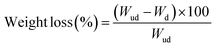Open Access Article
Open Access ArticleTest-tube model for rapid and accelerated biodegradation of poly(lactic acid)/poly(butylene succinate) blended bioplastic obtained from solution casting
Shreetam
Parida
a,
Nivethitha
Ashok
a and
Rajendra
Kurapati
 *ab
*ab
aSchool of Chemistry, Indian Institute of Science Education and Research Thiruvananthapuram, Maruthamala P.O., Vithura, Thiruvananthapuram, Kerala 695551, India. E-mail: rkurapati@iisertvm.ac.in
bThe Centre for Advanced Materials Research with International Engagement (CAMRIE), Indian Institute of Science Education and Research Thiruvananthapuram, Maruthamala P.O., Vithura, Thiruvananthapuram, Kerala 695551, India
First published on 27th October 2025
Abstract
Developing an alternative accelerated biodegradation test to the standard tests is crucial for the swift assessment of bioplastics to reduce the time duration and cost. Designing a blended bioplastic with an acceptable extent of miscibility in the polymeric phase and its rapid and accelerated real-time degradation is a persistent challenge because of its different chemical and crystalline properties. Hereby, we developed miscible blended bioplastic films of biopolymers poly(lactic acid) (PLA) and poly(butylene succinate) (PBS) with varied ratios by a simple solution casting method. The as prepared bioplastic blends of PLA/PBS (50/50 and 20/80) showed high miscibility with fascinating morphology as confirmed by optical and electron microscopy. Furthermore, the PLA/PBS films were validated by physicochemical methods including infrared spectroscopy, powder X-ray diffraction, thermogravimetric analyses and wettability by the contact angle measurements. All the results confirmed the successful preparation of blended bioplastics of PLA/PBS. Next, we developed a test-tube model for rapid and accelerated biodegradation (RAB) of the developed bioplastic blends of PLA/PBS in the form of films and powder (microparticles) using mobilised enzymes such as proteinase K and lipase in the aqueous buffer. The extent of degradation of the blends was followed using the physicochemical methods, along with the weight loss measurements. All the RAB results suggested that changes in surface area (from films to microparticle powder) affected the extent of enzymatic degradation of the bioplastic films and powder.
1. Introduction
Excessive plastic use and the gathering of waste have had a severely negative effect on environmental and ecological factors.1 Plastic packaging materials made from synthetic polymers are mostly single-use and generally discarded once they are used. In total, 40% of petrochemical-based plastics are being used for packaging purposes and nearly 60% of plastic packaging products are used to pack food and beverages.1 About 95% of plastic packaging products are wasted after a single use and end up in landfills, where they disintegrate into micro- or nanoplastics.1 It has also been found that microplastics and their particles are found in milk, meat, and other edible items and have a direct impact on human health.2,3 One of the solutions to reduce the environmental risk of non-degradable plastic is by using alternative biodegradable bioplastics made from renewable materials (e.g., biomass from corn and sugarcane), which undergo biodegradation by the action of microorganisms found in the soil.4 However, biodegradable plastics have replaced conventional plastics to a very limited extent. Biodegradable polymers such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(caprolactone) (PCL), poly(butylene adipate-co-terephthalate) (PBAT), and poly(butylene succinate) (PBS) have been used exclusively for manufacturing biodegradable plastics.5 Furthermore, the European Bioplastics Organization forecasted that PLA and PBS would be the most demanded polymers for bioplastic applications by 2026, due to their applications in the biomedical and pharmaceutical industries.6,7 In general, biopolymers are known to have lower mechanical strength compared to synthetic polymers.8 For example, the usage of PLA in biomedical applications and packaging is limited because of its high brittleness.9 Therefore, blending PLA with other biopolymers such as PBS could enhance the flexibility and processability while maintaining the biodegradability. Being a biodegradable polyester, PBS has high processing ability, thereby PLA/PBS blended bioplastics are used in various applications. However, making blends of PLA/PBS is limited by incompatibility due to the lower chain entanglement across the interface. To improve the compatibility and flexibility of bioplastic blends, third components such as other biopolymer materials (e.g., bamboo powder, wood flour and cellulose fibres) were used to obtain the PLA/PBS blends.9 Blend miscibility or compatibility persisted as a challenge, which is the key requirement for packaging, biomedical and biodegradation purposes.10–13 Therefore, developing a PLA/PBS blend with good miscibility is a challenge without adding a third biopolymer, as mentioned. PLA/PBS blends have been achieved by using thermo-mechanical methods such as melt-extrusion, batch mixing by screw extrusion, compression moulding, etc.14,15On the other hand, though various biodegradable plastics are emerging as alternatives to conventional non-biodegradable plastics, testing for the biodegradation of such biodegradable plastic materials involves a variety of standard tests with many parameters, thereby requiring a longer time, and is expensive.10 The various standard tests for checking biodegradability are conducted under specified conditions, including realistic parameters and varied periods from 14 days to 24 months.16 Furthermore, the International Standard Organisation (ISO) recommends many standard tests for assessing the biodegradability of biodegradable plastics, including in soil, seawater, marine sediment, etc., which are regularly updated.17 Also, the European Union (EU) recommended a series of standard biodegradation methods or tests. According to the European Chemical Agency (ECHA) report, there are three generalized categories of aerobic biodegradation, namely inherently degradable (minimum degradation is 70% within 14 days), readily degradable (minimum degradation is 60% within 28 days) and ultimately degradable (minimum degradation is 90% within 6 months for aqueous, 24 months for soil or seawater/sediment).16,18
However, assessing the biodegradability of biodegradable plastics following standard methods is a big hurdle as it takes a long duration (months to years) following various realistic conditions (soil, seawater, etc.), which will result in an increase in the cost of newly developed biodegradable plastics.19 Therefore, alternative methods such as accelerated biodegradation tests have been developed to obtain rapid biodegradability of the biodegradable bioplastics. The accelerated tests involve treating the bioplastic directly with isolated microbial enzymes (from soil or seawater, etc.), varied size of the bioplastic particles (to increase the surface area), incubation at higher temperatures, and varied pH (to improve the enzymatic catalysis), organic solvents, pre-treatments, adding enzyme modifiers, etc.19–23 Development of such rapid and accelerated biodegradation (RAB) tests is crucial for assessing the biodegradability of newly developed biodegradable bioplastics in a rapid manner. However, rapid biodegradation under accelerated conditions is still challenging to design. Such accelerated degradation methods could help to make a faster assessment of the degradation behaviour of newly developed bioplastics or biodegradable plastics.
PLA/PBS is a commonly used bioplastic blend for biomedical and packaging applications, but the biodegradability of PLA/PBS blended bioplastics is poorly understood. Therefore, the biodegradation of PLA/PBS blends needs to be understood thoroughly. Hence, designing a test tube model of “rapid and accelerated biodegradation (RAB)” for studying the degradation of bioplastics (PLA/PBS blends) using accelerated conditions, varying particle size, higher temperature, and enzyme modifiers would be interesting in this regard.
Unlike physical or chemical methods, enzymatic degradation leverages the natural capabilities of microbial enzymes to break down plastics efficiently. Microbial enzymes, capable of using plastics as their sole carbon source, have shown significant potential in accelerating the degradation process compared to traditional methods. Degradation of plastics by microbial enzymes offers a strategy to depolymerise waste petro-plastics into monomers for recycling or to mineralise them into carbon dioxide (CO2), water (H2O), and new biomass while producing higher-value bioproducts.21,24,25 Herein, we developed the bioplastic of PLA/PBS using a simple solution casting method (varying the composition of PLA and PBS, PLA/PBS-80/20, 50/50, 20/80) with acceptable miscibility and the plastic was characterised using spectroscopic and thermal gravimetric techniques. Next, the obtained bioplastic was subjected to rapid accelerated biodegradation (RAB) by varying particle size, at higher temperature via enzymatic hydrolysis by proteinase K from Tritirachium album, lipase acrylic resin, and lipase from Pseudomonas sp.
2. Materials and methods
2.1. Materials
Poly(lactic acid) of PLAX175 grade was purchased from Banka Bioloo Ltd, Hyderabad, India, with a molecular weight of 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 943 g per mole obtained from gel permeation chromatography (GPC). BioPBSTM (FZ79AC) grade poly(butylene succinate) was purchased from Tanhim Enterprise Pvt. Ltd, Greater Noida West, India, possessing a density of 1.26 g cm−3, and a molecular weight of 65
943 g per mole obtained from gel permeation chromatography (GPC). BioPBSTM (FZ79AC) grade poly(butylene succinate) was purchased from Tanhim Enterprise Pvt. Ltd, Greater Noida West, India, possessing a density of 1.26 g cm−3, and a molecular weight of 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 g per mole obtained from GPC. Chloroform was purchased from Merck Millipore. Dimethyl formamide (DMF, HPLC grade, assay > 99.9%) and KBr (>99.9% assay) were purchased from Sigma Aldrich. DSC alumina crucibles (Tzero pans) were purchased from TA Instruments, USA. Proteinase K (600 mAnson-U per mL; lyophilized powder, molecular biology grade) enzyme derived from Tritirachium album, lipase acrylic resin expressed in Aspergillus niger, and lipase from Pseudomonas sp. (type XIII, lyophilized powder) were all purchased from Sigma Aldrich. Co., USA.
840 g per mole obtained from GPC. Chloroform was purchased from Merck Millipore. Dimethyl formamide (DMF, HPLC grade, assay > 99.9%) and KBr (>99.9% assay) were purchased from Sigma Aldrich. DSC alumina crucibles (Tzero pans) were purchased from TA Instruments, USA. Proteinase K (600 mAnson-U per mL; lyophilized powder, molecular biology grade) enzyme derived from Tritirachium album, lipase acrylic resin expressed in Aspergillus niger, and lipase from Pseudomonas sp. (type XIII, lyophilized powder) were all purchased from Sigma Aldrich. Co., USA.
2.2. PLA and PLA/PBS blended bioplastic film preparation
Pristine PLA and a blend of PLA/PBS (80/20, 50/50, 20/80) were taken at 4, 5, 8, 10, and 15 wt% and dissolved in chloroform under continuous stirring for either 2 h or 4 h at 65 °C to obtain miscible solutions.13,26 Next, those miscible solutions were immediately cast on Petri-dishes (diameter 14.5 cm). Following solvent evaporation, the sample was incubated slowly in an incubator overnight at room temperature. The obtained PLA/PBS films were slowly delaminated from the Petri plates from the corner (to avoid cracking and stretching) and dried in an oven at 65 °C for 48 h to remove extra solvent retained in the film matrix. It was observed that, except for the 4 wt% solution, the other weight percentage solutions resulted in hard, uneven and brittle blends. However, the 4 wt% solution under continuous stirring for 4 h at 65 °C formed very flat and smooth films, including for the pristine PLA and blends of PLA/PBS of 80/20, 50/50 and 20/80, as shown in Fig. S1.2.3. Preparation of PLA and blended bioplastics of PLA/PBS powder
Cryogenic grinding was applied to obtain the powder form of the bioplastic blends using liquid nitrogen. Briefly, the prepared PLA film and PLA/PBS blended films were kept under cryogenic conditions by dipping in liquid nitrogen, and the films were immediately subjected to grinding using a BOSCH® mixer (Bosch TrueMixx Radiance Mixer Grinder 600 Watt, MGM4334RIN, Red) until all the films turned into a powder. Liquid nitrogen makes the PLA, PBS and PLA/PBS blends glassy or brittle, thereby helping in the powder formation. The prepared powders were segregated or separated using stainless steel sieves (sieve size range: Indian standard 469/1972, A.S.T.M. mesh number: 60 and 35 with coarse sizes 200 micron and 500 micron, respectively) to obtain microparticles in the range of 200–350 µm and 500–700 µm.2.4. Rapid and accelerated biodegradation (RAB) of PLA and blended PLA/PBS bioplastic
2.5. General characterisation methods
The thickness of the pristine and PLA/PBS blended bioplastics was measured using a KLA Tencor, D-600 model profilometer under room temperature conditions, maintaining a 2 mg stylus force and a 2.5 µm surface range with a 0.05 mm s−1 speed. Fourier transform infra-red spectroscopy (FTIR) analyses of the obtained bioplastic films of PLA, PBS, blended PLA/PBS (80/20, 50/50 and 20/80) and powder samples were analysed using the IR grade KBr pellet method in an IR Prestige-21 Fourier Transform Infrared spectrophotometer, Shimadzu, Japan. Samples were analysed until 50 scans under a frequency range of 400–4000 cm−1. Similarly, degraded pristine PLA, pristine PBS, and PLA/PBS blends were also analysed.Next, scanning electron microscope (SEM) analysis of the bioplastic films and powders was performed by mounting the samples (films and powders) on carbon tape placed on aluminium stubs, which hold the films or powders properly for sputtering and analysis. The samples were made conductive with gold sputtering before imaging in a Nova NanoSEM 450 (NPE206 EDS/WDS) instrument, maintaining 15 kV to observe the clear surface images of the films and powders. Degraded pristine PLA, pristine PBS, and PLA/PBS blends were also characterised to get a clear view of the degraded samples according to the real-time frames.
The films and powders were subjected to X-ray irradiation in the specimen holder in a PanAnalytical PXRD (wide angle) system with a Cu Kα source (λ = 1.5405 Å, 45 kV, 30 mA). The 2θ angles were set in the range of 5°–60°, provided with a 1° per minute scan rate at room temperature conditions for all the samples. Degraded pristine PLA, pristine PBS, and PLA/PBS blends were also diffracted under the same conditions to understand the structural changes.
Thermogravimetry analysis (TGA) of the PLA, PBS, PLA/PBS (80/20), PLA/PBS (50/50), and PLA/PBS (20/80) bioplastic films was carried out using the STA200 Thermal Analysis System (HITACHI Japan). All the samples were placed in an alumina crucible by taking 1–2 mg of each of the samples and the temperature was set from 28°–600 °C at a rate of 10 °C min−1 under inert conditions maintained by N2 gas as the supply gas (100 ml min−1) and protecting gas (100 ml min−1) to observe the TGA degradation curves and phase miscibility and composition.
Differential scanning calorimetry (DSC) was also carried out using the DSC Q20 (TA Instruments, USA) system for all samples to understand the phase miscibility and crystallinity of the blended films. A nitrogen atmosphere was maintained throughout the experiments. Samples were ramped from 40–200 °C with a 20 °C min−1 rate and cooled down with the same rate to avoid any errors or moisture in the sample. Then the ramp was set from 5–200 °C with a 10 °C min−1 rate to produce the data. The glass transition temperatures (Tg), cold crystalline temperatures (Tcc), and melting temperatures (Tm) were observed for all the samples.
Polymer concentrations were maintained at 8 mg mL−1 in dimethyl formamide (DMF) purchased from Sigma Aldrich (HPLC grade, assay > 99.9%) and stirred until they dissolved completely. The solution was later filtered using a Merck filter (porosity 0.45 µm) before being taken for sample injection in the HPLC vials. Samples were subjected to analysis in an Infinity GPC/SEC system from Agilent Technologies, US, with a flow rate of solvent (DMF was stabilised with 0.1% LiCl) of 0.5 mL min−1 and a purge run of 600 s. Columns were heated at 50 °C to run all of the undegraded PLA, PBS and degraded PLA and PBS samples. Number average molecular weight (Mn), weight average molecular weight (Mw), and polydispersity index PD were obtained for the injected samples.
The water contact angle (WCA) of the undegraded and degraded PLA, PBS, PLA/PBS (80/20), PLA/PBS (50/50), and PLA/PBS (20/80) films were observed using the sessile drop method in the Drop Shape Analyser KRUSS instrument, Germany, with the gravitational acceleration of 9.80665 m s−2. WCA for all the samples was observed in the range of 70–100 °C. Degraded pristine PLA, pristine PBS, and PLA/PBS blends were also observed to understand the changes in the nature of the film surfaces after hydrolysis.
2.6. Results and discussion
At the beginning, the miscibility of the PLA/PBS blends of 80/20, 50/50, and 20/80 was deciphered with the help of physicochemical analyses. First, FTIR spectroscopy was employed to understand the interaction between the functional groups in the PLA and PBS, as shown in Fig. 2. Briefly, the absorption at 2922 cm−1 is attributed to the stretching of the C–H bond of the main polymer chain of PLA and PBS. Next, the intense vibrations at 1715 cm−1 are related to the ester carbonyl (–O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the PLA and vibrations at 1328 cm−1 correspond to the elongation vibration of the –COO bond of PLA. Furthermore, 1160 cm−1 is a characteristic C–O–C stretching of the repeating unit (–OCH2CH2) of PBS. As the PBS percentages increase, OH stretching was also increased gradually in PLA/PBS (20/80) due to the availability of free –OH groups in the blend, thereby confirming the interaction between the PLA and PBS chains in the blend.29,35,36 Also, C–O–C stretching was only observed in pristine PBS and not found upon adding PLA to obtain PLA/PBS blends. In PLA/PBS (50/50), the C
O) in the PLA and vibrations at 1328 cm−1 correspond to the elongation vibration of the –COO bond of PLA. Furthermore, 1160 cm−1 is a characteristic C–O–C stretching of the repeating unit (–OCH2CH2) of PBS. As the PBS percentages increase, OH stretching was also increased gradually in PLA/PBS (20/80) due to the availability of free –OH groups in the blend, thereby confirming the interaction between the PLA and PBS chains in the blend.29,35,36 Also, C–O–C stretching was only observed in pristine PBS and not found upon adding PLA to obtain PLA/PBS blends. In PLA/PBS (50/50), the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency was observed with less intensity, unlike other blends, and replicated spectra were observed for PLA/PBS (80/20 and 20/80) (Fig. 2a).
O stretching frequency was observed with less intensity, unlike other blends, and replicated spectra were observed for PLA/PBS (80/20 and 20/80) (Fig. 2a).
 | ||
Fig. 2 The FTIR spectra (a), PXRD pattern (b), TGA curves (c) and Td![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) max trends (d) of the prepared pristine PLA, pristine PBS films and PLA/PBS blended films, respectively. max trends (d) of the prepared pristine PLA, pristine PBS films and PLA/PBS blended films, respectively. | ||
Next, powder XRD (PXRD) was employed to understand the crystallinity of the plastic films (Fig. 2b). In particular, 2θ angles located at 16.8°, 19.3°, and 22.8° are attributed to pristine PLA according to a previous report.12,33,37 Furthermore, three peaks located at 19.7°, 21.7°, and 22.8° are related to the pristine PBS with the (020), (021), and (110) planes, respectively, as reported earlier.29 Upon obtaining the PLA/PBS blends, the resulting films of PLA/PBS (80/20) possess similar crystallinity to the pristine PLA due to a higher percentage of PLA compared to PBS. However, the PLA/PBS films (50/50 and 20/80) showed the characteristic peaks for both PLA and PBS because of the successful formation of the blended plastic films, which were not present in the pristine PLA or PBS films. Hence, the PLA/PBS (50/50) and PLA/PBS (20/80) blends showed higher miscibility than the PLA/PBS (80/20) blend. Furthermore, thermogravimetric analysis (TGA/DTG, Fig. 2c and d) data show that both the pristine films and PLA/PBS blends show a single degradation curve, confirming successful blend formation between PLA and PBS.29 This is not commonly observed for the blended plastic obtained using mechanical and other film-forming methods.12,33,38 However, PLA/PBS (50/50) showed minor deviation as observed in the DTG plot (shown in Fig. S3, SI). Importantly, the PLA/PBS blends were found to have lower Td-max temperature compared to the pristine PLA or PBS films, confirming that the PLA/PBS blends have less thermal stability than the pristine films of PLA and PBS, which may result in higher degradability of the blends over the pristine films.28,39 Overall, the FTIR, PXRD and TGA results are in agreement with blend formation between PLA and PBS.
Next, DSC was also employed to further confirm the miscibility of PLA and PBS in the obtained blend plastic films by finding the glass transition temperature (Tg), melting temperature (Tm), and cold crystalline temperature (Tcc) of the pristine polymers and blends (Fig. 3a). From the DSC plots, it is observed that the Tm of all the PLA/PBS blends is reduced compared to the pristine films (PLA or PBS), except PLA/PBS (80/20), since it shows two Tm at 110 °C and 147 °C, suggesting the immiscibility of PLA and PBS. However, the PLA/PBS (50/50) and PLA/PBS (20/80) blends show only one Tm at 110 °C, confirming the impressive phase miscibility between PLA and PBS at these two particular ratios, and also suggesting the uniform crystallinity in the blended films in comparison with reported works with other blending techniques.39,40 Furthermore, water contact angle (WCA) measurements (Fig. 3b) reveal that the surface hydrophilicity decreased as the percentage of PBS was increased in the blends, which could be attributed to the presence of more ester groups in PBS that make the blend more hydrophobic.29,35,41 Hence, PLA/PBS blends could be fascinating for packaging applications. Finally, SEM analysis was used to understand the morphology of the obtained blended films of PLA/PBS (Fig. 3c–f). First, pristine PLA films were found to have a smooth and shielded surface with spherulite-like morphology.37 However, the PLA/PBS blend films show a rough morphology with cavities, and these features increase as the percentage of PBS increases in the blends, starting from 80/20 to 20/80 of PLA/PBS.33,41 In addition, the blended films of PLA/PBS were found to have smoother and denser morphology than those obtained using other methods, including a melt compounding process.13,29,37
2.6.2.1. RAB study of PLA/PBS blended bioplastic films and powders using mobilized proteinase K. RAB is one of the in vitro controlled real-time degradation experiments with an accelerated condition to anticipate the maximum extent of degradation of synthetic biopolymers and their blends.28,35,43,44 Weight loss analysis of the samples was carried out through the difference in mass before and after treatment to evaluate the degradation of the film samples under RAB conditions. It was found that the PLA/PBS (50/50) blend has the highest weight loss after 7 days of treatment compared to other blends and pristine PLA (Fig. 5a), which was also supported by observing the complete breakage of the blend film into powder-like particles (Fig. 4b and Fig. S4a). The highest degradation of the PLA/PBS (50/50) blend film could be because of the uneven surface with a porous nature (SEM images, Fig. 3d). Therefore, the porous nature of the PLA/PBS (50/50) films is more likely to cause the highest degradation, thereby leading to weight loss. On the other side, 7 days of treatment with microbial enzyme caused the pristine PLA film and PLA/PBS (80/20 and 20/80) blends to be brittle and broken into large pieces (especially PLA/PBS-80/20, Fig. 4b and Fig. S4a). Next, weight loss measured after 12 h, 3 days and 7 days of treatment (Fig. 5a) for the pristine PLA film was very low, which could be attributed to the PLA film's smoothness and higher Tm compared to the PLA/PBS blends.45,46 This is more evident for the PLA/PBS blend (50/50), having the lowest Tm as per DSC plots and rough surfaces (Fig. 3d).46 In the case of powder samples (size 500–700 µm, Fig. 5b) degradation under RAB conditions using proteinase K, the PLA/PBS-80/20 blend powder underwent the highest degradation compared to the other blends and PLA. This could be attributed to the higher surface area of the microparticles. Similar degradation of powder samples of PLA/PBS blend particles (size 200–355 µm, Fig. 5c) was found to occur, where degradability was reduced as the percentage of PBS was decreased in the blend. Overall, the blend powder samples with 200–355 µm particles were degraded slightly more than those with particles of 500–700 µm. This could be due to the higher surface area available for 200–355 µm particles over 500–700 µm particles. Next, GPC was performed to estimate the molecular weight loss of the PLA plastic films and powders after treatment with proteinase K for 7 days, where the analysis was not possible for the blends, done only for the pristine PLA films and powder samples (Fig. 5d–f). The results confirmed that the total chain degradation or hydrolysis was ∼47% within the first 12 h of treatment, and reached nearly saturation (49%) by 72 h for PLA in the film (Fig. 5d and Table S3). Notably, chain loss or degradation for the PLA powder samples (for both 500–700 µm particles and 200–355 µm particles, Fig. 5e and f, and Table S4) was gradually increased until 7 days, unlike in the film samples, where the chain loss was saturated after 3 days. Also, GPC confirmed that the 500–700 µm range PLA powder samples were degraded to 46% and the 200–355 µm range PLA powder degraded to 43% by 7 days. Here, the larger particles (500–700 µm) were degraded slightly more than the smaller range particles (200–355 µm). This is possibly due to variation in the shape and crystallinity of the microparticles, which might have been induced by cryogenic grinding or due to the uneven enzyme distribution in the specific experiments. The mechanism of enzymatic degradation is shown in Scheme S1.
Next, FTIR analysis was performed to understand the structural changes or functional groups of the PLA/PBS blends after treating with proteinase K (Fig. 6) for 7 days. First, a drastic reduction in the C–H stretching and ester–O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequencies was observed for the degraded pristine PLA film (named PLA-D, Fig. 6a), which confirms the cleavage of the ester linkage in the polymeric chain.42,45 Next, FTIR analysis for the degraded PLA/PBS blends (labelled as PLA/PBS-D) shows the presence of broad vibrations at ∼3300 to 3500 cm−1, which might correspond to the presence of free –OH groups, which were formed after the enzymatic hydrolysis of PLA alone (Fig. S4, SI). However, these broad bands were absent in the undegraded PLA/PBS (untreated at 0 h sample). Similarly, in the case of degraded PLA/PBS (20/80), broadening of –OH and shifting of the C
O stretching frequencies was observed for the degraded pristine PLA film (named PLA-D, Fig. 6a), which confirms the cleavage of the ester linkage in the polymeric chain.42,45 Next, FTIR analysis for the degraded PLA/PBS blends (labelled as PLA/PBS-D) shows the presence of broad vibrations at ∼3300 to 3500 cm−1, which might correspond to the presence of free –OH groups, which were formed after the enzymatic hydrolysis of PLA alone (Fig. S4, SI). However, these broad bands were absent in the undegraded PLA/PBS (untreated at 0 h sample). Similarly, in the case of degraded PLA/PBS (20/80), broadening of –OH and shifting of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O frequencies support that the PLA/PBS blends underwent enzymatic hydrolysis.29,33,44,46 Similar changes were observed for the powder samples of PLA and the PLA/PBS blends after treating with protease K for 7 days (Fig. 6b and Fig. S5a), confirming the enzymatic hydrolysis of PLA in the PLA/PBS blend powder samples.
O frequencies support that the PLA/PBS blends underwent enzymatic hydrolysis.29,33,44,46 Similar changes were observed for the powder samples of PLA and the PLA/PBS blends after treating with protease K for 7 days (Fig. 6b and Fig. S5a), confirming the enzymatic hydrolysis of PLA in the PLA/PBS blend powder samples.
To support this, PXRD was also employed, where the PXRD results show no change in the crystallinity of the PLA film and PLA powder samples before and after degradation for 7 days (Fig. S5b).33 However, there was a change in the crystallinity only for PLA/PBS (50/50) blend samples after enzymatic treatment for 7 days (Fig. S5b–f). However, PLA/PBS (50/50) shows some splitting or shoulder peak at 19.8°, which might be due to the structural changes in the blend after 7 days (Fig. S5e). The crystallinity changes observed mainly for the PLA/PBS (50/50) blend could be attributed to its lowest melting temperature (Tm, Fig. 3a) and having a highly rough surface morphology compared to the other blends (Fig. 3d–f).34 Furthermore, SEM and OM images also supported the degradation of the film samples of the PLA and PLS/PBS blends after up to 7 days of enzymatic treatment (Fig. 7 and Fig. S6, S7). Optical images demonstrate that broken films were observed in the PLA and PLS/PBS blend film samples, suggesting that degradation had occurred (Fig. 7a–d).
In addition, more porous and damaged areas in the films of PLA and the PLA/PBS blends were observed in the SEM images (Fig. 7e–h). However, such significant morphological changes were not observed for the powder samples of PLA and the PLA/PBS blends, which could be due to the uneven surface morphology with an irregular shape, making it challenging to identify the reduction of size and morphology of the powder particles (Fig. S8). Furthermore, water contact angle (WCA) measurements were performed, and the results confirmed that the blended films were flat and flexible in the beginning, i.e. before biodegradation (Fig. 4b). However, the PLA films and PLA/PBS blend films became brittle and curvy (except PLA/PBS-50/50) after enzymatic treatment, and therefore we could not measure the WCA of the blends (Fig. S9a–e). The WCA of the PLA/PBS (50/50) film was reduced to 31% after 12 h of degradation (Fig. S9c), resulting in an increase in hydrophilicity by 12 h of degradation, which further supports the degradation of PLA/PBS (50/50) occurring to a greater extent than the other blended films.35 Therefore, such a PLA/PBS (50/50) blend could be interesting with higher biodegradability over the other blends.
2.6.2.2. Degradation of pristine PLA using lipase immobilised in acrylic resin in an organic solvent. Next, the impact of organic solvents on the enzymatic degradation of PLA was studied using toluene as the solvent under RAB conditions.22,47 The results confirmed that the prepared PLA film did not dissolve completely throughout incubation, and the resulting film was recovered for GPC analysis. The molecular weight loss after 72 h was found to be only 12.45%, which is nearly 4 times less than that in the proteinase K-mobilised enzyme treated under RAB conditions (Table S6). The plausible reason could be that immobilised enzymes might be unable to bind to the PLA films or have low solubility in toluene, resulting in a lower percentage of degradation.48 As the PLA film alone degraded poorly in the organic solvent, the PLA/PBS blends were not studied under such conditions.
2.6.2.3. Degradation of pristine PBS and PLA/PBS blends using lipase. As proteinase K targets the PLA chain in the PLA/PBS blend plastic, lipase from Pseudomonas sp. can degrade the PBS chain in the PLA/PBS blends.30–32 Hence, we attempted to understand the degradation of PBS in pristine PBS films and PLA/PBS blend films by treating them with lipase under RAB conditions (Fig. 4). PBS or PLA/PBS blend films were incubated in 0.1 M phosphate buffer at pH 7.2 (Fig. 8a and b).29 It was found that PLA/PBS (80/20) films degraded to a larger extent than pristine PBS and PLA/PBS films (50/50 and 20/80, Fig. 8c) after 7 days. However, pristine PBS, PLA/PBS (50/50) and PLA/PBS (20/80) showed similar degradation after 7 days. PLA/PBS (80/20) degraded up to ∼28% on the 7th day of study, which may be attributed to the Tm data of the blend. PLA/PBS (20/80) degraded to a higher extent on the 3rd day of study, as it has a higher percentage of PBS in it.
Next, FTIR spectra of the PBS and PLA/PBS blends were analysed to understand their enzymatic hydrolysis. First, the degraded PBS film showed a broadened –OH stretching frequency and shifting of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O frequency from ∼1725–1715 cm−1 to ∼1620–1633 cm−1 (Fig. 8d), which could be due to the hydrolysis of the ester group to generate free COOH groups and the C–H stretching frequency also vanished after degradation.29
O frequency from ∼1725–1715 cm−1 to ∼1620–1633 cm−1 (Fig. 8d), which could be due to the hydrolysis of the ester group to generate free COOH groups and the C–H stretching frequency also vanished after degradation.29
Additionally, optical images of the degraded samples revealed that the morphology of the films was not changed much except for the PLA/PBS (50/50) blend after treatment for 7 days (Fig. 8e–h), where the PLA/PBS (50/50) film was found to be broken with a porous nature after the degradation. In support of this, SEM analysis of the PLA/PBS (50/50) blend suggested that the film was broken down into smaller films and had several pores (Fig. 8i and j). Subsequently, it turned entirely to microparticles after 1 minute of sonication (Fig. 8k). The highest degradation found in the PLA/PBS (50/50) blend film compared to other blends could be attributed to the film's rough morphology, arrangement of the two polymers in the matrix, and crystallinity of the mixed blend (Tm plot) compared to other films.34,43
3. Conclusion
First, we developed a simple solution casting method for preparing a PLA/PBS blended biodegradable bioplastic with high miscibility (especially for a 50/50 and 20/80 ratio) based on thermal gravimetric (DSC and TGA) and electron microscopy analyses. Other physicochemical characterisation using FTIR, PXRD, including water contact angle measurements, and optical microscopy results also confirmed the successful preparation of the blended bioplastic of PLA/PBS. We believe that the developed solution casting method could be applied to make miscible bioplastic blends of PLA/PBS, which have many biomedical and packaging applications. Second, we developed an efficient test-tube method to assess the biodegradability of the obtained PLA/PBS bioplastic blends in the form of films and powders by treating with appropriate microbial enzymes (proteinase K and lipase) under rapid accelerated biodegradation (RAB) conditions (pH, high temperature incubation, microparticles and organic solvent). Overall, the PLA/PBS (50/50) blended bioplastic films degraded to a greater extent, 24.2% within 7 days with proteinase K in aqueous medium, compared to the other PLA/PBS blends. Furthermore, the powder forms of the pristine PLA and PLA/PBS blended bioplastic underwent RAB by proteinase K, and it was found that surface area plays a vital role in enhancing the biodegradation. In addition, the pristine PLA film could not be degraded significantly using immobilised (acrylic resin) lipase in a non-aqueous medium, which deciphered the advantages of aqueous medium for better biodegradability of bioplastic blends. Finally, we examined the biodegradation of PBS and PLA/PBS blends by lipase (Pseudomonas sp.) under RAB conditions. The results confirm that PLA/PBS (80/20) degraded faster than the PLA/PBS (20/80) and PLA/PBS (50/50) blends and pristine PBS. Overall, our test-tube model RAB studies demonstrated that the PLA/PBS blend bioplastic films showed faster degradation (within 7 days) compared to the blends obtained from other techniques (melt blending, mechanical extrusion, etc.). The test-tube model RAB degradation could be applied for understanding the rapid accelerated biodegradation of other bioplastic blends by simply treating them with appropriate microbial enzymes under accelerated conditions (pH, temperature, size of the plastic, solvent, etc.). Our RAB model could be a simple test-tube model to predict the biodegradability of the blended bioplastic by incubating with the selected microbial enzyme (e.g., isolated from microbes present in the soil). Also, the RAB model could be used to screen and compare newly developed blended or biodegradable bioplastics in a shorter time than the standardised biodegradation tests. However, correlating the accelerated or rapid biodegradation of bioplastic with real-time or practical degradation is difficult. Since practical or environmental degradation conditions will be very different from the accelerated or RAB model, the biodegradation of the bioplastic will be studied.Author contributions
Shreetam Parida: experimental, methodology, characterisations, data analyses and original draft; Nivethitha Ashok: experimental and formal analysis; Dr Rajendra Kurapati: project conceptualisation, methodology, supervision and draft revision.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ma00690b.Any additional data required for replication or further inquiry can be obtained upon request.
Acknowledgements
The authors are thankful to the Department of Biotechnology (DBT), Govt of India, for supporting and funding the project with reference number “BT/PR48101/BCE/8/1808/2023”. The authors acknowledge IISER Thiruvananthapuram, Kerala, India, for providing the necessary laboratory and characterisation facilities. They are also thankful to Dr Bhoje Gowd E., CSIR-NIIST, Thiruvananthapuram, Polymer Science division, for water contact angle measurements and necessary suggestions.Notes and references
- K. J. Groh, T. Backhaus, B. Carney-Almroth, B. Geueke, P. A. Inostroza, A. Lennquist, H. A. Leslie, M. Maffini, D. Slunge, L. Trasande, A. M. Warhurst and J. Muncke, Sci. Total Environ., 2019, 651, 3253–3268 CrossRef CAS PubMed.
- W.-H. Hsu, Y.-Z. Chen, Y.-T. Chiang, Y.-T. Chang, Y.-W. Wang, K.-T. Hsu, Y.-Y. Hsu, P.-T. Wu and B.-H. Lee, Nat. Commun., 2025, 16, 5026 CrossRef CAS PubMed.
- P. Shreetam, A. Nivethitha and K. Rajendra, Ann. Biomed. Sci. Eng., 2024, 8, 004–010 CrossRef.
- M. Mangal, C. V. Rao and T. Banerjee, Polym. Int., 2023, 72, 984–996 CrossRef CAS.
- M. Nofar, D. Sacligil, P. J. Carreau, M. R. Kamal and M. C. Heuzey, Int. J. Biol. Macromol., 2019, 125, 307–360 CrossRef CAS PubMed.
- N.-A. Taib, M. Rahman, D. Huda, K. Kuok, S. Hamdan, M. K. Bakri, M. Julaihi and A. Khan, Polym. Bull., 2022, 1, 1–35 Search PubMed.
- BIOPLASTICS MARKET UPDATE 2021 | European Bioplastics, 2021, https://docs.european-bioplastics.org/publications/market_data/Report_Bioplastics_Market_Data_2021_short_version.pdf.
- Z. H. W. Easton, M. A. J. Essink, L. Rodriguez Comas, F. R. Wurm and H. Gojzewski, Macromol. Chem. Phys., 2023, 224, 2200421 CrossRef CAS.
- H. Cai, C. Cao, Y. Zheng, D. Liu, X. Xia, X. Sun, X. Liu, L. Xiao, Q. Qian and Q. Chen, Macromol. Mater. Eng., 2023, 308, 2200581 CrossRef CAS.
- E. C. Van Roijen and S. A. Miller, Resour., Conserv. Recycl., 2022, 181, 106236 CrossRef CAS.
- N. C. Giri, V. Verma and B. P. Patro, in Assessing the Effects of Emerging Plastics on the Environment and Public Health, ed. S. H. Joo, IGI Global, Hershey, PA, USA, 2022, pp. 249–283 DOI:10.4018/978-1-7998-9723-1.ch011.
- R. Wang, S. Wang, Y. Zhang, C. Wan and P. Ma, Polym. Eng. Sci., 2009, 49, 26–33 CrossRef CAS.
- T. Zhao, J. Yu, X. Zhang, W. Han, S. Zhang, H. Pan, Q. Zhang, X. Yu, J. Bian and H. Zhang, Polym. Bull., 2023, 81, 1–24 Search PubMed.
- W. Pivsa-Art, S. Pivsa-Art, K. Fujii, K. Nomura, K. Ishimoto, Y. Aso, H. Yamane and H. Ohara, J. Appl. Polym. Sci., 2015, 132, 41856 CrossRef.
- K. Shi, Z. Bai, T. Su and Z. Wang, Int. J. Biol. Macromol., 2019, 126, 436–442 CrossRef CAS PubMed.
- L. Filiciotto and G. Rothenberg, ChemSusChem, 2021, 14, 56–72 CrossRef CAS PubMed.
- A. Folino, D. Pangallo and P. S. Calabrò, J. Environ. Chem. Eng., 2023, 11, 109424 CrossRef CAS.
- J.-G. Rosenboom, R. Langer and G. Traverso, Nat. Rev. Mater., 2022, 7, 117–137 CrossRef PubMed.
- S. Andréia da Silva, D. J. L. Faccin and N. S. M. Cardozo, ACS Sustainable Chem. Eng., 2024, 12, 11856–11865 CrossRef.
- J. Šerá, L. Serbruyns, B. De Wilde and M. Koutný, Polym. Degrad. Stab., 2020, 171, 109031 CrossRef.
- S. Chinaglia, M. Tosin and F. Degli-Innocenti, Polym. Degrad. Stab., 2018, 147, 237–244 CrossRef CAS.
- M. H. Aris, M. S. M. Annuar and T. C. Ling, Polym. Degrad. Stab., 2016, 133, 182–191 CrossRef CAS.
- A. N. Mistry, B. Kachenchart, A. Wongthanaroj, A. Somwangthanaroj and E. Luepromchai, Polym. Degrad. Stab., 2022, 202, 110051 CrossRef CAS.
- Z. Montazer, M. B. Habibi Najafi and D. B. Levin, Can. J. Microbiol., 2019, 65, 224–234 CrossRef CAS PubMed.
- J. Kaushal, M. Khatri and S. K. Arya, Cleaner Eng. Technol., 2021, 2, 100083 CrossRef.
- H. Jeong, J. Rho, J.-Y. Shin, D. Y. Lee, T. Hwang and K. J. Kim, Biomed. Eng. Lett., 2018, 8, 267–272 CrossRef PubMed.
- S. Grima, V. Bellon-Maurel, P. Feuilloley and F. J. Silvestre, J. Polym. Environ., 2000, 8, 183–195 CrossRef CAS.
- Y. Kikkawa, H. Abe, T. Iwata, Y. Inoue and Y. Doi, Biomacromolecules, 2002, 3, 350–356 CrossRef CAS PubMed.
- X. Hu, T. Su, P. Li and Z. Wang, Polym. Bull., 2018, 75, 533–546 CrossRef CAS.
- K. Mukai, Y. Doi, Y. Sema and K. Tomita, Biotechnol. Lett., 1993, 15, 601–604 CrossRef CAS.
- Y. Tokiwa and T. Suzuki, Nature, 1977, 270, 76–78 CrossRef CAS PubMed.
- C. W. Lee, Y. Kimura and J.-D. J. M. R. Chung, Macromol. Res., 2008, 16, 651–658 CrossRef CAS.
- F.-L. Chang, B. Hu, W.-T. Huang, L. Chen, X.-C. Yin, X.-W. Cao and G.-J. He, Polymer, 2022, 259, 125336 CrossRef CAS.
- H. Cai, V. Davé, R. A. Gross and S. P. Mccarthy, J. Polym. Sci., Part B: Polym. Phys., 1996, 34, 2701–2708 CrossRef CAS.
- S. Li and S. McCarthy, Macromolecules, 1999, 32, 4454–4456 CrossRef CAS.
- A. Gowman, T. Wang, A. Rodriguez-Uribe, A. K. Mohanty and M. Misra, ACS Omega, 2018, 3, 15205–15216 CrossRef CAS PubMed.
- J. W. Park and S. S. Im, J. Appl. Polym. Sci., 2002, 86, 647–655 CrossRef CAS.
- E. Hassan, Y. Wei, H. Jiao and Y. Muhuo, J. Fibre Bioeng. Inf., 2013, 6, 85–94 CrossRef.
- B. Ahn, S. Kim, Y. Kim and J. Yang, J. Appl. Polym. Sci., 2001, 82, 2808–2826 CrossRef CAS.
- T. Qiu, M. Song, L. Zhao and M. Processes, Mech. Adv. Mater., 2016, 2, 7 Search PubMed.
- W. Yu, L. Sun, M. Li, M. Li, W. Lei and C. Wei, Polymers, 2023, 15, 4305 CrossRef CAS PubMed.
- R.-S. Rose, K. H. Richardson, E. J. Latvanen, C. A. Hanson, M. Resmini and I. A. Sanders, Int. J. Mol. Sci., 2020, 21, 1176 CrossRef CAS PubMed.
- Y. Tokiwa and B. P. Calabia, Biotechnol. Lett., 2004, 26, 1181–1189 CrossRef CAS PubMed.
- A. Rosato, A. Romano, G. Totaro, A. Celli, F. Fava, G. Zanaroli and L. J. P. Sisti, Polymers, 2022, 14, 1850 CrossRef CAS PubMed.
- H. Fukuzaki, M. Yoshida, M. Asano and M. Kumakura, Eur. Polym. J., 1989, 25, 1019–1026 CrossRef CAS.
- M. S. Reeve, S. P. McCarthy, M. J. Downey and R. A. J. M. Gross, Macromolecules, 1994, 27, 825–831 CrossRef CAS.
- C. DelRe, Y. Jiang, P. Kang, J. Kwon, A. Hall, I. Jayapurna, Z. Ruan, L. Ma, K. Zolkin, T. Li, C. D. Scown, R. O. Ritchie, T. P. Russell and T. Xu, Nature, 2021, 592, 558–563 CrossRef CAS PubMed.
- D. Edith and J.-L. Six, J. Appl. Surf. Sci., 2006, 253, 2758–2764 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |