Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Plant extract-mediated green-synthesized CuO nanoparticles for environmental and microbial remediation: a review covering basic understandings to mechanistic study
Mashrafi Bin
Mobarak
 *a,
MD. Foysal
Sikder
b,
Khandakar Sidratul
Muntaha
b,
Shariful
Islam
b,
S. M. Fazle
Rabbi
*a,
MD. Foysal
Sikder
b,
Khandakar Sidratul
Muntaha
b,
Shariful
Islam
b,
S. M. Fazle
Rabbi
 b and
Fariha
Chowdhury
b and
Fariha
Chowdhury
 *c
*c
aInstitute of Glass and Ceramic Research and Testing (IGCRT), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka 1205, Bangladesh. E-mail: mashrafibinmobarak@gmail.com
bDepartment of Applied Chemistry and Chemical Engineering, Bangabandhu Sheikh Mujibur Rahman Science and Technology University, Gopalganj 8100, Bangladesh
cBiomedical and Toxicological Research Institute (BTRI), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka 1205, Bangladesh. E-mail: chowdhuryfariha@gmail.com
First published on 19th March 2025
Abstract
This review provides a comprehensive overview of nanoparticles, with a particular focus on plant extract-mediated green-synthesized copper oxide nanoparticles (CuO NPs). This article is one of the simplest to read as it aims at beginner researchers, who may not have advanced knowledge on topics like nanoparticles, including metal and metal oxide nanoparticles, their classification, and techniques to prepare them. Various synthesis procedures are discussed, emphasizing green synthesis methods that utilize plant extracts as reducing and stabilizing agents. Subsequently, the mechanisms involved in the formation of CuO NPs are highlighted. Their significant applications with a mechanistic overview on environmental remediation, especially in the eradication of textile dyes and pharmaceutical wastes, and their antimicrobial properties are elucidated. By carefully scrutinizing the information available in the literature, this article aims to equip novice researchers with a foundational understanding of nanoparticles, their synthesis, and their practical applications, fostering further exploration in the field of nanotechnology.
1. Introduction
Research on nanomaterials, which have at least one dimension within the nanoscale region (1–100 nm), known as nanomaterials, has become one of the most intriguing disciplines of science. As the surface areas of nanomaterials becomes larger compared to their size, they exhibit superior physical and chemical properties compared with their bulk counterparts.1 From electronics to medicine, nanomaterials have been offering enhanced performance, efficiency, and functionality, enabling targeted drug delivery, improved energy storage, and production of light-weight materials with a higher strength.2 Among various types of nanomaterials, transition metal oxide (MO) NPs have garnered significant attention owing to their comprehensive applicability in functional smart material fabrication.3 The MO NPs beget unique phenomena such as quantum confinement effects, altered surface energies, interface effects, and increased surface-to-volume ratios, which are not observed in non-NPs of MO.4Copper(II) oxide (CuO) has gained great interest in recent years as a p-type semiconductor with its monoclinic structure, superior conductivity, photovoltaic features, and large stability among nanomaterials.5 Realizing and utilizing these exceptional properties broadened the scope of applications of CuO NPs as effective anti-bacterial and anti-fungal agents,6,7 materials for environmental remediation via photodegradation or adsorptive removal of toxic substances,8,9 catalysts for increasing the rate of oxidation reactions,10 sensors for the identification of gases and biomolecules,11,12 and energy-storing materials, such as batteries or supercapacitors with the desirable electrochemical characteristics.13
There are several existing methods widely used for the synthesis of CuO NPs, which include chemical precipitation, sol–gel, hydrothermal, and biological methods.14 The traditional chemical and physical methods of CuO NP synthesis often involve toxic reagents and complex procedures. To reduce or eliminate the use of toxic chemicals, finding an alternative source is crucial, and the use of plant extracts for the green synthesis of CuO NPs presents a sustainable alternative.15 In this greener approach, the phytochemicals that are present in the plant extract act as a reducing and stabilizing agent for the CuO NPs. Through this process, use of harmful chemicals can be avoided, and thus, an eco-friendly synthesis procedure can be established. Various plant sources such as Averrhoa carambola,6Psidium guajava,16Catha edulis,17 and Moringa oleifera18 have been exploited for this purpose.
The process through which CuO NPs are synthesized using plant extract is a very intricate method, as it involves several biochemical interactions. The biomolecules from the plant extract facilitate the conversion of the metal precursor into the desired NPs by acting as reducing agents. Additionally, they also stabilize the formed NPs by preventing agglomeration. This process not only yields NPs with controlled size and morphology but also enhances their functional properties.19 The plant extract-mediated synthesized CuO NPs exhibit significant potential in environmental applications, particularly in the photodegradation and adsorptive removal of textile dyes and pharmaceutical wastes.15 The narrow band gap of CuO NPs, which is typically around 2.1 to 2.71 eV, is beneficial since it enables efficient charge separation and a high redox potential. These features coupled with a large surface area allow the CuO NPs to efficiently photocatalyze the complex organic pollutants and decompose them into less harmful by-products through advanced oxidation processes.20 Studies have demonstrated that CuO NPs can effectively degrade pollutants by generating reactive oxygen species (ROS) which react with pollutant molecules, leading to their mineralization.21 Furthermore, the plant extract-mediated CuO NPs have shown promising antimicrobial properties against a range of bacterial and fungal strains.22 The mechanism of CuO NP's antimicrobial activity mainly involves the disruption of microbial cell wall and the generation of ROS that induce oxidative stress within the microbial cell.14 This dual functionality, environmental remediation and antimicrobial activity make plant extract-mediated CuO NPs stand out as a valuable material in both wastewater treatment and healthcare applications.
The present review seeks to give a detailed account of the biogenic synthesis of CuO NPs via plant extracts, starting with the fundamentals of the NPs and going further to explore their synthesis, degradation/adsorptive capabilities and antimicrobial properties. Starting with the latest research enhanced with fundamental principles, this article emphasizes the opportunities that green nanotechnology could provide for the NP synthesis as a way of achieving environmental sustainability.
2. Basics into nanoparticles
The prefix “nano” originates from the Greek word for “dwarf” and signifies one billionth (10−9) of a unit. Although there are various definitions for NPs, to date, a single and universally accepted definition is not yet established.23 The International Union of Pure and Applied Chemistry (IUPAC) defines a NP as “a particle of any shape with dimensions ranging from 1 to 100 nanometers (nm) (1 nm = 1 × 10−9meters)”.24,25 The American Society for Testing Materials (ASTM) offers a slightly broader definition: “a particle with at least one dimension larger than 1 nanometer and smaller than 100 nanometers, and which may or may not exhibit properties that are dependent on its size”.26 Mainly there are two common definitions for NP size. One definition specifies a diameter range of 1–100 nm.27 To help visualize this scale, a human hair is roughly 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nm thick, while an atom has a radius of about 0.1 nm, and a DNA double helix has a radius of 2 nm.23 Particles smaller than 1 nm are typically referred to as atomic clusters. Interestingly, some particles larger than 100 nm can still exhibit properties associated with NPs.28
000 nm thick, while an atom has a radius of about 0.1 nm, and a DNA double helix has a radius of 2 nm.23 Particles smaller than 1 nm are typically referred to as atomic clusters. Interestingly, some particles larger than 100 nm can still exhibit properties associated with NPs.28
Richard P. Feynman, the American physicist, envisioned the idea of nanotechnology and is regarded as the “father of nanotechnology”. In one of his famous talks “There's Plenty of Room at the Bottom,” in 1959, Feynman famously posed the question, “Why cannot we write the entire 24 volumes of the Encyclopedia Britannica on the head of a pin?” which initiated the concept of nanotechnology.23,29 The existence of NPs is dependent on the chemical and electromagnetic properties of the material and the forms in which they exist include aerosols (solid or liquid particles suspended in air), suspensions (solid particles dispersed in liquids), and emulsions (mixtures of two immiscible liquids).30,31 The structure of NPs can range from simple and uniform to complex and layered, depending on whether they are composed of a single material or a combination of materials. Complex NPs may have multiple layers including a surface layer, a shell, and a core.32
2.1. Impact on properties for nano-size
NPs exhibit distinct physicochemical properties from their bulk counterparts of the same material.33 These unique properties stem from two primary reasons: surface effects and quantum confinement.29 As the particle size decreases, the number of surface atoms significantly increases out of the total number of particles that contributes towards the geometry. This increase in surface area is extremely significant for determining several properties. Another reason is that, at the nanoscale, the movement of electrons is confined to distinct energy states as a result of confinement into a restricted volume. This effect, known as quantum confinement, modifies the electronic, optical, and magnetic properties of the material.34 The effects on other properties include improved reactivity, catalysis, and heat conductivity when the particle size is reduced to the nanoscale. A larger amount of surface electrons facilitate heat transfer, increasing the thermal conductivity. Smaller NPs show a lower melting point, attributed again to surface effects. Since NPs possess more of the surface atoms, they have a proportionately higher surface energy, resulting in their higher chemical reactivity. This would increase their reactivity as effective catalysts, with smaller NPs being more catalytic.35Metallic and semiconductor NPs display unique optical properties for two primary reasons: quantum confinement and localized surface plasmon resonance (LSPR). The latter phenomenon arises from the collective oscillation of electrons on the NP surface when the NP is excited by light. This phenomenon affects the interaction of light with the NP.36–38 The energy difference between a material's valence band (filled electron states) and conduction band (empty electron states) is termed the band gap. In NPs, the band gap typically increases compared to bulk materials. This altered band gap affects how light interacts with the NP, leading to different absorption and emission behaviors.39 The increased band gap in NPs can also influence electrical properties by affecting the mobility of charge carriers (electrons and holes). In some metallic NPs, a higher band gap can lead to a decrease in electrical conductivity. For instance, Cu NPs may lose conductivity at certain sizes, while conversely, insulating materials such as SiO2 can exhibit some degree of conductivity at the nanoscale. The uneven distribution of electrons in some NPs can induce magnetic properties.35 Additionally, the large surface-to-volume ratio of NPs can influence the magnetic coupling between neighboring particles, affecting their overall magnetic behavior. The size and shape are also crucial factors in determining the magnetic properties of NPs.31
2.2. Classification of nanomaterials
The classification of nanomaterials can be done based on their origin, dimensionality (number of dimensions within the nanoscale) and chemical composition of the nanostructures.35,40,412.2.1.1. Natural nanomaterials. Nanomaterials that are formed through natural processes and found in nature fall into this category. The examples are volcanic ash, viruses, protein structures, wings of insects, spider silk, milk and blood which is natural colloid, and ocean spray.41
2.2.1.2 Synthetic nanomaterials. Synthetic or artificial nanomaterials are engineered in laboratory or industrial settings to achieve desired morphologies and functionalities. The formation of these NPs is achieved by various processes such as chemical synthesis, physical vapor deposition and self-assembly. The examples of synthetic nanomaterials are quantum dots, carbon-based materials such as graphene and polymeric NPs.29,41
2.2.2.1. 0D (zero-dimensional). When all three dimensions (x, y and z) of a material are confined to the nanoscale, it is termed 0D material and is falls under this category. The examples are fullerene which is a tiny spherical cage made entirely of carbon.
2.2.2.2. 1D (one-dimensional). Materials with one dimension extending beyond the nanoscale, and other two dimensions within the nanoscale range are termed 1D nanomaterials. Nanowires, nanotubes, nanofibers, and nano rods are examples of 1D nanomaterials.
2.2.2.3. 2D (two-dimensional). Materials with thin films or plate-like structures which have one dimension in the nanometer range are depicted as 2D nanomaterials. Graphene, a single layer of carbon atoms arranged in a honeycomb lattice, is a prime example of a 2D nanomaterial.
2.2.2.4. 3D (three-dimensional). Materials, whose building blocks or constituents are within the nanometer range, even if the overall dimensions of the material are larger, are considered 3D nanomaterials. These materials are composed of numerous nanosized crystals arranged in various configurations. The examples of this category are bundles of nanowires and nanotubes.
2.2.3.1. Carbon-based nanomaterials. The carbon-based nanomaterials are made entirely out of carbon and have so many exceptional properties such as high conductivity (thermal and electrical), extreme strength, and biocompatibility. This category includes fullerenes, graphenes, carbon nanotubes (CNTs), and carbon nanofibers. CNTs are basically rolled-up graphene sheets. They have amazing strength-to-weight ratios.
2.2.3.2. Metal nanomaterials. The subdivision of bulk metals into small assemblies of clusters creates these nanomaterials, which have more novel optical and electrical behaviors because of the quantum confinement effects. Nanomaterials can be synthesized using several metals including gold, silver, and iron.
2.2.3.3. Metal oxide nanomaterials. Metal oxides are formed from their respective metals, and exhibit modified properties, especially improved reactivity and efficiency. They are utilized in supercapacitors, gas sensors, and various solar cell types. Notable examples are CuO, ZnO, NiO, Fe3O4, and TiO2.
2.2.3.4. Organic nanomaterials. Organic nanomaterials consist of organic molecules such as proteins, liposomes, lipids, or polymers. Their biodegradable and non-toxic nature makes them particularly suitable for applications in drug delivery and tissue engineering.
2.2.3.5. Inorganic nanomaterials. This extensive category includes both metal and metal oxide nanomaterials, typically produced by precipitating inorganic salts. Ceramic nanomaterials, recognized for their heat resistance and chemical stability, are a key example and are commonly used in drug delivery.
The visual representation of nanomaterial classification is shown in Fig. 1.
2.3. Synthesis methods of NPs
The synthesis of nanomaterials is a fascinating field which basically has two main approaches: bottom–up and top–down.29 Each approach offers unique advantages and is suited for different types of NPs.2.3.1.1. Sol–gel method. This versatile technique involves forming a liquid suspension (sol) containing precursors. These precursors transform into a gel network and eventually into NPs through controlled reactions. It is particularly useful for metal oxides.42
2.3.1.2. Precipitation method. This method begins by dissolving a desired metal salt in a solution. By adding another chemical, the dissolved ions come together as solid NPs via a precipitation reaction. This method is simple but offers less control over size and shape.43
2.3.1.3. Reduction method. This method involves reducing a metal salt to its elemental state using a reducing agent. For example, the Turkevich method (citrate reduction) is a popular way to synthesize spherical gold NPs.44
2.3.1.4 Chemical vapor deposition method. Chemical Vapor Deposition, or CVD, is a technique for creating thin films on surfaces. In this process, a heated substrate is exposed to a combination of gaseous chemicals inside a chamber at normal temperature. These chemicals react upon contact with the hot substrate, forming a thin layer of desired material on its surface. The resulting film is then collected for use. The success of CVD depends on the temperature of the substrate. While CVD produces high-quality, uniform, and strong films, it requires specialized equipment and can generate hazardous gaseous byproducts.45
2.3.1.5. Spinning method. Spinning disc reactors (SDRs) are one of the important tools for NP creation. Inside the SDR, a disc spins at high speeds, while a liquid precursor and water are pumped in. This fast-paced environment fuses atoms and molecules together forming NPs. The superior feature of SDRs is the tunability; by adjusting factors such as spin speed and liquid flow, the size and properties of the NPS can be controlled.46
2.3.1.6. Pyrolysis process. Industrial NP production often relies on pyrolysis, a burning process. Here, a precursor, either liquid or vapor, is fed into a furnace and incinerated at high pressures. The resulting gases are then filtered to collect the NPs. Some variations use lasers or plasma instead of flames for even higher temperatures. Pyrolysis is popular for its simplicity, efficiency, affordability, and ability to continuously produce large quantities of NPs.47
2.3.1.7 Molecular condensation method. Molecular condensation, also known as atomic or molecular beam condensation, involves evaporating or sputtering a bulk material in a vacuum chamber to produce a vapor. This vapor then condenses into NPs upon collision with an inert gas like helium or argon. The NP size and morphology can be controlled by adjusting parameters such as gas pressure, flow rate, and temperature. This method is commonly used to produce metallic NPs in a pure, uncontaminated form.48
2.3.1.8. Sonochemical synthesis. Sonochemical synthesis uses high-intensity ultrasound to drive chemical reactions and produce NPs. The ultrasound creates cavitation bubbles in a liquid solution that rapidly collapse, generating localized hot spots with extreme temperatures and pressures. This allows for the rapid nucleation and growth of NPs from dissolved precursors. Sonochemical methods can produce a variety of NP compositions including metals, metal oxides, and semiconductors. Key advantages are the ability to control particle size and the mild reaction conditions compared to other techniques.49
2.3.1.9. Electrochemical synthesis. Electrochemical synthesis of NPs involves reducing metal ions in solutions at an electrode surface to form NPs. This is done by applying a potential difference between two electrodes immersed in an electrolyte solution containing metal salts. The reduced metal atoms nucleate and grow into NPs on the electrode surface. Parameters such as applied potential, electrolyte composition, and reaction time can be tuned to control the NP size and morphology. Electrochemical methods are simple, cost-effective, and can be scaled up for industrial production of NPs.50
2.3.1.10. Biosynthesis. Biosynthesis offers an environment friendly method for creating NPs. This technique utilizes bacteria, plant extracts, and fungi (along with precursors) as a safe alternative to traditional chemicals for reducing and stabilizing the NPs during synthesis. The resulting bio-synthesized NPs possess unique and improved properties, making them well suited for biomedical applications.51
2.3.2.1. Mechanical milling. Mechanical milling offers a cost-effective approach for transforming bulk materials into nanoscale components. This technique excels in creating homogeneous mixtures of different materials, making it particularly useful for producing nanocomposites. Its applications span a wide range including the development of reinforced aluminum alloys, durable coatings, and advanced nanoalloys. Moreover, carbon nanomaterials produced through mechanical milling represent a promising new class of materials with potential for addressing environmental, energy storage, and energy conversion challenges.53
2.3.2.2. Lithography. Lithography is a critical technique for constructing nanoscale structures using focused light or particle beams. This method can be categorized into two primary approaches: masked and maskless lithography. Masked lithography involves transferring intricate patterns onto a large surface using a predefined template or mask. The examples of masked lithography are photolithography, nanoimprint lithography, and soft lithography. In contrast, maskless lithography offers greater flexibility by directly writing arbitrary patterns without the need for a mask. This approach encompasses techniques such as scanning probe lithography, focused ion beam lithography, and electron beam lithography. Moreover, by combining focused ion beam implantation with wet chemical etching, it is possible to create complex three-dimensional micro- and nano-structures.54,55
2.3.2.3. Laser ablation. Laser ablation is a green synthesis technique that produces NPs by vaporizing a target material with high-energy laser pulses.56 This versatile method can create a wide array of nanomaterials including metals, carbon-based structures, and ceramic composites. Unlike traditional methods, it eliminates the need for stabilizing chemicals. Pulsed laser ablation in liquids is particularly promising for producing uniformly sized NP solutions without the use of surfactants. By carefully controlling laser parameters such as energy, wavelength, and the addition of salts, fine-tuning of size and distribution of the resulting NPs is possible.57,58
2.3.2.4. Sputtering. Sputtering is a technique for creating NPs by bombarding a solid target with high-energy particles.59 This process effectively generates thin films. Energetic gas ions impact the target, dislodging tiny clusters of atoms. The specific configuration of ions and their energy determine the size of these ejected clusters. Sputtering can be achieved through various methods including magnetron, radio-frequency diode, and DC diode sputtering. Typically conducted in a vacuum chamber, sputtering involves introducing a gas and applying a high voltage to the target. This creates ions which collide with the target, ejecting atoms. Magnetron sputtering has been successfully used to produce layered WSe2 nanofilms on SiO2 and carbon paper substrates. A key advantage of sputtering is its ability to produce NPs with minimal impurities and at a lower cost than that of electron-beam lithography.60–62
2.3.2.5. Electrospinning. Electrospinning is considered as one of the easiest top–down methods for creating nanostructured materials, particularly nanofibers. It works with a wide range of materials, most commonly polymers. A major innovation in this field is coaxial electrospinning. This technique utilizes a spinneret with two tiny tubes, one inside the other. By pumping different liquids through these tubes, core–shell nanofibers using an electric field can be created. One liquid can be viscous, while the other is less so, allowing for a core-and-shell structure. Coaxial electrospinning shines as a simple and efficient top–down method for producing large quantities of core–shell ultrathin fibers, sometimes reaching centimeters in length. This versatile technique has been used to develop core–shell and hollow materials from various classes including polymers, inorganic materials, organics, and even hybrids.63–65Fig. 2 shows the brief overview of the synthesis approaches of NPs.
3. Brief introduction of metal oxide NPs
In chemistry, an oxide refers to a compound containing oxygen, bonded to at least one other element. Another way to view this is as a combination of an element and the oxygen ion (O2−). In these compounds, oxygen typically has an oxidation state of −2. MOs can be categorized based on the number of oxygen atoms bonded to the metal and the metal's specific oxidation state. Notably, MOs exhibit a wide variety of structures, ranging from simple molecules to complex polymers and crystalline arrangements.66,67 Recently, inorganic MO NPs have gained significant interest compared to organic MO NPs. Their high surface area-to-volume ratio, compared to bulk materials, offers several advantages. These advantages include enhanced catalytic activity, exceptional loading capacity, the ease of surface functionalization, applications in drug delivery and biomedicine, and tunable mechanical properties. Among various inorganic nanomaterials (NMs), MO NPs are particularly attractive and widely used due to several factors. First, well-established and simple synthesis methods exist for MO NPs. Second, their physicochemical properties can be easily controlled. Finally, MO NPs possess fascinating and tunable characteristics, making them versatile for various applications.68 MO NPs have a wide range of applications across various scientific and medical fields. Some of the key applications include energy storage and conversion (batteries,69 supercapacitors,70 solar cells,71etc.), biomedical applications (tissue engineering, cancer treatment, gene therapy, wound healing,72etc.), environmental and chemical applications (gas sensors,73 catalysis,74 antibacterial and antifungal applications,75etc.), electronics and optics (antennas, rectifiers, optoelectronics76etc.), and resistive switching and magnetic applications (resistive switching devices, magnetic storage devices,77etc.). These applications highlight the versatility and potential of MO NPs in various fields, from energy and biomedical applications to environmental and electronics fields.While both metal and metal oxide NPs offer exciting possibilities in nanotechnology, MO NPs possess several advantages that make them a more versatile and tunable platform for various applications. Unlike pure metals, which can be prone to oxidation or degradation in certain environments, MO NPs offers superior chemical stability. This makes them more durable and reliable for long-term applications.78 Moreover, the addition of oxygen to a metal introduces a new dimension for manipulating properties. By varying the metal type, oxidation state, and particle size, it is possible to achieve a wider range of electrical, optical, and catalytic properties in MO NPs compared to their pure metal counterparts.79 On top of that, MO NPs possess surfaces rich in hydroxyl groups (OH−) that readily react with other molecules. This allows for easier surface functionalization, enabling them to be tailored for specific purposes such as drug delivery or binding to target biomolecules.80 When it comes to biocompatibility, some MO NPs exhibit better biocompatibility than pure metals. This makes them more suitable for applications in medicine and diagnostics, where interaction with biological systems is crucial.78 However, the environmental impact of both types of NPs needs careful evaluation. Some MO NPs is less toxic or easier to manage in the environment than their pure metal counterparts.
The research on MO NPs is constantly evolving, and new applications are being discovered all the time. The MO NPs that are being actively researched include zinc oxide NPs (ZnO NPs), iron oxide NPs (Fe2O3 and Fe3O4 NPs), titanium dioxide NPs (TiO2 NPs), silicon dioxide NPs (SiO2 NPs), copper oxide NPs (CuO NPs), and aluminum oxide NPs (Al2O3 NPs). These MO NPs have been extensively studied due to their unique properties and potential applications in various fields including biomedicine, environmental remediation, and energy storage.
Zinc oxide (ZnO) NPs are characterized by their wide bandgap, excellent electrical conductivity, and strong antibacterial properties. These attributes make them highly versatile, finding applications in various fields. In electronics, ZnO NPs are employed in the fabrication of transparent conductive films and sensors. Their antibacterial nature has led to their incorporation into wound dressings, textiles, and personal care products. Additionally, ZnO NPs are used in sunscreens due to their ability to block UV radiation.81,82
Titanium dioxide (TiO2) NPs are another class of MO NPs with remarkable properties and wide-ranging applications. TiO2 exists in three primary crystalline structures: anatase, rutile, and brookite. Among these, anatase and rutile are the most commonly used forms. TiO2 NPs possess excellent photocatalytic activity, which means they can utilize sunlight to break down pollutants and organic compounds. This property has made them indispensable in environmental remediation and water purification. Additionally, TiO2 NPs exhibit high refractive index and opacity, making them ideal pigments for paints, coatings, and plastics. Their non-toxicity and UV-blocking capabilities have also led to their extensive use in sunscreens and cosmetics.83,84
Iron oxide NPs, primarily in the forms of magnetite (Fe3O4) and hematite (Fe2O3), have garnered significant attention due to their magnetic properties. These NPs exhibit superparamagnetism, meaning they can be easily magnetized and demagnetized. This characteristic makes them invaluable in various biomedical applications including drug delivery, magnetic resonance imaging (MRI) contrast agents, and hyperthermia treatment for cancer. Beyond biomedicine, iron oxide NPs find applications in catalysis, environmental remediation, and data storage. Their magnetic properties also enable their use in sensors and actuators.85,86
Aluminum oxide (Al2O3), commonly known as alumina, is a versatile material that exists in various crystalline forms, with the most common being α-alumina. When reduced to the nanoscale, it exhibits unique properties that make it highly desirable for numerous applications. Al2O3 NPs possess exceptional hardness, a high melting point, excellent chemical stability, and remarkable electrical insulation properties. These characteristics make them ideal for applications in ceramics, abrasives, catalysts, and as reinforcing agents in composite materials. Additionally, alumina NPs have shown promise in biomedical fields due to their biocompatibility and potential for drug delivery.87–89
Silicon dioxide (SiO2), or silica, is another widely used material, with its nanoscale counterpart offering distinct advantages. SiO2 NPs are characterized by their large surface area, high porosity, and excellent thermal stability. These properties make them invaluable in various industries. In the electronics sector, silica NPs are essential components of semiconductors, optical fibers, and sensors. Their biocompatibility and non-toxicity have led to their application in drug delivery systems, biosensors, and tissue engineering. Moreover, silica NPs find use in catalysis, water treatment, and as additives in cosmetics and personal care products.90–92
Copper oxide NPs, specifically cupric oxide (CuO), exhibit interesting properties that have attracted significant research interest. They possess excellent electrical conductivity, high catalytic activity, and antimicrobial properties. These characteristics make CuO NPs promising candidates for various applications. In the energy sector, they are employed in lithium-ion batteries, solar cells, and fuel cells. Their catalytic properties find use in environmental remediation, such as the degradation of pollutants. Additionally, CuO NPs have shown potential as antibacterial agents for wound healing and water purification.9,14,93 In the following part of this review, details regarding the CuO NPs, their plant extract-mediated green synthesis, application and mechanism are discussed elaborately.
4. CuO NPs: the material of interest
CuO NPs have emerged as a captivating subject in materials science due to their exceptional properties as p-type semiconductors with a narrow band gap. Unlike their bulk counterparts, CuO NPs exhibit remarkable physical and chemical characteristics attributed to their large surface area and size-dependent effects.94,95 This has sparked intense research interest in their potential applications across diverse fields. CuO NPs are particularly promising as electrode materials for next-generation lithium-ion batteries (LIBs) owing to their high theoretical capacity, safety, and environmental friendliness.96,97 Their strong light absorption, low heat emission, and favorable electrical properties make them suitable for solar cell fabrication. Additionally, these nanostructures excel in various applications, including gas and biosensing, nanofluids, photodetection, energy storage, environmental remediation, and catalysis.5,98 The versatility of CuO NPs extends to the creation of organic–inorganic nanocomposites with enhanced properties such as thermal and electrical conductivity, mechanical strength, and durability. They effectively catalyze the oxidation of harmful gases and organic compounds, and their superhydrophobic nature shows promise in self-cleaning coatings, water treatment, and oil–water separation. To harness the full potential of CuO NPs, researchers have developed methods to synthesize nanostructures in various shapes and sizes, from zero-dimensional NPs to complex three-dimensional architectures.99–101 While cuprous oxide (Cu2O) shares some similarities, its distinct properties and lower stability compared to CuO make the latter more suitable for practical applications. Compared to other MO NPs, CuO NPs offer unique advantages in magnetism, superhydrophobicity, and catalysis. Although its application in LIBs is less explored than some counterparts, its favorable attributes make it a promising candidate.102,1034.1 Crystal structure of CuO NPs
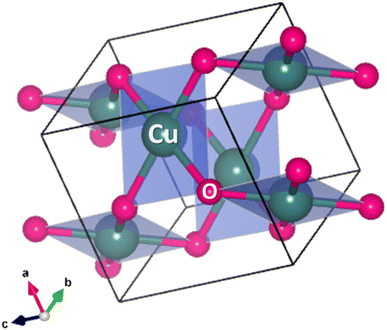
CuO has a distinct crystal structure characterized by its monoclinic symmetry. The crystal system belongs to the space group C2/c, and its unit cell parameters have been refined through various structural analyses.104 In the CuO crystal structure, each Cu atom is coordinated by four O atoms. This arrangement forms a nearly square planar configuration, where the Cu atom is at the center and the O atoms are located at the corners of a distorted tetrahedron. The coordination geometry leads to the formation of ribbons of parallelograms in the [110] direction, while two types of –Cu–O–Cu– chains can be observed in the [101] direction.105 CuO exhibits a characteristic structural distortion due to the strong Jahn-Teller effect commonly observed in divalent copper compounds. This distortion results in a square planar arrangement of oxygen atoms around the copper ion. The Cu–O bond distances within this plane are slightly longer than those found in cuprous oxide. The remaining Cu–O distances perpendicular to this plane are significantly longer, definitively excluding an octahedral coordination geometry. Oxygen atoms in CuO are coordinated to four copper atoms in a distorted tetrahedral configuration. While the copper ion in CuO is undeniably in the +2 oxidation state, the bonding within the compound is generally accepted to be a combination of ionic and covalent characters.5 The crystallographic parameters of a typical CuO compound are presented in Table 1 and a crystal structure was drawn (Fig. 3) based on the presented crystallographic information using the VESTA version 3 software.106,107| Sl. no. | Crystallographic parameter | ICDD card no #00-041-0254 |
|---|---|---|
| 1 | Mineral name | Tenorite |
| 2 | Crystal system | Monoclinic |
| 3 | Space group | C2/c |
| 4 | Space group number | 15 |
| 5 | Unit cell parameters | a = 4.6850 Å, b = 3.4230, c = 5.1320 Å, α = γ = 90°, β = 99.52° |
| 6 | Calculated density | 6.52 g cm−3 |
| 7 | Measured density | 6.45 g cm−3 |
| 8 | Volume of unit cell | 81.17 Å3 |
| 9 | Number of symmetry-independent molecules (Z) | 4.00 |
| 10 | Reference intensity ratio (RIR) | 2.80 |
4.2 Synthesis of CuO NPs
The synthesis of CuO NPs can be broadly classified into three main categories: physical, chemical, and biological synthesis.108 The fundamentals of these synthesis categories have been described in the “2.3. Synthesis methods of NPs” section.The sol–gel method is a popular choice for synthesizing NPs due to its simplicity and efficiency. It offers precise control over particle size, making it ideal for applications requiring specific dimensions. Research has shown that the sol–gel method can produce CuO NPs with sizes ranging from 10 to 100 nm.119 Wang et al. synthesized CuO NPs with a size of ∼100 nm following the sol–gel technique and investigated the gas sensing efficacy of the synthesized NPs.120 The calcination time and temperature play crucial roles in determining the physical properties of CuO NPs synthesized by the sol–gel method. By carefully adjusting these parameters, NP characteristics can be fine-tuned. For instance, Jayaprakash et al. successfully synthesized both uncapped and capped CuO NPs using ethylene diaminetetraacetic acid (EDTA) as a capping agent. The capping agent helped control the size and shape of the NPs, demonstrating the versatility of the sol–gel method.121
Co-precipitation is a widely used technique for the synthesis of CuO NPs. It involves the simultaneous precipitation of copper and oxygen ions from a solution, leading to the formation of CuO NPs. Different sized and shaped CuO NPs have been prepared using various copper precursors such as copper(II) nitrate, copper(II) sulfate, copper(II) chloride, and copper(II) acetate.43,122 Capping agents, often used as both reducing and stabilizing agents, are typically introduced alongside the precursor materials at the beginning of the chemical synthesis process for various NPs.123
The hydrothermal/solvothermal method involves heating a reaction mixture containing copper precursors and a solvent (typically water or an organic solvent) in a sealed autoclave under high-temperature and -pressure conditions. The elevated conditions facilitate the dissolution of the precursors, promote nucleation, and control the growth of the CuO NPs. Hydrothermal/solvothermal synthesis offers several advantages for the preparation of CuO NPs, such as controlled particle size and morphology, high purity, crystallinity, and uniformity. Zhang et al. synthesized CuO nanoplatelets following a simple hydrothermal technique where the thickness of the NPs was 65–80 nm.124 In the work of Zhao et al., nanosheets of CuO were synthesized in large amounts using the hydrothermal technique, and the thickness of the sheets ranged from 40 to 50 nm.125 CuO microspheres consisting of nanosheets were prepared by Wang et al. following the solvothermal method, which also included annealing after the synthesis.126 Gopalakrishnan et al. reported the synthesis of 1D CuO NPs through a surfactant- and template-free solvothermal technique that resulted in nanowires of 15 nm diameter and 90 nm length.127
Microwave-assisted synthesis utilizes electromagnetic waves to heat the reaction mixture directly, leading to faster reaction times and improved control over particle size and morphology compared to traditional heating methods. As the polar molecules present in the reaction mixture absorb the microwave energy, they experience rapid oscillations, leading to localized heating and enhanced molecular motion. This increased kinetic energy facilitates the nucleation and growth of CuO NPs, resulting in smaller, more uniform particles with desirable properties. Furthermore, microwave-assisted synthesis often produces NPs with unique properties compared to those synthesized using conventional methods. The rapid heating and localized energy deposition can influence the nucleation and growth kinetics, resulting in particles with different sizes, shapes, and surface morphologies. These properties can have significant implications for the performance of CuO NPs in various applications such as catalysis, sensing, and energy storage.128 Different morphologies of CuO were achieved by changing the alkali source by Jung et al. following the microwave-assisted synthesis procedure.129 Feather- and flower-like CuO nanocrystals were prepared by Zhang et al. adapting the microwave-assisted synthesis technique.130
The microbial synthesis of CuO NPs employs certain microorganisms such as bacteria, fungi, and algae. These biological entities produce CuO NPs either extracellularly or intracellularly. While the exact mechanisms of NP formation using biological agents remain unclear, it is believed that specific biomolecules are involved in this process. Additionally, intracellular and extracellular NP syntheses differ, with the cell wall of microorganisms likely playing a crucial role in the former and extracellular enzymes in the latter. Due to its faster production rate and simpler synthesis process, extracellular NP synthesis has become more prevalent than intracellular methods.131 Singh et al. synthesized CuO NPs by exploiting132Escherichia coli (E. coli). A report from Ghorbani et al. depicts the formation of CuO NPs of size averaging 49 nm, achieved through the extracellular synthesis by Salmonella typhimurium.133 Chilean white-rot fungus (Stereum hirsutum) was used by Cuevas et al. for extracellular biosynthesis of spherical CuO NPs (5 to 20 nm).134 Brown seaweed (Sargassum polycystum) was utilized for the synthesis of CuO NPs, and its antimicrobial and anticancer activities were examined.135
The use of plant extracts for the synthesis of CuO NPs is the most widely accepted and implemented “green synthesis” technique. Using plant extracts to make NPs has advantages over other biological methods such as microbial synthesis. The primary raw materials for PEM-CuO NP synthesis, such as leaves, fruits, flowers and fruit peels, are readily available and abundant in nature. Moreover, these materials often require minimal pre-treatment and relatively straightforward extraction process, which lowers the cost of the synthesis.136,137 Furthermore, plant-based synthesis generally avoids harsh chemicals and can utilize agricultural waste, minimizing environmental impact.138 In contrast, the production cost of the microbial synthesis method of nanoparticles is higher due to the maintenance of microbial cultures, including media preparation and sterilization, compared to plant extract preparation.139 Additionally, genetically modified microorganisms can raise ethical concerns and may be subject to stricter regulations. Moreover, separating and purifying nanoparticles from microbial biomass can be a complex and costly process.
However, the exact composition of plant extracts can vary depending on many factors such as season, geographical location, and plant species, potentially affecting the reproducibility and consistency of nanoparticle synthesis. This variability can make it difficult to achieve precise control over size, shape and uniformity compared to some microbial methods.140 However, microbial cultures can be grown in controlled environments, leading to high yields and consistent nanoparticle production, and can be readily scaled up for industrial production using bioreactors. Microorganisms, especially genetically modified ones, can offer greater control over the size, shape, and stability of synthesized nanoparticles, as their metabolic pathways can be manipulated to produce nanoparticles with specific characteristics.
Both plant extract-mediated and microbial synthesis offer unique advantages and limitations. On the one hand, plant-based methods are more cost-effective, environmentally friendly and require fewer chemicals. On the other hand, microbial-mediated synthesis offers greater control over nanoparticle properties, but they come with higher costs and potential ethical considerations. The choice between these methods depends on the specific requirements of the application, including the desired nanoparticle properties, production scale, cost constraints, and environmental considerations. However, considering the popularity and widespread use of plant extract-mediated synthesis, this review aims to discuss the implementation of the synthesis of CuO NPs using plant extracts, and hence, detailed discussions are presented in the following section. Fig. 4 graphically summarizes the synthesis methods of CuO NPs.
5. Plant extract-mediated synthesis of CuO NPs
While both PEM and microorganism-assisted synthesis procedures are considered green methods for producing CuO NPs, PEM synthesis is often more prominently termed “green” due to several factors. Plant extracts offer a more advantageous approach to synthesizing CuO NPs than microorganisms. While the latter can produce NPs, challenges such as toxicity, isolation, and incubation limit their practicality. Plant extracts, however, are non-toxic, readily available, and can complete the NP synthesis process within a few hours at room temperature.108 The bioactive compounds including flavonoids, phenols, and terpenoids in plant extracts act as reducing and stabilizing agents, converting metal salts into NPs.141 These compounds generate electrons that reduce the metal ions, leading to NP formation.142 Plant-based NP synthesis is a simple, safe, and energy-efficient method that produces stable NPs.143 Additionally, this method can be scaled up more easily for industrial production, making it suitable for commercial applications.144 Furthermore, plants are often considered more natural and environmentally friendly than microorganisms, which makes PEM synthesis more likely to be termed the “greener” approach. Table 2 summarizes the recent reports on plant sources and precursors used for PEM-CuO NP synthesis, along with the resulting size and morphology.| Sl. no. | Plant source | Plant part | Precursor used | Particle size | Morphology | Ref. |
|---|---|---|---|---|---|---|
| 1 | Averrhoa carambola | Leaf | Copper sulfate pentahydrate | 98 ± 26 nm | Irregular, mostly spherical | 6 |
| 2 | Aloe vera | Leaf | Copper nitrate | 20–30 nm | Spherical | 231 |
| 3 | Calotropis gigantean | Leaf | Copper nitrate | 20 nm | Spherical | 232 |
| 4 | Rubus glaucus | Leaf and fruit | Copper nitrate trihydrate | 43.3 and 52.5 nm | Spherical | 233 |
| 5 | Ixiro coccinea | Leaf | Copper sulfate pentahydrate | 80–110 nm | Spherical | 234 |
| 6 | Malva sylvestris | Flower | Copper nitrate trihydrate | 26 nm | Spherical | 235 |
| 7 | Azadirachta indica | Leaf | Copper acetate tetrahydrate | 12 nm | Spherical | 236 |
| 8 | Eupatorium odoratum | Leaf | Copper sulfate pentahydrate | — | Spherical | 237 |
| 9 | Acanthospermum hispidum | Leaf | Copper sulfate pentahydrate | — | Spherical | 237 |
| 10 | Albizia lebbeck | Leaf | Copper sulfate pentahydrate | <100 nm | Spherical | 238 |
| 11 | Kalopanax pictus | Leaf | Copper sulfate pentahydrate | 26–67 nm | Spherical | 239 |
| 12 | Rosa sahandina | Fruit | Copper sulfate pentahydrate | <50 nm | Spherical | 240 |
| 13 | Hylotelephium telephium | Flower | Copper nitrate hexahydrate | 83 nm | Spherical | 241 |
| 14 | Pterolobium hexapetalum | Leaf | Copper sulfate pentahydrate | 10–50 nm | Spherical | 242 |
| 15 | Tabernaemontana divaricate | Leaf | Copper sulfate solution | 48 nm | Spherical | 243 |
| 16 | Coriandrum sativum | Seed | Copper chloride | 18.2 nm | Irregular | 244 |
| 17 | Acalypha indica | Leaf | Copper sulfate pentahydrate | 29 nm | Spherical | 245 |
| 18 | Albizia lebbeck | Leaf | Copper sulfate pentahydrate | 100 nm | Spherical | 246 |
| 19 | Pterocarpus marsupium | Wood | Copper sulfate monohydrate | 20–50 nm | Spherical | 247 |
| 20 | Eichhornia crassipes | Leaf | Copper sulfate | 15–30 nm | Spherical | 248 |
| 21 | Aloe barbadensis | Leaf | Copper sulfate | 15–30 nm | Versatile and spherical | 249 |
| 22 | Terminalia catappa | Leaf | Copper sulfate pentahydrate | 103–29 nm | Spherical | 250 |
| 23 | Oak | Fruit | Copper nitrate trihydrate | 34 nm | Quasi-cubic | 251 |
| 24 | Beta vulgaris | Root | Copper sulfate pentahydrate | 11.4 to 63.9 nm | Predominantly spherical and irregular | 252 |
| 25 | Citrofortunella microcarpa | Leaf | Copper nitrate trihydrate | 54–68 nm | Spherical-like | 253 |
5.1. Mechanism of CuO formation in PEM synthesis route
Generally, the PEM synthesis of metal oxide NPs can be explained through two primary mechanisms: chelation-based complex formation and bioreduction. Both of the mechanisms were widely discussed in the literature.145According to the chelation mechanism, the bioactive compounds (extracts such as flavonoids, phenols, and other phytochemicals) present in the plant extract coordinate with metal precursor ions and form stable intermediate complexes.146 Upon thermal decomposition during calcination, these complexes lead to the formation of metal oxide NPs. Several studies support this mechanism, including the work by Matinise et al., which demonstrated that antioxidants in Moringa oleifera leaf extract chelate zinc(II) ions, forming zinc–organic complexes that subsequently transform into ZnO NPs upon heat treatment. This conclusion was further reinforced by FTIR spectral analysis, which confirmed the presence of bioactive functional groups in the synthesized NPs.147–149
However, the bioreduction mechanism suggests that the phytochemicals act as reducing agents that convert the metal ions into their zero-valent states. These reduced metal atoms then react with the dissolved oxygen present in the reaction medium and lead to the formation of metal oxides. Singh et al. proposed this route for the synthesis of ZnO quantum dots using Eclipta alba leaf extract, wherein zinc acetate was reduced by phytochemicals before reacting with oxygen to form ZnO. Furthermore, this mechanism suggests that plant-derived compounds also aid in NP stabilization by preventing agglomeration.150
For the PEM synthesis of CuO NPs, a similar mechanism can be proposed. The phytochemicals present in the extract may either chelate copper ions to form copper–organic complexes that decompose into CuO during calcination or reduce Cu2+ to its metallic state, followed by oxidation to CuO. The presence of functional groups from plant metabolites in the synthesized CuO NPs, as identified by spectroscopic analyses, further supports these proposed pathways. Nagore et al. have reported the synthesis of CuO NPs using Polyalthia longifolia, which involved the formation of a complex between copper ions and phytochemicals to form CuO NPs via a nucleation process and this process continued until the NPs obtained a stable size and shape.151 Nagajyothi et al. synthesized CuO NPs from black beans (Phaseolus vulgaris) and proposed that the water in the system produced OH−, which reacted with the metal precursor (copper sulfate heptahydrate) to form Cu(OH)2. Then, the phytochemicals present in the black bean aqueous extract encapsulated the CuO NPs by reduction.152 Aroob et al. utilized Seriphidium oliverianum leaf extract to synthesize CuO NPs. From the study, they concluded that various functional groups in flavonoids were responsible for the formation of the NPs. The enol flavonoids were converted to keto flavonoids and accordingly reduced the Cu ions by H atoms released in the process.21 Alhalili reported the green synthesis of CuO NPs by the leaf extract of Eucalyptus globulus. He abridged the synthesis mechanism by concluding that the metal complex formed with the polyphenols in the extract solution, which eventually was reduced to CuO NPs.19 From the work of Nagore et al.,151 Sutradhar et al.,153 and Veisi et al.,154 the fact is established that when the reduction of Cu ions produces Cu metal atoms, they are converted to CuO NP by air O2 or dissolved oxygen present in the solution at a moderate temperature of approximately 80–85 °C. All of these studies showed that polyol or polyphenols present in the phytochemicals were responsible for the reduction and encapsulation of Cu ions, eventually forming CuO NPs.
Based on the concept of chelation mechanism, a generalized reaction scheme of PEM-CuO NPs synthesis can be proposed. Fig. 5 schematically illustrates the formation mechanism of CuO NPs.
Step-1: Chelation of Cu2+ by plant extract compounds
| Cu2+ + phytochemicals → Cu-phytochemical complex |
For example, if flavonoids (FL) are involved:
| Cu2+ + FL → Cu-FL complex |
Step-2: thermal decomposition of Cu-Phytochemical Complex.
Meanwhile, the basic understanding of bioreduction mechanism aids in creating the reaction scheme as follows.
Step-1: Reduction of Cu2+ to Cu0 by plant extract compounds
| Cu2+ + phytochemicals → Cu0 + oxidized phytochemicals |
For example, using ascorbic acid (C6H8O6) as a reducing agent:
| Cu2+ + C6H8O6 → Cu0 + C6H6O6 + 2H+ |
Step-2: Oxidation of Cu0 to CuO in the presence of oxygen and water.
Reaction with dissolved oxygen in the solution will result in CuO:
Reaction in the presence of water will result in:
5.2. Characterization techniques of CuO NPs
The characterization of CuO NPs is crucial to understand their structural, morphological, and optical properties.155 The success of the plant-mediated CuO NP synthesis can be confirmed through the X-ray diffraction (XRD) analysis. XRD provides information about the crystal structure, lattice parameters, and phase purity of the synthesized CuO NPs. It can be used to confirm the formation of the desired CuO phase and identify any impurities, since metallic Cu or Cu2O can co-exist with the CuO NPs.7 Further clarification or confirmation of CuO formation can be achieved through the X-ray photoelectron spectroscopic (XPS) technique. XPS determines the elemental composition and oxidation states of the elements present in the CuO NPs. It can be used to confirm the presence of copper and oxygen in the desired ratios and to investigate the surface chemistry of the NPs.6 In addition to that, the presence of impurities or the elements of capping or stabilizing agents can be detected at the surface level of CuO NPs.When the formation of CuO phase is confirmed, the next vital characterization would be to carry out transmission electron microscopy (TEM) and/or scanning electron microscopy (SEM). TEM provides high-resolution images of the morphology, size distribution, and shape of the CuO NPs. It can be used to visualize individual NPs and identify defects or agglomeration.7 Scanning electron microscopy (SEM) offers a lower resolution than that of TEM, but can be used to examine the surface morphology and topography of the CuO NPs.6,156 It is particularly useful for studying larger samples or for obtaining information about the particle distribution in a powder. Fourier transform infrared (FTIR) spectroscopy can be used to identify the functional groups present in the CuO NPs, such as the Cu–O bond. It can also be used to study the interactions between the NPs and any stabilizing or capping agents.
Ultraviolet-Visible (UV-Vis) Spectroscopy can be used to measure the optical properties of the CuO NPs, including their absorption and bandgap.157 It can be used to assess the electronic structure of the NPs and their potential applications in optoelectronic devices.7 Energy-dispersive X-ray spectroscopy (EDX) can be used to determine the elemental composition of the CuO NPs. By analyzing the X-rays emitted from the sample, EDX can confirm the presence of copper and oxygen in CuO NPs in terms of atom and mass percentage. This information is essential for understanding the stoichiometry of the material. Apart from that, the presence of other elements can be detected and quantified through EDX analysis. If integrated with the elemental mapping technique, the distribution of elements throughout a particular area of the NP can also be measured.17
The zeta potential measurement can also be employed for the assessment of the surface charge of the NPs. The value of zeta potential is crucial for understanding the stability of the NP dispersion and its interactions with other particles or surfaces.6,158 Atomic Force Microscopy (AFM) provides high-resolution images of the NP surface topography. It can reveal information about the size, shape, and surface morphology of the CuO NPs.159,160 Photoluminescence (PL) spectroscopy measures the emission of light from the NPs when they are excited by a light source. This technique can provide insights into the electronic structure and defect states within the CuO NPs.161 Thermogravimetric Analysis (TGA) determines the thermal stability of the NPs. By heating the sample and measuring the weight loss, TGA can identify the presence of any organic components or impurities.156 Raman spectroscopy provides information about the vibrational modes of the CuO NPs. This technique can help to identify the specific crystal structure and detect any phase transformations or defects.162
5.3. Influence of experimental parameters on CuO NP formation
The plant extract-mediated synthesis of CuO NPs is heavily influenced by various factors such as reaction conditions (pH, temperature, and time), precursor choice, and plant extract concentration. These parameters collectively determine the NP size, shape, and overall properties. By carefully controlling these variables, NPs with specific characteristics for a wide range of applications can be prepared.On the contrary, studies in the literature also depict the effective formation of CuO NPs at higher pH values and report pH 7–9 as the optimum pH range.165,166 This may be because, when the pH falls below 7, creating acidic conditions, the effectiveness of the plant extract diminishes in acidic environments, which can result in the denaturation or degradation of the bioactive compounds responsible for the reduction and stabilization of the NPs. As a result, larger NPs tend to form under these conditions.164,167,168
6. Textile effluent remediation by PEM-CuO NPs
The textile industry is a significant contributor to environmental pollution, primarily through the discharge of untreated or inadequately treated wastewater. This effluent is laden with various harmful substances that pose severe risks to aquatic ecosystems and human health.181 Textile manufacturing processes, particularly dyeing and finishing, are notorious for generating large volumes of wastewater.182 An estimated 200 liters of water are required to produce just one kilogram of cloth, and dyes, heavy metals, surfactants, and other toxic compounds contaminate a substantial portion of this water.183 The effluents often contain complex mixtures of pollutants, including organic and inorganic substances, which can severely degrade water quality. Studies indicate that textile dyeing alone accounts for approximately 20% of all freshwater pollution globally.184 While combating such pollution caused by textile dye-laden effluent, researchers utilized numerous photocatalysts and adsorbents. The PEM or simply the green-synthesized CuO NPs are potential materials to win such combats. There are a myriad of studies that report the efficacy of CuO NPs in the eradication of textile effluents.15 CuO usually removes toxic chemicals in two of the most preferred ways: adsorption, by their high surface area, and photocatalysis, by generating reactive oxygen species (ROS) when exposed to light.185 They are most preferred because of their cost-effectiveness and simple design.186 Adsorption by CuO NPs can be both physical and chemical surface interactions. It is an endothermic and spontaneous process. The adsorbent can be reused several times with high efficiency.187 The adsorption capacity depends on the degree of functionalization.188 CuO NPs can be activated under visible/solar light, ultraviolet (UV) light, and simulated sunlight.189Table 3 lists the treatment of various dyes in aqueous solutions by PEM-CuO NPs.| Plant source | Plant part | Removal process | Conditions for maximum output | Removal efficiency | Ref. |
|---|---|---|---|---|---|
| Justicia gendarussa | Leaf | Photocatalysis | CuO = 20 mg, dye = 100 mL of 10 ppm, time = 5 h, under sunlight irradiation | 97% of methylene blue (MB) | 254 |
| Brassica rapa | Leaf | Adsorption | CuO = 2.0 g L−1 at room temperature for 10 mg L−1 dye, time = 60 min | >92% of Amaranth dye, 80% Congo red (CR) and 47% Bismarck brown R (BBR) | 255 |
| Eucalyptus globulus | Leaf | Adsorption | Concentration = 0.04 g/50 mL, pH = 4.5, temperature = 25 °C | 95 mg g−1Qmax (maximum adsorption capacity) for Methyl orange (MO) | 19 |
| Punica granatum | Leaf | Adsorption | CuO = 12 g L−1, time = 24 h, temperature = 298 K for 50 mg L−1 dye | 95.80% of Safranin-O dye; Qmax = 189.54 mg g−1 | 256 |
| Ephedra alata | Whole plant | Adsorption | CuO = 0.02 g, dye concentration = 10 mg L−1, pH = 7, temperature = 373 K | 133.75 mg g−1Qmax for MB | 257 |
| Seriphidium oliverianum | Leaf | Photocatalysis | CuO = 10 mg for 10 mg L−1 dye, time = 60 min, using sunlight | 65.231% ± 0.242 of Methyl green (MG) and 65.078% ± 0.392 of MO | 21 |
| Psidium guajava | Leaf | Photocatalysis | CuO = 0.1 mg mL−1, pollutant = 10 mL mixture of 1 mM, using visible light | 91% of MB | 258 |
| 89% of Methyl red (MR) | |||||
| 80% of MO | |||||
| 97% of Eosin yellow EY) | |||||
| Psidium guajava | Leaf | Photocatalysis | CuO = 10 mg for both 40 ppm dye (20 mL), time = 2 h, irradiation under direct sunlight | 93% of Nile blue, 81% of reactive yellow 160 (RY160) | 259 |
| Aloe-vera | Leaf | Photocatalysis | CuO = 25 mg, dye = 100 mL of 10 ppm, time = 24 min, using UV light | Maximum degradation of 96.1% of MO dye | 260 |
| Ocimum tenuiflorum | Leaf | Photocatalysis | CuO = 25 mg, dye = 100 mL of 10 ppm, pH = 4, using UV light (125 W) | 96.4 ± 0.83% of MO dye | 261 |
| Wedelia urticifolia | Leaf | Adsorption | CuO = 10 to 40 mg, dye = 10 to 25 ppm, time = 0.5 h to 6 h, temperature = RT, stirring speed = 120 rpm, pH = solution pH | 99% of RhB | 262 |
| Aloe barbadensis | Leaf | Adsorption | CuO = 0.05 to 0.5 g L−1, dye = 100 mg L−1, at pH = alkaline, time = 210 min, stirring speed = 150 rpm | 98.89% of MB, Qmax = 95.5 mg g−1 | 263 |
| Ocimum americanum | Leaf | Photocatalysis | CuO = 1 mg mL−1, dye = 0.1 mg mL−1, time = 200 min, using sunlight | 75.69% of EY, 34.12% of Rhodamine 123 (Rh123), 71.06% of MB | 264 |
| Carica papaya | Fruit | Photocatalysis | CuO = 1 mg, dye = 10 mL of 0.001 M, time = 1 h | 18.45% of MO under sunlight | 265 |
| 30.98% of MO under UV light | |||||
| Lemon tea extract | — | CuO = 1 mg, dye = 10 mL of 0.001 M, time = 1 h | 31.95% of MO under sunlight | ||
| 45.23% of MO under UV light | |||||
| Annona muricata | Leaf | Photocatalysis | CuO = 20 mg/100 mL of dyes, time = 1 h, using sunlight | 90% of reactive red 120 (RR120) | 266 |
| 95% of MO | |||||
| Ferulago angulate (schlecht) boiss | Whole plant | Photocatalysis | CuO = 0.05 g, dye = 50 mL of 100 µM, time = 2.5 h, under fluorescent lamp (λ > 400 nm, 80 W) | 84% of RhB | 267 |
| Citrofortunella microcarpa | Leaf | Photocatalysis | CuO = 10 mg L−1, dye = 10 ppm, pH = 5, time = 10 min, using UV light | 98% of RhB | 253 |
| Punica granatum | Leaf | Adsorption | CuO = 0.4 g L−1, Equilibrium time = 120 min, temperature = 298 K | 96% of Neutral red (NR) dye; Qmax = 283 mg g−1 | 268 |
7. Pharmaceutical waste remediation by PEM-CuO NPs
Pharmaceutical waste encompasses a variety of antibiotics, painkillers, and anticancer drugs that pollute the environment and disrupt the natural functioning of ecosystems.190 The increase in environmental pharmacological substances and their potential harmful effects on biological systems are global issues, especially challenging for countries with high population growth. Evidence shows that these substances threaten genetic, species, and community diversity191 and cause chronic damage, behavioral changes, accumulation in tissues, reproductive damage, and inhibition of cell proliferation, and can be incorporated into food chains.192 Plants can accumulate pharmaceutical compounds in their seeds, roots, and leaves. This accumulation has been linked to a reduction in grain yield by up to 50%. Once in water, these compounds can alter the behavior of fish and other organisms, inhibit reproduction in crustaceans, and even cause the death of exposed organisms.193The application of NPs for the eradication of these pharmaceutical wastes is previously reported in the literature.194–196 Photocatalytic degradation and adsorptive removal are more predominant than other removal techniques. However, the exploitation of PEM-CuO NPs for such cause is a newer concept, and previous reports show its potential applicability.197 In Table 4, we compile the application of PEM-CuO NPs and their composites for the treatment of pharmaceutical waste.
| Plant source | Plant part | Precursor of CuO NPs | NP name | Pharmaceutical waste | Treatment process | Treatment efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Ocimum sanctum | Leaf | Copper acetate | CuO | Doxycycline hydrochloride | Adsorptive removal | Q max = 8.56 mg g−1 at 100 mg L−1, 170 min, pH 6 at 298 K | 197 |
| Platanus occidentalis | Leaf | Copper sulphate | CuO | Paracetamol | Adsorptive removal | Q max = 64.52 mg g−1 | 269 |
| Green tea extract | Leaf | Copper sulphate | CuO | Ciprofloxacin | Adsorptive removal | 92% (con. 0.01 mg L−1, 0.75 g L−1, pH 4, 180 min | |
| Tragia involucrata | Leaf | Copper nitrate trihydrate | g-C3N4/CuO | Ciprofloxacin | Photodegradation | 93% | 270 |
| Parthenium hysterophorus | Whole plant | Copper(II) sulphate | CuO | Rifampicin | Photodegradation | 98.43% (65 °C, 50 mg dosage, 10 mg L−1 drug, pH 2, time 8 min | 271 |
| Euphorbia polygonifolia | Aerial parts | Copper(II) sulfate pentahydrate | Fe3O4@CuO | Metronidazole | Photodegradation | 89% | 272 |
| Ciprofloxacin | 94% | ||||||
| Cephalexin | 96% | ||||||
| Psidium guajava | Leaf | Copper sulphate | CuO/Fe2O3 | Tetracycline | Photodegradation | 88% in 80 min | 273 |
| Macadama nut shell extract | Nut shell | Copper nitrate hexahydrate | CuO NPs | Tetracycline | Photodegradation | 74% in 120 min | 274 |
| Ferula persica | — | Copper sulphate | CuO–CdO-bentonite | Levofloxacin | Photodegradation | 96.11% at catalyst dosage = 0.4 g L−1, concentration = 10 mg L−1 and pH = 8 | 275 |
8. Mechanism of pollutant eradication by PEM-CuO NPs through photodegradation and adsorption
CuO NPs have been used as potential photocatalysts in the degradation of organic pollutants including toxic dyes and pharmaceutical wastes due to their unique properties including high surface area, adjustable bandgap and good photocatalytic characteristics. This photodegradation process mainly involves the generation of reactive oxygen species (ROS) under light irradiation, which subsequently break down the pollutants.Upon irradiation with ultraviolet (UV) light or visible light, the energy of the incident photons can excite the electrons of CuO NPs from their valence band (VB) to the conduction band (CB), generating electron–hole pairs.198,199 These holes serve as powerful oxidizing agents and oxidize water molecules to generate ROS such as hydroxyl radicals (OH˙−). At the same time, the electrons in the conduction band can reduce the adsorbed oxygen on the CuO NP surface to form superoxide radicals (O2˙−). These ROS decompose the molecules of organic pollutants such as dyes and pharmaceutical wastes through oxidation reactions.200–203 Once the molecules of organic pollutants are attached to the surface of CuO NPs, the ROS species bind the molecule. This breaks the chemical bond of organic molecules and ultimately degrades the toxic pollutants into less harmful products such as carbon dioxide and water. The probable reaction mechanism for the photodegradation of toxic organic pollutants using CuO NPs as photocatalysts is as follows:
| CuO + hν→ CuO (ecb− + hvb+) |
| H2O + h+ → H+ + OH˙− |
| O2 + 2e− → O2˙− |
| O2˙− + H+ → HO2˙− |
| HO2˙− → H2O2 + O2 |
| H2O2 → 2OH˙− |
| OH˙− + organic pollutants → degradation products (CO2 + H2O) |
CuO NPs show promising efficiency for the photodegradation of toxic organic dyes and pharmaceutical wastes due to their excellent photocatalytic properties. While the formation of ROS and the oxidation of organic pollutants are crucial to the degradation process, other factors such as particle size and concentration of NPs, bandgap energy, solution pH, temperature and reaction time also affect the photocatalytic degradation activity.204 The pH of the solution plays a significant role in the adsorption capacity and degradation efficiency. At lower pH values, CuO NPs typically carry a positive charge, which enhances their interaction with negatively charged molecules of pollutants. However, at higher pH values, the surface becomes negatively charged, which can repel negatively charged pollutants and reduce the degradation efficiency. Moondeep et al. have reported a similar phenomenon, in which, at higher pH values, the CuO NP surface is negatively charged, which improves its interaction with cationic dyes. Meanwhile, at lower pH values, the positively charged surface of CuO NPs enhances their interaction with anionic dye.205 Along with the pH of the solution, the concentration of CuO NPs in the reaction mixture also plays a crucial role in photocatalytic efficiency. An adequate concentration of CuO is necessary to optimize the light absorption and pollutant interaction. A much lower concentration may not provide sufficient active sites for adsorption, while too high concentrations can cause turbidity that can lead to light scattering and reduced effective surface area for reactions.206 Moreover, the temperature and time can influence the photocatalytic activity to some extent. At higher temperatures, the reaction rates increase with the increase in molecular collisions. However, excessively high temperatures can reduce dissolved oxygen concentration, leading to increased electron–hole recombination, which hinders photocatalytic activity.207 Additionally, optimal irradiation times allow sufficient generation of ROS, facilitating the degradation process. After reaching a plateau at a favorable time, further exposure does not significantly enhance the degradation efficiency.206 Therefore, it is necessary to optimize these parameters to improve the photocatalytic activity. Fig. 6 illustrates the underlying mechanism of photocatalysis by PEM-CuO NPs.
The high surface area and surface charge of CuO NPs contribute to their adsorption capabilities along with the photocatalytic degradation capacity. This mechanism of adsorptive removal mainly involves the interaction between the toxic organic pollutant molecules and the CuO NPs in different physicochemical ways. The surface charge of CuO NPs can vary with pH, which influences the interaction of CuO NPs with the pollutant molecules. If the pollutant molecules are charged, they can be attracted to the oppositely charged surface of the CuO NPs through electrostatic forces. At higher pH levels, negative charges on CuO surfaces increase, which enhances the electrostatic attraction with cationic pollutant molecules, whereas, at lower pH levels, the positively charged surface of CuO NPs attracts the anionic pollutant molecules.208 Moreover, the capping or stabilizing molecules on PEM-CuO NPs may contain plant phytochemicals and the functional groups present in them contribute to toxic pollutant molecule adsorption through hydrogen bonding and van der Waals interactions.209 Furthermore, the aromatic hydrocarbons present in organic pollutants can facilitate the adsorption procedure by interacting with the π-electron cloud of the CuO NPs through π–π interactions.210 Similar to photodegradation, the organic pollutant adsorption process is influenced by the concentration of pollutants and the available active sites on the CuO NPs.
9. Antimicrobial activity of PEM-CuO NPs
The concept of antimicrobial activity involves the ability of certain agents to kill or inhibit disease-causing microbes. This activity can be exhibited by various antimicrobial agents including those that are antibacterial, antifungal, or antiviral.211 Metal NPs, especially those of silver, gold, and copper, have been thoroughly investigated for their antibacterial properties, and the results indicate that they are quite successful in treating a wide range of bacterial types.212 Inorganic metal oxide NPs such as CuO, ZnO, MgO, TiO2, and SiO2 have significant antimicrobial features.213 CuO NPs have demonstrated broad-spectrum antibacterial activities against a range of bacteria as well as antifungal activities.214Table 5 and Table 6 provide data on the antibacterial and antifungal activities of PEM-CuO NPs, respectively.| Bacterial strain and type | Plant source | Dosagea of CuO NPs | Antimicrobial efficiency in terms of zone of inhibition (ZOI) | Ref. |
|---|---|---|---|---|
| a Dosage for which highest ZOI found was selected for inclusion. | ||||
| Bacillus subtilis (Gram-positive) | Morinda citrifolia | 25 µL | 13.6 ± 1.1 mm | 168 |
| Pterolobium hexapetalum | 50 µg mL−1 | 15 ± 0.29 mm | 242 | |
| Abutilon indicum | 5 mg | 15 ± 0.11 mm | 276 | |
| Allium sativum | 150 µg mL−1 | 10.90 ± 0.62 | 277 | |
| Portulaca oleracea | 200 µg mL−1 | 16.7 ± 0.6 mm | 278 | |
| Escherichia coli (Gram-negative) | Averrhoa carambola | 100 µg mL−1 | 24 mm | 6 |
| Ailanthus altissima | 100 µg mL−1 | 18 mm | 279 | |
| Malus domestica | 100 µg mL−1 | 17 mm | 280 | |
| Aerva javanica | 50 µg mL−1 | 5 ± 1 mm | 281 | |
| Ruellia tuberosa | 75 µg mL−1 | ∼11 mm | 282 | |
| Staphylococcus aureus (Gram-positive) | Averrhoa carambola | 100 µg mL−1 | 16 mm | 6 |
| Malus domestica | 100 µg mL−1 | 19 mm | 280 | |
| Ruellia tuberosa | 75 µg mL−1 | ∼18 mm | 282 | |
| Cardiospermum halicacabum | 900 µg mL−1 | 22.6 ± 1.5 mm | 283 | |
| Cordia sebestena | 1000 µg mL−1 | 24 mm | 284 | |
| Salmonella typhi (Gram-negative) | Averrhoa carambola | 100 µg mL−1 | 16 mm | 6 |
| Moringa oleifera | — | ∼12.5 mm | 285 | |
| Solanum melongena | 150 µg mL−1 | 15 mm | 286 | |
| Falcaria vulgaris | 0.5 mg mL−1 | 18 ± 0.4 mm | 287 | |
| Aloe vera | 1 mg mL−1 | 13 ± 0.02 mm | 288 | |
| Streptococcus pneumoniae (Gram-positive) | Psidium guajava | 80 µg mL−1 | ∼15 mm | 16 |
| Citrus reticulata | 50 µg mL−1 | 18 mm | 289 | |
| Citrus sinensis | 27 mm | |||
| Citrus limon | 17 mm | |||
| Antigonon leptopus | 50 µg/50 µL | 24 mm | 290 | |
| Klebsiella pneumonia (Gram-negative) | Azadirachta indica | — | ∼35 mm | 291 |
| Simmondsia chinensis | — | ∼25 mm | ||
| Mentha spicata | 100 µg mL−1 | 17.4 ± 0.6 mm | 292 | |
| Allium sativum | 150 µg mL−1 | 10.65 ± 0.63 mm | 277 | |
| Cassia fistula | 1 mg | 34 ± 1.59 mm | 293 | |
| Melia azedarach | 1 mg | 39 ± 2.00 mm | ||
| Staphylococcus epidermidis (Gram-positive) | Fumaria indica | 5 mg/5 mL | 3.98 mm | 294 |
| Zanthoxylum armatum | 100 µL | 11.66 ± 0.33 mm | 295 | |
| Zanthoxylum armatum | 19.66 ± 0.33 mm | |||
| Berberis lycium | 16.66 ± 0.33 mm | |||
| Momordica charantia | 1.25 mg/50 µL | 23 ± 2.0 mm | ||
| Pseudomonas aeruginosa (Gram-negative) | Adhatoda vasica | 1 mg mL−1 | 12 mm | 296 |
| Ficus religiosa | 1 mg mL−1 | 13 mm | 297 | |
| Leucas aspera | 500 µg | 10 mm | 298 | |
| Morinda tinctoria | 500 µg | 10 mm | ||
| Acanthospermum hispidum | 500 µg mL−1 | 19 mm | 299 | |
| Fungal strain | Plant source | Dosage | Antifungal efficiency | Ref. |
|---|---|---|---|---|
| a Zone of inhibition (ZOI). b Percentage of inhibition; the dosage for which the highest efficiency was found, was selected for inclusion. | ||||
| Aspergillus niger | Manilkara hexandraf | 10 µL mL−1 | 9 mm | 300 |
| Malus domestica | 0.05 mgL−1 | 92% | 301 | |
| Cissus quadrangularis | 1000 ppm | ∼85% | 302 | |
| Syzygium alternifolium | 40 µg mL−1 | ∼7 mm | 303 | |
| Tinospora cordifolia | 100 µg mL−1 | ∼11 mm | 304 | |
| Brassica oleracea | 100 µg mL−1 | 9 mm | 305 | |
| Bougainvillea | 5 mg mL−1 | ∼19 mm | 306 | |
| Eichhornia crassipes | 100 µg mL−1 | 18.33 ± 1 mm | 248 | |
| Candida albicans | Tinospora cordifolia | 100 µg mL−1 | ∼15 mm | 304 |
| Ephedra alata | 6 mg mL−1 | 16.2 mm | 307 | |
| Allium sativum | 150 µg mL−1 | 10.05 ± 0.63 mm | 277 | |
| Aspergillus flavus | Allium sativum | 150 µg mL−1 | 9.30 ± 0.58 mm | 277 |
| Spinacia oleracea | — | ∼14 mm | 308 | |
| Eichhornia crassipes | 100 µg mL−1 | ∼16 mm | 248 | |
| Cissus quadrangularis | 1000 ppm | ∼85% | 302 | |
| Fusarium oxysporium | Eichhornia crassipes | 100 µg mL−1 | ∼18 mm | 248 |
| Heliotropium bacciferum | 100 µg mL−1 | 71.11% | 309 | |
| Tamarix aphylla | 100 mg L−1 | 88% | 310 | |
| Aspergillus fumigatus | Allium sativum | 150 µg mL−1 | 9.95 ± 0.65 mm | 277 |
| Eichhornia crassipes | 100 µg mL−1 | ∼14 mm | 248 | |
| Saccharomyces cerevisiae | Ephedra alata | 6 mg mL−1 | 18.4 mm | 307 |
| Fusarium culmorum | Eichhornia crassipes | 100 µg mL−1 | ∼22 mm | 248 |
| Botrytis cinerea | Heliotropium bacciferum | 100 µg mL−1 | 50.74% | 309 |
| Rhizoctonia solani | Heliotropium bacciferum | 100 µg mL−1 | 81.48% | 309 |
10. Mechanism of the antimicrobial activity of PEM-CuO NPs
Recently, NPs have received immense attention for various biomedical applications, especially as antimicrobial agents. A broad spectrum of microorganisms have been reported to be susceptible to different NPs. CuO NPs have emerged as promising antimicrobial agents due to their salient physicochemical properties such as large surface area, small particle size, stability, electrostatic attraction, hydrophobic interactions and van der Waals forces. In addition, the abundance of copper makes it quite cheap to produce CuO NPs on a large scale. Trissa et al. synthesized CuO NPs using the leaf extract of Averrhoa carambola and investigated their antibacterial activity against Gram-positive and Gram-negative bacterial strains.6 Vijay et al. synthesized CuO NPs utilizing Aloe vera leaf extract and explored their bactericidal activity against fish pathogens.215 Both of the studies found prominent antimicrobial activity.While the precise mechanism for the antimicrobial activity of PEM-CuO NPs is elusive, several studies have proposed that the antimicrobial property is primarily attributed to the interaction of the NPs with the outer layer of the microbial cell wall. Some studies reported in the literature suggested that the Cu2+ ions released from CuO NPs bind to the negatively charged cell membrane due to electrostatic and van der Waals forces.216,217 This interaction eventually led to the formation of the pits by disrupting the integrity of the cell membrane. The produced Cu2+ enters the cell and damages the nucleic acid of DNA molecules as well as hampers cell processes such as enzyme activity and metabolism, which eventually lead to cellular breakdown. Other studies proposed that the generation of reactive oxygen species (ROS) is the primary initiator of antimicrobial activity. ROS can oxidize cell components by damaging DNA, proton efflux pumps and proteins. It can also cause enzyme breakdown, reduce the quantity of the antioxidant glutathione, and ultimately cause cell death.218–220
Another probable mechanism is the electrostatic adhesion of CuO NPs to the cell membrane of microbes. When CuO NPs get attached to the cell, the shape of the plasma membrane changes and causes the leakage of internal cellular components, resulting in cell death. Along with these proposed mechanisms, the size, shape, and concentration of NPs affect the antimicrobial activity significantly. Fig. 7 shows the probable mechanism of antimicrobial activity of CuO NPs. The antimicrobial mechanism involving membrane disruption and ROS generation is quite ubiquitous for both bacteria and fungi. However, the distinct cell wall structures and metabolic processes of bacteria and fungi tailor the mechanism differently. The bacterial cell wall is made up of peptidoglycan, while the fungal cell wall is composed of chitin and glucan. These differences in the cell wall structure affect the mode of action of CuO NPs. In the case of bactericidal activity, CuO NPs attached to the bacterial cell wall damage the cell structure and interact with DNA, causing cell damage and preventing cell replication. In contrast, for fungicidal activity, CuO NPs inhibit fungal growth by damaging the mycelial structure and interacting with fungal enzymes, which disrupt the cellular processes.
11. Challenges and limitations in PEM-CuO NPs synthesis
Although PEM-CuO NPs offer a sustainable as well as environment-friendly alternative to conventional methods, it also faces some challenges and limitations.11.1 Difficulty in controlling size-shape-morphology and reproducibility concerns
The most crucial challenge faced by such synthesis procedure is the difficulty in controlling the size, shape and morphology of the synthesized CuO NPs due to the complexity of the plant extracts.221 Plant extracts are complex mixtures of various biomolecules that play crucial roles in the synthesis CuO NPs by reducing metal ions, preventing aggregation, and controlling nanoparticle growth. However, the exact composition and concentration of these biomolecules can vary significantly depending on several factors such as environmental conditions during plant growth, temperature, light, water availability, and nutrient levels.Furthermore, extraction methods as well as the choice of solvents, temperatures, and extraction times can significantly impact the types and amounts of compounds extracted.164 This variability in composition can make it difficult to precisely control the size, shape, and morphology of the synthesized CuO NPs. One of the biggest challenges in reproducibility is that even minor changes in size, shape, or nanoparticle surface chemistry can significantly affect their stability, interactions with biological media, and overall biodistribution.222 Additionally, the complex interactions between different biomolecules and synthesis parameters pose challenges in optimizing the synthesis process, and the presence of residual organic molecules from the plant extract can further complicate the characterization of the resulting CuO NPs.223
11.2 Hurdles in optimizing the reaction parameters
The optimization of reaction parameters for the PEM NP synthesis is a monumental task as it requires careful control of several key parameters for achieving desired properties of NPs. For example, the precursor salt concentration of CuO NPs directly impacts the availability of copper ions for reduction. Higher concentrations can accelerate the nucleation and growth, which leads to the formation of larger particles. On the contrary, lower concentrations result in slower growth and smaller particles.5 Thus, balancing sufficient copper ions for NP formation with the risk of uncontrolled growth and aggregation is very crucial.The pH of the reaction mixture influences the redox potential of reducing agents in the plant extract and the surface charge of the resulting CuO NPs.224 This affects the reduction rate, NP stability, and morphology. Therefore, the adjustment of pH with acid and base requires careful consideration for specific plant extract and the desired CuO NP properties. Temperature significantly affects the reaction kinetics. Although it is understood that a higher temperature accelerates reduction, excessively high temperatures result in rapid and uncontrolled growth, yielding larger and less uniform particles.
The reaction time regulates the exposure duration of copper ions to the reducing agents. Insufficient time may lead to incomplete reduction, while excessive time can result in overgrowth or aggregation. Balancing the plant extract concentration is also essential for controlling size and preventing aggregation. Higher concentrations can accelerate reduction and improve stabilization but may also increase nucleation sites, potentially leading to smaller particles. The stirring rate ensures uniform reactant distribution and promotes efficient mixing, enhancing the uniformity of NP formation. Optimal stirring ensures proper mixing without excessive shear forces that could affect NP growth.225
11.3 Difficulty in mass production
Industrial production of PEM-CuO NPs is a very convoluted task due to challenges in maintaining consistent plant extract quality (due to natural variations), ensuring uniform mixing and reaction conditions in larger reactors, managing mass and heat transfer limitations, implementing sophisticated process control and monitoring, addressing economic factors, guaranteeing reproducibility, and complying with regulations. Controlling the inherently variable plant extracts, scaling reactor design, and maintaining consistent product quality at a larger volume are also the key hurdles to overcome for successful industrial production of CuO NPs using this green synthesis method.22611.4 Limitation in product lifespan and consistency
The PEM-CuO NPs face stability and shelf-life challenges due to residual organic matter, which may originate from the biomolecules in the plant extract. This residue can oxidize or degrade, resulting in an inconsistent production. Additionally, the inherent tendency of NPs to aggregate, undergo Ostwald ripening, and experience surface degradation, particularly in the case of CuO due to factors such as oxygen, moisture, and temperature, further degrades the material. The pH of the storage medium, the choice of solvent, and exposure to light also contribute to this problem. Preventive measures include thorough purification to remove organic residues, surface modification with stabilizing agents, controlled storage in inert atmospheres at low temperatures away from light, optimizing pH and solvent, and encapsulation within protective matrices can ensure better stability but upsurges the cost.22711.5 Toxicity and environmental safety concerns
Although the plant extract-mediated route for CuO NP synthesis eliminates the need for toxic substances as reducing or stabilizing agents, the CuO NPs themselves can still pose toxicity and environmental risks. CuO NPs in general can cause harm through oxidative stress, copper ion release, and direct physical interaction with cells. The PEM-CuO NPs raises concerns due to the presence of potentially toxic residual organic components and possible allergens coming from the plant extract. When compared with the chemically synthesized counterpart, the PEM-CuO NPs exhibit lower toxicity. The work of Saif et al. demonstrated that chemically synthesized CuO NPs show a much higher toxicity, with an EC50 value of 0.102 ± 0.019 mg L−1, while PEM-CuO NPs show an EC50 value of 0.69 ± 0.226 mg L−1, with more than five times lower toxicity.228 This difference may have caused by the plants producing stable NPs, where phenolic compounds such as flavonoids or tannins act as stabilizing and coating agents, which leads to lower dissolution of PEM-CuO NPs than chemically synthesized CuO NPs.To reduce the toxic effects of PEM-CuO NPs, optimizing the NP concentration, selection of appropriate dosage, considering real environmental conditions while measuring dissolution, encapsulation, and composite making with magnetic nanocomposites should be considered.229,230
12. Conclusion and future prospects
The future of PEM-CuO NP synthesis is promising, especially considering increasing environmental concerns. PEM-CuO NP synthesis offers a sustainable and environmentally friendly approach to nanomaterial production methodology, where plant materials act as reducing agents and limit the use of toxic chemicals needed in the conventional synthesis procedures. These NPs exhibit unique properties including small particle size, high surface area, stability, excellent catalytic activity and notable antimicrobial properties, making them suitable for various applications including dye degradation, water purification, drug delivery, antimicrobial activity and even tissue engineering.However, it is crucial to address the toxicological aspects of these NPs to ensure their safe applications in diversified fields. As there is no established evidence regarding their toxicity, further in vitro and in vivo research is necessary to understand the mechanisms behind any kind of adverse effects. Considering the potential functionalities of CuO NPs, it is imperative to prioritize safety by conducting thorough toxicological assessments. Researchers can focus on these areas to enhance the viability and safety of plant-based NP synthesis and ultimately contribute to the advancement of nanotechnology. Further, this review article can provide a fundamental understanding of plant-mediated NPs to aid in their research.
Data availability
This review article does not contain any original data generated or analyzed by the authors.Author contributions
M. Bin Mobarak: conceptualization, methodology, writing – original draft, review & editing, supervision; MD. F. Sikder: writing – original draft; K. Muntaha: writing – original draft; S. Islam: writing – original draft; S. Rabbi: writing – original draft; F. Chowdhury: supervision, writing – original draft, review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors greatly appreciate the support from Bangladesh Council of Scientific and Industrial Research (BCSIR) through R&D project (ref. no. 39.02.0000.011.14.157.2022/172; Date: 10.11.2022). We extend our heartfelt gratitude to Dr Samina Ahmed, Chairman of BCSIR, for her invaluable support and mentorship.References
- P. G. Bhavyasree and T. S. Xavier, Curr. Res. Green Sustain. Chem., 2022, 5, 100249 CrossRef CAS.
- A. K. Soni and R. K. Jha, Cureus, 2024, 16(4), e59234 Search PubMed.
- Q. Zhang, K. Zhang, D. Xu, G. Yang, H. Huang, F. Nie, C. Liu and S. Yang, Prog. Mater. Sci., 2014, 60, 208–337 CrossRef CAS.
- H. Zheng, J. Z. Ou, M. S. Strano, R. B. Kaner, A. Mitchell and K. Kalantar‐zadeh, Adv. Funct. Mater., 2011, 21, 2175–2196 CrossRef CAS.
- T. H. Tran and V. T. Nguyen, Int. Sch. Res. Not., 2014, 2014, 1–14 CrossRef.
- T. Saha, M. B. Mobarak, M. N. Uddin, M. S. Quddus, M. R. Naim and N. S. Pinky, Mater. Chem. Phys., 2023, 127979 CrossRef CAS.
- F. Chowdhury, M. B. Mobarak, M. Hakim, M. N. Uddin, M. S. Hossain, U. S. Akhter, D. Islam, S. Ahmed and H. Das, New J. Chem., 2024, 48, 17038–17051 RSC.
- B. Shaabani, E. Alizadeh-Gheshlaghi, Y. Azizian-Kalandaragh and A. Khodayari, Adv. Powder Technol., 2014, 25, 1043–1052 CrossRef CAS.
- J. O. Ighalo, P. A. Sagboye, G. Umenweke, O. J. Ajala, F. O. Omoarukhe, C. A. Adeyanju, S. Ogunniyi and A. G. Adeniyi, Environ. Nanotechnol. Monit. Manag., 2021, 15, 100443 CAS.
- A. F. Zedan, A. T. Mohamed, M. S. El-Shall, S. Y. AlQaradawi and A. S. AlJaber, RSC Adv., 2018, 8, 19499–19511 RSC.
- D. M. Chethana, T. C. Thanuja, H. M. Mahesh, M. S. Kiruba, A. S. Jose, H. C. Barshilia and J. Manjanna, Ceram. Int., 2021, 47, 10381–10387 CrossRef CAS.
- F. Peng, Y. Sun, Y. Lu, W. Yu, M. Ge, J. Shi, R. Cong, J. Hao and N. Dai, Nanomaterials, 2020, 10, 774 CrossRef CAS PubMed.
- V. Senthilkumar, Y. S. Kim, S. Chandrasekaran, B. Rajagopalan, E. J. Kim and J. S. Chung, RSC Adv., 2015, 5, 20545–20553 RSC.
- S. Naz, A. Gul, M. Zia and R. Javed, Appl. Microbiol. Biotechnol., 2023, 107, 1039–1061 CrossRef CAS PubMed.
- H. N. Cuong, S. Pansambal, S. Ghotekar, R. Oza, N. T. T. Hai, N. M. Viet and V.-H. Nguyen, Environ. Res., 2021, 111858 Search PubMed.
- S. Sathiyavimal, S. Vasantharaj, V. Veeramani, M. Saravanan, G. Rajalakshmi, T. Kaliannan, F. A. Al-Misned and A. Pugazhendhi, J. Environ. Chem. Eng., 2021, 9, 105033 CrossRef CAS.
- W. W. Andualem, F. K. Sabir, E. T. Mohammed, H. H. Belay and B. A. Gonfa, J. Nanotechnol., 2020, 2020(1), 2932434 Search PubMed.
- G. Kalaiyan, S. Suresh, K. M. Prabu, S. Thambidurai, M. Kandasamy, N. Pugazhenthiran, S. K. Kumar and T. Muneeswaran, J. Environ. Chem. Eng., 2021, 9, 104847 CrossRef CAS.
- Z. Alhalili, Arab. J. Chem., 2022, 15, 103739 CrossRef CAS.
- M. I. Said and A. A. Othman, RSC Adv., 2021, 11, 37801–37813 RSC.
- S. Aroob, S. A. Carabineiro, M. B. Taj, I. Bibi, A. Raheel, T. Javed, R. Yahya, W. Alelwani, F. Verpoort and K. Kamwilaisak, Catalysts, 2023, 13, 502 CrossRef CAS.
- N. Verma and N. Kumar, ACS Biomater. Sci. Eng., 2019, 5, 1170–1188 CrossRef CAS PubMed.
- S. Bayda, M. Adeel, T. Tuccinardi, M. Cordani and F. Rizzolio, Molecules, 2019, 25, 112 CrossRef PubMed.
- J. Jeevanandam, A. Barhoum, Y. S. Chan, A. Dufresne and M. K. Danquah, Beilstein J. Nanotechnol., 2018, 9, 1050–1074 CrossRef CAS PubMed.
- M. Vert, Y. Doi, K.-H. Hellwich, M. Hess, P. Hodge, P. Kubisa, M. Rinaudo and F. Schué, Pure Appl. Chem., 2012, 84, 377–410 CrossRef CAS.
- M. Sajid and J. Płotka-Wasylka, Microchem. J., 2020, 154, 104623 CrossRef CAS.
- I. Khan, K. Saeed and I. Khan, Arab. J. Chem., 2019, 12, 908–931 CrossRef CAS.
- ScoEaNIHR ES and European Commission, Scientific Committee on Emerging and Newly Identified Health Risks (SCENHR), 2010 Search PubMed.
- N. Baig, I. Kammakakam and W. Falath, Mater. Adv., 2021, 2, 1821–1871 RSC.
- M. Puolamaa, Risk Assessment Methodologies for Nanotechnologies, European Asylum Support Office, Malta, 2006 Search PubMed.
- N. Joudeh and D. Linke, J. Nanobiotechnol., 2022, 20, 262 CrossRef PubMed.
- S. A. M. Ealia and M. P. Saravanakumar, in IOP Conference Series: Materials Science and Engineering, IOP Publishing, 2017, vol. 263, p. 032019 Search PubMed.
- K. Schwirn, L. Tietjen and I. Beer, Environ. Sci. Eur., 2014, 26, 1–9 CrossRef.
- M. Asoro, J. Damiano and P. J. Ferreira, Microsc. Microanal., 2009, 15, 706–707 CrossRef.
- A. B. Asha and R. Narain, in Polymer Science and Nanotechnology, Elsevier, 2020, pp. 343–359 Search PubMed.
- W. A. A. Mohamed, H. Abd El-Gawad, S. Mekkey, H. Galal, H. Handal, H. Mousa and A. Labib, Nanotechnol. Rev., 2021, 10, 1926–1940 CrossRef CAS.
- M. Sui, S. Kunwar, P. Pandey and J. Lee, Sci. Rep., 2019, 9, 16582 CrossRef PubMed.
- J. Krajczewski, K. Kołątaj and A. Kudelski, RSC Adv., 2017, 7, 17559–17576 RSC.
- M. Singh, M. Goyal and K. Devlal, J. Taibah Univ. Sci., 2018, 12, 470–475 CrossRef.
- S. A. Afolalu, S. B. Soetan, S. O. Ongbali, A. A. Abioye and A. S. Oni, in IOP Conference Series: Materials Science and Engineering, IOP Publishing, 2019, vol. 640, p. 012065 Search PubMed.
- B. Mekuye and B. Abera, Nano Select, 2023, 4, 486–501 CrossRef CAS.
- M. Niederberger, Acc. Chem. Res., 2007, 40, 793–800 CrossRef CAS PubMed.
- K. Phiwdang, S. Suphankij, W. Mekprasart and W. Pecharapa, Energy Proc., 2013, 34, 740–745 CrossRef CAS.
- J. Kimling, M. Maier, B. Okenve, V. Kotaidis, H. Ballot and A. Plech, J. Phys. Chem. B, 2006, 110, 15700–15707 CrossRef CAS PubMed.
- M. Adachi, S. Tsukui and K. Okuyama, Jpn. J. Appl. Phys., 2003, 42, L77 CrossRef CAS.
- L. M. Cafiero, G. Baffi, A. Chianese and R. J. J. Jachuck, Ind. Eng. Chem. Res., 2002, 41, 5240–5246 CrossRef CAS.
- R. D'Amato, M. Falconieri, S. Gagliardi, E. Popovici, E. Serra, G. Terranova and E. Borsella, J. Anal. Appl. Pyrolysis, 2013, 104, 461–469 CrossRef.
- C. Suryanarayana and B. Prabhu, in Nanostructured Materials, Elsevier, 2007, pp. 47–90 Search PubMed.
- H. Xu, B. W. Zeiger and K. S. Suslick, Chem. Soc. Rev., 2013, 42, 2555–2567 RSC.
- F. Trivinho-Strixino, J. S. Santos and M. S. Sikora, in Nanostructures, Elsevier, 2017, pp. 53–103 Search PubMed.
- C. L. Keat, A. Aziz, A. M. Eid and N. A. Elmarzugi, Bioresour. Bioprocess., 2015, 2, 47 CrossRef.
- X. Fu, J. Cai, X. Zhang, W.-D. Li, H. Ge and Y. Hu, Adv. Drug Deliv. Rev., 2018, 132, 169–187 CrossRef CAS PubMed.
- T. P. Yadav, R. M. Yadav and D. P. Singh, Nanosci. Nanotechnol., 2012, 2, 22–48 CrossRef.
- K. Xu and J. Chen, Appl. Nanosci., 2020, 10, 1013–1022 CrossRef CAS.
- V. Garg, R. G. Mote and J. Fu, Appl. Surf. Sci., 2020, 526, 146644 CrossRef CAS.
- V. Amendola and M. Meneghetti, Phys. Chem. Chem. Phys., 2009, 11, 3805–3821 RSC.
- M. Kim, S. Osone, T. Kim, H. Higashi and T. Seto, KONA Powder Part. J., 2017, 34, 80–90 CrossRef CAS.
- N. G. Semaltianos, Crit. Rev. Solid State Mater. Sci., 2010, 35, 105–124 CrossRef CAS.
- P. Ayyub, R. Chandra, P. Taneja, A. K. Sharma and R. Pinto, Appl. Phys. A, 2001, 73, 67–73 CrossRef CAS.
- M. Nie, K. Sun and D. D. Meng, J. Appl. Phys., 2009, 106(5), 054314 CrossRef.
- J. Liang, Q. Liu, T. Li, Y. Luo, S. Lu, X. Shi, F. Zhang, A. M. Asiri and X. Sun, Green Chem., 2021, 23, 2834–2867 RSC.
- H. Wender, P. Migowski, A. F. Feil, S. R. Teixeira and J. Dupont, Coord. Chem. Rev., 2013, 257, 2468–2483 CrossRef CAS.
- W. E. Teo and S. Ramakrishna, Nanotechnology, 2006, 17, R89 CrossRef CAS PubMed.
- F. Fadil, N. D. N. Affandi, M. I. Misnon, N. N. Bonnia, A. M. Harun and M. K. Alam, Polymers, 2021, 13, 2087 CrossRef CAS PubMed.
- A. Nadaf, A. Gupta, N. Hasan, S. Ahmad, P. Kesharwani and F. J. Ahmad, RSC Adv., 2022, 12, 23808–23828 RSC.
- M. M. Montemore, M. A. Van Spronsen, R. J. Madix and C. M. Friend, Chem. Rev., 2018, 118, 2816–2862 CrossRef PubMed.
- U. O. Aigbe and A. O. Osibote, J. Hazard. Mater. Adv., 2024, 100401 Search PubMed.
- R. Kumar, G. R. Pulikanti, K. R. Shankar, D. Rambabu, V. Mangili, L. R. Kumbam, P. S. Sagara, N. Nakka and M. Yogesh, in Metal Oxides for Biomedical and Biosensor Applications, Elsevier, 2022, pp. 205–231 Search PubMed.
- Z. Quan, E. Ni, S. Hayashi and N. Sonoyama, J. Mater. Chem. A, 2013, 1, 8848–8856 RSC.
- C. An, Y. Zhang, H. Guo and Y. Wang, Nanoscale Adv., 2019, 1, 4644–4658 RSC.
- S. Akin and S. Sonmezoglu, in Emerging Materials for Energy Conversion and Storage, Elsevier, 2018, pp. 39–79 Search PubMed.
- S. Murthy, P. Effiong and C. C. Fei, in Metal Oxide Powder Technologies, Elsevier, 2020, pp. 233–251 Search PubMed.
- Y.-F. Sun, S.-B. Liu, F.-L. Meng, J.-Y. Liu, Z. Jin, L.-T. Kong and J.-H. Liu, Sensors, 2012, 12, 2610–2631 CrossRef CAS PubMed.
- J. C. Védrine, Catalysts, 2017, 7, 341 CrossRef.
- A. Raghunath and E. Perumal, Int. J. Antimicrob. Agents, 2017, 49, 137–152 CrossRef CAS PubMed.
- M. S. Chavali and M. P. Nikolova, SN Appl. Sci., 2019, 1, 1–30 Search PubMed.
- F. Verbakel, S. C. J. Meskers, D. M. De Leeuw and R. A. J. Janssen, J. Phys. Chem. C, 2008, 112, 5254–5257 CrossRef CAS.
- A. M. Negrescu, M. S. Killian, S. N. Raghu, P. Schmuki, A. Mazare and A. Cimpean, J. Funct. Biomater., 2022, 13, 274 Search PubMed.
- Z. Alhalili, Molecules, 2023, 28, 3086 CrossRef CAS PubMed.
- H. Tamura, K. Mita, A. Tanaka and M. Ito, J. Colloid Interface Sci., 2001, 243, 202–207 CrossRef CAS.
- E. Y. Shaba, J. O. Jacob, J. O. Tijani and M. A. T. Suleiman, Appl. Water Sci., 2021, 11, 48 CrossRef CAS.
- F. Islam, S. Shohag, M. J. Uddin, M. R. Islam, M. H. Nafady, A. Akter, S. Mitra, A. Roy, T. B. Emran and S. Cavalu, Materials, 2022, 15, 2160 CrossRef CAS PubMed.
- J. Wang, Z. Wang, W. Wang, Y. Wang, X. Hu, J. Liu, X. Gong, W. Miao, L. Ding and X. Li, Nanoscale, 2022, 14, 6709–6734 RSC.
- M. A. Irshad, R. Nawaz, M. Z. Ur Rehman, M. Adrees, M. Rizwan, S. Ali, S. Ahmad and S. Tasleem, Ecotoxicol. Environ. Saf., 2021, 212, 111978 CrossRef CAS PubMed.
- A. V. Samrot, C. S. Sahithya, J. Selvarani, S. K. Purayil and P. Ponnaiah, Curr. Res. Green Sustain. Chem., 2021, 4, 100042 CrossRef CAS.
- N. Ajinkya, X. Yu, P. Kaithal, H. Luo, P. Somani and S. Ramakrishna, Materials, 2020, 13, 4644 CAS.
- S. V. Gudkov, D. E. Burmistrov, V. V. Smirnova, A. A. Semenova and A. B. Lisitsyn, Nanomaterials, 2022, 12, 2635 CAS.
- R. C. Congreve, C. P. Quezada and V. Kokkarachedu, in Nanoparticles in Modern Antimicrobial and Antiviral Applications, ed. V. Kokkarachedu and R. Sadiku, Springer International Publishing, Cham, 2024, pp. 265–288 Search PubMed.
- M. Rahmati and M. Mozafari, J. Cell. Physiol., 2019, 234, 3321–3335 CAS.
- C. Y. Rahimzadeh, A. A. Barzinjy, A. S. Mohammed and S. M. Hamad, PLoS One, 2022, 17, e0268184 CrossRef CAS PubMed.
- A. Rodriguez-Otero, V. Vargas, A. Galarneau, J. Castillo, J. H. Christensen and B. Bouyssiere, Processes, 2023, 11, 3373 CrossRef CAS.
- R. S. Dubey, Y. Rajesh and M. A. More, Mater. Today: Proc., 2015, 2, 3575–3579 CAS.
- S. Sagadevan, S. Vennila, A. R. Marlinda, Y. Al-Douri, M. Rafie Johan and J. Anita Lett, Appl. Phys. A, 2019, 125, 489 CrossRef CAS.
- S. Anandan and S. Yang, J. Exp. Nanosci., 2007, 2, 23–56 CrossRef CAS.
- O. Lupan, V. Postica, V. Cretu, N. Wolff, V. Duppel, L. Kienle and R. Adelung, Phys. Status Solidi Rapid Res. Lett., 2016, 10, 260–266 CrossRef CAS.
- C. Wang, D. Higgins, F. Wang, D. Li, R. Liu, G. Xia, N. Li, Q. Li, H. Xu and G. Wu, Nano Energy, 2014, 9, 334–344 CrossRef CAS.
- M. K. Song, S. Park, F. M. Alamgir, J. Cho and M. Liu, Mater. Sci. Eng., R, 2011, 72, 203–252 CrossRef.
- M. H. Saleem, U. Ejaz, M. Vithanage, N. Bolan and K. H. M. Siddique, Clean Technol. Environ. Policy, 2024, 1–26 CAS.
- L.-B. Luo, X.-H. Wang, C. Xie, Z.-J. Li, R. Lu, X.-B. Yang and J. Lu, Nanoscale Res. Lett., 2014, 9, 637 CrossRef PubMed.
- H. Xu, W. Wang, W. Zhu, L. Zhou and M. Ruan, Cryst. Growth Des., 2007, 7, 2720–2724 CrossRef CAS.
- S. K. Shinde, D. P. Dubal, G. S. Ghodake and V. J. Fulari, RSC Adv., 2015, 5, 4443–4447 RSC.
- J. C. Park, J. Kim, H. Kwon and H. Song, Adv. Mater., 2009, 21, 803–807 CrossRef CAS.
- R. Bunea, A. K. Saikumar and K. Sundaram, Mater. Sci. Appl., 2021, 12, 315–329 CAS.
- T. Ito, H. Yamaguchi, K. Okabe and T. Masumi, J. Mater. Sci., 1998, 33, 3555–3566 CrossRef CAS.
- D. Su, X. Xie, S. Dou and G. Wang, Sci. Rep., 2014, 4, 5753 CrossRef CAS PubMed.
- S. Åsbrink and L.-J. Norrby, Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem., 1970, 26, 8–15 CrossRef.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- A. Waris, M. Din, A. Ali, M. Ali, S. Afridi, A. Baset and A. U. Khan, Inorg. Chem. Commun., 2021, 123, 108369 CrossRef CAS.
- M. I. Amal, J. T. Wibowo, L. Nuraini, G. Senopati, M. Y. Hasbi and G. Priyotomo, in IOP Conference Series: Materials Science and Engineering, IOP Publishing, 2019, vol. 578, p. 012039 Search PubMed.
- E. Ayoman and S. G. Hosseini, J. Therm. Anal. Calorim., 2016, 123, 1213–1224 CrossRef CAS.
- K. S. Khashan, G. M. Sulaiman and F. A. Abdulameer, Arab. J. Sci. Eng., 2016, 41, 301–310 CrossRef CAS.
- R. E. Russo, X. Mao, H. Liu, J. Gonzalez and S. S. Mao, Talanta, 2002, 57, 425–451 Search PubMed.
- H. Zeng, X. Du, S. C. Singh, S. A. Kulinich, S. Yang, J. He and W. Cai, Adv. Funct. Mater., 2012, 22, 1333–1353 CrossRef CAS.
- M. Censabella, V. Iacono, A. Scandurra, K. Moulaee, G. Neri, F. Ruffino and S. Mirabella, Sensor. Actuator. B Chem., 2022, 358, 131489 CrossRef CAS.
- Y.-C. Liang and T.-H. Li, Nanomaterials, 2022, 12, 2634 CrossRef CAS PubMed.
- M. Verma, V. Kumar and A. Katoch, Mater. Sci. Semicond. Process., 2018, 76, 55–60 CAS.
- R. Al-Gaashani, S. Radiman, N. Tabet and A. R. Daud, J. Alloys Compd., 2011, 509, 8761–8769 CAS.
- C. Xu, Y. Liu, G. Xu and G. Wang, Mater. Res. Bull., 2002, 37, 2365–2372 CAS.
- M. E. Grigore, E. R. Biscu, A. M. Holban, M. C. Gestal and A. M. Grumezescu, Pharmaceuticals, 2016, 9, 75 Search PubMed.
- F. Wang, H. Li, Z. Yuan, Y. Sun, F. Chang, H. Deng, L. Xie and H. Li, RSC Adv., 2016, 6, 79343–79349 CAS.
- J. Jayaprakash, N. Srinivasan and P. Chandrasekaran, Spectrochim. Acta Mol. Biomol. Spectrosc., 2014, 123, 363–368 CAS.
- A. A. Gvozdenko, S. A. Siddiqui, A. V. Blinov, A. B. Golik, A. A. Nagdalian, D. G. Maglakelidze, E. N. Statsenko, M. A. Pirogov, A. A. Blinova and M. N. Sizonenko, Sci. Rep., 2022, 12, 12843 CAS.
- R. Javed, M. Zia, S. Naz, S. O. Aisida, N. ul Ain and Q. Ao, J. Nanobiotechnol., 2020, 18, 1–15 Search PubMed.
- M. Zhang, X. Xu and M. Zhang, Mater. Lett., 2008, 62, 385–388 CAS.
- J. G. Zhao, S. J. Liu, S. H. Yang and S. G. Yang, Appl. Surf. Sci., 2011, 257, 9678–9681 CAS.
- T. Wang and Q. Xiao, Mater. Chem. Phys., 2013, 139, 603–608 CAS.
- M. Gopalakrishnan and A. K. S. Jeevaraj, Mater. Sci. Semicond. Process., 2014, 26, 512–515 CrossRef CAS.
- A. Kumar, Y. Kuang, Z. Liang and X. Sun, Mater. Today Nano, 2020, 11, 100076 CrossRef.
- A. Jung, S. Cho, W. J. Cho and K.-H. Lee, Korean J. Chem. Eng., 2012, 29, 243–248 CrossRef CAS.
- M. Zhang, X. Xu and M. Zhang, J. Dispersion Sci. Technol., 2008, 29, 508–513 CrossRef CAS.
- S. Honary, E. Gharaei-Fathabad, H. Barabadi and F. Naghibi, J. Nanosci. Nanotechnol., 2013, 13, 1427–1430 CrossRef CAS PubMed.
- A. V Singh, R. Patil, A. Anand, P. Milani and W. N. Gade, Curr. Nanosci., 2010, 6, 365–369 CrossRef.
- H. R. Ghorbani, F. P. Mehr and A. K. Poor, Orient. J. Chem., 2015, 31, 527–529 CrossRef.
- R. Cuevas, N. Durán, M. C. Diez, G. R. Tortella and O. Rubilar, J. Nanomater., 2015, 2015, 789089 CrossRef.
- S. V. P. Ramaswamy, S. Narendhran and R. Sivaraj, Bull. Mater. Sci., 2016, 39, 361–364 CrossRef CAS.
- T. Mustapha, N. Misni, N. R. Ithnin, A. M. Daskum and N. Z. Unyah, Int. Res. J. Publ. Environ. Health, 2022, 19, 674 Search PubMed.
- P. Rauwel, S. Küünal, S. Ferdov and E. Rauwel, Adv. Mater. Sci. Eng., 2015, 2015, 1–9 Search PubMed.
- S. Antunes Filho, M. S. Dos Santos, O. A. L. Dos Santos, B. P. Backx, M.-L. Soran, O. Opriş, I. Lung, A. Stegarescu and M. Bououdina, Molecules, 2023, 28, 3060 CrossRef CAS PubMed.
- A. Azad, H. Zafar, F. Raza and M. Sulaiman, Pharm. Fronts., 2023, 5, e117–e131 CrossRef.
- T. Mustapha, N. Misni, N. R. Ithnin, A. M. Daskum and N. Z. Unyah, Int. J. Environ. Res. Publ. Health, 2022, 19, 674 CrossRef CAS PubMed.
- M. Asemani and N. Anarjan, Green Process. Synth., 2019, 8, 557–567 CAS.
- R. Sivaraj, P. K. Rahman, P. Rajiv, S. Narendhran and R. Venckatesh, Spectrochim. Acta Mol. Biomol. Spectrosc., 2014, 129, 255–258 CrossRef CAS PubMed.
- P. Narasaiah, B. K. Mandal and N. C. Sarada, in IOP Conference Series: Materials Science and Engineering, IOP Publishing, 2017, vol. 263, p. 022012 Search PubMed.
- M. A. Gacem and K. A. Abd-Elsalam, in Green Synthesis of Silver Nanomaterials, ed. K. A. Abd-Elsalam, Elsevier, 2022, pp. 669–698 Search PubMed.
- V. Selvanathan, M. Aminuzzaman, L.-H. Tey, S. A. Razali, K. Althubeiti, H. I. Alkhammash, S. K. Guha, S. Ogawa, A. Watanabe and M. Shahiduzzaman, Materials, 2021, 14, 6379 Search PubMed.
- M. Bandeira, M. Giovanela, M. Roesch-Ely, D. M. Devine and J. da Silva Crespo, Sustain. Chem. Pharm., 2020, 15, 100223 Search PubMed.
- O. J. Nava, P. A. Luque, C. M. Gómez-Gutiérrez, A. R. Vilchis-Nestor, A. Castro-Beltrán, M. L. Mota-González and A. Olivas, J. Mol. Struct., 2017, 1134, 121–125 CrossRef CAS.
- M. Fazlzadeh, R. Khosravi and A. Zarei, Ecol. Eng., 2017, 103, 180–190 Search PubMed.
- N. Matinise, X. G. Fuku, K. Kaviyarasu, N. Mayedwa and M. Maaza, Appl. Surf. Sci., 2017, 406, 339–347 Search PubMed.
- A. K. Singh, P. Pal, V. Gupta, T. P. Yadav, V. Gupta and S. P. Singh, Mater. Chem. Phys., 2018, 203, 40–48 Search PubMed.
- P. Nagore, S. Ghotekar, K. Mane, A. Ghoti, M. Bilal and A. Roy, BioNanoSci, 2021, 11, 579–589 Search PubMed.
- P. C. Nagajyothi, P. Muthuraman, T. V. M. Sreekanth, D. H. Kim and J. Shim, Arab. J. Chem., 2017, 10, 215–225 CrossRef CAS.
- P. Sutradhar, M. Saha and D. Maiti, J. Nanostruct. Chem., 2014, 4, 86 CrossRef.
- H. Veisi, B. Karmakar, T. Tamoradi, S. Hemmati, M. Hekmati and M. Hamelian, Sci. Rep., 2021, 11, 1983 CrossRef CAS PubMed.
- Y. B. Chan, V. Selvanathan, L.-H. Tey, M. Akhtaruzzaman, F. H. Anur, S. Djearamane, A. Watanabe and M. Aminuzzaman, Nanomaterials, 2022, 12, 3589 CrossRef CAS PubMed.
- M. Bin Mobarak, Md. S. Hossain, F. Chowdhury and S. Ahmed, Arab. J. Chem., 2022, 15, 104117 CrossRef CAS.
- Y. J. Wong, H. Subramaniam, L. Shing Wong, A. C. T. A. Dhanapal, Y. B. Chan, M. Aminuzzaman, L.-H. Tey, A. K. Janakiraman, S. Kayarohanam and S. Djearamane, Green Process. Synth., 2024, 13, 20240164 CrossRef.
- S. Bhattacharjee, J. Contr. Release, 2016, 235, 337–351 CrossRef CAS PubMed.
- M. M. Modena, B. Rühle, T. P. Burg and S. Wuttke, Adv. Mater., 2019, 31, 1901556 Search PubMed.
- Z. T. Khodair, M. W. M. Alzubaidy, A. M. S. Almohaidi, A. A. Sultan, S. M. H. AL-Shimmary and S. S. Albusultan, AIP Conf. Proc., 2019, 2190, 020006 CrossRef CAS.
- S. Dagher, Y. Haik, A. I. Ayesh and N. Tit, J. Lumin., 2014, 151, 149–154 Search PubMed.
- K. R. Reddy, J. Mol. Struct., 2017, 1150, 553–557 CrossRef CAS.
- T. Achamo, E. A. Zereffa, H. C. A. Murthy, V. P. Ramachandran and R. Balachandran, Green Chem. Lett. Rev., 2022, 15, 598–614 CrossRef CAS.
- D. Letchumanan, S. P. Sok, S. Ibrahim, N. H. Nagoor and N. M. Arshad, Biomolecules, 2021, 11, 564 CrossRef CAS PubMed.
- A. Antonio-Pérez, L. F. Durán-Armenta, M. G. Pérez-Loredo and A. L. Torres-Huerta, Micromachines, 2023, 14, 1882 CrossRef PubMed.
- S. A. Akintelu, A. S. Folorunso, F. A. Folorunso and A. K. Oyebamiji, Heliyon, 2020, 6, e04508 CrossRef PubMed.
- K. M. Rajesh, B. Ajitha, Y. Ashok Kumar Reddy, Y. Suneetha and P. Sreedhara Reddy, Mater. Today: Proc., 2016, 3, 1985–1991 Search PubMed.
- M. Priya, R. Venkatesan, S. Deepa, S. S. Sana, S. Arumugam, A. M. Karami, A. A. Vetcher and S.-C. Kim, Sci. Rep., 2023, 13, 18838 CrossRef CAS PubMed.
- A. Joshi, A. Sharma, R. K. Bachheti, A. Husen and V. K. Mishra, in Nanomaterials and Plant Potential, ed. A. Husen and M. Iqbal, Springer International Publishing, Cham, 2019, pp. 221–237 Search PubMed.
- K. Rajendran and S. Sen, J. Photochem. Photobiol. B Biol., 2016, 159, 82–87 CrossRef CAS PubMed.
- G. M. Sulaiman, A. T. Tawfeeq and M. D. Jaaffer, Biotechnol. Prog., 2018, 34, 218–230 CrossRef CAS PubMed.
- K. Ali, Q. Saquib, B. Ahmed, M. A. Siddiqui, J. Ahmad, M. Al-Shaeri, A. A. Al-Khedhairy and J. Musarrat, Process Biochem., 2020, 91, 387–397 CrossRef CAS.
- S. Taghavi Fardood, A. Ramazani, P. A. Asiabi and S. W. Joo, J. Struct. Chem., 2018, 59, 1737–1743 CrossRef CAS.
- S. T. Fardood and A. Ramazani, J. Appl. Chem. Res, 2018, 12, 8–15 Search PubMed.
- J. Sackey, A. C. Nwanya, A. K. H. Bashir, N. Matinise, J. B. Ngilirabanga, A. E. Ameh, E. Coetsee and M. Maaza, Mater. Chem. Phys., 2020, 244, 122714 CrossRef CAS.
- J. K. Patra and K.-H. Baek, J. Nanomater., 2014, 2014, 417305 CrossRef.
- M. I. Din, F. Arshad, A. Rani, A. Aihetasham, M. Mukhtar and H. Mehmood, Biomed. Mater., 2017, 9, 41–48 Search PubMed.
- Y. B. Chan, M. Aminuzzaman, L.-H. Tey, Y. F. Win, A. Watanabe, S. Djearamame and M. Akhtaruzzaman, Materials, 2023, 16, 5421 CrossRef CAS PubMed.
- H. Siddiqui, M. S. Qureshi and F. Z. Haque, Optik, 2016, 127, 4726–4730 CrossRef CAS.
- A. Al-Yunus, W. Al-Arjan, H. Traboulsi, R. Schuarca, P. Chando, I. D. Hosein and M. Hessien, Nanomaterials, 2024, 14, 308 CrossRef CAS PubMed.
- M. B. Mobarak, M. S. Hossain, Z. Yeasmin, M. Mahmud, M. M. Rahman, S. Sultana, S. M. Masum and S. Ahmed, J. Mol. Struct., 2022, 1252, 132142 CrossRef.
- M. B. Mobarak, N. S. Pinky, F. Chowdhury, M. S. Hossain, M. Mahmud, M. S. Quddus, S. A. Jahan and S. Ahmed, J. Saudi Chem. Soc., 2023, 101690 CrossRef.
- D. A. Yaseen and M. Scholz, Int. J. Environ. Sci. Technol., 2019, 16, 1193–1226 CrossRef CAS.
- S. Velusamy, A. Roy, S. Sundaram and T. Kumar Mallick, Chem. Rec., 2021, 21, 1570–1610 CrossRef CAS PubMed.
- B. Abebe, H. C. A. Murthy and E. Amare, J. Encapsulation Adsorpt. Sci., 2018, 8, 225–255 CrossRef CAS.
- G. Z. Kyzas, M. Kostoglou, N. K. Lazaridis, D. A. Lambropoulou and D. N. Bikiaris, Chem. Eng. J., 2013, 222, 248–258 CrossRef CAS.
- J. O. Ighalo, P. A. Sagboye, G. Umenweke, O. J. Ajala, F. O. Omoarukhe, C. A. Adeyanju, S. Ogunniyi and A. G. Adeniyi, Environ. Nanotechnol. Monit. Manag., 2021, 15, 100443 CAS.
- M. Manyangadze, N. H. M. Chikuruwo, T. B. Narsaiah, C. S. Chakra, M. Radhakumari and G. Danha, S. Afr. J. Chem. Eng., 2020, 31, 25–32 Search PubMed.
- R. Kumar, J. Kaur, M. Rawat, A. A. Alarfaj, R. Acevedo, M. Cascione, V. De Matteis and J. Singh, Hum. Ecol. Risk Assess., 2023, 29, 927–937 CrossRef CAS.
- F. Manole, P. Marian, G. M. Mekeres and A. N. Csep, Pharmacophore, 2023, 14, 106–110 CrossRef.
- M. C. Néstor and C. Mariana, in Ecopharmacovigilance: Multidisciplinary Approaches to Environmental Safety of Medicines, ed. L. M. Gómez-Oliván, Springer International Publishing, Cham, 2019, pp. 235–253 Search PubMed.
- C. B. Patneedi and K. D. Prasadu, Rasayan J. Chem., 2015, 8, 67–70 CAS.
- M. Camotti Bastos, M. Soubrand, T. Le Guet, É. Le Floch, E. Joussein, M. Baudu and M. Casellas, Geoderma, 2020, 375, 114498 CrossRef CAS.
- F. Quddus, A. Shah, F. J. Iftikhar, N. S. Shah and A. Haleem, Catalysts, 2023, 13, 511 CrossRef CAS.
- S. Singh, V. Kumar, A. G. Anil, D. Kapoor, S. Khasnabis, S. Shekar, N. Pavithra, J. Samuel, S. Subramanian and J. Singh, J. Environ. Manage., 2021, 300, 113569 CrossRef CAS PubMed.
- S. Latif, A. Liaqat, M. Imran, A. Javaid, N. Hussain, T. Jesionowski and M. Bilal, Environ. Res., 2023, 216, 114500 CrossRef CAS PubMed.
- N. Dhiman, Waste Manage. Bull., 2024, 2, 216–228 CrossRef.
- Y.-K. Phang, M. Aminuzzaman, M. Akhtaruzzaman, G. Muhammad, S. Ogawa, A. Watanabe and L.-H. Tey, Sustainability, 2021, 13, 796 CrossRef CAS.
- Y. B. Chan, M. Aminuzzaman, Y. F. Win, S. Djearamane, L. S. Wong, S. K. Guha, H. Almohammadi, M. Akhtaruzzaman and L.-H. Tey, Catalysts, 2024, 14, 486 CrossRef CAS.
- A. Bhattacharjee and M. Ahmaruzzaman, RSC Adv., 2016, 6, 41348–41363 RSC.
- H. Akhter, S. Sarker Ritu, S. Siddique, F. Chowdhury, R. Tasmiyah Chowdhury, S. Akhter and M. Hakim, RSC Adv., 2024, 14, 36209–36225 RSC.
- M. Ganeshbabu, J. S. Priya, G. M. Manoj, N. P. N. Puneeth, C. Shobana, H. Shankar and R. K. Selvan, Int. J. Biol. Macromol., 2023, 253, 127027 CrossRef CAS PubMed.
- M. Chauhan, N. Kaur, P. Bansal, R. Kumar, S. Srinivasan and G. R. Chaudhary, J. Nanomater., 2020, 2020, 6123178 Search PubMed.
- X. Hu, X. Hu, Q. Peng, L. Zhou, X. Tan, L. Jiang, C. Tang, H. Wang, S. Liu, Y. Wang and Z. Ning, Chem. Eng. J., 2020, 380, 122366 CrossRef CAS.
- F. El-Sayed, M. S. A. Hussien, M. I. Mohammed, V. Ganesh, T. H. AlAbdulaal, H. Y. Zahran, I. S. Yahia, H. H. Hegazy, M. S. Abdel-wahab, M. Shkir, S. Valarasu and M. A. Ibrahim, Nanomaterials, 2022, 12, 1060 CrossRef CAS PubMed.
- A. R. Nyachhyon and G. Neupane, Sci. World, 2024, 17, 106–113 CrossRef.
- S. Khan, T. Noor, N. Iqbal and L. Yaqoob, ACS Omega, 2024, 9, 21751–21767 CrossRef CAS PubMed.
- A. I. Khedr and M. H. H. Ali, Sci. Rep., 2024, 14, 29156 CrossRef CAS PubMed.
- D. M. Nzilu, E. S. Madivoli, D. S. Makhanu, S. I. Wanakai, G. K. Kiprono and P. G. Kareru, Sci. Rep., 2023, 13, 14030 CrossRef CAS PubMed.
- N. Thakur, P. Kumar, N. Thakur, K. Kumar, A. Tapwal and P. Sharma, Biomater. Polym. Horiz., 2022, 1(4) DOI:10.37819/bph.1.331.
- G. G. Nascimento, J. Locatelli, P. C. Freitas and G. L. Silva, Braz. J. Microbiol., 2000, 31, 247–256 Search PubMed.
- S. Shahzadi, N. Zafar and R. Sharif, Bact. Pathog. Antibact. Control, 2018, 51 DOI:10.5772/intechopen.72526.
- K. Sobha, K. Surendranath, V. Meena, K. T. Jwala, N. Swetha and K. S. M. Latha, Biotechnol. Mol. Biol. Rev, 2010, 5, 1–12 CAS.
- M. Ahamed, H. A. Alhadlaq, M. A. M. Khan, P. Karuppiah and N. A. Al-Dhabi, J. Nanomater., 2014, 2014, 637858 CrossRef.
- P. P. N. V. Kumar, U. Shameem, P. Kollu, R. L. Kalyani and S. V. N. Pammi, BioNanoSci, 2015, 5, 135–139 CrossRef.
- A. George, D. M. A. Raj, A. D. Raj, A. A. Irudayaraj, J. Arumugam, H. J. Prabu, S. J. Sundaram, N. A. Al-Dhabi, M. V. Arasu and M. Maaza, Surf. Interfaces, 2020, 21, 100761 CrossRef CAS.
- I. Sondi and B. Salopek-Sondi, J. Colloid Interface Sci., 2004, 275, 177–182 CrossRef CAS PubMed.
- E. Takele Assefa, G. Shumi, K. Mohammed Gendo, G. Kenasa and N. Roba, Results Chem., 2024, 8, 101606 CrossRef CAS.
- E. F. El-Belely, M. M. Farag, H. A. Said, A. S. Amin, E. Azab, A. A. Gobouri and A. Fouda, Nanomaterials, 2021, 11, 95 CrossRef CAS PubMed.
- F. Chowdhury, M. B. Mobarak, M. Hakim, M. N. Uddin, M. S. Hossain, U. S. Akhter, D. Islam, S. Ahmed and H. Das, New J. Chem., 2024, 48, 17038–17051 RSC.
- A. Azad, H. Zafar, F. Raza and M. Sulaiman, Pharm. Front., 2023, 5, e117–e131 CrossRef.
- J. O. Adeyemi, A. O. Oriola, D. C. Onwudiwe and A. O. Oyedeji, Biomolecules, 2022, 12, 627 CrossRef CAS PubMed.
- M. H. Haido, A. H. Matti, S. M. Taher and M. Haido, Cureus, 2024, 16(5), e61146 Search PubMed.
- J. Borgatta, Y. Shen, C. Tamez, C. Green, J. K. Hedlund Orbeck, M. S. Cahill, C. Protter, C. Deng, Y. Wang, W. Elmer, J. C. White and R. J. Hamers, J. Agric. Food Chem., 2023, 71, 9644–9655 CrossRef CAS PubMed.
- S. Ying, Z. Guan, P. C. Ofoegbu, P. Clubb, C. Rico, F. He and J. Hong, Environ. Technol. Innovat., 2022, 26, 102336 CrossRef CAS.
- A. Hosseingholian, S. D. Gohari, F. Feirahi, F. Moammeri, G. Mesbahian, Z. S. Moghaddam and Q. Ren, Mater. Today Sustain., 2023, 24, 100500 Search PubMed.
- H. T. Phan and A. J. Haes, J. Phys. Chem. C, 2019, 123, 16495–16507 CrossRef CAS PubMed.
- S. Saif, A. Tahir, T. Asim and Y. Chen, Nanomaterials, 2016, 6, 205 CrossRef PubMed.
- S. Anwaar, F. Altaf, T. Anwar, H. Qureshi, E. H. Siddiqi, W. Soufan and W. Zaman, Sci. Rep., 2024, 14, 1–15 CrossRef PubMed.
- A. S. Ibrahim, G. A. Ali, A. Hassanein, A. M. Attia and E. R. Marzouk, Sustainability, 2022, 14, 4914 CrossRef CAS.
- P. P. N. Kumar, U. Shameem, P. Kollu, R. L. Kalyani and S. V. N. Pammi, BioNanoScience, 2015, 5, 135–139 CrossRef.
- J. K. Sharma, M. S. Akhtar, S. Ameen, P. Srivastava and G. Singh, J. Alloys Compd., 2015, 632, 321–325 CrossRef CAS.
- B. Kumar, K. Smita, L. Cumbal, A. Debut and Y. Angulo, J. Saudi Chem. Soc., 2017, 21, S475–S480 CrossRef CAS.
- K. Vishveshvar, M. V. Aravind Krishnan, K. Haribabu and S. Vishnuprasad, BioNanoSci, 2018, 8, 554–558 CrossRef.
- A. S. Beheshtian, M. H. Givianrad, H.-A. Rafiee-Pour and P. A. Azar, Opt. Quant. Electron., 2023, 55, 463 CrossRef CAS.
- D. Rehana, D. Mahendiran, R. S. Kumar and A. K. Rahiman, Biomed. Pharmacother., 2017, 89, 1067–1077 CrossRef CAS PubMed.
- M. Gowri, N. Latha and M. Rajan, BioNanoSci, 2019, 9, 545–552 CrossRef.
- G. Jayakumarai, C. Gokulpriya, R. Sudhapriya, G. Sharmila and C. Muthukumaran, Appl. Nanosci., 2015, 5, 1017–1021 CrossRef CAS.
- S. A. Moon, B. K. Salunke, P. Saha, A. R. Deshmukh and B. S. Kim, Korean J. Chem. Eng., 2018, 35, 702–708 CrossRef CAS.
- P. C. Nagajyothi, P. Muthuraman, T. V. M. Sreekanth, D. H. Kim and J. Shim, Arab. J. Chem., 2017, 10, 215–225 CrossRef CAS.
- G. Karunakaran, M. Jagathambal, G. S. Kumar and E. Kolesnikov, JOM, 2020, 72, 1264–1272 CrossRef CAS.
- E. Nagaraj, K. Karuppannan, P. Shanmugam and S. Venugopal, J. Clust. Sci., 2019, 30, 1157–1168 CrossRef CAS.
- R. Sivaraj, P. K. S. M. Rahman, P. Rajiv, H. A. Salam and R. Venckatesh, Spectrochim. Acta Mol. Biomol. Spectrosc., 2014, 133, 178–181 Search PubMed.
- A. Pramanik, A. K. Datta, D. Das, D. V. Kumbhakar, B. Ghosh, A. Mandal, S. Gupta, A. Saha and S. Sengupta, Cytol. Genet., 2018, 52, 299–308 CrossRef.
- R. Sivaraj, P. K. S. M. Rahman, P. Rajiv, H. A. Salam and R. Venckatesh, Spectrochim. Acta Mol. Biomol. Spectrosc., 2014, 133, 178–181 CrossRef CAS PubMed.
- G. Jayakumarai, C. Gokulpriya, R. Sudhapriya, G. Sharmila and C. Muthukumaran, Appl. Nanosci., 2015, 5, 1017–1021 CrossRef CAS.
- G. S. Rajgovind, D. K. Gupta, N. D. Jasuja and S. C. Joshi, J. Microb. Biochem. Technol., 2015, 7(3), 140–144 Search PubMed.
- P. Vanathi, P. Rajiv and R. Sivaraj, Bull. Mater. Sci., 2016, 39, 1165–1170 CrossRef CAS.
- S. Gunalan, R. Sivaraj and R. Venckatesh, Spectrochim. Acta Mol. Biomol. Spectrosc., 2012, 97, 1140–1144 CrossRef CAS PubMed.
- M. Kamali, F. Samari and F. Sedaghati, Mater. Sci. Eng. C, 2019, 103, 109744 CrossRef CAS PubMed.
- M. Sorbiun, E. Shayegan Mehr, A. Ramazani and S. Taghavi Fardood, Int. J. Environ. Res., 2018, 12, 29–37 CrossRef CAS.
- R. Chandrasekaran, S. A. Yadav and S. Sivaperumal, J. Clust. Sci., 2020, 31, 221–230 CrossRef CAS.
- M. Rafique, F. Shafiq, S. S. Ali Gillani, M. Shakil, M. B. Tahir and I. Sadaf, Optik, 2020, 208, 164053 CrossRef CAS.
- S. Vasantharaj, P. Shivakumar, S. Sathiyavimal, P. Senthilkumar, S. Vijayaram, M. Shanmugavel and A. Pugazhendhi, Appl. Nanosci., 2021, 1–8 Search PubMed.
- B. Fatima, S. I. Siddiqui, R. Ahmed and S. A. Chaudhry, DWT, 2019, 164, 192–205 CrossRef CAS.
- T. B. Vidovix, H. B. Quesada, R. Bergamasco, M. F. Vieira and A. M. S. Vieira, Environ. Technol., 2022, 43, 3047–3063 CrossRef CAS PubMed.
- A. Atri, M. Echabaane, M. Bouzid, A. B. Lamine and R. B. Chaâbane, Research Square, 2023 DOI:10.21203/rs.3.rs-2710235/v1 , preprint.
- N. Sreeju, A. Rufus and D. Philip, J. Mol. Liq., 2017, 242, 690–700 CrossRef CAS.
- J. Singh, V. Kumar, K.-H. Kim and M. Rawat, Environ. Res., 2019, 177, 108569 CrossRef CAS PubMed.
- S. Sharma and K. Kumar, J. Dispersion Sci. Technol., 2021, 42, 1950–1962 CrossRef CAS.
- S. Sharma, K. Kumar, N. Thakur, S. Chauhan and M. S. Chauhan, J. Environ. Chem. Eng., 2021, 9, 105395 CrossRef CAS.
- M. Y. Rather and S. Sundarapandian, J. Clust. Sci., 2022, 33, 925–933 CrossRef CAS.
- S. P. Thakur and V. Kumar, J. Environ. Health Sci. Eng., 2019, 17, 367–376 CrossRef PubMed.
- D. B. Manikandan, M. Arumugam, S. Veeran, A. Sridhar, R. Krishnasamy Sekar, B. Perumalsamy and T. Ramasamy, Environ. Sci. Pollut. Res., 2021, 28, 33927–33941 CrossRef CAS PubMed.
- A. Ikram, S. Jamil and M. Fasehullah, Mater. Innov., 2022, 2, 115–122 CrossRef CAS.
- S. Kayalvizhi, A. Sengottaiyan, T. Selvankumar, B. Senthilkumar, C. Sudhakar and K. Selvam, Optik, 2020, 202, 163507 CrossRef CAS.
- E. Shayegan Mehr, M. Sorbiun, A. Ramazani and S. Taghavi Fardood, J. Mater. Sci.: Mater. Electron., 2018, 29, 1333–1340 CrossRef CAS.
- T. B. Vidovix, E. F. D. Januário, M. F. Araújo, R. Bergamasco and A. M. S. Vieira, Environ. Prog. Sustain. Energy, 2022, 41, e13864 CrossRef CAS.
- K. G. Akpomie and J. Conradie, Sci. Rep., 2023, 13, 859 CrossRef CAS PubMed.
- M. Jeevarathinam and I. V. Asharani, Inorg. Chem. Commun., 2024, 169, 113113 CrossRef CAS.
- D. M. Nzilu, E. S. Madivoli, D. S. Makhanu, S. I. Wanakai, G. K. Kiprono and P. G. Kareru, Sci. Rep., 2023, 13, 14030 CrossRef CAS PubMed.
- K. Pakzad, H. Alinezhad and M. Nasrollahzadeh, Appl. Organomet. Chem., 2020, 34, e5910 CrossRef CAS.
- S. Kaushal, A. Kumar, H. Bains and P. P. Singh, Environ. Sci. Pollut. Res., 2022, 30, 37092–37104 CrossRef PubMed.
- Y. Tangjaideborisu, P. Yugala, C. Phanawansombat, P. Shanmugam, S. Boonyuen and P. N. Nakorn, Research Square, 2024 DOI:10.21203/rs.3.rs-5265919/v1 , preprint.
- M. Mahjoore, M. Honarmand and A. Aryafar, Environ. Sci. Pollut. Res., 2023, 30, 44439–44456 CrossRef CAS PubMed.
- F. Ijaz, S. Shahid, S. A. Khan, W. Ahmad and S. Zaman, Trop. J. Pharmaceut. Res., 2017, 16, 743–753 CrossRef CAS.
- K. Velsankar, A. K. RM, R. Preethi, V. Muthulakshmi and S. Sudhahar, J. Environ. Chem. Eng., 2020, 8, 104123 CrossRef CAS.
- A. M. Eid, A. Fouda, S. E.-D. Hassan, M. F. Hamza, N. K. Alharbi, A. Elkelish, A. Alharthi and W. M. Salem, Catalysts, 2023, 13, 348 CrossRef CAS.
- A. Awwad and M. Amer, Chem. Int., 2020, 6, 210–217 CAS.
- M. S. Jadhav, S. Kulkarni, P. Raikar, D. A. Barretto, S. K. Vootla and U. S. Raikar, New J. Chem., 2018, 42, 204–213 RSC.
- F. Amin, B. Khattak, A. Alotaibi, M. Qasim, I. Ahmad, R. Ullah, M. Bourhia, A. Gul, S. Zahoor and R. Ahmad, Evid. base Compl. Alternative Med., 2021, 2021, 1–12 CrossRef PubMed.
- S. Vasantharaj, S. Sathiyavimal, M. Saravanan, P. Senthilkumar, K. Gnanasekaran, M. Shanmugavel, E. Manikandan and A. Pugazhendhi, J. Photochem. Photobiol. B Biol., 2019, 191, 143–149 CrossRef CAS PubMed.
- S. K. Karuppannan, R. Ramalingam, S. M. Khalith, M. J. H. Dowlath, G. D. Raiyaan and K. D. Arunachalam, Biocatal. Agric. Biotechnol., 2021, 31, 101904 CrossRef CAS.
- S. Prakash, N. Elavarasan, A. Venkatesan, K. Subashini, M. Sowndharya and V. Sujatha, Adv. Powder Technol., 2018, 29, 3315–3326 CrossRef CAS.
- H. Ibne Shoukani, S. Nisa, Y. Bibi, A. Ishfaq, A. Ali, S. Alharthi, K. tul Kubra and M. Zia, Sci. Rep., 2024, 14, 21246 CrossRef CAS PubMed.
- J. Komara, J. P. Karumuri and B. S. S. Naik, Hybrid Adv., 2024, 7, 100304 CrossRef.
- H. Mirhadi, A. Momeni and M. H. Meshkatalsadat, Nano Biomed. Eng., 2024, 16, 473–483 CrossRef.
- S. Jabeen, V. U. Siddiqui, S. Bala, N. Mishra, A. Mishra, R. Lawrence, P. Bansal, A. R. Khan and T. Khan, ACS Omega, 2024, 9, 30190–30204 CrossRef CAS PubMed.
- H. EL-Moslamy, H. Shokry, H. Rezk and R. Abdel-Fattah, J. Nanomater. Mol. Nanotechnol, 2017, 13, 21–23 Search PubMed.
- M. Sravanthi, D. Muni Kumar, B. Usha, M. Ravichandra, M. Mahendra Rao, K. P. J. Hemalatha and M. Ravichandra, Int. J. Adv. Res, 2016, 4, 589–602 CrossRef CAS PubMed.
- T. Khairy, D. H. Amin, H. M. Salama, I. M. A. Elkholy, M. Elnakib, H. M. Gebreel and H. A. E. Sayed, Sci. Rep., 2024, 14, 25020 CrossRef CAS PubMed.
- B. Malaikozhundan, V. N. Lakshmi and R. Krishnamoorthi, Mater. Today Commun., 2022, 33, 104348 CrossRef CAS.
- M. Naseer, R. Ramadan, J. Xing and N. A. Samak, Int. Biodeterior. Biodegrad., 2021, 159, 105201 CrossRef CAS.
- A. Hamid, S. Haq, S. Ur Rehman, K. Akhter, W. Rehman, M. Waseem, S. Ud Din, Z. Ul-Abdin, M. Hafeez, A. Khan and A. Shah, Chem. Pap., 2021, 75, 4189–4198 CrossRef CAS.
- A. U. Mirza, M. S. Khan, S. A. A. Nami, A. Kareem, S. Rehman, S. A. Bhat and N. Nishat, Chem. Biodiversity, 2019, 16, e1900145 CrossRef PubMed.
- P. G. Bhavyasree and T. S. Xavier, Heliyon, 2020, 6(2), e03323 CrossRef CAS PubMed.
- P. G. Bhavyasree and T. S. Xavier, Chem. Eng. J. Adv., 2021, 8, 100152 CrossRef.
- R. Radhakrishnan, F. A. Khan, A. Muthu, A. Manokaran, J. S. Savarenathan and K. Kasinathan, Lett. Appl. NanoBioScience, 2021, 10, 2706–2714 Search PubMed.
- S. Pansambal, K. Deshmukh, A. Savale, S. Ghotekar, O. Pardeshi, G. Jain, Y. Aher and D. Pore, J. Nanostruct., 2017, 7, 165–174 CAS.
- A. A. Lawrence and J. T. J. Prakash, Int. J. Sci. Res. Phys. Appl. Sci., 2018, 6, 57–68 Search PubMed.
- M. A. Choudhary, R. Manan, M. Aslam Mirza, H. Rashid Khan, S. Qayyum and Z. Ahmed, Int. J. Mater. Sci. Eng, 2018, 4, 1–6 Search PubMed.
- D. Devipriya and S. M. Roopan, Mater. Sci. Eng. C, 2017, 80, 38–44 CrossRef CAS PubMed.
- P. Yugandhar, T. Vasavi, P. Uma Maheswari Devi and N. Savithramma, Appl. Nanosci., 2017, 7, 417–427 CrossRef CAS.
- S. Saha, S. Das and R. Basu, EJPMR, 2019, 6, 340–346 Search PubMed.
- C. S. Sundaram, J. S. Kumar, S. S. Kumar, P. L. N. Ramesh, T. Zin and U. M. Rao, Med. J. Malaysia, 2020, 75, 677–684 CAS.
- M. Shammout and A. Awwad, MW Shammout and AM Awwad. A novel route for the synthesis of copper oxide nanoparticles using Bougainvillea plant flowers extract and antifungal activity evaluation. Chemistry International, 2021, vol. 7, pp. 71–78 Search PubMed.
- A. Atri, M. Echabaane, A. Bouzidi, I. Harabi, B. M. Soucase and R. B. Chaâbane, Heliyon, 2023, 9(2), e13484 CrossRef CAS PubMed.
- G. Thandapani, K. Arthi, P. Pazhanisamy, J. J. John, C. Vinothini, V. Rekha, K. Santhanalakshmi and V. Sekar, Mater. Today Commun., 2023, 34, 105248 CrossRef CAS.
- E. Hamdy, H. El-Gendi, A. Al-Askar, A. El-Far, P. Kowalczewski, S. Behiry and A. Abdelkhalek, Open Chem., 2024, 22, 20240028 CrossRef CAS.
- I. H. Shah, M. Ashraf, A. R. Khan, M. A. Manzoor, K. Hayat, S. Arif, I. A. Sabir, M. Abdullah, Q. Niu and Y. Zhang, 3 Biotech, 2022, 12, 128 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |