Open Access Article
Open Access ArticleGlucose reduced nano-Se mitigates Cu-induced ROS by upregulating antioxidant genes in zebrafish larvae†
Suganiya
Umapathy
and
Ieshita
Pan
 *
*
Institute of Biotechnology, Department of Medical Biotechnology, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences, Thandalam, Chennai, 602 105, Tamil Nadu, India. E-mail: bony.iesk@gmail.com; ieshitapan.sse@saveetha.com
First published on 27th February 2025
Abstract
This study compares the therapeutic efficiency of bovine serum albumin-stabilized selenium nanoparticles in reducing oxidative stress and improving cellular health. The nanoparticles were synthesized using mussel-extracted selenium with two reducing agents: D-glucose and orange. Inductively coupled plasma-optical emission spectroscopy and X-ray diffraction analyses confirmed the presence of selenium. The reducing agent and duration influenced the nanoparticle size. Reduction with D-glucose for 1 hour revealed that the particles exhibited an average size of 10 nm. Copper sulfate-induced malformations such as yolk sac and pericardial edema were observed with 25 μg ml−1 of orange-reduced nanoparticles, while D-glucose-reduced nanoparticles mitigated these malformations at 25 μg ml−1. Treatment with stabilized Se-NPs reduced with D-glucose for 30 minutes showed 33% dose-dependent radical scavenging activities, upregulated approximately 2-fold of superoxide dismutase, catalase, glutathione reductase, and glutathione peroxidase encoding genes and restored homeostasis by decreasing lipid peroxidation (27.32 nmol mg−1 ml−1) and nitric oxide levels (6.71 μM). They also had the potential to restore cognitive properties such as larval movement (93.40 m) without altering larval behaviour. Live cell imaging indicated a significant decrease in cellular reactive oxygen species and lipid peroxidation levels in the gut and liver. These findings suggest that Se-NPs reduced for 30 minutes with D-glucose are promising candidates for oxidative stress-induced neurodegeneration.
Introduction
Mitochondria are dynamic, membrane-bound organelles that play a pivotal role in orchestrating cellular energy production in almost all eukaryotic cells. They are central to sustaining life by generating the ATP required for various cellular functions and regulating several metabolic processes, including redox homeostasis, calcium signaling, and cellular apoptosis.1,2 Mitochondria are one of the most crucial organelles involved in the structure and function of neuronal networks in the brain. Disruption of mitochondrial homeostasis can directly progress oxidative damage, leading to neurodegeneration.3 Major contributors to mitochondrial dysfunction are mitochondrial fission, fusion, and mitophagy, leading to neurodegenerative diseases including Alzheimer's Disease (AD), Parkinson's Disease (PD), Amyotrophic Lateral Sclerosis (ALS), prion diseases etc.4,5 Excessive fusion leads to the formation of elongated mitochondrial tubules, while increased fission initiates the fragmentation of mitochondria.6 These events trigger oxidative stress, halt the production of energy, and cause abnormal signaling in cellular pathways.7 Reactive oxygen species (ROS) can be both beneficial for and detrimental to human health. Generally, ROS contributes to several redox-regulating processes within cells to maintain cellular homeostasis. However, overproduction and accumulation of ROS lead to oxidative stress, damaging cell structures and causing various diseases.8,9 In both healthy and pathological situations, mitochondria are the primary source of ROS. Superoxide and hydroxyl radicals are the primary oxygen-free radicals.10 Cellular metabolisms like lipoxygenases (LOX) and cyclooxygenases (COX), produces superoxide anion radicals.11 The pathophysiology of chronic illnesses such as cancer, diabetes, neurodegenerative diseases, and cardiovascular diseases is heavily influenced by oxidative stress. Oxidative stress increases the levels of pro-oxidant factors, leading to structural alterations in mitochondrial DNA and functional alterations by aberrant gene expression.12Nanotechnology has received significant attention due to the well-established fact that its combination with biotechnology creates a platform with enormous potential and significance in terms of its variety of applications.13 Different kinds of nanoparticles (NPs) can be formulated to maximize their functions; these include semiconductor, metal, metal oxide, organic, and inorganic NPs. Semiconductor nanoparticles, such as quantum dots, are used in controlled drug release due to their responsive optical and electronic properties.14 Treatment with metal oxide NPs i.e., CeO2-NPs decreased post-injury neuronal death and damage while improving CAT, SOD activity levels, and glutathione![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glutathione-disulfide ratios notably.15 Silver NPs efficiently inhibited lipid peroxidation (LPO) by scavenging ROS, showing their antioxidant effectiveness.16 Organic NPs, such as liposomes, enhance drug bioavailability by encapsulating hydrophobic drugs,17 while inorganic NPs offer stability and targeted delivery.18 Diverse approaches can be implemented in the production of NPs, including green synthesis and conventional chemical synthesis.19 Selenium (Se) is an essential micronutrient that is involved in the proper functioning of all organisms. It is a cofactor of many enzymes including glutathione peroxidase and thioredoxin reductase. Selenoproteins, such as selenocysteine and selenomethionine, are crucial forms naturally present in prokaryotes and eukaryotes. Se is an effective radical scavenging agent against oxidative stress.20 Deficiency of Se can lead to chronic diseases such as diabetes, cardiovascular disease, obesity, respiratory diseases, neurodegenerative diseases, and cancer.21 To overcome this, inorganic forms of Se (selenite and selenate) are being used as dietary supplementation.20 Selenium nanoparticles (Se-NPs) are known to have high bioavailability and a low toxicity profile.22–24 They can reduce the accumulation of free radicals and prevent oxidative stress.25 One of the major contributors to free radicals is an increase in NO and malondialdehyde (MDA) levels, which triggers LPO.26,27 Copper NPs trigger oxidative stress by elevating LPO while disrupting antioxidant enzymes.28 Se-NPs significantly reduce MDA levels and restore antioxidant enzyme activity.29,30 Se-NPs potentially eliminated oxidative stress induced by streptozotocin by decreasing the LPO and NO levels in the pancreas.31 Therefore, nano-Se plays a crucial role as an antioxidant defence system.32 Moreover, Se-NPs can cross the blood–brain barrier showing their potential in modulating inflammation and brain-related diseases.33,34 Some of the emerging in vitro and in vivo studies using PC12 cell lines and rat models highlighted the therapeutic efficacy of Se-NPs in mitigating oxidative stress and neurodegenerative diseases such as AD and PD.35–38 Though Se-NPs have many therapeutic benefits, most were chemically synthesized using sodium selenite which may induce mild toxic effects.39–42
glutathione-disulfide ratios notably.15 Silver NPs efficiently inhibited lipid peroxidation (LPO) by scavenging ROS, showing their antioxidant effectiveness.16 Organic NPs, such as liposomes, enhance drug bioavailability by encapsulating hydrophobic drugs,17 while inorganic NPs offer stability and targeted delivery.18 Diverse approaches can be implemented in the production of NPs, including green synthesis and conventional chemical synthesis.19 Selenium (Se) is an essential micronutrient that is involved in the proper functioning of all organisms. It is a cofactor of many enzymes including glutathione peroxidase and thioredoxin reductase. Selenoproteins, such as selenocysteine and selenomethionine, are crucial forms naturally present in prokaryotes and eukaryotes. Se is an effective radical scavenging agent against oxidative stress.20 Deficiency of Se can lead to chronic diseases such as diabetes, cardiovascular disease, obesity, respiratory diseases, neurodegenerative diseases, and cancer.21 To overcome this, inorganic forms of Se (selenite and selenate) are being used as dietary supplementation.20 Selenium nanoparticles (Se-NPs) are known to have high bioavailability and a low toxicity profile.22–24 They can reduce the accumulation of free radicals and prevent oxidative stress.25 One of the major contributors to free radicals is an increase in NO and malondialdehyde (MDA) levels, which triggers LPO.26,27 Copper NPs trigger oxidative stress by elevating LPO while disrupting antioxidant enzymes.28 Se-NPs significantly reduce MDA levels and restore antioxidant enzyme activity.29,30 Se-NPs potentially eliminated oxidative stress induced by streptozotocin by decreasing the LPO and NO levels in the pancreas.31 Therefore, nano-Se plays a crucial role as an antioxidant defence system.32 Moreover, Se-NPs can cross the blood–brain barrier showing their potential in modulating inflammation and brain-related diseases.33,34 Some of the emerging in vitro and in vivo studies using PC12 cell lines and rat models highlighted the therapeutic efficacy of Se-NPs in mitigating oxidative stress and neurodegenerative diseases such as AD and PD.35–38 Though Se-NPs have many therapeutic benefits, most were chemically synthesized using sodium selenite which may induce mild toxic effects.39–42
This study hypothesizes that Se-NPs could mitigate copper-induced neurotoxicity through multiple mechanisms, including reducing oxidative stress, restoring mitochondrial function, and reactivating antioxidant enzyme systems. Copper-induced toxicity is known to generate excessive ROS, leading to LPO, mitochondrial dysfunction, and inhibition of key enzymes such as SOD, CAT, GSH, GPx, and AChE. By counteracting these effects, using Se-NPs holds promise as a therapeutic strategy for addressing oxidative stress-related neurodegenerative conditions. In this study, we synthesized Se-NPs using selenium obtained from mussels. Mussels are known for their unique ability to bioaccumulate selenium, a micronutrient with potential antioxidant and health benefits. We employed various reducing agents, namely D-glucose and orange with BSA as a stabilizer. These reducing agents were applied at two different time intervals to assess their efficiency in reducing the size and preventing aggregation of the nanoparticles. The synthesized Se-NPs were characterized and subsequently tested for their antioxidant and neuroprotective properties against copper sulfate (CuSO4)-induced stress in vivo in zebrafish larvae. The study aims to evaluate the potential of Se-NPs in restoring enzymatic activity and neurotransmitter function disrupted by copper toxicity while highlighting their broader applications in mitigating oxidative stress-induced damage.
Results and discussion
Synthesis and characterization of mussel extracted Se
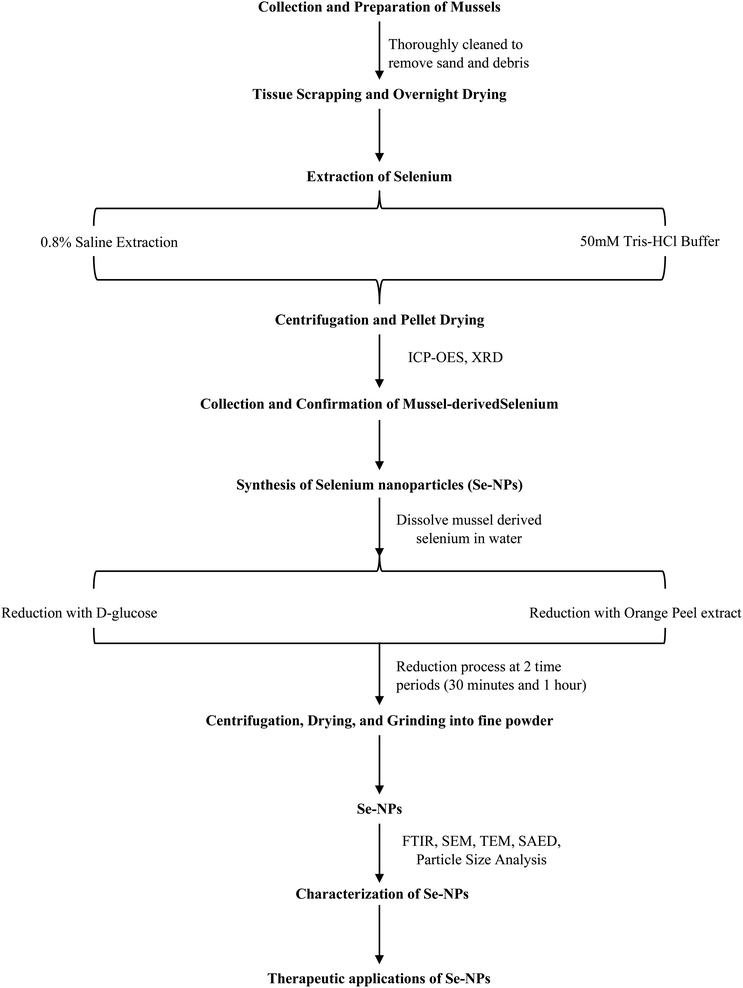
Different extraction methods such as 0.8% saline and Tris–HCl buffer (pH-7.4) extraction methods were utilized to determine the most suitable method for extracting Se from mussels. The results from ICP-OES showed a sharp peak at 196.03 nm with a concentration of 0.93 mg L−1 for the saline extraction method and 0.51 mg L−1 for the buffer extraction method (Fig. 1a and b). Similarly, a study conducted by Tyburska et al. also reported a peak at 196.03 for selenium.43 For Se extracted using the saline method, the XRD data revealed peaks at 26.51°, 31.58°, 45.33°, 56.31°, 66.13°, and 75.16° angles corresponding to the planes (100), (110), (111), (112), (210), and (113), respectively. The nature was observed to be 85% crystalline and 15% amorphous. Conversely, Se extracted using the buffer method showed peaks at 22.16° and 26.77°, corresponding to the planes (100) and (110), respectively (Fig. 1c and d). The nature was observed to be 70% crystalline and 30% amorphous. A study conducted in 2019, by Hassanien et al., focused on dye degradation and also observed XRD peaks aligning with the corresponding planes for Se-NPs confirming the presence of Se.44Synthesis and characterization of mussel extracted Se-NPs
Se was reduced using D-glucose and orange peel extract to synthesize Se-NPs. The reduction process was carried out at two different time periods (30 minutes and 1 hour) to determine the efficiency of reducing agents and they were stabilized using BSA to prevent aggregation. A study by El Badawy et al. reported that stabilization kinetics impact the aggregation pattern of nanoparticles.45 SEM imaging at 5 × 10−3 magnification was done to determine the morphology of Se-NPs. The SEM results showed that the nanoparticles revealed a near-spherical shape for both non-stabilized and stabilized Se-NPs. Compared to non-stabilized Se-NPs, stabilized nanoparticles exhibited less aggregation. The mean area and length of the nanoparticles were calculated using ImageJ software, which was found to be 20 ± 3 and 10 ± 3 nm for stabilized Se-NPs reduced with D-glucose for 30 minutes and 1 hour (Fig. 1e and f). In contrast, the size of non-stabilized Se-NPs reduced with orange for 30 minutes and 1 hour was observed to be 33 ± 6 and 29 ± 7 nm (ESI Fig. 1a and b†). In 2016, a study by Nie et al. on the synthesis of highly uniform Se-NPs using glucose as a reductant reported that the nanoparticles were spherical and were about 240 nm in size.46 Glucose-reduced Se-NPs were spherical with a size ranging from 280–295 nm.46 Se-NPs reduced with orange were reported to be spherical with a size range of 16–95 nm.47 Similarly, the size of stabilized Se-NPs reduced with orange peel extract for 30 minutes and 1 hour was observed to be 19 ± 4 and 18 ± 7 nm (Fig. 1g and h), respectively, while the sizes of non-stabilized Se-NPs reduced with orange peel extract for 30 minutes and 1 hour were found to be 29 ± 8 and 26 ± 9 nm (ESI Fig. 1c and d†), respectively. A study on the green synthesis of Se-NPs using orange peel by Salem et al. reported that the nanoparticles were spherical and the size ranged between 16 and 95 nm.47 Se-NPs synthesized from mussel-extracted Se were found to be similar in shape and were reduced better with D-glucose. Based on the size reduction and aggregate formation it was assumed that the stabilized reduction process produced better nanoparticles which can be an advantage to pass through the blood–brain barrier. TEM imaging confirms the near-spherical shape. The mean area of Se-NPs reduced with D-glucose was 23.95 ± 6.18 nm (Fig. 1i) and Se-NPs reduced with orange was 53.50 ± 21.96 nm (Fig. 1k). A study by Van der Horst et al. on electrochemical sensor applications using bismuth-silver nanoparticles showed that the TEM size of silver nanoparticles ranged between 10 and 20 nm with spherical shapes, accompanied by some aggregates.48 The SAED pattern showed concentric diffraction rings interspersed with discrete spots, which are characteristic of a polycrystalline material with localized crystalline domains. This indicates that the sample contains multiple crystallites with varying orientations, contributing to ring formation (Fig. 1j and l). Particle size analysis showed that Se-NPs reduced with D-glucose and orange were monodispersed with a size of 310.1 ± 73.2 nm and 378 ± 102.1 nm at refractive index 1.59 (ESI Fig. 1e†). Zeta potential was measured at −1.2 mV and 0.4 mV, indicating that the particles have a very low surface charge or are nearly neutral and tend to aggregate. The sharp peak implies a uniform distribution of zeta potential values (ESI Fig. 1f and g†). Similar patterns were observed in a study by Bhattacharyya et al. on the one-pot fabrication of silver nanoparticles.49The total yield of Se was observed to be approximately 10% higher in the 0.8% saline extraction method, compared to the buffer extraction method (Fig. 1m). This percentage was calculated based on the dry weight of the extracted selenium in milligrams. The chemical attributes of the stabilized Se-NPs were determined using FTIR. The smooth and sharp peaks observed were at 3276.55–3278.35 cm−1 (medium, sharp C–H stretching alkene), 2920.69–2850.78 cm−1 (medium, sharp C–H stretching alkane), 1626.11–1626.07 cm−1 (medium, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching di-substituted alkene), 1531.68–1530.69 cm−1 (strong, N–O stretching nitro-compound), 1404.61–1402.19 cm−1 (strong, S
C stretching di-substituted alkene), 1531.68–1530.69 cm−1 (strong, N–O stretching nitro-compound), 1404.61–1402.19 cm−1 (strong, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching sulfonyl chloride), 1228.67–1228.37 cm−1 (strong, C–O stretching alkyl aryl ether), 1038–1035.12 cm−1 (strong, S
O stretching sulfonyl chloride), 1228.67–1228.37 cm−1 (strong, C–O stretching alkyl aryl ether), 1038–1035.12 cm−1 (strong, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching sulfoxide), and 518.39–404.31 cm−1 (strong, metal–ligand stretching) for stabilized Se-NPs reduced for 30 minutes and 1 hour with D-glucose (Fig. 1n). Stabilized Se-NPs reduced for 30 minutes and 1 hour with orange showed peaks at 3278.91–3273.23 cm−1 (medium, sharp C–H stretching alkene), 2924.34–2851.30 cm−1 (medium, sharp C–H stretching alkane), 1627.70–1627.66 cm−1 (medium, C
O stretching sulfoxide), and 518.39–404.31 cm−1 (strong, metal–ligand stretching) for stabilized Se-NPs reduced for 30 minutes and 1 hour with D-glucose (Fig. 1n). Stabilized Se-NPs reduced for 30 minutes and 1 hour with orange showed peaks at 3278.91–3273.23 cm−1 (medium, sharp C–H stretching alkene), 2924.34–2851.30 cm−1 (medium, sharp C–H stretching alkane), 1627.70–1627.66 cm−1 (medium, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching di-substituted alkene), 1532.01–1515.51 cm−1 (strong, N–O stretching nitro-compound), 1404.68–1392.67 cm−1 (strong, S
C stretching di-substituted alkene), 1532.01–1515.51 cm−1 (strong, N–O stretching nitro-compound), 1404.68–1392.67 cm−1 (strong, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching sulfonyl chloride), 1236.15–1228.28 cm−1 (strong, C–O stretching alkyl aryl ether), 1033.83–1031.98 cm−1 (strong, S
O stretching sulfonyl chloride), 1236.15–1228.28 cm−1 (strong, C–O stretching alkyl aryl ether), 1033.83–1031.98 cm−1 (strong, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching sulfoxide), and 472.40–410.13 cm−1 (strong, metal–ligand stretching) (Fig. 1o). The obtained peaks indicated the presence of the metal, stabilizer, and the reducing agents used (ESI Table 1†). The time of reduction and variation in the reducing agent did not significantly affect the position of functional groups in the nano-Se. Similar peaks were observed in the study reported by Alagesan & Venugopal for green-synthesized Se-NPs.50
O stretching sulfoxide), and 472.40–410.13 cm−1 (strong, metal–ligand stretching) (Fig. 1o). The obtained peaks indicated the presence of the metal, stabilizer, and the reducing agents used (ESI Table 1†). The time of reduction and variation in the reducing agent did not significantly affect the position of functional groups in the nano-Se. Similar peaks were observed in the study reported by Alagesan & Venugopal for green-synthesized Se-NPs.50
Developmental toxicity assessment
The developmental toxicity assessment of Se-NPs (5–25 μg ml−1) was conducted using zebrafish embryos. The concentration was selected based on a previously reported study that obtained a similar model and showed regulated biological effects without exacerbating the system.51 In the control group, no abnormal morphological changes were observed. Groups treated with stabilized Se-NPs reduced with D-glucose for 30 minutes and 1 hour (5–25 μg ml−1) for 0–72 hpf showed no malformation, compared to the stress-exposed group which showed malformations such as pericardial edema (PSE) and yolk sac edema (YSE) (Fig. 2a and b). On the other hand, groups treated with stabilized Se-NPs (5–20 μg ml−1) reduced with orange for 30 minutes and 1 hour showed no malformations, while 25 μg ml−1 showed malformations such as bent spine (BS) and YSE (Fig. 2c and d). Compared to stabilized Se-NPs reduced with orange for 30 minutes and 1 hour, stabilized nano-Se reduced with D-glucose for 30 minutes and 1 hour showed a higher survival rate at 96 hpf, reducing CuSO4-induced stress (Fig. 2a1–d1). Bulk selenium showed a lower survival rate compared to nano-selenium (ESI Fig. 2†). A previous study on green synthesized Se-NPs and their toxicity profile by Kalishwaralal et al. reported that at 15–25 μg ml−1, malformations such as tail malformation and PSE were observed.51 Exposure to hydrogen peroxide (5 mM) until 120 hours post-fertilization induced stress, significantly reducing the survival rate of zebrafish embryos and larvae.52 Furthermore, zebrafish embryos and larvae treated with BSA-synthesized Se-NPs at concentrations of 20–25 μg ml−1 showed mortality.51 Therefore, mussel-extracted Se-NPs reduced with glucose can be a potential alternative source with lower toxicities.In vitro antioxidant analysis of stabilized Se-NPs
In vivo enzymatic assay of stabilized Se-NPs
The group exposed to CuSO4 showed a significant reduction in GSH activity (4.67 nmol mg−1 protein) compared to the control group (7.89 nmol mg−1 protein). However, treatment with 25 μg ml−1 of stabilized nano-Se reduced with D-glucose for 30 minutes and 1 hour significantly increased GSH activity in CuSO4-exposed zebrafish larvae. The GSH levels increased to 7.02 and 6.49 nmol mg−1 of protein, respectively (Fig. 4i and j). A similar effect was observed when using stabilized Se-NPs reduced with orange peel extract at a concentration of 15 μg ml−1 for 30 minutes and 1 hour. However, the GSH activity only increased to 6.17 and 5.72 nmol mg−1 of protein (ESI Fig. 3i and j†). Therefore, stabilized nano-Se has potential antioxidant properties, which can reduce oxidative stress and protect cellular homeostasis. El-Borady et al. reported that Se-NPs increased GSH levels by decreasing ROS levels.60
Expression of antioxidant genes
Deficiency of selenium, a key component of antioxidant enzymes, modulates selenoproteins in turn triggering ROS production, leading to disruption of cellular homeostasis.62,63 An antioxidant gene expression study was conducted in zebrafish larvae exposed to CuSO4 and treated with stabilized Se-NPs reduced for 30 minutes and 1 hour with D-glucose and orange peel extract. Compared to the control group, the CuSO4-induced stress group showed significant downregulation of SOD (0.2-fold), CAT (0.1-fold), glutathione-disulfide reductase (GSR) (0.1-fold), and glutathione peroxidase (GPX) (0.4-fold) expression. In contrast, treatment with stabilized Se-NPs reduced for 30 minutes and 1 hour with D-glucose and stabilized Se-NPs reduced for 30 minutes and 1 hour with orange peel extract significantly (p < 0.05) upregulated the SOD (2.2-fold), CAT (1.7-fold), GSR (2.1-fold), and GPX (2.5-fold) expression, confirming its potential in influencing the expression of antioxidant genes by mitigating ROS (Fig. 4m). This protective effect may likely be mediated through the nuclear factor erythroid 2-related factor 2 (NRF-2) pathway.64,65 NRF-2 is a transcription factor that plays a pivotal role in cellular defense against oxidative stress. Under oxidative stress conditions, NRF-2 dissociates from Keap1 and binds to antioxidant response elements.66 Real-time PCR studies reported by Handa et al. showed that upon utilization of selenium, anti-oxidative enzymes encoding gene expression were elevated.67Localization of cellular ROS
To evaluate the intracellular ROS level, DCFDA fluorescent staining was performed on zebrafish larvae (96 hours post fertilization; 96 hpf). The control group showed a mean fluorescence intensity (MFI) of 8.9. Zebrafish larvae exposed to CuSO4 had ROS levels of 61.75 MFI. While overall cellular ROS was detected in the larvae, maximum localization was found in the gut and liver regions. Treatment with stabilized Se-NPs significantly (p < 0.05) reduced cellular ROS levels in CuSO4-induced zebrafish larvae, compared to the untreated stress group (Fig. 5a–d). The 25 μg ml−1 stabilized Se-NPs treated with D-glucose for 30 minutes and 1 hour showed lower ROS levels at concentrations of 10.1 and 12.2 MFI (Fig. 5a1 and b1). In contrast, stabilized Se-NPs reduced with orange for 30 minutes and 1 hour showed lower ROS levels at concentrations of 15 μg ml−1 with 17.67 and 18.27 MFI (Fig. 5c1 and d1). Therefore, stabilized Se-NPs treated with D-glucose for 30 minutes could be a potential antioxidant therapeutic for ROS-mediated neurodegeneration. Raju et al. conducted a similar protocol to assess the ROS levels in zebrafish larvae exposed to H2O2 stress, showing higher intensity in the stress group, compared to the treatment groups.68Determination of live cell lipid peroxidation
To determine the LPO levels in zebrafish larvae (96 hpf), DPPP fluorescent staining was conducted. The control group exhibited lower LPO levels at 10.67 MFI. Zebrafish larvae exposed to CuSO4 showed increased LPO levels of 24.68 MFI. Treatment with stabilized Se-NPs significantly (p < 0.05) reduced LPO levels in CuSO4-induced stress groups compared to the untreated stress group (Fig. 5e–h). However, treatment with stabilized Se-NPs reduced with D-glucose for 30 minutes and 1 hour showed significantly lower intensity at a concentration of 25 μg ml−1 with 11.35 and 13.17 MFI (Fig. 5e1 and f1). In contrast, stabilized Se-NPs reduced with orange for 30 minutes and 1 hour showed lower intensity at a concentration of 15 μg ml−1 with 15.78 and 16.37 MFI (Fig. 5g1 and hl). Raju et al. performed a similar protocol to assess the LPO levels in zebrafish larvae exposed to H2O2 stress, showing similar upregulation in intensity in the stress group, compared to the treatment groups.68Estimation of AChE activity
Compared to the control group, zebrafish larvae exposed to CuSO4 showed a significant decrease in AChE levels dropping from 1.14 μmol ml−1 to 0.76 μmol ml−1. Treatment with 25 μg ml−1 stabilized Se-NPs reduced with D-glucose for 30 minutes and 1 hour, in CuSO4-exposed zebrafish larvae, resulted in a dose-dependent increase in AChE levels, reaching 1.04 and 0.97 μmol ml−1 (Fig. 6a and b), respectively. Conversely, treatment with stabilized Se-NPs reduced with orange peel extract for 30 minutes and 1 hour only restored the AChE concentration up to 0.95 and 0.90 μmol ml−1 respectively (Fig. 6c and d). This suggests that stabilized Se-NPs reduced with D-glucose for 30 minutes significantly restored the AChE concentration. A study focusing on pentylenetetrazole-induced oxidative stress demonstrated that pentylenetetrazole-exposed mice treated with Se-NPs showed improved AChE levels compared to untreated mice.69 Therefore, stabilized Se-NPs regulated CuSO4-induced neuron damage and protected the central nervous system (CNS).Estimation of locomotor activity
To measure the locomotor activity of zebrafish larvae and determine cognitive alterations in the CNS, neurobehavior will be assessed based on the distance travelled by the zebrafish larvae (in meters). Treatment with 25 μg ml−1 of stabilized Se-NPs, reduced with glucose for 30 minutes and 1 hour, improved cognitive behaviour by restoring the covered distance to 93.40 m and 78.79 m in the CuSO4 exposed zebrafish larva group (Fig. 6e–n). Conversely, treatment with stabilized Se-NPs reduced with orange for 30 minutes and 1 hour at a concentration of 15 μg ml−1 only improved cognitive behaviour by 46.48 m and 44.16 m (Fig. 6o–x). The control group exhibited normal cognitive behaviour with an estimated travelled distance of 102.60 m (Fig. 6y). Following CuSO4 exposure, the zebrafish larvae could only travel 16.2 m due to stress effects (Fig. 6z), which was restored by approximately 91% with D-glucose reduced nano-Se. It is already established that zinc oxide nanoparticles increase oxidative stress and impair cognitive function by decreasing the expression of cyclic adenosine monophosphate response element binding protein (CREB), phosphorylated CREB, and synapsin I age-dependently.70 At the neuromuscular junction, acetylcholine is primarily released by the motor neuron, acting as a neurotransmitter and regulating fast synaptic currents essential for precise locomotion control.71 Li et al. reported that alterations in the central nervous system can be determined by the behavioral changes in animals,72 indicating that stabilized Se-NPs did not affect the central nervous system. D-glucose reduced nano-Se restores the AChE concentration which is directly associated with the formation and release of acetylcholine through synaptic vesicles and controls cognitive properties without altering behavior.Conclusion
In the ETC, mitochondria are a significant source of ROS. Increased ROS production can occur due to impaired mitochondrial function disrupting cellular homeostasis by reducing antioxidant defences and increasing lipid peroxidation. This can lead to various illnesses, including neurodegeneration. Enzymes such as SOD, CAT, and GSH act as defense mechanisms to neutralize ROS. One of the essential proteins involved in the production of glutathione is selenoproteins. Deficiency of these selenoproteins also plays a role in decreasing antioxidant enzymes. However, there are several commercial drugs available, such as coenzyme Q10, N-acetylcysteine, edaravone, non-steroidal anti-inflammatory drugs, corticosteroids, memantine, riluzole, selegiline, levodopa, carbidopa, donepezil, etc., that can regulate oxidative stress and enhance neuroprotection. Many researchers have used nanoparticles as carriers to deliver drugs to the target site. These nanoparticles can be considered therapeutic due to their high precision, less toxic profile, and ability to cross the blood–brain barrier. Se-NPs have attracted significant interest as carriers for various applications, especially in medicine. Generally, they are biocompatible and do not exhibit toxicity compared to other forms of Se. The strong antioxidant properties of Se-NPs make them effective ROS scavengers that protect cells from oxidative damage.In our study, mussel-extracted Se was used as a rich source of Se for the synthesis of Se-NPs. Saline extraction yielded a higher amount compared to the buffer extraction method. FTIR peaks indicated the presence of the metal, stabilizer, and reducing agents used. The size of the nanoparticles depended on the reducing agent. D-Glucose efficiently produced nearly spherical nano-Se of 20 nm size with potential therapeutic approaches. A comparison between reducing agents showed that a 30-minute reduction with D-glucose produced potent nano-Se in reducing oxidative stress and improving survival rates in CuSO4 stress-induced zebrafish larvae. In the stress group, in vivo treatment with 5–25 μg ml−1 of stabilized Se-NPs reduced with D-glucose showed no toxic effects and survival rates were improved in a concentration-dependent manner. On the other hand, orange-reduced stabilized Se-NPs were found to be non-toxic only up to 15 μg ml−1. Moreover, YSE was found to be developed above those concentrations in stress-induced zebrafish larvae in vivo. All stabilized Se-NPs showed concentration-dependent DPPH scavenging activity and concentration-independent ABTS scavenging activity. However, the highest scavenging capacity was recorded with nano-Se reduced with D-glucose for 30 minutes. In vivo enzymatic analysis of stabilized Se-NPs reduced with D-glucose for 30 minutes showed a remarkable improvement in antioxidant enzyme levels while decreasing LPO and NO levels in zebrafish larvae. They restored the enzyme levels by producing SOD, CAT, GSH, and GST, which may be an NRF2-dependent mechanism, and significantly reduced the MDA and NO levels in a concentration-dependent manner. The qPCR confirmed that nearly a 2-fold enhancement of antioxidant genes was mitigating cellular ROS. Live cell imaging also supported the therapeutic properties of D-glucose-reduced nano-Se. Additionally, D-glucose reduced nano-Se enhanced AChE levels in a dose-dependent manner. Locomotor analysis confirmed that D-glucose reduced, and stabilized Se-NPs did not disrupt CNS function and improved cognitive functions in stress-induced zebrafish larvae. Thus, treatment with 25 μg ml−1 of D-glucose reduced nano-Se, upregulated the antioxidant gene expression, and downregulated LPO and NO levels with an enhancement of AChE production which directly influences the release of acetylcholine from synaptic vesicles and regulates cognitive function without altering the behavior of the larvae. Therefore, D-glucose-reduced Se-NPs could be a potential therapeutic option to reduce oxidative stress and improve cognitive function in oxidative stress-related diseases and disorders. This characteristic makes them safer for therapeutic applications.
Future advancements in combined therapy could involve the integration of Se-NPs with other therapeutic agents to enhance their efficacy. Combining Se-NPs with traditional antioxidants, anti-inflammatory drugs, or targeted delivery systems may synergize their effects and provide a more effective approach to regulate oxidative stress and stress-related diseases. Further research is necessary on Se-NPs to make significant advancements in the management and treatment of various debilitating conditions, as they have the ability to enhance endogenous antioxidant enzymes and cross the blood–brain barrier.
Materials and methods
Chemicals used
Sodium chloride (NaCl) was purchased from Merck (CAS no: 7647-14-5). Tris(hydroxymethyl) aminomethane was purchased from Thermo Fisher Scientific (CAS no: 77-86-1). L-Ascorbic acid (CAS No: 50-81-7). Hydrogen peroxide (H2O2; CAS: 7722-84-1) and hydrochloric acid (CAS no: 7647-01-0) were purchased from Sigma-Aldrich. Magnesium chloride (CAS no: 7786-30-3). Calcium chloride (CAS no: 10043-52-4), sodium phosphate dibasic (CAS no: 7558-79-4), potassium phosphate monobasic (CAS no: 7778-77-0), potassium chloride (CAS no: 7447-40-7), nitroblue tetrazolium salt (NBT; CAS no: 298-83-9), L-methionine (CAS no: 63-68-3), riboflavin (CAS no: 83-88-5), thiobarbituric acid (TBA; CAS no: 504-17-6), trichloroacetic acid (TCA; Cc: 76-03-9), Griess reagent (EC: 215-981-2), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB; CAS no: 69-78-3), potassium phosphate dibasic (CAS no: 7758-11-4), and 1-chloro 2,4- dinitrobenzene (CAS no: 97-00-7) were purchased from Sigma-Aldrich. BSA was purchased from Sisco Research Laboratories (SRL; CAS no: 9048-46-8). 2,2-Diphenyl-1-picrylhydrazyl (DPPH; CAS no: 1898-66-4), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS salt; CAS No: 30931-67-0), dichlorofluorescein diacetate (DCFHDA; CAS no: 2044-85-1), 2,2-diphenyl-1-picrylhydrazyl (DPPH; CAS no: 1898-66-4), potassium persulfate (CAS no: 7727-21-1), and ethylenediaminetetraacetic acid (EDTA; CAS no: 60-00-4) were purchased from Sisco Research Laboratories (SRL). RDP Trio™ Reagent was collected from HiMedia (SKU: MB566) and AURA 2x One-Step RT-PCR Master Mix was collected from AURA Biotechnologies Pvt Ltd (ABT-18S). Glassware was purchased from Borosil® and 96-well ELISA plates were purchased from Thermo Fisher Scientific®.Extraction of selenium from mussels
Extraction with 0.8% saline. A 0.8% saline solution was freshly prepared by dissolving 8 g of sodium chloride (NaCl) in 1000 ml of distilled water. Next, 8.5 g of mussel tissue was weighed and ground with 100 ml of the 0.8% saline solution using a mortar and pestle. The resulting mixture was centrifuged at 5000 rpm for 30 minutes at room temperature (37 °C). This centrifugation step was repeated until no pellet was observed. The collected pellet was dried at 60 °C in a hot air oven. Once completely dry, the pellet was ground into a fine powder using a mortar and pestle.73
Extraction with 50 mM Tris–HCl buffer pH 7.4. A Tris–HCl buffer (pH 7.4) was freshly prepared by dissolving 2.65 g of Tris base and 4.44 g of HCl in 1000 ml of distilled water. Next, 8.5 g of mussel tissue was weighed and ground with 100 ml of the Tris–HCl buffer using a mortar and pestle. Following the method previously described for selenium purification, fine Se powder was obtained.73
Synthesis of Se-NPs
A green synthesis approach was used to produce Se-NPs by reducing mussel-derived Se with D-glucose and crude orange peel extract (schematically represented in Chart 1). Fresh oranges were purchased from local markets and washed, and their peels were collected. The collected orange peels were blended with 100 g in 500 ml of water using a mixer, filtered, and stored at 4 °C as needed. 0.2 g of mussel-derived selenium was dissolved in 50 ml of distilled water using a sonicator. In a round bottom flask, 50 ml of the dissolved mussel-derived Se was combined with 15 ml of 0.25 M D-glucose and 15 ml of crude orange peel extract separately. Both reduction processes were carried out at two different intervals, 30 minutes and 1 hour, with and without a stabilizer (5% BSA), using a heating mantle. The resulting mixture was centrifuged at 5000 rpm for 30 minutes at 37 °C (room temperature). The collected pellets were then dried at 60 °C in a hot air oven. Once completely dried, the pellets were ground into a fine powder using a mortar and pestle.47,74 These synthesized Se-NPs were used for further experimental investigations.Characterization of mussel extracted Se and Se-NPs
The Se-NPs underwent a comprehensive analysis utilizing various analytical techniques. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) was conducted using a PerkinElmer OPTIMA 5300 DV ICP-OES to determine trace element concentrations.75 X-ray diffraction (XRD) and crystallography studies were carried out using a BRUKER D8 ADVANCE POWDER XRD. The measurements were conducted over a 2θ range spanning from 10 to 80°.76 Fourier-transform infrared (FTIR) spectroscopy was used to examine the presence of functional groups. This analysis was performed using a Nicolet Summit FTIR instrument (Thermo Fisher Scientific) operating in diffuse reflectance mode. The detectors used were DTGS and KBr, and 16 scans were recorded over a wavenumber range from 400 to 4000 cm−1.76 The resulting spectra were plotted with the wavenumber (cm−1) on the X-axis and transmittance (%) on the Y-axis using OriginPro 8.5 software. Scanning Electron Microscope (SEM) analysis was conducted using a Hitachi Model: S-3400N to elucidate the surface morphology of the Se-NPs. The SEM was operated at 15 kV in high vacuum (HV) mode with semiconductor secondary electron (SE) detection. The acquired images were further analyzed for size distribution using ImageJ software.76 TEM and SAED (FEI Tecnai G2 20 S-TWIN TEM) were performed at 200 kV. Particle size analysis and zeta potential measurement were done using a nanoPartica SZ-100V2 (Horiba).76In vitro antioxidant studies
To evaluate the in vitro antioxidant potential of stabilized Se-NPs, DPPH and ABTS assays were conducted following methods outlined in previous studies.77,78 Stock solutions containing 50 μM ascorbic acid and 1 mg ml−1 of Se-NPs were prepared from which working solutions of 5, 10, 15, 20 and 25 μg ml−1 were derived. These concentrations were fixed based on previously reported studies79 and were consistently utilized throughout the study. Three individual experiments were performed.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was incubated for 24 hours in the dark at room temperature. To achieve an absorbance of 0.7 ± 2 at 734 nm, 20× PBS was used to dilute the ABTS solution. 50 μM ascorbic acid was used as a positive control along with stabilized Se-NPs (5, 10, 15, 20 and 25 μg ml−1). These were added to a 96-well ELISA plate along with the ABTS solution. The plate was then incubated at room temperature in the dark for an hour. The samples were measured at 734 nm using a Thermo Fisher Scientific® microplate reader.
1) was incubated for 24 hours in the dark at room temperature. To achieve an absorbance of 0.7 ± 2 at 734 nm, 20× PBS was used to dilute the ABTS solution. 50 μM ascorbic acid was used as a positive control along with stabilized Se-NPs (5, 10, 15, 20 and 25 μg ml−1). These were added to a 96-well ELISA plate along with the ABTS solution. The plate was then incubated at room temperature in the dark for an hour. The samples were measured at 734 nm using a Thermo Fisher Scientific® microplate reader.
In vivo developmental toxicity studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (male
1 (male![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) female). To prevent the female fish from swallowing the eggs, a mesh was placed at the bottom of the spawning tank. Embryos were collected from the breeding unit 30 minutes after the onset of light. The embryos were rinsed with freshly prepared E3 medium80 and kept at 26 ± 1 °C (OECD, 2013) until the experiments were conducted.
female). To prevent the female fish from swallowing the eggs, a mesh was placed at the bottom of the spawning tank. Embryos were collected from the breeding unit 30 minutes after the onset of light. The embryos were rinsed with freshly prepared E3 medium80 and kept at 26 ± 1 °C (OECD, 2013) until the experiments were conducted.
In vivo antioxidant studies
For enzymatic assays, the CuSO4-exposed larvae treated with stabilized Se-NPs (5–25 μg ml−1) were homogenized (n = 20, all experiments were conducted in triplicate) in a solution containing 100 mM Tris–HCl buffer (pH 7.8 at 4 °C) with 150 mM potassium chloride and 1 mM EDTA, at 96 hpf. The homogenized sample was centrifuged at 10000 rpm for 15 minutes and the supernatant was used for further enzymatic analysis.85 The Bradford method was used for protein estimation.86Antioxidant gene expression by real-time polymerase chain reaction (RT-PCR)
RNA was extracted from the experimentally homogenized zebrafish larvae using RDP Trio™ Reagent. The primers for antioxidant enzymes and housekeeping genes were designed using NCBI's Primer-BLAST (Table 1). Expression of the genes was analyzed using AURA 2x One-Step RT-PCR Master Mix. The reverse transcription process starts with a single cycle at a temperature ranging between 44 and 50 °C lasting for 15 minutes, followed by enzyme activation, at 95 °C for 3 minutes. The denaturation step (repeated for 40 cycles) was at 95 °C for 10 seconds. Annealing was done at 60 °C for 45 seconds, and the extension process was performed at 72 °C for 15 seconds. The fold change was calculated using the 2−ΔΔct method.77| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Reference |
|---|---|---|---|
| SOD | GGTCCGCACTTCAACCCTCA | TACCCAGGTCTCCGACGTGT | NCBI: NM_131294.1 |
| CAT | AACTGTGGAAGGAGGGTCGC | CGCTCTCGGTCAAAATGGGC | NCBI: XM_021470442.1 |
| GSR | GATGGGCACCATAGCTAACCC | CATGAGCAGGAAGCAACACCC | NCBI: XM_005169592.4 |
| GPx | AACTACACTCAGCTTGCGGC | TCCGCTTCACTTCCAGGCTC | NCBI: NM_001030070.2 |
| β-actin | AAGCTGTGACCCACCTCACG | GGCTTTGCACATACCGGAGC | NCBI: NM_131031.2 |
Estimation of acetylcholinesterase (AChE)
The larvae were analyzed for cognitive impairments after exposure to CuSO4 and treatment with stabilized Se-NPs. The homogenized larvae were centrifuged at 5000 rpm for 15 minutes. The supernatant was mixed with an action mixture containing 3.3 mM DTNB and incubated for 20 minutes. The absorbance was recorded at 412 nm in 1-minute intervals following the acetylcholine iodide addition.72,77Cognitive behavior analysis
The locomotor abnormalities of the zebrafish larvae were evaluated by analyzing the swimming behavior pattern.90 At the end of the exposure period (7 dpf) each exposure group (n = 3 larvae/well; experiments were conducted in triplicate) was placed in a white chambered ice tray (2.5 × 3.5 cm) containing 2 ml of E3 medium prepared without methylene blue, for 10 minutes of acclimatization. The locomotion of larvae was recorded at the beginning of the light cycle under noise-free conditions using a commercial smartphone camera after acclimatization. The video was recorded for 60 seconds at 60 frames per second and locomotion was plotted using UMA Tracker software.91Estimation of ROS levels in zebrafish larvae
All groups including the control, stress group and stabilised Se-NP treated group larvae were anaesthetized (n = 6 per group) using tricaine. The larvae were stained with DCFDA (20 μg ml−1) dye and incubated for 1 hour in the dark at room temperature. After incubation, the stained larvae were observed pictographically under a fluorescence microscope (CKX53 Microscope, Japan) and analyzed using ImageJ software.92Estimation of LPO levels in zebrafish larvae
All groups including the control, stress group and stabilised Se-NP treated group larvae were anaesthetized (n = 6 per group) using tricaine. The larvae were stained with DPPP (25 μg ml−1) dye and incubated for 30 minutes at room temperature. After incubation, the stained larvae were observed pictographically under a fluorescence microscope (CKX53 Microscope, Japan) and analyzed using ImageJ software.92Statistical analysis
All experiments in this study were conducted in triplicate and are presented as mean ± standard deviation (SD). The data were analyzed using one-way analysis of variance (ANOVA) and Dunnett's multiple comparison test using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA).93 Significant results were denoted by the symbol “*” and were considered significant with p < 0.05.Ethical statement
All experiments were conducted in accordance with the ethical guidelines for the care and utilization of animals, as approved by the Institutional Ethical Committee under protocol number SU/CLAR/RD/001/2023. All experiments were performed in compliance with OECD guidelines. All animal procedures were performed in accordance with the guidelines for care and use of laboratory animals of the Saveetha Institute of Medical and Technical Sciences, Saveetha University, and approved by the Institutional Animal Ethics Committee of Saveetha Medical College. No human subjects were used throughout the study.Data availability
All data generated or analyzed during this study are included in this published article. Data for this article, including qPCR primers, are available at NCBI (NCBI: NM_131294.1; NCBI: XM_021470442.1; NCBI: XM_005169592.4; NCBI: NM_001030070.2; NCBI: NM_131031.2) at https://www.ncbi.nlm.nih.gov/; SOD: https://www.ncbi.nlm.nih.gov/nuccore/NM_131294.1; CAT: https://www.ncbi.nlm.nih.gov/nuccore/XM_021470442.1; GSR: https://www.ncbi.nlm.nih.gov/nuccore/XM_005169592.4; GPx: https://www.ncbi.nlm.nih.gov/nuccore/NM_001030070.2; Beta actin: https://www.ncbi.nlm.nih.gov/nuccore/NM_131031.2Author contributions
SU is responsible for conducting experiments and result analysis and obtaining figures and IP is responsible for conceptualization and result validation. Both the authors wrote the original draft and verified the final manuscript.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We express our gratitude to the Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences, Saveetha University, for providing the necessary infrastructure to carry out this work successfully.References
- L. D. Osellame, T. S. Blacker and M. R. Duchen, Best Pract. Res., Clin. Endocrinol. Metab., 2012, 26, 711–723 CrossRef CAS PubMed.
- L.-D. Popov, Cell. Signal., 2023, 109, 110794 CrossRef CAS PubMed.
- F. A. Bustamante-Barrientos, N. Luque-Campos, M. J. Araya, E. Lara-Barba, J. de Solminihac, C. Pradenas, L. Molina, Y. Herrera-Luna, Y. Utreras-Mendoza, R. Elizondo-Vega, A. M. Vega-Letter and P. Luz-Crawford, J. Transl. Med., 2023, 21, 613 CrossRef.
- D. M. Niyazov, S. G. Kahler and R. E. Frye, Mol. Syndromol., 2016, 7, 122–137 CrossRef CAS PubMed.
- R. N. L. Lamptey, B. Chaulagain, R. Trivedi, A. Gothwal, B. Layek and J. Singh, Int. J. Mol. Sci., 2022, 23, 1851 CrossRef CAS PubMed.
- W. Chen, H. Zhao and Y. Li, Signal Transduct. Targeted Ther., 2023, 8, 333 CrossRef PubMed.
- T. Vezza, P. Díaz-Pozo, F. Canet, A. M. de Marañón, Z. Abad-Jiménez, C. García-Gargallo, I. Roldan, E. Solá, C. Bañuls, S. López-Domènech, M. Rocha and V. M. Víctor, World J. Mens Health, 2022, 40, 399 CrossRef PubMed.
- S. K. Bardaweel, M. Gul, M. Alzweiri, A. Ishaqat, H. A. ALSalamat and R. M. Bashatwah, Eurasian J. Med., 2018, 50, 193–201 CrossRef CAS PubMed.
- G. Pizzino, N. Irrera, M. Cucinotta, G. Pallio, F. Mannino, V. Arcoraci, F. Squadrito, D. Altavilla and A. Bitto, Oxid. Med. Cell. Longev., 2017, 2017, 1–13 Search PubMed.
- Tsatsakis, Docea, Calina, Tsarouhas, Zamfira, Mitrut, Sharifi-Rad, Kovatsi, Siokas, Dardiotis, Drakoulis, Lazopoulos, Tsitsimpikou, Mitsias and Neagu, J. Clin. Med., 2019, 8, 1295 CrossRef CAS PubMed.
- K. H. Al-Gubory, C. Garrel, P. Faure and N. Sugino, Reprod. Biomed. Online, 2012, 25, 551–560 CrossRef CAS PubMed.
- M. Sharifi-Rad, N. V. Anil Kumar, P. Zucca, E. M. Varoni, L. Dini, E. Panzarini, J. Rajkovic, P. V. Tsouh Fokou, E. Azzini, I. Peluso, A. Prakash Mishra, M. Nigam, Y. El Rayess, M. El Beyrouthy, L. Polito, M. Iriti, N. Martins, M. Martorell, A. O. Docea, W. N. Setzer, D. Calina, W. C. Cho and J. Sharifi-Rad, Front. Physiol., 2020, 11, 694 CrossRef.
- S. E. Laouini, A. Bouafia, A. V. Soldatov, H. Algarni, M. L. Tedjani, G. A. M. Ali and A. Barhoum, Membranes, 2021, 11, 468 CrossRef CAS PubMed.
- C. E. Probst, P. Zrazhevskiy, V. Bagalkot and X. Gao, Adv. Drug Deliv. Rev., 2013, 65, 703–718 CrossRef CAS PubMed.
- Z. S. Bailey, E. Nilson, J. A. Bates, A. Oyalowo, K. S. Hockey, V. S. S. S. Sajja, C. Thorpe, H. Rogers, B. Dunn, A. S. Frey, M. J. Billings, C. A. Sholar, A. Hermundstad, C. Kumar, P. J. VandeVord and B. A. Rzigalinski, J. Neurotrauma, 2020, 37, 1452–1462 CrossRef PubMed.
- F. Karimi, N. Rezaei-savadkouhi, M. Uçar, A. Aygun, R. N. Elhouda Tiri, I. Meydan, E. Aghapour, H. Seckin, D. Berikten, T. Gur and F. Sen, Food Chem. Toxicol., 2022, 169, 113406 CrossRef CAS PubMed.
- R. Gil-Gonzalo, D. A. Durante-Salmerón, S. Pouri, E. Doncel-Pérez, A. R. Alcántara, I. Aranaz and N. Acosta, Pharmaceutics, 2024, 16(8), 1036 CrossRef CAS PubMed.
- K. R. Trabbic, K. A. Kleski and J. J. Barchi, ACS Bio Med Chem Au, 2021, 1, 31–43 CrossRef CAS PubMed.
- A. M. El Shafey, Green Process. Synth., 2020, 9, 304–339 Search PubMed.
- M. Kieliszek, Molecules, 2019, 24, 1298 CrossRef CAS PubMed.
- L. Di Renzo, P. Gualtieri, G. Frank and A. De Lorenzo, Nutrients, 2023, 15, 2253 CrossRef PubMed.
- F. Barzegarparay, H. Najafzadehvarzi, R. Pourbagher, H. Parsian, S. M. Ghoreishi and S. Mortazavi-Derazkola, Biomass Convers. Biorefin., 2024, 14, 25369–25378 CrossRef CAS.
- F. Chen, X. H. Zhang, X. D. Hu, P. D. Liu and H. Q. Zhang, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 937–948 CrossRef CAS PubMed.
- N. Bisht, P. Phalswal and P. K. Khanna, Adv. Mater., 2022, 3, 1415–1431 RSC.
- J. T. Tendenedzai, E. M. N. Chirwa and H. G. Brink, Sci. Rep., 2023, 13, 20379 CrossRef CAS PubMed.
- G. Rusip, S. Ilyas, I. N. E. Lister, C. N. Ginting and I. Mukti, F1000Research, 2022, 10, 1061 Search PubMed.
- O. A. Mahdy, S. Z. Abdel-Maogood, M. Abdelsalam and M. A. Salem, BMC Vet. Res., 2024, 20, 60 CrossRef CAS.
- N. Liu, L. Tong, K. Li, Q. Dong and J. Jing, Molecules, 2024, 29, 2414 CrossRef CAS PubMed.
- S. Salaramoli, H. Amiri, H. R. Joshaghani, M. Hosseini and S. I. Hashemy, Metab. Brain Dis., 2023, 38, 2055–2064 CrossRef CAS PubMed.
- N. Zakeri, M. R. Kelishadi, O. Asbaghi, F. Naeini, M. Afsharfar, E. Mirzadeh and S. kasra Naserizadeh, PharmaNutrition, 2021, 17, 100263 CrossRef CAS.
- O. M. El-Borady, M. S. Othman, H. H. Atallah and A. E. Abdel Moneim, Heliyon, 2020, 6, e04045 CrossRef PubMed.
- M. Iranifam, M. Fathinia, T. Sadeghi Rad, Y. Hanifehpour, A. R. Khataee and S. W. Joo, Talanta, 2013, 107, 263–269 CrossRef CAS PubMed.
- H. Amani, R. Habibey, F. Shokri, S. J. Hajmiresmail, O. Akhavan, A. Mashaghi and H. Pazoki-Toroudi, Sci. Rep., 2019, 9, 6044 CrossRef PubMed.
- T. Yin, L. Yang, Y. Liu, X. Zhou, J. Sun and J. Liu, Acta Biomater., 2015, 25, 172–183 CrossRef CAS PubMed.
- L. Qiao, Y. Chen, X. Song, X. Dou and C. Xu, Int. J. Nanomed., 2022, 17, 4807–4827 CrossRef CAS PubMed.
- Z. Li, H. Liang, Y. Wang, G. Zheng and L. Yang, ACS Appl. Nano Mater., 2024, 7, 20411–20424 CrossRef CAS.
- M. Zhu, Y. Zhang, C. Zhang, L. Chen and Y. Kuang, J. Biomater. Appl., 2023, 38, 109–121 CrossRef CAS PubMed.
- H. Zhao, J. Song, T. Wang and X. Fan, Nanomed.: Nanotechnol. Biol. Med., 2024, 59, 102755 CrossRef CAS PubMed.
- B. Bálint, K. Balogh, M. Mézes and B. Szabó, Eur. J. Soil Biol., 2021, 107, 103361 CrossRef.
- S. Shoeibi, P. Mozdziak and A. Golkar-Narenji, Top. Curr. Chem., 2017, 375, 88 CrossRef PubMed.
- T. M. Sakr, M. Korany and K. V. Katti, J. Drug Deliv. Sci. Technol., 2018, 46, 223–233 CrossRef CAS.
- S. Hariharan and S. Dharmaraj, Inflammopharmacology, 2020, 28, 667–695 CrossRef CAS PubMed.
- A. Tyburska, K. Jankowski, A. Ramsza, E. Reszke, M. Strzelec and A. Andrzejczuk, J. Anal. At. Spectrom., 2010, 25, 210–214 RSC.
- R. Hassanien, A. A. I. Abed-Elmageed and D. Z. Husein, ChemistrySelect, 2019, 4, 9018–9026 CrossRef CAS.
- A. M. El Badawy, K. G. Scheckel, M. Suidan and T. Tolaymat, Sci. Total Environ., 2012, 429, 325–331 CrossRef CAS PubMed.
- T. Nie, H. Wu, K.-H. Wong and T. Chen, J. Mater. Chem. B, 2016, 4, 2351–2358 RSC.
- S. S. Salem, M. S. E. M. Badawy, A. A. Al-Askar, A. A. Arishi, F. M. Elkady and A. H. Hashem, Life, 2022, 12, 893 CrossRef CAS PubMed.
- C. Van der Horst, B. Silwana, E. Iwuoha and V. Somerset, Anal. Lett., 2015, 48, 1311–1332 CrossRef CAS.
- A. Bhattacharyya, R. Prasad, A. A. Buhroo, P. Duraisamy, I. Yousuf, M. Umadevi, M. R. Bindhu, M. Govindarajan and A. L. Khanday, J. Nanosci., 2016, 2016, 1–7 CrossRef.
- V. Alagesan and S. Venugopal, Bionanoscience, 2019, 9, 105–116 CrossRef.
- K. Kalishwaralal, S. Jeyabharathi, K. Sundar and A. Muthukumaran, Artif. Cells, Nanomed., Biotechnol., 2016, 44, 471–477 CrossRef CAS PubMed.
- S.-H. Cha, J.-H. Lee, E.-A. Kim, C. H. Shin, H.-S. Jun and Y.-J. Jeon, RSC Adv., 2017, 7, 46164–46170 RSC.
- J. Vyas and S. Rana, Int. J. Curr. Pharm. Res., 2017, 9, 147 CrossRef CAS.
- X. Zhai, C. Zhang, G. Zhao, S. Stoll, F. Ren and X. Leng, NanoBiotechnology, 2017, 15, 4 CrossRef PubMed.
- S. Naz, R. Hussain, Z. Guangbin, A. M. M. Chatha, Z. U. Rehman, S. Jahan, M. Liaquat and A. Khan, Front. Vet. Sci., 2023, 10 Search PubMed.
- M. A. Khan, D. Singh, A. Arif, K. K. Sodhi, D. K. Singh, S. N. Islam, A. Ahmad, K. Akhtar and H. R. Siddique, Life Sci., 2022, 305, 120792 CrossRef CAS PubMed.
- M. D. L. Á. Sariñana-Navarrete, Á. Morelos-Moreno, E. Sánchez, G. Cadenas-Pliego, A. Benavides-Mendoza and P. Preciado-Rangel, Agronomy, 2023, 13, 652 CrossRef.
- M. Lesnichaya, E. Karpova and B. Sukhov, Colloids Surf., B, 2021, 197, 111381 CrossRef CAS PubMed.
- S. S. Anuse, V. Sumathi, C. Uma, D. Sangeetha, P. Sivagurunathan and D. J. M. Kumar, Uttar Pradesh J. Zool., 2022, 115–120 CrossRef.
- O. M. El-Borady, M. S. Othman, H. H. Atallah and A. E. Abdel Moneim, Heliyon, 2020, 6, e04045 CrossRef PubMed.
- P. Horky, B. Ruttkay-Nedecky, M. Kremplova, O. Krystofova, R. Kensova, D. Hynek, P. Babula, O. Zitka, L. Zeman, V. Adam and R. Kizek, Int. J. Electrochem. Sci., 2013, 8, 6162–6179 CrossRef CAS.
- J. Mu, L. Lei, Y. Zheng, J. Liu, J. Li, D. Li, G. Wang and Y. Liu, Antioxidants, 2023, 12, 796 CrossRef CAS PubMed.
- G. Barchielli, A. Capperucci and D. Tanini, Antioxidants, 2022, 11, 251 CrossRef CAS PubMed.
- X. Wang, C. X. Hai, X. Liang, S. X. Yu, W. Zhang and Y. L. Li, J. Ethnopharmacol., 2010, 127, 424–432 CrossRef CAS PubMed.
- J. Fang, H. Yin, Z. Yang, M. Tan, F. Wang, K. Chen, Z. Zuo, G. Shu, H. Cui, P. Ouyang, H. Guo, Z. Chen, C. Huang, Y. Geng and W. Liu, Ecotoxicol. Environ. Saf., 2021, 208, 111610 CrossRef CAS PubMed.
- M. Hammad, M. Raftari, R. Cesário, R. Salma, P. Godoy, S. N. Emami and S. Haghdoost, Antioxidants, 2023, 12, 1371 CrossRef CAS PubMed.
- N. Handa, S. K. Kohli, A. Sharma, A. K. Thukral, R. Bhardwaj, E. F. Abd_Allah, A. A. Alqarawi and P. Ahmad, Environ. Exp. Bot., 2019, 161, 180–192 CrossRef CAS.
- S. V. Raju, A. Mukherjee, P. Sarkar, P. K. Issac, C. Lite, B. A. Paray, M. K. Al-Sadoon, A. R. Al-Mfarij and J. Arockiaraj, Fish Physiol. Biochem., 2021, 47, 1073–1085 CrossRef CAS PubMed.
- K. M. Mohamed, M. S. Abdelfattah, M. El-khadragy, W. A. Al-Megrin, A. Fehaid, R. B. Kassab and A. E. Abdel Moneim, Green Process. Synth., 2023, 12(1) CAS.
- L. Tian, B. Lin, L. Wu, K. Li, H. Liu, J. Yan, X. Liu and Z. Xi, Sci. Rep., 2015, 5, 16117 CrossRef CAS PubMed.
- M. Rima, Y. Lattouf, M. Abi Younes, E. Bullier, P. Legendre, J.-M. Mangin and E. Hong, Sci. Rep., 2020, 10, 15338 CrossRef CAS PubMed.
- F. Li, J. Lin, X. Liu, W. Li, Y. Ding, Y. Zhang, S. Zhou, N. Guo and Q. Li, Ann. Transl. Med., 2018, 6, 173 CrossRef PubMed.
- U. Kristan, P. Planinšek, L. Benedik, I. Falnoga and V. Stibilj, Chemosphere, 2015, 119, 231–241 CrossRef CAS PubMed.
- T. Nie, H. Wu, K. H. Wong and T. Chen, J. Mater. Chem. B, 2016, 4, 2351–2358 RSC.
- J. Machat, V. Otruba and V. Kanicky, J. Anal. At. Spectrom., 2002, 17, 1096–1102 RSC.
- C. Ramamurthy, K. S. Sampath, P. Arunkumar, M. S. Kumar, V. Sujatha, K. Premkumar and C. Thirunavukkarasu, Bioprocess Biosyst. Eng., 2013, 36, 1131–1139 CrossRef CAS PubMed.
- P. K. Issac and K. Velumani, Bionanoscience, 2024, 14(5), 5310–5326 CrossRef.
- N. S. Dumore and M. Mukhopadhyay, J. Mol. Struct., 2020, 1205, 127637 CrossRef CAS.
- A. J. Kora, IET Nanobiotechnol., 2018, 12, 658–662 CrossRef PubMed.
- S. Y. Williams and B. J. Renquist, J. Vis. Exp., 2016,(107), e53297 Search PubMed.
- F. Gao, Z. Yuan, L. Zhang, Y. Peng, K. Qian and M. Zheng, Nanomaterials, 2023, 13, 2629 CrossRef CAS PubMed.
- K. Kalishwaralal, S. Jeyabharathi, K. Sundar and A. Muthukumaran, Artif. Cells, Nanomed., Biotechnol., 2015, 1–7 Search PubMed.
- Y. Wang, J. Tian, F. Shi, X. Li, Z. Hu and J. Chu, Microbiol. Immunol., 2021, 65, 410–421 CrossRef CAS PubMed.
- P. K. Issac, A. Guru, M. Velayutham, R. Pachaiappan, M. V. Arasu, N. A. Al-Dhabi, K. C. Choi, R. Harikrishnan and J. Arockiaraj, Life Sci., 2021, 283, 119864 CrossRef CAS PubMed.
- C. Lite, A. Guru, M. Juliet and J. Arockiaraj, Environ. Toxicol., 2022, 37, 1988–2004 CrossRef CAS PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- M. Velayutham, B. Ojha, P. K. Issac, C. Lite, A. Guru, M. Pasupuleti, M. V. Arasu, N. A. Al-Dhabi and J. Arockiaraj, Cell Biol. Int., 2021, 45, 2331–2346 CrossRef CAS PubMed.
- P. K. Issac, J. J. Santhi, V. A. Janarthanam and K. Velumani, Bionanoscience, 2024, 14(2), 903–918 CrossRef.
- K. Schulz, S. Kerber and M. Kelm, Nitric Oxide, 1999, 3, 225–234 CrossRef CAS PubMed.
- M. Krishnan and S. C. Kang, Neurotoxicol. Teratol., 2019, 74, 106811 CrossRef CAS PubMed.
- O. Yamanaka and R. Takeuchi, J. Exp. Biol., 2018, 221(16), jeb182469 CrossRef PubMed.
- G. Sudhakaran, A. Chandran, A. R. Sreekutty, S. Madesh, R. Pachaiappan, B. O. Almutairi, S. Arokiyaraj, Z. A. Kari, G. Tellez-Isaias, A. Guru and J. Arockiaraj, Molecules, 2023, 28, 5350 CrossRef CAS PubMed.
- N. Qamar, P. John and A. Bhatti, Int. J. Nanomed., 2020, 15, 3497–3509 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4na00644e |
| This journal is © The Royal Society of Chemistry 2025 |