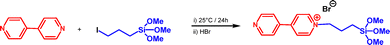Open Access Article
Open Access ArticleAntibacterial nitrogen-containing mesostructured SBA-15-type materials: insight into functional groups and surface polarity
Mohamed Amine
Benzaouia
a,
Othmane
Dardari
a,
Ghanem
Hamdoun
a,
Nadia
Katir
 a and
Abdelkrim
El Kadib
a and
Abdelkrim
El Kadib
 *ab
*ab
aEuromed University of Fes, UEMF, Morocco. E-mail: a.elkadib@ueuromed.org
bHassan II Academy of Science and Technology, Rabat, Morocco
First published on 8th October 2025
Abstract
Several nitrogen-containing organofunctionalized SBA-15-type materials have been successfully prepared and characterized. Besides the well-known aminopropyl-modified silica, propyl-dimethyl-octadecyl-ammonium and propyl-pyridyl-pyridinium have also been used for tethering the framework through Pluronic-templated sol–gel hydrolysis and co-condensation. Regardless of the nature of the starting precursors, their functionalities and/or their bulkiness, well-ordered silica mesostructures with high surface areas, pore sizes and pore volumes were obtained, with the flexibility or rigidity fingerprint of the precursor being transcribed at the mesoscale. The accessibility to nitrogen functionalities has been assessed through adsorption of the anionic helianthine dye pollutant. Their antibacterial activity was next evaluated against Gram negative Escherichia coli and Gram positive Staphylococcus aureus, revealing primary dependence on the loading of ammonium and pyridinium groups and correlating well with their accessibility. A substantial increase in the antibacterial activity was noticed through trimethylsilylation of residual surface silanols, providing consequently an easy route towards more potent antibacterial materials, through switching surface polarity, without introducing any metal additive that could add cost and compromise their sustainability. We also demonstrate the utility of these nitrogen-containing solids to uptake and release pharmaceuticals, as exemplified by the entrapment of the well-established quercetin antioxidant, opening the way towards synergistic biomaterials.
Introduction
Since their discovery, nanostructured silica-based materials created through self-assembly and sol–gel polymerization have attracted significant attention.1–5 Their high surface area, uniform pore size, versatile surface functionalization and excellent thermal stability render them ideal nanoreactors for various applications ranging from adsorption and catalysis to sensing and health.6–9 In the realm of antibacterial materials, mesoporous silica serves not only as a host for bioactive silver, copper and gold nanoparticles and other alloys and mixed oxides, but also can be used as a carrier for conjugating metal-free ammonium-based antibacterial materials.10–12 This option is largely preferred considering the detrimental effect of metal nanoparticles, including their side effects and toxicity for humans as well as their negative impact for the surrounding environment and the ecosystem.13–15One of the fundamental assets of mesostructured SBA-15-type materials lies in their versatile surface chemistry associated with the presence of abundant surface silanols and the wealth of available precursors that are prone to react with them through stable siloxane bridges.16–19 Surface functionalization of SBA-15 with nitrogen-containing groups is expected to provide potent antioxidant, antiviral and antibacterial materials,7,20–24 as well as more sophisticated ceramic and three-dimensional bio-based nanostructures,25–28 making it a valuable route to be explored towards advanced antimicrobial formulation and coating.29 Yet, little is known regarding the structure–reactivity relationship of these immobilized systems compared to the non-immobilized ammonium functionalities.30 Precedents in the literature have claimed that the hydrophobicity imparted by the grafted reagent prevents or at least minimize surface bacterial adhesion and hence the antibacterial resistive biofilm formation.31–34 Unfortunately, the preparation of these rationally designed scaffolds is tedious and accounts heavily for the final cost of the antibacterial formulation.35 Surprisingly, no reports have hitherto explored trivial silanol passivation near the antibacterial reagent to further repel contact by exacerbating surface hydrophobicity. We consequently set out to engage an in-depth study to gain further understanding on the precise effect that these functional groups can exert on the biological activity. With this aim, we have prepared a variety of modified SBA-15-type materials, the common thread being the presence of nitrogen-containing groups (either amine or ammonium and aliphatic or aromatic) in the material framework. We further expanded the library of studied materials to their trimethylsilylated-surface congeners to unveil the possible role of the remaining surface silanols during the interaction of cells with the solid surface. We have also explored the entrapment of quercetin as a representative active pharmaceutical ingredient, opening indeed another channel towards synergistic drug transporters. Through accurate comparison, this study substantiates the importance of the accessibility to the functional group and further established a clear correlation between the outer-sphere surface polarity of the surface and its biological response.
Results and discussion
Synthesis and characterization of 1-(3-trimethoxysilylpropyl)-4-(4-pyridyl)pyridinium bromide
Besides the commercially available 3-aminopropyltriethoxysilane and 3-(trihydroxysilyl)propyldimethyloctadecyl ammonium (NQSi), we have also attempted to use another pyridinium-based cationic species for SBA-15 surface modification. For such a purpose, we have prepared 1-(3-trimethoxysilylpropyl)-4-(4-pyridyl)pyridinium bromide through nucleophilic substitution attack of 4,4′-bipyridine towards iodopropyltrimethoxysilane at room temperature for 24 hours, followed by exchanging an iodide counter ion to bromide, for the reasons of solubility (Scheme 1). It should be noted that the direct use of bromopropyltrimethoxysilane against 4,4′-bipyridine failed probably because of the poor leaving ability of bromide compared to iodide. The targeted product (PyrSi) was isolated as an orange-colored powder in 90% yield and was spectroscopically characterized. Its FTIR, 1H and 13C NMR, and UV-vis analyses (Fig. S1–S3) were found to be comparable to those previously reported in the literature.21,22Sol–gel mesoporous organosilica synthesis and characterization
Nitrogen-containing SBA-15-type organosilicas were prepared by co-condensing tetraethylorthosilicate (TEOS) as a silica source with a selected nitrogen-containing organomodifier (aminoproyltriethoxylsilane APTES, 1-(3-trimethoxysilylpropyl)-4-(4-pyridyl)pyridinium bromide PyrSi or 3-(trihydroxysilyl)propyl dimethyl octadecyl ammonium chloride NQSi), using the amphiphilic Pluronic P123 as a structure-directing agent (Fig. 1). Surfactant removal was then achieved through Soxhlet extraction using acidified ethanol.36 The amount of the organosilane (NQSi or PyrSi) was varied to achieve either X = 1% or 5% grafting, as it will be denoted for each material (SBA@NQX% and SBA@PyrX%). Silica prepared using aminoproyltriethoxylsilane will be denoted as SBA@NH25%. Pristine silica SBA-15 was also prepared following the same procedure, using only TEOS as the silica source and will be denoted as SBA@OH to reflect the presence of residual silanols on its surface. Hexamethyldisilazane (Me3Si)2NH was used as a trimethylsilylating agent towards the remaining surface silanols,37 resulting in their transformation into hydrophobic trimethylsilyl groups. These surface passivated materials will be denoted as SBA@NQX%-SiMe3 and SBA@PyrX%-SiMe3. They were used to comparatively elucidate the effect of the surface polarity on the biological response.Fourier transform infrared (FTIR) spectroscopy of SBA@NQX% and SBA@PyrX% revealed a characteristic Si–O–Si peak at 1045 cm−1 (Fig. S4). At a lower organic loading of 1%, the Si–C band of the functionalizing agents could not be observed because of the marginal amount engaged in the initial solution. However, at 5% loading, such signature could be recognized at around 950 cm−1, along with an increase in the intensity of the C–H bond. A further increase in the intensity of the Si–C band was noticed for trimethylsilylated SBA@NQ1%-SiMe3 and SBA@Pyr1%-SiMe3 materials, consistent with the introduction of additional methyl bridged to silicon groups.
Solid-state cross-polarization magic angle spinning nuclear magnetic resonance (CP-MAS29Si NMR) shows the presence of high intensity peaks at −100 ppm and −109 ppm assigned, respectively, to Q3 and Q4 signals of the silica framework, while Q2 seems to be minor and embedded within the shoulder of the fused Q3 and Q4 peaks. Besides, a weak T-type signal of the terminal silicon belonging to the linker could be recognized at −67 ppm for SBA@Pyr1% and was hardly observable at −74 ppm for SBA@NQ1%. However, the intensity of these T-type signals further increase at 5% loading and could be distinguished at −63 and −74 ppm for SBA@NQ5% and at −54 and −67 ppm for SBA@Pyr5% (Fig. 2a). This brings convincing evidence for the covalent bonding on the co-condensed silicon-containing NQSi and PyrSi precursors to the silica framework (Fig. 2). Solid-state CP MAS 13C NMR of SBA@NQ5% confirmed the presence of an aliphatic skeleton (10 ppm to 68 ppm) of the NQSi chain (Fig. S5). Solid-state 1H NMR of SBA@Pyr5% showed two broad signals around 10 ppm. The COSY experiment established a clear correlation between these signals, thereby confirming their attribution to the pyridine–pyridinium moieties incorporated into the mesoporous silica network (Fig. S6). In the trimethylsilylated SBA@NQ1%-SiMe3 and SBA@Pyr1%-SiMe3 materials, two trends have been observed: (i) the presence of intense signals at 13.26 and 13.65 ppm in solid-state CP-MAS 29Si NMR spectra, unambiguously attributed to Si–O–SiMe3; (ii) treatment of the surface causes more framework condensation as illustrated by the increased intensity of the Q4-type signal at −109 ppm at the expense of the Q3-type signal at −100 ppm (Fig. 2b).
Thermogravimetric analysis (TGA) reveals distinct behavior across different materials in terms of water evaporation and thermal stability, reflecting the difference imparted by the nature of nitrogen-containing organic groups (Fig. S7). All materials remove physisorbed water below 100 °C, with the water content being dependent on their organic functionalities. From 100 °C to 250 °C, organosilica materials maintain their stability, with no major changes observed up to 320 °C. Beyond this temperature, thermal degradation occurs, with SBA@NQ5% showing a more pronounced weight loss of 21%, compared to only 8% weight loss observed for SBA@Pyr5%. This discrepancy can be tentatively explained by the great thermal stability of aromatic derivatives compared to the aliphatic ones that are more prone to C–C oxidative scission at elevated temperatures.38 Nitrogen elemental analysis further corroborates the presence of covalently linked nitrogen species that could normally leach out of the solution if only physisorbed (Table 1). The amount of nitrogen fits nearly the expected theoretical value, with an increase in its content (respectively by 4.1 and 4.27) with the increasing organic loading from 1% to 5% in SBA@NQX% and SBA@PyrX%. This means that nearly 85% of the engaged amount of the co-condensing reagent was assembled within the mesostructured framework during the early stage of the hydrolysis–condensation. The bulkiness of the NQSi reagent and its remarkably crowded nature did not hinder its quantitative grafting on the silica walls. This could be attributed to its hydrolysable Si(OH)3 groups, known to react faster compared to conventional trialkoxysilyl groups that need an induction time for triggering hydrolysis and further condensation.
| Materials | S area (m2 g−1) | D (nm) | V (cm3 g−1) | C BET | N (mmol g−1) | Grafting density (molecule nm−2) | % Residue at 600 °C |
|---|---|---|---|---|---|---|---|
| SBA@OH | 720 | 5.6 | 0.8 | 140 | — | — | 81 |
| SBA@NH25% | 440 | 5.1 | 0.5 | 230 | 0.36 | 0.37 | 79 |
| SBA@Pyr1% | 666 | 5.9 | 0.7 | 253 | 0.11 | 0.05 | 78 |
| SBA@Pyr5% | 613 | 5 | 0.7 | 277 | 0.47 | 0.23 | 82 |
| SBA@NQ1% | 745 | 6.4 | 1 | 158 | 0.1 | 0.08 | 80 |
| SBA@NQ5% | 515 | 6 | 0.8 | 50 | 0.41 | 0.48 | 74 |
| SBA@Pyr1%-SiMe3 | 365 | 5.5 | 0.5 | 49 | 0.02 | 0.016 | 83 |
| SBA@NQ1%-SiMe3 | 274 | 5.9 | 0.5 | 36 | 0.028 | 0.061 | 68 |
Nitrogen physisorption analysis shows an adsorption–desorption isotherm profile typical of SBA-15-type porous materials (Fig. 3 and Table 1). High surface areas of 745 m2 g−1 for SBA@NQ1% and 666 m2 g−1 for SBA@Pyr1% were obtained, being nearly comparable to the one of the pristine SBA@OH (720 m2 g−1). The increased surface area of SBA@NQ1% and its expanded pore diameter to 6.4 nm, in spite of its bulkiness, suggests that the latter acts as a swelling reagent for the micellar medium.39 For the two materials, increasing the amount of the co-condensing agent from 1% to 5% do not alter the mesostructure ordering but decreases the specific surface area from 745 to 515 m2 g−1 for SBA@NQ5% and from 666 to 613 m2 g−1 in the case of SBA@Pyr5%. More dramatic decrease in the specific surface area occurs upon surface trimethylsilylation, going down to 274 m2 g−1 for SBA@NQ1%-SiMe3 and 365 m2 g−1 for SBA@Pyr1%-SiMe3 (Fig. 3 and Fig. S8). This agrees with the quantitative surface trimethylsilyation evidenced by the great intensity of the corresponding signals at 13.26 and 13.65 ppm in solid-state 29Si NMR. Consequently, bulkier trimethylsilyl groups are introduced to substitute discrete silanols, more plausibly at the pore entrance during the diffusion of the hydrophobic hexamethyldisilazane, thereby reducing the specific surface areas of the corresponding materials. A close look on the CBET factor reveals a diverging pattern depending on the organic groups. CBET has often taken as an indicator for the surface polarity of the solid surface, especially with regard to chemical functionalization.39,40 Its decrease reflects a lower interaction with nitrogen, and hence a more inert and hydrophobic solid surface. Native SBA@OH displays a CBET value of 140. For SBA@Pyr1%, the recorded value of 253 indicates substantial surface polarity probably owing to the strong donating ability of the pyridine moieties, both as a hydrogen donor and an electron donor. Stronger interactions are further observed at 5% loading for SBA@Pyr5%, reflected by CBET = 277 presumably because more polar pyridine–pyridinium groups are immobilized on the surface at the expense of surface silanols. In turn, NQSi coverage seems to impart the material surface increased hydrophobicity, consistent with the length of the octadecyl spacer. At 1% loading, no significant alteration occurs for SBA@NQ1% compared to the pristine SBA@OH (CBET of 158 versus 140). At a lower loading of 1%, the grafting density of NQSi was equal to 0.08 molecule nm−1, offering plausibly more empty and hence available space to interact with residual surface silanol groups. In contrast, a severe decrease to a CBET value of 50 was observed for SBA@NQ5%, where we assume that at 5% loading, the surface was totally covered and hence becomes more hydrophobic. Incredibly, trimethylsilylated materials further decrease the CBET values to 49 for SBA@Pyr1%-SiMe3 and 36 for SBA@NQ1%-SiMe3, allowing us to draw two additional conclusions: (i) the highest polarity of SBA@PyrX% does not only rely solely on the pyridine–pyridinium groups, but also in their proximal geometry and plausible interaction with surface silanols. The consumption of these groups in SBA@PyrX%-SiMe3 suppresses the stronger interaction previously seen for SBA@PyrX%; (ii) trimethylsilylated SBA@NQ1%-SiMe3 behaves quite similarly to SBA@NQ5%. We in fact assume that, at high loading, NQSi distribution density along with the steric hindrance of the substituents at the nitrogen center hinders access to the exposed silanols, although they are still present on the surface. One might indeed conclude that the surface polarity of the corresponding materials could be tuned either by adjusting the loading of the tether or by trimethylsilylation.
Scanning electron microscopy (SEM) analysis allows visualization of the textured network at the microscale. Non-functionalized SBA@OH consists of uniform elongated aggregates with rod-like morphology, typical of Pluronic-templated mesoporous materials (Fig. S9). In the cases of SBA@NQ1% and SBA@Pyr1%, the images reveal rod-like structures, confirming the preservation of the mesostructured form of SBA-15 (Fig. 4). However, the way the rod-like structure aggregates to each other to build an extended secondary structure changes from one material to another. In the case of SBA@NQ1%, rods are twisted and this becomes more pronounced at 5% loading as it can be observed in the onset image (Fig. 4a). In contrast, those of SBA@Pyr1% linearly extend to form a more continuous rigid network. The resulting organization at the microscale can be attributed to the interplay of the used co-condensing reagent with the Pluronic and the nucleating siloxanes during the assembly of the mesostructure as it has already been reported for similar self-structuring precursors.39,41 Transmission electron microscopy (TEM) images of the corresponding samples reveal well-defined nanoscale ordering, typical of the mesostructured silica network, with the dark regions corresponding to the dense silica walls, while the periodically arranged bright lines reflects the presence of uniform, ordered pores, in agreement with nitrogen sorption analysis. Besides, in the case of SBA@NQ1%, the twisted architecture could be easily recognized, further corroborating the original organization of the rods at the microscale. Such a twisted structure could not be seen in the case of SBA@Pyr1% where only direct anisotropic rods could be observed (Fig. 4b). In the whole, SEM and TEM images conclude that the co-condensing reagents used could be anchored on the silica framework with no detrimental effect on the pore uniformity or the regularity of the assembled network. Besides, the rigidity or flexibility fingerprint of the starting precursor transcribes in the hybrid at the microscale and even at the nanoscale.42
Accessibility test of functional groups
Given the complexity of the used NQSi and PyrSi precursors, a question arises concerning the accessibility of the corresponding cationic groups to the surrounding guests, including dyes and cells, once confined inside of the mesostructured framework. This accessibility is of prime importance regarding their use as antibacterial and antiviral coating materials. We have indeed embarked to assess their adsorptive capacity for anionic dyes that could intuitively reflect the availability of the anchored functionalities inside of the mesopores. For such a purpose, we have selected helianthine (methyl orange) as a representative anionic dye.At neutral pH of 6.5, unmodified silica SBA@OH failed to adsorb the anionic dye, recording less than 3% uptake, which exclude the involvement of the remaining silanols in interacting with the adsorbate. A significant uptake of 28% could be reached with SBA@NH25%, due to the possible hydrogen bonding with the primary amine groups. Surprisingly, a lower performance of 4% was recorded for SBA@Pyr5% because of the lower propensity of the exposed pyridine for hydrogen bonding. This lower uptake is also indicative of the inaccessibility to the cationic pyridinium fragment that seems to be buried inside the silica framework, and not exposed to be partnered with the anionic dye (Fig. 5). However, the uptake could be increased to 43% upon alkylation of the terminal pyridine through methylation to the bipyridinium decorating surface (denoted herein as SBA@BPyr5%). Although this post-modification induces severe reduction in the specific surface area (SBET = 246 m2 G−1), transforming external pyridine groups into pyridinium provides the driving force for electrostatic interactions with the dye, allowing for its substantial removal from aqueous solution. Outstanding performance has been observed for SBA@NQ5%, reaching nearly fully removal (99%), which correspond to 16.7 mg g−1 of the dye. Interestingly, at a basic pH of 9.5, all the materials display poor adsorption ability, except SBA@NQ5%. The main reason for this decrease is probably the deprotonation occurring on the surface of these materials rendering them negatively charged, leading indeed to electrostatic repulsion with methyl orange (MO). The increased performance observed at an acidic pH of 2 for SBA@OH, SBA@NH2 and SBA@Pyr5% confirms our assumptions of the occurrence of adsorption through an ionic exchange mechanism. SBA@NQ5% maintains its highest adsorption capacity owing to the quaternary nature of its nitrogen center, hindering it from protonation or deprotonation and hence making it insensitive to pH variation. These experiments further demonstrate the full accessibility of the ammonium groups in SBA@NQ5% in spite of the presence of such lengthy dodecyl substituent that could hinder access to the nitrogen center. At an active site basis, the number of occupied nitrogen sites of SBA@NQ5% for adsorbing a methyl orange concentration of Co = 10−4 mol L−1 was found to be 12.3%, meaning that a large portion of cationic groups is still available for interaction with the dye (Table S1). A further increase of the initial concentrations to 5.10−4 mol L−1 and 10−3 mol L−1 decreases the removal efficiency to 92% and 75%, respectively. This corresponds to maximum adsorption capacities of 75 mg g−1and 123 mg g−1, respectively (Fig. S10), meaning that 54% of the active nitrogen site are interacting for C0 = 5.10−4 mol L−1 and even 90% for a C0 = 10−3 mol L−1 (Table S1). This reflects the great exposure of the cationic site in SBA@NQ5%. Oppositely, one could also conclude on the inaccessibility of the pyridinium in SBA@Pyr5% that seems to be more oriented to the silica surface, probably through hydrogen bonding or week interactions with the neighboring surface silanols.
Antibacterial activity
Next, the antibacterial activity of these materials against Escherichia coli and Staphylococcus aureus was investigated. At the first glance, non-modified SBA@OH displays the lowest inhibitory effect, indicating that the pristine silica surface lacks appreciable antibacterial activity and consequently highlighting surface-functionalization as a key enabler to impart these materials with the requested bio-activity. More improvement was rather observed using both SBA@NQX% and SBA@PyrX%. In the case of Staphylococcus aureus, the former achieves inhibition zones of 5.9 and 8.4 mm at 1% and 5% loading, respectively. The latter achieves 7.4 and 9.7% mm, respectively, at 1% and 5% loading, outperforming indeed its aliphatic congeners (Fig. 6a and b). A similar trend was also observed for the inhibition of Escherichia coli. The increase in the two cases of the inhibitory effect depending on the content of the nitrogen-containing groups clearly indicates their involvement in the biological activity. Comparing the performance of SBA@NH25% and the one of SBA@NQ1% reveals the superior inhibitory effect caused by ammonium compared to amine functionalities, although the nitrogen content of the former is more than three times superior to the content of the later. At nearly similar nitrogen loading, the inhibitory effect of ammonium is doubled compared to the primary amine, certainly because ammonium groups favor electrostatic interaction with bacteria, a preliminary step toward their destruction. The even greatest activity of SBA@PyrX% also suggests the importance of the pyridinium–pyridine motif that outperforms the performance recorded for aliphatic ammonium groups of SBA@NQX%. The involvement of the exposed pyridine, solely or in combination with the fused pyridinium, seems to be the most plausible mechanism, as the selective accessibility of the buried quaternary pyridinium has been challenged by the methyl orange adsorption test, as it has been discussed early.To further investigate the importance of the surface polarity for eradicating bacterial cells, we further used the trimethylsilylated variants and benchmarked each sample with its non-passivated partner. The results clearly point to the positive effect imparted by the presence of trimethylsilyl groups, although this post-grating has been found to severely reduce the specific surface areas and could indeed be expected to limit access to the mesopores. At the same amount of the tether, the passivated material outperforms the non-passivated ones. Noteworthy is the activity reached with only 1% amount that has been next passivated, being higher that the inhibitory effect reached by introducing 5% leading. However, a steady-state was reached at 5% loading for the inhibition of Staphylococcus aureus, with the trimethylsilylation route being less effective to further boost the antibacterial performance. This result could be due to the marginal effect of the trimethylsilylation against SBA@NQ5%, which at this loading display already a CBET value of 50, indicative of its pronounced hydrophobicity (Fig. 6c and d). In fact, at such loading, the vertical branching allows dimethylammonium to be much more close to each other while the remaining octadeyl behaves as a brush, exalting more pronounced hydrophobicity. In the case of SBA@Pyr5%-SiMe3, while no variation in CBET was observed, the most plausible explanation could be rooted in the reduced specific surface area of the corresponding material, being less favorable to the penetration of heavy objects like bacteria. Mechanistically speaking, bacteria could be drawn to the pores through electrostatic interactions. The positively charged ammonium in NQSi or pyridine pyridinium in PyrSi tethers strongly interacts with the negatively charged bacterial cell membranes, thereby facilitating its adhesion to the silica surface. Once within the pores, these interactions not only promoted bacterial attachment but also positioned the cells in close proximity to the antimicrobial functional groups. The hydrophobic backbone of the functionalized silica penetrated the bacterial peptidoglycan layer, which forms part of the cell wall. This penetration disrupted the integrity of the peptidoglycan network, compromising the cell wall's structural stability. As a result, the bacterial cell membrane became destabilized, leading to a loss of essential cellular components and ultimately causing cell lysis. This disruption of the cell walls and membrane integrity was the primary mechanism that led to bacterial death, as the cells were no longer able to maintain their osmotic balance or defend against external stressors. The improved antibacterial activity observed herein is in agreement with the enhanced bioactivity of the silica surface upon coating with fatty acids, attributed to the full coverage of the pristine silica surface.43,44 The dependence of activity on both cationic charge density and hydrophobicity supports this proposed mechanism,45–47 but further experiments are necessary to further clarify the biological action. In the whole, the improvement of the antibacterial activity of 1% loading after trimethylsilylation (SBA@NQ1%-SiMe3 and SBA@Pyr1%-SiMe3) is one of the most selling points of these materials. Considering the wide availability of the passivating reagents and their cost-effectiveness compared to the cost of the starting precursors, more scalable materials could be designed by trivial tuning of their surface polarity.
Uptake and release of quercetin
In this part, we have selected only three nitrogen-containing organosilica to study their uptake, release and the anti-oxidant activity once entrapping quercetin. The ability of mesostructured silica to host active pharmaceuticals has been already demonstrated and further explored for designing controlled-release systems.48 However, more research should be done to further explore the combination of synergistic action “per se and loaded pharmaceutical”.49 At a pH of 5.5, conventional SBA@NH25% achieves a lower uptake of 6%, while SBA@Pyr5% and SBA@NQ5% were able to host 16% and 20% of the quercetin drug, respectively. This trend perfectly concordats with the accessibility test as previously discussed (Fig. 7). A maximum adsorption capacity of 45 mg g−1, corresponding to an efficient removal of 30% uptake, was rather observed for SBA@OH, thereby highlighting the occurrence of hydrogen bonding between Si–OH of the host and polar groups of the pharmaceutical. At a pH of 9.5, all the materials display an enhanced uptake of the pharmaceutical, with SBA@NQ5%, recording the highest value (75%) efficiency, with an adsorption capacity of 114 mg g−1 (Fig. 7). This spectacular uptake is due to the formation of anionic phenolate species upon quercetin deprotonation at pH > 7.5, favoring cationic–anionic exchange between the positively charged SBA@NQ5% and its guest. SBA@Pyr5% and SBA@NQ5% loaded at pH = 5.5 trigger quercetin release, with SBA@Pyr5% enabling to escape more pharmaceutical (20%) compared to 11% release in the case of SBA@NQ5% (Fig. S11). This indicates that the loaded quercetin probably interacts through different ways with its adsorbent, with presumably less interactions occurring within the pores of SBA@Pyr5% compared to tighter interplay in the case of SBA@NQ5%. SBA@NH25% failed to release even a trace amount of the drug and consequently did not show any measurable antioxidant activity. In contrast, SBA@NQ5%_Q and SBA@Pyr5%_Q display great antioxidant activity, with IC50 values of 0.7 and 0.48, respectively. These preliminary results demonstrate that these materials act synergistically as active antibacterial drugs and also as a reservoir for controlled release of antioxidants. | ||
| Fig. 7 Removal of quercetin using silica and the modified silica surface. (a) Percentage of quercetin removal, and (b) adsorption capacity on organosilicas at different pH values. | ||
Experimental section
Starting reagents
Commercially available reagents and solvents were purchased from Across and Sigma-Aldrich and used without further purification. 3-(Trihydroxysilyl)propyldimethyloctadecyl ammonium (CAS number 27668-52-6) was purchased from across with 5% of purity in water.Methods and materials
Fourier transform infrared (FTIR) spectra were obtained with a PerkinElmer Spectrum 100FT-IR spectrometer equipped with ATR accessory. The FTIR spectra were recorded in the 4000–600 cm−1 range with a resolution of 4 cm−1 and an accumulation of 32 scans. Solid-state 13C CP-MAS NMR and 29Si NMR spectra were recorded using a JEOL JNM-ECZR 600 MHz spectrometer, operating at 150 MHz for 13C and 243 MHz for 29Si. Cross-polarization (CP) was optimized individually for each sample to enhance signal sensitivity. Samples were packed into 3.2 mm zirconia rotors and spun at a magic angle spinning (MAS) speed of 22 kHz to reduce dipolar broadening and improve spectral resolution. The recycling delay was optimized based on the T1 relaxation time for each sample to ensure efficient signal recovery between scans. For the 13C CP-MAS NMR spectra, a ramped CP sequence was employed with high-power proton decoupling during acquisition. The contact time was individually optimized for each sample. Additionally, high-resolution 1H NMR and 2D 1H homocorrelation spectra were acquired to address the limitations in sensitivity for 13C and 29Si, taking advantage of the enhanced resolution achieved with a higher MAS speed of 22 kHz. All spectra were processed using standard methods, including Fourier transformation, baseline correction, and line broadening, to enhance resolution and signal clarity. Diffuse reflectance UV-visible spectroscopy (DRUV) was measured in the 200–800 nm wavelength range using a PerkinElmer Lambda 1050 spectrometer equipped with an integrating sphere. Thermogravimetric analysis (TGA) was performed using a Discovery TGA analyzer at a heating rate of 15 °C min−1 from room temperature to 600 °C under a nitrogen atmosphere. Textural properties were evaluated through nitrogen adsorption–desorption isotherms using a TriStar II Plus (Micromeritics) analyzer. Measurements were conducted at 77.3 K following a degassing step at 120 °C for 12 hours to eliminate surface moisture and physisorbed species. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method. SEM images were obtained using a JEOL JSM 6700F. Energy dispersive X-ray spectroscopy (EDS-SEM) analyses of the powders were performed using an Oxford EDS system. TEM images were obtained using a JEOL JEM 2010 at an activation voltage of 200 kV. We tested the antibacterial properties of organosilica by using a total viable colony count method.50 The study focused on two foodborne pathogens, namely Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive). For the experiment, the bacterial strains were aseptically cultured in 20 mL of tryptic soy broth (TSB) for E. coli and brain heart infusion (BHI) broth for S. aureus. The cultures were incubated at 37 °C for 16 hours. After incubation, the bacterial suspension was diluted to a concentration of 105–106 CFU mL−1, and 20 mL of the diluted suspension was transferred into a 100 mL conical flask containing 100 mg of the film sample. The flask was incubated at 37 °C with gentle shaking for 12 hours. A control flask containing only the diluted broth (without the film) was also included. Viable bacterial counts (expressed as Log10 CFU mL−1) were recorded at 2, 4, 8, and 10-hour intervals by plating the samples onto agar plates. Each measurement was performed in triplicate at each time point to ensure accuracy.Preparation of 1-(3-trimethoxysilylpropyl)-4-(4-pyridyl)pyridinium bromide
An equimolar amount of the commercially available 4,4′-bipyridine (500 mg, 3.2 mmol) reacted with 3-iodopropyltrimethoxysilane (1 mL, 4.8 mmol) in 10 mL of acetonitrile for 24 h at room temperature. The temperature was then increased to 85 °C and the reaction mixture was stirred for an additional 24 hours. The obtained orange solid was subjected to filtration and washed three times with 10 mL of toluene. Ionic exchange of an iodide ion by bromide was done through using 10 mL of HBr in water (4 M). The mixture was then filtered and washed twice with 5 mL of HBr (4 M), followed by drying at 80 °C for 4 hours.Preparation of pristine and nitrogen-containing SBA-15-type organosilica
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at a pH of 5.5 through dispersing SBA@NQ5%_Q and SBA@Pyr5%_Q at Cm = 2 mg mL−1. The released quercetin was calculated using an UV-vis spectrophotometer by monitoring the intensity change of the adsorption bond at λ = 367 nm.51
1 at a pH of 5.5 through dispersing SBA@NQ5%_Q and SBA@Pyr5%_Q at Cm = 2 mg mL−1. The released quercetin was calculated using an UV-vis spectrophotometer by monitoring the intensity change of the adsorption bond at λ = 367 nm.51
Antibacterial activity
The disc diffusion method was chosen to study the antibacterial activity of organosilica powder against Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria. The powders were compacted into a circular disc of 2 mm in diameter. After sterilization, the circles were spread on the Mueller Hinton agar surface using a bacterial density of approximately 1.5 × 106 CFU mL−1. The samples were kept for 24 h at 37 °C and the diameters of the inhibition zones around the circles were measured.Conclusions
With the sake of designing more powerful, metal-free, antibacterial materials, we have herein designed a set of nitrogen-containing SBA-15-type mesoporous materials. Pyridyl-pyridinium and dodecylammonium were successfully embedded within the material framework, without disrupting the mesostructure ordering. The effect of the tether amount (1% versus 5%) was investigated and proved to be pivotal for inhibiting the bacterial growth. Interestingly, substantial amplification was also observed by trivial switching the silica surface polarity from hydrophilic to hydrophobic through trimethylsilylation. This surface functionalization provides a way for improving the antibacterial performance while minimizing the amount of the costly active ingredient used. Besides, nitrogen functionalities offer an additional role of interacting with entrapped drugs as exemplified by the uptake and release of antioxidant-based pharmaceuticals. With the well-established versatility of mesoporous materials and the wealth of available precursors, surface-chemistry opens new channels of possibilities for the design of cost-effective metal-free antiviral and antibacterial materials, with expanded possibilities towards multifunctional and synergistic drug transporters.Conflicts of interest
There are no conflicts to declare.Data availability
Data are available in the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ma00714c.Acknowledgements
Hassan II academy of Science and Technology is acknowledged for the financial support from the Nano-Bio-Mat project.Notes and references
- A. C. Kresge, M. E. Leonowicz, W. J. Roth, J. Vartuli and J. Beck, Nature, 1992, 359, 710–712 CrossRef.
- H.-P. Lin and C.-Y. Mou, Science, 1996, 273, 765–768 CrossRef CAS PubMed.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS PubMed.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. Chu, D. H. Olson, E. W. Sheppard and S. B. McCullen, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS.
- B. L. Newalkar, N. V. Choudary, P. Kumar, S. Komarneni and T. S. G. Bhat, Chem. Mater., 2002, 14, 304–309 CrossRef CAS.
- L. Paul, S. Mukherjee, S. Chatterjee, A. Bhaumik and D. Das, ACS Omega, 2019, 4, 17857–17863 CrossRef CAS PubMed.
- P. Verma, Y. Kuwahara, K. Mori, R. Raja and H. Yamashita, Nanoscale, 2020, 12, 11333–11363 RSC.
- V. Mamaeva, C. Sahlgren and M. Lindén, Adv. Drug Delivery Rev., 2013, 65, 689–702 CrossRef CAS PubMed.
- A. Bernardos, E. Piacenza, F. Sancenon, M. Hamidi, A. Maleki, R. J. Turner and R. Martínez-Máñez, Small, 2019, 15, 1900669 CrossRef PubMed.
- Y. Song, H. Jiang, B. Wang, Y. Kong and J. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 1792–1801 CrossRef CAS PubMed.
- M. Sanchez-Milla, R. Gomez, J. Perez-Serrano, J. Sanchez-Nieves and F. J. de la Mata, Mater. Sci. Eng., C, 2020, 109, 110526 CrossRef CAS PubMed.
- J. Zhang, W. Guo, Q. Li, Z. Wang and S. Liu, Environ. Sci.: Nano, 2018, 5, 2482–2499 RSC.
- M. Sharifi, M. K. Farahani, M. Salehi, A. Atashi, M. Alizadeh, R. Kheradmandi and S. Molzemi, ACS Biomater. Sci. Eng., 2022, 9, 106–138 CrossRef PubMed.
- A. F. Fakhardo, E. I. Anastasova, S. R. Gabdullina, A. S. Solovyeva, V. B. Saparova, V. V. Chrishtop, E. D. Koshevaya, E. F. Krivoshapkina, P. V. Krivoshapkin and G. O. Kiselev, ACS Appl. Bio. Mater., 2019, 2, 4427–4435 CrossRef CAS PubMed.
- L. Chen, S. Zhang, Y. Duan, X. Song, M. Chang, W. Feng and Y. Chen, Chem. Soc. Rev., 2024, 53, 1167–1315 RSC.
- A. Stein, B. J. Melde and R. C. Schroden, Adv. Mater., 2000, 12, 1403–1419 CrossRef CAS.
- J. M. Rosenholm and M. Lindén, Chem. Mater., 2007, 19, 5023–5034 CrossRef CAS.
- S. Fiorilli, B. Onida, B. Bonelli and E. Garrone, J. Phys. Chem. B, 2005, 109, 16725–16729 CrossRef CAS PubMed.
- S. Rahmani, K. Bouchmella, J. Budimir, L. Raehm, M. B. Cardoso, P. Trens, J.-O. Durand and C. Charnay, ACS Omega, 2019, 4, 1479–1486 CrossRef CAS.
- M. Álvaro, B. Ferrer, V. Fornés and H. García, Chem. Commun., 2001, 2546–2547 RSC.
- N. Fattori, C. M. Maroneze, H. A. Magosso, Y. V. Kholin and Y. Gushikem, J. Colloid Interface Sci., 2012, 384, 137–142 CrossRef CAS PubMed.
- Y. Zhu, H. Li, Q. Zheng, J. Xu and X. Li, Langmuir, 2012, 28, 7843–7850 CrossRef CAS PubMed.
- F. Sevimli and A. Yılmaz, Microporous Mesoporous Mater., 2012, 158, 281–291 CrossRef CAS.
- M. Thanigachalam and A. V. M. Subramanian, Biomater Transl., 2023, 4, 151 Search PubMed.
- H. Guo, M. Guo, Z. Xia and Z. Shao, Biomater Transl., 2024, 5, 33 Search PubMed.
- W. Ma, H. Lu, Y. Xiao and C. Wu, Org. Res., 2025, 1, 025040004 Search PubMed.
- N. Prabhakar, E. Långbacka, E. Özliseli, J. Mattsson, A. Mahran, I. Suleymanova, C. Sahlgren, J. M. Rosenholm, M. Åkerfelt and M. Nees, Small Sci., 2024, 4, 2400084 CrossRef CAS PubMed.
- M. Michailidis, I. Sorzabal-Bellido, E. A. Adamidou, Y. A. Diaz-Fernandez, J. Aveyard, R. Wengier, D. Grigoriev, R. Raval, Y. Benayahu and R. A. D’Sa, ACS Appl. Mater. Interfaces, 2017, 9, 38364–38372 CrossRef CAS PubMed.
- J. Huang, R. R. Koepsel, H. Murata, W. Wu, S. B. Lee, T. Kowalewski, A. J. Russell and K. Matyjaszewski, Langmuir, 2008, 24, 6785–6795 CrossRef CAS PubMed.
- B. Gottenbos, H. C. van der Mei, F. Klatter, P. Nieuwenhuis and H. J. Busscher, Biomaterials, 2002, 23, 1417–1423 CrossRef CAS PubMed.
- Z. Li, D. Lee, X. Sheng, R. E. Cohen and M. F. Rubner, Langmuir, 2006, 22, 9820–9823 CrossRef CAS PubMed.
- J. J. Oosterhof, K. J. Buijssen, H. J. Busscher, B. F. van der Laan and H. C. van der Mei, Appl. Environ. Microbiol., 2006, 72, 3673–3677 CrossRef CAS PubMed.
- J. Song, H. Kong and J. Jang, Colloids Surf., B, 2011, 82, 651–656 CrossRef CAS PubMed.
- K. Kuciński and G. Hreczycho, ChemSusChem, 2019, 12, 1043–1048 CrossRef PubMed.
- S. G. de Ávila, L. C. C. Silva and J. R. Matos, Microporous Mesoporous Mater., 2016, 234, 277–286 CrossRef.
- J. D. Webb, T. Seki, J. F. Goldston, M. Pruski and C. M. Crudden, Microporous Mesoporous Mater., 2015, 203, 123–131 CrossRef CAS.
- J. C. Markwart, A. Battig, M. M. Velencoso, D. Pollok, B. Schartel and F. R. Wurm, Molecules, 2019, 24, 3901 CrossRef CAS PubMed.
- A. El Kadib, N. Katir, A. Finiels, A. Castel, N. Marcotte, K. Molvinger, C. Biolley, P. Gaveau, M. Bousmina and D. Brunel, Dalton Trans., 2013, 42, 1591–1602 RSC.
- A. El Kadib, P. Hesemann, K. Molvinger, J. Brandner, C. Biolley, P. Gaveau, J. J. Moreau and D. Brunel, J. Am. Chem. Soc., 2009, 131, 2882–2892 CrossRef CAS PubMed.
- Y. Brahmi, N. Katir, A. Castel and A. El Kadib, Microporous Mesoporous Mater., 2013, 177, 75–81 CrossRef CAS.
- M. Ferenc, N. Katir, K. Miłowska, M. Bousmina, J.-P. Majoral, M. Bryszewska and A. El Kadib, J. Mater. Chem. B, 2015, 3, 2714–2724 RSC.
- E. Pędziwiatr-Werbicka, K. Miłowska, M. Podlas, M. Marcinkowska, M. Ferenc, Y. Brahmi, N. Katir, J. P. Majoral, A. Felczak and A. Boruszewska, Chem. – Eur. J., 2014, 20, 9596–9606 CrossRef PubMed.
- M. Ferenc, N. Katir, K. Milowska, M. Bousmina, Y. Brahmi, A. Felczak, K. Lisowska, M. Bryszewska and A. El Kadib, Micropor. Mesopor. Mat, 2016, 231, 47–56 CrossRef CAS.
- K. Ciepluch, N. Katir, A. El Kadib, A. Felczak, K. Zawadzka, M. Weber, B. Klajnert, K. Lisowska, A.-M. Caminade and M. Bousmina, Mol. Pharm., 2012, 9, 448–457 CrossRef CAS PubMed.
- N. Katir, J. P. Majoral, A. El Kadib, A.-M. Caminade and M. Bousmina, Eur. J. Org. Chem., 2012, 269–273 CrossRef CAS.
- M. Boundor, B. Bielska, N. Katir, N. Wronska, K. Lisowska, M. Bryszewska, K. Miłowska and A. El Kadib, ACS Appl. Polym. Mater., 2023, 5, 9952–9963 CrossRef CAS.
- M. Vallet-Regi and A. Ramila, Chem. Mater., 2001, 13, 308–311 CrossRef CAS.
- M. Cokol, H. N. Chua, M. Tasan, B. Mutlu, Z. B. Weinstein, Y. Suzuki, M. E. Nergiz, M. Costanzo, A. Baryshnikova and G. Giaever, Mol. Syst. Biol., 2011, 7, 544 CrossRef PubMed.
- J.-W. Liu, T. Fu, Y. He, L.-J. Wang, K.-R. Ding and H.-Z. Zhao, Chem. Eng. J., 2024, 497, 154770 CrossRef CAS.
- I. Trendafilova, A. Szegedi, J. Mihály, G. Momekov, N. Lihareva and M. Popova, Mater. Sci. Eng., C, 2017, 73, 285–292 CrossRef CAS PubMed.
- O. P. Sharma and T. K. Bhat, Food Chem., 2009, 113, 1202–1205 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |