Open Access Article
Open Access ArticleFabrication of water-dispersible dye/polymer matrix-stabilized β-FeOOH (Rh-B/F127@β-FeOOH) nanoparticles: synthesis, characterization and therapeutic applications
Neela Mohan
Chidambaram†
 *a,
Palanisamy
Rajkumar†
b,
P. Arul
Prakash
c,
G. M.
Rathika
d,
K.
Prabhu
e,
Senthil Muthu Kumar
Thiagamani
*a,
Palanisamy
Rajkumar†
b,
P. Arul
Prakash
c,
G. M.
Rathika
d,
K.
Prabhu
e,
Senthil Muthu Kumar
Thiagamani
 *f,
M. Khalid
Hossain
*f,
M. Khalid
Hossain
 *g,
Manikandan
Ayyar
*g,
Manikandan
Ayyar
 *h,
Lalitha
Gnanasekaran
i and
Jinho
Kim
*b
*h,
Lalitha
Gnanasekaran
i and
Jinho
Kim
*b
aDepartment of Chemistry, Srimad Andavan Arts and Science College (Autonomous), Affiliated to Bharathidasan University, Tiruchirappalli 620 005, Tamil Nadu, India. E-mail: neela.chem@gmail.com
bDepartment of Mechanical Engineering, Yeungnam University, Gyeongsan-si, 38451, Gyeongbuk, Republic of Korea. E-mail: rajphysics@yahoo.com; jinho@ynu.ac.kr
cPG & Research Department of Biotechnology, National College (Autonomous), Affiliated to Bharathidasan University, Tiruchirappalli 620 001, Tamil Nadu, India. E-mail: arulmvp@gmail.com
dDepartment of Chemistry, K. Ramakrishnan College of Technology (Autonomous), Tiruchirappalli 621 112, Tamil Nadu, India. E-mail: gmrathika@gmail.com
ePG & Research Department of Biotechnology, Srimad Andavan Arts and Science College (Autonomous), Affiliated to Bharathidasan University, Tiruchirappalli 620 005, Tamil Nadu, India. E-mail: prabhurajk726@gmail.com
fDepartment of Mechanical Engineering, Kalasalingam Academy of Research and Education, Anand Nagar, Krishnankoil, Tamil Nadu 626126, India. E-mail: tsmkumar@klu.ac.in
gInstitute of Electronics, Atomic Energy Research Establishment, Bangladesh Atomic Energy Commission, Dhaka 1349, Bangladesh. E-mail: khalid.baec@gmail.com
hDepartment of Chemistry, Centre for Material Chemistry, Karpagam Academy of Higher Education, Coimbatore 641021, Tamil Nadu, India. E-mail: manikandan.frsc@gmail.com
iInstituto de Alta Investigación, Universidad de Tarapacá, Arica-1000000, Chile. E-mail: lalitha1887@gmail.com
First published on 24th January 2025
Abstract
In this study, dye/polymer matrix-stabilized β-FeOOH nanomaterials were fabricated for therapeutic applications. Rh-B/F127@β-FeOOH nanomaterials were synthesized using two different methods: co-precipitation (CoP) and hydrothermal (HT) methods. The as-synthesized nanoparticles were characterized using various spectroscopic techniques, including FT-IR, UV-Vis, PL, XRD, HR-TEM, and XPS analysis. The functional groups and optical properties were confirmed by FT-IR spectroscopy, UV-Vis and fluorescence spectroscopy. The Rh-B/F127@β-FeOOH nanomaterials exhibited both rod-like and sphere-like morphology, as confirmed by HR-TEM analysis. Unlike the nanorods, the nanospheres produced multi-colored emissions at 407, 446, 482 and 520 nm. The oxidative states and elements were confirmed by XPS spectroscopy. MTT assays were used to analyze the cytotoxicity of the nanospheres against A549 cells. The reactive oxygen species (ROS) generation and apoptotic cell death caused by the β-FeOOH nanospheres were evaluated by flow cytometry. Cell cycle analysis indicated that the treatment of nanospheres-induced S-phase cell cycle arrest in A549 cells. The synthesized nanospheres induced late-stage apoptosis in the A549 cell line, with a cell death rate of up to 30.37% at the IC50 concentration. Additionally, the antioxidant activities of the synthesized nanorods showed a high scavenging activity against free radicals, as examined by different assays such as such as DPPH, RP, and FRAP. The above results suggest that the synthesized nanorods and nanospheres are promising and efficient material for therapeutic applications.
1. Introduction
Recently, nanomedicines have been developed to boost cancer treatment, creating new potential for improved effectiveness. This is an important breakthrough as cancer is the leading cause of death worldwide. Compared to other cancer types, lung cancer is one of the most frequent malignancies and causes about 19% of deaths worldwide. Nanoparticle research aims to minimize the adverse effects of cancer and allow the precise targeting of tumors without any toxic side effects.1Several research studies have attempted to investigate novel materials with great efficacy and low side effects for cancer treatment.2–4 Combining novel nanomaterials with specific therapeutic technique has shown promise for cancer diagnosis and therapy. However, there are still many challenges to overcome for the development of new materials for use in the preclinical and clinical research of tumors to improve the therapeutic efficacy.5
Over the past few decades, nanostructured metal oxide materials have attracted considerable attention due to their distinctive intrinsic characteristics, including tunable surface chemistry, morphology, structure, large surface-volume area, and high chemical stability.6 Among the various iron-based oxide materials, β-FeOOH (also referred to as akaganeite) with a hollandite-type structure has been utilized as an effective semiconductor, with a band gap of 2.12 eV. The monoclinic crystal-structured akaganeite (β-FeOOH) has a specific gravity (SG) of 3.52 and unit cell parameters of a = 10.587 Å, b = 3.0357 Å, c = 10.527 Å, and β = 90.14.7 Its framework is made up of iron oxyhydroxide and includes tunnels that are partially occupied by chloride anions in environments containing chloride.8 The tunnel structure make it a versatile material that can be used in various areas of applications, like in catalysts, electrodes, ion-exchange materials, and adsorbents.9–12
β-FeOOH nanoparticles are commonly synthesized through various methods, like hydrolysis,13 co-hydrolysis,14 hydrothermal technique,15–18 thermolysis,19 precipitation and co-precipitation method,20 surfactant-templated synthesis,21 electric explosion,22 and green synthesis.23 Also, nanocrystalline akaganeite exhibits an elevated surface area and a narrow pore-size distribution, showcasing diverse crystal morphologies, such as rod and nanotubes,17 needles,21 spindles,24 and cigar-shaped.10 The different shapes of β-FeOOH materials have ignited significant research interest regarding their nanoscale derivatives, which have been used in magnetic storage devices, catalysis, and sensors.25 Besides, low-toxic β-FeOOH nanomaterials have recently been investigated for biological applications, such as MRI (magnetic resonance imaging) for medical diagnosis and therapies.16,26,27
The use of organic dyes, like rhodamine-B (Rh-B) and its derivatives, as fluorescent labels has also become prevalent in biological applications due to their excellent physical and chemical properties, such as high fluorescent quantum yield, broad emission wavelength, and sensitivity.28 However, Rh-B dyes have inherent limitations, such as hydrophobicity, leading to self-bleaching, and poor selectivity, causing the non-specific staining of tissues.29 To address these issues, researchers have encapsulated Rh-B dyes into biocompatible polymeric carriers to enhance their stability.30–32 Nonetheless, Rh-B encapsulation within nanoparticles (NPs) often results in aggregation and quenching, even when using polymeric carriers.33
One strategy to mitigate aggregation and quenching is by chemically conjugating Rh-B molecules to polymer chains. This approach immobilizes the RB molecules, preventing aggregation and self-quenching. Polymers offer numerous functional groups along their chains, making them suitable for such functionalization or modification.34 However, many of the polymers that have been explored for this purpose to date lack biocompatibility and have poorly understood toxicity mechanisms.35
In the recent past, amphiphilic triblock copolymers, particularly focusing on Pluronic copolymers like Pluronic F-127, have attracted significant attention in various interdisciplinary fields.36 Pluronic F-127, being a water-soluble triblock copolymer composed of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO), possesses several advantageous properties including stability, good mechanical properties, biocompatibility, and a non-toxic nature.37 Pluronic F-127 has also attracted significant attention due to its ability to serve as a vehicle for drug delivery38–40 and as a template for nanoparticle synthesis.41 Due to its biocompatibility, low toxicity against cells and body fluids, and weak immunogenic properties, it is highly suitable for biomedical applications.37,40
The aim of this present study was to synthesize high-quality Rh-B/F127@β-FeOOH nanorods and nanospheres for therapeutic applications by two different methods, namely co-precipitation (CoP) and hydrothermal (HT) methods, using dye–micelle (Rh-B/F127) matrix inclusion solutions. The optical, structural, and morphological properties of the obtained Rh-B/F127@β-FeOOH nanoparticles were explored by FT-IR, UV-Vis, PL, XRD, SEM, HR-TEM, and XPS analyses. The synthesized Rh-B/F127@β-FeOOH nanoparticles exhibited good anticancer activities. The cytotoxicity activity of the nanospheres was studied using an MTT assay. For the first time, we demonstrate the Rh-B/F127@β-FeOOH nanospheres-induced apoptosis effect on A549 cells with the help of cell cycle analysis, ROS generation, DAPI, intracellular uptake, and AO/EtBr staining tests. The antioxidant activity of the nanorods was examined by various assays, such as DPPH, RP, and PFRAP assay.
2. Experimental
2.1 Materials
FeCl3·6H2O (≥98%), FeCl2·4H2O (≥96%) and urea (98%) were purchased from Merck. Sodium hydroxide (NaOH, 99%) was obtained from LobaChemie. Rhodamine-B (99%) was purchased from Alfa Aesar. The F127 copolymer was purchased from Sigma-Aldrich. All the chemicals were utilized in their original state without any additional purification steps.2.2 Methods
Rh-B/F127@β-FeOOH nanorods and nanospheres were prepared by co-precipitation (CoP) and hydrothermal (HT) methods using ferric chloride and rhodamine dye/F127 (Rh-B/F127) matrix solutions.2.2.1.1 Co-precipitation (CoP) method. First, 1 mM of FeCl3·6H2O and 1 mM urea (acting as fuel) were dissolved in 50 mL of water and heated up to 50 °C for 1 h. Then, the obtained solution was filtered using Whatman No. 40 filter paper to remove the insoluble impurities. Then the solution was heated at 80 °C and maintained at this temperature for 30 min. To this solution, 10 mL of 3 M NaOH solution was added, keeping the pH at 11. After that, the whole solution was maintained at 80 °C for 2 h to allow the growth of β-FeOOH NPs. The prepared NPs were separated by centrifugation and washed with ethanol. To eliminate by-products, the process was repeated at least twice. Finally, the obtained NPs were dried for 3 h at 80 °C.
For the Rh-B@β-FeOOH nanomaterials, 20 mL of rhodamine-B (2.0 mg dissolved in 20 mL water) was initially added instead of urea, followed by the same synthesis procedure as mentioned above.
2.2.1.2 Hydrothermal (HT) method. The synthesis of β-FeOOH nanoparticles was performed by a hydrothermal method without the addition of any precipitating agents. First, 1 mM of FeCl3·6H2O and 1 mM of urea were dissolved in 50 mL of water, and the solution was heated up to 50 °C and maintained there for 1 h. The obtained solution was then filtered using Whatman No. 40 filter paper. The obtained solution was then transferred to a Teflon-lined stainless-steel autoclave for hydrothermal treatment at 180 °C for 2 h and then cooled down to room temperature. The obtained nanoparticles were separated by centrifugation and washed with ethanol at least twice to remove any by-products. Finally, the obtained nanospheres were dried at 80 °C for 3 h.
For the Rh-B@β-FeOOH nanomaterials, 20 mL of rhodamine-B (2.0 mg dissolved in 20 mL water) was initially added instead of urea, followed by the same protocol as described above.
2.2.2.1 Preparation of the dye–micelle inclusion solution. The rhodamine-B/F127 (Rh-B/F127) inclusion matrix solution was initially prepared as follows:42 first, 1 g of Pluronic F-127 copolymer and 2.0 mg of Rh-B dye were mixed with 100 mL water and then stirred for 2 h to form a dye–polymer inclusion solution.
2.2.2.2 Preparation of Rh-B/F127@β-FeOOH nanorods by the co-precipitation (CoP) method. First, 1 mM of FeCl3·6H2O was mixed with 50 mL of dye–polymer matrix solution, and then the mixture was heated up to 50 °C and maintained there for 1 h to form a dye–polymer–metal-ion complex. Then, the obtained dye–polymer–metal-ion complex solution was filtered using Whatman No. 40 filter paper to remove any insoluble impurities. Then, 10 mL of 3 M NaOH solution was introduced into the reaction mixture at 80 °C, maintaining the pH at ∼11. After that, the whole solution was maintained at 100 °C for 2 h to allow the formation of Rh-B/F127@β-FeOOH nanorods. The prepared NRs were separated using centrifugation and washed with ethanol. To eliminate any by-products, the process was repeated at least twice. Finally, the obtained NPs were dried for 3 h at 120 °C.
2.2.2.3 Preparation of Rh-B/F127@β-FeOOH nanospheres by the hydrothermal (HT) method. The synthesis of Rh-B/F12@β-FeOOH nanospheres was performed by a hydrothermal method without the addition of any surfactant or precipitating agents. In brief, 1 mM of FeCl3·6H2O was mixed with 50 mL of dye–polymer matrix solution, and the mixture was heated up to 50 °C and maintained there for 1 h to form a dye–polymer–metal-ion complex. The obtained dye–polymer–metal-ion complex solution was then filtered to remove any insoluble impurities using Whatman No. 40 filter paper. The dye–micelle–metal-ion complex solution was then transferred to a Teflon-lined stainless-steel autoclave and heated at 180 °C for 2 h. The obtained Rh-B/F127@β-FeOOH nanospheres were isolated by centrifugation and washed with ethanol at least twice to remove any by-products. Finally, the obtained nanospheres were dried at 120 °C for 3 h.
2.3 Characterization techniques
Various spectroscopic methods were used to characterize the synthesized Rh-B/F127@β-FeOOH nanorods and nanospheres. UV-visible (Shimadzu) and fluorescence spectroscopy (Shimadzu) were used to investigate the optical (absorption and emission) properties. Infrared spectroscopy (FT-IR) was used to confirm the existence of functional groups in the samples. X-Ray photoelectron spectroscopy (XPS) measurements were performed to determine the phase purity and oxidative states of the elements. To accurately interpret other elemental peaks in the XPS spectrum, C-correction in XPS analysis was applied, which uses the C 1s peak from adventitious carbon on a sample surface as a reference point to calibrate the binding energy scale. This is usually accomplished by setting the C–C bond in the C 1s peak to a standard binding energy of 284.8 eV and aligning all other peaks accordingly. Scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HR-TEM) were used to examine the surface morphology and particle sizes of the samples. A confocal laser scanning microscope was used for intracellular fluorescence imaging. Flow cytometry (FACSverse Cytometer, Becton, Dickinson and Company (BD)) was used to determine the ratio of cell populations in various cell cycle phases, intercellular uptake, ROS production, and apoptosis cell death.2.4 Anticancer activity of Rh-B/F127@β-FeOOH nanospheres
 | (1) |
2.5 In vitro antioxidant assays of Rh-B/F127@β-FeOOH nanorods
 | (2) |
3. Results and discussion
3.1 Formation of dye/copolymer matrix-stabilized β-FeOOH NPs
We developed rhodamine-B/F127 polymer matrix-stabilized β-FeOOH (Rh-B/F127@β-FeOOH) nanorods and nanospheres by co-precipitation and hydrothermal methods. To synthesize Rh-B/F127@β-FeOOH nanorods, a co-precipitation approach was conducted. In this reaction system, NaOH solution was introduced into the reaction mixture at 80 °C. The color of the mixture suddenly changed from light pink to yellowish-brown, and then the temperature increased to 100 °C to form a dark-brown precipitate, which indicated the formation of an iron oxyhydroxide (β-FeOOH) colloidal suspension. Eqn (3) depicts the synthesis route for the Rh-B/F127@β-FeOOH nanorods.48| Fe3+ + 3OH− → Fe(OH)3 → FeOOH + H2O | (3) |
Also, a simple hydrothermal approach was used to synthesize Rh-B/F127@β-FeOOH nanospheres at a high temperature (180 °C) for 2 h. In this reaction, no precipitating agents were used. The chemical reaction that occurred in this reaction can be represented as follows (eqn (4)):49
| Fe3+ + 2H2O → FeOOH + 3H+ | (4) |
Scheme 1 shows a schematic representing the synthesis routes for the Rh-B/F127@β-FeOOH nanomaterials using co-precipitation (CoP) and hydrothermal (HT) methods.
3.2 Fourier-transform infrared (FT-IR) analysis
The capping of the dye/copolymer matrix and its interaction with the surface of β-FeOOH NPs was performed for the FT-IR spectroscopy investigations. The presence of functional group over the surface of the pure β-FeOOH nanoparticles was confirmed using the FT-IR bands Fig. 1(a) and (b) displays the FT-IR spectra of β-FeOOH, Rh-B@β-FeOOH, and Rh-B/F127@β-FeOOH nanoparticles.
Fig. 1(a1) and (b1) shows the presence of functional groups over the surface of β-FeOOH nanoparticles, as confirmed by the FT-IR bands at ∼3440, ∼1630, ∼1450, ∼820, and ∼620 cm−1. The bands at ∼3450 cm−1 corresponded to the presence of N–H stretching modes in amide groups. The absorption peak around 1655 cm−1 represented the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the amide, while C–N stretching frequency appeared at 1450 cm−1. The FT-IR bands at ∼1600 cm−1 were ascribed to the –OH stretching vibration, while the bands at 922 and 620 cm−1 were ascribed to the –OH bending modes in β-FeOOH.50 The FT-IR spectrum indicated that the urea was coordinated with the β-FeOOH nanorods surface through the oxygen atom of the C
O stretching of the amide, while C–N stretching frequency appeared at 1450 cm−1. The FT-IR bands at ∼1600 cm−1 were ascribed to the –OH stretching vibration, while the bands at 922 and 620 cm−1 were ascribed to the –OH bending modes in β-FeOOH.50 The FT-IR spectrum indicated that the urea was coordinated with the β-FeOOH nanorods surface through the oxygen atom of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group or the nitrogen atom of the N–H group, resulting in the formation of urea-capped β-FeOOH nanoparticles.
O group or the nitrogen atom of the N–H group, resulting in the formation of urea-capped β-FeOOH nanoparticles.
Several specific absorption bands appeared in the FT-IR spectra of the rhodamine-B capped β-FeOOH nanorods. As shown in Fig. 1(a2) and (b2), the intense peaks at ∼1410 cm−1 corresponded to the asymmetric and symmetric C–N stretches. The appearance of an intense peak may be due to the large dipole moment of carboxylate. The bands at ∼3420 cm−1 were ascribed to the existence of N–H stretching. The appearance of bands at ∼1630 cm−1 was attributed to the –OH stretching vibration, while the bands at 840 and 626 cm−1 were ascribed to the –OH bending modes in β-FeOOH.50 The overall results confirmed the formation of Rh-B-capped β-FeOOH NPs.
In order to prove the conjugation of the dye/polymer matrix over the β-FeOOH NPs, FT-IR was carried out. Fig. 1(a3) and (b3) clearly shows the presence of a peak located at ∼1100 cm−1, which was accredited to the stretching vibration of C–O–C in the polymer chain of the F127 copolymer, and was ascribed to the newly formed ester bond between the F127 copolymer and Rh-B dye. In addition, the absence, as shown in Fig. 1(a2) and (b2), of a band at 1450 cm−1 confirmed the formation of the Rh-B/F127 polymer matrix. The presence of a broadband at ∼3620 cm−1 was accredited to the stretching vibration of –OH groups in the F127 polymer. The characteristic peaks at 650 cm−1 corresponded to the Fe–O stretching vibration mode in β-FeOOH.50 These findings confirmed the formation of Rh-B/F127@β-FeOOH NPs containing both Rh-B and F127 copolymer, as predicted by the preparation process.
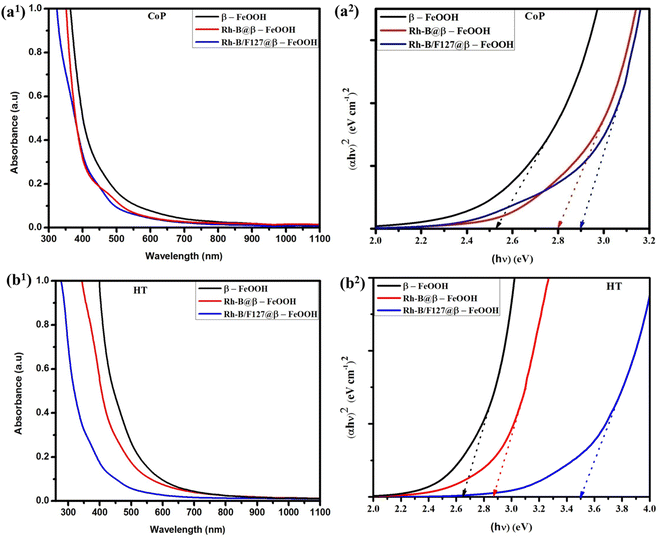
3.3 UV-Vis analysis
The absorption spectra of the synthesized β-FeOOH, Rh-B@β-FeOOH, and Rh-B/F127@β-FeOOH nanomaterials are shown in Fig. 2. As can be seen in Fig. 2(a1) and (b1), an onset absorption band can be clearly observed in the visible region. This result is consistent with data from other studies.11 In both cases, the absorption peaks of Rh-B and the Rh-B/F127-capped β-FeOOH nanomaterials were blue-shifted compared to the pristine β-FeOOH nanoparticles. The appearance of the blue-shift may be due to the formation of β-FeOOH NPs by the dye/copolymer matrix. The band gap (Eg) for the synthesized samples was ascertained by extrapolation from the absorption edge, which is given by the following equation:51| (αhν)n = A(hν − Eg) | (5) |
| S. no. | Samples | Bandgap (eV) | |
|---|---|---|---|
| Nanorods | Nanospheres | ||
| 1 | β-FeOOH | 2.52 | 2.60 |
| 2 | Rh-B@β-FeOOH | 2.80 | 2.86 |
| 3 | Rh-B/F127@β-FeOOH | 2.90 | 3.50 |
3.4 Photoluminescence analysis
The PL spectra of the synthesized β-FeOOH nanoparticles were recorded at an excitation wavelength of 360 nm and the corresponding emission spectra are shown in Fig. 3. The synthesized nanorods displayed three different emissions with lower intensities located at 437, 477, and 518 nm, respectively and their respective spectra are presented in Fig. 3(a).Unlike the nanorods, the nanospheres emitted five different emissions at 360, 407, 446, 482 and 520 nm upon excitation at the 320 nm wavelength (Fig. 3(b1) and (b2)). The existence of an emission peak at 360 nm (due to UV emission) may be related to the recombination of electron–hole pairs in the free excitons.52 The emission bands located at 407, 446, 482, and 520 nm led to violet, deep-blue, a deep greenish-blue (cyan color), and green emissions, respectively. The emission peak that appeared at 407 nm may be due to LMCT related to the HOMO of the ligand and the LUMO centered over Fe3+ metal ions.53 The other peaks at 446, 482, and 520 nm could be ascribed to (i) 6A1g → 4T1g and 6A1g → 4T2g transitions involving d–d orbitals of Fe3+ ions, and may be to the crystal field splitting of a FeO6 octahedron with an Oh symmetry54,55 or (ii) ligand effects between the ligand functional groups and Fe3+ centers at the NPs' surfaces.56
In addition, compared to the fluorescence spectra of Rh-B@β-FeOOH nanoparticles, the Rh-B@F127 matrix-stabilized β-FeOOH NPs exhibited slightly higher emission intensities with 2–4 nm blue-shifts. This phenomenon may be attributed to either (i) surface modification by the growth of the Rh-B/F127 matrix over the surfaces of the β-FeOOH nanoparticles, which may reduce the sizes of the nanomaterials, or (ii) the interaction between the dye/F127 matrix and the internal surface of the β-FeOOH nanoparticles which may increase electron–hole recombination over the surfaces of the β-FeOOH NPs.
3.5 XPS analysis
XPS analysis confirmed the oxidation states and elemental compositions of the Rh-B/F127@β-FeOOH nanospheres. The core-level XPS spectra and their corresponding energy levels are presented in Fig. 4(a)–(f). The survey spectrum (Fig. 4(a)) showed the presence of Fe 2p, O 2p, and C 1s peaks. As shown in Fig. 4(c), the Fe 2p core was split into Fe 2p3/2 and Fe 2p1/2 peaks. The binding energies of Fe 2p3/2 and Fe 2p1/2 were located at 712 and 726 eV, respectively, which were in good agreement with the reported literature for β-FeOOH.15,57,58 Small shake-up satellite peaks at 718.9 and 733.4 eV could also be detected in the XPS spectra, which were characteristic of Fe3+ in the octahedral sites of the synthesized β-FeOOH nanospheres.16 As shown in Fig. 4(b), the presence of C 1s was shown by a peak that appeared at 283 eV due to the presence of rhodamine-B and the F127 matrix over the β-FeOOH nanospheres. From Fig. 4(d), the presence of oxygen atoms was further confirmed from the O 1s core-level spectrum, with two bands appearing at 530.1 and 531.5 eV, which were assigned to Fe–O–Fe and Fe–O–H bonds, respectively.59 The above result indicates that the oxidation state of oxygen in the present case was −2. The XPS spectrum of Fe 3p is shown in Fig. 4(e). From Fig. 4(f), the binding energy of Cl 2p at 200.00 eV suggested the presence of Cl atoms, which would be located in the hollandite channels of β-FeOOH.60 The XPS results provide evidence for the formation of Rh-B/F127@β-FeOOH nanospheres with hollandite structures. The binding energies, oxidation states, and percentage compositions of the elements present in the nanospheres are listed in Table 2.| S. no. | Core-levels | Splitting | Binding energy (eV) | Splitting width (eV) | Oxidation state | % composition (at%) |
|---|---|---|---|---|---|---|
| 1 | Fe 2p | Fe 2p3/2 | 712.0 | 13.0 | +3 | 20.6 |
| Fe 3p1/2 | 725.0 | |||||
| 2 | O 1s | O 1s (Fe–OH) | 532.0 | 2.0 | −2 | 78.3 |
| O 1s (Fe–O) | 530.0 | |||||
| 3 | C 1s | — | 283.0 | — | — | — |
| 4 | Cl 2p | — | 200.0 | — | — | 1.1 |
3.6 Crystal structural analysis
X-Ray diffraction analysis (XRD) was used to further confirm the crystal structures of the as-produced nanorods and nanospheres, and the corresponding XRD patterns are shown in Fig. 5(a) and (b). Obviously, all the diffraction peaks of the prepared sample were in good agreement with the reference data for the β-FeOOH phase.20 The peaks appearing at 2θ = 11.81°, 16.71°, 26.50°, 33.75°, 34.95°, 38.95°, 46.07°, 52.01°, 55.71°, 61.20°, 64.18°, and 68.58° could be ascribed to the (110), (200), (130), (400), (211), (301), (411), (600), (251), (002), (541), and (132) reflection planes.61 The observed peaks aligned with the tetragonal structure of the pure β-FeOOH phase (JCPDS 34-1266) and thus could be accurately indexed. There were no additional peaks from other phases, like Fe2O3, or Fe3O4, observed. The absence of peaks from other iron oxide phases suggested that the synthesized β-FeOOH nanoparticles were successfully prepared without any impurities. The widening of the diffraction peaks further confirmed that the nanoparticles had nanocrystalline structures, indicating their small size and high surface area.The approximate crystallite size “D” of the synthesized nanoparticles was estimated using the following Scherrer's equation:62
 | (6) |
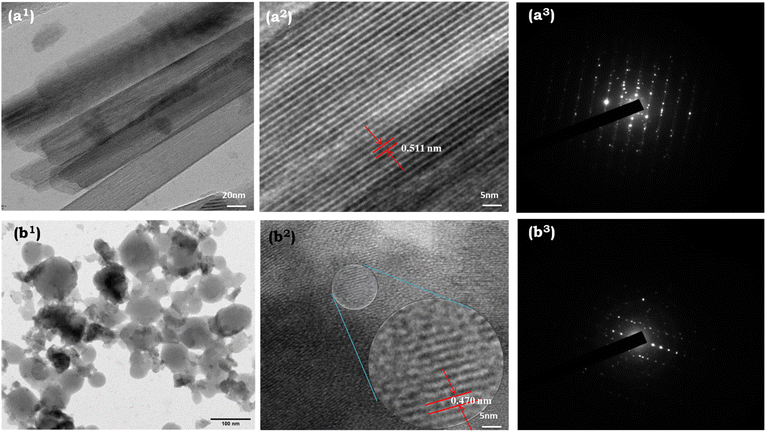
3.7 HR-TEM analysis
HR-TEM analysis was next used to investigate the morphology and size of the nanoparticles. Fig. 6 shows the HR-TEM images of the as-obtained Rh-B/F127@β-FeOOH nanomaterials synthesized using co-precipitation and hydrothermal methods. The typical TEM image (Fig. 6(a1), synthesized by CoP) displayed a rod-like morphology with widths of 17–20 nm and lengths of 0.5–1.0 μm. The SAED pattern of single nanorods is shown in Fig. 6(a3). The alignment of bright spots in parallel affirmed the single-crystalline nature of the nanorods. In addition, clear lattice fringe could be observed from Fig. 6(a2), which revealed the single-crystalline nature of the samples. The interplanar distance measured approximately 0.511 nm, aligning with the d-spacing observed in the (200) plane of β-FeOOH.As shown in Fig. 6(b1), the TEM images revealed that the hydrothermally prepared β-FeOOH nanoparticles were nearly spherical in morphology with an average diameter of 45.0 nm, which was very close to the crystallite size (nm) calculated from the XRD pattern. Fig. 6(b3) shows the SAED pattern of the nanospheres, indicating the high crystallinity of the β-FeOOH NPs. Fig. 6(b2) clearly illustrates the interatomic separation was 0.470 nm, which was good agreement with the interatomic separation in the (101) plane.
3.8 Anticancer activity of the Rh-B/F127@β-FeOOH nanospheres
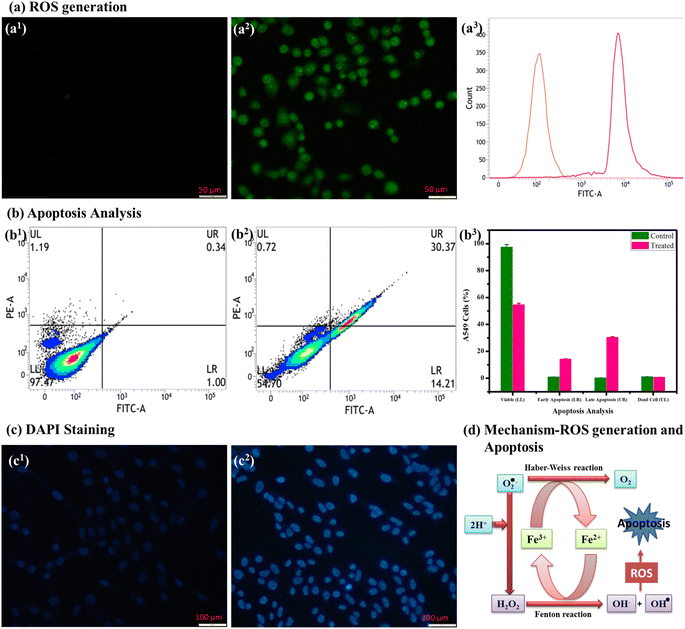
To the best of our knowledge, no study has yet evaluated the anticancer activity against Rh-B/F127@β-FeOOH nanospheres. Here, we aimed to evaluate the potential anticancer activity of β-FeOOH nanospheres against A459 cells. Flow cytometry was used to investigate the cellular uptake, cell cycle arrest, ROS generation, and apoptotic cell death, by Rh-B/F127@β-FeOOH nanospheres using annexin-V and propidium iodide (PI) staining.The A549 and L132 cells were treated with various concentrations ranging from 0–500 μg mL−1 for 24 and 48 h. The concentration-dependent cell viabilities are shown in Fig. 7, which clearly indicate that the cell viability decreased with increasing the nanoparticles' concentration. As can be seen in Fig. 7, the L132 cells displayed more than 80% cell viability at 300 μg mL−1 for 24 h (Fig. 7(a1)) after being exposed to Rh-B/F127@β-FeOOH nanospheres, whereas the A549 cells showed less than 45.0% cell viability (Fig. 7(a2)). The calculated IC50 concentration of Rh-B/F127@β-FeOOH nanospheres was 260 μg mL−1. Previous studies have investigated the cytotoxicity of iron oxide nanoparticles and their nanocomposites. For instance, Bhushan et al. (2018) demonstrated that α-Fe2O3/Co3O4 nanocomposites exhibited greater toxicity than α-Fe2O3 toward MCF-7 cells after 24 h,63 while Alkinani et al. (2024) demonstrated that Fe3O4@Glu–gingerol NPs displayed selective cytotoxicity, with a markedly lower IC50 value in A549 cancer cells (190 μg mL−1) versus normal cells (554 μg mL−1).64 Another study reported that iron oxide nanoparticles showed 50% viability in A549 cells at a concentration of 970 μg mL−1 after 24 h.65 In contrast, our result showed that the Rh-B/F127@β-FeOOH nanospheres achieved 50% viability in A549 cells at a significantly lower concentration of 260 μg mL−1. These findings suggested that the Rh-B/F127@β-FeOOH nanospheres hold promise as a cytotoxic agent against cancer cell lines. The above results confirmed that the hydrothermally synthesized nanospheres have good biocompatibility and can be used as a therapeutic agent.
FACSVerse cytometry is a capable method to verify the ratio of a cell population in various phases, such as Go/G1, S, and G2/M, by performing quantitative measurements of the nuclear DNA content in each cell cycle. In our study, propidium iodide (PI) was used to stain the nuclear DNA without disturbing the function of the nucleus. Fig. 7(c1)–(c3) show the cell cycle distribution for the untreated and treated A549 cells measured at 24 h incubation. The quantitative results (histogram) for cell cycle arrest are summarized in Fig. 7(c3). As shown in Fig. 7(c1), the cell cycle analysis indicated that the untreated cells exhibited a distribution of 52.68% in G1/G0, 22.97% in G2/M, and 22.78% in the S phase, respectively. Treatment with the Rh-B/F127@β-FeOOH nanospheres induced a significant accumulation of cells in the S phase (increasing from 22.78% to 27.77%), along with a decrease in G2/M population (decreasing from 22.97% to 15.54%). Notably, the G0/G1 phase exhibited minimal changes (Fig. 7(c2)). These findings indicated that the Rh-B/F127@β-FeOOH nanospheres could impair A549 cell proliferation by inducing S-phase cycle arrest, resulting in a reduced G2/M phase population.
One of the main mechanisms of cell death brought on by nanoparticles-induced oxidative stress has been identified as apoptosis. Among the various apoptotic pathways, the intrinsic mitochondrial apoptotic pathway has been found to play a pivotal role in nanoparticles-induced cell death since mitochondria are one of the major target organelles for nanoparticles-induced oxidative stress. The overproduction of ROS levels reduces the ROS–antioxidant equilibrium and initiates cell death by apoptosis. Previous literature has indicated that an increase in the intracellular free iron levels affects the normal ROS–antioxidant balance by promoting ROS production through Fenton and/or Haber–Weiss reactions.67
Until now, numerous iron-based nanomaterials have been developed as Fenton agents. The Fenton reaction is commonly associated with Fe2+ or Fe3+, which can produce hydroxyl radical (˙OH) through peroxidase mimicking the catalytic decomposition of H2O2. It is well known that superoxide-driven Fenton reactions and/or Haber–Weiss reactions provide an effective method for generating OH˙ in A549 cells. If superoxide and H2O2 are generated in cells, they might induce significant energy and selective cancer cell death. In this catalytic reaction, the superoxide, O2˙− serves as a precursor for H2O2 and a reductant for Fe3+ (Fe3+ → Fe2+). Finally, the Fenton reaction generates hydroxyl radicals (˙OH) from H2O2 through the oxidation of Fe2+ (Fe2+ → Fe3+) (as shown in Fig. 8(d)).67 Generally, in living systems, a “leakage” of electrons from the electron-transport chain produces superoxide radicals and hydrogen peroxide in both intracellular and extracellular volumes. The overproduction of ROS through β-FeOOH nanospheres leads to mitochondria membrane destruction and DNA fragmentation and thereby induces cancer cell apoptosis. The mechanism in the present study allows efficient tumor-specific treatment without producing significant side effects in healthy cells (IC50 value > 500 μg mL−1).
| Fe3+ + O2˙− → Fe2+ + O2 (Haber–Weiss reaction) |
| Fe2+ + H2O2 → Fe3+ + OH− + ˙OH− (Fenton reaction) |
The annexin-V assay demonstrated a marked induction of late apoptosis in A549 cells treated with Rh-B/F127@β-FeOOH nanospheres for 24 h. Specifically, the percentage of late apoptotic cells increased from 0.34% (untreated controls) to 30.37% at the IC50 concentration, indicating a late apoptotic effect (Fig. 8(c)). The histogram in Fig. 8(c3) shows the quantification% of cell death.
Fig. 9(a) shows the bright field images of A549 cell lines without the incubation of nanoparticles, wherein their cellular boundaries are clearly visible. Fig. 9(b) shows the A549 cells with Rh-B/F127@β-FeOOH nanospheres visualized under a fluorescence microscope, wherein the results showed green, yellow-orange, and red emissions that originated from the live cell membrane and early-stage and later-stage apoptosis of A549 cells, respectively. Green fluorescence appeared in the normal viable control cells because AO can only bind to viable cell membranes. The yellow-orange-colored bodies originated from early apoptosis, which may be due to nuclear shrinkage and blebbing (condensed chromatin), whereas red fluorescence showed the later-stage apoptotic cells. The above results verified the Rh-B/F127@β-FeOOH nanospheres-induced apoptosis of A549 cells, which occurred for any one of the following reasons: (i) changes in chromatin condensation, (ii) fragmented nuclei, and (ii) membrane blebbing.69
3.9 Antioxidant activity of Rh-B/F127@β-FeOOH nanorods
Reactive oxygen species (ROS) are unfavorable by-products of cellular respiration that are essential for cell signaling but can also lead to oxidative damage in living cells. ROS are molecules, ions, and free radicals that are extremely active due to their unpaired valence shell electrons. An excess of ROS in the body has been linked to a number of disorders, such as cancer, aging, cataracts, diabetic milieus, cardiovascular disease, and cognitive dysfunction. Antioxidants are crucial in stopping free radicals before they damage cells and other biological targets and for preventing a number of diseases because of their capability to scavenge free radicals.The antioxidant activity of iron-based nanoparticles, like Fe2O3 and Fe3O4 NPs, has been well-established in previous studies70,71 and is rooted in their capacity to neutralize free radicals through hydrogen atom transfer or electron donation. This mechanism protects biomolecules from oxidative damage.
Based on the above literature, the antioxidant capacities of Rh-B/F127@β-FeOOH nanorods showed potential free-radical-scavenging activity against all three separate assays: DPPH, ferric reducing antioxidant power (FRAP), and reducing power assay (RP), with each of them performed at different concentrations from 100 to 500 μg mL−1 and the results are depicted in Fig. 10(a)–(c). Ascorbic acid was used as a positive control in the same concentration range.
The antioxidant activity of Rh-B/F127@β-FeOOH nanorods was assessed using the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) scavenging assay, as shown in Fig. 10(a). The DPPH assay provided a rapid and straightforward method for evaluating the nanorods' free-radical-scavenging capacity. The antioxidant activity is attributed to electron transfer from oxygen atoms on the particle surface to the nitrogen atom of DPPH molecules, resulting in stable molecule formation.72Fig. 10(a) illustrates the dose-dependent (100–500 μg mL−1) DPPH-scavenging activity against Rh-B/F127@β-FeOOH nanorods. The results exhibited their potent inhibitory action and dose-dependent free-radical-scavenging capability. The values of the DPPH radical scavenging efficiencies against Rh-B/F127@β-FeOOH nanorods were 62.42% (100 μg mL−1), 66.64% (150 μg mL−1), 70.86% (200 μg mL−1), 81.06% (300 μg mL−1), and 88.56% (500 μg mL−1). The free-radical-scavenging activity of Rh-B/F127@β-FeOOH nanorods was slightly higher compared with the standard (52.4–71.1%) inhibition. Therefore, our results demonstrate that Rh-B/F127@β-FeOOH nanorods have substantial antioxidant potential, consistent with or even exceeding that reported in previous studies.
The ability of an antioxidant to contribute an electron can be quantified by the reducing power assay. However, the reducing power assay may not accurately measure the overall antioxidant capacity of a compound as it only evaluates its ability to donate electrons, neglecting other important aspects, such as its stability and ability to neutralize free radicals. This assay relies on the interaction of samples with potassium ferricyanide (Fe3+) to generate potassium ferrocyanide (Fe2+). Subsequently, a reaction with ferric chloride (FeCl3) results in the formation of a ferric–ferrous complex, exhibiting a maximum absorbance at 700 nm, as illustrated in Fig. 10(b). For the measurement of the reductive ability, we investigated Fe3+ to Fe2+ transformation in the presence of β-FeOOH NRs following the standard method.73 The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity. It was observed that, the antioxidant activity and the reducing power of β-FeOOH NRs increased with their increasing concentration.
The FRAP assay can evaluate antioxidant potency by measuring the reduction of ferric ions (Fe3+) to ferrous ions (Fe2+) via electron transfer.74 This process, described by the reaction Fe3+ + e− → Fe2+, was significantly enhanced by the presence of β-FeOOH nanorods. The ferric reducing power of the Rh-B/F127@β-FeOOH nanorods was evaluated using the FRAP assay. Fig. 10(c) shows a dose-dependent (100–500 μg mL−1) increase in the reducing power was observed. The obtained results showed significant inhibition at 500 μg mL−1. Notably, the Rh-B/F127@β-FeOOH nanorods exhibited a superior ferric-ions-reducing capacity compared to ascorbic acid (vitamin C) at 500 μg mL−1, indicating their robust antioxidant potential.
The above results strongly suggest that the β-FeOOH nanorods have a strong antioxidant capacity and could potentially be used as a natural alternative to ascorbic acid in certain applications. Further analyses are required to examine the underlying mechanisms behind this enhanced reducing power and to explore the potential benefits of using β-FeOOH nanorods in various industries and applications.
4. Conclusions
In summary, dye/polymer matrix-stabilized β-FeOOH nanomaterials were developed and characterized and showed potential to serve as therapeutic agents. The as-prepared nanomaterials were characterized using various techniques, including FT-IR, UV-Vis, PL, XRD, HR-TEM, and XPS. The hydrothermally synthesized Rh-B/F127@β-FeOOH nanospheres produced multi-colored emissions. The XRD results provided additional confirmation of the crystal structure. The oxidative states and presence of various elements were confirmed by XPS spectroscopy. The obtained β-FeOOH nanomaterials showed rod-like and spherical-like morphologies, as confirmed by HR-TEM analysis. The cytotoxicity activity of β-FeOOH nanospheres against A549 cells was analyzed by MTT assay. Flow cytometry was used to analyze the reactive oxygen species (ROS) generation and apoptotic cell death caused by the β-FeOOH nanospheres. The induction of apoptosis in A549 cells was revealed by annexin-V with propidium iodide (PI) and DAPI staining. From the cell cycle analysis, it was observed that the β-FeOOH nanospheres blocked the cell cycle development of A549 cells in the S phases supported by an increase in the S-phase cell population. The antioxidant activities of the nanorods were confirmed by different assays, such as DPPH, FRAP, and PFRAP. The results showed that the nanorods have a high scavenging activity against free radicals. Overall, the present study suggests that the synthesized Rh-B/F127@β-FeOOH nanoparticles are attractive and efficient candidates for various biomedical applications, including anticancer and antioxidant activities.Data availability
The authors confirm that the data will be available on request.Author contributions
CNM, MA: construction plan, writing-original draft preparation, graphical and software work. PAP: cytotoxicity interpretation and anticancer activity. PRK, JK: XPS and HR-TEM analysis. GMR, SMKT, LG, MKH: visualization and format checking. KP: graphical and software work. All authors have given approval to the final version of the manuscript.Conflicts of interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We acknowledge Alagappa University, Karaikudi for providing HR-TEM and XPS analyses. A549 cell lines were obtained from the National Center for Cell Science (NCCS), Pune, India.References
- C. Bae, H. Kim, Y.-M. Kook, C. Lee, C. Kim, C. Yang, M. H. Park, Y. Piao, W.-G. Koh and K. Lee, Induction of ferroptosis using functionalized iron-based nanoparticles for anti-cancer therapy, Mater. Today Bio, 2022, 17, 100457 CrossRef CAS PubMed
.
- H. Zhao, Y. Dong, P. Jiang, G. Wang and J. Zhang, Highly dispersed CeO2 on TiO2 nanotube: a synergistic nanocomposite with superior peroxidase-like activity, ACS Appl. Mater. Interfaces, 2015, 7, 6451–6461 CrossRef CAS PubMed
.
- Z. Zhang, J. Hao, W. Yang, B. Lu, X. Ke, B. Zhang and J. Tang, Porous Co3O4 nanorods–reduced graphene oxide with intrinsic peroxidase-like activity and catalysis in the degradation of methylene blue, ACS Appl. Mater. Interfaces, 2013, 5, 3809–3815 CrossRef CAS PubMed
.
- M. Hu, K. Korschelt, P. Daniel, K. Landfester, W. Tremel and M. B. Bannwarth, Fibrous nanozyme dressings with catalase-like activity for H2O2 reduction to promote wound healing, ACS Appl. Mater. Interfaces, 2017, 9, 38024–38031 CrossRef CAS PubMed
.
- J. A. Champion, Y. K. Katare and S. Mitragotri, Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers, J. Controlled Release, 2007, 121, 3–9 CrossRef CAS PubMed
.
- D. Chen, W. Wei, R. Wang, J. Zhu and L. Guo, α-Fe2O3 nanoparticles anchored on graphene with 3D quasi-laminated architecture: in situ wet chemistry synthesis and enhanced electrochemical performance for lithium ion batteries, New J. Chem., 2012, 36, 1589–1595 RSC
.
- Y. Yang, M. Liu, H. Zhu, Y. Chen, G. Mu, X. Liu and Y. Jia, Preparation, characterization, magnetic property, and Mössbauer spectra of the β-FeOOH nanoparticles modified by nonionic surfactant, J. Magn. Magn. Mater., 2008, 320, 132–136 CrossRef
.
- N. G. Holm, New evidence for a tubular structure of β-iron(III) oxide hydroxide-akaganéite, Origins Life Evol. Biospheres, 1985, 15, 131–139 CrossRef CAS
.
- H. Morimoto, K. Takeno, Y. Uozumi, K. I. Sugimoto and S. I. Tobishima, Synthesis of composite electrode materials of FeOOH-based particles/carbon powder and their high-rate charge–discharge performance in lithium cells, J. Power Sources, 2011, 196, 6512–6516 CrossRef CAS
.
- J. Cai, J. Liu, Z. Gao, A. Navrotsky and S. L. Suib, Synthesis and anion exchange of tunnel structure akaganeite, Chem. Mater., 2001, 13, 4595–4602 CrossRef CAS
.
- M. Mohapatra, L. Mohapatra, S. Anand and B. K. Mishra, One-Pot Synthesis of High Surface Area Nano-Akaganeite Powder and Its Cation Sorption Behavior, J. Chem. Eng. Data, 2010, 55, 1486–1491 CrossRef CAS
.
- T. Wang, P. Zhang, S. Ni, Y. Huang, K. Qiu, J. Li, M. Zhang, H. Yin and J. Li, Synthesis of nano-akaganeite powder and its chromium adsorption behaviour, Ferroelectrics, 2019, 540, 184–192 CrossRef CAS
.
- E. A. Deliyanni, D. N. Bakoyannakis, A. I. Zouboulis, K. A. Matis and L. Nalbandian, Akaganeite-type beta-FeO(OH) nanocrystals: preparation and characterization, Microporous Mesoporous Mater., 2001, 42, 49–57 CrossRef CAS
.
- L. Zeng, W. Ren, J. Zheng, A. Wu and P. Cui, Synthesis of water-soluble FeOOH nanospindles and their performance for magnetic resonance imaging, Appl. Surf. Sci., 2012, 258, 2570–2575 CrossRef CAS
.
- Y. X. Zhang and Y. Jia, A facile solution approach for the synthesis of akaganéite (β-FeOOH) nanorods and their ion-exchange mechanism toward As (V) ions, Appl. Surf. Sci., 2014, 290, 102–106 CrossRef CAS
.
- X. Zhang, J. Ge, B. Lei, Y. Xue and Y. Du, High quality β-FeOOH nanostructures constructed by a biomolecule-assisted
hydrothermal approach and their pH-responsive drug delivery behaviours, CrystEngComm, 2015, 17, 4064–4069 RSC
.
- W. R. Richmond, J. M. Cowley, G. M. Parkinson and M. Saunders, An electron microscopy study of β-FeOOH (akaganéite) nanorods and nanotubes, CrystEngComm, 2006, 8, 36–40 RSC
.
- M. Tadic, I. Milosevic, S. Kralj, M. Mbodji and L. Motte, Silica-Coated and Bare Akaganeite Nanorods: Structural and Magnetic Properties, J. Phys. Chem. C, 2015, 119, 13868–13875 CrossRef CAS
.
- Y. Xiong, Y. Xie, S. Chen and Z. Li, Fabrication of self-supported patterns of aligned β-FeOOH nanowires by a low-temperature solution reaction, Chem.–Eur. J., 2003, 9, 4991–4996 CrossRef CAS PubMed
.
- K. Cho, B. Shin, H. K. Park, B. G. Cha and J. Y. Kim, Size-controlled synthesis of uniform akaganeite nanorods and their encapsulation in alginate microbeads for arsenic removal, RSC Adv., 2014, 4, 21777–21781 RSC
.
- M. Nesterova, J. Moreau and J. F. Banfield, Model biomimetic studies of templated growth and assembly of nanocrystalline FeOOH, Geochim. Cosmochim. Acta, 2003, 67, 1177–1187 CrossRef CAS
.
- Y. Hao, X. Gao and P. W. Chen, One-step synthesis of FeO(OH) nanoparticles by electric explosion of iron wire underwater, Def. Technol., 2022, 18, 133–139 CrossRef
.
- H. Xiong, Y. Liao, L. Zhou, Y. Xu and S. Wang, Biosynthesis of Nanocrystal Akaganéite from FeCl2 Solution Oxidized by Acidithiobacillus ferrooxidans Cells, Environ. Sci. Technol., 2008, 42, 4165–4169 CrossRef CAS PubMed
.
- N. Zhang, Q. Sheng, Y. Zhou, S. Dong and J. Zheng, Synthesis of FeOOH@PDA-Ag nanocomposites and their application for electrochemical sensing of hydrogen peroxide, J. Electroanal. Chem., 2016, 781, 315–321 CrossRef CAS
.
- Y. X. Yang, M. L. Liu, H. Zhu, Y. R. Chen, G. J. Mu, X. N. Liu and Y. Q. Jia, Preparation, characterization, magnetic property, and Mossbauer spectra of the β-FeOOH nanoparticles modified by nonionic surfactant, J. Magn. Magn. Mater., 2008, 320, 132–136 CrossRef
.
- X. Chen, Y. Zeng, Z. Chen, S. Wang, C. Xin, L. Wang, C. Shi, L. Lu and C. Zhang, Synthesis and electrochemical property of FeOOH/graphene oxide composites, Front. Chem., 2020, 8, 328 CrossRef CAS PubMed
.
- T. T. V. Phan, N. Q. Bui, S. W. Cho, S. Bharathiraja, P. Manivasagan, M. S. Moorthy, S. Mondal, C. S. Kim and J. Oh, Photoacoustic imaging-guided photothermal therapy with tumor-targeting HA-FeOOH@PPy nanorods, Sci. Rep., 2018, 8, 8809 CrossRef PubMed
.
- X.-L. Shi, G.-J. Mao, X.-B. Zhang, H.-W. Liu, Y.-J. Gong, Y.-X. Wu, L.-Y. Zhou, J. Zhang and W. Tan, Rhodamine-based fluorescent probe for direct bio-imaging of lysosomal pH changes, Talanta, 2014, 130, 356–362 CrossRef CAS PubMed
.
- H. Liu, Y. Wang, H. Li, Z. Wang and D. Xu, Luminescent rhodamine B doped core-shell silica nanoparticle labels for protein microarray detection, Dyes Pigm., 2013, 98, 119–124 CrossRef CAS
.
- Y.-H. Zhu, J.-L. Wang, H.-B. Zhang, M. I. Khan, X.-J. Du and J. Wang, Incorporation of a rhodamine B conjugated polymer for nanoparticle trafficking both in vitro and in vivo, Biomater. Sci., 2019, 7, 1933–1939 RSC
.
- J. Kennedy, E. Larrañeta, M. T. C. McCrudden, C. M. McCrudden, A. J. Brady, S. J. Fallows, H. O. McCarthy, A. Kissenpfennig and R. F. Donnelly,
In vivo studies investigating biodistribution of nanoparticle-encapsulated rhodamine B delivered via dissolving microneedles, J. Controlled
Release, 2017, 265, 57–65 CrossRef CAS PubMed
.
- X. Wang, X.-Q. Chen, H.-S. Peng, X.-F. Wei, X.-J. Wang, K. Cheng, Y.-A. Liu and W. Yang, Facile synthesis of polypyrrole-rhodamine B nanoparticles for self-monitored photothermal therapy of cancer cells, J. Mater. Chem. B, 2020, 8, 1033–1039 RSC
.
- C.-Y. Hsu and Y.-L. Liu, Rhodamine B-anchored silica nanoparticles displaying white-light photoluminescence and their uses in preparations of photoluminescent polymeric films and nanofibers, J. Colloid Interface Sci., 2010, 350, 75–82 CrossRef CAS PubMed
.
- C. Jian, C. Gong, S. Wang, S. Wang, X. Xie, Y. Wei and J. Yuan, Multifunctional comb copolymer ethyl cellulose-g-poly(ε-caprolactone)-rhodamine B/folate: synthesis, characterization and targeted bonding application, Eur. Polym. J., 2014, 55, 235–244 CrossRef CAS
.
- J. Qiao, P. Dong, X. Mu, L. Qi and R. Xiao, Folic acid-conjugated fluorescent polymer for up-regulation folate receptor expression study via targeted imaging of tumor cells, Biosens. Bioelectron., 2016, 78, 147–153 CrossRef CAS PubMed
.
- A. Cabana, A. Ait-Kadi and J. Juhász, Study of the gelation process of polyethylene oxide–polypropylene oxide – polyethylene oxide copolymer (poloxamer 407) aqueous solutions, J. Colloid Interface Sci., 1997, 19, 307–312 CrossRef PubMed
.
- H. Vu-Quang, M. S. Vinding, T. Nielsen, M. G. Ullisch, N. C. Nielsen and D. T. Nguyen,
et al., Pluronic F127-folate coated super paramagnetic iron oxide nanoparticles as contrast agent for cancer diagnosis in magnetic resonance imaging, Polymers, 2019, 11, 743 CrossRef CAS PubMed
.
- J. Gilbert, J. Hadgraft, A. Bye and L. Brookes, Drug release from Pluronic F-127 gels, Int. J. Pharm., 1986, 32, 223–228 CrossRef CAS
.
- Y. Kadama, U. Yerramillia and A. Bahadur, Solubilization of poorly water-soluble drug carbamezapine in Pluronic® micelles: effect of molecular characteristics, temperature and added salt on the solubilizing capacity, Colloids Surf., B, 2009, 72, 141–147 CrossRef PubMed
.
- Z. Wei, J. Hao, S. Yuan, Y. Li, W. Juan, X. Sha and X. Fang, Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization, Int. J. Pharm., 2009, 376, 176–185 CrossRef CAS PubMed
.
- T. Sakai and P. Alexandridis, Single-Step Synthesis and Stabilization of Metal Nanoparticles in Aqueous Pluronic Block Copolymer Solutions at Ambient Temperature, Langmuir, 2004, 20, 8426–8430 CrossRef CAS PubMed
.
- Y. Wu, J. Liu, J. Ma, Y. Liu, Y. Wang and D. Wu, Ratiometric Nanothermometer Based on Rhodamine Dye-Incorporated F127-Melamine-Formaldehyde Polymer Nanoparticle: Preparation, Characterization, Wide-Range Temperature Sensing, and Precise Intracellular Thermometry, ACS Appl. Mater. Interfaces, 2016, 8, 14396–14405 CrossRef PubMed
.
- D. Chowrasia, C. Karthikeyan, L. Choure, M. Gupta, M. Arshad and P. Trivedi, Synthesis, characterization and anticancer activity of some fluorinated 3,6-diaryl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazoles, Arabian J. Chem., 2017, 10, S2424–S2428 CrossRef CAS
.
- S. Karthik, R. Sankar, K. Varunkumar and V. Ravikumar, Romidepsin induces cell cycle arrest, apoptosis, histone hyperacetylation and reduces matrix metalloproteinases 2 and 9 expression in bortezomib sensitized non-small cell lung cancer cells, Biomed. Pharmacother., 2014, 68, 327–334 CrossRef CAS PubMed
.
- A. Serpen, E. Capuano, V. Fogliano and V. Gökmen, A new procedure to measure the antioxidant activity of insoluble food components, J. Agric. Food Chem., 2007, 55, 7676–7681 CrossRef CAS PubMed
.
- R. Pulido, L. Bravo and F. Saura-calixto, Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay, J. Agric. Food Chem., 2000, 44, 3396–3402 CrossRef PubMed
.
- I. E. F. Benzie and J. J. Strain, The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay, Anal. Biochem., 1996, 239, 70–76 CrossRef CAS PubMed
.
- A. Ghanbariasad, S. M. Taghizadeh, P. L. Show, S. Nomanbhay, A. Berenjian, Y. Ghasemi and A. Ebrahiminezhad, Controlled synthesis of iron oxyhydroxide (FeOOH) nanoparticles using secretory compounds from Chlorella vulgaris microalgae, Bioengineered, 2019, 10, 390–396 CrossRef CAS PubMed
.
- C. Luna, M. Ilyn, V. Vega, V. M. Prida, J. González, R. Mendoza-Reséndez, M. de Lardizabal and S. Sebastián, Size distribution and frustrated antiferromagnetic coupling effects on the magnetic behavior of ultrafine akaganéite (β-FeOOH) nanoparticles, J. Phys. Chem. C, 2014, 118, 21128 CrossRef CAS
.
- S. Bashir, R. W. McCabe, C. Boxall, M. S. Leaver and D. Mobbs, Synthesis of α- and β-FeOOH iron oxide nanoparticles in non-ionic surfactant medium, J. Nanopart. Res., 2009,(11), 701–706 CrossRef CAS
.
- V. Renuga, C. Neela Mohan, M. S. M. Jaabir, P. A. Prakash and M. Navaneethan, Synthesis and Surface Passivation of CuInS2/MnS/ZnS Core–Multishell Nanocrystals, their Optical, Structural, and Morphological Characterization, and their Bioimaging Applications, Ind. Eng. Chem. Res., 2018, 57, 15703–15721 CrossRef CAS
.
- A. Lassoued, B. Dkhil, A. Gadri and S. Ammar, Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method, Results Phys., 2017, 7, 3007–3015 CrossRef
.
- I. P. Pozdnyakov, A. V. Kolomeets, V. F. Plyusnin, A. A. Melnikov, V. O. Kompanets, S. V. Chekalin, N. Tkachenko and H. Lemmetyinen, Photophysics of Fe(III)–tartrate and Fe(III)–citrate complexes in aqueous solutions, Chem. Phys. Lett., 2012, 530, 45–48 CrossRef CAS
.
- X. S. Xu, T. V. Brinzari, S. Lee, Y. H. Chu, L. W. Martin, A. Kumar, S. McGill, R. C. Rai, R. Ramesh, V. Gopalan, S. W. Cheong and J. L. Musfeldt, Optical properties and magnetochromism in multiferroic BiFeO3, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 134425 CrossRef
.
- J. H. Jung, M. Matsubara, T. Arima, J. P. He, Y. Kaneko and Y. Tokura, Optical Magnetoelectric Effect in the Polar GaFeO3 Ferrimagnet, Phys. Rev. Lett., 2004, 93, 037403 CrossRef CAS PubMed
.
- M. F. Kircher, U. Mahmood, R. S. King, R. Weissleder and L. Josephson, A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation, Cancer Res., 2003, 63, 8122–8125 CAS
.
- G. Park, Y. –I. Kim, Y. H. Kim, M. Park, K. Y. Jang, H. Song and K. M. Nam, Preparation and phase transition of FeOOH nanorods: strain effects on catalytic water oxidation, Nanoscale, 2017, 9, 4751–4758 RSC
.
- T. Yamashita and P. Hayes, Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS
.
- F. Yang, S. Zhang, H. Li, S. Li, K. Cheng, J. S. Li and D. C. W. Tsang, Corn straw-derived biochar impregnated with α-FeOOH nanorods for highly effective copper removal, Chem. Eng. J., 2018, 348, 191–201 CrossRef CAS
.
- X. Song and J. F. Boily, Variable hydrogen bond strength in akaganeite, J. Phys. Chem. C, 2012, 116, 2303–2312 CrossRef CAS
.
- Y. Li, X. Li, Y. Hao, A. Kakimov, D. Li, Q. Sun, L. Kou, Z. Tian, L. Shao, C. Zhang, J. Zhang and X. Sun, β-FeOOH interlayer with abundant oxygen vacancy toward boosting catalytic effect for lithium sulfur batteries, Front. Chem., 2020, 8, 309 CrossRef CAS PubMed
.
- C. Neela Mohan, V. Renuga and A. Manikandan, Influence of silver precursor concentration on structural, optical and morphological properties of Cu1−xAgxInS2 semiconductor nanocrystals, J. Alloys Compd., 2017, 729, 407–417 CrossRef CAS
.
- M. Bhushan, Y. Kumar, L. Periyasamy and A. K. Viswanath, Antibacterial applications of α-Fe2O3/Co3O4 nanocomposites and study of their structural, optical, magnetic and cytotoxic characteristics, Appl. Nanosci., 2018, 8, 137–153 CrossRef CAS
.
- T. A. Alkinani, F. A. Bajgiran, M. Rezaei, A. M. Maivan, F. J. Golrokh, M. Bejarbaneh, S. R. Mojdehi, S. Gorji, R. Ghasemian, M. D. J. Pustin Sarai, F. Akbari, S. Dehghan, F. Mirzaee, N. H. Abdulrahman and A. Salehzadeh, Evaluation the cytotoxic effect of Fe3O4@Glu-gingerol on lung adenocarcinoma cell line (A549) with biological mechanisms, Heliyon, 2024, 10, e23419 CrossRef CAS PubMed
.
- S. Das, S. Diyali, G. Vinothini, B. Perumalsamy, B. Gowdhami, T. Ramasamy and B. Biswas, Synthesis, morphological analysis, antibacterial activity of iron oxide nanoparticles and the cytotoxic effect on lung cancer cell line, Heliyon, 2020, 6, 04953 Search PubMed
.
- J. A. Pietenpol and Z. A. Stewart, Cell cycle checkpoint signaling: cell cycle arrest versus apoptosis, Toxicology, 2002, 181–182, 475–481 CrossRef CAS PubMed
.
- M. A. Voinov, J. O. Sosa Pagán, E. Morrison, T. I. Smirnova and A. I. Smirnov, Surface-Mediated Production of Hydroxyl Radicals as a Mechanism of Iron Oxide Nanoparticle Biotoxicity, J. Am. Chem. Soc., 2011, 133, 35–41 CrossRef CAS PubMed
.
- J. Raju, J. M. R. Patlolla, M. V. Swamy and C. V. Rao, Diosgenin, a Steroid Saponin of Trigonella foenum graecum (Fenugreek), Inhibits Azoxymethane-Induced Aberrant Crypt Foci Formation in F344 Rats and Induces Apoptosis in HT-29 Human Colon Cancer Cells, Cancer Epidemiol., Biomarkers Prev., 2004, 13, 1392–1398 CrossRef CAS
.
- S. Y. Choi, N. Yang, S. K. Jeon and T. H. Yoon, Semi-Quantitative Estimation of Cellular SiO2 Nanoparticles Using Flow Cytometry Combined with X-ray Fluorescence Measurements, Cytometry, 2014, 85, 771–780 CrossRef PubMed
.
- S. Majeed, M. Danish, I. M. N. Mohamad, S. H. Sekeri, M. T. Ansari, A. Nanda and G. Ahmad, Bacteria mediated synthesis of iron oxide nanoparticles and their antibacterial, antioxidant, cytocompatibility properties, J. Cluster Sci., 2021, 32, 1083–1094 CrossRef CAS
.
- A. Bouafia, S. E. Laouini, A. Khelef, M. L. Tedjani and F. Guemari, Effect of ferric chloride concentration on the type of magnetite (Fe3O4) nanoparticles biosynthesized by aqueous leaves extract of artemisia and assessment of their antioxidant activities, J. Cluster Sci., 2021, 32, 1033–1041 CrossRef CAS
.
- N. Mohamed, O. E. Hessen and H. S. Mohammed, Thermal stability, paramagnetic properties, morphology and antioxidant activity of iron oxide nanoparticles synthesized by chemical and green methods, Inorg. Chem. Commun., 2021, 128, 108–572 CrossRef
.
- M. Ullah, D.-S. Kim and K.-H. Park, Evaluating antioxidant activity of phenolic mediated Fe3O4 nanoparticles using Usnea longissima methanol extract, Results Chem., 2022, 4, 10066 Search PubMed
.
- N. A. Zakariya, S. Majeed, W. Hafizah and W. Jusof, Investigation of antioxidant and antibacterial activity of iron oxide nanoparticles (IONPS) synthesized from the aqueous extract of Penicillium spp, Sens. Int., 2022, 3, 100164 CrossRef
.
Footnote |
| † Neela Mohan Chidambaram and Palanisamy Rajkumar contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |