Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A review of core–shell metal–organic frameworks: preparation and biomedical applications
Simindokht
Zarei-Shokat
,
Sheyda
Abedi-Banaei
,
Amir
Kashtiaray
,
Zahra
Yazdi
,
Haniyehalsadat
Amirhosseini
and
Ali
Maleki
 *
*
Catalysts and Organic Synthesis Research Laboratory, Department of Chemistry, Iran University of Science and Technology, Tehran 16846-13114, Iran. E-mail: maleki@iust.ac.ir
First published on 22nd May 2025
Abstract
Core–shell metal–organic frameworks (MOFs) are an advanced class of hybrid materials, and their synthesis methods are crucial for determining their functionality in biomedical applications. Various synthetic strategies have been developed to create these hierarchical structures, each offering unique advantages in controlling shell thickness, core composition, crystallinity, and surface properties. Recent advancements in preparing core–shell MOF structures have focused on developing composite structures that involve MOFs integrated with other materials, such as nanoparticles, metal sulfides, and metal oxides. In this context, we explore different classes of core@MOF, MOF@shell, and MOF@MOF structures. Additionally, we discuss the practical applications of core–shell MOFs in anti-cancer and antibacterial activities, bioimaging, biosensing, and synergistic therapy, as well as their therapeutic potential.
1. Introduction
“Similar microporous structures zeolite but using organic building blocks and metal have the potential for more precise rational design by controlling the shape, size, and functionalization of the pores. We are announcing the creation of MOFs intended to selectively bind aromatic guest molecules”. This is the initial description of the MOF structure by Yaghi in 1995.1 Interest in this area was sparked in the late 1999 when Yaghi et al. synthesized their MOFs based on the concept of reticular design and named MOF-5, also known as IRMOF-1; the compound has the formula Zn4O(BDC)3 and is a cubic MOF, representing the start of this type of material's history.2 MOFs are remarkable organic–inorganic hybrid compounds characterized by one-, two-, or three-dimensional (1D, 2D, and 3D) structural topologies. Composed of inorganic metal ions or clusters alongside organic ligands, MOFs exhibit exceptional properties, such as high surface area, micro- and mesoporosity, and significant potential for further chemical functionalization.3 Their applicability in the biomedical sector has surged in recent years, solidifying MOFs as a pivotal component in advancing innovative solutions.4 Core–shell structures have attracted considerable interest because of their distinct characteristics and varied compositions, and the core–shell MOF includes three structures: core@MOF, MOF@shell, and MOF@MOF.4 The core and the shell can consist of silica,5 polymers,6,7 nanoparticles,8 quantum dots (BQ),9 carbon materials,8 and MOFs,6 which act as either shells or cores. The distinctive structure of core–shell MOFs consists of a functional core encased by a specialized shell, offering numerous synergistic advantages. Conventional MOFs frequently experience structural instability when exposed to aqueous environments or biological fluids.10 In contrast, the shell surrounding core–shell MOFs serves as a protective barrier, preventing early degradation of the core and preserving structural integrity under physiological conditions, such as fluctuating pH levels or the presence of enzymes. Furthermore, the shell layer can be tailored to respond to specific biological triggers, like pH changes, redox conditions, or enzymes, facilitating site-specific and on-demand drug release.11,12 Applying a biocompatible shell such as a zeolitic imidazolate framework (ZIF), a polymer coating, or silica can reduce potential toxic interactions, improving tolerability and safety profiles in vivo. Core–shell designs can also incorporate multiple functions within a single system. For instance, the core can act as a storage reservoir for drug molecules, while the shell can include imaging agents (such as Gd3+ for MRI or fluorescent dyes), targeting ligands, or responsive components.13–15 This multifunctionality supports theranostic applications, merging diagnostics and therapy into one delivery system. In summary, core–shell MOFs offer a customizable, multi-layered platform that overcomes the limitations of traditional MOFs.16–19 In 2007, William et al. were the first to describe a core–shell structure using nanoscale metal–organic frameworks (NMOFs) and silica. In this structure, the NMOFs, serving as the core, consisted of an Ln(BDC)1.5(H2O)2 composition, where Ln represents Eu3+, Gd3+, or Tb3+ and BDC stands for 1,4-benzenedicarboxylate. The silica formed the shell around the core, highlighting the central role of NMOFs in this structure.20 The methods for creating core–shell structures differ because of their diverse range and various uses. According to the latest research advancements, the methods for creating core–shell MOFs can be grouped into multiple classifications: surface modification methods, post-modification methods, in situ growth methods, self-template sacrifice methods, epitaxial growth methods, and one-pot methods. Various techniques have been explored for producing core–shell MOFs and their composite formations. The in situ growth method, with its more straightforward preparation process, presents a challenge in achieving a consistent coating of the external MOF shell onto the internal core. The selection of each method is of utmost importance, as it is guided by the specific needs and circumstances in which the framework will be employed, and can significantly impact the outcome of the work.21 The core–shell MOF's design boasts an extensive specific surface area and a high pore volume, making it incredibly versatile. The pore size can be easily customized to fit various needs and allows for rapid cargo transfer and diffusion. Plus, with its exceptional biocompatibility and biodegradability, this structure stands out as a sustainable choice for different applications. Moreover, it demonstrates the structural benefits of multiple characteristics, including a roomy cavity, substantial loading capacity, and protective properties. As a result, core–shell MOFs are considered ideal materials for biomedical applications.22 This article will discuss the latest progress in core–shell MOF materials, covering their production, modification, and medical uses like delivering drugs, monitoring biological processes, imaging, and treating cancer. Core–shell MOFs present exciting opportunities in drug delivery by encapsulating therapeutic molecules through methods like pore encapsulation, covalent binding, and surface adsorption. Utilizing van der Waals forces, electrostatic interactions, π–π stacking, and hydrogen bonding, these frameworks effectively transport and release medications. These materials are suitable for drug delivery and are not only non-responsive to stimuli but also responsive to various stimuli, including pH, magnetic field, light, and temperature. This is important for enhancing the effectiveness of drugs and minimizing their side effects. The present utilization of MOFs is restricted to a few classifications, including ZIF, Materials Institute Lavoisier (MIL), and University of Oslo (UIO). Moreover, recent instances documented in the literature have shown the potential of MOFs for biomedical uses. The use of core–shell MOFs in treatment systems has been widely researched. Instead of relying solely on traditional methods like chemotherapy (CT), photothermal therapy (PTT), photodynamic therapy (PDT), or immunotherapy, core–shell MOFs can serve as carriers for therapeutic agents and as active treatment components. Even more exciting is the potential for imaging-guided multimodal therapies, where chemo/PTT, chemo/PDT, and many others work synergistically to combat tumors more effectively. To achieve this, we can modify the structure of core–shell MOFs or load them with targeted molecules and imaging agents, making the fight against cancer even more precise. The future of cancer treatment is bright, and core–shell MOFs could play a pivotal role. Ultimately, we will investigate the potential opportunities and obstacles to using MOFs in biomedical applications. Compared to other extensive evaluations concerning the uses of MOFs in biomedicine,21,23 this feature article will focus on specific research on core–shell MOFs in the four areas mentioned above in recent years, offering researchers a thorough grasp of the present state and exciting advancements of MOFs in this area from diverse viewpoints.2. Preparation strategies of core–shell MOFs
2.1. Preparation strategies of core@MOF and MOF@shell
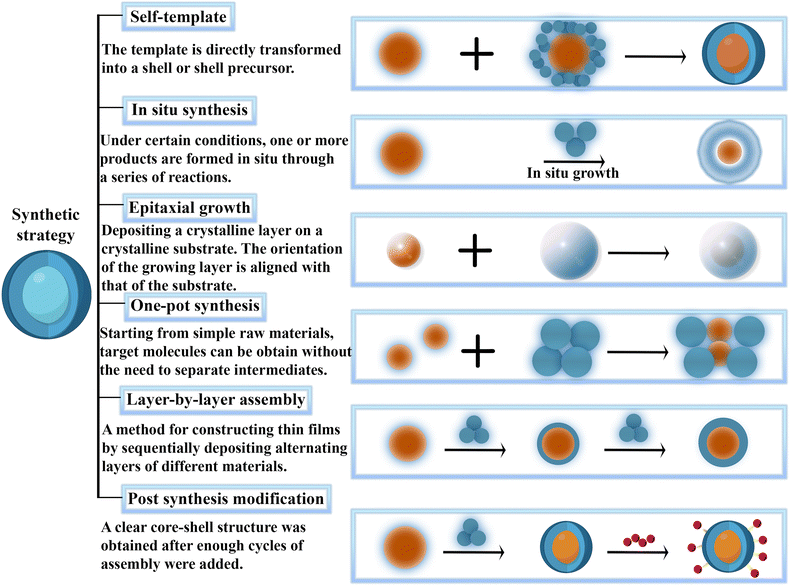
The placement position of the MOF in the core–shell arrangement may be different.21 The MOF serves as a protective layer for the core, ensuring it remains intact and preventing the core from leaching and accumulating independently during the entire reaction process. It creates a stable and continuous environment that is highly conducive to the reaction. In addition, when MOFs are employed as the outer materials, producing a consistent single crystal or polycrystalline MOF layer becomes feasible by controlling the circumstances. The attainment of uniformity requires other usual arrangements of MOF composites. The core@MOF structure is classified based on various core compositions: metal nanoparticles@MOF, metal oxides@MOF, metal sulfides@MOF, and, other nanoparticles@MOF. The composites at the core of MOFs are formed by uniformly coating the outer layer of another material with the MOF shell through a combination of physical and chemical techniques. This procedure entails carefully coating the MOF shell to guarantee consistency and durability. The materials comprising metal nanoparticles@metal–organic frameworks with core–shell configurations exhibit customizable shapes and evenly dispersed nanoparticles. These materials have garnered significant attention in biomedicine because of their exceptional physical and chemical properties, versatility, stability, and dispersibility. The metal nanoparticle core is effectively encapsulated within a MOF shell, serving as a robust substrate for the nanoparticles. The core–shell structure of metal sulfide@MOF has been the subject of extensive attention up to this point. Most metal sulfides have a relatively small bandgap, which is a notable advantage compared to most metal oxides. Semiconductor materials composed of sulfide@MOF are widely utilized in biology and medicine to serve as targeted attachments for various entities, including proteins, cells, acidic components of the nucleus, viruses, and so on. Metal oxides with semiconductor properties, such as TiO2, ZnO, and Fe3O4, have been extensively researched and proven valuable in the field of biomedicine. Integrating MOFs with metal oxides can greatly enhance biomedical efficacy. The synthesis of these structures is of particular importance due to their applications in biomedicine, and there are a wide range of synthetic methods to achieve these structures, which include one-pot approaches, in situ growing methods, self-template methods and layer-by-layer (LBL) assembly, (Fig. 1) and each of these methods have their unique feature according to the structure of core@MOF (Table 1).21,24,25| Structure | Composition | Growth mechanism | Ref. |
|---|---|---|---|
| Core@MOF | (Co-MOF)@CoNiO2 | In situ | 26 |
| Core@MOF | (Ti/Ce)UiO-X MOFs@TiO2 | In situ | 27 |
| Core@MOF | CeO2@ZIF-8 | In situ | 28 |
| Core@MOF | Co/MnOx@quasi-MOF-74 | In situ | 29 |
| Core@MOF | Fe3O4@MIL-100(Fe) | In situ | 30 |
| Core@MOF | Fe3O4@MIL-101-SO3 | In situ | 31 |
| Core@MOF | MOF-Ti@PANI | In situ | 32 |
| Core@MOF | Pd@ZIF-8 | In situ | 33 |
| Core@MOF | Pt/CeO2@UIO-66-NH2 | In situ | 34 |
| Core@MOF | RhB@ZIF-71 | In situ | 35 |
| Core@MOF | TixFe1−xOy@Fe2O3 | In situ | 36 |
| Core@MOF | Z67@CNF | In situ | 37 |
| Core@MOF | Au@MOF-5 | One-pot | 38 |
| Core@MOF | Au@MOF-74 | One-pot | 39 |
| Core@MOF | CdS@ZIF-8 | One-pot | 40 |
| Core@MOF | Fe3O4/CNC@ZIF-8 | One-pot | 41 |
| Core@MOF | Pt@ZIF-8 | One-pot | 42 |
| Core@MOF | Ru@HKUST-1 | One-pot | 43 |
| Core@MOF | SiO2@50Benz-Cys | One-pot | 44 |
| Core@MOF | TGZ@eM | One-pot | 45 |
| MOF@Shell | Co-SA/P | In situ | 46 |
| MOF@Shell | HKUST-1@Ag | In situ | 47 |
| MOF@Shell | ZrMOF@MnO2 | In situ | 48 |
| MOF@Shell | CoS2/WS2 | One-pot | 49 |
| MOF@Shell | DNAzyme@Cu/ZIF-8 | One-pot | 50 |
| MOF@Shell | DPGG@UiO-66 | One-pot | 51 |
| MOF@Shell | PCN-222@Zr-BPDC | One-pot | 52 |
| MOF@Shell | pt@ZIF-8 | One-pot | 53 |
| MOF@Shell | ZIF-67@SiO2 | One-pot | 54 |
| MOF@Shell | MOF-235@PEG@silica | Self-template | 55 |
| MOF@MOF | MIL-88B@MIL-88A | Epitaxial growth | 56 |
| MOF@MOF | Co-MOF-74@Mn-MOF-74 | Epitaxial growth | 57 |
| MOF@MOF | Cu-MOF@Co-MOF | Epitaxial growth | 58 |
| MOF@MOF | Fe-MIL-88B@Fe-MIL-88C | Epitaxial growth | 59 |
| MOF@MOF | IRMOF-2@MOF-5 Janus particles | Epitaxial growth | 60 |
| MOF@MOF | MnFe-X PBAs | Epitaxial growth | 61 |
| MOF@MOF | NH2-MIL-88B(Fe)@ZIF-8 | Epitaxial growth | 62 |
| MOF@MOF | Ni-MOF-74@Co-MOF-74 | Epitaxial growth | 63 |
| MOF@MOF | UiO-66-(OH)2@UiO-66-NH2 | Epitaxial growth | 64 |
| MOF@MOF | UiO-66@67-bpy | Epitaxial growth | 65 |
| MOF@MOF | UIO-66@UIO-67 | Epitaxial growth | 66 |
| MOF@MOF | UiO-66-NH2@Ni-MOF | Epitaxial growth | 67 |
| MOF@MOF | UiO-67@Ni-MOF | Epitaxial growth | 68 |
| MOF@MOF | UiO67@Rh@UiO66 | Epitaxial growth | 69 |
| MOF@MOF | ZIF-67@Co-MOF-74 | Epitaxial growth | 70 |
| MOF@MOF | ZIF-67@ZIF-8 | Epitaxial growth | 71 |
| MOF@MOF | ZIF-L@ZIF-67 | Epitaxial growth | 72 |
| MOF@MOF | Zn-MOF@Co-MOF | Epitaxial growth | 73 |
| MOF@MOF | NH2-MIL-101(Al)@ZIF-8 | Epitaxial growth | 74 |
| MOF@MOF | ZIF-L(Co)@ZIF-8 | Heteroepitaxial | 75 |
| MOF@MOF | PMOF-3@HHU-9 | One pot | 76 |
| MOF@MOF | NJU-Bai21@HHU-9 | One pot | 76 |
| MOF@MOF | PMOF-3@NJUBai21 | One pot | 76 |
| MOF@MOF | ZIF-11@ZIF-8 | Post-modifications | 77 |
By integrating MOFs as the core element and effectively layering them with complementary materials, we can fully harness the MOF core's and outer shell's exceptional biomedical properties. This innovative approach enhances functionality and opens up new avenues for advanced composite materials in biomedical applications. Employing MOFs as the primary materials because of their exceptional elasticity facilitates a robust chemical connection between the MOF core and the shell, allowing for integrating specific functions that enhance shell assembly. Furthermore, using a sturdier porous material for the outer layer can protect the relatively unstable MOF without blocking its pores, thus maintaining its significant surface area and porous structure, crucial for enhancing biomedical applications. The MOF@shell structure can be mainly classified as MOF@metal sulfides, MOF@COF, and MOF@other materials.24 Researchers have shown significant interest in combining MOFs with metal sulfides. It is feasible to minimize the leaching and aggregation of MOF cores due to their instability by creating core–shell structures. In the scenario involving MOF@COF, the covalent organic framework (COF) is a porous crystalline polymer. COFs are composed of light elements interconnected through robust covalent bonds using reticular chemistry. They possess significant potential across a wide range of applications. The advantages of core–shell MOFs@COFs are as follows: (1) the adjustable properties of MOFs and COFs make it highly efficient to establish strong reinforcement interactions between the MOF cores and COF shells, ensuring sufficient protection for the COF layer to avoid detachment. (2) COFs have a higher photoconductivity and charge mobility than MOFs with low conductivity because they can adequately store the p arrays. There are significantly fewer records of composites with MOFs as the central components compared to those that feature MOFs as the outer layer. This may be attributed to the challenge of reactants penetrating the shell for further reactions when the MOF is utilized as the core. The issues can be tackled by considering the following factors: (1) utilizing a highly absorbent outer layer allows reactants to flow through its pores effortlessly, facilitating a seamless one-step reaction process. (2) Tweaking the thickness of the shell layer can boost the movement and mixing of reactants, leading to a significant increase in electron transfer efficiency. (3) The core and shell synergize, forming a strong covalent bond. The MOF core creates a contained setting for the reaction system. Simultaneously, the shell, which has a customizable pore size, can selectively permit beneficial substances to enter while preventing the escape of active components, enhancing their effectiveness, or allowing for a specific degree of separation. The creation of these formations is significant because of their use in biomedicine. Various synthetic methods produce these formations, such as the one-pot, in situ growing, and self-template methods (Fig. 1),21,24,78 and these methods are similar to the synthesis of core@MOF structures. All these methods are compared and reviewed in Table 2.
| Preparation method | Key characteristics | Advantages | Disadvantages | Examples/notes | Ref. |
|---|---|---|---|---|---|
| Self-template method | The MOF core acts as both a template and a precursor and transforms into the shell | Efficient use of material and no need for external template | Restricted to convertible MOFs and necessitates exact conditions | ZnO@ZIF-8 | 79 |
| One-pot synthesis | Core and shell precursors added simultaneously; self-assembly leads to core–shell formation | Time-saving; fewer steps | Less control over distinct core/shell structures | UiO-66@SiO2/less common for precise architectures | 80 |
| Layer-by-layer (LbL) | Precursors are introduced in stages, with washing conducted in between to create accurate layers of the shell | High control over shell thickness and composition | Time-consuming; labor-intensive | Fe3O4@UiO-66-NH2/often used in thin films or surface coatings | 81 |
| Epitaxial growth | Shell grows directly on the core with lattice match | Produces seamless, highly ordered interfaces | Requires strict lattice match; fewer material combinations | UiO-66@ZIF-8/often seen in isoreticular MOFs | 82 |
| Post-synthetic method | Shell material deposited onto core MOF post-synthesis | Flexibility in material choice; works with fragile cores | Might cause partial damage to the core or incomplete coating | ZIF-67@Co-MOF-74/used for hybrid/heterostructures | 83 |
| In situ method | The core formed on the template surface and then the shell built around it | Can form hollow/complex structures | Requires template removal if undesired | Pt@UiO-66-NH2/useful in hierarchical or porous structures | 84 |
2.2. Preparation strategies of MOF@MOF
The initial preparation of core–shell structured MOF@MOF single crystals through epitaxial growth was first proposed by Kitagawa et al. in 2009.92 This marked the beginning of the narrative surrounding these types of porous coordination polymer (PCP) materials. The formation of MOF@MOF hybrid materials involves the addition of different organic ligands after crystal nucleation, allowing for combining two or more distinct types of MOFs into a single unified hybrid material comprising MOF-on-MOF. Usually, these hybrid structures are composed of two types of architectures: a MOF can be completely enclosed by another MOF, resulting in a core–shell structure known as MOF@MOF. Additionally, a MOF can be strategically grown on the surface of another MOF in either an isotropic or anisotropic manner, leading to a layered MOF-on-MOF configuration.93 The focus here is on core–shell structures, as these materials offer enhanced performance across a wider range due to increased structural diversity and more opportunities for improving surface properties, sometimes resulting in the emergence of new features.94 To successfully develop MOF@MOF hybrids, aligning the lattice of the second metal building unit with that of the initial MOF core is essential. For instance, MOFs from the MIL, ZIF and UiO-66 and various other MOF structures are commonly used as the foundation and integrated into an additional MOF layer. The MOF@MOF framework boasts a range of significant advantages, such as its flexible structures, extensive surface area, and high porosity. It features abundant exposed active sites and demonstrates excellent biocompatibility and remarkable biological activity. Its robust chemical and colloidal stability ensures reliability, while efficient surface modification enhances its functionality. Furthermore, functional groups like –NH2 and –COOH in MOFs contribute to their impressive capabilities. They have been utilized as practical bases that enhance the ability to immobilize antibiotics and biomolecules, including glucose, antibodies, and aptamers.93 The methods for creating these structures will now be examined, which include epitaxial growth, post-synthetic modification, and one-pot synthesis (Fig. 1).952.2.3.1. Selective transmetalation method. The selective transmetalation method is an alternative approach to creating MOF@MOF composites through post-synthetic modification. One of the strategies for post-synthetic modification involves replacing the structural metal ions that are part of the MOF connectivity. Lah et al. researched four isostructural MOFs that exhibit different levels of stability, specifically M6(BTB)4(BP)3 (referred to as ITHD crystals) where M represents Zn(II) (1), Co(II) (2), Cu(II) (3), and Ni(II) (4), BTB represents 1,3,5-benzenetribenzoate, and BP represents 4,4′-dipyridyl. The development of core–shell heterostructures via epitaxial growth involves a core crystal that is thermodynamically stable paired with a less stable shell crystal, which is a process of significant importance. The innovative kinetically regulated transmetalation processes we’re proposing will revolutionize the creation of core–shell heterostructures, specifically 1@2, 1@3, and 1@4. By immersing the thermodynamically unstable structure 1 in solutions containing Co(II), Cu(II), and Ni(II), we are leading the way in a selective transmetalation technique that will redefine how we synthesize core–shell MOFs@MOFs.94,101
2.2.3.2. Post-synthetic ligand exchange. Rosi et al. were the first to report the creation of MOFs@MOFs with varied structures and directional porosity gradients using post-synthetic ligand exchange, underscoring the critical function of organic ligands in MOF preparation. This research paves the way for potential breakthroughs in the field. Following this, Guo and colleagues showcased the successful creation of core–shell structures of ZIF-67@MOFs (with MOFs being Co-MOF-74, Co-BDC, Co-NH2BDC, and CoBTC) through a postsynthetic ligand-exchange technique. The system model included the synthesis of ZIF-67 using Co(NO3)2·6H2O and 2-methylimidazole (2-MI). ZIF-67@Co-MOF-74 with a core–shell structure was created by exchanging 2-methylimidazole (2-MI) with 2,5-dihydroxyterephthalic acid (DHTP). This exchange occurred because DHTP exhibited a more vital coordinating ability than 2-MI. When ZIF-67 encountered DHTP solutions, DHTP took the place of surface-bound 2-MI to bind with cobalt, resulting in the formation of Co-MOF-74 crystals as the outer layer of ZIF-67@Co-MOF-74.The thickness of the Co-MOF-74 shell can be modified by altering the ZIF-67/DHTP mass ratio. The color of ZIF-67@Co-MOF-74 shifted from purple to purple-gray when the concentration of DHTP was increased, indicating the successful preparation of ZIF-67@Co-MOF-74.42,50 Matzger et al. conducted a meticulous investigation into the creation of hierarchical core–shell MOFs@MOFs through post-synthetic ligand exchange. This rigorous approach was undertaken to gain a comprehensive understanding of the post-synthetic ligand exchange process. The cubic MOF-5 model, which utilized benzene-1,4-dicarboxylate (BDC) and zinc acetate clusters to create large pores, was a key focus of the study. Another significant aspect was the selection of 1,4-dicarboxylic acid benzene-2,3,5,6-d4 (H2BDC-d4) as the additional ligand. The research involved immersing MOF-5 in a tetrahydrofuran (THF) solution with a 0.01 M concentration of H2BDC-d4 (H2BDC-d4/BDC = 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 18 hours. Raman maps of the sliced crystals revealed the formation of core–shell heterostructures, with BDC-d4 predominantly located at the outer layer, penetrating to a depth of about 80 μm. Extending the soaking period to 72 hours or increasing the temperature to 45 °C resulted in a more significant depth increase of approximately 160 μm. The research has significant implications for the field of materials science and chemistry. Using dimethylformamide (DMF) as the exchange solvent, which has smaller diffusion coefficients, produced some intriguing results. Through Raman analysis and PXRD measurements, we discovered significant cracking in the crystals after just three days of incubation in a 0.1 M H2BDC-d4 solution. These findings not only highlight the impact of the solvent choice but also open new avenues for understanding the behavior of these materials under specific conditions. However, the integrity of MOF-5 was maintained, indicating that crystal exchange was caused by ligand diffusion. UMCM-8, which shares the same metal nodes as MOF-5, underwent a fascinating transformation when linked by H2BDC and 2,6-naphthalene dicarboxylic acid for ligand exchange. Remarkably, this process led to the preparation of an anticipated core–shell structure following a post-synthetic H2BDC-d4 ligand exchange in a low-concentration environment (0.005 M). The UiO-66 MOF has shown an impressive ability for post-synthetic ligand exchange, resulting in an intriguing core–shell structure. This process highlights the critical role of ligand diffusion, achieved by substituting 10 mol equivalents of H2BDCd4 in water at 85 °C for just one hour. These findings shed light on the complexities of post-synthetic modifications and pave the way for innovative designs in hierarchical core–shell MOFs.93,102
1) for 18 hours. Raman maps of the sliced crystals revealed the formation of core–shell heterostructures, with BDC-d4 predominantly located at the outer layer, penetrating to a depth of about 80 μm. Extending the soaking period to 72 hours or increasing the temperature to 45 °C resulted in a more significant depth increase of approximately 160 μm. The research has significant implications for the field of materials science and chemistry. Using dimethylformamide (DMF) as the exchange solvent, which has smaller diffusion coefficients, produced some intriguing results. Through Raman analysis and PXRD measurements, we discovered significant cracking in the crystals after just three days of incubation in a 0.1 M H2BDC-d4 solution. These findings not only highlight the impact of the solvent choice but also open new avenues for understanding the behavior of these materials under specific conditions. However, the integrity of MOF-5 was maintained, indicating that crystal exchange was caused by ligand diffusion. UMCM-8, which shares the same metal nodes as MOF-5, underwent a fascinating transformation when linked by H2BDC and 2,6-naphthalene dicarboxylic acid for ligand exchange. Remarkably, this process led to the preparation of an anticipated core–shell structure following a post-synthetic H2BDC-d4 ligand exchange in a low-concentration environment (0.005 M). The UiO-66 MOF has shown an impressive ability for post-synthetic ligand exchange, resulting in an intriguing core–shell structure. This process highlights the critical role of ligand diffusion, achieved by substituting 10 mol equivalents of H2BDCd4 in water at 85 °C for just one hour. These findings shed light on the complexities of post-synthetic modifications and pave the way for innovative designs in hierarchical core–shell MOFs.93,102
2.2.3.3. Internal extended growth method. In numerous instances, crafting MOFs@MOFs involves using comparable frameworks, often drawing on the same metal nodes or strikingly similar ligands. However, the challenge lies in blending MOFs with entirely different components, as it requires overcoming the barrier of interface energy. However, Kitagawa et al. devised an innovative and versatile internal extended growth technique for creating MOFs@MOFs with distinct crystal structures. They created a hybrid MOF-on-MOF heterostructure NH2-UiO-66@NH2-MIL-125 by incorporating NH2-UiO-66(Zr) into the precursor of NH2-MIL-125(Ti) with the assistance of the structure-directing agent poly(vinylpyrrolidone) (PVP) while using microwave heating. Furthermore, they effectively built different MOF-on-MOF heterostructures, including MIL-101 (Cr)@NH2-MIL-125 (Ti) and a combination of NH2-UiO-66 (Zr)/MOF-76 (Tb)/NH2-MIL125 (Ti) using this approach. The suggested internal extended growth method not only shows potential but also promises the adaptable creation of multicompartment MOFs@MOFs, thereby opening new avenues for research and applications.94,103
2.2.3.4. Surfactant-mediated overgrowth method. An alternative method for creating MOFs@MOFs with different topologies involved using surfactant-mediated overgrowth. Tsung et al. meticulously reported the synthesis of core–shell UiO-66@ZIF-8 using cetyltrimethylammonium bromide (CTAB) in an aqueous solution. The method involved a careful mixing of UiO-66 core crystals, ZIF-8 precursors, and CTAB. The nucleation of ZIF-8 was promoted by subjecting the reaction system to ultrasonication for 5 minutes, after which it was stirred for 3 hours to produce the core–shell UiO-66@ZIF-8. The incorporation of CTAB was found to be essential, as core–shell UiO-66@ZIF-8 could only be synthesized using it. The quantity of CTAB was also a key factor; insufficient CTAB led to incomplete ZIF-8 coverage, whereas the appropriate amount enabled the coating of ZIF-8 with uniform nucleation and overgrowth. The surfactant type significantly influenced the overgrowth of UiO-66@ZIF8. The addition of compounds such as tetradecyltrimethylammonium bromide (TTAB), methyl pyridinium bromide (CPB), and sodium dodecyl sulfate (SDS) led to the development of the polycrystalline and fractured ZIF-8 coating as they were not able to accurately regulate nucleation and overgrowth. PVP hindered the synthesis of UiO-66@ZIF-8 due to the requirement for appropriate interaction between PVP and the two MOFs in water. The duration of ultrasonication also had a significant impact on the nucleation of ZIF-8. Without ultrasonication, a fractured ZIF-8 layer formed. An unfinished ZIF-8 shell was noted when the sonication duration exceeded 15 minutes. After being sonicated for one hour, a yolk–shell heterostructure was created. After the above discussions, it was hypothesized that the material's growth mechanism occurred in the following manner: Initially, suitable ZIF-8 nuclei were generated under specific ultrasonic conditions. Subsequently, these nuclei adhered to the surface of UiO-66 in a specific orientation due to the presence of CTAB. The small ZIF-8 nuclei, which were evenly distributed, ultimately expanded into the shell. The unique structures of UiO-66 and ZIF-8 provide additional evidence that the use of surfactants in overgrowth can surpass the challenge of creating MOFs@MOFs with mismatched lattices due to structural similarity.94,104
2.2.3.5. Retrosynthetic design. MOF@MOF materials have significant potential. Preparing and applying these materials promise significant benefits, but their complexity can be quite daunting. This is mainly due to our limited control over the formation process, which makes this task challenging. Zhou et al. proposed using a retrosynthetic design method that considers surface modification and stability to create MOF@MOF architectures carefully. A core substrate of PCN-222(Zr) with high chemical stability was used to perform surface functionalization. The substrate was initially subjected to an excess of benzene-1,4-dicarboxylic acid linker for 24 hours. After that, a Zn(NO3)2 solution was added to the reaction system for a duration of 24 hours to promote the slow development of the MOF-5 shell. The verification of PCN-222@MOF-5 formation was established through optical microscopy pictures. The purple-colored needle-shaped PCN-222 and the colorless cubic MOF-5 confirmed the formation. Citric acid (CA) was utilized to alter the surface of PCN-222 via the carboxylate groups in CA, highlighting the importance of post-modification. The synthesis of composites PCN-222@MOF-5 resulted from this, showing that surface functionalization could link two different MOFs. When considering stability, core substrates such as UiO-66(Zr), UiO-67(Zr), PCN-160(Zr), MOF-808(Zr), PCN-222(Zr), PCN-250(Fe), and MIL-125(Ti) were utilized due to their stability as stable MOFs. The series MOF5(Zn), HKUST-1(Cu), and (Yb), which exhibit relatively low stability, were employed as shell MOFs to verify the general applicability of a retrosynthetic stability assessment. The innovative construction of the MOF@MOF has been successfully achieved, as evidenced by vibrant optical imaging and clear PXRD patterns. This confirms the effectiveness of the approach. Furthermore, modifying the allocation of core and shell MOFs allows for creating various arrangements, including evenly mixed, center-focused, and asymmetric distributions with equal parts. The suggested concepts of altering surfaces and evaluating stability in retrosynthesis are expected to be used in a controlled manner to design and prepare more MOFs@MOFs in the future.94,105
3. Biomedical applications
MOFs have special characteristics that render them very attractive for use in medical applications. Their porous formations make them ideal for administering various medications. The ability to choose from various metal ions and organic ligands allows for developing MOFs that possess natural anti-tumor properties. This enables the creation of synergistic systems between MOFs and drugs. Furthermore, MOFs can serve as precursors or templates for various anticancer substances.106 Moreover, controlled preparation methods can lead to the preparation of core–shell structures in MOFs. The original characteristics of MOFs are preserved by these formations, and they also exhibit additional structural characteristics. When MOF core shells are compared to pure MOFs, they contain empty spaces inside, which enables higher drug loading and improved mass transfer capabilities. The combination of MOFs with other functional materials in core–shell structures results in a synergistic effect, where the final outcome exceeds the combined individual components.107 In this feature article, our focus will be on the utilization of core–shell MOFs in biomedicine, specifically in the fields of bioimaging, biosensing, drug delivery, and synergistic therapy in recent years.3.1. Bioimaging
Detection and monitoring of lesion areas, as well as the transport of bioactive molecules, are crucial in treating serious diseases like cancer. These aspects rely on diagnostic tools like bioimaging.107 Bioimaging enables the visualization of an organism's structure, understanding biological functions, and observing biological processes in real-time without physical intervention; it is capable of examining structures at the subcellular level up to entire multicellular organisms.108 Biomedical imaging techniques used in current clinical practices include magnetic resonance (MRI) imaging, computed tomography (CT), and fluorescence (FL) imaging. These techniques can be combined to produce high-resolution images crucial for making informed medical decisions. At the heart of this process are contrast agents. These agents play a pivotal role in differentiating between anatomical variances and enhancing the distinction among bodily tissues, thereby enabling the production of high-quality images that are vital for medical decision-making. Furthermore, contrast agents should have a long enough circulatory duration when administered intravenously. Nanoparticles (NPs) ranging from 1 to 100 nanometers have proven to be useful tools for imaging inside living organisms for biological diagnosis. They allow for long-term imaging at low doses. MOFs are also seen as potential nanomaterials for bioimaging. These frameworks, which consist of crystalline porous materials composed of inorganic units containing transition metal cations/clusters and connecting organic ligands, offer unique properties that make them suitable for bioimaging applications. Core–shell MOFs with nano-dimensions are utilized as contrast agents for highly effective imaging methods. Their biodegradability enables quick decomposition and cleaning after use. This section will explore the application of core–shell MOF structures in various bioimaging models.107 | ||
| Fig. 2 (a) Creating the HUC-PEG nanocomposite followed by the formation of its MOF-POP and demonstration of the HUC-PEG theranostic platform for conducting CT/photothermal imaging-guided therapy in both in vitro and in vivo settings. (b) The mechanism of interface-enhanced phototherapy for HUC-PEG. Reproduced from ref. 114 with permission from Elsevier, copyright 2020. | ||
3.2. Biosensing
A biosensor functions as a device that identifies variations in a biological substance, producing an electrical signal that corresponds to these changes. Biosensor receptors can consist of materials like enzymes, nucleic acids, antigens, and other elements.116 Biosensors can oversee specific biological processes and identify illnesses by recognizing changes in the signal of particular substances, playing a crucial role in medical treatment.11 MOFs demonstrate exceptional sensitivity in sensing due to their ability to change color and also offer advantages such as modifiable structures, extensive surface area, porosity, numerous exposed active sites, and outstanding biocompatibility.117 Moreover, the practical applications of MOFs in improving the binding of antibiotics and biomolecules like glucose, antibodies, and aptamers are significant. Their –NH2 or –COOH functional groups have been successfully utilized as frameworks, and their core–shell configurations offer further promise for biosensor uses by enhancing optical characteristics, durability, and selectivity.93,118 Creating advanced and innovative biosensors that provide effective detection beyond traditional methods is currently a difficult task.119 Liu et al. enhanced a uniform voltammetric (HVC) sensor using an electrochemical approach to identify Pb2+. The Me@UiO-66-NH2 system functions as the responsive material prompted by the smart target. The UiO-66-NH2 MOF, serving as a nanocarrier, encapsulates a signal probe (methylthionine chloride) and is coated with COOH complementary sequences (CP) and Pb2+ aptamers. The HVC sensor optimized with Me@UiO-66-NH2 shows remarkable analytical performance under specific conditions. It displays exceptional analytical abilities with a minimal limit of detection (LOD) of 0.166 pM and a wide linear range from 5.0 pM to 500.0 nM. These findings demonstrate a low LOD and an extensive linear range, highlighting superb analytical performance. This MOF framework serves as a signal transmitter and a ground-breaking platform for creating aptamer-based bioelectronic devices boasting remarkable sensitivity. It preserves selectivity within intricate sample matrices and has the potential for multiplex detection. Incorporating this pioneering MOF–aptamer system into portable sensors facilitates on-site monitoring of heavy metals in water and guarantees food safety.120 Lu et al. created a composite material called UiO-67@Ni-MOF and utilized it to detect glucose. They proved the formation of the mentioned structure with SEM, TEM, and EDS elemental mapping analyses (Fig. 3b and c). The impressive surface area and robust electrocatalytic capabilities of UiO-67 significantly boosted electron transfer in UiO-67@Ni-MOF, enhancing its performance and efficiency. In addition, the Ni-MOF showed remarkable electrochemical catalytic capabilities for glucose, making it a standout performer in the field. The results of the amperometry test indicated that when conditions were optimal, the sensor demonstrated quick response (under 5 seconds), a wide linear range (5 mM to 3.9 mM), and a low detection limit (0.98 mM). Furthermore, it demonstrated remarkable consistency, boasting a relative standard deviation of just 1.1%, alongside an impressive overall reliability with a relative standard deviation of 1.9%. Plus, it showcased exceptional durability over time. The developed electrochemical biosensor demonstrated rapid response times, a broad detection range, and a low limit of detection (LOD) (Fig. 3a).121 | ||
| Fig. 3 (a) Illustration provided in the schematic shows the process of creating core–shell UiO-67@Ni-MOF composites through internal extended growth while being regulated by polyvinylpyrrolidone. The modified electrode with UiO-67@Ni-MOF is used for nonenzymatic glucose sensing. (b) (1) SEM images of UiO-67, (2) Ni-MOF, and (3) TEM images of UiO-67. (c), (1) SEM image, (2) TEM image, and (3) EDS elemental mapping of prepared UiO-67@Ni-MOF. Reproduced from ref. 121 with permission from Elsevier, copyright 2020. | ||
3.3. Drug delivery systems
The current era sees both cancer and bacterial illnesses posing a significant danger to human health, emphasizing the continuous necessity for research, public health campaigns, and advancements in healthcare. The targeted transportation of drugs for these illnesses has become a crucial challenge. Throughout history, drugs have played an essential role in combating diseases, and the continuous advancement of science and technology has led to the creation of increasingly effective drugs. Nonetheless, the adverse effects of drugs and their breakdown in the body's environment significantly impede their clinical usage. To overcome this hurdle, the use of nanocarriers, which can load and release drugs at specific sites within the body, has been proposed and demonstrated as an effective treatment strategy.110 The nanoscale core–shell MOF structures have high porosity and a large surface area, which make them potential drug carriers. MOFs' molecularly precise porous structure and organic–inorganic hybrid nature provide them with unique adsorption properties, making core–shell MOFs ideal for drug loading. MOFs can effectively deliver drugs with varying molecular weights, hydrophilicity, and sizes at predetermined ratios.106 Here, we address the significance of drug delivery in the individual treatments of cancer and bacterial diseases. | ||
| Fig. 4 (a) Designing a DUCNP@Mn-MOF framework for the multifaceted fusion therapy of cancer and understanding how the nanoparticles (DUCNP@Mn-MOF/FOE) hinder the growth of tumor cells. (b) (1) Body weight changes in mice. (2) Using DUCNP@Mn-MOF/FOE treatment leads to successful inhibition of tumor growth in living organisms. The tumor volumes for each group (n = 5; ****p < 0.0001) were examined. (c) In vivo FL imaging was conducted on mice bearing tumors in their right hind leg following various administration groups. Reproduced from ref. 125 with permission from American Chemical Society, copyright 2023. | ||
 | ||
| Fig. 5 (a) Preparation of ONP@ZnO2@ZIF-67 and understanding how the nanosystem works for identifying and treating infections. (b) Macrophages expressed ONOO− after being infected with methicillin-resistant Staphylococcus aureus (MRSA) at various time points. (c) Images of FL signals from macrophages stimulated by 3-morpholino sydnonimine hydrochloride (SIN-1, ONOO− donor), MRSA, and antibiotic-resistant Escherichia coli (ARE) were captured. (1) Seen using a confocal laser scanning microscope. (2) Viewed using an In vivo imaging system (IVIS). (d) The FL intensity of probes is used to assess the level of bacterial infection at wound locations. Reproduced from ref. 129 with permission from Elsevier, copyright 2022. | ||
3.4. Synergistic therapy
Nowadays, research is concentrating on creating multimodal treatment approaches to achieve better antitumor results. Conventional cancer treatments like surgery, radiation, and chemotherapy can be life-saving, but they often come with a harsh price. Patients frequently face a whirlwind of side effects, grapple with high rates of recurrence, and even develop resistance to multiple drugs.130 As a growing method for treating cancer, combined therapy using core–shell MOFs, which involves multiple models of phototherapy, has gained considerable interest because of its minimal side effects, inherent non-invasiveness, and precise targeting when the tumor sites are exposed to localized radiation. The combination of various medical treatments in synergistic cancer therapy has demonstrated improved therapeutic outcomes and significantly decreased toxicity compared to individual therapy.131 In the fight against cancer, use of core–shell MOFs in biomedicine has become a hopeful and advanced method for delivering drugs or cargo. Their exceptional characteristics, including efficient loading of drugs/cargo and degradation triggered by the tumor microenvironment, have given rise to optimism within the medical field. Core–shell MOFs, particularly those that combine several theranostic components, have substantial promise for effective cancer therapy.132 MOFs provide significant benefits for creating versatile treatment platforms due to their adjustable structure and expansive surface area. Exploring different combinations of therapies using MOF core–shell structures, such as chemo/photodynamic therapy, chemo/photothermal therapy, photothermal therapy/photodynamic therapy, and chemo/photothermal therapy/photodynamic therapy, has demonstrated encouraging therapeutic outcomes for treating tumors. This part will explore the most recent example of combined therapy using MOF core–shell structures.21 | ||
| Fig. 6 (a) Schematic diagram depicting the preparation of ICG-PFH/MOF/DNA-DOX and the mechanism of synergistic photo-chemotherapy. Reproduced from ref. 138 with permission from Elsevier, copyright 2024. (b) The strategy of synthesis and chemotherapy are integrated with PDT in the UiO-66-NH2. Reproduced from ref. 137 with permission from Elsevier, copyright 2022. | ||
4. Conclusion and perspectives
Core–shell MOFs represent an exciting and versatile class of materials with significant potential for biomedical applications. The core–shell design allows for precise tailoring of their structures, compositions, and functionalities, resulting in improved control over their physicochemical properties, including stability, biocompatibility, and targeted delivery. Recent advancements in synthetic methodologies such as one-pot synthesis, in situ synthesis, post-synthetic modification, LBL assembly, self-templating, and epitaxial growth have expanded the capabilities of core–shell MOFs. These innovations enable their application in various biomedical applications, including drug delivery, bioimaging, biosensing, and synergistic therapy.Despite their significant potential, several challenges must be addressed before core–shell MOFs can be widely used in clinical applications. Key issues include large-scale production, reproducibility, and long-term biocompatibility, all of which require further investigation. Additionally, the potential toxicity of certain metal components and their degradation products must be carefully evaluated through rigorous preclinical studies. It is also essential to understand how these materials interact with biological systems at the molecular and cellular levels in order to optimize their safety and effectiveness.
Looking ahead, integrating advanced computational modeling and machine learning into the design of core–shell MOFs could accelerate the discovery of novel structures specifically tailored for biomedical applications. Additionally, interdisciplinary collaborations among materials scientists, chemists, biologists, and clinicians will be essential for overcoming current limitations and fully realizing the potential of these materials in precision medicine. With ongoing research efforts and technological innovation, core–shell MOFs have the potential to transform the field of biomedical engineering and enhance healthcare outcomes worldwide.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the partial support from the Research Council of the Iran University of Science and Technology (IUST).References
- O. M. Yaghi, G. Li and H. Li, Nature, 1995, 6558, 703–706 CrossRef.
- H. Li, M. Eddaoudi, M. O’Keeffe and O. M. Yaghi, Nature, 1999, 6759, 276–279 CrossRef.
- S. Ibrar, N. Z. Ali, E. O. Ojegu, O. B. Odia, L. Ikhioya and I. Ahmad, Appl. Organomet. Chem., 2023, 3.4, 294–307 Search PubMed.
- G. Cai, P. Yan, L. Zhang, H. C. Zhou and H. L. Jiang, Chem. Rev., 2021, 20, 12278–12326 CrossRef PubMed.
- N. Zhang, B. Zhu, F. Peng, X. Yu, Y. Jia, J. Wang, L. Kong, Z. Jin, T. Luo and J. Liu, Chem. Commun., 2014, 50(57), 7686–7689 RSC.
- K. Koh, A. G. Wong-Foy and A. J. Matzger, Chem. Commun., 2009, 6162–6164 RSC.
- K. A. McDonald, J. I. Feldblyum, K. Koh, A. G. Wong-Foy and A. J. Matzger, Chem. Commun., 2015, 60, 11994–11996 RSC.
- O. Kwon, J. Y. Kim, S. Park, J. H. Lee, J. Ha, H. Park, H. R. Moon and J. Kim, Nat. Commun., 2019, 10(1), 3620 CrossRef PubMed.
- E. Ozyilmaz, O. Caglar, I. Sargin and G. Arslan, Mater. Today Commun., 2022, 30, 103066 CrossRef CAS.
- V. Shrivastav, P. Dubey, S. Sundriyal, U. K. Tiwari and A. Deep, Coord. Chem. Rev., 2024, 499, 215497 CrossRef.
- X. Ge, R. Wong, A. Anisa and S. Ma, Biomaterials, 2022, 281, 121322 CrossRef CAS PubMed.
- M. X. Wu, J. Gao, F. Wang, J. Yang, N. Song, X. Jin, P. Mi, J. Tian, J. Luo, F. Liang and Y. W. Yang, Small, 2018, 14(17), 1704440 CrossRef PubMed.
- J. Yang, D. Dai, X. Zhang, L. Teng, L. Ma and Y. W. Yang, Theranostics, 2023, 13(1), 295 CrossRef CAS PubMed.
- M. X. Wu and Y. W. Yang, Adv. Mater., 2017, 29(23), 1606134 CrossRef PubMed.
- P. Shams, H. Panahi, M. Tahan, F. Jahansooz and A. Heidaripour, J. Med. Nanomater. Chem., 2023, 267–275 Search PubMed.
- J. Gao, H. M. Yu, M. Wu, Q. Chen, Y. Yang, Y. Qu, M. Sun, J. C. Qin, L. Ma and Y. W. Yang, Mater. Today Chem., 2022, 23, 100716 CrossRef CAS.
- J. Yang, D. Dai, Z. Cai, Y. Q. Liu, J. C. Qin, Y. Wang and Y. W. Yang, Acta Biomater., 2021, 15(134), 664–673 CrossRef PubMed.
- M. X. Wu, H. J. Yan, J. Gao, Y. Cheng, J. Yang, J. R. Wu, B. J. Gong, H. Y. Zhang and Y. W. Yang, ACS Appl. Mater. Interfaces, 2018, 10(40), 34655–34663 CrossRef CAS PubMed.
- M. Sajjadnejad and S. M. Haghshenas, J. Med. Nanomater. Chem., 2023, 69–91 Search PubMed.
- W. J. Rieter, K. M. L. Taylor and W. Lin, J. Am. Chem. Soc., 2007, 129(32), 9852–9853 CrossRef CAS PubMed.
- J. Tang, C. Huang, Y. Liu, T. Wang, M. Yu, H. Hao, W. Zeng, W. Huang, J. Wang and M. Wu, Coord. Chem. Rev., 2023, 490, 215211 CrossRef CAS.
- H. Shen, J. Liu, J. Lei and H. Ju, Chem. Commun., 2018, 66, 9155–9158 RSC.
- J. Yang and Y. W. Yang, Small, 2020, 16(10), 1906846 CrossRef CAS PubMed.
- M. Liu, Z. Xing, Z. Li and W. Zhou, Coord. Chem. Rev., 2021, 446, 214123 CrossRef CAS.
- J. W. Osterrieth, D. Wright, H. Noh, C. W. Kung, D. Vulpe, A. Li, J. E. Park, R. P. Van Duyne, P. Z. Moghadam, J. J. Baumberg and O. K. Farha, J. Am. Chem. Soc., 2019, 141(9), 3893–3900 CrossRef CAS PubMed.
- L. Wang, D. Jia, L. Yue, K. Zheng, A. Zhang, Q. Jia and J. Liu, ACS Appl. Mater. Interfaces, 2020, 12(42), 47526–47538 CrossRef CAS PubMed.
- P. Parnicka, W. Lisowski, T. Klimczuk, A. Mikolajczyk and A. Zaleska-Medynska, Appl. Catal., B, 2022, 310, 121349 Search PubMed.
- X. Huang, S. Yan, D. Deng, L. Zhang, R. Liu and Y. Lv, ACS Appl. Mater. Interfaces, 2021, 13(2), 3471–3480 CrossRef CAS PubMed.
- W. G. Cui, Y. T. Li, H. Zhang, Z. C. Wei, B. H. Gao, J. J. Dai and T. L. Hu, Appl. Catal., B, 2020, 278, 119262 CrossRef CAS.
- A. Ringaci, A. V. Yaremenko, K. G. Shevchenko, S. D. Zvereva and M. P. Nikitin, Chem. Eng. J., 2021, 418, 129386 Search PubMed.
- S. K. Elsaidi, M. A. Sinnwell, D. Banerjee, A. Devaraj, R. K. Kukkadapu, T. C. Droubay, Z. Nie, L. Kovarik, M. Vijayakumar, S. Manandhar and M. Nandasiri, Nano Lett., 2017, 17(11), 6968–6973 CrossRef CAS PubMed.
- L. Wang, C. Li, R. Wang, Y. Lin, K. Xiong, B. Wang and C. Hao, J. Mol. Liq., 2023, 376, 121373 CrossRef CAS.
- Y. Li, W. S. Lo, F. Zhang, X. Si, L. Y. Chou, X. Y. Liu, B. P. Williams, Y. H. Li, S. H. Jung, Y. S. Hsu and F. S. Liao, J. Am. Chem. Soc., 2021, 143(13), 5182–5190 CrossRef CAS PubMed.
- Y. Long, S. Song, J. Li, L. Wu, Q. Wang, Y. Liu, R. Jin and H. Zhang, ACS Catal., 2018, 8(9), 8506–8512 CrossRef CAS.
- Y. Zhang, M. Gutiérrez, A. K. Chaudhari and J. C. Tan, ACS Appl. Mater. Interfaces, 2020, 12(33), 37477–37488 CrossRef CAS PubMed.
- C. H. Li, C. L. Huang, X. F. Chuah, D. S. Raja, C. T. Hsieh and S. Y. Lu, Chem. Eng. J., 2019, 361, 660–670 CrossRef CAS.
- E. H. Kwon, M. Kim, C. Y. Lee, M. Kim and Y. D. Park, ACS Appl. Mater. Interfaces, 2022, 14(8), 10637–10647 CrossRef CAS PubMed.
- L. He, Y. Liu, J. Liu, Y. Xiong, J. Zheng, Y. Liu and Z. Tang, Angew. Chem., Int. Ed., 2013, 52(13), 3741–3745 CrossRef CAS PubMed.
- Y. Zhang, Y. Hu, G. Li and R. Zhang, Mikrochim. Acta, 2019, 186, 1 CrossRef PubMed.
- M. Zeng, Z. Chai, X. Deng, Q. Li, S. Feng, J. Wang and D. Xu, Nano Res., 2016, 9, 2729–2734 CrossRef CAS.
- C. Hou, L. Fu, Y. Wang, W. Chen, F. Chen, S. Zhang and J. Wang, Cellulose, 2021, 28(14), 9253–9268 CrossRef CAS.
- C. L. Whitford, C. J. Stephenson, D. A. Gomez-Gualdron, J. T. Hupp, O. K. Farha, R. Q. Snurr and P. C. Stair, J. Phys. Chem. C, 2017, 121(45), 25079–25091 CrossRef CAS.
- P. Pachfule, X. Yang, Q. L. Zhu, N. Tsumori, T. Uchida and Q. Xu, J. Mater. Chem. A, 2017, 5(10), 4835–4841 RSC.
- X. Ma, J. Zhang, C. Zhang, X. Yang, A. Yu, Y. Huang, S. Zhang and G. Ouyang, ACS Appl. Mater. Interfaces, 2021, 13(33), 40070–40078 CrossRef CAS PubMed.
- L. U. Zhang, Z. Wang, Y. Zhang, F. Cao, K. Dong, J. Ren and X. Qu, ACS Nano, 2018, 12(10), 10201–10211 CrossRef CAS PubMed.
- J. Wan, Z. Zhao, H. Shang, B. Peng, W. Chen, J. Pei, L. Zheng, J. Dong, R. Cao, R. Sarangi and Z. Jiang, J. Am. Chem. Soc., 2020, 142(18), 8431–8439 CrossRef CAS PubMed.
- D. Li, X. Cao, Q. Zhang, X. Ren, L. Jiang, D. Li, W. Deng and H. Liu, J. Mater. Chem. A, 2019, 7(23), 14108–14117 RSC.
- Y. Liu, C. S. Gong, L. Lin, Z. Zhou, Y. Liu, Z. Yang, Z. Shen, G. Yu, Z. Wang, S. Wang and Y. Ma, Theranostics, 2019, 9(10), 2791 CrossRef CAS PubMed.
- C. Yao, Q. Wang, C. Peng, R. Wang, J. Liu, N. Tsidaeva and W. Wang, Chem. Eng. J., 2024, 479, 147924 CrossRef CAS.
- Z. Wang, J. Niu, C. Zhao, X. Wang, J. Ren and X. Qu, Angew. Chem., 2021, 133(22), 12539–12545 CrossRef.
- W. Zhu, G. Xiang, J. Shang, J. Guo, B. Motevalli, P. Durfee, J. O. Agola, E. N. Coker and C. J. Brinker, Adv. Funct. Mater., 2018, 28(16), 1705274 CrossRef.
- X. Yang, S. Yuan, L. Zou, H. Drake, Y. Zhang, J. Qin, A. Alsalme and H. C. Zhou, Angew. Chem., 2018, 130(15), 3991–3996 CrossRef.
- C. Li, M. Zhang, X. Di, D. Yin, W. Li and C. Liang, Chin. J. Catal., 2016, 37(9), 1555–1561 CrossRef CAS.
- D. Xu, Y. Qin, X. Cao, Y. Huang, J. Wang, X. Liu, F. Liu and X. Li, Colloids Surf., A, 2024, 696, 134306 CrossRef CAS.
- T. Si, X. Lu, H. Zhang, S. Wang, X. Liang and Y. Guo, Chin. Chem. Lett., 2022, 33(8), 3869–3872 CrossRef CAS.
- D. Kim, G. Lee, S. Oh and M. Oh, Chem. Commun., 2019, 55(1), 43–46 RSC.
- X. Gu, C. Huang, Z. Xu, H. Wu, R. Dong, R. Liu, J. Chen and H. Zhu, J. Solid State Chem., 2021, 294, 121803 CrossRef CAS.
- Y. Zhou, B. Tang, S. Wang and J. Long, Int. J. Hydrogen Energy, 2020, 45(32), 15785–15795 CrossRef CAS.
- G. Lee, S. Lee, S. Oh, D. Kim and M. Oh, J. Am. Chem. Soc., 2020, 142(6), 3042–3049 CrossRef CAS PubMed.
- P. Á. Szilágyi, M. Lutz, J. Gascon, J. Juan-Alcañiz, J. Van Esch, F. Kapteijn, H. Geerlings, B. Dam and R. Van De Krol, CrystEngComm, 2013, 15(30), 6003–6008 RSC.
- M. X. Wu, Y. Wang, G. Zhou and X. Liu, ACS Appl. Mater. Interfaces, 2020, 12(49), 54285–54305 CrossRef CAS PubMed.
- C. Dong, R. Tian, Y. Zhang, K. Liu, G. Chen, H. Guan and Z. Yin, Chem. Eng. J., 2022, 442, 136094 CrossRef CAS.
- H. Li, F. Yue, H. Xie, C. Yang, Y. Zhang, L. Zhang and J. Wang, CrystEngComm, 2018, 20(7), 889–895 RSC.
- S. J. Yin, H. Chen, S. Wang, Y. Wang and F. Q. Yang, Heliyon, 2023, 9(5), 16245 CrossRef PubMed.
- M. X. Wu, C. Wei, X. H. Wang, Q. Q. Xia, H. Wang and X. Liu, Inorg. Chem., 2022, 61(11), 4705–4713 CrossRef CAS PubMed.
- A. E. Abdelmaoula, J. Shu, Y. Cheng, L. Xu, G. Zhang, Y. Xia, M. Tahir, P. Wu and L. Mai, Small Methods, 2021, 5(8), 2100508 CrossRef CAS PubMed.
- Q. Zhang, L. Fan, W. Liu, Y. Xie, J. Li and G. Huang, J. Ind. Eng. Chem., 2023, 124, 311–322 CrossRef CAS.
- M. Lu, Y. Deng, Y. Li, T. Li, J. Xu, S. W. Chen and J. Wang, Anal. Chim. Acta, 2020, 1110, 35–43 CrossRef CAS PubMed.
- Y. Zhang, B. Wei and H. Liang, ACS Appl. Mater. Interfaces, 2023, 15(2), 3442–3454 CrossRef CAS PubMed.
- C. Guo, J. Guo, Y. Zhang, D. Wang, L. Zhang, Y. Guo, W. Ma and J. Wang, CrystEngComm, 2018, 20(47), 7659–7665 RSC.
- K. Li, Y. Zhang, P. Wang, X. Long, L. Zheng, G. Liu, X. He and J. Qiu, J. Alloys Compd., 2022, 903, 163701 CrossRef CAS.
- H. Park, S. Oh, S. Lee, S. Choi and M. Oh, Appl. Catal., B, 2019, 246, 322–329 CrossRef CAS.
- D. Li, X. Cheng, R. Xu, Y. Wu, X. Zhou, C. Ma and Y. Yu, J. Mater. Chem. A, 2019, 7(34), 19929–19938 RSC.
- L. Zhang, J. Wang, X. Ren, W. Zhang, T. Zhang, X. Liu, T. Du, T. Li and J. Wang, J. Mater. Chem. A, 2018, 6(42), 21029–21038 RSC.
- Y. Liu, C. Wu, Z. Zhou, W. Liu, H. Guo and B. Zhang, J. Membr. Sci., 2022, 659, 120787 CrossRef CAS.
- H. Tan, X. Zhao, L. Du, B. Wang, Y. Huang, Y. Gu and Z. Lu, Small, 2024, 20(3), 2305881 CrossRef CAS PubMed.
- S. R. Hosseini, M. Omidkhah, Z. M. Lighvan, S. Norouzbahari and A. Ghadimi, Sep. Purif. Technol., 2023, 307, 122679 CrossRef CAS.
- Z. Zhao, J. Ding, R. Zhu and H. Pang, J. Mater. Chem. A, 2019, 7(26), 15519–15540 RSC.
- W. Zhan, Q. Kuang, J. Zhou, X. Kong, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2013, 135(5), 1926–1933 CrossRef CAS PubMed.
- X. Zhang, Q. Han and M. Ding, RSC Adv., 2015, 5(2), 1043–1050 RSC.
- R. Chen, C. Tao, Z. Zhang, X. Chen, Z. Liu and J. Wang, ACS Appl. Mater. Interfaces, 2019, 11(46), 43156–43165 CrossRef CAS PubMed.
- J. Zhuang, L. Y. Chou, B. T. Sneed, Y. Cao, P. Hu, L. Feng and C. K. Tsung, Small, 2015, 11(41), 5551–5555 CrossRef CAS PubMed.
- C. Guo, J. Guo, Y. Zhang, D. Wang, L. Zhang, Y. Guo, W. Ma and J. Wang, CrystEngComm, 2018, 20, 7659–7665 RSC.
- H. Wang, X. Yuan, Y. Wu, G. Zeng, H. Dong, X. Chen, L. Leng, Z. Wu and L. Peng, Appl. Catal., B, 2016, 186, 19–29 CrossRef CAS.
- F. Meng, S. Zhang, L. Ma, W. Zhang, M. Li, T. Wu, H. Li, T. Zhang, X. Lu, F. Huo and J. Lu, Adv. Mater., 2018, 30(49), 1803263 CrossRef PubMed.
- S. Ding, J. Wan, Y. Ma, Y. Wang, M. Pu, X. Li and J. Sun, J. Hazard. Mater., 2021, 411, 125194 CrossRef CAS PubMed.
- W. Bao, M. Liu, J. Meng, S. Liu, S. Wang, R. Jia, Y. Wang, G. Ma, W. Wei and Z. Tian, Nat. Commun., 2021, 12(1), 6399 CrossRef CAS PubMed.
- X. Meng, J. Deng, F. Liu, T. Guo, M. Liu, P. Dai, A. Fan, Z. Wang and Y. Zhao, Nano Lett., 2019, 19(11), 7866–7876 CrossRef CAS PubMed.
- L. Zhang, J. Zhang, X. Li, C. Wang, A. Yu, S. Zhang, G. Ouyang and Y. Cui, Appl. Surf. Sci., 2021, 538, 148054 CrossRef CAS.
- D. Lin, P. Duan, W. Yang, Y. Liu and Q. Pan, Inorg. Chem. Front., 2020, 7, 1643–1650 RSC.
- S. Ehrling, C. Kutzscher, P. Freund, P. Müller, I. Senkovska and S. Kaskel, Microporous Mesoporous Mater., 2018, 263, 268–274 CrossRef CAS.
- S. Furukawa, K. Hirai, K. Nakagawa, Y. Takashima, R. Matsuda, T. Tsuruoka, M. Kondo, R. Haruki, D. Tanaka, H. Sakamoto and S. Shimomura, Angew. Chem., 2009, 121(10), 1798–1802 CrossRef.
- C. Guo, F. Duan, S. Zhang, L. He, M. Wang, J. Chen, J. Zhang, Q. Jia, Z. Zhang and M. Du, J. Mater. Chem., 2022, 10(2), 475–507 RSC.
- M. X. Wu, Y. Wang, G. Zhou and X. Liu, ACS Appl. Mater. Interfaces, 2020, 12(49), 54285–54305 CrossRef CAS PubMed.
- C. Liu, J. Wang, J. Wan and C. Yu, Coord. Chem. Rev., 2021, 432, 213743 CrossRef CAS.
- Y. Shan, L. Chen, H. Pang and Q. Xu, Small Struct., 2021, 2(2), 2000078 CrossRef CAS.
- X. Yang, S. Yuan, L. Zou, H. Drake, Y. Zhang, J. Qin, A. Alsalme and H. C. Zhou, Angew. Chem., 2018, 130(15), 3991–3996 CrossRef.
- M. Liu, Z. Xing, Z. Li and W. Zhou, Coord. Chem. Rev., 2021, 446, 214123 Search PubMed.
- W. Guo, W. Xia, K. Cai, Y. Wu, B. Qiu, Z. Liang, C. Qu and R. Zou, Small, 2017, 13(41), 1702049 CrossRef PubMed.
- L. Figueroa-Quintero, D. Villalgordo-Hernández, J. J. Delgado-Marín, J. Narciso, V. K. Velisoju, P. Castaño, J. Gascón and E. V. Ramos-Fernández, Small Methods, 2023, 7(4), 2201413 CrossRef CAS PubMed.
- X. Song, T. K. Kim, H. Kim, D. Kim, S. Jeong, H. R. Moon and M. S. Lah, Chem. Mater., 2012, 24(15), 3065–3073 CrossRef CAS.
- J. A. Boissonnault, A. G. Wong-Foy and A. J. Matzger, ACS Appl. Mater. Interfaces, 2017, 139(42), 14841–14844 Search PubMed.
- Y. Gu, Y. Wu, L. Li, W. Chen, F. Li and S. Kitagawa, Angew. Chem., Int. Ed., 2017, 129(49), 15864–15868 CrossRef.
- J. Zhuang, L. Y. Chou, B. T. Sneed, Y. Cao, P. Hu, L. Feng and C. K. Tsung, Small, 2015, 11, 5551–5555 CrossRef CAS PubMed.
- L. Feng, S. Yuan, J. L. Li, K. Y. Wang, G. S. Day, P. Zhang, Y. Wang and H. C. Zhou, ACS Cent. Sci., 2018, 4, 1719–1726 CrossRef CAS PubMed.
- P. Gao, Y. Chen, W. Pan, N. Li, Z. Liu and B. Tang, Angew. Chem., Int. Ed., 2021, 133(31), 16901–16914 CrossRef.
- M. Nazari, A. S. Saljooghi, M. Ramezani, M. Alibolandi and M. Mirzaei, J. Mater. Chem. B, 2022, 10(43), 8824–8851 RSC.
- R. Weissleder and M. Nahrendorf, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(47), 14424–14428 CrossRef CAS PubMed.
- H. S. Wang, Coord. Chem. Rev., 2017, 349, 139–155 CrossRef CAS.
- L. Zhang, M. Liu, Z. Fang and Q. Ju, Coord. Chem. Rev., 2022, 468, 214641 CrossRef CAS.
- L. Zhang, C. Liu, Y. Gao, Z. Li, J. Xing, W. Ren, L. Zhang, A. Li, G. Lu, A. Wu and L. Zeng, Adv. Healthcare Mater., 2018, 7(24), 1870086 CrossRef.
- H. X. Zhao, Q. Zou, S. K. Sun, C. Yu, X. Zhang, R. J. Li and Y. Y. Fu, Chem. Sci., 2016, 7(8), 5294–5301 RSC.
- T. Wang, S. Li, Z. Zou, L. Hai, X. Yang, X. Jia, A. Zhang, D. He, X. He and K. Wang, J. Mater. Chem. B, 2018, 6(23), 3914–3921 RSC.
- X. Zheng, L. Wang, Y. Guan, Q. Pei, J. Jiang and Z. Xie, Biomaterials, 2020, 235, 119792 CrossRef CAS PubMed.
- H. Alijani, A. Noori, N. Faridi, S. Z. Bathaie and M. F. Mousavi, J. Solid State Chem., 2020, 292, 121680 CrossRef CAS.
- Y. Wang, K. Liu, M. Zhang, T. Xu, H. Du, B. Pang and C. Si, Carbohydr. Polym., 2023, 313, 120851 Search PubMed.
- Y. Cui, J. Zhang, H. He and G. Qian, Coord. Chem. Rev., 2018, 47(15), 5740–5785 CAS.
- E. Dezhakam, R. F. Vayghan, S. Dehghani, T. Kafili-Hajlari, A. Naseri, M. Dadashpour, B. Khalilzadeh and G. S. Kanberoglu, Sci. Rep., 2024, 14(1), 29850 CrossRef CAS PubMed.
- S. Khan, W. C. Cho, A. Sepahvand, S. Haji Hosseinali, A. Hussain, M. M. Nejadi Babadaei, M. Sharifi, M. Falahati, L. A. Jaragh-Alhadad, T. L. Ten Hagen and X. Li, J. Nanobiotechnol., 2023, 21(1), 136 CrossRef CAS PubMed.
- T. Liu, R. Zhou, C. Zhang, Y. Yi and G. Zhu, Chem. Commun., 2023, 59(25), 3771–3774 RSC.
- M. Lu, Y. Deng, Y. Li, T. Li, J. Xu, S. W. Chen and J. Wang, Anal. Chim. Acta, 2020, 1110, 35–43 CrossRef CAS PubMed.
- R. Taheri-Ledari, S. Zarei-Shokat, F. S. Qazi, M. Ghafori-Gorab, F. Ganjali, A. Kashtiaray, M. Mahdavi, M. Safavi and A. Maleki, ACS Appl. Mater. Interfaces, 2023, 17(12), 17703–17717 CrossRef PubMed.
- L. He, K. Pang, W. Liu, Y. Tian, L. Chang, X. Liu, M. Zhao, Y. Liu, Y. Li, X. Jiang and R. Song, J. Mater. Chem. B, 2019, 7(7), 1050–1055 RSC.
- X. Cai, Y. Zhao, L. Wang, M. Hu, Z. Wu, L. Liu, W. Zhu and R. Pei, J. Mater. Chem. B, 2021, 9(33), 6646–6657 RSC.
- X. Zhao, S. He, B. Li, B. Liu, Y. Shi, W. Cong, F. Gao, J. Li, F. Wang, K. Liu, C. Sheng, J. Su and H. G. Hu, Nano Lett., 2023, 23(3), 863–871 CrossRef CAS PubMed.
- T. C. Livesey, L. A. M. Mahmoud, M. G. Katsikogianni and S. Nayak, Pharmaceutics, 2023, 15(1), 274 CrossRef CAS PubMed.
- L. Yan, A. Gopal, S. Kashif, P. Hazelton, M. Lan, W. Zhang and X. Chen, Chem. Eng. J., 2022, 435, 134975 CrossRef CAS.
- Y. Tu, C. Lei, F. Deng, Y. Chen, Y. Wang and Z. Zhang, New J. Chem., 2021, 45(19), 8701–8713 RSC.
- K. Huang, F. Li, K. Yuan, Y. Yang, H. Chang, Y. Liang, X. Yan, J. Zhao, T. Tang and S. Yang, Appl. Mater. Today, 2022, 28, 101513 CrossRef.
- C. Liang, L. Xu, G. Song and Z. Liu, Chem. Soc. Rev., 2016, 45(22), 6250–6269 RSC.
- Z. Bao, K. Li, P. Hou, R. Xiao, Y. Yuan and Z. Sun, Mater. Chem. Front., 2021, 5, 1632–1654 RSC.
- Y. Zeng, G. Xu, X. Kong, G. Ye, J. Guo, C. Lu, A. Nezamzadeh-Ejhieh, M. S. Khan, J. Liu and Y. Peng, Int. J. Pharm., 2022, 627, 122228 CrossRef CAS PubMed.
- L. He, Q. Ni, J. Mu, W. Fan, L. Liu, Z. Wang, L. Li, W. Tang, Y. Liu, Y. Cheng and L. Tang, J. Am. Chem. Soc., 2020, 142(14), 6822–6832 CrossRef CAS PubMed.
- L. He, M. Brasino, C. Mao, S. Cho, W. Park, A. P. Goodwin and J. N. Cha, Small, 2017, 13(24), 1700504 CrossRef PubMed.
- Y. Shao, B. Liu, Z. Di, G. Zhang, L.-D. Sun, L. Li and C.-H. Yan, ACS Appl. Mater. Interfaces, 2020, 142, 3939–3946 CAS.
- K. Lu, C. He and W. Lin, ACS Appl. Mater. Interfaces, 2014, 136(48), 16712–16715 CAS.
- Q. Ding, Z. Xu, L. Zhou, C. Rao, W. Li, M. Muddassir, H. Sakiyama, B. Li, Q. Ouyang and J. Liu, J. Colloid Interface Sci., 2022, 621, 180–194 CrossRef CAS PubMed.
- X. Lin, H. Wu, J. Zhang, X. Chen, X. Gao and Y. Liu, Chem. Eng. J., 2024, 480, 147865 CrossRef CAS.
- X. Yang, S. Yuan, L. Zou, H. Drake, Y. Zhang, J. Qin, A. Alsalme and H. C. Zhou, Angew. Chem., Int. Ed., 2018, 130(15), 3991–3996 CrossRef.
- S. Z. Ren, B. Wang, X. H. Zhu, D. Zhu, M. Liu, S. K. Li, Y. S. Yang, Z. C. Wang and H. L. Zhu, ACS Appl. Mater. Interfaces, 2020, 12(22), 24662–24674 CrossRef CAS PubMed.
- S. Khan, M. Falahati, W. C. Cho, Y. Vahdani, R. Siddique, M. Sharifi, L. A. Jaragh-Alhadad, S. Haghighat, X. Zhang, T. L. Ten Hagen and Q. Bai, Adv. Colloid Interface Sci., 2023, 321, 103007 CrossRef CAS PubMed.
- Z. Wang, W. Yu, N. Yu, X. Li, Y. Feng, P. Geng, M. Wen, M. Li, H. Zhang and Z. Chen, Chem. Eng. J., 2020, 400, 125877 CrossRef CAS.
- S. Li, X. Shi, H. Wang and L. Xiao, J. Biomed. Mater. Res., Part B, 2021, 109(6), 841–852 CrossRef CAS PubMed.
- J. Liu, T. Liu, P. Du, L. Zhang and J. Lei, Angew. Chem., Int. Ed., 2019, 58, 7808–7812 CrossRef CAS PubMed.
- Z. Zhou, J. Zhao, Z. Di, B. Liu, Z. Li, X. Wu and L. Li, Nanoscale, 2021, 13(1), 131–137 RSC.
- S. Rojas, A. Arenas-Vivo and P. Horcajada, Coord. Chem. Rev., 2019, 388, 202–226 CrossRef CAS.
- J. Y. Zeng, M. K. Zhang, M. Y. Peng, D. Gong and X. Z. Zhang, Adv. Funct. Mater., 2018, 28(8), 1705451 CrossRef.
- L. He, N. Zheng, Q. Wang, J. Du, S. Wang, Z. Cao, Z. Wang, G. Chen, J. Mu, S. Liu and X. Chen, Adv. Sci., 2023, 10(1), 2205208 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |