DOI:
10.1039/D5LP00074B
(Review Article)
RSC Appl. Polym., 2025,
3, 1124-1144
Biomolecule-modified synthetic polymers for wound healing and orthopaedic applications
Received
19th March 2025
, Accepted 26th May 2025
First published on 30th May 2025
Abstract
Synthetic polymers play an important role in medical devices such as implants, wound dressings and catheters since the first use of polyethylene in bone and cartilage implants in the 1940s. Since then, many more synthetic polymers have been used in medical devices for applications ranging from orthopaedic implants, wound care products, heart valves, and stents to tissue grafts. However, nearly all the polymers used in these devices are bioinert, except for some that are biodegradable such as polyesters. These polymers generally do not confer bioactivity on their own and need additional stimuli such as through modification with biomolecules or blending with bioadditives. We term polymers chemically modified with biomolecules “Biohybrid Polymers” and they represent a new class of biomaterials that are purely synthetic. These biomaterials possess properties required for use in applications such as tissue engineering and medical device fabrication. In this review, we explore the different types of biohybrid polymers that have been reported for use in skin, bone and cartilage tissue engineering with brief descriptions of their chemical synthesis methods. The materials are categorised based on their targeted applications in wound care or orthopaedics to help readers understand what are the potential materials that may be used for each type of tissue being regenerated.
1 Introduction
The use of non-biological materials for medical implants began in the 19th century mainly in the form of metallic implants such as gold facial implants. Synthetic polymers began finding their way into medical implants in the 1940s mainly through the use of polyethylene for bone and cartilage implants.1 This largely coincided with the first practical industrial production of polyethylene in 1939. Since the discovery of Ziegler Natta catalysts in the 1950s for the sustainable production of high density polyethylene (HDPE), there has been widespread use of this inert plastic in medical devices.2 PE in various forms (HDPE, UHMWPE, and XPE) finds uses in medical applications such as stents, joint prostheses and facial implants today. Apart from polyethylene, other popular synthetic polymers for medical implants include silicone, polymethyl methacrylate (PMMA), polyether ether ketone (PEEK), polyamide, poly(tetrafluoroethylene) (PTFE) and polyesters such as polycaprolactone (PCL), polylactide (PLA) and poly(lactic-co-glycolic acid) (PLGA).3–9
In general, properties required in a particular device are key to making the right material choice.10–13 For example, in selecting a material for the spacer of knee joint prostheses, considerations for material durability towards repeated abrasive forces from knee joint movements, are essential. Hence, typical choices for tibial plate spacers of knee prostheses are UHMWPE and XPE (ultra-high molecular weight PE and crosslinked PE respectively). On the other hand, in a bone void filler meant to be a temporary defect filling material whilst the bone regrows, a biodegradable polymer such as polycaprolactone would be desired. For soft tissue implants, silicone, expanded poly(tetrafluoroethylene) (ePTFE) and PLGA are common materials used. Regardless of the material choice in implants, one common criterion essential for the use of such materials in the human body is the need to be biocompatible and not trigger excessive immunological responses physiologically.14,15 Furthermore, medical devices are governed by strict regulatory requirements defined by regulatory bodies such as the Medical Device Regulation (MDR), the U.S. Food and Drug Administration (FDA) and the National Medical Products Administration (NMPA). Testing involves the evaluation of devices for compliance with standards such as ISO10993, where toxicity, chemical leachable and degradation byproducts, just to name a few, are important test criteria necessary for regulatory approval.16 In view of this, most synthetic materials used in medical implants tend to be fairly inert and mainly fall under the few material categories listed above.
Despite the challenges in using new materials for medical implants due to regulatory controls, there has been much research into enhancing the bioactivities of existing commonly used synthetic polymers to improve the biological properties of these implant materials. Methods used to enhance the bioactivities of synthetic polymers include direct chemical modification of polymer structures with biomolecules, surface modification of bulk materials with biomolecules, incorporation of cells and addition of bioadditives such as collagen or calcium-based minerals to bulk materials. The addition of bioadditives to enhance the bioactivities of bulk materials is more of a blending process and there are multiple reviews covering the types of biomaterials that can be blended into bulk polymers such as PE, PCL, PLA and PLGA to improve their bioactivity such as osteoinduction or anti-oxidation.17–19 Likewise, the incorporation of cells and growth factors into materials to enhance the bioactivities of both synthetic and natural materials has been a hot topic of late with several reviews covering this area.20,21 In this review, we will mainly focus on chemical modification of synthetic polymer structures with biomolecules to enhance a material's bioactivity and explore the use of these new biomaterials in medical devices. Of particular interest to us is tissue regeneration to enhance healing outcomes in wound care products, and bone and cartilage implants. Hence, this review shall focus on biohybrid polymers and their uses in wound healing and orthopedic applications (Scheme 1).
 |
| | Scheme 1 Synthetic polymers for wound healing and orthopedic applications. | |
1.1 Skin tissue
Skin is the largest organ in the body and the essential ‘first line of defense’ in the human immune system.22 Skin injuries vary from small incisions that self-heal in a few days, to severe degree burns, resulting in life-threatening situations for the patient.23 Factors that influence the wound healing process include the type of wounds (superficial, partial thickness and full-thickness), degree of tissue damage caused by injuries e.g. burns (first, second, and third-degree), trauma, amount of moisture around the wound and the presence of infections or secondary skin damage.24 The key requirements of materials for skin tissue regeneration are biocompatibility, appropriate mechanical properties, permeability, capability of cell proliferation on their surfaces and possibility of integration with surrounding tissue. Synthetic polymers have gained prominent attention in tissue engineering due to a number of beneficial characteristics25,26 such as (i) processable mechanical properties, from soft to stiff matters, (ii) structure-tunable properties, (iii) suitability for versatile material processing methods, (iv) simplicity of manufacturing, (v) commercially available and cost-effectiveness, and (vi) potentially allow the release of biomolecules. Though they may lack the inherent bioactivity present in natural biopolymers, their inert properties and possible modification with biomolecules make them interesting materials for skin care applications. Biomolecules that are suitable for skin tissue engineering applications should be able to stimulate skin regeneration by enhancing skin cell adhesion, migration and proliferation. Examples of such are integrin-binding molecules such as the arginylglycylaspartic acid (RGD), laminin-derived glycopeptides that attach to the basement membrane of the extracellular matrix (ECM), glycosaminoglycans such as hyaluronan, for maintaining tissue homeostasis and angiogenesis.27–29 By incorporating such biomolecules capable of encouraging skin tissue regeneration in synthetic polymers, one can envisage a hybrid biosynthetic polymer that is capable of repairing skin tissues whilst being thermomechanically stable for conventional material processing into useful wound care products. For full thickness wound healing, angiogenesis is an important factor, on top of granulation tissue formation, to prevent skin necrosis. Biomolecules that can stimulate angiogenesis would be useful in this case.
1.2 Bone tissue
Synthetic polymers are becoming increasingly popular for use as scaffold materials in bone regeneration due to their wide availability, tunable mechanical properties, excellent processability, well-defined chemical structures and long shelf life.30 Biodegradable scaffolds have gained popularity in the last decade due to their ability to biodegrade in vivo, rendering a second surgical event for scaffold removal unnecessary. A wide range of bioresorbable synthetic polymers have been developed for bone tissue engineering. When bone tissue damage is permanent and regeneration is difficult, non-biodegradable polymers such as polyethylene (PE) can be used to partially replace the injured bone. Osteoinductive biomolecules are critical for the preparation of bone tissue scaffolds that are capable of repairing bone defects. Some of these include glycosaminoglycans such as heparan sulfate, osteopontin derived peptides such as the SVVYLGR sequence and collagen-derived peptide such as RGD.31 Angiogenesis is critical to the success of bone tissue regeneration using a scaffold, as non-vascularized bone tissues can undergo osteonecrosis. Hence, biomolecules that are capable of angiogenesis may also be potential candidates for the modification of synthetic polymers targeted at bone tissue regeneration.
1.3 Cartilage tissue
Cartilage is present throughout the human body and supports connective tissues in parts of the human body such as bronchial tubes, intervertebral discs, meniscus, and the joints between bones. It is characterized by a tough, viscoelastic and lubricated structure. Damage or degeneration of cartilage tissue often results in limited movement, pain and even permanent disability. However, cartilage regeneration remains a challenge in regenerative medicine, as cartilage has limited healing capacity due to its avascular structure and lack of innervation.32 Synthetic polymeric materials have emerged to regenerate or replace cartilage tissues and represent a promising alternative.33 Synthetic polymers for use in cartilage repair ought to be able to withstand shear forces caused by joint movements for implant durability. At the same time, biomolecules targeting cartilage regeneration ought to be chondroinductive.34
Chemical modifications of various types of synthetic polymers to form biohybrid polymers and their uses in different wound care or orthopedic applications are described in the sections below. A summary of the types of synthetic polymers that may potentially be used in medical device fabrication, their applications and possible chemical modification methods to form biohybrid materials, are provided in Tables 1 and 2.
Table 1 Specific applications in wound healing and orthopaedics of each polymer
| Polymers |
Applications |
| PEG |
Wound healing: PEG derivatives are commonly used to form hydrogels with excellent moisture-retaining properties. When chemically modified with antioxidants like ferulic or lauric acid and RGD peptides, PEG promotes anti-inflammatory effects and enhances cell adhesion for faster wound closure. The CPT system (chitosan–PEG–tyramine) facilitates hemostasis and forms an adhesive matrix over bleeding wounds. |
| PCL |
Wound healing: PEG-functionalized PCL nanofibers integrated with bioactive molecules such as colostrum and gelatin mimic the extracellular matrix (ECM), aiding burn healing and delivering growth factors for skin regeneration. |
|
Bone: Chemically modified PCL with osteoinductive factors like BMP-2, osteogenic growth peptides (OGP), and RGD peptides enhances osteoblast adhesion and differentiation, making it effective for bone repair. |
|
Cartilage: PCL conjugated with peptides like E7 or grafted with collagen mimics ECM structure and supports glycosaminoglycan (GAG) retention, promoting chondrocyte activity and cartilage matrix formation. |
| PLA |
Wound healing: PLA modified with chitosan and PNVP enhances dermal fibroblast proliferation and tissue adhesion, supporting wound closure. |
|
Bone: Incorporation of osteoinductive peptides such as RGD and BMP-2 improves bone regeneration by promoting stem cell recruitment and mineralization |
|
Cartilage: PLA nanofibers with lignin provide structural mimicry to native cartilage, aiding scaffold integration and chondrocyte support. |
| PLGA |
Wound healing: PLGA systems functionalized with nitric oxide donors (e.g., GSNO) or peptide brushes promote antibacterial activity and rapid tissue regeneration in infected or burn wounds. |
|
Bone: BMP-2 immobilization on PLGA scaffolds stimulates bone tissue ingrowth, while collagen mimetic peptides like P15 enhance mineral deposition. |
|
Cartilage: PLGA-based hydrogels loaded with kartogenin (KGN) and HA nanoparticles foster chondrocyte proliferation and cartilage matrix development. |
| PE |
Wound healing: Brush copolymers of PE with bioactive ligands improve wound integration by encouraging cellular adhesion and proliferation. |
|
Bone: UHMWPE materials modified with RGD or collagen-like sequences promote osteogenic differentiation and reduce fibrous encapsulation, enhancing implant success. |
Table 2 Representative modifications to form biohybrid polymers for wound healing and orthopaedic applications
| Polymer |
Tissue |
Modification |
Properties |
Ref. |
| PEG |
Skin |
Poly(ethylene glycol) methyl ether methacrylate (MPEGMA) with lipoic acid (LA) and ferulic acid (FA) covalently linked to PEG |
Antioxidant skin care |
37
|
| PEG-grafted chitosan scaffold |
Wound healing |
43
|
| Reinforced PEG-chitosan hydrogel |
Cell attachment in wound healing |
49
|
| Chitosan–PEG–tyramine (CPT) conjugate hydrogel |
Wound healing |
50
|
| RGD-containing PEGDA (RGD–PEGDA) |
Cell adhesion in wound healing |
51
|
| Cyclic RGDfE peptide-containing PEGDA (cRGD–PEGDA) |
Cell adhesion in wound healing |
35
|
| PEG acetylene (4-PEG-Ace) conjugates with RGD diazide (RGD-2N3) |
Cell adhesion in wound healing |
35
|
| Norbornene-terminated PEG and peptide conjugation |
Cell adhesion in wound healing |
53
|
| PCL |
Skin |
Colostrum conjugated PCL–PEG |
Skin regeneration |
56
|
| mPEG–PCL–gelatin |
Burn wound treatment |
58
|
| Gelatin–PCLA conjugation |
Excisional wound healing |
59
|
| Bone |
PCL immobilized with osteogenic growth peptide |
Osteogenic effect |
60
|
| PCL conjugated with BMP-2 |
Upregulation of gene expression of osteogenic markers |
62
|
| SpBMP-9/pFibro co-functionalized PCL films |
Promote osteoblast differentiation |
63
|
| PCL surface modified with YIGSR and WKYMVm |
Osteogenic differentiation and vasculogenesis |
64
|
| Chitosan-g-PCL conjugated with biosilica |
Enhanced cell mineralization and ALP activity |
65
|
| PCL blended with PCL/RGD or PCL/(GPHyp)3 brush copolymer |
Osteogenic effect |
53
|
| mPEG–PCL and RGD-conjugated mPEG–PCL (mPEG–PCL–RGD) hydrogel |
Promote new bone formation |
66
|
| Cartilage |
PCL scaffold grafted with collagen |
Stimulating chondrogenesis |
67
|
| Peptide-PCL conjugation |
Bind and localize the GAGs within a scaffold for cartilage tissue engineering |
68
|
| E7 peptide–PCL conjugation |
Chondrogenic effect and reduced tissue inflammation |
71
|
| PLA |
Skin |
Chitosan, PLA and poly(N-vinyl pyrrolidone) conjugation |
Human dermal fibroblasts guiding |
77
|
| Bone |
PLA conjugated with RGD |
Osteogenic effect |
78
|
| PLA immobilized with BMP-2 derived peptide (SSVPT) |
Osteoconductive and bone regeneration |
79
|
| RGD-conjugated triblock copolymer RGD/PEG–PLA–PGL in HA/PLGA nanocomposite |
Bone regeneration |
82
|
| PLA blended with PLA/RGD or PLA/(GPHyp)3 brush copolymer |
Osteogenic effect and reduced tissue inflammation |
53
|
| Chitosan grafted with PLLA |
Osteoblast proliferation |
83
|
| Cartilage |
Lignin grafted PLA nanofibers |
Mimic cartilage tissue matrix for tissue regeneration |
84
|
| PLGA |
Skin |
S-Nitrosoglutathione-PLGA (GSNO–PLGA) conjugation |
Infected cutaneous wound treatment |
90
|
| PLGA-peptide brush copolymer |
Burn wound treatment |
91
|
| Bone |
PLGA immobilized with BMP-2 |
Osteogenic differentiation and bone regeneration |
92
|
| Collagen mimetic peptide (P15) and OGP immobilized on HA/PLGA substrate |
Osteogenic differentiation and new bone mineralization |
93
|
| Porous PLGA with glycol chitosan immobilized with BMP-2 |
Calcium mineralization and osteogenic differentiation |
95
|
| Cartilage |
HA–PLGA conjugation |
Cartilage regeneration |
97
|
| PLGA–HA nanoparticles |
Cartilage cell binding |
98
|
| PLGA–KGN–HA hydrogel |
Cartilage regeneration |
99
|
| PE |
Skin |
Non-woven cloth coated by covalently-incorporated PE-biomolecules brush polymers |
Full-thickness excisional wounds treatment |
102
|
| Bone |
UHMWPE blended with peptide covalently-incorporated PE brush copolymer |
Osteogenic effect and reduced tissue inflammation |
54
|
2 Poly(ethylene glycol) (PEG)
Poly(ethylene glycol) (PEG) is widely used in pharmaceutical and biomedical applications mainly due to its controllable structural and compositional properties. Also, the high hydrophilicity of PEG allows it to be used in hydrogel preparations. Hydrogels containing PEG bear similarities to the extracellular matrix (ECM) and are thus suitable for cell growth and proliferation. However, PEG itself lacks bioactivity due to its inert nature and is thus often additionally functionalized with ECM peptide motifs.35 Apart from blending with biomaterials as the conventional strategy,36 modification of PEG through direct conjugation with biomolecules has gained much popularity.
2.1 Skin tissue
Neugebauer's team37 reported an amphiphilic copolymer of poly(ethylene glycol) methyl ether methacrylate (MPEGMA) and alkyne functionalized 2-hydroxyethyl methacrylate (AlHEMA) made by controlled atom transfer radical polymerization (ATRP) (Fig. 1). This copper-catalyzed reaction was carried out via ATRP initiators ethyl α-bromoisobutyrate (EiBBr) and the bromoester derivative of 4-n-butylresorcinol (4nBREBr2). Two antioxidant substances used in skin care, (±)-α-lipoic acid (LA) and ferulic acid (FA), were covalently linked to the alkyne-functionalized copolymers using a “click” chemistry reaction. The “click” reaction has received great interest due to its efficiency, stereospecificity and oxygen/water insensitivity. FA is used as a photoprotective substance with minimal toxicity, anti-inflammatory and anti-cancer properties, for its skin anti-aging effects.38 LA is a natural antioxidant compound that has a positive effect in skin anti-cancer therapy.39 The antioxidants LA and FA could inhibit the generation of reactive oxygen species or nitrogen. As this antioxidant-conjugated compound is designed for potential applications in cosmetology, metal residues from the copper catalyst would become a drawback due to cytotoxicity of copper.9 The TC50 value for Cu2+ in medical implants is 0.9 ppm for 48 h exposure and 0.5 ppm for 72 h exposure.40 Toxicology data for medical devices made of novel materials are essential for regulatory approval of these devices. Hence, effective and efficient removal of copper during material scale up production would be an important consideration for manufacturing this material for commercial applications since it is important to keep the residual copper content below TC50 values. Extraction techniques have been investigated to remove copper-based catalysts in ATRP such as adsorption via alumina columns, liquid–liquid phase separation or an ion-exchange resin treatment based on different monomers and polymerization conditions. Among them, precipitation in the basic medium might be the most efficient and economic approach for these poly(ethylene glycol)-based macromolecules.41 Alternative approaches, such as reversible addition–fragmentation chain transfer (RAFT) polymerization, have received attention in bioconjugation. Examples of peptide-, protein-, and nucleic acid-polymer conjugated by RAFT have been reported.42 The absence of a metal catalyst is a significant advantage of RAFT. It is also a versatile method that allows for polymerization in a wide range of solvent conditions and monomers.
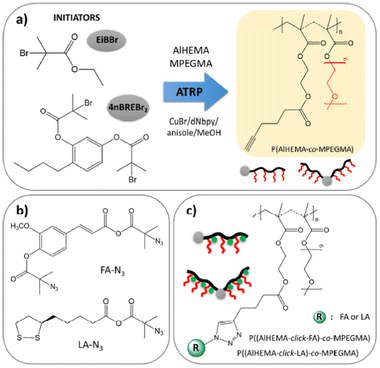 |
| | Fig. 1 (a) ATRP synthetic route to obtain copolymers, (b) “click”-able antioxidants and (c) antioxidant-conjugated polymers (reproduced from ref. 37 with permission from MDPI, copyright 2020). | |
A number of investigations also focused on PEG-grafted polysaccharide scaffolds. For example, Kumar's team43 developed a PEG-grafted chitosan scaffold that serves as growth factor delivery vehicle for enhanced wound healing (Fig. 2a). Chitosan is a linear polysaccharide that consists of β-(1 → 4)-linked 2-acetamido-2-deoxy-D-glucopyranose and 2-amino-2-deoxy-D-glucopyranose units and is generally obtained by chitin deacetylation. Chitin is a naturally abundant polysaccharide produced by biosynthesis and is found in crustaceans such as crabs, shellfish and shrimps. In terms of the amount, it is second to cellulose. Chitosan has been used in wound treatment due to its antibacterial, hemostatic and mucoadhesive effects. It is known to promote fibroblast formation and facilitate the early stages of healing.44–48 Hsieh's team49 synthesized the reinforced PEG-chitosan hydrogel through the formation of ester and amide linkages (Fig. 2b). The positive effect on cell attachment indicated its potential use for wound dressings. Further modification beyond PEG-grafted chitosan was also attempted. Park's team50 reported a chitosan–PEG–tyramine (CPT) conjugate hydrogel for hemostasis in wound healing (Fig. 2c). In this study, one terminus of the PEG chain was grafted onto chitosan and another terminus was bonded to the biomolecules.
 |
| | Fig. 2 (a) A PEG-grafted chitosan random copolymer (reproduced from ref. 43 with permission from Springer Nature, copyright 2019). (b) Reinforced PEG–chitosan (reproduced from ref. 49 with permission from Wiley-VCH, copyright 2013). (c) Chitosan–PEG–CPT (reproduced from ref. 50 with permission from Elsevier, copyright 2012). | |
Cell-adhesive peptides (CAPs) are another major target for PEG modification in materials for wound care products. Both linear and arm-shaped CAP-conjugated PEG made by chemical routes have been reported. Marchant's team, developed a method to create a peptide-incorporated PEGDA macromer (RGD–PEGDA) where Arg-Gly-Asp (RGD) is attached with two PEG monoacrylates (Fig. 3a).51 Additionally, they also reported the use of cyclic RGDfE peptide c[RGDfE(SSSKK-NH2)] conjugated with Acr-PEG-NHS to form cRGD–PEGDA (Fig. 3b).35 The RGD peptide functions as an integrin binder to facilitate cell adhesion, migration, proliferation and immunomodulation. Integrins are responsible for re-epithelialization and granulation tissue formation in wound healing. RGD-recognizing integrins αVβ6 and αVβ8 regulate inflammation through TGF-β activation and contribute to granulation tissue remodeling and angiogenesis.29 Furthermore, through the binding of αVβ3 integrins of macrophages, the RGD peptide can enhance anti-inflammatory M2 macrophage polarization.52
 |
| | Fig. 3 Synthesis of RGD-incorporated PEGDA (RGD–PEGDA) (a) by conjugation of diaminopropionic acid (Dap)-capped GRGDSP and Acr-PEG-NHS (reproduced from ref. 51 with permission from American Chemical Society, copyright 2006). (b) Constructed by a hydrophilic tail with a spacer of three serine residues (SSS) and a linker of two lysine residues (KK), and cRGD-incorporated PEGDA (cRGD–PEGDA) (reproduced from ref. 35 with permission from Elsevier, copyright 2010). | |
West's team, reported on the synthesis of cell-adhesive PEG hydrogels via click chemistry between 4-arm PEG acetylene (4-PEG-Ace) and cell-adhesive peptide RGD diazide (CAP-2N3) (Fig. 4).35 CAP-2N3 was prepared via a solid phase peptide and 4-PEG-Ace was developed by acetylation of tetrahydroxy terminated 4-arm PEG. PEG hydrogel networks were formed by the click reaction which is the copper-catalysed reaction between CAP-2N3 and 4-PEG-Ace. The gelation time varied from 2 to 30 min, depending on the temperature and concentrations of the catalyst and precursor. However, this metal complex (Cu, Na)-mediated hydrogel preparation process would raise issues of cytotoxicity due to difficulty in the purification of the gel network. New materials are required to undergo the chemical characterisation test in accordance with ISO10993, to establish biocompatibility of the product. The product is subjected to repeated extractions to simulate physiological conditions of the implanted device. Toxicology studies would be conducted on the extracts and metal residues from catalysts may potentially impact the toxicology outcomes of the test. This would in turn affect the regulatory approval of the device using this material. Hence, effective removal of metal catalysts from the biohybrid materials is critical. To ensure the safety and quality of the final medical products, developers should consider a suitable purification technique to remove the catalyst residues or adopt alternative metal catalyst-free approaches such as RAFT polymerization in the early material development stage.
 |
| | Fig. 4 Cell-adhesive PEG hydrogels prepared via click chemistry between 4-arm-PEG acetylene (4-PEG-Ace) and cell adhesive peptide diazide (CAP-2N3) in copper(II) sulfate and sodium ascorbate (reproduced from ref. 35 with permission from Elsevier, copyright 2010). | |
Motivated by covalent-bonding modification between PEG and biomolecules, Teo's team developed RGD peptide or collagen peptide mimics, (GPHyp)3-conjugated PEG with a norbornene terminus (Fig. 5).53,54 Collagen peptide mimics with a specific amino acid sequence of proline, hydroxyl proline and glycine (GPHyp)3, exhibit strong affinity to type II collagen, which is the main component of the tissue extracellular matrix (ECM).55 These norbornene-terminated PEG and peptide conjugates functioned as tissue-regenerative motifs for subsequent polymerization using ring opening metathesis polymerization. Thus, PEG as an inert synthetic polymer can be covalently modified with different bioactive moieties to deliver various bioactivities such as cell adhesion, proliferation, hemostasis and anti-inflammation for wound care material fabrication.
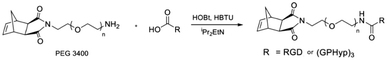 |
| | Fig. 5 Synthesis of PEGylated peptides macromonomers (reproduced from ref. 53 with permission from American Chemical Society, copyright 2022). | |
3 Poly(caprolactone) (PCL)
Polycaprolactone (PCL) is an aliphatic polyester that has been studied extensively since 2001 for its first application in skin tissue engineering.22 Besides, PCL has load bearing properties suitable for use in bone tissue regeneration. However, PCL itself does not promote bioactivity and its hydrophobicity limits cell adhesion and proliferation. For example, PCL lacks appropriate biofunctions such as biological recognition sites or surface modifications required for tissue regeneration. To bio-functionalize such polymer surfaces, additional post-fabrication such as physisorption or modification using biomolecules is needed.
3.1 Skin tissue
Singh's team developed nanofibrous dressings fabricated using colostrum conjugated PCL–PEG through plasma induced graft polymerization.56 Carboxyl groups that are functionalized as chemical arms were grafted onto PCL (Fig. 6a). Growth factors present in the colostrum conferred bioactivity to stimulate skin repair and regeneration. Gelatin is a promising material to promote skin wound healing as it functions as a natural substrate for the extracellular matrix (ECM). The use of gelatin in wound care can potentially compensate for tissue breakage around wound beds.57 A skin substitute constructed by mPEG–PCL-grafted–gelatin (Bio-Syn) was developed by Koul's team (Fig. 6b).58 The gelatin backbone was grafted with mPEG–PCL via a Michael addition reaction. This mPEG–PCL–gelatin copolymer could be further compounded with hyaluronan/chondroitin sulfate/sericin to construct Bio-Syn PCL composites for wound care. In vivo Wistar rat studies on 2nd degree burn wound model revealed accelerated wound contraction. This finding suggests that the Bio-Syn PCL composite scaffold may serve as a useful dermal substitute for burn care. Lee's team59 synthesized poly(ε-caprolactone-co-lactide) (PCLA)–modified gelatin (Gel–PCLA) via the NHS/EDC reaction (Fig. 7). This Gel–PCLA conjugate can spontaneously form and assemble the hydrogel onto excisional wound treatment. It also showed efficacy in protecting wounds from external contamination. By adjusting the CL/LA feed ratio to control the molecular weight of PCLA blocks, this PCLA gel can be tuned flexibly in terms of thermal responsive properties, gelation window, rheology and biodegradability. The methods adopted by Koul's and Lee's teams for the conjugation of gelatin onto synthetic polymers are straightforward, making them promising targets for large scale manufacturing into medical devices.
 |
| | Fig. 6 (a) Colostrum conjugated PCL–PEG (reproduced from ref. 56 with permission from Elsevier, copyright 2022) and (b) reaction scheme for the synthesis of mPEG–PCL–g-gelatin (reproduced from ref. 58 with permission from Royal Society of Chemistry, copyright 2018). | |
 |
| | Fig. 7 Synthesis of PCLA–Gel conjugates (reproduced from ref. 59 with permission from American Chemical Society, copyright 2019). | |
3.2 Bone tissue
Pan's team modified the surfaces of 3D printed PCL scaffolds with osteogenic growth peptide (OGP) (NH2-ALKRQGRTLYGFGG-OH) by surface amination and electrostatic self-assembly methods (Fig. 8).60 They reported that OGP-modified PCL facilitated adhesion, proliferation of rat bone marrow-derived stem cells (BMSCs) and induced osteogenic differentiation of BMSCs into osteoblasts in vitro. The implantation of the PCL/OGP scaffold in a rat cranial defect model promoted bone formation and accelerated mineralization. It should be noted that the OGP used in this method consists of a 14-animo acid sequence that is prepared through the modification of a native peptide isolated from human and rodent serum. Thus, there would be a tedious cell processing step prior to the obtainment of OGP. Therefore, the method is not very practical from an industrial perspective for product manufacturing and storage due to the active biologics handling.
 |
| | Fig. 8 PCL scaffolds for bone tissue engineering. (a) Schematic diagram of PCL/OGP scaffold preparation via surface modification (reproduced from ref. 60 with permission from Elsevier, copyright 2020). | |
Growth factors such as bone morphogenetic proteins (BMPs) have been extensively studied for their ability to promote new bone formation. Adverse effects such as uncontrolled bone formation have been reported from the use of bone grafts containing BMP-2.61 To reduce the initial dosage of BMP-2 and prevent initial burst release, Hollister's team directly attached BMP-2 to PCL scaffolds using aminolysis followed by chemical conjugation.62 The immobilization efficiency was found to be higher in chemical conjugation compared to physical adsorption. The release of conjugated BMP-2 was observed to be obviously slower compared to that from BMP-2-adsorbed PCL scaffolds. Osteogenic markers for gene expression were upregulated in bone marrow stromal cells cultured on BMP-2 conjugated scaffolds, but not on scaffolds with BMP-2 physically adsorbed. As BMPs are typically expensive to produce, Faucheux's team developed a peptide derived from BMP-9 (SpBMP-9) and grafted it onto the PCL film together with the peptide derived from fibronectin motifs (pFibro) that has cell adhesion properties.63 The SpBMP-9/pFibro co-functionalized PCL films were found to enhance cell adhesion and favor osteoblast differentiation of mesenchymal progenitor cells. Their study revealed the effectiveness of SpBMP-9 on cell adhesion, intracellular signaling and osteoblastic differentiation.
To induce vascularisation for bone regeneration, He's team developed two bioactive peptides, YIGSR and WKYMV-containing PCL/poliglecaprone (PGC) nanofibrous scaffolds using the strategy of immobilization onto a polyglycolic acid (PGA)-binding motif (SCNSSSYSWYCWFGGSSPS).64 WKYMVm (Trp-Lys-Tyr-Met-Val-D-Met) is reported to induce endothelial progenitor cells (EPCs) recruitment and vasculogenesis, while YIGSR (Tyr-Ile-Gly-Ser-Arg) can enhance adhesion, spreading, and proliferation of cells. In vitro bioassays confirmed tube formation of EPCs with the induction of osteogenic differentiation. Robust recruitment of EPCS followed by early vasculogenesis stimulation was also observed in the rat defect model. Although the peptides are short in this method, the PGA binding motif used to conjugate the peptides is too complex. Moreover, the immobilization method is uncontrollable and thus the biocomponent conjugation is hard to quantify precisely. Batch-to-batch variation tends to occur in biomaterials modified using immobilization. This can raise quality control issues during product manufacturing later on.
Hybrid polymers that combine both the complementary properties of PCL and biomolecules have also been reported. The polycationic nature of chitosan (CHS) enhances adhesion to negatively charged molecules such as growth factors and proteoglycans. CHS also plays a key role in cellular adhesion due to its structural similarity with glycosaminoglycans (GAGs), a major component in the bone extracellular matrix. However, the use of CHS has been hindered by its poor mechanical properties and brittleness. Muller's team reported the synthesis of a chitosan-graft-PCL (CHS-g-PCL) composite by the conjugation of carboxylic acid on PCL to chitosan via amidation.65 The surface of CHS-g-PCL was then modified with osteogenic biosilica using surface-immobilized enzyme silicatein. This hybrid CHS/PCL composite was found to enhance both cell mineralization and ALP activity of osteoblastic cells. Recently, Teo's team developed the synthesis of biohybrid brush-type PCL-peptide copolymers constructed by pendant side arms of PCL and PEGylated peptides, including linear Arg-Gly-Asp (RGD) and collagen mimic peptide (GPHyp)3 (Fig. 9).53 The brush copolymers were used as bioadditives, blended with commercial PCL and subsequently 3D printed via the fused filament fabrication method. These bioactive PCL materials are responsible for osteogenic differentiation in vitro as demonstrated in ALP assays. Briefly, the biohybrid brush copolymers were synthesised using Ring Opening Metathesis Polymerization (ROMP). The synthetic macromers and bioactive macromeres were prepared individually depending on the properties and applications of the final materials. Hence, the materials prepared using this method could be customized. Currently, the scalability of the biohybrid copolymers was validated for a 100 g production.
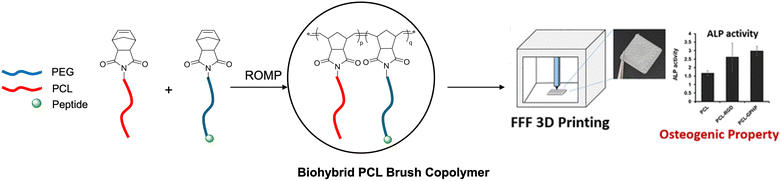 |
| | Fig. 9 3D printed biohybrid PCL brush copolymer with osteogenic properties (reproduced from ref. 53 with permission from American Chemical Society, copyright 2022). | |
Chun's team reported a thermoresponsive in situ forming hydrogel fabricated by methoxy polyethylene glycol–polycaprolactone (mPEG–PCL) and RGD-conjugated mPEG–PCL (mPEG–PCL–RGD) (Fig. 10). The remarkable expression of osteogenic markers of BMSCs was observed in the mPEG–PCL–RGD system and the in vivo rabbit calvarial defect model showed that the hydrogels significantly promoted new bone formation.66 The method to prepare this thermosensitive hydrogel in situ is clinically feasible and the precursors can be scaled up easily for manufacturing.
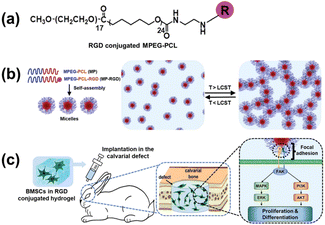 |
| | Fig. 10 A diagram showing the MP hydrogels containing RGD for promoting bone regeneration in a rabbit calvarial defect model by controlling osteogenic differentiation. (a) Preparation process of the RGD-conjugated MP hydrogel. (b) Sol–gel transition of the hydrogels. (c) Implantation of the composite hydrogel into the calvarial defects (reproduced from ref. 66 with permission from Royal Society of Chemistry, copyright 2020). | |
3.3 Cartilage tissue
Wang's team reported collagen-grafted 3D PCL scaffolds for chondrogenesis where 3D PCL scaffolds fabricated through electrohydrodynamic printing (E-Jetting) were functionalized with collagen through a polydopamine-mediated grafting process. The collagen-grafted PCL scaffold became hydrophilic, supporting cell attachment and allowed chondrocytes to preserve their healthy phenotypes. Chondrogenesis evaluation demonstrated a positive role of collagen in chondrogenesis simulation. This study showed the effectiveness of the collagen-grafted PCL scaffold in promoting cartilage regeneration.67 While adding ECM biomolecules like proteins and glycosaminoglycans (GAGs) enhances scaffold biological function, replicating native tissues by precisely controlling the hierarchical arrangement of these biomolecules remains challenging. Stevens's team developed peptide–polycaprolactone (PCL) conjugates that are covalently linked to glycosaminoglycans (GAGs), including hyaluronic acid (HA)-binding peptide–PCL (HAbind–PCL) and chondroitin sulfate (CS)–binding peptide–PCL (CSbind–PCL), incorporating bioactive sequences to target and concentrate GAGs within scaffolds for cartilage tissue engineering (Fig. 11a).68 GAGs like HA and CS are abundant in the extracellular matrix (ECM) and on cell membranes, playing critical roles in various cell–cell, cell–ECM, and protein interactions.69 The peptides developed in this study distinctly and non-covalently attach to HA and CS, mimicking dynamic interactions between native ECM components and proteins, which may enhance their functionality. HAbind–PCL (Fig. 11b) includes the RYPISRPRKR peptide from the HA-binding domain of the link protein, enhancing the interaction between HA and the proteoglycan aggrecan in the articular cartilage. CSbind–PCL (Fig. 11c) incorporates the YKTNFRRYYRF sequence identified through phage display, which binds to CS and prevents the neurite outgrowth inhibition.70 Maleimide-functionalized PCL was synthesized by the modification of the hydroxyl terminal group of PCL with a heterobifunctional linker, p-maleimidophenyl isocyanate. The peptides, which have a bioactive sequence connected to cysteine and a glycine spacer at the N-terminus, were subsequently conjugated to PCL by Thiol-Michael addition click reaction of the cysteine thiol group and maleimide group. To fabricate fibrous scaffolds, CSbind–PCL and HAbind–PCL were mixed with unmodified PCL in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) and then electrospun. The peptides specifically bind GAGs within the scaffold, which organize growth factors, chemokines, and cytokines to direct cell growth, differentiation, and migration in a variety of biological processes, including development and inflammation.
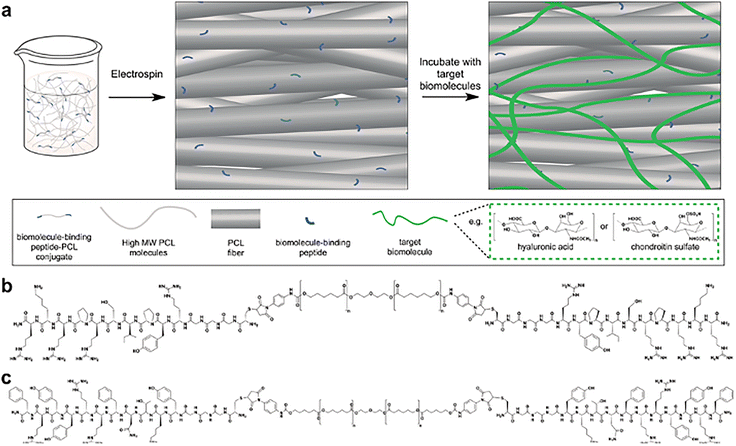 |
| | Fig. 11 (a) High-molecular-weight poly(ε-caprolactone) (PCL) that is surface functionalized with peptide–PCL conjugates by co-electrospinning. The chemical structures of (b) the hyaluronic acid (HA)-binding peptide–PCL conjugate (HAbind–PCL), featuring the specific binding sequence RYPISPRPKR and (c) the chondroitin sulphate (CS)-binding peptide–PCL conjugate (CSbind–PCL), containing the specific binding sequence YKTNFRRYYRF (reproduced from ref. 68 with permission from Wiley-VCH, copyright 2010). | |
Ao's team discovered a peptide sequence (E7) consisting of seven amino acids (EPLQLKM) with strong specificity for bone marrow-derived mesenchymal stem cells (MSCs) through phage display technology. They reported the creation of an MSC-homing device using synthetic E7 peptide conjugated to PCL electrospun meshes. Knee joints of rats were implanted with these E7-conjugated PCL electrospun meshes together with a microfracture, to stimulate mobilization of endogenous MSCs.71 In comparison to RGD-conjugated PCL meshes, the E7-conjugated PCL meshes showed a significantly higher expression of MSC surface markers and attracted fewer inflammatory cells in vivo. A particular peptide, named E7, was discovered from hBMMSC-affinity clones using an affinity assay. Electrospun PCL meshes were first prepared, and aminated PCL meshes were created by immersing the electrospun PCL in a 1,6-hexanediamine/isopropanol solution. To conjugate the E7 peptide to the aminated PCL meshes, sulfo-SMCC (sulfosuccinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate) was used as a cross-linker and the sulfo-SMCC solution was applied to the aminated meshes, and then washed with a conjugation buffer. The E7 peptide, dissolved in the same buffer, was subsequently applied to the sulfo-SMCC-treated PCL scaffolds. The cell proliferation, spreading, and adhesion of hBMMSCs within peptide-conjugated meshes were evaluated with two types of PCL meshes. The hBMMSCs attached adhered to fibers and interconnected between the fibers to form a three-dimensional network, demonstrating the effective attachment and adhesion properties of the E7-conjugated PCL meshes (Fig. 12).
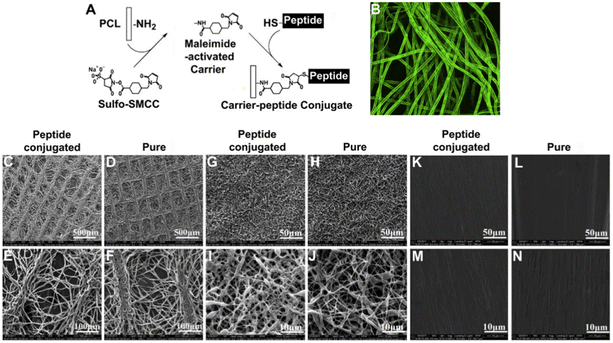 |
| | Fig. 12 SEM images of FITC-labeled peptides conjugated electrospun PCL fibers meshes (A). Confocal microscopy image of homogeneous green fluorescent PCL fibers (B). The structure of peptide-conjugated and pure PCL meshes with lattice (C–F), thick non-woven (G–J), and lower porosity patterns (K–N) examined via SEM (reproduced from ref. 71 with permission from Elsevier, copyright 2010). | |
4 Polylactide (PLA)
Polylactide (PLA) is a biodegradable polyester derived from materials such as rice and corn. A plethora of medical devices made with PLA have been approved by US Food and Drug Administration (FDA) for various applications. L-Lactic acid (LLA) and D-lactic acid (DLA) are two isomers of lactic acid.72,73 Despite known disadvantages such as poor cell interaction, slow rate of degradation, shrinkage,74 hydrophobicity and acidity of byproducts that may trigger an inflammatory response, PLA scaffolds have been employed widely in tissue engineering applications.75 PLA scaffolds can be fabricated through common material processing methods such as gas forming and electrospinning.74,76 PLA-based biomaterials have been widely used in bone tissue engineering applications due to their excellent mechanical properties, biodegradability and good processability. Surface modification is a promising strategy to improve osseointegration of the scaffold with surrounding bones.
4.1 Skin tissue
Modification of PLA with biomolecules was attempted by researchers to enhance its bioactivity for skin tissue engineering. Sidorenko's team77 synthesized covalent molecular brushes comprising chitosan, PLA and poly(N-vinyl pyrrolidone) (PNVP) for stimulating human dermal fibroblasts (Fig. 13). PLA was grafted by interfacial ring opening polymerization of lactide initiated from chitosan and PNVP is grafted by radical polymerization. Hydrophobic PLA and hydrophilic PNVP formed the hybrid chitosan-based molecular brushes. This is an interesting way of polymer modification with important biomaterials such as chitosan, for wound care material fabrication.
 |
| | Fig. 13 Molecular structures of Chitosan, PLA and poly(N-vinyl pyrrolidone) (reproduced from ref. 77 with from American Chemical Society, copyright 2020). | |
4.2 Bone tissue
Lin's team reported the surface pretreatment of PLA by atmospheric pressure plasma jet followed by chemical conjugation to RGD through amide bond formation.78 The RGD-immobilized PLA substrates promoted cell proliferation and showed enhanced osteogenic induction. Quan's team immobilized an oligopeptide (SSVPT, Ser-Ser-Val-Pro-Thr) derived from BMP-2 on 3D printed porous PLA via dopamine coating.79 BMP-2 is a multifunctional growth factor under the family of transforming growth factor β (TGF-β). The BMP-2 derived peptide functions as the receptor binding sites on BMP-2 for bone formation via strong stimulation of bone precursor cells. The scaffold revealed high osteoconductive properties to rat marrow mesenchymal stem cell proliferation. Bone regeneration was accelerated in the rat cranial bone defect model, where micro-CT scan showed bone growing from one side edge towards the center of the implantation site. Compared to direct immobilization of the peptide onto scaffold materials, the oligopeptide (SSVPT) in this work was firstly chemically bonded to polydopamine (PDA) to form an SSVPT–PDA conjugate as the coating layer to be immobilized on to the PLA scaffold. Thus, the final fabricated PLA–PDA–SSVPT scaffold is anticipated to be more effective and quality consistent compared to direction immobilization. Chatterjee's team modified the 3D printed PLA scaffold surface by the grafting of polyethyleneimine with citric acid with subsequent calcium phosphate deposition.80 Citric acid is found in bones and plays a key role in the precipitation of the apatite phase. Adhesion and proliferation of mesenchymal stem cells were promoted on surface functionalization with enhanced osteogenesis and mineralization.
RGD-coated hydroxyapatite (HA) disks have been reported to inhibit new bone formation due to the competing effects of RGD and adsorbed proteins for integrin receptors that negatively affects implant integration.81 To overcome this problem, Chen's team developed porous scaffolds by introducing RGD-conjugated triblock copolymer RGD/PEG–PLA–PGL into the HA/PLGA nanocomposite (Fig. 14a).82 The incorporation of the copolymer improved cell adhesion and growth of osteoblasts dramatically and showed larger new bone formation and better fusion interface in the rabbit defect model. Teo's team blended bioactive PLA-peptide copolymers constructed by pendant side chains of PLA and PEGylated RGD or (Gly-Pro-Hyp)3 with commercial PLA for 3D printing.53 Chen made the RGD conjugation through the amine group of RGD, instead of the coupling method from RGD's carboxylic group adopted by Teo. Interestingly, both RGD-modified bioactive PLA materials promoted osteogenic differentiation in vitro and reduced tissue inflammation in murine models, revealing diverse modification strategies through different functional groups of the RGD motif. There are reports about PLA implants causing surrounding tissues inflammation due to the acidic products arising from their degradation, so the ability of bioactive PLA to manage inflammatory response is beneficial. Chitosan-graft-PLA polymers were prepared by the “grafting to” approach via amine coupling chemistry to provide hybrid biomaterials with improved mechanical stability and bioactivity (Fig. 14b).83 The hybrid copolymers exhibited improved cell adhesion and higher proliferation of pre-osteoblastic cells.
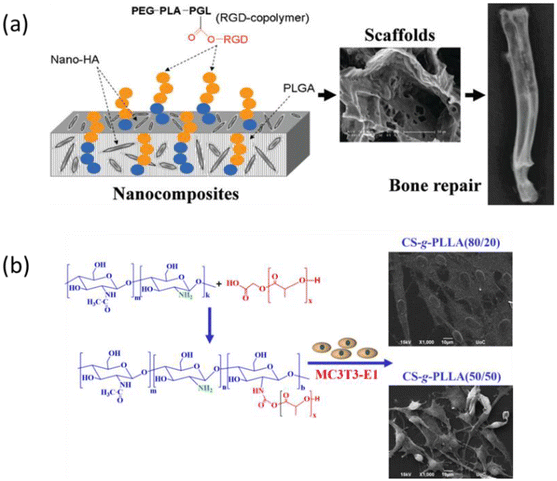 |
| | Fig. 14 Hybrid polymers of PLA for bone repair. (a) Porous scaffold of RGD-conjugated copolymer with the PLGA/HA composite. The scaffold was able to regenerate new bone with better fusion interface in a rabbit model (reproduced from ref. 82 with permission from American Chemical Society, copyright 2011). (b) Chitosan-g-PLLA copolymers prepared via the “grafting to” approach with good pre-osteoblastic cell adhesion (reproduced from ref. 83 with permission from MDPI, copyright 2020). | |
4.3 Cartilage tissue
Kai's team reported lignin grafted PLA (lignin-PLA) nanofibers designed to promote cell growth and create a native-like environment that mimics the cartilage tissue matrix for regenerative purposes (Fig. 15).84 The lignin–PLA nanofibers were synthesized by grafting PLA onto lignin through ring opening polymerization, followed by electrospinning. These lignin–PLA nanofibers enhanced the miscibility of lignin within fibers, providing enhanced mechanical properties, and demonstrated good biocompatibility with multiple cell lines in vitro.
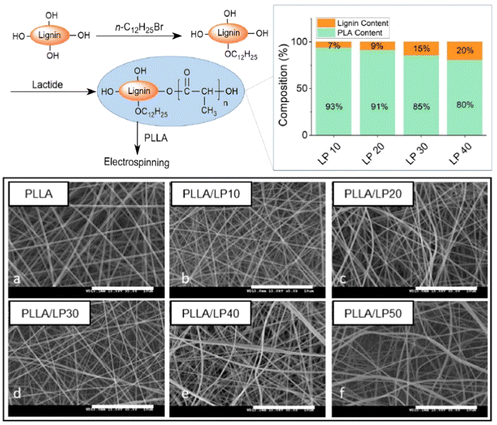 |
| | Fig. 15 Synthesis protocol for lignin-PLA (LP) copolymers, along with their respective copolymer composites, and the morphology of electrospun PLLA/LP nanofibers (reproduced from ref. 84 with permission from BMC, copyright 2022). | |
5 Poly(lactic-co-glycolic acid) (PLGA)
Biodegradable polyester PLGA has been used in skin regeneration materials with various techniques85–88 such as 3D printing, electrospinning, gas forming, phase separation, and fiber bonding. PLGA is one of the few synthetic materials that have been used in medical devices approved by the FDA for clinical applications. However, PLGA on its own, similar to PLA, is limited by its hydrophobicity for skin tissue application and is known to be proinflammatory upon degradation. Furthermore, PLGA does not mimic the important properties of tissues and cartilage.89 Thus strategies to improve scaffold properties such as biocompatibility, cell proliferation and collagen deposition, have been investigated.87
5.1 Skin tissue
Yoo's team90 reported a S-nitrosoglutathione (GSNO)-conjugated PLGA polymer (Fig. 16) for infected cutaneous wound treatment. NO exhibits beneficial effects on inflammation modulation during the wound healing process. Through the formation of reactive nitrogen species, NO can induce bacterial cell death through the destruction of bacterial cell membranes, proteins, and DN. This property allows it to become a potent antibacterial agent for broad-spectrum activity in infected wound treatments. The polymer GSNO–PLGA was synthesized via coupling of PLGA and the primary amine group of GSNO. The loading efficiency of GSNO was remarkably improved during the nanoparticle fabrication process because the hydrophilic GSNO was covalently conjugated to hydrophobic PLGA to minimize the loss of GSNO. Although DMSO is non-toxic, it is still important to remove solvent traces from medical device materials and complete removal of DMSO in large scale productions can be challenging.
 |
| | Fig. 16 Synthesis of S-nitrosoglutathione-conjugated PLGA (GSNO–PLGA) (reproduced from ref. 90 with permission from MDPI, copyright 2020). | |
Recently, Teo's team has reported chemically-bonded PLGA-peptide brush copolymers obtained via ring opening metathesis polymerization for use in dermal matrices (Fig. 17).91 The device was termed “bioactive PLGA dermal matrix”. The overall stability of this PLGA in the physiological system and the subsequent localized therapeutic efficacy at the wound sites are enhanced via covalent bonding of biomolecules to synthetic polymers. In vivo porcine burn model revealed that this bioactive PLGA dermal matrix healed partial thickness burn wounds within 21 days with minimal inflammation, as compared to clinical gold standard Biobrane™, which showed visible redness in wound even after 21 days, with measurable inflammation in tissue biopsy. Further studies on the use of bioactive PLGA in other medical devices such as bioactive wound dressings for various wound types, resorbable sutures, skin patch for scar prevention in incisional wounds, are underway in Teo's lab.
 |
| | Fig. 17 Bioactive PLGA dermal scaffold for burn wound treatment constructed by 3D printing (reproduced from ref. 91 with permission from American Chemical Society, copyright 2023). | |
5.2 Bone tissue
Cho's team reported a biologically inspired strategy to prepare a functionalized PLGA scaffold.92 Polydopamine (pDA), commonly observed in the mussel adhesive protein, was coated onto the 3D PLGA scaffold followed by immobilization of the BMP-2 derived peptide via catechol chemistry (Fig. 18a). Scaffolds were found to greatly enhance in vitro osteogenic differentiation and calcium mineralization of human adipose-derived stem cells and promoted in vivo bone formation in mouse calvarial bone defects. As with earlier discussion, the immobilization method is uncontrollable and can result in batch-to-batch variation. Quality consistency cannot be guaranteed during scale up production. Zhang's team used a similar pDA-assisted immobilization strategy to attach collagen mimetic peptide (P15) and osteogenic growth peptide (OGP) onto the HA/PLGA substrate (Fig. 18b).93 Cell adhesion and proliferation were promoted via the incorporation of the collagen mimetic peptide while the OGP induced osteodifferentiation of cells. Shao's team loaded bioactive molecules GFOGER and BMP-9 in the 3D printed porous PLGA scaffold and observed enhanced new bone mineral deposition in the rabbit model.94 The biomolecules up-regulated the expression of Runx7, OCN, COL-1 and SP7 to promote new bone regeneration. Lee's team demonstrated surface modification of PLGA with glycol chitosan via the immobilization of BMP-2 using EDC/NHS chemistry.95 The modified PLGA-GCH-BMP-2 scaffold revealed improved in vitro calcium mineralization and osteogenic differentiation of human adipose derived stem cells using osteogenic medium. Wick's team developed a novel multicomponent nano-hydroxyapatite–PLGA-collagen biomaterial (nHAP–PLGA-collagen) with similar mechanical properties to human cancellous bone.96 nHAP–PLGA was first prepared using nanoparticles of HAP as the initiator and collagen was conjugated to nHAP–PLGA by N-hydroxysuccinimide modification. The expression of ALP, osteocalcin and mineral deposition were achieved via human mesenchymal stem cells cultivated onto the 3D scaffold. Notably, the kinetics of minerals deposition was similar to osteogenesis in vivo.
 |
| | Fig. 18 Polydopamine-assisted peptide immobilization of the PLGA scaffold. (a) BMP-2 peptide immobilized PLGA scaffold with improved osteogenic differentiation and in vivo bone formation in the mouse model (reproduced from ref. 92 with permission from American Chemical Society, copyright 2013). (b) Collagen mimetic peptide (P15) and osteogenic growth peptide (OGP) immobilized onto the HA/PLGA substrate increased cell adhesion and differentiation (reproduced from ref. 93 with permission from American Chemical Society, copyright 2016). | |
5.3 Cartilage tissue
Hyaluronic acid (HA) plays a crucial role in cartilage tissue regeneration. The CD44 receptor, found on chondrocyte surfaces, has a strong affinity for HA. As a result, injectable HA-based microgels, functionalized with active molecules, can precisely target chondrocytes. Park's team developed a HA-coated microporous PLGA scaffold for this purpose (Fig. 19).97 A PLGA–PEG di-block copolymer with amine groups (PLGA–PEG–NH2) was prepared by coupling the reaction of PEG diamine with NHS-activated PLGA. This copolymer was then blended with PLGA in varying amounts, dissolved in methylene chloride, and cast into films, which were dried at room temperature and under vacuum. To conjugate hyaluronic acid (HA) to the PLGA blend, carboxylic groups of HA were activated using HOBt and EDC in PBS (pH 6.8). The PLGA films (or scaffolds) were applied in HA solution for 6 hours with gentle stirring to allow chemical conjugation. Afterward, the HA/PLGA scaffolds were washed and vacuum-dried. These HA/PLGA scaffolds enhanced chondrocyte attachment, proliferation, and differentiation, aiding in cartilage repair. RT-PCR and histological analyses confirmed enhanced collagen type II expression and morphological tissue changes indicative of cartilage formation.
 |
| | Fig. 19 HA surface modification scheme of biodegradable polymer scaffolds (reproduced from ref. 97 with permission from the Elsevier, copyright 2005). | |
Cruz's team developed hyaluronic acid (HA) conjugated poly(lactide-co-glycolide) (PLGA) nanoparticles (NPs) to enhance the target specificity of the drug to the osteoarthritic knee joint (Fig. 20). PLGA-grafted sodium hyaluronate was prepared using dimethoxy-PEG (PLGA–HA) and compared to PLGA NPs without grafting as a control. The NPs were loaded with near-infrared (NIR) dye and 20 nm size gold nanoparticles for in vitro, in vivo, and ex vivo testing. The results showed that NPs were of appropriate size for cellular uptake and did not exhibit cytotoxicity to chondrocytes.98
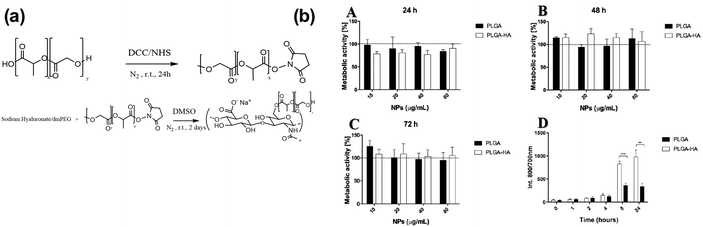 |
| | Fig. 20 (a) Schematic of the synthesis process for the PLGA–HA polymer, (b) a cell metabolic activity (MTS) assay was performed to evaluate the effects of two different types of nanoparticles (NPs) on the C28/I2 cell line at various concentrations. Metabolic cell activity: (A) 24 h, (B) 48 h, and (C) 72 h. Positive control used 50% DMSO, while the negative control involved only cells, indicated by the black horizontal line. (D) Binding assay: PLGA and PLGA–HA NPs were incubated with the C28/I2 cell line at 40 μg mL−1 was performed at different time intervals. No significant difference (p > 0.05) was found between PLGA–HA NPs and PLGA NPs at 0, 1, 2, and 4 h. Significant differences (p < 0.05) were observed between PLGA–HA NPs and PLGA NPs after 8 h of cell incubation, with more significant differences (p < 0.001) after 24 hours (n = 3) (reproduced from ref. 98 with permission from MDPI, copyright 2022). | |
Gu's team reported a novel strategy of cross-linkable matrix integrated with nanoparticles loaded with Kartogenin (KGN) to create the natural hyaline cartilage by a simple method (Fig. 21).99 Kartogenin (KGN) induces the differentiation of MSCs into chondrocytes and promotes cartilage repair. Biodegradable poly(lactic-co-glycolic acid) (PLGA) was applied to encapsulate KGN nanoparticles by an emulsion-based formulation method. A UV light photo-crosslinkable monomer, acrylated hyaluronic acid (m-HA), was used to prepare a hydrogel scaffold to provide a supportive matrix structure for cell regeneration, improve nanoparticle stability, and reduce burst release. Hyaluronic acid (HA) was modified by reacting it with methacrylic anhydride (MA) to introduce a double bond, resulting in m-HA. HA was first dissolved in cold distilled water overnight, followed by the addition of MA to the solution. The pH of the reaction solution was maintained at 8–9 and stirred at 4 °C for 24 h. The purified m-HA was then obtained by dialysis for 48 h followed by lyophilization. KGN-loaded PLGA nanoparticles are incorporated into the HA matrix to provide continuous release of KGN, which supports rapid defect filling and the formation of hyaline cartilage.
 |
| | Fig. 21 (A) A diagram illustrating KGN-loaded PLGA nanoparticles, the molecular structures of KGN, and acrylated hyaluronic acid (m-HA). (B) Surgical procedure for repairing cartilage defects. (C) Hyaline cartilage formation through chondrogenesis with a photo-crosslinked HA scaffold containing KGN-loaded nanoparticles (reproduced from ref. 99 with permission from American Chemical Society, copyright 2016). | |
6 Polyethylene (PE)
The properties of polyethylene (PE) such as non-toxicity, thermal stability, high mechanical strength and inertness of PE allow it to be widely used in biomaterial applications. Yet, foreign body responses from PE-based implants caused by prolonged inflammation have received much attention from the clinical and materials research community.100 Extensive research has been conducted in order to improve the biocompatibility of polyethylene.101 Surface modification and incorporation of bioactive motifs have been used to improve bioactivity and biocompatibility of implants fabricated with PE.
6.1 Skin tissue
By incorporating biomolecules such as integrin-binding RGD, collagen mimic (GPHyp)3, laminin-derived peptide (AGQWHRVSVRWGC) (A5G81) and hyaluronic acid (HA), into PE-brush copolymers, a series of skin regenerative PE-based non-woven dressings were developed via dip coating techniques by Teo's team (Fig. 22).102 Cost-effectiveness of both polyethylene and non-woven cloth allow such materials to be used as disposable dressings. Due to the non-biodegradation, PE-based bioadditives also prevent undesirable adverse effects from byproduct degradation affecting the physiological conditions. Air and moisture permeability of these bioactive PE-coated non-woven dressings facilitate exudate management because of the porosity design. In vivo porcine wound model studies revealed that these non-woven dressings accelerated the wound healing process in full thickness wounds compared to foam dressings for chronic wound management, Allevyn™ in clinical standard practice.
 |
| | Fig. 22 Schematic illustration of bioactive PE-coated non-woven dressings for full-thickness wound treatment (reproduced from ref. 102 with permission from Elsevier, copyright 2024). | |
6.2 Bone tissue
Teo's team synthesized a bioactive PE-peptide brush copolymer with side arms of PE and PEGylated biomolecules including RGD and (GPHyp)3. The copolymers were formulated with UHMWPE, extruded into filaments and 3D-printed into sheets via fused filament fabrication methods. The bioactive PE scaffold was promoted with osteogenic activity whilst inflammatory response was reduced relative to pure UHMWPE as determined by the in vitro alkaline phosphatase (ALP) assay and using an in vivo murine model (Fig. 23).54
 |
| | Fig. 23 Schematic illustration of bioactive PE-peptide brush copolymers with enhanced osteogenic activity (reproduced from ref. 54 with permission from Royal Society of Chemistry, copyright 2023). | |
7 Conclusions
The overall outlook of biohybrid polymers for tissue engineering or medical use is exciting and promising. Numerous organic and/or polymer synthesis strategies have been reported by various groups around the world for the development of biomolecule-modified synthetic polymers. Skin, bone and cartilage have been successfully regenerated using these synthetic biomaterials for applications in wound healing and orthopedic devices. Such chemical modification of synthetic polymers with biomolecules to create bioactive materials provides an alternative to simple blending of biomolecules with polymers, which can be challenging if the polymer is highly hydrophobic. With the constant development of advanced organic synthesis methodologies and new catalysts that enable previously impossible or low-yielding reactions between biomolecules and synthetic polymers being reported, the formation of wide ranging biohybrid polymers with varying bioactivities is made possible now. The ability to regenerate tissues using these biohybrid polymers is exciting and paves the way for regenerative medicine using medical devices made of such materials. Such acellular tissue regeneration methods may also reduce treatment costs and risk of cancer or nerve damage from the use of stem cells or growth factors in treatment. Furthermore, with an overall increase in medical awareness and the education level of global population, there is a rising demand for better treatment outcomes or even personalized medicine. The use of advanced manufacturing methods to prepare implantable medical devices has gained significant interest both as a means to produce customized implants and to prepare implants with high complexity that cannot be produced using conventional methods. Customized implants are particularly useful in orthopedics to repair irregular shaped bone defects. Advanced manufacturing methods using biohybrid materials to produce customized tissue regenerative implants would thus require the materials to be able to withstand the processing conditions of such manufacturing methods in terms of rheological properties and thermal stability.103
8 Challenges and future perspectives
The challenge for biomaterial development for use in medical devices often lies in the scalability of the materials both in terms of raw material or reagent cost and number of synthesis steps. For future biomaterial development for medical device fabrication, it is important to note the downstream implications in manufacturing when designing a material synthesis strategy or selecting a biomolecule, to allow the intended medical device to be commercially viable. Tedious multi-step synthesis with challenging purification steps or the use of highly complex biomolecules is unfavorable in this respect as it would increase material manufacturing costs significantly which gets passed on to the cost of the medical device. With increasing concerns over ballooning healthcare costs by governments worldwide, new medical devices need to be reasonably priced for widespread adoption. Without clinical adoption of the intended medical device, the proposed biomaterial would be stuck in the research phase with little benefit to patients. Furthermore, new materials are subjected to stringent chemical characterization tests for biocompatibility.
Purification of materials and eventual clearance of biocompatibility and toxicology tests of the intended device made from these materials are further challenges for biohybrid polymer development. The use of metal catalysts that are difficult to remove effectively from the final material can pose a challenge to obtaining favorable testing outcomes in toxicological tests of the medical device fabricated from these materials. Hence, in order to fully exploit the clinical benefits of biohybrid materials, the intended use of biomaterials in medical device production should be considered as early as possible in the material design phase to avoid disappointment during the material scale up process or device fabrication stage. For example, it would be useful to keep material synthesis within five steps, select metal catalysts that can be easily removed from the end product and avoid the use of solvents with very high boiling points such as DMSO and DMF for ease of solvent removal during large scale production, or solvents with low flash points, due to safety considerations during large scale manufacturing.
Regulatory considerations for implants using novel materials at the design phase of the implant would be extremely helpful to avoid disappointment at the end. Important tests to include during the implant safety tests include ISO 10993-18, chemical characterization, on top of standard ISO10993 tests such as cytotoxicity, pyrogenicity, skin sensitization, intracutaneous reactivity and acute systemic toxicity. Chemical leachables and extracts in polar and apolar solvents are tested for toxic substances. Toxicology studies are critical in ascertaining the safety limits of the implant based on the duration and quantity of use. For example, the toxicology data obtained from chemical characterization studies in ISO10993-18 would advise how many sheets of wound dressings of a particular size a patient can receive within a specified duration, before the toxicity limit is reached. With appropriate safety tests being conducted by certified testing laboratories and guidance of the research team by an experienced regulatory consultant for a specific country of interest in regulatory clearance, it is possible for medical devices derived from such novel materials to obtain regulatory approval. A successful example of a wound care product derived from a novel biohybrid material having obtained FDA 510(k) approval would be PuraDerm gel (FDA 510(k) no. K193085). The PLGA–RGD based skin substitute reported by Teo et al. has completed First-in-Human studies in 10 patients and is currently being prepared for regulatory submission in Singapore and USA.92
Besides regulatory considerations, mechanism of action for the novel materials, how they interact with the physiological system, can be studied to help advice future material and medical device development teams, on the use of such materials for tissue repair. There have been studies on individual biomolecules’ interaction with specific cells in the body and how they may potentially facilitate tissue repair. However, whether the same mechanism applies when the biomolecule is on the synthetic polymer and finally, as a medical device on the patient's body, is new and would require further investigation. For example, it is reported that RGD interacts with α5β1 integrin for granulation tissue formation, αVβ6 and αVβ8 for immunomodulation.29 However, whether the mechanism of action remains the same after RGD is bound to a synthetic polymer and fabricated into a wound dressing, requires further investigation.
Finally, with appropriate design of these biohybrid polymers both for clinical benefits and eventual material and device production, we believe there is much potential for biohybrid polymers to improve clinical outcomes for wound care products and orthopedic implants, especially in regenerative medicine, which is gaining popularity amongst patients and clinicians. The future of regenerative medicine and tissue engineering using biohybrid polymers is bright if material design considerations for manufacturing viability can be considered at early phases of material development.
Author contributions
The manuscript was written through contributions of all authors. E. J. Park, J. Guo, Y. C. Teo and P. Teo wrote the main manuscript text and prepared figures. P. Teo provided the concept and supervised the writing. All authors have given approval to the final version of the manuscript. E. J. Park and J. Guo contributed equally to this work.
Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.
Conflicts of interest
The authors declare no competing financial interest.
Acknowledgements
This work is supported by generous research grants from Agency for Science, Technology and Research (A*STAR) Singapore, grant number M24N9b0123.
References
- J. L. Frodel and S. Lee, The use of high-density polyethylene implants in facial deformities, Arch. Otolaryngol. Head Neck Surg., 1998, 124(11), 1219–1223 CrossRef CAS PubMed.
- J. P. Claverie and F. Schaper, Ziegler-Natta catalysis: 50 years after the Nobel Prize, MRS Bull., 2013, 38, 213–218 CrossRef CAS.
- I. K. Açarı,
et al., Chemistry and engineering of brush type polymers: Perspective towards tissue engineering, Adv. Colloid Interface Sci., 2022, 305, 102694–102719 CrossRef PubMed.
- B. D. Ulery, L. S. Nair and C. T. Laurencin, Biomedical Applications of Biodegradable Polymers, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 832–864 CrossRef CAS PubMed.
- Z. Terzopoulou,
et al., Biocompatible Synthetic Polymers for Tissue Engineering Purposes, Biomacromolecules, 2022, 23, 1841–1863 CrossRef CAS PubMed.
- S. D'Onofri and F. Bignotti, Surface modification of polyamide 12 angioplasty balloons byphotochemical reaction with an aromatic azide, Polym. Adv. Technol., 2019, 30, 51–57 CrossRef.
- C. Adamzyk,
et al., Bone tissue engineering using polyetherketoneketone scaffolds combined with autologous mesenchymal stem cells in a sheep calvarial defect model, J. Craniomaxillofac. Surg, 2016, 44(8), 985–994 CrossRef PubMed.
- P. Gentile,
et al., An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering, Int. J. Mol. Sci., 2014, 15, 3640–3659 CrossRef CAS PubMed.
-
L. W. McKeen, Plastics Used in Medical Devices, in Handbook of Polymer Applications in Medicine and Medical Devices, ed. W. Andrew, Elsevier, 1st edn, 2013 Search PubMed.
- J. Vienken and C. Boccato, Do medical devices contribute to sustainability? The role of innovative polymers and device design, Int J Artif Organs, 2024, 47(4), 240–250 CrossRef PubMed.
- M. Maaz Arif,
et al., Polymer-based biomaterials for chronic wound management: Promises and challenges, Int. J. Pharm., 2021, 598, 120270 CrossRef CAS PubMed.
- J. Giron,
et al., Biomaterials for bone regeneration: an orthopedic and dentistry overview, Braz. J. Med. Biol. Res., 2021, 54(9), e11055 CrossRef CAS PubMed.
- C. Yang,
et al., Tissue engineering strategies hold promise for the repair of articular cartilage injury, Biomed Eng Online, 2024, 23(1), 92 CrossRef PubMed.
- L. Crawford,
et al., Biocompatibility Evolves: Phenomenology to Toxicology to Regeneration, Adv. Healthc. Mater., 2021, 10(11), e2002153 CrossRef PubMed.
- W. Al-Zyoud,
et al., Biocompatibility Testing for Implants: A Novel Tool for Selection and Characterization, Materials, 2023, 16(21), 6881 CrossRef CAS PubMed.
-
ISO 10993-1:2018 Biological evaluation of medical devices—Part 1: Evaluation and testing within a risk management process, ed. ISO, 2018, ISO, Switzerland Search PubMed.
- H. Ma,
et al., 3D-printed bioceramic scaffolds: From bone tissue engineering to tumortherapy, Acta Biomater., 2018, 79, 37–59 CrossRef CAS PubMed.
- F. Pupilli,
et al., Design Strategies and Biomimetic Approaches for Calcium Phosphate Scaffolds in Bone Tissue Regeneration, Biomimetics, 2022, 7(3), 112 CrossRef CAS PubMed.
- Z. Li,
et al., Vitamin E highly cross-linked polyethylene reduces mid-term wear in primary total hip replacement: a meta-analysis and systematic review of randomized clinical trials using radiostereometric analysis, EFORT Open Rev., 2021, 6(9), 759–770 CrossRef PubMed.
- A. K. Gaharwah, I. Singh and A. Khademhosseini, Engineered biomaterials for in situ tissue regeneration, Nat. Rev. Mater., 2020, 2084 Search PubMed.
- J. Qin,
et al., Recent Advances in Bioengineered Scaffolds for Cutaneous Wound Healing, Front. Bioeng. Biotechnol., 2022, 10, 841583 CrossRef PubMed.
- M. Sheikholeslam,
et al., Biomaterials for Skin Substitutes, Adv. Healthc. Mater., 2018, 7(5), 1700897 CrossRef PubMed.
- G. Kaur,
et al., Biomaterials-Based Regenerative Strategies for Skin Tissue Wound Healing, ACS Appl. Bio Mater., 2022, 5(5), 2069–2106 CrossRef CAS PubMed.
- P. Lou,
et al., Extracellular vesicle-based therapeutics for the regeneration of chronic wounds: current knowledge and future perspectives, Acta Biomater., 2021, 119, 42–56 CrossRef CAS PubMed.
- Z. A.-O. Terzopoulou,
et al., Biomacromolecules, 2022, 23(5), 1841–1863 CrossRef CAS PubMed.
- İ.,K. Açarı,
et al. Chemistry and engineering of brush type polymers: Perspective towards tissue engineering, Adv. Colloid Interface Sci., 2022, 305, 102694 CrossRef PubMed.
- Y. Wang,
et al., Hyaluronan oligosaccharides promote diabetic wound healing by increasing angiogenesis, Pharmacol. Rep., 2016, 68(6), 1126–1132 CrossRef CAS PubMed.
- Y. Zhu,
et al., Potent laminin-inspired antioxidant regenerative dressing accelerates wound healing in diabetes, Proc. Natl. Acad. Sci. U. S. A., 2018, 115(26), 6816–6821 CrossRef CAS PubMed.
- L. Koivisto,
et al., Integrins in Wound Healing, Adv. Wound Care, 2014, 3(12), 762–783 CrossRef PubMed.
- A. Szwed-Georgiou,
et al., Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems, ACS Biomater. Sci. Eng., 2023, 9(9), 5222–5254 CrossRef CAS PubMed.
- Y. Hamada,
et al., Angiogenic activity of osteopontin-derived peptide SVVYGLR, Biochem. Biophys. Res. Commun., 2003, 310(1), 153–157 CrossRef CAS PubMed.
- D. J. Huey, J. C. Hu and K. A. Athanasiou, Unlike bone, cartilage regeneration remains elusive, Science, 2012, 338(6109), 917–921 CrossRef CAS PubMed.
- M. Wang,
et al., Articular cartilage repair biomaterials: strategies and applications, Mater. Today Bio, 2024, 24, 100948 CrossRef CAS PubMed.
- H. J. Liao, H. T. Chen and C. H. Chang, Peptides for Targeting Chondrogenic Induction and Cartilage Regeneration in Osteoarthritis, Cartilage, 2024, 19476035241276406 CrossRef PubMed.
- J. Zhu, Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering, Biomaterials, 2010, 31(17), 4639–4656 CrossRef CAS PubMed.
- A. El-Ghalbzouri,
et al., The use of PEGT/PBT as a dermal scaffold for skin tissue engineering, Biomaterials, 2004, 25(15), 2987–2996 CrossRef CAS PubMed.
- J. Odrobinska and D. Neugebauer, PEG Grafted Polymethacrylates Bearing Antioxidants as a New Class of Polymer Conjugates for Application in Cosmetology, Materials, 2020, 13(16), 3455 CrossRef CAS PubMed.
- K. Zdunska,
et al., Antioxidant Properties of Ferulic Acid and Its Possible Application, Skin Pharmacol. Physiol., 2018, 31(6), 332–336 CrossRef CAS PubMed.
- D. Farhat and H. Lincet, Lipoic acid a multi-level molecular inhibitor of tumorigenesis, Biochim. Biophys. Acta, Rev. Cancer, 2020, 1873(1), 188317 CrossRef CAS PubMed.
- J. C. Wataha, C. T. Hanks and R. G. Craig, In vitro synergistic, antagonistic, and duration of exposure effects of metal cations on eukaryotic cells, J. Biomed. Mater. Res., 1992, 26(10), 1297–1309 CrossRef CAS PubMed.
- I. Ydens,
et al., Removal of copper-based catalyst in atom transfer radicalpolymerization using different extraction techniques, e-Polymers, 2004, 4(1), 039 Search PubMed.
- M. S. Messina,
et al., Preparation of Biomolecule-Polymer Conjugates by Grafting-From Using ATRP, RAFT, or ROMP, Prog. Polym. Sci., 2020, 100, 101186 CrossRef CAS PubMed.
- A. Vijayan, S. A and G. S. V. Kumar, PEG grafted chitosan scaffold for dual growth factor delivery for enhanced wound healing, Sci. Rep., 2019, 9(1), 19165 CrossRef CAS PubMed.
- C. You,
et al., Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation, Sci. Rep., 2017, 7(1), 10489 CrossRef PubMed.
- J. K. Patra,
et al., Nano based drug delivery systems: recent developments and future prospects, J. Nanobiotechnol., 2018, 16(1), 71 CrossRef PubMed.
- H. Ueno,
et al., Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs, Biomaterials, 1999, 20(15), 1407–1414 CrossRef CAS PubMed.
- E. Khor and L. Y. Lim, Implantable applications of chitin and chitosan, Biomaterials, 2003, 24(13), 2339–2349 CrossRef CAS PubMed.
- S. Senel and S. J. McClure, Potential applications of chitosan in veterinary medicine, Adv. Drug Delivery Rev., 2004, 56(10), 1467–1480 CrossRef CAS PubMed.
- S.-H. Chen,
et al., Synthesis and Characterization of Reinforced Poly(ethylene glycol)/Chitosan Hydrogel as Wound Dressing Materials, Macromol. Mater. Eng., 2013, 298(4), 429–438 CrossRef CAS.
- E. Lih,
et al., Rapidly curable chitosan-PEG hydrogels as tissue adhesives for hemostasis and wound healing, Acta Biomater., 2012, 8(9), 3261–3269 CrossRef CAS PubMed.
- J. Zhu,
et al., Extracellular Matrix-like Cell-Adhesive Hydrogels from RGD-Containing Poly(ethylene glycol) Diacrylate, Macromolecules, 2006, 39(4), 1305–1307 CrossRef CAS.
- H. Wang,
et
al., A Photoresponsive Hyaluronan Hydrogel Nanocomposite for Dynamic Macrophage Immunomodulation, Adv. Healthc. Mater., 2019, 8(4), e1801234 CrossRef PubMed.
- Y. C. Teo,
et al., Bioactive PCL-Peptide and PLA-Peptide Brush Copolymers for Bone Tissue Engineering, ACS Appl. Bio Mater., 2022, 5(10), 4770–4778 CrossRef CAS PubMed.
- J. Guo,
et al., Bioactive polyethylene synthesized by ring opening metathesis polymerization for potential orthopaedic applications, Polym. Chem., 2023, 14(15), 1743–1751 RSC.
- T. Luo and K. L. Kiick, Collagen-like peptides and peptide-polymer conjugates in the design of assembled materials, Eur. Polym. J., 2013, 49(10), 2998–3009 CrossRef CAS PubMed.
- T. Kaur, A. Joshi and N. Singh, Natural cocktail of bioactive factors conjugated on nanofibrous dressing for improved wound healing, Biomater. Adv., 2022, 143, 213163 CrossRef CAS PubMed.
- H. Cao,
et al., Gelatin-based biomaterials and gelatin as an additive for chronic wound repair, Front. Pharmacol., 2024, 15, 1398939 CrossRef CAS PubMed.
- S. Bhowmick,
et al., Nanofibrous artificial skin substitute composed of mPEG-PCL grafted gelatin/hyaluronan/chondroitin sulfate/sericin for 2(nd) degree burn care: in vitro and in vivo study, RSC Adv., 2018, 8(30), 16420–16432 RSC.
- M. H. Turabee, T. Thambi and D. S. Lee, Development of an Injectable Tissue Adhesive Hybrid Hydrogel for Growth Factor-Free Tissue Integration in Advanced Wound Regeneration, ACS Appl. Bio Mater., 2019, 2(6), 2500–2510 CrossRef CAS PubMed.
- Q. Wang,
et al., Osteogenic growth peptide-loaded 3D-printed PCL scaffolds for the promotion of osteogenesis through the ERK pathway, Mater. Des., 2020, 193, 108811 CrossRef CAS.
- A. W. James,
et al., A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2, Tissue Eng., Part B, 2016, 22(4), 284–297 CrossRef CAS PubMed.
- H. Zhang,
et al., Chemically-Conjugated Bone Morphogenetic Protein-2 on Three-Dimensional Polycaprolactone Scaffolds Stimulates Osteogenic Activity in Bone Marrow Stromal Cells, Tissue Eng., Part A, 2010, 16(11), 3441–3448 CrossRef CAS PubMed.
- S. Beauvais,
et al., Modulation of MAPK signalling by immobilized adhesive peptides: Effect on stem cell response to BMP-9-derived peptides, Acta Biomater., 2016, 31, 241–251 CrossRef CAS PubMed.
- L. Li,
et al., Dual-Peptide-Functionalized Nanofibrous Scaffolds Recruit Host Endothelial Progenitor Cells for Vasculogenesis to Repair Calvarial Defects, ACS Appl. Mater. Interfaces, 2020, 12(3), 3474–3493 CrossRef CAS PubMed.
- M. Wiens,
et al., Characterization and osteogenic activity of a silicatein/biosilica-coated chitosan-graft-polycaprolactone, Acta Biomater., 2014, 10(10), 4456–4464 CrossRef CAS PubMed.
- H. J. Kim,
et al., Injectable hydrogels based on MPEG–PCL–RGD and BMSCs for bone tissue engineering, Biomater. Sci., 2020, 8(15), 4334–4345 RSC.
- Y. Cai,
et al., Collagen grafted 3D polycaprolactone scaffolds for enhanced cartilage regeneration, J. Mater. Chem. B, 2013, 1(43), 5971–5976 RSC.
- L. W. Chow,
et al., Peptide-directed spatial organization of biomolecules in dynamic gradient scaffolds, Adv. Healthc. Mater., 2014, 3(9), 1381–1386 CrossRef CAS PubMed.
- O. Guillame-Gentil,
et al., Engineering the extracellular environment: Strategies for building 2D and 3D, Adv. Mater., 2010, 22(48), 5443–5462 CrossRef CAS PubMed.
- K. C. Butterfield, A. W. Conovaloff and A. Panitch, Development of affinity-based delivery of NGF from a chondroitin sulfate biomaterial, Biomatter, 2011, 1(2), 174–181 CrossRef PubMed.
- Z. Shao,
et al., Polycaprolactone electrospun mesh conjugated with an MSC affinity peptide for MSC homing in vivo, Biomaterials, 2012, 33(12), 3375–3387 CrossRef CAS PubMed.
- B. Gupta, N. Revagade and J. Hilborn, Poly(lactic acid) fiber: An overview, Prog. Polym. Sci., 2007, 32(4), 455–482 CrossRef CAS.
- M. L. Becker and J. A. Burdick, Introduction: Polymeric Biomaterials, Chem. Rev., 2021, 121(18), 10789–10791 CrossRef CAS PubMed.
- W. Cui,
et al., Evaluation of electrospun fibrous scaffolds of poly(dl-lactide) and poly(ethylene glycol) for skin tissue engineering, Mater. Sci. Eng., C, 2009, 29(6), 1869–1876 CrossRef CAS.
-
X. Lin, et al., Poly(Lactic Acid)-Based Biomaterials: Synthesis, Modification and Applications, in Biomedical Science, Engineering and Technology, ed. N. G. Dhanjoo, IntechOpen, Rijeka, 2012, ch. 11 Search PubMed.
- K. L. Kim,
et al., Enhanced dermal wound neovascularization by targeted delivery of endothelial progenitor cells using an RGD-g-PLLA scaffold, Biomaterials, 2009, 30(22), 3742–3748 CrossRef CAS PubMed.
- M. Chawathe,
et al., Adaptive Hybrid Molecular Brushes Composed of Chitosan, Polylactide, and Poly(N-vinyl pyrrolidone) for Support and Guiding Human Dermal Fibroblasts, ACS Appl. Bio Mater., 2020, 3(7), 4118–4127 CrossRef CAS PubMed.
- F.-C. Kung,
et al., Dual RGD-immobilized poly(L-lactic acid) by atmospheric pressure plasma jet for bone tissue engineering, Colloids Surf., B, 2019, 178, 358–364 CrossRef CAS PubMed.
- X. Zhang,
et al., Immobilization of BMP-2-derived peptides on 3D-printed porous scaffolds for enhanced osteogenesis, Biomed. Mater., 2020, 15(1), 015002 CrossRef CAS PubMed.
- R. Jaidev and K. Chatterjee, Surface functionalization of 3D printed polymer scaffolds to augment stem cell response, Mater. Des., 2019, 161, 44–54 CrossRef.
- M. Hennessy,
et al., The effect of RGD peptides on osseointegration of hydroxyapatite biomaterials, Biomaterials, 2008, 29(21), 3075–3083 CrossRef PubMed.
- P. Zhang,
et al., RGD-Conjugated Copolymer Incorporated into Composite of Poly(lactide-co-glycotide) and Poly(l-lactide)-Grafted Nanohydroxyapatite for Bone Tissue Engineering, Biomacromolecules, 2011, 12(7), 2667–2680 CrossRef CAS PubMed.
- M. Kaliva,
et al., Biodegradable Chitosan-graft-Poly(l-lactide) Copolymers For Bone Tissue Engineering, Polymers, 2020, 12 DOI:10.3390/polym12020316.
- R. Liang,
et al., PLA-lignin nanofibers as antioxidant biomaterials for cartilage regeneration and osteoarthritis treatment, J. Nanobiotechnol., 2022, 20(1), 327 CrossRef CAS PubMed.
- B. Duan,
et al., A nanofibrous composite membrane of PLGA–chitosan/PVA prepared by electrospinning, Eur. Polym. J., 2006, 42(9), 2013–2022 CrossRef CAS.
- S. G. Kumbar,
et al., Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering, Biomaterials, 2008, 29(30), 4100–4107 CrossRef CAS PubMed.
- X. Zhu,
et al., Electrospun Fibrous Mats with High Porosity as Potential Scaffolds for Skin Tissue Engineering, Biomacromolecules, 2008, 9(7), 1795–1801 CrossRef CAS PubMed.
- C. Ru,
et al., Suspended, Shrinkage-Free, Electrospun PLGA Nanofibrous Scaffold for Skin Tissue Engineering, ACS Appl. Mater. Interfaces, 2015, 7(20), 10872–10877 CrossRef CAS PubMed.
- G. E. Park,
et al., Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds, Biomaterials, 2005, 26(16), 3075–3082 CrossRef CAS PubMed.
- J. Lee,
et al., Nitric Oxide-Releasing S-Nitrosoglutathione-Conjugated Poly(Lactic-Co-Glycolic Acid) Nanoparticles for the Treatment of MRSA-Infected Cutaneous Wounds, Pharmaceutics, 2020, 12(7), 618 CrossRef CAS PubMed.
- Y. C. Teo,
et al., 3D Printed Bioactive PLGA Dermal Scaffold for Burn Wound Treatment, ACS Mater. Au, 2023, 3(3), 265–272 CrossRef CAS PubMed.
- E. Ko,
et al., Polydopamine-Assisted Osteoinductive Peptide Immobilization of Polymer Scaffolds for Enhanced Bone Regeneration by Human Adipose-Derived Stem Cells, Biomacromolecules, 2013, 14(9), 3202–3213 CrossRef CAS PubMed.
- Z. Wang,
et al., Improved Cell Adhesion and Osteogenesis of op-HA/PLGA Composite by Poly(dopamine)-Assisted Immobilization of Collagen Mimetic Peptide and Osteogenic Growth Peptide, ACS Appl. Mater. Interfaces, 2016, 8(40), 26559–26569 CrossRef CAS PubMed.
- X. Song,
et al., Bioinspired Protein/Peptide Loaded 3D Printed PLGA Scaffold Promotes Bone Regeneration, Front. Bioeng. Biotechnol., 2022, 10, 832727 CrossRef PubMed.
- J. Amirian,
et al., Porous BMP-2 immobilized PLGA/Glycol chitosan scaffold with enhanced hydrophilicity, mineralization and osteogenesis, Mater. Lett., 2022, 308, 131140 CrossRef.
- D. B. Bhuiyan,
et al., Bone regeneration from human mesenchymal stem cells on porous hydroxyapatite-PLGA-collagen bioactive polymer scaffolds, Bio-Med. Mater. Eng., 2017, 28, 671–685 CAS.
- H. S. Yoo,
et al., Hyaluronic acid modified biodegradable scaffolds for cartilage tissue engineering, Biomaterials, 2005, 26(14), 1925–1933 CrossRef CAS PubMed.
- L. A.-O. X. Zerrillo,
et al., PLGA Nanoparticles Grafted with Hyaluronic Acid to Improve Site-Specificity and Drug Dose Delivery in Osteoarthritis, Nanomaterials, 2022, 12(13), 2248 CrossRef CAS PubMed.
- D. Shi,
et al., Photo-Cross-Linked Scaffold with Kartogenin-Encapsulated Nanoparticles for Cartilage Regeneration, ACS Nano, 2016, 10(1), 1292–1299 CrossRef CAS PubMed.
- E. Zolotarevova,
et al., Distribution of polyethylene wear particles and bone fragments in periprosthetic tissue around total hip joint replacements, Acta Biomater., 2010, 6(9), 3595–3600 CrossRef CAS PubMed.
- A. Gigante,
et al., Effectiveness of Vitamin-E-Doped
Polyethylene in Joint Replacement: A Literature Review., J. Funct. Biomater, 2015, 6(3), 889–900 CrossRef CAS PubMed.
- J. Guo,
et al., Bioactive polyethylene-coated nonwovens for wound healing application, Next. Mater., 2024, 3, 100088 CrossRef.
- A. Abbas,
et al., Performance of bioresorbable peptide incorporated polymers produced for fused deposition modelling (FDM), Next. Mater., 2024, 5, 100223 CrossRef.
Footnote |
| † These authors contributed equally to this work. |
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article ,
Yew Chin
Teo
,
Yew Chin
Teo
 and
Peili
Teo
and
Peili
Teo
 *
*
























