Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Engineering of two-in-one Fe@Eu nanoparticles through hydrothermal synthesis: bimetallic hybrids for theranostic applications
Evangelia
Tsitsou
 a,
Danai
Prokopiou
a,
Danai
Prokopiou
 a,
Athina
Papadopoulou
a,
Athina
Papadopoulou
 a,
Alexandros K.
Bikogiannakis
b,
Georgios
Kyriakou
a,
Alexandros K.
Bikogiannakis
b,
Georgios
Kyriakou
 b,
Elias
Sakellis
cd,
Nikos
Boukos
c,
Marios
Kostakis
b,
Elias
Sakellis
cd,
Nikos
Boukos
c,
Marios
Kostakis
 e,
Nikolaos S.
Thomaidis
e,
Nikolaos S.
Thomaidis
 e and
Eleni K.
Efthimiadou
e and
Eleni K.
Efthimiadou
 *a
*a
aInorganic Chemistry Laboratory, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis, Zografou 157 71, Greece. E-mail: efthim@chem.uoa.gr
bDepartment of Chemical Engineering, University of Patras, Caratheodory 1, Patras, GR 265 04, Greece
cInstitute of Nanoscience and Nanotechnology, NCSR “Demokritos”, Aghia Paraskevi Attikis, 153 41, Greece
dSection of Condensed Matter Physics, Department of Physics, National and Kapodistrian University of Athens, Athens, 157 84, Greece
eLaboratory of Analytical Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis, Zografou 157 71, Greece
First published on 3rd September 2025
Abstract
This study focuses on the synthesis of bimetallic Fe@Eu hybrid nanoparticles (NPs), combining structural and morphological characterization with an in vitro biological evaluation. By merging the superparamagnetic behavior of iron oxide nanoparticles (IONPs) with the distinctive optical properties of europium, these hybrid NPs emerge as strong candidates for a range of biomedical applications, particularly in cancer imaging and therapy. Magnetic IONPs were synthesized via co-precipitation and surface-modified with citrate to enhance colloidal stability. Europium was then introduced at varying Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu molar ratios (1
Eu molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25). Structural and morphological characterization confirmed the successful fabrication of the hybrids. DLS analysis demonstrated the excellent colloidal stability required for biomedical deployment. FT-IR, pXRD, and XPS verified the formation of magnetite and the successful incorporation of europium, which appeared as europium hydroxide nanorods. TEM elemental mapping further confirmed the co-existence of iron and europium within the same nanostructures. PL measurements revealed dual fluorescence capabilities, corroborated by widefield optical fluorescence microscopy, reinforcing their potential in multimodal imaging. In vitro studies showed efficient cellular internalization with minimal cytotoxicity. Mechanistic insights pointed to mild cell cycle disruption, moderate ROS generation, and apoptosis induction as part of the NPs’ biological activity.
0.25). Structural and morphological characterization confirmed the successful fabrication of the hybrids. DLS analysis demonstrated the excellent colloidal stability required for biomedical deployment. FT-IR, pXRD, and XPS verified the formation of magnetite and the successful incorporation of europium, which appeared as europium hydroxide nanorods. TEM elemental mapping further confirmed the co-existence of iron and europium within the same nanostructures. PL measurements revealed dual fluorescence capabilities, corroborated by widefield optical fluorescence microscopy, reinforcing their potential in multimodal imaging. In vitro studies showed efficient cellular internalization with minimal cytotoxicity. Mechanistic insights pointed to mild cell cycle disruption, moderate ROS generation, and apoptosis induction as part of the NPs’ biological activity.
Introduction
Metal-based nanomaterials have gained particular interest in recent years, as they possess chemical and optical properties that make them ideal for use in a variety of applications,1 such as photonics, sensors, catalysis, and biomedicine.2 Divalent and trivalent metal ions from almost all metal salts are used as starting materials, which are reduced to the desired metallic nanoparticles (NPs) with appropriate reducing agents.3 Among different metallic ions, iron emerges as an element of major importance due to its biocompatibility and essential role in biological systems, making it highly advantageous for the development of functional NPs.4,5 At the nanoscale, iron is used in its oxide forms. These iron oxide nanoparticles (IONPs) exhibit the so-called “quantum size effect”, whereby their optical, magnetic, and electrical properties are influenced by interactions at the level of individual atoms or molecules, rather than by bulk-averaged quantum effects.6,7Surface modification on IONPs is carried out using various organic, inorganic or polymeric materials activating numerous properties and leading to a plethora of applications. Incorporation of bioactive molecules, such as nucleotides, proteins, antibodies, and drugs, makes IONPs particularly useful in the biomedical field.7,8 Magnetic NPs have been studied for biomedical applications since the 1990s, while IONPs have already been approved by the Food and Drug Administration (FDA) for use in medicine and food.9 Regardless of the type of application, IONPs possess properties that enhance their effectiveness, such as superparamagnetic nature with reduced size, small size, increased surface area to volume ratio, excellent dispersibility in solutions, reduced susceptibility to oxidation, magnetic stability, relatively easy synthesis, ease of surface modification, long blood retention time, biodegradability, and low toxicity.7,8,10,11 For instance, magnetic NPs as contrast agents present some positive characteristics, in contrast to conventional agents, such as shorter waiting time until examination, shorter duration of residence of the agent inside the patient's body (while reducing its side effects), and better detection of various tissue types, since they improve the overall image quality of the body's cells.10,12,13 Depending on the size of the IONPs and the material used as a coating, they also target a different site for imaging. There are IONPs that have been approved for clinical use as magnetic resonance imaging (MRI) contrast agents; however, several of them have been already discontinued.9,12
When NPs consist of two different metals, they are called bimetallic. Bimetallic NPs (BNPs) are expected to have properties that are a combination of the properties of the two constituent metals, while they may also exhibit new properties that are a result of the synergy of the two metals. Their properties naturally differ from those of the pure elements, with the optical, electronic, thermal, and catalytic properties largely depending on the size of the formed NPs.14,15 BNPs are an interesting field of research, as they add an additional degree of freedom due to the two different metals that compose them, resulting in different properties and, therefore, additional applications.14,16–18 Nanomaterials doped with rare earth elements exhibit some exceptional chemical and optical characteristics, such as high photofluorescence intensity, long fluorescence lifetime, emission from the visible to near-ultraviolet spectrum, high stability against photochemical degradation, sharp emission peaks with a narrow bandwidth, constant light intensity, and low toxicity.19–22 For the synthesis of these BNPs, two important features must be taken into account. First, lanthanide ions are characterized by a small molecular absorption cofactor, so that direct excitation does not always lead to strong fluorescence. This is corrected by the use of organic substituents (chromophores), which absorb light and transfer the energy to the excited states of the ions, which in turn exhibit fluorescence with a large Stokes shift. Second, since IONPs are known to be strong luminescence quenchers, it is necessary to coat the IONPs with a layer that suppresses the occurrence of this phenomenon. This coating also contributes to the immobilization of the lanthanides, improved chemical reactivity, and better colloidal stability of the NPs.23–26
Europium-doped iron oxide NPs (Eu-IONPs) have emerged as promising candidates, combining the superparamagnetic properties of iron oxides with the distinctive luminescent characteristics of europium ions. This dual functionality enables their use in multimodal imaging techniques, such as MRI and fluorescence imaging, enhancing diagnostic accuracy and therapeutic monitoring. Recent studies have focused on the synthesis and characterization of Eu-IONPs to optimize their physicochemical properties for biological applications.27–33 For instance, Zhang et al.31 reported the synthesis of biocompatible superparamagnetic Eu-IONP clusters using a straightforward one-pot method. These NPs exhibited excellent water solubility, colloidal stability, and enhanced T1-weighted MRI contrast, demonstrating their potential as multifunctional nanoprobes for in vivo imaging. Similarly, Costa et al.32 developed hybrid magneto-luminescent iron oxide nanocubes functionalized with europium complexes. Their comprehensive evaluation of hemolytic properties and protein corona formation provided valuable insights into the biocompatibility and surface interactions of these NPs, which are crucial for their safe application in nanobiotechnology. Furthermore, Lunin et al.33 introduced spindle-like Eu-IONPs synthesized through a facile ferrihydrite crystallization procedure. These NPs not only enhanced MRI contrast, but also exhibited shape-induced cytotoxicity, highlighting the influence of NP morphology on biological interactions and therapeutic potential. Two more groups, de Espindola et al.34 and Prasad et al.,35 highlighted the dual functionality of their NPs as a key feature enabling their potential applications. The combination of magnetic and optical properties allows for multi-modal imaging and the possibility of simultaneous diagnosis and therapy (theranostics).
Building upon these advancements, our study aims to synthesize bimetallic Fe@Eu NPs with varying Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu ratios using the co-precipitation method. Structural, morphological, colloidal, and fluorescence properties were systematically characterized. In vitro assays, including cytotoxicity and cellular internalization, were performed to evaluate bioactivity. The synergistic interplay of iron and europium was shown to enhance multifunctionality, highlighting Fe@Eu NPs as promising platforms for biomedical imaging and therapeutic applications.
Eu ratios using the co-precipitation method. Structural, morphological, colloidal, and fluorescence properties were systematically characterized. In vitro assays, including cytotoxicity and cellular internalization, were performed to evaluate bioactivity. The synergistic interplay of iron and europium was shown to enhance multifunctionality, highlighting Fe@Eu NPs as promising platforms for biomedical imaging and therapeutic applications.
Experimental
Materials
Ferrous chloride (FeCl2·4H2O) and ferric chloride (FeCl3·6H2O) were purchased from Acros Organics. Ammonia solution (NH4OH 30%; aquatic, Mr = 17.03 g mol−1) and europium(III) nitrate pentahydrate (Eu(NO3)3·5H2O; Mr = 428.05 g mol−1) were purchased from Sigma-Aldrich. Tri-sodium citrate (Na3C6H5O7·2H2O; Mr = 294.10 g mol−1, d = 1.76 g cm−3) was obtained from Fischer Scientific. Ethylene glycol (C2H5O2; Mr = 62.07 g mol−1, d = 1.11 kg L−1) was obtained from Merck. Nitric acid (HNO3, 65% G.R.; Mr = 63.01 g mol−1, d = 1.4 kg L−1) was purchased from Lach-Ner, s.r.o. High glucose DMEM (Dulbecco's Modified Eagle's Medium, with L-glutamine, with sodium pyruvate, sterile filtered), penicillin–streptomycin solution 100× (sterile filtered), L-glutamine 100× 200 mM (sterile filtered), and trypsin-EDTA 1× in solution (without Ca, without Mg, with phenol red, sterile filtered) were purchased from Biosera. DPBS (phosphate-buffered saline, without Ca, without Mg, sterile filtered) and FBS Good (fetal bovine serum) were obtained by PAN Biotech. MTT (3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide) and PI (propidium iodide) were acquired from Cayman Chemical. RNAse A was obtained from abmGood. DMSO (dimethyl sulfoxide) was obtained from Fisher Chemical. DNR (daunorubicin hydrochloride) was provided by Pharmacia & Upjohn. Ethanol euro denatured 99% was purchased from VWR Chemicals. H2DCFDA (2′,7′-dichlorodihydrofluorescein diacetate) was obtained from Thermo Fisher Scientific. Annexin V binding buffer 10×, Annexin V/FITC (fluorescein isothiocyanate), and 7-AAD (7-aminoactinomycin D) were acquired from BD Biosciences.Instrumentation
Dynamic light scattering (DLS) measurements of the samples were performed on a Malvern Instruments Zetasizer Nano Series with a multipurpose titrator. In the data presented in this study, each value represents the average value of 3 measurements, with 20 runs per measurement. The Fourier transform infrared (FT-IR) spectra of the samples were obtained using a Shimadzu IR Affinity-1 (QATR 10 single reflection ATR accessory) spectrometer and the spectra were scanned over the range 4000–400 cm−1. The ultraviolet-visible (UV-Vis) absorption spectra in the wavelength range of 200–600 nm were obtained on a Shimadzu UV-2600i UV-Vis spectrophotometer. For the transmission electron microscopy (TEM) analysis, the images were taken using an FEI Talos F200i field-emission (scanning) transmission electron microscope (Thermo Fisher Scientific Inc., Waltham, MA, USA), operating at 200 kV and equipped with a windowless energy-dispersive spectroscopy microanalyzer (6T/100 Bruker, Hamburg, Germany). Photoluminescence (PL) spectra in the excitation/emission wavelength range of 300–900 were obtained using a Shimadzu RF-5301PC spectrofluorophotometer.The powder X-ray diffraction (pXRD) spectra of the samples were obtained using a Bruker D8 Advance diffractometer in Bragg–Brentano geometry, operating at a voltage of 40 kV and a current of 25 mA. As an X-ray source, a Cu anode was used with Cu-Ka1 radiation (λ = 1.5418 Å), while Cu-Ka2 interferences are considered negligible due to the use of a Göbel mirror. Diffraction patterns were monitored in the 2θ angle range of 15–65 degrees with a step size equal to 0.04° s−1 and a scan speed equal to 1.6 s per step.
X-ray photoelectron spectroscopy (XPS) measurements were conducted within an ultra-high vacuum chamber at an operating pressure below 1.7 × 10−9 mbar.36 A Leybold LH EA11 hemispherical energy analyzer, operated at 100 eV pass energy, was employed to analyze the kinetic energy of emitted photoelectrons excited by a non-monochromatic AlKα source emitting at 1486.6 eV. Sample powders were pressed against thin lead sheets and introduced into the chamber without further treatment. Spectra were analyzed using XPSPEAK 4.1 software, following the application of a Shirley background. The reported binding energies were charge-corrected with reference to adventitious carbon at 284.8 eV. Surface atomic ratios were calculated using experimental relative sensitivity factors.
The absorption of the solutions in the MTT assay was measured on an Eliza Microplate Reader Biobase spectrophotometer; the test wavelength was set at 540 nm and the reference wavelength at 620 nm. Widefield optical fluorescence microscopy of the NPs was carried out using an OMAX Trinocular Compound EPI-fluorescence microscope (model: M834FLR) with a 1.3 MP CMOS camera (blue filters: excitation 410–490 nm, emission 515 nm; green filters: excitation 490–540 nm, emission 590 nm), while for cells it was carried out using a EUROMEX bScope Trino Plan Fluo Microscope (model: BS.3153-PLI) with a 6.1 MP 1/1.8” SONY EXMOR CMOS SENSOR Euromex Scientific Camera (sCMEX-6) (blue filters: excitation 450–495 nm, emission 515; green filters: excitation 495–555 nm, emission 595 nm; violet filters: excitation 380–415 nm, emission 475 nm; ultraviolet filters: excitation 320–380 nm, emission 435 nm). Intracellular iron and Europium quantification was performed via inductively coupled plasma-mass spectrometry using an Agilent 7900 ICP-MS instrument. Helium was used as the collision gas to minimise polyatomic interferences, and the isotopes used for quantification were 56Fe and 151Eu.The flow cytometry experiments were conducted on a Becton Dickinson FACSCalibur flow cytometer and the obtained data were processed using FlowJo software.
Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe(III) ratio of 1
Fe(III) ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 contributes to the formation of iron oxide nuclei, which consist of magnetite at a percentage higher than 95%. After the temperature reached 90 °C and the two salts were solubilized, 8 mL of concentrated ammonia solution (NH3) was added into the flask to ensure the alkaline environment needed for the formation and precipitation of magnetite. At the end of the reaction, the color of the mixture turned black, indicating the formation of magnetite. When the reaction mixture cooled down to room temperature, the precipitate was magnetically separated and washed thrice with dd. water, in order to remove excess reagents. The synthesized magnetic IONPs were dispersed in water and stored at 4 °C. The overall reaction of the synthesis is presented below (reaction (R3)).38
2 contributes to the formation of iron oxide nuclei, which consist of magnetite at a percentage higher than 95%. After the temperature reached 90 °C and the two salts were solubilized, 8 mL of concentrated ammonia solution (NH3) was added into the flask to ensure the alkaline environment needed for the formation and precipitation of magnetite. At the end of the reaction, the color of the mixture turned black, indicating the formation of magnetite. When the reaction mixture cooled down to room temperature, the precipitate was magnetically separated and washed thrice with dd. water, in order to remove excess reagents. The synthesized magnetic IONPs were dispersed in water and stored at 4 °C. The overall reaction of the synthesis is presented below (reaction (R3)).38| Fe3O4 + 2H+→ γ-Fe2O3 + Fe2+ + H2O | (R1) |
 | (R2) |
| 2Fe3+ + Fe2+ + 8OH− → Fe3O4 + 4H2O | (R3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe(III) ratio of 1
Fe(III) ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and the mixture was left until complete dissolution. After the dissolution of the salts and while the temperature was still at 80 °C, 2.5 mL of concentrated ammonia solution (NH3) was added into the reaction flask. At this stage, 1 g (3.4 mmol) tri-sodium citrate was dissolved in 2 mL of distilled water and added into the reaction flask after another 30 min. The addition of tri-sodium citrate was followed by an increase in temperature (95 °C) and the reaction was left to complete for 90 min. When the reaction mixture cooled down to room temperature, the precipitate was magnetically separated and washed thrice with dd. water. The synthesized magnetic IONPs@citrate was dispersed in water and stored at 4 °C. The overall reaction of the synthesis is presented below (reaction (R4)).39
2 and the mixture was left until complete dissolution. After the dissolution of the salts and while the temperature was still at 80 °C, 2.5 mL of concentrated ammonia solution (NH3) was added into the reaction flask. At this stage, 1 g (3.4 mmol) tri-sodium citrate was dissolved in 2 mL of distilled water and added into the reaction flask after another 30 min. The addition of tri-sodium citrate was followed by an increase in temperature (95 °C) and the reaction was left to complete for 90 min. When the reaction mixture cooled down to room temperature, the precipitate was magnetically separated and washed thrice with dd. water. The synthesized magnetic IONPs@citrate was dispersed in water and stored at 4 °C. The overall reaction of the synthesis is presented below (reaction (R4)).39| 2FeCl3 + FeCl2 + 8NH3 + 4H2O + (HOOC)(CH2)C(OH)(COOH)(CH2)(COOH) → citrate – Fe3O4 + 8NH4Cl | (R4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Eu ratios of 1
Eu ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.25 having water as the dispersion medium (IONPs@citrate@Eu (Fe
0.25 having water as the dispersion medium (IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 3, in H2O), IONPs@citrate@Eu (Fe
3, in H2O), IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1, in H2O), IONPs@citrate@Eu (Fe
1, in H2O), IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.25, in H2O)).
These syntheses were based on the work of Espindola et al.34 with some modifications. Particularly, in the case of an Fe
0.25, in H2O)).
These syntheses were based on the work of Espindola et al.34 with some modifications. Particularly, in the case of an Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu ratio of 1
Eu ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, in a 50 mL round bottom flask, placed inside an oil bath and on a heated stirrer, 20 mL of dd. water was added. The flask was heated at around 70 °C in the presence of bubbling N2 gas. Then, 870 μL of IONPs@citrate was added into the flask and after 10 min 2–3 drops of concentrated ammonia solution (NH3) was added as well. Ammonia contributes to the alkaline environment needed for deprotonation of the carboxyl groups of citrate on the IONPs’ surface (–COOH → –COO−), so that europium can be more easily attached to them. After another 10–15 min, 0.15 g (0.35 mmol) Eu(NO3)3·5H2O (Fe
3, in a 50 mL round bottom flask, placed inside an oil bath and on a heated stirrer, 20 mL of dd. water was added. The flask was heated at around 70 °C in the presence of bubbling N2 gas. Then, 870 μL of IONPs@citrate was added into the flask and after 10 min 2–3 drops of concentrated ammonia solution (NH3) was added as well. Ammonia contributes to the alkaline environment needed for deprotonation of the carboxyl groups of citrate on the IONPs’ surface (–COOH → –COO−), so that europium can be more easily attached to them. After another 10–15 min, 0.15 g (0.35 mmol) Eu(NO3)3·5H2O (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu ratio = 1
Eu ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was added into the reaction flask and the reaction was left to complete for 24 h. When the reaction mixture cooled down to room temperature, the precipitate was magnetically separated and washed thrice with dd. water. The synthesized magnetic IONPs@citrate@Eu (Fe
3) was added into the reaction flask and the reaction was left to complete for 24 h. When the reaction mixture cooled down to room temperature, the precipitate was magnetically separated and washed thrice with dd. water. The synthesized magnetic IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, in H2O) were dispersed in water and stored at 4 °C.
3, in H2O) were dispersed in water and stored at 4 °C.
Similar procedures were followed for the syntheses of the magnetic IONPs@citrate@Eu with Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu ratios of 1
Eu ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25. However, for these two ratios, 750 μL of concentrated ammonia was added for the deprotonation of citrate molecules and the reactions were completed after 2 h. Moreover, for sample IONPs@citrate@Eu (Fe
0.25. However, for these two ratios, 750 μL of concentrated ammonia was added for the deprotonation of citrate molecules and the reactions were completed after 2 h. Moreover, for sample IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in H2O), the amount of added Eu(NO3)3·5H2O was 0.054 g (0.13 mmol), whilst for sample IONPs@citrate@Eu (Fe
1, in H2O), the amount of added Eu(NO3)3·5H2O was 0.054 g (0.13 mmol), whilst for sample IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in H2O) the amount was 0.014 g (0.033 mmol).
0.25, in H2O) the amount was 0.014 g (0.033 mmol).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Eu ratios of 1
Eu ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.25 having ethylene glycol as the dispersion medium (IONPs@citrate@Eu (Fe
0.25 having ethylene glycol as the dispersion medium (IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1, in EG), IONPs@citrate@Eu (Fe
1, in EG), IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.25, in EG)).
These syntheses were based on the work of Martinson et al.40 with some modifications. Particularly, in the case of an Fe
0.25, in EG)).
These syntheses were based on the work of Martinson et al.40 with some modifications. Particularly, in the case of an Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu ratio of 1
Eu ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in a 25 mL round bottom flask, placed inside an oil bath and on a heated stirrer, 10 mL of ethylene glycol (EG) was added. The flask was heated at around 80 °C in the presence of bubbling N2 gas. Then, 435 μL of IONPs@citrate and 200 μL of NaOH 1 N were added into the flask. In this case, NaOH was used instead of NH3 for the deprotonation of the carboxyl groups of citrate molecules on the IONPs’ surface. After 30 min, 0.027 g (0.063 mmol) Eu(NO3)3·5H2O dissolved in 500 μL ethanol (EtOH) was added into the reaction flask and the reaction was left to complete for 2 h. When the reaction mixture cooled down to room temperature, the precipitate was magnetically separated and washed thrice with dd. water. The synthesized magnetic IONPs@citrate@Eu (Fe
1, in a 25 mL round bottom flask, placed inside an oil bath and on a heated stirrer, 10 mL of ethylene glycol (EG) was added. The flask was heated at around 80 °C in the presence of bubbling N2 gas. Then, 435 μL of IONPs@citrate and 200 μL of NaOH 1 N were added into the flask. In this case, NaOH was used instead of NH3 for the deprotonation of the carboxyl groups of citrate molecules on the IONPs’ surface. After 30 min, 0.027 g (0.063 mmol) Eu(NO3)3·5H2O dissolved in 500 μL ethanol (EtOH) was added into the reaction flask and the reaction was left to complete for 2 h. When the reaction mixture cooled down to room temperature, the precipitate was magnetically separated and washed thrice with dd. water. The synthesized magnetic IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in EG) were dispersed in water and stored at 4 °C.
1, in EG) were dispersed in water and stored at 4 °C.
The same procedure was followed for the synthesis of the magnetic IONPs@citrate@Eu with an Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu ratio of 1
Eu ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25. Nevertheless, in this case, the amount of added Eu(NO3)3·5H2O was equal to 0.0068 g (0.016 mmol).
0.25. Nevertheless, in this case, the amount of added Eu(NO3)3·5H2O was equal to 0.0068 g (0.016 mmol).
Ethylene glycol, with its two hydroxyl groups, serves as an effective complexing agent for metal ions of varying sizes. Its use in synthesis helps prevent selective precipitation and ensures better homogeneity among components. In the presence of nitrate anions, a phenomenon known as the “glycol effect” occurs.41 Nitrates tend to form strong hydrogen bonds with ethylene glycol in the polar domain, dominating its coordination sphere and reducing interactions with other ions.42 This interaction also initiates an exothermic redox reaction between nitrate and the hydroxyl groups, producing nitrogen oxide gases (NOx) and oxidizing ethylene glycol to glyoxylate.43 In this study, ethylene glycol was used specifically to minimize the disruptive effects of nitrate ions during NP synthesis.
All syntheses were performed via a one-pot co-precipitation process.
In vitro biological evaluation
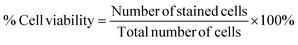 | (1) |
 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 4% (w/v) potassium ferrocyanide (K4Fe(CN)6·3H2O) and 4 M HCl in PBS – for 30 min at room temperature. The acidic environment promotes partial dissolution of the internalized NPs by providing protons, which liberate Fe(III) ions from the NP matrix. These free Fe(III) ions subsequently react with ferrocyanide to form the characteristic Prussian blue complex, marking the sites of iron accumulation within the cells. After the staining procedure, cells were rinsed with PBS and counterstained with 0.02% nuclear red solution for at least 5 min to visualize cell nuclei, which appear red and provide contrast against the blue-stained regions indicating iron presence. Finally, the cells were washed again with PBS, imaged under an optical microscope, and documented using a 1.3MP CMOS camera.44
1 mixture of 4% (w/v) potassium ferrocyanide (K4Fe(CN)6·3H2O) and 4 M HCl in PBS – for 30 min at room temperature. The acidic environment promotes partial dissolution of the internalized NPs by providing protons, which liberate Fe(III) ions from the NP matrix. These free Fe(III) ions subsequently react with ferrocyanide to form the characteristic Prussian blue complex, marking the sites of iron accumulation within the cells. After the staining procedure, cells were rinsed with PBS and counterstained with 0.02% nuclear red solution for at least 5 min to visualize cell nuclei, which appear red and provide contrast against the blue-stained regions indicating iron presence. Finally, the cells were washed again with PBS, imaged under an optical microscope, and documented using a 1.3MP CMOS camera.44
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 events per sample were analyzed using FlowJo software.
000 events per sample were analyzed using FlowJo software.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 events per sample. The resulting data were further processed and analyzed using FlowJo software.
000 events per sample. The resulting data were further processed and analyzed using FlowJo software.
Results and discussion
Structural and morphological characterization
The UV-Vis spectra of each synthesis are presented in Fig. S1 of the SI. In all the absorption spectra of the NPs, three major peaks are observed. The first peak around 200 nm is attributed to IL transitions, the second peak around 380 nm is attributed to LMCT transitions, and the third peak around 490 nm is attributed to d–d or pair excitation processes. Among the three observed peaks, the one corresponding to LMCT transitions is the most clearly observed, giving important information about the structure of the NPs, and the one corresponding to d–d or pair excitation processes is the least clearly observed, probably due to overlapping with LMCT transitions. Based on the theory that the LMCT peak blueshifts with reducing size and redshifts with increasing size of the NPs, we can draw two major conclusions. Firstly, the addition of tri-sodium citrate redshifts the spectrum, indicating the attachment on the IONPs’ surface and resulting in NPs of bigger size. Secondly, the addition of europium results in Fe@Eu NPs of either a slightly bigger or a slightly smaller size. This indicates that europium managed to be incorporated in IONPs@citrate, but probably changes its structure. No characteristic absorption peak due to europium is observed in the spectra, since, as already mentioned, magnetite strongly absorbs light in the wavelength range of 250–900 nm. However, analysis of the UV-Vis spectra reveals that a lower theoretical europium content in the Fe@Eu NPs corresponds to reduced absorption intensity.
Table 1 summarizes the DLS characteristics of the synthesized NPs (distribution curves of size and ZP are shown in Fig. S2 of the SI). The data indicate that varying the europium content in Fe@Eu NPs influences their colloidal stability. Specifically, IONPs, IONPs@citrate, and IONPs@citrate@Eu with an Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu ratio of 1
Eu ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (synthesized in H2O and in EG) exhibit good colloidal stability since ZP is |30–50| mV. In contrast, IONPs@citrate@Eu with Fe
1 (synthesized in H2O and in EG) exhibit good colloidal stability since ZP is |30–50| mV. In contrast, IONPs@citrate@Eu with Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu ratios of 1
Eu ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (synthesized in H2O) and 1
3 (synthesized in H2O) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 (synthesized in H2O and in EG) are characterized by aggregation, thus reducing the colloidal stability. These observations regarding the Fe@Eu NPs are visually supported and align with experimental results. Across all samples, the DH and PdI values are increased, indicating a tendency for NP aggregation. However, the exact size of the Fe@Eu NPs will be further characterized using TEM and pXRD analyses.
0.25 (synthesized in H2O and in EG) are characterized by aggregation, thus reducing the colloidal stability. These observations regarding the Fe@Eu NPs are visually supported and align with experimental results. Across all samples, the DH and PdI values are increased, indicating a tendency for NP aggregation. However, the exact size of the Fe@Eu NPs will be further characterized using TEM and pXRD analyses.
| Sample | D H (nm) | PdI | ZP (mV) |
|---|---|---|---|
| IONPs | 1.038 ± 74.32 | 0.644 ± 0.032 | −44.1 ± 2.21 |
| IONPs@citrate | 221.9 ± 2.691 | 0.329 ± 0.018 | −40.6 ± 0.709 |
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, in H2O) 3, in H2O) |
993.2 ± 50.71 | 0.510 ± 0.027 | 24.2 ± 0.702 |
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in H2O) 1, in H2O) |
2.156 ± 178.4 | 0.693 ± 0.062 | 34.9 ± 0.503 |
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in EG) 1, in EG) |
2.964 ± 809.5 | 0.744 ± 0.183 | 39.7 ± 0.404 |
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in H2O) 0.25, in H2O) |
2.553 ± 382.3 | 0.835 ± 0.084 | 9.20 ± 1.13 |
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in EG) 0.25, in EG) |
2.448 ± 462.4 | 0.719 ± 0.110 | 14.3 ± 0.854 |
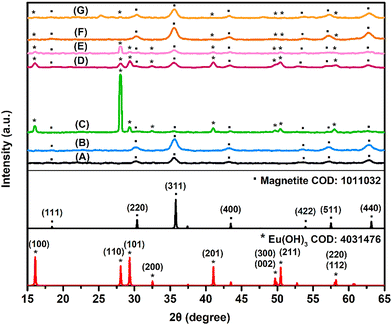
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and the symmetrical stretching vibration of the carboxylic anions (–COO−) of citrate, respectively.64 Especially, the second peak might be an indication of the presence of a –COO–Fe bond, which is attributed to the reaction between the hydroxyl radicals on magnetite's surface and the carboxylic groups of citrate.65 With the addition of europium on the NPs’ surface, these two peaks show a slight shift. Apart from supporting the presence of the chemisorbed carboxylate groups of citrate on the NPs, according to the literature, these two peaks give information about the way citrate molecules bind to the magnetite surface.64 When the mathematical difference of these two peaks is bigger than 200 cm−1, the carboxylic acids form monodendate binding with the magnetite surface and, when the difference is smaller than 200 cm−1, the binding is bidendate.66 According to this, citrate anions form monodendate binding only in the IONPs@citrate sample, whereas in all IONPs@citrate@Eu samples the binding is bidendate. The peaks at 3605 and 3610 cm−1 are of particular interest, because, according to the literature, they are attributed to the presence of Eu(OH)3 rods.67
O) and the symmetrical stretching vibration of the carboxylic anions (–COO−) of citrate, respectively.64 Especially, the second peak might be an indication of the presence of a –COO–Fe bond, which is attributed to the reaction between the hydroxyl radicals on magnetite's surface and the carboxylic groups of citrate.65 With the addition of europium on the NPs’ surface, these two peaks show a slight shift. Apart from supporting the presence of the chemisorbed carboxylate groups of citrate on the NPs, according to the literature, these two peaks give information about the way citrate molecules bind to the magnetite surface.64 When the mathematical difference of these two peaks is bigger than 200 cm−1, the carboxylic acids form monodendate binding with the magnetite surface and, when the difference is smaller than 200 cm−1, the binding is bidendate.66 According to this, citrate anions form monodendate binding only in the IONPs@citrate sample, whereas in all IONPs@citrate@Eu samples the binding is bidendate. The peaks at 3605 and 3610 cm−1 are of particular interest, because, according to the literature, they are attributed to the presence of Eu(OH)3 rods.67
The formation of Eu(OH)3 is probably driven by the alkaline conditions during the addition of europium nitrate. It is well established that amines and ammonia promote the precipitation of trivalent rare-earth hydroxides72 according to the following reaction (reaction (R5)):
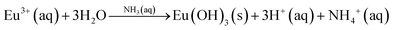 | (R5) |
X-ray diffraction measurements contribute to the determination of the average crystallite size of the synthesized NPs. The size is calculated using the Debye–Scherrer equation (eqn (3)):
 | (3) |
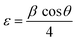 | (4) |
| Sample | D (nm) | ε (×10−3) |
|---|---|---|
| IONPs | 8.86 | 4.34 |
| IONPs@citrate | 8.31 | 4.37 |
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, in H2O) 3, in H2O) |
11.14 | 3.57 |
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in H2O) 1, in H2O) |
8.06 | 4.82 |
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in EG) 1, in EG) |
9.32 | 4.34 |
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in H2O) 0.25, in H2O) |
8.51 | 4.27 |
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in EG) 0.25, in EG) |
8.77 | 4.24 |
Analyzing the data in Table 2 and comparing the Fe@Eu NPs with IONPs@citrate, it can be seen that, in some cases, D is increased and accompanied by a decreased ε, while, in others, the exact opposite is observed. The elevation or reduction of these values does not appear to have any specific correlation to the amount of europium present in the Fe@Eu NPs. This means that larger europium ions substitute smaller iron ions in the magnetite lattice or they are incorporated in the defects formed internally or on the surface of the lattice. Probably, these alterations of the crystal lattice are not arranged in a consistent order, but rather randomly.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in H2O) are representative examples. XPS scans of the samples revealed the presence of Eu, Fe, O, and C. In general, the XPS data show the presence of magnetite in all samples and its coexistence with Eu(OH)3 in samples IONPs@citrate@Eu with Fe
0.25, in H2O) are representative examples. XPS scans of the samples revealed the presence of Eu, Fe, O, and C. In general, the XPS data show the presence of magnetite in all samples and its coexistence with Eu(OH)3 in samples IONPs@citrate@Eu with Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu ratios of 1
Eu ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 in H2O. Apart from the Eu 3d region, the O 1s region is also in very good agreement with the above findings (Table 3).
0.25 in H2O. Apart from the Eu 3d region, the O 1s region is also in very good agreement with the above findings (Table 3).
| Sample | Eu/Fea | Eu/Ob | Fe/Oc | Fe3+/Fe2+ | O (M–Ox)/O (M–OH)d |
|---|---|---|---|---|---|
| a Surface atomic ratio of europium and iron. b Surface atomic ratio of europium and oxygen. c Surface atomic ratio of iron and oxygen. d Ratio of peaks belonging to metal oxide species (M–Ox) and hydroxide and metal hydroxide species (M–OH, –OH) in the O 1s region. | |||||
| IONPs@citrate | n.a. | n.a. | 0.44 | 2.26 | 2.94 |
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, in H2O) 3, in H2O) |
4.44 | 0.82 | 0.18 | 2.94 | 0.62 |
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in H2O) 1, in H2O) |
3.88 | 0.65 | 0.17 | 2.80 | 0.79 |
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in H2O) 0.25, in H2O) |
1.80 | 0.54 | 0.30 | 2.62 | 1.76 |
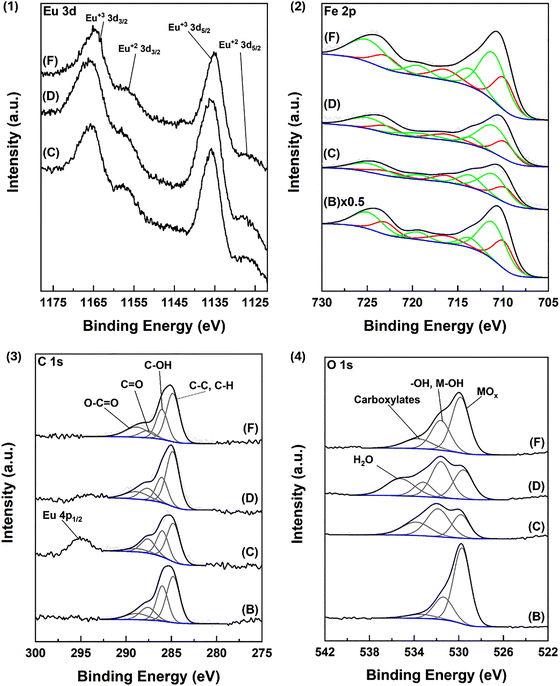
XPS scans confirm the presence of europium in the Eu(OH)3 form for all Fe@Eu samples (Fig. 2(1)). The main (3d5/2) peak is centered at 1135.6 eV for Eu(OH)3 as reported previously.76 The small peak that resolves at ca. 1127 eV can be attributed to Eu in the 2+ oxidation state, indicative of defects on the surface.67,77 For IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in H2O and Fe
1, in H2O and Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, in H2O), the 3+ oxidation state peaks show a slightly higher FWHM and a slightly different peak shape (see the 3d3/2 peak shape). These observations likely indicate the presence of a small amount of Eu2O3 in the sample, as the Eu+3 peaks for europium(III) oxide are slightly shifted to higher binding energies.67 The spectrum maxima do not show a notable shift, and therefore the main component should be considered to be Eu(OH)3. For IONPs@citrate@Eu (Fe
3, in H2O), the 3+ oxidation state peaks show a slightly higher FWHM and a slightly different peak shape (see the 3d3/2 peak shape). These observations likely indicate the presence of a small amount of Eu2O3 in the sample, as the Eu+3 peaks for europium(III) oxide are slightly shifted to higher binding energies.67 The spectrum maxima do not show a notable shift, and therefore the main component should be considered to be Eu(OH)3. For IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in H2O), the peaks shift to smaller binding energies, namely 1134.9 eV for the 3d5/2 component. Due to the low ratio of Eu/Fe in this sample, it is likely that the Eu–Fe interactions are stronger, reducing the binding energy of the peak.
0.25, in H2O), the peaks shift to smaller binding energies, namely 1134.9 eV for the 3d5/2 component. Due to the low ratio of Eu/Fe in this sample, it is likely that the Eu–Fe interactions are stronger, reducing the binding energy of the peak.
The main Fe 2p photoelectron peak shows two main contributions centered at ca. 710 and 724 eV. Peak deconvolution is performed as reported by Atrei et al.78 During deconvolution, identical constraints were applied to all spectra. The results reveal contributions from both Fe²⁺ and Fe³⁺ species, confirming the presence of magnetite (Fe3O4) in every sample. In Fig. 2(2), peaks assigned to Fe²⁺ are shown in red, while those assigned to Fe³⁺ are in green. The Fe²⁺ state is characterized by the 2p3/2 peak at 709.8 eV, a satellite at 716.4 eV, and the 2p1/2 peak at 723.0 eV. The Fe³⁺ state includes the 711.1 eV peak (octahedral sites), the 713.7 eV peak (tetrahedral sites), a satellite at 719.4 eV, and the 2p1/2 component at 724.9 eV. The Fe³⁺/Fe²⁺ ratio increases with higher Eu/Fe content, reaching 2.94 for IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, H2O), compared to 2.26 for the control IONPs@citrate. This corresponds to a total Fe valence of +8.1 in the magnetite structure, consistent with the expected value of +8.
3, H2O), compared to 2.26 for the control IONPs@citrate. This corresponds to a total Fe valence of +8.1 in the magnetite structure, consistent with the expected value of +8.
The C 1s spectra (Fig. 2(3)) show contributions from four peaks. Adventitious carbon is set at 284.8 eV, with C–OH species appearing at 286.0 eV. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species are present at 287.6 eV and O–C
O species are present at 287.6 eV and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O contributions are centered at 288.8 eV.79 Oxygenated species arise from the exposure of the sample to air and background gases present in the UHV system. The peak at ca. 294 eV is attributed to Eu and specifically the 4p1/2 bound state, indicating that the contribution from this peak weakens with decreasing Eu content in the sample.80
O contributions are centered at 288.8 eV.79 Oxygenated species arise from the exposure of the sample to air and background gases present in the UHV system. The peak at ca. 294 eV is attributed to Eu and specifically the 4p1/2 bound state, indicating that the contribution from this peak weakens with decreasing Eu content in the sample.80
The O 1s region (Fig. 2(4)) deconvolutes into contributions from M–Ox (FexOy, EuxOy) groups at 529.7 eV, –OH, M–OH species at ca. 531.5 eV from surface hydroxyl groups, and Eu–OH. For the Eu doped samples, this peak shows a slight shift to higher binding energies due to the contributions from the Eu–OH species. Another peak resolves at ca. 533.5 eV for surface impurities and carboxylate species present through sample preparation. Finally, for IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1
Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in H2O), one more peak can be resolved in the region at 535.2 eV. Peaks at such binding energies could indicate the presence of water in the sample.67,78,81
1, in H2O), one more peak can be resolved in the region at 535.2 eV. Peaks at such binding energies could indicate the presence of water in the sample.67,78,81
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, nanorods can be clearly observed, confirming the previous results. Mapping and elemental analysis were also performed for the samples. EDX spectra (Fig. S4 of the SI) demonstrated the existence of Fe, Eu, and O, confirming that europium is successfully incorporated in all samples. From the mapping, it can be validated that the nanorods mainly consist of Eu and O and only a small amount of Fe. In contrast, in the case of the nanospheres, there is a uniform distribution of Eu, Fe, and O. The ratio of Fe
1 ratio, nanorods can be clearly observed, confirming the previous results. Mapping and elemental analysis were also performed for the samples. EDX spectra (Fig. S4 of the SI) demonstrated the existence of Fe, Eu, and O, confirming that europium is successfully incorporated in all samples. From the mapping, it can be validated that the nanorods mainly consist of Eu and O and only a small amount of Fe. In contrast, in the case of the nanospheres, there is a uniform distribution of Eu, Fe, and O. The ratio of Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu initially added for the syntheses is different from the obtained one. From Table 4, it can be observed that only a small amount of Eu can be incorporated in the magnetite structure, something that is probably attributed to the defects of the magnetite lattice and the larger radius of Eu. An important conclusion that can be drawn here is that the size of the Fe@Eu NPs is much smaller than the one measured with DLS.
Eu initially added for the syntheses is different from the obtained one. From Table 4, it can be observed that only a small amount of Eu can be incorporated in the magnetite structure, something that is probably attributed to the defects of the magnetite lattice and the larger radius of Eu. An important conclusion that can be drawn here is that the size of the Fe@Eu NPs is much smaller than the one measured with DLS.
| Sample | Z | Element | Mass fraction (%) | Obtained Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu Eu |
Size distribution (± SD) [nm] |
|---|---|---|---|---|---|
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, in H2O) 3, in H2O) |
26 | Fe | 94.55 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06 0.06 |
11.8 ± 1.8 |
| 63 | Eu | 5.45 | |||
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in H2O) 1, in H2O) |
26 | Fe | 88.37 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.13 0.13 |
11.1 ± 1.8 |
| 63 | Eu | 11.63 | |||
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in EG) 1, in EG) |
26 | Fe | 64.77 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.54 0.54 |
10.8 ± 1.6 |
| 63 | Eu | 35.23 | |||
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in H2O) 0.25, in H2O) |
26 | Fe | 94.71 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06 0.06 |
11.1 ± 2.5 |
| 63 | Eu | 5.29 | |||
IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in EG) 0.25, in EG) |
26 | Fe | 92.25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08 0.08 |
9.4 ± 2.0 |
| 63 | Eu | 7.75 |
From the PL spectra of the Fe@Eu NPs (Fig. 4(1)), two main clear peaks are detected after excitation at 350 nm. Specifically, a broad peak is observed around 470 nm, along with a weaker peak near 825 nm in the near-infrared (NIR) region. There is a high probability that the black core of magnetite absorbs a lot of the excitation light or there are a lot of formed defects on the host lattice, leading to a reduced emission intensity of europium.89 This could explain the very broad peak centered around 470 nm, indicating green photoluminescence and, thus, the presence of Eu(II) in the Fe@Eu NPs, and overlapping the red photoluminescence of Eu(III) present in the precursor molecule. Gaussian fitting of each curve reveals that the broad main peak is composed of multiple underlying individual peaks. In more detail, the peak around 470 nm can be attributed to the 4f65d1 → 4f7 transition of Eu(II) or it can be an indication that aggregates are formed, leading to Fe@Eu NPs of different sizes emitting light of higher energy. However, further analysis of this curve presents a broad peak between 590 and 650 nm attributed to the 5D0 → 7F1–3 transitions of Eu(III). The peak of small intensity around 825 nm is attributed to the rarely observed 5D0 → 7F6 transition of Eu(III).
In vitro biological evaluation
The percentage of cell viability is determined by comparing the absorbance values of NP-treated cells to those of untreated controls. Subsequently, the cytotoxic potential of the NPs can be quantitatively evaluated through the construction of a dose–response curve, which illustrates the correlation between NP concentration and cell viability. NPs of very small size, around 10 nm, and their high positive charge improve penetration through the negatively charged bilipid membrane distributing iron molecules in the cells.93 The levels of toxicity are correlated with surface modification, byproducts of the NPs, chemical composition of the different cell lines, oxidation state of IONPs as well as the interaction between IONPs and proteins.94 In general, administration of IONPs has been related to extensive cytotoxicity leading, for instance, to apoptosis, mitochondrial disorder, DNA damage, and excessive ROS production.94,95 Nevertheless, coating of NPs with an amphiphilic layer enhances colloidal stability and improves their biodistribution, preventing agglomeration caused by intramolecular interactions or interactions with biological molecules and prolonging their circulation time.94,96 Overall, the highest administered concentration of NPs is expected to be more toxic because of the metabolic differences.
Results in Fig. 5(1) demonstrate that, in most cases, cytotoxicity is dose-dependent in HEK-293 cells reaching 70% even after incubation with 100 μMFe for 24 h. In particular, IONPs@citrate, which is the precursor sample for the following syntheses, present viability around 70% or higher, whilst the same tendency is also observed in bare NPs. As for Fe@Eu NPs (C)–(G), viability seems to exceed 80%, indicating not only that these NPs are biocompatible with the specific cell line, but also that the addition of Eu leads to higher percentages of viability. HEK-293 is a cell line of human origin and, thus, the biocompatibility of the NPs is a positive first step for their application as a theranostic agent. From Fig. 5(2), without reference to dose-dependency, it can be observed that viability exceeds 70% in most cases underlying the biocompatibility of NPs with HeLa cells as well. However, a major change is observed after the administration of (C), which presents dose-dependent toxicity and leads to viability approximately equal to 50% after incubation with 100 μMFe. The size of all NPs is around 10 nm and their surface charge is positive, meaning that all of them can be easily internalized in cells. However, in HeLa cells probably the integrity of (C) is diminished, leading to high levels of available free iron molecules in the cells and increased toxicity.
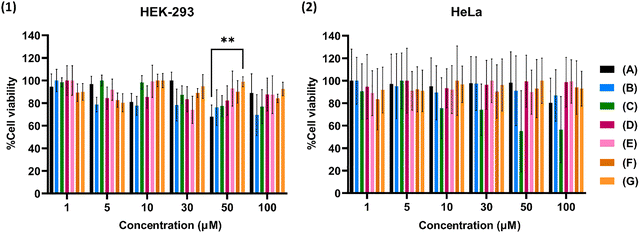 | ||
Fig. 5 MTT cytotoxicity assay of (1) HEK-293 and (2) HeLa cell lines. Cells were treated with 1, 5, 10, 30, 50 and 100 μMFe for 24 h. The calculation of the percentages was conducted using eqn (1), including the control, and values represent the mean value ± SD of three different triplicate experiments. The interaction is considered very significant for **p ≤ 0.01 (multiple comparisons were performed between the different NPs in the mean value of each concentration). NPs: (A) IONPs, (B) IONPs@citrate, (C) IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, in H2O), (D) IONPs@citrate@Eu (Fe 3, in H2O), (D) IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in H2O), (E) IONPs@citrate@Eu (Fe 1, in H2O), (E) IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in EG), (F) IONPs@citrate@Eu (Fe 1, in EG), (F) IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in H2O), and (G) IONPs@citrate@Eu (Fe 0.25, in H2O), and (G) IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in EG). 0.25, in EG). | ||
This experiment was conducted for further determination of cytotoxicity, investigating the proliferation and migration of the cells through the scratched area. As can be observed from Fig. 6, there is a significant difference between the two cell lines with HEK-293 being more affected by the administration of the Fe@Eu NPs. In particular, comparing the images in Fig. 6(1) from (C) to (G) with the control, it can be concluded that cell proliferation is reduced, since 72 h after administering NPs a certain percentage of the wound is still open. However, more than 70% of the wound is healed in all cases. As for HeLa cells (Fig. 6(2)), proliferation does not appear to be very disturbed taking into consideration that the wound has reached almost 100% closure after 28 h of incubation. The only differences appear in cases (C) and (E), where healing slows down, leading to reduced proliferation.
 | ||
Fig. 6 Representative images of the wound healing assay of (1) HEK-293 and (2) HeLa cells after administration of 100 μMFe Fe@Eu NPs for 24 h. The calculation of the %wound closure was conducted using eqn (2). Scale bar: 0.1 mm. NPs: (C) IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, in H2O), (D) IONPs@citrate@Eu (Fe 3, in H2O), (D) IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in H2O), (E) IONPs@citrate@Eu (Fe 1, in H2O), (E) IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in EG), (F) IONPs@citrate@Eu (Fe 1, in EG), (F) IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in H2O), and (G) IONPs@citrate@Eu (Fe 0.25, in H2O), and (G) IONPs@citrate@Eu (Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 1 Eu = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, in EG). 0.25, in EG). | ||
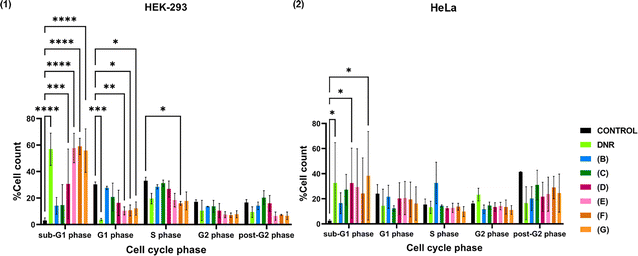
HEK-293 and HeLa cells were treated with Fe@Eu NPs at a concentration of 100 μMFe for 24 h. This concentration was selected based on prior MTT assay results, which confirmed that the NPs maintain biocompatibility at this dose. The DNA content in the various phases of the cell cycle was then analyzed to determine any perturbations caused by NP exposure. As a positive control, DNR was employed, a chemotherapeutic agent well known for its potent cytostatic and cytotoxic effects. DNR intercalates into DNA, generates reactive oxygen species (ROS), inhibits topoisomerase II activity, and forms DNA adducts, all of which contribute to structural and functional damage to DNA and the cell membrane.103,104 In response to this damage, cells typically undergo cell cycle arrest, providing time for DNA repair mechanisms to be activated. This experimental setup enables the comparison of Fe@Eu NP-induced effects to those of a well-established cytotoxic agent.
From the results in Fig. 9, it can be stated that cell cycle distribution presents differences in both cell lines, HEK-293 and HeLa, in a divergent way after the administration of Fe@Eu NPs. In particular, observing the control group in Fig. 9(1) and Fig. S5(1), a normal cell cycle in HEK-293 consists of an increased population in G1 and S phases, while it decreases to half in G2 and post-G2 phases and is almost nil in the sub-G1 phase. As for HeLa cells, observing the control group in Fig. 9(2) and Fig. S5(2), a normal cell cycle consists of an almost evenly distributed population in G1, S and G2 phases, whilst it reaches almost zero point in the sub-G1 phase and evolves in the post-G2 phase. Cytostatic DNR affects the cell cycle of HEK-293 by extremely increasing the DNA content in the sub-G1 phase, meaning that a large percentage of cells is driven to apoptosis before they even enter the cell cycle.105,106 As a result, the distribution of cells in the other phases is reduced, especially in the G1 phase. As for HeLa cells, cell cycle distribution follows a different pathway after the administration of DNR with DNA content being increased in sub-G1 and G2 phases.
Comparing the results of the negative and positive controls with those of the administered Fe@Eu NPs, some interesting results were observed. In particular, administration of (B) to HEK-293 leads to cell cycle distribution similar to that of the negative control, meaning that the cell cycle is not disturbed. This is probably attributed to the surface modification of IONPs, which contributes to avoidance of agglomeration and release of iron ions in the cell. The same tendency is also observed after administering (C). Nevertheless, this pattern ceases to be valid for NPs (D)–(G), for which the sub-G1 phase increases dramatically and the population in the next phases is diminished, as observed in DNR. Since (D) and (E) are colloidally stable, cell cycle distribution could be correlated with the presence of rods in the sample or the release of increased iron ions. For (F) and (G), agglomeration of Fe@Eu NPs is an extra reason for the disturbance of the cell cycle. As for HeLa cells, it seems that administration of all NPs results in increased DNA content in sub-G1 and post-G2 phases, meaning that a large percentage of cells is driven to apoptosis at the beginning and the end of the cycle. Only a small percentage of cells remain unaffected, continuing the cell cycle under normal conditions. According to the literature, increased reactive oxygen species (ROS) generation has been previously associated with elevated levels of apoptosis in cancer cells. Substances leading to increased levels of ROS and apoptosis are considered good candidates for anticancer agents and, thus, cancer therapy.107
By analyzing the results in Fig. 10 (and Fig. S6 of the SI), it can be confirmed that IONPs induce the generation of ROS in both cell lines. According to the literature, both cell lines internalize NPs via endocytosis, but the magnitude of ROS induction can differ due to variations in metabolic activity, iron handling capacity, and baseline antioxidant defense between the two cell types. In particular, HeLa cells are reported to upregulate antioxidant mechanisms at earlier time points, which may transiently mitigate oxidative stress but become insufficient upon prolonged exposure (24 h), resulting in elevated ROS levels. Conversely, HEK-293 cells, while showing a more acute ROS response initially, appear to possess more effective early cytoprotective responses that stabilize ROS levels over time.113 In the present study, treatment of HEK-293 cells with NPs of 100 μMFe results in lower ROS levels compared to HeLa cells under the same conditions. Notably, after administration of formulation (B), ROS levels increased by approximately 50% beyond the baseline in HEK-293 cells, whereas the increase in HeLa cells was around 80%. This suggests that the presence of intracellular IONPs – along with moderate particle dissolution and subsequent iron ion release – may more strongly impact the redox balance in HeLa cells. The same trend appears with increasing europium content, indicating a formulation-dependent modulation of oxidative stress. Of particular interest are formulations (C) and (D), which cause significant ROS elevation, especially in HeLa cells. This effect may be attributed to differences in NP size, morphology, rate of intracellular ion release, or the interaction with cellular antioxidant systems and can support the selective pro-oxidant and pro-apoptotic effects of these formulations. In the case of formulation (C), it can also be observed that administration to HEK-293 does not elevate the ROS percentage significantly reinforcing their biosafety. The consistently higher ROS levels observed in HeLa cells can also be explained by their transformed phenotype. As a cancer-derived cell line, HeLa cells typically exhibit elevated metabolic rates and mitochondrial activity, rendering them more vulnerable to oxidative stress. This vulnerability is further exacerbated by their NP accumulation intracellularly, resulting in enhanced ROS generation. Formulations (E), (F), and (G) also increase the levels of ROS, without this elevation being very significant biologically. The possibility of ROS elevation being responsible for the apoptosis and the disturbance of the cell cycle is extremely high, given that apoptosis and ROS production have been previously correlated with the presence of iron and interference with the Fenton reaction.
As shown in Fig. 11 (and Fig. S7 in the SI), treatment with the NPs induces high levels of apoptosis in both cell lines. In particular, the percentage of total apoptotic HEK-293 cells ranges between 40 and 70%, with most of the population in early apoptosis. A similar pattern is observed in HeLa cells, with total apoptotic cells ranging from 45% to 75%, and the majority of population concurrently entering early apoptosis. The percentage of necrotic cells may be negligible in both cell lines; however, the present experiment confirms that the apoptotic pathway is the one followed and leads the cells to cell death after 24 h of incubation. As already stated in the literature and confirmed by our study, the presence of iron inside the cells contributes to the elevation of ROS due to the Fenton reaction and, as a consequence, cells are driven to apoptosis.
Overall, HEK-293 cells appear to internalize Fe@Eu NPs more efficiently than HeLa cells, as indicated by the consistently higher intracellular concentrations of both Fe (Fig. 12 (1) and (2)) and Eu (Fig. 12 (3) and (4)). Notably, the accumulation of Fe and Eu in HeLa cells reaches only about one-fourth to one-third of the levels observed in HEK-293 cells.
Considering the combined findings, including ICP-MS data, several important conclusions can be drawn regarding the behavior of the synthesized Fe@Eu NPs in healthy versus cancerous cell lines. Specifically, treatment with 100 μMFe of NPs resulted in more efficient internalization in HEK-293 cells, accompanied by only moderate cytotoxicity. In contrast, despite lower internalization levels in HeLa cells, the NPs triggered more pronounced biological responses. These included a significant reduction in cell viability, enhanced in vitro fluorescence, elevated ROS production, and increased apoptosis after 24 hours of exposure.
These observations suggest that, although fewer NPs accumulate in HeLa cells, the internalized concentrations of Fe and Eu are sufficient to disrupt key metabolic pathways and induce substantial oxidative stress. The elevated oxidative stress levels may also underlie the enhanced fluorescence observed in HeLa cells following NP treatment.
Conclusions
In summary, this work presents the synthesis of optomagnetic Fe@Eu NPs composed of iron and europium at varying Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu molar ratios (1
Eu molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25), following a rapid, straightforward, and environmentally friendly co-precipitation method. Surface modification of the IONPs with citrate improved colloidal stability, though the introduction of europium at different amounts induced slight aggregation. Morphologically, the NPs were predominantly spherical-like, with an average diameter of approximately 10 nm; however, nanorod formation was observed at higher europium content. Structural characterization confirmed that europium was incorporated into the magnetite crystal structure or deposited on the particle surface. A particularly notable property of these NPs is their dual fluorescence capability under UV irradiation, enhancing their potential as multimodal imaging agents.
0.25), following a rapid, straightforward, and environmentally friendly co-precipitation method. Surface modification of the IONPs with citrate improved colloidal stability, though the introduction of europium at different amounts induced slight aggregation. Morphologically, the NPs were predominantly spherical-like, with an average diameter of approximately 10 nm; however, nanorod formation was observed at higher europium content. Structural characterization confirmed that europium was incorporated into the magnetite crystal structure or deposited on the particle surface. A particularly notable property of these NPs is their dual fluorescence capability under UV irradiation, enhancing their potential as multimodal imaging agents.
Biological evaluation demonstrated the overall biocompatibility of the Fe@Eu NPs in both healthy (HEK-293) and cancerous (HeLa) cell lines following 24-hour treatment. After cellular uptake, the NPs were found to interfere with the Fenton reaction, resulting in ROS generation, apoptosis induction, and mild disruption of the cell cycle. The magnitude of these effects varied with the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu ratio, suggesting that the biological activity of the NPs can be tuned by adjusting their composition. This tunability supports their versatility across different biomedical applications.
Eu ratio, suggesting that the biological activity of the NPs can be tuned by adjusting their composition. This tunability supports their versatility across different biomedical applications.
Comparative analysis between HEK-293 and HeLa cells provided valuable insights into cellular responses and therapeutic relevance. In HEK-293 cells, formulations (B) and (C) caused minimal disruption to the cell cycle, closely resembling the untreated control. This suggests that the surface-modified IONPs effectively limited intracellular iron release and prevented excessive oxidative stress, maintaining cellular homeostasis in healthy tissues. In contrast, formulations (D) through (G) induced significant cell cycle arrest at the sub-G1 phase – a marker of apoptosis – along with a reduction in cells progressing through subsequent phases. These effects are likely due to increased iron ion release, agglomeration, or the presence of anisotropic (rod-like) structures.
In HeLa cells, all nanoparticle variants led to notable increases in sub-G1 and post-G2 DNA contents, indicating robust apoptotic activity. These findings are consistent with previous reports linking high intracellular ROS levels to apoptosis in cancer cells. ROS assays further supported this, revealing statistically significant ROS increases in HeLa cells, particularly in response to formulations (C) and (D). These effects are attributed to the enhanced oxidative potential introduced by europium incorporation, which may modulate surface chemistry and iron ion dynamics.
Further analysis of NPs’ interference in various pathways of programmed cell death was conducted utilizing flow cytometry. It was confirmed that substantial levels of early apoptosis across both cell lines were produced, with minimal necrosis observed. Apoptosis levels ranged from 40 to 70% in HEK-293 and 45–75% in HeLa cells, confirming that apoptosis is the primary mode of cell death, likely driven by intracellular iron-mediated ROS production.
Importantly, formulation (C) emerged as the most selective and promising candidate, demonstrating minimal cytotoxicity toward healthy HEK-293 cells while inducing significant apoptotic activity in HeLa cancer cells. This dual selectivity highlights a potential therapeutic window, making formulation (C) especially attractive for targeted cancer therapy with reduced risk to surrounding healthy tissue. Collectively, these findings underscore the multifunctional nature of Fe@Eu NPs: strong fluorescence emission suitable for imaging combined with selective induction of apoptosis in cancer cells. Their favorable biocompatibility in non-cancerous cells, alongside their capacity to selectively induce oxidative stress in cancerous environments, position these nanostructures as promising candidates for further development in cancer diagnostics and therapeutics.
Author contributions
Evangelia Tsitsou: data curation, formal analysis, investigation, visualization, writing – original draft. Danai Prokopiou: investigation. Athina Papadopoulou: investigation. Alexandros K. Bikogiannakis: investigation, formal analysis. Georgios Kyriakou: investigation, resources. Elias Sakellis: investigation. Nikos Boukos: resources. Marios Kostakis: investigation, Nikolaos S. Thomaidis: resources. Eleni K. Efthimiadou: project administration, funding acquisition, conceptualization, supervision, resources, methodology, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this manuscript are available from the author upon request.Supplementary information is available. See DOI: https://doi.org/10.1039/d5ma00659g.
Acknowledgements
This work was conducted with the financial support through the Special Account for Research Grants (S. A. R. G.) from the National and Kapodistrian University of Athens (N. K. U. A.). All powder diffraction data were collected at the National and Kapodistrian University of Athens X-Ray Diffraction Core Facility. The creation of the graphical abstract was carried out using the BioRender.com platform, ChemDraw Ultra 12.0 program, and Microsoft PowerPoint.References
- N. Baig, I. Kammakakam and W. Falath, Mater. Adv., 2021, 2, 1821–1871 RSC.
- B. Fu, J. Sun, Y. Cheng, H. Ouyang, G. Compagnini, P. Yin, S. Wei, S. Li, D. Li, V. Scardaci and H. Zhang, Adv. Funct. Mater., 2021, 31, 2107363 CrossRef CAS.
- L. A. Kolahalam, I. V. Kasi Viswanath, B. S. Diwakar, B. Govindh, V. Reddy and Y. L. N. Murthy, Mater. Today Proc., 2019, 18, 2182–2190 CrossRef.
- J. Silver, in Chemistry of Iron, ed. J. Silver, Springer, Netherlands, Dordrecht, 1993, pp. 1–29 Search PubMed.
- D. Nicholls, Comprehensive Inorganic Chemistry, Pergamon Press, Exeter, 1973, vol. 24 Search PubMed.
- A. S. Teja and P.-Y. Koh, Prog. Cryst. Growth Charact. Mater., 2009, 55, 22–45 CrossRef CAS.
- N. Ajinkya, X. Yu, P. Kaithal, H. Luo, P. Somani and S. Ramakrishna, Materials, 2020, 13, 4644 CrossRef CAS PubMed.
- M. Wegmann and M. Scharr, Precision Medicine, Elsevier, 2018, pp. 145–181 Search PubMed.
- M. G. Montiel Schneider, M. J. Martín, J. Otarola, E. Vakarelska, V. Simeonov, V. Lassalle and M. Nedyalkova, Pharmaceutics, 2022, 14, 204 CrossRef CAS PubMed.
- A. V. Samrot, C. S. Sahithya, J. Selvarani A, S. K. Purayil and P. Ponnaiah, Curr. Res. Green Sustainable Chem., 2021, 4, 100042 CrossRef CAS.
- M. R. Ghazanfari, M. Kashefi, S. F. Shams and M. R. Jaafari, Biochem. Res. Int., 2016, 2016, 1–32 CrossRef PubMed.
- G. S. Demirer, A. C. Okur and S. Kizilel, J. Mater. Chem. B, 2015, 3, 7831–7849 RSC.
- M. Bustamante-Torres, D. Romero-Fierro, J. Estrella-Nuñez, B. Arcentales-Vera, E. Chichande-Proaño and E. Bucio, Polymers, 2022, 14, 752 CrossRef CAS.
- A. K. Sharma, Nanotheranostics, 2023, 7, 258–269 CrossRef PubMed.
- A. Zaleska-Medynska, M. Marchelek, M. Diak and E. Grabowska, Adv. Colloid Interface Sci., 2016, 229, 80–107 CrossRef CAS PubMed.
- K. Loza, M. Heggen and M. Epple, Adv. Funct. Mater., 2020, 30, 1909260 CrossRef CAS.
- R. Pujales-Paradela, T. Granath, M. T. Seuffert, T. Kasper, K. Müller-Buschbaum and K. Mandel, J. Mater. Chem. C, 2022, 10, 1017–1028 RSC.
- M. Runowski, T. Grzyb and S. Lis, J. Nanopart. Res., 2012, 14, 1188 CrossRef PubMed.
- C. Wang, L. Xu, X. Li and Q. Lin, Chem. Phys. Chem., 2012, 13, 3765–3772 CrossRef CAS PubMed.
- S. S. Syamchand and G. Sony, J. Lumin., 2015, 165, 190–215 CrossRef CAS.
- J. Qin, G. Liang, Y. Feng, B. Feng, G. Wang, N. Wu, Y. Zhao and J. Wei, Nanoscale, 2020, 12, 6096–6103 RSC.
- Z. Li, Y. Zhang, B. Shuter and N. Muhammad Idris, Langmuir, 2009, 25, 12015–12018 CrossRef CAS PubMed.
- R. R. Silva, A. P. Duarte, R. M. Sábio, J. M. A. Caiut, M. Gressier, M.-J. Menu, A. Franco and S. J. L. Ribeiro, ChemistrySelect, 2016, 1, 5923–5928 CrossRef CAS.
- C. R. De Silva, S. Smith, I. Shim, J. Pyun, T. Gutu, J. Jiao and Z. Zheng, J. Am. Chem. Soc., 2009, 131, 6336–6337 CrossRef CAS PubMed.
- S. Han, Y. Tang, H. Guo, S. Qin and J. Wu, Nanoscale Res. Lett., 2016, 11, 273 CrossRef PubMed.
- S. Chakraborty, K. S. Sharma, A. Rajeswari, K. V. Vimalnath, H. D. Sarma, U. Pandey, J. Jagannath, R. S. Ningthoujam, R. K. Vatsa and A. Dash, J. Mater. Chem. B, 2015, 3, 5455–5466 RSC.
- L. Yang, Z. Zhou, H. Liu, C. Wu, H. Zhang, G. Huang, H. Ai and J. Gao, Nanoscale, 2015, 7, 6843–6850 RSC.
- J. C. Park, G. T. Lee, H.-K. Kim, B. Sung, Y. Lee, M. Kim, Y. Chang and J. H. Seo, ACS Appl. Mater. Interfaces, 2018, 10, 25080–25089 CrossRef CAS.
- J. Zhao, X. Li, X. Wang and X. Wang, Nanoscale Res. Lett., 2019, 14, 200 CrossRef PubMed.
- L. P. Singh, N. V. Jadhav, S. Sharma, B. N. Pandey, S. K. Srivastava and R. S. Ningthoujam, J. Mater. Chem. C, 2015, 3, 1965–1975 RSC.
- T. Zhang, Z. Wang, H. Xiang, X. Xu, J. Zou and C. Lu, ACS Appl. Mater. Interfaces, 2021, 13, 33850–33861 CrossRef CAS PubMed.
- L. S. da Costa, L. U. Khan, L. S. Franqui, F. de, S. Delite, D. Muraca, D. S. T. Martinez and M. Knobel, J. Mater. Chem. B, 2021, 9, 428–439 RSC.
- A. V. Lunin, I. L. Sokolov, I. V. Zelepukin, I. V. Zubarev, M. N. Yakovtseva, E. N. Mochalova, J. M. Rozenberg, M. P. Nikitin and E. L. Kolychev, RSC Adv., 2020, 10, 7301–7312 RSC.
- A. de Espindola, C. S. Chagas, E. Barbosa, C. E. Castro, F. L. A. Fonseca, P. S. Haddad and C. Molina, J. Magn. Magn. Mater., 2022, 545, 168751 CrossRef CAS.
- A. I. Prasad, A. K. Parchur, R. R. Juluri, N. Jadhav, B. N. Pandey, R. S. Ningthoujam and R. K. Vatsa, Dalton Trans., 2013, 42, 4885 RSC.
- C. Drivas, S. Kennou and G. Kyriakou, Appl. Surf. Sci., 2025, 682, 161672 CrossRef CAS.
- D. Prokopiou, M. Pissas, G. Fibbi, F. Margheri, B. Kalska-Szostko, G. Papanastasiou, M. Jansen, J. Wang, A. Laurenzana and E. Efthimiadou, Toxicol. In Vitro, 2021, 72, 105094 CrossRef PubMed.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS.
- J. Liu, C. Dai and Y. Hu, Environ. Res., 2018, 161, 49–60 CrossRef CAS PubMed.
- K. D. Martinson, I. S. Kondrashkova and V. I. Popkov, Russ. J. Appl. Chem., 2017, 90, 1214–1218 CrossRef CAS.
- W. Chen, F. Li and J. Yu, Mater. Lett., 2006, 60, 57–62 CrossRef CAS.
- A. Mariani, M. Campetella, C. Fasolato, M. Daniele, F. Capitani, L. Bencivenni, P. Postorino, S. Lupi, R. Caminiti and L. Gontrani, J. Mol. Liq., 2017, 226, 2–8 CrossRef CAS.
- S. M. Masoudpanah, S. M. Mirkazemi, S. Shabani and P. T. Dolat Abadi, Ceram. Int., 2015, 41, 9642–9646 CrossRef CAS.
- M. Theodosiou, E. Sakellis, N. Boukos, V. Kusigerski, B. Kalska-Szostko and E. Efthimiadou, Sci. Rep., 2022, 12, 8697 CrossRef CAS PubMed.
- F. Margheri, C. Luciani, M. L. Taddei, E. Giannoni, A. Laurenzana, A. Biagioni, A. Chillà, P. Chiarugi, G. Fibbi and M. Del Rosso, Oncotarget, 2014, 5, 1538–1553 CrossRef PubMed.
- I. Y. Kim, T. G. Lee, V. Reipa and M. B. Heo, Nanomaterials, 2021, 11, 1943 CrossRef CAS PubMed.
- Z. Darzynkiewicz, G. Juan and E. Bedner, Curr. Protoc. Cell Biol., 1999, 1, 8.4.1–8.4.18 Search PubMed.
- J. A. Pérez-Arizti, J. L. Ventura-Gallegos, R. E. Galván Juárez, M. del, P. Ramos-Godinez, Z. Colín-Val and R. López-Marure, Chem. – Biol. Interact., 2020, 317, 108966 CrossRef PubMed.
- K. Bode, C. Link, P. Krammer and H. Weyd, BIO-Protoc., 2020, 10, e3737 CAS.
- R. K. Shukla, A. Kumar, A. Pandey, S. S. Singh and A. Dhawan, J. Biomed. Nanotechnol., 2011, 7, 100–101 CrossRef CAS PubMed.
- E. Eruslanov and S. Kusmartsev, in Advanced Protocols in Oxidative Stress II, ed. D. Armstrong, Humana Press, Totowa, NJ, 2010, vol. 594, pp. 57–72 Search PubMed.
- M. Pedrino, P. Brassolatti, A. C. Maragno Fattori, J. Bianchi, J. M. de Almeida Rodolpho, K. F. de Godoy, M. Assis, E. Longo, K. Nogueira Zambone Pinto Rossi, C. Speglich and F. de Freitas Anibal, Toxicol. Mech. Methods, 2022, 32, 213–223 CrossRef CAS PubMed.
- S. Elmore, Toxicol. Pathol., 2007, 35, 495–516 CrossRef CAS PubMed.
- M. Zimmermann and N. Meyer, in Mammalian Cell Viability, ed. M. J. Stoddart, Humana Press, Totowa, NJ, 2011, vol. 740, pp. 57–63 Search PubMed.
- I. Vermes, C. Haanen, H. Steffens-Nakken and C. Reutellingsperger, J. Immunol. Methods, 1995, 184, 39–51 CrossRef CAS PubMed.
- D. Stephenson, T. Nemkov, S. M. Qadri, W. P. Sheffield and A. D’Alessandro, Front. Physiol., 2022, 13, 828087 CrossRef PubMed.
- C. L. Williams, H. M. Neu, S. L. J. Michel and D. S. Merrell, Acinetobacter baumannii, in Methods in Molecular Biology, ed. I. Biswas and P. Rather, Springer New York, Humana Press, New York, NY, 2019, vol. 1946, pp. 195–205 Search PubMed.
- Y. P. He, Y. M. Miao, C. R. Li, S. Q. Wang, L. Cao, S. S. Xie, G. Z. Yang, B. S. Zou and C. Burda, Phys. Rev. B, 2005, 71, 125411 CrossRef.
- S. Bhattacharjee, J. Controlled Release, 2016, 235, 337–351 CrossRef CAS PubMed.
- R. Xu, Particuology, 2008, 6, 112–115 CrossRef CAS.
- M. Danaei, M. Dehghankhold, S. Ataei, F. Hasanzadeh Davarani, R. Javanmard, A. Dokhani, S. Khorasani and M. Mozafari, Pharmaceutics, 2018, 10, 57 CrossRef PubMed.
- A. S. Lawrie, A. Albanyan, R. A. Cardigan, I. J. Mackie and P. Harrison, Vox Sang., 2009, 96, 206–212 CrossRef CAS PubMed.
- D. Predoi, Dig. J. Nanomater. Bios., 2007, 2, 169–173 Search PubMed.
- M. Morel, F. Martínez and E. Mosquera, J. Magn. Magn. Mater., 2013, 343, 76–81 CrossRef CAS.
- Y. Wei, B. Han, X. Hu, Y. Lin, X. Wang and X. Deng, Procedia Eng., 2012, 27, 632–637 CrossRef CAS.
- G. B. Deacon and R. J. Phillips, Coord. Chem. Rev., 1980, 33, 227–250 CrossRef CAS.
- J.-G. Kang, Y. Jung, B.-K. Min and Y. Sohn, Appl. Surf. Sci., 2014, 314, 158–165 CrossRef CAS.
- I. L. Ardelean, L. B. N. Stoencea, D. Ficai, A. Ficai, R. Trusca, B. S. Vasile, G. Nechifor and E. Andronescu, J. Nanomater., 2017, 2017, 1–9 CrossRef.
- K. Chinnaraj, A. Manikandan, P. Ramu, S. A. Antony and P. Neeraja, J. Supercond. Nov. Magn., 2015, 28, 179–190 CrossRef CAS.
- C. S. Ciobanu, E. Andronescu and D. Predoi, Dig. J. Nanomater. Bios., 2011, 6, 1239–1244 Search PubMed.
- R. Gabbasov, M. Polikarpov, V. Cherepanov, M. Chuev, I. Mischenko, A. Lomov, A. Wang and V. Panchenko, J. Magn. Magn. Mater., 2015, 380, 111–116 CrossRef CAS.
- V. G. Pol, O. Palchik, A. Gedanken and I. Felner, J. Phys. Chem. B, 2002, 106, 9737–9743 CrossRef CAS.
- R. Marin and D. Jaque, Chem. Rev., 2021, 121, 1425–1462 CrossRef CAS PubMed.
- N. S. Leel, M. Kiran, M. K. Kumawat, P. A. Alvi, V. S. Vats, D. Patidar, B. Dalela, S. Kumar and S. Dalela, J. Lumin., 2023, 263, 119981 CrossRef CAS.
- A. V. Thorat, T. Ghoshal, P. Carolan, J. D. Holmes and M. A. Morris, J. Phys. Chem. C, 2014, 118, 10700–10710 CrossRef CAS.
- D. F. Mullica, C. K. C. Lok, H. O. Perkins, G. A. Benesh and V. Young, J. Electron Spectrosc. Relat. Phenom., 1995, 71, 1–20 CrossRef CAS.
- N. S. Leel, S. Kumar, P. A. Alvi, B. Dalela, A. Sharma, S. Kumar and S. Dalela, J. Magn. Magn. Mater., 2024, 603, 172233 CrossRef CAS.
- A. Atrei, B. Lesiak-Orlowska and J. Tóth, Appl. Surf. Sci., 2022, 602, 154366 CrossRef CAS.
- X. Chen, X. Wang and D. Fang, Fuller. Nanotub. Carbon Nanostruct., 2020, 28, 1048–1058 CrossRef CAS.
- D. Briggs, Handbook of X-ray Photoelectron Spectroscopy, ed. C. D. Wagner, W. M. Riggs, L. E. Davis, J. F. Moulder and G. E. Muilenberg, Perkin-Elmer Corp., Physical Electronics Division, Eden Prairie, Minnesota, USA, 1981, vol. 3, v-v Search PubMed.
- A. K. Friedman, W. Shi, Y. Losovyj, A. R. Siedle and L. A. Baker, J. Electrochem. Soc., 2018, 165, H733–H741 CrossRef CAS.
- K. Binnemans, Coord. Chem. Rev., 2015, 295, 1–45 CrossRef CAS.
- A. Ugale, T. N. Kalyani and S. J. Dhoble, Lanthanide-Based Multifunctional Materials, Elsevier, 2018, pp. 59–97 Search PubMed.
- S. Chaudhary, P. Sharma, S. Kumar, S. A. Alex, R. Kumar, S. K. Mehta, A. Mukherjee and A. Umar, J. Mol. Liq., 2018, 269, 783–795 CrossRef CAS.
- H. Terraschke and C. Wickleder, Chem. Rev., 2015, 115, 11352–11378 CrossRef CAS PubMed.
- C. D. Mungmode, D. H. Gahane and S. V. Moharil, Bikaner, India, 2018, p. 060037.
- D. Kim, T. H. Kim, T. E. Hong, J.-S. Bae, C. H. Kim, J. Kim, S.-J. Kim, K.-W. Jeon and J.-C. Park, Materials, 2020, 13, 1859 CrossRef CAS PubMed.
- L. Saravanan, R. Jayavel, S. S. Aldeyab, J. S. Zaidi, K. Ariga and A. Vinu, J. Nanosci. Nanotechnol., 2011, 11, 7783–7788 CrossRef CAS PubMed.
- Y.-F. Xu, D.-K. Ma, M.-L. Guan, X.-A. Chen, Q.-Q. Pan and S.-M. Huang, J. Alloys Compd., 2010, 502, 38–42 CrossRef CAS.
- E.-J. Cho and S.-J. Oh, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, R15613–R15616 CrossRef CAS.
- I. Choudhary, R. Shukla, A. Sharma and K. K. Raina, J. Mater. Sci.: Mater. Electron., 2020, 31, 20033–20042 CrossRef.
- R. Meenakshi, V. T. Jisha and S. S. Soumya, J. Sol-Gel Sci. Technol., 2024, 111, 540–552 CrossRef CAS.
- M. V. Shestovskaya, A. L. Luss, O. A. Bezborodova, V. V. Makarov and A. A. Keskinov, Pharmaceutics, 2023, 15, 2406 CrossRef CAS PubMed.
- N. Singh, G. J. S. Jenkins, R. Asadi and S. H. Doak, Nano Rev., 2010, 1, 5358 CrossRef PubMed.
- O. A. Alabi, A. H. Silva, M. P. Rode, C. dal Pizzol, A. M. de Campos, F. B. Filippin-Monteiro, A. A. Bakare and T. B. Creczynski-Pasa, Toxicol. Ind. Health, 2021, 37, 77–89 CrossRef CAS PubMed.
- T. Mai and J. Z. Hilt, J. Nanoparticle Res., 2017, 19, 253 CrossRef.
- L. G. Rodriguez, X. Wu and J.-L. Guan, Cell Migration, Humana Press, New Jersey, 2004, vol. 294, pp. 023–030 Search PubMed.
- J. E. N. Jonkman, J. A. Cathcart, F. Xu, M. E. Bartolini, J. E. Amon, K. M. Stevens and P. Colarusso, Cell Adhes. Migr., 2014, 8, 440–451 CrossRef PubMed.
- R. Meguro, Y. Asano, S. Odagiri, C. Li, H. Iwatsuki and K. Shoumura, Arch. Histol. Cytol., 2007, 70, 1–19 CrossRef CAS PubMed.
- M. Monici, Biotechnology Annual Review, Elsevier, 2005, vol. 11, pp. 227–256 Search PubMed.
- N. M. Cordina, N. Sayyadi, L. M. Parker, A. Everest-Dass, L. J. Brown and N. H. Packer, Sci. Rep., 2018, 8, 4521 CrossRef PubMed.
- T. S. Blacker and M. R. Duchen, Free Radicals Biol. Med., 2016, 100, 53–65 CrossRef CAS PubMed.
- D. Gewirtz, Biochem. Pharmacol., 1999, 57, 727–741 CrossRef CAS PubMed.
- H. M. Al-Aamri, H. Ku, H. R. Irving, J. Tucci, T. Meehan-Andrews and C. Bradley, BMC Cancer, 2019, 19, 179 CrossRef PubMed.
- E. Lukášová, M. Řezáčová, A. Bačíková, L. Šebejová, J. Vávrová and S. Kozubek, FEBS Open Bio, 2019, 9, 870–890 CrossRef PubMed.
- Y.-P. Hsiao, H.-L. Huang, W.-W. Lai, J.-G. Chung and J.-H. Yang, J. Dermatol. Sci., 2009, 54, 175–184 CrossRef CAS PubMed.
- M. M. Al-Oqail, N. N. Farshori, E. S. Al-Sheddi, S. M. Al-Massarani, Q. Saquib, M. A. Siddiqui and A. A. Al-Khedhairy, Oxid. Med. Cell. Longev., 2021, 2021, 6695634 CrossRef PubMed.
- S. Shukla, A. Jadaun, V. Arora, R. K. Sinha, N. Biyani and V. K. Jain, Toxicol. Rep., 2015, 2, 27–39 CrossRef CAS PubMed.
- U. S. Gaharwar, R. Meena and P. Rajamani, J. Appl. Toxicol., 2017, 37, 1232–1244 CrossRef CAS PubMed.
- M. Havrdova, K. Hola, J. Skopalik, K. Tomankova, M. Petr, K. Cepe, K. Polakova, J. Tucek, A. B. Bourlinos and R. Zboril, Carbon, 2016, 99, 238–248 CrossRef CAS.
- C. Xue, J. Wu, F. Lan, W. Liu, X. Yang, F. Zeng and H. Xu, J. Nanosci. Nanotechnol., 2010, 10, 8500–8507 CrossRef CAS PubMed.
- S. Fu, S. Wang, X. Zhang, A. Qi, Z. Liu, X. Yu, C. Chen and L. Li, Colloids Surf. B Biointerfaces, 2017, 154, 239–245 CrossRef CAS PubMed.
- Y. Liu, Y. Meng, Y. Zhu, L. Gu, A. Ma, R. Liu, D. Liu, S. Shen, S. Zhang, C. Xu, J. Zhang and J. Wang, Regener. Biomater., 2024, 11, rbae065 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |