Open Access Article
Open Access ArticleGreen procedures for synthesizing potential hNMDA receptor allosteric modulators through reduction and one-pot reductive acetylation of nitro(hetero)arenes using a superparamagnetic Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocatalyst†
Hossein
Mousavi
 *,
Behzad
Zeynizadeh
*,
Behzad
Zeynizadeh
 and
Farhad
Sepehraddin
and
Farhad
Sepehraddin
Department of Organic Chemistry, Faculty of Chemistry, Urmia University, Urmia, Iran. E-mail: 1hossein.mousavi@gmail.com
First published on 10th March 2025
Abstract
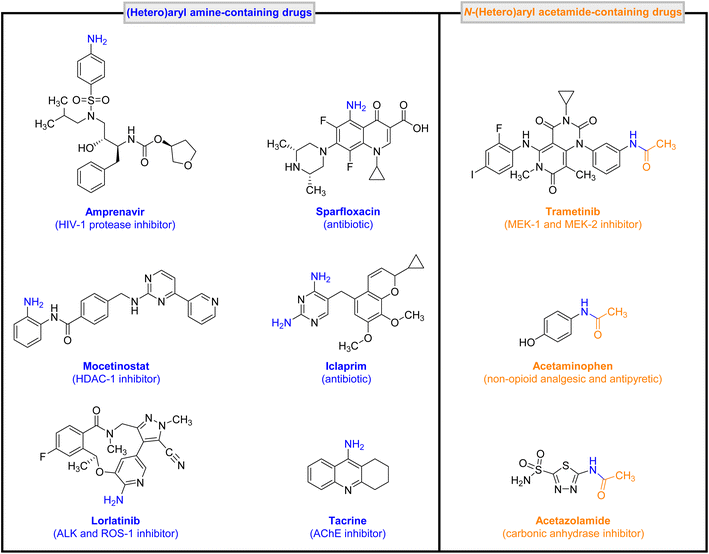
The conversion of nitro(hetero)arenes to corresponding (hetero)aryl amines and other practical organic compounds plays a crucial role in various sciences, especially environmental remediation and public health. In the current research work, diverse green and efficient strategies for the convenient reduction (hydrogenation) and one-pot two-step reductive acetylation of nitro(hetero)arenes using a core–shell-type mesoporous zirconocene-containing magnetically recoverable nanocomposite (viz. Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2)) as a powerful nanocatalytic system have been developed. In the presented organic transformations, the superparamagnetic Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite exhibited satisfactory turnover numbers (TONs) and turnover frequencies (TOFs), along with acceptable reusability. On the other hand, we investigated the potential biological effect of the synthesized (hetero)aryl amines and N-(hetero)aryl acetamides against the transmembrane domain (TMD) of the human N-methyl-D-aspartate (hNMDA) receptor based on molecular docking studies. Furthermore, the drug-likeness properties of our hit compound (viz. N-(3-(1-hydroxyethyl)phenyl)acetamide) have been scrutinized by in silico ADMET analyses.
1. Introduction
Numerous (hetero)aromatic nitro compounds are toxic and hazardous.1 Therefore, exploring green, efficient, economical, and simple synthetic strategies for conversion of the mentioned nitro-containing compounds to the corresponding (hetero)aryl amines and/or other valuable organic compounds, including N-(hetero)aryl acetamides, is of great significance for environmental remediation, public health, and also medicinal chemistry (Fig. 1).2In the new century, “Green Chemistry” has become one of the hottest and most significant terms in all branches of chemistry.3 Based on green chemistry principles, designing effective, eco-friendly, recoverable, and reusable heterogeneous catalytic systems is still an ultimate goal for numerous critical organic transformations. During the last three decades, zirconium (Zr)-containing catalytic systems have played an essential role in organic synthesis.4 It is worth noting that zirconium is one of the earth-abundant transition metals (EATMs),5 and also based on a paper, which was reported by Bystrzanowska, Petkov, and Tobiszewski in 2019, results of the multicriteria decision analysis (MCDA) approach demonstrated that Zr-containing heterogeneous catalytic systems are environmentally desirable compared to rhodium (Rh)-, copper (Cu)-, cadmium (Cd)-, palladium (Pd)-, gold (Au)-, cobalt (Co)-, and also nickel (Ni)-based systems.6 On the other hand, the recoverability and reusability of the catalyst are crucial factors from the green chemistry point of view. In this regard, one of the best choices is the use of magnetic nanoparticles (such as Fe3O4, CuFe2O4, ZrFe2O4, MgFe2O4, CoFe2O4, NiFe2O4, MnFe2O4, etc.) as a core part of a core–shell-type catalytic system.7 In addition to the catalyst, according to the principles of green chemistry, the solvent is another main factor in organic synthesis. Undoubtedly, water is the most preferable choice compared to all common solvents because of its unique features, such as abundance, non-toxicity, sustainability, etc.8
Neurodegenerative diseases (NDs) are among the alarming and significant health challenges facing contemporary society.9 In a situation where most of them are still incurable, many endeavors have been made to overcome or control them. N-methyl-D-aspartate (NMDA) receptors, known as ionotropic glutamate-gated cation channels with high calcium (CaII) permeability, are members of the immense family of glutamate receptors and are linked to synapse development, synaptic plasticity, and cognitive functions, which are vital for diverse aspects of brain function. Notably, research has highlighted the significance of NMDA receptors in various NDs (such as Alzheimer's disease (AD), Parkinson's disease (PD), and Huntington's disease (HD)) and neuro-developmental disorders including intellectual disability, schizophrenia, autism, and epilepsy.10,11 Also, NMDA receptors have a confirmed role in ischemic brain injury and neuropathic pain.11
In recent years, the computer-aided drug design (CADD) strategy has been recognized as a powerful approach that utilizes particular computational methods and algorithms to speed up and enhance the drug discovery and development process and helps researchers by identifying and validating potential drug targets via analyzing biological data and understanding the interactions between proteins, enzymes, receptors, and many other molecules concerned in disease pathways.12
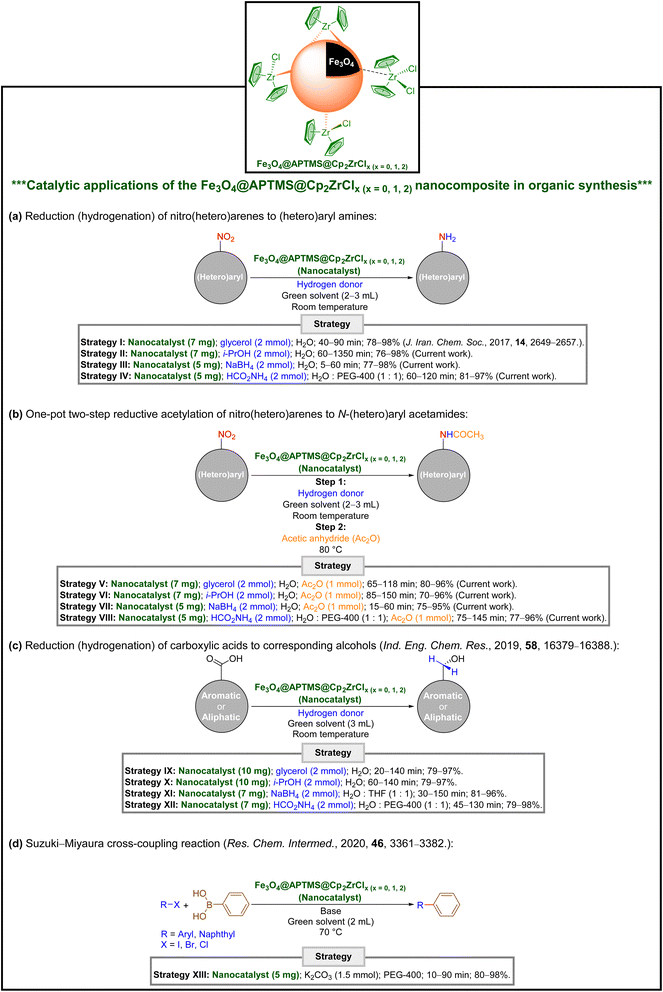
In continuation of our research on various types of catalytic organic transformations13–16 and also due to the importance of introducing a new environmentally benign approach to the conversion of nitro(hetero)arenes to valuable organic compounds, herein we wish to report diverse green and efficient strategies for the convenient reduction (hydrogenation) and one-pot two-step reductive acetylation of nitro(hetero)arenes using a superparamagnetic Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite as an efficient core–shell-type mesoporous zirconocene-containing recoverable nanocatalyst (Fig. 2). Besides, we have investigated the potential medicinal effect of all synthesized (hetero)aryl amines and N-(hetero)aryl acetamides against the transmembrane domain (TMD) of the human N-methyl-D-aspartate (hNMDA) receptor using molecular docking studies. We have also examined the drug-likeness properties of our hit compound (viz. N-(3-(1-hydroxyethyl)phenyl)acetamide) through in silico ADMET analyses.
2. Results and discussion
2.1. Preparation of the superparamagnetic core–shell-type Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite
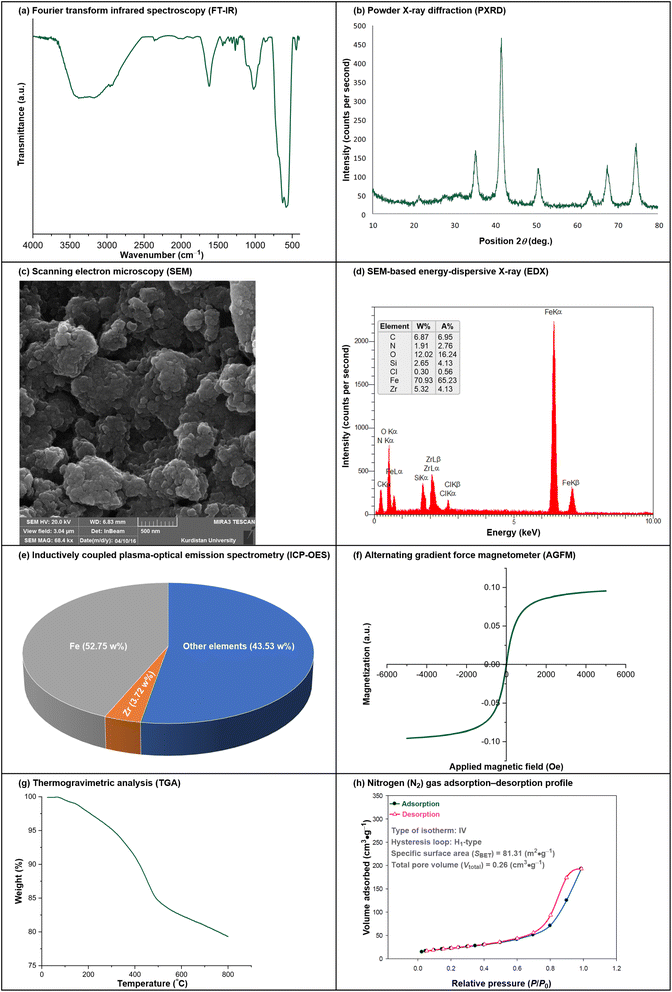
We started our work by preparing the superparamagnetic Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite according to our previously published papers (Fig. 3).14–16 It is worthwhile to note that the structure of the mentioned core–shell-type magnetic system was characterized by Fourier transform infrared (FT-IR) spectroscopy, powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), SEM-based energy-dispersive X-ray (EDX) spectroscopy, inductively coupled plasma-optical emission spectrometry (ICP-OES), alternating gradient force magnetometry (AGFM), thermogravimetric analysis (TGA), and nitrogen (N2) gas adsorption–desorption analysis (Fig. 4), which was reported and discussed in our previously published papers.14–16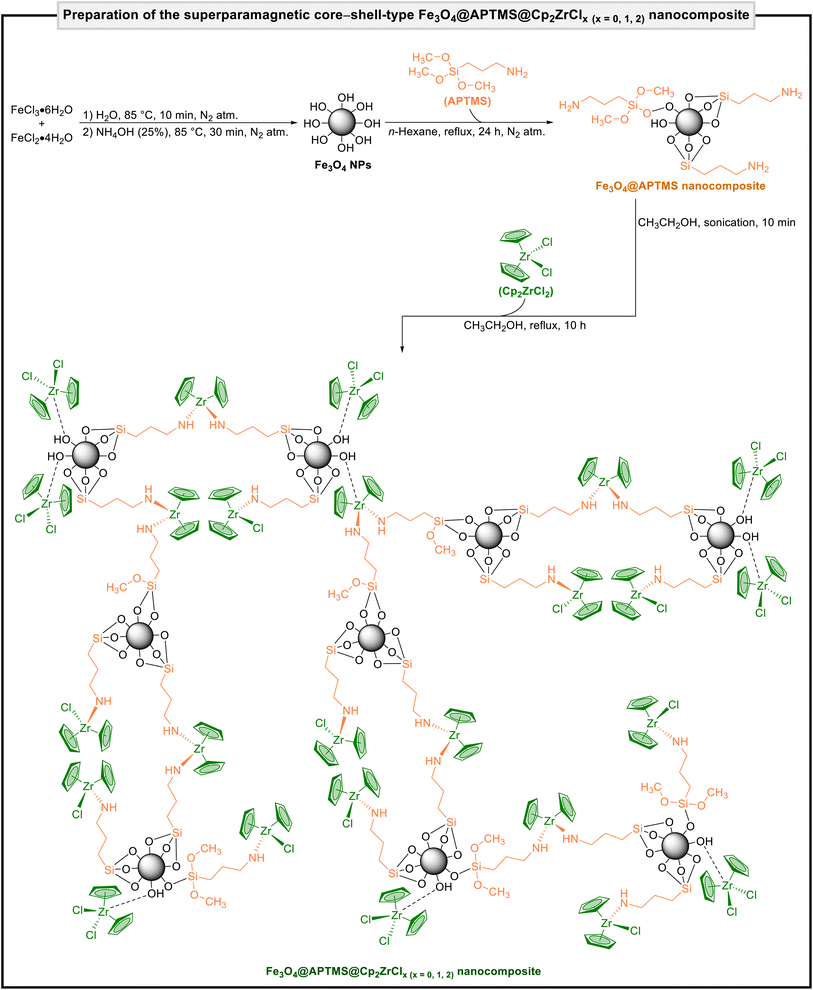 | ||
| Fig. 3 Preparation of the superparamagnetic core–shell-type Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite. | ||
In the FT-IR spectra of the as-prepared Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite (Fig. 4, section a), the broad peak in the region of 3500 cm−1 to 3000 cm−1 is related to the free hydroxyl and stretching vibration of amine functional groups. Additionally, the three peaks at 2958 cm−1, 2925 cm−1, and 2851 cm−1 correspond to the C–H stretching vibrations of the existing –CH (aromatic), –CH2–, and –CH3 (in Si–O–CH3) functional groups. The strong absorption peak at 1621 cm−1 is associated with the bending vibration of free H2O that adsorbed on the surface of Fe3O4 nanoparticles, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of the cyclopentadienyl (Cp) ligand, and N–H bending vibrations, which overlapped in one peak. Also, the two peaks at 1437 cm−1 and 1407 cm−1 can be related to the C–O (stretching), scissoring mode of the –CH2– groups (bending), and stretching vibration of Si–CH2. Notably, the region from 1345 cm−1 to 1212 cm−1 can be related to stretching vibrations of C–N and C–O, as well as out-of-plane wagging and twisting bending vibrations of the –CH2– groups along with the bending vibration of Si–CH2. The two relatively weak peaks at 1188 cm−1 and 1164 cm−1, along with the strong peak at 1097 cm−1, can correspond to Si–OCH3. The peaks at 1020 cm−1, 998 cm−1, 945 cm−1, 863 cm−1, and 838 cm−1 are related to the out-of-plane bending vibrations of the aromatic C–H bonds belonging to the structure of the cyclopentadienyl (Cp) ligand. The peak attributed to the in-plane rocking-type bending vibrations of the –CH2– groups appears at 690 cm−1. Furthermore, the three strong peaks at 628 cm−1, 581 cm−1, and 564 cm−1 are associated with the splitting ν1 vibration of Fe2+–O2−, while the peak at 446 cm−1 belongs to the splitting ν2 vibration of Fe3+–O2−.
C stretching vibration of the cyclopentadienyl (Cp) ligand, and N–H bending vibrations, which overlapped in one peak. Also, the two peaks at 1437 cm−1 and 1407 cm−1 can be related to the C–O (stretching), scissoring mode of the –CH2– groups (bending), and stretching vibration of Si–CH2. Notably, the region from 1345 cm−1 to 1212 cm−1 can be related to stretching vibrations of C–N and C–O, as well as out-of-plane wagging and twisting bending vibrations of the –CH2– groups along with the bending vibration of Si–CH2. The two relatively weak peaks at 1188 cm−1 and 1164 cm−1, along with the strong peak at 1097 cm−1, can correspond to Si–OCH3. The peaks at 1020 cm−1, 998 cm−1, 945 cm−1, 863 cm−1, and 838 cm−1 are related to the out-of-plane bending vibrations of the aromatic C–H bonds belonging to the structure of the cyclopentadienyl (Cp) ligand. The peak attributed to the in-plane rocking-type bending vibrations of the –CH2– groups appears at 690 cm−1. Furthermore, the three strong peaks at 628 cm−1, 581 cm−1, and 564 cm−1 are associated with the splitting ν1 vibration of Fe2+–O2−, while the peak at 446 cm−1 belongs to the splitting ν2 vibration of Fe3+–O2−.
The PXRD plot of the as-prepared Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite is presented in Fig. 4 (section b). In addition to the peaks corresponding to the Fe3O4 NPs, some distinct PXRD peaks related to silicon (Si) appeared at 2θ = 27.69° (broad), 68.87°, and 76.67°. Furthermore, the peak at 2θ = 22.13°, associated with the (002) lattice spacing of carbon-based materials with an amorphous nature, may correspond to the Cp ligand and aliphatic chains. Notably, several distinct PXRD planes of zirconium (Zr), including (100), (002), (101), (103), (200), (201), (004), and (202), overlap with the peaks of Fe3O4 NPs and Si.
The scanning electron microscopy (SEM) image of the as-prepared Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite (Fig. 4, section c) shows the morphology and size distribution of the particles. Also, the SEM-based energy-dispersive X-ray (EDX) diagram (Fig. 4, section d) of the mentioned nanocomposite confirmed the presence of the carbon (C), nitrogen (N), oxygen (O), silicon (Si), chlorine (Cl), ferrite (Fe), and zirconium (Zr) elements. Furthermore, the inductively coupled plasma-optical emission spectrometry (ICP-OES) analysis (Fig. 4, section e) demonstrated the exact amounts of Fe (52.75 w%) and Zr (3.72 w%) in the as-prepared core–shell-type Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite structure.
The analysis using an alternating gradient force magnetometer (AGFM) showed that the as-synthesized Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite exhibits superparamagnetic behavior and sufficient saturation magnetization (Ms) for effective magnetic recycling performance (Fig. 4, section f). On the other hand, the thermogravimetric analysis (TGA) shows that the zirconocene-containing catalytic system exhibits excellent thermal stability for the organic transformations carried out at temperatures ranging from room temperature to 80 °C (Fig. 4, section g). The TGA diagram (Fig. 4, section g) indicates that the structure of this nanocomposite remains completely stable up to 80 °C, with only a 0.19% weight loss observed at this temperature. Interestingly, the superparamagnetic zirconium-containing nanocomposite experiences a 21% weight loss at 800 °C.
The nitrogen (N2) gas adsorption–desorption analysis (Fig. 4, section h) of the as-synthesized Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite reveals an isotherm shape approximately classified as type IV with a H1-type hysteresis loop. Also, the mentioned analysis demonstrated that the specific surface area (SBET) and total pore volume (Vtotal) values of the as-prepared mesoporous Zr-containing nanocomposite were 81.31 m2 g−1 and 0.26 cm3 g−1, respectively (Fig. 4, section h).
2.2. Reduction (hydrogenation) of nitro(hetero)arenes to the corresponding (hetero)aryl amines
It should be noted that Zeynizadeh and Sepehraddin reported the first catalytic application of the as-prepared Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite for the reduction (hydrogenation) of nitro-containing (hetero)aromatic compounds to the corresponding amines using glycerol as a reducing (hydrogen donor) agent in water at room temperature (Fig. 2 and Table 1).14 In the current work, we tried to investigate the effect of other easily accessible reducing (hydrogen donors) agents, namely isopropanol (i-PrOH), sodium borohydride (NaBH4), and ammonium formate (HCO2NH4), on the stated organic transformation in the presence of the mentioned superparamagnetic core–shell-type zirconocene-containing nanocatalyst. For this purpose, we carried out several tests to find the optimal reaction conditions (Table S1†). After this stage, we investigated the scope and limitations of the new and green reduction (hydrogenation) protocols using various types of nitro(hetero)arenes (Table 1). As shown in Table 1, the experimental results indicate that substrates with electron-donating and electro-withdrawing groups are also well tolerated under optimized reaction conditions. Besides, the turnover numbers (TONs) and turnover frequencies (TOFs) of the Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocatalyst in these reactions were calculated and are listed in Table 1. Importantly, as indicated in Table 1, the values of TONs and TOFs were satisfactory and acceptable. It is important to note that the exact mechanism for the reduction (hydrogenation) of nitro(hetero)arenes to the corresponding (hetero)aryl amines using introduced hydrogen donors in the presence of the as-prepared Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite as the heterogeneous mesoporous nanocatalyst is unclear. Despite this issue, we introduced different plausible mechanisms (two mechanisms to illustrate the role and behavior of glycerol or i-PrOH (Schemes 1 and 2) and also one reaction mechanism (Scheme 3) for NaBH4 or HCO2NH4) in the presence of the mentioned zirconocene-containing nanocatalyst based on our observations and literature data.15,17| Entry | Substrate | Product | Strategy | |||
|---|---|---|---|---|---|---|
| Strategy I | Strategy II | Strategy III | Strategy IV | |||
| a (a) The strategy I (except entries 4 and 17) was reported in ref. 14. (b) TON (turnover number) = [(mol of product formed)/(mol of catalyst used)]. (c) TOF (turnover frequency) = [(mol of product formed)/(mol of catalyst used) × (time)]. (d) The TON and TOF values were calculated based on the existing amount of zirconium (Zr) in the structure of the as-prepared Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite based on ICP-OES analysis. | ||||||
| 1 |

|

|
Time = 40 min | Time = 60 min | Time = 10 min | Time = 60 min |
| Yield = 96% | Yield = 97% | Yield = 95% | Yield = 97% | |||
| TON = 336.84 | TON = 340.35 | TON = 467.98 | TON = 477.83 | |||
| TOF = 8.42 | TOF = 5.67 | TOF = 46.80 | TOF = 7.96 | |||
| 2 |

|

|
Time = 60 min | Time = 75 min | Time = 10 min | Time = 60 min |
| Yield = 98% | Yield = 93% | Yield = 97% | Yield = 90% | |||
| TON = 343.86 | TON = 326.31 | TON = 477.83 | TON = 443.35 | |||
| TOF = 5.73 | TOF = 4.35 | TOF = 47.78 | TOF = 7.39 | |||
| 3 |

|

|
Time = 55 min | Time = 100 min | Time = 15 min | Time = 75 min |
| Yield = 96% | Yield = 90% | Yield = 95% | Yield = 89% | |||
| TON = 336.84 | TON = 315.79 | TON = 467.98 | TON = 438.42 | |||
| TOF = 6.12 | TOF = 3.16 | TOF = 31.20 | TOF = 5.84 | |||
| 4 |

|

|
Time = 50 min | Time = 60 min | Time = 5 min | Time = 60 min |
| Yield = 91% | Yield = 98% | Yield = 98% | Yield = 95% | |||
| TON = 319.30 | TON = 343.86 | TON = 482.76 | TON = 467.98 | |||
| TOF = 6.38 | TOF = 5.73 | TOF = 96.55 | TOF = 7.80 | |||
| 5 |

|

|
Time = 50 min | Time = 80 min | Time = 10 min | Time = 70 min |
| Yield = 95% | Yield = 84% | Yield = 93% | Yield = 90% | |||
| TON = 333.33 | TON = 294.73 | TON = 458.13 | TON = 443.35 | |||
| TOF = 6.67 | TOF = 3.68 | TOF = 45.81 | TOF = 6.33 | |||
| 6 |

|

|
Time = 60 min | Time = 120 min | Time = 15 min | Time = 90 min |
| Yield = 90% | Yield = 82% | Yield = 93% | Yield = 87% | |||
| TON = 315.79 | TON = 287.72 | TON = 458.13 | TON = 443.35 | |||
| TOF = 5.26 | TOF = 2.40 | TOF = 30.54 | TOF = 4.93 | |||
| 7 |

|

|
Time = 85 min | Time = 120 min | Time = 20 min | Time = 120 min |
| Yield = 85% | Yield = 78% | Yield = 90% | Yield = 81% | |||
| TON = 298.24 | TON = 273.68 | TON = 443.35 | TON = 399.01 | |||
| TOF = 3.51 | TOF = 2.28 | TOF = 22.17 | TOF = 3.32 | |||
| 8 |

|

|
Time = 45 min | Time = 70 min | Time = 15 min | Time = 70 min |
| Yield = 93% | Yield = 89% | Yield = 94% | Yield = 93% | |||
| TON = 326.31 | TON = 312.28 | TON = 463.05 | TON = 458.13 | |||
| TOF = 7.25 | TOF = 4.46 | TOF = 30.87 | TOF = 6.54 | |||
| 9 |
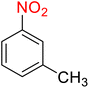
|
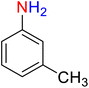
|
Time = 45 min | Time = 100 min | Time = 20 min | Time = 80 min |
| Yield = 87% | Yield = 89% | Yield = 93% | Yield = 87% | |||
| TON = 305.26 | TON = 312.28 | TON = 458.13 | TON = 428.57 | |||
| TOF = 6.78 | TOF = 3.12 | TOF = 22.91 | TOF = 5.36 | |||
| 10 |

|

|
Time = 90 min | Time = 120 min | Time = 20 min | Time = 100 min |
| Yield = 95% | Yield = 84% | Yield = 90% | Yield = 89% | |||
| TON = 333.33 | TON = 294.74 | TON = 443.35 | TON = 438.42 | |||
| TOF = 3.70 | TOF = 2.46 | TOF = 22.17 | TOF = 4.38 | |||
| 11 |
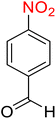
|

|
Time = 60 min | Time = 120 min | Time = 30 min | Time = 85 min |
| Yield = 88% | Yield = 88% | Yield = 86% | Yield = 86% | |||
| TON = 308.77 | TON = 308.77 | TON = 423.64 | TON = 423.64 | |||
| TOF = 5.15 | TOF = 2.57 | TOF = 14.12 | TOF = 4.98 | |||
| 12 |

|

|
Time = 60 min | Time = 120 min | Time = 35 min | Time = 120 min |
| Yield = 90% | Yield = 81% | Yield = 84% | Yield = 85% | |||
| TON = 315.79 | TON = 284.21 | TON = 413.79 | TON = 418.72 | |||
| TOF = 5.26 | TOF = 2.37 | TOF = 11.82 | TOF = 3.49 | |||
| 13 |

|

|
Time = 75 min | Time = 120 min | Time = 40 min | Time = 120 min |
| Yield = 90% | Yield = 76% | Yield = 77% | Yield = 81% | |||
| TON = 315.79 | TON = 266.67 | TON = 379.31 | TON = 399.01 | |||
| TOF = 4.21 | TOF = 2.22 | TOF = 9.48 | TOF = 3.32 | |||
| 14 |

|

|
Time = 65 min | Time = 120 min | Time = 40 min | Time = 85 min |
| Yield = 78% | Yield = 84% | Yield = 81% | Yield = 87% | |||
| TON = 273.68 | TON = 294.74 | TON = 399.01 | TON = 428.57 | |||
| TOF = 4.21 | TOF = 2.46 | TOF = 9.97 | TOF = 5.04 | |||
| 15 |

|

|
Time = 40 min | Time = 85 min | Time = 30 min | Time = 90 min |
| Yield = 87% | Yield = 87% | Yield = 86% | Yield = 89% | |||
| TON = 305.26 | TON = 305.26 | TON = 423.64 | TON = 438.42 | |||
| TOF = 7.63 | TOF = 3.59 | TOF = 14.12 | TOF = 4.87 | |||
| 16 |

|

|
Time = 40 min | Time = 100 min | Time = 30 min | Time = 90 min |
| Yield = 92% | Yield = 88% | Yield = 82% | Yield = 85% | |||
| TON = 322.81 | TON = 308.77 | TON = 403.94 | TON = 418.72 | |||
| TOF = 8.07 | TOF = 3.09 | TOF = 13.46 | TOF = 4.65 | |||
| 17 |

|

|
Time = 90 min | Time = 135 min | Time = 60 min | Time = 120 min |
| Yield = 95% | Yield = 89% | Yield = 80% | Yield = 90% | |||
| TON = 333.33 | TON = 312.28 | TON = 394.09 | TON = 443.35 | |||
| TOF = 3.70 | TOF = 2.31 | TOF = 6.57 | TOF = 3.69 | |||
| 18 |

|

|
Time = 65 min | Time = 75 min | Time = 25 min | Time = 60 min |
| Yield = 88% | Yield = 90% | Yield = 87% | Yield = 91% | |||
| TON = 308.77 | TON = 315.79 | TON = 428.57 | TON = 448.27 | |||
| TOF = 4.75 | TOF = 4.21 | TOF = 17.14 | TOF = 7.47 | |||
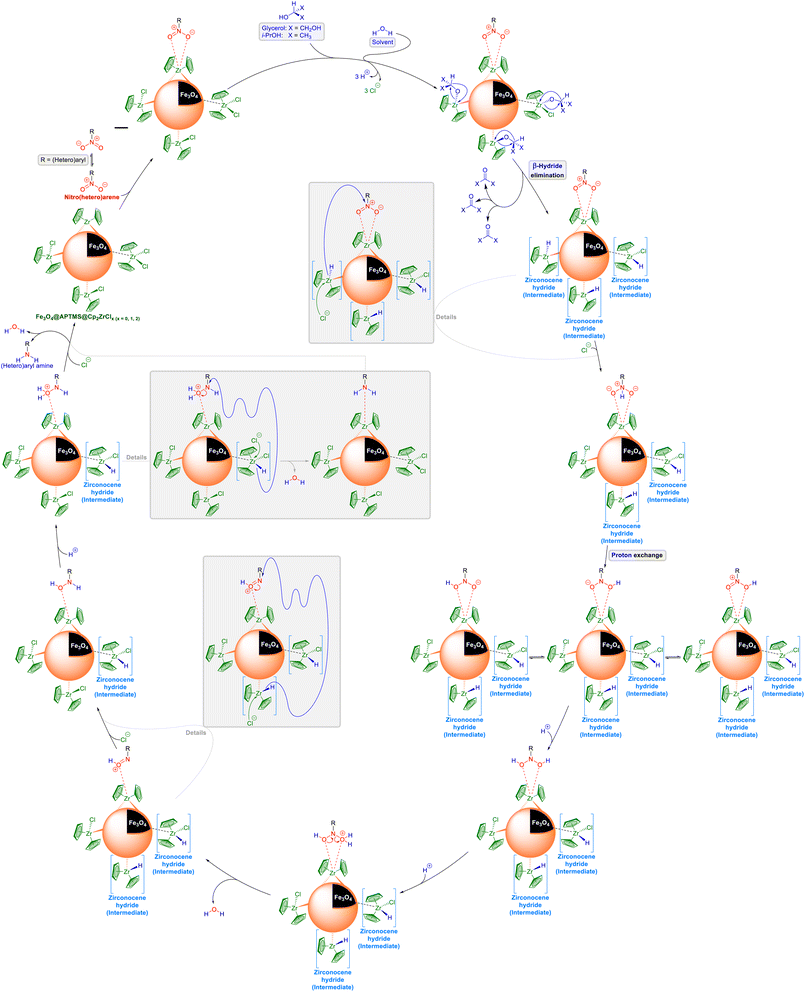
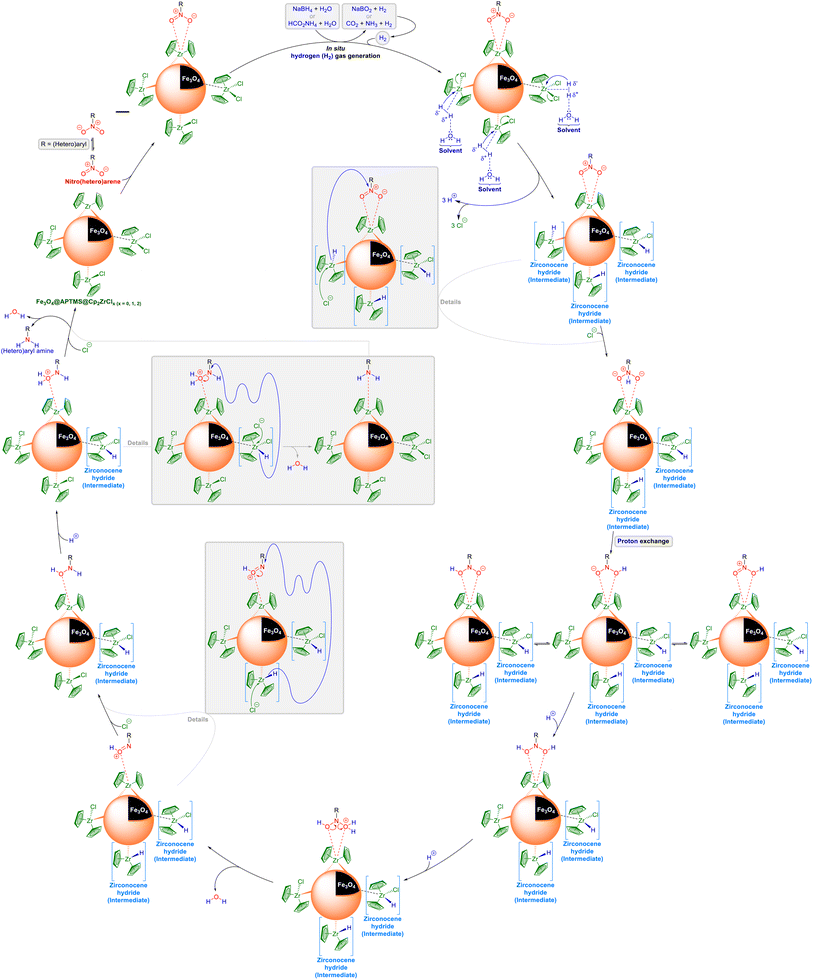
As illustrated in Schemes 1 and 2, when the nitro(hetero)arene reduction (hydrogenation) reaction was carried out with glycerol or i-PrOH as the reducing (hydrogen donor) agent in the presence of the as-prepared Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocatalyst in water, we suspect that this reaction may have occurred via two different pathways, including direct hydrogen transfer and the hydridic route, based on literature data. In the first pathway (Scheme 1), viz. direct hydrogen transfer, glycerol or i-PrOH is activated by attaching to the zirconium metal. Subsequently, by performing a Meerwein–Ponndorf–Verley (MPV)-like reaction, hydrogen is transferred directly to the nitro(hetero)arene molecule. Conversely, in the second pathway (Scheme 2), viz. hydridic route, after the activation of glycerol or i-PrOH by attaching to the zirconium metal, the β-hydride elimination causes the formation of a zirconocene hydride intermediate, which is capable of the reduction (hydrogenation) of nitro(hetero)arene to the corresponding (hetero)aryl amine through the transfer hydrogenation process. On the other hand and as shown in Scheme 3, when the reduction (hydrogenation) reaction was conducted with NaBH4 or HCO2NH4 as the reducing (hydrogen donor) agent in the presence of the as-prepared Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocatalyst in the water solvent (or the H2O:PEG-400 mixture solvent), we observed the production of hydrogen (H2) gas inside the reaction vessel. Based on this observation, we believe that the in situ-generated H2 gas diffused in the reaction environment and was then activated by the zirconium (Zr) metal and the electron pairs of oxygen belonging to the water molecule. After that, replacing zirconium-attached chlorine with partially negative charge hydrogen caused the formation of the zirconocene hydride intermediate, which was one of the fundamental intermediates in the mentioned reduction (hydrogenation) reaction. Subsequently, the step-by-step hydrogen transfer process from the zirconocene hydride intermediate to the nitro(hetero)arene molecule, which was followed by the proton exchange and then the elimination of two water molecules, led to the reduction (hydrogenation) of the nitro(hetero)arene molecule to the corresponding (hetero)aryl amine.
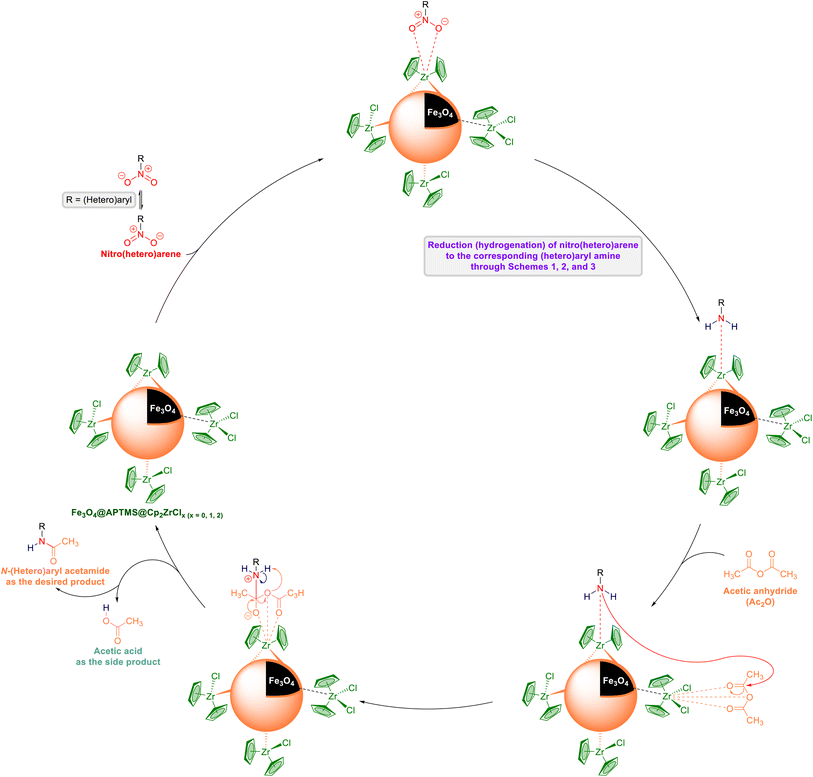
2.3. One-pot two-step reductive acetylation of nitro(hetero)arenes to the corresponding N-(hetero)aryl acetamides
Developing effective and straightforward synthetic strategies for constructing amides, which are prevalent functional and structural elements in drugs, agrochemicals, peptides, proteins, and many others, is highly valuable in organic synthesis.18 From the green chemistry point of view, one-pot reactions are always the best choice for chemists due to their simplicity, time- and cost-effectiveness, and many others.19 In this regard, and following successful reduction (hydrogenation) strategies for nitro(hetero)arenes, we decided to introduce new one-pot two-step reductive acetylation approaches for the efficient and green synthesis of N-(hetero)aryl acetamides from nitro(hetero)arenes. For this purpose, in the second step of the mentioned one-pot organic transformation (viz. acetylation), we used acetic anhydride (Ac2O) as an acetylating agent under different reaction temperature conditions (80 °C) rather than step one (room temperature). Under these reaction conditions, we successfully prepared diverse N-(hetero)aryl acetamide derivatives (Table 2). Notably, based on the amount of catalyst used, our protocols have acceptable reaction times, yields, TONs, and TOFs (Table 2). Furthermore, a plausible mechanism for the current one-pot two-step reductive acetylation reactions is depicted in Scheme 4. Briefly, after the reduction (hydrogenation) of the nitro(hetero)arene molecule to the corresponding (hetero)aryl amine, which is illustrated in Schemes 1–3, the in situ obtained (hetero)aryl amine compound was acetylated through attacking Ac2O, which was activated by the as-prepared Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocatalyst (Scheme 4).| Entry | Substrate | Product | Strategy | |||
|---|---|---|---|---|---|---|
| Strategy V | Strategy VI | Strategy VII | Strategy VIII | |||
| a (a) TON (turnover number) = [(mol of product formed)/(mol of catalyst used)]. (b) TOF (turnover frequency) = [(mol of product formed)/(mol of catalyst used) × (time)]. (c) The TON and TOF values were calculated based on the existing amount of zirconium (Zr) in the structure of the as-prepared Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite based on ICP-OES analysis. (d) In entry 2, the amount of acetic anhydride (Ac2O) used was 2 mmol. | ||||||
| 1 |

|

|
Time = 75 min | Time = 85 min | Time = 15 min | Time = 75 min |
| Yield = 96% | Yield = 96% | Yield = 95% | Yield = 96% | |||
| TON = 336.84 | TON = 336.84 | TON = 467.98 | TON = 472.90 | |||
| TOF = 4.49 | TOF = 3.96 | TOF = 31.20 | TOF = 6.30 | |||
| 2 |
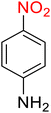
|

|
Time = 87 min | Time = 120 min | Time = 25 min | Time = 100 min |
| Yield = 93% | Yield = 81% | Yield = 91% | Yield = 89% | |||
| TON = 326.31 | TON = 284.21 | TON = 448.27 | TON = 438.42 | |||
| TOF = 3.75 | TOF = 2.37 | TOF = 17.93 | TOF = 4.38 | |||
| 3 |

|
|
Time = 67 min | Time = 95 min | Time = 20 min | Time = 85 min |
| Yield = 92% | Yield = 88% | Yield = 92% | Yield = 91% | |||
| TON = 322.81 | TON = 308.77 | TON = 453.20 | TON = 448.27 | |||
| TOF = 4.82 | TOF = 3.25 | TOF = 22.66 | TOF = 5.27 | |||
| 4 |

|

|
Time = 70 min | Time = 125 min | Time = 25 min | Time = 88 min |
| Yield = 85% | Yield = 86% | Yield = 90% | Yield = 85% | |||
| TON = 298.24 | TON = 301.75 | TON = 443.35 | TON = 418.72 | |||
| TOF = 4.26 | TOF = 2.41 | TOF = 17.71 | TOF = 4.76 | |||
| 5 |

|

|
Time = 118 min | Time = 150 min | Time = 27 min | Time = 120 min |
| Yield = 86% | Yield = 80% | Yield = 86% | Yield = 83% | |||
| TON = 301.75 | TON = 280.70 | TON = 423.64 | TON = 408.87 | |||
| TOF = 2.56 | TOF = 1.87 | TOF = 15.69 | TOF = 3.41 | |||
| 6 |

|

|
Time = 115 min | Time = 150 min | Time = 55 min | Time = 145 min |
| Yield = 80% | Yield = 70% | Yield = 75% | Yield = 77% | |||
| TON = 280.70 | TON = 245.61 | TON = 369.46 | TON = 379.31 | |||
| TOF = 2.44 | TOF = 1.64 | TOF = 6.72 | TOF = 2.61 | |||
| 7 |

|

|
Time = 65 min | Time = 115 min | Time = 35 min | Time = 110 min |
| Yield = 88% | Yield = 85% | Yield = 85% | Yield = 88% | |||
| TON = 308.77 | TON = 298.24 | TON = 418.71 | TON = 433.50 | |||
| TOF = 4.75 | TOF = 2.59 | TOF = 11.96 | TOF = 3.94 | |||
| 8 |

|
|
Time = 70 min | Time = 120 min | Time = 40 min | Time = 115 min |
| Yield = 86% | Yield = 81% | Yield = 80% | Yield = 82% | |||
| TON = 301.75 | TON = 284.21 | TON = 394.09 | TON = 403.94 | |||
| TOF = 4.31 | TOF = 2.37 | TOF = 9.85 | TOF = 3.51 | |||
| 9 |

|

|
Time = 90 min | Time = 150 min | Time = 60 min | Time = 140 min |
| Yield = 84% | Yield = 85% | Yield = 81% | Yield = 88% | |||
| TON = 294.74 | TON = 298.24 | TON = 399.01 | TON = 433.50 | |||
| TOF = 3.27 | TOF = 1.99 | TOF = 6.65 | TOF = 3.10 | |||
2.4. A comparative study
The practicality of the current diverse green procedures for the convenient reduction (hydrogenation) and one-pot two-step reductive acetylation of nitro(hetero)arenes in the presence of the superparamagnetic core–shell-type Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite as a powerful catalytic system is highlighted by a comparison of the obtained results with those of some of the previously reported methods on the mentioned organic transformations (Table 3). The stated comparative study demonstrated that the current green synthetic strategies have a relatively suitable place in terms of efficiency compared to the previous protocols.| Part A | ||||
|---|---|---|---|---|
| Entry | Reaction conditions | Time | Yield | Ref. |
| a Present work. | ||||
| A1 | Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) (7 mg); glycerol (2 mmol); H2O; r. t. | 40 min | 96% | 14 |
| A2 | Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) (7 mg); i-PrOH (2 mmol); H2O; r. t. | 60 min | 97% | |
| A3 | Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) (5 mg); NaBH4 (2 mmol); H2O; r. t. | 10 min | 95% | |
| A4 | Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) (5 mg); HCO2NH4 (2 mmol); H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG-400 (1 PEG-400 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); r. t. 1); r. t. |
60 min | 97% | |
| A5 | Fe3O4/f-MWCNT-CS-Glu/NiII (5 mg); NaBH4 (2 mmol); H2O; 60 °C | 4 min | 98% | 13a |
| A6 | Fe3O4@SiO2@KCC-1@MPTMS@CuII (10 mg); NaBH4 (2 mmol); H2O; 60 °C | 5 min | 98% | 13d |
| A7 | (7 wt%) Pd/C (30 mg); NaBH4 (2 mmol); H2O; reflux | 7 min | 93% | 13f |
| A8 | CuFe2O4 (48 mg); NaBH4 (2 mmol); H2O; reflux | 50 min | 95% | 13i |
| A9 | Fe2Se2CO9 (3 mol%); NH2NH2·H2O (2 mmol); H2O; 110 °C | 15 min | 89% | 20 |
| A10 | Ni(OH)2@PANI-1 (3.2 mol%); NaBH4 (10 mmol); H2O; reflux | 1.5 h | 85% | 21 |
| A11 | Cu-BTC@Fe3O4 (15 mg); NaBH4 (4 mmol); CH3CH2OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3 H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 45 °C 1); 45 °C |
3 h | 99% | 22 |
| A12 | IT-MHAP-Ag (60 mg); NaBH4 (5 mmol); H2O; reflux | 25 min | 98% | 23 |
| A13 | PSeCN/Ag (20 mg); NaBH4 (5 mmol); H2O; 75 °C | 25 min | 99% | 24 |
| A14 | CoOCN (20 mg); NH2NH2·H2O (2 mmol); H2O; 100 °C | 5 h | 84% | 25 |
| A15 | Fe3O4@SiO2@KIT-6@2-ATP@CuI (20 mg); NaBH4 (5 mmol); H2O; r. t. | 60 min | 89% | 26 |
| A16 | [C4(DABCO)2]·NiCl4 (80 mg); NaBH4 (3.5 mmol); H2O; 70 °C | 1 min | 98% | 27 |
| A17 | Ru-N,P-CBM (0.02 mol% of Ru); NaBH4 (5 mmol); CH3CH2OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); r. t. 1); r. t. |
60 min | 98% | 28 |
| A18 | MBC-PVIm/Pd (30 mg); NaBH4 (3 mmol); H2O; 50 °C | 30 min | 99% | 29 |
| A19 | SiO2/Fe3O4-SiO2-NH2/Cu-Ag (5 mg); NaBH4 (2 mmol); H2O; 70 °C | 5 min | 83% | 30 |
| A20 | rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) (20 mg); NH2NH2·H2O (2 mmol); CH3CH2OH; reflux | 10 min | 98% | 31 |
| A21 | Se0 (20 mol%); NaBH4 (4 mmol); NaOH (1 mmol); H2O; 100 °C | 3 h | 88% | 32 |
| A22 | Fe3O4/GO–IL–Pd (50 mg); NaBH4 (2 mmol); H2O; 60 °C | 10 min | 95% | 33 |
| A23 | Fe3O4/Biochar–Pd (10 mg); NaBH4 (5 mmol); CH3CH2OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3 H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 70 °C 1); 70 °C |
20 min | 96% | 34 |
| A24 | Cu-NPs (5 mol%); NaBH4 (4 mmol); CH3CH2OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); r. t. 3); r. t. |
2 h | 99% | 35 |
| Part B | ||||
|---|---|---|---|---|
| Entry | Reaction conditions | Time | Yield | Ref. |
| B1 | Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) (7 mg); glycerol (2 mmol); H2O; r. t.; Ac2O (1 mmol); 80 °C | 75 min | 96% | |
| B2 | Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) (7 mg); i-PrOH (2 mmol); H2O; r. t.; Ac2O (1 mmol); 80 °C | 85 min | 96% | |
| B3 | Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) (5 mg); NaBH4 (2 mmol); H2O; r. t.; Ac2O (1 mmol); 80 °C | 15 min | 95% | |
| B4 | Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) (5 mg); HCO2NH4 (2 mmol); H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG-400 (1 PEG-400 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); r. t.; Ac2O (1 mmol); 80 °C 1); r. t.; Ac2O (1 mmol); 80 °C |
75 min | 96% | |
| B5 | Fe3O4/f-MWCNT-CS-Glu/NiII (5 mg); NaBH4 (2 mmol); Ac2O (1 mmol); H2O; r. t. | 5 min | 97% | 13a |
| B6 | Fe3O4@SiO2@KCC-1@MPTMS@CuII (10 mg); NaBH4 (2 mmol); Ac2O (1 mmol); H2O; 60 °C | 7 min | 95% | 13d |
| B7 | (7 wt%) Pd/C (30 mg); NaBH4 (2 mmol); Ac2O (1 mmol); H2O; reflux | 8 min | 88% | 13f |
| B8 | Cu(Hdmg)2 (10 mol%); NaBH4 (3 mmol); EtOAc; 60 °C | 170 min | 97% | 13h |
| B9 | [C4(DABCO)2]·NiCl4 (80 mg); NaBH4 (3.5 mmol); H2O; Ac2O (1 mmol); 70 °C | 2 min | 98% | 27 |
| B10 | rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) (20 mg); NH2NH2·H2O (2 mmol); Ac2O (2 mmol); CH3CH2OH; reflux | 15 min | 97% | 31 |
| B11 | (2 wt%) Pd/(5 wt%) Sn–Al2O3 (50 mg); H2 atmosphere; Ac2O (1 mmol); H2O; r. t. | 3 h | 98% | 36 |
| B12 | f-ZCu (100 mg); NH2NH2·H2O (2 mmol); CH3CO2H; 110 °C | 6 h | 82% | 37 |
2.5. Recoverability and reusability experiments
In the next phase of this work, we assessed the recyclability and reusability of the as-prepared core–shell-type Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite. As shown in Table 4, we conducted the desired reaction strategies over five consecutive runs without observing a significant loss in the catalytic activity of the zirconocene-containing nanocatalytic system.| Run number | Reaction strategy | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | |||||||||
| Time (min) | Yield (%) | Time (min) | Yield (%) | Time (min) | Yield (%) | Time (min) | Yield (%) | Time (min) | Yield (%) | Time (min) | Yield (%) | Time (min) | Yield (%) | Time (min) | Yield (%) | |
| 1 | 40 | 96 | 60 | 97 | 10 | 95 | 60 | 97 | 75 | 96 | 85 | 96 | 15 | 95 | 75 | 96 |
| 2 | 40 | 95 | 60 | 97 | 10 | 95 | 60 | 97 | 75 | 95 | 85 | 96 | 15 | 95 | 75 | 96 |
| 3 | 45 | 94 | 65 | 96 | 12 | 94 | 65 | 96 | 80 | 94 | 90 | 94 | 20 | 94 | 80 | 94 |
| 4 | 45 | 94 | 65 | 96 | 12 | 94 | 65 | 95 | 80 | 92 | 90 | 91 | 20 | 92 | 80 | 91 |
| 5 | 50 | 93 | 70 | 95 | 14 | 93 | 70 | 94 | 85 | 90 | 95 | 90 | 25 | 91 | 90 | 90 |
2.6. Molecular docking and ADMET studies
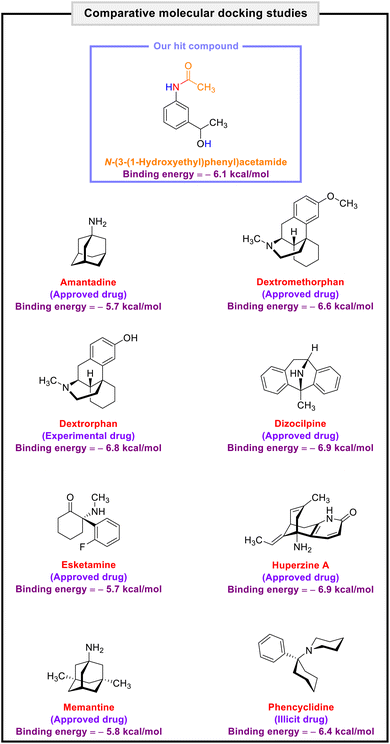
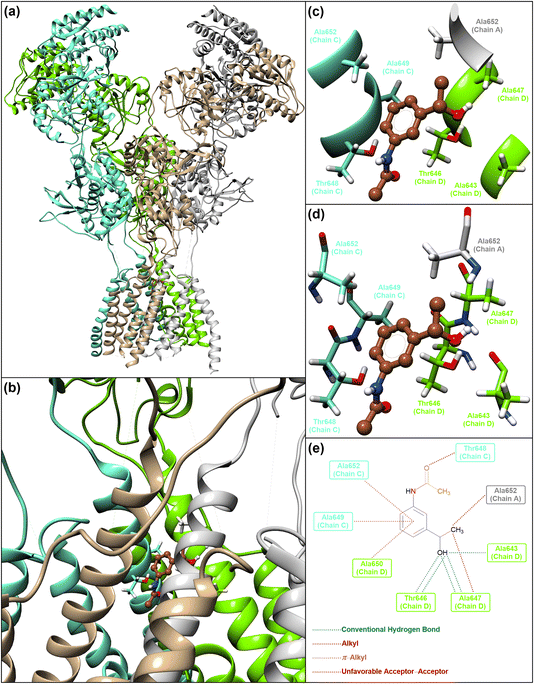
It is worth noting that NMDA receptors have a heterotetrameric structure consisting of an amino-terminal domain (ATD), a ligand-binding domain (LBD), and a transmembrane domain (TMD). To explore the protein–ligand interactions between the transmembrane domain (TMD) of the human N-methyl-D-aspartate (hNMDA) receptor (PDB ID: 7EU7) and our obtained compounds (viz. (hetero)aryl amines and N-(hetero)aryl acetamides), we carried out a molecular docking process using AutoDock Vina as an open-source program for doing molecular docking along with UCSF Chimera as a graphical user interface. The obtained binding energies of the molecular docking investigations ranged from −4.1 kcal mol−1 to −5.3 kcal mol−1 for (hetero)aryl amines (Fig. 5) and −4.9 kcal mol−1 to −6.1 kcal mol−1 for N-(hetero)aryl acetamides (Fig. 6). From the binding energy point of view, the accomplished results verified that N-(3-(1-hydroxyethyl)phenyl)acetamide with a binding energy of −6.1 kcal mol−1 is slightly better than other compounds. As shown in Fig. 7, N-(3-(1-hydroxyethyl)phenyl)acetamide was able to form five conventional hydrogen bonds with Ala643D (2.844 Å), Thr646D (2.073 Å and 3.324 Å), and Ala647D (3.015 Å and 3.070 Å) of the hNMDA receptor TMD. The mentioned compound also has interactions with residues Ala652A (alkyl), Thr648C (unfavorable acceptor–acceptor), Ala649C (π–alkyl), Ala652C (π–alkyl), Ala647D (alkyl), and Ala650D (π–alkyl) (Fig. 7). It is worthwhile to note that we conducted comparative molecular docking studies between N-(3-(1-hydroxyethyl)phenyl)acetamide as our hit compound and related medicinal compounds (including Amantadine, Dextromethorphan, Dextrorphan, Dizocilpine, Esketamine, and Huperzine A), and N-(3-(1-hydroxyethyl)phenyl)acetamide appears to have a distinct advantage for this purpose, from the binding energy point of view (Fig. 8). Understanding the absorption, distribution, metabolism, excretion, and toxicity (ADMET) parameters is essential for developing new drugs and drug-like molecules.38 In this context, we utilized the free web tool SwissADME, provided by the Swiss Institute of Bioinformatics (http://www.swissadme.ch/),39 to assess the ADME properties of N-(3-(1-hydroxyethyl)phenyl)acetamide. The in silico data related to physicochemical properties and lipophilicity, water solubility, pharmacokinetics, and drug-likeness and medicinal chemistry of the obtained N-(3-(1-hydroxyethyl)phenyl)acetamide compound have been collected and are shown in Fig. 9. Interestingly, the attained results demonstrated that N-(3-(1-hydroxyethyl)phenyl)acetamide generally possesses drug-like behavior because it could successfully pass the four of five fundamental drug-likeness filters, including Lipinski (Pfizer), Ghose (Amgen), Veber (GSK), and Egan (Pharmacia). Furthermore, the mentioned organic compound gratifyingly passed pan assay interference structures (PAINS) and Brenk filters. Also, the bioavailability radar of the synthesized N-(3-(1-hydroxyethyl)phenyl)acetamide is depicted in Fig. 9. On the other hand, the BOILED-Egg plot of N-(3-(1-hydroxyethyl)phenyl)acetamide shows high gastrointestinal (GI) absorption and has blood–brain barrier (BBB) permeability, and the red dot as a P-glycoprotein non-substrate (PGP–) demonstrates predictions that the mentioned organic molecule cannot be effluxed from the central nervous system (CNS) by PGP (Fig. 9). Besides, the in silico toxicity evaluation was carried out using an online server, Deep-PK (https://biosig.lab.uq.edu.au/deeppk/).40 As shown in Fig. 9, the results of the in silico Ames mutagenesis, avian, and bee toxicity tests were safe for the mentioned synthesized compound.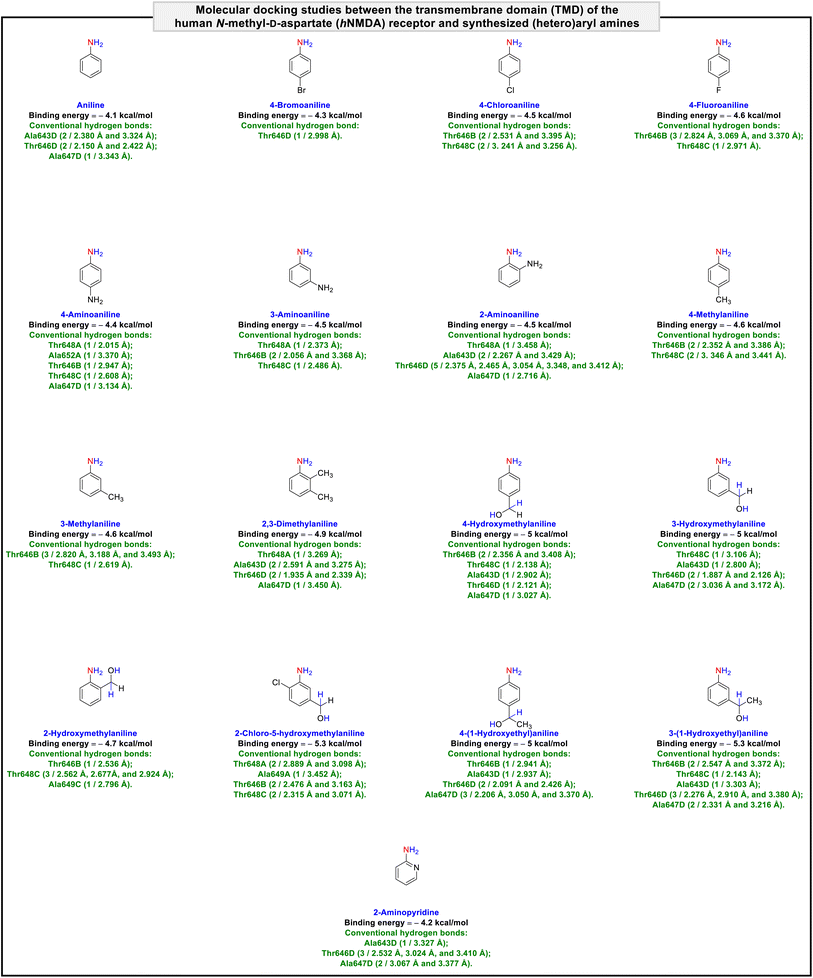 | ||
| Fig. 5 Molecular docking studies between the transmembrane domain (TMD) of the human N-methyl-D-aspartate (hNMDA) receptor and synthesized (hetero)aryl amines. | ||
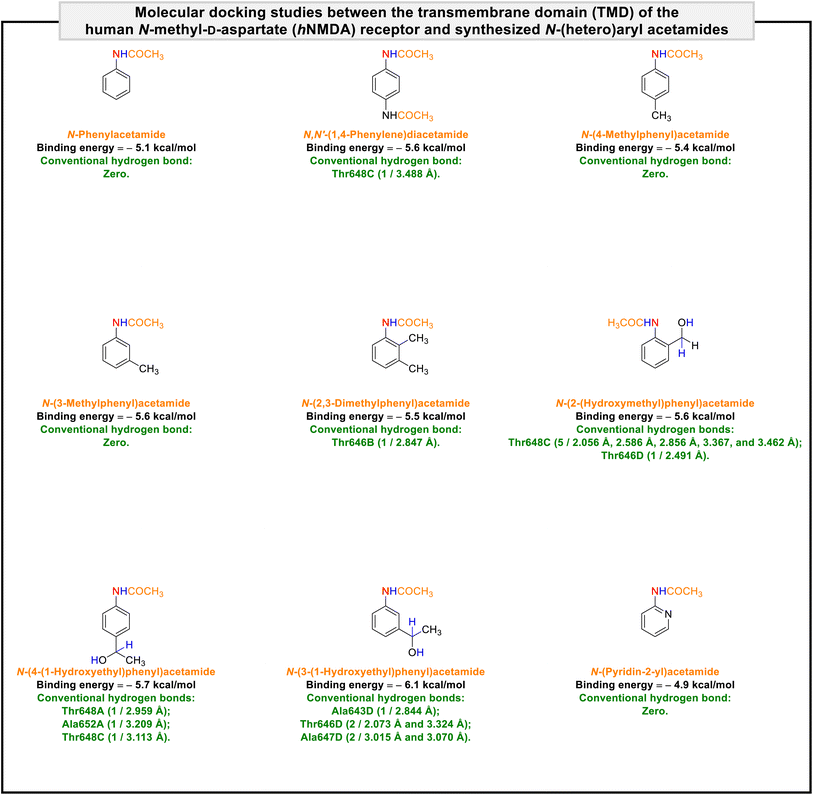 | ||
| Fig. 6 Molecular docking studies between the transmembrane domain (TMD) of the human N-methyl-D-aspartate (hNMDA) receptor and synthesized N-(hetero)aryl acetamides. | ||
 | ||
| Fig. 7 Close-up views (3D and 2D) of N-(3-(1-hydroxyethyl)phenyl)acetamide in the TMD of the hNMDA receptor. | ||
3. Experimental
3.1. Reagents, samples, and apparatus
All starting materials, reagents, and solvents were commercially available (purchased from Merck, Sigma-Aldrich, and Fluka companies) and used directly without further purification. The FT-IR spectra were recorded using a Thermo Nicolet Nexus 670 spectrometer. The 1H and 13C{H} NMR spectra were recorded on a Bruker Avance 300 MHz spectrometer at 300 MHz and 75 MHz, respectively. The crystalline structure of the as-prepared zirconocene-containing nanocomposite was investigated by PXRD on a Philips PANalytical X'Pert Pro diffractometer (Netherlands). The SEM image and EDX diagram of the as-prepared Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite were acquired using an FESEM-TESCAN MIRA3 electron microscope. The exact amounts of Fe and Zr were detected by inductively coupled plasma-optical emission spectrometry (ICP-OES). The magnetic properties of the mentioned zirconocene-containing nanocomposite were analyzed on an alternating gradient force magnetometer (AGFM) at room temperature. The thermogravimetric analysis (TGA) was performed with a Shimadzu DTG-60 instrument under a nitrogen atmosphere. The nitrogen (N2) gas adsorption–desorption isotherms were measured using a Belsorp-Max (BEL Japan, Inc). Thin-layer chromatography (TLC) was used to determine the purity of products and monitor the reaction over a silica gel 60 F254 aluminum sheet.3.2. Preparation of the superparamagnetic core–shell-type Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite
The superparamagnetic core–shell-type Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite was prepared accurately through our previous research papers.14–163.3. General procedure for the green reduction (hydrogenation) of nitro(hetero)arenes to (hetero)aryl amines catalyzed by the as-prepared Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite
As a representative example, in a round-bottom flask (10 mL) equipped with a magnetic stirrer, a mixture of nitrobenzene (1 mmol) and H2O (2–3 mL) was prepared. Then, 5 mg of the as-prepared superparamagnetic core–shell-type Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite was added, and the mixture was stirred. In the next step, NaBH4 (2 mmol) was added, and the resulting mixture was continuously stirred at room temperature for ten minutes. After the completion of the reaction, the mentioned zirconocene-containing catalytic system was separated from the reaction pot using an external magnet. The reaction mixture was extracted with ethyl acetate (EtOAc) (2 × 5 mL) and then dried over anhydrous sodium sulfate (Na2SO4). Lastly, the solvent was evaporated under reduced pressure followed by further purification using short-column chromatography over silica gel which afforded pure liquid aniline in 95% yield.3.4. General procedure for the convenient one-pot two-step reductive acetylation of nitro(hetero)arenes to N-(hetero)aryl acetamides catalyzed by the as-prepared Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite
As an example, in a round-bottom flask (10 mL) equipped with a magnetic stirrer, a mixture of nitrobenzene (1 mmol) and H2O (2–3 mL) was prepared, and then, 5 mg of the as-prepared core–shell-type Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite was added, and the mixture was stirred. In the next step, NaBH4 (2 mmol) was added, and the resulting mixture was continuously stirred at room temperature for ten minutes. After completion of the reduction (hydrogenation) step, acetic anhydride (Ac2O) (1 mmol) was added to the reaction mixture, followed by stirring for a further five minutes at 80 °C. After the completion of the acetylation reaction, the mentioned superparamagnetic zirconocene-containing catalytic system was separated from the reaction pot using an external magnet. The reaction mixture was extracted with ethyl acetate (EtOAc) (2 × 5 mL) and then dried over anhydrous sodium sulfate (Na2SO4). Lastly, the solvent was evaporated under reduced pressure followed by further purification using short-column chromatography over silica gel affording the pure acetanilide in 95% yield.3.5. In silico molecular docking and ADMET
The molecular docking simulation was carried out using AutoDock Vina (version 1.1.2) as an open-source program incorporating UCSF Chimera (version 1.15) as a graphical user interface on an Apple MacBook Pro (Retina, 13-inch, Mid-2014, equipped with a 2.8 GHz dual-core Intel Core i5 processor, 8 GB 1600 MHz DDR3 memory, Intel Iris 1536 MB graphics, and 500 GB Apple SSD SM0512F storage). The 3D crystal structure of the human N-methyl-D-aspartate (hNMDA) receptor (PDB ID: 7EU7) was downloaded from the Protein Databank (https://www.rcsb.org/). Before the molecular docking process, the protein structure (PDB ID: 7EU7) was prepared using UCSF Chimera. The 2D structures of the synthesized (hetero)aryl amines and N-(hetero)aryl acetamides were generated using ChemBioDraw Ultra (version 14.0.0.117). Then, the conversion of the 2D skeletons to the related 3D structures of the ligands and their energy minimization processes (by the MM2 force field calculation method) was carried out using ChemBio3D Ultra (version 14.0.0.117). Also, structure editing steps (including dock prep and minimize structure) for the 3D ligands were repeated using UCSF Chimera. The 3D structures of medicinal compounds, which are shown in Fig. 8, were downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov/). All the 3D structures of the mentioned medicinal compounds underwent the aforementioned structure editing steps using UCSF Chimera. All the protein–ligand interactions were analyzed using UCSF Chimera and BIOVIA Discovery Studio (version v21.1.0.20298). The 3D figures of the protein–ligand interactions were visualized using UCSF Chimera, and the related 2D projects were drawn using ChemBioDraw Ultra. The in silico ADME and toxicity analyses were investigated using SwissADME (http://www.swissadme.ch/) and Deep-PK (https://biosig.lab.uq.edu.au/deeppk/), respectively.4. Conclusions
In this paper, diverse green and efficient reaction strategies for the simple reduction (hydrogenation) and one-pot two-step reductive acetylation of nitro(hetero)arenes using a core–shell-type Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) magnetically recoverable nanocomposite as a powerful nanocatalytic system have been reported. Notably, in the presented protocols, the as-prepared superparamagnetic Fe3O4@APTMS@Cp2ZrClx (x = 0, 1, 2) nanocomposite had satisfactory turnover numbers (TONs) and turnover frequencies (TOFs) and also acceptable reusability. In this paper, the potential biological effect of the synthesized (hetero)aryl amines and N-(hetero)aryl acetamides, as a series of small organic molecules, against the transmembrane domain (TMD) of the human N-methyl-D-aspartate (hNMDA) receptor based on molecular docking studies has also been investigated. Besides, the drug-likeness properties of N-(3-(1-hydroxyethyl)phenyl)acetamide, as the hit compound, using in silico ADMET analyses, have been explored. Notably, research to find and develop new and green synthetic strategies for pharmaceutically interesting small organic molecules is underway in our research group.Data availability
Data will be made available on request.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors gratefully appreciate the financial support of this work from the Research Council of Urmia University.References
- (a) D.-S. Zhang, Y. Liu, X. Ren, F. Geng, Y.-Z. Zhang, Y. Baikeli, M. Yang, Z. Liu, Y. Wang, X. Zhang and L. Geng, Colloids Interface Sci. Commun., 2023, 56, 100730 CrossRef CAS; (b) M. Shahid, Z. H. Farooqi, R. Begum, M. Arif, W. Wu and A. Irfan, Crit. Rev. Anal. Chem., 2020, 50, 513–537 CrossRef CAS PubMed; (c) P. Kovacic and R. Somanathan, J. Appl. Toxicol., 2014, 34, 810–824 CrossRef CAS PubMed; (d) S. Sun, Z. Zhang, S. Li, J. Le, H. Qian, X. Yin, Y. Liu, W. Yang and Y. Chen, J. Mater. Sci., 2023, 58, 5587–5598 CrossRef CAS; (e) S. Aghajani, M. Mohammadikish and M. Khalaji-Verjani, Langmuir, 2023, 39, 8484–8493 CrossRef CAS PubMed; (f) R. Sharma, P. S. Sagara, D. Sharma and A. Chaudhary, J. Organomet. Chem., 2024, 1018, 123294 CrossRef CAS; (g) M. Mirhosseyni, G. Mohammadi Ziarani and A. Badiei, J. Mol. Struct., 2025, 1321, 139763 CrossRef CAS; (h) M. Bakhtiarian, M. Safaei and M. M. Khodaei, J. Mol. Struct., 2025, 1321, 139913 Search PubMed; (i) S. A. Haladu, K. A. Elsayed, A. A. Manda, T. S. Kayed, F. Ercan, S. M. Magami, S. Akhtar, A. ben Ahmed, M. A. Hafez and A. Elhassan, J. Mol. Struct., 2025, 1321, 139930 CrossRef CAS; (j) M. Arif, A. Rauf, H. Raza, S. Ben Moussa, S. M. Haroon, A. Y. A. Alzahrani and T. Akhter, Int. J. Biol. Macromol., 2024, 275, 133633 Search PubMed; (k) N. Y. Baran, M. Çalişkan, A. Özpala and T. Baran, Int. J. Biol. Macromol., 2024, 262, 130134 CrossRef CAS PubMed; (l) C. C. Naik, D. P. Kamat and S. K. Gaonkar, Int. J. Biol. Macromol., 2024, 286, 131752 CrossRef PubMed; (m) A. Khalil, A. Khan, T. Kamal, A. A. P. Khan, S. B. Khan, M. T. S. Chani, K. A. Alzahrani and N. Ali, Int. J. Biol. Macromol., 2024, 262, 129986 Search PubMed; (n) N. Y. Baran, J. Organomet. Chem., 2024, 1008, 123047 CrossRef; (o) R. Mondal, R. Ghanta, T. Chowdhury, A. Bhaumik and T. Chattopadhyay, J. Mol. Struct., 2025, 1322, 140287 CrossRef CAS; (p) V. Aggarwal, E. Bala, S. Saima, S. Pathan, S. Guleria, S. Sharma, M. Selvaraj and P. K. Verma, Synlett, 2024, 35, 245–267 CrossRef CAS; (q) M. Mahendran, Y. Mariappan, R. Thamizhselvan and S. Vembu, Mat. Adv., 2024, 5, 3890–3903 RSC; (r) S. Iniyan, A. Vijayaprabhakaran, C. Sebastian and M. Kathiresan, Mat. Adv., 2024, 5, 7006–7015 Search PubMed; (s) S. Taimur, S. Rehman, M. Ellahi, S. Rizwan, H. Razzaq and T. Yasin, Mat. Adv., 2024, 5, 9428–9444 RSC; (t) F. Eshrati, H. Ghafuri, P. Hanifehnejad and H. Dogari, Mat. Adv., 2025, 6, 278–297 Search PubMed; (u) B.-B. Xing, Y.-S. Wang, T. Zhang, J.-Y. Liu, H. Jiao and L. Xu, Mat. Adv., 2025, 3, 756–765 RSC; (v) M. A. Pandit, D. S. H. Kumar, M. Varkolu and K. Muralidharan, Nanoscale Adv., 2025, 7, 1143–1153 RSC; (w) R. Mozafari, M. Mohammadi, S. Moradi and M. Ghadermazi, RSC Adv., 2025, 15, 1358–1374 RSC; (x) W. Guo, Y. Zheng, W. Xiang and Y. Zhang, RSC Sustain., 2025, 3, 243–254 RSC.
- (a) M. C. Manjula, S. Manjunatha, K. L. Nagashree, M. Shivanna, M. P. Rao, N. Nanda and P. Ramachandra, ChemistrySelect, 2023, 8, e202300936 CrossRef CAS; (b) S. Sghajani and M. Mohammadikish, Langmuir, 2022, 38, 8686–8695 Search PubMed; (c) J. Song, Z.-F. Huang, L. Pan, K. Li, X. Zhang, L. Wang and J.-J. Zou, Appl. Catal. B Environ., 2018, 227, 386–408 Search PubMed; (d) S. Kumar and S. K. Maurya, J. Org. Chem., 2023, 88, 8690–8702 CrossRef CAS PubMed; (e) X. Li, J. An, Z. Gao, C. Xu, Y. Cheng, S. Li, L. Li and B. Tang, Chem. Sci., 2023, 14, 3554–3561 RSC; (f) S. Karimi, M. Gholinejad, R. Khezri, J. M. Sansano, C. Nájera and M. Yus, RSC Adv., 2023, 13, 8101–8113 Search PubMed; (g) H. Jiang, G. Yuan, Z. Cui, Z. Zhao, Z. Dong, J. Zhang, Y. Cong and X. Li, Ind. Eng. Chem. Res., 2023, 62, 13355–13367 Search PubMed; (h) H. Yu, J. Liu, Q. Wan, G. Zhao, E. Gao, J. Wang, B. Xu, G. Zhao and X. Fan, Mol. Catal., 2023, 540, 113045 CrossRef CAS; (i) Z. Ma, J. Chen, M. Chen, L. Dong, W. Mao, Y. Long and J. Ma, Mol. Catal., 2023, 547, 113372 CrossRef CAS; (j) S. Taheri, M. M. Heravi and A. Saljooqi, Sci. Rep., 2023, 13, 17566 CrossRef CAS PubMed; (k) A. Maurya, U. K. Patel, S. Kumar and A. Agarwal, RSC Adv., 2024, 14, 29505–29517 RSC; (l) F. Doraghi, Y. Mohammadkhani Kalooei, N. Mehdi Zadeh Darban, B. Larijani and M. Mahdavi, J. Organomet. Chem., 2024, 1019, 123313 CrossRef CAS; (m) P. Ding, E. Fayad, O. A. A. Ali and H.-L. Qin, Tetrahedron, 2024, 167, 134269 Search PubMed; (n) C. A. Romero-Soto, A. L. Iglesias, A. F. Velázquez-Ham, J. P. Camarena-Díaz, E. Correa-Ayala, J. L. Gomez-Lopez, D. Chávez, A. Ochoa-Terán, G. Aguirre, A. L. Rheingold, D. B. Grotjahn, M. Parra-Hake and V. Miranda-Soto, RSC Adv., 2024, 14, 24019–24030 RSC; (o) Y. Wei, S. Wang, Y. Zhang, M. Li, J. Hu, Y. Liu, J. Li, L. Yu, R. Huang and D. Deng, ACS Catal., 2023, 13, 15824–15832 CrossRef CAS; (p) J. Wu, W. Lang, H. Li, K. Du, J. Deng, S. Zhao, W. Zhang, Z. Peng and Z. Liu, ACS Sustainable Chem. Eng., 2023, 11, 14960–14968 Search PubMed; (q) J. Ma, X. Mao, C. Hu, X. Wang, W. Gong, D. Liu, R. Long, A. Du, H. Zhao and Y. Xiong, J. Am. Chem. Soc., 2024, 146, 970–978 Search PubMed; (r) A. Singh, O. Singh, A. Maji, S. Singh, N. Singh, P. K. Maji and K. Ghosh, Mol. Catal., 2024, 555, 113835 CrossRef CAS; (s) W. Gao, Y. Gao, B. Liu, J. Kang, Z. Zhang, M. Zhang and Y. Zou, RSC Adv., 2024, 14, 5055–5060 Search PubMed; (t) Z. Kefayati, M. Malmir and M. M. Heravi, Res. Chem. Intermed., 2024, 50, 127–146 CrossRef CAS; (u) A. S. Hazari, M. L. Frisch, Y. Wen, M. D. Stankovic and C. P. Berlinguette, J. Am. Chem. Soc., 2024, 146, 28153–29160 Search PubMed; (v) X. Zhuang, H. Song, J. Wang, Z. Zhang, J. Wang, B. Sun, W. Su and C. Jin, Green Chem., 2024, 26, 9682–9689 RSC.
- (a) J. B. Zimmerman, P. T. Anastas, H. C. Erythropel and W. Leitner, Science, 2020, 367, 397–400 CrossRef CAS PubMed; (b) H. C. Erythropel, J. B. Zimmerman, T. M. de Winter, L. Petitjean, F. Melnikov, C. H. Lam, A. W. Lounsbury, K. E. Mellor, N. Z. Janković, Q. Tu, L. N. Pincus, M. M. Falinski, W. Shi, P. Coish, D. L. Plata and P. T. Anastas, Green Chem., 2018, 20, 1929–1961 RSC; (c) T. Keijer, V. Bakker and J. C. Slootweg, Nat. Chem., 2019, 11, 190–195 CrossRef CAS PubMed; (d) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC; (e) H. Mousavi, Int. J. Biol. Macromol., 2021, 186, 1003–1166 CrossRef CAS PubMed; (f) M. Rimaz, Z. Jalalian, H. Mousavi and R. H. Prager, Tetrahedron Lett., 2016, 57, 105–109 CrossRef CAS; (g) M. Rimaz, J. Khalafy and H. Mousavi, Res. Chem. Intermed., 2016, 42, 8185–8200 CrossRef CAS; (h) A. s. Alqahtani and S. Elbeltagi, J. Organomet. Chem., 2025, 1027, 123508 CrossRef CAS; (i) S. Swami, N. Sharma, G. Sharma and R. Shrivastava, RSC Adv., 2025, 15, 2361–2415 RSC; (j) N. Hassanzadeh, M. G. Dekamin and E. Valiey, Nanoscale Adv., 2025, 7, 99–123 RSC.
- (a) L. Peng, Y. Zhao, T. Yang, Z. Tong, Z. Tang, A. Orita and R. Qiu, Top. Curr. Chem., 2022, 380, 44 CrossRef PubMed; (b) L.-P. Mo and Z.-H. Zhang, Curr. Org. Chem., 2011, 15, 3800–3823 CrossRef CAS; (c) K. Nikoofar and Z. Khademi, Res. Chem. Intermed., 2016, 42, 3929–3977 CrossRef CAS; (d) N. Suzuki, S. Ito, M. Kawate and T. Takemoto, J. Organomet. Chem., 2023, 1001, 122883 CrossRef CAS; (e) J. Okuda, J. Organomet. Chem., 2023, 1000, 122833 CrossRef CAS; (f) M. Chegeni and M. Mehri, J. Organomet. Chem., 2023, 984, 122585 CrossRef CAS; (g) W. Asim, A. S. Waheeb, M. A. Awad, A. M. Kadhum, A. Ali, S. H. Mallah, M. A. Iqbal and M. M. Kadhim, J. Mol. Struct., 2022, 1250, 131925 CrossRef CAS; (h) N. Moeini, M. Ghadermarzi and S. Molaei, J. Mol. Struct., 2022, 1251, 131982 CrossRef CAS; (i) H. Maati, O. Amadine, Y. Essamlali, B. Rezki, I. Ayouch, H. Mahi, K. Abdelouahdi and M. Zahouily, J. Mol. Struct., 2023, 1294, 136347 CrossRef CAS; (j) A. S. Al-Wasidi, J. Organomet. Chem., 2024, 1022, 123403 CrossRef CAS; (k) L. K. Dilmukhametova, M. G. Shaibakova and I. R. Ramazanov, Russ. Chem. Bull., 2024, 73, 1894–1899 CrossRef CAS; (l) M. Rimaz, J. Khalafy, H. Mousavi, S. Bohlooli and B. Khalili, J. Heterocycl. Chem., 2017, 54, 3174–3186 CrossRef CAS; (m) M. Rimaz, H. Mousavi, P. Keshavarz and B. Khalili, Curr. Chem. Lett., 2015, 4, 159–168 CrossRef; (n) M. Rimaz, H. Mousavi, M. Behnam and B. Khalili, Curr. Chem. Lett., 2016, 5, 145–154 CrossRef; (o) M. Rimaz, H. Mousavi, B. Khalili and L. Sarvari, J. Iran. Chem. Soc., 2019, 16, 1687–1701 CrossRef CAS; (p) M. Rimaz, H. Mousavi, B. Khalili and F. Aali, J. Chin. Chem. Soc., 2018, 65, 1389–1397 CrossRef CAS; (q) S.-J. Yao, J.-M. Lin, L.-Z. Dong, Y.-L. Li, N. Li, J. Li and Y.-Q. Lan, Sci. Bull., 2024, 69, 1418–1426 CrossRef CAS PubMed; (r) A. S. Al-Wasidi, J. Organomet. Chem., 2025, 1022, 123403 CrossRef; (s) J. Wu, M. Yang, D. Leng, Q. Hu, Y. Yao, H. Sun and Z. Gao, RSC Adv., 2025, 15, 1747–1753 RSC.
- D. Wang and D. Astruc, Chem. Soc. Rev., 2017, 46, 816–854 RSC.
- M. Bystrzanowska, P. Petkov and M. Tobiszewski, ACS Sustainable Chem. Eng., 2019, 7, 18434–18443 CrossRef CAS.
- (a) D. Wang and D. Astruc, Chem. Rev., 2014, 114, 6949–6985 CrossRef CAS PubMed; (b) M. G. Sibi, D. Verma and J. Kim, Catal. Rev., 2020, 62, 163–311 CrossRef; (c) Q. Sun, X.-Q. Zhang, Y. Wang and A.-H. Lu, Chin. J. Catal., 2015, 36, 683–691 CrossRef CAS; (d) M. B. Gawande, A. Goswami, T. Asefa, H. Guo, A. V. Biradar, D.-L. Peng, R. Zboril and R. S. Varma, Chem. Soc. Rev., 2015, 44, 7540–7590 RSC; (e) B. Zuo, W. Li, X. Wu, S. Wang, Q. Deng and M. Huang, Chem.–Asian J., 2020, 15, 1248–1265 CrossRef CAS PubMed; (f) Z. Li, M. Li, Z. Bian, Y. Kathiraser and S. Kawi, Appl. Catal. B Environ., 2016, 188, 324–341 CrossRef CAS; (g) H. Veisi, M. Pirhayati, P. Mohammadi, T. Tamoradi, S. Hemmati and B. Karmakar, RSC Adv., 2023, 13, 20530–20556 Search PubMed; (h) N. Kaviani, S. Behrouz, A. A. Jafari and M. N. Soltani Rad, RSC Adv., 2023, 13, 30293–30305 RSC; (i) M. Mohammadi, A. Ghorbani-Choghamarani and S. M. Ramish, J. Mol. Struct., 2023, 1292, 136115 CrossRef CAS; (j) M. Y. Saleh, A. K. O. Aldulaimi, S. M. Saeed and A. H. Adhab, J. Mol. Struct., 2025, 1321, 139597 Search PubMed; (k) D. Singh, A. Kumar, Y. Jadeja, S. V. Menon, P. Singh, S. M. Ibrahim, M. Singh, M. K. Abosaoda, S. B. AlReshaidan and M. A. El-Meligy, J. Mol. Struct., 2025, 1321, 140133 CrossRef CAS; (l) H. V. Le, H. X. Le, V. T. B. Nguyen, T. T. Le, H. N. Nguyen, T. T. H. Nguyen, T. T. Nguyen, P. H. Ho, K. D. Nguyen and D. P. Tran, ChemistrySelect, 2024, 9, e202402007 Search PubMed; (m) Y. Wang, X. Wang, Y. Dong, M. Peng, L. Guo, M. Cui, Y. He, J. Yi, H. Ma, H. Zhang and H. Fan, Green Chem., 2024, 26, 6131–6138 RSC; (n) H. P Mata, J. M. Anghinoni, E. C. Silva, P. Macchion, S. S. Ferreira, J. F. de Souza, E. J. Lenardão and A. R. Fajardo, J. Organomet. Chem., 2025, 1025, 123478 Search PubMed; (o) M.-S. Mashhoori and R. Sandaroos, J. Organomet. Chem., 2025, 1025, 123486 CrossRef CAS; (p) Q. Qiu, J. R. Song, H. Zheng, M. Shahriari, A. F. El-kott, A. G. Alkhathami and K. Morcy, J. Organomet. Chem., 2025, 1023, 123423 CrossRef CAS; (q) K. Dolatyari and M. M. Khodaei, J. Organomet. Chem., 2024, 1021, 123352 CrossRef CAS; (r) L. Bhosale, R. Gurav, A. Gurav, S. Sankpal and S. Hangirgekar, J. Organomet. Chem., 2024, 1021, 123341 CrossRef CAS; (s) H. Jia, Y. Cui, X. Yuan, Y. Xu and Y. Li, J. Organomet. Chem., 2024, 1021, 123354 CrossRef CAS.
- (a) C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC; (b) D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551 RSC; (c) V. Hessel, N. N. Tran, M. R. Asrami, Q. D. Tran, N. V. C. Long, M. Escribà-Gelonch, J. O. Tejada, S. Linke and K. Sunmacher, Green Chem., 2022, 24, 410–437 Search PubMed; (d) C. J. Clarke, W.-C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 Search PubMed; (e) F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clarck, T. J. Farmer, A. J. Hunt, C. R. McElory and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 7 CrossRef; (f) R. N. Butler and A. C. Coyne, Chem. Rev., 2010, 110, 6302–6337 CrossRef CAS PubMed; (g) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725–748 CrossRef CAS PubMed; (h) M.-O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415–1427 RSC.
- (a) R. Tenchov, J. M. Sasso and Q. A. Zhou, ACS Chem. Neurosci., 2024, 15, 2665–2694 CrossRef CAS PubMed; (b) K. Singh, A. Kaur, B. Goyal and D. Goyal, ACS Chem. Neurosci., 2024, 15, 2545–2564 CrossRef CAS PubMed; (c) M. Farzan, B. Abedi, I. Bhia, A. Madanipour, M. Farzan, M. Bhia, A. Aghaei, I. Kheirollahi, M. Motallebi, H. Amini-Khoei and Y. Nuri Ertas, ACS Chem. Neurosci., 2024, 15, 2966–2981 CrossRef CAS PubMed; (d) S. Willems, D. Zaienne and D. Merk, J. Med. Chem., 2021, 64, 9592–9638 CrossRef CAS PubMed; (e) O. Hansson, Nat. Med., 2021, 27, 954–963 CrossRef CAS PubMed; (f) D. M. Wilson, M. R. Cookson, L. Van Den Bosch, H. Zetterberg, D. M. Holtzman and I. Dewachter, Cell, 2023, 186, 693–714 CrossRef CAS PubMed; (g) J. Wang, Y. Cao, Y. Lu, H. Zhu, J. Zhang, J. Che, R. Zhuang and J. Shao, Eur. J. Med. Chem., 2024, 264, 115998 CrossRef CAS PubMed; (h) Q. Li, Q. Liao, S. Qi, H. Huang, S. He, W. Lyu, J. Liang, H. Qin, Z. Cheng, F. Yu, X. Dong, Z. Wang, L. Han and Y. Han, Eur. J. Med. Chem., 2024, 271, 116386 CrossRef CAS PubMed; (i) Z. Cai, Z. Yang, H. Li and Y. Fang, Bioorg. Chem., 2024, 147, 107386 CrossRef CAS PubMed; (j) J. Zhang, P. Jiang, S. Wang, M. Li, Z. Hao, W. Guan, J. Pan, J. Wu, Y. Zhang, H. Li, L. Chen, B. Yang and Y. Liu, Bioorg. Chem., 2024, 153, 107819 CrossRef CAS PubMed.
- (a) T.-S. Chou, M. Epstein, R. G. Fritzemeier, N. S. Akins, S. Paladugu, E. Z. Ullman, D. C. Liotta, S. F. Traynelis and H. Furukawa, Nature, 2024, 632, 209–217 CrossRef CAS PubMed; (b) K. Michalski and H. Furukawa, Sci. Adv., 2024, 10, eadl5952 CrossRef CAS PubMed; (c) C. Geoffroy, R. Berraud-Pache, N. Chéron, I. McCort-Tranchepain, J. Doria, P. Paoletti and L. Mony, ACS Chem. Neurosci., 2024, 15, 3321–3343 CrossRef CAS PubMed; (d) Y. Zhang, F. Ye, T. Zhang, S. Lv, L. Zhou, D. Du, H. Lin, F. Guo, C. Luo and S. Zhu, Nature, 2021, 596, 301–305 CrossRef CAS PubMed; (e) M. R. Wilcox, A. Nigam, N. G. Glasgow, C. Narangoda, M. B. Phillips, D. S. Patel, S. Mesbahi-Vasey, A. L. Turcu, S. Vázquez, M. G. Kurnikova and J. W. Johnson, Nat. Commun., 2022, 13, 4114 CrossRef CAS PubMed; (f) A. Camargo, A. P. Dalmagro, G. A. Altê, A. L. B. Zeni, C. I. Tasca and A. L. S. Rodrigues, Chem. Biol. Interact., 2023, 375, 110440 CrossRef CAS PubMed; (g) P. Song, C. Ma, S. Wang, Y. Zhang, L. Xu, F. Fang, X. Huang, Y. Zhang, Q. Mao, C. Shi, M. Cheng and Y. Xu, Bioorg. Med. Chem., 2025, 119, 118063 CrossRef CAS PubMed; (h) Z. Wang, Y. He, G. Chen and X. Bao, New J. Chem., 2025, 49, 2319–2334 RSC.
- H. Mousavi, M. Rimaz and B. Zeynizadeh, ACS Chem. Neurosci., 2024, 15, 1828–1881 CrossRef CAS PubMed.
- (a) V. T. Sabe, T. Ntombela, L. A. Jhamba, G. E. M. Maguire, T. Govender, T. Naicker and H. G. Kruger, Eur. J. Med. Chem., 2021, 224, 113705 CrossRef CAS PubMed; (b) P. B. Cox and R. Gupta, ACS Med. Chem. Lett., 2022, 13, 1016–1029 CrossRef CAS PubMed; (c) A. Tirehdast, S. Sheikhi-Mohammareh, H. Sabet-Sarvestani, M. G. Organ, V. Semeniuchenko and A. Shiri, RSC Adv., 2024, 14, 29122–29133 RSC; (d) H. Mousavi, B. Zeynizadeh and M. Rimaz, Bioorg. Chem., 2023, 135, 106390 CrossRef CAS PubMed; (e) D. E. Arthur, Inform. Med. Unlocked, 2023, 41, 101301 CrossRef; (f) N. H. Siddiquee, M. I. Hossain, M. E. K. Talukder, S. A. A. Nirob, M. Shourav, I. Jahan, U. H. A. Tamanna, P. Das, R. Akter, M. hasan, M. Abdullah-Al-Mamun and O. Saha, Inform. Med. Unlocked, 2023, 45, 101458 CrossRef; (g) S. S. Marufa, T. Rahman, M. M. Rahman, M. Rahman, S. J. Khan, R. Jahan, H. Nishino, M. S. Alam and A. Haque, RSC Adv., 2024, 14, 35198–35214 RSC.
- (a) H. Mousavi, B. Zeynizadeh and M. Hasanpour Galehban, Nanoscale Adv., 2024, 6, 3961–3977 RSC; (b) M. Hasanpour Galehban, B. Zeynizadeh and H. Mousavi, J. Mol. Struct., 2023, 1271, 134017 CrossRef; (c) M. Hasanpour Galehban, B. Zeynizadeh and H. Mousavi, RSC Adv., 2022, 12, 16454–16478 RSC; (d) M. Hasanpour Galehban, B. Zeynizadeh and H. Mousavi, RSC Adv., 2022, 12, 11164–11189 RSC; (e) B. Zeynizadeh, H. Mousavi and F. Mohammad Aminzadeh, J. Mol. Struct., 2022, 1255, 131926 CrossRef CAS; (f) B. Zeynizadeh, F. Mohammad Aminzadeh and H. Mousavi, Res. Chem. Intermed., 2021, 47, 3289–3312 CrossRef CAS; (g) R. Bakhshi, B. Zeynizadeh and H. Mousavi, J. Chin. Chem. Soc., 2020, 67, 623–637 CrossRef CAS; (h) B. Zeynizadeh, H. Mousavi and S. Zarrin, J. Chin. Chem. Soc., 2019, 66, 928–933 CrossRef CAS; (i) B. Zeynizadeh, F. Mohammad Aminzadeh and H. Mousavi, Res. Chem. Intermed., 2019, 45, 3329–3357 CrossRef CAS; (j) B. Zeynizadeh, F. Mohammad Aminzadeh and H. Mousavi, Green Process. Synth., 2019, 8, 742–755 CAS; (k) B. Zeynizadeh, R. Younesi and H. Mousavi, Res. Chem. Intermed., 2018, 44, 7331–7352 CrossRef CAS; (l) H. Mousavi, B. Zeynizadeh, R. Younesi and M. Esmati, Aust. J. Chem., 2018, 71, 595–609 CrossRef CAS.
- B. Zeynizadeh and F. Sepehraddin, J. Iran. Chem. Soc., 2017, 14, 2649–2657 CrossRef CAS.
- B. Zeynizadeh, F. Sepehraddin and H. Mousavi, Ind. Eng. Chem. Res., 2019, 58, 16379–16388 CrossRef CAS.
- B. Zeynizadeh, H. Mousavi and F. Sepehraddin, Res. Chem. Intermed., 2020, 46, 3361–3382 CrossRef CAS.
- (a) H. K. Kadam and S. G. Tilve, RSC Adv., 2015, 5, 83391–83470 RSC; (b) A. Mannu, A. Grabulosa and S. Baldino, Catalysts, 2020, 10, 162 CrossRef CAS; (c) H. M. Marçon and J. C. Pastre, RSC Adv., 2022, 12, 7980–7989 RSC; (d) J. Song, M. Hua, X. Huang, A. Visa, T. Wu, H. Fan, M. Hou, Z. Zhang and B. Han, Green Chem., 2021, 23, 1259–1265 RSC; (e) C. A. Akinnawo, N. Bingwa and R. Meijboom, New J. Chem., 2021, 45, 7878–7892 RSC; (f) J. Song, B. Zhou, H. Zhou, L. Wu, Q. Meng, Z. Liu and B. Han, Angew. Chem., Int. Ed., 2015, 54, 9399–9403 CrossRef CAS PubMed; (g) T. Wang, H. Xu, J. Hu and Y. Zhang, New J. Chem., 2020, 44, 14686–14694 RSC; (h) Z. Wang, C. Xie, X. Li, J. Nie, H. Yang and Z. Zhang, Chem. Commun., 2022, 58, 4067–4070 RSC; (i) Y. Sha, Z. Xiao, H. Zhou, K. Yang, Y. Song, N. Li, R. He, K. Zhi and Q. Liu, Green Chem., 2017, 19, 4829–4837 RSC; (j) Y. Wang, J. Huang, S. Lu, P. Li, X. Xia, C. Li and F. Li, New J. Chem., 2020, 44, 20308–20315 RSC; (k) T. Wang, A. Hu, G. Xu, C. Liu, H. Wang and Y. Xia, Catal. Lett., 2019, 149, 1845–1855 CrossRef CAS; (l) L. Ye, Y. Han, Z. Yu, M. Zhang, J. Xiong, R. Zhang, X. Li, Y. Qiao and X. Lu, Fuel, 2022, 328, 125233 CrossRef CAS; (m) Y. Zhang, X. Shen, L. Hu, Z. Wu, X. Wang and J. Jiang, Biomass Convers. Biorefin., 2024, 14, 2045–2061 CrossRef CAS; (n) Q. Peng, H. Wang and Y. Xia, Catal. Lett., 2023, 153, 720–731 CrossRef CAS; (o) Q. Peng, X. Li, X. Wang, Y. Li, Y. Xia, X. Liu and H. Wang, Sustain. Energy Fuels, 2021, 5, 4069–4079 RSC; (p) G. Deshmukh and R. Murugavel, Synlett, 2025, 36, 75–81 CrossRef CAS; (q) H. Zarei, S. Sobhani and J. M. Sansano, Appl. Organomet. Chem., 2024, 38, e7740 CrossRef CAS; (r) S. Ma, X. Wang, J. Cao, H. Chen, Y. Lu and R. Wang, ChemistrySelect, 2024, 9, e202400730 CrossRef CAS; (s) S. Pachisia, R. Kishan, S. Yadav and R. Gupta, Inorg. Chem., 2021, 60, 2009–2022 CrossRef CAS PubMed; (t) O. A. Luongo, M. Lemmerer, S. L. Albers and J. Streuff, Angew. Chem., Int. Ed., 2024, 63, e202413182 CrossRef CAS PubMed.
- (a) P. Patinl, Q. Zheng, K. Kurpiewsk and A. Dömling, Nat. Commun., 2023, 14, 5807 CrossRef PubMed; (b) A. Darvishi, H. Bakhshi and A. Heydari, RSC Adv., 2022, 12, 16535–16543 RSC; (c) E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606–631 RSC; (d) V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471–479 CrossRef CAS PubMed; (e) S. Kumari, A. V. Carmona, A. K. Tiwari and P. C. Trippier, J. Med. Chem., 2020, 63, 12290–12358 CrossRef CAS PubMed; (f) S. S. R. Gupta, A. V. Nakhate, G. P. Deshmukh, S. Periasamy, P. S. Samudrala, S. K. Bhargava and M. L. Kantam, ChemistrySelect, 2018, 3, 8436–8443 CrossRef CAS; (g) M. Lubberink, W. Finnigan and S. L. Flitsch, Green Chem., 2023, 25, 2958–2970 RSC; (h) Y. Ge, W. Huang, S. Ahrens, A. Spannenberg, R. Jackstell and M. Beller, Nat. Synth., 2024, 3, 202–213 CrossRef CAS; (i) Q. Yan, Q.-J. Yuan, A. Shatskiy, G. R. Alvey, E. V. Stepanova, J.-Q. Liu, M. D. Kärkäs and X.-S. Wang, Org. Lett., 2024, 26, 3380–3385 CrossRef CAS PubMed.
- (a) B. H. Rostein, S. Zaretsky, V. Rai and A. K. Yudin, Chem. Rev., 2014, 114, 8323–8359 CrossRef PubMed; (b) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2014, 112, 3083–3135 CrossRef PubMed; (c) F. Sutanto, S. Shaabani, C. G. Neochoritis, T. Zarganes-Tzitzikas, P. Patil, E. Ghonchepour and A. Dömling, Sci. Adv., 2021, 7, eabd9307 CrossRef CAS PubMed; (d) D. Hurtado-Rodríguez, A. Salinas-Torres, H. Rojas, D. Becerra and J.-C. Castillo, RSC Adv., 2022, 12, 35158–35176 RSC; (e) H. R. Chaudhary and D. M. Patel, RSC Adv., 2024, 14, 31072–31116 RSC; (f) C. Zavarise, J.-C. Cintrat, E. Romero and A. Sallustrau, RSC Adv., 2024, 14, 39253–39267 RSC; (g) M. Rimaz, H. Mousavi, M. Behnam, L. Sarvari and B. Khalili, Curr. Chem. Lett., 2017, 6, 55–68 CrossRef; (h) M. Rimaz, B. Khalili, G. Khatyal, H. Mousavi and F. Aali, Aust. J. Chem., 2017, 70, 1274–1284 CrossRef CAS; (i) M. Rimaz, H. Mousavi, L. Nikpey and B. Khalili, Res. Chem. Intermed., 2017, 43, 3925–3937 CrossRef CAS; (j) M. Rimaz, H. Mousavi, L. Ozzar and B. Khalili, Res. Chem. Intermed., 2019, 45, 2673–2694 CrossRef CAS; (k) D. Zeleke and T. Damena, Res. Chem., 2024, 7, 101283 CAS; (l) N. Yaghmaeiyan, M. Mirzaei and A. Bamoniri, Res. Chem., 2023, 5, 100696 CAS; (m) S. Handique and P. Sharma, Res. Chem., 2023, 5, 100781 CAS; (n) S. S. Bhalodiya, M. P. Parmar, D. B. Upadhyay, C. D. Patel, D. P. Vala, D. Rajani and H. M. Patel, Res. Chem., 2024, 7, 101304 CAS; (o) M. Rimaz and H. Mousavi, Turk. J. Chem., 2013, 37, 252–261 CAS; (p) M. Behrouzi, K. Rabiei and S. Ghasemzadeh, J. Organomet. Chem., 2024, 1022, 123413 CrossRef CAS; (q) F. Pirani and H. Eshghi, J. Organomet. Chem., 2024, 1020, 123339 CrossRef CAS; (r) M. Tandi, V. Sharma, B. Gopal and S. Sundriyal, RSC Adv., 2025, 15, 1447–1489 RSC.
- C. Sharma, A. K. Srivastava, A. Soni, S. Kumari and R. K. Joshi, RSC Adv., 2020, 10, 32516–32521 RSC.
- V. S. Sypu, M. Bhaumik, K. Raju and A. Maity, J. Colloid Interface Sci., 2021, 581, 979–989 CrossRef CAS PubMed.
- S. Yang, Z.-H. Zhang, Q. Chen, M.-Y. He and L. Wang, Appl. Organomet. Chem., 2018, 32, e4132 CrossRef.
- M. Pashaei and E. Mehdipour, Appl. Organomet. Chem., 2018, 32, e4226 CrossRef.
- M. Piri, M. M. Heravi, A. Elhampour and F. Nemati, J. Mol. Struct., 2021, 1242, 130646 CrossRef CAS.
- A. K. Srivastava, H. Khandaka and R. K. Joshi, SynOpen, 2023, 7, 121–129 CrossRef CAS.
- Z. Moradi and A. Ghorbani-Choghamarani, Sci. Rep., 2023, 13, 7645 CrossRef CAS PubMed.
- N. Seyedi, F. Shirini and H. Tajik, J. Mol. Struct., 2023, 1285, 135547 CrossRef CAS.
- M. Dabiri, A. Mnachekanian Salmasi, N. Salarinejad and S. Kazemi Movahed, J. Mol. Struct., 2023, 1287, 135609 CrossRef CAS.
- P. Mohammadi, M. M. Heravi, L. Mohammadi and A. Saljooqi, Sci. Rep., 2023, 13, 17375 CrossRef CAS PubMed.
- M. Z. Sarker, M. M. Rahman, H. Minami, M. S. I. Sarker and H. Ahmad, Colloids Surf. A Physicochem. Eng. Asp., 2023, 668, 131447 CrossRef CAS.
- M. Bayzidi and B. Zeynizadeh, RSC Adv., 2022, 12, 15020–15037 RSC.
- A. Capperucci, M. Clemente, A. Cenni and D. Tanini, ChemSusChem, 2023, 16, e202300086 CrossRef CAS PubMed.
- F. Dadvar and D. Elhamifar, Nanoscale Adv., 2024, 6, 5398–5408 RSC.
- F. Gholamrezaei, S. Y. Ebrahimipour and E. Ghonchepour, J. Organomet. Chem., 2024, 1019, 123314 CrossRef CAS.
- B. Kaboudin, B. Burunli, M. Nazerian, T. Zhang and Y. Gu, J. Organomet. Chem., 2025, 1025, 123477 CrossRef CAS.
- V. Bilakanti, N. Gutta, V. K. Velisoju, M. Dumpalapally, S. Inkollu, N. Nama and V. Akula, React. Kinet. Mech. Catal., 2020, 130, 347–362 CrossRef CAS.
- N. K. Lamba, P. Choudhary, Twinkle, J. Kaushik, S. K. Choudhary and S. K. Sonkar, Langmuir, 2024, 40, 18961–18967 CrossRef CAS PubMed.
- (a) H. van de Waterbeemd and E. Gifford, Nat. Rev. Drug Discov., 2003, 2, 192–204 CrossRef CAS PubMed; (b) E. V. Feinberg, E. Joshi, V. S. Pande and A. C. Cheng, J. Med. Chem., 2020, 63, 8835–8848 CrossRef CAS PubMed; (c) C.-Y. Jia, G.-F. Hao and G.-F. Yang, Drug Discov. Today, 2020, 25, 248–258 CrossRef CAS PubMed; (d) L. L. G. Ferreira and A. D. Andricopulo, Drug Discov. Today, 2019, 24, 1157–1165 CrossRef CAS PubMed; (e) L. Guan, H. Yang, Y. Cai, L. Sun, P. Di, W. Li, G. Liu and Y. Tang, Med. Chem. Commun., 2019, 10, 148–157 RSC; (f) S. Q. Pantaleão, P. O. Fernandes, J. E. Gonçalves, V. G. Maltarollo and K. M. Honorio, ChemMedChem, 2022, 17, e202100542 CrossRef PubMed; (g) R. Sayad, A. Bouzina, Y. O. Bouone, D. Beldjezzia, A. Djemel, M. Ibrahim-Ouali, N.-E. Aouf and Z. Aouf, RSC Adv., 2024, 14, 24585–24603 RSC; (h) A. Bouzina, Y. O. Bouone, O. Sekiou, M. Aissaoui, T.-S. Ouk, A. Djemel, R. Mansouri, M. Ibrahim-Ouali, Z. Bouslama and N.-E. Aouf, RSC Adv., 2023, 13, 19567–19584 RSC; (i) S. S. Ramasamy, K. Adhigaman, V. Nandakumar, A. Sundarasamy, S. Jagadeesan, M. Saravanakumar, J. G. Malecki, N. Easwaran and S. Thangaraj, J. Mol. Struct., 2025, 1321, 140218 CrossRef CAS.
- (a) A. Daina, O. Michielin and V. Zoete, Sci. Rep., 2017, 7, 42717 CrossRef PubMed; (b) B. Bakchi, A. D. Krishna, E. Sreecharan, V. B. J. Ganesh, M. Niharika, S. Maharshi, S. B. Puttagunta, D. K. Sigalapalli, R. R. Bhandare and A. B. Shaik, J. Mol. Struct., 2022, 1259, 132712 CrossRef CAS.
- Y. Myung, A. G. C. de Sá and D. B. Ascher, Nucleic Acids Res., 2024, 52, W469–W475 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4na00882k |
| This journal is © The Royal Society of Chemistry 2025 |