Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A new mesoporous Ce–Mn-LDH-based Co-MOF nano-composite for the green synthesis of tetrazoloquinazolines†
Samira
Javadi
and
Davood
Habibi
 *
*
Department of Organic Chemistry, Faculty of Chemistry and Petroleum Sciences, Bu-Ali Sina University, Hamedan, Iran. E-mail: davood.habibi@gmail.com; dhabibi@basu.ac.ir; Fax: +98-81-31408025; Tel: +98-81-38380922
First published on 21st November 2024
Abstract
Tannic acid (TA), as a plant polyphenol, has many active sites for chelation with metals, so TA-oligomers (TA-Olig) were used for the first time as ligands on the surface of Ce–Mn-LDH to prepare the layered double hydroxide-based metal–organic framework (Ce–Mn-LDH@CPTMS@TA-Olig@Co-MOF = E) nanocomposite. In this regard, a homogeneous water/ethanol solution was prepared by sol–gel method using polyethylene glycol and ammonia solution, and then TA was converted into a set of oligomers in the presence of formaldehyde. In the next step, Ce–Mn-LDH was prepared in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 of Ce to Mn, modified with 3-chloropropylmethoxysilane, functionalized by TA-Olig, and then cobalt salt was used to prepare E. Finally, the structure of E was determined by FT-IR, ICP, XRD, BET, EDX, SEM, SEM-mapping, TEM, and TGA-DTA techniques and used as a new and potent nanocatalyst for the synthesis of tetrazoloquinazolines a(1–12). One of the advantages of this nanocatalyst is the active surface to form products in a limited time in mild conditions with high efficiency and easy separation. Also, the catalytic activity is maintained even after four consecutive runs.
4 of Ce to Mn, modified with 3-chloropropylmethoxysilane, functionalized by TA-Olig, and then cobalt salt was used to prepare E. Finally, the structure of E was determined by FT-IR, ICP, XRD, BET, EDX, SEM, SEM-mapping, TEM, and TGA-DTA techniques and used as a new and potent nanocatalyst for the synthesis of tetrazoloquinazolines a(1–12). One of the advantages of this nanocatalyst is the active surface to form products in a limited time in mild conditions with high efficiency and easy separation. Also, the catalytic activity is maintained even after four consecutive runs.
1. Introduction
The spherical structure of colloidal particles, with diameters ranging from 0.01 to 1 μm, has been extensively studied in fields such as catalysis, drug delivery, medicine, material science, and biology.1Among the various approaches for preparing stable colloidal particles, the Stöber method has been developed. A key strategy in this method is the preparation of silica-containing colloidal particles using the sol–gel process, which creates a homogeneous system for colloidal particles with a uniform structure, facilitating the synthesis of various types of polymer and mineral spheres, such as resorcinol-formaldehyde spheres.2,3 Coordination polymers (CPs) or metal–organic frameworks (MOFs) are networks composed of organic linkers and metal centers. They exhibit diverse structures and tunable particle sizes, which have attracted significant attention over the last two decades.4,5
Metal-phenol coordination polymers (MPCPs) are a subset of metal–organic coordination polymers that are typically prepared using plant polyphenols, such as TA, as organic ligands. The interaction between metal species and catechol groups facilitates their formation. Owing to their high surface area, MPCPs find applications in biomedicine, sensors, and catalysis.6,7 TA is a natural polyphenol from the phenolic acid group, consisting of a central glucose unit with ten gallic acid molecules attached to it.8,9 A unique feature of TA is the strong interaction between catechol groups and metal ions, which leads to extensive chemical compatibility, especially in metal ion complexes.10
The design of nanoporous solid materials from two-dimensional materials, such as transition metal dichalcogenides (TMDs), layered double hydroxides (LDHs), graphene oxide, etc., has attracted much attention nowadays.11–13 LDHs, or pseudo hydrotalcite, are a group of two-dimensional layered solids in which divalent and trivalent metal cations, as well as interlayer anions, are present in their semi-hydrophilic structure.14
The integration of two MOF and LDH structures into a composite with unique properties is a smart design to increase the catalytic performance.15 In this method, the uniform growth of MOF crystals on LDH plates results in the formation of organic-mineral hybrid materials with high porosity and surface area, which are used in the fields of catalysis, energy storage, absorption, and optoelectronics.16,17 Therefore, synthesizing these composites via a simple, cost-effective, and environmentally friendly method is of great importance.18 In most studies, these composites have been used as absorbers for the removal of metal pollutants from the environment due to their high porosity. In this work, we have utilized them as a green and powerful catalyst for the synthesis of quinazolines. For example, Soltani and colleagues, in 2021, utilized MOF growth on NiCo-LDH to synthesize a NiCo-LDH/MOF nanocomposite for removing metal ions from an aqueous medium.19 Mallakpour and colleagues modified the surface of LDH with chitosan and TA to absorb reactive dyes.20
Quinazolines are nitrogen-containing heterocycles that were prepared by Gabriel in 1903, with other names such as phenazine, 6-benzopyrimidine, and benzo-1,3-diazine also known. Quinazolines are nitrogen-containing cyclic heterocycles that were prepared by Gabriel in 1903, with other names such as phenazine, 6-benzopyrimidine, and benzo-1,3-diazine also known.21 Quinazolines and tetrazole-pyrimidines indeed have a wide field of biological activities,22,23 and are known for their anti-cancer, anti-diabetic, anti-inflammatory, anti-microbial, anti-hypertensive, and anti-convulsant.24,25 Therefore, the synthesis of tetrazole-pyrimidines was reported by Ba0.5Sr0.5Fe12O19@PU-SO3H,26p-TsOH,27 Fe3O4@C/Ph-SO3H,28 TsOH,29 AlCl3,30 and Fe3O4@PEG-400-SO3H.31
In this work, based on the principles of green chemistry, TA was used to prepare corresponding TA-Olig with abundant catechol groups to be chelated to metal to form a coordinated metal-phenolic compound in an easy and green way. So, Ce–Mn-LDH was used as a scaffold for the preparation of the stable metal-phenolic coordinated compound (Scheme 1).
Finally, E was characterized by FT-IR, ICP, XRD, EDX, SEM, SEM-mapping, TEM, and TGA-DTA techniques and used as a potent nanocatalyst for the green synthesis of tetrazolo-[1,5-a]quinazolines a(1–12) from the three component condensation reaction of 5-amino-tetrazole, aldehydes, and diketones under solvent-free conditions at 70 °C with short reaction times and high yields (Scheme 2).
2. Experimental
2.1. Material
All chemicals [Ce(NO3)3·6H2O, Mn(NO3)2·4H2O, Co(NO3)2·6H2O, TA, Na2CO3, NaOH, toluene, and ethanol] were purchased from the Aldrich and Merck chemical companies and used as received.2.2. Equipment
Melting points were determined by A BUCHI 510 device in open capillary tubes. FT-IR spectra were recorded on a PerkinElmer GX spectrophotometer using KBr. 1H and 13C NMR spectra were recorded on a BRUKER AVANCE 300 MHz instrument in DMSO-d6. Crystalline structures of products were identified on a Bruker D8 Advance XRD instrument with Cu Kα as the incident radiation (λ = 1/56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 054 Å) (40 kV and 100 mA). The catalyst particles' morphology was determined via the SEM images on a Philips XL-30 operated at 30 kV accelerating voltage. Qualitative detection of the Ce and Mn elements was performed by EDX in an ESEM (SIGM, Germany) instrument. The structure morphologies of the catalyst particles were determined via the TEM images using an EM10C instrument with an accelerating voltage of 100 kV. TGA-DTA analysis was performed with a heating rate of 30 °C min−1 over a temperature range of 25–1200 °C using the PerkinElmer Pyris Diamond apparatus. The BET-BJH analyses were performed by BELSORP MINI II. The ICP measurements were performed using a PerkinElmer 5300 DV ICP/OES. Progress of the reaction progress was monitored by TLC (silica gel SIL G/UV 254 plates), and ultrasonication was performed by ultrasonic device 2200 ETH SONICA.
054 Å) (40 kV and 100 mA). The catalyst particles' morphology was determined via the SEM images on a Philips XL-30 operated at 30 kV accelerating voltage. Qualitative detection of the Ce and Mn elements was performed by EDX in an ESEM (SIGM, Germany) instrument. The structure morphologies of the catalyst particles were determined via the TEM images using an EM10C instrument with an accelerating voltage of 100 kV. TGA-DTA analysis was performed with a heating rate of 30 °C min−1 over a temperature range of 25–1200 °C using the PerkinElmer Pyris Diamond apparatus. The BET-BJH analyses were performed by BELSORP MINI II. The ICP measurements were performed using a PerkinElmer 5300 DV ICP/OES. Progress of the reaction progress was monitored by TLC (silica gel SIL G/UV 254 plates), and ultrasonication was performed by ultrasonic device 2200 ETH SONICA.
2.3. Synthesis of E
E was synthesized as follows:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH 5
EtOH 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was prepared in 1 hour at room temperature. Then, TA (1 g) was added to the solution. After homogenization (about 30 min), a saturated solution of CH2O in water (0.3 mL) was added and stirred for 24 hours. Then, A was autoclaved for 24 at 120 °C, centrifuged, and dried at 80 °C.3
1) was prepared in 1 hour at room temperature. Then, TA (1 g) was added to the solution. After homogenization (about 30 min), a saturated solution of CH2O in water (0.3 mL) was added and stirred for 24 hours. Then, A was autoclaved for 24 at 120 °C, centrifuged, and dried at 80 °C.3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) in water (50 mL). Solution number two was prepared by dissolving Na2CO3 (10.6 g, 0.1 mol) and NaOH (4 g, 0.1 mol) (molar ratio: 1
4) in water (50 mL). Solution number two was prepared by dissolving Na2CO3 (10.6 g, 0.1 mol) and NaOH (4 g, 0.1 mol) (molar ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in water (50 mL), heated at 60 °C for 24 h and added dropwise to solution number one until the pH of the new solution reached 10. Then, the prepared dark brown solid (B) was separated, washed several times with water, and oven-dried at 80 °C.
1) in water (50 mL), heated at 60 °C for 24 h and added dropwise to solution number one until the pH of the new solution reached 10. Then, the prepared dark brown solid (B) was separated, washed several times with water, and oven-dried at 80 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (5
EtOH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture by ultrasonication for about 10 min. A (0.5 g) was also dispersed in H2O
1) mixture by ultrasonication for about 10 min. A (0.5 g) was also dispersed in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (ratio 5
EtOH (ratio 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by ultrasonication for about 10 min, and was then added dropwise to C, refluxed at 110 °C for 48 h, and D separated by centrifugation and dried at 80 °C.
1) by ultrasonication for about 10 min, and was then added dropwise to C, refluxed at 110 °C for 48 h, and D separated by centrifugation and dried at 80 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (5
EtOH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by ultrasonication for 10 min and Co(NO3)2·6H2O (0.29 g, 5 mmol) was added. The mixture refluxed at 110 °C for 24 h, autoclaved at 120 °C for 24 h, and E was collected and washed with H2O/EtOH several times and dried at 80 °C.
1) by ultrasonication for 10 min and Co(NO3)2·6H2O (0.29 g, 5 mmol) was added. The mixture refluxed at 110 °C for 24 h, autoclaved at 120 °C for 24 h, and E was collected and washed with H2O/EtOH several times and dried at 80 °C.
2.4. General procedure for the synthesis of a(1–12)
A mixture of 5-amino-tetrazole (85 mg, 1 mmol), aldehyde (1 mmol), 1,3-diketone (280 mg, 1 mmol), and E (10 mg) was stirred in solvent-free condition at 70 °C for the specified time. After completion of the reaction (TLC, n-hexane/acetone 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the mixture was cooled to room temperature, and hot ethanol (5 mL) was added to dissolve products. E was easily separated from the ethanolic solution by centrifugation, the product was washed with ethanol (3 × 20 mL), dried under reduced pressure, and characterized by comparing their IR, NMR spectra, and melting points with authentic samples.26
3), the mixture was cooled to room temperature, and hot ethanol (5 mL) was added to dissolve products. E was easily separated from the ethanolic solution by centrifugation, the product was washed with ethanol (3 × 20 mL), dried under reduced pressure, and characterized by comparing their IR, NMR spectra, and melting points with authentic samples.26
3. Results and discussion
3.1. Characterization of the catalyst
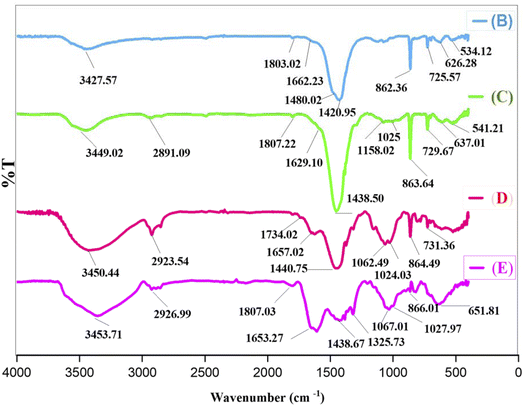
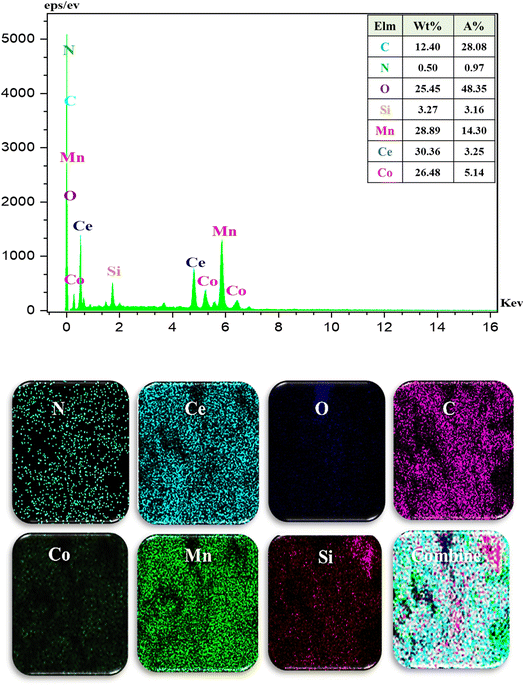
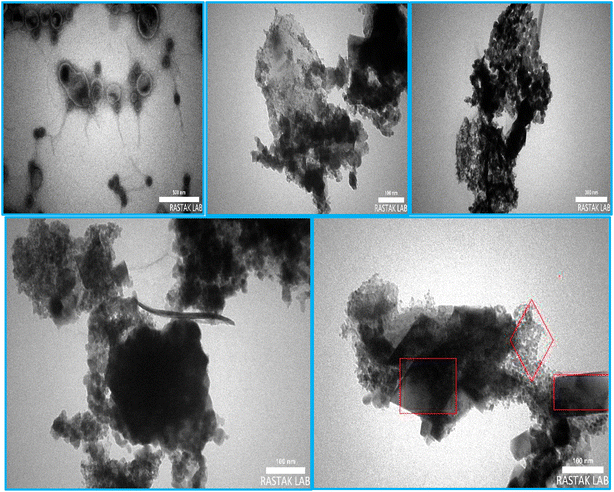
E was characterized by Fourier-Transform Infrared (FT-IR), X-ray Diffraction (XRD), Energy Dispersive X-ray (EDX), EDX-Mapping, Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Thermo-Gravimetric-Differential Thermal Analysis (TGA-DTA), Brunauer–Emmett–Teller (BET)–Barrett–Joyner–Halenda (BJH) and Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) techniques.Fig. 2 represents the FT-IR spectra of B, C, D, and E.
Curve B exhibits three peaks at 626, 534, and 862 cm−1, corresponding to Ce–O, Mn–O, and Ce–O–Mn bonds, respectively. The broad peak at about 3427 cm−1 is attributed to the OH stretching vibrations, and the peak at 1662 cm−1 is associated with the bending vibrations of interlayer water. Additionally, the peaks at 1803 and 1480 cm−1 are linked to stretching and asymmetric vibrations of C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.
O bonds, respectively.
Curve C shows peaks at 1025 and 1158 cm−1, indicative of Si–O–M and Si–O–Si bonds.
In curve D, the shift of peaks to 1657 and 1734 cm−1, as well as the broadening of the peak at 3449 cm−1, indicates the functionalization of C with A.
In curve E, the presence of a Co–O peak at about 651 cm−1, confirms the successful synthesis of E.
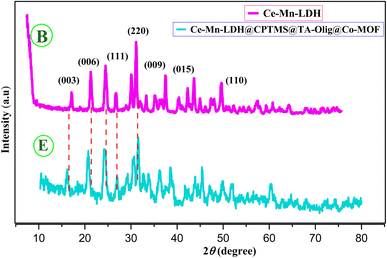
In graph E, the peak intensities are diminished, and the emergence of peaks at around 41.5°, 44.2°, and 47.3° shows the formation of Co-MOF (Co-PTA). The XRD pattern of the E composite indicates the simultaneous presence of both B and E in the composite structure and the growth of Co-MOF (Co-PTA) crystals on the Ce–Mn-LDH sheets.36
The FESEM images (Fig. 5, second row) clearly show the presence and successful growth of Co-MOF (Co-PTA) nanoparticles on surface of the Ce–Mn-LDH ultrathin nanosheets.
| S BET (m2 g−1) | V t (cm3 g−1) | D BJH (nm) | |
|---|---|---|---|
| B | 27.833 | 0.1899 | 27.296 |
| E | 20.878 | 0.0701 | 13.445 |
The significant decrease in pore volume from B (0.1899 cm3 g−1) to E (0.0701 cm3 g−1), indicates that the pores in B are being filled or blocked by the MOF crystals, which is consistent with the successful synthesis of E.
The decrease in pore diameter in E (13.445 nm) compared to B indicates the formation of Co-MOF (Co-PTA) crystals in the pores of B (27.296 nm), leading to smaller pore sizes.
The E isotherm shows some mesoporosity (pores with diameters between 2 and 50 nm), which is typical for materials with MOF structures. This porosity is probably due to the presence of MOF crystals, which create smaller and more uniform pores.
The B isotherm shows the absence of mesoporosity in B, the pores being larger and more irregular, which is consistent with the observed larger pore diameter.
3.2. Optimization
To optimize the synthesis of 10a, the one-pot condensation reaction of 5-amino-tetrazole, 4-chlorobenzaldehyde, and dimedone was carried out in various temperatures, catalyst amounts, and solvents in the presence of E. The best optimal conditions were about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole of 5-aminotetrazole, 4-chlorobenzaldehyde, and dimedone with 10 mg of E at 70 °C under solvent-free conditions (Table 2).
1 mole of 5-aminotetrazole, 4-chlorobenzaldehyde, and dimedone with 10 mg of E at 70 °C under solvent-free conditions (Table 2).
| Entry | Amount of catalyst | Solvent | Temp (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Conditions: 5-aminotetrazol (1 mmol), 4-chlorobenzaldehyde (1 mmol), dimedone (1 mmol), solvent-free. b Isolated pure yield. | |||||
| 1 | 10 mg | EtOH | r.t. | 45 | 70 |
| 2 | 10 mg | CHCl3 | r.t. | 60 | 40 |
| 3 | 10 mg | CH2Cl2 | r.t. | 80 | 35 |
| 4 | 10 mg | CH3CN | r.t. | 70 | 54 |
| 5 | 10 mg | Solvent-free | r.t. | 30 | 50 |
| 6 | 10 mg | EtOH | Reflux | 20 | 80 |
| 7 | 10 mg | CHCl3 | Reflux | 60 | 68 |
| 8 | 10 mg | CH2Cl2 | Reflux | 70 | 58 |
| 9 | 10 mg | CH3CN | Reflux | 25 | 80 |
| 10 | 10 mg | Solvent-free | 60 | 20 | 85 |
| 11 | 10 mg | Solvent-free | 70 | 5 | 90 |
| 12 | 10 mg | Solvent-free | 80 | 10 | 88 |
| 13 | 10 mg | Solvent-free | 90 | 10 | 88 |
| 14 | 20 mg | Solvent-free | 70 | 30 | 71 |
| 15 | 30 mg | Solvent-free | 70 | 25 | 77 |
| 16 | No catalyst | Solvent-free | 70 | 120 | 65 |
3.3. Synthesis of a(1–12)
Based on the obtained results from the model reaction (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of 5-amino-tetrazole, 4-chlorobenzaldehyde, and dimedone, in ethanol with 10 mg of E at 70 °C), all synthesized compounds were characterized and approved with comparison of their spectroscopic data and melting points with authentic samples (Table 3).
1 molar ratio of 5-amino-tetrazole, 4-chlorobenzaldehyde, and dimedone, in ethanol with 10 mg of E at 70 °C), all synthesized compounds were characterized and approved with comparison of their spectroscopic data and melting points with authentic samples (Table 3).
a Reaction conditions: 5-amino tetrazole (1 mmol), 4-chloroaldehyde (1 mmol), dimedone (1 mmol), E (10 mg) in solvent-free condition, at 70 °C.
b Isolated yield.
c TON (turnover number): based on the ICP measurements at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the content of Co in the catalyst is about 30.54, so the mmole of Co in 10 mg of the catalyst is 0.3 mmole g−1. For entry 1, since the yield is about 78%, the effective mmole the TON is 0.78 ÷ 0.3 = 2.6.
d TOF (turnover frequency): TOF = TON ÷ time. For entry 1, since the time is about 30 min, the TOF is 2.6 ÷ 30 = 0.08. 4, the content of Co in the catalyst is about 30.54, so the mmole of Co in 10 mg of the catalyst is 0.3 mmole g−1. For entry 1, since the yield is about 78%, the effective mmole the TON is 0.78 ÷ 0.3 = 2.6.
d TOF (turnover frequency): TOF = TON ÷ time. For entry 1, since the time is about 30 min, the TOF is 2.6 ÷ 30 = 0.08.
|
|---|

|
3.4. Spectral data of a(1–12)
3.5. Comparison of the catalyst activities
Table 4 shows the comparison of the previous methods (entries 1–10) used for the synthesis of a(1–12) with our proposed method (entry 11). As can be seen, our proposed method has a short time (5 min) with about 90% yield.| Entry | Catalyst | Conditions | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | p-TSOH | Solvent-free, 120 °C | 2 h | 84 | 26 |
| 2 | Fe3O4@C/Ph SO3H | H2O, 80 °C | 1 h | 96 | 27 |
| 3 | g-C3N4/NHSO3H | i-PrOH, 80 °C | 4 h | 95 | 28 |
| 4 | NaN3, Hg(OAc)2 | HOAc, 100 °C | 6 h | 67 | 29 |
| 5 | AlCl3 | CH3CN, reflux | 3 h | 92 | 30 |
| 6 | I2 | i-PrOH, reflux | 10 min | 92 | 42 |
| 7 | p-TSA | Solvent-free, 70 °C | 6 min | 88 | 43 |
| 8 | MNPs@SiO2-Pr ANDSA | EtOH/H2O, 100 °C | 5 min | 94 | 44 |
| 9 | PyTFA | μW, 90 °C | 25 min | 93 | 45 |
| 10 | Our catalyst | Solvent-free, 70 °C | 5 min | 90 | This work |
3.6. Proposed mechanism
Scheme 3 shows the possible mechanism for the synthesis of a(1–12) by E.43,443.7. Recyclability of E
The recovery ability of the E was investigated in the model reaction after four consecutive runs (Fig. 9). At the end of the reaction, E was washed with water and ethanol, dried at 80 °C, and reused in the next runs. As a result, no significant loss of activity was observed.In addition, the FT-IR spectrum of E before and after the consecutive runs of recovery showed a very nice similarity (Fig. 10).
4. Conclusion
In this study, for the first time, TA oligomers were used as a green ligand for the synthesis of MOFs to modify the surface of the Ce–Mn-LDH. These oligomers have many active sites for chelation with metal by creating metal-phenol-coordinated polymers. Also, FT-IR, ICP, XRD, BET, EDX, SEM, SEM-mapping, TEM, and TGA-DTA techniques showed that the LDH is a suitable surface for the growth of TA oligomers and the formation of metal–organic framework crystals. The catalytic performance of this composite E was evaluated in the synthesis of tetrazolo[1,5-a]quinazolines. The high yield of products in a short time and the ability to recycle without side products are the advantages of this composite E.Data availability
All data generated or analyzed during this study are included in the ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The support by the Bu-Ali Sina University is gratefully acknowledged.References
- J. Qiu, P. H. Camargo, U. Jeong and Y. Xia, Synthesis, transformation, and utilization of monodispersed colloidal spheres, Acc. Chem. Res., 2019, 52, 3475–3487 CrossRef CAS PubMed.
- P. P. Ghimire and M. Jaroniec, Renaissance of Stöber method for synthesis of colloidal particles: new developments and opportunities, J. Colloid Interface Sci., 2021, 584, 838–865 CrossRef CAS PubMed.
- J. Wei, G. Wang, F. Chen, M. Bai, Y. Liang, H. Wang, D. Zhao and Y. Zhao, Sol-gel synthesis of metal–phenolic coordination spheres and their derived carbon composites, Angew. Chem., Int. Ed., 2018, 57, 9838–9843 CrossRef CAS PubMed.
- G. Liang, B. Feng, G. Wang, N. Wua, Y. Deng, A. A. Elzatahry, A. Alghamdi, Y. Zhao and J. Wei, Facile synthesis of metal-polyphenol-formaldehyde coordination polymer colloidal nanoparticles with sub-50 nm for T1-weighted magnetic resonance imaging, Chin. Chem. Lett., 2021, 32, 842–848 CrossRef.
- H. J. Lee, Y. J. Cho, W. Cho and M. Oh, Controlled isotropic or anisotropic nanoscale growth of coordination polymers: formation of hybrid coordination polymer particles, ACS Nano, 2013, 7, 491–499 CrossRef PubMed.
- H. Huang, J. Qin, G. Wang, Z. Guo, X. Yu, Y. Zhao and J. Wei, Synthesis of spiny metal–phenolic coordination crystals as a sensing platform for sequence-specific detection of nucleic acids, CrystEngComm, 2018, 20, 7626–7630 RSC.
- J. Qin, N. Guo, J. Yang and Y. Chen, Recent advances of metal–polyphenol coordination polymers for biomedical applications, Biosensors, 2023, 13, 776 CrossRef CAS PubMed.
- N. Aelen, M. I. Popa, O. Nova, G. Lisa and L. Balait, Tannic acid incorporation in chitosan-based microparticles and in vitro controlled release, J. Mater. Sci.: Mater. Med., 2009, 20, 1095–1102 CrossRef PubMed.
- M. A. Gwa, B. M. Hong, J. M. Seok, S. A. Park and W. H. Park, Effect of tannic acid on the mechanical and adhesive properties of catechol-modified hyaluronic acid hydrogels, Int. J. Biol. Macromol., 2021, 191, 699–705 CrossRef PubMed.
- B. Kaczmare, Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials-A minireview, Materials, 2020, 13, 3224 CrossRef PubMed.
- Q. Huang, J. Zhao, M. Liu, J. Chen, X. Zhu, T. Wu, J. Tian, Y. Wen, X. Zhang and Y. Wei, Preparation of polyethylene polyamine@tannic acid encapsulated MgAl-layered double hydroxide for the efficient removal of copper(II) ions from aqueous solution, J. Taiwan Inst. Chem. Eng., 2018, 82, 92–101 CrossRef CAS.
- L. G. Bach, M. R. Islam, X. T. Cao, J. M. Park and K. T. Lim, A novel photoluminescent nanohybrid of poly(ε-caprolactone) grafted Mg/Al layered double hydroxides and Tb3+ ions: synthesis and characterization, J. Alloys Compd., 2014, 582, 22–28 CrossRef CAS.
- R. Dou, J. Ma, D. Huang, C. Fan, W. Zhao, M. Peng and S. Komarneni, Bisulfite assisted photocatalytic degradation of methylene blue by Ni-Fe-Mn oxide from MnO4 intercalated LDH, Appl. Clay Sci., 2018, 161, 235–241 CrossRef CAS.
- S. Javadi and D. Habibi, The new sulfonic-acid-based Ce-Mn-layered double hydroxide as a capable nano-catalyst for the green-synthesis of spiro[indoline-pyran] ones and spiro[acenaphthylene-pyran]ones, Res. Chem. Intermed., 2023, 49, 2005–2024 CrossRef CAS.
- Y. Yang, X. Yan, X. Hu, R. Feng and M. Zhou, In-situ growth of ZIF-8 on layered double hydroxide: effect of Zn/Al molar ratios on their structural, morphological, and adsorption properties, J. Colloid Interface Sci., 2017, 505, 206–212 CrossRef CAS PubMed.
- W. D. Zhang, Q. T. Hu, L. L. Wang, J. Gao, H. Y. Zhu, X. Yan and Z. G. Gu, In-situ generated Ni-MOF/LDH heterostructures with abundant phase interfaces for enhanced oxygen evolution reaction, Appl. Catal., B, 2021, 286, 119906 CrossRef CAS.
- S. Chongd, S. Bhattacharje, P. Bhanj and A. Bhaumi, Porous organic–inorganic hybrid materials for catalysis, energy and environmental applications, Chem. Commun., 2022, 58, 3429–3460 RSC.
- R. Soltani, R. Pelalak, M. Pishnamazi, A. Marjani, S. M. Sarkar, A. B. Albadarin and S. Shirazian, Novel bimodal micro-mesoporous Ni50Co50-LDH/UiO-66-NH2 nano-composite for Tl(I) adsorption, Arabian J. Chem., 2021, 14, 103058 CrossRef CAS.
- R. Soltani, R. Pelalak, M. Pishnamazi, A. Marjani and S. Shirazian, A water-stable functionalized NiCo-LDH/MOF nanocomposite: green synthesis, characterization, and its environmental application for heavy metals adsorption, Arabian J. Chem., 2021, 14, 103052 CrossRef CAS.
- S. Mallakpour, Z. Radfar and M. Feiz, Chitosan/tannic acid/ZnFe layered double hydroxides and mixed metal oxides nanocomposite for the adsorption of reactive dyes, Carbohydr. Polym., 2023, 305, 120528 CrossRef CAS PubMed.
- P. R. Patel, H. Joshi, U. Shah, Ma. Bapna and B. Patel, New generation of quinazolinone derivatives as potent antimicrobial agents, Asian Pacific Journal of Health Sciences, 2021, 8, 61–66 CrossRef.
- M. Asif, Chemical characteristics, synthetic methods, and biological potential of quinazoline and quinazolinone derivatives, Int. J. Med. Chem., 2014, 27, 395637 Search PubMed.
- J. Devasi, A. Nizam and V. L. Vasanth, Azole-based antibacterial agents: a review on multistep synthesis strategies and biology, Polycyclic Aromat. Compd., 2022, 42(8), 5474–5495 CrossRef.
- L. M. Antypen, S. I. Kovalenk, O. M. Antypenk, A. M. Kats and O. M. Achkaso, Design and evaluation of novel antimicrobial and anticancer agents among tetrazolo[1,5-c] quinazoline-5-thione S-derivatives, Sci. Pharm., 2013, 81, 15–42 CrossRef PubMed.
- A. Maleki, M. Aghaei and T. Kari, Facile synthesis of 7-aryl-benzo[h]tetrazolo[5,1-b]-quinazoline-5,6-dione fused polycyclic compounds by using a novel magnetic poly-urethane catalyst, Polycyclic Aromat. Compd., 2019, 39, 266–278 CrossRef CAS.
- L. Wu, Synthesis and biological evaluation of novel 1,2-naphthoquinones possessing tetrazolo[1,5-a]pyrimidine scaffolds as potent antitumor agents, RSC Adv., 2015, 5, 24960–24965 RSC.
- A. Hassankhani, B. Gholipour, S. Rostamni, E. Zarenezha, N. Nouruz, T. Kavetsky, R. Khalilo and M. Shokouhimeh, Sustainable design and novel synthesis of highly recyclable magnetic carbon containing aromatic sulfonic acid: Fe3O4@C/Ph-SO3H as green solid acid promoted regioselective synthesis of tetrazoloquinazolines, Appl. Organomet. Chem., 2021, 35, e6346 CrossRef CAS.
- S. Shen, H. Zhang, C. Yu, C. Yao, T. Li, B. Qin, J. Lu and D. Wang, Solvent-free combinatorial synthesis of tetrazolo[1,5-a]thiopyrano[3,4-d]pyrimidine derivatives, Res. Chem. Intermed., 2013, 39, 1799–1806 CrossRef CAS.
- P. Kour, V. P. Singh, B. Khajuria, T. Singh and A. Kumar, Al(III) chloride catalyzed multi-component domino strategy: synthesis of library of dihydrotetrazolo[1,5-a] pyrimidines and tetrahydrotetrazolo[1,5-a]quinazolinones, Tetrahedron Lett., 2017, 58, 4179–4185 CrossRef CAS.
- F. Bonyasi, M. Hekmati and H. Veisi, Preparation of core/shell nanostructure Fe3O4@PEG400-SO3H as heterogeneous and magnetically recyclable nanocatalyst for one-pot synthesis of substituted pyrroles by Paal-Knorr reaction at room temperature, J. Colloid Interface Sci., 2017, 496, 177–187 CrossRef CAS PubMed.
- R. Jia, C. Zhao, Z. Huang, X. Liu, D. Wang, Z. Hui and X. Xu, An in-situ growth strategy of NiCo-MOF nanosheets with more activity sites for asymmetric supercapacitors, Ionics, 2020, 26, 6309–6318 CrossRef CAS.
- X. Tao, Y. Han, C. Sun, L. Huang and D. Xu, Plasma modification of NiAlCe–LDH as improved photocatalyst for organic dye wastewater degradation, Appl. Clay Sci., 2019, 172, 75–79 CrossRef CAS.
- Y. Wanga, X. Liu, N. Zhang, G. Qiu and R. Ma, Cobalt-doped Ni-Mn layered double hydroxide nanoplates as high-performance electrocatalyst for oxygen evolution reaction, Appl. Clay Sci., 2018, 165, 277–283 CrossRef.
- T. Zhang, Z. C. Liu, Y. C. Ye, Y. Wang, H. Q. Yang, H. X. Gao and W. M. Yang, Dry reforming of ethane over FeNi/Al–Ce–O catalysts: composition-induced strong metal–support interactions, Engineering, 2022, 173–185 CrossRef CAS.
- A. N. Wagassa, L. T. Tufa, J. Lee, E. A. Zereffa and T. A. Shifa, Controllable doping of Mn into Ni0.075-xMnxAl0.025(OH)2(CO3)0.0125·yH2O for efficient adsorption of fluoride ions, Global Challenges, 2023, 7, 2300018 CrossRef PubMed.
- M. Wang, X. Yao, Y. Chen, B. Lin, N. Li, K. Zhi, Q. Liu and H. Zhou, A novel tannic acid-based carbon-supported cobalt catalyst for transfer hydrogenation of biomass derived ethyl levulinate, Front. Chem., 2022, 10, 964128 CrossRef CAS PubMed.
- L. Y. Zeng and C. Cai, Iodine catalyzed one-pot multicomponent synthesis of a library of compounds containing tetrazolo[1,5-a]pyrimidine core, J. Comb. Chem., 2010, 12, 35–40 CrossRef CAS PubMed.
- M. Farahi, B. Karami, A. Jokar and K. Eskandari, An environmentally benign synthesis of pyrimidine-fused coumarin and triazole motifs via a catalytic domino reaction, Org. Prep. Proced. Int., 2017, 49, 514–524 CrossRef CAS.
- C. Zhang and W. Li, Regioselective synthesis of 6-aryl-benzo[h][1,2,4]-triazolo[5,1-b]quinazoline-7,8-diones as potent antitumoral agents, Bioorg. Med. Chem. Lett., 2013, 23, 5002–5005 CrossRef PubMed.
- A. Hassankhani, B. Gholipour and S. Rostamni, An efficient regioselective three-component synthesis of tetrazoloquinazolines using g-C3N4 covalently bonded sulfamic acid, Polyhedron, 2020, 175, 114217 CrossRef.
- A. Hassankhani and E. Mosaddegh, An efficient synthesis of tetrahydrotetrazolo[1,5-a]quinazoline derivatives by a three-component reaction of 5-aminotetrazole, aryl-aldehydes, and dimedone, Sci. Iran., 2015, 22, 942–947 Search PubMed.
- R. Ghorbani-Vaghei, S. Alavinia and N. Sarmast, Fe3O4@SiO2@propyl-ANDSA: a new catalyst for the synthesis of tetrazoloquinazolines, Appl. Organomet. Chem., 2018, 32, e4038 CrossRef.
- C. Raju, K. Madhaiyan, R. Uma, R. Sridhar and S. Ramakrishna, Antimicrobial and anti-oxidant activity evaluation of tetrazolo[1,5-a]pyrimidines: a simple diisopropyl-ammonium trifluoroacetate mediated synthesis, RSC Adv., 2012, 2, 11657 RSC.
- M. V. Murlykina, A. D. Morozova, I. M. Zviagin, Y. I. Sakhno, S. M. Desenko and V. A. Chebanov, Aminoazole-based diversity-oriented synthesis of heterocycles, Front. Chem., 2018, 6, 527 CrossRef PubMed.
- S. Akrami, B. Karami and M. Farahi, A new and green approach for regiospecific synthesis of novel chromeno-triazolopyrimidin using tungstic acid immobilized MCM-41 as a reusable catalyst, J. Heterocycl. Chem., 2020, 57, 2446–2454 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4na00643g |
| This journal is © The Royal Society of Chemistry 2025 |