Natural product-inspired [3 + 2] cycloaddition-based spirooxindoles as dual anticancer agents: synthesis, characterization, and biological evaluation by in vitro and in silico methods†
Narayanasamy
Nivetha
a,
Jevid
Don Hamid
a,
Akshaya
Simha N
b,
Devanand
Devegowda
c,
Ramith
Ramu
b and
Sivan
Velmathi
 *a
*a
aOrganic and Polymer Synthesis Laboratory, Department of Chemistry, National Institute of Technology, Tiruchirappalli, 620015, Tamil Nadu, India. E-mail: velmathis@nitt.edu
bDepartment of Biotechnology and Bioinformatics, JSS Academy of Higher Education and Research, Mysuru, 570015, Karnataka, India
cCenter of Excellence in Molecular Biology and Regenerative Medicine, Department of Biochemistry, JSS Medical College, JSS Academy of Higher Education and Research, Mysuru, 570015, Karnataka, India
First published on 11th October 2024
Abstract
Breast and colorectal cancers are the most common tumors, with high recurrence and low survival rates. We designed and synthesized a series of spirooxindole pyrrolidinyl derivatives, which were further evaluated for anti-proliferative activity using MDA-MB-468 and HCT 15 cell lines. The best inhibitor of this class, compound 6f, showed a very good inhibition potency, both on the MDA-MB-468 and HCT 15 cells as confirmed by molecular docking and molecular dynamic studies that predicted its binding mode into the active site of the targets. In summary, this study provided a new anti-proliferative derivative 6f which is worthy of further research.
1. Introduction
Breast cancer is the most prevalent cancer among women in both developed and developing nations. GLOBOCAN's data from 2020 indicates that there were approximately 2.261 million new cases of breast cancer and 0.684 million deaths caused by it.1 Breast tumors often originate from the excessive growth of ductal cells, and through repeated exposure to various cancer-causing factors, they can evolve into non-cancerous growths or even aggressive, metastatic carcinomas.2Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States, with an estimated 153![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020 individuals diagnosed in 2023.3 The disease is influenced by environmental and genetic factors, leading to the development of cancer-like traits in colon lining cells. These traits are triggered by genetic and epigenetic changes that activate cancer-promoting genes while deactivating tumor-suppressing genes. Most colorectal cancers originate from stem cells or stem cell-like cells at the base of colon crypts.4 High levels of fatty acid synthase (FAS) in CRC tissues are linked to factors like lymph node metastasis, TNM (tumor, node, metastasis) stage, and poor patient prognosis.
020 individuals diagnosed in 2023.3 The disease is influenced by environmental and genetic factors, leading to the development of cancer-like traits in colon lining cells. These traits are triggered by genetic and epigenetic changes that activate cancer-promoting genes while deactivating tumor-suppressing genes. Most colorectal cancers originate from stem cells or stem cell-like cells at the base of colon crypts.4 High levels of fatty acid synthase (FAS) in CRC tissues are linked to factors like lymph node metastasis, TNM (tumor, node, metastasis) stage, and poor patient prognosis.
Caspase-3, a key player in apoptosis, is used in cancer treatment. Targeting Caspase-3 could improve cancer cell responsiveness to chemotherapy and radiotherapy while limiting invasion and metastasis.5 B-cell lymphoma 2 (BCL2) is an antiapoptotic protein found in solid tumors like breast, prostate, colorectal, lung, stomach, and ovarian cancers. It is upregulated by estrogen in breast cancer, indicating estrogen receptor functionality and impacting disease outcomes. BCL2 serves as a reliable prognostic marker, especially for hormone receptor (HR)-positive breast cancer, according to recent research.6
Oxindoles, a class of compounds with unique structural, physiological, and biological properties, are crucial in chemical and medicinal chemistry.7–12 Spirooxindoles are key building blocks for drugs, particularly as organic substitutes in the synthesis of various medicines. Their rigidity and 3D nature make them attractive targets in organic chemistry and pharmaceutical research. Multicomponent reactions (MCRs) are essential tools for generating multiple molecules in a single step, used in various fields to demonstrate their superiority over other processes.13–16 However, in chemical synthesis, solvents are crucial, and eco-friendly solvents are essential. Ethanol is a cost-effective and efficient solvent for producing bioactive compounds with minimal waste, making MCRs effective methods for synthesis.17–22
Additionally, an effective synthetic method for the regioselective and stereoselective construction of various organic hybrids, including spirooxindoles, was the 1,3-dipolar cycloaddition of non-stabilized azomethine ylides with activated olefins.23–25 Recently, there has been a lot of interest in this synthetic technique that is eco-friendly and has achieved significant advancement in this field.26–28
Among the most significant nitrogen-containing molecules, spirooxindole-based compounds exhibit structural rigidity associated with the spirocarbon present in the heterocycles, especially in the treatment of cancer.29–31 These analogs can effectively bind with the key amino acids of the targets. An interesting natural product with anticancer activity is spirotryprostatin A (I) (Fig. 1).32 According to Gollner's group, BI-0252 (II) is a promising compound that was highly selective and orally active with in vivo efficacy even after single-dose treatment.33 Santos and colleagues reported a novel spiropyrazoline-based oxindole scaffold (III) and assessed it's in vitro cytotoxicity against the MCF-7 breast cancer cell line. The MDA-MB-231 cell line was used to test the hit that had a high efficacy towards the breast cancer line.34 Barkat's group reported a series of functionalized spirooxindoles and the p-Br substituted benzene (IV) had highly potent activity towards breast cancer cells.35 The research group also reported the synthesis of spirooxindole pyrrolothiazoles with a 3-cinnamoyl moiety. A compound with a dichloro substituent (V) was found to be the most active component of the series against HCT-116, HepG2, and PC-3 cancer cells based on the results of the cytotoxicity activity experiment.36 In light of these findings, we have rationally designed and synthesized new vanillin tethered spirooxindole hybrids as anticancer agents.
 | ||
| Fig. 1 Reported spirooxindoles with anticancer activity and our rationally designed target spirooxindoles. | ||
2. Results and discussion
2.1. Chemistry
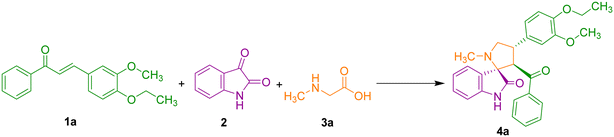
In the present investigation, we wish to report a greener solvent mediated synthesis and anticancer activity of new class of vanillin tethered spirooxindole hybrids employing a multicomponent 1,3-dipolar cycloaddition reaction. Sarcosine 3a and isatin 2 involved in the preparation of azomethine ylide by decarboxylative condensation further reacts with exocyclic dipolarophiles 1a–f to furnish the spirocycloadduct. The synthetic strategy for the preparation of 4 is described in Scheme 1.Solvent optimization for this three-component reaction was examined by the reaction of an equimolar mixture of vanillin chalcone 1a, isatin 2 and sarcosine 3a under reflux in different solvents including acetonitrile, tetrahydrofuran, DMF, 1,4-dioxane, water and methanol in appropriate times (Table 1, entries 1–6). Under these conditions, the corresponding vanillin tethered spirooxindole pyrrolidine was furnished in moderate yields. To enhance the yield of the product and in a move towards green synthesis, it is planned to perform the reaction in ethanol. Thus, an equimolar mixture of 1a, 2 and 3a was subjected to heating in ethanol at 80 °C for 2 h (Table 1, entry 7). After the completion of the reaction as evidenced by TLC analysis, product 4a was isolated in excellent yield (83%). This reveals ethanol as a suitable solvent for this reaction in terms of improved yield and shorter reaction time.
| Entry | Solvent | Temperature (°C) | Time (h) | Isolated yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), 2 (1.0 mmol) and 3a (1.0 mmol) in 10 mL solvent. b Isolated yield. c NR = no reaction. | ||||
| 1 | Acetonitrile | 84 | 4 | 65 |
| 2 | Tetrahydrofuran | 68 | 4 | 60 |
| 3 | DMF | 155 | 6 | 57 |
| 4 | 1,4-Dioxane | 103 | 4 | 69 |
| 5 | Water | 100 | 5 | NRc |
| 6 | Methanol | 67 | 2 | 71 |
| 7 | Ethanol | 80 | 2 | 83 |
Consequently, our synthetic methodology began with the three component 1,3-dipolar cycloaddition reaction of 1a–f with azomethine ylide formed from isatin 2 and sarcosine 3a under heating at 80 °C in ethanol, which led to the formation of vanillin tethered spirooxindole pyrrolidines 4a–f (Scheme 2).
The structure of all the vanillin tethered spirooxindole pyrrolidines 4b–f agrees with the spectral data as described for a representative case (4a). The FT-IR spectrum of compound 4a showed NH stretching at 3284 cm−1, and the oxindole ring and phenyl ring show C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in 1712 and 1610 cm−1. In the 1H NMR spectrum, a triplet at δ 1.28 is attributed to CH3 protons. A singlet at δ 2.06 corresponds to N–CH3 protons. The methylene protons (H-5) of the pyrrolidine ring appeared as two triplets at δ 3.37 and 3.43. A singlet and a quartet at δ 3.76 and 3.95 correspond to methoxy and ethoxy protons. The methine protons (H-4 & H-3) of the pyrrolidine ring appeared as a multiplet at δ 4.28–4.33 and as a doublet at δ 4.43, respectively. The 12 aromatic protons resonated between the region of δ 6.48–7.42. The NH proton of the oxindole ring appeared as a singlet at δ 10.48. In the 13C NMR spectrum, compound 4a showed the presence of 28 carbons including the CH3, N–CH3, O–CH3 and O–CH2 carbons at δ 15.24, 34.91, 55.92 and 64.21, respectively. Furthermore, the pyrrolidine ring C-3, C-4 and C-5 carbons resonated at δ 62.04, 60.31 and 44.27. The spiro carbon (C-2) appeared at δ 73.10 and the carbonyl carbons at δ 179.53 and 197.74. The HRMS analysis was also in good accordance with the proposed structure. The spirocycloadduct 4a exhibited a peak at m/z 457.2130 [M + H]+ in the mass spectrum, which exactly matches with the calculated m/z 457.2127 [M + H]+.
O stretching in 1712 and 1610 cm−1. In the 1H NMR spectrum, a triplet at δ 1.28 is attributed to CH3 protons. A singlet at δ 2.06 corresponds to N–CH3 protons. The methylene protons (H-5) of the pyrrolidine ring appeared as two triplets at δ 3.37 and 3.43. A singlet and a quartet at δ 3.76 and 3.95 correspond to methoxy and ethoxy protons. The methine protons (H-4 & H-3) of the pyrrolidine ring appeared as a multiplet at δ 4.28–4.33 and as a doublet at δ 4.43, respectively. The 12 aromatic protons resonated between the region of δ 6.48–7.42. The NH proton of the oxindole ring appeared as a singlet at δ 10.48. In the 13C NMR spectrum, compound 4a showed the presence of 28 carbons including the CH3, N–CH3, O–CH3 and O–CH2 carbons at δ 15.24, 34.91, 55.92 and 64.21, respectively. Furthermore, the pyrrolidine ring C-3, C-4 and C-5 carbons resonated at δ 62.04, 60.31 and 44.27. The spiro carbon (C-2) appeared at δ 73.10 and the carbonyl carbons at δ 179.53 and 197.74. The HRMS analysis was also in good accordance with the proposed structure. The spirocycloadduct 4a exhibited a peak at m/z 457.2130 [M + H]+ in the mass spectrum, which exactly matches with the calculated m/z 457.2127 [M + H]+.
To explore the generality and the substrate scope of the 1,3-dipolar cycloaddition reaction, sarcosine 3a was replaced with L-proline 3b. The corresponding vanillin tethered spirooxindole pyrrolizidines 5a–f were obtained in excellent yields by the reaction of vanillin-based chalcones 3a–f with isatin 2 and L-proline 3b in ethanol for 2 h (Scheme 3).
Compounds 5a–f were characterized by IR, 1H, 13C and HRMS analysis. In the FT-IR spectrum, the NH stretching frequency is located at 3203 cm−1. The oxindole ring and phenyl ring carbonyls exhibited stretching frequencies in 1718 and 1680 cm−1. The 1H NMR spectrum of compound 5a exhibited a triplet at δ 1.27, which corresponds to methyl protons. A series of multiplets at δ 1.68–1.77, 1.85–1.89, 2.33–2.37 and 2.57–2.61 are attributed to H-6, H-7 and H-8 methylene protons of the pyrrolizidine ring. The methoxy protons resonated as a singlet at δ 3.78. The methine proton (H-4) of the pyrrolizidine ring was observed as a triplet at δ 3.83. A quartet at δ 3.94 corresponds to ethoxy protons of the phenyl ring and methine protons (H-3) of the pyrrolizidine ring. The N–CH proton of the pyrrolizidine ring appeared as a doublet at δ 4.84. The aromatic protons were observed in the region of δ 6.54–7.46. A singlet at δ 10.22 corresponds to an NH proton. In the 13C NMR spectrum, the peaks at δ 15.26, 55.95 and 64.17 correspond to methyl, methoxy and ethoxy carbons. The pyrrolizidine ring carbons appeared at δ 27.11, 30.06, 48.03, 52.55, 63.38 and 71.76, which correspond to C-7, C-6, C-4, C-8, C-3 and C-5 carbons, respectively. The peaks at δ 72.98, 179.90 and 197.37 are attributed to spiro carbon (C-2) and two carbonyl carbons. All aromatic carbons resonated in the range of δ 110.01–149.42. Additionally, the molecular ion peak at m/z 483.2288 [M + H]+ in the mass spectrum confirms compound 5a formation.
Inspired by the outcomes, we further decided to study the 1,3-dipolar cycloaddition reaction of vanillin-based chalcones 1a–f with isatin 2 and L-thioproline 3c in the presence of ethanol at reflux for 2 h to furnish vanillin tethered spirooxindole pyrrolothiazoles 6a–f (Scheme 4). The reaction proceeded well with both electron withdrawing and donating functionalities with good yields.
All the synthesized compounds were characterized by IR, 1H, 13C NMR and HRMS as described for compound 6a. The stretching frequencies at 3311, 1728 and 1676 cm−1 correspond to NH, oxindole and phenyl ring carbonyls, respectively. In the 1H NMR spectrum, compound 6a resonated with a triplet at δ 1.28 due to CH3 protons. The methylene protons of the pyrrolothiazole ring appeared as a doublet at δ 3.04, 3.34 and 3.72, corresponding to H-6 and H-8 protons, respectively. The methoxy and ethoxy protons were observed as a singlet at δ 3.81 and a quartet at δ 3.95. The methine protons of the pyrrolothiazole ring appeared as multiplets at δ 3.82–3.87 (H-4) and 4.14–4.18 (H-3) and as a doublet at δ 4.80 (H-5). The twelve aromatic protons resonated in the range of δ 6.54–7.46. A singlet at δ 10.22 corresponds to an NH proton. In the 13C NMR spectrum, the CH3, O–CH3 and O–CH2 carbons of the phenyl ring resonated at δ 15.25, 56.01 and 64.17, respectively. The pyrrolothiazole ring carbons appeared at δ 36.36, 51.20, 53.90, 61.81 and 74.86 which corresponds to C-6, C-4, C-8, C-3 and C-5 carbons. The peak at δ 73.80 was due to spiro carbon (C-2), and the peaks at δ 178.70 and 195.95 correspond to carbonyl carbons. The peaks located between δ 109.98 and 149.49 are attributed to aromatic carbons. Additionally, the formation of the spiro compound 6a was confirmed by HRMS analysis. The molecular ion peak at m/z 501.1848 [M + H]+ corresponds to compound 6a, which evidences the formation of the desired product.
The plausible mechanism proposed to rationalize the regioselective and diastereoselective formation of vanillin tethered spirooxindole heterocycles is described in Scheme 5. The nucleophilic attack of the amino group in the amino acids 3a–c on the C-3 carbonyl of isatin 2 commences the reaction, followed by dehydration. Carbon dioxide is evicted under the reaction conditions forming a reactive and non-stable azomethine ylide (II). Subsequently, the reaction of azomethine ylide with dipolarophiles 1a–f may occur in path A or B. However, the exclusive formation of vanillin tethered spirooxindoles discloses that path A is more favorable than path B. Presumably, the secondary orbital interaction and non-steric hindrance between the oxindole and chalcone carbonyls present in the same face during the approach of 1,3-dipole (II) towards the dipolarophiles leads to the formation of spiro compounds 4/5/6a–f. Probably, this may be due to the electron rich carbon of the azomethine ylide (II) added to the more electron deficient β-carbon of dipolarophiles 1a–f. On the other hand, the carbonyl of 1,3-dipole (II) and dipolarophiles 1a–f are opposite to each other, which undergoes electrostatic repulsion. As a result, the regioisomers 4′/5′/6′a–f are not favorable.
2.2. Biological studies
| Name of the compounds | BCL2 | Caspase 3 | FAS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Binding affinity (kcal mol−1) | Total intermolecular interactions | Total hydrogen bonds | Binding affinity (kcal mol−1) | Total intermolecular interactions | Total hydrogen bonds | Binding affinity (kcal mol−1) | Total intermolecular interactions | Total hydrogen bonds | |
| 4a | −9.9 | 10 | 2 | −8.3 | 5 | 2 | −9.7 | 10 | 1 |
| 4b | −9.1 | 8 | 3 | −8.4 | 7 | 3 | −9.8 | 7 | 2 |
| 4c | −8.7 | 9 | 2 | −8.0 | 6 | 1 | −10.0 | 6 | 1 |
| 4d | −8.6 | 9 | 3 | −8.2 | 8 | 2 | −10.5 | 8 | 1 |
| 4e | −8.9 | 11 | 1 | −8.7 | 6 | 3 | −9.3 | 11 | 1 |
| 4f | −9.4 | 12 | 2 | −8.8 | 10 | 2 | −9.7 | 10 | 1 |
| 5a | −9.3 | 10 | 3 | −8.5 | 7 | 1 | −10.5 | 10 | 1 |
| 5b | −9.0 | 13 | 1 | −8.5 | 8 | 3 | −9.4 | 9 | 2 |
| 5c | −10.2 | 10 | 2 | −8.5 | 9 | 2 | −10.5 | 8 | 1 |
| 5d | −10.1 | 12 | 1 | −8.6 | 8 | 1 | −10.2 | 7 | 2 |
| 5e | −9.1 | 11 | 2 | −8.8 | 8 | 2 | −10.3 | 8 | 1 |
| 5f | −9.3 | 9 | 3 | −8.4 | 9 | 3 | −9.0 | 9 | 1 |
| 6a | −9.4 | 9 | 1 | −8.8 | 11 | 2 | −9.7 | 9 | 2 |
| 6b | −9.2 | 10 | 2 | −9.0 | 8 | 3 | −9.7 | 10 | 2 |
| 6c | −9.6 | 12 | 3 | −8.5 | 10 | 2 | −9.2 | 11 | 1 |
| 6d | −9.9 | 12 | 2 | −8.7 | 9 | 1 | −10.0 | 8 | 1 |
| 6e | −10.5 | 11 | 2 | −9.0 | 10 | 2 | −9.2 | 10 | 1 |
| 6f | −11.2 | 16 | 4 | −10.5 | 11 | 4 | −10.8 | 13 | 1 |
| 5-Fluorouracil | −7.7 | 4 | 1 | −4.3 | 5 | 5 | −5.7 | 3 | — |
With respect to BCL2, compound 6f formed 16 non-bonded interactions out of which four were hydrogen bonds with Arg105 (2.17 Å, 2.70 Å & 2.58 Å) and Asp70 (3.57 Å). Other than hydrogen bonds, compound 6f formed hydrophobic interactions such as pi–anion interactions with Asp 108 (4.97 Å), pi–pi stacked with Tyr67 (4.98 Å), pi–pi T shaped with Phe71 (4.91 Å), alkyl interactions with Ala108 (4.27 Å), Val92 (5.10 Å), and Leu96 (3.68 Å) and pi–alkyl interactions with Tyr67 (4.88 Å), Phe112 (4.75 Å), Leu96 (4.47 Å) and Ala108 (4.71 Å). Meanwhile, 5-fluorouracil formed only 4 intermolecular interactions out of which 1 was a hydrogen bond with Tyr161 (3.50 Å). Other than a hydrogen bond, it formed halogen interactions with Ala59 (3.07 Å), pi–pi T shaped with Phe63 (4.79 Å) and pi–alkyl interactions with Val107 (5.04 Å); there were less intermolecular interactions, lower binding affinity and less hydrophobic interactions compared to compound 6f. The results of the current study were in accordance with the previous report37 wherein the bioactive secondary metabolite, friedelin from Wedelia trilobata, interacted with the same amino acids (binding affinity = −10.8 kcal mol−1) which have been mentioned above with a lesser binding energy compared to compound 6f which is the lead compound of the current study. The 3D and 2D depictions of compound 6f–BCL2 and 5-fluorouracil–BCL2, respectively, are presented in Fig. 2.
With respect to caspase 3, compound 6f formed 11 non-bonded interactions, out of which four were hydrogen bonds with Ser209 (2.06 Å, 3.48 Å), Ser251 (2.81 Å), and Asn208 (3.32 Å). Other than hydrogen bonds, compound 6f formed hydrophobic interactions such as pi–pi T shaped with Phe252 (5.18 Å) and Phe256 (5.92 Å), alkyl interactions with Lys210 (4.35 Å), and pi–alkyl interactions with Trp206 (5.17 Å), Trp214 (5.49 Å) and Phe252 (5.12 Å). Meanwhile, 5-fluorouracil also formed 5 non-bonded interactions which were all hydrogen bonds with Asn208 (2.26 Å & 2.75 Å), Trp214 (1.82 Å), Arg207 (2.46 Å) and Phe250 (2.42 Å). Although it formed 5 more hydrogen bonds, there were less intermolecular interactions, lower binding affinity and no hydrophobic interactions compared to compound 6f. The results of the present study were in accordance with the previous report,38 wherein flavonoids such as nicotiflorin, naringenin, hesperidin, and kaempferol from Acalypha indica interacted with binding affinities of −6.81, −6.45, −6.33, and −6.10 kcal mol−1, respectively with caspase 3. Although the compounds interacted with the same amino acids as in the current study, the binding affinity and the number of intermolecular interactions are much lower compared to those of compound 6f which is the lead molecule in the current study. The 3D and 2D depictions of compound 6f–caspase 3 and 5-fluorouracil–caspase 3 are presented in Fig. 3.
With respect to fatty acid synthase (FAS), compound 6f formed 13 non-bonded interactions, among which was a hydrogen bond with Glu2251 (2.33 Å). Other than hydrogen bonds, compound 6f formed hydrophobic interactions such as pi–sigma interactions with His2481 (3.63 Å), pi–pi stacked interactions with Phe2423 (5.66 Å), pi–pi T shaped interactions with Tyr2347 (5.17 Å & 5.20 Å), alkyl interactions with Val2344 (4.66 Å) and Leu2427 (4.27 Å) and pi–alkyl interactions with Tyr2347 (5.03 Å & 5.06 Å), Tyr2351 (5.07 Å), Leu2222 (5.05 Å) and Ile2250 (4.58 Å & 5.09 Å). Meanwhile, 5-fluorouracil formed 3 intermolecular interactions without any hydrogen bond and hydrophobic interactions, such as halogen bonding with fluorine of Tyr2351 (3.29 Å) and pi–alkyl interactions with Ala2363 (4.30 Å & 4.35 Å). The outcomes of the current study were in accordance with the earlier report,39 where the drug orlistat interacted with the residues like Ile2250, Asp2338, Tyr2307, Ala2367, Glu2366, Phe2370, Leu2427 and Phe2423. The residues Tyr2343, Ser2308, Arg2482 and His2481 were found to form four hydrogen bond interactions. However, orlistat had a very low binding affinity (−2.97 kcal mol−1) compared to compound 6f which is the lead molecule in the current study. The 3D and 2D representation of compound 6f–caspase 3 and 5-fluorouracil–caspase 3 is presented in Fig. 4.
During the validation of molecular docking simulation, the RMSD values between the original and re-docked co-crystal ligands DRO1166, tripeptide Trp-Glu-His-Asp with an acetyl group & 7FA500 were found to be 0.9824, 0.7684 & 0.8694, which proved that the docking protocol was right (Fig. 5). In the molecular docking simulation, both 5-fluorouracil and compound 6f were bound to the same inhibitor binding site of the 3 targets, where the co-crystal inhibitor ligands DRO1166, tripeptide Trp-Glu-His-Asp with an acetyl group & 7FA500 were previously bound. The outcomes of the docking of the co-crystal ligands with their respective protein are presented in Fig. 5via 3D representations.
Molecular dynamics (MD) simulation was used to explain the dynamic behaviour of the protein–ligand complex with respect to time in a solvated environment. In this simulation study, the Rg, SASA, ligand RMSD, total number of hydrogen bonds maintained by the ligands during the simulation, and secondary structure pattern variation between the protein and their complexes are all examined. Through determining the presence of a ligand in the binding pocket, the RMSD of the protein–ligand complex provides insight into the stability of the complex during the simulation. Root mean square distances are computed by the Rg keeping the rotating axis in mind as it considers the different masses. Every time step of the trajectory is considered during the simulation, considering the capacity, shape, and folding. With respect to the protein structure, RMSF focuses on regions that vary the most/least from the mean. Protein–ligand complex hydrophobic core circumference is calculated using SASA. Furthermore, ligand hydrogen bonds emerged during the complete simulation duration of the molecular docking research under investigation. Plots were made with the understanding that only the intermolecular hydrogen bonds between the ligands and the relevant protein were considered during the study. In this study, nine simulations were performed at 100 ns each simulation using BCL-2, caspase 3, and FAS in conjunction with the representative medications, compound 6f and 5-fluorouracil.
During the entire simulation, compound 6f and 5-fluorouracil stayed in the BCL2 inhibitor binding cleft. The RMSD figure showed that compound 6f showed an increase in stability after 10 ns, but 5-fluorouracil was not stable until 30 ns. Compound 6f was not found using any of the odd variants in the RMSF analysis case. This proved that throughout the simulation, the partial inhibitor stayed constant. The RMSF pattern of the apoprotein and the protein–compound 6f complex is comparable. Extremely minimal fluctuation was seen outside of the loop region (500 residues) and terminal regions during the modelling of the protein–compound 6f combination. The protein–5-fluorouracil complex exhibited more fluctuations. Furthermore, compound 6f was more closely connected, as shown in the Rg figure. The available SASA reduced as the simulation period was extended because the molecule successfully occupied the inhibitor binding site. At 50 ns, hydrogen bonds were assessed in BCL2–compound 6f and BCL2–compound 6f complexes. Furthermore, an examination of the hydrogen bonds created by the ligands reveals that compound 6f generated 7 hydrogen bonds and 5-fluorouracil generated 5 hydrogen bonds.
However, there were more variances in the protein–5-fluorouracil complex. The Rg plot also showed that compound 6f had a tighter coupling to the protein than 5-fluorouracil. This interaction resulted in a considerable reduction in the SASA value of the protein–compound 6f complex. Due to the chemical successfully occupying the inhibitor binding cleft, the accessible SASA reduced as the simulation time was prolonged. The hydrogen bonds formed by the ligands are also examined, and it is shown that compound 6f produced four more hydrogen bonds than 5-fluorouracil. By these results, we can predict that compound 6f and 5-fluorouracil inhibited BCL2 thereby exhibiting anti-cancer activity. The MD simulation results were in accordance with the previous study,37 wherein similar MD trajectories were obtained. The molecular dynamics simulation results of p53 with compound 6f and 5-fluorouracil are depicted in Fig. 6.
Compound 6f and 5-fluorouracil persisted in the binding site of caspase 3 during the entire simulation. In contrast to 5-fluorouracil, which was not stable until 30 ns, compound 6f demonstrated an improvement in stability after 10 ns, according to the RMSD plot. With respect to RMSF analysis, compound 6f was not detected with any of the odd variations. This demonstrated that the partial inhibitor remained stable during the simulation. The protein–compound 6f complex and apoprotein both have a similar RMSF pattern. During the simulation of the protein–compound 6f complex, very less variation was seen outside of the loop region (200 residues) and terminal sections. The protein–5-fluorouracil complex, however, showed greater variations. Additionally, the Rg plot demonstrates that compound 6f was more tightly coupled to the protein than 5-flurouracil. The SASA value of the protein–compound 6f complex was significantly reduced because of this binding. The available SASA reduced as the simulation period was extended because the molecule successfully occupied the inhibitor binding site. At 50 ns, hydrogen bonds were assessed in caspase 3–compound 6f and caspase 3–compound 6f complexes. Furthermore, an examination of the hydrogen bonds created by the ligands reveals that compound 6f generated 5 hydrogen bonds and 5-fluorouracil generated 3 hydrogen bonds.
However, there were more variances in the protein–5-fluorouracil complex. The Rg plot also showed that compound 6f had a tighter coupling to the protein than 5-fluorouracil. This interaction resulted in a considerable reduction in the SASA value of the protein–compound 6f complex. Due to compound 6f successfully occupying the inhibitor binding cleft, the accessible SASA reduced as the simulation period was prolonged. The hydrogen bonds formed by the ligands are also examined, and it is shown that compound 6f produced four more hydrogen bonds than 5-fluorouracil. By these results, we can predict that compound 6f and 5-fluorouracil activated caspase 3, thereby exhibiting anti-tumor activity. The molecular dynamics simulation results of caspase 3 with compound 6f and 5-fluorouracil are depicted in Fig. 7. The outcomes were in accordance with the earlier report;38 herein, the MD simulation study was confined to RMSD and RMSF. The present study described all the MD simulation parameters, which indicated that this study is accurate in comparison to previous studies.
Compound 6f and 5-fluorouracil remained in the inhibitor binding cleft of FAS during the entire simulation. In contrast to 5-fluorouracil, which is not stable until 30 ns, compound 6f demonstrated an improvement in stability after 10 ns, in accordance with the RMSD plot. In the outcomes of RMSF analysis, compound 6f was not detected with any of the odd variations. This exhibited that the partial inhibitor remained stable during the simulation. The protein–6f complex and apoprotein both have an identical RMSF pattern. During the simulation of the protein–6f complex, slight variation was seen outside the loop region (450 residues) and terminal sections. The protein–5-fluorouracil complex, however, showed greater variations. Additionally, compound 6f was more tightly coupled with the protein than 5-fluorouracil, according to the Rg plot. The SASA value of the protein–compound 6f complex was significantly reduced because of this binding. The available SASA reduced as the simulation period was extended because the molecule successfully occupied the inhibitor binding site. At 50 ns, hydrogen bonds were assessed in FAS–compound 6f and FAS–compound 6f complexes. Furthermore, an examination of the hydrogen bonds created by the ligands reveals that compound 6f generated 7 hydrogen bonds and 5-fluorouracil generated 3 hydrogen bonds.
However, there were more variances in the protein–5-fluorouracil complex. The Rg plot also showed that compound 6f had a tighter coupling to the protein than 5-fluorouracil. This interaction resulted in a considerable reduction in the SASA value of the protein–compound 6f complex. Due to compound 6f successfully occupying the inhibitor binding site, the accessible SASA declined as the simulation period was prolonged. The hydrogen bonds formed by the ligands are also examined, and it is shown that compound 6f formed 7 hydrogen bonds and 5-fluorouracil formed 3 hydrogen bonds. By these results, we can predict that compound 6f and 5-fluorouracil inhibited FAS, thereby exhibiting anti-cancer activity. The molecular dynamics simulation results of FAS with compound 6f and 5-fluorouracil are depicted in Fig. 8. The outcomes were in accordance with the earlier report,40 wherein the MD simulation study was restricted to RMSD and RMSF. Meanwhile, the present study explained all the MD simulation parameters like RMSD, RMSF, Rg, SASA and ligand hydrogen bonds, which suggested the accuracy of the current study compared to the previous study. MD trajectory values of compound 6f and 5-fluorouracil complexed with BCL2, caspase 3 and FAS are depicted in Table 3.
| MD trajectories | BCL2 | Caspase 3 | Fatty acid synthase (FAS) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BCL2 | BCL2–compound 6f complex | BCL2–5-fluorouracil complex | Caspase 3 | Caspase 3–compound 6f | Caspase 3–5-fluorouracil | FAS | FAS–compound 6f | FAS–5-fluorouracil | |
| RMSD (nm) | 0.3 | 0.3 | 0.4 | 0.3 | 0.3 | 0.35 | 0.30 | 0.30 | 0.35 |
| RMSF (nm) | 0.45 | 0.45 | 0.65 | 0.45 | 0.45 | 0.78 | 0.35 | 0.35 | 0.75 |
| Rg (nm) | 2.25 | 2.25 | 2.25 | 2.0 | 2.0 | 2.0 | 1.8 | 1.8 | 1.8 |
| SASA (nm2) | 155 | 155 | 165 | 220 | 220 | 235 | 115 | 115 | 135 |
| Ligand H-bonds (max.) | — | 7 | 5 | — | 5 | 3 | 7 | 2 | |
Since the current study deals with the anti-cancer mechanism of spirooxindole pyrrolidinyl derivatives, the results of in silico analyses (molecular docking and molecular dynamics simulation) were compared to those of the standard drug 5-fluorouracil. 5-Fluorouracil (5-FU) has been a crucial component in cancer treatment since 1957, particularly in colon cancer therapy, and it is also utilized for breast cancer and other malignancies such as those affecting the head and neck. Structurally resembling pyrimidine molecules found in DNA and RNA, 5-FU is an organic compound with a heterocyclic aromatic nature. It mimics uracil but substitutes a hydrogen atom at the C-5 position with a fluorine atom. Although there is only one known crystal structure for pure 5-FU, it crystallizes with four molecules in the asymmetric unit, forming a hydrogen-bonded sheet structure. Its structural similarity allows 5-FU to disrupt nucleoside metabolism, potentially integrating into RNA and DNA, thereby inducing cell death through cytotoxic effects.41 Molecular dynamics studies are performed to validate the molecular docking studies & to study the stability of protein–ligand complexes. Hence, in the current study, to compare the stability of compound 6f–protein complexes, 5-fluorouracil was selected for molecular dynamics simulation.
| Protein–ligand complexes | Types of binding energies | ||||
|---|---|---|---|---|---|
| van der Waals energy (kJ mol−1) | Electrostatic energy (kJ mol−1) | Polar solvation energy (kJ mol−1) | SASA energy (kJ mol−1) | Binding energy (kJ mol−1) | |
| BCL2–compound 6f | −300.51 ± 0.11 | −7.27 ± 0.36 | −370.25 ± 1.1 | −17.37 ± 0.50 | −554.96 ± 0.75 |
| BCL2–5-fluorouracil | −95.25 ± 0.14 | −8.36 ± 0.25 | −38.72 ± 0.44 | −8.55 ± 0.12 | −110.70 ± 0.84 |
| Caspase 3–compound 6f | −280.25 ± 0.18 | −12.15 ± 0.19 | −345.21 ± 0.23 | −15.54 ± 0.24 | −498.54 ± 0.57 |
| Caspase 3–5-fluorouracil | −90.45 ± 0.22 | −13.14 ± 0.15 | −45.15 ± 0.17 | −6.15 ± 0.11 | −124.15 ± 0.88 |
| FAS–compound 6f | −265.15 ± 0.22 | −15.15 ± 0.17 | −321.18 ± 0.32 | −20.19 ± 0.17 | −459.26 ± 1.12 |
| FAS–5-fluorouracil | −88.24 ± 0.14 | −17.25 ± 0.47 | −56.15 ± 0.17 | −6.88 ± 0.21 | −109.51 ± 0.82 |
2.3. In vitro studies
| Cell lines | Compounds | |
|---|---|---|
| IC50 (μM) | ||
| Compound 6f | 5-Fluorouracil* | |
| Values are expressed as mean ± SD. Means in the same column with distinct superscripts (a, b) are significantly different (p ≤ 0.05) as separated by the Duncan multiple range test. *5-Fluorouracil was used as the positive control. | ||
| MDA-MB-468 | 361.33 ± 1.75a | 550.56 ± 2.85b |
| HCT-15 | 273.71 ± 1.98a | 578.98 ± 2.08b |
In the current study, the effect of compound 6f treatment in the MDA-MB-468 cell line was observed. When MDA-MB-468 cells were treated with compound 6f at a concentration of 200 μM, there was a substantial increase in the sub-G0/G1 cell population, which is an indicator of apoptosis, which rose to 44.5% compared to the 1.78% observed in the cells treated only with the vehicle control. Additionally, there was a concurrent reduction in the populations of cells in the S and G2/M phases of the cell cycle, as shown in Fig. 9(A). Notably, the percentage of cells in the G0/G1 phase initially increased with the 50 μM dose of compound 6f but reduced with the 100 μM and 200 μM doses. The results of cell cycle analysis were in accordance with the reported study.42 According to their study, when MCF-7 cells were treated with 200 μM of vitamin D3, it led to a notable rise in the sub-G0/G1 cell population, with a percentage of 46.66%, in contrast to the vehicle-treated control cells with only 2.57%. At the same time, there was a drop in the number of cells during the S and G2/M phases of the cell cycle. Initially, the percentage of cells in the G0/G1 phase went up when treated with 50 μM of vitamin D3, but it declined when exposed to higher concentrations of vitamin D3, specifically at 100 μM and 200 μM.
 | ||
| Fig. 9 Effects of compound 6f and 5-fluorouracil on the cell cycle of (A) MDA-MB-468 cells and (B) HCT 15 cells. | ||
With respect to HCT 15 cells, when they were treated with 200 μM of compound 6f, it resulted in G2/M growth arrest, with a rate of 43.5% compared to the control cells, which exhibited only 12.25% in this phase. At the same time, there was a decrease in the populations of cells in the G0/G1 and S phases of the cell cycle when they were treated with compound 6f, as seen in Fig. 9(B). 100 μM of 5-fluorouracil, the positive control, also caused G2/M phase arrest with a reduction of the percentage of cells in the G0/G1 and S phases, as displayed in Fig. 9(B). Notably, no significant changes were observed in the sub-G0/G1 cell population under these experimental conditions. The results were in accordance with the reported outcomes.42 With respect to their study, at 200 μM concentration, vitamin D3 caused G2/M growth arrest. Additionally, reduction in G0/G1 and S phase cell populations were observed in the cells treated with vitamin D3.
| Cell lines | Compounds | |
|---|---|---|
| IC50 (μM) | ||
| Compound 6f | 5-Fluorouracil* | |
| Values are expressed as mean ± SD. Means in the same column with distinct superscripts (a, b) are significantly different (p ≤ 0.05) as separated by the Duncan multiple range test. *5-Fluorouracil was used as the positive control. | ||
| MDA-MB-468 | 407.4756 ± 2.23a | 495.45 ± 2.56b |
| HCT-15 | 365.1521 ± 2.75a | 425.56 ± 2.40b |
2.4. Cell death assay
Dual staining with acridine orange and ethidium bromide is a well-recognised technique to visualize the effect of pharmacological agents in the process of cell death.46 With respect to this staining method, green cells indicate live cells, whereas red cells indicate dead ones. With respect to both MDA-MB-468 and HCT 15 cells, the highest percentage of dead cells was observed at 500 μM (LD50 = 280.87 ± 2.23 μM) of compound 6f which could be due to the activation of caspase 3. However, at 1000 μM with respect to MDA-MB-468 cells and 1000 μM and 2000 μM with respect to HCT 15 cells (LD50 = 312.37 ± 2.75 μM), the dead cell percentage decreased which could be due to other mechanisms of cell death. 5-Fluorouracil (100 μM) is used as a positive control in the present study. The result of cell death (apoptosis assay) is depicted as LD50 values in Table 7, and Fig. 10(A and B) shows the stained images of the MDA-MB-468 and HCT 15 cells, respectively.| Cell lines | Compounds | |
|---|---|---|
| LD50 (μM) | ||
| Compound 6f | 5-Fluorouracil* | |
| Values are expressed as mean ± SD. Means in the same column with distinct superscripts are significantly different (p ≤ 0.05) as separated by the Duncan multiple range test. *5-Fluorouracil was used as the positive control. | ||
| MDA-MB-468 | 280.87 ± 2.23a | 495.45 ± 2.56b |
| HCT-15 | 312.37 ± 2.75a | 425.56 ± 2.40b |
3. Conclusion
A series of unexplored vanillin tethered spirooxindole pyrrolidines 4a–f, pyrrolizidines 5a–f, and pyrrolothiazoles 6a–f were synthesized employing greener solvent ethanol via a multicomponent 1,3-dipolar cycloaddition strategy. The synthesized compounds were evaluated for their in silico and in vitro anticancer activity. Among all the compounds, compound 6f was found to be a potent and stable modulator of the 3 targets including BCL2, caspase 3, and FAS. Hence, compound 6f under in vitro conditions increased the % proliferation inhibition in a dose-dependent manner across MDA-MB-468 and HCT 15 cell lines when administered at different concentrations. With respect to cell cycle analysis, when MDA-MB-468 cells were treated with compound 6f at a concentration of 200 μM, there was a significant increase in the sub-G0/G1 cell population, which is an indicator of apoptosis, which rose to 44.5% compared to the 1.78% observed in the control cells treated with only the vehicle. Additionally, there was a concurrent decrease in the populations of cells in the S and G2/M phases. Notably, the percentage of cells in the G0/G1 phase initially increased with the 50 μM dose of compound 6f but decreased with the 100 μM and 200 μM doses. With respect to HCT 15 cells, when they were treated with 200 μM of compound 6f, it resulted in G2/M growth arrest, with a rate of 43.5% compared to the control cells, which exhibited only 12.25% in this phase. At the same time, there was a decrease in the populations of cells in the G0/G1 and S phases in the compound 6f-treated cells. With respect to caspase 3 assay, a gradual increase in % caspase 3 expressing cells corresponding to the dosage was observed in the case of MDA-MB-468 cells. In contrast, at 2000 μM of compound 6f, there was a decrease in the % caspase 3 expressing cells with respect to HCT 15 cells. With respect to cell death assay, the highest percentage of dead cells was observed at 500 μM of compound 6f across both MDA-MB-468 and HCT 15 cell lines. Hence, from the above results it could be concluded that the spirocycloadduct 6f could emerge as a potent dual anti-cancer drug.4. Experimental section
A detailed experimental discussion of synthesis, characterization of the compounds and biological studies are provided in the ESI.†Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its ESI.† Any raw data files needed in another format are available from the corresponding author upon reasonable request.Author contributions
N. N. and S. V. conceived the experimental design; N. N. and J. D. H.: synthesis of the compounds; A. S. N., D. D., and R. R. carried out the biological studies; N. N.: compound characterization; N. N., A. S. N., and D. D.: writing – original draft; R. R. and S. V.: resources, supervision and writing – reviewing & editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors S. V. and N. N. would like to extend our sincere thanks to DST-FIST-SR/FSTCS-II/2018/64 for funding the HRMS instrument and NMR Auto Sampler facilities.References
- R. L. Siegel, K. D. Miller, N. S. Wagle and A. Jemal, Cancer statistics, 2023, Ca-Cancer J. Clin., 2023, 73, 17–48 CrossRef PubMed.
- C. Sonnenschein and A. M. Soto, Carcinogenesis explained within the context of a theory of organisms, Prog. Biophys. Mol. Biol., 2016, 122, 70–76 CrossRef CAS PubMed.
- R. L. Siegel, N. S. Wagle, A. Cercek, R. A. Smith and A. Jemal, Colorectal cancer statistics, 2023, Ca-Cancer J. Clin., 2023, 73, 233–254 Search PubMed.
- E. J. Kuipers, W. M. Grady, D. Lieberman, T. Seufferlein, J. J. Sung, P. G. Boelens, C. J. Van de Velde and T. Watanabe, Colorectal cancer, Nat. Rev. Dis. Primers, 2015, 1, 15065 CrossRef.
- M. Zhou, X. Liu, Z. Li, Q. Huang, F. Li and C. Y. Li, Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells, Int. J. Cancer, 2018, 143, 921–930 CrossRef CAS.
- Y. H. Eom, H. S. Kim, A. Lee, B. J. Song and B. J. Chae, BCL2 as a subtype-specific prognostic marker for breast cancer, J. Breast Cancer, 2016, 19, 252–260 CrossRef PubMed.
- F. M. Moghaddam and B. Aghamiri, Facile one-pot, multi-component reaction to synthesize spirooxindole-annulated thiopyran derivatives under environmentally benevolent conditions, Heliyon, 2022, 8, e10666 CrossRef.
- F. M. Moghaddam, B. Aghamiri and M. Jalalinik, A facile one-pot, four-component synthesis of (Z)-isomer of rhodamine-oxindole derivatives under environmentally benevolent conditions, Synth. Commun., 2022, 52, 175–184 CrossRef CAS.
- L. M. Zhou, R. Y. Qu and G. F. Yang, An overview of spirooxindole as a promising scaffold for novel drug discovery, Expert Opin. Drug Discovery, 2020, 15, 603–625 CrossRef CAS PubMed.
- S. S. Panda, A. S. Girgis, M. N. Aziz and M. S. Bekheit, Spirooxindole: A versatile biologically active heterocyclic scaffold, Molecules, 2023, 28, 618 CAS.
- S. Nasri, M. Bayat and F. Mirzaei, Recent strategies in the synthesis of spiroindole and spirooxindole scaffolds, Top. Curr. Chem., 2021, 379, 1–37 Search PubMed.
- F. M. Moghaddam, L. Kavoosi and B. Aghamiri, Facile one-pot, domino reaction to synthesize (Z)-2-imino-5-(1-alkyl-2-oxoindolin-3-ylidene)thiazolidine-4-one derivatives and its DFT study for the E/Z confirmation structure, Synth. Commun., 2023, 54, 1–11 Search PubMed.
- A. Domling, W. Wang and K. Wang, Chemistry and biology of multicomponent reactions, Chem. Rev., 2012, 12, 3083–3135 Search PubMed.
- P. Slobbe, E. Ruijter and R. V. Orru, Recent applications of multicomponent reactions in medicinal chemistry, MedChemComm, 2012, 3, 1189–1218 CAS.
- H. A. Younus, M. Al-Rashida, A. Hameed, M. Uroos, U. Salar, S. Rana and K. M. Khan, Multicomponent reactions (MCR) in medicinal chemistry: a patent review, Expert Opin. Ther. Pat., 2021, 31, 267–289 CAS.
- S. Gulati, S. E. John and N. Shankaraiah, Microwave-assisted multicomponent reactions in heterocyclic chemistry and mechanistic aspects, Beilstein J. Org. Chem., 2021, 17, 819–865 CAS.
- F. M. Kerton and R. Marriott, Alternative solvents for green chemistry, R. Soc. Chem., 2nd edn, 2013 Search PubMed.
- C. Capello, U. Fischer and K. Hungerbuhler, What is a green solvent? a comprehensive framework for the environmental assessment of solvents, Green Chem., 2007, 9, 927–934 CAS.
- D. Prat, J. Hayler and A. Wells, A survey of solvent selection guides, Green Chem., 2014, 16, 4546–4551 CAS.
- C. J. Clarke, W. C. Tu, O. Levers, A. Brohl and J. P. Hallett, Green and sustainable solvents in chemical processes, Chem. Rev., 2018, 118, 747–800 CrossRef CAS.
- K. Hackl and W. Kunz, Some aspects of green solvents, C. R. Chim., 2018, 21, 572–580 CrossRef.
- Y. Fan and F. Picchioni, Modification of starch: a review on the application of “green” solvents and controlled functionalization, Carbohydr. Polym., 2020, 241, 116350–116369 CrossRef CAS PubMed.
- M. A. Rani, S. Vivek Kumar, K. Malathi, M. Muthu, A. I. Almansour, R. Suresh Kumar and R. Ranjith Kumar, A one-pot multicomponent 1, 3-dipolar cycloaddition strategy: combinatorial synthesis of dihydrothiophenone-engrafted dispiro hybrid heterocycles, ACS Comb. Sci., 2017, 19, 308–314 CrossRef.
- S. Purushothaman, R. Prasanna and R. Raghunathan, Regioselective synthesis of spiropyrrolidine/spiropyrrolizidine/spirothiazolidine-grafted macrocycles through 1,3-dipolar cycloaddition methodology, Tetrahedron, 2013, 69, 9742–9750 CrossRef CAS.
- M. Poornachandran and R. Raghunathan, Synthesis of dispirooxindolecycloalka[d] pyrimidino[2,3-b]-thiazole pyrrolidine/thiapyrrolizidine ring systems, Tetrahedron, 2006, 62, 11274–11281 CrossRef CAS.
- N. Arumugam, A. I. Almansour, R. Suresh Kumar, A. J. Mohammad, A. Al-Aizari, S. I. Alaqeel, S. Kansız, V. Siva Krishna, D. Sriram and N. Dege, Regio- and diastereoselective synthesis of spiropyrroloquinoxaline grafted indole heterocyclic hybrids and evaluation of their anti-Mycobacterium tuberculosis activity, RSC Adv., 2020, 10, 23522–23531 RSC.
- K. Martina, S. Tagliapietra, V. V. Veselov and G. Cravotto, Green protocols in heterocycle syntheses via 1,3-dipolar cycloadditions, Front. Chem., 2019, 7, 95 CrossRef.
- A. Dandia, R. Singh, S. Khan, S. Kumari and P. Soni, A rational eco-compatible design strategy for regio- and diastereoselective synthesis of novel dispiropyrrolidine/ thiapyrrolizidine hybrids, Tetrahedron Lett., 2015, 56, 4438–4444 CrossRef CAS.
- B. Yu, D. Q. Yu and H. M. Liu, Spirooxindoles: promising scaffolds for anticancer agents, Eur. J. Med. Chem., 2015, 97, 673–698 CrossRef CAS.
- R. Ghosh, J. B. Vitor, E. Mendes, A. Paulo and P. C. Acharya, Stereoselective synthesis of spirooxindole derivatives using one-pot multicomponent cycloaddition reaction and evaluation of their antiproliferative efficacy, ACS Omega, 2020, 5, 27332–27343 CrossRef CAS PubMed.
- J. Yang, Y. Hu, Q. Li, F. Yu, J. Cao, D. Fang, Z. Huang and D. Shi, Efficient and regioselective synthesis of novel functionalized dispiropyrrolidines and their cytotoxic activities, ACS Comb. Sci., 2014, 16, 139–145 CAS.
- C. B. Cui, H. Kakeya and H. Osada, Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase, Tetrahedron, 1996, 52, 12651–12666 CAS.
- A. Gollner, D. Rudolph, H. Arnhof, M. Bauer, S. M. Blake, G. Boehmelt, X. L. Cockroft, G. Dahmann, P. Ettmayer, T. Gerstberger, J. Karolyi-Oezguer, D. Kessler, C. Kofink, J. Ramharter, J. Rinnenthal, A. Savchenko, R. Schnitzer, H. Weinstabl, U. Weyer-Czernilofsky, T. Wunberg, D. B. Wunberg and D. B. McConnell, Discovery of Novel Spiro[3H-indole-3,2-pyrrolidin]-2(1H)-one compounds as Chemically Stable and Orally Active Inhibitors of the MDM2-P53 Interaction, J. Med. Chem., 2016, 59, 10147–10162 CAS.
- A. Monteiro, L. M. Goncalves and M. M. Santos, Synthesis of novel spiropyrazoline oxindoles and evaluation of cytotoxicity in cancer cell lines, Eur. J. Med. Chem., 2014, 79, 266–272 CAS.
- G. Lotfy, H. El Sayed, M. M. Said, Y. M. A. Aziz, A. Al-Dhfyan, A. M. Al-Majid and A. Barakat, Regio- and stereoselective synthesis of novel spiro-oxindole via 1,3-dipolar cycloaddition reaction. Anti-cancer and molecular docking studies, J. Photochem. Photobiol., B, 2018, 180, 98–108 CrossRef CAS PubMed.
- A. Barakat, M. S. Islam, H. M. Ghawas, A. M. Al-Majid, F. F. El-Senduny, F. A. Badria, Y. A. Elshaier and H. A. Ghabbour, Design and synthesis of new substituted spirooxindoles as potential inhibitors of the MDM2–p53 interaction, Bioorg. Chem., 2019, 86, 598–608 CrossRef CAS PubMed.
- H. G. Gowtham, F. Ahmed, S. Anandan, C. S. Shivakumara, A. Bilagi, S. Pradeep, C. Shivamallu, A. A. Shati, M. Y. Alfaifi, S. E. Elbehairi and R. R. Achar, In silico computational studies of bioactive secondary metabolites from wedelia trilobata against anti-apoptotic B-cell lymphoma-2 (BCL2) protein associated with cancer cell survival and resistance, Molecules, 2023, 28, 1588 CrossRef CAS PubMed.
- E. Febrina, A. Asnawi, R. Abdulah, K. Lestari and U. Supratman, Identification of flavonoids from Acalypha indica L.(Euphorbiaceae) as caspase-3 activators using molecular docking and molecular dynamics, Int. J. Appl. Pharm., 2022, 14, 162–166 CrossRef CAS.
- P. R. Deepa, S. Vandhana, S. Muthukumaran, V. Umashankar, U. Jayanthi and S. Krishnakumar, Chemical inhibition of fatty acid synthase: molecular docking analysis and biochemical validation in ocular cancer cells, J. Ocul. Biol. Dis. Infor., 2010, 117–128 CrossRef CAS.
- A. John, V. Umashankar, S. Krishnakumar and P. R. Deepa, Comparative modeling and molecular dynamics simulation of substrate binding in human fatty acid synthase: enoyl reductase and β-ketoacyl reductase catalytic domains, Genomics Inform., 2015, 13, 15 CrossRef.
- N. Zhang, Y. Yin, S. J. J. Xu and W. S. Chen, 5-Fluorouracil: mechanisms of resistance and reversal strategies, Molecules, 2008, 13, 1551–1569 CrossRef CAS.
- P. K. Veeresh, C. G. Basavaraju, S. Dallavalasa, P. G. Anantharaju, S. M. Natraj, O. A. Sukocheva and S. V. Madhunapantula, Vitamin D3 Inhibits the Viability of Breast Cancer Cells In Vitro and Ehrlich Ascites Carcinomas in Mice by Promoting Apoptosis and Cell Cycle Arrest and by Impeding Tumor Angiogenesis, Cancers, 2023, 15, 4833 CrossRef CAS.
- I. Ivasechko, A. Lozynskyi, J. Senkiv, P. Roszczenko, Y. Kozak, N. Finiuk, O. Klyuchivska, N. Kashchak, N. Manko, Z. Maslyak and D. Lesyk, Molecular design, synthesis and anticancer activity of new thiopyrano [2,3-d] thiazoles based on 5-hydroxy-1,4-naphthoquinone (juglone), Eur. J. Med. Chem., 2023, 252, 115304 CrossRef CAS.
- J. Bai, Y. Li and G. Zhang, Cell cycle regulation and anticancer drug discovery, Cancer Biol. Med., 2017, 14, 348 CrossRef CAS.
- X. Tong, R. Tang, M. Xiao, J. Xu, W. Wang, B. Zhang, J. Liu, X. Yu and S. Shi, Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research, J. Hematol. Oncol., 2022, 15, 1–32 Search PubMed.
- V. H. Pushpa, M. G. Kuruburu, M. K. Jayanthi, N. A. Simha, A. T. Babakr, R. Sreenivasan, R. Ramu and S. V. Madhunapantula, Bioactive profiling and evaluation of anti-proliferative and anti-cancerous properties of Shivagutika, an Indian polyherbal formulation synchronizing in vitro and in silico approaches, Front. Chem., 2023, 11 DOI:10.3389/fchem.2023.1195209.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4md00634h |
| This journal is © The Royal Society of Chemistry 2025 |