Open Access Article
Open Access ArticlePoly(3-hexylthiophene) nanoparticles as visible-light photoinitiators and photosensitizers in 3D printable acrylic hydrogels for photodynamic therapies†
Rocío Natera
Abalos
a,
Ilaria Abdel
Aziz
 b,
Matías
Caverzan
a,
Arianna Sosa
Lochedino
a,
Luis E.
Ibarra
b,
Matías
Caverzan
a,
Arianna Sosa
Lochedino
a,
Luis E.
Ibarra
 c,
Antonela
Gallastegui
b,
Carlos A.
Chesta
a,
M. Lorena
Gómez
c,
Antonela
Gallastegui
b,
Carlos A.
Chesta
a,
M. Lorena
Gómez
 a,
David
Mecerreyes
a,
David
Mecerreyes
 bd,
Rodrigo E.
Palacios
bd,
Rodrigo E.
Palacios
 *a and
Miryam
Criado-Gonzalez
*a and
Miryam
Criado-Gonzalez
 *be
*be
aInstituto de Investigaciones en Tecnologías Energéticas y Materiales Avanzados (IITEMA), Universidad Nacional de Río Cuarto (UNRC) and Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Campus Universitario, 5800 Río Cuarto, Argentina. E-mail: rpalacios@exa.unrc.edu.ar
bPOLYMAT, University of the Basque Country UPV/EHU, Joxe Mari Korta Center. Avda. Tolosa 72, 20018, Donostia-San Sebastián, Spain. E-mail: miryam.criado@ehu.eus
cInstituto de Biotecnología Ambiental y Salud (INBIAS), Universidad Nacional de Río Cuarto (UNRC) and Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Campus Universitario, 5800 Río Cuarto, Argentina
dIkerbasque, Basque Foundation for Science, 48013 Bilbao, Spain
eInstitute of Polymer Science and Technology (ICTP-CSIC), 28006 Madrid, Spain
First published on 3rd March 2025
Abstract
The design of smart photoelectrodes capable of stimulating the localized production of reactive oxygen species (ROS) on demand is of great interest for redox medicine therapies. In this work, poly(3-hexylthiophene) semiconducting polymer nanoparticles (P3HT SPNs) are used with a dual role to fabricate light-responsive hydrogels. First, P3HT SPNs act as visible-light photoinitiators to induce the photopolymerization of acrylic monomers such as acrylamide (AAm), 2-(hydroxyethyl) acrylate (HEA), and poly(ethylene glycol) diacrylate (PEGDA). This leads to the formation of acrylic hydrogels loaded with the P3HT SPNs, as demonstrated by photo-rheology and infrared spectroscopy. Furthermore, P3HT SPNs are also successfully used as photoinitiators for digital light processing (DLP) 3D printing purposes to fabricate shape-defined intelligent hydrogels. Interestingly, P3HT SPNs retain their photoelectrochemical properties when embedded within the polymer hydrogels, showing photocurrent densities that range from ∼0.2 to ∼1.1 μA cm−2 depending on the intensity of the visible light-lamp (λ = 467 nm). Second, they can be used as photosensitizers (PS) to generate reactive oxygen species (ROS), 12–15 μM H2O2, on demand. The acrylic hydrogels containing P3HT SPNs do not exhibit cytotoxic effects under normal physiological conditions in the darkness against mouse glioma 261 (GL261) cells and S. aureus bacteria. However, they induce a ∼50% reduction GL261 cancer cell viability and a ∼99% S. aureus cell death in contact with them upon illumination (λ = 467 nm) due to the localized overproduction of ROS, which makes them attractive candidates for photodynamic therapies (PDT).
New conceptsIn this work, we demonstrate the concept of smart light-responsive hydrogels for photodynamic therapies (PDT) through the employment of poly(3-hexylthiophene) semiconducting polymer nanoparticles (P3HT SPNs). The excellent optoelectronic properties of P3HT SPNs are exploited with a dual role to act as (i) efficient and highly biocompatible visible light photoinitiators (PIS) for the polymerization of acrylic hydrogels and (ii) photosensitizers (PS) to trigger the overproduction of ROS (12–15 μM H2O2). Unlike traditional PIS, P3HT SPNs do not precise toxic co-initiators, while they retain their photocurrent properties when embedded within the acrylic hydrogels for proven PDT and future in situ bioprinting purposes. Furthermore, P3HT SPNs are used as PIS (λ > 405 nm) during the digital light processing (DLP) 3D printing of high-resolution hydrogels towards personalized scaffolds for post-surgical tissue defects reparation. The P3HT SPNs containing hydrogels succesfully reduce a ∼50% mouse glioma (GL261) cancer cell viability and a ∼99% S. aureus bacteria viability in a spatial-temporal controlled manner upon visible light illumination (λ > 467 nm) due to the localized overproduction of ROS. In conclusion, the intelligent P3HT acrylic hydrogels are a new class of promising materials for PDT against both cancer and bacteria, offering unique advantages over traditional materials reported in the literature. |
1. Introduction
Reactive oxygen species (ROS) are biologically relevant oxidants for redox medicine. While a moderate increase in the ROS levels can regulate the fates of endothelial cells promoting tissue regeneration, excessive ROS production (i.e., hydrogen peroxide (H2O2)) triggers oxidative stress resulting in cell dysfunction and apoptosis.1–3 This is of paramount importance in the case of cancer cells that produce higher levels of ROS than normal cells. This elevated ROS can be targeted by anticancer therapies that stimulate additional ROS production, pushing cancer cells to their limit and eventually killing them.4,5 This is the mechanism behind photodynamic therapy (PDT), a minimally invasive clinical treatment that combines photosensitizers (PS) in conjunction with appropriate light wavelengths and molecular oxygen to generate enough ROS to induce cell apoptosis.6 Mainly due to superb spatial-temporal control of light, PDT enables enhanced tumor therapeutic efficacy and targeting compared to conventional cancer therapies, minimizing side effects in healthy tissues. Moreover, PDT can also be applied after surgical resection to eliminate remaining cancer cells at the site, which is key for glioblastomas (GBM), one of the most aggressive tumors of the central nervous system with resistance to conventional radio- and chemotherapies.7,8 Another interesting aspect to be considered is the possibility of replacing and regenerating the post-surgical tissue defects for faster patient recovery.9,10 This can be achieved with the employment of printable hydrogel-based scaffolds that can mimic the shape and mechanical properties of the damaged tissues while providing ideal conditions for nutrient transportation and cell ingrowth ensuring a faster regeneration.11–13 In recent years, 3D printing technologies have emerged as powerful manufacturing methods to fabricate low-cost, customizable, and complex 3D biomedical scaffolds with micrometric precision.14,15 When stimuli-responsive materials are employed the process is referred to as 4D-printing.16,17 Among them, digital light processing (DLP) 4D printing is attracting increasing attention due to the high resolution of the intelligent printed scaffolds. For such a purpose, light-induced printable inks require a resin containing acrylic monomers and photoinitiator systems (PIS) able to generate free radicals to initiate a fast photopolymerization upon light activation.18,19 While water-soluble type I and type II PIS are the most commonly used PIS for biomedical applications, they present some limitations. Type I PIS require UV light for activation and type II PIS a co-initiator, which usually is toxic (e.g., amines, onium salts), that donates electrons or hydrogen, hampering their use in clinical trials.20,21 Very recently, we reported the employment of conjugated polymer (CP) nanoparticles as visible-light panchromatic PIS of acrylic monomers in water.22–24 Among different types of CPs, poly(3-hexylthiophene) (P3HT) is the gold standard of p-type semiconducting polymers for (bio-opto)electronic applications. It is commercially available at a competitive price and possesses excellent optoelectronic properties to act as an efficient and highly biocompatible phototransducer when processed both in the form of thin films and as nanoparticles.25–28 Nevertheless, to the best of our knowledge, the development of 3D printable hydrogels containing P3HT has not been reported nor has their application for PDT against cancer. These features make P3HT semiconducting polymer nanoparticles (SPNs) valuable players to be explored as both photoinitiator systems (PIS) and photosensitizers (PS) for redox active hydrogels. In this work we show that P3HT SPNs can act as (i) visible light PIS for the photopolymerization and 3D printing of stimuli-responsive acrylic polymer hydrogels relevant for biomedicine, and (ii) active PS to trigger the overproduction of ROS from hydrogels for PDT against GBM cells and S. aureus bacteria (Scheme 1).2. Results and discussion
The ability of P3HT SPNs to generate radicals was harnessed for use as photoinitiator systems (PIS) for the polymerization of acrylic monomers (AAm, HEA, and PEGDA) in aqueous media leading to the formation of hydrogels (Scheme 1). The employment of P3HT SPNs as PIS does not require co-initiators, while they also act as cross-linkers when excited in the visible and near UV range (Fig. S2B, ESI†). To explore this, first, the effect of the P3HT SPNs concentration (x = 5, 80 and 105 mg mL−1) on the formation of acrylic polymer hydrogels, PEGDA-SPNsx, was studied by photo-rheology (Fig. 1A). This technique allowed us to determine the gelation point (G′ = G′′), where the crossover between the liquid-like state (G′′ > G′) and the solid-like state (G′ > G′′) takes place, thus pointing out the formation of the PEGDA-SPNsx hydrogels. At the lowest SPNs concentration (5 mg mL−1), the gelation process of PEGDA-SPNs5 was very slow (∼80 min). As the concentration of SPNs increased, the gelation occurred faster. At the intermediate SPNs concentration (80 mg mL−1), the formation of PEGDA-SPNs80 hydrogels occurred in ∼45 min due to both the higher number of radicals produced during the irradiation process and the higher number of crosslinks. At the highest SPNs concentration (105 mg mL−1), the gelation process of PEGDA-SPNs105 hydrogels was also favored but slower (∼65 min) than in the previous case. This can be attributed to different factors such as radical-radical annihilation due to a higher number of radicals produced, and/or the prevention of the growth of long polymer chains to form a gel due to the presence of a higher number of SPNs in the media. In all cases, hydrogels with good consistency, colored in red and translucent were formed. The mechanical properties of the synthesized hydrogels were further characterized by oscillatory rheology (Fig. 1B). The elastic modulus (G′) of the synthesized hydrogels increased with the SPNs concentration, from 3 × 102 Pa for PEGDA-SPNs5 to 1 × 104 Pa for PEGDA-SPNs105 and remained stable over the whole frequency range. Besides, PEGDA-SPNs5 hydrogels remained stable (G′ > G′′) up to 200% strain, whereas the linear viscoelastic regime decreased for PEGDA-SPNs80 and PEGDA-SPNs105 hydrogels up to 70% strain due to their higher crosslinking degree and lower flexibility (Fig. S3A, ESI†).The effectiveness of P3HT SPNs as PIS of other acrylic monomers was also investigated by photo-rheology (Fig. 1C and Fig. S3B, ESI†). In the case of the acrylamide monomer (AAm), the formation of PAAm-SPNs80 hydrogels was much faster (∼20 min) than in the case of the PEGDA-SPNs80 hydrogels (∼45 min). This can be explained by the fact that in the case of PEGDA (Mn 525 g mol−1) the chains to be crosslinked by radical polymerization were longer than in the case of AAm (MW = 71.1 g mol−1) which restricted the formation of crosslinking points slowing down the gelation process. In the third case, the gelation of a mixture comprising a diacrylate polymer (PEGDA) and an acrylic monomer (HEA) was studied. The formation of PEGDA-co-HEA-SPNs80 hydrogels was faster (∼27 min) than PEGDA-SPNs80 hydrogels but slower than PAAm-SPNs80 hydrogels, as expected due to the combination of both the presence of initial longer chains of PEGDA (Mn 525 g mol−1), but in lower quantity (25%v/v) than in the case of PEGDA-SPNs80 hydrogels (50%v/v) as they are mixed with a monomer (HEA) of lower Mw (116.1 g mol−1), leading to an intermediate production of intramolecular and intermolecular crosslinking points. The mechanical properties of the hydrogels were further studied by oscillatory rheology (Fig. 1D). Both PEGDA-SPNs80 and PEGDA-co-HEA-SPNs80 hydrogels (G′ > G′′) were stable over the whole frequency range. PEGDA-co-HEA-SPNs80 hydrogels exhibited a higher elastic modulus (G′ ∼ 3 × 105 Pa) than PEGDA-SPNs80 hydrogels (G′ ∼ 1 × 104) Pa due to their lower crosslinking degree. The effect of SPNs increased concentration in the mechanical properties of PEGDA-co-HEA-SPNs105 hydrogels was also investigated (Fig. S4, ESI†). Both PEGDA-co-HEA-SPNs80 and PEGDA-co-HEA-SPNs105 hydrogels exhibited good flexibility when manipulated with a tweezer (see Fig. S4A, ESI†). However, G′ experienced a decrease from ∼3 × 105 Pa for PEGDA-co-HEA-SPNs80 to ∼3 × 104 Pa for PEGDA-co-HEA-SPNs105 hydrogels (Fig. S4B, ESI†). In agreement with the crosslinking dynamics, we attribute this result to self-deactivation of radicals and/or to the formation of less crosslinked hydrogels, due to a higher number of SPNs that hinders the growth of long polymer chains. These less crosslinked hydrogels broke earlier during increasing oscillation strains, as corroborated by the lower yield strain ( , point at which G′ = G′′) values of PEGDA-co-HEA-SPNs105
, point at which G′ = G′′) values of PEGDA-co-HEA-SPNs105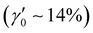 than PEGDA-co-HEA-SPNs80
than PEGDA-co-HEA-SPNs80 hydrogels (Fig. S4C, ESI†).
hydrogels (Fig. S4C, ESI†).
To confirm the ability of P3HT SPNs to photoinduce the polymerization of those acrylic monomers and/or polymers in aqueous solution, polymerization kinetics were followed by FT-IR, monitoring the consumption of the vinyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band located at ∼6180 cm−1 (Fig. 1E). In all cases, the prepolymer mixture contained a concentration of SPNs of 80 mg mL−1 and were irradiated with a blue LED (390 nm, 10 mW cm−2). In agreement with photo-rheology results, PAAm-SPNs80 showed the fastest polymerization kinetic reaching a 100% conversion in ∼30 min, just followed by PEGDA-co-HEA-SPNs80 that achieved a full conversion after 40 min of irradiation. Finally, PEGDA-SPNs80 exhibited the slowest polymerization kinetic reaching a 80% conversion in 80 minutes. This is characteristic of polymeric materials synthesised with diacrylate monomers such as PEGDA, which can also act as a crosslinker, and affect the conversion due to differences in mobility and accessibility to C
C band located at ∼6180 cm−1 (Fig. 1E). In all cases, the prepolymer mixture contained a concentration of SPNs of 80 mg mL−1 and were irradiated with a blue LED (390 nm, 10 mW cm−2). In agreement with photo-rheology results, PAAm-SPNs80 showed the fastest polymerization kinetic reaching a 100% conversion in ∼30 min, just followed by PEGDA-co-HEA-SPNs80 that achieved a full conversion after 40 min of irradiation. Finally, PEGDA-SPNs80 exhibited the slowest polymerization kinetic reaching a 80% conversion in 80 minutes. This is characteristic of polymeric materials synthesised with diacrylate monomers such as PEGDA, which can also act as a crosslinker, and affect the conversion due to differences in mobility and accessibility to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds by the polymeric chains in formation. Thus, it could be confirmed that SPNs have the capacity to act as PIS, photoiniciating the radical polymerization of AAm, PEGDA and HEA monomers in aqueous media, with high monomer conversion yields (80–100%), which led to the formation of hydrogels.
C double bonds by the polymeric chains in formation. Thus, it could be confirmed that SPNs have the capacity to act as PIS, photoiniciating the radical polymerization of AAm, PEGDA and HEA monomers in aqueous media, with high monomer conversion yields (80–100%), which led to the formation of hydrogels.
The swelling properties of the hydrogels were further characterized by weight changes when immersed in PBS (pH 7.4) over time (Fig. 1F). The swelling of both PEGDA-SPNs80 and PEGDA-co-HEA-SPNs80 hydrogels occurred immediately in less than 1 minute. However, the swelling degree was higher for PEGDA-co-HEA-SPNs80 (∼600 wt%) than for PEGDA-SPNs80 (∼170 wt%) hydrogels. This can be attributed to the more polar nature of HEA, with hydroxyl end groups, than PEGDA, thus allowing PEGDA-co-HEA-SPNs80 hydrogels to hold more water. This behavior has also been observed for other hydrogel systems obtained by copolymerization of HEA with a difunctional monomer from ethylene glycol and thioetheracrylate (EGnSA) using a commercial photinitiator (Darocur).29
The design of materials with the ability to be processed into 3D scaffolds with complex structures is actively searched in the biomedical field for personalized medicine purposes.30 In this regard, the ability of P3HT SPNs to be used as PIS for digital light printing (DLP) processes was also assessed (Fig. 2A). First, the optimal printing conditions were investigated (100 μm layer height and 120 s irradiation per layer) to obtain high-resolution 3D printed scaffolds with shape-defined morphologies and the characteristic red colour of P3HT SPNs (Fig. 2B). The optical properties of the hydrogels were characterized by UV-vis spectrophotometry (Fig. 2C). Both PEGDA-SPNs80 and PEGDA-co-HEA-SPNs80 hydrogels showed similar absorption spectra with a maximum located at 460 nm and a shoulder at 605 nm, which are typical of P3HT SPNs.28 These results demonstrate that P3HT SPNs embedded in the final hydrogels retain their optical properties after being irradiated to act as PIS. It was also observed that the optical absorption intensity increased with the concentration of SPNs within the hydrogels (Fig. S5, ESI†). The hydrogels were also capable of emitting fluorescence after visible-light excitation (λexc = 480 nm) (Fig. 2D). Both hydrogels showed similar fluorescence spectra with a maximum emission peak at 640 nm, which is also characteristic of P3HT SPNs. These results confirm the optical responsivity of the hydrogels in the visible range making them 4D-printable. This effect was proven visually by UV-light excitation of the previously printed butterfly, which exhibited fluorescence as shown in the picture included in the inset of Fig. 2D and Fig. S6 (ESI†).
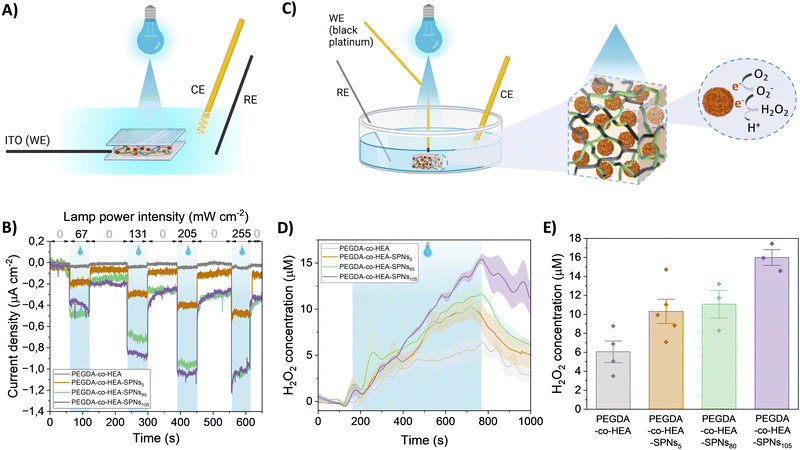
The photoelectrochemical properties of PEGDA-co-HEA-SPNsx hydrogels were characterized in a three-electrode electrochemical cell using PBS as the electrolyte, a Pt bundle as a counter electrode (CE) and Ag/AgCl as the reference electrode (RE). The hydrogels were sandwiched between a transparent ITO electrode, used as the working electrode (WE), and a glass slide to maintain the hydrogel in electrical contact with the ITO. All the electrodes were immersed in PBS, as schematically reported (Fig. 3A). A photocathodic behavior was recorded when irradiating with a blue LED (λ = 467 nm), with an increase in current for increasing irradiation intensity (Fig. 3B). The photocathodic behavior of P3HT is ascribed to oxygen reduction reactions, as photogenerated electrons from the polymer reduce available species in the electrolyte, i.e. oxygen.31,32 Additionally, the current density increased with increasing concentration of SPNs. Increasing the concentration from 5 to 80, doubled the recorded current, while increasing from 80 to 105 did not show a further increase. Interestingly, the PEGDA-co-HEA hydrogels used as a control did not exhibit photocurrent properties.
As previously reported for P3HT thin films and nanoparticles, the metastable photogenerated O2− undergoes a fast dismutation in aqueous media resulting in the formation of H2O2. Hence, we employed a Black Platinum working electrode to characterize the production of H2O2. Black Platinum electrodes have been reported to have a higher sensitivity and selectivity towards H2O2.33–35 The schematic of the electrochemical setup is reported in Fig. 3C, with Pt as CE, Ag/AgCl as RE, and PBS as electrolyte. H2O2 generation was recorded only during photostimulation (λ = 467 nm, 67 mW cm−2) (Fig. 3D), whereas when the illumination was switched off, the concentration dropped. An H2O2 generation up to ∼12 μM, ∼13 μM and ∼15 μM was recorded for the 5, 80 and 105 mg mL−1 nanoparticles concentrations, respectively (Fig. 3E). We chose the lowest irradiation density according to cytotoxicity tests carried out on GL261 cells. The H2O2 production did not reflect the concentration dependence demonstrated for the photocathodic recordings. As the photocurrent recording is not selective towards specific chemical species, but rather to the electrons that actually reach the WE, we hypothesize that different reactive species in addition to H2O2 were produced.
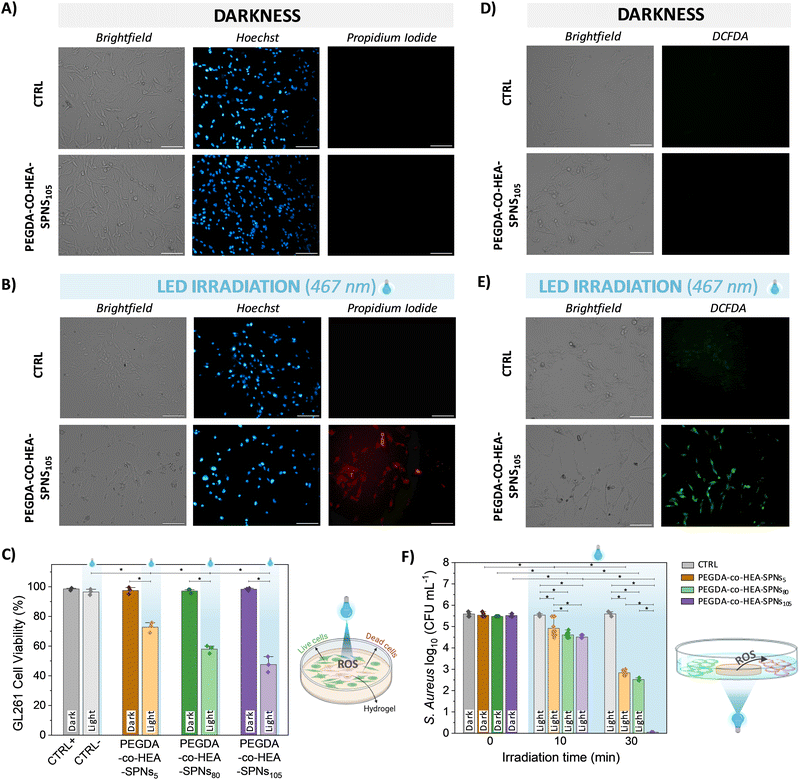
Prior to photo-stimulation in vitro cell tests, the cytotoxicity of the PEGDA-co-HEA-SPNsx hydrogels was tested in contact with mouse glioma 261 (GL261) cells (Fig. 4A and C and Fig. S7A, ESI†). GL261 cells were stained with Hoechst (cell nuclei, blue fluorescence) and with propidium iodide (death cell nuclei, red fluorescence). Results indicate that PEGDA-co-HEA-SPNsx hydrogels were not cytotoxic in darkness exhibiting a GL261 cell viability higher than 95% in all cases, without significant differences with respect to the control (CTRL). Then, the hydrogels were irradiated with a LED (λ = 467 nm; 60 mW cm−2, 40 J cm−2) for 10 min (Fig. 4B and Fig. S7B, ESI†). After irradiation, it was observed a decrease in the viability of GL261 cancer cells in contact with the PEGDA-co-HEA-SPNsx hydrogels, whereas those in contact with silicone disks used as a control remained unaffected. To better quantify the decrease in cell viability, flow cytometry experiments were performed (Fig. 4C and Fig. S8, ESI†). The results indicate that cell viability (CV) decreased with increasing SPNs concentration within the hydrogels, reaching values of 73 ± 3% CV for PEGDA-co-HEA-SPNs5, 58 ± 3% CV for PEGDA-co-HEA-SPNs80 and 48 ± 5% CV for PEGDA-co-HEA-SPNs105 hydrogels, proving their effectiveness for PDT against GBM. One of the primary mechanisms of PDT involves the production of ROS.6 Upon photoirradiation with a blue LED light (λ = 467 nm), the PEGDA-co-HEA-SPNsx hydrogels containing photo-active SPNs started to produce ROS, ∼12–15 μM H2O2 (Fig. 3D and E), that effectively reach GL261 cancer cells in contact with the hydrogels, as proven through fluorescence microscopy by incubating GL261 cells with the 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCF-DA) probe (Fig. 4D, E and Fig. S9, ESI†). Notably, PDT produced cytotoxic ROS only when the hydrogels were activated by light for spatial and temporal controlled PDT against GL261 cancer cells. Furthermore, PDT against Gram-positive S. aureus bacteria was also assessed (Fig. 4F). PEGDA-co-HEA-SPNsx hydrogels were not anti-microbial per se in the darkness, as no colony forming units (CFU) reduction was observed with respect to the silicone disks used as a control. Nevertheless, upon illumination with a blue LED light (λ = 467 nm, 40 mW cm−2) a decrease in the S. aureus CFU viability, induced by ROS production from PEGDA-co-HEA-SPNsx hydrogels, was observed. The S. aureus viability decreased with the increasing SPNs concentration in the PEGDA-co-HEA-SPNsx hydrogels, as well as with the irradiation time. A ∼ 18% reduction in the S. aureus viability was achieved for PEGDA-co-HEA-SPNs105 hydrogels after 10 min irradiation, and it increased up to ∼99% after 30 min irradiation. This photoinduced anti-microbial property plays also a key role in PDT when the photoactive materials and photo-stimulation probes need to be implanted within the body to avoid infection during and after the implantation. Overall, these results demonstrated the efficacy of the PEGDA-co-HEA-SPNsx hydrogels developed herein for cancer and anti-microbial photodynamic therapies.
3. Materials and methods
3.1. Materials
3-Hexylthiophene ≥98.0% (3HT) was supplied by TCI, iron(III) chloride and methanol ≥99.9% were purchased from Fluka. Tetrahydrofuran (THF), poly(ethylene glycol) diacrylate (PEGDA) >99% and Mn 575, acrylamide (AAm) >99.9%, 2-(hydroxyethyl) acrylate (HEA) >96%, pluronic F-127 (PF-127), Darocur 1173, and phosphate buffer saline (PBS) were purchased from Sigma-Aldrich. Dulbecco's modified Eagle's medium (DMEM), Penicillin 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units mL−1, streptomycin 10
000 units mL−1, streptomycin 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg mL−1 solution, glutamine (GlutaMAXTM 100×), trypsin-EDTA Solution 10×, sodium pyruvate 100 mM solution, Hoechst 33342, and Propidium iodide, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) were purchased from Gibco. Fetal bovine serum (FBS) was purchased from Internegocios S.A. Broth and tryptine-soy agar (TS) were provided by Britania, and D-glucose by Cicarelli. All reagents were used as received.
000 μg mL−1 solution, glutamine (GlutaMAXTM 100×), trypsin-EDTA Solution 10×, sodium pyruvate 100 mM solution, Hoechst 33342, and Propidium iodide, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) were purchased from Gibco. Fetal bovine serum (FBS) was purchased from Internegocios S.A. Broth and tryptine-soy agar (TS) were provided by Britania, and D-glucose by Cicarelli. All reagents were used as received.
3.2. Synthesis and characterization of P3HT nanoparticles
Prior nanoparticles synthesis, poly(3-hexylthiophene) (P3HT) was synthesized by oxidative chemical copolymerisation of 3-hexylthiophene, using FeCl3 as an oxidising agent (4 equiv. respect 3HT monomer) and acetonitrile as solvent, at room temperature overnight, as reported previously.26 The synthesized P3HT possessed a Mn 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 g mol−1, as determined by size exclusion chromatography using polystyrene standards, and a regioregularity of 75%, as determined by 1H NMR (Fig. S1, ESI†). Then, P3HT nanoparticles (SPNs) were obtained by flash nanoprecipitation following a previously reported protocol.28 Briefly, a stock solution of P3HT (1 mg mL−1 in THF) containing PF-127 (1 mg mL−1 in THF) as surfactant, in a ratio 1
400 g mol−1, as determined by size exclusion chromatography using polystyrene standards, and a regioregularity of 75%, as determined by 1H NMR (Fig. S1, ESI†). Then, P3HT nanoparticles (SPNs) were obtained by flash nanoprecipitation following a previously reported protocol.28 Briefly, a stock solution of P3HT (1 mg mL−1 in THF) containing PF-127 (1 mg mL−1 in THF) as surfactant, in a ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (%v/v) of P3HT:PF-127, was solubilized at 60 °C for 1 h. Then, 1 mL of this solution was injected into 10 mL of Milli-Q water under stirring at 1300 rpm and 85 °C. Subsequently, the as formed SPNs dispersion was transferred to a beaker to evaporate the THF at 50 °C for 90 min. Finally, the aqueous suspension of SPNs was stored at 4 °C in the darkness for further experiments. The average size of the SPNs was determined by transmission electron microscopy (TEM) using a Talos F200i field emission gun instrument equipped with a Bruker X-Flash100 XEDS spectrometer. SPNs were deposited over carbon-coated copper grids, and dried at room. TEM images were analyzed with the software ImageJ showing an average diameter of 60 ± 6 nm (Fig. S2A, ESI†).
9 (%v/v) of P3HT:PF-127, was solubilized at 60 °C for 1 h. Then, 1 mL of this solution was injected into 10 mL of Milli-Q water under stirring at 1300 rpm and 85 °C. Subsequently, the as formed SPNs dispersion was transferred to a beaker to evaporate the THF at 50 °C for 90 min. Finally, the aqueous suspension of SPNs was stored at 4 °C in the darkness for further experiments. The average size of the SPNs was determined by transmission electron microscopy (TEM) using a Talos F200i field emission gun instrument equipped with a Bruker X-Flash100 XEDS spectrometer. SPNs were deposited over carbon-coated copper grids, and dried at room. TEM images were analyzed with the software ImageJ showing an average diameter of 60 ± 6 nm (Fig. S2A, ESI†).
3.3. Synthesis of hydrogels
Hydrogels were synthesized by radical polymerization of acrylic monomers (AAm and HEA) and/or polymers (PEGDA) using P3HT SPNs as the photoinitiator system (PIS) in aqueous media. To that aim, the prepolymer mixture in a ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (%v/v) of monomer:SPNs was loaded in a syringe and injected in a glass container (10 mm × 30 mm) of 1 mm thickness sealed with a silicon joint. Then, this vessel was irradiated with a Kessil (PR160L) lamp with a central wavelength of 390 nm (irradiance of 10 mW cm−2), where it absorbs the PIS and not the other components. Three different prepolymer mixtures were prepared, 50%w/w AAm aqueous solution and SPNs in a ratio 1
1 (%v/v) of monomer:SPNs was loaded in a syringe and injected in a glass container (10 mm × 30 mm) of 1 mm thickness sealed with a silicon joint. Then, this vessel was irradiated with a Kessil (PR160L) lamp with a central wavelength of 390 nm (irradiance of 10 mW cm−2), where it absorbs the PIS and not the other components. Three different prepolymer mixtures were prepared, 50%w/w AAm aqueous solution and SPNs in a ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (%v/v), PEGDA and SPNs in a ratio 1
1 (%v/v), PEGDA and SPNs in a ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (%v/v), and PEGDA, HEA and SPNs in a ratio 0.5
1 (%v/v), and PEGDA, HEA and SPNs in a ratio 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (%v/v). The resulting hydrogels were named as PAAm-SPNsx, PEGDA-SPNsx, and PEGDA-co-HEA-SPNsx respectively (where x stands for the SPNs concentration). As a control, hydrogels were prepared by photopolymerization of these acrylic monomers and/or polymer using Darocur 1173 (1%v/v) as a photoinitiator in aqueous media, in a ratio 1
1 (%v/v). The resulting hydrogels were named as PAAm-SPNsx, PEGDA-SPNsx, and PEGDA-co-HEA-SPNsx respectively (where x stands for the SPNs concentration). As a control, hydrogels were prepared by photopolymerization of these acrylic monomers and/or polymer using Darocur 1173 (1%v/v) as a photoinitiator in aqueous media, in a ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (%v/v) of monomer:aqueous Darocur, instead of SPNs.
1 (%v/v) of monomer:aqueous Darocur, instead of SPNs.
3.4. Photopolymerization kinetics
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band at 6180 cm−1. The prepolymeric mixtures were loaded into the sealed vessel described above and the spectrum at time 0 was recorded. The mixture was then irradiated with the Kessil lamp (390 nm, 10 mW cm−2). At different time intervals (10, 20, 30, 40, 50, 60, and 80 min), the irradiation was cut off and the FT-IR spectra were recorded. This process was repeated until high conversion (∼80% or more) was observed, depending on the pre-polymeric mixture under study.
C band at 6180 cm−1. The prepolymeric mixtures were loaded into the sealed vessel described above and the spectrum at time 0 was recorded. The mixture was then irradiated with the Kessil lamp (390 nm, 10 mW cm−2). At different time intervals (10, 20, 30, 40, 50, 60, and 80 min), the irradiation was cut off and the FT-IR spectra were recorded. This process was repeated until high conversion (∼80% or more) was observed, depending on the pre-polymeric mixture under study.
3.5. Digital light processing (DLP) 3D printing
The prepolymeric mixture was poured into the resin vat of a DLP 3D printer (Anycubic Photon Mono X 6, λ = 405 nm, 2 mW cm−2) and hydrogel structures were printed (layer height = 100 μm, exposure time = 120 s). The printed patterns were first designed with Autodesk Inventor 2021 software.3.6. Physical–chemical characterization of hydrogels
 | (1) |
3.7. Electrochemical characterization of photo-responsive hydrogels
The electrochemical characterization of the hydrogels was carried out in a three electrodes electrochemical cell, with PBS as electrolyte, a platinum bundle as counter electrode (CE), Ag/AgCl (Sat) as reference electrode (RE). A VMP3 Biologic potentiostat was employed for electrochemical recording. The cell was kept in a Faraday cage to reduce electrical noise. For all the electrochemical measurement, the hydrogels were soaked in PBS overnight at 4 °C in the dark, and the measurements were carried out the day after soaking.3.8. In vitro cell culture tests
Prior to cell seeding, PEGDA-co-HEA-SPNsx (x = 5, 80 and 105 mg mL−1) hydrogels were formed by photopolymerization (390 nm, 10 mW cm−2) in silicon molds of 10 mm diameter and 1 mm thickness for 6000 s. Then, PEGDA-SPNsx (x = 5, 80 and 105 mg mL−1) hydrogels were placed in a 24-well plate and washed with 1 mL of PBS under sterile conditions for 3 days to remove non-reacted monomers by replacing the washing PBS solution daily.3.9. Antimicrobial tests
3.10. Statistical analysis
Statistical analyses were performed using one-way ANOVA, at a significance level of *p < 0.05, using Tukey's multiple comparison test.4. Conclusions
In this work, P3HT SPNs were successfully employed both as photoinitiators of acrylic hydrogels and as photosensitizers for PDT. First, P3HT SPNs induced the visible light-photopolymerization of acrylic monomers (AAm, HEA, and PEGDA) leading to the formation of PAAm-SPNs, PEGDA-SPNs, and PEGDA-co-HEA-SPNs hydrogels. They also acted as active photosensitizers when embedded within the hydrogels to trigger the production of H2O2, a kind of ROS used for photodynamic therapies. The effect of SPNs concentration on the photopolymerization kinetics, photocurrent properties, and ROS production of the hydrogels was studied, and results pointed out an enhanced localized PDT performance with increasing SPNs concentration as expected. PEGDA-co-HEA-SPNs105 hydrogels decreased a ∼50% GL261 cancer cells viability upon irradiation with a visible blue LED light. Moreover, PEGDA-co-HEA-SPNs105 hydrogels also presented anti-microbial properties with ∼99% reduction S. aureus viability after 30 min irradiation with a visible blue LED light. All in all, these results show that the intelligent P3HT acrylic hydrogels developed in this work are promising materials for PDT against both cancer cells and bacteria.Author contributions
R. N. A. experimental research, formal analysis, and writing. I. A. A. photocurrent and ROS production experiments, formal analysis, and writing. M. C. in vitro cell tests and formal analysis. A. S. L. in vitro antimicrobial tests and formal analysis. L. E. I. in vitro cell tests, formal analysis, and writing. A. G. experimental research. C. A. C. supervision. L. M. G. supervision. D. M. funding acquisition, writing, and reviewing. R. E. P. supervision, writing, and reviewing. M. C-G. conceptualization, rheological experiments, formal analysis, supervision, funding acquisition, writing, review, and editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the grant PID2020–119026GB-I00 funded by MCIN/AEI/10.13039/501100011033. M. C.-G. thanks “Ayuda RYC2022-036380-I financiada por MICIU/AEI/10.13039/501100011033 y por el FSE +” and the Emakiker program of POLYMAT (UPV/EHU). This work was funded by the European Union under GA no. 101129945 “IONBIKE 2.0”, and the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 101034379. Authors also appreciate financial support from SECyT UNRC (PPI 2024), ANPCyT (PICT 2020/3803) and CONICET (PIP 11220200102377CO01). R. N. A., M. C., A. S. L. thanks CONICET for his doctoral scholarships. C. A. C., M. L. G., and R. E. P. are permanent research staff of CONICET.References
- A. N. Kolodkin, R. P. Sharma, A. M. Colangelo, A. Ignatenko, F. Martorana, D. Jennen, J. J. Briedé, N. Brady, M. Barberis, T. D. G. A. Mondeel, M. Papa, V. Kumar, B. Peters, A. Skupin, L. Alberghina, R. Balling and H. V. Westerhoff, NPJ Syst. Biol. Appl., 2020, 6, 34 CrossRef PubMed.
- M. Criado-Gonzalez and D. Mecerreyes, J. Mater. Chem. B, 2022, 10, 7206–7221 RSC.
- B. A. Cesca, M. D. Caverzan, M. J. Lamberti and L. E. Ibarra, Int. J. Mol. Sci., 2024, 25, 7525 CrossRef CAS PubMed.
- A. Rahman, S. Pallichankandy, F. Thayyullathil and S. Galadari, Free Radicals Biol. Med., 2019, 134, 527–544 CrossRef CAS PubMed.
- B. Perillo, M. Di Donato, A. Pezone, E. Di Zazzo, P. Giovannelli, G. Galasso, G. Castoria and A. Migliaccio, Exp. Mol. Med., 2020, 52, 192–203 CrossRef CAS PubMed.
- G. Li, C. Wang, B. Jin, T. Sun, K. Sun, S. Wang and Z. Fan, Cell Death Discovery, 2024, 10, 466 CrossRef PubMed.
- M. Miretti, M. A. González Graglia, A. I. Suárez and C. G. Prucca, J. Photochem. Photobiol., 2023, 13, 100161 CrossRef.
- L. E. Ibarra, M. L. Vilchez, M. D. Caverzán and L. N. Milla Sanabria, J. Neurosci. Res., 2021, 99, 1024–1047 CrossRef CAS PubMed.
- H. Yang, R. Liu, Y. Xu, L. Qian and Z. Dai, Nano-Micro Lett., 2021, 13, 35 CrossRef PubMed.
- X. Wang, T. Li, H. Ma, D. Zhai, C. Jiang, J. Chang, J. Wang and C. Wu, NPG Asia Mater., 2017, 9, e376–e376 CrossRef CAS.
- R. C. Advincula, J. R. C. Dizon, E. B. Caldona, R. A. Viers, F. D. C. Siacor, R. D. Maalihan and A. H. Espera, MRS Commun., 2021, 11, 539–553 CrossRef CAS PubMed.
- J. Chakraborty, J. Fernández-Pérez, M. Takhsha Ghahfarokhi, K. A. van Kampen, T. ten Brink, J. Ramis, M. Kalogeropoulou, R. Cabassi, C. de Julián Fernández, F. Albertini, C. Mota, S. Ghosh and L. Moroni, Cell Rep. Phys. Sci., 2024, 5, 101819 CrossRef CAS.
- A. Hernández-Sosa, R. A. Ramírez-Jiménez, L. Rojo, F. Boulmedais, M. R. Aguilar, M. Criado-Gonzalez and R. Hernández, Polymers, 2022, 14, 2229 CrossRef PubMed.
- M. Caprioli, I. Roppolo, A. Chiappone, L. Larush, C. F. Pirri and S. Magdassi, Nat. Commun., 2021, 12, 2462 CrossRef CAS PubMed.
- M. Criado-Gonzalez, A. Dominguez-Alfaro, N. Lopez-Larrea, N. Alegret and D. Mecerreyes, ACS Appl. Polym. Mater., 2021, 3, 2865–2883 CrossRef CAS PubMed.
- H. B. D. Tran, C. Vazquez-Martel, S. O. Catt, Y. Jia, M. Tsotsalas, C. A. Spiegel and E. Blasco, Adv. Funct. Mater., 2024, 34, 2315238 CrossRef CAS.
- C. A. Spiegel, M. Hackner, V. P. Bothe, J. P. Spatz and E. Blasco, Adv. Funct. Mater., 2022, 32, 2110580 CrossRef CAS.
- N. Ballard and J. M. Asua, Prog. Polym. Sci., 2018, 79, 40–60 CrossRef CAS.
- M. Regato-Herbella, D. Mantione, A. Blachman, A. Gallastegui, G. C. Calabrese, S. E. Moya, D. Mecerreyes and M. Criado-Gonzalez, ACS Macro Lett., 2024, 13, 1119–1126 CrossRef CAS PubMed.
- F. Dumur, Eur. Polym. J., 2022, 169, 111120 CrossRef CAS.
- E. R. Zhiganshina, M. V. Arsenyev, D. A. Chubich, D. A. Kolymagin, A. V. Pisarenko, D. S. Burkatovsky, E. V. Baranov, A. G. Vitukhnovsky, A. N. Lobanov, R. P. Matital, D. Y. Aleynik and S. A. Chesnokov, Eur. Polym. J., 2022, 162, 110917 CrossRef CAS.
- A. Gallastegui, R. M. Spada, G. Cagnetta, R. A. Ponzio, S. R. Martínez, C. M. Previtali, M. L. Gómez, R. E. Palacios and C. A. Chesta, Macromol. Rapid Commun., 2020, 41, 2070019 CrossRef CAS.
- G. E. Cagnetta, A. Gallastegui, S. R. Martínez, D. Mantione, M. Criado-Gonzalez, M. Regato-Herbella, L. Lezama, R. E. Palacios, M. L. Gómez, D. Mecerreyes and C. A. Chesta, Macromolecules, 2024, 57, 78–87 CrossRef.
- G. E. Cagnetta, S. R. Martínez, L. E. Ibarra, A. Gallastegui, J. F. Martucci, R. E. Palacios, C. A. Chesta and M. L. Gómez, Biomater. Adv., 2023, 149, 213399 CrossRef CAS PubMed.
- F. Lodola, V. Rosti, G. Tullii, A. Desii, L. Tapella, P. Catarsi, D. Lim, F. Moccia and M. R. Antognazza, Sci. Adv., 2019, 5, eaav4620 CrossRef CAS PubMed.
- M. Criado-Gonzalez, L. Bondi, C. Marzuoli, E. Gutierrez-Fernandez, G. Tullii, C. Ronchi, E. Gabirondo, H. Sardon, S. Rapino, M. Malferrari, T. Cramer, M. R. Antognazza and D. Mecerreyes, ACS Appl. Mater. Interfaces, 2023, 15, 35973–35985 CrossRef CAS PubMed.
- G. Tullii, E. Gutierrez-Fernandez, C. Ronchi, C. Bellacanzone, L. Bondi, M. Criado-Gonzalez, P. Lagonegro, F. Moccia, T. Cramer, D. Mecerreyes, J. Martín and M. R. Antognazza, Nanoscale, 2023, 15, 18716–18726 RSC.
- M. Criado-Gonzalez, C. Marzuoli, L. Bondi, E. Gutierrez-Fernandez, G. Tullii, P. Lagonegro, O. Sanz, T. Cramer, M. R. Antognazza and D. Mecerreyes, Nano Lett., 2024, 24, 7244–7251 CrossRef CAS PubMed.
- M. Regato-Herbella, I. Morhenn, D. Mantione, G. Pascuzzi, A. Gallastegui, A. B. Caribé dos Santos Valle, S. E. Moya, M. Criado-Gonzalez and D. Mecerreyes, Chem. Mater., 2024, 36, 1262–1272 CrossRef CAS PubMed.
- E. Yarali, M. J. Mirzaali, A. Ghalayaniesfahani, A. Accardo, P. J. Diaz-Payno and A. A. Zadpoor, Adv. Mater., 2024, 36, 2402301 CrossRef CAS PubMed.
- I. Abdel Aziz, M. Malferrari, F. Roggiani, G. Tullii, S. Rapino and M. R. Antognazza, iScience, 2020, 23, 101091 CrossRef CAS PubMed.
- L. Bondi, C. Marzuoli, E. Gutiérrez-Fernández, G. Tullii, J. Martín, B. Fraboni, D. Mecerreyes, M. R. Antognazza and T. Cramer, Adv. Electron. Mater., 2023, 9, 2300146 CrossRef CAS.
- B. Ilic, D. Czaplewski, P. Neuzil, T. Stanczyk, J. Blough and G. J. Maclay, J. Mater. Sci., 2000, 35, 3447–3457 CrossRef CAS.
- S. Ben-Amor, E. Vanhove, F. Sékli Belaïdi, S. Charlot, D. Colin, M. Rigoulet, A. Devin, N. Sojic, J. Launay, P. Temple-Boyer and S. Arbault, Electrochim. Acta, 2014, 126, 171–178 CrossRef CAS.
- S. E. Stanca, F. Hänschke, A. Ihring, G. Zieger, J. Dellith, E. Kessler and H. G. Meyer, Sci. Rep., 2017, 7, 1074 CrossRef CAS PubMed.
- S.-E. Stanca, O. Vogt, G. Zieger, A. Ihring, J. Dellith, A. Undisz, M. Rettenmayr and H. Schmidt, Commun. Chem., 2021, 4, 98 CrossRef CAS PubMed.
- A. Ahmad, A. Khan, N. Manzoor and L. A. Khan, Microb. Pathog., 2010, 48, 35–41 CrossRef CAS PubMed.
- S. R. Martínez, L. E. Ibarra, R. A. Ponzio, M. V. Forcone, A. B. Wendel, C. A. Chesta, M. B. Spesia and R. E. Palacios, ACS Infect. Dis., 2020, 6, 2202–2213 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR spectrum of P3HT; TEM image and UV-vis spectrum of P3HT SPNs; rheological properties of hydrogels; fluorescence pictures of hydrogels; fluorescence microscopy images GL261 cell viability and ROS production tests; flow cytometry results. See DOI: https://doi.org/10.1039/d4mh01802h |
| This journal is © The Royal Society of Chemistry 2025 |