 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Applications of bionanomaterials in neurodegenerative diseases
Abhijit
Biswas
 *,
Pravin
Hivare
*,
Pravin
Hivare
 ,
Raghu
Solanki
,
Raghu
Solanki
 ,
Sharad
Gupta
,
Sharad
Gupta
 and
Dhiraj
Bhatia
and
Dhiraj
Bhatia
 *
*
Department of Biological Sciences and Engineering, Indian Institute of Technology Gandhinagar, Palaj 382355, Gujarat, India. E-mail: abhijit.biswas@iitgn.ac.in; pravin.hivare@iitgn.ac.in; raghu.solanki@iitgn.ac.in; sharad@iitgn.ac.in; dhiraj.bhatia@iitgn.ac.in
First published on 23rd April 2025
Abstract
Neurodegenerative diseases (NDs) are mainly characterized by progressive neuronal loss, and pose a significant healthcare burden due to limited treatment options and the inefficacy of drugs at the target site within the brain. The blood–brain barrier (BBB) presents a major challenge by restricting drug delivery, necessitating innovative therapeutic strategies. In this review article, we explore the growing field of bionanomaterials for ND treatments. Biomaterials and biofunctionalized nanomaterials mimic biological systems and offer unique properties that help overcome existing drug delivery limitations. Different bionanomaterials such as metallic nanoparticles, liposomes, exosomes, carbon-based nanomaterials, dendrimers, polymeric nanomaterials and short peptides have been explored in the treatment of NDs. We assess clinical and preclinical evidence on bionanomaterials and discuss their therapeutic potential. Metallic nanoparticles like gold, silver, and cerium oxide (CeO2) exhibit neuroprotective effects and hold promise for enabling drug delivery across the BBB. Liposomes and exosomes, natural vesicles, are efficient and biocompatible drug carriers, while dendrimers and synthetic polymers offer targeted drug delivery and controlled release capabilities. We further investigated the role of quantum dots (QDs) and explored diagnostic imaging and targeted therapies in NDs with tunable fluorescence properties. Peptides, short protein chains, can specifically target protein misfolding, a key pathological feature in many NDs. Micelles, assemblies of surfactant molecules, are being developed for enhanced drug delivery across the BBB. Despite significant advancements in the use of bionanomaterials for ND applications, several challenges remain, particularly in their application to neuroscience. We further investigate the importance of elucidating nanoparticle toxicity profiles and optimizing BBB penetration as a crucial factor for clinical translation. Future research should focus on the development of biocompatible and targeted bionanomaterials with enhanced therapeutic efficacy. This review highlights the potential of bionanomaterials to revolutionize ND treatment by facilitating targeted drug delivery and advanced therapeutic interventions.
1. Introduction
The ability of various biomaterials and biofunctionalized nanomaterials to interact and modulate biological systems at the cellular and molecular milieu with extremely high specificities has been validated in many diseases.1–4 Many treatments, which are primarily based on a combination of medication and psychotherapy, are already approved to treat neurodegenerative diseases (NDs). Still, most of them merely address the symptoms that are related to the disorders.5,6 The blood–brain barrier's (BBB) limiting properties, which prevent about 99% of all “foreign substances” from entering the brain, are the main obstacle to the absence of pathogenesis-targeting therapeutics.7–9 For this reason, there is a tremendous need to develop efficient therapeutics, which can only be achieved by a deep understanding of the materials involved and the mechanisms of each disease. In 2021, the World Health Organization (WHO) reported that more than 3 billion people are suffering from NDs worldwide.NDs consist mainly of Alzheimer's disease (AD), Parkinson's disease (PD), frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), and Huntington's disease (HD) with progressive neuronal loss in the central nervous system (CNS) or peripheral nervous system (PNS) (Fig. 1).
These conditions, driven by diverse mechanisms like protein misfolding, severely impair cognitive function, motor capabilities, and overall quality of life, making them a leading cause of disability and ill health globally. Fig. 1 shows the signs and symptoms of various NDs. More than 1 in 3 people are affected by neurological conditions, which has increased by 18% since 1990.10,11
The effective treatments for slowing down the progression of NDs mainly depend on the diagnosis stage (early/late stage). A differential diagnosis is often required to detect NDs, including blood marker detection, behavioral symptoms, and imaging detection from PET, MRI, or brain activity using TMS or EEG.12 In recent years, nanomedicines have emerged as an excellent tool for diagnosing and treating different NDs. Because of its unique shape, size, and properties, nanomaterial-based nanomedicine has cell-specific targeting activity and lower cytotoxicity. Fig. 2 shows the criteria for developing efficient and safe nanoparticle-based nanomedicines for diagnosing and treating NDs. Nanomaterials generally have sizes between 10 nm and 100 nm.
 | ||
| Fig. 2 Criteria for developing efficient and safe nanoparticle-based nanomedicine for diagnosing and treating NDs. | ||
Different nanomaterials are studied to diagnose and treat NDs, such as metallic nanoparticles (e.g., AuNP, SiNP, CeO, Fe2O3, CNPs), quantum dots, liposomes, peptides, DNA-based nanomaterials, and dendrimers.13–17
In Table 1, preclinically and clinically evaluated different nanomaterials used in ND treatment are shown. This review delves into the application of various nanomaterials in neuroscience, focusing on their potential to revolutionize the diagnosis and treatment of NDs. We explore the following nanomaterials and their specific applications: metallic nanoparticles (for drug delivery, imaging, and neuroprotection), liposomes (lipid-based nanocarriers for targeted drug delivery across the BBB), exosomes (extracellular vesicles for cell-to-cell communication and therapeutic delivery), dendrimers (highly branched polymers for drug delivery and imaging), quantum dots (fluorescent nanoparticles for imaging and sensing), peptides (peptide-based drug delivery systems and therapeutic peptides), and micelles (self-assembled nanostructures for drug delivery and imaging) in neuroscience.
| Nanoparticle formulations | Drug name | Target disease | Ref. |
|---|---|---|---|
| PEG–PLGA | Riluzole | Amyotrophic lateral sclerosis (ALS) | 18 |
| Poly(n-butylcyanoacrylate) | Rivastigmine | Alzheimer's disease | 19 and 20 |
| CeO2 NP | L-DOPA | Parkinson's disease | 20 |
| (MPB-PE) and (PDP-PE) couples | D-Penicillamine | Alzheimer's disease | 21 |
| Gold nanocrystals | Molecular surgery | Alzheimer's disease | 22 |
| Polymer NP | Copaxone®/Glatopa (Teva) | Multiple sclerosis | 23 |
| Liposomes and polymers | Riluzole | Amyotrophic lateral sclerosis (ALS) | 18 |
| Gold nanocrystals | CNM-Au8 | Multiple sclerosis | 24 |
| PLGA | L-DOPA | Parkinson's disease | 25 |
| Cerium oxide | Photothermal therapy | Stroke | 26 |
| Thermotherapy and magnetic iron-oxide NPs + reduced dose radiotherapy | Nano-thermotherapy | Multiform | 23 |
2. Benefit of bionanomaterials compared to conventional treatment strategies
A revolutionary advancement in material science, bionanomaterials combine biological molecules with nanoscale elements to enhance performance in industrial, environmental, and medical contexts. Their benefits over existing systems stem from a number of important factors:2.1 Better biocompatibility
Bionanomaterials, particularly those that incorporate biomolecules like proteins, peptides, and polysaccharides, exhibit better biocompatibility, reducing the likelihood of toxicity and immunological rejection associated with conventional materials.27,282.2 Superior mechanical properties
Bionanomaterials often exhibit higher strength, flexibility, and durability than traditional polymers, metals, or ceramics used in biomedical applications because of their nanoscale reinforcement.29,302.3 Improved drug delivery performance
Compared to conventional drug delivery methods, nanocarriers made of bionanomaterials enable the targeted and regulated release of medications, reducing systemic toxicity and enhancing therapeutic efficacy.312.4 Enhanced antibacterial and antiviral activities
Many bionanomaterials, such as those containing graphene, chitosan, or silver, have strong antibacterial qualities that make them superior to conventional antimicrobial coatings or drugs that can face resistance issues.32,332.5 Applications in regenerative medicine
Where traditional scaffolds fall short in providing optimal bioactivity, bionanomaterials can help cells adhere, develop, and differentiate. This makes them appropriate for tissue engineering and regenerative medicine applications.31,32,342.6 Sustainable and eco-friendly
Compared to their synthetic equivalents, a variety of bionanomaterials have a lower environmental impact because they are derived from natural sources or biodegradable polymers.35Complicated regulatory approval procedures: bionanomaterials must pass stringent regulatory reviews due to the fact that they frequently contain novel nano-bio interactions. This delays the market release of bionanomaterials and raises development costs.
Lack of standardization: it is difficult to establish universal standards due to variations in synthesis methods, purity, and physicochemical characteristics, which limits scalability and reproducibility.
Insufficient industrial infrastructure: new processing technologies must be invested in because existing manufacturing facilities are not always equipped to handle the production of nanomaterials on a big scale.
Long-term safety and toxicity concerns: even though several bionanomaterials exhibit short-term biocompatibility, further research is necessary to determine the long-term effects on the environment and human health.
Market competition and adoption challenges: the market is still dominated by established materials, and the transition to bionanomaterials will require significant financial outlays, training, and modifications to existing regulations, all of which may make adoption more difficult.34
3. Important physicochemical characteristics of bionanomaterials for ND treatment
The physicochemical properties of nanomaterials are essential for their efficacy in addressing neurological disorders. These properties influence the capacity of nanomaterials to penetrate the blood–brain barrier (BBB), efficiently transport therapeutic agents, and reduce toxicity. The following are the primary physicochemical features and their significance in achieving therapeutic objectives for neurological diseases:3.1 Size
In engineered nanomaterials, dimension plays a vital role that influences the movement and distribution of nanomaterials within the bloodstream, their ability to cross physiological drug barriers, targeted localization in specific sites and cells, and even the triggering of cellular responses. The size of a non-spherical nanomaterial is represented as an equivalent diameter of a spherical particle, where the defined physical properties, like diffusivity, are comparable to those of the nanomaterial within the same setting.36 A commonly used illustration is the hydrodynamic diameter of a molecule, which refers to the effective size determined from the diffusion coefficient through the Stokes–Einstein equation.373.2 Shape
The configuration of nanomaterials significantly impacts their effectiveness in drug delivery, degradation, transport, targeting, and internalization. The efficiency of drug delivery vehicles is greatly affected by the management of the shapes of these carriers, while the process of phagocytosis of these vehicles by macrophages also relies on the shape of the carriers. Additionally, by adjusting the shapes of drug-loaded nanomaterials, we can influence their flow and adhesion within the circulatory system as well as the duration of their in vivo circulation.36,383.3 Surface properties
The composition of the surface is fundamentally important to the outer layers but not to the bulk materials. Surface energy plays a crucial role in the dissolution, aggregation, and accumulation of nanomaterials. The surface charge, which can influence receptor binding and the penetration of physiological barriers, determines the dispersion stability or aggregation of nanomaterials and is typically assessed using zeta potential.10,113.4 Composition and purity
The makeup of a nanomaterial influences how it moves, is delivered, and distributes within the body. In the biomedical use of nanomaterials, it may be necessary to merge two or more types of nanomaterials to create a complex, such as a chelate, a conjugate, or a capsule. As a result, analyzing the chemical composition of the nanomaterial complex becomes more complex than assessing a single entity.39,403.5 Stability
Pharmaceutical stability refers to the ability to maintain the same characteristics over time following the production of a pharmaceutical. Like traditional single-molecule drugs, the stability of nanomedicines can be influenced by several factors, including temperature, humidity, solvents, pH levels, particle or molecular size, exposure to various forms of ionizing and non-ionizing radiation, enzymatic breakdown, and even the presence of other excipients and impurities. The stability of nanomaterials may affect their associated toxicity; for example, several studies have indicated that the cytotoxicity of quantum dots might be triggered during their synthesis, storage, or even in vivo through oxidative or photolytic degradation of the quantum dots.38,41,424. Nanomaterials in neurodegenerative diseases
4.1 Metallic nanoparticles
Metallic nanoparticles are usually colloidal metal nanoparticles with a size range of 10 nm to 1000 nm.43,44 In biomedical applications, metal nanoparticles remain conjugated and/or absorbed with different therapeutic molecules on their surface.45–47 Nowadays, these types of nanomaterials are emerging as potential therapeutics in different fields, including NDs. Fig. 3 depicts various nanoparticles that have applications in NDs. Metal nanoparticles can be easily prepared and fabricated, their shape and size can be modulated, and they can conjugate different therapeutically active molecules.47–49 In NDs, various nanomaterials have been studied; among them, gold nanoparticles (AuNPs),50 silica nanoparticles (SiNPs),51 and cerium oxide (CeO2)51,52 are notable.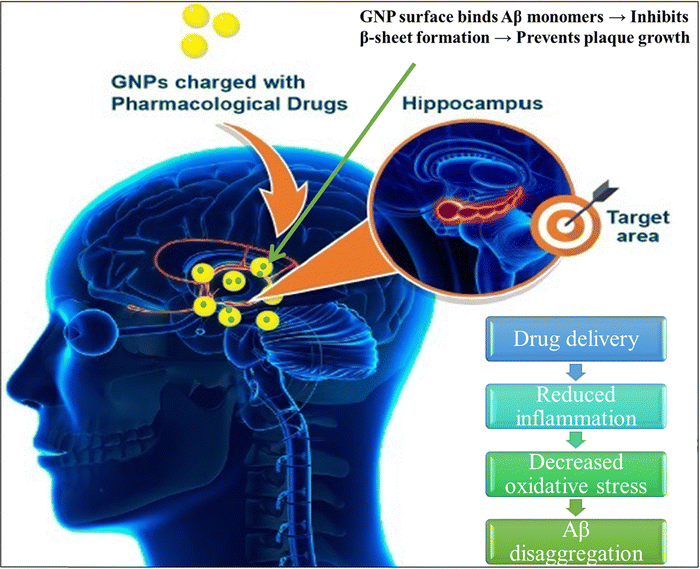 | ||
| Fig. 4 Gold nanoparticles (AuNPs) conjugated with pharmaceutical drugs can effectively target specific areas, increasing drug delivery, lowering inflammation, and decreasing oxidative stress, while also improving interaction with amyloid-β (Aβ). Reprinted from ref. 57. | ||
Several AuNP-based drug deliveries in the brain have been studied.60 The potential of AuNP-coupled amyloid in BBB crossing61 demonstrated that insulin-targeted AuNPs could target BBB insulin in receptors, which is an effective approach for in vivo therapeutics and imaging.60 Although AuNPs show potential applications in various NDs it is essential to thoroughly evaluate possible negative effects. The size of AuNPs has been recognized as a key factor for affecting their toxicity, as smaller nanoparticles enhance surface reactivity and the ability to trigger cellular stress. Also, they are high cost and there are regulatory challenges.50
| Nanoparticles | Cell | Model | Function | Ref. |
|---|---|---|---|---|
| SiNPs | PC-12 | Neuronal cells | At increased levels of H2O2, exclusive release of metal chelator happens, which helps to overcome the side effects after long term exposure | 69 |
| SiNPs | HUVEC | Central nervous system | Promote cytotoxicity and inflammation in HUVECs, which is related to the activation of potassium channels | 70 |
| SiNPs | SH-SY5Y | Brain cell | Showed dose-dependent neurotoxicity probably due to association with oxidative stress and induced apoptosis | 71 |
| Colloidal SiNPs | SH-SY5Y | Neuronal cells | Si-PLLA NPs reduce significantly GSH levels after 6 h and 24 h treatment | 72 |
| MSNPs | PC-12 | Alzheimer's disease | Significantly reduced the cell viability, resulting in apoptosis and in a dose-dependent manner increased the intracellular ROS in both cell lines | 70 |
The application of SiNPs in clinical settings is still not fully developed. This is attributed to several challenges and limitations associated with SiNPs, such as the insufficient research on the immunogenicity and toxicity of various SiNP forms, their pharmacodynamics and pharmacokinetics, as well as their biodistribution and elimination from the human body. Moreover, scaling up the production of SiNPs at an industrial level while maintaining strict oversight of their properties and consistency is a complex task. Additionally, based on the surface characteristics of SiNPs, they can interact with the phospholipids in red blood cell membranes, resulting in hemolysis. Furthermore, studies have indicated that SiNPs might decrease the levels of endogenous ROS, potentially leading to increased growth of malignant melanoma. The accumulation of SiNPs in normal organs, particularly the liver and spleen, is another concern that must be resolved prior to advancing SiNPs into clinical applications.73
4.2 Liposomes
For several years, liposomes have been used as nanocarriers for drug delivery.93,94 Liposomes are spherical vesicles consisting of lipid bilayers, and they have low toxicity and are highly bio-compatible.95 Liposomes can deliver both lipophilic and hydrophobic drugs.94 This nanocarrier type shows promising ND potential by delivering drugs via the BBB (Table 3). Liposomes can be modified by various ligands of interest, like peptides and PEG. Gamma-aminobutyric acid (GABA)-based liposomes can be used in drug delivery to the brain.96 In such a delivery system, osmotic shock can open the BBB temporarily to boost liposome-mediated drug delivery.97 GABA-based liposomes are used in many NDs, epilepsy, stress, and anxiety.98,99 In a recent study, liposomes containing RVG peptide and transferrin (Tf) on their surface showed higher transfection ability than the unmodified one.96 This type of delivery system can efficiently deliver drugs to the brain by using a brain-targeting ligand. Tomitaka et al. developed a magneto liposome-based system that showed promising results in X-ray computed tomography.77 However, a few drawbacks are needed to address the better use of liposomes including instability, cost of production, prolonged leakage, and low in vivo circulation time.100| Drug name | Target | Phase | Ref. |
|---|---|---|---|
| Cytarabine | Brain and central nervous system tumors, leukemia lymphoma | I | NCT00003073 |
| 2B3-101(glutathione) PEGylated liposomal doxorubicin hydrochloride | Brain tumor | I | NCT00019630 |
| Brain metastases | II | NCT01386580 | |
| Methotrexate, cytarabine | Central nervous system metastases | II | NCT00992602 |
| Doxorubicin | Brain metastases from breast cancer | II | NCT00465673 |
| Brain tumor | I | NCT00019630 | |
| Primary brain lymphoma | II | NCT01848652 | |
| Marqibo | Brain tumors | I | NCT01222780 |
| Talineuren | Parkinson's disease | I | NCT04976127 |
| ITV DepoCyt and temozoiomide | Astrocytoma brain tumor diffuses intrinsic pontine glioma | I | NCT03086616 |
| Total tumor mRNA | Adult glioblastoma | I | NCT04573140 |
| Bupivacaine | Migraine, cluster headache sphenopalatine ganglion neuralgia | II | NCT04930887 |
4.3 Exosomes
Exosomes are extracellular vesicles having a size in the range of 30–150 nm produced by all cells and work as endogenous nanoparticles.94,101 Exosomes are excellent nanocarriers as they are non-immunogenic and can carry a variety of payloads, e.g., proteins, nucleic acids, and lipids. Apart from other diseases, exosomes are widely used in NDs. Exosomes derived from mesenchymal stem cells (MSC-expos) can migrate to the brain's specific diseased regions of PD94 and AD.102 Dendritic cell-derived exosomes showed immunomodularity during stroke, and macrophage-derived exosomes showed neuroinflammation. Lydia et al. engineered exosomes, which can carry siRNA to mice brains.103 Bone marrow dendritic cell (BMDC)-derived exosomes with interleukins are used to minimize immunogenicity. Dendritic cells (DCs) were engineered to express Lamp2b, an exosomal membrane protein connected to the neuron-specific RVG peptide, in order to enhance brain targeting.104 Engineered exosomes could carry siRNA to neurons, oligodendrocytes, or microglia, which may result in cell-specific gene suppression.98 Exosome-mediated therapeutics are facing several challenges, including the isolation of high-purity exosomes, the lack of proper protocols for large-scale separation, inadequate storage conditions, and prohibitively high costs.994.4 Dendrimers
A dendrimer is a polymeric nanomaterial that has emerged as an excellent candidate in nanomedicine because of its distinctive structural characteristics: well-defined, globular, controllable structure, highly branched, low polydispersity, and multivalency.105 Dendrimers are used to carry a variety of biologically active molecules like oligonucleotides, antibodies, and antigens.106,107 Dendrimers open a promising field in NDs as therapeutics and a theragnostic tool (Table 4). Polyamidoamine (PAMAM) is one of the most valuable and interesting dendrimers with potential applications in treating brain disease.107 The following features make PAMAM an excellent nanomaterial: high aqueous solubility, high stability, presence of cavities, surface tunability, and small size. Carbamazepine (CBZ) is an anti-epilepsy drug showing excellent autophagy and neurodegeneration resistance in vivo. However, the major drawback is poor water solubility.107 PAMAM generation 4.5 carboxyl terminated dendrimers solve the solubility issue.108,109 These formulations have potential applications in treating different NDs associated with aggregated protein toxicity, such as PD, AD, HD, and ALS.110 G4 PAMAM dendrimers having a surface modification with PEG and targeted wheat germ agglutinin (WGA) and transferrin (Tf) conjugated with doxorubicin (DOX) have great potential in the delivery of DOX payloads at the brain tumor compared to unmodified dendrimers.107 Singh et al. engineered amine terminated 4.0 G PAMAM dendrimer conjugated donepezil (Dz) nanoparticles. This formulation increases the half-life and brain uptake of drugs up to 4-fold with respect to an unmodified one.111 Nance et al. illustrated that hydroxyl group density is important in dendrimer-mediated drug delivery by crossing the CNS barriers and accumulating drugs at the targeted site.112 Based on these results, researchers have engineered PEGylated dendrimers and explored them in neurologic diseases as potential nanocarriers.94 Despite their excellent potential for drug delivery, several obstacles need to be overcome in this area, including (i) the troublesome synthesis and purification of desired dendrimers, leading to small yields, (ii) cytotoxicity issues, and (iii) the potential for membrane disruption upon interacting with cell membranes and forming nanoholes.113| Drug name | Formulation | Target disease | Function | Ref. |
|---|---|---|---|---|
| PAMAM—polyamidoamine; PEG-polyethylene glycol; pHBA—p-hydroxyl benzoic acid; WGA—wheat germ antigen. Tf—transferrin. | ||||
| Curcumin | Covalent conjugation with dendrimer via surface G3-succinamic acid | Glioma | Enhanced tumor-specific drug delivery114 | 115 |
| Doxorubicin | Encapsulated within PEGylated G4 PAMAM having Tf targeting ligand and WGA | Brain tumors | Enhanced the doxorubicin amount at the tumor site116 | 107 |
| Docetaxel | Encapsulated within G4 PAMAM having covalently attached pHBA | Glioblastoma | Enhanced drug delivery to the brain and glioblastoma-cell death117 | 116 |
| Carbamazepine | Encapsulated within a G4.5 dendrimer having a terminal carboxyl group | Neurodegenerative diseases | Reduced protein aggregation, increased drug solubility, autophagy. Reduced in vivo neurodegeneration118 | 110 |
| Paclitaxel | Covalently conjugated to G3 PAMAM having lauryl chains | Brain tumors | Rise toxicity and internalization in porcine brain endothelial cells119 | 120 |
| Venlafaxine | Covalently conjugated to PAMAM dendrimer-PEG hydrogels | Psychiatric | Increased release121 | 122 |
4.5 Quantum dots (QDs)
Quantum dots are unique nanoparticles that possess fluorescence and bright light emission.118,123 QDs are artificial semiconductor nanoparticles.97 High quantum yield, photostability, high emission, controllable size, and electrochemical features make QDs a distinct nanomaterial in drug delivery, diagnosis, targeting, sensing, and photo radiation treatment (PDT).124 Along with other biomedical applications, QDs are widely used in different NDs (e.g., AD and PD) because of their considerable potential in diagnosis and treatment properties in the central nervous system (CNS)114,125 (Table 5). QDs can cross the BBB because of their smaller size. The accumulation and transmission of α-synuclein (α-syn) strongly correlate to Parkinson's disease.14,117 Liu et al. demonstrated that graphene quantum dots (GQD) can inhibit α-syn fibrillization and directly interact with mature fibrils, which triggers their disaggregation. In vivo, GQD penetrates the BBB and protects it from α-syn preformed fibril-induced dopamine neuron loss.119 Shang et al. worked on labeling neuronal stem cells with QDs to monitor their uptake and biocompatibility. This study observed no significant change in viability, proliferation, or differentiation in human neuronal stem cells.121 Carbon quantum dots (CQDs) are another type of quantum dot having distinct properties, including biocompatibility, stability towards light, and small size (<10 nm), which helps them to enter the BBB. CQDs showed promising biomedical applications in treating brain tumors, bio-imaging, and NDs.97 The photostable luminescence and spectral brilliance of QDs suggest their diagnostic import in NDs, having been used to track α-syn aggregation in Parkinson's with unmatched imaging sensitivity. For now, we deem them essential for CNS investigative advancement, but their toxicological footprint and elimination kinetics require further study.| Quantum dots | Target disease | Observation | Ref. |
|---|---|---|---|
| GQD | Parkinson's disease | Inhibits accumulation of α-syn and mature fibrils, formation of Lewy bodies; prohibits neuronal loss, pathology transmission, mitochondria | 126 |
| GQD | Parkinson's disease | Dismantled α-syn proteins | 127 |
| GQD-tramiprosate | Alzheimer's disease | Synergistically inhibits amyloid β accumulation | 128 |
| Glycine-proline-glutamate on GQDs | Alzheimer's disease | Restrain Aβ aggregation, leading to learning capacity and enhanced memory | 129 |
| CdTe/CdS/ZnS QDs | Parkinson's disease | Stop the MPP + induced α-syn fibrillation, cell damage, reduced cytotoxicity, apoptosis, and oxidative stress | 130 |
| N-doped CQDs | Alzheimer's disease | Prevents amyloid β proteins photooxygenation and Cu2+ induced aggregation, | 131 |
| Branched polyethyleneimine | Alzheimer's disease | Prevents Aβ plaques | 132 |
| GQDs | Alzheimer's disease | Decrease the accumulation of Aβ1–42 peptides | 133 |
| CQDs | Alzheimer's disease | Suppress the cytotoxicity accumulation by inhibiting β-secretase 1 (BACE1) | 134 |
| N-Acetyl-L-cysteine-CdTe QDs | Alzheimer's disease | Inhibits the Aβ aggregation via blockage of active sites of peptides | 127 |
Although the positive outcomes of utilizing quantum dots (QDs) for drug delivery across the blood–brain barrier (BBB) are promising, there are significant concerns regarding the in vivo toxicity of QDs, primarily due to the inherent toxic properties of the materials. Furthermore, QDs can lead to neurological harm through several pathways, and these toxic effects could be linked to different mechanisms and a variety of neural factors.135,136
GQD: graphene quantum dots; CQD: carbon quantum dots.
4.6 Peptide-based nanomaterials
Peptides have a long history as therapeutics in successful disease management. A large number of peptides have been studied in the field of NDs.137 NDs are mainly caused by abnormal aggregation and deposition of proteins/peptides in neurons, which cause signal disruption and, consequently, neuronal death. Peptides are widely used as therapeutics because of their biocompatibility, low toxicity, and higher cellular membrane permeability.16 However, the major obstacle to using peptides in therapeutics is their stability in the biological milieu.138,139 Different strategies have been taken to overcome this issue, e.g., insertion of unnatural amino acids in the peptide backbone, backbone cyclization, and the use of D-amino acid.138,139 There are insufficient treatments for monitoring the NDs, and very few drugs have been approved because of the high rate of failure of drugs in clinical trials.140 Peptides are an important tool for monitoring protein aggregation and have been studied extensively (Table 6).118| Peptide | Target disease and mechanism | Applications |
|---|---|---|
| Oxytocin | It has a role in the early stages of the systemic inflammatory response,141 modulating LTP and LTD of synapses during early development142–144 | Post-traumatic stress disorder, ASD145–147 and schizophrenia145,148 |
| NPY | Regulation of immune cell function, neuroprotective149 | AD, PD, Machado–Joseph disease, HD,150–152 ALS153 |
| LEAP-2 and ghrelin | Regulate memory and spatial learning154,155 | AD having memory associated problem |
| PACAP | Have a role in microglia-expressed immune response receptors156 | PD, AD, HD157,158 |
| Neurolysin | Control the activity of other neuropeptides and regulate excitotoxicity and inflammation during ischaemic stroke159 | Ischaemic stroke |
| TLQP-62 | Controls neuroinflammation, developmental synaptic plasticity,160 and oxidative responses161 | Neuropsychiatric diseases |
| Prolactin | Plays roles in neuronal stem cell proliferation and neurogenesis. Expressed on astrocytes and microglia with impressive roles in inflammatory response162 | PD, AD163 |
Cell-penetrating peptides (CPPs) are a class of peptides having amino acid residues >30, emerging as a promising delivery vector of different drug molecules, including oligonucleotides and proteins.138,164 They can transport drug molecules across the BBB, making them valuable for neurodegenerative treatment. CPP can bind the cargo molecules both covalently and non-covalently138,165 (Fig. 5). CPP-nanoparticle combination can increase the half-life time of nanoparticle stability and allow higher drug loading efficacy. CPP and CPP-nanoparticle conjugates are widely studied in different neurogenerative diseases. An initial investigation concentrated on creating a fluorescent contrast agent for identifying Cat-D enzymatic activity, which serves as a marker for Aβ buildup in patients with Alzheimer's disease. To facilitate the passage of this contrast agent across the blood–brain barrier (BBB), a Tat penetrating peptide was employed. The absorption of the contrast agent by the cells and tissues of the AD mouse model validated successful migration through the BBB.166 Aβ1-6A2V, a variant of Aβ with an A2V substitution, was linked to Tat [Aβ1-6A2V-Tat (D)] and was effective in preventing Aβ aggregation in vitro. Research involving transgenic mice with Alzheimer's disease showed that it hindered the accumulation of cerebral amyloids and cognitive decline.167 A nanoparticle made from poly(lactide-co-glycolic acid) (PLGA) was developed, which is conjugated with a cyclic CPP (CRTIGPSVC peptide) and contains the S1 peptide (known for reducing Aβ production) along with curcumin, with the goal of enhancing spatial memory and recognition in a mouse model of Alzheimer's disease. Gold nanoparticles that are modified with cell-penetrating peptides have been utilized as delivery systems because of their distinctive theragnostic features, along with their excellent biocompatibility and small dimensions, which enable them to infiltrate tissues effectively. A transmembrane peptide–chondroitin sulfate–gold nanoparticle (Tat-CS@Au) was created, and its potential to combat Alzheimer's disease was assessed in vitro. The uptake of Tat-CS@Au by SH-SY5Y cells was verified. The findings further demonstrated that Tat-CS@Au diminished Aβ1–40 aggregation and considerably lowered apoptosis.168
 | ||
| Fig. 5 Schematic representation of covalent and non-covalent conjugation of CPP and nanomaterials. Reprinted with permission from ref. 169. | ||
L-DOPA, a dopamine precursor, is frequently used as it can traverse the blood–brain barrier more readily and be converted into dopamine in the brain. L-DOPA is widely acknowledged as the most efficient treatment for controlling the motor symptoms of Parkinson's disease. Transportan 10 (TP10) is a cationic cell-penetrating peptide (CPP) recognized for its exceptional ability to traverse cell membranes. A compound formed by linking TP10 with dopamine through a short poly(ethylene glycol) (PEG) spacer has demonstrated the capability to cross the blood–brain barrier (BBB) and shows a notable affinity for both dopamine D1 and D2 receptors. This compound has exhibited antiparkinsonian effects in animal studies. In preclinical investigations utilizing an MPTP-induced Parkinson's disease animal model, the concentration of TP10-dopamine found in the brain tissue increased, and the antiparkinsonian effects of TP10-dopamine surpassed those of L-DOPA. Kang et al. created a mitochondrial-targeting peptide known as CAMP (human cathelicidin antimicrobial peptide) that exhibits cell-penetrating properties and was effectively linked to the antioxidant protein human metallothionein 1a (hMT1A) for delivery to mitochondria. Treatment with CAMP-hMT1A decreased reactive oxygen species (ROS) production and revitalized mitochondrial function as well as the expression of tyrosine hydroxylase in cells from a Parkinson's disease model.170
Carnosine is a dipeptide (β-alanyl-L-histidine) composed of alanine and histidine. It is a natural antioxidant, synthesized in nerve tissue and human muscle.171 Carnosine can be easily absorbed in the digestive tract and cross the BBB. It has been used to treat different NDs like PD, AD, brain ischemia, and epilepsy.137,167 PolyQ is an ND caused by aggregation and misfolding of proteins because of abnormally expanded PolyQ stretch.172 HD, spinal and bulbar muscular atrophy, and dentatorubral pallidoluysian atrophy (DRPLA) are reported as polyQ diseases.137 NAPVSIPQ (NAP) is a neuroprotective peptide derived from neuroprotective protein (ADNP). This peptide is used in the treatment of PD. By blocking microglial activation, NAP reduces neurotoxicity and pro-inflammatory factors like nitric oxide (NO), IL-1, and tumor necrosis factors. β-Sheet breaker peptide is another class of peptide that can disrupt Aβ conformers, prevent amyloid formation, and restrain Aβ aggregation-related toxicity. This type of peptide is widely used for the treatment of PD.137 Vasoactive intestinal peptide (VIP) is a glucagon family peptide, known as neuroprotective peptide, and is used as a potent inhibitor for treating AD.173 The compatibility and efficiency of peptide-based nanomaterials in modulating protein aggregation kinetics, as demonstrated by β-sheet disruptors targeting Aβ conformers, suggests a role in ND therapeutics. These peptides exhibit minimal toxicity, yet their in vivo durability remains a formidable challenge to overcome.
Despite the tremendous potential applications of peptides in NDs, two major drawbacks need to be addressed regarding their clinical applications e.g., poor membrane permeabilization of peptides consisting of natural amino acids, and weak in vivo stability due to hydrolyzation of it by enzymes in vivo.138,174
4.7 Micelles
Micelles are aggregates of surfactant lipid molecules that form a colloidal solution while dispersing in liquid. Micelles have a hydrophobic core, and hydrophilic shells form a spherical shape.154,155,175 These types of assembly are widely used in drug delivery.176,177 The outside hydrophilic shell helps solubilize in solution, while the hydrophobic core carries drug molecules.178,179 The hydrophilic shell protects the drug and consequently increases its lifetime in the biological milieu. Due to their unique properties, micelles have potential applications in drug delivery in neuronal systems.180,181 Lactoferrin (Lf) is a natural protein derived from milk expressed in brain tissue, is attached to receptors, and can cross the BBB.97 Lf is used to prepare nanocarriers. Lf and micelle-conjugated linoleic acid are non-toxic; they have properties that activate the targeted nanoplatforms, which may bestow a probable solution to AD.182 Tripodo et al. showed that micelles consisting of curcumin-loaded inuliin-D-α-tocopherol succinate successfully translocated into a mesenchymal stromal cell, which opens a possibility for the treatment of ALS diseases.183 Zhan et al. demonstrated that candoxin-derived peptide ligand (CDX-inspired) micelle-based drug delivery systems can target brain delivery facilitated by a nicotine acetylcholine receptor ligand.184 A platinum-based chemotherapy drug was successfully delivered to glioblastoma through the BBB by forming micelles constructed using peptide-based ligands. This micelle construct has higher stability and accumulation of drugs at the animal model tumor site.185 A micellar system composed of β-amyloid protein derived targeted peptide and ROSS-perceptive amphiphilic polymer can target the brain and microglia. Similarly, there are many studies in which surface-modified micelles with targeting ligands were used for gene therapy for AD, traumatic brain injury, and synergistic chemotherapy of glioma.186 Some drawbacks still need to be addressed for better applicability, including poor solubility, weak in vivo stability, and less drug loading capacity.4.8. Polymer-based nanoparticles
Polymer-based nanoparticles have attracted considerable interest in the treatment of NDs because of their capability to penetrate the BBB, and facilitate targeted delivery. Lei et al. developed nanoparticles consisting of a PLGA–PEG skeleton loaded with fingolimod, which has been externally modified with mannose, with the aim of designing a glucose control strategy for the treatment of AD disease.187 Xia et al. developed a self-regulating multifunctional nano-modulator (siR/PIO@RP) that can intelligently target the damaged BBB to release therapeutic agents for combined Alzheimer's disease treatment. The siR/PIO@RP utilizes a feedback mechanism to autonomously adjust its distribution based on the physiological and pathological condition of the target area. This system is capable of executing complex tasks, surpassing the effectiveness of single-target therapeutic agents used in AD therapy, such as decreasing cerebral amyloid beta levels, mitigating neuroinflammation, and improving the function of the neurovascular unit (NVU).188 Lei et al. developed a nanocleaner composed of a PLGA core that is responsive to ROS and loaded with rapamycin, along with surface modifications using KLVFF peptide and acid-cleavable DAG peptide [R@(ox-PLGA)-KcD]. The presence of DAG can improve the targeting and uptake of the nanocleaner into neurovascular unit endothelial cells in AD lesions, and it subsequently detaches from the nanocleaner when exposed to the acidic environment of endosomes, facilitating the transcytosis of the nanocleaner from endothelial cells into the brain parenchyma. The exposed KLVFF can then bind and transport Aβ to microglia, reducing the neurotoxicity induced by Aβ.189A lysosome-targeting nano-chimera (endoTAC) is introduced based on a polyvalent receptor binding mechanism to enhance RAGE degradation and facilitate targeted drug delivery. The endoTAC demonstrates a strong affinity for RAGE and promotes its degradation through its polyvalent interactions. Furthermore, endoTAC exhibits increased accumulation in diseased brain tissue and shows potential as a precise delivery system for the brain. When loaded with simvastatin, the SV@endoTAC successfully reverses pathological characteristics both in vitro and in vivo. This study suggests that the combination of a lysosome-targeting chimera with an efficient drug delivery system could be a promising approach for Alzheimer's disease treatment.190 Although polymeric nanoparticles show potential for addressing neurodegenerative diseases, they come with obstacles, such as challenges in penetrating the blood–brain barrier (BBB), possible toxicity, and concerns regarding stability and biodistribution.
5. Case-specific applications of nanoparticles in neurodegenerative diseases
In this section, case-specific applications of various NPs (Section 4) are discussed for each ND, such as AD, PD, HD, and ALS, focusing on the specific abilities of NPs. We further addressed each ND's challenges, ranging from protein aggregation/misfolding, oxidative stress, neuroinflammation, and BBB penetration.The protein misfolding in AD forms plaques of Aβ and tangles of Tau protein, which leads to memory loss and neuronal cell death. The current treatment challenge for AD is the BBB to target Aβ plaques. As discussed in Section 4.1.1, AuNPs significantly reduce Aβ plaques, as shown by Huang et al.59 Cerium oxide (CeO2) nanoparticles (Section 4.1.4) have the ability to scavenge superoxide anions, hydrogen peroxide, and peroxynitrite, which can protect against different NDs. Dowding et al. showed that these NPs can protect against mitochondrial fragmentation and neuronal cell death in in vivo animal models of AD.51 Cimini et al. reported (PEG)-coated and antibody-conjugated CeO2 NPs coated with anti-Aβ antibody. These conjugated antioxidant nanoparticles (Aβ-CNPs-PEG) can target Aβ aggregates while activating brain-derived neurotrophic factor (BDNF) signaling.92 CeO2 nanoparticles suggest a dual role in AD treatment as these NPs play a neuroprotection and targeted therapy role.
In PD, the aggregation of α-syn protein, oxidative stress leads to dopaminergic neuron death in the substantia nigra region resulting in motor symptoms like tremors. Graphene quantum dots (GQDs) (Section 4.5) can penetrate the BBB, inhibiting α-syn fibrillization and disaggregating mature fibrils, protecting against dopaminergic neuron loss.128 Magnetic iron oxide nanoparticles, as mentioned in Section 4.1.3, show a promising approach for PD. Niu et al. developed magnetic Fe3O4 nanoparticles coated with oleic acid molecules as a nano-carrier showing cell-specific targeting via NGF receptor-mediated endocytosis and downregulating α-syn expression in PD models (in vitro and in vivo).191 The PD treatment can be enhanced with a combination of GQDs for α-syn and magnetic iron oxide nanoparticles for targeted delivery of therapeutics like Levodopa (L-DOPA) (FDA-approved with CeO2 NPs, as mentioned in Table 1).
In HD, mutation in the huntingtin gene results in protein misfolding and protein aggregation, which results in neuronal cell death and symptoms like muscle wasting and loss of sensory feeling. One of the main challenges in treating HD is protein aggregates and reducing oxidative stress while delivering the therapeutics to specific brain regions. Protein misfolding can be targeted using peptide-based nanomaterials, as mentioned in Section 4.6. Baig et al. thoroughly discussed the peptide's antioxidant, antimicrobial, and antithrombotic effects in NDs. Vasoactive intestinal peptides (28 amino acid neuropeptides) play a neuroprotective role in the pathogenesis of HD models (in vitro and in vivo).137
Mutation in SOD1 or TDP43 protein aggregation results in progressive motor neuron loss, muscle wasting, and speech difficulties in ALS. For effective treatment of ALS again, the therapeutics should reduce protein aggregates and help the survival of motor neurons. Section 2.3 dwells on exosomes, which have a natural role in cell-to-cell communication and the ability to carry therapeutic payloads. Bondi et al. showed that CeO2 nanoparticles (Section 4.1.4) enhanced survival and reduced disease severity in SOD1 G93A transgenic mice by mimicking superoxide dismutase activity. The combination of mesenchymal stem cell-derived exosomes for delivering SOD1-targeting siRNA and CeO2 NPs for ROS scavenging could play an essential role in ALS treatment.90
6. Challenges and future directions
Apart from the enormous applications of nanomaterials because of their unique physicochemical properties in diagnosing and treating different diseases, including NDs, it is important to address their toxicity in living systems. Cationic nanoparticles are more toxic than anionic nanoparticles, which may cause clotting and hemolysis.46 Nanoparticle-mediated nanomedicine can enter the body via different pathways, including the respiratory tract, skin, and inhalation.192 Upon entering the body, nanoparticles circulate through the blood, where they come into contact with plasma proteins, forming the protein corona responsible for the nanoparticles' pharmacological applicability.193 Different metallic and non-metallic nanoparticles, including AuNPs, carbon nanoparticles, liposomes, quantum dots, and peptides, are used for neurodegenerative disorder studies. All of these nanoparticles need to cross the BBB to reach the brain.193In vitro and in vivo studies of nanoparticles have shown that there appears to be some toxicity due to the increase in ROS, which further damages the genome and consequently creates oxidative stress conditions.In recent years, the extended amount of pollution, including nanoparticles, has had a pivotal role in the enhancement of neurodegenerative diseases like AD, PD, HD, and ALS.194 This review elucidates the potential of bionanomaterials to address NDs by leveraging engineered properties to overcome biological barriers, such as the BBB, as demonstrated by targeted delivery systems and imaging agents. In recent years, researchers have shown that different nanomaterials (e.g., metallic nanoparticles, quantum dots, peptides, micelle, carbon nanomaterials) have been used to overcome such barriers. Despite extensive studies, no clinically approved nanomaterial-based nanomedicine is available for treating NDs. Available medicine can only give specific relief. Yet, many NPs display critical limitations, including inconsistent BBB permeability, induction of oxidative stress, neurotoxicity, and instability in vivo, which currently hinder their therapeutic translation. Despite vast advancements, significant work is still needed to improve nanoparticles with higher therapeutic activity and consequent clinical use, consisting of detailed pharmacokinetics and bio-distribution.179 Thorough physicochemical characterization is essential to recognize the nanoparticle's final product and its clinical factors in the brain and the human body. Along with these therapeutic approaches, combining nanoparticle-based therapies with other therapeutic modalities, such as gene therapy or small molecule drugs, can enhance therapeutic efficacy. Understanding these factors should be considered in future studies, which can help us design prospective therapeutic targets for the NDs.
Author contributions
Abhijit Biswas: conceptualization, literature review and writing – original draft preparation. Pravin Hivare: literature review and writing – original draft preparation. Raghu Solanki: writing – review and editing. Sharad Gupta: writing – review and editing, and supervision. Dhiraj Bhatia: conceptualization, writing – review and editing, and supervision. All authors confirmed the final version of the manuscript.Data availability
Data for this article are available with permission from previous works.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We sincerely thank all the members of DB lab for critically reading the manuscript and giving their feedback. AB thanks DST for the National Postdoctoral Fellowship in Nanoscience and Technology. R. S. acknowledges the Science and Engineering Research Board (SERB), Government of India, for financial support through the National Post-Doctoral Fellowship (NPDF). DB thanks the SERB Ramanujan fellowship and IITGN, DBT, Gujcost, and GSBTM for funding support. SG thanks GSBTM for funding support.References
- Z. Tu, Y. Zhong, H. Hu, D. Shao, R. Haag, M. Schirner, J. Lee, B. Sullenger and K. W. Leong, Nat. Rev. Mater., 2022, 7, 557–574 CrossRef CAS PubMed.
- A. P. Singh, A. Biswas, A. Shukla and P. Maiti, Signal Transduction Targeted Ther., 2019, 4, 33 CrossRef PubMed.
- J. Li, H. Zeng, Z. Zeng, Y. Zeng and T. Xie, ACS Biomater. Sci. Eng., 2021, 7, 5363–5396 CrossRef CAS PubMed.
- Y. Huang, X. Guo, Y. Wu, X. Chen, L. Feng, N. Xie and G. Shen, Signal Transduction Targeted Ther., 2024, 9, 34 CrossRef PubMed.
- M. Kiaei, Basic Clin. Neurosci., 2013, 4, 3–4 Search PubMed.
- R. W. Levenson, V. E. Sturm and C. M. Haase, Annu. Rev. Clin. Psychol., 2014, 10, 581–606 Search PubMed.
- P. Hivare, C. Panda, S. Gupta and D. Bhatia, ACS Chem. Neurosci., 2021, 12, 363–377 CrossRef CAS PubMed.
- K. Li, Q. Ji, H. Liang, Z. Hua, X. Hang, L. Zeng and H. Han, J. Nanobiotechnol., 2023, 21, 181 CrossRef PubMed.
- R. N. L. Lamptey, B. Chaulagain, R. Trivedi, A. Gothwal, B. Layek and J. Singh, Int. J. Mol. Sci., 2022, 23, 1851 CrossRef CAS PubMed.
- J. D. Steinmetz, K. M. Seeher, N. Schiess, E. Nichols, B. Cao, C. Servili, V. Cavallera, E. Cousin, H. Hagins, M. E. Moberg, M. L. Mehlman, Y. H. Abate, J. Abbas, M. A. Abbasi, M. Abbasian, H. Abbastabar, M. Abdelmasseh, M. Abdollahi, M. Abdollahi, M.-A. Abdollahifar, R. Abd-Rabu, D. M. Abdulah, A. Abdullahi, A. Abedi, V. Abedi, R. A. A. Zuńiga, H. Abidi, O. Abiodun, R. G. Aboagye, H. Abolhassani, V. Aboyans, W. A. Abrha, A. Abualhasan, E. Abu-Gharbieh, S. Aburuz, L. H. Adamu, I. Y. Addo, O. M. Adebayo, V. Adekanmbi, T. A. Adekiya, W. Adikusuma, Q. E. S. Adnani, S. Adra, T. Afework, A. A. Afolabi, A. Afraz, S. Afzal, S. Aghamiri, A. Agodi, W. Agyemang-Duah, B. O. Ahinkorah, A. Ahmad, D. Ahmad, S. Ahmad, A. M. Ahmadzade, A. Ahmed, A. Ahmed, H. Ahmed, J. Q. Ahmed, L. A. Ahmed, M. B. Ahmed, S. A. Ahmed, M. Ajami, B. Aji, O. Ajumobi, S. E. Akade, M. Akbari, H. Akbarialiabad, S. Akhlaghi, K. Akinosoglou, R. O. Akinyemi, M. Akonde, S. M. A. Hasan, F. Alahdab, T. M. A. AL-Ahdal, R. M. Al-amer, M. Albashtawy, M. T. AlBataineh, K. A. Aldawsari, H. Alemi, S. Alemi, A. M. Algammal, A. A. S. Al-Gheethi, F. A. N. Alhalaiqa, R. K. Alhassan, A. Ali, E. A. Ali, L. Ali, M. U. Ali, M. M. Ali, R. Ali, S. Ali, S. S. S. Ali, Z. Ali, S. M. Alif, Y. Alimohamadi, A. A. Aliyi, M. Aljofan, S. M. Aljunid, S. Alladi, J. U. Almazan, S. Almustanyir, B. Al-Omari, J. S. Alqahtani, I. Alqasmi, A. Y. Alqutaibi, R. A.-S. Salman, Z. Altaany, J. A. Al-Tawfiq, K. A. Altirkawi, N. Alvis-Guzman, Y. M. Al-Worafi, H. Aly, S. Aly, K. H. Alzoubi, R. Amani, A. Amindarolzarbi, S. Amiri, M. H. Amirzade-Iranaq, H. Amu, D. A. Amugsi, G. A. Amusa, J. Amzat, R. Ancuceanu, D. Anderlini, D. B. Anderson, C. L. Andrei, S. Androudi, D. Angappan, T. W. Angesom, A. Anil, A. Ansari-Moghaddam, R. Anwer, M. Arafat, A. Y. Aravkin, D. Areda, H. Ariffin, H. Arifin, M. Arkew, J. Ärnlöv, M. Arooj, A. A. Artamonov, K. D. Artanti, R. T. Aruleba, A. A. Asadi-Pooya, T. F. Asena, M. Asghari-Jafarabadi, M. Ashraf, T. Ashraf, K. A. Atalell, S. S. Athari, B. T. T. Atinafu, P. Atorkey, M. M. W. Atout, A. Atreya, A. Aujayeb, A. Avan, B. P. A. Quintanilla, H. Ayatollahi, O. O. Ayinde, S. M. Ayyoubzadeh, S. Azadnajafabad, Z. Azizi, K. Azizian, A. Y. Azzam, M. Babaei, M. Badar, A. D. Badiye, S. Baghdadi, S. Bagherieh, R. Bai, A. A. Baig, S. Balakrishnan, S. Balalla, O. C. Baltatu, M. Banach, S. Bandyopadhyay, I. Banerjee, M. F. Baran, M. A. Barboza, M. Barchitta, M. Bardhan, S. L. Barker-Collo, T. W. Bärnighausen, A. Barrow, D. Bashash, H. Bashiri, H. A. Bashiru, A. Basiru, J. D. Basso, S. Basu, A.-M. M. Batiha, K. Batra, B. T. Baune, N. Bedi, A. Begde, T. Begum, B. Behnam, A. H. Behnoush, M. Beiranvand, Y. Béjot, A. Bekele, M. A. Belete, U. I. Belgaumi, M. Bemanalizadeh, R. G. Bender, B. Benfor, D. A. Bennett, I. M. Bensenor, B. Berice, P. J. G. Bettencourt, K. A. Beyene, A. Bhadra, D. S. Bhagat, K. Bhangdia, N. Bhardwaj, P. Bhardwaj, A. Bhargava, S. Bhaskar, A. N. Bhat, V. Bhat, G. K. Bhatti, J. S. Bhatti, R. Bhatti, A. Bijani, B. Bikbov, M. M. Bilalaga, A. Biswas, S. Bitaraf, V. R. Bitra, T. Bjørge, V. Bodolica, A. O. Bodunrin, A. Boloor, D. Braithwaite, C. Brayne, H. Brenner, A. Briko, M. L. B. Vega, J. Brown, C. M. Budke, D. Buonsenso, K. Burkart, R. A. Burns, Y. Bustanji, M. H. Butt, N. S. Butt, Z. A. Butt, L. S. Cabral, F. L. C. dos Santos, D. Calina, I. R. Campos-Nonato, C. Cao, H. Carabin, R. Cárdenas, G. Carreras, A. F. Carvalho, C. A. Castańeda-Orjuela, A. Casulli, F. Catalá-López, A. L. Catapano, A. Caye, L. Cegolon, M. Cenderadewi, E. Cerin, P. R. Chacón-Uscamaita, J. S. K. Chan, G. S. Chanie, J. Charan, V. K. Chattu, E. C. Abebe, H. Chen, J. Chen, G. Chi, F. Chichagi, S. B. Chidambaram, R. Chimoriya, P. R. Ching, A. Chitheer, Y. Y. Chong, H. Chopra, S. G. Choudhari, E. K. Chowdhury, R. Chowdhury, H. Christensen, D.-T. Chu, I. S. Chukwu, E. Chung, K. Coberly, A. Columbus, J. Comachio, J. Conde, P. A. Cortesi, V. M. Costa, R. A. S. Couto, M. H. Criqui, N. Cruz-Martins, M. A. D. Ohadi, S. Dadana, O. Dadras, X. Dai, Z. Dai, E. D’Amico, H. A. Danawi, L. Dandona, R. Dandona, A. H. Darwish, S. Das, S. Das, A. M. Dascalu, N. R. Dash, M. Dashti, F. P. D. la Hoz, A. de la Torre-Luque, D. D. Leo, F. E. Dean, A. Dehghan, A. Dehghan, H. Dejene, D. Demant, A. K. Demetriades, S. Demissie, X. Deng, H. D. Desai, V. G. C. Devanbu, K. Dhama, S. D. Dharmaratne, M. Dhimal, D. D. da Silva, D. Diaz, M. Dibas, D. D. Ding, M. Dinu, M. A. Dirac, M. Diress, T. C. Do, T. H. P. Do, K. D. K. Doan, M. Dodangeh, M. F. Doheim, K. G. Dokova, D. Dongarwar, H. L. Dsouza, J. Dube, S. Duraisamy, O. C. Durojaiye, S. Dutta, A. M. Dziedzic, H. A. Edinur, N. Eissazade, M. Ekholuenetale, T. C. Ekundayo, N. E. Nahas, I. E. Sayed, M. A. E. Najafi, I. Elbarazi, N. M. Elemam, F. J. Elgar, I. Y. Elgendy, H. R. Elhabashy, M. Elhadi, L. T. Elilo, R. G. Ellenbogen, O. A. A. Elmeligy, M. A. Elmonem, M. Elshaer, I. Elsohaby, M. Emamverdi, T. I. Emeto, M. Endres, C. I. Esezobor, S. Eskandarieh, A. Fadaei, A. F. Fagbamigbe, A. Fahim, A. Faramarzi, J. Fares, M. F. Kouhanjani, A. Faro, F. Farzadfar, A. Fatehizadeh, M. Fathi, S. Fathi, S. A. F. Fatima, A. Feizkhah, S.-M. Fereshtehnejad, A. J. Ferrari, N. Ferreira, G. Fetensa, N. Firouraghi, F. Fischer, A. C. Fonseca, L. M. Force, A. Fornari, B. Foroutan, T. Fukumoto, M. A. Gadanya, A. M. Gaidhane, Y. Galali, N. Galehdar, Q. Gan, A. P. Gandhi, B. Ganesan, W. M. Gardner, N. Garg, S.-Y. Gau, R. K. Gautam, T. Gebre, M. Gebrehiwot, G. G. Gebremeskel, H. G. Gebreslassie, L. Getacher, B. G. Yazdi, F. Ghadirian, F. Ghaffarpasand, R. Ghanbari, M. Ghasemi, R. M. Ghazy, S. Ghimire, A. Gholami, A. Gholamrezanezhad, E. Ghotbi, S. Ghozy, A. Gialluisi, P. S. Gill, L. M. Glasstetter, E. V. Gnedovskaya, A. Golchin, M. Golechha, P. Goleij, D. Golinelli, M. Gomes-Neto, A. C. Goulart, A. Goyal, R. J. Gray, M. Grivna, H. A. Guadie, B. Guan, G. Guarducci, S. Guicciardi, D. A. Gunawardane, H. Guo, B. Gupta, R. Gupta, S. Gupta, V. B. Gupta, V. K. Gupta, R. A. Gutiérrez, F. Habibzadeh, V. Hachinski, R. Haddadi, M. Hadei, N. R. Hadi, N. Haep, T. G. Haile, A. Haj-Mirzaian, B. J. Hall, R. Halwani, S. Hameed, M. Hamiduzzaman, A. Hammoud, H. Han, N. Hanifi, G. J. Hankey, M. A. Hannan, J. Hao, H. Harapan, H. E. Hareru, A. Hargono, N. I. Harlianto, J. M. Haro, N. N. Hartman, A. I. Hasaballah, F. Hasan, H. Hasani, M. Hasanian, A. Hassan, S. Hassan, S. Hassanipour, H. Hassankhani, M. B. Hassen, J. Haubold, S. I. Hay, K. Hayat, M. I. Hegazy, G. Heidari, M. Heidari, R. Heidari-Soureshjani, H. Hesami, K. Hezam, Y. Hiraike, H. J. Hoffman, R. Holla, K. P. Hopf, N. Horita, M. M. Hossain, M. B. Hossain, S. Hossain, H. Hosseinzadeh, M. Hosseinzadeh, S. Hostiuc, C. Hu, J. Huang, M. N. Huda, J. Hussain, N. R. Hussein, H.-H. Huynh, B.-F. Hwang, S. E. Ibitoye, M. Ilaghi, O. S. Ilesanmi, I. M. Ilic, M. D. Ilic, M. Immurana, F. Iravanpour, S. M. S. Islam, F. Ismail, H. Iso, G. Isola, M. Iwagami, C. C. D. Iwu, M. Iyer, A. Jaan, L. Jacob, F. Jadidi-Niaragh, M. Jafari, M. Jafarinia, A. Jafarzadeh, K. Jahankhani, N. Jahanmehr, H. Jahrami, A. Jaiswal, M. Jakovljevic, R. D. G. Jamora, S. Jana, N. Javadi, S. Javed, S. Javeed, S. K. Jayapal, S. Jayaram, H. Jiang, C. O. Johnson, W. D. Johnson, M. Jokar, J. B. Jonas, A. Joseph, N. Joseph, C. E. Joshua, M. Jürisson, A. Kabir, Z. Kabir, G. G. Kabito, V. Kadashetti, F. Kafi, R. Kalani, F. Kalantar, F. Kaliyadan, A. Kamath, S. Kamath, T. Kanchan, A. Kandel, H. Kandel, K. K. Kanmodi, M. Karajizadeh, J. Karami, S. D. Karanth, I. M. Karaye, A. Karch, A. Karimi, H. Karimi, A. K. Behnagh, H. Kasraei, N. J. Kassebaum, J. H. Kauppila, H. Kaur, N. Kaur, G. A. Kayode, F. Kazemi, L. Keikavoosi-Arani, C. Keller, M. Keykhaei, M. A. Khadembashiri, Y. S. Khader, M. A. Khafaie, H. Khajuria, A. Khalaji, F. Khamesipour, M. Khammarnia, M. Khan, M. A. Khan, Y. H. Khan, M. Z. K. Suheb, S. Khanmohammadi, T. Khanna, K. Khatab, H. Khatatbeh, M. M. Khatatbeh, S. Khateri, M. N. Khatib, H. R. K. Kashani, M. S. Khonji, F. Khorashadizadeh, M. Khormali, J. Khubchandani, S. Kian, G. Kim, J. Kim, M. S. Kim, Y. J. Kim, R. W. Kimokoti, A. Kisa, S. Kisa, M. Kivimäki, S. Kochhar, A.-A. Kolahi, K. N. Koly, F. Kompani, W. J. Koroshetz, S. Kosen, M. K. Arami, A. Koyanagi, M. A. Kravchenko, K. Krishan, V. Krishnamoorthy, B. K. Defo, M. A. Kuddus, A. Kumar, G. A. Kumar, M. Kumar, N. Kumar, N. B. Kumsa, S. Kundu, M. D. Kurniasari, D. Kusuma, A. Kuttikkattu, H. H. Kyu, C. L. Vecchia, M. A. Ladan, C. Lahariya, T. Laksono, D. K. Lal, T. Lallukka, J. Lám, F. H. Lami, I. Landires, B. Langguth, S. Lasrado, K. Latief, K. Latifinaibin, K. M.-M. Lau, M. B. Laurens, B. K. Lawal, L. K. D. Le, T. T. T. Le, C. Ledda, M. Lee, S. Lee, S. W. Lee, W.-C. Lee, Y. H. Lee, M. Leonardi, T. L. Lerango, M.-C. Li, W. Li, V. S. Ligade, S. S. Lim, C. Linehan, C. Liu, J. Liu, W. Liu, C.-H. Lo, W. D. Lo, S. W. Lobo, G. Logroscino, G. Lopes, P. D. Lopukhov, L. Lorenzovici, S. Lorkowski, J. A. Loureiro, J. Lubinda, G. Lucchetti, R. L. Saute, Z. F. Ma, M. Mabrok, M. Machoy, F. Madadizadeh, M. M. A. E. Razek, A. A. Maghazachi, N. Maghbouli, S. Mahjoub, M. Mahmoudi, A. Majeed, J. N. Malagón-Rojas, E. M. Rad, K. Malhotra, A. A. Malik, I. Malik, T. H. Mallhi, D. C. Malta, A. Manilal, V. Mansouri, M. A. Mansournia, B. P. Marasini, H. R. Marateb, S. F. Maroufi, J. Martinez-Raga, S. Martini, F. R. Martins-Melo, M. Martorell, W. März, R. R. Marzo, J. Massano, Y. Mathangasinghe, E. Mathews, R. J. Maude, A. Maugeri, P. K. Maulik, M. Mayeli, M. Mazaheri, C. McAlinden, J. J. McGrath, J. K. Meena, M. M. Mehndiratta, M. A. M. Mendez-Lopez, W. Mendoza, O. Mendoza-Cano, R. G. Menezes, M. Merati, A. Meretoja, A. Merkin, A. M. Mersha, T. Mestrovic, T. Mi, T. Miazgowski, I. M. Michalek, E. T. Mihretie, L. H. N. Minh, R. Mirfakhraie, A. Mirica, E. M. Mirrakhimov, M. Mirzaei, A. Misganaw, S. Misra, P. Mithra, B. A. Mizana, A. Mohamadkhani, N. S. Mohamed, E. Mohammadi, H. Mohammadi, S. Mohammadi, S. Mohammadi, M. Mohammadshahi, M. Mohammed, S. Mohammed, S. Mohammed, S. Mohan, H. Mojiri-forushani, N. Moka, A. H. Mokdad, S. Molinaro, H. Möller, L. Monasta, M. Moniruzzaman, F. Montazeri, M. Moradi, Y. Moradi, M. Moradi-Lakeh, P. Moraga, N. Morovatdar, S. D. Morrison, A. Mosapour, J. F. Mosser, E. Mossialos, M. Motaghinejad, P. Mousavi, S. E. Mousavi, S. Mubarik, L. Muccioli, F. Mughal, G. D. Mukoro, A. Mulita, F. Mulita, F. Musaigwa, A. Mustafa, G. Mustafa, S. Muthu, A. J. Nagarajan, P. Naghavi, G. R. Naik, F. Nainu, T. S. Nair, H. H. R. Najmuldeen, N. N. Ansari, G. Nambi, H. N. Areshtanab, S. Nargus, B. R. Nascimento, A. Y. Naser, A. J. J. Nashwan, H. Nasoori, A. Nasreldein, Z. S. Natto, J. Nauman, B. P. Nayak, A. Nazri-Panjaki, M. Negaresh, H. Negash, I. Negoi, R. I. Negoi, S. M. Negru, S. A. Nejadghaderi, M. H. Nematollahi, O. D. Nesbit, C. R. J. Newton, D. H. Nguyen, H. T. H. Nguyen, H. Q. Nguyen, N.-T. T. Nguyen, P. T. Nguyen, V. T. Nguyen, R. K. Niazi, T. K. Nikolouzakis, V. Niranjan, L. A. Nnyanzi, E. A. Noman, N. Noroozi, B. Norrving, J. J. Noubiap, C. A. Nri-Ezedi, G. Ntaios, V. Nuńez-Samudio, D. Nurrika, B. Oancea, I. A. Odetokun, M. J. O’Donnell, R. E. Ogunsakin, J. O. Oguta, I.-H. Oh, H. Okati-Aliabad, S. R. Okeke, A. P. Okekunle, O. C. Okonji, P. G. Okwute, A. T. Olagunju, M. T. Olaiya, M. D. Olana, M. I. Olatubi, G. M. M. Oliveira, I. I. Olufadewa, B. O. Olusanya, A. O. Bali, S. Ong, O. E. Onwujekwe, M. Ordak, A. U. Orji, D. V. Ortega-Altamirano, U. L. Osuagwu, N. Otstavnov, S. S. Otstavnov, A. Ouyahia, M. O. Owolabi, M. P. P. Aaa, K. Pacheco-Barrios, J. R. Padubidri, P. K. Pal, P. N. Palange, C. Palladino, R. Palladino, R. F. Palma-Alvarez, F. Pan, D. Panagiotakos, S. Panda-Jonas, A. Pandey, A. Pandey, J. D. Pandian, H. U. Pangaribuan, I. Pantazopoulos, S. Pardhan, P. P. Parija, R. R. Parikh, S. Park, A. Parthasarathi, A. Pashaei, J. Patel, S. Patil, D. Patoulias, S. Pawar, P. Pedersini, U. Pensato, D. M. Pereira, J. Pereira, M. O. Pereira, M. F. P. Peres, N. Perico, S. Perna, I.-R. Petcu, F. E. Petermann-Rocha, H. T. Pham, M. R. Phillips, G. D. Pinilla-Monsalve, M. A. Piradov, E. Plotnikov, D. Poddighe, B. Polat, R. Poluru, C. D. Pond, G. R. Poudel, A. Pouramini, A. M. Pourbagher-Shahri, M. Pourfridoni, N. Pourtaheri, P. Y. Prakash, S. Prakash, V. Prakash, E. J. S. Prates, N. Pritchett, H. Purnobasuki, N. H. Qasim, I. Qattea, G. Qian, V. Radhakrishnan, P. Raee, H. R. Shahraki, I. Rafique, A. Raggi, P. R. Raghav, M. M. Rahati, F. Rahim, Z. Rahimi, M. Rahimifard, M. O. Rahman, M. H. U. Rahman, M. Rahman, M. A. Rahman, A. M. Rahmani, S. Rahmani, H. R. Youshanlouei, M. Rahmati, S. R. Moolambally, A. Rajabpour-Sanati, H. Ramadan, S. K. Ramasamy, P. Ramasubramani, S. Ramazanu, N. Rancic, I. R. Rao, S. J. Rao, D. Rapaka, V. Rashedi, A. M. Rashid, M.-M. Rashidi, M. R. Alavijeh, A. Rasouli-Saravani, S. Rawaf, C. Razo, E. M. M. Redwan, A. R. Bana, G. Remuzzi, N. Rezaei, N. Rezaei, N. Rezaei, M. Rezaeian, T. G. Rhee, A. Riad, S. R. Robinson, M. Rodrigues, J. A. B. Rodriguez, L. Roever, E. L. B. Rogowski, M. Romoli, L. Ronfani, P. Roy, K. R. Pramanik, E. Rubagotti, M. A. Ruiz, T. C. Russ, K. S. Sunnerhagen, A. M. A. Saad, Z. Saadatian, K. Saber, M. SaberiKamarposhti, S. Sacco, B. Saddik, E. Sadeghi, S. Sadeghian, U. Saeed, U. Saeed, M. Safdarian, S. Z. Safi, R. Sagar, D. Sagoe, F. S. Sharif-Askari, N. S. Sharif-Askari, A. Sahebkar, S. S. Sahoo, M. A. Sahraian, S. A. Sajedi, J. W. Sakshaug, M. A. Saleh, H. S. Omran, M. R. Salem, S. Salimi, H. S. Kafil, S. Samadzadeh, S. Samargandy, Y. L. Samodra, V. P. Samuel, A. M. Samy, N. Sanadgol, R. K. Sanjeev, F. Sanmarchi, D. F. Santomauro, I. N. Santri, M. M. Santric-Milicevic, A. Saravanan, A. Sarveazad, M. Satpathy, M. Saylan, M. Sayyah, N. Scarmeas, M. P. Schlaich, A. Schuermans, M. Schwarzinger, D. C. Schwebel, S. Selvaraj, A. K. Sendekie, P. Sengupta, S. Senthilkumaran, D. Serban, M. T. Sergindo, Y. Sethi, S. SeyedAlinaghi, A. Seylani, M. Shabani, M. Shabany, M. Shafie, S. Shahabi, A. Shahbandi, S. Shahid, F. Shahraki-Sanavi, H. R. Shahsavari, M. J. Shahwan, M. A. Shaikh, K. S. Shaji, S. Sham, A. T. T. Shama, M. A. Shamim, M. Shams-Beyranvand, M. A. Shamsi, M. Shanawaz, M. Sharath, S. Sharfaei, A. Sharifan, M. Sharma, R. Sharma, B. B. Shashamo, M. Shayan, R. A. Sheikhi, S. Shekhar, J. Shen, S. M. Shenoy, P. H. Shetty, D. S. Shiferaw, M. Shigematsu, R. Shiri, A. Shittu, K. M. Shivakumar, F. Shokri, S. Shool, S. A. Shorofi, S. Shrestha, A. B. S. Tankwanchi, E. E. Siddig, I. D. Sigfusdottir, J. P. Silva, L. M. L. R. Silva, E. Sinaei, B. B. Singh, G. Singh, P. Singh, S. Singh, S. B. Sirota, S. Sivakumar, A. A. M. Sohag, R. Solanki, H. Soleimani, S. Solikhah, Y. Solomon, Y. Solomon, S. Song, Y. Song, H. Sotoudeh, M. Spartalis, B. A. Stark, J. R. Starnes, A. V. Starodubova, D. J. Stein, T. J. Steiner, L. J. Stovner, M. Suleman, R. S. Abdulkader, A. Sultana, J. Sun, D. Sunkersing, A. Sunny, H. Susianti, C. K. Swain, M. D. Szeto, R. Tabarés-Seisdedos, S. M. Tabatabaei, S. Tabatabai, M. Tabish, M. Taheri, A. Tahvildari, A. Tajbakhsh, M. Tampa, J. J. L. Tamuzi, K.-K. Tan, H. Tang, M. Tareke, I. U. Tarigan, N. Y. Tat, V. Y. Tat, R. T. Oliaee, S. M. Tavangar, A. Tavasol, Y. M. Tefera, A. Tehrani-Banihashemi, W. A. Temesgen, M.-H. Temsah, M. Teramoto, A. H. Tesfaye, E. G. Tesfaye, R. Tesler, O. Thakali, P. Thangaraju, R. Thapa, R. Thapar, N. K. Thomas, A. G. Thrift, J. H. V. Ticoalu, T. Tillawi, R. Toghroli, M. Tonelli, M. R. Tovani-Palone, E. Traini, N. M. Tran, N.-H. Tran, P. V. Tran, S. J. Tromans, T. C. Truelsen, T. T. T. T. Truyen, A. Tsatsakis, G. M. Tsegay, E. E. Tsermpini, A. R. Tualeka, D. G. Tufa, C. S. Ubah, A. J. Udoakang, I. Ulhaq, M. Umair, S. Umakanthan, K. K. Umapathi, B. Unim, B. Unnikrishnan, A. G. Vaithinathan, A. Vakilian, S. V. Tahbaz, R. Valizadeh, J. V. den Eynde, P. Vart, S. B. Varthya, T. J. Vasankari, S. Vaziri, B. Vellingiri, N. Venketasubramanian, G.-I. Verras, D. Vervoort, J. H. Villafańe, L. Villani, A. F. V. Veloz, M. Viskadourou, S. K. Vladimirov, V. Vlassov, S. R. Volovat, L. T. Vu, I. S. Vujcic, B. Wagaye, Y. Waheed, W. Wahood, M. T. Walde, F. Wang, S. Wang, Y. Wang, Y.-P. Wang, M. Waqas, A. Waris, K. G. Weerakoon, R. G. Weintraub, A. H. Weldemariam, R. Westerman, J. L. Whisnant, D. P. Wickramasinghe, N. D. Wickramasinghe, B. Willekens, L. B. Wilner, A. S. Winkler, C. D. A. Wolfe, A.-M. Wu, S. W. Hanson, S. Xu, X. Xu, A. Yadollahpour, S. Yaghoubi, G. Yahya, K. Yamagishi, L. Yang, Y. Yano, Y. Yao, S. S. Yehualashet, A. Yeshaneh, M. Yesiltepe, S. Yi, A. Yiğit, V. Yiğit, D. K. Yon, N. Yonemoto, Y. You, M. Z. Younis, C. Yu, H. Yusuf, S. Zadey, M. Zahedi, F. Zakham, N. Zaki, A. Zali, G. Zamagni, R. Zand, G. G. Z. Zandieh, M. Zangiabadian, A. Zarghami, M. S. Zastrozhin, M. G. M. Zeariya, Z. B. Zegeye, F. Zeukeng, C. Zhai, C. Zhang, H. Zhang, Y. Zhang, Z.-J. Zhang, H. Zhao, Y. Zhao, P. Zheng, H. Zhou, B. Zhu, A. Zhumagaliuly, M. Zielińska, Y. T. Zikarg, M. Zoladl, C. J. L. Murray, K. L. Ong, V. L. Feigin, T. Vos and T. Dua, Lancet Neurol., 2024, 23, 344–381 CrossRef PubMed.
- D. M. Wilson, M. R. Cookson, L. Van Den Bosch, H. Zetterberg, D. M. Holtzman and I. Dewachter, Cell, 2023, 186, 693–714 CrossRef CAS PubMed.
- N. Shusharina, D. Yukhnenko, S. Botman, V. Sapunov, V. Savinov, G. Kamyshov, D. Sayapin and I. Voznyuk, Diagnostics, 2023, 13, 573 CrossRef CAS PubMed.
- D. Bhatia, S. Arumugam, M. Nasilowski, H. Joshi, C. Wunder, V. Chambon, V. Prakash, C. Grazon, B. Nadal, P. K. Maiti, L. Johannes, B. Dubertret and Y. Krishnan, Nat. Nanotechnol., 2016, 11, 1112–1119 CrossRef CAS PubMed.
- P. Hivare, J. Gadhavi, D. Bhatia and S. Gupta, Traffic, 2022, 23, 391–410 CrossRef CAS PubMed.
- A. Gangrade, P. Hivare, S. Gupta and D. Bhatia, in Hydrogels for Tissue Engineering and Regenerative Medicine, ed. J. M. Oliveira, J. Silva-Correia and R. L. Reis, Academic Press, 2024, pp. 367–385 Search PubMed.
- P. Hivare, A. Gangrade, G. Swarup, K. Bhavsar, A. Singh, R. Gupta, P. Thareja, S. Gupta and D. Bhatia, Nanoscale, 2022, 14, 8611–8620 RSC.
- P. Hivare, A. Rajwar, S. Gupta and D. Bhatia, ACS Appl. Bio Mater., 2021, 4, 3350–3359 CrossRef CAS PubMed.
- G. Y. Wang, S. L. Rayner, R. Chung, B. Y. Shi and X. J. Liang, Mater. Today Bio, 2020, 6, 100055 CrossRef CAS PubMed.
- B. Wilson, M. K. Samanta, K. Santhi, K. P. S. Kumar, N. Paramakrishnan and B. Suresh, Brain Res., 2008, 1200, 159–168 CrossRef CAS PubMed.
- A. C. Kaushik, S. Bharadwaj, S. Kumar and D.-Q. Wei, Sci. Rep., 2018, 8, 9169 CrossRef PubMed.
- Z. Cui, P. R. Lockman, C. S. Atwood, C.-H. Hsu, A. Gupte, D. D. Allen and R. J. Mumper, Eur. J. Pharm. Biopharm., 2005, 59, 263–272 CrossRef CAS PubMed.
- M. J. Kogan, N. G. Bastus, R. Amigo, D. Grillo-Bosch, E. Araya, A. Turiel, A. Labarta, E. Giralt and V. F. Puntes, Nano Lett., 2006, 6, 110–115 CrossRef CAS PubMed.
- T. T. Nguyen, T. T. Dung Nguyen, T. K. Vo, N.-M.-A. Tran, M. K. Nguyen, T. Van Vo and G. Van Vo, Biomed. Pharmacother., 2021, 143, 112117 CrossRef CAS PubMed.
- K. Jagaran and M. Singh, Int. J. Mol. Sci., 2021, 22, 9082 CrossRef CAS PubMed.
- D. Snead and D. Eliezer, Exp. Neurobiol., 2014, 23, 292–313 CrossRef PubMed.
- X. Tian, T. Fan, W. Zhao, G. Abbas, B. Han, K. Zhang, N. Li, N. Liu, W. Liang, H. Huang, W. Chen, B. Wang and Z. Xie, Bioact. Mater., 2021, 6, 2854–2869 CAS.
- T. R. Kyriakides, A. Raj, T. H. Tseng, H. Xiao, R. Nguyen, F. S. Mohammed, S. Halder, M. Xu, M. J. Wu, S. Bao and W. C. Sheu, Biomed. Mater., 2021, 16, 042005 CrossRef CAS PubMed.
- A. Sinha, F. Z. Simnani, D. Singh, A. Nandi, A. Choudhury, P. Patel, E. Jha, R. S. Chouhan, N. K. Kaushik, Y. K. Mishra, P. K. Panda, M. Suar and S. K. Verma, Mater. Today Bio, 2022, 17, 100463 CrossRef CAS PubMed.
- H. R. Bakhsheshi-Rad, E. Hamzah, M. Kasiri-Asgarani, S. N. Saud, F. Yaghoubidoust and E. Akbari, Vacuum, 2016, 131, 106–110 CrossRef CAS.
- N. Joudeh and D. Linke, J. Nanobiotechnol., 2022, 20, 262 CrossRef PubMed.
- J. K. Patra, G. Das, L. F. Fraceto, E. V. R. Campos, M. D. P. Rodriguez-Torres, L. S. Acosta-Torres, L. A. Diaz-Torres, R. Grillo, M. K. Swamy, S. Sharma, S. Habtemariam and H.-S. Shin, J. Nanobiotechnol., 2018, 16, 71 CrossRef PubMed.
- S. Sharmin, M. M. Rahaman, C. Sarkar, O. Atolani, M. T. Islam and O. S. Adeyemi, Heliyon, 2021, 7, e06456 CrossRef CAS PubMed.
- A. Chakraborty, A. Diwan and J. Tatake, AIMS Microbiol., 2023, 9, 444–466 CAS.
- Nanotechnology for Telecommunications – 1st Edition - Sohail Anwar – M, https://www.routledge.com/Nanotechnology-for-Telecommunications/Anwar-Raja-Qazi-Ilyas/p/book/9781138113817?srsltid=AfmBOorZg4JfAtuQiCOCMpTPsWqX0M16qcimbyOJK83pZauq8tjBQpcw, (accessed April 29, 2025).
- Y. Zhang, K. Poon, G. S. P. Masonsong, Y. Ramaswamy and G. Singh, Pharmaceutics, 2023, 15, 922 CrossRef CAS PubMed.
- J. A. Champion, Y. K. Katare and S. Mitragotri, J. Controlled Release, 2007, 121, 3–9 CrossRef CAS PubMed.
- C. M. Maguire, M. Rösslein, P. Wick and A. Prina-Mello, Sci. Technol. Adv. Mater., 2018, 19, 732–745 CrossRef CAS PubMed.
- P.-C. Lin, S. Lin, P. C. Wang and R. Sridhar, Biotechnol. Adv., 2014, 32, 711–726 CrossRef PubMed.
- R. M. Crist, J. H. Grossman, A. K. Patri, S. T. Stern, M. A. Dobrovolskaia, P. P. Adiseshaiah, J. D. Clogston and S. E. McNeil, Integr. Biol., 2013, 5, 66–73 CrossRef CAS PubMed.
- M. L. Etheridge, S. A. Campbell, A. G. Erdman, C. L. Haynes, S. M. Wolf and J. McCullough, Nanomedicine, 2013, 9, 1–14 CrossRef CAS PubMed.
- C. J. Briscoe and D. S. Hage, Bioanalysis, 2009, 1, 205–220 CrossRef CAS PubMed.
- R. Hardman, Environ. Health Perspect., 2006, 114, 165–172 CrossRef PubMed.
- K. McNamara and S. A. M. Tofail, Adv. Phys.: X, 2017, 2, 54–88 CAS.
- H. R. Badwaik, L. Kumari, K. Nakhate, V. S. Verma and K. Sakure, in Studies in Natural Products Chemistry, ed. A. U. Rahman, Elsevier, 2019, vol. 63, pp. 415–460 Search PubMed.
- J. Conde, G. Doria and P. Baptista, J. Drug Delivery, 2012, 2012, 751075 Search PubMed.
- D. Chenthamara, S. Subramaniam, S. G. Ramakrishnan, S. Krishnaswamy, M. M. Essa, F.-H. Lin and M. W. Qoronfleh, Biomater. Res., 2019, 23, 20 CrossRef CAS PubMed.
- V. V. Mody, R. Siwale, A. Singh and H. R. Mody, J. Pharm. BioAllied Sci., 2010, 2, 282–289 CrossRef CAS PubMed.
- V. Chandrakala, V. Aruna and G. Angajala, Emergent Mater., 2022, 5, 1593–1615 CrossRef CAS PubMed.
- A. A. Yetisgin, S. Cetinel, M. Zuvin, A. Kosar and O. Kutlu, Molecules, 2020, 25, 2193 CrossRef CAS PubMed.
- M.-C. Chiang, Y.-P. Yang, C. J. B. Nicol and C.-J. Wang, Int. J. Mol. Sci., 2024, 25, 2360 CrossRef CAS PubMed.
- J. M. Dowding, W. Song, K. Bossy, A. Karakoti, A. Kumar, A. Kim, B. Bossy, S. Seal, M. H. Ellisman, G. Perkins, W. T. Self and E. Bossy-Wetzel, Cell Death Differ., 2014, 21, 1622–1632 CrossRef CAS PubMed.
- X. Jiang and H. Gao, Neurotoxicity of nanomaterials and nanomedicine, Elsevier/Academic Press, London, 2017 Search PubMed.
- J. Zhang, L. Mou and X. Jiang, Chem. Sci., 2020, 11, 923–936 RSC.
- E. O. Mikhailova, J. Funct. Biomater., 2021, 12, 70 CrossRef CAS PubMed.
- R. Khursheed, K. Dua, S. Vishwas, M. Gulati, N. K. Jha, G. M. Aldhafeeri, F. G. Alanazi, B. H. Goh, G. Gupta, K. R. Paudel, P. M. Hansbro, D. K. Chellappan and S. K. Singh, Biomed. Pharmacother., 2022, 150, 112951 CrossRef CAS PubMed.
- L. Zhao, T. Lan, G. Jiang and B. Yan, Inorg. Chem. Commun., 2022, 141, 109486 CrossRef CAS.
- G. de, B. Silveira, A. P. Muller, R. A. Machado-de-Ávila and P. C. L. Silveira, Neural Regener. Res., 2021, 16, 2425–2426 CrossRef PubMed.
- N. Puranik, D. Yadav and M. Song, Int. J. Mol. Sci., 2023, 24, 14044 CrossRef CAS PubMed.
- Y. Huang, Y. Chang, L. Liu and J. Wang, Molecules, 2021, 26, 4301 CrossRef CAS PubMed.
- O. Betzer, M. Shilo, R. Opochinsky, E. Barnoy, M. Motiei, E. Okun, G. Yadid and R. Popovtzer, Nanomedicine, 2017, 12, 1533–1546 CrossRef CAS PubMed.
- J. Ruff, S. Hüwel, M. J. Kogan, U. Simon and H.-J. Galla, Nanomedicine, 2017, 13, 1645–1652 CrossRef CAS PubMed.
- M. Jaroniec, Nat. Chem., 2009, 1, 166 CrossRef CAS PubMed.
- D. E. Nayab, F. U. Din, H. Ali, W. A. Kausar, S. Urooj, M. Zafar, I. Khan, K. Shabbir and G. M. Khan, J. Nanobiotechnol., 2023, 21, 477 CrossRef PubMed.
- G. Quan, X. Pan, Z. Wang, Q. Wu, G. Li, L. Dian, B. Chen and C. Wu, J. Nanobiotechnol., 2015, 13, 89 CrossRef PubMed.
- Nanoengineered mesoporous silica nanoparticles for smart delivery of doxorubicin | J. Nanopart. Res., https://link.springer.com/article/10.1007/s11051-014-2515-y, (accessed April 30, 2025).
- C. M. Nday, E. Halevas, G. E. Jackson and A. Salifoglou, J. Inorg. Biochem., 2015, 145, 51–64 CrossRef CAS PubMed.
- Y. Song, D. Du, L. Li, J. Xu, P. Dutta and Y. Lin, ACS Appl. Mater. Interfaces, 2017, 9, 20410–20416 CrossRef CAS PubMed.
- A. K. Singh, S. S. Singh, A. S. Rathore, S. P. Singh, G. Mishra, R. Awasthi, S. K. Mishra, V. Gautam and S. K. Singh, ACS Biomater. Sci. Eng., 2021, 7, 3737–3753 CrossRef CAS PubMed.
- J. Geng, M. Li, L. Wu, C. Chen and X. Qu, Adv. Healthcare Mater., 2012, 1, 332–336 CrossRef CAS PubMed.
- L. Yang, Q. Yan, J. Zhao, J. Li, X. Zong, L. Yang and Z. Wang, Toxicol. Lett., 2013, 223, 16–24 CrossRef CAS PubMed.
- F. Wang, C. Jiao, J. Liu, H. Yuan, M. Lan and F. Gao, Toxicol. In Vitro, 2011, 25, 1548–1556 CrossRef CAS PubMed.
- F. Koch, A.-M. Möller, M. Frenz, U. Pieles, K. Kuehni-Boghenbor and M. Mevissen, Toxicol. In Vitro, 2014, 28, 990–998 CrossRef CAS PubMed.
- Multidisciplinary Role of Mesoporous Silica Nanoparticles in Brain Regeneration and Cancers: From Crossing the Blood–Brain Barrier to Treatment – Mendiratta – 2019 – Particle & Particle Systems Characterization - Wiley Online Library, https://onlinelibrary.wiley.com/doi/full/10.1002/ppsc.201900195, (accessed April 30, 2025).
- R. Qiao, C. Fu, H. Forgham, I. Javed, X. Huang, J. Zhu, A. K. Whittaker and T. P. Davis, Adv. Drug Delivery Rev., 2023, 197, 114822 CrossRef CAS PubMed.
- F. D’Agata, F. A. Ruffinatti, S. Boschi, I. Stura, I. Rainero, O. Abollino, R. Cavalli and C. Guiot, Molecules, 2017, 23, 9 CrossRef PubMed.
- T. Birol, N. A. Benedek, H. Das, A. L. Wysocki, A. T. Mulder, B. M. Abbett, E. H. Smith, S. Ghosh and C. J. Fennie, Curr. Opin. Solid State Mater. Sci., 2012, 16, 227–242 CrossRef CAS.
- A. Tomitaka, H. Arami, S. Gandhi and K. M. Krishnan, Nanoscale, 2015, 7, 16890–16898 RSC.
- D. Dhar, S. Ghosh, S. Das and J. Chatterjee, Nanomedicine, 2022, 17, 107–132 CrossRef CAS PubMed.
- S. Lakshmanan, G. K. Gupta, P. Avci, R. Chandran, M. Sadasivam, A. E. S. Jorge and M. R. Hamblin, Adv. Drug Delivery Rev., 2014, 71, 98–114 CrossRef CAS PubMed.
- A. Tomitaka, A. Kaushik, B. D. Kevadiya, I. Mukadam, H. E. Gendelman, K. Khalili, G. Liu and M. Nair, Drug Discovery Today, 2019, 24, 873–882 CrossRef CAS PubMed.
- C. N. Loynachan, G. Romero, M. G. Christiansen, R. Chen, R. Ellison, T. T. O’Malley, U. P. Froriep, D. M. Walsh and P. Anikeeva, Adv. Healthcare Mater., 2015, 4, 2100–2109 CrossRef CAS PubMed.
- S. Niu, L.-K. Zhang, L. Zhang, S. Zhuang, X. Zhan, W.-Y. Chen, S. Du, L. Yin, R. You, C.-H. Li and Y.-Q. Guan, Theranostics, 2017, 7, 344–356 CrossRef CAS PubMed.
- E. K. Schneider-Futschik and F. Reyes-Ortega, Pharmaceutics, 2021, 13, 1157 CrossRef CAS PubMed.
- C. Xu and X. Qu, NPG Asia Mater., 2014, 6, e90–e90 CrossRef CAS.
- B. A. Rzigalinski, C. S. Carfagna and M. Ehrich, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2017, 9, 1444 Search PubMed.
- T. Pirmohamed, J. M. Dowding, S. Singh, B. Wasserman, E. Heckert, A. S. Karakoti, J. E. S. King, S. Seal and W. T. Self, Chem. Commun., 2010, 46, 2736–2738 RSC.
- E. G. Heckert, A. S. Karakoti, S. Seal and W. T. Self, Biomaterials, 2008, 29, 2705–2709 CrossRef CAS PubMed.
- C. Korsvik, S. Patil, S. Seal and W. T. Self, Chem. Commun., 2007, 1056–1058 RSC.
- W. Yang, M. Zhang, J. He, M. Gong, J. Sun and X. Yang, Regener. Biomater., 2022, 9, rbac037 CrossRef CAS PubMed.
- M. L. Bondì, E. F. Craparo, G. Giammona and F. Drago, Nanomedicine, 2010, 5, 25–32 CrossRef PubMed.
- Z. Mazibuko, Y. E. Choonara, P. Kumar, L. C. Du Toit, G. Modi, D. Naidoo and V. Pillay, J. Pharm. Sci., 2015, 104, 1213–1229 CrossRef CAS PubMed.
- A. Cimini, B. D’Angelo, S. Das, R. Gentile, E. Benedetti, V. Singh, A. M. Monaco, S. Santucci and S. Seal, Acta Biomater., 2012, 8, 2056–2067 CrossRef CAS PubMed.
- R. Tenchov, R. Bird, A. E. Curtze and Q. Zhou, ACS Nano, 2021, 15, 16982–17015 CrossRef CAS PubMed.
- A. Vashist, P. Manickam, A. D. Raymond, A. Y. Arias, N. Kolishetti, A. Vashist, E. Arias and M. Nair, ACS Appl. Bio Mater., 2023, 6, 2614–2621 CrossRef CAS PubMed.
- H. Nsairat, D. Khater, U. Sayed, F. Odeh, A. Al Bawab and W. Alshaer, Heliyon, 2022, 8, e09394 CrossRef CAS PubMed.
- B. Dos Santos Rodrigues, S. Arora, T. Kanekiyo and J. Singh, Brain Res., 2020, 1734, 146738 CrossRef PubMed.
- A. Waris, A. Ali, A. U. Khan, M. Asim, D. Zamel, K. Fatima, A. Raziq, M. A. Khan, N. Akbar, A. Baset and M. A. S. Abourehab, Nanomaterials, 2022, 12, 2140 CrossRef CAS PubMed.
- K. Sun, X. Zheng, H. Jin, F. Yu and W. Zhao, Pharmaceutics, 2022, 14, 2252 CrossRef CAS PubMed.
- A. Cano, Á. Muñoz-Morales, E. Sánchez-López, M. Ettcheto, E. B. Souto, A. Camins, M. Boada and A. Ruíz, Pharmaceutics, 2023, 15, 298 CrossRef CAS PubMed.
- L. Sercombe, T. Veerati, F. Moheimani, S. Y. Wu, A. K. Sood and S. Hua, Front. Pharmacol., 2015, 6, 286 Search PubMed.
- L. M. Doyle and M. Z. Wang, Cells, 2019, 8, 727 CrossRef CAS PubMed.
- W. A. Eimer and R. Vassar, Mol. Neurodegener., 2013, 8, 2 CrossRef CAS PubMed.
- L. Alvarez-Erviti, Y. Seow, H. Yin, C. Betts, S. Lakhal and M. J. A. Wood, Nat. Biotechnol., 2011, 29, 341–345 CrossRef CAS PubMed.
- M. Xu, T. Feng, B. Liu, F. Qiu, Y. Xu, Y. Zhao and Y. Zheng, Theranostics, 2021, 11, 8926–8944 CrossRef CAS PubMed.
- V. Leiro, S. Duque Santos, C. D. F. Lopes and A. Paula Pêgo, Adv. Funct. Mater., 2018, 28, 1700313 CrossRef.
- J. Wang, B. Li, L. Qiu, X. Qiao and H. Yang, J. Biol. Eng., 2022, 16, 18 CrossRef PubMed.
- M. Florendo, A. Figacz, B. Srinageshwar, A. Sharma, D. Swanson, G. L. Dunbar and J. Rossignol, Molecules, 2018, 23, 2238 CrossRef PubMed.
- D. E. Igartúa, C. S. Martinez, S. Del, V. Alonso and M. J. Prieto, AAPS PharmSciTech, 2020, 21, 110 CrossRef PubMed.
- D. E. Igartúa, F. González-Lizárraga, C. S. Martinez, S. del, V. Alonso, C. L. Ávila, R. Chehín, N. S. Chiaramoni and M. J. Prieto, OpenNano, 2023, 11, 100140 CrossRef.
- D. E. Igartúa, C. S. Martinez, C. F. Temprana, S. D. V. Alonso and M. J. Prieto, Int. J. Pharm., 2018, 544, 191–202 CrossRef PubMed.
- A. K. Singh, A. Gothwal, S. Rani, M. Rana, A. K. Sharma, A. K. Yadav and U. Gupta, ACS Omega, 2019, 4, 4519–4529 CrossRef CAS.
- E. Nance, F. Zhang, M. K. Mishra, Z. Zhang, S. P. Kambhampati, R. M. Kannan and S. Kannan, Biomaterials, 2016, 101, 96–107 CrossRef CAS PubMed.
- F. Palan and B. Chatterjee, J. Drug Delivery Sci. Technol., 2022, 73, 103474 CrossRef CAS.
- M. D. Villalva, V. Agarwal, M. Ulanova, P. S. Sachdev and N. Braidy, Nanomedicine, 2021, 16, 1595–1611 CrossRef CAS PubMed.
- N. H. Gamage, L. Jing, M. J. Worsham and M. M. Ali, J. Nanomed. Nanotechnol., 2016, 7, 393 Search PubMed.
- R. Swami, I. Singh, H. Kulhari, M. K. Jeengar, W. Khan and R. Sistla, J. Nanopart. Res., 2017, 19, 358 CrossRef.
- M. Maqbool, J. Gadhavi, A. Singh, P. Hivare, S. Gupta and N. Hoda, Org. Biomol. Chem., 2021, 19, 1589–1603 RSC.
- O. S. Wolfbeis, Chem. Soc. Rev., 2015, 44, 4743–4768 RSC.
- J. Liu, F. Erogbogbo, K.-T. Yong, L. Ye, J. Liu, R. Hu, H. Chen, Y. Hu, Y. Yang, J. Yang, I. Roy, N. A. Karker, M. T. Swihart and P. N. Prasad, ACS Nano, 2013, 7, 7303–7310 CrossRef CAS PubMed.
- H. M. Teow, Z. Zhou, M. Najlah, S. R. Yusof, N. J. Abbott and A. D’Emanuele, Int. J. Pharm., 2013, 441, 701–711 CrossRef CAS PubMed.
- W. Shang, X. Zhang, M. Zhang, Z. Fan, Y. Sun, M. Han and L. Fan, Nanoscale, 2014, 6, 5799–5806 RSC.
- H. Yang and S. T. Lopina, J. Biomed. Mater. Res., Part A, 2005, 72, 107–114 CrossRef PubMed.
- M.-X. Zhao and E.-Z. Zeng, Nanoscale Res. Lett., 2015, 10, 171 CrossRef PubMed.
- A. A. H. Abdellatif, M. A. Younis, M. Alsharidah, O. Al Rugaie and H. M. Tawfeek, Int. J. Nanomed., 2022, 17, 1951–1970 CrossRef PubMed.
- B. Hasannejadasl, F. P. Janbaz, E. Choupani, M. Fadaie, M. A. Hamidinejad and D. Ahmadvand, Thrita J. Neu, 2021, 9, e100105 Search PubMed.
- D. Kim, J. M. Yoo, H. Hwang, J. Lee, S. H. Lee, S. P. Yun, M. J. Park, M. Lee, S. Choi, S. H. Kwon, S. Lee, S.-H. Kwon, S. Kim, Y. J. Park, M. Kinoshita, Y.-H. Lee, S. Shin, S. R. Paik, S. J. Lee, S. Lee, B. H. Hong and H. S. Ko, Nat. Nanotechnol., 2018, 13, 812–818 CrossRef CAS PubMed.
- L. Xiao, D. Zhao, W.-H. Chan, M. M. F. Choi and H.-W. Li, Biomaterials, 2010, 31, 91–98 CrossRef CAS PubMed.
- Y. Liu, L.-P. Xu, Q. Wang, B. Yang and X. Zhang, ACS Chem. Neurosci., 2018, 9, 817–823 CrossRef CAS PubMed.
- C. Liu, H. Huang, L. Ma, X. Fang, C. Wang and Y. Yang, R. Soc. Open Sci., 2019, 6, 190271 CrossRef CAS PubMed.
- L. Wang, X. Li, Y. Han, T. Wang, Y. Zhao, A. Ali, N. N. El-Sayed, J. Shi, W. Wang, C. Fan and N. Chen, Sci. China: Chem., 2016, 59, 1486–1491 CrossRef CAS.
- Y. J. Chung, B. I. Lee and C. B. Park, Nanoscale, 2019, 11, 6297–6306 RSC.
- R. Malishev, E. Arad, S. K. Bhunia, S. Shaham-Niv, S. Kolusheva, E. Gazit and R. Jelinek, Chem. Commun., 2018, 54, 7762–7765 RSC.
- Y. Liu, L.-P. Xu, W. Dai, H. Dong, Y. Wen and X. Zhang, Nanoscale, 2015, 7, 19060–19065 RSC.
- X. Han, Z. Jing, W. Wu, B. Zou, Z. Peng, P. Ren, A. Wikramanayake, Z. Lu and R. M. Leblanc, Nanoscale, 2017, 9, 12862–12866 RSC.
- Y. Hu, X. Wang, Y. Niu, K. He and M. Tang, Nanoscale Adv., 2024, 6, 3733–3746 RSC.
- G. Xu, S. Mahajan, I. Roy and K.-T. Yong, Front. Pharmacol., 2013, 4, 140 Search PubMed.
- M. H. Baig, K. Ahmad, M. Saeed, A. M. Alharbi, G. E. Barreto, G. M. Ashraf and I. Choi, Biomed. Pharmacother., 2018, 103, 574–581 CrossRef CAS PubMed.
- A. Biswas, K. Chakraborty, C. Dutta, S. Mukherjee, P. Gayen, S. Jan, A. M. Mallick, D. Bhattacharyya and R. Sinha Roy, ACS Appl. Mater. Interfaces, 2019, 11, 4719–4736 CrossRef CAS PubMed.
- A. M. Mallick, A. Biswas, S. Mishra, S. Jadhav, K. Chakraborty, A. Tripathi, A. Mukherjee and R. S. Roy, Chem. Sci., 2023, 14, 7842–7866 RSC.
- G. P. Morris, I. A. Clark and B. Vissel, Acta Neuropathol. Commun., 2014, 2, 135 Search PubMed.
- M. Y. Hamasaki, H. V. Barbeiro, D. F. Barbeiro, D. M. G. Cunha, M. K. Koike, M. C. C. Machado and F. Pinheiro da Silva, J. Neuroimmunol., 2016, 290, 33–35 CrossRef CAS PubMed.
- K. T. Rajamani, S. Wagner, V. Grinevich and H. Harony-Nicolas, Front. Synaptic Neurosci., 2018, 10, 17 CrossRef PubMed.
- N. De Fruyt, A. J. Yu, C. H. Rankin, I. Beets and Y. L. Chew, Int. J. Biochem. Cell Biol., 2020, 125, 105801 CrossRef CAS PubMed.
- B. J. Marlin and R. C. Froemke, Dev. Neurobiol., 2017, 77, 169–189 CrossRef CAS PubMed.
- F. Muscatelli, M. G. Desarménien, V. Matarazzo and V. Grinevich, Curr. Top. Behav. Neurosci., 2018, 35, 239–268 Search PubMed.
- A. J. Guastella and I. B. Hickie, Biol. Psychiatry, 2016, 79, 234–242 CrossRef CAS PubMed.
- K. J. Parker, O. Oztan, R. A. Libove, R. D. Sumiyoshi, L. P. Jackson, D. S. Karhson, J. E. Summers, K. E. Hinman, K. S. Motonaga, J. M. Phillips, D. S. Carson, J. P. Garner and A. Y. Hardan, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 8119–8124 CrossRef CAS PubMed.
- N. Y. Shin, H. Y. Park, W. H. Jung, J. W. Park, J.-Y. Yun, J. H. Jang, S. N. Kim, H. J. Han, S.-Y. Kim, D.-H. Kang and J. S. Kwon, Neuropsychopharmacology, 2015, 40, 1919–1927 CrossRef CAS PubMed.
- C. Li, X. Wu, S. Liu, Y. Zhao, J. Zhu and K. Liu, Front. Neurosci., 2019, 13, 869 CrossRef PubMed.
- Roles of Neuropeptide Y in Neurodegenerative and Neuroimmune Diseases - PubMed, https://pubmed.ncbi.nlm.nih.gov/31481869/, (accessed April 30, 2025).
- J. Duarte-Neves, L. Pereira de Almeida and C. Cavadas, Neurobiol. Dis., 2016, 95, 210–224 CrossRef CAS PubMed.
- X.-Y. Chen, Y.-F. Du and L. Chen, Front. Mol. Neurosci., 2018, 11, 493 CrossRef CAS PubMed.
- C. M. Clark, R. M. Clark, J. A. Hoyle and T. C. Dickson, J. Neurochem., 2021, 156, 273–289 CrossRef CAS PubMed.
- A. Bose, D. Roy Burman, B. Sikdar and P. Patra, IET Nanobiotechnol., 2021, 15, 19–27 CrossRef PubMed.
- N. A. N. Hanafy, M. El-Kemary and S. Leporatti, Cancers, 2018, 10, 238 CrossRef PubMed.
- L. Carniglia, D. Ramírez, D. Durand, J. Saba, J. Turati, C. Caruso, T. N. Scimonelli and M. Lasaga, Mediators Inflammation, 2017, 2017, 5048616 Search PubMed.
- R. Yang, X. Jiang, R. Ji, L. Meng, F. Liu, X. Chen and Y. Xin, Cell. Mol. Biol. Lett., 2015, 20, 265–278 CAS.
- N. Cabezas-Llobet, L. Vidal-Sancho, M. Masana, A. Fournier, J. Alberch, D. Vaudry and X. Xifró, Mol. Neurobiol., 2018, 55, 8263–8277 CrossRef CAS PubMed.
- S. Nozohouri, S. Jayaraman, B. Vaidya, V. Karamyan and T. Abbruscato, FASEB J., 2019, 33, lb82–lb82 Search PubMed.
- S. Thakker-Varia, J. Behnke, D. Doobin, V. Dalal, K. Thakkar, F. Khadim, E. Wilson, A. Palmieri, H. Antila, T. Rantamaki and J. Alder, Stem Cell Res., 2014, 12, 762–777 CrossRef CAS PubMed.
- C. Li, M. Li, H. Yu, X. Shen, J. Wang, X. Sun, Q. Wang and C. Wang, ACS Chem. Neurosci., 2017, 8, 2005–2018 CrossRef CAS PubMed.
- H. Duc Nguyen, B. Pal Yu, N. H. M. Hoang, W. H. Jo, H. Young Chung and M.-S. Kim, Neuroendocrinology, 2022, 112, 427–445 CrossRef CAS PubMed.
- H. Duc Nguyen, B. Pal Yu, N. H. M. Hoang, W. H. Jo, H. Young Chung and M.-S. Kim, Neuroendocrinology, 2022, 112, 427–445 CrossRef CAS PubMed.
- A. Biswas, M. Maloverjan, K. Padari, A. Abroi, M. Rätsep, S. K. T. S. Wärmländer, J. Jarvet, A. Gräslund, V. Kisand, R. Lõhmus and M. Pooga, Pharmaceutics, 2023, 15, 396 CrossRef CAS PubMed.
- Y. Zhang, P. Guo, Z. Ma, P. Lu, D. Kebebe and Z. Liu, J. Nanobiotechnol., 2021, 19, 255 CrossRef CAS PubMed.
- R. Ta, M. Suchy, J. H. K. Tam, A. X. Li, F. S. Martinez-Santiesteban, T. J. Scholl, R. H. E. Hudson, R. Bartha and S. H. Pasternak, Contrast Media Mol. Imaging, 2013, 8, 127–139 CrossRef CAS PubMed.
- G. Di Fede, M. Catania, E. Maderna, M. Morbin, F. Moda, L. Colombo, A. Rossi, A. Cagnotto, T. Virgilio, L. Palamara, M. Ruggerone, G. Giaccone, I. Campagnani, M. Costanza, R. Pedotti, M. Salvalaglio, M. Salmona and F. Tagliavini, Sci. Rep., 2016, 6, 20949 CrossRef CAS PubMed.
- Y. Feng, X. Li, D. Ji, J. Tian, Q. Peng, Y. Shen and Y. Xiao, Int. J. Biol. Macromol., 2023, 230, 123125 CrossRef CAS PubMed.
- M. Pirhaghi, F. Mamashli, F. Moosavi-Movahedi, P. Arghavani, A. Amiri, B. Davaeil, M. Mohammad-Zaheri, Z. Mousavi-Jarrahi, D. Sharma, Ü. Langel, D. E. Otzen and A. A. Saboury, Mol. Pharm., 2024, 21, 2097–2117 CrossRef CAS PubMed.
- I. Rusiecka, J. Ruczyński, A. Kozłowska, E. Backtrog, P. Mucha, I. Kocić and P. Rekowski, Bioconjugate Chem., 2019, 30, 760–774 CrossRef CAS PubMed.
- J. H. Cararo, E. L. Streck, P. F. Schuck and G. D. C. Ferreira, Aging Dis., 2015, 6, 369–379 CrossRef PubMed.
- P. O. Bauer and N. Nukina, J. Neurochem., 2009, 110, 1737–1765 CrossRef CAS PubMed.
- J. Fahrenkrug, N. Popovic, B. Georg, P. Brundin and J. Hannibal, J. Mol. Neurosci., 2007, 31, 139–148 CrossRef CAS PubMed.
- A. Biswas, K. Periyasamy, M. Maloverjan, L. Porosk, G. Arya, S. Mehta, H. Andla, R. Raid, V. Kisand, M. Rätsep, J. Wengel, A. Rebane and M. Pooga, Biomed. Pharmacother., 2025, 184, 117872 Search PubMed.
- D. R. Perinelli, M. Cespi, N. Lorusso, G. F. Palmieri, G. Bonacucina and P. Blasi, Langmuir, 2020, 36, 5745–5753 CrossRef CAS PubMed.
- Z. Ahmad, A. Shah, M. Siddiq and H.-B. Kraatz, RSC Adv., 2014, 4, 17028–17038 RSC.
- M. Ghezzi, S. Pescina, C. Padula, P. Santi, E. Del Favero, L. Cantù and S. Nicoli, J. Controlled Release, 2021, 332, 312–336 CrossRef CAS PubMed.
- P. Chithra, D. Bhatia and R. Solanki, Biomater. Adv., 2025, 170, 214221 CrossRef CAS PubMed.
- A. Kumar, S. K. Shahvej, P. Yadav, U. Modi, A. K. Yadav, R. Solanki and D. Bhatia, Pharmaceutics, 2025, 17, 379 CrossRef PubMed.
- L. I. Atanase, Polymers, 2021, 13, 477 CrossRef CAS PubMed.
- P. Kaur, A. Rajput, D. Singh, G. Singh, A. Mehra, S. Kaur, N. Bedi and S. Arora, Curr. Drug Delivery, 2024, 21, 65–79 CrossRef CAS PubMed.
- S. Kumari, S. M. Ahsan, J. M. Kumar, A. K. Kondapi and N. M. Rao, Sci. Rep., 2017, 7, 6602 CrossRef PubMed.
- G. Tripodo, T. Chlapanidas, S. Perteghella, B. Vigani, D. Mandracchia, A. Trapani, M. Galuzzi, M. C. Tosca, B. Antonioli, P. Gaetani, M. Marazzi and M. L. Torre, Colloids Surf., B, 2015, 125, 300–308 CrossRef CAS PubMed.
- C. Zhan, B. Li, L. Hu, X. Wei, L. Feng, W. Fu and W. Lu, Angew. Chem., Int. Ed., 2011, 50, 5482–5485 CrossRef CAS PubMed.
- P. Alfonso-Triguero, J. Lorenzo, A. P. Candiota, C. Arús, D. Ruiz-Molina and F. Novio, Nanomaterials, 2023, 13, 1619 CrossRef CAS PubMed.
- D. Wu, Q. Chen, X. Chen, F. Han, Z. Chen and Y. Wang, Signal Transduction Targeted Ther., 2023, 8, 217 CrossRef PubMed.
- T. Lei, Z. Yang, C. Jiang, X. Wang, W. Yang, X. Yang, R. Xie, F. Tong, X. Xia, Q. Huang, Y. Du, Y. Huang and H. Gao, ACS Nano, 2024, 18, 3234–3250 CrossRef CAS PubMed.
- X. Xia, Y. Wei, Q. Huang, Y. Zhou, X. Wang, Y. Shi, X. Yang, W. Yang, Y. Zhang, T. Lei, Y. Huang, H. Li, M. Qin and H. Gao, Acta Pharm. Sin. B, 2024, 14, 5464–5478 CrossRef CAS PubMed.
- T. Lei, Z. Yang, X. Xia, Y. Chen, X. Yang, R. Xie, F. Tong, X. Wang and H. Gao, Acta Pharm. Sin. B, 2021, 11, 4032–4044 CrossRef CAS PubMed.
- X. Wang, S. Chen, X. Xia, Y. Du, Y. Wei, W. Yang, Y. Zhang, Y. Song, T. Lei, Q. Huang and H. Gao, Adv. Mater., 2025, 37, 2411061 CrossRef CAS PubMed.
- S. Niu, L.-K. Zhang, L. Zhang, S. Zhuang, X. Zhan, W.-Y. Chen, S. Du, L. Yin, R. You, C.-H. Li and Y.-Q. Guan, Theranostics, 2017, 7, 344–356 CrossRef CAS PubMed.
- L. Xuan, Z. Ju, M. Skonieczna, P.-K. Zhou and R. Huang, MedComm, 2023, 4, e327 CrossRef CAS PubMed.
- A. Dhawan and V. Sharma, Anal. Bioanal. Chem., 2010, 398, 589–605 CrossRef CAS PubMed.
- L. Calderón-Garcidueñas and A. Ayala, Environ. Sci. Technol., 2022, 56, 6847–6856 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |


