Open Access Article
Open Access ArticleHow external forces affect the degradation properties of perfluorooctanoic acid in mechanochemical degradation: a DFT study†
Chuan
Wang
 *abc,
Cheng
Yang
a,
Jie
Wu
a,
Ziqiu
Wang
a and
Kun
Yang
a
*abc,
Cheng
Yang
a,
Jie
Wu
a,
Ziqiu
Wang
a and
Kun
Yang
a
aCollege of Ecology and Environment, Inner Mongolia University, Hohhot, Inner Mongolia Autonomous Region 010021, China. E-mail: wangchuan437@imu.edu.cn
bInner Mongolia Autonomous Region Key Laboratory for Pollution Control and Low-Carbon Resource Utilization Research, Inner Mongolia University, Hohhot, Inner Mongolia Autonomous Region 010021, China
cNational Institute of Chemistry, Department of Catalysis and Chemical Reaction Engineering, Hajdrihova 19, SI-1000 Ljubljana, Slovenia
First published on 11th June 2025
Abstract
Perfluorooctanoic acid (PFOA), recognized as a persistent organic pollutant, poses a serious threat to the environment and human health. Currently, mechanochemical degradation is considered a highly promising technology for the degradation of PFOA. This study systematically employs density functional theory (DFT) and the COGEF (COnstrained GEometry to simulate Forces) model to deeply investigate the impact of external forces on the degradation properties of PFOA molecules. Through quantum chemical calculations, we analyzed in detail the changes in the electronic structure, chemical reactivity, and decarboxylation reaction process of PFOA molecules under the influence of external forces. The results show that the application of external forces significantly alters the electronic density distribution of PFOA molecules, thereby enhancing their reactive activity, especially in terms of nucleophilicity and radical reactivity at the carboxylate end. Moreover, the application of external mechanical force reduces the Gibbs free energy change of the decarboxylation reaction, thereby making the reaction energetically favorable. This study not only theoretically elucidates the mechanism of mechanochemical degradation of PFOA, but also provides a basis for optimizing its mechanochemical degradation technology. In addition, this study also provides a systematic theoretical perspective for exploring the mechanisms of mechanochemical degradation.
1 Introduction
Perfluorooctanoic acid (PFOA), an artificially synthesized perfluorinated compound, has been extensively utilized in the production of various consumer goods, including non-stick cookware, waterproof apparel, and fire-fighting foams, owing to its exceptional chemical stability and unique surface-active properties.1–6 However, PFOA poses a severe threat to human health, with evidence indicating its potential to disrupt the endocrine system, impair reproductive development, damage the immune system, and its classification as a carcinogenic substance.7–13 The high stability of PFOA in the environment hinders its natural decomposition, allowing it to persist in aquatic systems, soils, and sediments. Its long-range transport and bioaccumulation through the food chain have led to the detection of PFOA in biota in the Southern Hemisphere, demonstrating its globally distributed characteristics.7,14–19 Consequently, the production and use of PFOA have been restricted worldwide, with PFOA and its related compounds being listed as controlled substances in the Persistent Organic Pollutants (POPs) regulation by the European Chemicals Agency (ECHA).As understanding of the environmental and health risks associated with PFOA deepens, research into its degradation is rapidly advancing. Investigators are exploring a variety of methods to effectively degrade this persistent pollutant.14,20–23 Given the high resistance of PFOA molecules to conventional organic oxidation methods, it is necessary to enhance the energy input of the degradation process through physical means, generating highly reactive free radicals to achieve defluorination and carbon chain mineralization of PFOA.24–26 The degradation process of PFOA typically involves the removal of CF2 units from the carboxylic acid end (or sulfonic acid end), forming short-chain perfluorocarboxylic acids, and continuing to remove CF2 units until ultimately converting to CO2, H+, and F−.19,27–32
In the field of environmental chemistry, mechanochemical degradation has emerged as an innovative technology that activates chemical reactions through mechanical force, facilitating the degradation of pollutants.33–37 Significant progress has been made in this area in recent years, particularly in the degradation of persistent organic pollutants (POPs). Research on mechanochemical degradation dates back to 1994 when Rowlands38 used mechanochemical methods to degrade organic pollutants such as polychlorinated biphenyls (PCBs) with satisfactory results. Since then, mechanochemical degradation has been widely applied to the degradation of chlorinated and brominated organic pollutants, including PCBs, dioxins, chlorobenzenes, pentachlorophenol, tetrabromobisphenol A, and decabromodiphenyl ether.33,34,39–52 Recently, the mechanochemical degradation of POPs through ball milling processes has garnered widespread interest among researchers due to its mild reaction conditions, low solvent demand, and green efficiency.53–56 Researchers have successfully degraded PFOA in soil and sediments using mechanochemical methods, achieving nearly 100% degradation efficiency.23,24,57–62 Mechanochemical degradation shows great potential in the treatment of solid PFOA, with advantages including: (1) mild reaction conditions that can be carried out at room temperature and pressure; (2) high degradation efficiency suitable for treating high-concentration and high-dose pollutants; (3) broad applicability for the degradation of pure PFOA, PFOA in soil, and PFOA fixed by adsorbents; (4) the reaction is conducted in a closed mill pot, ensuring a safe and controllable process. In the mechanochemical degradation of PFOA and other perfluorinated pollutants, strong bases such as KOH serve as efficient defluorinating agents, initially attacking the carboxylic or sulfonic groups in the fluorinated molecules, followed by detachment and mineralization processes, resulting in the formation of sulfates or carbonates. Fluoride ions combine with potassium ions, their positions replaced by hydroxide ions, and the carbon chain loses dihydroxylated carbon atoms under the induction of hydroxide ions, producing potassium formate and water. This peeling process of carbon atoms repeats until the carbon chain is completely destroyed.23,53,55,63,64
The introduction of quantum chemistry theory into the field of mechanochemistry enables the interpretation of the effects of external forces on the structural characteristics and energy states of systems from the microscale perspective of atoms and molecules. Quantum chemical models can quantitatively calculate the modification of reaction potential energy surfaces under external forces, thereby providing critical data support for researchers to analyze the regulatory mechanisms of external forces on reaction pathways based on transition state theory. In recent years, with the deepening cross-application of quantum chemistry methods in mechanochemistry, an independent disciplinary branch—quantum mechanochemistry—has gradually emerged.
The constrained geometries simulate external force (COGEF) model is currently widely adopted in this field to theoretically simulate the action of external forces.65 The core idea of this model embodies a bidirectional interaction mechanism: the application of external mechanical forces induces characteristic changes in molecular geometries; if the deformed molecular geometry is pre-acquired, the magnitude and direction of the equivalent external force can be back-calculated through the nuclear gradient of energy at this geometric structure. In recent years, the COGEF model has demonstrated broad applicability in mechanochemical calculations, with typical application scenarios including kinetic studies of force-responsive functional group reactions and energy barrier analysis of mechanochemically induced bond cleavage processes. By transforming macroscopic mechanical forces into quantitative changes in microscopic configurational parameters, this model provides an important research tool for establishing cross-scale mechanics-chemistry coupling theories.66–68
Although mechanochemical degradation has been proven to be a green and efficient method for pollutant degradation at the experimental level, there is a scarcity of literature that explains the impact of external forces on the degradation properties of pollutant molecules from a microscopic and theoretical perspective, as well as an in-depth exploration of the specific role and function of mechanical force in the degradation process of pollutants. Therefore, this study focuses on the degradation process of PFOA in a solvent-free ball milling system. We utilize quantum chemical computational methods to deeply analyze the impact of external forces on the properties of PFOA molecules, thereby exploring how mechanical force alters the degradation process of PFOA. The purpose of this paper is not only to elucidate the degradation mechanism of PFOA molecules but also to establish a methodological tool using quantum chemical calculations to explain the mechanistic principles of mechanochemical degradation. This will aid researchers in deeply understanding the reaction mechanisms of mechanochemical degradation processes at the atomic and molecular levels, which is of significant importance for the future application and optimization of mechanochemical degradation processes.
2 Theoretical computation and methods
2.1 The COGEF model
The COGEF (COnstrained GEometry to simulate Forces) model is a quantum chemical computation method designed to simulate the effects of mechanical forces on molecules.69,70 This model is predicated on the concept that the application of external forces to a molecule induces changes in its geometric configuration. Conversely, if the deformed molecular geometry is known, the magnitude of the external force can be calculated using the nuclear gradient of the energy within that geometric structure. Under the COGEF model, a set of fixed values is used to constrain the distance between two terminal atoms. After specifying the terminal atom distance R0, the energy-relaxed structure of the system is obtained using self-consistent field methods (constrained geometric optimization), from which the external force required to maintain the terminal atom distance R0 is calculated.The COGEF model can be implemented in most mainstream quantum chemical programs. It is not only capable of evaluating the strength of chemical bonds but also of obtaining the precise configuration of a molecule under force through geometric optimization. Subsequently, the electronic structure and chemical properties of the molecule in this state can be analyzed through potential energy surface and wave function analysis. The COGEF method has been validated as a powerful tool for predicting mechanochemical reactivity, and its application in the field of mechanochemistry is continuously advancing, providing a reliable theoretical tool and computational method for environmental pollution control, materials science, and chemical synthesis.
2.2 Conceptual density functional theory
Conceptual Density Functional Theory (CDFT), developed by R. G. Parr71 and others, is a theoretical framework that plays a significant role in the field of quantum chemistry and wave function analysis. The core idea of CDFT is to predict changes in molecular reactivity upon gain or loss of electrons through variations in electron density.72 CDFT is primarily used to predict and explain the reactivity and reactive sites of chemical substances, which is of great importance for studying chemical reactions.73 Based on the concept of electron density, CDFT introduces global and local reactivity descriptors to analyze the chemical reactivity of molecules. These descriptors include the Fukui function, dual descriptor, and relative nucleophilic/electrophilic indices, among others. In this study, we utilized CDFT to calculate global CDFT descriptors for PFOA molecules under force, such as chemical potential, hardness, Mulliken electronegativity, electrophilic index, and nucleophilic index. Additionally, we focused on the condensed Fukui functions of the main reactive atoms in the PFOA molecule to assess the impact of external forces on its molecular degradation properties.2.3 Theoretical functional and basis set selection
The theoretical approach of quantum chemical calculations in this study primarily employs the wB97XD functional,74 with the Def2TZVP75 basis set. The wB97XD functional was designed to account for non-covalent interactions, particularly weak interactions such as hydrogen bonding and van der Waals forces, thus demonstrating superior performance in handling interactions between carbon atoms in organic compounds. In this study, strong non-bonding interactions are present in the PFOA molecule, making the use of the wB97XD functional a suitable choice. The Def2TZVP basis set is an improved version of Dunning's cc-pVTZ basis set series, suitable for systems requiring high-precision calculations of electron correlation energies. Additionally, Def2TZVP incorporates extra polarization functions based on the original cc-pVTZ basis set, which better describe the electronic polarization effects of molecules. In this study, the F atom in the PFOA molecule exhibits a strong electronic polarization effect; therefore, the Def2TZVP basis set can accurately calculate the structure and chemical properties of PFOA.2.4 Computational software and workflow
In this study, molecular modeling and visualization were carried out using GaussView software.76 Quantum chemical calculations were performed using the Gaussian16 software,77 and wave function analysis relied on the Multiwfn software.78,79 All CDFT descriptor calculations were also completed using Multiwfn. The computational workflow for this study is outlined as follows (as shown in Fig. 1):3 Computational results and analysis
3.1 Molecular structure of PFOA
The constructed molecular structure of PFOA is depicted in Fig. 2a. Please refer to the atomic numbering in the figure, as these numbers will be frequently cited in subsequent research and analysis. It can be observed that the carbon atoms in the PFOA molecule are almost entirely encapsulated by fluorine atoms, and due to the electrostatic repulsion between the fluorine atoms, the entire PFOA molecule exhibits an elongated, chain-like structure. The PFOA molecule is replete with extremely stable C–F bonds, which confer upon PFOA its high stability in the environment, making it difficult to decompose. | ||
| Fig. 2 Molecular structure of PFOA: (a) F = 0 nN (in its original state without external force); (b) F = 0.025 nN. | ||
Table 1 summarizes some key geometric parameters of the PFOA molecule, providing a foundation for further analysis. In the PFOA molecule, the C–C bonds are comparatively weaker (with longer bond lengths and lower bond orders). However, the C atoms are surrounded by F atoms, which makes it difficult for external degradation reagents to attack the C–C bonds. Therefore, the degradation of the PFOA molecule needs to be based on the disruption of the C–F bonds. By comparing the bond length and bond order data in the PFOA molecule, it can be observed that there is significant variability among the C–F bonds. The C–F bonds located far from the carboxylate end (such as the C1–F20 bond) are shorter and have a higher bond order, indicating that these C–F bonds are more stable.
| Bond length | Bond order | |||
|---|---|---|---|---|
| Maximum value | Minimum value | Maximum value | Minimum value | |
| C–O | 1.34109(C8–O9) | 1.20112(C8–O11) | 1.90735(C8–O11) | 1.09110(C8–O9) |
| C–C | 1.55677(C4–C5) | 1.54735(C7–C8) | 0.88452(C1–C2) | 0.86069(C2–C3) |
| C–F | 1.35724(C6–F25) | 1.33661(C1–F19) | 1.03979(C1–F20) | 0.95179(C5–F14) |
3.2 Comparison of molecular properties under different force conditions
During the degradation of PFOA, the high chemical inertness of fluorine atoms typically results in degradation initiating at the carboxylate end. Consequently, in this study, we focused on the scenario where the oxygen atoms (O9 and O11) at the carboxylate end are subjected to external forces. It is important to note that the external forces must be applied in pairs (with equal magnitude and opposite direction) to a specific pair of atoms within the PFOA molecule; otherwise, they would merely induce overall translation of the molecule without affecting its configuration. In the COGEF model, the direction of the force must be aligned with the line connecting the pair of atoms, with a force magnitude of 0.025 nN, as illustrated in Fig. 2b. The computational results are presented in Table 2. Compared to its original state, the application of external force leads to an increase in the molecular energy. This is readily understood, as the external force perturbs the potential energy surface of the PFOA molecule, displacing it from its lowest energy position. Additionally, the imposed force performs work on the molecule, which is a factor contributing to the energy increase. Both the energy levels of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) increase, but the LUMO energy change is more significant, resulting in a reduction of the HOMO–LUMO gap under the influence of external force. This finding indicates that applying external force to the oxygen atoms can enhance the molecule's reactive activity.| External forces (nN) | Total energy (hartree) | HOMO (eV) | LUMO (eV) | Gap (eV) |
|---|---|---|---|---|
| F = 0 | −1953.4347 | −8.4996 | −1.1904 | 7.3091 |
| F = 0.025 | −1953.4301 | −8.4212 | −1.1447 | 7.2765 |
Subsequently, we calculated and analyzed the changes in the Conceptual Density Functional Theory (CDFT) parameters of the molecule under the influence of external force, with the results detailed in Table 3. External force can alter the overall chemical properties of the PFOA molecule to some extent, as evidenced by key indicators such as chemical potential, hardness, Mulliken electronegativity, electrophilic index, and nucleophilic index. When an external force is applied, the distance between oxygen atoms increases, and the electron density at the carboxylate end decreases, making the molecule more inclined to gain electrons. Consequently, the electrophilic index and Mulliken electronegativity increase, while the molecule's ability to lose electrons becomes more difficult, leading to a decrease in the nucleophilic index. These changes reveal how external forces can influence the chemical properties of a molecule by altering its electronic distribution.
| External forces (nN) | Softness (eV−1) | Electronegativity (eV) | Electrophilic index (eV) | Nucleophilic index (eV) |
|---|---|---|---|---|
| F = 0 | 0.0870 | 4.6376 | 0.9351 | 0.6219 |
| F = 0.025 | 0.0874 | 4.6616 | 0.9501 | 0.6078 |
Under two distinct force conditions, we calculated the three-dimensional real-space dual descriptor functions of the molecule, with the results depicted in Fig. 3. The blue and green areas in the figure respectively denote the nucleophilic and electrophilic sites of the PFOA molecule. It can be observed that both nucleophilic and electrophilic sites are primarily concentrated near the carboxyl group of the PFOA molecule. In both force states, the distribution of the dual descriptor functions does not exhibit significant macroscopic differences, indicating that the applied external forces do not markedly alter the spatial position of the reactive groups. This observation is of significant importance for understanding how external forces impact the chemical reactivity of molecules, as it reveals that the positions of the reactive sites on the PFOA molecule remain relatively stable even under the influence of external forces.
 | ||
| Fig. 3 Influence of external forces on the three-dimensional real-space dual descriptor functions: (a) F = 0 nN; (b) F = 0.025 nN. | ||
3.3 Analysis of the impact of external force intensity on molecular chemical properties
 | ||
| Fig. 4 Trends of basic molecular quantization parameters with varying strengths of external forces: (a) molecular energy; (b) HOMO energy; (c) LUMO energy; (d) HOMO–LUMO gap. | ||
Synthesizing these observations, we can conclude that increasing external force progressively elevates the energy of the molecular system, implying that the molecular properties become more dynamic. Concurrently, the augmentation of external force favors a reduction in the HOMO–LUMO gap value, making the molecule's reactivity more readily activated. These findings are of significant importance for understanding how external forces influence the electronic structure and chemical reactivity of molecules, providing valuable insights for further exploration of the mechanochemical degradation mechanisms.
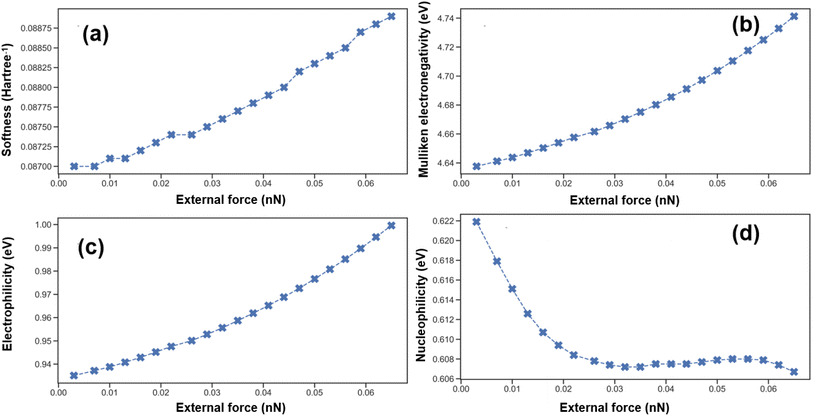 | ||
| Fig. 5 Trends of molecular global descriptors with varying strengths of external forces: (a) softness; (b) Mulliken electronegativity; (c) electrophilic index; (d) nucleophilic index. | ||
In summary, the application of external forces has a significant impact on the electronic properties and reactive activity of PFOA molecules. The increase in softness, electronegativity, and electrophilic index indicates that external forces make the molecule more dynamic and more likely to engage in chemical reactions. Conversely, the decrease in nucleophilic index may imply that the molecule's ability to donate electrons is inhibited in certain directions. These changes provide crucial clues for understanding how mechanical forces affect the chemical behavior of PFOA molecules.
 | ||
| Fig. 6 Trends of the condensed Fukui functions f+ for different atoms on the carboxylate group with external forces: (a) C8_f+; (b) O9_f+; (c) H10_f+; (d) O11_f+. | ||
As shown in Fig. 6, with the increase in external force, the condensed Fukui functions f+ of the atoms on the carboxylic group exhibit a clear upward trend, approximately linear in relation to the external force. This phenomenon suggests that the ability of these atoms to gain electrons is significantly enhanced with the application of an external force. The increase in the condensed Fukui function f+ implies an increased attraction to nucleophilic reagents, meaning they are more susceptible to nucleophilic attack. This trend reflects the subtle changes in the molecular structure under the influence of external forces, which may lead to a redistribution of electron density around these reactive atoms. Such changes in electron density provide more opportunities for reaction with nucleophilic reagents, thereby reducing the activation energy of the reaction. Consequently, under the influence of external forces, the introduction of a reducing agent can effectively promote the degradation process of PFOA. The addition of a reducing agent supplies extra electrons that can be captured by the atoms on the carboxylic group, thus triggering the degradation reaction of the PFOA molecule.
The Fukui function f0 represents the ability of an atom to engage in radical reactions, reflecting changes in electron distribution within a molecule in the absence of a net gain or loss of electrons. By calculating the condensed Fukui function f0 for the atoms on the carboxylic group of the PFOA molecule, we can assess their reactivity in radical reactions. The f0 values in Fig. 7 indicate that C8 and O11 are the most significant sites for radical reactions within the PFOA molecule. With the increase of external force, the condensed Fukui function f0 values for the atoms on the carboxylic group generally exhibit an upward trend, demonstrating that the reactivity of these atoms in radical reactions, which do not involve significant electron transfer, is enhanced under the influence of external force.
 | ||
| Fig. 7 Trends of the condensed Fukui functions f0 for atoms on the carboxylate group with external forces: (a) C8_f0; (b) O9_f0; (c) H10_f0; (d) O11_f0. | ||
The f− value represents the susceptibility of an atom to electrophilic attack. Based on the calculated f− values, we can identify the carbonyl oxygen atom O11 as the most significant electrophilic reaction site within the PFOA molecule. This is evidenced in Fig. 8, where the condensed Fukui functions f− for atoms C8 (carbon atom), O9 (hydroxyl oxygen atom), and O11 all exhibit a decreasing trend with the increase in external force. This trend indicates that the reactivity of these atoms as electrophilic reaction sites is actually diminishing with increasing external force.
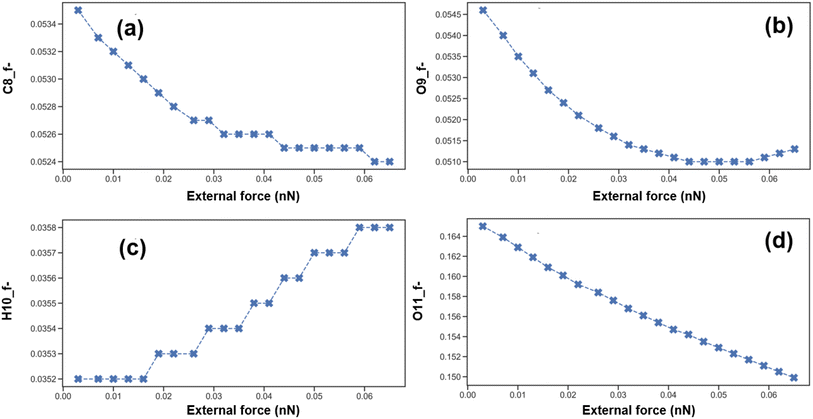 | ||
| Fig. 8 Trends of the condensed Fukui functions f− for atoms on the carboxylate group with external forces: (a) C8_f−; (b) O9_f−; (c) H10_f−; (d) O11_f−. | ||
However, the condensed Fukui function f− for the H10 atom displays a unique stepwise increasing trend, which may imply that the electrophilic reactivity of the 10H atom is enhanced under specific external forces. Nonetheless, considering that O11 is the most critical electrophilic reaction site in the molecule, we can conclude that, overall, the electrophilic reactivity at the carboxylate end of the PFOA molecule decreases with increasing external force. This suggests that the difficulty of oxidizing and degrading the PFOA molecule under external force increases, possibly due to the distortion of the molecular structure caused by the external force, which increases the activation energy required for the reaction and thus inhibits the occurrence of oxidative degradation reactions.
In this section, we conducted a detailed analysis of the Fukui functions ( f+, f0, f−) for key reactive atoms in the PFOA molecule to explore the impact of external forces on its chemical reactivity. For the PFOA molecule, external forces have a significant influence on its chemical reactivity. As the external force increases, the nucleophilic and radical reactivity of key atoms in the PFOA molecule increases, while the electrophilic reactivity declines. This indicates that by modulating external forces, we can alter the electronic structure and chemical reactivity of the PFOA molecule, thereby affecting its degradation process. These findings provide an important theoretical foundation for understanding the degradation mechanism of PFOA under the influence of external forces and offer potential avenues for the development of new PFOA treatment technologies.
3.4 Impact of external force on the PFOA decarboxylation reaction
The degradation process of PFOA (perfluorooctanoic acid) typically initiates at the carboxylic acid end of the molecule, as the carbon atom at this end is connected to the carboxylic acid group, which exhibits higher reactivity compared to the other perfluoroalkyl parts of the molecule. During the degradation process, the PFOA molecule first undergoes decarboxylation at the carboxylic acid end. Therefore, the focus of this section is to investigate the impact of external forces on the decarboxylation reaction of the PFOA molecule.In carboxylic acid molecules, the carbon atom in the carboxyl group is sp2 hybridized, with its three sp2 hybrid orbitals forming three σ bonds with the hydrocarbon group and two oxygen atoms, all of which lie in the same plane. The remaining p electron of the carbon atom forms a π bond with a p electron of the oxygen atom in the carbonyl group. The hydroxyl oxygen in the carboxyl group carries a pair of lone electrons, which can form a p–π conjugated system with the π bond in the carbonyl group. We conducted a comparative analysis of the conjugation effect on the carboxyl group in its initial state and under the influence of external forces. In the initial state, the bond lengths of C8–O9 and C8–O11 in the PFOA molecule are 1.34581 Å and 1.19268 Å, respectively, with a difference of 12.8%. Under the influence of an external force of 0.025 nN, the bond lengths of C8–O9 and C8–O11 become 1.41444 Å and 1.21603 Å, respectively, with a difference of 16.3%. This change indicates that the conjugation effect on the carboxyl group is weakened, and the stability of the carboxyl group decreases under the action of external forces, which is favorable for promoting the decarboxylation reaction.
We further calculated the changes in bond order of the C7–C8 bond, which breaks during the decarboxylation reaction, as the external force increases. The results are shown in Fig. 9. We observed that as the external force increases, the bond order of the C7–C8 bond tends to decrease. This indicates that the external force weakens the C7–C8 bond, making it more susceptible to cleavage. Consequently, the applied force facilitates the decarboxylation reaction of PFOA by lowering the strength of bonds, thus favoring the reaction to proceed.
 | ||
| Fig. 9 Trends of the bond order of the C7–C8 bond (the bond broken in the decarboxylation reaction) with external forces. | ||
Through quantum chemical simulations, we conducted an in-depth study of the decarboxylation process of PFOA. The analysis of the computational results revealed that during the decarboxylation reaction, the carboxylate group detaches from the PFOA molecule, forming two radicals with unpaired electrons—C7F15˙ and COOH˙. The spin density distribution of the radical products is depicted in Fig. 10. In Fig. 10a, it can be observed that the original PFOA molecule contains no unpaired electrons, with the spin density being zero at all locations. Fig. 10b and c display the spin density of the products under conditions without external force and with the application of 0.025 nN external force, respectively. In both scenarios, the products consist of two radicals. This indicates that the external force does not alter the types of products resulting from the decarboxylation reaction.
The Gibbs free energy change (ΔG) profile for the decarboxylation of PFOA is shown in Fig. 11. Under standard conditions, the decarboxylation of PFOA is a classically non-spontaneous reaction (ΔG > 0). However, our calculations reveal a striking reduction in ΔG upon applied mechanical force. When considering temperature effects on ΔG, the force-exerted system exhibits a significantly accelerated decline in ΔG. At 1000 K, the ΔG value reaches −0.001856 hartree (approximately −4.89 kJ mol−1), indicating a transition to a thermodynamically spontaneous process. This finding is particularly significant in the context of ball milling, where localized high-temperature “hotspots” are generated during mechanical activation. The combination of mechanical force and elevated temperature creates synergistic conditions that drive ΔG below zero, suggesting that mechanochemical activation can render PFOA decarboxylation an energetically favorable process.
4 Discussion
This study employs quantum chemical computational methods to explore the impact of external forces on the degradation properties of PFOA molecules. The computational results indicate that the application of external forces can significantly alter the electronic structure and chemical reactivity of PFOA molecules, thereby affecting their degradation process. The following is a discussion on the effects of external forces on the degradation properties of PFOA:4.1 Impact of external forces on the molecular structure and electronic properties
We observed that the application of external forces leads to changes in the electron density distribution of key atoms within the PFOA molecule. Particularly at the carboxylate end, external forces cause variations in the distance between oxygen atoms, which in turn affect the electron density of the carboxylate group. These changes not only influence the molecule's electronegativity and electrophilic/nucleophilic indices but also alter the molecule's softness, suggesting that external forces make PFOA molecules more susceptible to external influences and enhance their responsiveness to external stimuli.4.2 Promotion of the decarboxylation reaction by external forces
By simulating the decarboxylation process of PFOA, we observed that the application of external mechanical force reduces the Gibbs free energy change of the decarboxylation reaction, thereby making the reaction energetically favorable. This finding is consistent with the phenomenon observed in experiments where external forces promote reactions in mechanochemical degradation processes. External forces destabilize the carboxylate group by weakening the conjugation effect, thus facilitating the decarboxylation reaction.4.3 Theoretical calculations and real mechanochemical reactions
In mechanochemical reactions, it is true that the state of molecular forces may be complex. Therefore, we selected some representative cases, and within limited observations, it can be seen that external forces have a trend of enhancing the activity and degradability of PFOA. Under normal circumstances, the mechanochemical degradation process of pollutants such as PFOA requires the participation of co-grinding agents, which can further provide oxidants, reductants, highly active free radicals, and other favorable reaction conditions. In the discussion of this study, we did not provide a mechanochemical degradation reaction process involving other reactants, but through the analysis and discussion of the decarboxylation reaction of PFOA itself under external forces, we can still conclude that external forces have a favorable promotional effect on the degradation process.Although this study provides a theoretical analysis of the impact of external forces on the degradation properties of PFOA, the actual degradation effect remains the most discussed content in this field, and experimental verification is still necessary. Future research can explore the impact of different types and intensities of external forces on the degradation efficiency of PFOA and optimize mechanochemical degradation conditions in conjunction with experimental data. Additionally, studying the synergistic effects of external forces with other reaction conditions (such as temperature and type of additives) will also provide a more comprehensive understanding of PFOA degradation.
5 Conclusion
This study systematically investigates the impact of external forces on the degradation properties of PFOA molecules using quantum chemical computational methods. The main conclusions are as follows:(1) The application of external forces significantly alters the electronic structure of PFOA molecules, leading to changes in their chemical reactivity. The impact of external forces on molecular reactivity can be specifically represented by global molecular descriptors and CDFT indices. For PFOA molecules, when an external force is applied, it is specifically manifested as an increase in nucleophilicity and radical reactivity at the carboxylate end, while electrophilic reactivity decreases.
(2) External forces reduce the stability of the carboxylate group by weakening the conjugation effect on it. The application of external mechanical force reduces the Gibbs free energy change of the decarboxylation reaction, thereby making the reaction energetically favorable. The findings of this study provide an understanding of the mechanochemical degradation mechanism from the perspective of PFOA molecules themselves.
In summary, this study not only provides an in-depth understanding of the impact of external forces on the properties of PFOA molecules at the atomic and molecular levels but also offers a mechanistic explanation method for the widely applied mechanochemical degradation technology. This lays an important theoretical foundation for further application and optimization of mechanochemical degradation.
Data availability
Most of the data presented in this paper are included in the main manuscript and the ESI,† and additional data are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
As this research work draws to a close, the author would like to extend heartfelt thanks to the Inner Mongolia Natural Science Foundation (Project Number: 2025MS02029) and the Higher Education Innovative Team Development Plan (Project Number: NMGIRT2321). The financial support and resource guarantees provided by these projects have been invaluable, enabling the smooth progress of the research. Special gratitude is expressed to the High-Performance Computing Public Service Platform of Inner Mongolia Autonomous Region, China, and particularly to the “Hohhot Light” supercomputer for providing robust computational power and technical support. With strong computational power and robust technical support, all quantum chemical calculations in this study were efficiently completed. We thank Tian LU, the author of Multiwfn, for the helpful computational chemistry content on his personal blog (https://sobereva.com). Here, we would also like to express our gratitude to members of the computational chemistry forum (https://bbs.keinsci.com), such as sobereva (founder of the forum), wzkchem5, chands, zjxitcc etc., since the academic discussions with them have greatly contributed to the computational design and analysis presented in this paper.References
- O. Adu, X. Ma and V. K. Sharma, Bioavailability, phytotoxicity and plant uptake of per-and polyfluoroalkyl substances (PFAS): a review, J. Hazard. Mater., 2023, 447, 130805 CrossRef CAS PubMed.
- M. Ateia, A. Maroli and N. Tharayil, et al., The overlooked short- and ultrashort-chain poly- and perfluorinated substances: a review, Chemosphere, 2019, 220, 866–882 CrossRef CAS PubMed.
- N. Barbo, T. Stoiber and V. Naidenko O, et al., Locally caught freshwater fish across the United States are likely a significant source of exposure to PFOS and other perfluorinated compounds, Environ. Res., 2023, 220, 115165 CrossRef CAS PubMed.
- K. A. Barzen-Hanson, S. C. Roberts and S. Choyke, et al., Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater, Environ. Sci. Technol., 2017, 51(4), 2047–2057 CrossRef CAS PubMed.
- C. Chen, Y. Fang and D. Zhou, Selective pressure of PFOA on microbial community: enrichment of denitrifiers harboring ARGs and the transfer of ferric-electrons, Water Res., 2023, 233, 119813 CrossRef CAS PubMed.
- S. Deng, Y. Nie and Z. Du, et al., Enhanced adsorption of perfluorooctane sulfonate and perfluorooctanoate by bamboo-derived granular activated carbon, J. Hazard. Mater., 2015, 282, 150–157 CrossRef CAS PubMed.
- M. N. Ehsan, M. Riza and M. N. Pervez, et al., Environmental and health impacts of PFAS: sources, distribution and sustainable management in North Carolina (USA), Sci. Total Environ., 2023, 878, 163123 CrossRef CAS PubMed.
- L. J. L. Espartero, M. Yamada and J. Ford, et al., Health-related toxicity of emerging per- and polyfluoroalkyl substances: comparison to legacy PFOS and PFOA, Environ. Res., 2022, 212, 113431 CrossRef PubMed.
- Y. Li, J. Yao and J. Zhang, et al., First Report on the Bioaccumulation and Trophic Transfer of Perfluoroalkyl Ether Carboxylic Acids in Estuarine Food Web, Environ. Sci. Technol., 2022, 56(10), 6046–6055 CrossRef CAS PubMed.
- L. Liang, Y. Pan and L. Bin, et al., Immunotoxicity mechanisms of perfluorinated compounds PFOA and PFOS, Chemosphere, 2022, 291, 132892 CrossRef CAS PubMed.
- S. Poothong, E. Papadopoulou and J. A. Padilla-Sanchez, et al., Multiple pathways of human exposure to poly- and perfluoroalkyl substances (PFASs): from external exposure to human blood, Environ. Int., 2020, 134, 105244 CrossRef CAS PubMed.
- W. Zhang, S. Pang and Z. Lin, et al., Biotransformation of perfluoroalkyl acid precursors from various environmental systems: advances and perspectives, Environ. Pollut., 2021, 272, 115908 CrossRef CAS PubMed.
- G. Zheng, E. Schreder and J. C. Dempsey, et al., Per- and Polyfluoroalkyl Substances (PFAS) in Breast Milk: Concerning Trends for Current-Use PFAS, Environ. Sci. Technol., 2021, 55(11), 7510–7520 CrossRef CAS PubMed.
- R. A. Dickman and D. S. Aga, A review of recent studies on toxicity, sequestration, and degradation of per- and polyfluoroalkyl substances (PFAS), J. Hazard. Mater., 2022, 436, 129120 CrossRef CAS PubMed.
- Z. Du, S. Deng and Y. Bei, et al., Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents-a review, J. Hazard. Mater., 2014, 274, 443–454 CrossRef CAS PubMed.
- M. I. Gomis, R. Vestergren and D. Borg, et al., Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives, Environ. Int., 2018, 113, 1–9 CrossRef CAS PubMed.
- S. Kurwadkar, J. Dane and S. R. Kanel, et al., Per- and polyfluoroalkyl substances in water and wastewater: a critical review of their global occurrence and distribution, Sci. Total Environ., 2022, 809, 151003 CrossRef CAS PubMed.
- X. Lei, Q. Lian and X. Zhang, et al., A review of PFAS adsorption from aqueous solutions: current approaches, engineering applications, challenges, and opportunities, Environ. Pollut., 2023, 321, 121138 CrossRef CAS PubMed.
- L. Yang, L. He and J. Xue, et al., Persulfate-based degradation of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) in aqueous solution: review on influences, mechanisms and prospective, J. Hazard. Mater., 2020, 393, 122405 CrossRef CAS PubMed.
- J. K. Cui, P. P. Gao and Y. Deng, Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review, Environ. Sci. Technol., 2020, 54(7), 3752–3766 CrossRef CAS PubMed.
- A. Berhanu, I. Mutanda and T. L. Ji, et al., A review of microbial degradation of per- and polyfluoroalkyl substances (PFAS): biotransformation routes and enzymes, Sci. Total Environ., 2023, 859, 160010 CrossRef CAS PubMed.
- M. G. Alalm and D. C. Boffito, Mechanisms and pathways of PFAS degradation by advanced oxidation and reduction processes: a critical review, Chem. Eng. J., 2022, 450, 138352 CrossRef.
- S. C. E. Leung, P. Shukla and D. Chen, et al., Emerging technologies for PFOS/PFOA degradation and removal: a review, Sci. Total Environ., 2022, 827, 153669 CrossRef CAS PubMed.
- K. Zhang, J. Huang and G. Yu, et al., Destruction of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) by Ball Milling, Environ. Sci. Technol., 2013, 47(12), 6471–6477 CrossRef CAS PubMed.
- K. Zhang, Z. Cao and J. Huang, et al., Mechanochemical destruction of Chinese PFOS alternative F-53B, Chem. Eng. J., 2016, 286, 387–393 CrossRef CAS.
- J. Fang, S. Li and T. Gu, et al., Treatment of per- and polyfluoroalkyl substances (PFAS): a review of transformation technologies and mechanisms, J. Environ. Chem. Eng., 2024, 12(1), 111833 CrossRef CAS.
- E. B. Esfahani, F. A. Zeidabadi and S. Y. Zhang, et al., Photo-chemical/catalytic oxidative/reductive decomposition of per- and poly-fluoroalkyl substances (PFAS), decomposition mechanisms and effects of key factors: a review, Environ. Sci.:Water Res. Technol., 2022, 8(4), 698–728 RSC.
- T. Sidnell, R. J. Wood and J. Hurst, et al., Sonolysis of per- and poly fluoroalkyl substances (PFAS): a meta-analysis, Ultrason. Sonochem., 2022, 87, 105944 CrossRef CAS PubMed.
- R. K. Singh, S. Fernando and S. F. Baygi, et al., Breakdown Products from Perfluorinated Alkyl Substances (PFAS) Degradation in a Plasma-Based Water Treatment Process, Environ. Sci. Technol., 2019, 53(5), 2731–2738 CrossRef CAS PubMed.
- J. R. Groele, N. Sculley and T. M. Olson, et al., An investigation of plasma-driven decomposition of per- and polyfluoroalkyl substances (PFAS) in raw contaminated ground water, J. Appl. Phys., 2021, 130(5), 053304 CrossRef CAS.
- E. Gagliano, P. P. Falciglia and Y. Zaker, et al., Microwave regeneration of granular activated carbon saturated with PFAS, Water Res., 2021, 198, 117121 CrossRef CAS PubMed.
- Z. U. Zango, K. S. Khoo and A. Garba, et al., A review on superior advanced oxidation and photocatalytic degradation techniques for perfluorooctanoic acid (PFOA) elimination from wastewater, Environ. Res., 2023, 221, 115326 CrossRef CAS PubMed.
- A. Nasser and U. Mingelgrin, Birnessite-induced mechanochemical degradation of 2,4-dichlorophenol, Chemosphere, 2014, 107, 175–179 CrossRef CAS PubMed.
- S. Deng, Y. Bao and G. Cagnetta, et al., Mechanochemical degradation of perfluorohexane sulfonate: synergistic effect of ferrate(VI) and zero-valent iron, Environ. Pollut., 2020, 264, 114789 CrossRef CAS PubMed.
- I. Rabani, R. Zafar and K. Subalakshmi, et al., A facile mechanochemical preparation of Co3O4@g-C3N4 for application in supercapacitors and degradation of pollutants in water, J. Hazard. Mater., 2021, 407, 124360 CrossRef CAS PubMed.
- M.-F. He, W.-Q. Li and Z.-H. Xie, et al., Peracetic acid activation by mechanochemically sulfidated zero valent iron for micropollutants degradation: enhancement mechanism and strategy for extending applicability, Water Res., 2022, 222, 118887 CrossRef CAS PubMed.
- Z. Zhang, N. Wang and L. Zhu, et al., Synergistic effect between Fe and Bi2O3 on enhanced mechanochemical treatment of decabromodiphenyl ether, J. Environ. Chem. Eng., 2017, 5(1), 915–923 CrossRef CAS.
- S. Rowlands, A. Hall and P. Mccormick, et al., Destruction of toxic materials, Nature, 1994, 367, 223 CrossRef.
- P. Di Leo, M. D. R. Pizzigallo and V. Ancona, et al., Mechanochemical degradation of pentachlorophenol onto birnessite, J. Hazard. Mater., 2013, 244, 303–310 CrossRef PubMed.
- H. Wang, J. Huang and S. Zhang, et al., Study of degradation mechanism of dechlorane plus by mechanochemical reaction with aluminum and quartz sand, Chem. Eng. J., 2016, 292, 98–104 CrossRef CAS.
- Z. Chen, M. Tang and S. Lu, et al., Mechanochemical degradation of PCDD/Fs in fly ash within different milling systems, Chemosphere, 2019, 223, 188–195 CrossRef CAS PubMed.
- R. Wang, Z. Zhu and S. Tan, et al., Mechanochemical degradation of brominated flame retardants in waste printed circuit boards by Ball Milling, J. Hazard. Mater., 2020, 385, 121509 CrossRef CAS PubMed.
- S. Deng, L. Liu and G. Cagnetta, et al., Mechanochemically synthesized S-ZVIbm composites for the activation of persulfate in the pH-independent degradation of atrazine: effects of sulfur dose and ball-milling conditions, Chem. Eng. J., 2021, 423, 129789 CrossRef CAS.
- S. Wu, S. Yang and Q. Li, et al., Iron(II) sulfate crystals assisted mechanochemical modification of microscale zero-valent aluminum (mZVAl) for oxidative degradation of phenol in water, Chemosphere, 2021, 274, 129767 CrossRef CAS PubMed.
- X. Xu, Y. Chu and R. Chen, et al., Thermo-mechanochemical recycling of waste polypropylene into degradation products as modifiers for cleaner production and properties enhancement of bitumen, J. Clean. Prod., 2022, 379, 134792 CrossRef CAS.
- S. Deng, G. Cagnetta and G. Yu, et al., Mechanochemically synthesized sulfidated magnetite: a novel activator of permonosulfate for Fe(IV) dominated degradation of atrazine, Chem. Eng. J., 2023, 476, 146549 CrossRef CAS.
- K. Gobindlal, Z. Zujovic and J. Jaine, et al., Solvent-Free, Ambient Temperature and Pressure Destruction of Perfluorosulfonic Acids under Mechanochemical Conditions: Degradation Intermediates and Fluorine Fate, Environ. Sci. Technol., 2023, 57(1), 277–285 CrossRef CAS PubMed.
- L. Gong, J. Chen and Y. Hu, et al., Degradation of Chloroform by Zerovalent Iron: Effects of Mechanochemical Sulfidation and Nitridation on the Kinetics and Mechanism, Environ. Sci. Technol., 2023, 57(26), 9811–9821 CrossRef CAS PubMed.
- X. Xu, Y. Chu and Y. Luo, et al., Thermal-and-mechanochemical recycling of waste polypropylene into maleated-epoxided degradation products as warm-mix asphalt modifier: performance improvement and production mechanism analysis, J. Clean. Prod., 2023, 426, 139222 CrossRef CAS.
- N. Battye, D. Patch and I. Koch, et al., Mechanochemical degradation of per- and polyfluoroalkyl substances in soil using an industrial-scale horizontal ball mill with comparisons of key operational metrics, Sci. Total Environ., 2024, 928, 172274 CrossRef CAS PubMed.
- H. Hu, Q. Zhang and Q. Wang, et al., Rapid mechanochemical dechlorination of hexachlorobenzene with zero-valent silicon as a novel additive: the attempt of zero-valent nonmetallic, J. Environ. Chem. Eng., 2023, 11(6), 111398 CrossRef CAS.
- A. T. Mogharbel and C. L. Yestrebsky, Dechlorination comparison of octachlorodibenzofuran over ball-milled zero-valent magnesium with and without activated carbon in different solvent systems, J. Environ. Chem. Eng., 2019, 7(2), 102950 CrossRef CAS.
- G. Cagnetta, J. Robertson and J. Huang, et al., Mechanochemical destruction of halogenated organic pollutants: a critical review, J. Hazard. Mater., 2016, 313, 85–102 CrossRef CAS PubMed.
- P. Roesch, C. Vogel and F.-G. Simon, Reductive Defluorination and Mechanochemical Decomposition of Per- and Polyfluoroalkyl Substances (PFASs): From Present Knowledge to Future Remediation Concepts, Int. J. Environ. Res. Public Health, 2020, 17(19), 7242 CrossRef PubMed.
- J. Fang, S. Li and T. Gu, et al., Treatment of per- and polyfluoroalkyl substances (PFAS): a review of transformation technologies and mechanisms, J. Environ. Chem. Eng., 2024, 12(1), 111833 CrossRef CAS.
- I. G. Hakeem, P. Halder and S. Patel, et al., Current understanding on the transformation and fate of per- and polyfluoroalkyl substances before, during, and after thermal treatment of biosolids, Chem. Eng. J., 2024, 493, 152537 CrossRef CAS.
- N. Yang, S. Yang and Q. Ma, et al., Solvent-Free Nonthermal Destruction of PFAS Chemicals and PFAS in Sediment by Piezoelectric Ball Milling, Environ. Sci. Technol. Lett., 2023, 10(2), 198–203 CrossRef CAS PubMed.
- B. Yang, Y. Li and G. Yu, et al., Oxidative Degradation of PFOA/PFOS with Physicochemical Techniques, Prog. Chem., 2014, 26(7), 1265–1274 CAS.
- L. P. Turner, B. H. Kueper and K. M. Jaansalu, et al., Mechanochemical remediation of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) amended sand and aqueous film-forming foam (AFFF) impacted soil by planetary ball milling, Sci. Total Environ., 2021, 765, 142722 CrossRef CAS PubMed.
- L. Li, R. Guo and J. Gao, et al., Insight into mechanochemical destruction of PFOA by BaTiO3: an electron-dominated reduction process, J. Hazard. Mater., 2023, 450, 131028 CrossRef CAS PubMed.
- L. P. Turner, B. H. Kueper and D. J. Patch, et al., Elucidating the relationship between PFOA and PFOS destruction, particle size and electron generation in amended media commonly found in soils, Sci. Total Environ., 2023, 888, 164188 CrossRef CAS PubMed.
- R. Guo, L. Li and Z. Zhao, et al., Enhanced piezoelectric catalysis of BaTiO3 by ZVAl for mechanochemical defluorination of PFOA: promotion of electron transfer, J. Hazard. Mater., 2024, 465, 133040 CrossRef CAS PubMed.
- J. C. Verley, E. Mclennon and K. S. Rein, et al., Current trends and patterns of PFAS in agroecosystems and environment: a review, J. Environ. Qual., 2024, 20607 Search PubMed.
- M. Zhang, W. Wang and T. Gong, et al., Cutting-edge technologies and relevant reaction mechanism difference in treatment of long- and short-chain per- and polyfluoroalkyl substances: a review, Chemosphere, 2024, 354, 141692 CrossRef CAS PubMed.
- M. K. Beyer, Coupling of mechanical and chemical energy: proton affinity as a function of external force, Angew. Chem., Int. Ed., 2003, 42(40), 4913–4915 CrossRef CAS PubMed.
- S. Aydonat, A. H. Hergesell and C. L. Seitzinger, et al., Leveraging mechanochemistry for sustainable polymer degradation, Polym. J., 2024, 56(4), 249–268 CrossRef CAS.
- I. M. Klein, C. C. Husic and D. P. Kovács, et al., Validation of the CoGEF method as a predictive tool for polymer mechanochemistry, J. Am. Chem. Soc., 2020, 142(38), 16364–16381 CrossRef CAS PubMed.
- G. O’bryan, B. M. Wong and J. R. Mcelhanon, Stress sensing in polycaprolactone films via an embedded photochromic compound, ACS Appl. Mater. Interfaces, 2010, 2(6), 1594–1600 CrossRef PubMed.
- J. Ribas-Arino, M. Shiga and D. Marx, Understanding Covalent Mechanochemistry, Angew. Chem., Int. Ed., 2009, 48(23), 4190–4193 CrossRef CAS PubMed.
- T. Stauch and A. Dreuw, Advances in Quantum Mechanochemistry: Electronic Structure Methods and Force Analysis, Chem. Rev., 2016, 116(22), 14137–14180 CrossRef CAS PubMed.
- W. T. Yang and R. G. Parr, Hardness, Softness, and the Fukui Function in the Electronic Theory of Metals and Catalysis, Proc. Natl. Acad. Sci. U. S. A., 1985, 82(20), 6723–6726 CrossRef CAS PubMed.
- H. Chermette, Chemical reactivity indexes in density functional theory, J. Comput. Chem., 1999, 20(1), 129–154 CrossRef CAS.
- P. Geerlings, F. De Proft and W. Langenaeker, Conceptual density functional theory, Chem. Rev., 2003, 103(5), 1793–1873 CrossRef CAS PubMed.
- J.-D. Chai and M. Head-Gordon, Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections, Phys. Chem. Chem. Phys., 2008, 10(44), 6615–6620 RSC.
- F. Weigend, Accurate Coulomb-fitting basis sets for H to Rn, Phys. Chem. Chem. Phys., 2006, 8(9), 1057–1065 RSC.
- R. Dennington, T. A. Keith and J. M. Millam, GaussView Version 6 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 16 Rev. B.01, Wallingford, CT, 2016 Search PubMed.
- T. Lu and F. Chen, Multiwfn: a multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33(5), 580–592 CrossRef CAS PubMed.
- T. Lu, A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn, J. Chem. Phys., 2024, 161(8), 082503 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5mr00048c |
| This journal is © The Royal Society of Chemistry 2025 |