Asian J. Org. Chem., 2020, 9, 863 CrossRef CAS.
- J. Wang, M. E. Hoerrner, M. P. Watson and D. J. Weix, Angew. Chem., Int. Ed., 2020, 59, 13484 CrossRef CAS PubMed.
- L. A. T. Allen, R.-C. Raclea, P. Natho and P. J. Parsons, Org. Biomol. Chem., 2021, 19, 498 RSC.
- L. Bartali, A. Guarna, P. Larini and E. G. Occhiato, Eur. J. Org. Chem., 2007, 2007, 2152 CrossRef.
- S. Zhang, H. Neumann and M. Beller, Chem. Commun., 2019, 55, 5938 RSC.
- S. I. Shelash Al-Hawary, R. S. Zabibah, M. S. Alhassan, Y. Q. Almajidi, R. Gupta, D. N. Al-Saidi, F. S. Sheri, R. M. Romero-Parra and A. Shafiq, Polycyclic Aromat. Compd., 2023, 43, 1 CrossRef.
- P. So-ho, Y. Han-chul and J. Jae-chan, Nanomater. Chem., 2023, 1, 46 Search PubMed.
- R. Kshatriya, V. P. Jejurkar and S. Saha, Tetrahedron, 2018, 74, 811 CrossRef CAS.
- P. Yu, X. He, M. Baer, S. Beirinckx, T. Tian, Y. A. T. Moya, X. Zhang, M. Deichmann, F. P. Frey, V. Bresgen, C. Li, B. S. Razavi, G. Schaaf, N. von Wirén, Z. Su, M. Bucher, K. Tsuda, S. Goormachtig, X. Chen and F. Hochholdinger, Nat. Plants, 2021, 7, 481 Search PubMed.
- T. Yatabe, X. Jin, K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2015, 54, 13302 Search PubMed.
- T. Biglari and A. Jafarzadeh, Biol. Mol. Chem., 2024, 1, 94 Search PubMed.
- R. Javahershenas, V. A. Soloshonok, K. D. Klika and P. J. Jervis, Carbon Lett., 2024 DOI:10.1007/s42823-024-00835-w.
- K. Verma and R. Pratap, Nat. Prod. Rep., 2010, 27, 1571 RSC.
- R. Hajipour, Z. Khorsandi, F. Fakhari, M. Mortazavi and H. Farrokhpour, ChemistrySelect, 2018, 3, 6279 CrossRef.
- R. Wang and H. Wan, Res. Chem. Intermed., 2024, 50, 3079 CrossRef CAS.
- N. Martin, J. Synth. Chem., 2024, 3, 176 Search PubMed.
- X. Zhu, Y. Shi, H. Mao, Y. Cheng and C. Zhu, Adv. Synth. Catal., 2013, 355, 3558 CrossRef CAS.
- J. Cen, J. Li, Y. Zhang, Z. Zhu, S. Yang and H. Jiang, Org. Lett., 2018, 20, 4434–4438 CrossRef CAS PubMed.
- J. Choi and C. Park, Adv. Synth. Catal., 2018, 360, 3553–3562 CrossRef CAS.
- W. Wu, Y. Guo, X. Xu, Z. Zhou, X. Zhang, B. Wu and W. Yi, Org. Chem. Front., 2018, 5, 1713–1718 RSC.
- A. M. Amshawee, R. Ali, M. A. Hussain and M. Kazemi, Catal. Lett., 2025, 155, 77 CrossRef CAS.
- M. C. Willis and S. E. Flower, Synthesis, 2003, 2003, 1225 CrossRef.
- K. Fuchibe, I. Mukohara, A. Yamada, D. Miyazaki, R. Takayama and J. Ichikawa, Org. Lett., 2022, 24, 169 CrossRef CAS PubMed.
- X. Wang and Z. Dong, Eur. J. Org. Chem., 2022, e202200452 CrossRef CAS.
- Y.-M. Xiao, Y. Zhao, J.-Q. Li, J.-W. Yuan, L.-R. Yang, P. Mao and W.-P. Mai, New J. Chem., 2023, 47, 17092 RSC.
- M. Soleiman-Beigi, M. Kazemi, R. Aryan and L. Shiri, Lett. Org. Chem., 2014, 11, 321 CrossRef CAS.
- Y.-T. Huang, S.-Y. Lu, C.-L. Yi and C.-F. Lee, J. Org. Chem., 2014, 79, 4561 CrossRef CAS PubMed.
- J. Liu, C. Shao, Y. Zhang, G. Shi and S. Pan, Org. Biomol. Chem., 2014, 12, 2637 RSC.
- Z.-S. Zhang, Z. He, Y. Shi, M. Guan, D.-S. Zhao, D. Zhu, L.-T. Xiong, Y. Li, X. Deng and Z.-N. Cui, J. Agric. Food Chem., 2024, 72, 20299 CrossRef CAS PubMed.
- S. Siebenhaller, J. Gentes, A. Infantes, C. Muhle-Goll, F. Kirschhöfer, G. Brenner-Weiß, K. Ochsenreither and C. Syldatk, Front. Chem., 2018, 6, 24 Search PubMed.
- M. Kazemi and M. S. Beigi, Org. Chem.:Curr. Res., 2013, 2, 119 Search PubMed.
- M. Kim, S. Yu, J. G. Kim and S. Lee, Org. Chem. Front., 2018, 5, 2447 RSC.
- Y. Xiao, W. Lv, J. Yuan, L. Yang, P. Mao and W. Mai, ChemistrySelect, 2022, 7, e202200914 CrossRef CAS.
- C. Yan, Q. Wei, Q. Chen and L. Zhang, J. Mol. Struct., 2024, 1317, 138853 CrossRef CAS.
- J. Al-Shakarji and S. Kamel, Nanomater. Chem., 2024, 2, 168–179 Search PubMed.
- M. Rousta, D. Khalili, E. Ebrahimi and A. Khoy, Catal. Lett., 2024, 154, 5439 CrossRef.
- A. S. Prasad and B. Satyanarayana, J. Mol. Catal. A:Chem., 2013, 370, 205 CrossRef.
- T. Mohy El Dine, W. Erb, Y. Berhault, J. Rouden and J. Blanchet, J. Org. Chem., 2015, 80, 4532 Search PubMed.
- N. Martin and M. Toure, J. Synth. Chem., 2025, 4, 1–16 Search PubMed.
- N. Sharma, N. Srivastava, A. Kaushal, B. Das, A. Vashistha, L. Kumar, R. Kumar and A. K. Yadav, Chem. Biodiversity, 2023, 20, e202300647 CrossRef CAS PubMed.
- F. Odame, G. Woodcock, E. C. Hosten, K. Lobb and Z. R. Tshentu, J. Organomet. Chem., 2020, 922, 121359 CrossRef CAS.
- M. Singh, H. Verma, P. Bhandu, M. Kumar, G. Narendra, S. Choudhary, P. K. Singh and O. Silakari, J. Mol. Struct., 2023, 1272, 134128 CrossRef CAS PubMed.
- E. Rilvin-Derrick, N. Oram and J. Richardson, Synlett, 2020, 31, 369 CrossRef CAS.
- T. N. Reddy and D. P. de Lima, Asian J. Org. Chem., 2019, 8, 1227 CrossRef CAS.
- B. Dutta, S. Omar, S. Natour and R. Abu-Reziq, Catal. Commun., 2015, 61, 31 CrossRef CAS.
- J. Chen, H. Zhou and G. Liu, J. Inorg. Organomet. Polym. Mater., 2024 DOI:10.1007/s10904-024-03400-0.
| This journal is © The Royal Society of Chemistry 2025 |














