Open Access Article
Open Access ArticleEfficient racemization of the pharmaceutical compound Levetiracetam using solvent-free mechanochemistry†
Chrystal
Lopes
 a,
Lucia
Casali
b,
Franziska
Emmerling
a,
Lucia
Casali
b,
Franziska
Emmerling
 bc,
Tom
Leyssens
bc,
Tom
Leyssens
 d,
Valérie
Dupray
a,
Clement
Brandel
d,
Valérie
Dupray
a,
Clement
Brandel
 *a and
Yohann
Cartigny
*a
*a and
Yohann
Cartigny
*a
aUniv Rouen Normandie, Normandie Univ, SMS, UR 3233, F-76000 Rouen, France. E-mail: yohann.cartigny@univ-rouen.fr; clement.brandel@univ-rouen.fr
bFederal Institute for Materials Research and Testing, Richard-Willstätter-Straße 11, 12489 Berlin, Germany
cDepartment of Chemistry, Humboldt-Universität zu Berlin, 12489 Berlin, Germany
dInstitute of Condensed Matter and Nanosciences (IMCN), Université Catholique de Louvain, 1348 Louvain-La-Neuve, Belgium
First published on 31st October 2024
Abstract
We present the racemization of an active pharmaceutical ingredient Levetiracetam using a novel approach. We demonstrate the design of a 100% solvent-free process that proceeds by high energy milling inside a regular mixer mill. The kinetics of the racemization process is drastically improved compared to the solution-based approach and illustrates the tremendous potential of mechanochemistry. In this study, we highlight the importance of mixing efficiency regarding data reproducibility, and we show, in particular, that water contamination has a negative impact on the reaction rate. Moreover, in situ X-ray diffraction gives us first insights into the mechanisms involved in the solid state during the mechanochemical racemization process.
Introduction
More than 50% of commercialized active pharmaceutical ingredients (APIs) are chiral substances.1 The two enantiomers of a given API have identical physico-chemical properties but can act differently in asymmetric media such as living organisms: if one enantiomer of the API has the desired therapeutic effect, the counter-enantiomer could exhibit undesired effects.2 Access to enantiopure materials is therefore paramount, and the development of new methods for the production of pure enantiomers is a central concern in chemical research. Most often, the synthetic routes of the APIs are non-stereoselective and racemic mixtures (i.e., a mixture made of both enantiomers in equal proportions) are finally obtained. The counter-enantiomer is thus considered as an impurity and must be removed, for instance by using chiral HPLC.3,4 In this context, methods based on crystallization principles can also be used.5–7 Yet, whatever the separation route, the maximum theoretical yield is only 50%. It implies a loss of half of the product and does not fit with the 1st and 2nd principles of Anastas and Warner's green chemistry principles, both reporting the necessity to avoid waste.8Increasing the yield of the process to 100% is possible by converting the undesired enantiomer into a racemic mixture, which can be resolved once again. This transformation, called racemization, can be performed if the chiral entity can reach an achiral state either by using a racemizing agent or by using external parameters (heating, UV irradiation, dissolution, etc.).9–11 This reaction is generally done in solution as high molecular mobility is required for reactivity purposes, which is lacking in the solid state. Some processes take advantage of the racemization reaction to turn a racemic mixture into an enantiopure end state. Deracemization processes such as Viedma Ripening (VR) or Temperature Cycle Induced Deracemization (TCID) convert a racemic conglomerate crystal suspension in equilibrium with a saturated solution into an enantiopure material.12,13 In the case of VR, this is permitted by constant attrition of the suspension using glass beads that allows amplification of population imbalances between enantiomeric crystals.14 Also, the productivity and yield of the well-known Preferential Crystallization process15,16 can be improved by solution-racemization in a process known as Second Order Asymmetric Transformation.17,18 In this process, the constant interconversion of the enantiomers in the liquid state provides a steady mass input to feed the enantiopure seeds and offers control of the supersaturation of the counter enantiomer. Nevertheless, the enantiopurification methods described above all suffer from the same drawback: they require the use of substantial amounts of solvent(s) which can lead to a costly and toxic process. Furthermore, solvents are often hard to remove, leading to high carbon footprint processes. Adding a solvent to facilitate a reaction is not in agreement with the 5th principle of Anastas and Warner's green chemistry principles that aims at reducing the amount of added auxiliary to a reaction.8
Mechanochemistry is a promising approach to considerably reduce, or suppress, the amount of liquid substances used in chemical processes, and it has been applied for solvent-free organic synthesis and the preparation or screening of new solid phases of multicomponent materials.19,20 Some studies also highlight its potential regarding the prediction of API's degradation profiles.21–23 It is commonly accepted that transformations under high mechanical stress occur due to energy inputs that may decrease transition energies, alter transition states, create local and transient intense heating, etc.20,24 Reaction pathways under high mechanical stress may also differ from the solution pathways, yielding processes with different regioselectivities.25,26 Mechanochemical processes are usually performed via Neat-Grinding (NG, i.e., under dry conditions) using vibratory or planetary ball mills. The use of a minimal amount of solvent, a.k.a. Liquid-Assisted Grinding (LAG),27 is also common and often improves reactivity, or can be used for the formation of specific solvated solid phases.28 Friščić et al. introduced the η parameter to describe the ratio between the volume of solvent and the mass of solid (expressed in μL mg−1). These authors propose to use this parameter to differentiate mechanochemical (<1–2 μL mg−1) from solution-based processes (>1–2 μL mg−1).29
Besides numerous advantages, mechanochemistry is inherently confronted with mixing and homogenization issues. Ball milling often results in solid materials that exhibit a gummy-like texture, leading to sticky materials characterized by poor flowability. The materials are difficult to handle, and often stick to the wall of the grinding jar. The occurrence of such behavior during NG is erratic and is likely due to a number of factors. Solvent formation during the chemical reaction likely has a negative effect on mixing. It is also the case when the generated product has a rubber-like aspect. The so-called “snow-ball effect” has been coined to describe the typical coating of the grinding beads by pasty and sticky materials upon milling.30–32 The snow-ball effect may have a positive impact on reaction rates for a part of the powder but most often reduces mixing efficiency.33 Using solid lubricants, such as NaCl, SiO2 or even talc, mitigates this issue.32,34
The use of mechanochemistry in the field of enantiomer conversion and separation remains largely unexplored. Ikekawa et al.35 reported the racemization of the natural amino acid L-leucine by NG, but chemical degradation occurred and the yields were poor. Recently, we have published the first example of deracemization using LAG.36 The process may be considered as VR transferred in a high energy milling apparatus with much lower solvent loads and with much lower process duration (η ∼0.05 μL mg−1 with a process time of less than 2 hours in our work, while η ∼5 μL mg−1 and lasts more than 24 h in the case of VR).
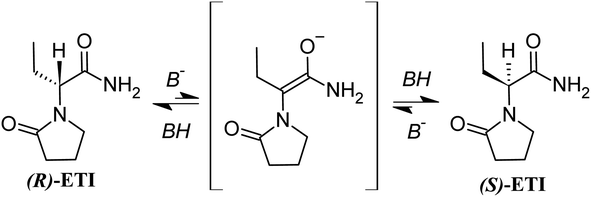
In the present study, we focus on the chiral API 2-(2-oxopyrrolidin-1-yl)butanamide (Scheme 1), which is used to treat epilepsy.37 The racemate is referred to as etiracetam ((RS)-ETI) with the pharmaceutically active S-enantiomer referred to as Levetiracetam ((S)-ETI). Concerning the solid landscape of Levetiracetam, no hydrate or polymorphic forms are known. In contrast, etiracetam crystallizes as a stable racemic compound and exhibits two enantiotropically related polymorphic forms, with a transition temperature equal to 30.5 °C. A racemic dihydrated solid form has also been reported.38,39 (S)-ETI being the active enantiomer and (R)-ETI having no pharmaceutical activity, (R)-ETI is typically transformed into (RS)-ETI by racemization with further (S)-ETI recovered via chiral resolution.
A solvent-based racemization route of Levetiracetam, which requires the use of sodium methanolate as a racemizing agent and refluxing the mixture for 12 hours, has been reported.40,41 The racemization mechanism has also been investigated in silico by Li et al.42 who concluded that the process requires proton abstraction using hydroxide ions, giving rise to an achiral intermediate (Scheme 1), before proton re-addition. In this paper, our aim is to evaluate the possibility to perform racemization of Levetiracetam without the use of a solvent through a mechanochemical NG process. This work focuses on the efficiency of mixing and process reproducibility to demonstrate the potential of mechanochemistry in the field of enantiomer conversion.
Materials and methods
Chemicals
(S)-2-(2-Oxopyrrolidin-1-yl)butanamide ((S)-ETI or Levetiracetam) was purchased from Xiamen Top Health Biochem. Tech. Co., Ltd. For reasons of accessibility, this enantiomer will be used for the racemization trials. Sodium chloride (NaCl, 99.5% purity), was purchased from VWR whereas sodium hydroxide (NaOH, 97% purity) and sodium methoxide (25/30% wt in methanol) were purchased from Alfa Aesar. A list gathering the purity and supplier of all used compounds is given in the ESI (Table S1).†Milling procedure
All milling experiments were performed with a Mixer Mill 400 from Retsch in a 10 mL jar containing one 12 mm diameter bead both composed of ZrO2. Milling was performed with a frequency of 30 Hz for variable duration expressed as tmilling. For experiments with controlled humidity, jar preparation was entirely performed within a glove bag in which relative humidity (RH) was controlled with N2 gas below 20%. The materials to be milled (i.e., (S)-ETI, NaOH and NaCl) were all stored for a minimum of one week inside a desiccator under P2O5 (Fig. S8 in the ESI†). The jar was sealed with parafilm inside the glove bag prior to milling (performed under ambient conditions). Once the milling stopped, the powder was immediately analyzed by cHPLC.cHPLC
cHPLC analyses were performed on an Ultimate 3000 system, with a UV detection at 210 nm. The milled material was dissolved in a mixture of heptane/IPA/TFA (80/20/0.1, v/v/v). The mobile phase was a heptane/IPA (90/10 v/v) mixture with a 1 mL min−1 flow rate. The stationary phase was a Chiralcel OD-H 5 μm 250 × 4.6 mm column from Daicel. The retention times were 16.3 and 20.1 min for (R)- and (S)-enantiomers, respectively.In situ powder X-ray diffraction (PXRD) measurements
Levetiracetam (100 mg) and NaCl (50 mg) with NaOH (7 mg) were placed in a customized Perspex jar43 of 12 mm diameter, and were then neat ground for 4 hours with a 10 mm zirconium ball at 50 Hz using a Fritsch Vibration Ball Mill (Pulverisette 23, Fritsch, Germany).In situ X-ray diffraction measurements were performed at 30 seconds intervals at the μSpot beamline (BESSY II, Helmholtz Centre Berlin for Materials and Energy).44 The experiments were conducted with a wavelength of 0.7314 Å using a double crystal monochromator (Si 111), and the resulting scattering images were integrated with the Dpdak-software.45 The data obtained were plotted simultaneously with the software OriginPro 2023 as a function of the scattering vector q (nm−1), in the x-axis, as it is conventionally used to compare diffractograms obtained at different wavelengths.
Results & discussion
Enabling mechanochemical racemization of levetiracetam
A solution-based reference racemization protocol was reported by Leyssens et al.40,41 and repeated here both at reflux and at ambient temperature. For this purpose, 200 mg of (S)-ETI was dissolved in 10 mL of methanol (MeOH) containing 0.05 eq. of sodium methoxide (MeONa). The quantity of MeOH has been chosen to afford a volume comparable to one of the jars used in milling experiments (10 mL jar, see the ESI†). Fig. 1 shows that the racemization experiment performed at reflux yields an enantiomeric excess (ee) of 88% after 240 minutes with no racemization occurring at ambient temperature after 24 h.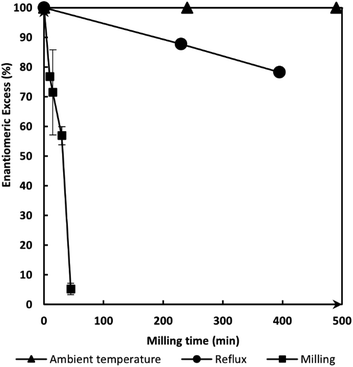 | ||
| Fig. 1 Evolution of the enantiomeric excess of ETI as a function of time: under milling (black squares), in solution at reflux (black circles), in solution at ambient temperature (black triangles). | ||
When performing the mechanochemical process through liquid assisted grinding (LAG), using 200 mg of (S)-ETI and 0.1 equivalent of MeONa added as a MeONa–methanol solution (η = 0.1 μL mg−1), a much faster process is observed. For each milling time (tmilling), experiments were repeated at least 4 times and the associated standard deviation is represented by the error bar. Fig. 1 (black squares) highlights that almost complete racemization (ee of 5%) was reached in ca. 60 minutes. These results show that the conversion rate of the racemization reaction can be considerably increased using a mechanochemical approach.
Although successful, the use of MeONa comes with some drawbacks: (i) it is usually formulated as a methanol solution which limits the approach to LAG only (no Neat-Grinding), (ii) the base solution is not stable under ambient conditions, as a reaction with atmospheric water occurs, (iii) the base shows health hazards, and its use in conjunction to pulverulent materials should be avoided.
Screening of alternative bases suitable for neat grinding racemization
We therefore decided to look for a base allowing NG racemization. Initially, attempts were performed in the absence of a racemizing agent. This did not lead to any enantio-conversion, hereby confirming the result of the DFT study of Li et al. and underlining the central role of proton carriers in the racemization mechanism.42 A screening was then performed to identify a racemizing agent suitable for NG. All the compounds tested are gathered in Table 1 alongside their pKa values. Milling experiments were systematically performed with tmilling = 30 min at 30 Hz using 1 eq. of base.| Base | pKa (measured in water at 25 °C)46 | Final %ee for tmilling = 30 min |
|---|---|---|
| a Estimated pKa. b For the first and second acidity respectively. c As there is no dissociation for magnesium dihydroxide, no pKa value can be measured or estimated. | ||
| 1,8-Diazabicyclo(5.4.0)undec-7-ene (DBU) | 13.0a | >99% |
| CaCO3 | 10.3 and 6.4b | >99% |
| MgCO3 | >99% | |
| K2CO3 | >99% | |
| Mg(OH)2 | —c | >99% |
| Ca(OH)2 | 12.7 | >99% |
| LiOH | 13.8 | >99% |
| NaOH | 14.8 | 70% |
Concerning the trials with the notably strong base DBU, no racemization occurred despite the rather high pKa value of 13. It is possible that the large molecular volume of this organic base hinders the proton abstraction of (S)-ETI. All the NG experiments performed with carbonate bases did not lead to racemization of (S)-ETI, with the ee upon milling remaining >99%. These bases have lower pKa values (10.3 and 6.4 for the first and second acidity respectively), which could explain the absence of racemization. Lithium, magnesium and calcium hydroxides failed to trigger the racemization reaction. Both the addition of solvent (water or methanol) or the use of a planetary, instead of vibration mill did not impact the outcome. In contrast, NG experiments performed with the strong base NaOH successfully triggered the racemization reaction (Table 1). 1H NMR analysis confirms the absence of chemical degradation even after 60 min of milling (see the ESI, Fig. S5†). Based on these results, it appears that the key parameter to trigger proton abstraction under mechanochemical conditions remains the strength of the base. Our results suggest that a pKa threshold corresponding to that of NaOH must be achieved (i.e., pKa ≥ 14.8). As a comparison, the pKa of MeONa is estimated at 15.5.
Racemization efficiency
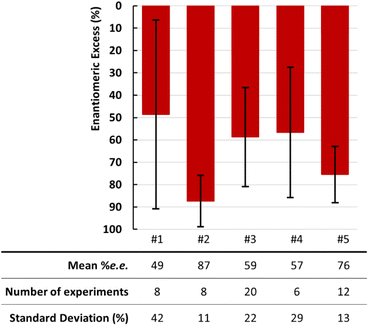
All experiments reported in this section were performed using 200 mg of (S)-ETI, 0.1 eq. of NaOH, and a grinding duration of tmilling = 60 min. Preliminary NG experiments (i.e., η = 0 μL mg−1) performed were found to be non-reproducible in terms of racemization efficiency: for a total of 8 experiments performed under the same conditions, the final ee varied from 0.4 to 96.7% with a standard deviation of 42 as shown by the error bars (Fig. 3, Exp #1).This lack of reproducibility likely finds its origin in the high hygroscopic character of solid NaOH,47 leading to uncontrolled amounts of water that may result in poor quality of mixing. Indeed, larger amounts of water can generate a paste-like material due to solubilization of the starting compounds.33 The subsequent low homogeneity during milling can be a source of lacking reproducibility.
To confirm the influence of water on the mixing quality, two sets of milling experiments were performed. A first set (Exp #2, 8 repetitions) is performed by adding 10 μL of water to the grinding jar (η(H2O) = 0.05 μL mg−1). The second set (Exp #3, 20 repetitions) is performed by filling the jar under controlled humidity using a glove bag (GB, hereafter) and with prior storage of all compounds under dry conditions (in the presence of P2O5 as a desiccant).
The impact of these parameters on the racemization efficiency is examined by comparing the results of Exp #1 to 3 (Fig. 2). In the case of Exp #2, the results show that the racemization kinetics is drastically reduced to a mean value of 87% ee when adding only 10 μL of water. As a consequence, the standard deviation is also reduced to 11% (compared to 42.2% for Exp #1). In parallel, these experiments were repeated using 50 μL of water (η(H2O) = 0.25 μL mg−1) resulting in the same outcome in terms of data reproducibility and racemization rate (Table S8, in the ESI†). In contrast, filling the jar using a GB (Exp #3) had almost no impact on the mean ee value compared to Exp #1, but the standard deviation decreased to 22%, showing improved reproducibility. Further experiments were performed by filling the jar under controlled humidity and by adding water. The racemization clearly slows down. These experiments confirm that water contamination has a negative impact on both racemization kinetics as well as reproducibility.
The literature shows several studies highlighting the advantage of adding an inert solid lubricant (such as NaCl, SiO2, sand, talc, etc.…) to the milling media25,32 to provide better homogeneity inside the jar. Since ETI generates H2O when racemizing (Scheme 1), thus decreasing the flowability of the powder, we therefore decided to perform NG experiments adding 100 mg of NaCl to investigate the influence of a solid lubricant on racemization kinetics. The 6 repetitions of Exp #4 did not involve the use of a GB to fill the jar. The results show a mean value of 57% and a standard deviation of 30% (i.e., between 13 and 87% ee). These data are similar to those obtained for Exp #3. Even if the use of NaCl is expected to improve mixing, the rather poor data reproducibility of Exp #4 can still be associated with water trapping by this salt. Therefore, we combined the use of NaCl and water control by filling the jars in the GB in a final series of experiments (Exp #5). This successfully makes the process more reproducible with a standard deviation of only 12%. However, the mean value of the ee remained at 75.5%, showing lower racemization kinetics compared to all other experiments (but Exp #2). This can be explained by the dilution effect, leading to a reduced number of contacts between (S)-ETI and the racemizing agent NaOH (i.e., due to the increase of (S)-ETI/NaCl and NaOH/NaCl contacts).
The results shown in Fig. 2 were correlated with a visual observation of the ground material just after grinding. For Exp #1 to 4, the materials were systematically found to be glued unevenly on the walls of the jar, especially at the edges, and a residual snow-balling was observed (Fig. S3, in the ESI†). Besides, NG experiments performed with a higher amount of NaOH (0.5 eq.) resulted in the material being entirely stuck to the grinding ball, thereby confirming the occurrence of a snow-balling effect (Fig. S7†). In contrast, the conditions of Exp #5 led to a uniform distribution of the powder and the presence of lumps and sticky material was strongly reduced. This confirms that data reproducibility is correlated with the mixing efficiency, which in turn is favored by a synergetic combination of low water content and the use of a solid lubricant.
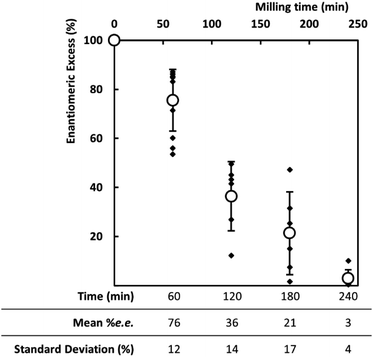
By using the optimal racemization and mixing conditions found in Exp #5, a kinetic follow-up of the racemization reaction was performed. For this, a series of 24 experiments has been performed and stopped after different tmilling (Fig. 3). For each milling time (except the one at 60 minutes for which 12 experiments were performed), statistics were made over 6 experiments. The optimized reproducibility of the results allows evaluation of the t1/2 of the racemization reaction at around 80 minutes, which shows much faster kinetics compared to solution-based racemization for which only 80% ee is reached after 400 min (Fig. 1). Due to the absence of exact temperature control, the data cannot be used to determine an accurate activation energy of the mechanochemical racemization process.
First insights into the racemization mechanism using in situ PXRD monitoring
To gain insight into the racemization mechanism, we performed in situ X-ray diffraction monitoring of the mechanochemical reaction using the conditions of Exp #4 (i.e., use of NaCl but no control of the humidity during both jar filling and milling) since it was easier to replicate these experimental conditions at the synchrotron facility.As shown in Fig. 4, the initial system ((S)-ETI) goes through different steps before ultimately leading to form I of (RS)-ETI. First, only the diffractograms of both (S)-ETI and NaCl are detected. Their intensities progressively decrease within the first 10 minutes, and become almost undetectable at 17 minutes. The disappearance of the diffraction peaks cannot be merely attributed to amorphization of (S)-ETI since the peaks of NaCl also decrease. Instead, the absence of a PXRD signal is more likely due to the occurrence of a snow-ball effect. After 17 minutes, the peaks of form I of (RS)-ETI and NaCl are suddenly detected, and remain until the end of the experiment. At the same time, it is also possible to observe the signal of Form II of (RS)-ETI, but the latter is weak and disappears progressively. When this experiment was reproduced (see Fig. S11 in the ESI†), the same trend was observed but the time after which the racemic phases appeared varied from one experiment to another, consistently with what was observed during sampling (Fig. 3).
Although further investigation would be needed to fully understand the kinetics, we can already extract unprecedented information on the mechanism of the mechanochemical racemization. In fact, according to the ex situ sampling data (Fig. 3), a continuous evolution of the ee as a function of grinding time from 100% to 0% is to be expected. Instead, both in situ datasets under the conditions of Exp #4 show a sudden appearance of (RS)-ETI crystals after the occurring snowball effect. The combination of both findings confirms that the racemization reaction should not occur in the crystalline state, but likely requires the formation of a disordered phase that is not detected by X-ray. Such a transient phase could contain a large amount of amorphous parts as a result of complex interactions between the ground material and trapped water.
Conclusions
In this paper, we show that Levetiracetam can easily be racemized using LAG and MeONa (in MeOH) as a racemizing agent. The mechanochemical racemization occurs much more rapidly than the solution-based process, thus reaching a racemic mixture in less than 100 min instead of an 80% ee after a mere 400 min. Moreover, we show that neat grinding can also efficiently lead to Levetiracetam racemization in only 240 min using NaOH as a base and conditions of Exp #5 (i.e., dry atmosphere and NaCl as a solid lubricant). The racemization kinetics drastically slow down when water is present, even in very small amounts. Water either comes from the racemization reaction or from atmospheric contamination, creating a paste-like material giving rise to a snowball effect. This leads to mixing issues and inhomogeneity created during neat grinding. To improve the reproducibility of the results, the humidity inside the milling jars was reduced, and a solid auxiliary (NaCl) added to prevent the formation of the pasty material and to support homogeneous mixing. The combined effect of these two adjustments enables satisfactory levels of reproducibility. Moreover, unprecedented information regarding the mechanism of mechanochemical racemization is gained by following the reaction in situ using X-ray diffraction. Our data show that the formation of a disordered phase is instrumental for the racemization to occur. Thus, we achieve a fast, reproducible and sustainable solvent-free route to racemization through high energy milling.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Region Normandie is gratefully acknowledged by the authors from Normandie Université for their support regarding the funding of the IPOCRAC project through RIN 100% #22E01374. The authors from UCLouvain would like to thank the FNRS for financial support (PDR T.0149.19 and T.262.20). LC and FE would also like to thank Lucia Parisek Jimenez for her contribution in performing the experiments.References
- L. A. Nguyen, H. He and C. Pham-Huy, Chiral Drugs: An Overview, Int. J. Biomed. Sci., 2006, 2(2), 85–100 CrossRef CAS.
- D. E. Drayer, Pharmacodynamic and Pharmacokinetic Differences between Drug Enantiomers in Humans: An Overview, Clin. Pharmacol. Ther., 1986, 40(2), 125–133, DOI:10.1038/clpt.1986.150.
- D. R. Taylor and K. Maher, Chiral Separations by High-Performance Liquid Chromatography, J. Chromatogr. Sci., 1992, 30(3), 67–85, DOI:10.1093/chromsci/30.3.67.
- J. Haginaka, Pharmaceutical and Biomedical Applications of Enantioseparations Using Liquid Chromatographic Techniques, J. Pharm. Biomed. Anal., 2002, 27(3), 357–372, DOI:10.1016/S0731-7085(01)00652-5.
- T. J. Ward and K. D. Ward, Chiral Separations: A Review of Current Topics and Trends, Anal. Chem., 2012, 84(2), 626–635, DOI:10.1021/ac202892w.
- H. Lorenz and A. Seidel-Morgenstern, Processes To Separate Enantiomers, Angew. Chem., Int. Ed., 2014, 53(5), 1218–1250, DOI:10.1002/anie.201302823.
- H. Lorenz, A. Perlberg, D. Sapoundjiev, M. P. Elsner and A. Seidel-Morgenstern, Crystallization of Enantiomers, Chem. Eng. Process., 2006, 45(10), 863–873, DOI:10.1016/j.cep.2005.11.013.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University PressOxford, 2000, DOI:10.1093/oso/9780198506980.001.0001.
- M. Reist, B. Testa, P.-A. Carrupt, M. Jung and V. R. Schurig, Enantiomerization, Diastereomerization, and Epimerization: Their Meaning and Pharmacological Significance, Chirality, 1995, 7(6), 396–400, DOI:10.1002/chir.530070603.
- D. C. Patel, R. M. Woods, Z. S. Breitbach, A. Berthod and D. W. Armstrong, Thermal Racemization of Biaryl Atropisomers, Tetrahedron: Asymmetry, 2017, 28(11), 1557–1561, DOI:10.1016/j.tetasy.2017.09.006.
- Q. Wang, A. Pietropaolo, M. Fortino, Z. Song, M. Bando, N. Naga and T. Nakano, Photo Racemization of 2,2′-Dihydroxy-1,1′-Binaphthyl Derivatives, Chirality, 2022, 34(2), 317–324, DOI:10.1002/chir.23400.
- C. Viedma, Chiral Symmetry Breaking During Crystallization: Complete Chiral Purity Induced by Nonlinear Autocatalysis and Recycling, Phys. Rev. Lett., 2005, 94(6), 065504, DOI:10.1103/PhysRevLett.94.065504.
- L.-C. Sögütoglu, R. R. E. Steendam, H. Meekes, E. Vlieg, T. Rutjes and F. P. J. Viedma Ripening, A Reliable Crystallisation Method to Reach Single Chirality, Chem. Soc. Rev., 2015, 44(19), 6723–6732, 10.1039/C5CS00196J.
- W. L. Noorduin, E. Vlieg, R. M. Kellogg and B. Kaptein, From Ostwald Ripening to Single Chirality, Angew. Chem., Int. Ed., 2009, 48(51), 9600–9606, DOI:10.1002/anie.200905215.
- G. Levilain and G. Coquerel, Pitfalls and Rewards of Preferential Crystallization, CrystEngComm, 2010, 12(7), 1983–1992, 10.1039/C001895C.
- G. Coquerel, Preferential Crystallization, in Novel Optical Resolution Technologies, ed. Sakai, K., Hirayama, N. and Tamura, R., Topics in Current Chemistry, Springer, Berlin, Heidelberg, 2007, pp 1–51, DOI:10.1007/128_2006_077.
- R. Oketani, M. Hoquante, C. Brandel, P. Cardinael and G. Coquerel, Resolution of an Atropisomeric Naphthamide by Second-Order Asymmetric Transformation: A Highly Productive Technique | Organic Process Research & Development, Org. Process Res. Dev., 2019, 23(6), 1197–1203, DOI:10.1021/acs.oprd.9b00133.
- G. Levilain, C. Rougeot, F. Guillen, J.-C. Plaquevent and G. Coquerel, Attrition-Enhanced Preferential Crystallization Combined with Racemization Leading to Redissolution of the Antipode Nuclei, Tetrahedron: Asymmetry, 2009, 20(24), 2769–2771, DOI:10.1016/j.tetasy.2009.11.015.
- T. Friščić, C. Mottillo and H. M. Titi, Mechanochemistry for Synthesis, Angew. Chem., 2020, 132(3), 1030–1041, DOI:10.1002/ange.201906755.
- Y. Xiao, C. Wu, X. Hu, K. Chen, L. Qi, P. Cui, L. Zhou and Q. Yin, Mechanochemical Synthesis of Cocrystal: From Mechanism to Application, Cryst. Growth Des., 2023, 23(6), 4680–4700, DOI:10.1021/acs.cgd.3c00183.
- R. P. Kaiser, E. F. Krake, L. Backer, J. Urlaub, W. Baumann, N. Handler, H. Buschmann, T. Beweries, U. Holzgrabe and C. Bolm, Ball Milling - a New Concept for Predicting Degradation Profiles in Active Pharmaceutical Ingredients, Chem. Commun., 2021, 57(90), 11956–11959, 10.1039/d1cc04716g.
- M. M. Amer, L. Backer, H. Buschmann, N. Handler, O. Scherf-Clavel, U. Holzgrabe and C. Bolm, Prediction of Degradation Profiles for Various Sartans under Solvent-Free Mechanochemical Conditions, Anal. Chem., 2024, 96(32), 13166–13173, DOI:10.1021/acs.analchem.4c02025.
- E. F. Krake, L. Backer, B. Andres, W. Baumann, N. Handler, H. Buschmann, U. Holzgrabe, C. Bolm and T. Beweries, Mechanochemical Oxidative Degradation of Thienopyridine Containing Drugs: Toward a Simple Tool for the Prediction of Drug Stability, ACS Cent. Sci., 2023, 9(6), 1150–1159, DOI:10.1021/acscentsci.3c00167.
- S. Pagola, Outstanding Advantages, Current Drawbacks, and Significant Recent Developments in Mechanochemistry: A Perspective View, Crystals, 2023, 13(1), 124, DOI:10.3390/cryst13010124.
- J. L. Howard, Q. Cao, L. Browne and D. Mechanochemistry, as an Emerging Tool for Molecular Synthesis: What Can It Offer?, Chem. Sci., 2018, 9(12), 3080–3094, 10.1039/C7SC05371A.
- F. Cuccu, L. De Luca, F. Delogu, E. Colacino, N. Solin, R. Mocci and A. Porcheddu, Mechanochemistry: New Tools to Navigate the Uncharted Territory of “Impossible” Reactions, ChemSusChem, 2022, 15(17), e202200362, DOI:10.1002/cssc.202200362.
- G. A. Bowmaker, Solvent-Assisted Mechanochemistry, Chem. Commun., 2012, 49(4), 334–348, 10.1039/C2CC35694E.
- S. Karki, T. Friščić, W. Jones and W. D. S. Motherwell, Screening for Pharmaceutical Cocrystal Hydrates via Neat and Liquid-Assisted Grinding, Mol. Pharmaceutics, 2007, 4(3), 347–354, DOI:10.1021/mp0700054.
- T. Friščić, S. L. Childs, S. A. A. Rizvi and W. Jones, The Role of Solvent in Mechanochemical and Sonochemical Cocrystal Formation: A Solubility-Based Approach for Predicting Cocrystallisation Outcome, CrystEngComm, 2009, 11(3), 418–426, 10.1039/B815174A.
- B. P. Hutchings, D. E. Crawford, L. Gao, P. Hu and S. L. James, Feedback Kinetics in Mechanochemistry: The Importance of Cohesive States, Angew. Chem., Int. Ed., 2017, 56(48), 15252–15256, DOI:10.1002/anie.201706723.
- G. Félix, N. Fabregue, C. Leroy, T.-X. Métro, C.-H. Chen and D. Laurencin, Induction-Heated Ball-Milling: A Promising Asset for Mechanochemical Reactions, Phys. Chem. Chem. Phys., 2023, 25(35), 23435–23447, 10.1039/D3CP02540C.
- J.-L. Do, C. Mottillo, D. Tan, V. Štrukil and T. Friščić, Mechanochemical Ruthenium-Catalyzed Olefin Metathesis, J. Am. Chem. Soc., 2015, 137(7), 2476–2479, DOI:10.1021/jacs.5b00151.
- M. Carta, S. L. James and F. Delogu, Phenomenological Inferences on the Kinetics of a Mechanically Activated Knoevenagel Condensation: Understanding the “Snowball” Kinetic Effect in Ball Milling, Molecules, 2019, 24(19), 3600, DOI:10.3390/molecules24193600.
- M. Salari, M. Rezaee, S. P. H. Marashi and S. H. Aboutalebi, The Role of the Diluent Phase in the Mechanochemical Preparation of TiO2 Nanoparticles, Powder Technol., 2009, 192(1), 54–57, DOI:10.1016/j.powtec.2008.11.011.
- A. Ikekawa and S. Hayakawa, Mechanochemical Racemization of L-Leucine, Bull. Chem. Soc. Jpn., 1984, 57(3), 889–890, DOI:10.1246/bcsj.57.889.
- C. Lopes, Y. Cartigny, C. Brandel, V. Dupray, C. Body, O. Shemchuk and T. Leyssens, A Greener Pathway to Enantiopurity: Mechanochemical Deracemization through Abrasive Grinding, Chem.–Eur. J., 2023, 29(35), e202300585, DOI:10.1002/chem.202300585.
- H. Alrabiah, Chapter Two - Levetiracetam, in Profiles of Drug Substances, Excipients and Related Methodology; Profiles of Drug Substances, Excipients, and Related Methodology, Academic Press, 2019, vol. 44, pp. 167–204, DOI:10.1016/bs.podrm.2019.02.003.
- C. Herman, V. Vermylen, B. Norberg, J. Wouters and T. Leyssens, The Importance of Screening Solid-State Phases of a Racemic Modification of a Chiral Drug: Thermodynamic and Structural Characterization of Solid-State Phases of Etiracetam, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2013, 69(4), 371–378, DOI:10.1107/S2052519213015054.
- C. Herman, B. Haut, L. Aerts and T. Leyssens, Solid–Liquid Phase Diagrams for the Determination of the Solid State Nature of Both Polymorphs of (RS)-2-(2-Oxo-Pyrrolidin-1-Yl)-Butyramide, Int. J. Pharm., 2012, 437(1–2), 156–161, DOI:10.1016/j.ijpharm.2012.07.047.
- O. Shemchuk, L. Song, K. Robeyns, D. Braga, F. Grepioni and T. Leyssens, Solid-State Chiral Resolution Mediated by Stoichiometry: Crystallizing Etiracetam with ZnCl2, Chem. Commun., 2018, 54(77), 10890–10892, 10.1039/C8CC06199H.
- G. Springuel and T. Leyssens, Innovative Chiral Resolution Using Enantiospecific Co-Crystallization in Solution, Cryst. Growth Des., 2012, 12(7), 3374–3378, DOI:10.1021/cg300307z.
- Z. Li, C. Wu, J. Liu, L. Li, C. Sun and T. Sun, Theoretical Studies on Racemization of Levetiracetam: Structural Movements, Character of Hydroxide Ion and Guidelines for Efficient Control, J. Mol. Liq., 2019, 288, 111055, DOI:10.1016/j.molliq.2019.111055.
- G. I. Lampronti, A. A. L. Michalchuk, P. P. Mazzeo, A. M. Belenguer, J. K. M. Sanders, A. Bacchi and F. Emmerling, Changing the Game of Time Resolved X-Ray Diffraction on the Mechanochemistry Playground by Downsizing, Nat. Commun., 2021, 12(1), 6134, DOI:10.1038/s41467-021-26264-1.
- I. Zizak, The mySpot Beamline at BESSY II, Journal of large-scale research facilities, 2016, 2, A102, DOI:10.17815/jlsrf-2-113.
- G. Benecke, C. Li, S. V. Roth, R. Gehrke, A. Rothkirch, T. Kracht, O. Paris, W. Wagermaier, A. Gourrier, M. Burghammer, C. Riekel and P. Fratzl, Directly Programmable Data Analysis Kit (DPDAK) for Online Analysis of High Throughput 2D Scattering Data, Emerging Themes in Analysis of Grazing Incidence Small-Angle Scattering Data, 2013, p. 18 Search PubMed.
- W. L. F. Armarego, Purification of Laboratory Chemicals: Part 2 Inorganic Chemicals, Catalysts, Biochemicals, Physiologically Active Chemicals, Nanomaterials, Butterworth-Heinemann, 2022 Search PubMed.
- J. M. Mäkynen, J. K. Jokiniemi, P. P. Ahonen, E. I. Kauppinen and R. Zilliacus, AHMED Experiments on Hygroscopic and Inert Aerosol Behaviour in LWR Containment Conditions: Experimental Results, Nucl. Eng. Des., 1997, 178(1), 45–59, DOI:10.1016/S0029-5493(97)00174-X.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4mr00103f |
| This journal is © The Royal Society of Chemistry 2025 |