 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A biocompatible β-cyclodextrin inclusion complex containing natural extracts: a promising antibiofilm agent†
Obaydah Abd Alkader
Alabrahim
 a,
Mostafa
Fytory
ab,
Ahmed M.
Abou-Shanab
c,
Jude
Lababidi
a,
Wolfgang
Fritzsche
d,
Nagwa
El-Badri
*c and
Hassan Mohamed El-Said
Azzazy
a,
Mostafa
Fytory
ab,
Ahmed M.
Abou-Shanab
c,
Jude
Lababidi
a,
Wolfgang
Fritzsche
d,
Nagwa
El-Badri
*c and
Hassan Mohamed El-Said
Azzazy
 *ad
*ad
aDepartment of Chemistry, School of Sciences & Engineering, The American University in Cairo, AUC Avenue, P.O. Box 74, New Cairo 11835, Egypt. E-mail: hazzazy@aucegypt.edu; Tel: +202 2615 2559
bMaterial Science and Nanotechnology Department, Faculty of Postgraduate Studies for Advanced Sciences (PSAS), Beni-Suef University, 62511, Beni-Suef, Egypt
cCenter of Excellence for Stem Cells and Regenerative Medicine, Zewail City of Science and Technology, Giza, 12578, Egypt. E-mail: nelbadri@zewailcity.edu.eg
dDepartment of Nanobiophotonics, Leibniz Institute of Photonic Technology, Jena 07745, Germany
First published on 13th January 2025
Abstract
Biofilms formed by several bacterial strains still pose a significant challenge to healthcare due to their resistance to conventional treatment approaches, including antibiotics. This study explores the potential of loading natural extracts with antimicrobial activities into β-cyclodextrin (βCD) nanoparticles, which are FDA-approved and have superior biocompatibility owing to their cyclic sugar structures, for biofilm eradication. An inclusion complex of βCD carrying Boswellia sacra essential oils (BOS) was prepared and characterized with regard to its physicochemical properties, antimicrobial efficacy, and antibiofilm activities. Encapsulation of BOS into βCD significantly enhanced the antimicrobial activity of BOS by 4-fold against Gram-positive (Staphylococcus aureus and Bacillus subtilis) and by 8-fold against Gram-negative (Escherichia coli and Pseudomonas putida) bacteria, with minimum inhibitory concentrations ranging from 2.5 to 5 mg mL−1. Furthermore, the BOS-βCD complex demonstrated a dual-action against bacterial biofilms where it prevented biofilm formation and disrupted established biofilms. This resulted in a significant reduction in biofilm biomass, with prevention and disruption rates reaching up to 93.78% and 82.17%, respectively. Additionally, the formula revealed an excellent biocompatibility profile with no induction of oxidative stress in human skin fibroblast cells. Our findings suggest that βCD nanoparticles loaded with BOS essential oils hold promise as an effective formula for preventing the formation of bacterial biofilms and combating preformed ones for use in relevant medical applications.
1. Introduction
Biofilms are among the major health concerns causing serious bacterial infections which can be developed by several strains of bacteria including Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), and Bacillus cereus (B. cereus).1,2 Biofilms are responsible for 1.96 million bacterial infections annually in the United States of America, resulting in higher health costs (>$18B) and more than 260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths.3 Biofilms can be defined as highly organized bacterial communities tightly surrounded by a self-produced protective layer made of extracellular matrix.1,2 Therefore, these sorts of biofilms have provoked the formation of multidrug resistant microbes, owing to their capability to maintain a well-protected environment for bacteria with secure support of nutrient supply and provide altered pH medium coupled with greater shear of mechanical forces in the surrounding environment, securing a well-habitable medium for bacteria to combat and develop antibiotic resistance.1,2,4,5 Moreover, biofilms can effectively develop resistance against antibiotics, combat host defense system, fight external stressors, and form a prime hindrance in delaying the healing of chronic wounds, establishing a challenging reservoir for harmful and opportunistic pathogens.1,2,6–8 About 80% of chronic microbial infections such as otitis, vaginitis, gingivitis, urethritis, conjunctivitis, colitis, prostatitis, and osteomyelitis are attributed to infections developed by bacterial biofilms.6,7,9 For instance, E.coli were detected in serious urinary tract infections with their capability to develop biofilms.10,11 Additionally, S. aureus were reported to develop colonies on medical devices developing biofilms and hence causing several nosocomial infections such as osteomyelitis, endocarditis, pneumonia, and sepsis.12,13 Thus, targeting infections developed by such biofilms still presents a major challenge which warrants the search of new approaches.
000 deaths.3 Biofilms can be defined as highly organized bacterial communities tightly surrounded by a self-produced protective layer made of extracellular matrix.1,2 Therefore, these sorts of biofilms have provoked the formation of multidrug resistant microbes, owing to their capability to maintain a well-protected environment for bacteria with secure support of nutrient supply and provide altered pH medium coupled with greater shear of mechanical forces in the surrounding environment, securing a well-habitable medium for bacteria to combat and develop antibiotic resistance.1,2,4,5 Moreover, biofilms can effectively develop resistance against antibiotics, combat host defense system, fight external stressors, and form a prime hindrance in delaying the healing of chronic wounds, establishing a challenging reservoir for harmful and opportunistic pathogens.1,2,6–8 About 80% of chronic microbial infections such as otitis, vaginitis, gingivitis, urethritis, conjunctivitis, colitis, prostatitis, and osteomyelitis are attributed to infections developed by bacterial biofilms.6,7,9 For instance, E.coli were detected in serious urinary tract infections with their capability to develop biofilms.10,11 Additionally, S. aureus were reported to develop colonies on medical devices developing biofilms and hence causing several nosocomial infections such as osteomyelitis, endocarditis, pneumonia, and sepsis.12,13 Thus, targeting infections developed by such biofilms still presents a major challenge which warrants the search of new approaches.
While current strategies exploited to treat biofilms have often involved antibiotics, alternative approaches are urgently needed. Natural extracts, particularly essential oils (EOs), have garnered significant interest attributing to their intrinsic antimicrobial and antibiofilm characteristics.14–17 The unique therapeutic benefits of Boswellia sacra EOs (BOS) are primarily attributed to their various content of promising bioactive components, including polyphenols, terpenes, and monoterpenoids.18 In a previous study, the GC-MS of BOS showed a detailed breakdown of the chemical constituents of BOS and revealed a total of 32 unique compounds that primarily belong to monoterpene in addition to the presence of sesquiterpenes, oxygenated monoterpenes, and esters.18 Monoterpenes dominated the GC profile with α-pinene (61.05%) showing the highest percentage, followed by D-limonene, δ-3-carene, camphene, O-cymene, and β-pinene.18 These compounds exhibited remarkable targeting ability towards the microbial cell membranes enhancing their permeability, thereby impairing critical cellular transport mechanisms and leading to bacterial cell death.19–22 By disrupting the lipid layers that protect microbial cells, terpenoids and similar compounds, which are fat-soluble, can more easily penetrate the cell membrane resulting in cell death.20 These findings matched the characteristics expected for BOS, aligning with previous research.23,24
Furthermore, studies have established insignificant cytotoxic effects and biocompatibility of different extracts of Boswellia species on normal cells, such as MCF10-2A (normal breast cells), HEK-293 (human embryonic kidney cells), WI-38 (normal lung cells), and HSF (human skin fibroblast cells).23,25–27 However, similar to other EOs, limitations such as poor bioavailability, targeted delivery, and stability hinder their therapeutic potential.28
Several nanocarriers were shown as promising platforms to combat bacterial biofilms by facilitating delivery of their encapsulated cargo of antimicrobials into biofilms and reducing their adhesion.29,30 Additionally, nanomaterials can effectively eradicate biofilms through induced physical disruption, oxidative stresses, and thermal damage inside the bacterial biofilm.31–35 The outstanding physicochemical properties of nanoparticles have facilitated their key role in fighting biofilms due to their unique surface charges, small sizes, and greater biological responses, hence serving as promising multi-functional antimicrobials.31,32 Many nanoparticulate-based systems have been remarkably used to encapsulate EOs, augmenting their bioavailability and stability along with facilitating their targeting accessibility to the biofilm site.29,30 Nevertheless, the anti-biofilm efficacy and therapeutic effects of EOs loaded into nanocarriers could be substantially improved by promoting their release sustainability and biofilm penetration ability.36 Hence, this targeting approach reduces off-target impacts on healthy tissue, thus offering a promising biocompatible strategy and playing a crucial factor in promoting wound healing and tissue regeneration.
Carbohydrate-based nanocarriers particularly those derived from cyclodextrins, have recently shown enhanced therapeutic properties and physicochemical characteristics, offering exceptional biocompatibility and versatility.14,37 The polymeric beta-cyclodextrin nanocarriers can be tailored to target the prime components of bacterial biofilms exploiting two major mechanisms. The first mechanism includes the development of molecular inclusion complexation with the essential virulence factor inducing signaling molecules inside the targeted bacterial biofilm. The second mechanism involves disrupting the matrix of the targeted biofilms via interactions with polysaccharides.38,39 On the other hand, in addition to their accreditation as FDA-approved carriers,14,18 beta-cyclodextrins have established superior biocompatibility imparted by their cyclic sugar structures, which is similar to components naturally found in human cells, which augments their further use as biocompatible drug delivery carriers.40 Hence, such nanocarriers can be specifically designed to target biofilms while minimizing undesirable effects on normal cells, making them ideal candidates for biocompatible, therapeutic, and wound-healing applications.29,30,41
This study aimed to develop and characterize a biocompatible inclusion complex of Boswellia sacra essential oils (BOS) loaded with hydroxypropyl-beta-cyclodextrins (BOS-βCD). The study investigated the physicochemical properties, morphology, and entrapment efficiency of the complex. Furthermore, the antimicrobial activity of BOS-βCD against various bacterial strains and biofilms was evaluated and compared to free BOS. Most importantly, in vitro cytotoxicity, apoptosis, cytoskeletal arrangement, and reactive oxygen species (ROS) generation were assessed to determine the biocompatibility of BOS-βCD on human skin fibroblast (HSF) cells. Additionally, biochemical analyses, including lactate production, glucose consumption, and total antioxidant capacity, were performed on HSF cells treated with BOS-βCD to further confirm the biocompatibility of BOS-βCD. This comprehensive investigation aimed to establish the BOS-βCD inclusion complex as a safe and effective antibacterial biofilm for potential therapeutic applications.
2. Materials and methods
2.1. Materials
2.2. Methods
On the other hand, an Ultra-high Resolution Transmission Electron Microscopy (UHR-TEM) was also employed to further assess the morphological features and encapsulation of BOS within BOS-βCD inclusion complex. The UHR-TEM (JEOL JEM-2100 Plus, Tokyo, Japan) was operated at an accelerating voltage of 200 kV. βCD and BOS-βCD were mixed, using minute quantity of each, with distilled water forming corresponding suspensions. Suspensions were then sonicated at 37 °C for 10 min. A droplet of each suspension was placed on a copper grid covered with a thin film of carbon (1 nm). The samples were consequently stained, using 2% uranyl acetate, and dried using a filter paper.14,42
To examine the chemical structures of BOS, βCD, and BOS-βCD, a Nicolet 380 FTIR spectrometer (Thermo Scientific, Madison, WI 53719, United States) was employed. The FTIR spectra were recorded in the range of 4000–400 cm−1. BOS analysis involved placing a drop of BOS on a KBr disc and aligning it with the infrared beam. Additionally, samples preparation of βCD and BOS-βCD involved their initial mixing with KBr in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ratio. These mixtures were then pressed into discs using a hydraulic press (15T manual press machine, China).14,28,44
100 ratio. These mixtures were then pressed into discs using a hydraulic press (15T manual press machine, China).14,28,44
To investigate the successful encapsulation of BOS within the cavities of BOS-βCD inclusion complex, 1H NMR spectra of BOS, βCD, and BOS-βCD (ESI Fig. S3–S5 and Table 1†) in addition to 2D-HNMR (NOESY) spectrum of BOS-βCD were obtained. To accomplish this, samples were dissolved in DMSO, and an NMR spectrometer was utilized at room temperature (400 MHz, BRUKER BioSpin GmbH, D-76287 Rheinstetten, Germany).14,45
Moreover, thermal characteristics of βCD, BOS, and BOS-βCD were assessed. The analysis was conducted using a thermogravimetric analysis device (Labsys Instruments, Paris, France). For this purpose, samples were heated from 25 °C to 600 °C at a constant rate of 10 °C min−1 under an atmosphere of nitrogen gas flow. The flow rate of the nitrogen was set at 50 mL min−1.14,28,42
 | (1) |
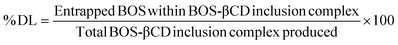 | (2) |
2.3. In vitro antimicrobial assessments
2.3.2.1. Prevention of biofilm formation assay. Bacterial inoculums of P. putida, E. coli, S. aureus, and B. subtilis were prepared at a concentration of 106 CFU mL−1, in which 100 μL of bacterial inoculum was added to each well of a 96-well plate. Serial dilutions of BOS-βCD were prepared for each bacterial strain examined, covering concentrations both below (½ MIC and ¼ MIC) and above (2 MIC and 4 MIC) the MIC values, determined by the microdilution assay, with 100 μL of each dilution added to the corresponding wells. Free BOS, free βCD, and benzalkonium chloride were treated similarly. The plates were incubated at 37 °C for 24 h at static conditions. After incubation, the contents of the wells were washed three times with sterile PBS to remove non-adherent bacteria. The remaining biofilm was stained with 200 μL of 0.1% crystal violet solution for 20 min at room temperature, followed by washing three times with distilled water to eliminate excess stain. Consequently, the plates were allowed to air-dry completely, and 200 μL of 95% ethanol was then added to each well to solubilize the crystal violet-stained biofilm. The absorbance of each well was measured at 570 nm to quantify the biofilm.48
2.3.2.2. Disruption of established biofilm assay. The initial bacterial inoculum of each bacterial strain of P. putida, E. coli, S. aureus, and B. subtilis were prepared, followed by overnight incubation at static conditions to facilitate biofilm formation. After incubation, the wells were washed with sterile PBS to remove any planktonic bacteria. Afterward, serial dilutions of the BOS-βCD were added directly to the wells for an additional overnight incubation period. Consequently, the contents of each well were removed, and the wells were washed again with sterile PBS to ensure that only the adherent biofilm, if present, remained. Hence after, 200 μL of 0.1% crystal violet solution was added to each well, following the previously described procedure for staining and quantifying the biofilm. Similar treatment conditions were applied for free BOS, free βCD, and benzalkonium chloride.48 The inhibition percentage was calculated using the following equation (eqn (3)):
 | (3) |
2.4. In vitro biocompatibility assays
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 dye (Molecular Probes, USA) and imaged using a Leica DMi8 inverted fluorescent microscope (Leica Microsystems, Wetzlar, Germany).
342 dye (Molecular Probes, USA) and imaged using a Leica DMi8 inverted fluorescent microscope (Leica Microsystems, Wetzlar, Germany).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 (Molecular Probes) for 15 min. Cells were visualized using a Leica DMi8 inverted fluorescent microscope (Leica Microsystems, Wetzlar, Germany).
342 (Molecular Probes) for 15 min. Cells were visualized using a Leica DMi8 inverted fluorescent microscope (Leica Microsystems, Wetzlar, Germany).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 dye, and images were obtained using Leica DMi8 inverted fluorescent microscope.
342 dye, and images were obtained using Leica DMi8 inverted fluorescent microscope.
2.5. Statistical studies
To assess data consistency and identify potential variations, the mean and standard deviation were determined for each measurement set. Three samples were prepared for each formulation. Statistical significance was determined using a p-value threshold of 5% or less. ANOVA was employed to detect statistically significant differences between the formulations. ImageJ software was used to determine the particle sizes.3. Results and discussion
3.1. BOS-βCD morphological examination
The obtained SEM images (ESI Fig. 1a and b†) depicted the morphology and size variations between βCD and BOS-βCD inclusion complex. While βCD demonstrated large ovoid alongside rectangular-shaped particles, the particles of BOS-βCD inclusion complex were presented with significant reductions in size accompanied by substantial morphological alterations. Remarkably, the particles of BOS-βCD inclusion complex exhibited agglomeration and several aggregates formation, indicating an interaction between larger and smaller particles occurred. Such observations suggested the potential complexation formation of BOS-βCD inclusion complex, possibly involving an amorphous product interacting with other components in the established complex. These findings are consistent with previous research.18,51Furthermore, UHR-TEM images (Fig. 1A and B) were examined to assess the encapsulation of BOS within the inclusion complex of BOS-βCD and to investigate the structural and morphological features of βCD and BOS-βCD. Notably, βCD displayed round vesicles (diameters: 82.54–412.84 nm) with larger micellar structures often formed. Conversely, the particles of BOS-βCD inclusion complex exhibited nearly spherical vesicles (diameters: 14.53–83.19 nm), with a thin membrane surrounding the encapsulated BOS, with a strong tendency to aggregate showing larger agglomerates (diameters: 141.53–401.61 nm), in which larger particles attracted smaller ones. These observations suggest a successful BOS encapsulation within the cavities of βCD forming their consequent BOS-βCD inclusion complex. On the other hand, both samples depicted several micellar structure formations that can be explained with the use of distilled water to dissolve both samples prior to their preparation for imaging, in which cyclodextrins manage to self-assemble under such conditions. Similar results were reported in previous studies.14,18,43
Significant differences in the particles size and distribution could be noticed among βCD and BOS-βCD inclusion complex. βCD exhibited a highly polydisperse system, with a PDI of 1.0 (>0.7), and a significantly heterogeneous particles size distribution with a Z-average of 1038.53 ± 221.26 nm. Conversely, BOS-βCD inclusion complex exhibited a PDI value of 0.38 ± 0.09 and Z-average of 323.37 ± 21.85 nm, reflecting a more uniform system with consistent particles size distribution. These findings can be explained by the well-known tendency of cyclodextrins, including βCD, and their developed inclusion complexes to self-assemble in water forming larger aggregates such as micelles, helping to dissolve lipophilic substances.14,42,52
3.2. FTIR analysis
FTIR spectra were obtained for investigating the BOS encapsulation within BOS-βCD, inclusion complex formation, and structural characteristics presented for BOS, βCD, and BOS-βCD inclusion complex (ESI Fig. S2†). BOS FTIR spectrum displayed characteristic peaks corresponding to O–H stretching (3435.1 cm−1), –CH2− group stretching (2926.3 cm−1), C–H stretching (2716.7 cm−1), H–O–H bending (1668.5 cm−1), C–H scissoring (1453.9 cm−1), C–O stretching (1364.7 cm−1), C–O–C stretching vibrations (1249.5 cm−1 and 1076.3 cm−1), and C–H bending of aromatics (882.7 cm−1 and 814.5 cm−1). Nevertheless, the spectrum of βCD showed prominent bands for O–H stretching (3463.7 cm−1), –CH2− group stretching (2912.2 cm−1), C–H stretching (2667.1 cm−1), H–O–H bending (1640.5 cm−1), C–H vibration (1443.7 cm−1), and symmetric and asymmetric C–O–C stretching vibrations (1146.3 cm−1 and 1011.9 cm−1). Interestingly, the FTIR spectrum of BOS-βCD inclusion complex showed that the characteristic peaks of BOS were obscured by more intense βCD's absorption bands, suggesting potential encapsulation of BOS within the βCD cavities and potential interactions occurred outside these cavities. Additionally, the shifts and broadening of O–H bands observed in BOS-βCD spectrum support this notion, in which inter-molecular hydrogen bonds might be occurred. Also, the broader –CH2 band further suggests the successful incorporation of the hydrophobic components of BOS into the BOS-βCD inclusion complex cavities. Overall, the findings presented herein confirm successful BOS encapsulation and formation of stable BOS-βCD complex. Similar results were previously reported.14,19,44,453.3. 2D-NMR NOESY spectroscopy
The encapsulation of BOS within the cavities of BOS-βCD inclusion complex was investigated, employing 1H NMR spectra of BOS, βCD, and BOS-βCD (ESI Fig. S3–S5 and Table 1†) in addition to 2D-HNMR (NOESY) spectrum of BOS-βCD (Fig. 1C). The 2D-NOESY spectroscopy method employs the Nuclear Overhauser Effect (NOE) to signify potential interactions imparted by the close proximity of BOS components (the guest molecule) and βCD (the host) within the developed BOS-βCD inclusion complex. While the NMR spectroscopy can refer to the successful formation of BOS-βCD inclusion complex, NOE provides a deeper understanding, in which it can detect the transfer of spin polarization established between nearby atoms in separate populations (BOS and BOS-βCD), reflecting interactions occurred within a short distance (around 0.4 nm).14,18,53,54 The indicated interactions are observed as crossed peaks (NOE correlations) in a 2D-NMR NOESY spectrum (Fig. 1C). Notably, the figure showed a significant correlation, imparted by the spatial proximity, between BOS's protons and specific βCD's protons (H3 and H5) within the inclusion complex BOS-βCD. These findings support the successful encapsulation of BOS components within the cavities of the obtained inclusion complex of BOS-βCD which further agrees with previous reports.14,18,55–573.4. TGA analysis
TGA curves for BOS, βCD, and BOS-βCD were obtained (Fig. 1D), depicting the weight loss as a function of temperature. For the TGA curve of BOS, the curve exhibited a rapid weight loss yet a well-stable thermal profile of BOS with 85.14% of total BOS loss, from 53.61 °C to 199.37 °C. The rapid loss of BOS is attributed to its high volatile nature and the rapid decomposition rates of EOs in general.14,18 On the other hand, the TGA curve of βCD exhibited an insignificant weight loss up to 297 °C, whereas a significant loss of 71.65% could be detected between 297 °C and 396 °C which can be explained by the thermal decomposition of the βCD molecules. More importantly, BOS-βCD curve showed a very similar thermal behavior to the TGA curve of βCD with some key differences. Both showed minimal weight loss initially, with a small amount of weight loss observed (1.5% at 98.9 °C) in the BOS-βCD inclusion complex owing to the evaporation of the water molecules. Additionally, the major weight loss (87.17%) happened at a slightly lower temperature range (288 °C to 385 °C) compared to βCD alone, indicating some influence from the BOS component. Thus, the encapsulation of BOS into BOS-βCD inclusion complex remarkably enhanced its thermal stability which was evidenced by the significant shift shown in the temperature at which the weight loss of the inclusion complex could be observed in comparison to BOS alone. These results are well matched with the previously published reports.44,583.5. Entrapment efficiency and drug loading capacity of BOS-βCD inclusion complex
BOS-βCD inclusion complex demonstrated excellent encapsulation efficiency (% EE) of 94.78 ± 1.87% and drug loading capacity (% DL) of 8.62 ± 0.17%. The exceptional % EE can be attributed to extended complexation process conducted alongside with the well-protected conditions applied on the obtained solution of the inclusion complex and drying process, positively affecting the encapsulation portion of the BOS, as previously reported.14,59 These findings are supported by previous reports which showed strong affinities of specific components presented in the GC-MS profile of BOS, such as α-pinene and limonene, to the βCD cavities.14,18,60 On the other hand, the determined %DL (8.62 ± 0.17%) of the obtained inclusion complex aligns with the previous reports established on similar complexes and thus validating the presented findings.14,423.6. In vitro antimicrobial and antibiofilm activities
| P. putida | S. aureus | E. coli | B. subtilis | |
|---|---|---|---|---|
| βCD | >200 | >200 | >200 | >200 |
| BOS | 20 | 20 | 20 | 20 |
| BOS-βCD | 2.5 | 5 | 2.5 | 5 |
3.6.2.1. Prevention of biofilm formation. For the biofilm prevention studies, the BOS-βCD complex showed varying efficacy across the examined strains (Fig. 2), reflecting how bacterial structure and physiology influence the biofilm formation. In particular, for S. aureus, the BOS-βCD demonstrated a significant biofilm prevention (90.88 ± 2.97%) at 20 mg mL−1, securing an effective hinder of the bacterial adhesion and aggregation that are key steps in biofilm formation.66 At lower concentrations, the prevention rates remained substantial, showing 90.90 ± 1.11% at 10 mg mL−1 and 85.40 ± 3.46% at 5 mg mL−1, indicating the ability of BOS-βCD to maintain its effectiveness even as concentrations decreased. This correlates well with the predetermined MIC findings, suggesting that concentrations at and above the MIC value are crucial for optimal biofilm prevention.67 Similarly, B. subtilis exhibited 82.55 ± 5.48% of its biofilm prevention at 20 mg mL−1, with notable reductions seen at complex concentrations of 10 mg mL−1 (77.31 ± 4.56%) and 5 mg mL−1 (61.66 ± 4.94%), reflecting a potential enhancement in the inclusion complex penetration through the peptidoglycan layer of the Gram-positive cell wall, disrupting the initial adhesion processes essential for biofilm development.68 On the other hand, the BOS-βCD complex reached 90.60 ± 3.53% prevention of P. putida biofilm utilizing a complex concentration of 10 mg mL−1, coupled with persistent efficacy shown at lower concentrations with prevention of 91.57 ± 2.80% at 5 mg mL−1 and 83.11 ± 9.20% at 2.5 mg mL−1. The enhanced solubility of BOS EO due to βCD might have allowed better penetration through the bacterium outer membrane, effectively disrupting adhesion mechanisms. This aligns with the predetermined MIC, reinforcing the notion that maintaining concentrations above the MIC is vital for combating biofilm formation.69,70 Furthermore, 93.78 ± 1.3% of E. coli biofilm was prevented at 10 mg mL−1 of BOS-βCD while a remarkable prevention of 92.51 ± 1.8% was achieved at 5 mg mL−1, reflecting the BOS-βCD effectiveness in inhibiting biofilm formation even with robust biofilm matrices.69,70 The trend observed here emphasizes that while prevention rates slightly decrease with lower concentrations of the complex utilized, they remain significant enough to suggest that the BOS-βCD complex effectively disrupts early biofilm formation stages, consistent with the observed MIC results. Notably, neither free βCD nor free BOS alone achieved this level of biofilm inhibition.
3.6.2.2. Disruption of established biofilm. The biofilm disruption/inactivation potential of BOS-βCD was examined against the same bacterial strains (P. putida, E. coli, S. aureus and B. subtilis), as shown in Fig. 3. For this purpose, the bacterial strains were cultured overnight for biofilm formation, then serial dilutions of BOS-βCD were added to examine their effects on the already formed biofilm. The individual compounds of free βCD and BOS EO could not reveal any inhibitory effect against the developed biofilms. For B. subtilis, the BOS-βCD achieved an inactivation effect of 75.26 ± 2.99% against the biofilm formed at 20 mg mL−1, decreasing slightly to 74.52 ± 5.74% at 10 mg mL−1 and 74.22 ± 2.41% at 5 mg mL−1. At concentrations below the predetermined MIC value (<5 mg mL−1), the biofilm inhibition effect dropped to 37.82 ± 6.43%, reflecting a significant reduction in the complex efficacy as the concentration decreased below the MIC value. The strong inhibition at higher concentrations suggests that BOS-βCD effectively disrupts the B. subtilis biofilm matrix, likely through terpene-mediated (present in BOS EO) disruption of cell walls and inhibition of quorum sensing pathways, both of which are critical for biofilm formation in Gram-positive bacteria.71 For S. aureus, the biofilm inhibition reached 82.17 ± 7.35% and 74.13% ± 3.73 at 20 mg mL−1 and 10 mg mL−1, respectively, whereas at 5 mg mL−1, the inhibition slightly decreased to 64.94 ± 4.13%. Conversely, the activity sharply declined at concentrations below 5 mg mL−1 (below MIC), with an inhibition of 34.94 ± 5.70% at 2.5 mg mL−1 and only 8.81 ± 11.38% at 1.25 mg mL−1. These findings suggest that BOS-βCD, at higher concentrations, could effectively impact the S. aureus biofilm structure by disrupting cellular adhesion and protein synthesis.18,72 For E. coli, biofilm inhibition rates reached 76.36 ± 6.41% at 10 mg mL−1 and 75.48 ± 9.48% at 5 mg mL−1. As concentration dropped to 2.5 mg mL−1 (MIC), the biofilm inhibition decreased to 61.15% ± 10.71, while maintaining a similar inhibition rate of 62.86 ± 16.55% at 1.25 mg mL−1. Below this, at 0.625 mg mL−1, the inhibition dropped to 42.56 ± 9.45%. These findings indicate that the effectiveness of BOS-βCD in penetrating the cell wall of E. coli might be correlated to the enhanced solubilization and targeting ability of BOS EO inside the βCD complex to reach intracellular targets involved in biofilm regulation.73 Finally, the BOS-βCD exhibited the highest biofilm inhibition effect against P. putida at 10 mg mL−1, reaching 78.61 ± 1.42%, which decreased to 68.40 ± 3.45% at 5 mg mL−1. At 2.5 mg mL−1 (MIC), the inhibition was 61.85 ± 10.95%, while a concentration of 1.25 mg mL−1 resulted in a significant drop to 40.06 ± 9.67%, and the lowest concentration of 0.625 mg mL−1 showed only 5.86 ± 11.33% inhibition. These findings suggest that the BOS-βCD effectively impacted the biofilm formed by P. putida, likely by interfering with the biofilm-promoting factors. The current results are consistent with previously published studies, in which Kfoury et al. reported that the encapsulation of EOs in cyclodextrins could increase their aqueous solubility up to 16-fold and reduce their photodegradation rates (augmenting stability) up to 44-fold. These improvements led to enhanced antimicrobial and antibiofilm activities.73 Moreover, Jaroš et al. demonstrated that the boswellic acids derived from BOS EO could significantly inhibit the biofilm formed by three bacterial strains (Staphylococcus epidermidis, Enterococcus faecalis, and E. coli.), making them potent agents against bacterial infections.74
3.7. Biocompatibility assessments
On the other hand, the MTT cytotoxicity assay (Fig. 4) demonstrated a significant enhancement (p < 0.05) of HSF metabolic activity upon treatment with BOS-βCD. Hence, these findings suggest a favorable safety profile for BOS-βCD when applied to normal HSF cells. Assays for apoptosis resistance and cellular metabolic activity revealed that the BOS-βCD did not induce programmed cell death and might even have stimulated metabolic processes in HSF cells.
Findings of this study are consistent with the results published by Singh et al. which evaluated the safety of repeated oral administration of BOS to rats over 90 days which suggested that BOS is safe in rats up to a dose of 500 mg per kg body weight.75 Moreover, Bian et al. findings demonstrated that the formation of a naringin-βCD inclusion complex and incorporating it in chitosan hydrogel for wound healing applications exhibited biocompatibility in MTT assay against fibroblast L929 cells.76 Another research group reported that cannabidiol-βCD inclusion complex enhanced the properties of PVA-borax hydrogels for wound management applications, as it exhibited antibacterial and antioxidant properties without inducing cytotoxicity towards RAW 264.7 cells.77 These results further suggested that βCD, even when complexed with cannabidiol, naringin, or BOS, did not lead to apoptosis in cells. The BOS-βCD inclusion complex could present a promising platform for many biomedical applications, including drug delivery, based on its remarkable antimicrobial profile and established biocompatibility.
Immunofluorescence staining of β-actin, a major cytoskeletal component, did not reveal any significant alterations in the organization of the filamentous network (Fig. 5B). Conversely, α-SMA, a marker for contractile stress fibers, was downregulated in treated cells compared to control. The observed decrease in α-SMA suggests potential modulation of cytoskeleton that could affect cellular contractility.78 Interestingly, this decrease in α-SMA coincides with the enhanced metabolic activity observed by the MTT assay. This could be indicative of a shift towards a more proliferative phenotype in BOS-βCD treated cells. Proliferating cells generally exhibit lower levels of α-SMA as they prioritize growth and division over contractility.78,79 These effects align with the requirements that may affect the wound healing process, where reduced contractility allows cells to migrate and participate in tissue repair, and proliferative cells contribute to faster wound closure and tissue regeneration. Therefore, modulation of cellular behavior by BOS-βCD supports their safety profile by promoting both cell migration and proliferation.80–82 Further investigation is needed to confirm and elucidate the specific mechanisms by which BOS-βCD influences α-SMA expression and cellular proliferation.
Also, the antioxidant capacity in the HSF-treated cells was significantly reduced as compared to the control cells (Fig. 6B). Similarly, the study of Bayiha et al. demonstrated that budesonide-βCD inclusion complex offered protective effects against ROS generation, decreased PI3K/Akt activation, and reduced phosphorylated/unphosphorylated HDAC2, highlighting the protective and anti-cytotoxic activity of βCD.83
Glucose consumption and lactate production of HSF cells were further measured to assess the impact of BOS-βCD on HSF cell metabolism. HSF cells treated with BOS-βCD resulted in a significant increase in cellular glucose uptake compared to control cells, as evidenced by lower glucose levels in the conditioned medium (Fig. 6A). This elevated glucose uptake was accompanied by enhanced lactate production in the treated cells (Fig. 6B). These findings suggest that BOS-βCD treatment might stimulate the metabolic activity of HSF cells, potentially shifting them toward a more glycolytic phenotype.
The enhanced metabolic activity observed on BOS-βCD treated cells likely plays a role in the potential increase in proliferation.84 Increased glucose uptake and lactate production suggest a shift towards a more glycolytic metabolic phenotype, which is a hallmark of rapidly dividing cells.85 This metabolic reprogramming allows proliferating cells to generate the building blocks and energy necessary for rapid growth and division.86,87 Furthermore, the observed decrease in antioxidant capacity, as indicated in Fig. 6C, could be a consequence of this metabolic shift, or it could represent a separate effect of BOS-βCD inclusion complex. These findings highlight the potential role of BOS-βCD to optimize their use in relevant biomedical applications by supporting energy metabolism, regulating oxidative stress, and enhancing immune responses, thereby promoting effective tissue regeneration. The potential long-term effects of BOS-βCD on HSF cell metabolism and proliferation require further investigation.
4. Conclusions
Biofilms pose a significant challenge due to their resistance to conventional treatments. Boswellia sacra essential oils (BOS) have shown significant antibacterial, anticancer, and antioxidant properties. This study explored the encapsulation of BOS into hydroxypropyl-beta-cyclodextrin (βCD), an FDA-approved polymeric carrier, as a potential approach for targeting bacterial infections related to biofilms. BOS-βCD demonstrated remarkable physicochemical properties, high encapsulation efficiency (94.78 ± 1.87%), and superior antimicrobial activity compared to free BOS by 4-fold against S. aureus and B. subtilis and by 8-fold against E. coli and P. putida, with minimum inhibitory concentrations ranging from 2.5 to 5 mg mL−1. Furthermore, BOS-βCD effectively inhibited initial bacterial adhesion, reaching a prevention effect of 93.78 ± 1.3%, and disrupted established biofilms, achieving an inhibition rate of 82.17 ± 7.35%, highlighting its potential as a dual-action agent for biofilm management. These findings demonstrate the potential of BOS-βCD as a promising agent to combat biofilm-associated infections by both preventing biofilm formation and disrupting established biofilms. The BOS-βCD complex also exhibited noticeable biocompatibility, where neither cytotoxicity, oxidative stress, nor disruption of the cytoskeletal protein β-actin, were observed in HSF cells treated with BOS-βCD. The complex also enhanced metabolic activity and proliferation of HSF cells.In conclusion, BOS-βCD complexes have the potential of effectively preventing the formation of bacterial biofilms as well as preformed biofilms developed by four common bacterial strains and combating other biofilm-associated healthcare challenges. The combination of potent antimicrobial activity and excellent biocompatibility supports the use of BOS-βCD as an antibacterial and an antibiofilm dual-agent which could potentially improve outcomes for patients affected by biofilm-induced infections.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
All authors declare no competing interests.Acknowledgements
This work was supported by a DUP grant from the American University in Cairo to Professor Hassan M. E. Azzazy and a grant from the Science and Technology Development Fund (STDF; FLUG grant No. # 46721) to Professor Nagwa El-Badri.References
- X. Pan, W. Liu, Q. Du, H. Zhang and D. Han, Recent Advances in Bacterial Persistence Mechanisms, Int. J. Mol. Sci., 2023, 24(18), 14311 CrossRef CAS.
- L. K. Vestby, T. Grønseth, R. Simm and L. L. Nesse, Bacterial Biofilm and its Role in the Pathogenesis of Disease, Antibiotics, 2020, 9(2), 59, DOI:10.3390/antibiotics9020059.
- D. J. Hassett, M. T. Borchers and R. J. Panos, Chronic obstructive pulmonary disease (COPD): Evaluation from clinical, immunological and bacterial pathogenesis perspectives, J. Microbiol., 2014, 52(3), 211–226, DOI:10.1007/s12275-014-4068-2.
- S. M. McCarty, C. A. Cochrane, P. D. Clegg and S. L. Percival, The role of endogenous and exogenous enzymes in chronic wounds: A focus on the implications of aberrant levels of both host and bacterial proteases in wound healing, Wound Repair Regen., 2012, 20(2), 125–136, DOI:10.1111/j.1524-475X.2012.00763.x.
- A. K. Ojha, W. R. Jacobs and G. F. Hatfull, Genetic Dissection of Mycobacterial Biofilms, in Mycobacteria Protocols, ed. T. Parish and D. M. Roberts, Springer, New York: New York, NY, 2015, pp. 215–226 Search PubMed.
- D. Leaper, O. Assadian and C. E. Edmiston, Approach to chronic wound infections, Br. J. Dermatol., 2015, 173(2), 351–358, DOI:10.1111/bjd.13677.
- I. Negut, V. Grumezescu and A. M. Grumezescu, Treatment Strategies for Infected Wounds, Molecules, 2018, 23(9), 2392, DOI:10.3390/molecules23092392.
- N. Hoffmann, B. Lee, M. Hentzer, T. B. Rasmussen, Z. Song, H. K. Johansen, M. Givskov and N. Høiby, Azithromycin Blocks Quorum Sensing and Alginate Polymer Formation and Increases the Sensitivity to Serum and Stationary-Growth-Phase Killing of Pseudomonas aeruginosa and Attenuates Chronic P. aeruginosa Lung Infection in Cftr−/− Mice, Antimicrob. Agents Chemother., 2007, 51(10), 3677–3687, DOI:10.1128/aac.01011-06.
- M. Burmølle, T. R. Thomsen, M. Fazli, I. Dige, L. Christensen, P. Homøe, M. Tvede, B. Nyvad, T. Tolker-Nielsen, M. Givskov, C. Moser, K. Kirketerp-Møller, H. K. Johansen, N. Høiby, P. Ø. Jensen, S. J. Sørensen and T. Bjarnsholt, Biofilms in chronic infections – a matter of opportunity – monospecies biofilms in multispecies infections, FEMS Immunol. Med. Microbiol., 2010, 59(3), 324–336, DOI:10.1111/j.1574-695X.2010.00714.x.
- M. Rojas-Lopez, R. Monterio, M. Pizza, M. Desvaux and R. Rosini, Intestinal pathogenic Escherichia coli: Insights for vaccine development, Front. Microbiol., 2018, 9, 440, DOI:10.3389/fmicb.2018.00440.
- C. Schroll, K. B. Barken, K. A. Krogfelt and C. Struve, Role of type 1 and type 3 fimbriae in Klebsiella pneumoniae biofilm formation, BMC Microbiol., 2010, 10(1), 179, DOI:10.1186/1471-2180-10-179.
- A. H. Siddiqui and J. Koirala, Methicillin-Resistant Staphylococcus aureus, in StatPearls, StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC, Treasure Island (FL) ineligible companies, Disclosure: Janak Koirala declares no relevant financial relationships with ineligible companies, 2024 Search PubMed.
- S. Jarraud, C. Mougel, J. Thioulouse, G. Lina, H. Meugnier, F. Forey, X. Nesme, J. Etienne and F. Vandenesch, Relationships between Staphylococcus aureus Genetic Background, Virulence Factors, agr Groups (Alleles), and Human Disease, Infect. Immun., 2002, 70(2), 631–641, DOI:10.1128/iai.70.2.631-641.2002.
- O. A. A. Alabrahim and H. M. E.-S. Azzazy, Antimicrobial Activities of Pistacia lentiscus Essential Oils Nanoencapsulated into Hydroxypropyl-beta-cyclodextrins, ACS Omega, 2024, 9(11), 12622–12634, DOI:10.1021/acsomega.3c07413.
- M. S. Criollo-Mendoza, L. A. Contreras-Angulo, N. Leyva-López, E. P. Gutiérrez-Grijalva, L. A. Jiménez-Ortega and J. B. Heredia, Wound Healing Properties of Natural Products: Mechanisms of Action, Molecules, 2023, 28(2), 598, DOI:10.3390/molecules28020598.
- V. Jaramillo, E. Díaz, L. N. Muñoz, A. F. González-Barrios, J. Rodríguez-Cortina, J. C. Cruz and C. Muñoz-Camargo, Enhancing Wound Healing: A Novel Topical Emulsion Combining CW49 Peptide and Lavender Essential Oil for Accelerated Regeneration and Antibacterial Protection, Pharmaceutics, 2023, 15(6), 1739, DOI:10.3390/pharmaceutics15061739.
- O. A. A. Alabrahim, J. M. Lababidi, W. Fritzsche and H. M. E.-S. Azzazy, Beyond Aromatherapy: Can Essential Oils Loaded Nanocarriers Revolutionize Cancer Treatment?, Nanoscale Adv., 2024, 6, 5511–5562, 10.1039/D4NA00678J.
- O. A. A. Alabrahim, S. Alwahibi and H. M. E.-S. Azzazy, Improved Antimicrobial Activities of Boswellia sacra Essential Oils Nanoencapsulated into Hydroxypropyl-beta-cyclodextrins, Nanoscale Adv., 2024, 6, 910–924, 10.1039/D3NA00882G.
- J. Rakmai, B. Cheirsilp, A. Torrado-Agrasar, J. Simal-Gándara and J. C. Mejuto, Encapsulation of yarrow essential oil in hydroxypropyl-beta-cyclodextrin: physiochemical characterization and evaluation of bio-efficacies, CyTA-J. Food, 2017, 15(3), 409–417, DOI:10.1080/19476337.2017.1286523.
- P. Dhar, P. Chan, D. T. Cohen, F. Khawam, S. Gibbons, T. Snyder-Leiby, E. Dickstein, P. K. Rai and G. Watal, Synthesis, Antimicrobial Evaluation, and Structure–Activity Relationship of α-Pinene Derivatives, J. Agric. Food Chem., 2014, 62(16), 3548–3552, DOI:10.1021/jf403586t.
- A. C. Rivas da Silva, P. M. Lopes, M. M. Barros de Azevedo, D. C. Costa, C. S. Alviano and D. S. Alviano, Biological activities of α-pinene and β-pinene enantiomers, Molecules, 2012, 17(6), 6305–6316, DOI:10.3390/molecules17066305.
- L. de Sousa Eduardo, T. C. Farias, S. B. Ferreira, P. B. Ferreira, Z. N. Lima and S. B. Ferreira, Antibacterial activity and time-kill kinetics of positive enantiomer of α-pinene against strains of Staphylococcus aureus and Escherichia coli, Curr. Top. Med. Chem., 2018, 18(11), 917–924, DOI:10.2174/1568026618666180712093914.
- H. M. E.-S. Azzazy, A. Abdelnaser, H. Al Mulla, A. M. Sawy, S. N. Shamma, M. Elhusseiny, S. Alwahibi, N. K. Mahdy and S. A. Fahmy, Essential Oils Extracted from Boswellia sacra Oleo Gum Resin Loaded into PLGA–PCL Nanoparticles: Enhanced Cytotoxic and Apoptotic Effects against Breast Cancer Cells, ACS Omega, 2023, 8(1), 1017–1025, DOI:10.1021/acsomega.2c06390.
- S. Al-Saidi, K. B. Rameshkumar, A. Hisham, N. Sivakumar and S. Al-Kindy, Composition and antibacterial activity of the essential oils of four commercial grades of Omani luban, the oleo-gum resin of Boswellia sacra FLUECK, Chem. Biodivers., 2012, 9(3), 615–624, DOI:10.1002/cbdv.201100189.
- M. M. Suhail, W. Wu, A. Cao, F. G. Mondalek, K.-M. Fung, P.-T. Shih, Y.-T. Fang, C. Woolley, G. Young and H.-K. Lin, Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells, BMC Compl. Alternative Med., 2011, 11(1), 129, DOI:10.1186/1472-6882-11-129.
- A. A. Abd-Rabou and A. E. Edris, Frankincense essential oil nanoemulsion specifically induces lung cancer apoptosis and inhibits survival pathways, Cancer Nanotechnol., 2022, 13(1), 22, DOI:10.1186/s12645-022-00128-9.
- B. Alotaibi, W. A. Negm, E. Elekhnawy, T. A. El-Masry, W. S. Elseady, A. Saleh, K. N. Alotaibi and S. A. El-Sherbeni, Antibacterial, Immunomodulatory, and Lung Protective Effects of Boswelliadalzielii Oleoresin Ethanol Extract in Pulmonary Diseases: In Vitro and In Vivo Studies, Antibiotics, 2021, 10(12), 1444, DOI:10.3390/antibiotics10121444.
- O. A. A. Alabrahim and H. M. E.-S. Azzazy, Synergistic anticancer effect of Pistacia lentiscus essential oils and 5-Fluorouracil co-loaded onto biodegradable nanofibers against melanoma and breast cancer, Discover Nano, 2024, 19(1), 27, DOI:10.1186/s11671-024-03962-5.
- J. Nandhini, E. Karthikeyan and S. Rajeshkumar, Nanomaterials for wound healing: Current status and futuristic frontier, Biomed. Technol., 2024, 6, 26–45, DOI:10.1016/j.bmt.2023.10.001.
- M. Liu, X. Wei, Z. Zheng, Y. Li, M. Li, J. Lin and L. Yang, Recent Advances in Nano-Drug Delivery Systems for the Treatment of Diabetic Wound Healing, Int. J. Nanomed., 2023, 18(null), 1537–1560, DOI:10.2147/IJN.S395438.
- J. M. V. Makabenta, A. Nabawy, C.-H. Li, S. Schmidt-Malan, R. Patel and V. M. Rotello, Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections, Nat. Rev. Microbiol., 2021, 19(1), 23–36, DOI:10.1038/s41579-020-0420-1.
- R. Y. Pelgrift and A. J. Friedman, Nanotechnology as a therapeutic tool to combat microbial resistance, Adv. Drug Delivery Rev., 2013, 65(13), 1803–1815, DOI:10.1016/j.addr.2013.07.011.
- F. A. Al-Wrafy, A. A. Al-Gheethi, S. K. Ponnusamy, E. A. Noman and S. A. Fattah, Nanoparticles approach to eradicate bacterial biofilm-related infections: A critical review, Chemosphere, 2022, 288, 132603, DOI:10.1016/j.chemosphere.2021.132603.
- W. Xie, S. Zhang, F. Pan, S. Chen, L. Zhong, J. Wang and X. Pei, Nanomaterial-based ROS-mediated strategies for combating bacteria and biofilms, J. Mater. Res., 2021, 36(4), 822–845, DOI:10.1557/s43578-021-00134-4.
- C. Sahli, S. E. Moya, J. S. Lomas, C. Gravier-Pelletier, R. Briandet and M. Hémadi, Recent advances in nanotechnology for eradicating bacterial biofilm, Theranostics, 2022, 12(5), 2383–2405, DOI:10.7150/thno.67296.
- V. Dupuis, C. Cerbu, L. Witkowski, A.-V. Potarniche, M. C. Timar, M. Żychska and C. M. Sabliov, Nanodelivery of essential oils as efficient tools against antimicrobial resistance: a review of the type and physical-chemical properties of the delivery systems and applications, Drug Delivery, 2022, 29(1), 1007–1024, DOI:10.1080/10717544.2022.2056663.
- D. D. Gadade and S. S. Pekamwar, Cyclodextrin Based Nanoparticles for Drug Delivery and Theranostics, Adv. Pharm. Bull., 2020, 10(2), 166–183, DOI:10.34172/apb.2020.022.
- P. Saha and M. R. Rafe, Cyclodextrin: A prospective nanocarrier for the delivery of antibacterial agents against bacteria that are resistant to antibiotics, Heliyon, 2023, 9, e19287, DOI:10.1016/j.heliyon.2023.e19287.
- A. Scala, A. Piperno, G. Grassi, L. M. Scolaro and A. Mazzaglia, Chapter 12 - Nanoconstructs Based on Cyclodextrins for Antimicrobial Applications, in Nano- and Microscale Drug Delivery Systems, ed. A. M. Grumezescu, Elsevier, 2017, pp. 229–244 Search PubMed.
- G. Kali, S. Haddadzadegan and A. Bernkop-Schnürch, Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products, Carbohydr. Polym., 2024, 324, 121500, DOI:10.1016/j.carbpol.2023.121500.
- S. Sharma, L. Mulrey, M. Byrne, A. K. Jaiswal and S. Jaiswal, Encapsulation of Essential Oils in Nanocarriers for Active Food Packaging, Foods, 2022, 11(15), 2337, DOI:10.3390/foods11152337.
- J. A. Kamimura, E. H. Santos, L. E. Hill and C. L. Gomes, Antimicrobial and antioxidant activities of carvacrol microencapsulated in hydroxypropyl-beta-cyclodextrin, LWT-Food Sci. Technol., 2014, 57(2), 701–709, DOI:10.1016/j.lwt.2014.02.014.
- L. E. Hill, C. Gomes and T. M. Taylor, Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications, LWT-Food Sci. Technol., 2013, 51(1), 86–93, DOI:10.1016/j.lwt.2012.11.011.
- C. Yuan, Y. Wang, Y. Liu and B. Cui, Physicochemical characterization and antibacterial activity assessment of lavender essential oil encapsulated in hydroxypropyl-beta-cyclodextrin, Ind. Crops Prod., 2019, 130, 104–110, DOI:10.1016/j.indcrop.2018.12.067.
- G. Zhang, C. Yuan and Y. Sun, Effect of Selective Encapsulation of Hydroxypropyl-β-cyclodextrin on Components and Antibacterial Properties of Star Anise Essential Oil, Molecules, 2018, 23(5), 1126, DOI:10.3390/molecules23051126.
- S. D. Sarker, L. Nahar and Y. Kumarasamy, Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals, Methods, 2007, 42(4), 321–324, DOI:10.1016/j.ymeth.2007.01.006.
- A. Ebrahimi, M. Hemati, Z. Shabanpour, S. Habibian Dehkordi, S. Bahadoran, S. Lotfalian and S. Khubani, Effects of benzalkonium chloride on planktonic growth and biofilm formation by animal bacterial pathogens, Jundishapur J. Microbiol., 2015, 8(2), e16058, DOI:10.5812/jjm.16058.
- G. A. O’Toole, Microtiter dish biofilm formation assay, J. Vis. Exp., 2011, 47, 2437, DOI:10.3791/2437.
- P. Kumar, A. Nagarajan and P. D. Uchil, Analysis of Cell Viability by the MTT Assay, Cold Spring Harb. Protoc., 2018, 2018(6), pdb.prot095505, DOI:10.1101/pdb.prot095505.
- M. V. Berridge and A. S. Tan, Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction, Arch. Biochem. Biophys., 1993, 303(2), 474–482, DOI:10.1006/abbi.1993.1311.
- A. G. Guimarães, M. A. Oliveira, R. d. S. Alves, P. d. P. Menezes, M. R. Serafini, A. A. de Souza Araújo, D. P. Bezerra and L. J. Quintans Júnior, Encapsulation of carvacrol, a monoterpene present in the essential oil of oregano, with β-cyclodextrin, improves the pharmacological response on cancer pain experimental protocols, Chem.-Biol. Interact., 2015, 227, 69–76, DOI:10.1016/j.cbi.2014.12.020.
- T. Loftsson, M. Másson and M. E. Brewster, Self-Association of Cyclodextrins and Cyclodextrin Complexes, J. Pharm. Sci., 2004, 93(5), 1091–1099, DOI:10.1002/jps.20047.
- B. Gao, G. Wang, B. Li and L. Wu, Self-Inclusion and Dissociation of a Bridging β-Cyclodextrin Triplet, ACS Omega, 2020, 5(14), 8127–8136, DOI:10.1021/acsomega.0c00363.
- M. I. Rodríguez-López, M. T. Mercader-Ros, C. Lucas-Abellán, J. A. Pellicer, A. Pérez-Garrido, H. Pérez-Sánchez, M. J. Yáñez-Gascón, J. A. Gabaldón and E. Núñez-Delicado, Comprehensive Characterization of Linalool-HP-β-Cyclodextrin Inclusion Complexes, Molecules, 2020, 25(21), 5069, DOI:10.3390/molecules25215069.
- N. Khan, A. K. Singh and A. Saneja, Preparation, Characterization, and Antioxidant Activity of L-Ascorbic Acid/HP-β-Cyclodextrin Inclusion Complex-Incorporated Electrospun Nanofibers, Foods, 2023, 12(7), 1363, DOI:10.3390/foods12071363.
- S. Saha, A. Roy, K. Roy and M. N. Roy, Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules, Sci. Rep., 2016, 6(1), 35764, DOI:10.1038/srep35764.
- M. I. Rodríguez-López, M. T. Mercader-Ros, S. López-Miranda, J. A. Pellicer, A. Pérez-Garrido, H. Pérez-Sánchez, E. Núñez-Delicado and J. A. Gabaldón, Thorough characterization and stability of HP-β-cyclodextrin thymol inclusion complexes prepared by microwave technology: A required approach to a successful application in food industry, J. Sci. Food Agric., 2019, 99(3), 1322–1333, DOI:10.1002/jsfa.9307.
- S. Jiang, T. Zhao, Y. Wei, Z. Cao, Y. Xu, J. Wei, F. Xu, H. Wang and X. Shao, Preparation and characterization of tea tree oil/hydroxypropyl-β-cyclodextrin inclusion complex and its application to control brown rot in peach fruit, Food Hydrocolloids, 2021, 121, 107037, DOI:10.1016/j.foodhyd.2021.107037.
- R. N. Marreto, E. E. C. V. Almeida, P. B. Alves, E. S. Niculau, R. S. Nunes, C. R. S. Matos and A. A. S. Araújo, Thermal analysis and gas chromatography coupled mass spectrometry analyses of hydroxypropyl-β-cyclodextrin inclusion complex containing Lippia gracilis essential oil, Thermochim. Acta, 2008, 475(1), 53–58, DOI:10.1016/j.tca.2008.06.015.
- J. Rakmai, B. Cheirsilp, J. C. Mejuto, A. Torrado-Agrasar and J. Simal-Gándara, Physico-chemical characterization and evaluation of bio-efficacies of black pepper essential oil encapsulated in hydroxypropyl-beta-cyclodextrin, Food Hydrocolloids, 2017, 65, 157–164, DOI:10.1016/j.foodhyd.2016.11.014.
- T. Loftsson and M. E. Brewster, Pharmaceutical applications of cyclodextrins: basic science and product development, J. Pharm. Pharmacol., 2010, 62(11), 1607–1621, DOI:10.1111/j.2042-7158.2010.01030.x.
- A. Ait-Ouazzou, L. Cherrat, L. Espina, S. Lorán, C. Rota and R. Pagán, The antimicrobial activity of hydrophobic essential oil constituents acting alone or in combined processes of food preservation, Innovat. Food Sci. Emerg. Technol., 2011, 12(3), 320–329, DOI:10.1016/j.ifset.2011.04.004.
- T. D. Tavares, J. C. Antunes, J. Padrão, A. I. Ribeiro, A. Zille, M. T. P. Amorim, F. Ferreira and H. P. Felgueiras, Activity of Specialized Biomolecules against Gram-Positive and Gram-Negative Bacteria, Antibiotics, 2020, 9(6), 314, DOI:10.3390/antibiotics9060314.
- D. Ghosh, S. Yadav, S. Bag, A. I. Mallick and P. De, Antibacterial activity of hydrophobicity modulated cationic polymers with enzyme and pH-responsiveness, J. Mater. Chem. B, 2024, 12(11), 2894–2904, 10.1039/D3TB02801A.
- A. Arrais, E. Bona, V. Todeschini, A. Caramaschi, N. Massa, M. Roncoli, A. Minervi, E. Perin and V. Gianotti, Thymus vulgaris Essential Oil in Beta-Cyclodextrin for Solid-State Pharmaceutical Applications, Pharmaceutics, 2023, 15(3), 914, DOI:10.3390/pharmaceutics15030914.
- K. Schilcher and A. R. Horswill, Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies, Microbiol. Mol. Biol. Rev., 2020, 84(3) DOI:10.1128/mmbr.00026-19.
- A. Omar, T. E. El-Banna, F. I. Sonbol and M. M. El-Bouseary, Potential antivirulence and antibiofilm activities of sub-MIC of oxacillin against MDR S. aureus isolates: an in-vitro and in-vivo study, BMC Microbiol., 2024, 24(1), 295, DOI:10.1186/s12866-024-03429-8.
- M. Rohde, The Gram-Positive Bacterial Cell Wall, Microbiol. Spectr., 2019, 7(3) DOI:10.1128/microbiolspec.gpp3-0044-2018.
- B. dos Santos Lima, S. Shanmugam, J. de Souza Siqueira Quintans, L. J. Quintans-Júnior and A. A. de Souza Araújo, Inclusion complex with cyclodextrins enhances the bioavailability of flavonoid compounds: a systematic review, Phytochem. Rev., 2019, 18(5), 1337–1359, DOI:10.1007/s11101-019-09650-y.
- Á. Sarabia-Vallejo, M. d. M. Caja, A. I. Olives, M. A. Martín and J. C. Menéndez, Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects, Pharmaceutics, 2023, 15(9), 2345, DOI:10.3390/pharmaceutics15092345.
- A. Naik and R. Premanath, Anti-quorum sensing activity of essential oils against multidrug-resistant Klebsiella pneumoniae: a novel approach to control bacterial virulence, Braz. J. Microbiol., 2024, 3909–3920, DOI:10.1007/s42770-024-01546-0.
- R. Elawady, A. G. Aboulela, A. Gaballah, A. A. Ghazal and A. N. Amer, Antimicrobial Sub-MIC induces Staphylococcus aureus biofilm formation without affecting the bacterial count, BMC Infect. Dis., 2024, 24(1), 1065, DOI:10.1186/s12879-024-09790-3.
- M. Kfoury, L. Auezova, H. Greige-Gerges and S. Fourmentin, Encapsulation in cyclodextrins to widen the applications of essential oils, Environ. Chem. Lett., 2019, 17(1), 129–143, DOI:10.1007/s10311-018-0783-y.
- P. Jaroš, E. Timkina, J. Michailidu, D. Maršík, M. Kulišová, I. Kolouchová and K. Demnerová, Boswellic Acids as Effective Antibacterial Antibiofilm Agents, Molecules, 2022, 27(12), 3795, DOI:10.3390/molecules27123795.
- P. Singh, K. M. Chacko, M. Aggarwal, B. Bhat, R. Khandal, S. Sultana and B. T. Kuruvilla, A-90 day gavage safety assessment of Boswellia serrata in rats, Toxicol. Int., 2012, 19(3), 273 CrossRef.
- D. Bian, Y. Pilehvar, S. Kousha and J. Bi, Bioactive Wound Healing 3D Structure Based on Chitosan Hydrogel Loaded with Naringin/Cyclodextrin Inclusion Nanocomplex, ACS Omega, 2024, 9(9), 10566–10576, DOI:10.1021/acsomega.3c08785.
- P. Sangsanoh, S. Chaiarwut, C. Choipang, J. Niyompanich, O. Suwantong, K. Lirdprapamongkol, J. Svasti, P. Chuysinuan, S. Techasakul and P. Supaphol, Cannabidiol/β-Cyclodextrin Inclusion Complex-Loaded Poly(Vinyl Alcohol) Semi-solid Hydrogels for Potential Use in Wound Management, J. Polym. Environ., 2023, 31(9), 3982–3997, DOI:10.1007/s10924-023-02845-7.
- A. V. Shinde, C. Humeres and N. G. Frangogiannis, The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling, Biochim. Biophys. Acta, Mol. Basis Dis., 2017, 1863(1), 298–309, DOI:10.1016/j.bbadis.2016.11.006.
- N. P. Talele, J. Fradette, J. E. Davies, A. Kapus and B. Hinz, Expression of α-Smooth Muscle Actin Determines the Fate of Mesenchymal Stromal Cells, Stem Cell Rep., 2015, 4(6), 1016–1030, DOI:10.1016/j.stemcr.2015.05.004.
- T. Wild, A. A. Aljowder, A. Aljawder, J. Marotz and F. Siemers, From Wound to Scar: Scarring Explained—Pathophysiology of Wound Healing, in Scars: A Practical Guide for Scar Therapy, ed. S. P. Nischwitz, L.-P. Kamolz and L. K. Branski, Springer International Publishing, Cham, 2024, pp. 11–27 Search PubMed.
- L. Huelsboemer, L. Knoedler, A. Kochen, C. T. Yu, H. Hosseini, K. S. Hollmann, A. E. Choi, V. A. Stögner, S. Knoedler, H. C. Hsia, B. Pomahac and M. Kauke-Navarro, Cellular therapeutics and immunotherapies in wound healing – on the pulse of time?, Mil. Med. Res., 2024, 11(1), 23, DOI:10.1186/s40779-024-00528-5.
- D. G. Greenhalgh, Wound Healing, in Burn Care for General Surgeons and General Practitioners, ed. D. G. Greenhalgh, Springer International Publishing, Cham, 2016, pp. 95–116 Search PubMed.
- J. C. Bayiha, B. Evrard, D. Cataldo, P. De Tullio and M.-P. Mingeot-Leclercq, The Budesonide-Hydroxypropyl-β-Cyclodextrin Complex Attenuates ROS Generation, IL-8 Release and Cell Death Induced by Oxidant and Inflammatory Stress. Study on A549 and A-THP-1 Cells, Molecules, 2020, 25(21), 4882 CrossRef CAS.
- N. S. Chandel, Metabolism of Proliferating Cells, Cold Spring Harbor Perspect. Biol., 2021, 13(10), a040618, DOI:10.1101/cshperspect.a040618.
- J. Zhu and C. B. Thompson, Metabolic regulation of cell growth and proliferation, Nat. Rev. Mol. Cell Biol., 2019, 20(7), 436–450, DOI:10.1038/s41580-019-0123-5.
- A. G. Jimenez, C. Cooper-Mullin, N. B. Anthony and J. B. Williams, Cellular metabolic rates in cultured primary dermal fibroblasts and myoblast cells from fast-growing and control Coturnix quail, Comp. Biochem. Physiol., Part A:Mol. Integr. Physiol., 2014, 171, 23–30, DOI:10.1016/j.cbpa.2014.02.006.
- S. Wang, Y. Liang and C. Dai, Metabolic Regulation of Fibroblast Activation and Proliferation during Organ Fibrosis, Kidney Dis., 2022, 8(2), 115–125, DOI:10.1159/000522417.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4na00916a |
| This journal is © The Royal Society of Chemistry 2025 |






