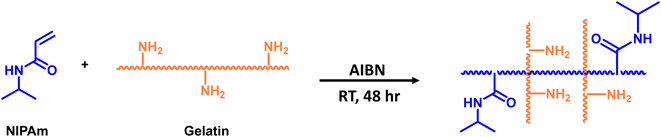
Open Access Article
Open Access ArticleFunctional self-healing aldehyde-derived nanoparticle-crosslinked gelatin/PNIPAm-based adhesive gels†
P. A.
Parvathy
ab,
Sriparna
De
c,
Manjinder
Singh
d,
Gaurav
Manik
 d and
Sushanta K.
Sahoo
*ab
d and
Sushanta K.
Sahoo
*ab
aMaterials Science and Technology Division, CSIR-National Institute for Interdisciplinary Science and Technology, Thiruvananthapuram 695019, India. E-mail: sushanta@niist.res.in
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, India
cDepartment of Allied Health Sciences, Brainware University, Kolkata 700125, India
dDepartment of Polymer and Process Engineering, Indian Institute of Technology, Roorkee 247667, India
First published on 14th March 2025
Abstract
The combination of lower critical solution temperature (LCST) and upper critical solution temperature (UCST) polymers provides varying dissolution to the system, with tunable physiochemical properties. In the current work, injectable, fluorescent, and thermo-responsive poly-N-isopropylacrylamide (PNIPAm)-gelatin nanocomposite gels were prepared by combining non-covalent interactions and Schiff base chemistry. The incorporation of aldehyde-based nanoparticles (2.5–10%) facilitated the crosslinking of the matrix via the formation of imine linkages with gelatin, which significantly reduced the pore size of the gels from 30–40 micron to 4–5 μm. The temperature-dependent viscoelastic properties showed that the storage modulus increased significantly above 40 °C, which confirmed the thermosensitive behaviour of the gels owing to the combined effect of PNIPAm and gelatin. Higher storage modulus over loss modulus for all crosslinked gels indicates the elastic behaviour of the gels. The introduction of imine linkages offered instant self-healing features and the nanoparticles provide photoluminescence to the polymeric gel system. Furthermore, the tackiness offered by gelatin enhances the adherence to human skin and the gels are found to be biocompatible towards fibroblast cell lines (L929) and are promising for drug delivery systems, injectable materials, and optically trackable adhesives. The findings provide new insights into the multifunctional properties of thermoresponsive PNIPAm-gelatin nanocomposite gels with dynamic imine linkages for biological applications.
1. Introduction
Injectable, self-healing, responsive gels encompass dynamic and reversible three-dimensional network architectures capable of reinstating their original structure and functionality after damage.1,2 They have garnered significant attention due to their favourable properties, such as bio-compatibility, simple formation, ease of handling, adaptability.3–5 Additionally, they have emerged as versatile intelligent materials for biomedical applications, including drug delivery,6 regenerative medicine,1 and tissue engineering.7 The chemical strategies to prepare self-healing hydrogels involve the introduction of either non-covalent interactions, dynamic covalent interactions or a combination of both. Non-covalent interactions include electrostatic interactions,8 metal coordination,9 hydrophobic interactions10 and hydrogen bonding,11 whereas dynamic covalent interactions include the introduction of imine bonds (Schiff base),12 disulfide linkages,13 Diels–Alder reaction,14 boronate ester,15 and acyl hydrazone.16 Among these, imine bonds are considered more stable in various media, such as solvent, hydrolytic, alkaline and neutral media, and they are recyclable.17–21Nano-crosslinked hydrogels contain dynamic interactions, integrating the inherent characteristics of nanoparticles as well as demonstrating the distinct benefits of the matrix components through dynamic bonds.22 The incorporation of nanoparticles as crosslinkers enhances the strength and injectability of the hydrogels by fortifying the structure, and also imparts multi-functionality, such as response to external stimuli (pH, temperature, and light), anti-bacterial and anti-oxidant properties.23–25 Nanomaterials possess highly specific surfaces that facilitate physical and chemical interaction with polymers and exhibit unique nanoscale characteristics for medicinal applications, including drug administration, tumour therapy, and tissue regeneration.22 The nanomaterials used in dynamic hydrogel fabrication include metals and metallic oxides,26 nanoclays,27 carbon-based nanomaterials,28 black phosphorus,29 and polymeric nanomaterials.30 Furthermore, the fluorescent properties of nanomaterials helps to provide optical tracing characteristics to the gel system.
A variety of polymeric materials have been used as structural components in the development of self-healing injectable gels. Natural polymers often used in such gels include gelatin, collagen, hyaluronic acid, alginate, and chitosan, due to their features such as inherent biomedical qualities, ease of availability, and biocompatibility.31,32 Gelatin is a UCST-type polymer, which exhibits a gel nature below the UCST (40 °C) and a liquid nature above the UCST33–36 (hydrophobic to hydrophilic transition). Collagen-derived gelatin has exceptional biocompatibility and is used in several biomedical applications.37 Thus, gelatin has been reported to be miscible with other polymers to achieve optimal gelling characteristics and maintain stability at physiological temperatures.38 The thermo-responsive properties of gelatin have been investigated for drug delivery systems.35,36 NIPAm is a prominent thermo-responsive polymer that has a LCST of 32 °C, which is near the physiological temperature. The ability to adjust the LCST up to the desired value and modify the structure with various synthetic or bio-based monomers/polymers enhances the demand for PNIPAm-based materials. PNIPAm-based hydrogels are extensively investigated as biomedical and smart materials, such as drug delivery systems, wound healing materials, and cell culturing systems.39–43 The combination of a LCST and UCST polymer, known as a “schizophrenic polymer44”, a type of dual thermo-responsive polymer with complex dissolution behaviour, helps in applications such as drug delivery and theranostics.45
Aggarwal et al. prepared fluorescent self-healing injectable hydrogels from gelatin, cross-linked via Schiff base chemistry using dopamine-terephthaldehyde (TA)-based carbon dots. The gels were biocompatible (>90%) and exhibited antibacterial activity towards E. coli and S. aureus.46 Sun et al. synthesised self-healing hydrogels from gallic acid coupled with P(NIPAm-co-AH) and oxidised sodium alginate. An amine-modified PNIPAm copolymer served as a crosslinking point via Schiff base reaction and the gels showed adhesive properties to different substrates (rubber, metal, heart, spleen, etc.).47 Khodaei et al. prepared self-healing hydrogels from aldehyde-modified carrageenan and dopamine using Schiff base chemistry, and the gel was found to possess a better storage modulus of 800 Pa and a shear thinning effect.48
The aim of this work is to develop thermo-responsive self-healable-fluorescent bio-adhesive gels through the integration of a LCST polymer (PNIPAm), a UCST polymer (gelatin) and aldehyde-grafted carbon-based nanoparticles, while crosslinking through the formation of reversible imine bonds. The gel system integrates physical (hydrogen bonding) and dynamic covalent interactions through Schiff base chemistry utilizing TA-based nanoparticles (TNs) with gelatin, which has not been reported so far. The formation of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N chromophore through the conjugation of nanoparticles imparts self-healing, intrinsic photoluminescence properties, and tunable pore size, endowing it with potential as a bio-adhesive to trace and monitor the affected skin or wounds. The conjugation of fluorescent TN augmented the photoluminescence (PL) intensity of the gels, which is essential for optical tracing.
N chromophore through the conjugation of nanoparticles imparts self-healing, intrinsic photoluminescence properties, and tunable pore size, endowing it with potential as a bio-adhesive to trace and monitor the affected skin or wounds. The conjugation of fluorescent TN augmented the photoluminescence (PL) intensity of the gels, which is essential for optical tracing.
2. Experimental
2.1 Materials
NIPAm (97.0%) and gelatin were procured from Sigma Aldrich. Azobisisobutyronitrile (AIBN) (98.0%) was purchased from Himedia and TA (>98.0%) was obtained from TCI chemicals. The solvent methanol was obtained from Merck (99.5%) and absolute ethanol from Analytical CSS reagents. The chemicals were used without further purification. Mouse fibroblast cells (L929) were procured from the National Centre for Cell Science (NCCS, 36 Pune, India), along with the cell culture media. Fetal bovine serum (FBS), and Dulbecco's modified Eagle's 40 medium (DMEM) (99%) were obtained from Himedia and MTT reagent (97.5%) was procured from Sigma Aldrich.2.2 Preparation of the PNIPAm-gelatin polymer system (P5G5)
NIPAm (1 g) and AIBN (0.014 g) were mixed into a 1 wt% gelatin solution (100 mL, solvent combination of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 1
water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) under a nitrogen environment at room temperature for 48 h to get the polymeric solution (Scheme 1).49 Different ratios of NIPAm
9) under a nitrogen environment at room temperature for 48 h to get the polymeric solution (Scheme 1).49 Different ratios of NIPAm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gelatin were employed to prepare the polymeric solution as depicted in Table 1. Based on its better miscibility, P5G5 was selected for preparing the solution and then concentrated to one fourth of the initial volume by removing solvent for the next step.
gelatin were employed to prepare the polymeric solution as depicted in Table 1. Based on its better miscibility, P5G5 was selected for preparing the solution and then concentrated to one fourth of the initial volume by removing solvent for the next step.
| Sample | NIPAm (%) | Gelatin (%) |
|---|---|---|
| P4G6 | 40 | 60 |
| P5G5 | 50 | 50 |
| P6G4 | 60 | 40 |
2.3 Synthesis of the PNIPAm-gelatin nanocomposite gels
The nanocomposite gels were prepared via a two-step process (Scheme 2). TA-based nanoparticles were synthesized by employing a solvothermal process. 0.2 g of TA was dissolved in 40 mL methanol and autoclaved at 180 °C for 12 h. The yellow colour solution obtained after the process was filtered using a syringe filter (0.22 μm), and the solvent was evaporated to get TN nanoparticles in powder form. Nanocomposite hydrogels were synthesized via mixing the polymeric solutions with different weight percentages of TN nanoparticles dissolved in ethanol (Table 2). The pH of the solution was adjusted to 8 and the reaction was proceeded at 70 °C for 24 h. The obtained yellow gel was subjected to further characterization.| Sample | TN (wt%) |
|---|---|
| P5G5-TN 2.5 | 2.5 |
| P5G5-TN 5 | 5 |
| P5G5-TN 7.5 | 7.5 |
| P5G5-TN 10 | 10 |
2.4 Characterization
TA and TN nanoparticles were characterised using FT-IR [ATR-FT-IR spectrophotometer (PerkinElmer)] in the range of 4000 cm−1 to 400 cm−1 and 1H-NMR [500 MHz Bruker Avance DPX spectrometer using acetone-d6 as a solvent]. UV-Vis analysis of the TA and TN solutions and crosslinked gels was conducted using a UV-2700i UV-vis spectrophotometer (Shimadzu) and photoluminescence (solution) was studied using a Yvon Fluorolog 3 spectrofluorimeter using a 450 W xenon flash lamp as the excitation source. The particle size of the TN was measured with a ZetaPals DLS/zeta potential analyser (0.5 mg ml−1 in acetone). Furthermore, the particle size of the TN was determined using high-resolution transmission electron microscopy (FEI, Tecnai G2, T30 S-TWIN).The polymerization of PNIPAm and its interaction with gelatin and crosslinking were confirmed with FT-IR-ATR analysis of NIPAm, gelatin and the crosslinked gels. X-ray diffraction analysis was performed under Cu Kα radiation in a Malvern Panalytical diffractometer at a diffraction angle (2θ) of 10° and 90°, respectively. SEM (Carl Zeiss EVO MA18 FE SEM) was used to analyse the morphology of the freeze-dried gels, which are operating at an accelerating voltage of 15 kV. Differential scanning calorimetry (DSC) of the cross-linked gels in water was performed using a Discovery series DSC 25, TA instrument at a heating rate of 2 °C min−1 under a N2 atmosphere. The temperature-dependent viscoelastic properties of the polymer and crosslinked gels were studied using a Rheometer (MCR102, ANTONPAAR, USA), in the temperature range of 25–60 °C. The images of the gels and TA and TN solutions under UV were taken using a SPECTROLINE Model CM-10A fluorescence analysis cabinet (365 nm illumination). Adhesion of the gels was tested using different substrates, such as metal, plastic, glass and skin.
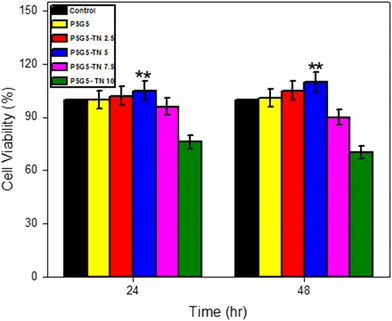
2.5 MTT assay
The National Centre for Cell Science (NCCS) in Pune, India, provided the mouse L929 fibroblast cells utilized in this study to evaluate cell growth and survival on the composites. The cells were grown in DMEM, given synthetic formulations, and their cytotoxicity was monitored. In vitro cytotoxicity was investigated using the MTT assay.50 Each well's optical density (OD) was measured, and the following formula was used to assess the relative cell viability:To examine the effect of the materials on cell growth, the investigation was conducted in triplicate.
3. Results and discussion
3.1 TA nanoparticle characteristics
TA was chosen as the organic compound for preparing the nanoparticles, which can cross-link with the –NH2 groups of gelatin through Schiff base chemistry.46,51 The FT-IR-ATR spectra shown in Fig. 1a verified the retention of the aldehyde peak of TA in the TNs at 1682 cm−1 after the solvothermal process. The signal associated with the aldehydic proton in the TNs presented at 10 ppm in the 1H NMR (Fig. 1b). DLS analysis yields the hydrodynamic diameter of the TN particles at around 160 nm (Fig. 1c). The size for nanoparticle aggregation was further validated using TEM imaging (Fig. 1d). The particle dimensions were determined to be between 60 and 70 nm at the aggregation corners. The aggregation of the particles can be attributed to hydrogen bond interactions between the aldehyde groups and π–π stacking. The particle sizes seen in DLS data are often augmented due to the solvent's impact. A comparison of the XRD patterns is shown in the ESI (Fig. S1†), indicating that the degree of modification in the TNs relative to TA is minimal.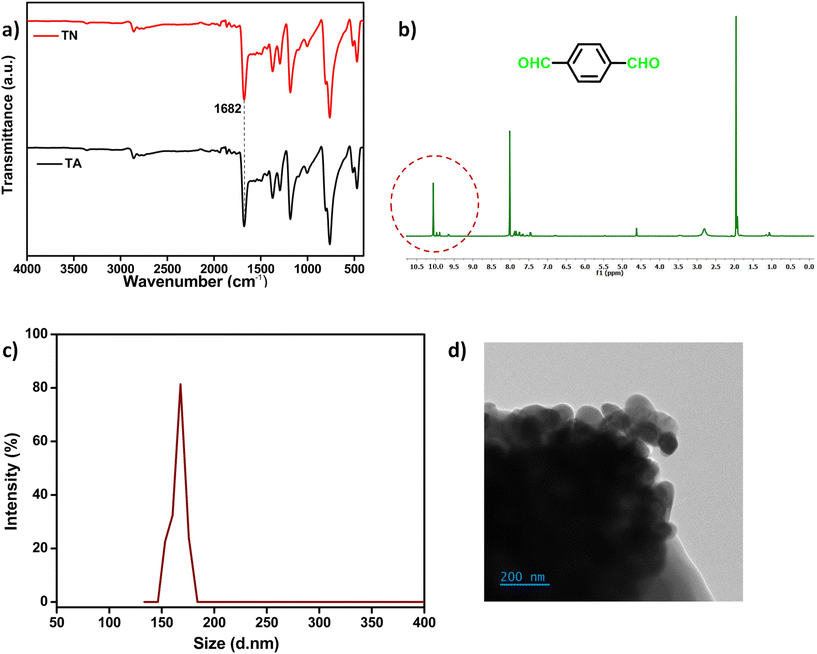 | ||
| Fig. 1 (a) FT-IR-ATR comparison of TA and the TNs, (b) 1H NMR spectra of the TNs in acetone-d6, (c) DLS spectra of the TNs in acetone (0.5 mg ml−1) and (d) TEM image of the TN particles. | ||
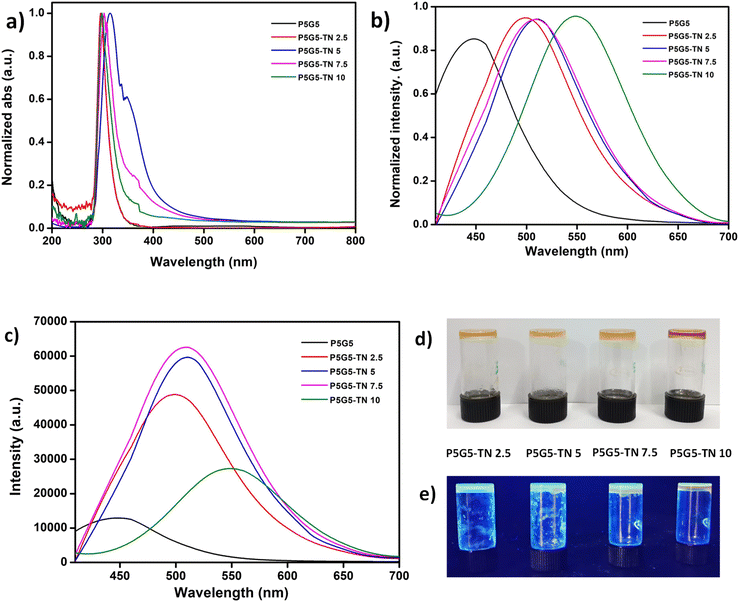
Fig. 2a and b illustrate the absorbance and photoluminescence spectra of the nanoparticles. The broad absorption peak seen at 330 nm is attributed to the n–π* transition in the TNs (Fig. 2a).52 The peak intensity and broadness increased compared to TA, owing to the development of discrete energy levels during the solvothermal process. PL analysis confirmed the fluorescent behaviour of the nanoparticles. The emission spectra (exc. 380 nm) can be correlate with UV analysis, where the intensity of the TN solution changed significantly compared to TA (Fig. 2b). An image depicting a comparison of the TN and TA solutions under UV light is given in the ESI (Fig. S2†).
3.2 PNIPAm-gelatin nanocomposite gel
Radical polymerization was used to synthesize the PNIPAm polymer using AIBN as the initiator, in which gelatin was introduced to interact with the polymer through physical means. Furthermore, the gels were synthesized by Schiff-base chemistry involving the –CHO groups of TN and the –NH2 groups of gelatin. Under acidic pH conditions, the crosslinking reaction between the –CHO and –NH2 functional groups results in the formation of hemiacetals, while in alkali medium, the reaction predominantly involves the formation of Schiff bases.53 Here, the preparation of P(NIPAm-gelatin)-TN was conducted at pH 8 and the polymeric solution transitioned to a sticky gel after crosslinking, along with a change from transparent to a yellow hue. FT-IR analysis was conducted to confirm the polymerization and crosslinking (Fig. 3a and b). In the FT-IR data of P5G5 (Fig. 3a), the broader absorption band around 3548 cm−1 indicates hydrogen bond formation between the –OH and –NHCO groups. Moreover, the bands usually occurring at 1656 cm−1 and 1556 cm−1 in the spectrum of NIPAm shifted to 1644 cm−1 and 1541 cm−1, respectively, indicating hydrogen bonding interactions between gelatin and PNIPAm.49,54 The peak corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 1619 cm−1 in NIPAm got reduced in P5G5 confirming the polymerization.49 The hydrogen bonding between NIPAm and gelatin enables the polymeric solution to be highly miscible. The Schiff base formation was confirmed via conducting the reaction of TNs with gelatin and the corresponding FT-IR-ATR analysis is depicted in Fig. S3.† The aldehydic peak (1682 cm−1) arising from the TNs completely disappeared in the gelatin-TN system. The amide I and amide II bands, located at approximately 1632 and 1530 cm−1, respectively, are typical spectral features for gelatin. Since the peak for imine –C
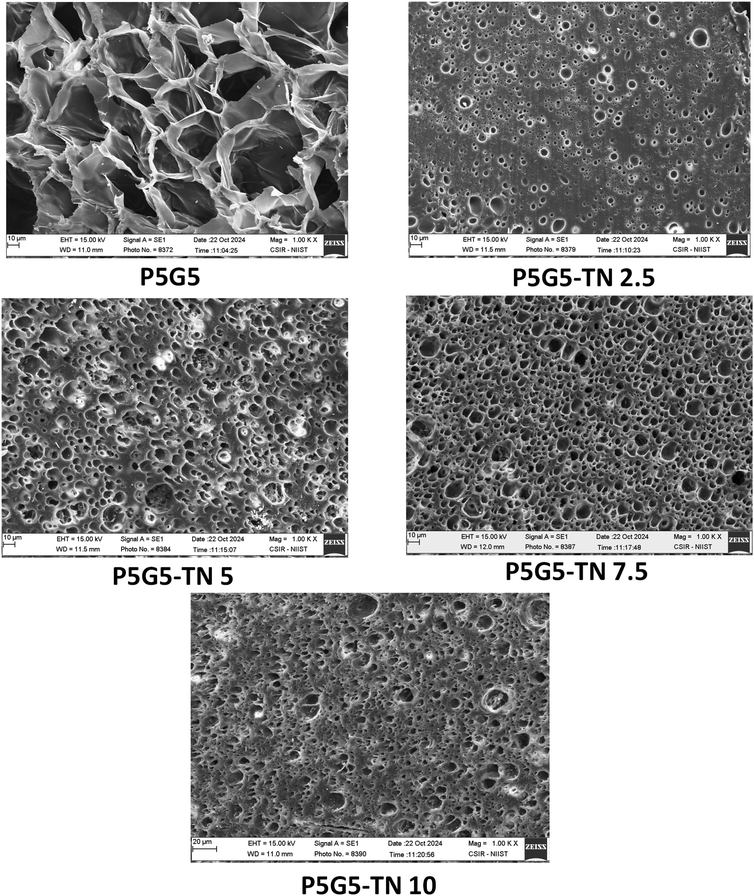
C at 1619 cm−1 in NIPAm got reduced in P5G5 confirming the polymerization.49 The hydrogen bonding between NIPAm and gelatin enables the polymeric solution to be highly miscible. The Schiff base formation was confirmed via conducting the reaction of TNs with gelatin and the corresponding FT-IR-ATR analysis is depicted in Fig. S3.† The aldehydic peak (1682 cm−1) arising from the TNs completely disappeared in the gelatin-TN system. The amide I and amide II bands, located at approximately 1632 and 1530 cm−1, respectively, are typical spectral features for gelatin. Since the peak for imine –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– stretching vibration lies in the range of 1640–1690 cm−1, it overlaps with the strong band for amide I in gelatin. These results are consistent with those of Joy et al.,55 Qian et al.56 and Dong et al.57 A similar fact was observed in the case of the crosslinked gels. No aldehydic peak was observed in the nanocomposite gels. TN doesn't alter the peak positions except that it reduces the broadness of the peak for amide at 1530 cm−1 and the peak at 3548 cm−1. The XRD pattern of the gels depicted in Fig. 3c clarifies the amorphous nature of the gels. The nanoparticles effectively crosslinked with the gelatin system and the presence of sharp peaks in P5G5-TN10 indicates a surplus amount of TNs that could not take part in crosslinking.
N– stretching vibration lies in the range of 1640–1690 cm−1, it overlaps with the strong band for amide I in gelatin. These results are consistent with those of Joy et al.,55 Qian et al.56 and Dong et al.57 A similar fact was observed in the case of the crosslinked gels. No aldehydic peak was observed in the nanocomposite gels. TN doesn't alter the peak positions except that it reduces the broadness of the peak for amide at 1530 cm−1 and the peak at 3548 cm−1. The XRD pattern of the gels depicted in Fig. 3c clarifies the amorphous nature of the gels. The nanoparticles effectively crosslinked with the gelatin system and the presence of sharp peaks in P5G5-TN10 indicates a surplus amount of TNs that could not take part in crosslinking.
 | ||
| Fig. 3 (a) FT-IR-ATR analysis of the NIPAm, gelatin, and P5G5 and (b) crosslinked gels, and (c) the XRD pattern comparison. | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N formed as a result of Schiff base reaction. The photoluminescence spectrum shows a red shift of the peak maxima in the nanocomposite gels (450 to 549 nm, ext. 380 nm) compared to P5G5, which further confirms the presence of an imine bond (Fig. 5b). Similar results were observed by Aggarwal et al.,46 where imine bonds caused a red shift in gelatin/CD composite hydrogels. The intensity of the spectrum of the gels continuously increased until 7.5 wt% TNs (P5G5-TN 7.5) and got reduced in P5G5-TN 10 (Fig. 5c). The sudden drop in intensity may be due to the aggregation of excess TN in the system and these results can be correlated with the XRD spectra and TEM image. The nanocomposite gel appeared brownish yellow in colour (a characteristic of –C
N formed as a result of Schiff base reaction. The photoluminescence spectrum shows a red shift of the peak maxima in the nanocomposite gels (450 to 549 nm, ext. 380 nm) compared to P5G5, which further confirms the presence of an imine bond (Fig. 5b). Similar results were observed by Aggarwal et al.,46 where imine bonds caused a red shift in gelatin/CD composite hydrogels. The intensity of the spectrum of the gels continuously increased until 7.5 wt% TNs (P5G5-TN 7.5) and got reduced in P5G5-TN 10 (Fig. 5c). The sudden drop in intensity may be due to the aggregation of excess TN in the system and these results can be correlated with the XRD spectra and TEM image. The nanocomposite gel appeared brownish yellow in colour (a characteristic of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N chromophoric moieties60) in vis-light and turned to fluorescent with UV 365 nm illumination (Fig. 5d and e).
N chromophoric moieties60) in vis-light and turned to fluorescent with UV 365 nm illumination (Fig. 5d and e).
 | ||
| Fig. 7 (a) Storage modulus (G′), (b) loss modulus and (c) complex viscosity (η*) analysis of the nanocomposite gels, and (d) tackiness and injectability. | ||
Imines are a class of dynamic covalent bonds that break and repair by themselves. This ability makes the gels self-healable instantly (Fig. 8a and b), for which the mechanism is depicted in Fig. 8c. The adhesion of the gels to different substrates, such as metal, plastic and glass, is depicted in Fig. 8d–f. Fig. 8g and h illustrate the adhesion of gels to the skin, from which they may be easily removed, facilitating their use in bandages.
4. Conclusions
We developed thermo-responsive nanocomposite gels by utilizing the PNIPAm-gelatin system and crosslinking the amine groups of gelatin with terephthaldehyde nanoparticles via Schiff base reaction. The physiochemical properties of the gels stem from the temperature response of PNIPAm and gelatin, together with the dynamic covalent imine bonds produced during the Schiff-base reaction. The gels exhibit fluorescence imparted by TN, injectability, adhesiveness and self-healing properties provided by the imine linkages. The elevated transition in moduli (G′ and G′′) and viscosity curves in the range 45–54 °C showcase the modified LCST and combined temperature response of PNIPAm and crosslinked gelatin in the gel system. The formation of a crosslinked network dropped the pore size of the gels to 4–5 μm, and the system demonstrated adhesion to various substrates. The developed biocompatible self-healing adhesive gels can be used for various applications, including tissue engineering, drug delivery systems, wound healing materials, and soft robotics. Moreover, the photoluminescence characteristic can facilitate the monitoring of localized wounds.Data availability
Data will be made available on request.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Author P. A. Parvathy is grateful to Department of Science and Technology, Government of India for the Inspire fellowship grant (IF190279). Dr Sriparna De would like to acknowledge the SEED Research grant, Brainware University (BWU/PRJ/SMG/22-23/001) for providing research support and facilities.References
- P. Bertsch, M. Diba, D. J. Mooney and S. C. G. Leeuwenburgh, Self-Healing Injectable Hydrogels for Tissue Regeneration, Chem. Rev., 2023, 123(2), 834–873, DOI:10.1021/acs.chemrev.2c00179.
- S. Nejati and L. Mongeau, Injectable, Pore-Forming, Self-Healing, and Adhesive Hyaluronan Hydrogels for Soft Tissue Engineering Applications, Sci. Rep., 2023, 13(1), 1–12, DOI:10.1038/s41598-023-41468-9.
- J. Chen and X. Zou, Self-Assemble Peptide Biomaterials and Their Biomedical Applications, Bioact. Mater., 2019, 4, 120–131, DOI:10.1016/j.bioactmat.2019.01.002.
- J. Xu, Y. Liu and S. Hsu, Hydrogels Based on Schi Ff Base Linkages For, Molecules, 2019, 24(3005), 1–21 Search PubMed.
- L. Xu, R. Qin, J. Zhang, J. Liu, S. Liu, F. Li, A. Gong, Q. Hanliang, F. Du and M. Zhang, Mussel-Inspiredin Situfabrication of a Photothermal Composite Hydrogel for MR-Guided Localized Tumor Ablation, RSC Adv., 2021, 11(32), 19461–19469, 10.1039/d1ra00903f.
- Y. Cheng, H. Zhang, H. Wei and C. Y. Yu, Injectable Hydrogels as Emerging Drug-Delivery Platforms for Tumor Therapy, Biomater. Sci., 2024, 12(5), 1151–1170, 10.1039/d3bm01840g.
- A. Atwal, T. P. Dale, M. Snow, N. R. Forsyth and P. Davoodi, Injectable Hydrogels: An Emerging Therapeutic Strategy for Cartilage Regeneration, Adv. Colloid Interface Sci., 2023, 321, 103030, DOI:10.1016/j.cis.2023.103030.
- M. Diba, H. Wang, T. E. Kodger, S. Parsa and S. C. G. Leeuwenburgh, Highly Elastic and Self-Healing Composite Colloidal Gels, Adv. Mater., 2017, 29(11), 1604672, DOI:10.1002/adma.201604672.
- E. Khare, N. Holten-Andersen and M. J. Buehler, Transition-Metal Coordinate Bonds for Bioinspired Macromolecules with Tunable Mechanical Properties, Nat. Rev. Mater., 2021, 6(5), 421–436, DOI:10.1038/s41578-020-00270-z.
- E. E. Meyer, K. J. Rosenberg and J. Israelachvili, Recent Progress in Understanding Hydrophobic Interactions, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(43), 15739–15746, DOI:10.1073/pnas.0606422103.
- S. Li, J. Yu, M. Zhang, Z. Ma, N. Chen, X. Li, J. Ban, J. Xie, Z. Chen, J. Ma, C. Tian, Y. Qin, J. Wang, W. Gao, L. Long, J. Zhao, X. Hou and X. Yuan, An Intermediate Unit-Mediated, Continuous Structural Inheritance Strategy for the Dilemma between Injectability and Robustness of Hydrogels, Adv. Funct. Mater., 2022, 32(14), 1–12, DOI:10.1002/adfm.202110617.
- M. E. Belowich and J. F. Stoddart, Dynamic Imine Chemistry, Chem. Soc. Rev., 2012, 41(6), 2003–2024, 10.1039/c2cs15305j.
- B. Lewis, J. M. Dennis and K. R. Shull, Effects of Dynamic Disulfide Bonds on Mechanical Behavior in Glassy Epoxy Thermosets, ACS Appl. Polym. Mater., 2023, 5(4), 2583–2595, DOI:10.1021/acsapm.2c02194.
- S. M. Morozova, Recent Advances in Hydrogels via Diels–Alder Crosslinking: Design and Applications, Gels, 2001, 37(7), 161 Search PubMed.
- L. Terriac, J. J. Helesbeux, Y. Maugars, J. Guicheux, M. W. Tibbitt and V. Delplace, Boronate Ester Hydrogels for Biomedical Applications: Challenges and Opportunities, Chem. Mater., 2024, 36(14), 6674–6695, DOI:10.1021/acs.chemmater.4c00507.
- Y. Liu, Y. Liu, Q. Wang, Y. Han, H. Chen and Y. Tan, Doubly Dynamic Hydrogel Formed by Combining Boronate Ester and Acylhydrazone Bonds, Polymers, 2020, 12(2), 1–15, DOI:10.3390/polym12020487.
- L. Li, X. Peng, D. Zhu, J. Zhang and P. Xiao, Recent Progress in Polymers with Dynamic Covalent Bonds, Macromol. Chem. Phys., 2023, 224(20), 1–25, DOI:10.1002/macp.202300224.
- T. Huang, W. Zhang, S. Yang, L. Wang and G. Yu, Imine-Linked Covalent Organic Frameworks: Recent Advances in Design, Synthesis, and Application, SmartMat, 2024, 1–40, DOI:10.1002/smm2.1309.
- X. Wang, H. J. Zhang, Y. Yang, Y. Chen, X. Zhu and X. You, Biopolymer-Based Self-Healing Hydrogels: A Short Review, Giant, 2023, 16, 100188, DOI:10.1016/j.giant.2023.100188.
- R. Bui and M. A. Brook, Dynamic Covalent Schiff-Base Silicone Polymers and Elastomers, Polymer, 2019, 160, 282–290, DOI:10.1016/j.polymer.2018.11.043.
- Z. Liu, Y. Tang, Y. Chen, Z. Lu and Z. Rui, Dynamic Covalent Adhesives and Their Applications: Current Progress and Future Perspectives, Chem. Eng. J., 2024, 497, 154710, DOI:10.1016/j.cej.2024.154710.
- Q. Wang, Y. Zhang, Y. Ma, M. Wang and G. Pan, Nano-Crosslinked Dynamic Hydrogels for Biomedical Applications, Mater. Today Bio, 2023, 20, 100640, DOI:10.1016/j.mtbio.2023.100640.
- M. Wu, J. Chen, W. Huang, B. Yan, Q. Peng, J. Liu, L. Chen and H. Zeng, Injectable and Self-Healing Nanocomposite Hydrogels with Ultrasensitive PH-Responsiveness and Tunable Mechanical Properties: Implications for Controlled Drug Delivery, Biomacromolecules, 2020, 21(6), 2409–2420, DOI:10.1021/acs.biomac.0c00347.
- Y. H. Yeo and W. H. Park, Dual-Crosslinked, Self-Healing and Thermo-Responsive Methylcellulose/Chitosan Oligomer Copolymer Hydrogels, Carbohydr. Polym., 2021, 258, 117705, DOI:10.1016/j.carbpol.2021.117705.
- F. Gang, H. Yan, C. Ma, L. Jiang, Y. Gu, Z. Liu, L. Zhao, X. Wang, J. Zhang and X. Sun, Robust Magnetic Double-Network Hydrogels with Self-Healing, MR Imaging, Cytocompatibility and 3D Printability, Chem. Commun., 2019, 55(66), 9801–9804, 10.1039/c9cc04241e.
- R. Xing, K. Liu, T. Jiao, N. Zhang, K. Ma, R. Zhang, Q. Zou, G. Ma and X. Yan, An Injectable Self-Assembling Collagen-Gold Hybrid Hydrogel for Combinatorial Antitumor Photothermal/Photodynamic Therapy, Adv. Mater., 2016, 28(19), 3669–3676, DOI:10.1002/adma.201600284.
- Z. Dou, H. Tang, K. Chen, D. Li, Q. Ying, Z. Mu, C. An, F. Shao, Y. Zhang, Y. Zhang, H. Bai, G. Zheng, L. Zhang, T. Chen and H. Wang, Highly Elastic and Self-Healing Nanostructured Gelatin/Clay Colloidal Gels with Osteogenic Capacity for Minimally Invasive and Customized Bone Regeneration, Biofabrication, 2023, 15(2), 025001, DOI:10.1088/1758-5090/acab36.
- J. Liu, G. Song, C. He and H. Wang, Self-Healing in Tough Graphene Oxide Composite Hydrogels, Macromol. Rapid Commun., 2013, 34(12), 1002–1007, DOI:10.1002/marc.201300242.
- Y. Miao, Y. Chen, J. Luo, X. Liu, Q. Yang, X. Shi and Y. Wang, Black Phosphorus Nanosheets-Enabled DNA Hydrogel Integrating 3D-Printed Scaffold for Promoting Vascularized Bone Regeneration, Bioact. Mater., 2023, 21, 97–109, DOI:10.1016/j.bioactmat.2022.08.005.
- W. Ma, W. Cao, T. Lu, Z. Jiang, R. Xiong, S. K. Samal and C. Huang, Healable, Adhesive, and Conductive Nanocomposite Hydrogels with Ultrastretchability for Flexible Sensors, ACS Appl. Mater. Interfaces, 2021, 13(48), 58048–58058, DOI:10.1021/acsami.1c20271.
- E. Pocurull, N. Fontanals, M. Calull and C. Aguilar, Natural and Synthetic Polymers for Biomedical and Environmental Applications, Polymers, 2019, 591–641, DOI:10.1016/B978-0-12-816911-7.00020-7.
- L. Li, F. Yu, L. Zheng, R. Wang, W. Yan, Z. Wang, J. Xu, J. Wu, D. Shi, L. Zhu, X. Wang and Q. Jiang, Natural Hydrogels for Cartilage Regeneration: Modification, Preparation and Application, J. Orthop. Transl., 2019, 17(2), 26–41, DOI:10.1016/j.jot.2018.09.003.
- T. Kopač, Mathematical Model for Characterization of Temperature-Responsive Polymers: A Study on the Rheological Behavior of Gelatin and Poly(N-Isopropylacrylamide), Polym. Test., 2024, 133, DOI:10.1016/j.polymertesting.2024.108402.
- M. Le, W. Huang, K. F. Chen, C. Lin, L. Cai, H. Zhang and Y. G. Jia, Upper Critical Solution Temperature Polymeric Drug Carriers, Chem. Eng. J., 2022, 432, 134354, DOI:10.1016/j.cej.2021.134354.
- Y. H. Cheng, E. Chavez, K. L. Tsai, K. C. Yang, W. T. Kuo, Y. P. Yang, S. H. Chiou and F. H. Lin, Effects of Thermosensitive Chitosan-Gelatin Based Hydrogel Containing Glutathione on Cisd2-Deficient Chondrocytes under Oxidative Stress, Elsevier Ltd., 2017, vol. 173. DOI:10.1016/j.carbpol.2017.05.069.
- Y. H. Cheng, Y. C. Ko, Y. F. Chang, S. H. Huang and C. J. L. Liu, Thermosensitive Chitosan-Gelatin-Based Hydrogel Containing Curcumin-Loaded Nanoparticles and Latanoprost as a Dual-Drug Delivery System for Glaucoma Treatment, Exp. Eye Res., 2019, 179, 179–187, DOI:10.1016/j.exer.2018.11.017.
- R. Andreazza, A. Morales, S. Pieniz and J. Labidi, Gelatin-Based Hydrogels: Potential Biomaterials for Remediation, Polymers, 2023, 15(4), 1–12, DOI:10.3390/polym15041026.
- S. Chatterjee and P. C. L. Hui, Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems, Polymers, 2021, 13(13), 2086, DOI:10.3390/polym13132086.
- Y. Li, J. Luo, G. Xie, D. Zhu, C. Zhao, X. Zhang, M. Liu, Y. Wu, Y. Guo and W. Yu, Recent Progress on Regulating the LCST of PNIPAM-Based Thermochromic Materials, ACS Appl. Polym. Mater., 2024, 7, 1–11, DOI:10.1021/acsapm.4c03406.
- N. A. Shaibie, N. A. Ramli, N. D. F. Mohammad Faizal, T. Srichana and M. C. I. Mohd Amin, Poly(N-Isopropylacrylamide)-Based Polymers: Recent Overview for the Development of Temperature-Responsive Drug Delivery and Biomedical Applications, Macromol. Chem. Phys., 2023, 224(20), 1–12, DOI:10.1002/macp.202300157.
- S. Dolui, B. Sahu, S. A. Mohammad and S. Banerjee, Multi-Stimuli Responsive Sequence Defined Multi-Arm Star Diblock Copolymers for Controlled Drug Release, JACS Au, 2023, 3(8), 2117–2122, DOI:10.1021/jacsau.3c00339.
- I. Sanzari, E. Buratti, R. Huang, C. G. Tusan, F. Dinelli, N. D. Evans, T. Prodromakis and M. Bertoldo, Poly(N-Isopropylacrylamide) Based Thin Microgel Films for Use in Cell Culture Applications, Sci. Rep., 2020, 10(1), 1–14 CrossRef PubMed.
- S. A. Mohammad, S. Dolui, D. Kumar, M. M. Alam and S. Banerjee, Anisotropic and Self-Healing Copolymer with Multiresponsive Capability via Recyclable Alloy-Mediated RDRP, Macromol. Rapid Commun., 2021, 42(12), 1–7, DOI:10.1002/marc.202100096.
- V. Bütün, S. Liu, J. V. M. Weaver, X. Bories-Azeau, Y. Cai and S. P. Armes, A Brief Review of “schizophrenic” Block Copolymers, React. Funct. Polym., 2006, 66(1), 157–165, DOI:10.1016/j.reactfunctpolym.2005.07.021.
- Y. Nan, C. Zhao, G. Beaudoin and X. X. Zhu, Synergistic Approaches in the Design and Applications of UCST Polymers, Macromol. Rapid Commun., 2023, 44(23), 1–16, DOI:10.1002/marc.202300261.
- M. Aggarwal, H. Panigrahi, D. K. Kotnees and P. Das, Multifunctional Self-Healing Carbon Dot-Gelatin Bioadhesive: Improved Tissue Adhesion with Simultaneous Drug Delivery, Optical Tracking, and Photoactivated Sterilization, Biomacromolecules, 2024, 25(5), 3178–3189, DOI:10.1021/acs.biomac.4c00313.
- W. Sun, J. Zhu, Z. Cui, C. Zhou, S. Guo, W. Li and J. Qin, Self-Healing Hydrogel Prepared from Gallic Acid Coupled P(NIPAM-Co-AH) and Oxidized Sodium Alginate for Diabetic Wound Repairing, React. Funct. Polym., 2024, 201, 105951, DOI:10.1016/j.reactfunctpolym.2024.105951.
- T. Khodaei, J. Nourmohammadi, A. Ghaee and Z. Khodaii, An Antibacterial and Self-Healing Hydrogel from Aldehyde-Carrageenan for Wound Healing Applications, Carbohydr. Polym., 2023, 302, 120371, DOI:10.1016/j.carbpol.2022.120371.
- J. W. Guo, C. F. Wang, J. Y. Lai, C. H. Lu and J. K. Chen, Poly(N-Isopropylacrylamide)-Gelatin Hydrogel Membranes with Thermo-Tunable Pores for Water Flux Gating and Protein Separation, J. Membr. Sci., 2021, 618, 118732, DOI:10.1016/j.memsci.2020.118732.
- P. A. Parvathy, S. De, M. Singh, G. Manik and S. K. Sahoo, RAFT Polymerization Assisted P (NIPAm- Co -AAc) -AEMR Integrated PVA Hydrogels: Dual Responsive Features, Texture Analysis, and Cytotoxicity Studies, React. Funct. Polym., 2024, 205, 106052, DOI:10.1016/j.reactfunctpolym.2024.106052.
- L. Raju, A. R. Stesho Crystalin Lazuli, N. K. Udaya Prakash and E. Rajkumar, Chitosan-Terephthaldehyde Hydrogels – Effect of Concentration of Cross-Linker on Structural, Swelling, Thermal and Antimicrobial Properties, Materialia, 2021, 16, 101082, DOI:10.1016/j.mtla.2021.101082.
- S. Kumar and J. Koh, Physiochemical and Optical Study of Chitosan-Terephthaldehyde Derivative for Biomedical Applications, Int. J. Biol. Macromol., 2012, 51(5), 1167–1172, DOI:10.1016/j.ijbiomac.2012.09.001.
- S. Farris, J. Song and Q. Huang, Alternative Reaction Mechanism for the Cross-Linking of Gelatin with Glutaraldehyde, J. Agric. Food Chem., 2010, 58(2), 998–1003, DOI:10.1021/jf9031603.
- H. W. Yang, A. W. Lee, C. H. Huang and J. K. Chen, Characterization of Poly(N-Isopropylacrylamide)-Nucleobase Supramolecular Complexes Featuring Bio-Multiple Hydrogen Bonds, Soft Matter, 2014, 10(41), 8330–8340, 10.1039/c4sm01496k.
- J. Joy, A. Gupta, S. Jahnavi, R. S. Verma, A. R. Ray and B. Gupta, Understanding the in Situ Crosslinked Gelatin Hydrogel, Polym. Int., 2016, 65(2), 181–191, DOI:10.1002/pi.5042.
- Y. Qian, K. Zhang and F. Chen, Cross-Linking of Gelatin and Chitosan Complex Nanofibers for Tissue-Engineering Scaffolds, 2012, pp. 1099–1113 Search PubMed.
- Y. Dong, H. Chen, P. Qiao and Z. Liu, Development and Properties of Fish Gelatin/Oxidized Starch Double Network Film Catalyzed by Thermal Treatment and Schiff’ Base Reaction, Polymers, 2019, 11(12), 1–14, DOI:10.3390/polym11122065.
- P. Promdontree, A. Ounkaew, Y. Yao, H. Zeng, R. Narain and S. Ummartyotin, Temperature-Responsive Injectable Composite Hydrogels Based on Poly(N-Isopropylacrylamide), Chitosan, and Hemp-Derived Cellulose Nanocrystals, Polymers, 2024, 16(21), 2984, DOI:10.3390/polym16212984.
- A. Trubetskaya, J. Leppiniemi, S. Lipponen, S. Lombardo, W. Thielemans, T. Maloney, T. Pääkkönen, K. K. Kesari, J. Ruokolainen, V. P. Hytönen and E. Kontturi, Thermoresponsive and Biocompatible Poly(N-Isopropylacrylamide)-Cellulose Nanocrystals Hydrogel for Cell Growth, Mater. Adv., 2023, 5(2), 570–583, 10.1039/d3ma00495c.
- M. Qi, L. Zheng, C. Li, Y. Xiao, J. Liu, S. Wu and B. Zhang, The Yellowing Mechanism of Polyesteramide Based on Poly(Ethylene Terephthalate) and Polyamide 6, J. Appl. Polym. Sci., 2021, 138(10), 1–10, DOI:10.1002/app.49986.
- P. R. Chen, P. L. Kang, W. Y. Su, F. H. Lin and M. H. Chen, The Evaluation of Thermal Properties and in Vitro Test of Carbodiimide or Glutaraldehyde Cross-Linked Gelatin for PC 12 Cells Culture, Biomed. Eng., 2005, 17(2), 101–107, DOI:10.4015/S1016237205000160.
- P. A. Parvathy, S. De, M. Singh, G. Manik and S. K. Sahoo, RAFT Polymerization Assisted P(NIPAm-Co-AAc)-AEMR Integrated PVA Hydrogels: Dual Responsive Features, Texture Analysis, and Cytotoxicity Studies, React. Funct. Polym., 2024, 205, 106052, DOI:10.1016/j.reactfunctpolym.2024.106052.
- S. De, A. Ghosh, D. Mandal, K. Sarkar, A. P. Samanta, M. Basak, A. Saha, D. Bhattacharya, S. Nandi, J. Sarkar, M. Mandal, K. Acharya, P. Ghosh and D. Chattopadhyay, Lysine-Mediated Yttrium Oxide Nanoparticle–Incorporated Nanofibrous Scaffolds with Tunable Cell Adhesion, Proliferation, and Antimicrobial Potency for In Vitro Wound-Healing Applications, ACS Appl. Bio Mater., 2024, 6414–6429, DOI:10.1021/acsabm.4c00551.
- Q. Weng, J. Yi, X. Chen, D. Luo, Y. Wang, W. Sun, J. Kang and Z. Han, Controllable Synthesis and Biological Application of Schiff Bases from d -Glucosamine and Terephthalaldehyde, ACS Omega, 2020, 5(38), 24864–24870, DOI:10.1021/acsomega.0c03591.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5lp00038f |
| This journal is © The Royal Society of Chemistry 2025 |