Open Access Article
Open Access ArticleAntibacterial peptidomimetics based on guanidine-functionalized di-tertiary amides
Ghayah
Bahatheg
 *ab,
Rajesh
Kuppusamy
*ac,
Lissy M.
Hartmann
d,
Charles G.
Cranfield
*ab,
Rajesh
Kuppusamy
*ac,
Lissy M.
Hartmann
d,
Charles G.
Cranfield
 d,
David StC.
Black
d,
David StC.
Black
 a,
Mark
Willcox
c and
Naresh
Kumar
a,
Mark
Willcox
c and
Naresh
Kumar
 *a
*a
aSchool of Chemistry, The University of New South Wales (UNSW), Sydney, NSW 2052, Australia. E-mail: g.bahatheg@unsw.edu.au; r.kuppusamy@unsw.edu.au; n.kumar@unsw.edu.au
bDepartment of Chemistry, Faculty of Science, University of Jeddah, Jeddah 21589, Saudi Arabia
cSchool of Optometry and Vision Science, The University of New South Wales (UNSW), Sydney, NSW 2052, Australia
dSchool of Life Sciences, University of Technology Sydney, PO Box 123, Ultimo 2007, Australia
First published on 1st October 2025
Abstract
Tertiary amides such as peptoids are a novel class of peptidomimetics that offer enhanced structure, activity, and stability compared to many naturally occurring antimicrobial peptides. Guanidino compounds have gained interest in medicinal chemistry as cell-penetrating molecules. This work investigates the changes in the antibacterial activity of modified guanidino groups on the structure of active guanidino tertiary amides by incorporating lipophilic, hydrophobic, and extra cationic groups, thereby combining the properties of the tertiary amide in the peptoid backbone with the important role of addition of extra cationic and lipophilic residues, such as those in AMPs, but supported by guanidine backbones. A library of active antibacterial bromo-phenyl and dichloro-phenyl-based guanidinium tertiary amides, including three series, was designed. These compounds exhibited MICs of 1–2 μg mL−1, 4–8 μg mL−1, and 16.5–35.6 μg mL−1 against S. aureus, E. coli, and P. aeruginosa, respectively. Tertiary amides with their guanidine bearing an alkylated cationic group of 3C (19a and 20a) and 6C (19b and 20b) length resulted in the most active molecules against all tested strains. Additionally, at 8× MIC, compound 19b was the most effective S. aureus biofilm disruptor, disrupting 75% of the biofilm, while compound 19g was the most active molecule against E. coli biofilm, with 50% disruption. The membrane permeability and QCM-D studies suggested that the designed cationic tertiary amides could depolarize and disrupt the bacterial cell membrane. The most potent peptoids were non-toxic, with HC50 of more than 50 μg mL−1.
Introduction
New classes of antibacterial drugs are in urgent demand to stem the extreme resistance of pathogenic bacterial cells against conventional antibiotics, which is negatively affecting the progress of therapeutic accomplishments.1,2 The World Health Organization has classified resistance to traditional antibiotics as one of the three major public health threats in the 21st century.1 Traditional antibiotics generally act on specific cellular targets, providing sufficient time for the development of unpredictable resistance. Additionally, bacterial biofilms enhance bacterial resistance and are particularly challenging for traditional antibiotics to overcome.3Natural antimicrobial peptides (AMPs) have gained interest as alternatives to conventional antibiotics. Their structures are rich in cationic and hydrophobic residues, allowing them to quickly damage the anionic surface of microbial cytoplasmic membranes.4 Despite AMPs showing unique antimicrobial potency against different pathogens, including bacteria, fungi, and viruses,5,6 the clinical use of them is limited.7 These limitations are due to their chemical instability, protease susceptibility, and sometimes poor permeability into the target cell membrane.6,8 The chemical stability of the drugs is critical for localisation, efficacy, and safety. Here, the tertiary amides, as in peptoid-based scaffolds, can enhance the stability in the presence of protease action.9
Peptoids or N-substituted glycines are a class of peptidomimetics that with demonstrated activity against a broad spectrum of microbes.7 This high potency of peptoids is due to their rigid tertiary amide structure (the side chain connected to nitrogen instead of alpha carbonyl carbon) compared to AMPs, which makes their structures chemically and physically stable.7,10 These advantages of peptoids as tertiary amides bearing different cationic and/or hydrophobic residues have increased the attention of researchers to design more of these structures. Similarly, tertiary amide-based fungicides such as isoflucypram, metalaxyl, benalaxyl, and ofurace have been commercialized.11 Despite their potential, relatively few studies have explored the design and optimization of tertiary amide-based peptoid-inspired peptidomimetics for antibacterial applications. Salinomycins commonly used in veterinary medicine its tertiary amide derivatives showed good antibacterial activity against both Gram-positive and Gram-negative bacteria.11
Incorporating a guanidine group into molecules is a practical method to enhance antibacterial activity.12 Insertion of guanidino (arginine-type) instead of amino (lysine-type) groups into antibacterial tertiary amide backbones of the peptoids improves their antibacterial properties.13,14 Recently, guanidinium and lipophilic molecules have gained interest in medicinal chemistry as cellular delivery vehicles due to their cell-penetrating abilities.13,15–19 The effectiveness of the guanidino group can be attributed to the delocalization of the charge of the guanidino group due to the resonance process, which increases the stability of the positive charge on the guanidine part, leading to an increase in the electrostatic interaction with bacterial membrane phospholipids.20,21 Considering the potential of the guanidino group on the molecule activity, researchers have designed antibacterial compounds containing arginine-type monomers instead of lysine-type monomers 1 (Fig. 1).22 The same group in a different study added more than ten different residues into their structures and reported that the best active compounds against a broad spectrum of Gram-positive and Gram-negative bacteria were guanidino derivatives (NhArg-Nmfp-Nmfp)4 and (NhArg-Nphe-Nphe)4.23
Lipophilicity is another feature that can help impart antibacterial activity by increasing the membrane permeability of the molecules.24 Fatty acid residues are a fundamental factor in the potent activity of some antibiotics such as daptomycin and the polymyxin families.25,26
The attachment of a lipid tail to lysine-type compounds increased the antibacterial activity, and the most active compound was MG10 (Fig. 1) attached to the longest lipid tail of 16C with an MIC of 6.3 μg mL−1 against S. aureus and E. coli.27 Additionally, the antibacterial compound 1 H-(NLys-Nspe-Nspe)4-NH2 shows increased activity when fatty tails (Npent, Ndec, and Ntridec residues) are added, and the Ntridec-14mer lipopeptoid 2 (Fig. 1) with the longest lipophilic tail showed the most activity against B. subtilis with MIC of 1.8 μg mL−1 and an MIC on 14 μg mL−1 against E. coli.28 Moreover, Ntridec-14mer lipidic molecule showed an ability to disrupt S. aureus and P. aeruginosa pre-established biofilms.29,30 Other researchers have inserted alkyl chains with variable length to the sides and the centre of short antibacterial and antibiofilm compound 3 (Fig. 1), and shown that the length of the alkyl chains modulates antibacterial activity and mammalian cell toxicity.31
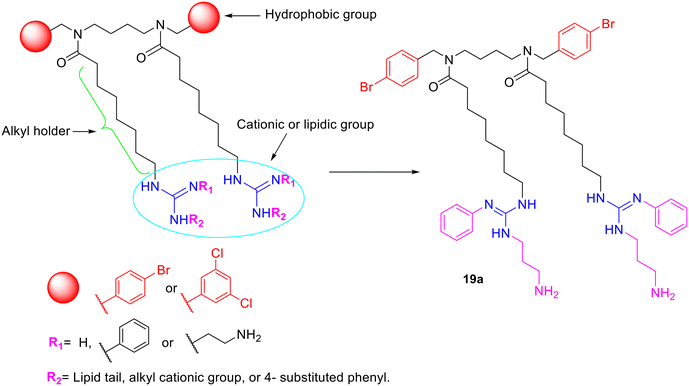
It has been reported that guanidino molecules have a higher antibacterial potential than other molecules.32 In this work, active amino tertiary amides 12a and 12b32,33 were subjected to a guanidination reaction using substituted guanidine reagents. The guanidine reagents were first prepared by i) directly connecting a lipophilic tail or aromatic group to the guanidine moiety, ii) thioureas containing a phenyl group and amino-alkyl or phenyl group, or iii) thioureas with an ethylamine and substituted phenyl moieties. These guanidine reagents were reacted with amino tertiary amides to give three different series (Fig. 2).
Results and discussion
Synthesis
 | ||
| Scheme 2 Preparation of the substituted thiourea reagents, A] using phenyl isothiocyanate 7, and B] using N-Boc-ethylamine isothiocyanate 9. | ||
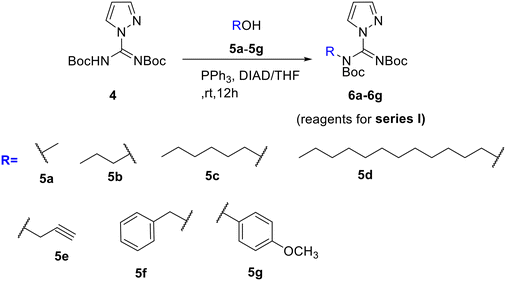
To design the first series of the guanidine reagents, different alcohols 5a–5g were added to N,N′-di-Boc-1H-pyrazole-1-carboxamidine under Mitsunobu reaction conditions to yield carboxamidine derivatives 6a–6g (Scheme 1).
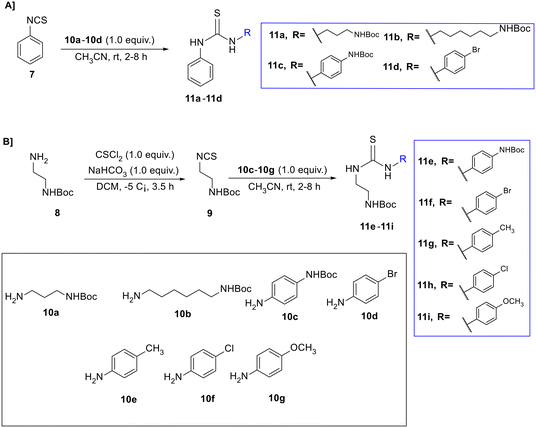
The second and third series were designed to create different thiourea reagents bearing one or two cationic sides and substituted or unsubstituted phenyl rings, in order to explore the effect of cationic and hydrophobic groups on the antibacterial activity. The second series was formed by adding different N-Boc-amino compounds 10a–10d to phenyl isothiocyanate 7, producing thiourea reagents 11a–11d (Scheme 2A).
To synthesize the third series, first, compound N-Boc-ethylenedi-amine 8 was reacted with thiophosgene (CSCl2) in the presence of sodium bicarbonate (NaHCO3) and dichloromethane (DCM) as a solvent to produce N-Boc-ethylamine isothiocyanate 9. This was followed by connecting compound 9 (cationic group) with the 4-substituted phenyl reagents (10c–10g) as hydrophobic groups to afford the thioureas 11e–11i (Scheme 2B).
Synthesis of the lipophilic and cationic tertiary amide
To synthesize the first series of substituted guanidine tertiary amides, the amino tertiary amides 12a33 and 12b32 were reacted with compounds 6a–6g to form the Boc-protected guanidino tertiary amides 13a–13g and 14a–14g. This was followed by the removal of the Boc group using trifluoroacetic acid in DCM, which gave the desired substituted guanidino tertiary amides series IA and IB (15a–15g and 16a–16g) in good yields (Scheme 3). Additionally, to design the third series of substituted guanidino tertiary amides, the amino tertiary amides (12a and 12b) and compounds 11a–11i in DMF were treated with anhydrous triethylamine Et3N before the addition of mercuric chloride in small portions at 0 °C to produce the Boc-protected guanidino tertiary amides 17a–17i and 18a–18i (Scheme 4). After that, Boc groups were eliminated using TFA in DCM to form the second series A and B (19a–19d and 20a–20c) and the third series A and B (19e–19i and 20e–20i) of substituted guanidino tertiary amides in moderate yield (Scheme 4). The procedures of synthesis and characterization data of the lipophilic and cationic tertiary amides are available in the experimental section of the SI S1–S70.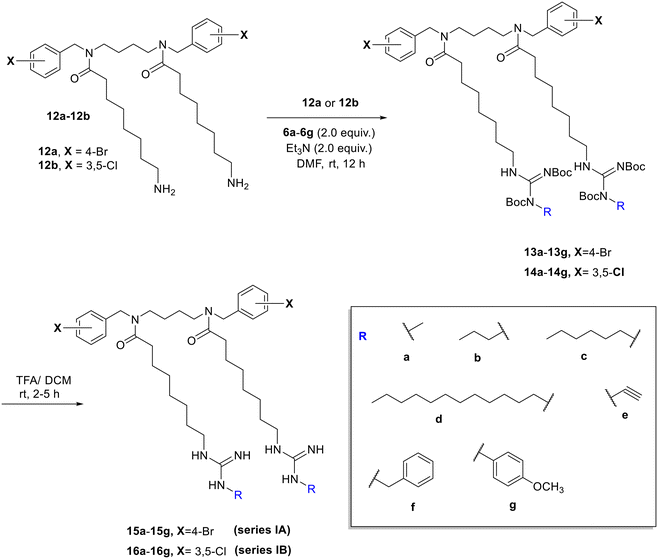 | ||
| Scheme 3 The preparation of the first series of substituted guanidine lipophilic tertiary amides using substituted carboxamidine reagents (6a–6g). | ||
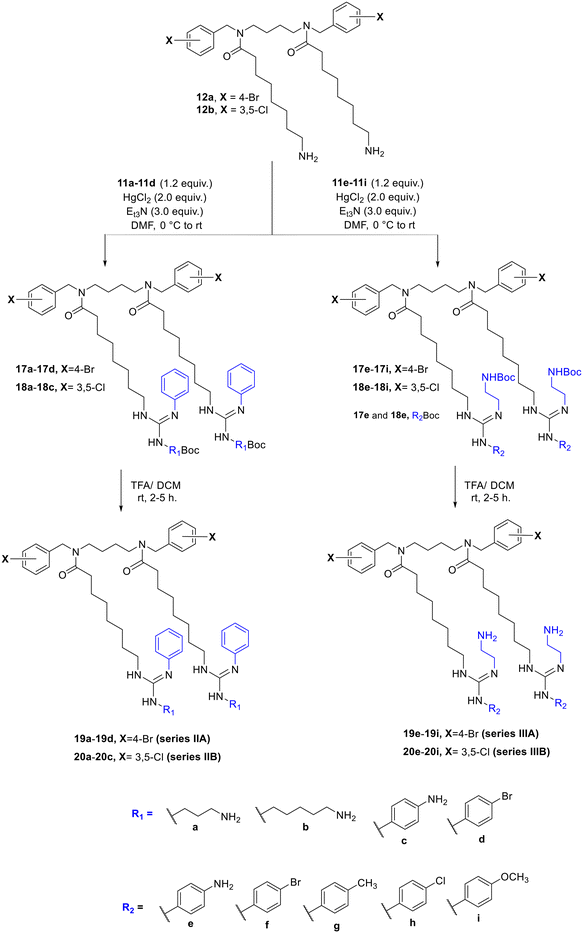 | ||
| Scheme 4 The preparation of the second and third series of substituted guanidine cationic tertiary amides using substituted thiourea reagents (11a–11i). | ||
Antibacterial activity
The antibacterial activities of the substituted guanidino lipophilic and cationic tertiary amides were evaluated against six bacterial strains: Gram-positive bacteria S. aureus (SA38, ATCC 6538, ATCC 25923); and Gram-negative bacteria (E. coli K12, E. coli ATCC 25922), and P. aeruginosa (PA01). The activity of the substituted guanidino tertiary amides was compared with the activity of MSI-78 (Pexiganan, an AMP). The MIC and toxicity values are reported in Table 1 (see the experiment protocol in the SI).| ID | Series | MIC (μg mL−1) | HC (μg mL−1) | Alog P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus 38 | S. aureus ATCC 6538 | S. aureus ATCC 25923 | E. coli K12 | E. coli ATCC 25922 | PA01 | HC10 | HC50 | |||
a NT (not tested); PA01 (P. aeruginosa strain PA01); bolded MICs represent the most active molecules against S. aureus (MIC < 2.5 μg mL−1), E. coli (MIC < 5 μg mL−1), and PA01 (MIC < 36 μg mL−1); HC = hemolytic concentration giving 10% or 50% hemolysis; Alog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P is a measure of lipophilicity/hydrophobicity. P is a measure of lipophilicity/hydrophobicity.
|
||||||||||
| 15a | IA | 1.6 | 1.6 | 1.6 | 6.2 | 6.2 | >205 | >9.8 | 20.5 | 6.87 |
| 15b | 1.7 | 1.7 | 1.7 | 13.8 | 13.8 | 219 | 10.5 | 21.9 | 8.63 | |
| 15c | 3.7 | 3.7 | 7.5 | 60 | 60 | >240 | 24 | 48 | 11.30 | |
| 15d | >282 | NT | NT | >282 | NT | NT | 112.9 | 451.7 | 16.63 | |
| 15e | 1.7 | 1.7 | 1.7 | 27 | 13.5 | 217 | 17.4 | >21.5 | 7.33 | |
| 15f | 3.8 | 3.8 | 3.8 | 15.2 | 15.2 | 243 | 24.3 | >48.6 | 10.32 | |
| 15g | 16 | NT | 16 | 16 | 16 | NT | 25.8 | >51.6 | 10.01 | |
| 16a | IB | 1.6 | 1.6 | 1.6 | 6.2 | 6.2 | 200 | 9.6 | 16 | 7.75 |
| 16b | 1.7 | 1.7 | 1.7 | 13.4 | 13.4 | 214 | 10.3 | 17.1 | 9.51 | |
| 16c | 7.3 | 7.3 | 7.3 | >235 | NT | NT | 18.8 | 23.5 | 12.18 | |
| 16d | >277 | NT | NT | >277 | NT | NT | 110.9 | >444 | 17.51 | |
| 16e | 1.7 | 1.7 | 1.7 | 13.2 | 6.6 | 216 | 16.9 | 16.9 | 8.21 | |
| 16f | 7.4 | 7.4 | 7.4 | 119 | NT | NT | >23.8 | >47.6 | 11.20 | |
| 16g | 15.8 | 15.8 | 15.8 | >253 | NT | NT | 6.1 | 12.2 | 10.89 | |
| 19a | IIA | 1 | 1 | 1 | 4.1 | 2 | 16.5 | 26.5 | >52.9 | 9.03 |
| 19b | 2.2 | 2.2 | 1.1 | 4.4 | 2.2 | 35.6 | 28.6 | 57.2 | 11.84 | |
| 19c | 17.6 | 17.6 | 17.6 | >282 | NT | >282 | 13.5 | 28.2 | 12.66 | |
| 19d | >313 | NT | NT | >313 | NT | >313 | 125.5 | >502 | 15.86 | |
| 20a | IIB | 2 | 2 | 2 | 4 | 4 | 32.4 | 12.5 | 20.8 | 9.91 |
| 20b | 1.1 | 1.1 | 1.1 | 4.4 | 4.4 | 35.6 | 13.5 | 22.5 | 12.72 | |
| 20c | 17.3 | 17.3 | 17.3 | >277 | NT | >277 | 27.8 | 55.4 | 13.54 | |
| 19e | IIIA | 8.3 | 8.3 | 8.3 | 8.3 | 8.3 | 33 | 53.1 | >106 | 7.88 |
| 19f | 4.6 | 9.3 | 9.3 | 9.3 | 18.5 | 74.2 | >59.4 | >119 | 11.08 | |
| 19g | 2.1 | 2.1 | 4.1 | 4.1 | 4.1 | 66.1 | 21.2 | 26.5 | 10.57 | |
| 19h | 4.3 | 4.3 | 4.3 | 17.3 | 17.3 | 68.6 | >54.9 | >110 | 10.75 | |
| 19i | 2.1 | 2.1 | 2.1 | 4.2 | 2.1 | 34 | 21.8 | 27.3 | 9.22 | |
| 20e | IIIB | 8.1 | 8.1 | 8.1 | 8.1 | 8.1 | 32.5 | 26.0 | >52.1 | 8.76 |
| 20f | 4.5 | 9.1 | 9.1 | 72.9 | 146 | 292.2 | >58.4 | >117 | 11.96 | |
| 20g | 4 | 4 | 4 | 8.1 | 16.2 | 64.8 | 12.5 | 20.7 | 11.45 | |
| 20i | 2.1 | 2.1 | 2.1 | 4.2 | 4.2 | 66.8 | 12.9 | 21.4 | 10.10 | |
| MSI-78 (ref. 34–38) | 1.3–2.6 | — | 4.0 | 1.3–2.6 | 8.0–16.0 | 1.3–2.6 | — | — | 4.52 | |
In general, the majority of the synthesized tertiary amides had excellent activity against S. aureus strains with MICs ranging from 1 μg mL−1 to 8 μg mL−1. Additionally, compared to the previously synthesized tertiary amides,32,33 where only two compounds had excellent activity against E. coli strains with MIC of 6.2 μg mL−1, in this work, a range of active tertiary amides had excellent activity against Gram-negative bacteria, especially E. coli, with an average MIC of 4–8 μg mL−1.
In the first series with IA (bromo derivatives) and IB (chloro derivatives) of alkyl-substituted guanidino tertiary amides 15a–15d and 16a–16d, the antibacterial activity against S. aureus and E. coli K12 decreased as the length of the alkyl chain increased. The guanidino methyl, propyl, and propyne tertiary amides (15a, 15b, 15e, 16a, 16b, and 16e) were the most active lipo-tertiary amides against S. aureus with MICs around 1.6 μg mL−1, while hexyl derivatives 15c and 16c showed a slight reduction in the activity by two-fold and four-fold against S. aureus with MICs of 3.7 and 7.3 μg mL−1 respectively. The attachment of the dodecyl lipid chain (12C) to the guanidine part in compounds 15d and 16d caused a loss of antibacterial activity against S. aureus.
The alteration in activity resulting from the insertion of an aromatic (phenyl and 4-methoxyphenyl) hydrophobic group instead of an alkyl tail was investigated in compounds 15f–15g and 16f–16g. The bromo tertiary amide 15f was slightly more active against S. aureus than chloro tertiary amide 16f with MICs of 3.8 and 7.4 μg mL−1, respectively. The insertion of the methoxy group (OCH3) on the guanidino phenyl ring in compounds 15g and 16g reduced their activity, with MICs around 16 μg mL−1. In the same series (IA and IB), as with the activity against S. aureus, the activity against E. coli was reduced by increasing the alkyl chain. The methylated guanidino compounds 15a and 16a showed activity with MICs of 6.2 μg mL−1, while this activity was decreased for propyl and propyne guanidino compounds 15b, 16b and 16e with MICs of approximately 13.5 μg mL−1 and 15e with MIC of up to 27 μg mL−1. The activity continued to decrease by increasing the alkyl tail length; the hexyl guanidino bromo tertiary amide 15c had MIC of 60 μg mL−1, whereas the hexyl guanidino chloro tertiary amide 16c and dodecyl guanidino tertiary amides (15d and 16d) were inactive against E. coli K12.
The bromo tertiary amides 15f and 15g (with phenyl and 4-methoxy phenyl attached to the guanidino group) showed moderate activity against E. coli with MICs around 15.5 μg mL−1, whereas the dichloro tertiary amides 16f and 16g (with phenyl and 4-methoxy phenyl attached to the guanidine group) were inactive against E. coli K12.
Alog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values represent the lipophilicity of the guanidino lipo-tertiary amides (15 and 16). The activity decreased with an increase in the lipophilicity of the molecule (Alog
P values represent the lipophilicity of the guanidino lipo-tertiary amides (15 and 16). The activity decreased with an increase in the lipophilicity of the molecule (Alog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values). This is clear from the low Alog
P values). This is clear from the low Alog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of the most active methylated guanidino compounds 15a and 16a (Alog
P values of the most active methylated guanidino compounds 15a and 16a (Alog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 6.9 and 7.8) compared to the high Alog
P = 6.9 and 7.8) compared to the high Alog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of dodecyl guanidino tertiary amides 15d and 16d (Alog
P values of dodecyl guanidino tertiary amides 15d and 16d (Alog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 16.7 and 17.5).
P = 16.7 and 17.5).
Additionally, all the designed lipo-tertiary amides of series IA and IB were inactive (MIC ≥ 200 μg mL−1) against PA01. Fig. 3A summarizes the activity of these lipophilic tertiary amides against S. aureus 38 and E. coli K12.
 | ||
| Fig. 3 The antibacterial activity of series I (A), II (B), and III (C) against S. aureus 38 and E. coli K12 as represented by the MIC values. | ||
In the second series II, two groups were attached to the guanidine to form series IIA (19a–19d) and series IIB (20a–20d); one side of the guanidine groups has a cationic group, and the other side a phenyl group.
The activities of the bromophenyl substituted-guanidino tertiary amides series IIA (19a–19d) and dichlorophenyl substituted-guanidino tertiary amides series IIB (20a–20c) were evaluated against all the bacterial strains. Compound 19d was an interesting compound that lost antimicrobial activity when the cationic side was replaced with an additional hydrophobic group.
The compounds 19a, 19b, 20a, and 20b of series IIA and IIB were the most active molecules against the bacterial strains among all the designed lipophilic and cationic tertiary amides with MICs of 1–2 μg mL−1 against S. aureus, 2.3–3.9 μg mL−1 against E. coli and 16.5–35.5 μg mL−1 against P. aeruginosa PA01. Compound 19a was the most active compound against P. aeruginosa PA01 with MIC of 16.5 μg mL−1.
The best activities of these compounds, 19a–b and 20a–b, could be attributed to the free cationic group that was attached by the 3C and 6C alkyl chains to the bottom side of the guanidine group. The change of the alkyl linker from 3C (19a and 20a) to 6C (19b and 20b) did not change the activity. In the case of compounds 19c and 20c, the attachment of an aryl cationic group (aniline) decreased the activity against S. aureus with MIC around 17.5 μg mL−1 compared to 1–2 μg mL−1 for 19a–b and 20a–b. Moreover, compounds 19c and 20c lost the activity against Gram-negative bacteria compared to the active compounds 19a–b and 20a–b. The replacement of the cationic group NH2 on 19c with Br produced compound 19d. This replacement reduced the cationicity and increased the hydrophobicity of 19d, with a high Alog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value of 15.9, which resulted in the loss of activity against all bacterial strains, highlighting the importance of maintaining a balance between the cationic and hydrophobic components in these tertiary amides. Fig. 3B shows a comparison between the MIC values of series II derivatives against S. aureus 38 and E. coli K12.
P value of 15.9, which resulted in the loss of activity against all bacterial strains, highlighting the importance of maintaining a balance between the cationic and hydrophobic components in these tertiary amides. Fig. 3B shows a comparison between the MIC values of series II derivatives against S. aureus 38 and E. coli K12.
In series III compounds 19e–19i and 20e–20i, the cationic group (ethylamine group) was linked to the sides of the guanidine groups, and the bottom side was attached to different 4-substituted phenyl rings (4-(bromo, methyl, chloro, or methoxy)-phenyl). As shown in Fig. 3C and Table 1, the compounds 19f–19i and 20f–20i showed the same range of activity against S. aureus with MICs of 2.1–4.6 μg mL−1, which indicates that the activity is attributed to the cationic group regardless of the type of hydrophobic group. Also, the lipophilicity did not play a role in the activity of compounds 19f–19i and 20f–20i, which is evident from the very similar Alog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of these tertiary amides (Table 1). Compounds that had methyl or methoxy groups (19g, 19i, 20g, and 20i) showed more activity against E. coli strains with 4.1, 4.2, 8.1, and 4.2 μg mL−1, respectively, compared to 19f, 19h, and 20f (which contain additional chlorine and bromine atoms) with MIC of 9.3, 17.3, and 72.9 μg mL−1, respectively (Table 1 and Fig. 3C).
P values of these tertiary amides (Table 1). Compounds that had methyl or methoxy groups (19g, 19i, 20g, and 20i) showed more activity against E. coli strains with 4.1, 4.2, 8.1, and 4.2 μg mL−1, respectively, compared to 19f, 19h, and 20f (which contain additional chlorine and bromine atoms) with MIC of 9.3, 17.3, and 72.9 μg mL−1, respectively (Table 1 and Fig. 3C).
Compounds 19e and 20e have two cationic sides, with an additional one (NH2) which is attached to the para position of the phenyl ring. These two compounds, 19e and 20e, exhibited equal activity against S. aureus and E. coli, with MICs of approximately 8 μg mL−1. The best active compounds in series III against PA01 were 19e, 20e (aniline derivatives), and 19i, with MICs ranging from 32.5 μg mL−1 to 34 μg mL−1. In contrast, other compounds in the same groups showed less activity, with MICs of 64.8–74.2 μg mL−1, except for 20f, which was inactive against PA01. Furthermore, the activity of the active compounds in series I, II, and III was evaluated against other Gram-positive and Gram-negative bacterial strains, and their activity was similar to that against S. aureus 38 and E. coli K12.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values in Table 1.
P values in Table 1.
Series II contains the most active structures 19a–b and 20a–b that include guanidine groups bearing a phenyl ring and cationic alkyl tails. In this series, when the cationic alkyl chain of 19a–b and 20a–b was replaced by an aryl cationic group (aniline) (19c and 20c), the activity decreased due to the increase in lipophilicity. Additionally, the replacement of the NH2 group on the aniline moiety by a bromine atom (19d) resulted in complete loss of antibacterial activity (with the highest Alog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value of 15.8).
P value of 15.8).
In series III, all peptoids had a good activity (the MIC values in this group were slightly similar or very close), especially against Gram-positive bacteria, because all these compounds contain the same cationic group (ethylamine group) on one side of the guanidine, and the other side contained a substituted phenyl group. The lipophilicity did not play a clear role in the activity of this series of tertiary amides. Methyl and methoxy-phenyl derivatives of this series were the most active derivatives against S. aureus and E. coli, while aniline analogs were the most active derivatives against PA01. The SAR of the substituted guanidine tertiary amides is outlined in Fig. 4.
Hemolytic activity assay
The toxicity of all substituted guanidino tertiary amides was assessed by their ability to lyse horse red blood cells (Thermo Fisher Scientific, HB250) and is represented as their HC10 (concentration producing a maximum 10% hemolysis) and HC50 (concentration producing a maximum 50% hemolysis) values (Table 1). In general, all bromo tertiary amides of series IA, IIA, and IIIA showed lower toxicity against mammalian blood cells and higher therapeutic doses compared to chloro tertiary amides series IB, IIB, and IIIB. Additionally, the most active cationic substituted guanidino tertiary amides 19a and 19b of series II exhibited lower levels of hemolysis (1–5%) at 25 μg mL−1 compared to the active lipophilic guanidino tertiary amides 15a–b, 16a–b of series I. The most active compounds against all tested bacterial strains with the lowest toxicity were compounds 19a and 19b, which had MICs of 1–2 μg mL−1 against S. aureus 38 and therapeutic indices (TI) of 52.9 and 24.9 times of their MICs, respectively. Also, they had therapeutic indices (TIs) of 13 times their MICs against E. coli, and TIs of 3.2 and 1.6 against PA01, respectively (TI = HC/MIC). Table 2 shows the therapeutic index (TI) and selectivity ratio (SR) of the active tertiary amides against S. aureus 38, E. coli K12, and PA01 (see the experiment protocol in the SI).| ID | HD/HC (μg mL−1) | MIC (μg mL−1) | Therapeutic index (TI) | Selectivity ratio (SR) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HD10/HC10 | HD50/HC10 | S. aureus 38 | E. coli K12 | PA01 | TIS | TIE | TIP | SRS | SRE | SRP | |
| a HD10 (10% haemolytic dose), HD50 (50% haemolytic dose), TI = HD50/MIC, SR = HD10/MIC. TIS, TIE, TIP, SRS, SRE, and SRP (therapeutic indices and the selectivity ratios against S. aureus 38, E. coli K12, and PA01, respectively). | |||||||||||
| 15a | 9.8 | 20.5 | 1.6 | 6.2 | 205 | 12.8 | 3.3 | 0.1 | 6.1 | 1.6 | 0.1 |
| 15b | 10.5 | 21.9 | 1.7 | 13.8 | 219 | 12.8 | 1.6 | 0.1 | 6.2 | 0.7 | 0.1 |
| 15c | 24 | 48 | 3.7 | 60 | 240 | 12.9 | 0.8 | 0.2 | 6.5 | 0.4 | 0.1 |
| 15e | 17.4 | 21.5 | 1.7 | 27 | 217 | 12.6 | 0.8 | 0.1 | 10.2 | 0.6 | 0.1 |
| 15f | 24.3 | 48.6 | 3.8 | 15.2 | 243 | 12.7 | 3.2 | 0.2 | 6.4 | 1.6 | 0.1 |
| 16a | 9.6 | 16 | 1.6 | 6.2 | 200 | 10 | 2.6 | 0.1 | 6 | 1.5 | 0.1 |
| 16b | 10.3 | 17.1 | 1.7 | 13.4 | 214 | 10.1 | 1.3 | 0.1 | 6.1 | 0.7 | 0.1 |
| 16e | 16.9 | 16.9 | 1.7 | 13.2 | 216 | 9.9 | 1.3 | 0.1 | 9.9 | 1.3 | 0.1 |
| 19a | 26.5 | 52.9 | 1 | 4.1 | 16.5 | 52.9 | 12.9 | 3.2 | 26.5 | 6.4 | 1.6 |
| 19b | 28.6 | 57.2 | 2.3 | 4.4 | 35.6 | 24.8 | 13 | 1.6 | 12.4 | 6.5 | 0.8 |
| 20a | 12.5 | 20.8 | 2 | 4 | 32.4 | 10.4 | 5.2 | 0.6 | 6.2 | 3.1 | 0.4 |
| 20b | 13.5 | 22.5 | 1.1 | 4.4 | 35.6 | 20.4 | 5.1 | 0.6 | 12.3 | 3.1 | 0.4 |
| 19e | 53.1 | 106 | 8.3 | 8.3 | 33 | 12.7 | 12.7 | 3.2 | 6.4 | 6.4 | 1.6 |
| 19f | 59.4 | 119 | 4.6 | 9.3 | 74.2 | 25.8 | 12.8 | 1.6 | 12.9 | 6.4 | 0.8 |
| 19g | 21.2 | 26.5 | 2.1 | 4.1 | 66.1 | 12.6 | 6.5 | 0.4 | 10.1 | 5.2 | 0.3 |
| 19h | 54.9 | 110 | 4.3 | 17.3 | 68.6 | 25.6 | 6.4 | 1.6 | 12.7 | 3.2 | 0.8 |
| 19i | 21.8 | 27.3 | 2.1 | 4.2 | 34 | 13 | 6.5 | 0.8 | 10.4 | 5.2 | 0.6 |
| 20f | 58.4 | 117 | 4.5 | 72.9 | 292.2 | 26 | 1.6 | 0.4 | 12.9 | 0.8 | 0.2 |
| 20g | 12.5 | 20.7 | 4 | 8.1 | 64.8 | 5.2 | 2.5 | 0.3 | 3.1 | 1.5 | 0.2 |
| 20i | 12.9 | 21.4 | 2.1 | 4.2 | 66.8 | 10.2 | 5.1 | 0.3 | 6.1 | 3.1 | 0.2 |
Antibiofilm activity
The bacterial biofilm has developed various mechanisms to resist the activity of traditional antibiotics and antimicrobial agents, which has made the treatment of biofilm-associated acute infections a critical issue in this era.39–41 Conventional antibiotics and antimicrobials are inactive against infections caused by bacteria in their biofilm state.41 Therefore, the antibiofilm activity of the active tertiary amides was investigated against pre-established biofilms of Gram-positive (S. aureus 38) and Gram-negative bacteria (E. coli K12).The active tertiary amides with additional cationic groups 19a, 20a, 19b, 20b, 19e, 19g, 19i, and 20i were selected to measure their ability to disrupt the pre-established biofilms of S. aureus 38 using the crystal violet (CV) staining assay (Fig. 5A–C). All the compounds were tested at three concentrations: 2×, 4×, and 8× MIC. At all concentrations of 2×, 4×, and 8× MIC, the best disruptor of S. aureus 38 pre-established biofilms was aminohexyl guanidinium bromo-tertiary amides 19b with 42%, 51%, and 75% disruption of the biofilm, respectively. This activity could be a result of the free long tail (hexyl) that holds the cationic group. Additionally, at 2× MIC, the short version of compound 19b (compound 19a), featuring an amino-propyl tail, was the second with 18% disruption of S. aureus 38 biofilms. The remaining compounds, 20a, 20b, 19e, 19g, 19i, and 20i, at 2× MIC eradicated less than 10% of the biofilms of S. aureus 38. At 4× MIC, aniline guanidino bromo-tertiary amide 19e was the best biofilm disruptor after 19b, with 42% reduction in the S. aureus 38 biomass (Fig. 5A). At 4× MIC, the remaining compounds 19a, 20a, 20b, 19g, 19i, and 20i disrupted 37%, 27%, 28%, 18%, 22%, and 38% of the S. aureus 38 biomass, respectively (Fig. 5B). Compound 20b (aminohexyl guanidinium chloro-tertiary amide) showed a considerable reduction of the S. aureus 38 biofilms with 55% reduction at 8× MIC compared to 9% at 2× MIC and compound 19e reduced more of the S. aureus 38 biomass (55%). At 8× MIC, the short version of 19b and 20b, compounds 19a and 20a, disrupted around 50% of the S. aureus 38 pre-established biofilms, while the ethylamine guanidino tertiary amides 19g, 19i, and 20i (with methyl and methoxy groups) have the lowest activity against S. aureus 38 biofilm with around 35% disruption. Interestingly, the majority of bromo derivatives, especially at low concentrations, disrupt the S. aureus 38 biofilms faster than chloro derivatives (Fig. 5C) (see the experiment protocol in the SI).
Moreover, the antibiofilm activity of the same active cationic tertiary amides 19a, 20a, 19b, 20b, 19e, 19g, 19i, and 20i was investigated against eradicating established biofilms of E. coli K12 (Fig. 5D–F). At 2× MIC, compounds 19b (amino-hexyl guanidino bromo-phenyl-based tertiary amide) and 20a (amino-propyl guanidino dichloro-phenyl-based tertiary amide) were the best compounds with approximately 20% disruption of E. coli biomass, while compounds 19a (amino-propyl of bromo-tertiary amide) and 19g (methylated phenyl of bromo-tertiary amide) came in the second place with 15% disruption. The remaining tertiary amides 20b, 19e, 19i, and 20i showed less than 10% reduction in the E. coli biofilms (Fig. 5D).
At 4× MIC, compounds 19b and 20a disrupted 35% of E. coli biomass, whereas compounds 19a and 19g (aminoethyl and methylated phenyl guanidines) disrupted 30% of it. Additionally, at 4× MIC, the other tested tertiary amides 20b and 19e reduced 19% and 15% of the biomass of E. coli K12, respectively, while methoxy phenyl guanidino tertiary amides 19i and 20i disrupted only 7% of the E. coli K12 biofilms (Fig. 5E). At the highest concentration (8× MIC), compound 19g (with aminoethyl cationic group and methyl phenyl group connected to the guanidine part) and 20i (with aminoethyl cationic group and methoxy phenyl group connected to the guanidine part) were the best disruptors, with 50% and 45% disruption of the E. coli K12 biofilm, respectively. On the other hand, compounds 19a, 20a, and 19b showed a 40% reduction of the E. coli K12 biomass at 8× MIC. The remaining compounds (20b, 19e, and 19i) disrupted about 30% of the E. coli K12 biomass (Fig. 5F).
Cytoplasmic membrane depolarization
To examine the potential disruption effect of tertiary amides on the bacterial cytoplasmic membrane, the diSC3-5 (3,3′-dipropyl-thiadicarbocyanine iodide) as a membrane potential-sensitive dye was used (see the experiment protocol in the SI). The dye directly partitions into the membrane of bacterial cells and aggregates throughout the membrane, causing self-quenching of fluorescence. If the substituted guanidino tertiary amide has the ability to disrupt the bacterial cytoplasmic membrane, the dye will be released because of the bacterial cell membrane depolarizes or is damaged. An increase in fluorescence will be observed due to the increased flow of the dye out of the damaged bacterial membranes. Compounds 19a and 19b, which had the best activity against both Gram-positive and Gram-negative strains and the lowest toxicity, were selected to study their ability to damage the bacterial cytoplasmic membrane.The two compounds 19a and 19b were added at 0.5×, 1×, 2×, and 4× MIC, which caused damage to the cell membranes of S. aureus 38 and E. coli K12 in a time and concentration-dependent manner (Fig. 6). In general, at 4× MIC levels, both compounds 19a and 19b affected the S. aureus 38 and E. coli K12 membranes within 3 minutes with an increase in the fluorescence. At the lowest concentration of 0.5× and 0.1× MIC, compound 19a (aminopropyl guanidino tertiary amide) affected the cell membrane within 3 min (Fig. 6A), while 19b (aminohexyl guanidino tertiary amide) did not cause any changes in the fluorescence at the lowest concentrations (0.5× and 0.1× MIC) (Fig. 6B). The fast effect of compound 19a on the bacterial cell membrane at 0.5× MIC compared to 19b could be due to the effect of the low lipophilicity (Alog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 9) of the shorter side chain length of the cationic tail of compound 19a compared to that of compound 19b (Alog
P = 9) of the shorter side chain length of the cationic tail of compound 19a compared to that of compound 19b (Alog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 12). As shown in Fig. 6A, compound 19a increased the fluorescence gradually and in a steady line at 0.5×, 1× and 2× until it reached the peak at 4× MIC within 12–15 minutes due to its effect on the S. aureus 38 cytoplasmic membrane. The addition of compound 19b at 2× and 4× MIC on the S. aureus 38 cytoplasmic membrane caused an increase in fluorescence level before reaching the peak of fluorescence at 4× MIC within 9 minutes up to 15 minutes (Fig. 6B). Similar to the effect of 19a on the S. aureus 38 membrane, compound 19a affected the E. coli K12 membrane at 0.5× MIC, maintaining a constant fluorescence level from 3 minutes to 15 minutes. In contrast, at 1×, 2×, and 4× MIC, the fluorescence increased slightly over time (Fig. 6E).
P = 12). As shown in Fig. 6A, compound 19a increased the fluorescence gradually and in a steady line at 0.5×, 1× and 2× until it reached the peak at 4× MIC within 12–15 minutes due to its effect on the S. aureus 38 cytoplasmic membrane. The addition of compound 19b at 2× and 4× MIC on the S. aureus 38 cytoplasmic membrane caused an increase in fluorescence level before reaching the peak of fluorescence at 4× MIC within 9 minutes up to 15 minutes (Fig. 6B). Similar to the effect of 19a on the S. aureus 38 membrane, compound 19a affected the E. coli K12 membrane at 0.5× MIC, maintaining a constant fluorescence level from 3 minutes to 15 minutes. In contrast, at 1×, 2×, and 4× MIC, the fluorescence increased slightly over time (Fig. 6E).
In the case of the impact of compound 19b on the E. coli K12 at 0.5× MIC, there was no noticeable change in the fluorescence, whilst at 1× and 2× MIC, the fluorescence was increased slightly by increasing the time before reaching the maximum release of the dye at its 4× MIC (Fig. 6F). Moreover, to investigate the responsible mechanism for cell killing, the effects of cationic tertiary amides 19a and 19b on the viability of S. aureus 38 and E. coli K-12 cells have been analysed (Fig. 6C, D, G, and H). In general, the cell viability of compounds 19a and 19b against S. aureus 38 and E. coli K12 resembled the results observed in membrane disruption experiments. At 4× MIC, compound 19a showed almost 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reductions in S. aureus 38 bacterial numbers within 9 min, and these results coincide with the results of the dye release assay as well, while compound 19b showed more than 5
log reductions in S. aureus 38 bacterial numbers within 9 min, and these results coincide with the results of the dye release assay as well, while compound 19b showed more than 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reductions in S. aureus 38 bacterial numbers within 15 min (Fig. 6C and D). In the case of E. coli K12 bacterial numbers, compound 19a and 19b caused about a 4
log reductions in S. aureus 38 bacterial numbers within 15 min (Fig. 6C and D). In the case of E. coli K12 bacterial numbers, compound 19a and 19b caused about a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log decrease in the bacterial numbers within 9 minutes, followed by a reduction of 3
log decrease in the bacterial numbers within 9 minutes, followed by a reduction of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log at 15 minutes for the aminohexyl tertiary amide 19b (Fig. 6G and H). Depending on these results, membrane permeabilization could be a possible mechanism for the substituted guanidino tertiary amides effects over a short time, but there may be longer-term effects that lead to bacterial cell killing. These influences might involve the release of autolysins, similar to the antimicrobial peptides melimine and Mel4.
log at 15 minutes for the aminohexyl tertiary amide 19b (Fig. 6G and H). Depending on these results, membrane permeabilization could be a possible mechanism for the substituted guanidino tertiary amides effects over a short time, but there may be longer-term effects that lead to bacterial cell killing. These influences might involve the release of autolysins, similar to the antimicrobial peptides melimine and Mel4.
Quartz Crystal Microbalance with Dissipation (QCM-D)
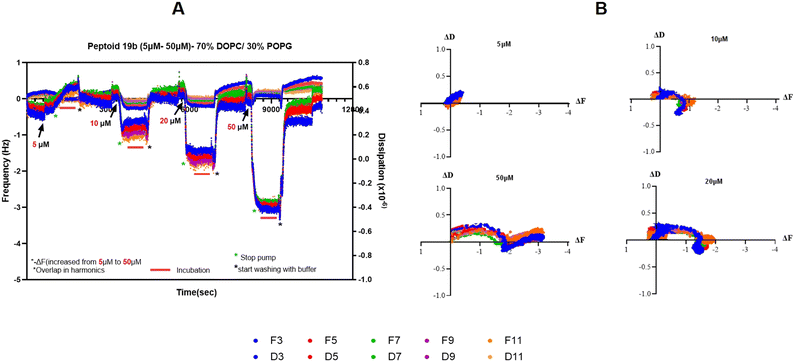
The mode of action of the most active tertiary amides, 19a and 19b (Fig. 7), was also studied using a nanogram-sensitive technique, Quartz Crystal Microbalance with Dissipation (QCM-D) (see the experiment protocol in the SI). The QCM-D method investigates the response of the antibacterial peptides towards lipid membranes.42 The QCM-D analysis shows the changes in two parameters over five harmonics or overtones (3rd, 5th, 7th, 9th, and 11th). Frequency (F) represents the mass of the membrane and additionally deposited compounds, and the change in frequency (ΔF) is proportional to mass changes. Dissipation (D) measures the viscoelasticity of the membrane and the change in dissipation (ΔD) provides insight into structural alterations by the addition of tertiary amides. In both cases, the impact on different harmonics signifies the effect of the substituted guanidino tertiary amides at different distances to the surface and interaction locations can thus be determined.42,43In this study, two models of membranes were employed: 100% POPC (a zwitterionic phosphatidylcholine membrane, as found in eukaryotic cells) and 70% POPC with 30% POPG (anionic phosphatidylglycerols mimicking bacterial cell membranes). During the experiment, the compounds (19a and 19b) were added at consecutively increasing concentrations of 5 μM, 10 μM, 20 μM, and 50 μM on the same membrane, incubated for 10 min each then membranes were washed with buffer for 20 min before the next concentration was added.
Interestingly, as shown in Fig. 8A, B and 10A (ΔF–t and ΔD–t plots), compound 19a (tertiary amides with modified guanidine that bears a propyl amine cationic tail) disrupted the negatively charged model membrane (70% POPC and 30% POPG) at 5 μM with a high positive change of frequency (+ΔF) and negative change of dissipation (−ΔD), while its analogue compound 19b (including hexyl amine as a cationic group) had a negative frequency change (−ΔF) which increased with increasing concentration, reaching the peak at 50 μM with a small positive change of dissipation (+ΔD) (Fig. 10A) across the concentrations. These differences in response between 19a and 19b to their addition to a negatively charged model membrane suggested a different mode or speed in the interaction with the bacterial membrane. This resembled the results from cytoplasmic depolarization studies, as shown in Fig. 6 where compound 19a is causing the release of the dye at lower concentration compared to 19b with higher reduction in the live bacteria number. The addition of compound 19a (5 μM) to the 70% DOPC and 30% POPG lipid layer was sufficient to disrupt the lipidic membrane, as shown in Fig. 8A and B. The addition of compound 19a (5 μM) led to a considerable decrease in the mass of the membrane affecting the overtone on the membrane surface (3rd) with +5.6 Hz, and slightly less for the inner overtones from +4.9 Hz (5th) to 4.1 Hz (11th), suggesting that the effect is strongest at the membrane surface. Additionally, the positive and high change of ΔF is combined with a decrease of viscoelasticity (increase of rigidity) signified by a negative change of dissipation (−ΔD) (Fig. 8B). Results showing positive ΔF values, negative ΔD values, and the spread of overtones suggested that compound 19a is disrupting the negatively charged lipidic membrane using the carpet or detergent-like mode of action.42 This reduction in mass by the addition of 5 μM of 19a was irreversible after washing (Fig. 8A), whereas rigidity is slightly reversible (Fig. 8B). Additionally, the dynamic effect of compound 19a in the interaction process on the 70% DOPC and 30% POPG lipidic membrane can be illustrated using ΔF vs. ΔD plots.44Fig. 8E shows the relationship between changes in frequency and dissipation caused by the addition of 19a on the DOPC/POPG model membrane at 5 μM. The ΔF–ΔD plot shows the loss of mass and an increase in rigidity of the membrane, indicating that compound 19a disrupts the bacterial-type membrane, similar to the antimicrobial peptide, aurein 1.2.45
In contrast, Fig. 8C and D shows the effect of 5 μM 19a on the zwitterionic phosphatidylcholine membrane (100% DOPC lipid layer), which differed from the membrane that contains a negative charge (Fig. 8A and B). Compound 19a (5 μM) on 100% POPC caused a significant increase in mass as indicated by the negative change in frequency values (−ΔF), which is most pronounced at the 3rd (−6.5 Hz) and 5th (−4.8 Hz) harmonics. These increases in the mass absorption came with a slight decrease in the rigidity of the membrane. After the wash, the mass gain was reversible, but the changes in the rigidity were not altered (Fig. 8C and D). Comparing the ΔF–ΔD plots of 19a (5 μM) between the two membrane types makes the difference in dynamic mechanism apparent (Fig. 8E and F).
This was followed by the addition of greater concentrations of compound 19a at 10 μM, 20 μM, and 50 μM to the same washed 100% POPC membrane. This caused a concentration dependent increase in the mass of the membrane (ΔF3rd harmonic = −8.6, −12.5, −17.9 Hz, respectively), combined with a decrease in the rigidity as indicated by the positive dissipation reaching its peak at 50 μM by +5 ppm. Additionally, 19a exhibited dynamic resistance to removal from the POPC membrane; however, continuous washing for four times led to the removal of a small portion of it from this membrane (Fig. 9A). The ΔF–ΔD plots in Fig. 9C show this increase in both the frequency and dissipation with a high spread of the harmonics in the frequency domain and increasing spread in the dissipation domain. Especially the change in the third and fifth harmonics, which observe activity towards the surface region, suggests that tertiary amide 19a aggregates on the POPC lipidic layer surface. A similar relationship between ΔF and ΔD was reported for peptide-Gly15Gly19-caerin1.1.45 The addition of 5 μM of 19b on the 100% POPC membrane showed an increase in the mass, as illustrated by a −2.7 Hz drop, and a minimal decrease in the rigidity of the 100% POPC membrane (Fig. 9B). The addition of higher concentrations of 19b to the washed POPC membrane increased in a concentration dependent manner leading to the maximum values at 50 μM (−7.1 Hz).
After washing, the mass addition and reduction of rigidity were mostly reversible. This suggests that minimal amounts of the compound remain bound even after washing, which also introduces minimal reduction of rigidity. Interestingly, compound 19b (Fig. 9B) showed a similar trend as 19a (Fig. 9A) in its effect on the 100% POPC model membrane. However, the change induced by 19b in both frequency and dissipation as well as the spread of the harmonics is greatly reduced suggesting reduced reactivity of the compound with the membrane. The overlap between the harmonics at low concentrations of 19b suggests that the substituted guanidino tertiary amide is penetrating the bilayer. Only at 50 μM does 19b begin to accumulate more on the surface, whereas compound 19a seems to aggregate largely on the surface of the bilayer at all tested concentrations (Fig. 9A). The ΔF–ΔD plots (Fig. 9C and D) show the mass increases with small changes in the rigidity, which could be a result of more pronounced binding to the surface for 19a at all concentrations, while 19b shows an increasing trend of insertion at low concentrations until it converts to surface binding for 50 μM.
Unlike 19a, the addition of 5 μM 19b (the aminohexyl guanidino tertiary amide) to the 70% POPC and 30% POPG lipidic membrane did not cause a noticeable change in mass and rigidity (Fig. 10A). The addition of 10 μM 19b to the washed POPC and POPG membrane caused a slight increase in mass with −1.2 Hz drop in the frequency. The mass increased as the concentration of 19b was increased to 20 μM and 50 μM, with −2.1 Hz and −3.2 Hz changes, respectively (Fig. 10A). These increases in the mass came with small changes in dissipation and a negligible decrease in the rigidity after the wash (Fig. 10A). Interestingly, 19b affected all the overtones of the membrane suggesting either transmembrane insertion of the substituted guanidino tertiary amide into the membrane or pore formation.42 These results can also be observed in the ΔF–ΔD plots (Fig. 10B), where the mass increase becomes stronger with increasing concentration. Additionally, the changes in mass were reversible after washing suggesting that the 19b can be largely removed from the membrane with only small remaining amounts of compound and effect on membrane rigidity.
Conclusion
The two guanidino sides of bromo-phenyl and dichloro-phenyl-based tertiary amides were modified by attaching one or both sides of two guanidino groups to a lipophilic tail (series I) (15a–15g and 16a–16g) or conjugation of cationic and hydrophobic groups (series II and III) (19a–19i and 20a–20i). In general, the bromo-phenyl modified guanidine derivatives were slightly active compared to dichloro-phenyl ones. This work produced 70% of the modified guanidinium bromo-phenyl and dichloro-phenyl-based tertiary amides with excellent activity against S. aureus strains with MICs ranging from 1 μg mL−1 to 8 μg mL−1 and 50% with MICs of 4–8 μg mL−1 against E. coli strains.The most active compound was 19a (bromo-phenyl-based tertiary amide with phenyl and propylamine tail on the guanidine group) with MICs of 1–2 μg mL−1, 4 μg mL−1, and 16.5 μg mL−1 against S. aureus strains, E. coli strains, and P. aeruginosa, respectively.
In the first series, the tertiary amides bearing the shortest lipophilic tails have the highest potency against Gram-positive and Gram-negative bacteria, except P. aeruginosa (all the series I tertiary amides were inactive against P. aeruginosa). In series II, the attachment of the alkyl (3C and 6C) cationic groups with guanidine (19a, 20a, 19b, and 20b) enhanced the activity compared to the attachment of the aryl cationic group in tertiary amides 19c and 20c (aniline-type). Additionally, these compounds 19a, 20a, 19b and 20b showed the best activity among all the designed compounds against all the tested strains with MIC of 1–2.3 μg mL−1 against S. aureus strains, around 4 μg mL−1 against E. coli strains, and 32–35 μg mL−1 against P. aeruginosa. In series III, 19i and 20i with a methoxy group showed the best activity against S. aureus and E. coli strains. The most active compounds of series III against P. aeruginosa were 19e and 20e (aniline derivatives). Most active tertiary amides disrupted between 40% to 50% of the S. aureus 38 biofilm and around 35% of E. coli K12 biofilm at their 8× MIC concentrations. The best S. aureus biofilm disruptor at 2×, 4×, and 8× MIC was compound 19b, which disrupted 42%, 51%, and 75% of the S. aureus biomass, respectively. Additionally, the most active compound against E. coli biofilm was 19g with 50% disruption at 8× MIC. The membrane permeability and the QCM-D studies revealed that these modified guanidinium tertiary amides could be good bacterial cell membrane disruptors. These most active cationic tertiary amides were non-toxic against blood cells with an HC50 of >50 μg mL−1. Finally, in a comparison between previously prepared amino tertiary amides 12a and 12b with MICs of 11 μg mL−1 and 5.4 μg mL−1 against S. aureus and 11 μg mL−1 and 21.5 μg mL−1 against E. coli, respectively, and HC50 of 10–20 μg mL−1. In this work, a library of active compounds against S. aureus strains with MICs between 1–2 μg mL−1 and around 4 μg mL−1 against E. coli, and with the lowest toxicity against mammalian red blood cells with HC50 of >50 μg mL−1 was obtained.
Author contributions
The synthesis and spectroscopic characterisation of compounds and MIC assays were conducted by G. B. The Biofilm disruption assay was conducted by G. B. The cytoplasmic membrane depolarisation assay was conducted by G. B. The haemolysis assay was conducted by G. B. The QCM-D experiment was conducted by L. H., C. G. C, and G. B. (data analysis). The manuscript was prepared by G. B. Supervision, N. K., R. K., M. W., and D. StC. B.; funding acquisition, G. B., N. K., M. W., and D. StC. B. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Data availability
Supplementary information: supplementary information (SI) including the experimental section for the chemistry part, general notes, general synthesis methods, and experimental data for the designed compounds; biology data including minimum inhibitory concentration (MIC), cytoplasmic membrane permeability assay, viable cell count assay, lysis of horse red blood cells, biofilm, and QCM-D; and the compound characterization data, including NMR, VT experiment, and mass spectra is available. See DOI: https://doi.org/10.1039/D5MD00688K.The data supporting this article have been included within the article and as part of the SI.
Acknowledgements
This work was supported by a Discovery Project from Australian Research Council grant (DP 180100845). We thank the NMR and BMSF facilities at the University of New South Wales (UNSW Australia) for their support in characterizing the synthesized compounds. The authors would like to acknowledge the Saudi Ministry of Education and Jeddah University for the Endowment Fund and financial support given to Ghayah Bahatheg.References
- J. Lin, K. Nishino, M. C. Roberts, M. Tolmasky, R. I. Aminov and L. Zhang, Mechanisms of antibiotic resistance, Front. Microbiol., 2015, 6, 1–24 Search PubMed.
- M. S. Butler and D. L. Paterson, Antibiotics in the clinical pipeline in October 2019, J. Antibiot., 2020, 73, 329–364 CrossRef CAS PubMed.
- J. Jass and H. M. Lappin-Scott, The efficacy of antibiotics enhanced by electrical currents against Pseudomonas aeruginosa biofilms, J. Antimicrob. Chemother., 1996, 38, 987–1000 CrossRef CAS PubMed.
- R. Rathinakumar, W. F. Walkenhorst and W. C. Wimley, Broad-spectrum antimicrobial peptides by rational combinatorial design and high-throughput screening: The importance of interfacial activity, J. Am. Chem. Soc., 2009, 131, 7609–7617 CrossRef CAS PubMed.
- Y. Sang and F. Blecha, Antimicrobial peptides and bacteriocins: alternatives to traditional antibiotics, Anim. Health Res. Rev., 2008, 9, 227–235 CrossRef.
- P. Kosikowska and A. Lesner, Antimicrobial peptides (AMPs) as drug candidates: a patent review (2003–2015), Expert Opin. Ther. Pat., 2016, 26, 689–702 CrossRef CAS.
- B. Mojsoska, R. N. Zuckermann and H. Jenssen, Structure-activity relationship study of novel peptoids that mimic the structure of antimicrobial peptides, Antimicrob. Agents Chemother., 2015, 59, 4112–4120 CrossRef CAS PubMed.
- D. J. Craik, D. P. Fairlie, S. Liras and D. Price, The Future of Peptide-based Drugs, Chem. Biol. Drug Des., 2013, 81, 136–147 CrossRef CAS.
- M. Won, C. Yoon, J. An, D. Kim and J. S. Kim, Mitochondria-Targeted Antibiotics toward Drug Resistant TNBC, ACS Appl. Bio Mater., 2025, 8, 7105–7112 CrossRef CAS.
- S. M. Miller, R. J. Simon, S. Ng, R. N. Zuckermann, J. M. Kerr and W. H. Moos, Comparison of the proteolytic susceptibilities of homologous L-amino acid, D-amino acid, and N-substituted glycine peptide and peptoid oligomers, Drug Dev. Res., 1995, 35, 20–32 CrossRef CAS.
- J. Stefańska, M. Antoszczak, K. Stępień, M. Bartoszcze, T. Mirski and A. Huczyński, Tertiary amides of Salinomycin: A new group of antibacterial agents against Bacillus anthracis and methicillin-resistant Staphylococcus epidermidis, Bioorg. Med. Chem. Lett., 2015, 25, 2082–2088 CrossRef PubMed.
- K. Andreev, C. Bianchi, J. S. Laursen, L. Citterio, L. Hein-Kristensen, L. Gram, I. Kuzmenko, C. A. Olsen and D. Gidalevitz, Guanidino groups greatly enhance the action of antimicrobial peptidomimetics against bacterial cytoplasmic membranes, Biochim. Biophys. Acta, Biomembr., 2014, 1838, 2492–2502 CrossRef CAS.
- P. A. Wender, D. J. Mitchell, K. Pattabiraman, E. T. Pelkey, L. Steinman and J. B. Rothbard, The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 13003–13008 CrossRef CAS.
- H. L. Bolt and S. L. Cobb, A practical method for the synthesis of peptoids containing both lysine-type and arginine-type monomers, Org. Biomol. Chem., 2016, 14, 1211–1215 RSC.
- W. Huang, J. Seo, J. S. Lin and A. E. Barron, Peptoid transporters: effects of cationic, amphipathic structure on their cellular uptake, Mol. BioSyst., 2012, 8, 2626–2628 RSC.
- D. K. Kölmel, D. Fürniss, S. Susanto, A. Lauer, C. Grabher, S. Bräse and U. Schepers, Cell penetrating peptoids (CPPos): Synthesis of a small combinatorial library by using IRORI MiniKans, Pharmaceuticals, 2012, 5, 1265–1281 CrossRef.
- C. Y. Huang, T. Uno, J. E. Murphy, S. Lee, J. D. Hamer, J. A. Escobedo, F. E. Cohen, R. Radhakrishnan, V. Dwarki and R. N. Zuckermann, Lipitoids - Novel cationic lipids for cellular delivery of plasmid DNA in vitro, Chem. Biol., 1998, 5, 345–354 CrossRef CAS.
- Y. Su, T. Doherty, A. J. Waring, P. Ruchala and M. Hong, Roles of arginine and lysine residues in the translocation of a cell-penetrating peptide from 13C, 31P, and 19F Solid-State NMR, Biochemistry, 2009, 48, 4587–4595 CrossRef CAS PubMed.
- A. Laniel, É. Marouseau, D. T. Nguyen, U. Froehlich, C. McCartney, P. L. Boudreault and C. Lavoie, Characterization of PGua4, a Guanidinium-Rich Peptoid that Delivers IgGs to the Cytosol via Macropinocytosis, Mol. Pharmaceutics, 2023, 20, 1577–1590 CrossRef CAS.
- A. S. Woods and S. Ferré, Amazing stability of the arginine-phosphate electrostatic interaction, J. Proteome Res., 2005, 4, 1397–1402 CrossRef CAS.
- J. Mavri and H. J. Vogel, Ion pair formation of phosphorylated amino acids and lysine and arginine side chains: a theoretical study, Proteins: Struct., Funct., Bioinf., 1996, 24, 495–501 CrossRef CAS.
- H. L. Bolt and S. L. Cobb, A practical method for the synthesis of peptoids containing both lysine-type and arginine-type monomers, Org. Biomol. Chem., 2016, 14, 1211–1215 RSC.
- H. L. Bolt, G. A. Eggimann, C. A. B. Jahoda, R. N. Zuckermann, G. J. Sharples and S. L. Cobb, Exploring the links between peptoid antibacterial activity and toxicity, MedChemComm, 2017, 8, 886–896 RSC.
- C. Peggion, F. Formaggio, M. Crisma, R. F. Epand, R. M. Epand and C. Toniolo, Trichogin: A paradigm for lipopeptaibols, J. Pept. Sci., 2003, 9, 679–689 CrossRef CAS.
- J. N. Steenbergen, J. Alder, G. M. Thorne and F. P. Tally, Daptomycin: A lipopeptide antibiotic for the treatment of serious Gram-positive infections, J. Antimicrob. Chemother., 2005, 55, 283–288 CrossRef CAS.
- H. Tsubery, I. Ofek, S. Cohen and M. Fridkin, N-terminal modifications of Polymyxin B nonapeptide and their effect on antibacterial activity, Peptides, 2001, 22, 1675–1681 CrossRef CAS.
- R. M. Green and K. L. Bicker, Evaluation of peptoid mimics of short, lipophilic peptide antimicrobials, Int. J. Antimicrob. Agents, 2020, 56, 106048 CrossRef CAS PubMed.
- N. P. Chongsiriwatana, T. M. Miller, M. Wetzler, S. Vakulenko, A. J. Karlsson, S. P. Palecek, S. Mobashery and A. E. Barron, Short alkylated peptoid mimics of antimicrobial lipopeptides, Antimicrob. Agents Chemother., 2011, 55, 417–420 CrossRef CAS.
- R. Kapoor, M. W. Wadman, M. T. Dohm, A. M. Czyzewski, A. M. Spormann and A. E. Barron, Antimicrobial peptoids are effective against Pseudomonas aeruginosa biofilms, Antimicrob. Agents Chemother., 2011, 55, 3054–3057 CrossRef CAS.
- J. S. Lin, L. A. Bekale, N. Molchanova, J. E. Nielsen, M. Wright, B. Bacacao, G. Diamond, H. Jenssen, P. L. Santa Maria and A. E. Barron, Anti-persister and Anti-biofilm Activity of Self-Assembled Antimicrobial Peptoid Ellipsoidal Micelles, ACS Infect. Dis., 2022, 8, 1823–1830 CrossRef CAS PubMed.
- J. Hoque, M. M. Konai, S. S. Sequeira, S. Samaddar and J. Haldar, Antibacterial and Antibiofilm Activity of Cationic Small Molecules with Spatial Positioning of Hydrophobicity: An in Vitro and in Vivo Evaluation, J. Med. Chem., 2016, 59, 10750–10762 CrossRef CAS PubMed.
- G. Bahatheg, R. Kuppusamy, M. Yasir, S. K. Mishra, D. S. C. Black, M. Willcox and N. Kumar, Functionalized Phenyl Peptoids with Enhanced Antibacterial Potency, ACS Infect. Dis., 2025, 11, 1648–1661 CrossRef CAS PubMed.
- G. Bahatheg, R. Kuppusamy, M. Yasir, S. Bridge, S. K. Mishra, C. G. Cranfield, D. StC. Black, M. Willcox and N. Kumar, Dimeric peptoids as antibacterial agents, Bioorg. Chem., 2024, 147, 107334 CrossRef CAS.
- R. Kuppusamy, M. Yasir, T. Berry, C. G. Cranfield, S. Nizalapur, E. Yee, O. Kimyon, A. Taunk, K. K. K. Ho, B. Cornell, M. Manefield, M. Willcox, D. S. C. Black and N. Kumar, Design and synthesis of short amphiphilic cationic peptidomimetics based on biphenyl backbone as antibacterial agents, Eur. J. Med. Chem., 2018, 143, 1702–1722 CrossRef CAS PubMed.
- Y. Ge, D. L. MacDonald, K. J. Holroyd, C. Thornsberry, H. Wexler and M. Zasloff, In vitro antibacterial properties of pexiganan, an analog of magainin, Antimicrob. Agents Chemother., 1999, 43, 782–788 CrossRef CAS.
- P. C. Fuchs, A. L. Barry and S. D. Brown, In vitro antimicrobial activity of MSI-78, a magainin analog, Antimicrob. Agents Chemother., 1998, 42, 1213–1216 CrossRef CAS.
- N. P. Chongsiriwatana, J. A. Patch, A. M. Czyzewski, M. T. Dohm, A. Ivankin, D. Gidalevitz, R. N. Zuckermann and A. E. Barron, Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 2794–2799 CrossRef CAS.
- H. Cohen, N. A. Wani, D. Ben Hur, L. Migliolo, M. H. Cardoso, Z. Porat, E. Shimoni, O. L. Franco and Y. Shai, Interaction of Pexiganan (MSI-78)-Derived Analogues Reduces Inflammation and TLR4-Mediated Cytokine Secretion: A Comparative Study, ACS Omega, 2023, 8, 17856–17868 CrossRef CAS.
- B. Amorena, E. Gracia, M. Monzón, J. Leiva, C. Oteiza, M. Pérez, J. L. Alabart and J. Hernández-Yago, Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro, J. Antimicrob. Chemother., 1999, 44, 43–55 CrossRef CAS PubMed.
- R. M. Donlan and J. W. Costerton, Biofilms: Survival mechanisms of clinically relevant microorganisms, Clin. Microbiol. Rev., 2002, 15, 167–193 CrossRef CAS.
- T. Bjarnsholt, O. Ciofu, S. Molin, M. Givskov and N. Høiby, Applying insights from biofilm biology to drug development-can a new approach be developed?, Nat. Rev. Drug Discovery, 2013, 12, 791–808 CrossRef CAS.
- T. John, B. Abel and L. L. Martin, The Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D) Technique Applied to the Study of Membrane-Active Peptides, Aust. J. Chem., 2018, 71, 543–546 CrossRef CAS.
- A. Mechler, S. Praporski, K. Atmuri, M. Boland, F. Separovic and L. L. Martin, Specific and selective peptide-membrane interactions revealed using quartz crystal microbalance, Biophys. J., 2007, 93, 3907–3916 CrossRef CAS PubMed.
- K. F. Wang, R. Nagarajan and T. A. Camesano, Differentiating antimicrobial peptides interacting with lipid bilayer: Molecular signatures derived from quartz crystal microbalance with dissipation monitoring, Biophys. Chem., 2015, 196, 53–67 CrossRef CAS PubMed.
- G. A. McCubbin, S. Praporski, S. Piantavigna, D. Knappe, R. Hoffmann, J. H. Bowie, F. Separovic and L. L. Martin, QCM-D fingerprinting of membrane-active peptides, Eur. Biophys. J., 2011, 40, 437–446 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |