Open Access Article
Open Access ArticleMechanochemical McMurry reaction†
Sayan K.
Jana‡
,
Sakshi Ajay
Shirsath‡
,
Debjyoti
Bhattacharjee‡
,
Pramod
Kumar‡
 and
Biplab
Maji
and
Biplab
Maji
 *
*
Department of Chemical Sciences, Indian Institute of Science Education and Research Kolkata, Mohanpur 741246, WB, India. E-mail: bm@iiserkol.ac.in
First published on 13th June 2025
Abstract
In this study, a mechanochemical adaptation of the McMurry coupling reaction was developed to synthesize ethylenes using Zn/TiCl4/Et3N as reagents. Leveraging solvent-free ball-milling conditions, the method achieved up to 97% yield across >23 substrates. The reaction was performed without inert gas protection in a Teflon milling jar and was found to be scalable and to accommodate various functional groups.
With the increase in methodological implications and promising growth in photochemistry,1 electrochemistry,2 flow chemistry,3 and mechanochemistry,4 chemists are becoming more inclined to pursue sustainable chemical syntheses. Though each methodology has its unique approach toward this goal, mechanochemistry is quite effective at mediating the reaction in the solid phase within a reduced reaction time.5 Traditional methods have emphasized solvents as a major component in facilitating the collisions of the reagents. However, at the same time, the use of solvents consistently has adverse effects on atom economy, reaction time, and energy requirements.6
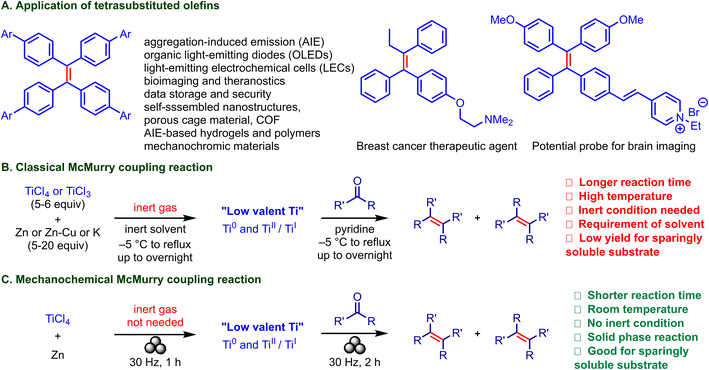
The formation of olefins is a crucial step in organic synthesis, enabling the creation of a wide range of natural and synthetic compounds used in healthcare, industry, and functional materials. Various strategies have been developed to achieve olefination reactions with precise control over stereoselectivity, regioselectivity, and chemoselectivity. Key methods include the Wittig reaction, Horner–Wadsworth–Emmons reaction, Julia–Kocienski olefination, and Tebbe olefination.7a,b Additionally, catalytic-scale olefination reactions such as olefin metathesis (e.g., Grubbs metathesis) and cross-coupling reactions (e.g., the Heck reaction) remain extensively studied and widely applied in modern synthesis.8a,b
In the 1980s, the emergence of the McMurry coupling reaction,9a–c involving the reductive coupling of two carbonyl compounds, established a pathway to synthesize alkenes, including tetra-aryl ethylenes (TAEs),10a–d which are of broad interest in material science,11 supramolecular chemistry,12a–c the pharmaceutical industry,10c,13 and biomedical applications (Fig. 1A). This thermal reaction is a two-step process and is mediated by low-valent-titanium-based reagents, which, due to their extreme sensitivity, are generated in situ using stable Ti(III) and Ti(IV) precursors and metal reductants (Zn, Zn–Cu, K, Li, etc) in an inert solvent under rigorous exclusion of air and moisture (Fig. 1B).14 The success of the reaction depends on the first step, the generation of the low-valent Ti species. Additionally, sparingly soluble carbonyl compounds hardly proceed to quantitative yields.15
In this regard, the mechanochemical McMurry coupling appears promising for the synthesis of TSEs. Deploying mechanical stress and shear could enable milder reaction conditions. Moreover, decreased reaction time and inert-gas-protection-free product formation without solvent could bring additional advantages. Ito and co-workers have recently unveiled the potential of mechanochemistry for various organic transformations.16a–c Metallic-zinc-mediated mechanochemical Simmons–Smith cyclopropanation via ball milling has also been reported by Browne.17 We reported the mechanochemical Clemmensen reduction reaction.18 In the current work, we developed a McMurry coupling reaction performed under ball-milling conditions and requiring neither inert gas nor solvent (Fig. 1C). The method enabled the coupling of a diverse range of carbonyls, including sparingly soluble substrates, allowing the synthesis of tetrasubstituted ethylenes (TSEs) in high yields.
Proceeding with our proposition, we chose benzophenone 1a as a model substrate for generating tetra-phenyl ethylene (TPE, 2a) using TiCl4 and zinc powder as the reducing reagent and trimethylamine base without solvent (Table 1). A one-pot, two-step ball-milling strategy was adopted for the reaction. The first step generated the low-valent Ti species (Ti4−nCl4−n) for the de-oxygenative reductive olefination of carbonyls.
| Entry | Variations from the above condition | 2a (% yield) |
|---|---|---|
| a Reaction conditions: TiCl4 (0.6 mmol) and Zn (1.2 mmol), two 10 mm-diameter SS balls in a 5-mL SS jar, T1 = 60 min, at 30 Hz. Then 1 (0.1 mmol), and Et3N (0.7 mmol), T2 = 120 min at 30 Hz. Yield was calculated using GC-FID, taking mesitylene as an internal standard. b 5-mL Teflon jar was used. | ||
| 1 | None | 99 |
| 2 | T2 = 90 min | 64 |
| 3 | T1 (90 min) and T2 (90 min) | 83 |
| 4 | T1 (60 min) and T2 (90 min) | 64 |
| 5 | TiCl4 (0.6 mmol), Zn (1.0 mmol) | 47 |
| 6 | TiCl4 (0.4 mmol), Zn (1.0 mmol) | 45 |
| 7 | Pyridine instead of NEt3 (T2 = 90 min) | 12 |
| 8 | K2CO3 instead of NEt3 (T2 = 90 min) | 13 |
| 9 | Without NEt3 | Trace |
| 10 | One 10 mm-diameter SS ball | 63 |
| 11 | Four 5 mm-diameter SS balls | 60 |
| 12 | 20 Hz oscillation frequency | 75 |
| 13 | Two 10 mm-diameter Zr balls | 94 |
| 14 | Two 10 mm-diameter Teflon balls | 70 |
| 15b | Two 10 mm-diameter Teflon balls | 97 |
All the reagents were weighed and mixed in air under ambient conditions. A mixture of TiCl4 (6 equiv.) and Zn (12 equiv.) was placed in a stainless steel (SS) jar with 10 mm-diameter SS balls and oscillated at 30 Hz for T1 (=60 min). Subsequently, Et3N (7 equiv.) and carbonyl compound were added to the resulting mixture, which was then milled again for T2 (=120 min). As a result, an excellent yield of 99% for 2 was achieved to our delight (Table 1, entry 1).
While screening the reaction conditions, we found that the milling times T1 and T2 played a crucial role in achieving a higher yield of product 2. Shortening T2 from 120 to 90 min decreased the yield to 64% (entry 2). With T2 at 90 min, changing T1 to 90 min increased the yield to 83% (entry 3), while reducing T1 to 60 further reduced the yield to 64% (entry 4). A similar effect was observed for the amounts of TiCl4 and Zn used, as decreasing them reduced the yield to 45–47% (entries 5 and 6) at T1 = 60 min and T2 = 90 min. The pivotal role of the base was also noticed, as changing it from triethylamine to pyridine drastically reduced the yield to 12% (entry 7). In addition, when using K2CO3 or not including base, no 2a formed (entries 8 and 9).
Next, we optimized key milling parameters, including the ball-to-reagent mass ratio, oscillation frequency, and milling medium, as they in general significantly influence reaction kinetics. Reducing the number of stainless steel (SS) balls negatively impacted the yield (entry 10). Increasing the ball number while decreasing its size and keeping the mass ratio constant also resulted in a lower yield (entry 11). Similarly, lowering the oscillation frequency diminished the reaction efficiency (entry 12).
To address corrosion observed during milling, we tested alternative solutions. Replacing SS balls with zirconium (Zr) balls gave a comparable yield but did not resolve the corrosion issue (entry 13). Using Teflon balls reduced corrosion but delivered a lower 70% yield (entry 14). We then designed a custom-made Teflon tube, which can be inserted into the SS milling jar (see ESI†). The tube, combined with Teflon balls, provided an excellent 97% yield of 2a with no corrosion (entry 15).
These optimized conditions encouraged us to further investigate the substrate scope for TAE synthesis (Fig. 2). Benzophenones containing various alkyl groups (3–5) and halogens (6–8) reacted smoothly under these conditions, delivering TAEs in excellent 91–98% yields. Fluorenone also reacted smoothly under these conditions, delivering 9 in an excellent 96% yield. However, a benzophenone derivative having a reducible cyano functional group failed to produce 10 under these conditions.
 | ||
Fig. 2
a
Reaction conditions: Table 1, entry 15. Isolated yield. E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratios were determined from 1H-NMR analysis of the crude reaction mixture. Z ratios were determined from 1H-NMR analysis of the crude reaction mixture. | ||
Furthermore, when acetophenones were treated under these ball-milling reaction conditions, tetrasubstituted olefins were produced in high yields, albeit in moderate to low E/Z ratios. Electron-neutral, electron-rich, and electron-deficient acetophenones reacted similarly well, producing olefins 11–18 in 81–96% yields with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E/Z ratios. The highest regioselectivity (5
1 E/Z ratios. The highest regioselectivity (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was seen for bromo-substituted acetophenone 17. The reaction also accommodated the free phenolic OH group to produce 18 in 76% yield. However, an indenone-based substrate failed to produce 19 under this condition, possibly due to the higher crowding in the product.
1) was seen for bromo-substituted acetophenone 17. The reaction also accommodated the free phenolic OH group to produce 18 in 76% yield. However, an indenone-based substrate failed to produce 19 under this condition, possibly due to the higher crowding in the product.
Various benzaldehydes were also tested as substrates. The reaction proceeded smoothly, and stilbenes 21–28 were isolated in high 70–93% yields. Importantly, exclusively E-selectivity was observed. In these cases, functional groups including Me (21), Ph (22), OMe (23), SMe (24), halogens (25–27), and CF3 (28) were tolerated. However, when cyanobenzyaldehyde was used as a substrate, the reaction failed to produce the desired product in an appreciable yield.
To demonstrate the practical utility of this protocol, we investigated a 10-fold scale up of the synthesis of 7 (Fig. 3a). The mechanochemical McMurry reaction was carried out on a scale of 1 mmol in a 10 mL stainless steel ball-milling jar with four 10 mm-diameter SS balls. It produced 7 in 67% yield. Compound 7 was reacted with boronic acid 29 to obtain the C4 symmetric compound 30, an important building block for synthesizing covalent organic frameworks (COFs, Fig. 3b).
 | ||
| Fig. 3 (a) Scaled-up mechanochemical synthesis. (b) Synthesis of COF building blocks. (c) McMurry coupling of a sparingly soluble substrate. | ||
Because of their importance in applications including organic light-emitting diodes, organic photovoltaics, organic semiconductors, and organic thin-film transistors, the functionalization of TAEs and the synthesis of conjugated alkenes have attracted considerable attention. We were therefore inspired to investigate the possibility of using our mechanoredox approach to promote deoxygenative dimerization of TAEs. In this regard, carbaldehyde 31 was chosen as the model. The mechanochemical reaction under these conditions produced the desired olefin 32 with a 45% yield (Fig. 3c). Notably, the low solubility of compound 31 demonstrates the utility of mechanochemical reactions for modulating challenging reactions in the solid state.
Conclusions
The potential of mechanochemistry as a sustainable method for organic synthesis has been demonstrated in this work. We established the synthesis of TAEs and TSEs in an environmentally friendly and cost-effective manner with excellent yields and broad functional group tolerance under solvent-free ball-milling conditions. By eliminating the need for solvents, costly catalysts, and sensitive reaction conditions, this technique allows for the synthesis of clean reaction products in less time than do conventional methods. These results demonstrated mechanochemistry as an environmentally benign and scalable route to green chemistry advancement.Data availability
The ESI† includes all experimental details, including optimization of the synthetic method, synthesis and characterization of all starting materials and products reported in this study, and NMR spectra of all products.Author contributions
S. K. J., D. B., and B. M. conceived and designed the project. S. K. J., S. A. S., D. B., and P. K. performed the experiments and contributed equally to this work. S. K. J., P. K., and B. M. wrote the manuscript. B. M. acquired the funding and directed the research.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. K. J. and P. K. acknowledge, respectively, PMRF and CSIR for PhD fellowships. The authors thank DST-ANRF (erstwhile SERB) (Grant No. SCP/2022/000352) for financial support.Notes and references
- N. Hoffmann, Chem. Rev., 2008, 108, 1052–1103 CrossRef CAS PubMed.
- M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS PubMed.
- H. L. D. Hayes and C. J. Mallia, Org. Process Res. Dev., 2024, 28, 1327–1354 CrossRef CAS.
- J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC.
- S. Pagola, Crystals, 2023, 13, 124 CrossRef CAS.
- X. Liu, Y. Li, L. Zeng, X. Li, N. Chen, S. Bai, H. He, Q. Wang and C. Zhang, Adv. Mater., 2022, 34, 2108327 CrossRef CAS PubMed.
- (a) B. Huang, Y. Shen, Z. Mao, Y. Liu and S. Cui, Org. Lett., 2016, 18, 4888–4891 CrossRef CAS PubMed; (b) K. Esfandiarfard, J. Mai and S. Ott, J. Am. Chem. Soc., 2017, 139, 2940–2943 CrossRef CAS PubMed.
- (a) O. M. Ogba, N. C. Warner, D. J. O'Leary and R. H. Grubbs, Chem. Soc. Rev., 2018, 47, 4510–4544 RSC; (b) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066 CrossRef CAS PubMed.
- (a) M. Ephritikhine and C. Villiers, in Modern Carbonyl Olefination, 2003, pp. 223–285, DOI:10.1002/3527601880.ch6; (b) J. E. McMurry and M. P. Fleming, J. Am. Chem. Soc., 1974, 96, 4708–4709 CrossRef CAS PubMed; (c) T. Takeda and A. Tsubouchi, J. Am. Chem. Soc., 1974, 96, 4708–4709 CrossRef PubMed.
- (a) P. Kumar and B. Maji, J. Mater. Chem. A, 2023, 11, 20752–20760 RSC; (b) R. P. Tanpure, A. R. Harkrider, T. E. Strecker, E. Hamel, M. L. Trawick and K. G. Pinney, Bioorg. Med. Chem., 2009, 17, 6993–7001 CrossRef CAS PubMed; (c) M. Banerjee, S. J. Emond, S. V. Lindeman and R. Rathore, J. Org. Chem., 2007, 72, 8054–8061 CrossRef CAS PubMed; (d) M. Zhang, Y. Yao, P. J. Stang and W. Zhao, Angew. Chem., Int. Ed., 2020, 59, 20090–20098 CrossRef CAS PubMed.
- B. C. Patra and S. Bhattacharya, Chem. Mater., 2021, 33, 8512–8523 CrossRef CAS.
- (a) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC; (b) M. L. Saha, X. Yan and P. J. Stang, Acc. Chem. Res., 2016, 49, 2527–2539 CrossRef CAS PubMed; (c) X. Yan, T. R. Cook, P. Wang, F. Huang and P. J. Stang, Nat. Chem., 2015, 7, 342–348 CrossRef CAS PubMed.
- K. Ohta, A. Kaise, F. Taguchi, S. Aoto, T. Ogawa and Y. Endo, Molecules, 2019, 24, 3966 CrossRef PubMed.
- X.-F. Duan, J. Zeng, J.-W. Lü and Z.-B. Zhang, J. Org. Chem., 2006, 71, 9873–9876 CrossRef CAS PubMed.
- T. Seo, in Palladium-Catalyzed Mechanochemical Cross-Coupling Reactions, ed. T. Seo, Springer Nature Singapore, Singapore, 2024, pp. 93–142, DOI:10.1007/978-981-97-1991-4_4.
- (a) K. Kondo, K. Kubota and H. Ito, Chem. Sci., 2024, 15, 4452–4457 RSC; (b) K. Kubota, T. Endo, M. Uesugi, Y. Hayashi and H. Ito, ChemSusChem, 2022, 15, e202102132 CrossRef CAS PubMed; (c) R. Takahashi, A. Hu, P. Gao, Y. Gao, Y. Pang, T. Seo, J. Jiang, S. Maeda, H. Takaya, K. Kubota and H. Ito, Nat. Commun., 2021, 12, 6691 CrossRef CAS PubMed.
- L. Pontini, J. A. Leitch and D. L. Browne, Green Chem., 2023, 25, 4319–4325 RSC.
- D. Bhattacharjee, S. K. Jana and B. Maji, Synthesis, 2024, 57, 84–90 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis and characterization details (PDF). See DOI: https://doi.org/10.1039/d5mr00065c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |