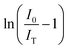Open Access Article
Open Access ArticleThermally stable Li+ co-doped ZnMoO4:Eu3+ phosphors for white LEDs, nitroaromatic sensing and low temperature non-contact optical thermometry applications†
Astha
Tyagi
 a,
M.
Rakshita
a,
M.
Rakshita
 b,
D.
Haranath
b,
D.
Haranath
 b and
Chikkadasappa
Shivakumara
b and
Chikkadasappa
Shivakumara
 *a
*a
aSolid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore-560012, India. E-mail: asthatyagi@iisc.ac.in; shiva@iisc.ac.in
bLuminescent Materials and Devices (LMD) Group, Department of Physics, National Institute of Technology, Warangal, 506004, Telangana, India. E-mail: rakshitaammulu1997@gmail.com; haranath@nitw.ac.in
First published on 15th July 2025
Abstract
The development of multifunctional luminescent materials with high thermal stability and sensitivity is crucial for advanced lighting, chemical sensing, and temperature monitoring applications. However, many existing red-emitting phosphors are limited by poor thermal stability, weak chemical selectivity, and narrow functional temperature windows. In this work, we present a Li+ co-doped ZnMoO4:Eu3+ phosphor (Zn0.88Eu0.09Li0.03MoO4, ZELMO) that overcomes these limitations through Li+ incorporation, which enhances crystallinity, facilitates charge compensation, and modifies the local crystal field around Eu3+ ions. ZELMO exhibits a two-fold increase in photoluminescence intensity, excellent color purity (93.6%), and thermal stability with 83% intensity retention at 423 K. It also demonstrates selective and ultra-sensitive detection of 4-nitrophenol, with a low detection limit of 12 nM. Moreover, ZELMO enables non-contact optical thermometry in the 15–295 K range, achieving a maximum relative sensitivity of 0.61% K−1. These results position ZELMO as a promising red-emitting phosphor for multifunctional use in solid-state lighting, environmental sensing, and low-temperature optical thermometry.
1. Introduction
The development of advanced luminescent materials has become essential for diverse applications, such as bio-imaging,1 temperature sensing,2 optical heating,3 display technologies,4 chemical sensing,5 solid-state lighting,6 and white light emission sources.7 Phosphor-based white light-emitting diodes (WLEDs) play a significant role due to their widespread use in general illumination and as backlights for liquid crystal displays (LCDs).8 Commercial WLEDs typically employ Ce3+-activated garnet yellow phosphors coated on blue-emitting LED chips.9–12 However, the lack of a red-emitting component in these systems often leads to a reduced color rendering index (CRI < 75) and higher correlated color temperature (CCT > 4500 K), adversely affecting the overall color quality.13,14 To overcome this limitation, the inclusion of red-emitting phosphors, especially Eu3+-activated oxide phosphors, has shown significant effectiveness. These materials are recognized for their narrow emission bands, excellent color purity, and remarkable quantum efficiency, making them ideal candidates for enhancing WLED performance.15–17Beyond lighting applications, the selective detection of nitroaromatic compounds has gained considerable attention due to their widespread use in explosives, industrial processes, and agriculture, as well as their potential environmental and health hazards.18,19 Exposure to 4-NP (para-nitrophenol) can result in critical health complications, including methemoglobinemia, damage to the kidneys and liver, persistent headaches, and nausea.20,21 Due to its toxicity and potential carcinogenicity, the U.S. Environmental Protection Agency has designated 4-NP as a “Priority Pollutant”.22 These factors highlight the urgent need for highly sensitive and selective detection methods. Luminescent materials, such as Eu3+-doped phosphors, offer a promising approach for addressing this challenge, enabling efficient and accurate detection of 4-NP.23
Accurate and efficient temperature sensing at low temperatures is essential for a wide range of disciplines, including fundamental sciences, engineering, manufacturing, metallurgy, and aerospace.24,25 Traditional temperature sensors, such as platinum resistance temperature detectors (RTDs),26 specialized thermistors,27 and silicon diodes,28 have been widely used due to their sensitivity and reliability. However, these sensors are typically large, require physical contact with the object being measured, and are unsuitable for remote temperature detection in large-area thermal distributions, fast-moving objects, or micro- and nanoscale systems.29 In contrast, luminescence-based ratiometric techniques offer a promising alternative for precise and efficient temperature measurements. These methods are non-invasive, self-calibrating, and provide high spatial resolution and sensitivity.30,31 Utilizing the temperature-dependent photoluminescence of rare-earth-doped materials, such as Eu3+-activated phosphors, this technique enables innovative, accurate, and scalable approaches to remote temperature sensing.32
Among potential host materials, ZnMoO4 stands out due to its excellent chemical stability,33 wide band gap,34 and highly tunable photoluminescence properties.35 These attributes, combined with its ability to accommodate rare-earth dopants like Eu3+, make ZnMoO4 a promising candidate for developing high-performance red phosphors.
In this study, we explore the optimization of Li+ co-doped ZnMoO4:Eu3+ for multiple applications, including its use as a red component in white LEDs, as well as for selective sensing of nitroaromatic compounds and temperature sensing. By systematically varying Eu concentration and co-doping with lithium, we identify the optimal dopant levels to enhance the photoluminescent properties. The material shows excellent sensitivity and selectivity for detecting nitroaromatic compounds, particularly para-nitrophenol, and demonstrates thermal stability at high temperatures, making it suitable for demanding applications. Additionally, temperature-dependent PL studies highlight its potential for temperature sensing. This work emphasizes the multifunctional potential of Eu-doped ZnMoO4 as a versatile material for optoelectronic devices, lighting technologies, and sensing applications.
2. Experiments
2.1. Materials and methods
The phosphors with compositions Zn(1−x)EuxMoO4 (0 ≤ x ≤ 0.11) and Zn(0.91−y)Eu0.09LiyMoO4 (0 ≤ y ≤ 0.05) were synthesized using the nitrate–citrate gel combustion method, a process well-suited for achieving homogeneous and fine powders. Zn(NO3)2·6H2O (99%, SD fine Chemicals), (NH4)6Mo7O24·4H2O (99%, SD fine Chemicals), Eu2O3 (99.9%, Sigma-Aldrich), and Li2CO3 (99%, Loba Chemie) were used as precursors, and citric acid (99.5%, Spectrochem) was used as a fuel for auto combustion. Zn(NO3)2·6H2O and (NH4)6Mo7O24·4H2O were dissolved in deionized water, while Eu2O3 and Li2CO3 were dissolved in dilute nitric acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). After combining the solutions, citric acid was added as a fuel. Heating at 150 °C resulted in gel formation, which combusted at ∼200 °C to yield powders. These were calcined at 700 °C for 3 hours to produce phase-pure phosphors.
1, v/v). After combining the solutions, citric acid was added as a fuel. Heating at 150 °C resulted in gel formation, which combusted at ∼200 °C to yield powders. These were calcined at 700 °C for 3 hours to produce phase-pure phosphors.
To perform the screening for nitroaromatic compounds, 50 mM solutions of various nitroaromatics were prepared in a pH 7 buffer solution (Merck, ETS). The synthesized Zn0.88Eu0.09Li0.03MoO4 phosphor was added at a concentration of 1 mg mL−1 to these solutions followed by 30 minutes of sonication to ensure uniformity prior to measurements. Similarly, for para-nitrophenol (4-NP) detection, the phosphor was mixed with PNP solutions (1 nM to 200 μM) prepared in the same pH 7 buffer, with sonication for 30 minutes. This preparation ensures reproducibility and highlights the selective sensing capability of the phosphor.
The Zn0.88Eu0.09Li0.03MoO4-polyvinyl alcohol (PVA) film was prepared by mixing a sonicated phosphor solution (0.2 g in deionized water) with a PVA solution (2 g in 50 mL deionized water), heating at 90 °C under stirring. The mixture was subsequently transferred onto a glass substrate and dried for 24 hours.
2.2. Characterization
Powder X-ray diffraction (XRD) analysis of the samples was performed using a PANalytical Empyrean diffractometer, operating at 45 kV and 30 mA with Cu Kα radiation (λ = 1.5418 Å). Data was acquired over a 2θ range of 10° to 80° with a scan rate of 2° min−1. Rietveld refinement was carried out using Fullprof Suite-2000 software to determine the structural parameters from the XRD patterns. X-ray photoelectron spectroscopy (XPS) study was conducted using a Thermo Scientific K-Alpha surface analysis instrument. The direct excitation 7Li magic angle spinning (MAS) experiments were conducted at room temperature using a 9.4 T solid-state NMR spectrometer (7Li Larmor frequency of 155.452 MHz) with a MAS frequency of 10 kHz. Fourier transform infrared (FTIR) spectroscopy was performed to analyze the functional groups present in the sample. The measurements were conducted in the range of 350–4000 cm−1 using a PerkinElmer Frontier spectrometer in attenuated total reflectance (ATR) mode. Diffuse reflectance spectra (DRS) were acquired using a PerkinElmer Lambda 750 spectrometer to evaluate the band gap of the compounds. Photoluminescence (PL) emission and excitation measurements were performed using a Jobin-Yvon FluoroMax spectrometer, which was equipped with a 450 W xenon lamp serving as the excitation source. The temperature-dependent PL studies in the range of 303 K to 573 K were conducted using a Horiba Scientific spectrofluorometer (model: Fluorolog-3). The temperature-dependent PL studies in the range of 15 K to 295 K were conducted using the WITec Alpha 300R photoluminescence Raman microscope and spectrometer. For lifetime measurements, the Edinburgh PL instrument was used with a microsecond flash lamp. Photoluminescence quantum yield (QY) measurements were carried out using an Edinburgh Instruments FLS1000 photoluminescence spectrometer equipped with a 450 W xenon arc lamp and an integrating sphere (model N-M01) for absolute measurements. The measurements were carried out at room temperature with an excitation wavelength of 394 nm. A BaSO4 coated reference was employed to obtain the baseline spectrum necessary for accurate QY calculation. The QY was calculated using the software provided by the instrument, following standard protocols.3. Results and discussion
3.1. Structural analysis
The X-ray diffraction (XRD) patterns of the synthesized compounds were analysed to study the crystal structure and the influence of dopants on the crystal lattice. The XRD patterns of the Zn1−xEuxMoO4 (0.01 ≤ x ≤ 0.11) phosphors are shown in Fig. 1(a). The diffraction peaks of all the synthesized compounds closely aligned with those in the JCPDS file no. 72-1486,36 with no evidence of secondary phases or impurities from the starting materials, indicating the formation of single-phase compounds. Additionally, it was observed that the low concentration of Eu3+ did not significantly influence the host lattice by replacing Zn2+ sites. This was confirmed by calculating the percentage difference in radii (Rr) using the following expression:37 | (1) |
 | ||
| Fig. 1 (a) XRD patterns of Zn(1−x)EuxMoO4 (0.01 ≤ x ≤ 0.11) phosphors. (b) Observed, calculated, and difference XRD patterns of ZnMoO4. (c) Crystal structure of ZnMoO4. | ||
Rietveld refinement analysis was conducted to determine the structural parameters of the host material using powder XRD data. The results confirmed that the synthesized compound crystallized in a triclinic structure with the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2). Fig. 1(b) illustrates the observed, calculated, and difference powder XRD patterns for the ZnMoO4 compound, showing excellent agreement between the observed and calculated data. The refined structural parameters for the sample are presented in Table S1 (ESI†). Furthermore, the VESTA program was employed to model the crystal structure of the ZnMoO4 host, utilizing atomic coordination and lattice parameters, as depicted in Fig. 1(c). It shows that the crystal structure of ZnMoO4 consists of a series of distorted Mo–O tetrahedra, Zn–O octahedra, and Zn–O pentahedra. The Eu3+ ions replaced the Zn2+ sites in the ZnMoO4 system.
(no. 2). Fig. 1(b) illustrates the observed, calculated, and difference powder XRD patterns for the ZnMoO4 compound, showing excellent agreement between the observed and calculated data. The refined structural parameters for the sample are presented in Table S1 (ESI†). Furthermore, the VESTA program was employed to model the crystal structure of the ZnMoO4 host, utilizing atomic coordination and lattice parameters, as depicted in Fig. 1(c). It shows that the crystal structure of ZnMoO4 consists of a series of distorted Mo–O tetrahedra, Zn–O octahedra, and Zn–O pentahedra. The Eu3+ ions replaced the Zn2+ sites in the ZnMoO4 system.
Fig. S1 (ESI†) presents the XRD patterns of Li+-co-doped Zn0.91Eu0.09MoO4 phosphors. The patterns align well with those of the Zn0.91Eu0.09MoO4 compound, confirming the retention of the triclinic crystal structure. No impurity peaks were observed for Li+ doping levels up to 5 mol%, indicating the successful incorporation of Li+ ions into the ZnMoO4 lattice.
3.2. XPS study
X-ray photoelectron spectroscopy (XPS) analysis was employed to provide in-depth insights into the surface chemical composition and electronic states of the synthesized samples Zn0.91Eu0.09MoO4 and Zn0.88Eu0.09Li0.03MoO4. The binding energy for each element was calibrated using the adventitious carbon peak at 284.8 eV as a reference for correcting any potential charge-induced shifts during the measurements.38Fig. 2(a) shows a survey scan of the Zn0.88Eu0.09Li0.03MoO4 phosphor, with characteristic peaks corresponding to Zn, Mo, O, Eu, and Li, confirming the successful incorporation of these elements into the ZnMoO4 matrix. Importantly, no impurities were detected in the XPS survey scan apart from the adventitious carbon peak, indicating the high purity of the synthesized phosphor materials. All the peaks in the survey scan were identified and assigned with reference to the National Institute of Standard Technology (NIST) XPS database.39 The XPS binding energy spectra of Zn(2p), Mo(3d), Eu(3d), Li(1s), and O(1s) for Zn0.91Eu0.09MoO4 and Zn0.88Eu0.09Li0.03MoO4 samples are shown in Fig. 2(b)–(f). In Fig. 2(b), the Zn 2p3/2 peak was detected at approximately 1022.0 eV, and the Zn 2p1/2 peak at around 1045.0 eV, consistent with Zn2+ in the ZnMoO4 matrix. These binding energies indicate that zinc remains in the +2 oxidation state in both samples. The presence of both spin–orbit split peaks confirms the stable chemical environment of Zn2+ across the samples, highlighting that Li co-doping has a negligible impact on the zinc oxidation state.40 In Fig. 2(c), the Mo 3d5/2 and Mo 3d3/2 peaks appeared around 232.4 eV and 235.6 eV, respectively, corresponding to Mo6+ states.40 In Fig. 2(d), both the Eu 3d5/2 and Eu 3d3/2 peaks were observed, confirming the presence of Eu3+ in the samples. The Eu 3d5/2 peak appeared at approximately 1134.5 eV, and the Eu 3d3/2 peak at around 1164.5 eV.41 The Li 1s peak (Fig. 2(e)) at 54.7 eV confirmed the successful incorporation of Li into the lattice.42 The O 1s spectra (Fig. 2(f)) were deconvoluted into two peaks to analyze the oxygen species; peak 1 (purple color) corresponds to lattice oxygen, and peak 2 (pink color) corresponds to oxygen vacancies. The peaks corresponding to lattice oxygen are observed at lower binding energy, while those corresponding to oxygen vacancies are at higher binding energy values. In the case of Zn0.91Eu0.09MoO4, the peaks were detected at around 530.48 eV and 531.71 eV, and in the case of the Zn0.88Eu0.09Li0.03MoO4 phosphor, the peaks were detected at around 530.49 eV and 532.10 eV.43 The percentage of oxygen vacancies was found to be 22.41% and 31.22% for Zn0.91Eu0.09MoO4 and Zn0.88Eu0.09Li0.03MoO4, respectively. This shows that there is an increase in the amount of oxygen vacancies upon co-doping with Li ions. These oxygen vacancies act as sensitizers, facilitating highly efficient radiative energy transfer to Eu3+ ions by strong mixing of the charge transfer band (CTB), leading to enhanced luminescence.44
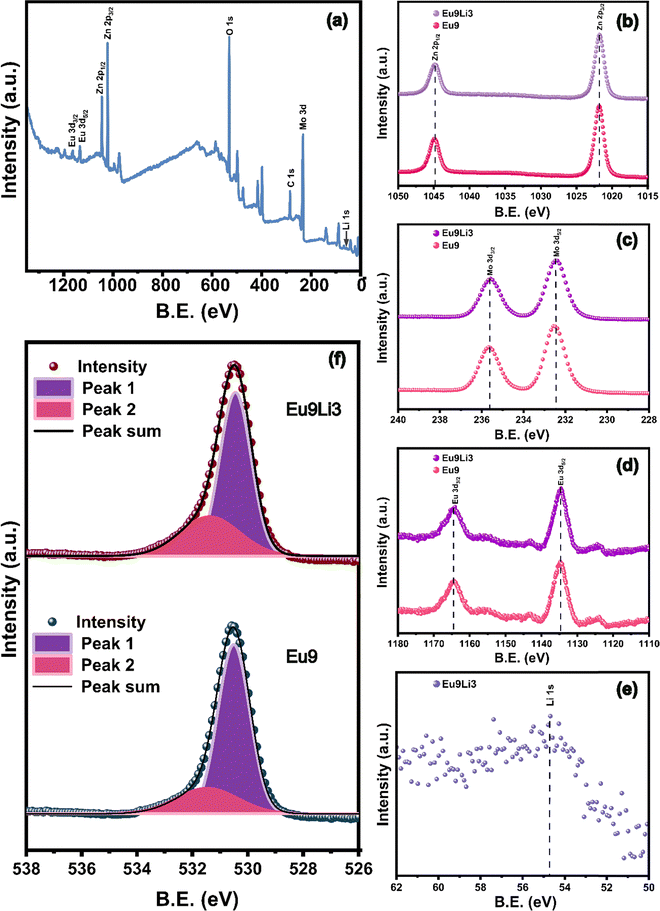 | ||
| Fig. 2 (a) XPS spectrum of the Zn0.88Eu0.09Li0.03MoO4 phosphor. High resolution XPS spectra of (b) Zn 2p, (c) Mo 3d, (d) Eu 3d, (e) Li 1s, and (f) O 1s. | ||
3.3. Solid state 7Li NMR
Fig. 3(a) presents the 7Li MAS-NMR spectra of Zn(0.91−y)Eu0.09LiyMoO4 (y = 0.01, 0.03, and 0.05) phosphors confirming the incorporation of Li ions in the ZnMoO4 lattice. Fig. 3(b) presents a zoomed-in view of the isotropic peak. All the samples exhibit two prominent peaks: one at approximately 3.6 ppm, which corresponds to diamagnetic Li sites in the sample, and another around −15.2 ppm, attributed to paramagnetic Li sites resulting from the presence of paramagnetic Eu3+ sites. However, as the lithium concentration increases beyond 3 mol%, the 7Li NMR spectrum shows a higher intensity of the diamagnetic peak, as further discussed in Section 3.7. | ||
| Fig. 3 (a) 7Li NMR spectra of Zn0.91−yEu0.09LiyMoO4 (y = 0.01, 0.03, and 0.05). (b) Magnified view of the isotropic peak for detailed observation. | ||
3.4. FESEM analysis
Fig. 4 shows the detailed analysis of the Zn0.88Eu0.09Li0.03MoO4 phosphor to identify the morphology and homogeneity of the dopants. As revealed in Fig. 4(a), the morphology of the obtained sample was microparticles with irregular shapes and varying sizes. Fig. 4(b)–(e) illustrate the elemental mapping of Zn, Mo, O, and Eu, respectively. These images confirm the homogeneous distribution of all the elements across the agglomerated particles, indicating successful and even incorporation of Eu3+ ions within the ZnMoO4 matrix. Fig. 4(f) shows the EDS image, which highlights specific areas of the sample where the EDS spectrum was obtained. Fig. 4(g) shows the EDS spectrum, which reveals characteristic peaks corresponding to these elements, with no significant impurities detected. The Al, C, and Au peaks in the EDS spectrum likely result from the aluminium stub, carbon tape, and gold sputter coating used for conductivity during SEM analysis.453.5. UV DRS and FTIR
The diffuse reflectance spectroscopy (DRS) spectra of the Zn(1−x)EuxMoO4 phosphors were recorded in the 200–800 nm range, as shown in Fig. 5(a). According to Zhai et al.,46 ZnMoO4 exhibits two distinct absorption bands: one in the 250–310 nm range, corresponding to the transition of electrons from the valence band to the conduction band of ZnMoO4, and another in the 310–390 nm range, which is associated with defects present in the ZnMoO4 lattice. Fig. 5(a) clearly shows that doping with Eu ions increases the population density of defects, as indicated by the enhanced absorption in the 310–390 nm region. The sharp bands at 394 nm, 464 nm, and 536 nm are attributed to the 7F0 → 5L6, 7F0 → 5D2, and 7F0 → 5D1 transitions of the Eu3+ ion, respectively.47 This confirms the successful incorporation of Eu3+ ions into the ZnMoO4 lattice. | ||
| Fig. 5 (a) and (b) DRS spectra and Kubelka–Munk plot of Zn(1−x)EuxMoO4 (x = 0.00, 0.01, 0.05, and 0.09) phosphors. (c) FTIR spectra of Zn(1−x)EuxMoO4 (0.01 ≤ x ≤ 0.11) phosphors. | ||
The Kubelka–Munk theory48 was employed to calculate the band gap of phosphor samples using the reflectance data.
 | (2) |
 is the diffuse reflectance of the sample.
is the diffuse reflectance of the sample.
The bandgap energy of the samples can be calculated using the Tauc relation49
| (αhν)1/n = B(hν − Eg) | (3) |
| (F(R∞)·hν)1/n = B(hν − Eg) | (4) |
The infrared vibrational modes of the bonds in the Zn(1−x)EuxMoO4 (0 ≤ x ≤ 0.11) phosphors were analyzed using FTIR spectroscopy. The FTIR spectra, recorded in transmittance mode within the wavenumber range of 385 to 4000 cm−1, are shown in Fig. 5(c). The phosphor samples showed prominent and identical IR absorption peaks at 948, 857, 727, and 426 cm−1. These peaks correspond to the ν1 stretching of Mo–O in bridging Mo–O–Mo linkages, the ν1 stretching of (MoO4)2− tetrahedra, the ν3 stretching of Mo–O, and the ν4 bending mode of Mo–O, respectively. The absence of any additional vibrational bands confirms the monophasic nature of the phosphors.
3.6. Photoluminescence properties
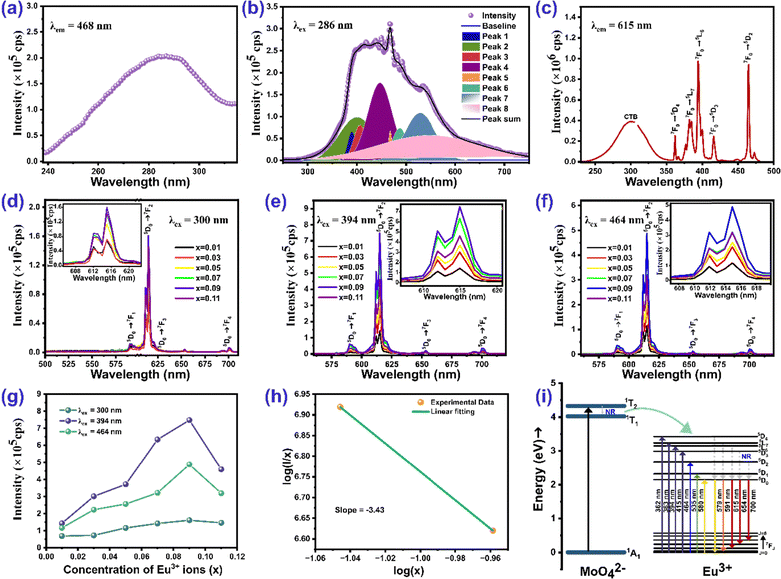
The PL excitation spectrum of the host ZnMoO4 acquired at 468 nm emission wavelength is shown in Fig. 6(a). The excitation spectrum shows a ligand to metal charge transfer (LMCT) broad band centered at 286 nm, which corresponds to O2− to Mo6+ charge transfer in the [MoO4]2− groups of ZnMoO4.53Fig. 6(b) exhibits the emission spectrum of ZnMoO4 acquired at emission wavelength 286 nm. The emission peak is deconvoluted into several Gaussian peaks in the range of 300 to 750 nm. The peak in the UV emission range at around 389 nm corresponds to the recombination of free excitons via donor–acceptor pair transitions from the conduction band (CB) to the valence band (VB). The peak at 407 nm is ascribed to electron transitions from the lower edge of the conduction band to the zinc vacancy (VZn) level.54 The blue emission at 447 nm arises from electron transitions from the donor level of zinc interstitials to the valence band.55 The emission band at 487 nm can be associated with various types of distorted regular MoO4 complexes.56 The green luminescence bands observed between 520 and 550 nm can be attributed to electron transitions from deep oxygen vacancy levels to the top of the valence band.54Fig. 6(c) shows the excitation spectra of the Zn0.91Eu0.09MoO4 phosphor at 615 nm emission wavelength. The broad band in the range of 240–350 nm arises because of the contribution from different electronic transitions, which are (i) charge transfer from O2− to Mo6+, (ii) charge transfer from the 2p orbital of O2− to the 4f orbital of Eu3+, and (iii) inter valence charge transfer from Eu3+ to Mo6+.57 The spectra contain other excitation peaks in the range of 350–500 nm arising due to the 4f–4f transition of the Eu3+ ion. Among these transitions, the most intense ones are located at 394 nm (7F0 → 5L6) and 464 nm (7F0 → 5D2), and the less intense ones are located at 362 nm (7F0 → 5D4), 382 nm (7F0 → 5G2), and 416 nm (7F0 → 5D3).37Fig. 6(d)–(f) show the emission spectrum of the Zn(1−x)EuxMoO4 (0 ≤ x ≤ 0.11) phosphor at excitation wavelengths 300 nm, 394 nm, and 464 nm, respectively. The peak position remains the same, whereas there is variation in the emission intensities for both the excitation wavelengths. The most intense emission peak occurs at 615 nm, corresponding to the 5D0 → 7F2 transition, and the other less intense peaks are at 590 nm (5D0 → 7F1), 653 nm (5D0 → 7F3) and 700 nm (5D0 → 7F4). The 5D0 → 7F1 emission at 590 nm is a magnetic dipole transition (MDT) and is generally unaffected by changes in the local environment surrounding the Eu3+ ion. In contrast, the 5D0 → 7F1 emission at 615 nm is an electric dipole transition (EDT) and is highly sensitive to the local environment, including factors like symmetry and the local field around the Eu3+ ion.37,57 The significantly greater intensity of the 5D0 → 7F2 transition at 615 nm (EDT) compared to the 5D0 → 7F1 transition at 590 nm (MDT) in ZnMoO4 suggests that Eu3+ ions at the Zn site lack inversion symmetry, indicating a low local symmetry around the europium ion in ZnMoO4. Fig. 6(g) shows that the emission intensity at 615 nm for all excitation wavelengths increases as the Eu ion concentration increases, reaching a peak at 9 mol% and then decreasing afterward.
To gain more insight into the concentration quenching of the Zn(1−x)EuxMoO4 (0 ≤ x ≤ 0.11) phosphors, the distance between Eu3+ ions, was calculated using the following equation.58
 | (5) |
 | (6) |
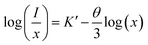 | (7) |
 . The critical concentration of Eu3+ was determined to be 9 mol%. The dependence of the emission intensity of the ZnMoO4:xEu3+ phosphor excited at 394 nm as a function of the corresponding concentration of Eu3+ for concentration greater than the critical concentration (x ≥ 0.09) was evaluated. The value of θ can be determined from the slope (−θ/3) of the plot, which was found to be 10.3, close to 10. This suggests that the energy migration between Eu3+ ions is primarily due to quadrupole–quadrupole interactions.
. The critical concentration of Eu3+ was determined to be 9 mol%. The dependence of the emission intensity of the ZnMoO4:xEu3+ phosphor excited at 394 nm as a function of the corresponding concentration of Eu3+ for concentration greater than the critical concentration (x ≥ 0.09) was evaluated. The value of θ can be determined from the slope (−θ/3) of the plot, which was found to be 10.3, close to 10. This suggests that the energy migration between Eu3+ ions is primarily due to quadrupole–quadrupole interactions.
The energy transfer mechanism from the host ZnMoO4 to Eu3+ ions is depicted in the schematic diagram in Fig. 6(i). Upon excitation at 286 nm, an electron in the oxygen 2p state (1A1) is excited to the 1T2 level of the molybdenum 4d state in MoO42−, after which it relaxes to the 1T1 excited state. The energy gained by MoO42− is then transferred to the 5D4 or higher energy levels of the Eu3+ ions within the host lattice via a resonance process.61 Upon excitation at 300 nm (charge transfer band), the emission spectra shown in Fig. 6(d) exclusively contain very sharp peaks corresponding to Eu3+. Compared with the emission peaks of Eu3+, the intrinsic blue-green emission from MoO42− groups is very weak, suggesting the existence of efficient energy transfer from the MoO42− cluster to the doped Eu3+ ion in ZnMoO4:Eu.
3.7. Photoluminescence properties of Li+ codoped Eu3+ ZnMoO4
To further investigate the effect of alkali ion co-doping on the Eu-doped phosphor materials, Zn0.91Eu0.09MoO4 was co-doped with Li+ at concentrations of 1 mol%, 3 mol%, and 5 mol%. The PL emission spectra of the Zn(0.91−y)Eu0.09LiyMoO4 (0 ≤ y ≤ 0.05) phosphors at 394 nm and 464 nm excitation wavelengths are shown in Fig. 7(a) and (b), respectively. It can be clearly seen that the spectral features (such as shape and position) remain unchanged even after Li+ co-doping. However, as shown in Fig. 7(c), the emission intensity at both excitation wavelengths increased with Li+ co-doping up to 3 mol%, after which a decline was observed at 5 mol% Li+ co-doping. A nearly twofold increase in PL intensity is observed for the Zn0.88Eu0.09Li0.03MoO4 phosphor, along with a rise in quantum yield from 14.5% to 27.2%. This enhancement at lower Li+ concentrations is likely due to the charge compensation effect.62 When Eu3+ is introduced into the ZnMoO4 matrix, it replaces Zn2+ ions, creating an additional positive charge with each substitution. These extra positive charges repel one another, which limits the further incorporation of Eu3+ ions into the host lattice. Excess Eu3+ ions located outside the lattice positions can also serve as luminescence quenching sites. As shown by the schematic representation in Fig. 7(f), to maintain overall charge neutrality, two Eu3+ ions substitute for three Zn2+ ions within the ZnMoO4 matrix, resulting in the formation of a Zn2+ vacancy ,
,  . These Zn2+ vacancies act as defect sites, leading to reduced overall luminescence intensity due to increased energy transfer from luminescent centers to vacancy defects. With the incorporation of Li+ ions, charge balance is achieved via the reaction,
. These Zn2+ vacancies act as defect sites, leading to reduced overall luminescence intensity due to increased energy transfer from luminescent centers to vacancy defects. With the incorporation of Li+ ions, charge balance is achieved via the reaction,  . Thus, the addition of Li+ lowers the probability of non-radiative transitions and significantly improves radiative transfer efficiency. However, because the ionic radii of the host metal and the alkali metal ion are different, even though the charge imbalance is addressed, alkali metal ions cannot fully resolve the volume imbalance issue. The problem of volume imbalance can be neglected only within a specific range of alkali metal ion concentration. As a result, when the Li+ doping concentration exceeds 3 mol%, it causes distortion of the crystal lattice, which subsequently reduces the phosphor's crystallinity and leads to a decrease in luminescence intensity.
. Thus, the addition of Li+ lowers the probability of non-radiative transitions and significantly improves radiative transfer efficiency. However, because the ionic radii of the host metal and the alkali metal ion are different, even though the charge imbalance is addressed, alkali metal ions cannot fully resolve the volume imbalance issue. The problem of volume imbalance can be neglected only within a specific range of alkali metal ion concentration. As a result, when the Li+ doping concentration exceeds 3 mol%, it causes distortion of the crystal lattice, which subsequently reduces the phosphor's crystallinity and leads to a decrease in luminescence intensity.
The formation of oxygen vacancies, which occurs when Zn2+ is replaced by Li+, is another crucial factor influencing the PL intensity of the ZnMoO4:Eu3+,Li+ phosphors. These oxygen vacancies are generated on the surface of the phosphor and function as sensitizers. Fig. 2(f) represents a notable increase in the number of oxygen vacancies in the case of co-doping with 3 mol% Li ions. The transfer of energy from the host to the Eu3+ ions through these sensitizers results in a substantial overlap of the charge transfer states. This significant overlap enhances the oscillator strength of the optical transition, thereby increasing the PL intensity.63 Lattice modification occurs through the formation of Eu⋯O⋯Li clusters, where the local field around the europium ion is altered due to a redistribution of electron density around the oxygen ion. This redistribution causes an increased pull of electrons toward the Eu3+ ion.64 The paramagnetic shift in the Li spectrum shown in Fig. 3 can be attributed to the formation of these clusters. The increase in the diamagnetic peak with increasing Li concentration could be indicative of a saturation point where excessive lithium ions start occupying sites away from Eu3+ ions.
The CIE chromaticity diagram is a valuable tool for assessing phosphor color emissions, where each emission is plotted based on its x and y chromaticity coordinates. In this study, the host ZnMoO4 phosphor exhibits emission in the blue region of the CIE diagram as shown in Fig. S2 (ESI†), indicative of its characteristic blue luminescence. As shown in Fig. 7(d), on doping with Eu3+, the emission shifts to the red region, reflecting the influence of Eu3+ ions in altering the color output. Interestingly, further co-doping with Li+ ions in the ZnMoO4:9%Eu3+ phosphor influences the chromaticity coordinates, leading to an increase in the x-coordinate and a decrease in the y-coordinate. This shift suggests an enhancement in color purity for the Li+ co-doped nanophosphor, as the changes in the coordinates correspond to a more refined emission color within the red spectrum. This chromatic adjustment highlights the role of Li+ co-doping in improving the visual quality of red-emitting phosphors by fine-tuning their chromatic properties. The color purity of the synthesized phosphors was calculated using the following equation.65
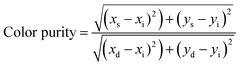 | (8) |
| CCT = −449n3 + 3525n2 − 6823n + 5520.33 | (9) |
 with the chromaticity epicenter defined at xe = 0.3320 and ye = 0.1858 for the 615 nm emission. Table S2 (ESI†) presents the color coordinates, CCT values and color purity for all ZnMoO4 phosphors doped with Eu3+ and co-doped with Eu3+ and Li+. The measured chromaticity coordinates of our phosphor closely approach the ideal red point (0.670, 0.330) and outperform those of the commercial Y2O2S:Eu3+ phosphor, which are (0.622, 0.351).66
with the chromaticity epicenter defined at xe = 0.3320 and ye = 0.1858 for the 615 nm emission. Table S2 (ESI†) presents the color coordinates, CCT values and color purity for all ZnMoO4 phosphors doped with Eu3+ and co-doped with Eu3+ and Li+. The measured chromaticity coordinates of our phosphor closely approach the ideal red point (0.670, 0.330) and outperform those of the commercial Y2O2S:Eu3+ phosphor, which are (0.622, 0.351).66
The decay profiles of Zn(0.91−y)Eu0.09LiyMoO4 (0 ≤ y ≤ 0.05) phosphors are presented in Fig. 7(e) with an excitation wavelength of 394 nm and emission wavelength of 615 nm. For the 5D0 → 7F2 transition, the decay curves of the phosphors are modeled using a bi-exponential function expressed as67
| I(t) = A1e(−t/τ1) + A2e(−t/τ2) | (10) |
 | (11) |
3.8. Judd–Ofelt analysis
Judd–Ofelt (JO) analysis is a crucial method to evaluate the luminescence properties and quantify the electronic transitions in the phosphors. The JO parameters (Ω2, Ω4) were extracted from the photoluminescence emission spectra, providing a detailed understanding of the local environment and asymmetry around the Eu3+ ions in the ZnMoO4 phosphor. The parameter Ω2 reflects the extent of asymmetry and covalency in the ligand field, while Ω4 correlates with the rigidity of the host lattice. The parameter Ω6 could not be obtained due to the absence of the 5D0 → 7F6 transition. The radiative emission rate (A0–J) for 5D0 → 7FJ (J = 1, 2, 4) transitions is determined by the integrated emission intensity (I0–J) and corresponding transition energy (hν0–J), correlating emission characteristics to radiative properties using the following equation:37 | (12) |
 | (13) |
 | (14) |
 | (15) |
 | (16) |
 | (17) |
| Phosphor | J–O intensity parameters (×10−20 cm2) | Ref. | |
|---|---|---|---|
| Ω 2 | Ω 4 | ||
| Na2ZnP2O7:0.01Eu3 | 3.37 | 1.22 | Fhoula et al.74 |
| KBaScSi3O9:Eu3+ | 1.05 | 0.30 | Nagaraj et al.75 |
| Y2O3:Eu3+ | 6.06 | 0.08 | Patwardhan et al.76 |
| NiNb2O6:Eu3+ | 10.02 | 2.73 | İlhan et al.77 |
| NaMgF3:Eu3+ | 34.9 | 6.03 | Nalumaga et al.78 |
| ZnMoO4:0.09Eu3+ | 38.79 | 7.43 | Present work |
| ZnMoO4:0.09Eu3+,0.03Li+ | 31.12 | 7.04 | Present work |
The stimulated emission cross-section (σe) is a critical parameter that characterizes the efficiency of energy extraction from a lasing medium. It is determined using the following formula:73
 | (18) |
The product of the stimulated emission cross-section (σe) and effective bandwidth (Δλeff) is an important parameter for predicting the amplification bandwidth of optical systems. Higher product values lead to enhanced amplifier performance. For ZnMoO4:Eu3+ (3 mol%), the maximum gain bandwidth observed was 26.15 × 10−28 cm3 for the 5D0 → 7F2 transition. Additionally, the product of radiative lifetime (τrad) and σe highlights substantial optical gain, establishing the 5D0 → 7F2 transition as a robust candidate for efficient laser applications and advanced display technologies.
3.9. Selective sensing of nitro aromatic compounds
In this study, selective sensing of nitroaromatic compounds was explored using phosphor emission quenching as a detection method. The Zn0.88Eu0.09Li0.03MoO4 (ZELMO) phosphor exhibited the highest emission intensity of all the synthesized samples. Consequently, this sample was selected for further sensing investigations.Fig. 8(a) provides a schematic illustration of the quenching mechanism, where adding para-nitrophenol (PNP) to an aqueous phosphor solution causes a marked reduction in phosphor emission on excitation at 394 nm. Fig. 8(b) shows the emission spectra at 394 excitation wavelength for a blank aqueous phosphor solution and solutions containing various nitroaromatic compounds, specifically nitrobenzene (NB), para-nitrobenzaldehyde (PNB), 3-nitrotoluene (3-NT), 2-nitrotoluene (2-NT), 3-nitrobenzoic acid (3-NBA), 4-nitrobenzoic acid (4-NBA), and para-nitrophenol (4-NP). Each compound was tested at a concentration of 50 mM, and PNP demonstrated a stronger quenching effect than other nitro aromatics.
Fig. 8(c) illustrates this selectivity in a bar graph, highlighting PNP's pronounced quenching effect on phosphor emission intensity relative to other compounds. Fig. 8(d) presents the quenching response of the phosphor across a concentration range of PNP from 1 nM to 200 μM, showing a decrease in emission intensity with increasing PNP concentration. To calculate the limit of detection (LOD) of PNP, the difference in fluorescence intensity (I0 − I) was plotted against the PNP concentration, as shown in Fig. 8(e). Here, I0 represents the fluorescence intensity of the blank (solvent without PNP), and I is the intensity of the PNP solution at varying concentrations. The detection limit for p-NP was calculated as 12 nM using the 3σ/slope method, where σ represents the standard deviation of the blank measurements.86 This detection limit is lower than the maximum allowable concentration of 0.43 μM in drinking water set by the US Environmental Protection Agency (EPA). Additionally, the performance of phosphors as a fluorescent sensor for p-NP was evaluated against other previously reported analytical methods, with comparative results summarized in Table 2. Finally, Fig. 8(f) examines the recyclability of the phosphor sensor over five sensing cycles with and without PNP, demonstrating minimal loss in emission intensity, indicating the phosphor's robustness and potential for repeated use in PNP detection.
| Sensing probe | Detection method | Detection limit (nM) | Ref. |
|---|---|---|---|
| rGO–MoS2/Fe3O4 | Electrochemical | 800 | Kalia et al.79 |
| Si NPs | Fluorescence | 74 | Liu et al.80 |
| rGO–HNT–AgNPs | Electrochemical | 48.6 | Hwa et al.81 |
| Cu0.5–Fe3O4@VXC-72/GCE | Electrochemical | 65 | Cao et al.82 |
| FeSe2 | Electrochemical | 30 | Chang et al.83 |
| PVP@BP | Electrochemical | 28 | Shen et al.84 |
| Si-CDs | Fluorescence | 23.45 | Zhu et al.85 |
| Zn0.88Eu0.09Li0.03MoO4 phosphor | Fluorescence | 12 | Present work |
3.10. Mechanism of sensing
The fluorescence quenching process can occur through several mechanisms, including dynamic quenching, static quenching, the inner filter effect (IFE), Förster resonance energy transfer (FRET), photo-induced electron transfer, aggregation-caused quenching (ACQ), collisional quenching, and ground-state complex formation, among others.87 To identify the most likely quenching pathways in this study, we analyzed the absorption spectra of the nitroaromatic compounds (Fig. 9(a)) and the excitation and emission spectra of the phosphor.The lack of overlap between the absorption spectrum of para-nitrophenol (PNP) and the emission spectrum of the phosphor, as shown in Fig. 9(b), indicates that Förster resonance energy transfer (FRET) is not involved in the quenching mechanism. In contrast, the emission spectrum of the phosphor shows substantial overlap with the absorption spectrum of PNP, pointing to the inner filter effect (IFE) as the primary mechanism for fluorescence quenching.
To further confirm the quenching pathway, time-resolved fluorescence decay experiments were performed. ZELMO exhibits a lifetime of 0.258 ms in the absence of PNP, which decreases only marginally to 0.246 ms upon addition of PNP (Fig. S3, ESI†). This minimal change in lifetime indicates that PNP does not participate in excited-state interactions with the phosphor, but instead reduces the detected fluorescence intensity through an inner filter effect.
The inner filter effect (IFE) occurs when the emission or excitation wavelengths of a fluorescent phosphor overlap with the absorption spectrum of a coexisting species (in this case, PNP). This overlap enables the absorber (PNP) to attenuate the fluorescence signal of the phosphor by absorbing emitted photons, effectively “filtering” the detected fluorescence without direct molecular interactions between the sensor and the analyte. IFE is particularly advantageous for sensing applications because it does not require physical binding or chemical modifications to the probe, unlike mechanisms such as static or dynamic quenching. As a result, IFE-based sensing is often simpler and more robust, avoiding structural or chemical perturbations of the probe, thus enhancing sensor stability and reusability.88
3.11. Thermal stability
The thermal stability of ZELMO was investigated by analyzing its emission behavior across a wide temperature range. The 3D color contour emission spectra at 394 nm excitation wavelength for the temperature range 15 K to 295 K (Fig. 10(a)) showed that the peak positions remained unchanged, indicating stable emission characteristics. However, a reduction in emission intensity was observed as the temperature increased. This observation was further confirmed by the normalized intensity bar graph (Fig. 10(b)), which demonstrated a steady decrease in emission intensities at 579 nm, 590 nm, 615 nm, 653 nm, and 700 nm within the same temperature range. In the higher temperature range of 303 K to 573 K, both the 3D contour plot (Fig. 10(c)) and the associated bar graph (Fig. 10(d)) showed a persistent decrease in emission intensity, with no shift in peak positions. This behavior suggests that the phosphor exhibits resistance to thermal quenching. The normalized integrated intensity versus temperature graph (Fig. 10(e)) further reveals that the phosphor retains 83% of its luminescence at 423 K and 69% at 573 K, highlighting its remarkable thermal stability.The activation energy (ΔE) for the thermal deactivation process was calculated using the following equation:89
 | (19) |
3.12. Low-temperature optical thermometry
This study explores the potential of the ZELMO phosphor for non-contact optical thermometry in the temperature range of 15 K to 295 K. The temperature-dependent photoluminescent properties of this phosphor were analyzed using the fluorescence intensity ratio (FIR) method, focusing on its emission bands at 579 nm (5D0 → 7F0), 615 nm (5D0 → 7F2), and 653 nm (5D0 → 7F3). Two specific fluorescence intensity ratios, 615 nm/579 nm, and 615 nm/653 nm, were selected for the subsequent calculations. The relationship between the FIR and temperature was determined using the following expression:90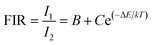 | (20) |
These intensity ratios were selected to examine how the dominant electric dipole transition of Eu3+ at 615 nm varies relative to other Eu3+ transitions as a function of temperature. With increasing temperature, a gradual decrease in the FIR ratio is observed. This trend is attributed to thermal expansion of the lattice, which increases the Eu3+ → O2− bond distance, thereby enhancing the covalency of the local environment.91 The increased covalency alters the asymmetry around the Eu3+ site, leading to a reduction in the relative intensity of the electric dipole transition and, consequently, a decrease in the FIR ratio. Moreover, this behavior supports the notion that the populations of the 7F0, 7F2, and 7F3 levels follow a Boltzmann distribution. The overall reduction in photoluminescence intensity at elevated temperatures is primarily due to thermal quenching, confirming thermal extinction as the dominant non-radiative mechanism.
The absolute sensitivity (Sa) and relative sensitivity (Sr), which quantify the absolute and relative variations in the fluorescence intensity ratio (FIR) with temperature changes, are calculated using the following equations:92
 | (21) |
 | (22) |
| Material | Temperature range (K) | S a-max (×10−3 K−1) | S r-max (% K−1) | Ref. |
|---|---|---|---|---|
| NaEuF4:Eu3+ | 298–523 | — | 0.43 | Tian et al.94 |
| Sr2GdSbO6:Eu3+ | 298–423 | 43 | 0.27 | Tai et al.95 |
| NaLa(MoO4)2:Eu3+,Li+ | 303–503 | — | 0.65 | Sonali et al.37 |
| YVO4:Eu3+ | 298–473 | 0.39 | 1.08 | Kolensnikov et al.96 |
| Na3Y(VO4)2:Eu3+ | 298–440 | 1 | 2.4 | Kachau et al.97 |
| ZnMoO4:Eu3+,Li+ | 15–295 | 13.9 | 0.61 | Present work |
The equation below is used to estimate the temperature uncertainty (ΔT):93
 | (23) |
3.13. Luminescence properties and environmental stability of a flexible phosphor polymer film
The luminescence properties of the ZELMO phosphor matrix embedded in a PVA polymer film are demonstrated through a series of images, providing insights into its emission characteristics, flexibility, and environmental stability. Fig. 12(a) presents the photoluminescence (PL) emission spectra of the film under excitation at 394 nm and 464 nm. The spectra reveal strong luminescence, attributed to the characteristic transitions of Eu3+ ions, confirming the efficient incorporation of the phosphor into the polymer matrix. Fig. 12(b) shows the film under both daylight and UV light, with the film demonstrating a distinct pink emission in the UV light. Notably, the film was also folded using forceps in UV light, highlighting its excellent flexibility without a significant loss in luminescent performance. This observation suggests that the phosphor–PVA composite can be effectively utilized in flexible optoelectronic devices, such as bendable sensors or display technologies.Fig. 12(c) illustrates the behavior of the film when immersed in a neutral pH 7 aqueous solution, under both daylight and UV light. The film maintains its characteristic pink luminescence in both conditions, indicating the stability of the phosphor under neutral aqueous environments. This property is essential for applications in sensors that may be exposed to aqueous surroundings, suggesting robust chemical stability. Fig. 12(d) and (e) represent the film immersed in an acidic solution (pH 2) and in an alkaline solution (pH 14) respectively, where the pink luminescence is similarly retained. This suggests that the phosphor–PVA film exhibits good resistance to acidic and basic conditions, further broadening its applicability in harsh environments.
4. Conclusion
In this study, we systematically investigated Eu3+-ion activated ZnMoO4 phosphor materials and optimized the photoluminescent properties for a range of multifunctional applications. By varying the Eu3+-ion concentrations and introducing lithium co-doping, we identified the optimal composition, Zn0.88Eu0.09Li0.03MoO4, with enhanced emission intensity and high color purity of 93.6%. The material's suitability as a red component for phosphor-based white LEDs (WLEDs) was established, highlighting its potential in solid-state lighting systems. Furthermore, the material exhibited excellent thermal stability, maintaining 83% of its luminescence intensity at 423 K, underscoring its reliability for diverse lighting applications. It also demonstrated exceptional sensitivity and selectivity for detecting para-nitrophenol (4-NP), with a detection range of 1 nM to 200 μM and a detection limit as low as 12 nM, confirming its utility in environmental monitoring and chemical sensing. Additionally, the phosphor showcased reliable performance for low-temperature sensing in the range of 15 K to 295 K, proving its versatility in non-contact optical thermometry. Overall, Li co-doped Eu-doped ZnMoO4 shows significant promise for applications in lighting, sensing, and environmental monitoring, offering a solid foundation for advancing rare-earth-doped phosphor technologies.Conflicts of interest
There are no conflicts to declare.Abbreviations
| LED | Light emitting diodes |
| WLED | White light emitting diodes |
| PL | Photoluminescence |
| ZELMO | Zn0.88Eu0.09Li0.03MoO4 |
| 4-NP | para-Nitrophenol |
Data availability
The datasets generated during and analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.Acknowledgements
The author, Astha Tyagi, sincerely acknowledges the Council of Scientific and Industrial Research (CSIR) for supporting this research through the CSIR fellowship. The authors, Astha Tyagi and C. Shivakumara, extend their gratitude to the Chemical Science Division at the Indian Institute of Science for providing access to the instrumental facility. They also appreciate the assistance of Ms Nikita Rao (SSCU, IISc) with the NMR experiments and the support of Mr K. Manjunath at the Central Facility, SSCU, IISc, in conducting low-temperature PL experiments. The authors, M. Rakshita and D. Haranath, are grateful to the Council of Scientific and Industrial Research (CSIR) and the Department of Science and Technology (DST), Government of India, for financial support under the projects CSIR-SRF #09/0922(11518)/2021-EMR-I and #CRG/2021/007142, respectively.References
- M. N. Da Silva, J. M. De Carvalho, M. C. De Abreu Fantini, L. A. Chiavacci and C. Bourgaux, ACS Appl. Nano Mater., 2019, 2, 6918–6927 CrossRef CAS.
- Y.-H. Han, C.-B. Tian, Q.-H. Li and S.-W. Du, J. Mater. Chem. C, 2014, 2, 8065–8070, 10.1039/c4tc01336k.
- R. S. Yadav, S. J. Dhoble and S. B. Rai, Sens. Actuators, B, 2018, 273, 1425–1434 CrossRef CAS.
- F. Zhang, H. Zhong, C. Chen, X. G. Wu, X. Hu, H. Huang, J. Han, B. Zou and Y. Dong, ACS Nano, 2015, 9, 4533–4542 CrossRef CAS PubMed.
- J. N. Hao and B. Yan, Chem. Commun., 2015, 51, 7737–7740 RSC.
- C. Mu, Z. Zhang, Y. Hou, H. Liu, L. Ma, X. Li, S. Ling, G. He and M. Zhang, Angew. Chem., 2021, 133, 12401–12405 CrossRef.
- Z. Tang, G. Zhang and Y. Wang, ACS Photonics, 2018, 5, 3801–3813 CrossRef CAS.
- P. Pust, P. J. Schmidt and W. Schnick, Nat. Mater., 2015, 14, 454–458 CrossRef CAS PubMed.
- C. Wang, Z. Zhao, Q. Wu, G. Zhu and Y. Wang, Dalton Trans., 2015, 44, 10321–10329 RSC.
- J. Ling, Y. Zhou, W. Xu, H. Lin, S. Lu, B. Wang and K. Wang, J. Adv. Ceram., 2020, 9, 45–54 CrossRef CAS.
- N. C. George, A. J. Pell, G. Dantelle, K. Page, A. Llobet, M. Balasubramanian, G. Pintacuda, B. F. Chmelka and R. Seshadri, Chem. Mater., 2013, 25, 3979–3995 CrossRef CAS.
- J. Ueda, P. Dorenbos, A. J. J. Bos, A. Meijerink and S. Tanabe, J. Phys. Chem. C, 2015, 119, 25003–25008 CrossRef CAS.
- Y. Tian, Y. Wei, Y. Zhao, Z. Quan, G. Li and J. Lin, J. Mater. Chem. C, 2016, 4, 1281–1294 RSC.
- J. Liang, L. Sun, S. Wang, Q. Sun, B. Devakumar and X. Huang, J. Alloys Compd., 2020, 836, 155469 CrossRef CAS.
- P. Dang, G. Li, X. Yun, Q. Zhang, D. Liu, H. Lian, M. Shang and J. Lin, Light: Sci. Appl., 2021, 10, 1–13 CrossRef PubMed.
- C. Ji, H. Zhang, W. Wang, H. Yuan and G. Zhao, J. Mater. Sci.: Mater. Electron., 2023, 34, 1–10 CrossRef.
- S. Wang, Y. Xu, T. Chen, W. Jiang, J. Liu, X. Zhang, W. Jiang and L. Wang, Chem. Eng. J., 2021, 404, 125912 CrossRef CAS.
- S. Bae, S. Gim, H. Kim and K. Hanna, Appl. Catal., B, 2016, 182, 541–549 CrossRef CAS.
- W. Yue, M. Chen, Z. Cheng, L. Xie and M. Li, J. Hazard. Mater., 2018, 344, 431–440 CrossRef CAS PubMed.
- C. Yin, J. Cai, L. Gao, J. Yin and J. Zhou, J. Hazard. Mater., 2016, 305, 15–20 CrossRef CAS PubMed.
- M. L. Wang, T. T. Jiang, Y. Lu, H. J. Liu and Y. Chen, J. Mater. Chem. A, 2013, 1, 5923–5933 RSC.
- X. Le, Z. Dong, Y. Liu, Z. Jin, T.-D. Huy, M. Le and J. Ma, J. Mater. Chem. A, 2014, 2, 19696–19706 RSC.
- W. Li, H. Zhang, S. Chen, Y. Liu, J. Zhuang and B. Lei, Biosens. Bioelectron., 2016, 86, 706–713 CrossRef CAS PubMed.
- D. Zhao, D. Yue, L. Zhang, K. Jiang and G. Qian, Inorg. Chem., 2018, 57, 12596–12602 CrossRef CAS PubMed.
- X. D. Wang, O. S. Wolfbeis and R. J. Meier, Chem. Soc. Rev., 2013, 42, 7834–7869 RSC.
- A. Shen, S. B. Kim, C. Bailey, A. W. K. Ma and S. Dardona, IEEE Sens. J., 2018, 18, 9105–9111 CAS.
- A. Wadhwa, J. Benavides-Guerrero, M. Gratuze, M. Bolduc and S. G. Cloutier, Materials, 2024, 17, 2489 CrossRef CAS PubMed.
- M. Mansoor, I. Haneef, S. Akhtar, A. De Luca and F. Udrea, Sens. Actuators, A, 2015, 232, 63–74 CrossRef CAS.
- Y. Cui, F. Zhu, B. Chen and G. Qian, Chem. Commun., 2015, 51, 7420–7431 RSC.
- X. Wang, Q. Liu, Y. Bu, C. S. Liu, T. Liu and X. Yan, RSC Adv., 2015, 5, 86219–86236 RSC.
- Z. Chen, K. Yin Zhang, X. Tong, Y. Liu, C. Hu, S. Liu, Q. Yu, Q. Zhao, W. Huang, Z. Chen, K. Y. Zhang, X. Tong, Y. Liu, C. Hu, S. Liu, Q. Yu, Q. Zhao and W. Huang, Adv. Funct. Mater., 2016, 26, 4386–4396 CrossRef CAS.
- M. G. Nikolić, Ž. Antić, S. Ćulubrk, J. M. Nedeljković and M. D. Dramićanin, Sens. Actuators, B, 2014, 201, 46–50 CrossRef.
- Q. N. Jia, S. Ren, G. W. Yuan and L. Wang, Appl. Mech. Mater., 2013, 343, 97–100 CAS.
- D. A. Spassky, A. N. Vasil’Ev, I. A. Kamenskikh, V. V. Mikhailin, A. E. Savon, Y. A. Hizhnyi, S. G. Nedilko and P. A. Lykov, J. Phys.: Condens. Matter, 2011, 23, 365501 CrossRef CAS PubMed.
- W. Ran, L. Wang, W. Zhang, F. Li, H. Jiang, W. Li, L. Su, R. Houzong, X. Pan and J. Shi, J. Mater. Chem. C, 2015, 3, 8344–8350 RSC.
- S. C. Abrahams, J. Chem. Phys., 1967, 46, 2052–2063 CrossRef CAS.
- Sonali, V. Chauhan, P. C. Pandey and C. Shivakumara, ACS Appl. Opt. Mater., 2024, 2, 41–56 CrossRef CAS.
- T. L. Barr and S. Seal, J. Vac. Sci. Technol., A, 1995, 13, 1239–1246 CrossRef CAS.
- J. R. Rumble, D. M. Bickham and C. J. Powell, Surf. Interface Anal., 1992, 19, 241–246 CrossRef CAS.
- D. Aman, S. Abdel-Azim, S. Said and S. G. Mohamed, RSC Adv., 2022, 12, 7120–7132 RSC.
- Y. Li, H. Li, Y. Li, D. Liu, J. Xie, H. Ma, H. Qu, J. Xu, Y. Han and L. Wang, Opt. Mater., 2023, 144, 114336 CrossRef CAS.
- B. V. R. Chowdari, G. V. Subba Rao and C. J. Leo, Mater. Res. Bull., 2001, 36, 727–736 CrossRef CAS.
- X. Li, Y. Wang, W. Liu, G. Jiang and C. Zhu, Mater. Lett., 2012, 85, 25–28 CrossRef CAS.
- Q. Du, G. Zhou, J. Zhou, X. Jia and H. Zhou, J. Alloys Compd., 2013, 552, 152–156 CrossRef CAS.
- B. Zhai, L. Yang and Y. M. Huang, Nanomaterials, 2019, 9, 99 CrossRef PubMed.
- B. Zhai, Q. Ma, L. Yang and Y. M. Huang, J. Nanomater., 2018, 2018, 1–10 CrossRef.
- G. R. Dillip and B. Deva Prasad Raju, J. Alloys Compd., 2012, 540, 67–74 CrossRef CAS.
- J. Tauc and A. Menth, J. Non-Cryst. Solids, 1972, 8–10, 569–585 CrossRef CAS.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi B, 1966, 15, 627–637 CrossRef CAS.
- L. X. Lovisa, Y. L. R. L. Fernandes, L. M. P. Garcia, B. S. Barros, E. Longo, C. A. Paskocimas, M. R. D. Bomio and F. V. Motta, Opt. Mater., 2019, 96, 109332 CrossRef CAS.
- W. Zhang, J. Yin, F. Min, L. Jia, D. Zhang, Q. Zhang and J. Xie, J. Mol. Struct., 2017, 1127, 777–783 CrossRef CAS.
- S. Jana, A. Mondal, J. Manam and S. Das, J. Alloys Compd., 2020, 821, 153342 CrossRef CAS.
- V. Chauhan, P. K. Pandey, P. Dixit, P. Deshmukh, S. Satapathy and P. C. Pandey, J. Lumin., 2022, 248, 118994 CrossRef CAS.
- R. Mimouni, A. Souissi, A. Madouri, K. Boubaker and M. Amlouk, Curr. Appl. Phys., 2017, 17, 1058–1065 CrossRef.
- D. H. Zhang, Q. P. Wang and Z. Y. Xue, Appl. Surf. Sci., 2003, 207, 20–25 CrossRef CAS.
- B. Askri, A. Mhamdi, N. Mahdhi and M. Amlouk, Phys. B, 2018, 539, 51–60 CrossRef CAS.
- S. K. Gupta, P. S. Ghosh, K. Sudarshan, R. Gupta, P. K. Pujari and R. M. Kadam, Dalton Trans., 2015, 44, 19097–19110 RSC.
- O. M. Ntwaeaborwa, S. J. Mofokeng, V. Kumar and R. E. Kroon, Spectrochim. Acta, Part A, 2017, 182, 42–49 CrossRef CAS PubMed.
- L. G. Van Uitert, J. Electrochem. Soc., 1967, 114, 1048 CrossRef CAS.
- B. Han, J. Zhang, Z. Wang, Y. Liu and H. Shi, J. Lumin., 2014, 149, 150–154 CrossRef CAS.
- K. Thomas, D. Alexander, S. Sisira, P. R. Biju, N. V. Unnikrishnan, M. A. Ittyachen and C. Joseph, J. Mater. Sci.: Mater. Electron., 2017, 28, 17702–17709 CrossRef CAS.
- S. Saha, S. Das, U. K. Ghorai, N. Mazumder, B. K. Gupta and K. K. Chattopadhyay, Dalton Trans., 2013, 42, 12965 RSC.
- A. De, B. Samanta, A. K. Dey, N. Chakraborty, T. K. Parya, S. Saha and U. K. Ghorai, ACS Appl. Nano Mater., 2022, 5, 331–340 CrossRef CAS.
- S. K. Gupta, K. Sudarshan, A. K. Yadav, R. Gupta, D. Bhattacharyya, S. N. Jha and R. M. Kadam, Inorg. Chem., 2018, 57, 821–832 CrossRef CAS PubMed.
- E. Sreeja, V. Vidyadharan, S. K. Jose, A. George, C. Joseph, N. V. Unnikrishnan and P. R. Biju, Opt. Mater., 2018, 78, 52–62 CrossRef CAS.
- Y. Hua, Sk. K. Hussain and J. S. Yu, Ceram. Int., 2019, 45, 18604–18613 CrossRef CAS.
- P. Halappa, B. Devakumar and C. Shivakumara, New J. Chem., 2018, 43, 63–71 RSC.
- P. Halappa, H. M. Rajashekar and C. Shivakumara, J. Alloys Compd., 2019, 785, 169–177 CrossRef CAS.
- C. Shivakumara, R. Saraf, S. Behera, N. Dhananjaya and H. Nagabhushana, Spectrochim. Acta, Part A, 2015, 151, 141–148 CrossRef CAS PubMed.
- S. Som, S. Das, S. Dutta, H. G. Visser, M. K. Pandey, P. Kumar, R. K. Dubey and S. K. Sharma, RSC Adv., 2015, 5, 70887–70898 RSC.
- R. Saraf, C. Shivakumara, S. Behera, H. Nagabhushana and N. Dhananjaya, RSC Adv., 2015, 5, 4109–4120 RSC.
- S. C. Lal, A. M. Aiswarya, K. S. Sibi and G. Subodh, J. Alloys Compd., 2019, 788, 1300–1308 CrossRef CAS.
- C. Manjunath, M. S. Rudresha, R. H. Krishna, B. M. Nagabhushana, B. M. Walsh, K. R. Nagabhushana and B. S. Panigrahi, Opt. Mater., 2018, 85, 363–372 CrossRef CAS.
- M. Fhoula and M. Dammak, J. Lumin., 2020, 223, 117193 CrossRef CAS.
- R. Nagaraj, A. Raja and S. Ranjith, J. Alloys Compd., 2020, 827, 154289 CrossRef CAS.
- M. A. Patwardhan, R. K. Jumale, R. L. Kohale, R. K. Joshi and S. A. Khapare, J. Opt., 2024, 53, 4969–4980 CrossRef.
- M. İlhan, M. K. Ekmekçi and İ. Ç. Keskin, RSC Adv., 2021, 11, 10451–10462 RSC.
- H. Nalumaga, J. J. Schuyt and G. V. M. Williams, J. Lumin., 2024, 266, 120251 CrossRef CAS.
- S. Kalia, R. Kumar, R. Sharma, S. Kumar, D. Singh and R. K. Singh, J. Phys. Chem. Solids, 2024, 184, 111719 CrossRef CAS.
- F. Liu, F. Liang, Z. Li, G. Kang, T. Wang, C. Chen and Y. Lu, Analyst, 2023, 148, 4030–4036 RSC.
- K.-Y. Hwa, T. S. K. Sharma and A. Ganguly, Inorg. Chem. Front., 2020, 7, 1981–1994 RSC.
- K. Cao, C. Si, H. Zhang, J. Hu and D. Zheng, J. Mater. Sci.: Mater. Electron., 2022, 33, 2386–2398 CrossRef CAS.
- X.-L. Cheng, X. Xia, Q.-Q. Xu, J. Wang, J.-C. Sun, Y. Zhang and S.-S. Li, Sens. Actuators, B, 2021, 348, 130692 CrossRef CAS.
- J. Shen, L. Liu, W. Huang and K. Wu, Anal. Chim. Acta, 2021, 1167, 338594 CrossRef CAS.
- W. Zhu, Y. Zhou, S. Liu, M. Luo, J. Du, J. Fan, H. Xiong and H. Peng, Food Chem., 2021, 348, 129126 CrossRef CAS PubMed.
- S. Hussain, S. De and P. K. Iyer, ACS Appl. Mater. Interfaces, 2013, 5, 2234–2240 CrossRef CAS.
- A. S. Tanwar, S. Patidar, S. Ahirwar, S. Dehingia and P. K. Iyer, Analyst, 2019, 144, 669–676 RSC.
- J. Sun, J. Zhao, L. Wang, H. Li, F. Yang and X. Yang, ACS Sens., 2018, 3, 183–190 CrossRef CAS PubMed.
- Y. Wei, L. Cao, L. Lv, G. Li, J. Hao, J. Gao, C. Su, C. C. Lin, H. S. Jang, P. Dang and J. Lin, Chem. Mater., 2018, 30, 2389–2399 CrossRef CAS.
- G. Zhang, M. S. Molokeev, Q. Ma, X. Yang, S. Han, Q. Chen, B. Zhong and B. Ma, CrystEngComm, 2020, 22, 5809–5817 RSC.
- Y. Bahrouni, I. Kachou, K. Saidi, T. Kallel, M. Dammak, I. Mediavilla and J. Jiménez, Mater. Adv., 2025, 6, 1307–1318 RSC.
- Y. Wu, S. Xu, Z. Xiao, F. Lai, J. Huang, J. Fu, X. Ye and W. You, Mater. Chem. Front., 2020, 4, 1182–1191 RSC.
- I. Kachou, K. Saidi, U. Ekim, M. Dammak, M. Çelikbilek Ersundu and A. E. Ersundu, Dalton Trans., 2024, 53, 2357–2372 RSC.
- Y. Tian, B. Tian, C. Cui, P. Huang, L. Wang and B. Chen, Opt. Lett., 2014, 39, 4164 CrossRef CAS PubMed.
- Y. Tai, R. Cui, J. Zhang, C. Wang, T. Zhao, B. Zhang and C. Deng, J. Rare Earths, 2024, 42, 1458–1469 CrossRef CAS.
- I. E. Kolesnikov, A. A. Kalinichev, M. A. Kurochkin, D. V. Mamonova, E. Y. Kolesnikov and E. Lähderanta, J. Phys. Chem. C, 2019, 123, 5136–5143 CrossRef CAS.
- I. Kachou, K. Saidi, R. Salhi and M. Dammak, RSC Adv., 2022, 12, 7529–7539 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional structural and optical characterization data to complement the main text. Fig. S1 shows the powder XRD patterns of Zn(0.91−y)Eu0.09LiyMoO4 phosphors with different lithium concentrations (y = 0.01, 0.03, and 0.05). Fig. S2 presents the CIE chromaticity diagram of the ZnMoO4 host material. Fig. S3 shows the PL decay curve of ZELMO with and without 4-NP. Table S1 lists the Rietveld refined structural parameters of ZnMoO4. Table S2 summarizes the CIE 1931 chromaticity coordinates, correlated color temperature (CCT), and color purity values of ZnMoO4:Eu3+,Li+ phosphors under 394 nm excitation. Table S3 provides detailed Judd–Ofelt analysis, including intensity parameters (Ω2 and Ω4), radiative transition probabilities, radiative lifetimes, branching ratios, and asymmetric ratios. Lastly, Table S4 contains the quantitative evaluation of optical properties such as effective bandwidth, stimulated emission cross-section, gain bandwidth, and optical gain for the ZnMoO4:Eu3+,Li+ phosphors. Table S5 shows comparative evaluation of the stimulated emission cross-sections (σe) corresponding to the transitions of Eu3+ ions in different host matrices. See DOI: https://doi.org/10.1039/d5ma00280j |
| This journal is © The Royal Society of Chemistry 2025 |