 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Touch transfer of microorganisms on polymer surfaces†
Meng-Chen
Chiang
 a,
Carla
Steppan
b,
Ted W.
Deisenroth
c,
Rupert
Konradi
a,
Carla
Steppan
b,
Ted W.
Deisenroth
c,
Rupert
Konradi
 c,
Todd
Emrick
c,
Todd
Emrick
 b and
Jessica D.
Schiffman
b and
Jessica D.
Schiffman
 *ad
*ad
aDepartment of Chemical Engineering, University of Massachusetts Amherst, Amherst, Massachusetts 01003-9303, USA. E-mail: schiffman@umass.edu
bDepartment of Polymer Science and Engineering, University of Massachusetts Amherst, Amherst, Massachusetts 01003-9303, USA
cBASF SE, Group Research, Carl Bosch Str 38, 67056 Ludwigshafen, Germany
dMaterials Science and Engineering Graduate Program, University of Massachusetts Amherst, Amherst, Massachusetts 01003-9303, USA
First published on 3rd June 2025
Abstract
The transfer of bacteria between dry, high-touch surfaces in healthcare settings is a key contributor to hospital-acquired infections (HAIs). In this study, we systematically investigated the relationship between the chemistry of polymer surfaces and the corresponding touch-transfer of microorganisms. The polymers investigated included polymer zwitterions, PEGylated polymers, poly(tetrafluoroethylene) (PTFE), and polystyrene (PS). Water contact angle measurements confirmed the breadth of surface energies of these polymers, ranging from <25° (polymer zwitterion) to >100° (PTFE). A touch transfer model was developed to study bacteria transfer by “finger touches” on an agar plate. The amount of Escherichia coli (E. coli) or Staphylococcus aureus (S. aureus) transferred after each touch was quantified via plate counting. For E. coli, the transfer rate was ∼29% on zwitterionic copolymer surfaces, whereas PS exhibited a much higher rate of ∼67%. For S. aureus, the transfer rate was ∼17% for the polymer zwitterion and ∼100% for PS. The low transfer rates from the polymer zwitterion were comparable to those of PTFE (∼19% for E. coli and ∼17% for S. aureus). These findings demonstrate the role of polymer composition and surface chemistry in bacterial transfer and provide insights for designing materials that effectively minimize microbial transmission in healthcare environments.
Introduction
Environmental hygiene, i.e., removing and/or killing potentially infectious microorganisms on medical equipment between uses, represents a public health concern.1,2 Annually in the United States, 1.7 million patients contract hospital-acquired infections (HAIs)3,4 from bacterial sources. Studies suggest that 20–40% of these bacterial diseases are transmitted by direct contact between the hands of healthcare workers or hospital visitors and “high-touch” surfaces, such as doorknobs and tables.1,4–7 As a result, there is a growing need to understand the potential for polymer coatings to mitigate bacterial transfer.Effective approaches to controlling the interactions of microorganisms with surfaces involve their design to be antimicrobial and/or antifouling. Antimicrobial surfaces typically use either biocide release or contact-killing methods for disinfection.8–10 Biocides, including metals (copper and silver), organics (antibiotics, chitosan, and cationic polymers), and biologics (enzymes, antimicrobial peptides, and bacteriophages), all effectively kill microorganisms.9,11 However, concerns associated with biocide use include the development of bacterial resistance and leaching.9,12–14 Contact-killing methods involve immobilizing biocides on surfaces that, in turn, disinfect microorganisms by their direct contact; however, the accumulation of microorganisms over time reduces the effectiveness of these surfaces.9 Therefore, antifouling surfaces that delay microorganism attachment in the absence of biocides are increasingly viewed as a more ideal and potentially sustainable approach.
Antifouling surfaces can be designed to inhibit microorganism attachment by tailoring surface physicochemical properties, such as charge density, roughness, stiffness, topography, thickness, and wettability.15–18 Surface chemistry influences the mechanism of attachment. For example, hydrophilic surfaces preferentially adsorb water, in turn preventing microorganism attachment or “fouling”. Due to their ability to reduce non-specific adsorption of biofoulants, polymer zwitterions represent a notable class of an antifouling polymers.19–21 Polymer zwitterions are characterized by their inner-salt composition, which results in overall charge-neutrality.22,23 As a result, polymer zwitterion coatings are hydrophilic with a tightly bound hydration layer that reduces biofoulant adhesion.20,24 While many studies have assayed the antifouling or repellent nature of a polymer coating in aqueous environments,16,25 few studies have quantified whether bacteria on a polymer surface may be lifted off of those surfaces by touching.26–29 For example, Zhao, et al.6 described a model to study factors influencing microbial transfer between a finger and metal, noting that touch force, microbial size, inoculation volume, repeated touches, and rubbing all affect transfer rates. Similarly, Behzadinasab, et al.30 examined the likelihood of SARS-CoV-2 virus transfer to the skin through contact with porous materials. However, systematic studies that explore the influence of polymer chemistry under ambient conditions are lacking. Specifically, we were interested if PEG- and polyzwitterion-modified surfaces behave differently under room temperature and dry conditions.
Here, for the first time, we describe an assay to systematically investigate the touch-transfer of microorganisms from polymer zwitterions and PEG-based coatings under dry conditions, as illustrated in Fig. 1. Most existing touch-transfer studies focus on bacterial viability after contact with known antimicrobial surfaces, such as those decorated with metal nanoparticles.31–35 Three reports have examined the transfer of bacteria or viruses between surfaces as a function of their porosity or mechanical contact.6,28,30 One report describes bacteria transfer from antifouling surfaces;29 we are motivated to understand the relationship between surface composition and touch-transfer performance. We selected poly(tetrafluoroethylene) (PTFE) and commercial poly(styrene) (PS) as low surface energy samples. In addition, we modified the PSvia benzophenone-induced grafting with hydrophilic polymers, including poly(sulfobetaine methacrylate) (PSBMA) and poly(ethylene glycol methacrylate) (PEGMA). The touch transfer of flagellated Escherichia coli (E. coli) and non-motile Staphylococcus aureus (S. aureus) was evaluated over nine touches.
Experimental
Materials
D-(+)-Glucose, calcium chloride (anhydrous), M9 minimum salt (M9 media), chloramphenicol (Bioreagent grade), tryptic soy broth (TSB media), Luria–Bertani broth (LB media), 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid (CPPA), 4,4′-azobis(4-cyanovaleryic acid) (ACVA), sulfobetaine methacrylate (SBMA), methyl methacrylate (MMA), diiodomethane, glycerol, [2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)-ammonium hydroxide (DMAPS), poly(ethylene glycol) methyl ether methacrylate (average Mn 500), sodium chloride (NaCl) (99%), were purchased from Sigma-Aldrich (St Louis, MO). Water (HPLC), anhydrous magnesium sulfate (MgSO4), and methanol (MeOH) were purchased from Fisher Scientific (Fair Lawn, NJ). 2,2,2-Trifluoroethanol (TFE) was purchased from Oakwood Chemical. Deuterated solvents (deuterium oxide, D2O; and 2,2,2-Trifluoroethanol-d3, TFE-d3) were purchased from Cambridge Isotope Laboratories. Spectra/Por7 dialysis membranes (3.5 kDa MWCO, pretreated RC tubing) were purchased from VWR. Spectinomycin dihydrochloride pentahydrate (USP grade) was purchased from Gold Biotechnology® (Olivette, MO). The Sylgard 184 silicone elastomer set (including base and curing agent) was purchased from Ellsworth Adhesives (Germantown, WI).Polymer synthesis and characterization
PSBMA, PSBMA-co-PMMA and PEGMA, shown in Fig. 1(b), were synthesized by reversible-addition fragmentation chain-transfer (RAFT) polymerization according to reported procedures.36 In general, monomers (including 3 mol% of a benzophenone-substituted methacylate,37 necessary for grafting-to PS) were dissolved in TFE with 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid (CPPA) as the chain-transfer agent (CTA) and 4,4′-azobis(4-cyanovaleryic acid) (ACVA) as the initiator. The initial monomer concentration was 1.0 M, and a monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CTA
CTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) initiator ratio of 50
initiator ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 was employed. Purification was accomplished either by repeated precipitation into cold MeOH or by dialysis against water (3.5 kDa MWCO membrane). The polymer products were characterized by proton nuclear magnetic resonance (1H NMR, Bruker Avance-500) in TFE-d3 or D2O. Molecular weights were estimated using gel permeation chromatography (GPC, Agilent 1200 series, equipped with three PSS PFG analytical linear M columns, 8 Å ∼ 300 mm, particle size 7 μm) performed on filtered (0.45 μm PTFE membrane) solutions eluting in 20 mM sodium trifluoroacetate in TFE with MeOH as the flow marker against PMMA standards.
0.2 was employed. Purification was accomplished either by repeated precipitation into cold MeOH or by dialysis against water (3.5 kDa MWCO membrane). The polymer products were characterized by proton nuclear magnetic resonance (1H NMR, Bruker Avance-500) in TFE-d3 or D2O. Molecular weights were estimated using gel permeation chromatography (GPC, Agilent 1200 series, equipped with three PSS PFG analytical linear M columns, 8 Å ∼ 300 mm, particle size 7 μm) performed on filtered (0.45 μm PTFE membrane) solutions eluting in 20 mM sodium trifluoroacetate in TFE with MeOH as the flow marker against PMMA standards.
Preparation of polymer coatings
Polystyrene (PS, ID: 8734K38) and polytetrafluoroethylene (PTFE, ID: 9266K81) sheets, each approximately 0.8 mm thick, were purchased from McMaster (Princeton, NJ). PS and PTFE sheets were cut into squares (22 mm × 22 mm) using a cutting plotter (Graphtec, Irvine, CA). Immediately prior to use, substrates were sonicated in DI water for 30 min, rinsed in methanol for 30 s, then dried under direct N2(g) flow; the clean substrates were stored in a glass Petri dish. For polymer-coated samples, cleaned PS substrates were spin-coated (Specialty Coating Systems G3P-8) with a 20 mg mL−1 TFE solution of PSBMA, PSBMA-co-PMMA or PEGMA and filtered through a 0.22 μm PTFE membrane at 2000 rpm for 60 s. After spin-coating, polymer films were grafted to PS substrates by UV irradiation at 365 nm (UVP CL-1000M crosslinker UV box, 5 mW m−2 power, equipped with 8 W, UVP 365 nm light tubes) at a distance of ∼8 cm from the light source for 15 min. All polymer-grafted PS substrates were stored in the dark prior to touch-transfer experiments. Non-coated substrates were used immediately after cleaning.Characterization of polymer coatings
The surface behavior of the polymer substrates was analyzed using contact angle goniometry (Biolin Scientific Attension Theta Optical Tensiometer). Static water contact angles were measured using a 2.5 μL droplet of water placed on the substrate. Contact angle was recorded over 60 s at 33.3 frames per second. Five separate measurements were performed on each substrate, probing different portions of the surface with each repetition. The static water contact angle was tested for each batch of modified PS substrates before and after 30 min immersion in DI water, allowing the substrates to dry under N2(g) flow after submersion. The surface energy was calculated for all substrates using Young's equation (eqn (1)) and the Owens, Wendt, Rabel and Kaelble (OWRK) model (eqn (2)) and static contact angle values obtained for water, diiodomethane, and glycerol:38γSV = γSL + γLV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (1) |
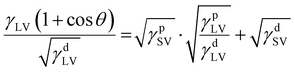 | (2) |
Preparation of microorganisms
Escherichia coli K12 MG1655 (E. coli) was purchased from DSMZ (Leibniz-Institut, Germany). Staphylococcus aureus SH1000 (S. aureus SH1000) was generously donated by Dr. Alexander Horswill (University of Colorado Anschutz Medical Campus). E. coli or S. aureus SH1000 was cultured in 5 mL of LB or TSB media (autoclaved at 120 °C for 15 min), respectively, with 50 μg mL−1 spectinomycin or 10 μg mL−1 chloramphenicol on a shake plate (250 rpm) for 16 h at 37 °C. The bacterial cultures were serially diluted to 104 cells per mL for use in the touch-transfer assay.Touch-transfer assay
As shown in Fig. 1 and S1,† a “finger” stamp was used to transfer the microbes from a contaminated surface across nine touches. The finger stamp was made in-house to mimic the stiffness of finger skin (∼60 kPa).39 Polydimethylsiloxane (PDMS) gels were made from a Sylgard 184 elastomer kit by mixing the base and curing agent at a weight ratio of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, according to a published protocol.18 The mixture was stirred vigorously for 15 min at room temperature, then poured into a sterile Petri dish (100 mm × 100 mm, Thermo Fisher Scientific), where air bubbles were removed by degassing. The PDMS solution was cured on a hot plate at 60 °C for 16 h, then circular coupons were cut (thickness = 2 mm, diameter = 19 mm) using a Spearhead 130 Power Punch MAXiset (Fluid Sealing Services, Wausau, WI). The PDMS gels were washed with 70% ethanol and dried under a stream of N2(g) prior to use. A 100 μL bacterial suspension (104 cells per mL) of either E. coli or S. aureus was deposited on top of the sterilized PDMS stamp. After 1 h of air-drying in a biosafety cabinet, residual media was removed carefully using a kimwipe. The contaminated PDMS stamp was then pressed onto the test substrate with a constant force of 1.96 N with a 200 g calibration weight (Hardware Factory Store, Oklahoma City, OK) for 30 s.
1, according to a published protocol.18 The mixture was stirred vigorously for 15 min at room temperature, then poured into a sterile Petri dish (100 mm × 100 mm, Thermo Fisher Scientific), where air bubbles were removed by degassing. The PDMS solution was cured on a hot plate at 60 °C for 16 h, then circular coupons were cut (thickness = 2 mm, diameter = 19 mm) using a Spearhead 130 Power Punch MAXiset (Fluid Sealing Services, Wausau, WI). The PDMS gels were washed with 70% ethanol and dried under a stream of N2(g) prior to use. A 100 μL bacterial suspension (104 cells per mL) of either E. coli or S. aureus was deposited on top of the sterilized PDMS stamp. After 1 h of air-drying in a biosafety cabinet, residual media was removed carefully using a kimwipe. The contaminated PDMS stamp was then pressed onto the test substrate with a constant force of 1.96 N with a 200 g calibration weight (Hardware Factory Store, Oklahoma City, OK) for 30 s.
Agar plates were prepared for the touch-transfer assay in square, sterile petri dishes (100 mm × 100 mm, Thermo Fisher Scientific). LB agar (10 g L−1 tryptone, 10 g L−1 NaCl, 5 g L−1 yeast extract, 15 g L−1 agar) or TSB agar (30 g L−1 tryptic soy broth; 15 g L−1 agar) solutions were prepared and autoclaved at 120 °C for 15 min. The solutions were then poured into the petri dishes and cooled to room temperature before use. The contaminated polymer test substrate was put in contact with the agar plate nine consecutive times using a constant force of 1.96 N (via a 200 g calibration weight) for 30 s per touch before being incubated for 24 h at 37 °C. After the incubation period, the resultant bacteria colonies were counted manually using ImageJ. Three replicates for each polymer surface were tested. The transfer probability (P) was calculated based on eqn (3):
Transfer probability,
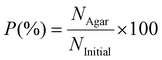 | (3) |
Statistical analysis
The significant difference between samples with different bacteria was determined using the two-tailed, unpaired Student t-test function in Microsoft Excel. The bacterial colony formation unit was reported as the mean ± standard error of three replicates. Statistical significance was set to p < 0.05.Results and discussion
Characteristics of polymer coatings
The relationship between polymer selection and microorganism transfer under dry conditions was tested on several polymer compositions. Commercially available PTFE, known for its ability to resist microbial attachment, and PS, one of the most commonly used polymers in plastics, were selected for their hydrophobicity and, in the case of PS, amenability to surface-grafting.40,41 To access hydrophilic surfaces, polymers containing a small percentage of benzophenone-containing comonomer were utilized to enable photo-induced grafting to PS. The polymeric zwitterions PSBMA and PSBMA-co-PMMA gave access to either a fully hydrophilic, zwitterionic surface (PSBMA) or a less hydrophilic, copolymer surface (PSBMA-co-PMMA). In addition, PEGMA grafting gave access to bacterial transfer under dry conditions on a hydrophilic polymer with no charge-containing subunits. Polymer structures were confirmed by 1H NMR in TFE-d3 or D2O, as shown in Fig. S2–4,† and the purified polymers were isolated as solid, light pink powders with estimated number-average molecular weight (Mn) values in the 15–80 kDa range and polydispersity index (PDI) values of 1.1–1.9 range, as shown in Table 1.| Polymer |
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y y |
M n (kDa) | PDI |
|---|---|---|---|
| PSBMA | — | 14.8 | 1.1 |
| PSBMA-co-PMMA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
21.3 | 1.2 |
| PEGMA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
89.1 | 1.9 |
Fig. 2 illustrates the range of hydrophilicity of the chosen substrates via data obtained from static water contact angle experiments. PTFE and PS demonstrated water contact angles of 112° and 95°, respectively, reflecting their hydrophobicity.42,43 In contrast, the PEGMA-modified PS substrate was much more hydrophilic, with a water contact angle of 41°. The PSBMA-coated substrates were also hydrophilic: PSBMA-co-PMMA had a water contact angle of 71° and for PSBMA the water contact angle was 24°. UV-induced surface grafting44 was found to produce robust, stable surface coatings, as evidenced by the consistent water contact angle before and after submersion in water for 12 h. In addition to water contact angle, surface energies were calculated from static contact angle data using additional probe liquids: glycerol and diiodomethane, as shown in Table 2.
 | ||
| Fig. 2 The static water contact angle values are shown in the bottom right corner for (a) PTFE, (b) PS, (c) PSBMA-co-MMA, (d) PEGMA, and (e) PSBMA. | ||
| Polymer | Water CA (°) | Glycerol CA (°) | Diiodomethane CA (°) | Surface energy (mN m−1) |
|---|---|---|---|---|
| PS | 94.7 ± 3.7 | 74.7 ± 1.5 | 45.8 ± 1.0 | 37.8 |
| PSBMA | 23.8 ± 2.7 | 28.4 ± 1.6 | 30.8 ± 0.8 | 62.5 |
| PEGMA | 40.9 ± 3.5 | 66.8 ± 0.2 | 39.7 ± 1.0 | 55.6 |
To confirm that polymer coatings lead to smoother substrates, preliminary surface roughness measurements were acquired using atomic force microscopy (AFM). Fig. S5† presents the topography, while Table S1† provides the surface roughness parameters, including root-mean-square roughness (Rq), average roughness (Ra), minimum roughness (Rmin), maximum roughness (Rmax), skewness (Rskw), and kurtosis (Rkur). A brief method is also provided. The Rq for PTFE, PS, and PSBMA-co-PMMA were ∼76, 176, and 3 nm, respectively. While studies have indicated that surface roughness can influence bacterial attachment,15,45 these representative measurements suggest that spin-coating ensures that the polymer-coated substrates have a low surface roughness (<10 nm). The impact of surface roughness on bacterial transfer would likely be minimal, as surface roughness is much smaller than the micron size bacteria.
Touch-transfer experiment using E. coli and S. aureus
The touch transfer assay was employed to investigate the dry contact transfer of bacteria from each polymer surface. E. coli and S. aureus were selected for their prominent roles in healthcare-associated infections (HAIs) and their distinct characteristics, such as differences in shape, sensing mechanisms, and membrane composition.3,46 The study was designed to explore how polymer composition and surface energy influence bacterial transfer under dry conditions.Key to assay development was to evaluate the number of bacterial colonies remaining after nine touches, as shown in Fig. S6(a).† Initial tests using a bacterial inoculation of 107 CFU mL−1 produced colony counts that were too high for accurate quantification, whereas an inoculation of 102 CFU mL−1 led to no detectable colonies after the second touch. After optimization, a concentration of 104 CFU mL−1 was chosen, and we investigated if the applied mass had an impact on transfer. Three different masses (50 g, 100 g, and 200 g) were tested (Fig. S6(b)†) and no difference in the bacterial transfer were found with these different weights. For this reason, the 200 g mass was selected to approximate the weight of holding a mobile phone.47
Moving forward with the optimized starting inoculation concentration and consistent applied weight, Fig. 3(a–e) shows the number of E. coli CFU transferred under dry conditions after nine sequential touches. Fig. S7† provides digital images of representative E. coli and S. aureus colonies that grew after a 24 h incubation period post-touch assay. Although PSBMA-co-PMMA and PEGMA exhibit an increase in E. coli transferred from the first to the second touch, the overall trend demonstrates a steady decrease in bacterial transfer with subsequent touches, reaching nearly zero CFUs by the ninth touch. The data from Fig. 3(a–e) has also been transformed into a heat map (Fig. 4(a–e)) as an alternative way to visualize the decrease in average colony count. When comparing the first touch across polymer substrates (Fig. S8(a)†), PSBMA-co-PMMA resulted in less E. coli transfer than seen for PS. This prompted us to investigate which touch number gave a statistically significant reduction in E. coli CFUs compared to the first touch. There was no statistical significance between the first touch and the subsequent touches on the PTFE surface. However, we found that it required seven touches for PS, six for PSBMA-co-PMMA, six for PEGMA, and seven for PSBMA to show a significant reduction in bacterial transfer. We summed the CFU across all touches to estimate the total number of bacteria transferred from the PDMS finger to the test substrates (Fig. 3(f)). We note that the number of E. coli that transferred by the ninth touch was nearly zero from the test substrates. Therefore, we make the assumption that all bacteria have transferred. PTFE, PS, PSBMA-co-PMMA, PEGMA, and PSBMA transferred a total of 195, 671, 293, 521, and 401 CFUs, respectively. PS the most, whereas PTFE transferred the least, which aligns with expectations because PTFE is known for its low biofouling properties due to its low-friction surface, which minimizes bacterial transfer.48 In contrast, PS had the highest transfer rate, likely due to hydrophobic interactions between bacteria and the PS surface, which resulted in a higher initial bacterial load transferred from the PDMS finger.49 While PEGMA (521 CFUs) and PSBMA (401 CFUs) transferred fewer E. coli than PS (671 CFUs), the differences were not statistically significant. Interestingly, PSBMA-co-PMMA exhibited the least bacterial transfer among the polymers studied. Both PTFE and PSBMA-co-PMMA transferred significantly fewer E. coli than PEGMA, with no significant difference between PTFE and PSBMA-co-PMMA.
 | ||
| Fig. 3 E. coli transfer over nine sequential touches on (a) PTFE, (b) PS, (c) PSBMA-co-PMMA, (d) PEGMA, and (e) PSBMA. Figure (f) provides the total number of E. coli transferred. Error bars represent standard error and an asterisk (*) denotes at least 95% confidence, whereas “n.s.” indicates no statistical significance. Images of representative agar plates are shown in Fig. S7(a).† | ||
 | ||
| Fig. 4 Heat maps of the average (a–e) E. coli and (f–j) S. aureus CFUs that transferred on (a) PTFE, (b) PS, (c) PSBMA-co-PMMA, (d) PEGMA, and (e) PSBMA. Data from Fig. 3 and 5. | ||
Fig. 5(a–e) present the transfer of S. aureus over sequential touches, which showed trends similar to E. coli—the number of transferred bacteria decreased with successive touches. The data from Fig. 5 has also been transformed into a heat map (Fig. 4(f–j)) as an alternative way to visualize the decrease in average colony counts. When comparing the first touch across materials, PS transferred statistically more S. aureus than PEGMA, as seen in Fig. S8(b).† As for E. coli, the number of touches required to show significantly fewer S. aureus that transferred compared to the first touch varied with polymer composition: PTFE showed a significant reduction by the third touch. PEGMA and PSBMA showed a statistical reduction of bacteria at the third and fourth touches. PS and PSBMA-co-PMMA exhibited a significant reduction by the ninth and eighth touches, respectively. To assess overall transferability, we summed the CFUs for S. aureus across all touches. PTFE, PS, PSBMA-co-MMA, PEGMA, and PSBMA resulted in the transfer of 171, 1042, 625, 239, and 175 CFUs, respectively. Statistically, PTFE transferred significantly fewer S. aureus than PS, PSBMA-co-MMA, and PEGMA, while PS transferred significantly more than PEGMA and PSBMA. Notably, PSBMA exhibited a comparable performance to PTFE in terms of S. aureus transfer. Interestingly, the number of bacteria transferred during the first touch predicted the overall transfer trend, as first-touch data mirrored the total bacterial transfer patterns (Fig. 3(f) and 5(f) compared to Fig. S8†). For example, E. coli colonies were transferred at a significantly lower level from PSBMA-co-PMMA than from PEGMA during the first touch, consistent with the total transfer data. Similarly, S. aureus colonies exhibited a lower transfer level from PSBMA than from PS in the first touch, which also aligned with the overall trend. Although bacterial colonies of both types were not at the lowest level from PTFE on the first touch, the number of colonies transferred reached nearly zero by the fourth touch, whereas other materials reached near-zero transfer at the eighth or ninth touch. Therefore, this resulted in the total bacterial transfer being the lowest for PTFE in both cases.
 | ||
| Fig. 5 S. aureus transfer over nine sequential touches on (a) PTFE, (b) PS, (c) PSBMA-co-PMMA, (d) PEGMA, and (e) PSBMA. (f) provides the total number of S. aureus transferred. Error bars represent standard error and an asterisk (*) denotes at least 95% confidence. Representative agar plates are shown in Fig. S7(b).† | ||
The transfer probability of bacteria from PDMS to test substrates is expressed by eqn (3), which divides the total number of transferred CFUs by 1000, representing the initial number of bacteria on the “finger”, and multiplying the result by 100% to obtain the percentage. This calculation gives the transfer probability, as seen in Fig. 6. This analysis was modeled after a study by Behzadinasab et al., which examined viral transfer to skin from porous solids.30Fig. 6(a) shows the results of this transfer probability for E. coli was ∼19.5% (PTFE), ∼67.0% (PS), ∼29.3% (PSBMA-co-PMMA), ∼52.1% (PEGMA), and ∼40.1% (PSBMA). In comparison, Fig. 6(b) shows the transfer probability of S. aureus to be ∼17.1%, ∼100%, ∼62.5%, ∼23.9%, and ∼17.5% for PTFE, PS, PSBMA-co-PMMA, PEGMA, and PSBMA, respectively. We note that an exciting outcome of the transfer rate calculation is the realization that the polymer zwitterion has performed comparably to PTFE, indicating that polymer zwitterions could potentially be used as alternatives to PTFE in some applications, for example, when the use of PTFE might cause the release of per- and polyfluoroalkyl substances (PFAS) of concern to human health.50 In addition to the low transfer rate, high-touch surfaces in healthcare settings could also be coated with zwitterionic polymers to reduce surface contamination and to add to the overall hygiene concept in limiting transmission by touching in between regular sterilization protocols.51,52
Compared to PS, the PSBMA-co-PMMA, PEGMA, and PSBMA surfaces exhibited lower transfer probabilities. Specifically, PSBMA-co-PMMA reduced E. coli and S. aureus transfer by 56% and 40%, respectively, while PEGMA reduced transfer by 22% and 77%. PSBMA reduced E. coli and S. aureus transfer by 40% and 83%, respectively, demonstrating that the polymer-coated substrates are more effective at preventing the transfer of S. aureus than E. coli. This difference potentially stems from variations in the bacterial cell envelope. Although both bacteria have hydrophobic membranes, S. aureus is generally more hydrophilic than E. coli, making it more likely to transfer on certain surfaces due to differences in hydrophilicity between the bacterial and substrate surfaces.53
Our findings indicate that PSBMA-co-PMMA is more effective at preventing E. coli transfer, while PSBMA better reduces S. aureus transfer. Since E. coli has a relatively hydrophobic outer membrane, polymers like PSBMA-co-PMMA, in which MMA comonomer units affect zwitterionic hydrophobicity, could affect its adhesion and transferability. Conversely, S. aureus has a thicker peptidoglycan layer and different surface protein compositions compared to E. coli, which might interact more with hydrophilic surfaces like PSBMA.46 Despite the varying performance of the polymer films, in general we find that increasing the hydrophilicity by grafting onto PS reduced bacteria overall transferability.
Conclusions
In this work, we evaluated the bacterial transfer on five different dry polymer surfaces. Use of commercial PTFE and PS, as well as PS modified with PSBMA-co-PMMA, PSBMA, and PEGMA, gave access to a range of surface compositions and energies (37.8–62.5 mN m−1). Over nine consecutive touches, a decreasing trend in countable bacterial colonies was observed. By summing the colonies at each touch, we estimated the likelihood of bacterial transfer from the initial contaminated surface. The use of PTFE resulted in the least amount of transfer of both bacterial types, while PS showed the greatest transfer probability. Among the coated substrates, PSBMA-co-PMMA showed the lowest E. coli transfer, with approximately 50% less than PS, and PSBMA demonstrated the lowest S. aureus transfer, reducing transfer by ∼80% compared to PS. These findings showed that zwitterionic polymers (PSBMA and PSBMA-co-PMMA) exhibit excellent performance on dry surfaces. We suggest that these polymer zwitterion surfaces hold potential to be used as antifouling coatings that offer broader versatility when PTFE or contact-killing surfaces, such as copper, are not suitable. This study opens exciting possibilities for future research, using optimized protocols to further examine commonly encountered bacterial transfer on dry surfaces that are relevant to hospitals and other settings.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the support of BASF SE through NORA (North American Research Alliance ).References
- V. C. C. Cheng, P. H. Chau, W. M. Lee, S. K. Y. Ho, D. W. Y. Lee, S. Y. C. So, S. C. Y. Wong, J. W. M. Tai and K. Y. Yuen, J. Hosp. Infect., 2015, 90, 220–225 CrossRef CAS PubMed.
- A. P. Harvey, E. R. Fuhrmeister, M. E. Cantrell, A. K. Pitol, J. M. Swarthout, J. E. Powers, M. L. Nadimpalli, T. R. Julian and A. J. Pickering, Environ. Sci. Technol. Lett., 2021, 8, 168–175 CrossRef CAS PubMed.
- M. Haque, M. Sartelli, J. McKimm and M. B. Abu Bakar, Infect Drug Resist., 2018, 11, 2321–2333 CrossRef PubMed.
- D. J. Weber, W. A. Rutala, M. B. Miller, K. Huslage and E. Sickbert-Bennett, Am. J. Infect. Control, 2010, 38, S25–S33 CrossRef PubMed.
- A. N. M. Kraay, M. A. L. Hayashi, N. Hernandez-Ceron, I. H. Spicknall, M. C. Eisenberg, R. Meza and J. N. S. Eisenberg, BMC Infect. Dis., 2018, 18, 540 CrossRef PubMed.
- P. Zhao and Y. Li, Environ. Sci. Technol., 2021, 55, 4148–4161 CrossRef CAS PubMed.
- B. Stephens, P. Azimi, M. S. Thoemmes, M. Heidarinejad, J. G. Allen and J. A. Gilbert, Curr. Pollut. Rep., 2019, 5, 198–213 CrossRef CAS PubMed.
- Z. Li, D. Lee, X. Sheng, R. E. Cohen and M. F. Rubner, Langmuir, 2006, 22, 9820–9823 CrossRef CAS PubMed.
- B. Song, E. Zhang, X. Han, H. Zhu, Y. Shi and Z. Cao, ACS Appl. Mater. Interfaces, 2020, 12, 21330–21341 CrossRef CAS PubMed.
- S. Agnihotri, S. Mukherji and S. Mukherji, Nanoscale, 2013, 5, 7328 RSC.
- M. M. Konai, B. Bhattacharjee, S. Ghosh and J. Haldar, Biomacromolecules, 2018, 19, 1888–1917 CrossRef CAS PubMed.
- S. Basu, B. M. Hanh, J. Q. Isaiah Chua, D. Daniel, M. H. Ismail, M. Marchioro, S. Amini, S. A. Rice and A. Miserez, J. Colloid Interface Sci., 2020, 568, 185–197 CrossRef CAS PubMed.
- G. Galli and E. Martinelli, Macromol. Rapid Commun., 2017, 38, 1600704 CrossRef PubMed.
- E. D. Brown and G. D. Wright, Nature, 2016, 529, 336–343 CrossRef CAS PubMed.
- S. Zheng, M. Bawazir, A. Dhall, H.-E. Kim, L. He, J. Heo and G. Hwang, Front. Bioeng. Biotechnol., 2021, 9, 643722 CrossRef PubMed.
- K. W. Kolewe, J. Zhu, N. R. Mako, S. S. Nonnenmann and J. D. Schiffman, ACS Appl. Mater. Interfaces, 2018, 10, 2275–2281 CrossRef CAS PubMed.
- K. W. Kolewe, S. R. Peyton and J. D. Schiffman, ACS Appl. Mater. Interfaces, 2015, 7, 19562–19569 CrossRef CAS PubMed.
- B. Barajas, I. S. Kurtz, A. J. Waldman and J. D. Schiffman, ACS Appl. Mater. Interfaces, 2023, 15, 52197–52206 CAS.
- L. Schardt, A. Martínez Guajardo, J. Koc, J. L. Clarke, J. A. Finlay, A. S. Clare, H. Gardner, G. W. Swain, K. Hunsucker, A. Laschewsky and A. Rosenhahn, Macromol. Rapid Commun., 2022, 43, 2100589 CrossRef CAS PubMed.
- Z. Chen, Langmuir, 2022, 38, 4483–4489 CrossRef CAS PubMed.
- J. B. Schlenoff, Langmuir, 2014, 30, 9625–9636 CrossRef CAS PubMed.
- Q. Shao and S. Jiang, Adv. Mater., 2015, 27, 15–26 CrossRef CAS.
- A. Laschewsky, Polymers, 2014, 6, 1544–1601 CrossRef.
- P. Bengani-Lutz, E. Converse, P. Cebe and A. Asatekin, ACS Appl. Mater. Interfaces, 2017, 9, 20859–20872 CrossRef CAS PubMed.
- A. Sathyan, I. Kurtz, P. Rathore, T. Emrick and J. D. Schiffman, ACS Appl. Bio Mater., 2023, 6, 2905–2915 CrossRef CAS PubMed.
- D. P. Regan, C. Fong, A. C. S. Bond, C. Desjardins, J. Hardcastle, S.-H. Hung, A. P. Holmes, J. D. Schiffman, M. S. Maginnis and C. Howell, ACS Appl. Mater. Interfaces, 2022, 14, 50543–50556 CrossRef CAS PubMed.
- E. Scott and S. F. Bloomfield, J. Appl. Bacteriol., 1990, 68, 271–278 CrossRef CAS PubMed.
- G. U. Lopez, C. P. Gerba, A. H. Tamimi, M. Kitajima, S. L. Maxwell and J. B. Rose, Appl. Environ. Microbiol., 2013, 79, 5728–5734 CrossRef CAS PubMed.
- S. Schmidt-Emrich, P. Stiefel, P. Rupper, H. Katzenmeier, C. Amberg, K. Maniura-Weber and Q. Ren, Materials, 2016, 9, 249 CrossRef PubMed.
- S. Behzadinasab, A. W. H. Chin, M. Hosseini, L. L. M. Poon and W. A. Ducker, Sci. Rep., 2021, 11, 22868 CrossRef CAS PubMed.
- T. Chang, M. Sepati, G. Herting, C. Leygraf, G. K. Rajarao, K. Butina, A. Richter-Dahlfors, E. Blomberg and I. Odnevall Wallinder, PLoS One, 2021, 16, e0247081 CrossRef CAS PubMed.
- M. Gunell, J. Haapanen, K. J. Brobbey, J. J. Saarinen, M. Toivakka, J. Mäkelä, P. Huovinen and E. Eerola, Nanotechnol. Sci. Appl., 2017, 10, 137–145 CrossRef CAS PubMed.
- T. L. Meister, Y. Brüggemann, B. Tamele, J. Howes, E. Steinmann and D. Todt, STAR Protoc., 2022, 3, 101188 CrossRef CAS PubMed.
- D. B. Weibel, A. Lee, M. Mayer, S. F. Brady, D. Bruzewicz, J. Yang, W. R. DiLuzio, J. Clardy and G. M. Whitesides, Langmuir, 2005, 21, 6436–6442 CrossRef CAS PubMed.
- J. K.-M. Knobloch, S. Tofern, W. Kunz, S. Schütze, M. Riecke, W. Solbach and T. Wuske, PLoS One, 2017, 12, e0187442 CrossRef PubMed.
- J. N. Pagaduan, N. Hight-Huf, A. Datar, Y. Nagar, M. Barnes, D. Naveh, A. Ramasubramaniam, R. Katsumata and T. Emrick, ACS Nano, 2021, 15, 2762–2770 CrossRef CAS PubMed.
- D. Chen, C.-C. Chang, B. Cooper, A. Silvers, T. Emrick and R. C. Hayward, Biomacromolecules, 2015, 16, 3329–3335 CrossRef CAS PubMed.
- J. K. Spelt, D. R. Absolom and A. W. Neumann, Langmuir, 1986, 2, 620–625 CrossRef CAS.
- A. Kalra, A. Lowe and A. Al-Jumaily, J. Mater. Sci. Eng., 2016, 5, 1–7 Search PubMed.
- L. C. Simões, M. Simões and M. J. Vieira, Antonie van Leeuwenhoek, 2010, 98, 317–329 CrossRef PubMed.
- Z. He, X. Lan, Q. Hu, H. Li, L. Li and J. Mao, Prog. Org. Coat., 2021, 157, 106285 CrossRef CAS.
- Y. Li, J. Q. Pham, K. P. Johnston and P. F. Green, Langmuir, 2007, 23, 9785–9793 CrossRef CAS PubMed.
- T. Yasuda, T. Okuno and H. Yasuda, Langmuir, 1994, 10, 2435–2439 CrossRef CAS.
- O. Prucker, T. Brandstetter and J. Rühe, Biointerphases, 2018, 13, 010801 CrossRef PubMed.
- C. Spengler, E. Maikranz, B. Glatz, M. A. Klatt, H. Heintz, M. Bischoff, L. Santen, A. Fery and K. Jacobs, Soft Matter, 2024, 20, 484–494 RSC.
- T. J. Silhavy, D. Kahne and S. Walker, Cold Spring Harbor Perspect. Biol., 2010, 2, a000414–a000414 Search PubMed.
- iPhone 13 Pro and 13 Pro Max – Technical Specifications, https://www.apple.com/by/iphone-13-pro/specs/, (accessed 28 January 2025).
- H. Terwisscha-Dekker, T. Hogenelst, R. Bliem, B. Weber and D. Bonn, Phys. Rev. E:Stat., Nonlinear, Soft Matter Phys., 2023, 107, 024801 CrossRef CAS PubMed.
- D. Rana and T. Matsuura, Chem. Rev., 2010, 110, 2448–2471 CrossRef CAS PubMed.
- R. Lohmann, I. T. Cousins, J. C. DeWitt, J. Glüge, G. Goldenman, D. Herzke, A. B. Lindstrom, M. F. Miller, C. A. Ng, S. Patton, M. Scheringer, X. Trier and Z. Wang, Environ. Sci. Technol., 2020, 54, 12820–12828 CrossRef CAS PubMed.
- L. Cobrado, A. Silva-Dias, M. M. Azevedo and A. G. Rodrigues, Eur. J. Clin. Microbiol. Infect. Dis., 2017, 36, 2053–2062 CrossRef CAS PubMed.
- F. T. Ogunsola and S. Mehtar, Antimicrob. Resist. Infect. Control, 2020, 9, 81 CrossRef PubMed.
- S. He, J. Wang, F. Yang, T.-L. Chang, Z. Tang, K. Liu, S. Liu, F. Tian, J.-F. Liang, H. Du and Y. Liu, Coatings, 2023, 13, 778 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5lp00110b |
| This journal is © The Royal Society of Chemistry 2025 |


