 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent progress in the synthesis of nanostructured Ti3C2Tx MXene for energy storage and wastewater treatment: a review
Qui Thanh
Hoai Ta†
 ab,
Jianbin
Mao†
c,
Ngo Thi
Chau
abd,
Ngoc Hoi
Nguyen
ab,
Jianbin
Mao†
c,
Ngo Thi
Chau
abd,
Ngoc Hoi
Nguyen
 ab,
Dieu Linh
Tran
ab,
Dieu Linh
Tran
 ab,
Thi My
Huyen Nguyen
a,
Manh Hoang
Tran
a,
Hoang
Van Quy
e,
Soonmin
Seo
ab,
Thi My
Huyen Nguyen
a,
Manh Hoang
Tran
a,
Hoang
Van Quy
e,
Soonmin
Seo
 *c and
Dai Hai
Nguyen
*c and
Dai Hai
Nguyen
 *ab
*ab
aInstitute of Advanced Technology, Vietnam Academy of Science and Technology, 1A TL29 Street, Thanh Loc Ward, District 12, Ho Chi Minh City 700000, Vietnam. E-mail: nguyendaihai0511@gmail.com; tathanhhoaiqui2292@gmail.com; chaungo2601@gmail.com; hoi83bmt@gmail.com; tdlinh92@gmail.com; myhuyen1001vn@gmail.com; tranmanhhoang1214@gmail.com
bGraduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Street, Cau Giay District, Hanoi 100000, Vietnam
cCollege of BioNano Technology, Gachon University, Gyeonggi 13120, Republic of Korea. E-mail: soonmseo@gachon.ac.kr; mg2895852@gmail.com
dFaculty of Pharmacy, Nguyen Tat Thanh University, 300A Nguyen Tat Thanh Street, Ward 13, District 4, Ho Chi Minh City, 700000, Vietnam
eDivision of Energy & Environmental Technology, Daegu–Gyeongbuk Institute of Science and Technology (DGIST), Daegu 42988, Republic of Korea. E-mail: quybk@dgist.ac.kr
First published on 24th April 2025
Abstract
MXene-based functional 2D materials hold significant potential for addressing global challenges related to energy and water crises. Since their discovery in 2011, Ti3C2Tx MXenes have demonstrated promising applications due to their unique physicochemical properties and distinctive morphology. Recent advancements have explored innovative strategies to enhance Ti3C2Tx into multifunctional materials, enabling applications in gas sensing, electromagnetic interference shielding, supercapacitors, batteries, water purification, and membrane technologies. Unlike previous reviews that primarily focused on the synthesis, properties, and individual applications of MXenes, this work provides a fundamental discussion of their role in wastewater treatment, recent advancements in energy harvesting, and their broader implications. Additionally, this review offers a comparative analysis of MXene-based systems with other state-of-the-art materials, providing new insights into their future development and potential applications.
1. Introduction
An increasing population and industry have brought massive global changes in the form of water crises and energy scarcity. Water is typically utilized in energy-production procedures, while energy is needed for the purification of water resources and water remediation; thus, water issues and energy dilemmas are relevant and interdependent.1–5 Over 1.3 billion civilians are living without access to electrical power, and around 1.6 million civilians lack clean water for daily activities.6,7 These issues will be further worsened due to global warming and the consistent demand for clean water and energy, which has been predicted to increase by over 50% over the next decade.8,9 Additionally, untreated wastewater is threatening human health and natural ecosystems. Moreover, current energy storage and conversion technologies are not suitable because of the inefficiency of these systems.10,11Scientific communities have studied materials and technology to solve the aforementioned issues, among which two-dimensional (2D) materials have attracted tremendous research attention owing to their unique properties and tunable structures. In particular, the discovery of carbides and nitrides (MXenes) in the 21st century further boosted the intensity of breakthrough research associated with carbon-based functional materials.12–15 MXenes are a group of 2D materials that have been explored since Gogotsi and co-workers reported them in 2011.14 Ti3C2Tx MXenes have synthesized by the etching of Al-containing MAX phases, where M is a pre-transition metal (Mo, Ta, Hf, Cr, Ti, V, etc.), A represents an A-group element (groups 13 and 14, or IIIA and IVA), and X stands for either C and/or N.16 The etched A element is usually replaced by a termination group (–F, –O, and –OH), giving MXene materials with a common structure such as Mn+1XnTx with n = 1 − 4.17,18
Ti3C2Tx MXenes are crucial materials due to their intrinsic properties, including high electrical conductivity, hydrophilicity, and excellent mechanical strength. Consequently, they have been widely utilized in various applications, such as electrodes, energy storage materials, and co-catalysts in photocatalysis. Ti3C2Tx films can be fabricated using multiple techniques, including vacuum-assisted filtration, spin-coating, rolling, printing, and spray-coating of exfoliated MXene solutions.19 Various properties and potential applications of Ti3C2Tx MXenes have been investigated in the literature.20–28 However, there has been limited research into the reuse and recycling of Ti3C2Tx materials from used supercapacitors and spent batteries. It is important to investigate the recycling of spent Ti3C2Tx MXene, as this would facilitate a broad range of applications and increase their environmental friendliness. Innovative methods for MXene-based multi-functional materials are expected to improve the synthesis cost of materials for energy generation and mitigate the global warming caused by wastewater. Driven by investigations into methods, efficiency, and stability, the synthesis of valuable MXene-based multi-functional materials is anticipated to bring about a sustainable future.
Numerous reviews have explored the diverse applications of MXenes, including their roles in electromagnetic interference shielding, gas sensing, photocatalysis, electrochemical energy storage systems, regenerative medicine, and next-generation rechargeable batteries.29–35 While earlier reviews have primarily emphasized the synthesis and singular applications of MXenes, the present work focuses on their integrated applications in energy and environmental domains. It highlights recent advancements, advanced characterization techniques, and scalability challenges associated with Ti3C2Tx MXenes. Furthermore, this review provides a fundamental discussion of electron transfer mechanisms, offers a critical comparison between MXene-based materials and other leading alternatives, and outlines key challenges and future directions for their practical deployment. The integration of these domains aligns with the global transition toward sustainable technologies. By examining the structure–function relationships of Ti3C2Tx MXene, this review seeks to demonstrate their multifunctionality and versatility, ultimately supporting the development of advanced platforms to address critical issues related to the global energy and water crises.
2. Synthesis and properties of MXene
2.1. Synthesis
Since their initial exploration, more than 160 MAX phases have been studied using density functional theory (DFT) calculations and experimental works. Two major dilemmas are encountered in this exploration: the identification of appropriate precursors and the development of feasible synthetic methods at the scale-up stage.36,37There are two major methods to prepare Ti3C2Tx MXenes: bottom-up and top-down methods. The top-down method is usually used to prepare MXene owing to its ease of scale-up, simple equipment, and cheapness compared to the bottom-up technique. Ti3C2Tx MXenes have been synthesized using HF acid as an etching agent to remove the Al layer (Fig. 1).38,39
 | ||
| Fig. 1 Schematic preparation processes of Ti3C2Tx MXene using a F-based method. Reproduced from ref. 39 with permission from Elsevier, copyright 2021. | ||
However, scientists have become concerned about the toxicity of HF, and efforts have been made to find other milder etching procedures instead of using HF, such as electrochemical etching, alkaline etching using NaOH, HCl and LiF, and molten salt.40–44 The methods allow for control of the nano- and micro-size of MXene, but require complicated systems with a small number of products. Therefore, the manufacture of Ti3C2Tx MXene has been dominated by etchants at the laboratory and factory scale. In particular, the use of hazardous HF acid to produce MXene seems to be difficult for mass production. To date, there are no viable alternatives to HF and in situ-formed HF as etchants; this challenge is in the embryonic stage. It is possible to synthesize the desired Ti3C2Tx MXene with proper granulometry, colloidal systems, and free-standing films, which are suitable for specific applications.45–47
Halogen-based etching has recently been used in the synthesis of Ti3C2Tx MXenes with halogen-terminated surfaces, as shown in Fig. 2. The rate and extent of removal can be controlled either optically or qualitatively owing to its colorimetric parameters, which offer direct quantitative feedback as compared to fluoride-based techniques. The plausible mechanism reveals that continual halogen (I2, Br2) injection offers high yields and efficiency (∼1% yield Ti3C2Tx at 1 mg mL−1).48
 | ||
| Fig. 2 (A–E) Halogen etching of Ti3AlC2 MAX phase. Reproduced from ref. 48 with permission from American Chemical Society, copyright 2021. | ||
In lieu of HF, science communities have modified the etchant to obtain MXenes with variety of physicochemical properties that are suitable for practical applications. Fig. 3 presents a timeline of the preparation routes of MXene since its discovery.
 | ||
| Fig. 3 Schematic of synthesis Ti3C2Tx MXene with variety of techniques. Reproduced from ref. 49 with permission from the Royal Society of Chemistry, copyright 2023. | ||
2.2. Properties
The unique properties of Ti3C2Tx MXenes typically depend on their composition, lateral size, etchant, and stacking order. When the Al layers are removed from the Al-containing MAX phase, Ti layers are exposed on two sides of the MXene layer, which are prone to bond with functional groups (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –F, and –OH) to decrease the total surface energy. Thus, it is important to fundamentally understand the effect of functional groups on the unique properties of Ti3C2Tx so as to achieve versatility in designing applications.50,51
O, –F, and –OH) to decrease the total surface energy. Thus, it is important to fundamentally understand the effect of functional groups on the unique properties of Ti3C2Tx so as to achieve versatility in designing applications.50,51
Unlike graphene, Ti3C2Tx MXenes have unique properties, such as high electronic conductivity, abundant terminal groups, and lamellar 2D structures. Electrical measurements were conducted on flakes and foam structures of metallic Ti3C2Tx MXenes, and gave values of around 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 S cm−1.52 The functional groups (F, OH, O) have an intrinsic oxidizing nature, which accelerates the redox reaction. Finally, the multilayered morphology allows high specific surface area and favors diffusion toward the active sites.47,53
000 S cm−1.52 The functional groups (F, OH, O) have an intrinsic oxidizing nature, which accelerates the redox reaction. Finally, the multilayered morphology allows high specific surface area and favors diffusion toward the active sites.47,53
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times its own weight.50 The hierarchical architecture of the MXene-bonded polyurethane/polyvinyl alcohol (PU/PVA) hydrogel has the potential to enhance its both strain and mechanical strength characteristics; the material exhibited a gauge factor of 5.7 at a strain of 191% after undergoing 5000 cycles.62
000 times its own weight.50 The hierarchical architecture of the MXene-bonded polyurethane/polyvinyl alcohol (PU/PVA) hydrogel has the potential to enhance its both strain and mechanical strength characteristics; the material exhibited a gauge factor of 5.7 at a strain of 191% after undergoing 5000 cycles.62
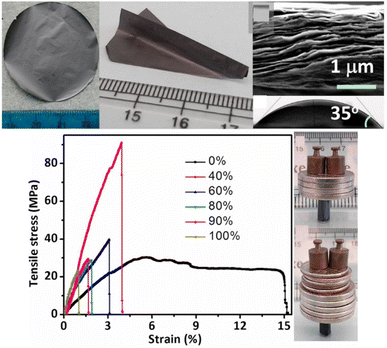 | ||
| Fig. 4 Mechanical properties of a metallic Ti3C2Tx MXene film. Reproduced from ref. 61 with permission from the National Academy of Sciences of the United States of America, copyright 2014. | ||
 | ||
| Fig. 5 Schematic illustrations depicting (a) the mechanisms of electrochemical charge storage and (b) the pathways for lithium diffusion within Ti3C2Tx MXene. Reproduced from ref. 66 with permission from Springer Nature, copyright 2019. Reproduced from ref. 65 with permission from the American Chemical Society, copyright 2012. | ||
Wei et al. prepared hollow Ti3C2Tx MXene by applying it onto the surface of poly(methyl methacrylate) (PMMA) nanospheres for the purpose of facilitating vanadium redox reactions.67 The heterostructure was then heated to prepare hollow MXene spheres, which were decorated into graphite-felt electrodes by dipping. The prepared electrodes were tested in vanadium redox flow batteries (VRFBs) to investigate their electrocatalytic properties using cyclic voltammetry, which were better than those observed for the pure carbon NP-based materials. At a high current density (300 mA cm−2), the electrolyte utilization efficiency was 62.9% and the energy efficiency was 75.0%, respectively. Interestingly, the battery displayed good stability and low energy efficiency decay at a current density of 200 mA cm−2 over 500 cycles. The excellent performance towards the V3+ and VO2+ redox reactions were due to its high electrical conductivity, flexibility, and chemical stability with natural hydrophilicity of the composites.67
The unique properties and intrinsic morphology metallic Ti3C2Tx of make it a reasonable choice for the manufacture of electrodes, which could be suitable for use in the field of energy storage applications. The dilemma in MXene commercialization is adaptation for large-scale industry, owing to the toxicity of the etching process and the harsh chemical conditions for its synthesis. At present, it seems that using the spark plasma sintering method to produce the MAX phase is inherently a batch process. The concerns about the etching procedure using HF should be investigated to control the particle size, defects, and toxicity.37
3. Applications of MXene-based functional materials
MXene-based materials offer a wide range of potential applications owing to their unique properties. These applications span many fields, including but not limited to sensor, electrochemical, optical, electronics, biomedical, and energy applications, as summarized in Fig. 6. In this section, their applications in energy and environment will be investigated in detail. | ||
| Fig. 6 Structure and potential applications of 2D MXenes. Reproduced from ref. 68 with permission from the American Association for the Advancement of Science, copyright 2021. | ||
3.1. Electrochemical energy storage
Du et al. investigated the use of an FeS2@MXene composite for lithium and sodium ion storage, which demonstrated remarkable rate capabilities. Their findings indicate that its specific capacity for lithium-ion storage is approximately 762 mA h g−1 at a current density of 10 A g−1, whereas the specific capacity for sodium-ion storage is around 563 mA h g−1 at a current density of 0.1 A g−1.70 Ali et al. studied Fe2O3/Ti3C2Tx anode materials for LIBs.71 Hybrids were synthesized by confining Fe2O3 NPs in Ti3C2Tx in different mixing ratios via a dry ball-milling system, and the resulting heterostructures showed high surface areas. The optimized composite with 50 wt% Fe2O3 displayed the highest performance and stability (270 mA h g−1 at 1 C). The nanocomposites synthesized by the ball-milling method exhibited uniform distribution, a more accessible surface, and minimum restacking and oxidation of the nanosheets, which increased their electrochemical performance (Fig. 7). Moreover, excellent volumetric capacitances were recorded for a Ti3C2Tx-based PVA hybrid in the electrolyte KOH, with values of 306 F Cm−3 and 528 F Cm−3 at 100 mV s−1 and 2 mV s−1, respectively.61 The use of the as-prepared composite with different electrolytes may widen its applications in the battery field.
 | ||
| Fig. 7 (a–d) Effect of preparation on the performance of Fe2O3/Ti3C2Tx anode materials for LIBs. Reproduced from ref. 71 with permission from American Chemical Society, copyright 2018. | ||
Gentile and co-workers reported that Ti3C2Tx MXenes were synthesized in high-concentration HF and after post-synthesis 300 °C thermal treatments could be used as an anode in sodium-ion batteries (SIBs) with good rate capability and outstanding stability over 360 cycles. The pure Ti3AlC2 MAX phase was synthesized using spark plasma sintering.41 In fact, a lower etching rate offered better structural order to accelerate the electrochemical process. The OH-rich and H-rich compounds have higher insertion–deinsertion potential and smaller capacitive contributions as compared to those rich in –F terminal groups.37
Ti3C2Tx MXene has massively lower capacity (115 mA h g−1) but superior capacity retention (100% at 500 cycles) and better rate capability (90 mA h g−1 at 1.0 A g−1) compared to SoA hard carbons (300 mA h g−1, 85 mA h g−1 at 1.5 A g−1, and 65% at 500 cycles), respectively.72 Ti3C2Tx MXene-based supercapacitors are commonly asymmetric devices with negative electrodes as layered structures owing to MXene being oxidized at high potentials (>0.6 V vs. SHE).45
In general, next-generation LIBs have utilized Si-rich composites as high-capacity anodes. However, Si has an excellent theoretical capacity but poor mechanical properties. To address this dilemma, the combination of Si and mesoporous carbon materials such as MXene enables the creation of a stable SEI. Xia and colleagues reported the preparation of a Si-based anode material in which Si p-NSs are wrapped with Ti3C2Tx MXene via an interfacial assembly method, as shown in Fig. 8.73 In the Si@Ti3C2Tx composite, the Ti3C2Tx MXene is characterized by an abundance of surface-terminating functional groups, which promotes robust interfacial interactions with the Si components, thereby enhancing the pseudocapacitive behavior and ensuring stable lithium storage. This interfacial synergy not only facilitates improved charge transfer kinetics, but also accommodates the volumetric changes that silicon undergoes during the lithiation and delithiation processes. Electrochemical characterization of the Si@Ti3C2Tx composite in a half-cell configuration revealed a notable reversible capacity of 1154 mA h g−1 after 150 cycles at a current density of 0.2 A g−1, accompanied by a remarkably low capacity decay rate of 0.026% per cycle. Additionally, the composite demonstrated exceptional long-term cycling stability, maintaining a capacity of 501 mA h g−1 over 2000 cycles at a current density of 1 A g−1. Comparison with other results indicates that the performances of the Si@Ti3C2Tx composites are in line with other carbon-based composites, providing evidence that Ti3C2Tx MXenes can be utilized to encapsulate Si and for other high-capacity conversion composite anodes.73–75
 | ||
| Fig. 8 Interfacial assembly Ti3C2Tx/Si for the enhancement of electrochemical Li storage activity. Reproduced from ref. 73 with permission from the American Chemical Society, copyright 2020. | ||
The Li–S system is one of the most critical systems in secondary batteries for next-generation electronics. Benefiting from the good dissolution of lithium polysulfide (LiPS) in the electrolyte, it avoids irreversible reactions that affect the cell integrity.76 Tang et al. synthesized a robust S@Ti3C2Tx composite combining LiPSS2 from LiF–HCl etched Ti3C2Tx MXene (Fig. 9). The optimized S@Ti3C2Tx composite displayed a uniform distribution of S in the characteristic multilayered Ti3C2Tx and had good electrochemical performance with ultralow capacity decay (0.014% after 1500 cycles) compared to pure S and Ti3C2Tx.77
 | ||
| Fig. 9 Cycling properties, rate capability, and charge–discharge profiles of the S@Ti3C2Tx composite. Reproduced from ref. 77 with permission from John Wiley and Sons, copyright 2019. | ||
Ti3C2Tx from spent batteries was utilized as recycled electrodes for SIBs/LIBs by Li et al.78 In their work, free-standing delaminated Ti3C2Tx electrodes were synthesized via a vacuum system using TMAOH solutions and annealed at high temperature to give the anode material. The free-standing annealed delaminated-Ti3C2Tx nanostructures displayed much better electrochemical properties than those of delaminated Ti3C2Tx samples owing to the elimination of functional groups and surface water molecules. The annealed delaminated-Ti3C2Tx electrodes displayed superior cycling stability at 1 A g−1 after 2000 cycles with a capacity retention of 93% (Fig. 10). The recycling process avoids the pyrometallurgical procedure typically used in current battery recycling. The product after heat treatment under a CO2 atmosphere is TiO2/C, which can be used in the fields of electrochemical oxygen or photocatalytic hydrogen evolution and photodegradation.
 | ||
| Fig. 10 Second life of Ti3C2Tx electrodes for LIBs/SIBs. Reproduced from ref. 78 with permission from Elsevier, copyright 2023. | ||
In addition to the active material, the separator is pivotal in improving the electrochemical performance of Li–S batteries. Research conducted by Yang and colleagues indicated that MXene-based composites effectively regulate polysulfide shuttling and maintain stability at elevated temperatures. MXene nanosheets with an average size of less than 5 nm were synthesized through a hydrothermal method following an etching process and subsequently incorporated onto g-C3N4 to serve as a functional separator layer in Li–S batteries. This configuration achieved a remarkable specific capacity of 1433 mA h g−1, accompanied by an exceptionally low capacity decay rate of 0.024% per cycle at a rate of 2 C over 1000 cycles. The enhanced electrochemical activity can be attributed to the abundant active sites present on the MXene, in conjunction with the pyridinic-N structure of g-C3N4.23,79
Gogotsi and co-coworkers synthesized flexible and conductive Ti3C2Tx/Co3O4 and Ti3C2Tx/NiCo2O4 composites for Li-ion storage by combining NiCo2O4 and Co3O4 with MXene using an alternating filtration method.81 As shown in Fig. 11, the hybrid film displayed an excellent reversible capacity of 1330 mA h g−1 at 0.1 C, along with enhanced rate capacity. The excellent electrical performance of the hybrid can be explained by the good metallic conductivity of MXene and the high theoretical capacity, good chemical stability, and low cost of Co3O4 nanoparticles, while ternary NiCo2O4 has two cations with good electrical conductivity. Table 1 presents a compilation of recent achievements in various MXene-based composites, along with their electrochemical performances.
 | ||
| Fig. 11 Schematic illustration of the combination of Ti3C2Tx MXene with transition metal oxide to obtain hybrid electrodes for energy storage. Reproduced from ref. 81 with permission from Elsevier, copyright 2016. | ||
| Material | Synthesis method | Electrochemical performance (capacitance) | Ref. |
|---|---|---|---|
| Ti3C2Tx MXene | HF etching | 75 F g−1 @ 2 A g−1 (3 M Na2SO4) | 96 |
| MXene/MoSe2 | LiF/HCl etching | 183 mA h g−1@1 A g−1 | 97 |
| MXene/graphene | Ultrasonic treatment | 405 F g−1 (6 M KOH) | 98 |
| MXene/CoF | HF etching | 1268 F g−1@1 A g−1 (0.1 M KOH) | 99 |
| MXene/Nb2C | Chemical etching | 53 F g−1@0.3 A g−1 (1 M PVA/H2SO4) | 100 |
| MXene/CNT/PANI | In situ polymerization and physical assembly | 429.4 F g−1@1 A g−1 (1.0 M H2SO4) | 101 |
| MXene/MnO2 | Mild chemical deposition method | 130.5 F g−1 @ 0.2 A g−1 (1 M Na2SO4) | 102 |
| MXene/BCN | Pyrolysis | 245 F g−1@1 A g−1 (1 M PVA/H2SO4) | 103 |
| MXene/PEDOT:PSS | Solution-blending filtration | 286 F g−1@2 mV s−1 (1 M H2SO4) | 104 |
| MXene/PANI@rGO | Solution etching | 45 F g−1 (PVA–PAA–NHS) | 105 |
| MXene/heteroatom-doped N | Polishing method | 390 F g−1 at 1 A g−1 (1 M H2SO4) | 106 |
| MXene/graphene@Ni | LiF/HCl etching | 254 F g−1@1 A g−1 | 107 |
| MXene/BC@PPy | Vacuum-filtration | 290 mF cm−2 | 108 |
Another study investigated the self-assembly of SnO2 nanowires on Ti3C2Tx nanosheets for fast energy storage via van der Waals interactions.82 The as-synthesized SnO2/Ti3C2Tx composite could avert the agglomeration of the SnO2 nanowires during the lithiation/delithiation process and prevent the active sites from being lost, which provided short Li+ diffusion pathways. The chemical reaction between Li/Li+ and SnO2/Ti3C2Tx can be summarized briefly as follows:
| SnO2 + 4Li+ + 4e− ↔ Sn + 2Li2O |
| Sn + zLi+ + ze− ↔ LizSn |
| Ti3C2Tx + zLi+ + ze− ↔ Ti3C2TxLiz |
Regarding the cathode, Ti3C2Tx MXenes have been extensively investigated as promising materials to enhance battery performance. An MXene/MoS2 composite cathode material was synthesized via a solvothermal approach and exhibited remarkable electrochemical performance. The Al/MXene/MoS2 battery demonstrated an initial capacity of 224 mA h g−1, which was maintained at 166 mA h g−1 after cycling. This performance is approximately 2.5 times greater than that of the Al/MoS2 battery, which recorded a capacity of 88 mA h g−1.83 The superior performance of the MXene/MoS2 composite is attributed to its significantly lower charge transfer resistance in comparison to the pure MoS2 cathode. The Ti3C2Tx MXene acts as a robust supporting framework, enhancing structural stability and reducing the pulverization of the MoS2 nanoflowers during the charge–discharge cycles. As anticipated, the MXene/MoS2 composite cathodes exhibited markedly lower charge transfer resistance and improved capacity retention compared to the MoS2-only cathodes. Additionally, the overlapping interlayer structure formed between the MXene multilayers and MoS2 nanoflowers increases the contact area, thereby facilitating enhanced electronic transport and further reducing charge transfer resistance.
Li and co-workers employed cetyltrimethylammonium bromide to expand the MXene interlayer spacing, followed by a selenization process to synthesize the composite cathode CTAB@Se/MXene. In aluminum-based batteries, the CTAB@Se/MXene composite demonstrated a high reversible specific discharge capacity of 583 mA h g−1 at a current density of 100 mA g−1.84 On the anode side, Al2Cl7− ions decompose into metallic Al and AlCl4−. During the charging process, oxidation reactions associated with the Ti2+/Ti3+ and Ti2+/Ti4+ redox couples in MXene take place on the cathode side, accompanied by the insertion of AlCl4− anions, as follows.
Anode:
| 4Al2Cl7− ↔ 7AlCl4− + Al |
Cathode:
| Ti3C2Tx + AlCl4− ↔ Ti3(AlCl4)C2Tx |
| ySe + 2zAlCl4− ↔ SeyClz + zAl2Cl7− |
Recently, the incorporation of MXene materials into TENGs has attracted increasing attention due to the unique properties of MXenes. These materials exhibit excellent electrical conductivity, mechanical flexibility, and surface functionalization capabilities, enhancing the overall performance of TENGs. For example, Cao et al. proposed an MXene liquid electrode to fabricate a stretchable and shape-adaptive TENG.85 In their study, the output voltage of the MXene-based TENGs reached up to 300 V. They highlighted the fact that the excellent fluidity and high electronegativity of the MXene liquid electrode provided the TENG with long-term reliability and stable electrical output.
Similarly, Du et al. demonstrated that the high electronegativity of MXenes effectively enhances the output performance of MXene-based TENGs.86 Their research presented an ultra-flexible and self-healable TENG with highly efficient electromagnetic interference shielding composed of modified Ti3C2Tx MXene (m-MXene)-based nanocomposite elastomers. Benefiting from the excellent electronegativity of m-MXene, the single-electrode TENG generated a high open-circuit voltage (Voc) ranging from −65 to 245 V, a short-circuit current (Isc) of 29 μA, and a peak power density of 1150 mW m−2, and was capable of powering twenty light-emitting diodes (LEDs).
Cai et al. explored the effect of surface chemistry on the work function of MXenes, which determines the performance of MXene-based TENGs.87 Their first-principles calculations revealed that surface functional groups significantly influence the work function of MXenes: –OH termination reduces the work function compared to a bare surface, while –F and –Cl increase it. Due to these exceptional properties, MXenes have been used as additives in TENGs via doping or blending methods. For instance, Luo et al. reported that MXene nanosheet doping promoted the crosslinking of a PVA hydrogel, improving its stretchability.88 The MXene nanosheets also formed microchannels on the surface, enhancing the conductivity of the hydrogel by improving ion transport and generating an additional triboelectric output via a streaming vibration potential mechanism.
Similarly, Gao's research illustrated that MXene doping enhanced the crystallinity of the composite films, resulting in a 450% improvement in tensile properties and an 80% reduction in wear volume during friction tests.89 The as-fabricated TENG using this composite film produced an open-circuit voltage of 397 V, a short-circuit current of 21 μA, and a transfer charge quantity of 232 nC, which were 4, 6, and 6 times higher, respectively, than those of a TENG made with pure PTFE film, as depicted in Fig. 12a and b. This work provided an innovative strategy to simultaneously improve the mechanical and electrical properties of TENGs.
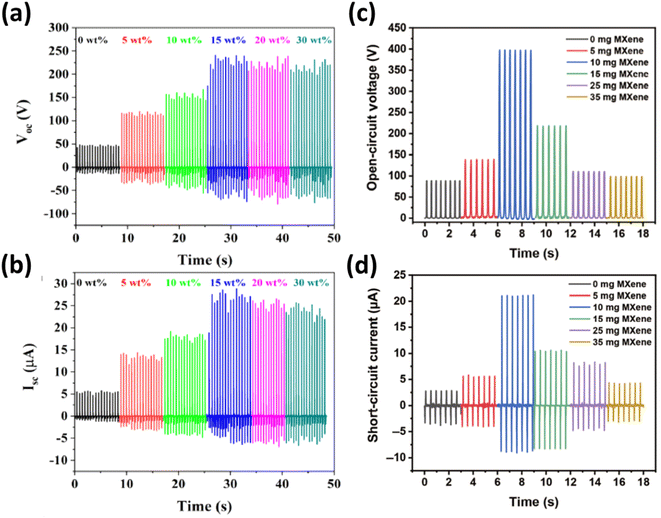 | ||
| Fig. 12 Output performance of the as-fabricated TENG: (a) open-circuit voltage; (b) short-circuit current. Output performance of the crumpled MXene-based TENG: (c) open circuit voltage; (d) short circuit voltage. Reproduced from ref. 94 with permission from Springer Nature, copyright 2021. Reproduced from ref. 95 with permission from Elsevier, copyright 2022. | ||
MXenes have also been shown to enhance dielectric properties and surface charge density. Bhatta et al. found that blending Ti3C2Tx nanosheets into a PVDF matrix substantially improved triboelectric performance.90 The dielectric modulation of PVDF nanofibers by incorporating conductive MXene nanosheets increased the dielectric constant by 270% and the surface charge density by 80%. Mirsepah et al. further demonstrated that MXene integration improved TENG performance.91 To prepare stretchable MXene-based triboelectric layers without compromising triboelectric properties, one approach involves compositing MXene with inherently stretchable materials. However, this method can lead to disadvantages, such as reduced electrical conductivity, limited stretchability, and slow response to external stimuli, limiting practical applications.
To address these issues, Cao et al. introduced a stretchable TENG using crumpled MXene films created by brush-coating MXene ink onto a pre-stretched latex substrate, followed by release.92 Additionally, Answer et al. incorporated a thin film of micron-sized ultrathin Ti3C2Tx MXene sheets (TMSs) into a polyethylene terephthalate (PET)-based tribo-negative electrode.93 After optimizing both triboelectric layers, the TMS-TENG achieved an open-circuit voltage of ∼390 V, a short-circuit current (Isc) of ∼96 μA, and a power density of 6.66 W m−2, as displayed in Fig. 12c and d.
In comparison to alternative materials for energy storage, MXenes with surface functional groups exhibit several beneficial characteristics, including high electrical conductivity and a layered structure that promotes swift ion intercalation and deintercalation. Their remarkable mechanical flexibility and hydrophilicity, coupled with the presence of titanium, contribute to pseudocapacitive behavior through the availability of numerous redox-active sites. However, several challenges must be addressed to fully exploit their capabilities. These challenges include the propensity for restacking due to van der Waals interactions, as well as oxidation instability in humid and aqueous environments. Furthermore, the synthesis process, which frequently involves hazardous and complex methods such as HF etching, presents obstacles regarding scalability and safety. Lastly, careful control of ion selectivity and compatibility across various electrolytes is essential to ensure optimal performance.
3.2. Water remediation
The application of Ti3C2Tx for environmental treatment and resource recovery has been of utmost importance in this field of research. In this section, the fundamentals of the removal of various pollutant via the utilization of Ti3C2Tx are addressed. The areas of focus are heavy metal ions in wastewater, dye degradation, and radionuclides. | ||
| Fig. 13 (a) Water quality before and after evaporation desalination. (b) Evaporation durability activity over 1 cycle and 20 cycles. (c and d) Schematic and optical photograph of the steam generation system. (e) Charging current density of the system from morning to night. (f) Water evaporation rate over one day. Reproduced from ref. 109 with permission from the Royal Society of Chemistry, copyright 2020. | ||
Tan and co-workers studied a Ti3C2Tx coating that improved the photothermal performance and fouling-resistance of a PVDF membrane in solar-assisted membrane distillation.110 The photothermal conversion was calculated to be 5.8 kW m−2, and after 21 h, the PVDF/Ti3C2Tx composite conferred a reduction of around 65% in flux decline in comparison with the uncoated membrane. The as-synthesized composite was able to prevent protein fouling and offer localized heating under light illumination with a large surface area from the multilayered structures.111,112 The photocatalytic performance can endow the photothermal membrane with self-cleaning functionality.
Li et al. synthesized biomimetic MoS2/GO/Ti3C2Tx nanocoatings with improved light-to-heat conversion (up to 93.2%) for solar steam generation.113 The bioinspired Ti3C2Tx nanocoatings resulted in a small loading of solar thermal composite (around 0.32 mg cm−2) but guaranteed high efficiency (1.33 kg m−2 h−1) as compared to another state-of-the-art device.
Zhang and co-workers revealed that carboxyl-terminated Ti3C2Tx MXene displays excellent removal capability for Eu(III) and U(VI) with high adsorption ability (345 mg g−1 for U and 97 mg g−1 for Eu).114 The aryl diazonium salt plays an important role in the stability of the catalyst in water after a one-week stability test, preventing the oxidation process of raw MXene. The key mechanism for improving the removal of radionuclide ions on the composite is the strong affinity of UO2+ and Eu2+ coordinated with the carboxyl terminations, creating inner-sphere surface complexes. Moreover, ion exchange and electrostatic interaction also partially contributed to the effective enrichment of radionuclides. In the same context, inner-sphere complex formation and chemical ion exchange properties are dominant in the adsorption of Ba2+/Sr2+ by Ti3C2Tx MXene.115 Since the electronegativity of Sr2+ (1.0) is greater than that of Ba2+ (0.9), Ba2+ tends to react with the negative surface charges of Ti3C2Tx (Fig. 14). The Ti3C2Tx surface charge becomes more negative with increasing pH value, which improves the free energy between adsorbent and adsorbates. Therefore, the Ti3C2Tx-based catalysts have potential in water purification of model fracking wastewater and radioactive wastewater.
With the economic drawbacks associated with traditional adsorbents, metallic MXene represents a suitable candidate for treating organic-dye-polluted wastewater. Research into the potential application of Ti3C2Tx MXenes for the removal of organic dyes such as methylene blue, 2,4-dinitrophenol, and rhodamine B has been reported based on their better adsorption than several other 2D materials and conventional adsorbents. In order to improve the effectiveness of MXenes, combination and functionalization through the grafting method were considered. The main mechanism of the interaction of Ti3C2Tx MXenes with pollutants has been reported to be single-layer based on the Freundlich and Langmuir isotherms.119,120 As shown in Fig. 15, a Ti3C2Tx–SO3H adsorbent was prepared by coupling-diazotization, and the adsorbent exhibited efficient removal of the cationic dye MB (111.11 mg g−1).120 The positive enthalpy changes (ΔH0; J mol−1) indicated that the adsorption of MB onto the catalysts is an endothermic process, while the negative Gibbs energy changes (ΔG0; J mol−1) demonstrated that the reaction was spontaneous. Moreover, the electrostatic interaction plays an essential role in the removal of methylene blue.
 | ||
| Fig. 15 Ti3C2Tx–SO3H composite for the removal of methylene blue in an alkaline environment. Reproduced from ref. 120 with permission from Elsevier, copyright 2019. | ||
The limitations of Ti3C2Tx MXenes for dye removal are their instability under air, CO2, and other environments. Moreover, a comprehensive assessment of the toxicity of Ti3C2Tx on humans and other organisms has not yet been performed, which limits the application of Ti3C2Tx in dye removal.119 Surface modification is an effective method for improving the biocompatibility of Ti3C2Tx and diminishing its cytotoxic effects on natural ecosystems. MXenes have been modified with dopamine, polyethylene glycol, hyaluronic acid, and glucose to improve their durability.121 In fact, collagen-combined Ti3C2Tx displayed lower toxicity and good cell viability over A375 human skin (malignant melanoma cells) using a zeta potential analyzer.122 An analysis of toxicity in vitro indicated that the modification of Ti3C2Tx MXene with collagen decreases oxidative stress and the generation of reactive oxygen species in non-malignant cells. Gu et al. reported a comparison of the toxicity of MXene quantum dots at the same mass using human endothelial cells (HUVECs). The results confirmed that Ti3C2Tx MXenes are more toxic than Nb2CTx MXene to HUVECs.123 The preparation procedure of Ti3C2Tx is still sophisticated and requires many reaction steps, hazardous acids, and specific precautions, and has a low production yield relative to the precursors, which prevent the scale-up of Ti3C2Tx for applied water treatment process. A summary the use of Ti3C2Tx MXenes and their composites for organic dye removal is provided in Table 2.
| Pollutant | Composite | Uptake/efficiency | Mechanism | Ref. |
|---|---|---|---|---|
| a MB: methylene blue, ST: safranine T, NR: neutral red, AB: acid blue 80, RhB: rhodamine B, CR: congo red, MO: methyl orange. | ||||
| MB | LiOH–Ti3C2Tx | 121 mg g−1 | Adsorption | 124 |
| MB | Ti3C2Tx | 100 mg g−1 | Adsorption | 124 |
| MB | NaOH–Ti3C2Tx | 189 mg g−1 | Adsorption | 124 |
| MB | KOH–Ti3C2Tx | 77 mg g−1 | Adsorption | 124 |
| ST | MXene-COOH@(PEI/PAA)n | 33 mg g−1 | Adsorption | 125 |
| NR | MXene-COOH@(PEI/PAA)n | 42 mg g−1 | Adsorption | 125 |
| MB | Ti3C2Tx | 140 mg g−1 | Adsorption | 126 |
| AB | Ti3C2Tx | 200 mg g−1 | Adsorption | 126 |
| MB | Phytic acid (PA)-Ti3C2Tx | 42 mg g−1 | Adsorption | 127 |
| RhB | Phytic acid (PA)-Ti3C2Tx | 22 mg g−1 | Adsorption | 127 |
| MB | Surface charged Ti3C2Tx | 2460 mg g−1 | Adsorption | 128 |
| MB | F-terminated Ti3C2Tx | 92% | Adsorption | 129 |
| MB | h Ti3C2Tx | 24 mg g−1 | Adsorption | 130 |
| MB | Ti3C2Tx | 39 mg g−1 | Adsorption | 131 |
| MB | Cellulose ester/Ti3C2Tx | 100% | Adsorption | 132 |
| CR | PEI/Ti3C2Tx | 3568 mg g−1 | Adsorption | 133 |
| MB | AAC/Ti3C2Tx | 311.5 | Adsorption | 134 |
| MO | Ti3C2Tx | 94 mg g−1 | Adsorption | 135 |
| Cr(VI) | Ti3C2Tx | 104 mg g−1 | Adsorption | 135 |
| MB | Ti3C2Tx–SO3H | 111 mg g−1 | Adsorption | 120 |
| MB | Ti3C2Tx/sodium alginate | 92 mg g−1 | Adsorption | 136 |
| MB | Ti3C2Tx/Fe3O4 | 1.71 mg g−1 | Reduction/adsorption | 137 |
| RhB | Ti3C2Tx/Co3O4 | 47 mg g−1 | Reduction/adsorption | 138 |
| MB | Ti3C2Tx/Co3O4 | 136 mg g−1 | Reduction/adsorption | 138 |
The multilayered morphology and extensive surface area of MXene-based materials significantly enhance pollutant adsorption by offering numerous active sites and interlayer spacing, which promote direct interactions with surface functional groups through mechanisms such as hydrogen bonding, electrostatic attraction, and chelation. Their superior electrical conductivity and highly reactive surfaces facilitate rapid adsorption kinetics and increased reaction rates through ion exchange and surface complexation. Nonetheless, a critical drawback of MXene-based materials is their susceptibility to oxidation when exposed to light and oxygen-rich environments, which can lead to restacking and aggregation. These processes considerably diminish the accessible surface area and the availability of active sites. Additionally, the potential environmental toxicity of MXenes necessitates comprehensive evaluation prior to their large-scale application and environmental release.
4. Conclusion and perspectives
This review summarizes the recent advances in the use of Ti3C2Tx MXene as a catalyst, adsorbent, and photocatalytic agent in energy storage and dye removal from wastewater. MXenes have been prepared using various etchants (HCl/LiF, NaF/LiF/KF), with a focus on direct HF and in-situ-formed HF etchants for effective products. After etching, organic molecules are associated with the intercalation and delamination of single-layered Ti3C2Tx MXene. Tip sonication offers a smaller flake size of Ti3C2Tx with defects relative to bath sonication, which should optimize the sonication parameters. Although successful results have been obtained, further in-depth exploration is required.Regarding the raw sources, the MAX phase is prepared at high temperatures using sophisticated machine systems. Toxic HF and other fluoride-containing etchants have been used to etch Ti3C2Tx MXene; more efforts are needed in this particular aspect. Connections between theoretical modeling calculations and the practical applications of Ti3C2Tx need to be established to provide the fundamentals for understanding the unique composition-related properties of Ti3C2Tx MXenes. Subsequently, the scale-up process between laboratories and industries could be solved and avoid risks. From the point of view of multilayered structures, the lamellar structure of the MXene-based materials has a significant effect on their results as either sorbents or electrodes. Furthermore, engineering high-surface-area materials using Ti3C2Tx MXene-derived composites is massively desirable for enhanced performance. Finally, further research efforts should be devoted to applications. Although massive enhancements have been achieved, the performance stability of Ti3C2Tx MXene-derived composite sorbents and electrodes still need to surpass that of conventional carbon-based materials.
In wastewater treatment, it is essential to elucidate a comprehensive and plausible mechanism underlying the interaction between adsorbates and Ti3C2Tx-based sorbents. This understanding is crucial for guiding the design and application of MXene materials to a broad range of contaminants. Despite employing similar raw materials and synthesis techniques, significant variations in sorption performance are often observed and achieved. These discrepancies underscore the need for a deeper investigation into the physicochemical interactions at the molecular level, including surface functional groups, interlayer spacing, and the role of terminal groups (–OH, –O, –F), which significantly influence adsorption capacity and selectivity.
For energy storage applications, the principal challenge in transitioning Ti3C2Tx MXene-derived composites from laboratory-scale research to commercial viability lies in the scalable production of materials with large surface areas, structural uniformity, and reproducible electrochemical performance. Overcoming synthesis-related inconsistencies, such as flake aggregation, oxidation during processing, and variability in surface terminations, will be crucial to ensuring consistent device performance. Moreover, the integration of Ti3C2Tx MXenes into TENG has emerged as a promising strategy to enhance energy conversion efficiency and expand the functionality of self-powered systems. This synergy has opened new opportunities in fields such as environmental sensing, wearable electronics, and sustainable energy harvesting. The unique combination of TENG technology with the tunable properties of MXenes represents a significant advancement, offering multifunctional platforms capable of simultaneously addressing energy and environmental challenges.
In conclusion, the application of Ti3C2Tx MXene in both environmental remediation and energy storage presents a transformative pathway to tackle pressing global issues such as energy depletion and water pollution. Although challenges remain in terms of scalability, stability, and mechanistic understanding, continued research efforts and innovative material design are well justified and hold substantial potential for real-world impact toward sustainable development in the 4.0 era.
Data availability
No data were used in the research described in the article.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.Acknowledgements
This research was supported by Project 2395 of the Ministry of Science and Technology of Vietnam and the Vietnam Academy of Science and Technology (VAST) under Grant Number THTEXS.02/24-26.References
- M. N. Tran, A. Skorynina, A. Addad, A. Fadel, K. Ben Tayeb, L. Karmazin, L. Thomas, M. Corda, Y. Wisse, O. Vovk, A. Y. Khodakov, B. Grandidier and V. Ordomsky, Appl. Catal., B, 2025, 363, 124834 CrossRef CAS.
- J. Wu, G. Yin, J. Liu, Z. Z. Yu and X. Li, Mater. Horiz., 2025 10.1039/D4MH01857E.
- S. A. Malin, Energy Res. Soc. Sci., 2025, 119, 103867 CrossRef.
- C. Li, D. Zhou, F. Zheng, Y. Wang and K. Bi, Chem. Eng. J., 2025, 505, 159765 CrossRef CAS.
- B. Felsmann, A. Guerrini, G. Hajdari, A. Kis and G. Romano, Appl. Energy, 2025, 380, 125076 CrossRef.
- A. Jägerskog, T. J. Clausen, T. Holmgren and K. Lexén, Energy and Water: The Vital Link for a Sustainable Future, Stockholm International Water Institute (SIWI), Stockholm, 2014 Search PubMed.
- Q. Ma, Y. Yu, M. Sindoro, A. G. Fane, R. Wang and H. Zhang, Adv. Mater., 2017, 29, 1605361 CrossRef PubMed.
- D. Y. Goswami and F. Kreith, in Energy Conversion, CRC Press, 2017, pp. 1–30 Search PubMed.
- A. Panday and H. O. Bansal, Int. J. Global Energy, 2014, 37, 304–318 CrossRef.
- Q. T. H. Ta, E. Cho, A. Sreedhar and J.-S. Noh, J. Catal., 2019, 371, 1–9 CrossRef CAS.
- P. S. Lee and X. Chen, Small, 2014, 10(17), 3432–3433 CrossRef CAS PubMed.
- Y. Gogotsi, MXenes: From Discovery to Applications of Two-Dimensional Metal Carbides and Nitrides, CRC Press, 2023 Search PubMed.
- Q. T. H. Ta, D. Thakur and J.-S. Noh, Chemosensors, 2023, 11, 477 CrossRef CAS.
- A. Sreedhar, Q. T. H. Ta and J.-S. Noh, Chemosphere, 2022, 135478 CrossRef CAS PubMed.
- S. G. Eswaran, M. Rashad, A. S. K. Kumar and A. F. M. E. Mahdy, Chem.–Asian J., 2025 DOI:10.1002/asia.202401181.
- Q. T. H. Ta, N. M. Tran, N. N. Tri, A. Sreedhar and J. S. Noh, Chem. Eng. J., 2021, 425, 131437 CrossRef CAS.
- K. Diedkova, A. D. Pogrebnjak, S. Kyrylenko, K. Smyrnova, V. V Buranich, P. Horodek, P. Zukowski, T. N. Koltunowicz, P. Galaszkiewicz and K. Makashina, ACS Appl. Mater. Interfaces, 2023, 15, 14033–14047 CAS.
- B. Anasori and Y. Gogotsi, Graphene 2D Nanomater., 2023, 1–3 Search PubMed.
- M. Alhabeb, K. Maleski, B. Anasori, P. Lelyukh, L. Clark and S. Sin, Chem. Mater., 2017 DOI:10.1021/acs.chemmater.7b02847.
- P. Praus, A. Smýkalová, R. Škuta, M. Koštejn, J. Pavlovský, J. Tokarský, K. Foniok, M. F. Edelmannová and K. Kočí, J. Photochem. Photobiol., A, 2023, 115260 Search PubMed.
- T. P. Nguyen, D. M. T. Nguyen, H. K. Le, D.-V. N. Vo, S. S. Lam, R. S. Varma, M. Shokouhimehr, C. C. Nguyen and Q. Van Le, Mol. Catal., 2020, 486, 110850 CrossRef CAS.
- N. T. T. Phuong, T. T. Nguyen, N. N. Nam and K. T. L. Trinh, Sens. Actuators, A, 2023, 114714 CrossRef CAS.
- K. Yang, C. Li, H. Qi, Y. Dai, Y. Cui and Y. He, J. Mater. Chem. A, 2023, 11, 10425–10434 RSC.
- Z. Cao, Y. B. Zhu, K. Chen, Q. Wang, Y. Li, X. Xing, J. Ru, L. G. Meng, J. Shu, N. Shpigel and L. F. Chen, Adv. Mater., 2024, 36, 2401271 CrossRef CAS PubMed.
- Z. Cao, H. Hu and D. Ho, Adv. Funct. Mater., 2022, 32, 2111805 CrossRef CAS.
- Z. Cao, G. Liang, D. Ho, C. Zhi and H. Hu, Adv. Funct. Mater., 2023, 33, 2303060 CrossRef CAS.
- J. Zhang, K. Wang, P. Lu, J. Gao, Z. Cao, F. Mo, D. Ho, B. Li and H. Hu, Adv. Funct. Mater., 2024, 34, 2310775 CrossRef CAS.
- Q. T. H. Ta, L. T. Nhiem, D. T. Y. Oanh, N. H. Hieu and P. K. T. Nguyen, Vietnam J. Chem., 2024 DOI:10.1002/VJCH.202400155.
- N. R. Hemanth, T. Kim, B. Kim, A. H. Jadhav, K. Lee and N. K. Chaudhari, Mater Chem Front, 2021, 5, 3298–3321 RSC.
- N. K. Chaudhari, ab Hanuel Jin, B. Kim, D. San Baek, S. Hoon Joo and K. Lee, J. Mater. Chem. A, 2017 10.1039/c7ta09094c.
- A. Sreedhar, P. Ravi and J.-S. Noh, J. Mater. Sci. Technol., 2024, 203, 237–254 CrossRef CAS.
- Y. Gan and Y. Xiong, RSC Adv., 2025, 15, 9555–9568 RSC.
- S. Zhang, L. Wang, Z. Feng, Z. Wang, Y. Wang, B. Wei, H. Liu, W. Zhao and J. Li, ACS Nano, 2025, 19, 9635 Search PubMed.
- I. Mubeen, S. Shah, E. Pervaiz and W. Miran, Mater. Sci. Energy Technol., 2024, 7, 180–194 CAS.
- Q. T. H. Ta, D. Thakur and J.-S. Noh, Chemosensors, 2023, 11, 477 CrossRef CAS.
- C. Wang, C. L. Tracy and R. C. Ewing, Appl. Phys. Rev., 2020, 7 DOI:10.1063/5.0019284.
- C. Ferrara, A. Gentile, S. Marchionna and R. Ruffo, Curr. Opin. Electrochem., 2021, 29, 100764 CrossRef CAS.
- U. Amara, I. Hussain, M. Ahmad, K. Mahmood and K. Zhang, Small, 2023, 19, 2205249 CrossRef CAS PubMed.
- N. M. Tran, Q. T. H. Ta and J.-S. Noh, Appl. Surf. Sci., 2021, 538, 148023 CrossRef.
- M. Benchakar, L. Loupias, C. Garnero, T. Bilyk, C. Morais, C. Canaff, N. Guignard, S. Morisset, H. Pazniak and S. Hurand, Appl. Surf. Sci., 2020, 530, 147209 CrossRef CAS.
- A. Gentile, C. Ferrara, S. Tosoni, M. Balordi, S. Marchionna, F. Cernuschi, M. Kim, H. Lee and R. Ruffo, Small Methods, 2020, 4, 2000314 CrossRef CAS.
- T. Li, L. Yao, Q. Liu, J. Gu, R. Luo, J. Li, X. Yan, W. Wang, P. Liu and B. Chen, Angew. Chem., Int. Ed., 2018, 57, 6115–6119 CrossRef CAS PubMed.
- Y. Wang, J. Zhang, X. Wang, M. Antonietti and H. Li, Angew. Chem., Int. Ed., 2010, 49, 3356–3359 CrossRef CAS PubMed.
- K. Mahabari, R. D. Mohili, M. Patel, A. H. Jadhav, K. Lee and N. K. Chaudhari, Nanoscale Adv., 2024, 6, 5388–5397 RSC.
- W. Yang, J. Yang, J. J. Byun, F. P. Moissinac, J. Xu, S. J. Haigh, M. Domingos, M. A. Bissett, R. A. W. Dryfe and S. Barg, Adv. Mater., 2019, 31, 1902725 CrossRef PubMed.
- N. J. Prakash and B. Kandasubramanian, J. Alloys Compd., 2021, 862, 158547 CrossRef.
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2015, 516, 78–81 CrossRef PubMed.
- A. Jawaid, A. Hassan, G. Neher, D. Nepal, R. Pachter, W. J. Kennedy, S. Ramakrishnan and R. A. Vaia, ACS Nano, 2021, 15, 2771–2777 CrossRef CAS PubMed.
- Y. An, Y. Tian, H. Shen, Q. Man, S. Xiong and J. Feng, Energy Environ. Sci., 2023, 16, 4191–4250 RSC.
- Y. Pei, X. Zhang, Z. Hui, J. Zhou, X. Huang, G. Sun and W. Huang, ACS Nano, 2021, 15, 3996–4017 CrossRef CAS PubMed.
- J. Liu, H. Zhang, R. Sun, Y. Liu, Z. Liu, A. Zhou and Z. Yu, Adv. Mater., 2017, 29, 1702367 CrossRef PubMed.
- A. Lipatov, M. Alhabeb, M. R. Lukatskaya, A. Boson, Y. Gogotsi and A. Sinitskii, Adv. Electron. Mater., 2016, 2, 1600255 CrossRef.
- F. Kong, X. He, Q. Liu, X. Qi, Y. Zheng, R. Wang and Y. Bai, Ceram. Int., 2018, 44, 11591–11596 CrossRef CAS.
- K. Wang, Y. Zhou, W. Xu, D. Huang, Z. Wang and M. Hong, Ceram. Int., 2016, 42, 8419–8424 CrossRef CAS.
- G. Li, K. Jiang, S. Zaman, J. Xuan, Z. Wang and F. Geng, Inorg. Chem., 2019, 58, 9397–9403 CrossRef CAS PubMed.
- M. Naguib, O. Mashtalir, M. R. Lukatskaya, B. Dyatkin, C. Zhang, V. Presser, Y. Gogotsi and M. W. Barsoum, Chem. Commun., 2014, 50, 7420–7423 RSC.
- D. Xiong, X. Li, Z. Bai and S. Lu, Small, 2018, 14, 1703419 CrossRef PubMed.
- V. N. Borysiuk, V. N. Mochalin and Y. Gogotsi, Nanotechnology, 2015, 26, 265705 CrossRef PubMed.
- A. Lipatov, H. Lu, M. Alhabeb, B. Anasori, A. Gruverman, Y. Gogotsi and A. Sinitskii, Sci. Adv., 2018, 4, eaat0491 CrossRef PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- Z. Ling, C. E. Ren, M.-Q. Zhao, J. Yang, J. M. Giammarco, J. Qiu, M. W. Barsoum and Y. Gogotsi, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 16676–16681 CrossRef CAS PubMed.
- H. Liu, C. Du, L. Liao, H. Zhang, H. Zhou, W. Zhou, T. Ren, Z. Sun, Y. Lu, Z. Nie, F. Xu, J. Zhu and W. Huang, Nat. Commun., 2022, 13(13), 1–11 Search PubMed.
- V. Augustyn, J. Come, M. A. Lowe, J. W. Kim, P. L. Taberna, S. H. Tolbert, H. D. Abruña, P. Simon and B. Dunn, Nat. Mater., 2013, 12(6), 518–522 CrossRef CAS PubMed.
- L. Fei, L. Lei, H. Xu, X. Guo, B. Chen, X. Han, X. Chen, Q. Huang and D. Wang, Carbon Energy, 2025, 7, e678 CrossRef CAS.
- Q. Tang, Z. Zhou and P. Shen, J. Am. Chem. Soc., 2012, 134, 16909–16916 CrossRef CAS PubMed.
- C. Choi, D. S. Ashby, D. M. Butts, R. H. DeBlock, Q. Wei, J. Lau and B. Dunn, Nat. Rev. Mater., 2019, 5(1), 5–19 CrossRef.
- L. Wei, C. Xiong, H. R. Jiang, X. Z. Fan and T. S. Zhao, Energy Storage Mater., 2020, 25, 885–892 CrossRef.
- A. V. Mohammadi, J. Rosen and Y. Gogotsi, Science, 2021, 372 DOI:10.1126/science.abf1581.
- J. Zhang, N. Kong, S. Uzun, A. Levitt, S. Seyedin, P. A. Lynch, S. Qin, M. Han, W. Yang and J. Liu, Adv. Mater., 2020, 32, 2001093 CrossRef CAS PubMed.
- C. F. Du, Q. Liang, Y. Zheng, Y. Luo, H. Mao and Q. Yan, ACS Appl. Mater. Interfaces, 2018, 10, 33779–33784 CrossRef CAS PubMed.
- A. Ali, K. Hantanasirisakul, A. Abdala, P. Urbankowski, M.-Q. Zhao, B. Anasori, Y. Gogotsi, B. Aïssa and K. A. Mahmoud, Langmuir, 2018, 34, 11325–11334 CrossRef CAS PubMed.
- C. Bommier, W. Luo, W.-Y. Gao, A. Greaney, S. Ma and X. Ji, Carbon N Y, 2014, 76, 165–174 CrossRef CAS.
- M. Xia, B. Chen, F. Gu, L. Zu, M. Xu, Y. Feng, Z. Wang, H. Zhang, C. Zhang and J. Yang, ACS Nano, 2020, 14, 5111–5120 CrossRef CAS PubMed.
- T. Shen, X. Xia, D. Xie, Z. Yao, Y. Zhong, J. Zhan, D. Wang, J. Wu, X. Wang and J. Tu, J. Mater. Chem. A, 2017, 5, 11197–11203 RSC.
- N. Liu, Z. Lu, J. Zhao, M. T. McDowell, H.-W. Lee, W. Zhao and Y. Cui, Nat. Nanotechnol., 2014, 9, 187–192 CrossRef CAS PubMed.
- E. M. Clarke and J. M. Wing, ACM Comput. Surv., 1996, 28, 626–643 CrossRef.
- H. Tang, W. Li, L. Pan, K. Tu, F. Du, T. Qiu, J. Yang, C. P. Cullen, N. McEvoy and C. Zhang, Adv. Funct. Mater., 2019, 29, 1901907 CrossRef.
- Y. Li, S. Arnold, S. Husmann and V. Presser, J. Energy Storage, 2023, 60, 106625 CrossRef.
- K. Yang, F. Zhao, J. Li, H. Yang, Y. Wang and Y. He, Adv. Funct. Mater., 2024, 34, 2410236 CrossRef CAS.
- Q. Zhao, Q. Zhu, J. Miao, P. Zhang, P. Wan, L. He and B. Xu, Small, 2019, 15, 1904293 CrossRef CAS PubMed.
- M.-Q. Zhao, M. Torelli, C. E. Ren, M. Ghidiu, Z. Ling, B. Anasori, M. W. Barsoum and Y. Gogotsi, Nano Energy, 2016, 30, 603–613 CrossRef CAS.
- Y. Liu, P. Zhang, N. Sun, B. Anasori, Q. Zhu, H. Liu, Y. Gogotsi and B. Xu, Adv. Mater., 2018, 30, 1707334 CrossRef PubMed.
- B. Tan, T. Lu, W. Luo, Z. Chao, R. Dong and J. Fan, Energ. Fuels, 2021, 35, 12666–12670 CrossRef CAS.
- Z. Li, X. X. Wang, W. Zhang and S. Yang, Chem. Eng. J., 2020, 398, 125679 CrossRef CAS.
- W. T. Cao, H. Ouyang, W. Xin, S. Chao, C. Ma, Z. Li, F. Chen and M. G. Ma, Adv. Funct. Mater., 2020, 30, 2004181 CrossRef CAS.
- Y. Du, X. Wang, X. Dai, W. Lu, Y. Tang and J. Kong, J. Mater. Sci. Technol., 2022, 100, 1–11 CrossRef CAS.
- X. Cai, Y. Xiao, B. Zhang, Y. Yang, J. Wang, H. Chen and G. Shen, Adv. Funct. Mater., 2023, 33, 2304456 CrossRef CAS.
- X. Luo, L. Zhu, Y. C. Wang, J. Li, J. Nie and Z. L. Wang, Adv. Funct. Mater., 2021, 31, 2104928 CrossRef CAS.
- Y. Gao, G. Liu, T. Bu, Y. Liu, Y. Qi, Y. Xie, S. Xu, W. Deng, W. Yang and C. Zhang, Nano Res., 2021, 14, 4833–4840 CrossRef CAS.
- T. Bhatta, P. Maharjan, H. Cho, C. Park, S. H. Yoon, S. Sharma, M. Salauddin, M. T. Rahman, S. S. Rana and J. Y. Park, Nano Energy, 2021, 81, 105670 CrossRef CAS.
- A. Mirsepah, L. Shooshtari, R. Mohammadpour, A. Esfandiar and A. Irajizad, Chem. Eng. J., 2024, 499, 155953 CrossRef CAS.
- Y. Cao, Y. Guo, Z. Chen, W. Yang, K. Li, X. He and J. Li, Nano Energy, 2022, 92, 106689 CrossRef CAS.
- S. Anwer, M. Umair Khan, B. Mohammad, M. Rezeq, W. Cantwell, D. Gan and L. Zheng, Chem. Eng. J., 2023, 470, 144281 CrossRef CAS.
- Y. Gao, G. Liu, T. Bu, Y. Liu, Y. Qi, Y. Xie, S. Xu, W. Deng, W. Yang and C. Zhang, Nano Res., 2021, 14, 4833–4840 CrossRef CAS.
- Y. Du, X. Wang, X. Dai, W. Lu, Y. Tang and J. Kong, J. Mater. Sci. Technol., 2022, 100, 1–11 CrossRef CAS.
- R. A. Murugesan and K. C. Nagamuthu Raja, Mater. Res. Bull., 2023, 163, 112217 CrossRef CAS.
- H. Huang, J. Cui, G. Liu, R. Bi and L. Zhang, ACS Nano, 2019, 13, 3448–3456 CrossRef CAS PubMed.
- S. Xu, G. Wei, J. Li, W. Han and Y. Gogotsi, J. Mater. Chem. A, 2017, 5, 17442–17451 RSC.
- I. Ayman, A. Rasheed, S. Ajmal, A. Rehman, A. Ali, I. Shakir and M. F. Warsi, Energ. Fuels, 2020, 34, 7622–7630 CrossRef CAS.
- K. Nasrin, V. Sudharshan, M. Arunkumar and M. Sathish, ACS Appl. Mater. Interfaces, 2022, 14, 21038–21049 CrossRef CAS PubMed.
- Y. Z. Cai, Y. S. Fang, W. Q. Cao, P. He and M. S. Cao, J. Alloys Compd., 2021, 868, 159159 CrossRef CAS.
- H. Jiang, Z. Wang, Q. Yang, M. Hanif, Z. Wang, L. Dong and M. Dong, Electrochim. Acta, 2018, 290, 695–703 CrossRef CAS.
- K. Nasrin, V. Sudharshan, K. Subramani, M. Karnan and M. Sathish, Small, 2022, 18, 2106051 CrossRef CAS PubMed.
- L. Li, N. Zhang, M. Zhang, X. Zhang and Z. Zhang, Dalton Trans., 2019, 48, 1747–1756 RSC.
- Y. Liu, H. Zhou, W. Zhou, S. Meng, C. Qi, Z. Liu and T. Kong, Adv. Energy Mater., 2021, 11, 2101329 CrossRef CAS.
- Y. Yao, X. Zhang, L. Tan, J. Pan, C. Zhan, W. Liu, Y. Feng, H. Li and L. Xiong, ACS Appl. Nano Mater., 2024, 7, 15143–15152 CrossRef CAS.
- S. Kumar, M. A. Rehman, S. Lee, M. Kim, H. Hong, J. Y. Park and Y. Seo, Sci. Rep., 2021, 11(1), 1–9 CrossRef PubMed.
- W. Cheng, J. Fu, H. Hu and D. Ho, Adv. Sci., 2021, 8, 2100775 CrossRef CAS PubMed.
- X. Zhao, L.-M. Peng, C.-Y. Tang, J.-H. Pu, X.-J. Zha, K. Ke, R.-Y. Bao, M.-B. Yang and W. Yang, Mater. Horiz., 2020, 7, 855–865 RSC.
- Y. Z. Tan, H. Wang, L. Han, M. B. Tanis-Kanbur, M. V. Pranav and J. W. Chew, J. Membr. Sci., 2018, 565, 254–265 Search PubMed.
- H. Wang, Y. Wu, X. Yuan, G. Zeng, J. Zhou, X. Wang and J. W. Chew, Adv. Mater., 2018, 30, 1704561 CrossRef PubMed.
- F. Jamil, H. M. Ali and M. M. Janjua, J. Energy Storage, 2021, 35, 102322 Search PubMed.
- K. Li, T.-H. Chang, Z. Li, H. Yang, F. Fu, T. Li, J. S. Ho and P.-Y. Chen, Adv. Energy Mater., 2019, 9, 1901687 Search PubMed.
- P. Zhang, L. Wang, K. Du, S. Wang, Z. Huang, L. Yuan, Z. Li, H. Wang, L. Zheng and Z. Chai, J. Hazard. Mater., 2020, 396, 122731 CrossRef CAS PubMed.
- B.-M. Jun, C. M. Park, J. Heo and Y. Yoon, J. Environ. Manage., 2020, 256, 109940 Search PubMed.
- J. A. Kumar, P. Prakash, T. Krithiga, D. J. Amarnath, J. Premkumar, N. Rajamohan, Y. Vasseghian, P. Saravanan and M. Rajasimman, Chemosphere, 2022, 286, 131607 CrossRef CAS PubMed.
- R. Rajan, M. Rajasimman and R. Natarajan, Chem. Prod. Process Model., 2010 DOI:10.2202/1934-2659.1517.
- Q. Thanh Hoai Ta, S. Park and J.-S. Noh, J. Colloid Interface Sci., 2017, 505, 437–444 CrossRef CAS PubMed.
- Y. Ibrahim, M. Meslam, K. Eid, B. Salah, A. M. Abdullah, K. I. Ozoemena, A. Elzatahry, M. A. Sharaf and M. Sillanpää, Sep. Purif. Technol., 2022, 282, 120083 CrossRef CAS.
- Y. Lei, Y. Cui, Q. Huang, J. Dou, D. Gan, F. Deng, M. Liu, X. Li, X. Zhang and Y. Wei, Ceram. Int., 2019, 45, 17653–17661 CrossRef CAS.
- A. M. Jastrzębska, A. Szuplewska, T. Wojciechowski, M. Chudy, W. Ziemkowska, L. Chlubny, A. Rozmysłowska and A. Olszyna, J. Hazard. Mater., 2017, 339, 1–8 CrossRef PubMed.
- A. Rozmysłowska-Wojciechowska, A. Szuplewska, T. Wojciechowski, S. Poźniak, J. Mitrzak, M. Chudy, W. Ziemkowska, L. Chlubny, A. Olszyna and A. M. Jastrzębska, Mater. Sci. Eng. Carbon, 2020, 111, 110790 CrossRef PubMed.
- M. Gu, Z. Dai, X. Yan, J. Ma, Y. Niu, W. Lan, X. Wang and Q. Xu, J. Appl. Toxicol., 2021, 41, 745–754 CrossRef CAS PubMed.
- Z. Wei, Z. Peigen, T. Wubian, Q. Xia, Z. Yamei and S. ZhengMing, Mater. Chem. Phys., 2018, 206, 270–276 CrossRef CAS.
- K. Li, G. Zou, T. Jiao, R. Xing, L. Zhang, J. Zhou, Q. Zhang and Q. Peng, Colloids Surf., A, 2018, 553, 105–113 CrossRef CAS.
- B.-M. Jun, J. Heo, N. Taheri-Qazvini, C. M. Park and Y. Yoon, Ceram. Int., 2020, 46, 2960–2968 CrossRef CAS.
- C. Cai, R. Wang, S. Liu, X. Yan, L. Zhang, M. Wang, Q. Tong and T. Jiao, Colloids Surf., A, 2020, 589, 124468 CrossRef CAS.
- B. Sun, X. Dong, H. Li, Y. Shang, Y. Zhang, F. Hu, S. Gu, Y. Wu, T. Gao and G. Zhou, Sep. Purif. Technol., 2021, 272, 118964 CrossRef CAS.
- N. M. Tran, Q. T. H. Ta, A. Sreedhar and J.-S. Noh, Appl. Surf. Sci., 2021, 537, 148006 CrossRef.
- C. Peng, P. Wei, X. Chen, Y. Zhang, F. Zhu, Y. Cao, H. Wang, H. Yu and F. Peng, Ceram. Int., 2018, 44, 18886–18893 CrossRef CAS.
- O. Mashtalir, K. M. Cook, V. N. Mochalin, M. Crowe, M. W. Barsoum and Y. Gogotsi, J. Mater. Chem. A, 2014, 2, 14334–14338 RSC.
- S. Zhang, S. Liao, F. Qi, R. Liu, T. Xiao, J. Hu, K. Li, R. Wang and Y. Min, J. Hazard. Mater., 2020, 384, 121367 CrossRef CAS PubMed.
- Y. Feng, H. Wang, J. Xu, X. Du, X. Cheng, Z. Du and H. Wang, J. Hazard. Mater., 2021, 416, 125777 CrossRef CAS PubMed.
- Y. Li, C. Pan, P. Kamdem and X.-J. Jin, Energy Fuels, 2020, 34, 10120–10130 CrossRef CAS.
- P. Karthikeyan, K. Ramkumar, K. Pandi, A. Fayyaz, S. Meenakshi and C. M. Park, Ceram. Int., 2021, 47, 3692–3698 CrossRef CAS.
- Z.-H. Zhang, J.-Y. Xu and X.-L. Yang, Mater. Chem. Phys., 2021, 260, 124123 CrossRef CAS.
- Z. Zhu, M. Xiang, L. Shan, T. He and P. Zhang, J. Solid State Chem., 2019, 280, 120989 CrossRef CAS.
- S. Luo, R. Wang, J. Yin, T. Jiao, K. Chen, G. Zou, L. Zhang, J. Zhou, L. Zhang and Q. Peng, ACS Omega, 2019, 4, 3946–3953 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |

