Open Access Article
Open Access ArticleTungsten oxide nanowire clusters anchored on porous carbon fibers as a sulfur redox mediator for lithium–sulfur batteries†
Tongzhen
Wang
a,
Xiaofei
Zhang
 a,
Jie
Yang
a,
Jiewu
Cui
a,
Jie
Yang
a,
Jiewu
Cui
 a,
Jian
Yan
a,
Jian
Yan
 a,
Jiaqin
Liu
a,
Jiaqin
Liu
 *b and
Yucheng
Wu
*b and
Yucheng
Wu
 *a
*a
aSchool of Materials Science and Engineering, Hefei University of Technology, Hefei 230009, China. E-mail: ycwu@hfut.edu.cn
bInstitute of Industry and Equipment Technology, Research Center of Advanced Composite Materials Design & Application of Anhui Province, Key Laboratory of Advanced Functional Materials and Devices of Anhui Province, Hefei University of Technology, Hefei, 230009, China. E-mail: jqliu@hfut.edu.cn
First published on 24th November 2024
Abstract
Addressing the sluggish redox kinetics of sulfur electrodes and mitigating the shuttle effect of intermediate lithium polysulfides (LiPS) are crucial for the advancement of high-energy lithium–sulfur batteries. Here, we introduce a pioneering flexible self-supporting composite scaffold that incorporates tungsten oxide nanowire clusters anchored on core–shell porous carbon fibers (WO3/PCF) for sulfur accommodation. The core of PCF serves as a robust electrode supporting scaffold, whereas the porous shell of PCF provides a 3D interconnected conductive network to accommodate sulfur, restrain polysulfide diffusion and buffer electrode expansion. The WO3 nanowire clusters not only entrap polysulfides but also function as a redox mediator to promote sulfur conversion, thus greatly mitigating the shuttle effect and boosting redox kinetics. The unique core–shell porous structure of PCF and the dual functionality of WO3 for LiPS capture and conversion contribute to the high capacity, exceptional cycling stability, and superior rate capability of the WO3/PCF/S cathode. Impressively, at a sulfur loading of 3.0 mg cm−2, it achieves an initial capacity of 1082 mA h·g−1 at 1 C with an ultralow decay rate of 0.039% over 1000 cycles. Even under a high sulfur loading of 6.1 mg cm−2, it maintains a reversible capacity of 536 mA h·g−1 after 1000 cycles with a decay rate of only 0.043% at 0.5 C.
Introduction
Lithium–sulfur batteries (LSBs) offer a high theoretical specific capacity of 1675 mA h g−1 and energy density of 2600 W h kg−1, significantly surpassing lithium-ion batteries (LIBs), driven by their complex sulfur-mediated redox reactions.1,2 Coupled with abundant sulfur reserves, cost-effectiveness, and environmental benefits, lithium–sulfur batteries are positioned as a leading contender for future sustainable energy solutions.2,3 Despite these advantages, the complex multiphase conversion chemistry of sulfur still faces several challenges, such as the insulating nature of sulfur and discharge products of Li2S2/Li2S, the notorious shuttle effect of lithium polysulfides (LiPS), slow sulfur redox kinetics, and electrode volume expansion during charging/discharging, leading to low coulombic efficiency, short cycle life, and rapid capacity fading.4,5To address these challenges, significant advancements have been made, such as the development of nanocomposite sulfur cathodes,6,7 functional separators/interlayers,8,9 solid-state electrolytes,10,11etc. Among these, sulfur cathode design remains critical for improving the performance of lithium–sulfur batteries, but it continues to face significant hurdles, particularly in managing severe LiPS shuttling and sluggish redox kinetics of sulfur. Research has primarily focused on strategies that physically confine sulfur species within porous carbon materials and chemically anchor and catalyze the redox reaction of LiPS on polar materials. Various polar materials (such as single atoms,12,13 metal oxides,14,15 metal sulfides,16,17 metal nitrides,18 metal carbides,19 metal phosphides,20etc.) have been incorporated into the carbon matrix to enhance LiPS absorption and act as electrocatalysts to accelerate redox kinetics, thus facilitating a stable LiPS “capture-conversion” process on the electrode surface.
For practical applications, lithium–sulfur batteries require a high sulfur loading on the cathode (>4 mg cm−2) and a high sulfur-to-carbon ratio (>70%).7,21 However, these requirements are constrained by sluggish charge/mass transfer kinetics within the electrode, which aggravates the challenges of slow sulfur redox kinetics, polysulfide shuttling, and electrode volume expansion. Conventional sulfur cathode materials, typically in powder form, require blending with conductive additives and binders, followed by coating onto a current collector. This process reduces the specific mass-energy density of batteries since conductive additives, binders, and the current collector do not contribute to capacity. Additionally, the electrochemically inert nature of binders obscures active sites and increases internal resistance, further hindering ion and electron transport.22,23 Therefore, innovating the design of the sulfur cathode structure by integrating active materials with current collectors and developing unique self-supporting sulfur cathode structures with high sulfur loading and “strong adsorption-rapid conversion” capabilities for LiPS is crucial for advancing the development of lithium–sulfur batteries.
Herein, we introduce a flexible, self-supporting composite scaffold featuring tungsten oxide nanowire clusters anchored on core–shell porous carbon fibers (WO3/PCF) for sulfur accommodation, which was achieved using a combined strategy of metal-assisted chemical etching followed by hydrothermal-annealing techniques. The core of PCF serves as a robust electrode supporting scaffold, while the porous carbon shell forms a 3D interconnected conductive network to accommodate active sulfur, restrain polysulfide diffusion, and buffer electrode volume expansion. The WO3 nanowire clusters not only entrap polysulfides but also function as a redox mediator to promote sulfur conversion, thereby significantly mitigating the shuttle effect and improving redox kinetics. Consequently, lithium–sulfur batteries based on the WO3/PCF/S cathode demonstrate superior electrochemical performance, including high capacity, exceptional cycle stability, superior rate capability, and low self-discharge.
Experimental section
Preparation of WO3/PCF
Porous carbon fibers (PCF) were synthesized using a metal-assisted chemical etching technique. Initially, carbon fiber cloth was sequentially cleaned ultrasonically in acetone, ethanol, and deionized water. Nickel hydroxide (Ni(OH)2) was then electrochemically deposited onto the carbon fiber surface in a three-electrode system, with the carbon fiber cloth as the working electrode, Ag/AgCl as the reference electrode, and platinum (Pt) as the counter electrode. The electrolyte consisted of 1 mol L−1 Ni(NO3)2 and 0.1 mol L−1 NaNO3. The electrodeposition current density and time were 6 mA cm−2 and 900 s, respectively. Following deposition, the samples were calcined at 800 °C at a heating rate of 5 °C min−1 in a nitrogen atmosphere for 4 hours. Following calcination, the samples were immersed in 3 mol L−1 hydrochloric acid at 80 °C for 6 hours and then rinsed with deionized water to yield PCF. The average mass density of PCF was approximately 7.0 mg cm−2.Subsequently, tungsten oxide (WO3) nanowire clusters were deposited onto the PCF surface via a hydrothermal method. A precursor solution containing 0.5 mol L−1 Na2WO4 and 0.3 mol L−1 H2C2O4 was prepared and adjusted to pH = 0.8 using 3 M HCl. Then, 2 g of (NH4)2SO4 was dissolved in 30 mL of the precursor solution. The PCF was immersed in this solution and placed in a 50 mL reactor, which was then heated at 180 °C for 12 hours. After the reaction, the sample was washed with deionized water, dried, and annealed at 400 °C with a heating rate of 3 °C min−1 under a nitrogen atmosphere for 4 hours to obtain WO3/PCF. The areal mass density of WO3/PCF was approximately 10.0 mg cm−2, and its thickness is around 370 μm (Fig. S1†).
Preparation of WO3/PCF/S
Sulfur was incorporated into the WO3/PCF host using a melt-diffusion method. The WO3/PCF host was mixed with excess sulfur powder in a reaction vessel and maintained at 155 °C for 12 hours. The temperature was then raised to 175 °C to remove surplus sulfur from the surface and control the final sulfur loading, producing the flexible self-supporting sulfur cathode of WO3/PCF/S. Similar procedures were followed for the preparation of WO3/CF/S and PCF/S cathodes.Visualized adsorption test
A 5 mM Li2S6 solution was first prepared by dissolving Li2S and sulfur at a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in a mixed solvent of 1,3-dioxolane (DOL)/1,2-dimethoxyethane (DME) (1
1 molar ratio in a mixed solvent of 1,3-dioxolane (DOL)/1,2-dimethoxyethane (DME) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) at 60 °C for 12 hours. For the static adsorption test, equal-sized WO3/PCF, WO3/CF, and PCF were immersed in 5 mL of the above Li2S6 solution. After standing for 8 hours, the adsorption capacity of the samples towards LiPS was evaluated by comparing the decolorization of the Li2S6 solution. All procedures were conducted in an argon-filled glove box. The adsorption capacities were subsequently quantified by analyzing the UV-vis absorption spectra of the Li2S6 solution post-adsorption.
1 in volume) at 60 °C for 12 hours. For the static adsorption test, equal-sized WO3/PCF, WO3/CF, and PCF were immersed in 5 mL of the above Li2S6 solution. After standing for 8 hours, the adsorption capacity of the samples towards LiPS was evaluated by comparing the decolorization of the Li2S6 solution. All procedures were conducted in an argon-filled glove box. The adsorption capacities were subsequently quantified by analyzing the UV-vis absorption spectra of the Li2S6 solution post-adsorption.
Materials characterization
The morphologies and microstructures were characterized by field-emission scanning electron microscopy (FESEM, ZEISS Sigma 300) and high-resolution transmission electron microscopy (HRTEM, JEM-2100F). Phase structures were identified by X-ray diffraction (XRD) analysis conducted on a Rigaku D/MAX2500V/PC with Cu-Kα radiation (λ = 0.154056 nm). Elemental composition and chemical states were examined using X-ray photoelectron spectroscopy (XPS, Escalab250Xi) and Raman spectroscopy (Raman, LabRAM HR Evolution, HORIBA Jobin-Yvon) with a 532 nm excitation laser. The specific surface area and pore size distribution were assessed using an automatic gas adsorption analyzer (Autosorb-IQ3, Quantachrome). Areal sulfur loading was determined using a Pyris 1 TGA (PerkinElmer) thermal analyzer from 0 to 600 °C at a heating rate of 5 °C min−1.Cell assembly and electrochemical measurements
CR2032-type cells were assembled with WO3/PCF/S, WO3/CF/S, and PCF/S as the cathodes, lithium metal (approximately 490 μm thick, Fig. S1†) as the anode, a Celgard 2500 polypropylene (PP) membrane as the separator, and an electrolyte of 1 mol L−1 LiTFSI with 1 wt% LiNO3 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of DOL/DME. The electrolyte-to-sulfur (E/S) ratio for Li–S cell assembly was set at 12 μL mg−1. All procedures were performed in a glove box under an argon atmosphere (O2, H2O < 0.1 ppm). Cyclic charge/discharge tests were conducted using a Land battery test system (CT2001A, Wuhan) within a voltage range of 1.7–2.8 V. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements were carried out using an electrochemical workstation (Autolab PGSTAT302N). CV was performed at a scan rate of 0.1 mV s−1 within a voltage range of 1.7–2.8 V, and EIS was performed in the frequency range from 100 kHz to 10 mHz with an amplitude of 5 mV s−1. For shuttle current evaluation, CR2032-type cells were assembled with 1.0 M LiTFSI in DME/DOL solution as the electrolyte. The cells underwent an initial two cycles at 0.05 C, followed by galvanostatic discharge to 1.7 V, charging to 2.38 V, and holding at this potential until the current stabilized, indicating the shuttle current. Symmetrical cells were assembled with WO3/PCF, WO3/CF, and PCF as electrodes, a Celgard 2500 as the separator, and 0.2 mol·L−1 Li2S6 solution as the electrolyte. CV of symmetrical cells was conducted at a scan rate of 0.3 mV s−1 from −1 to 1 V.
1 volume ratio of DOL/DME. The electrolyte-to-sulfur (E/S) ratio for Li–S cell assembly was set at 12 μL mg−1. All procedures were performed in a glove box under an argon atmosphere (O2, H2O < 0.1 ppm). Cyclic charge/discharge tests were conducted using a Land battery test system (CT2001A, Wuhan) within a voltage range of 1.7–2.8 V. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements were carried out using an electrochemical workstation (Autolab PGSTAT302N). CV was performed at a scan rate of 0.1 mV s−1 within a voltage range of 1.7–2.8 V, and EIS was performed in the frequency range from 100 kHz to 10 mHz with an amplitude of 5 mV s−1. For shuttle current evaluation, CR2032-type cells were assembled with 1.0 M LiTFSI in DME/DOL solution as the electrolyte. The cells underwent an initial two cycles at 0.05 C, followed by galvanostatic discharge to 1.7 V, charging to 2.38 V, and holding at this potential until the current stabilized, indicating the shuttle current. Symmetrical cells were assembled with WO3/PCF, WO3/CF, and PCF as electrodes, a Celgard 2500 as the separator, and 0.2 mol·L−1 Li2S6 solution as the electrolyte. CV of symmetrical cells was conducted at a scan rate of 0.3 mV s−1 from −1 to 1 V.
Calculation methods
Density functional theory (DFT) calculations were performed using the VASP 5.4 package.24,25 The electronic exchange–correlation interactions were described using the Perdew–Burke–Ernzerhof (PBE) functional under the Generalized Gradient Approximation (GGA).26 A 3 × 3 × 1 supercell was utilized to simulate the WO3 (002) surface. The Brillouin zone was sampled using a 2 × 2 × 1 Monkhorst–Pack grid for structural optimization and energy calculations.27 Convergence thresholds were set at 1 × 10−5 eV for energy and 0.01 eV Å−1 for atomic forces, with a plane-wave cutoff energy of 520 eV. A vacuum layer of 15 Å along the Z-axis direction was employed to prevent interactions between layers.The adsorption energy of S8 and Li2Sn (n = 1, 2, 4, 6, 8) on the WO3 (002) surface was calculated as
| Ead =ES8/Li2Sn@WO3 − EWO3 − ES8/Li2Sn |
Results and discussion
A metal-assisted chemical etching strategy was employed to controllably etch the carbon fiber (CF) skeleton, producing hierarchical porous carbon fiber (PCF) with a distinctive core–shell structure, as shown in Fig. 1a. In contrast to the initial CF (Fig. S2†), the surface of PCF was etched into a hierarchical porous carbon layer approximately 2 μm thick, while the inner core of PCF maintained a solid fiber structure, forming a core–shell structure (Fig. 2a). BET analysis reveals a high specific surface area of 323.3 m2 g−1 and a micro-mesoporous structure for the PCF shell (Fig. S3†). The specific surface area of the PCF shell was calculated by excluding the mass of the PCF core. The PCF core functions as a robust electrode supporting scaffold, while the porous PCF shell provides a 3D interconnected conductive network to accommodate active sulfur, buffer electrode volume expansion, and physically immobilize polysulfide, thus preventing sulfur loss within the PCF. Upon loading WO3 onto the PCF, the surface of the PCF was fully covered by “tower-like” WO3 nanowire clusters (Fig. 2b and d). These nanowires were approximately 1 μm in length and 15–25 nm in diameter. The clusters contain numerous voids, both within and between them, offering ample space for sulfur accommodation and enhancing electrolyte infiltration. EDS mapping of WO3/PCF (Fig. 2c) shows a uniform distribution of W, O and C elements, indicating that WO3 nanowires were evenly anchored on the PCF surface. A single WO3 nanowire (Fig. S4†) shows a tapered structure with a wide base and a sharp tip. The high-resolution TEM image (Fig. 2e and f) displays distinct lattice fringes with an interplanar spacing of 0.38 nm, corresponding to the (002) plane of WO3. The selected area electron diffraction (SAED) patterns (Fig. 2g) align well with the (002), (022) and (322) planes of WO3. Upon sulfur loading, active sulfur infiltrates the porous shell layer of PCF and uniformly encapsulates the WO3 nanowire surfaces (Fig. S5a and b†). EDS mapping confirms the even distribution of sulfur across WO3/PCF, without agglomeration observed on the surface or within the internal voids of the WO3 nanowire clusters (Fig. S5c†).The phase structures of PCF, WO3/PCF, and WO3/PCF/S were characterized using X-ray diffraction (XRD), as illustrated in Fig. 2h. The XRD pattern of PCF reveals a prominent peak of graphitized carbon at 2θ ≈ 26°. For WO3/PCF, additional diffraction peaks at 2θ ≈ 23.1°, 23.6°, 24.4°, 26.6°, 28.9°, 33.3°, 34.2°, 41.4°, 50.3°, and 55.8° align well with the characteristic peaks of WO3 (PDF No. 071-2141), confirming successful deposition of WO3 nanowire clusters on the PCF surface. Following sulfur loading onto WO3/PCF, new weak diffraction peaks emerged at 2θ ≈ 20.8° and 27.7° with other peaks overlapping WO3 peaks, consistent with elemental sulfur (PDF No. 53-1109), indicating effective sulfur incorporation into the WO3/PCF scaffold. The weak sulfur peaks suggest sulfur infiltration into the porous PCF shell and encapsulation on the WO3 nanowire surfaces, without sulfur agglomeration on the surface or within the internal voids of the WO3 nanowire clusters. The Raman spectra of PCF, WO3/PCF, and WO3/PCF/S (Fig. 2i) display characteristic peaks for D and G bands of carbon at 1346 and 1597 cm−1. The ID/IG ratio of WO3/PCF (1.07) and WO3/PCF/S was slightly lower than that of PCF (1.10), which ensures good conductivity that favors sulfur conversion.29 Notably, the Raman spectra of WO3/PCF and WO3/PCF/S display WO3 peaks at 694 cm−1, 803 cm−1, 941 cm−1, 252 cm−1, and 322 cm−1,30,31 along with sulfur peaks at 152 cm−1, 221 cm−1 and 475 cm−1 for WO3/PCF/S after sulfur loading.32 The elemental composition and chemical states of WO3/PCF were further investigated using X-ray photoelectron spectroscopy (XPS). The XPS survey spectrum (Fig. S6†) shows peaks at binding energies of 285.4, 531.5 and 37.2 eV, corresponding to the characteristic peaks of C 1s, O 1s and W 4f, respectively, indicating the presence of W, C and O elements in WO3/PCF. The C 1s spectrum (Fig. 2j) includes three fitted peaks corresponding to C–C (284.8 eV), C–O/C–O–C (286.3 eV), and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O (288.9 eV).33,34 The W 4f spectrum (Fig. 2k) features peaks at binding energies of 35.8 and 38.0 eV, corresponding to the W 4f7/2 and W 4f5/2 of W6+ in WO3, respectively.35,36 In the O 1s spectrum (Fig. 2l), the peak at a binding energy of 530.7 eV corresponds to the W–O bond, while peaks at 532.0 and 533.1 eV are attributed to hydroxyl or carbonate contaminants on the WO3/PCF surface.36 TGA analysis (Fig. S7†) reveals sulfur contents of approximately 23.8 and 30.4 wt% for WO₃/PCF/S and PCF/S cathodes, respectively, at an areal sulfur loading of 3.0 mg cm−2.
C–O (288.9 eV).33,34 The W 4f spectrum (Fig. 2k) features peaks at binding energies of 35.8 and 38.0 eV, corresponding to the W 4f7/2 and W 4f5/2 of W6+ in WO3, respectively.35,36 In the O 1s spectrum (Fig. 2l), the peak at a binding energy of 530.7 eV corresponds to the W–O bond, while peaks at 532.0 and 533.1 eV are attributed to hydroxyl or carbonate contaminants on the WO3/PCF surface.36 TGA analysis (Fig. S7†) reveals sulfur contents of approximately 23.8 and 30.4 wt% for WO₃/PCF/S and PCF/S cathodes, respectively, at an areal sulfur loading of 3.0 mg cm−2.
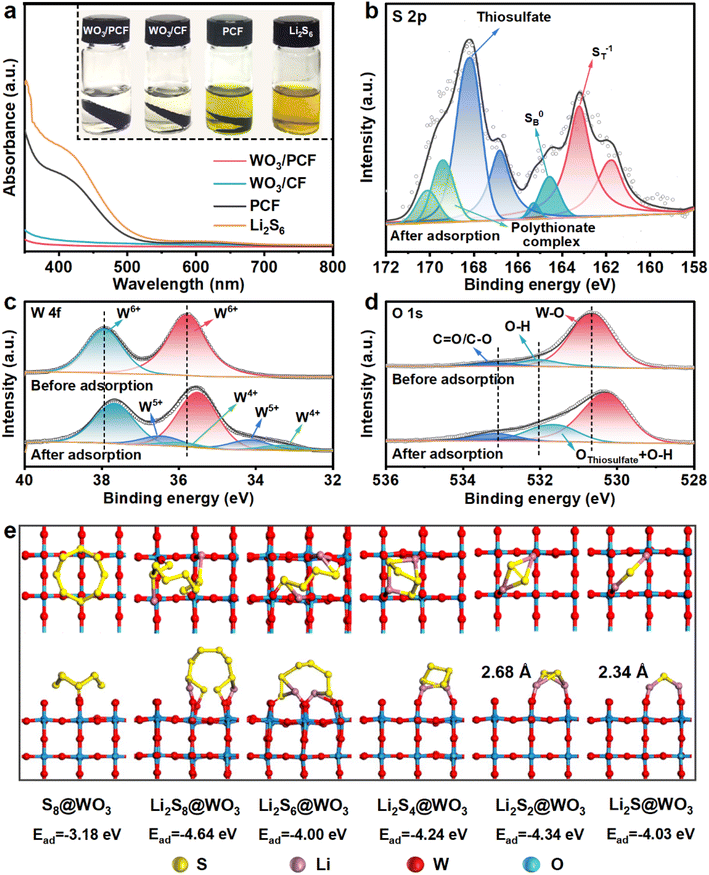
The adsorption capability of host materials towards LiPS was indicative of their ability to mitigate the shuttle effect. To assess the LiPS absorbing capacity, a visual adsorption test was conducted by immersing WO3/PCF, WO3/CF, and PCF in a Li2S6 solution and monitoring the decolorization of the Li2S6 solution over time (Fig. 3a). It was evident that after adsorption, the Li2S6 solution with WO3/PCF changed from deep yellow to almost clear. The degree of decolorization follows the order: WO3/PCF > WO3/CF > PCF. This observation was confirmed by the disappearance of the characteristic UV-vis peak for Li2S6 at 411 nm,37 demonstrating that WO3 has a strong adsorption capacity towards LiPS. The interaction mechanism between WO3 and LiPS was further explored by XPS analysis. After adsorption, the S 2p peak at 169.3 eV emerged in the survey spectrum of WO3/PCF-Li2S6 (Fig. S6†). The high-resolution S 2p spectrum (Fig. 3b) can be deconvoluted into four pairs of doublets: two pairs correspond to the terminal sulfur (ST−1) and bridging sulfur (SB0) of adsorbed Li2S6, while the other two pairs were attributed to thiosulfate and polythionate complexes,38,39 generated by the redox reaction of Li2S6 with WO3 and further reaction of thiosulfate with Li2S6. Additionally, the peaks in the W 4f and O 1s spectra (Fig. 3c and d) shift to lower binding energies, indicating electron transfer from Li2S6 to W and O atoms.40,41 Notably, two new pairs of peaks appear in the W 4f spectrum at 34.1/36.5 eV (corresponding to W5+) and 33.3/35.8 eV (corresponding to W4+), and the peak of O 1s at 531.7 eV was more intense than that before adsorption, both attributed to the formation of thiosulfate/polythionates.36 Based on the adsorption test results and XPS analysis, it can be inferred that WO3 interacts with soluble LiPS to form intermediate thiosulfate species, with LiPS donating electrons to WO3, reducing W6+ to W5+/W4+.36 Then, thiosulfate further interacts with higher-order LiPS, forming polythionate complexes and lower-order lithium polysulfides.42,43 Thus, these thiosulfate species formed on the WO3 surface function as a redox shuttle to catalyze LiPS conversion reactions, thereby suppressing the shuttle effect and enhancing the conversion kinetics of LiPS, as illustrated in Fig. 1b.
The interaction between LiPS and WO3 was further elucidated through density functional theory (DFT) calculations. The results reveal that S8 molecules lie flat on the WO3 surface with an adsorption energy of −3.18 eV, while Li2Sx (x = 1–8) molecules were adsorbed on the WO3 (002) surface via Li–O bonds with adsorption energies for Li2S8@WO3, Li2S6@WO3, Li2S4@WO3, Li2S2@WO3, and Li2S@WO3 being −4.64, −4.00, −4.24, −4.34, and −4.03 eV, respectively. During the adsorption process, long-chain Li2S8/Li2S6 undergo Li–S bond cleavage, while S–S bond lengths in short-chain Li2S2/Li2S increase from 2.23/2.09 Å to 2.68/2.34 Å (Fig. S8† and 3e). This strong chemisorption of WO3 towards LiPS, leading to the breaking of Li–S bonds or stretching of S–S bonds, facilitates the conversion of LiPS ↔ Li2S during charge/discharge cycles.44
To substantiate the high-efficiency sulfur conversion facilitated by WO3/PCF, a series of electrochemical performances were evaluated on WO3/PCF/S, WO3/CF/S and PCF/S cathodes. Cyclic voltammetry (CV) was performed within a voltage range of 1.7–2.8 V (Fig. S9†). All CV curves exhibit two reduction peaks and one oxidation peak, corresponding to reduction of sulfur to soluble long-chain LiPS (Li2Sx, 4 ≤ x ≤ 8) and further reduction to solid-state short-chain Li2S2/Li2S, and the reverse oxidation reactions.45,46 Notably, the redox peaks of WO3/PCF/S were sharper and more intense compared to other cathodes (Fig. 4a), indicating higher reaction activity and faster reaction kinetics. The reduction peaks shifted towards more positive potential and the oxidation peak shifted towards more negative potential, suggesting reduced electrode polarization and enhanced redox reversibility. Similarly, in the CV curves of symmetrical cells (Fig. 4b), all electrodes display four pairs of redox peaks. Peaks a, b and c correspond to the stepwise oxidation of Li2S6 to S8, while peaks d, e and f correspond to the stepwise reduction of S8 to Li2S6 on the working electrode. At peak g, Li2S6 was reduced to Li2S (or Li2S2), and at peak h, Li2S (or Li2S2) was oxidized to Li2S6.47,48 The WO3/PCF/S electrode exhibited higher peak currents and closer redox potentials, with the peak currents for the four pairs of redox peaks occurring at 0.075/−0.070 V (peak a/peak e), 0.236/−0.226 V (peak b/peak f), 0.339/−0.334 V (peak c/peak g) and 0.049/−0.046 V (peak d/peak h), indicating superior redox kinetics and reversibility.
The galvanostatic charge–discharge (GCD) curves of WO3/PCF/S, WO3/CF/S, and PCF/S cathodes at 1 C for the 1st, 100th, 200th, 300th, 500th, and 1000th cycles show two discharge plateaus and one charging plateau, consistent with the CV analysis (Fig. S10†). The WO3/PCF/S cathode demonstrates a higher overlap in its GCD curves, indicating superior cycling reversibility. The initial GCD curves (Fig. 4c) reveal that the WO3/PCF/S cathode has a voltage hysteresis (ΔV) of only 0.18 V, significantly lower than those of WO3/CF/S (0.25 V) and PCF/S (0.29 V), indicating minimal polarization and the fastest reaction kinetics. Additionally, QH is associated with the discharge plateau at 2.3 V, corresponding to the conversion of solid to soluble LiPS, while QL relates to the 2.0 V discharge plateau, indicating the conversion of soluble LiPS to solid Li2S2/Li2S. The QH/QL ratio reflects the catalytic conversion efficiency in sulfur redox reactions.49,50 The WO3/PCF/S cathode has a QL/QH ratio of 2.59, much higher than those of WO3/CF/S (2.01) and PCF/S (1.98), further demonstrating effective promotion of solid–liquid–solid sulfur conversion kinetics by WO3/PCF. Electrochemical Impedance Spectroscopy (EIS) was conducted to assess the internal interface and charge transfer resistances (Fig. 4d, Table S1†). Prior to cycling, the EIS spectra show a single semicircle attributed to the charge transfer resistance semicircle (Rct) of the cathode,51,52 with an Rct value of 19.25 Ω for WO3/PCF/S, significantly lower than those for WO3/CF/S (41.55 Ω) and PCF/S (98.35 Ω). After 200 cycles, all EIS spectra display two semicircles, with the emerging low-frequency semicircle indicating interfacial resistance (Rf), associated with the formation of an insulating Li2S2/Li2S layer on the lithium anode surface.40,53 The WO3/PCF/S cathode exhibits an Rf of 10.71 Ω and an Rct of 12.90 Ω, both significantly lower than those of WO3/CF/S and PCF/S, indicating that the WO3/PCF scaffold effectively mitigates the shuttle effect and reduces sulfur redox resistance during cycling.
WO3/PCF/S also shows exceptional cycling and rate performance. At a sulfur loading of 3.0 mg cm−2, it delivers an initial discharge capacity of 1082 mA h·g−1 at 1 C, with a decay rate of only 0.039% over 1000 cycles, significantly outperforming WO3/CF/S (991 mA h·g−1 and 0.065%) and PCF/S (641 mA h·g−1 and 0.070%) (Fig. 5a). At sulfur loadings of 2.2 mg cm−2 and 6.1 mg cm−2 (Fig. 5b), the WO3/PCF/S cathode maintains decay rates of 0.028% and 0.043% at 0.5 C over 1000 cycles, respectively, demonstrating excellent cycling stability at high sulfur loadings. Additionally, at 0.2 C, 2 C, and 5 C, the WO3/PCF/S cathode (3.0 mg cm−2 sulfur loading) shows capacity decay rates of 0.028%, 0.049%, and 0.058% over 1000 cycles, respectively, indicating stable cycling at high rates (Fig. 5c). Discharge capacities of WO3/PCF/S at 0.2, 0.5, 1, 2, 3 and 5 C were 1356, 1035, 924, 841, 747 and 672 mA h·g−1, respectively (Fig. 5d), superior to WO3/CF/S and PCF/S cathodes, demonstrating the exceptional rate performance of the WO3/PCF/S cathode. Self-discharge, assessed using the “cycle-rest” method, shows that at 0.5 C, the WO3/PCF/S cathode retains 89.6% of its capacity after resting for 3 days, outperforming WO3/CF/S (76.3%) and PCF/S (74.3%) (Fig. 5e). The suppression of the shuttle effect, quantified by measuring the shuttle current in potentiostatic mode,54,55 shows that WO3/PCF/S exhibits the lowest steady-state current (Fig. 5f), indicating effective suppression of the shuttle effect.
After cycling at 1 C for 200 cycles, the cathodes and separators from the disassembled cells were examined (insets of Fig. 6a–c). The separator from the cell with the WO3/PCF/S cathode shows a very faint yellow color, indicating effective suppression of the shuttle effect via physical constraint and adsorption catalysis of LiPS by PCF and WO3. Additionally, the cycled WO3/PCF/S cathode maintains its tower-like cluster structure, unlike the collapsed structure of WO3/CF/S and the porous structure of PCF/S. Furthermore, the undesired anode corrosion reaction between LiPS and the Li anode was significantly mitigated as well. After 200 cycles at 1 C, the Li anode that detached from the battery using the WO3/PCF/S cathode exhibited a smooth and compact surface without visible holes or cracks (Fig. 6d). In contrast, the Li anodes cycled with WO3/CF/S and PCF/S cathodes showed rough surfaces with notable porosity and fractures (Fig. 6e and f). Cross-sectional EDS mapping of F and S elements on the cycled Li anode reveals a well-defined interfacial layer. This layer is a dense, stable solid electrolyte interphase (SEI), formed from reactions involving polysulfides, the Li anode and electrolyte decomposition products (Fig. S11†), and primarily consists of inorganic compounds like Li2O, LiF, Li2SOx, and Li2S, along with certain organic molecules.56,57 Moreover, the cycled Li anode in the WO3/PCF/S cell displayed a much thinner SEI layer (15 vs. 36/52 μm) (Fig. 6g–i), indicating significantly reduced anode corrosion. This underscores the dual-anchoring role of PCF towards WO3 nanowire clusters and soluble polysulfides as well as the adsorption-catalysis role of WO3 in the reversible conversion of sulfur species. As illustrated in Fig. 1c, for the PCF, the physical confinement of the surface porous carbon layer cannot prevent soluble polysulfides escaping from the PCF, while for WO3/PCF, the WO3 nanowire clusters on the PCF can effectively capture the escaping polysulfides and facilitate the conversion of LiPS ↔ Li2S. Thus, such a flexible self-supporting WO3/PCF/S cathode achieves high capacity, exceptional cycle stability and superior rate capability, surpassing those reported in recent studies, as shown in Table S2.†
 | ||
| Fig. 6 (a–c) SEM and digital images of WO3/PCF/S, WO3/CF/S and PCF/S cathodes, separators and (d–i) Li metal anodes from the disassembled cells after 200 cycles at 1 C. | ||
Conclusions
In summary, we introduce a flexible, self-supporting composite scaffold featuring polar “tower-like” WO3 nanowire clusters anchored on core–shell porous carbon fibers (WO3/PCF) for sulfur accommodation. This distinctive architecture was fabricated using a combination of metal-assisted chemical etching followed by hydrothermal-annealing techniques. DFT theoretical calculations and extensive characterization reveal that the core of PCF acts as a robust electrode supporting scaffold, while the porous carbon shell forms a 3D interconnected conductive network that effectively accommodates active sulfur, suppresses polysulfide diffusion, and buffers electrode volume expansion. The WO3 nanowire clusters capture LiPS escaping from the porous carbon network via polar–polar interactions and act as a redox mediator to accelerate sulfur conversion through reacting with LiPS to form thiosulfate/polythionate species, thereby mitigating the shuttle effect and enhancing redox kinetics. The combined advantages of self-supporting PCF and the dual functionality of WO3 for LiPS capture and conversion contribute to the outstanding electrochemical performance of the WO3/PCF/S cathode, including high capacity (1356 mA h·g−1 at 0.2 C), superior rate capability (672 mA h·g−1 at 5 C), and exceptional cycling stability (an initial capacity of 1082 mA h·g−1 with an ultralow decay rate of 0.039% over 1000 cycles at 1 C), along with low self-discharge.Data availability
The data supporting this article have been included as part of the ESI.† All experimental data are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (52372187, 51972093, and U1910210), Higher Education Discipline Innovation Project “New Materials and Technology for Clean Energy” (No. B18018), Nature Science Research Project of Anhui Province (2008085ME129), and Key Research and Development Program of Anhui Province (2022h11020012).References
- W. H. Jin, X. Y. Zhang, M. Liu, Y. Zhao and P. Zhang, Energy Storage Mater., 2024, 67, 103223 CrossRef.
- H. Raza, S. Y. Bai, J. Y. Cheng, S. Majumder, H. Zhu, Q. Liu, G. P. Zheng, X. F. Li and G. H. Chen, Electrochem. Energy Rev., 2023, 6, 29 CrossRef CAS.
- Y. Z. Huang, L. Lin, C. K. Zhang, L. Liu, Y. K. Li, Z. S. Qiao, J. Lin, Q. L. Wei, L. S. Wang, Q. S. Xie and D. L. Peng, Adv. Sci., 2022, 9, 2106004 CrossRef CAS.
- J. Tan, L. L. Ma, Y. Wang, P. S. Yi, C. M. Ye, Z. Fang, Z. H. Li, M. X. Ye and J. F. Shen, Energy Environ. Mater., 2024, 7, e12688 CrossRef CAS.
- W. Q. Yao, K. Liao, T. X. Lai, H. Sul and A. Manthiram, Chem. Rev., 2024, 124, 4935–5118 CrossRef CAS PubMed.
- J. T. Kim, X. G. Hao, C. H. Wang and X. L. Sun, Matter, 2023, 6, 316–343 CrossRef CAS.
- M. Y. Wang, Z. C. Bai, T. Yang, C. H. Nie, X. Xu, Y. X. Wang, J. Yang, S. X. Dou and N. N. Wang, Adv. Energy Mater., 2022, 12, 2201585 CrossRef CAS.
- C. Yuan, X. F. Yang, P. Zeng, J. Mao, K. H. Dai, L. Zhang and X. L. Sun, Nano Energy, 2021, 84, 105928 CrossRef CAS.
- M. Waqas, Y. H. Niu, M. J. Tang, Y. S. Pang, S. Ali, Y. F. Dong, W. Q. Lv and W. D. He, Energy Storage Mater., 2024, 72, 103682 CrossRef.
- X. X. Zhu, L. G. Wang, Z. Y. Bai, J. Lu and T. P. Wu, Nano-Micro Lett., 2023, 15, 75 CrossRef.
- B. B. Gicha, L. T. Tufa, N. Nwaji, X. J. Hu and J. Lee, Nano-Micro Lett., 2024, 16, 172 CrossRef CAS PubMed.
- S. Maiti, M. T. Curnan, K. Kim, K. Maiti and J. K. Kim, Adv. Energy Mater., 2024, 2401911 CrossRef CAS.
- Y. Z. Song, L. W. Zou, C. H. Wei, Y. Zhou and Y. Hu, Carbon Energy, 2023, 5, e286 CrossRef CAS.
- J. Wu, T. Ye, Y. C. Wang, P. Y. Yang, Q. C. Wang, W. Y. Kuang, X. L. Chen, G. H. Duan, L. M. Yu, Z. Q. Jin, J. Q. Qin and Y. P. Lei, ACS Nano, 2022, 16, 15734–15759 CrossRef CAS PubMed.
- S. G. Deng, T. Z. Guo, J. Heier and C. F. Zhang, Adv. Sci., 2023, 10, 2204930 CrossRef CAS PubMed.
- H. C. Li, X. Y. Wang, H. W. Ma, D. Y. Guo, L. H. Wu, H. L. Jin and X. A, ChemElectroChem, 2024, 11, e202300696 CrossRef CAS.
- G. Y. Zhu, Q. Z. Wu, X. H. Zhang, Y. W. Bao, X. Zhang, Z. Y. Shi, Y. Z. Zhang and L. B. Ma, Nano Res., 2024, 17, 2574–2591 CrossRef CAS.
- J. L. Cheong, C. Hu, W. W. Liu, M. F. Ng, M. B. Sullivan and J. Y. Ying, Nano Energy, 2022, 102, 107659 CrossRef CAS.
- P. Wang, B. J. Xi, M. Huang, W. H. Chen, J. K. Feng and S. L. Xiong, Adv. Energy Mater., 2021, 11, 2002893 CrossRef CAS.
- Y. Yang, B. W. Sun, Z. Q. Sun, J. J. Xue, J. H. He, Z. H. Wang, K. N. Sun, Z. X. Sun, H. K. Liu and S. X. Dou, Coordin. Chem. Rev., 2024, 510, 215836 CrossRef CAS.
- Y. Hu, W. Chen, T. Y. Lei, Y. Jiao, J. W. Huang, A. J. Hu, C. H. Gong, C. Y. Yan, X. F. Wang and J. Xiong, Adv. Energy Mater., 2020, 10, 2000082 CrossRef CAS.
- Q. H. Q. Xiao, J. L. Yang, X. D. Wang, Y. R. Deng, P. Han, N. Yuan, L. Zhang, M. Feng, C. A. Wang and R. P. Liu, Carbon Energy, 2021, 3, 271–302 CrossRef CAS.
- Z. Y. Geng, T. Meng, F. Ma, W. C. Qin, X. H. Wang, H. F. Zhang and G. Guan, Adv. Funct. Mater., 2024, 34, 2309345 CrossRef CAS.
- G. Kresse and J. Furthmüller, Comp. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Condens. Matter Mater. Phys., 1976, 13, 5188–5192 CrossRef.
- L. B. Ni, S. Q. Duan, H. Y. Zhang, J. Gu, G. J. Zhao, Z. X. Lv, G. Yang, Z. Y. Ma, Y. Liu, Y. S. Fu, Z. Wu, J. Xie, M. Chen and G. W. Diao, Carbon, 2021, 182, 335–347 CrossRef CAS.
- C. Y. Zhang, G. W. Sun, Y. F. Bai, Z. Dai, Y. R. Zhao, X. P. Gao, G. Z. Sun, X. B. Pan, X. J. Pan and J. Y. Zhou, J. Mater. Chem. A, 2020, 8, 18358–18366 RSC.
- B. Zhang, C. Luo, Y. Q. Deng, Z. J. Huang, G. M. Zhou, W. Lv, Y. B. He, Y. Wan, F. Y. Kang and Q. H. Yang, Adv. Energy Mater., 2020, 10, 2000091 CrossRef CAS.
- S. Choi, D. H. Seo, M. R. Kaiser, C. Zhang, V. D. L. Timothy, Z. J. Han, A. Bendavid, X. Guo, S. Yick, A. T. Murdock, D. Su, B. R. Lee, A. Du, S. X. Dou and G. X. Wang, J. Mater. Chem. A, 2019, 7, 4596–4603 RSC.
- C. Nims, B. Cron, M. Wetherington, J. Macalady and J. Cosmidis, Sci. Rep., 2019, 9, 7971 CrossRef PubMed.
- S. G. Deng, X. T. Shi, Y. Zhao, C. Wang, J. H. Wu and X. Y. Yao, Chem. Eng. J., 2022, 433, 133683 CrossRef CAS.
- G. C. Ruan, Y. R. Shen, J. Yao, Y. W. Huang, S. Y. Yang, S. J. Hu, H. Q. Wang, Y. P. Fang and X. Cai, J. Power Sources, 2023, 566, 232922 CrossRef CAS.
- X. Y. Yang, H. L. Zu, L. Q Luo, H. W. Zhang, J. Y. Li, X. B. Yi, H. Liu, F. Y. Wang and J. J. Song, J. Alloy. Compd., 2020, 833, 154969 CrossRef CAS.
- S. K. Lee, H. Kim, S. Bang, S. T. Myung and Y. K. Sun, Molecules, 2021, 26, 377 CrossRef CAS.
- Y. Kong, X. Ao, X. Huang, J. L. Bai, S. Q. Zhao, J. Y. Zhang and B. B. Tian, Adv. Sci., 2022, 9, 2105538 CrossRef CAS.
- J. Q. Liu, K. H. Li, Q. Zhang, X. F. Zhang, X. Liang, J. Yan, H. H. Tan, Y. Yu and Y. C. Wu, ACS Appl. Mater. Interfaces, 2021, 13, 45547–45557 CrossRef CAS PubMed.
- Y. B. Wang, D. Y. Zhang, J. H. Han, Y. Yang, Y. Guo, Z. X. Bai, J. B. Cheng, P. L. K. Chu, H. Pang and Y. S. Luo, Chem. Eng. J., 2022, 433, 133629 CrossRef CAS.
- F. Ma, Z. Chen, K. Srinivas, Z. H. Zhang, Y. Wu, D. W. Liu, H. S. Yu, Y. Wang, X. S. Li, M. Q. Zhu, Q. Wu and Y. F. Chen, J. Energy Chem., 2024, 88, 260–271 CrossRef CAS.
- J. H. Li, Z. Y. Wang, K. X. Shi, Y. J. Wu, W. Z. Huang, Y. G. Min, Q. B. Liu and Z. X. Liang, Adv. Energy Mater., 2024, 14, 2303546 CrossRef CAS.
- X. Liang, C. Hart, Q. Pang, A. Garsuch, T. Weiss and L. F. Nazar, Nat. Commun., 2015, 6, 5682 CrossRef PubMed.
- H. Chen, W. D. Dong, F. J. Xia, Y. J. Zhang, M. Yan, J. P. Song, W. Zou, Y. Liu, Z. Y. Hu, J. Liu, Y. Li, H. E. Wang, L. H. Chen and B. L. Su, Chem. Eng. J., 2020, 381, 122746 CrossRef CAS.
- M. X. Wang, L. S. Fan, X. Sun, B. Guan, B. Jiang, X. Wu, D. Tian, K. N. Sun, Y. Qiu, X. J. Yin, Y. Zhang and N. Q. Zhang, ACS Energy Lett., 2020, 5, 3041–3050 CrossRef CAS.
- T. T. Sun, C. Huang, H. B. Shu, L. P. Luo, Q. Q. Liang, M. F. Chen, J. C. Su and X. Y. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 57975–57986 CrossRef CAS.
- Y. Y. Kong, L. Wang, M. Mamoor, B. Wang, G. M. Qu, Z. X. Jing, Y. P. Pang, F. B. Wang, X. F. Yang, D. D. Wang and L. Q. Xu, Adv. Mater., 2023, 36, 2310143 CrossRef PubMed.
- M. D. Zhang, J. W. Mu, Y. N. Li, Y. Y. Pan, Z. L. Dong, B. Chen, S. W. Guo, W. H. Yuan, H. W. Fang, H. Hu and M. B. Wu, J. Energy Chem., 2023, 78, 105–114 CrossRef CAS.
- T. Liu, S. N. Fu, S. S. X, J. Li, H. T. Cui, Y. Y. Liu, K. H. Liu, H. Y. Wei and M. R. Wang, Appl. Surf. Sci., 2024, 655, 159617 CrossRef CAS.
- Q. W. Ran, J. T. Liu, L. Li, Q. Hu, H. Y. Zhao, S. Komarneni and X. Q. Liu, Adv. Funct. Mater., 2024, 34, 2402872 CrossRef CAS.
- B. Wang, J. Y. Tang, S. Y. Jia, Z. Q. Xing, S. W. Chen, Y. Deng, X. K. Meng and S. C. Tang, Adv. Funct. Mater., 2024, 34, 2315836 CrossRef CAS.
- K. L. Liu, X. D. Zhang, F. J. Miao, Z. Wang, S. J. Zhang, Y. S. Zhang, P. Zhang and G. S. Shao, Small, 2021, 17, 2100065 CrossRef CAS.
- G. Z. Zhang, H. M. Deng, R. M. Tao, B. Q. Xiao, T. Y. Hou, S. Yue, N. Shida, Q. Cheng, W. X. Zhang and J. Y. Liang, Mater. Lett., 2020, 262, 127194 CrossRef CAS.
- M. Luo, Y. Bai, R. Sun, M. X. Qu, M. Y. Wang, Z. F. Yang, Z. H. Wang, W. Sun and K. N. Sun, J. Energy Chem., 2022, 73, 407–415 CrossRef CAS.
- P. Cheng, L. L. Shi, W. Q. Li, X. R. Fang, D. L. Chao, Y. G. Zhao, P. Cao, D. Q. Liu and D. Y. He, Small, 2023, 19, 2206083 CrossRef CAS.
- Z. F. Li, M. Zhang, Y. Liu, Y. Cao, Q. H. Zeng, Q. J. Mao, Z. H. Lu, Y. C. Jiang, A. Q. Chen, J. Z. Guan, H. H. Wang, L. Chen, R. Z. Li, Y. J. Zhou, J. C. Wang, X. F. Liu, X. Y. Liu and L. Y. Zhang, Adv. Funct. Mater., 2024, 2412579 CrossRef.
- H. J. Yang, Y. Qiao, Z. Chang, P. He and H. S. Zhou, Angew. Chem., Int. Ed., 2021, 60, 17726–17734 CrossRef CAS PubMed.
- L. S. Wu, J. P. Hu, S. J. Chen, X. R. Yang, L. Liu, J. S. Foord, P. Pobedinskas, K. Haenen, H. J. Hou and J. K. Yang, Electrochim. Acta, 2023, 466, 142973 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4na00829d |
| This journal is © The Royal Society of Chemistry 2025 |