 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of an anion exchange resin for enhanced PFAS adsorption in water treatment†
Leila
Khazdooz‡
,
Amin
Zarei‡
and
Alireza
Abbaspourrad
 *
*
Department of Food Science, College of Agriculture & Life Sciences, Cornell University, Stocking Hall, Ithaca, New York 14853, USA. E-mail: Alireza@cornell.edu
First published on 5th May 2025
Abstract
Per- and polyfluoroalkyl substances (PFASs) are a class of toxic, bioaccumulative, and persistent chemicals that pollute natural water sources and the environment, new materials and methods to remove them from water are needed. Anion exchange resins adsorb PFASs through strong electrostatic interactions with negatively charged PFAS molecules with high selectivity and removal efficiency from contaminated water. A series of cross-linked anion exchange resins in the form of polymeric beads were synthesized via inverse suspension polymerization using commercially available cationic monomers, (3-acrylamidopropyl)-trimethylammonium chloride (APTMAC) or diallyldimethylammonium chloride (DADMAC), and the crosslinker, N,N′-methylenebisacrylamide (BisAAm).The resins were evaluated for their ability to remove perfluorooctanoic acid (PFOA) from water and the DADMAC based resin (DR4) with 10 w/w% crosslinker showed the highest efficiency. DR4 exhibits high adsorption capacities of 3300 mg g−1 for PFOA, coupled with rapid adsorption kinetics, achieving equilibrium within an hour. Minimum interference effects from salts, pH variations, and natural organic matter (NOM) were observed and the competitive adsorption study indicated that the presence of other PFASs had no impact on the adsorption efficiency of DR4. DR4 was washed and reused, maintaining its performance after five consecutive PFOA adsorption and desorption cycles.
1. Introduction
Per- and polyfluoroalkyl substances (PFASs) have become ubiquitous in natural waters and because they are chemically and thermally stable as well as resistant to degradation, they are also found in drinking water.1 Recent research indicates that these compounds progressively bioaccumulate in human tissues such as the brain, liver, lungs, bones, and kidneys, leading to functional impairments.2–5 Furthermore, PFAS exposure can disrupt normal endocrine functions, weaken the immune system, impair fertility, elevate cholesterol levels, and decrease birth weight.6–10 The International Agency for Research on Cancer (IARC) and the Environmental Protection Agency have classified PFASs as potentially carcinogenic,11 highlighting their significant threat to both the environment and human health. Unfortunately, concentrations of two major PFASs, perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), have risen in natural water sources, with contaminated groundwater recording high levels exceeding 1000 ng L−1 for PFOA and PFOS,12 contrary to the EU Environmental Protection Agency's recommended advisory level of less than 70 ng L−1.13,14Adsorption emerges as one of the most efficient, environmentally sustainable, and economically viable techniques for removing PFASs from water.1,15–18 Multiple types of solid state adsorbents have been reported as effective for the removal of PFASs from aqueous solutions including anion-exchange resins,19–23 porous organic networks,14,24–26 and covalent organic frameworks (COFs).11,12,27–30 These materials typically offer large specific surface areas, suitable pore sizes, and specific functional groups that enhance adsorption efficiency. Some of these adsorbents, however, exhibit limitations such as low capacity, slow adsorption rates, and structural changes at different pH levels. Additionally, most of these porous materials are synthesized as a benchtop scale through several complicated steps.
Anionic exchange resins like IRA67, IRA400, and IRA910 are effective adsorbents for removing PFASs from water and are widely used in industry due to their cost-effectiveness, superior adsorption capacity, and high regeneration capability.20,22,31 However, they still suffer from slow adsorption kinetics and extended equilibrium times. A polymer with specialized multifunctional groups, including positive charges, hydrophobic carbon chains, hydrogen bonding sites, and fluorophilic interactions could address this problem.14,28,29,32–37 Another crucial factor in enhancing PFAS adsorption efficiency is improving water wettability through increased hydrophilicity. This enhancement facilitates the mass transfer of PFASs toward the adsorbent's capture sites.14 Considering these factors, we synthesized two cross-linked anion exchange resins starting with diallyldimethylammonium chloride (DADMAC) and (3-acrylamidopropyl)-trimethylammonium chloride (APTMAC), two commercially available cationic monomers, and cross-linked the resins using N,N′-methylenebisacrylamide (BisAAm) (Scheme 1). Polyvinyl alcohol (PVOH) was used as a porogen to enhance the porosity of the resins. Structurally, the quaternary ammonium groups impart a positive charge to the resins, while the NH groups provide hydrogen bonding sites. Compared to the APTMAC resin (AR), the DADMAC resin (DR) showed better affinity for removing PFOA from contaminated water. This is due to the incorporation of hydrogen bonding sites that, combined with the positive charge of quaternary ammonium moieties present in DADMAC, improved swelling in water which synergistically aids in the removal of PFASs. DR4 also showed high adsorption capacity (3300 mg g−1) for PFOA removal from aqueous solutions.
2. Materials and methods
2.1. Materials
Diallyldimethylammonium chloride (DADMAC) aqueous solution (65 w/w%), ammonium persulfate (APS) (≥98%), polyvinyl alcohol (PVOH) (fully hydrolyzed, MW ≈ 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), were purchased and used as received from Sigma Aldrich (St Louis, MO, USA). (3-Acrylamidopropyl)-trimethylammonium chloride (APTMAC) aqueous solution (75 w/w%) was purchased from Santa Cruz biotechnology (Dallas, TX, USA). N,N-Methylenebisacrylamide (BisAAm) (>99%) was purchased from Alfa Aesar (Haverhill, MA, USA). Dioctyl sulfosuccinate (AOT) (96%) was purchased from VWR (Radnor, PA, USA). Perfluorodecanoic acid (98%), perfluorooctanoic acid (95%), and perfluorooctane sulfonic acid (40% solution in water) were purchased from Sigma-Aldrich (St Louis, MO, USA). Perfluorohexanoic acid (98%) was purchased from AmBeed (Arlington Heights, IL, USA) and perfluorobutanoic acid (a 0.5 mol L−1 solution in water with LC-MS grade) was purchased from TCI (Montgomeryville, PA, USA). All solvents such as ethanol, methanol, analytical grade methanol, were purchased from Sigma-Aldrich (St Louis, MO, USA) and used as received. Mazola corn oil was purchased from a local market (Ithaca, NY, USA).
000 g mol−1), were purchased and used as received from Sigma Aldrich (St Louis, MO, USA). (3-Acrylamidopropyl)-trimethylammonium chloride (APTMAC) aqueous solution (75 w/w%) was purchased from Santa Cruz biotechnology (Dallas, TX, USA). N,N-Methylenebisacrylamide (BisAAm) (>99%) was purchased from Alfa Aesar (Haverhill, MA, USA). Dioctyl sulfosuccinate (AOT) (96%) was purchased from VWR (Radnor, PA, USA). Perfluorodecanoic acid (98%), perfluorooctanoic acid (95%), and perfluorooctane sulfonic acid (40% solution in water) were purchased from Sigma-Aldrich (St Louis, MO, USA). Perfluorohexanoic acid (98%) was purchased from AmBeed (Arlington Heights, IL, USA) and perfluorobutanoic acid (a 0.5 mol L−1 solution in water with LC-MS grade) was purchased from TCI (Montgomeryville, PA, USA). All solvents such as ethanol, methanol, analytical grade methanol, were purchased from Sigma-Aldrich (St Louis, MO, USA) and used as received. Mazola corn oil was purchased from a local market (Ithaca, NY, USA).
2.2. Characterization
Attenuated total reflectance Fourier transform infrared spectra (ATR–FTIR) were measured with a Shimadzu IRAffinity-1S spectrophotometer at the range from 400 to 4000 cm−1. Thermogravimetric Analysis (TGA) was performed using a Q500 Thermogravimetric Analyzer (TA Instruments, New Castle, DE, USA) under nitrogen atmosphere and a heating rate of 10 °C min−1 in the range of 25–800 °C. Scanning electron microscopy (SEM) was performed using a Zeiss Gemini 500 field emission SEM, samples were coated with a thin carbon layer using a Leica EM SCD 500 sputter system under an argon atmosphere at a pressure of 10−4 mbar. X-ray Photoelectron Spectroscopy (XPS) was conducted using a Scienta Omicron ESCA 2SR instrument under an operating pressure of approximately 1 × 10−9 Torr. Wide/survey scans were performed with a pass energy of 200 eV.2.3. Liquid chromatography/mass spectrometry (LC-MS) conditions
Liquid chromatography (LC, Agilent 1100 series) coupled with a mass spectrometer (MS) was used to analyze PFOA samples. A Phenomenex Luna Omega LC column (100 × 4.6 mm, 3 μm, polar Luna C18, 100 Å pore size) was used at a flow rate of 0.4 mL min−1. The samples were filtered through a 13 mm syringe filter polyvinylidene fluoride (PVDF) with a 0.22 μm pore size then were injected. Mobile phases A (Milli-Q water containing 20 mM ammonium acetate), and mobile phases B (methanol) were used for LC column elution. A gradient elution over a 14-minute run time was applied as follows: 0 to 1 min, 60% A; 1 to 5 min, 60% to 5% A; 5 to 10 min, 5% A; 10 to 12 min, 5% to 60% B; 12 to 14 min, 60% A. The injection volume was 10 μL.The mass spectrometer (Finnigan LTQ) was equipped with an electrospray interface (ESI) operating in positive ion mode. Sheath gas flow rate of 50 arbitrary units, capillary temperature at 350 °C, capillary voltage at 41.0 V, spray voltage at 4.00 kV, and tube lens at 125.0 V are the optimized parameters that are set for mass spectrometer. Quantitative analysis of samples with initial concentrations below 20 μg L−1 was performed by Pace Analytical Services Co. All details of the sample preparation methods and equipment used by Pace are based on our previous work.38 All measurements were conducted in triplicate.
2.4. Inverse suspension polymerization for resins synthesis
All resins were synthesized using inverse suspension polymerization according to our previous work.39,40 The formulation of each resin can be found in Table 1 and the specific quantities of the monomer, crosslinker, porogen, AOT, and APS for synthesis of each resin were summarized in Table S1.† All synthesized resins were characterized by FTIR, and TGA analysis (Fig. S1–S12†).| Resin name | AR1 | AR2 | AR3 | DR4 | DR5 | DR6 | DR7 |
|---|---|---|---|---|---|---|---|
a For all the resins synthesis, APS (2 mol% of monomer) as initiator, AOT (1 mol% of monomer) as a surfactant, MeOH (4 times mass of Bis-AAM), and oil/water ratio (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v 30 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) were used.
b Bis-AAM (mol % of APTMAC or DADMAC) was added as a crosslinker.
c PVOH (5 w/w% of monomer and Bis-AAM) was added as a porogen. v) were used.
b Bis-AAM (mol % of APTMAC or DADMAC) was added as a crosslinker.
c PVOH (5 w/w% of monomer and Bis-AAM) was added as a porogen.
|
|||||||
| APTMAC (mol%) | 100 | 100 | 100 | — | — | — | — |
| DADMAC (mol%) | — | — | — | 100 | 100 | 100 | 100 |
| Bis-AAM (mol%)b | 10 | 30 | 40 | 10 | 30 | 40 | 10 |
| Porogen (w/w%)c | 5 | 5 | 5 | 5 | 5 | 5 | — |
2.5. PFAS adsorption studies
The PFOA removal efficiency for each resin was calculated according to eqn (1), and the amount of PFOA adsorbed on the adsorbent was calculated by eqn (2),
 | (1) |
 | (2) |
A typical kinetic study for group I. A 500 mL plastic bottle was charged with 10 mg of resin DR4, and 100 mL of PFOA solution with 10 mg L−1 concentration. The mixture was shaken at room temperature by a rocking shaker at 80 rpm. At specified time points (5, 10, 15, 20, 30, 45, 60, 90, 120, and 180 min) 1 mL of each sample was collected, filtered, and analyzed by LC/MS. The kinetics of the PFOA adsorption by each group was fitted by Ho and McKay's pseudo-second-order adsorption model in a linearized form.38
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30% NaCl (1%)) and consequently washed with MQ-water (2 × 10 mL). The resin does not need any activation or any special regeneration before reusing.
30% NaCl (1%)) and consequently washed with MQ-water (2 × 10 mL). The resin does not need any activation or any special regeneration before reusing.
3. Results and discussion
3.1 Resin synthesis and characterization
We synthesized various anion exchange resins by inverse suspension polymerization of APTMAC and DADMAC. For all anion exchange resins BisAAm was used as a crosslinker. For the synthesis of these resins sodium di-2-ethylhexylsulfosuccinate (AOT) as an anionic surfactant was used to place most of the cationic monomers (APTMAC and DADMAC) on the surface of resins and increase the functionality of ammonium groups on the surface of the corresponding resins.40 The absence of a surfactant in inverse suspension polymerization can result in severe delamination due to phase separation within the aqueous droplets. To mitigate this issue, a surfactant is used to facilitate the participation of hydrophilic monomers at the interface, thereby ensuring their integration into the resin surface.40We synthesized three anion exchange resins (AR1, AR2, AR3) by using APTMAC and four anion exchange resins (DR4, DR5, DR6, DR7) by using DADMAC, the formulations of synthesized resins are summarized in Table 1, with additional details in Table S1.† To study the effect of crosslinker we increased the amount of Bis-AAM from 10 to 40% in APTMAC resins (AR1–AR3) and in DADMAC resins (DR4–DR6). In all mentioned synthesized resins PVOH (5 w/w% of both monomer and crosslinker) was used as a porogen to increase the porosity. To observe the effect of porosity in the resins, resin DR7 was synthesized using the same formulation as resin DR4 but without using porogen.
All synthesized resins were characterized by FT-IR and TGA. The FT-IR spectrum of resins confirmed the functional groups and showed the stretching vibrations of free N–H bonds in 3375–3248 cm−1 regions.41 A strong band at around 1643 cm−1 corresponds to the stretching C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the amide groups (Fig. S1–S6†). To assess the thermal stability of the synthesized resins, Thermogravimetric Analysis (TGA) was performed under a nitrogen atmosphere with a heating rate of 10 °C min−1, over a temperature range of 25 °C to 800 °C. All samples were dried overnight under vacuum at 60 °C; nevertheless, approximately 20% of its total mass was attributed to adsorbed atmospheric humidity. This relatively high moisture adsorption can be ascribed to the hydrophilic nature of the resin.40 The TGA thermograms showed a similar pattern for APTMAC resins (AR1–AR3) and also we observed a similar pattern for DADMAC resins (DR4–AR7). In all resins the first weight loss occurred around 300 °C which is related to the degradation of the crosslinking segments (Bis-AAM) within the polymer structure (Fig. S7–S12†).
O bond of the amide groups (Fig. S1–S6†). To assess the thermal stability of the synthesized resins, Thermogravimetric Analysis (TGA) was performed under a nitrogen atmosphere with a heating rate of 10 °C min−1, over a temperature range of 25 °C to 800 °C. All samples were dried overnight under vacuum at 60 °C; nevertheless, approximately 20% of its total mass was attributed to adsorbed atmospheric humidity. This relatively high moisture adsorption can be ascribed to the hydrophilic nature of the resin.40 The TGA thermograms showed a similar pattern for APTMAC resins (AR1–AR3) and also we observed a similar pattern for DADMAC resins (DR4–AR7). In all resins the first weight loss occurred around 300 °C which is related to the degradation of the crosslinking segments (Bis-AAM) within the polymer structure (Fig. S7–S12†).
We studied the performance of all synthesized resins for PFOA adsorption, and DR4 demonstrated the best PFOA adsorption. Therefore, we fully characterized the functional groups, thermal stability, and surface composition of DR4.
The FT-IR spectrum of the DR4 exhibited stretching vibrations of N–H bonds at 3375 and 3248 cm−1 (Fig. 1a), associated with the secondary amide groups of the crosslinker in the resin.41 The stretching vibrations of the aliphatic C–H bonds were observed around 2935 cm−1. A strong band at 1647 cm−1 corresponds to the stretching C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the amide groups. A band at 1527 cm−1 is attributed to the N–H bending vibration of the amide groups. The bending vibration of CH2 groups, indicative of the polymerization of DADMAC, was observed at 1473 cm−1. Lastly, a broad band in the 800–600 cm−1 region confirms the out-of-plane N–H wagging vibrations.41 The TGA thermograms and the rate of degradation as a function of temperature are illustrated in Fig. 1b. Typical of synthesized resins, approximately 20% of its total mass was attributed to adsorbed atmospheric humidity. The initial major weight loss, observed around 320 °C, corresponds to the degradation of the crosslinking segments (Bis-AAM) within the DR4 structure. The subsequent significant change in the degradation rate, noted at approximately 475 °C, signifies the degradation of the DADMAC subunit.42
O bond of the amide groups. A band at 1527 cm−1 is attributed to the N–H bending vibration of the amide groups. The bending vibration of CH2 groups, indicative of the polymerization of DADMAC, was observed at 1473 cm−1. Lastly, a broad band in the 800–600 cm−1 region confirms the out-of-plane N–H wagging vibrations.41 The TGA thermograms and the rate of degradation as a function of temperature are illustrated in Fig. 1b. Typical of synthesized resins, approximately 20% of its total mass was attributed to adsorbed atmospheric humidity. The initial major weight loss, observed around 320 °C, corresponds to the degradation of the crosslinking segments (Bis-AAM) within the DR4 structure. The subsequent significant change in the degradation rate, noted at approximately 475 °C, signifies the degradation of the DADMAC subunit.42
Finally, X-ray photoelectron spectroscopy (XPS) was used to investigate the chemical states of surface elements, confirming the presence of C, N, O, and Cl in the DR4 structure (Fig. 2a). The deconvolution of the C 1s spectrum revealed a distinct peak at a binding energy of 284.6 eV, indicating C–C bonds in the DR4 structure (Fig. 2b). The corresponding binding energies for C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds were observed at 285.8 eV and 286.5 eV, respectively. The N 1s spectrum showed two peaks at 399.1 eV and 401.9 eV, attributed to N–C in the crosslinker section and +N–C in quaternary ammonium moieties, respectively (Fig. 2c).43 A peak at 531.5 eV, implying the presence of oxygen, was assigned to the C
O bonds were observed at 285.8 eV and 286.5 eV, respectively. The N 1s spectrum showed two peaks at 399.1 eV and 401.9 eV, attributed to N–C in the crosslinker section and +N–C in quaternary ammonium moieties, respectively (Fig. 2c).43 A peak at 531.5 eV, implying the presence of oxygen, was assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups (Fig. 2d). Additionally, peaks observed at 196.6 eV, 198.2 eV, and 268.1 eV were identified as Cl 2p3/2, Cl 2p1/2, and Cl 2s, respectively, confirming the presence of chloride anions in the resin DR4 (Fig. 2e and f).43
O groups (Fig. 2d). Additionally, peaks observed at 196.6 eV, 198.2 eV, and 268.1 eV were identified as Cl 2p3/2, Cl 2p1/2, and Cl 2s, respectively, confirming the presence of chloride anions in the resin DR4 (Fig. 2e and f).43
 | ||
| Fig. 2 (a) High-resolution XPS analysis of resin DR4. Deconvoluted profiles of (b) C 1s, (Residual STD: 1.7) (c) N 1s, (Residual STD: 0.95) (d) O 1s (e) Cl 2p, (Residual STD: 1.2), and (f) Cl 2s. | ||
To assess the impact of adding a porogen during synthesis, the morphology of DR4 and DR7 resins was studied using scanning electron microscopy (SEM). The SEM images revealed that both resins exhibited spherical shapes beads in the range of 20 to 100 μm. However, DR4 displayed significant surface roughness and porosity attributable to the porogen (PVOH) used during its synthesis (Fig. 3a–c). In contrast, the surface of DR7, synthesized without the porogen, was completely smooth and lacked any roughness (Fig. 3d–f). This structural distinction accounts for the adsorption performance of DR4 for PFOA which is noticeably higher than DR7.
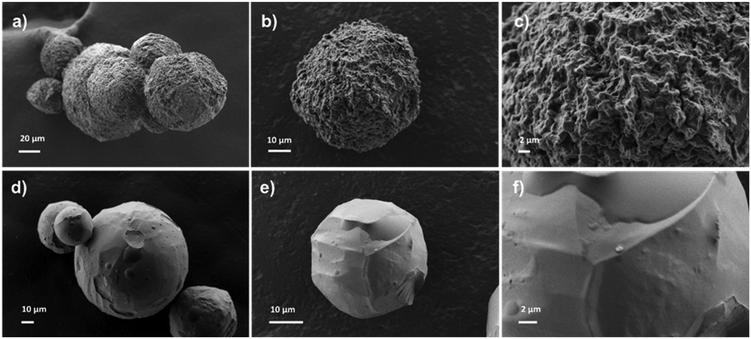 | ||
| Fig. 3 (a–c) SEM images of resin DR4 revealing spherical particle shapes and roughness. (d–f) SEM images of resin DR7 revealing spherical particle shapes and smooth surface. | ||
3.2 PFOA removal from aqueous solutions
After synthesizing the anion exchange resins, they were used to remove PFOA from aqueous solutions. In these experiments, 10 mg of each resin was separately added to 100 mL of an aqueous PFOA solution (5 mg L−1). After 1 hour of exposure at room temperature, the PFOA remaining in the solution was measured using LC-MS. The maximum removal efficiency was achieved using DR4, which removed 99.6% of the PFOA (Fig. 4a). Although both APTEMAC and DADMAC resins have positive sites in their structures to increase the affinity between the resins and PFOA, the DADMAC resins demonstrated higher removal efficiency than the APTEMAC resins.To explain the difference in adsorption, the swelling of APTEMAC and DADMAC resins was investigated, water swelling is enhanced by higher hydrophilicity of adsorbent particles and the presence of highly accessible functionalized capturing sites. Higher swelling accelerates the mass transfer of PFASs towards the adsorbents in aqueous solutions, thereby increasing adsorption of PFASs from water.14 The swelling of APTEMAC and DADMAC resins was investigated by comparing the behavior of each resin (100 mg) in water (Fig. 4b). The swelling percentage was determined using the equation: swelling = [(W2 − W1)/W1] × 100, where W1 represents the weight of the dry resin and W2 represents the weight of the swollen resin.39,44 DADMAC resins exhibited higher swelling than APTEMAC resins. This enhanced swelling likely accounts for the increased PFOA uptake observed with DADMAC resins. To test this, the proportion of the crosslinker was increased, which reduced the number of positive capturing sites and led to decreased swelling and, consequently, lower PFOA adsorption. The optimal amount of Bis-AAM was determined to be 10 mol%. Additionally, the use of PVOH as a porogen in the synthesis of DR4, compared to DR7, further improved both the swelling and adsorption efficiency of the resin.
3.3 PFOA adsorption using DR4
DR4 was determined to have the best adsorption of PFOA from water and therefore was used for all further studies. Energy-dispersive X-ray spectroscopy (EDS) and FT-IR were used to verify the presence of adsorbed PFOA on DR4. EDS mapping demonstrated a uniform distribution of fluorine across the surface of DR4 (Fig. S13†). Additionally, FT-IR analysis of the adsorbed PFOA on DR4 displayed a distinct band at 1690 cm−1, corresponding to the stretching vibration of the carbonyl functional group of PFOA (Fig. S14†). Furthermore, bands observed at approximately 1199 and 1143 cm−1 were attributed to the stretching vibrations of the CF3 and CF2 groups of PFOA, respectively.41To assess the resin DR4's performance, toward PFOA adsorption, the time-dependent and kinetic behavior were assessed for PFOA adsorption at various concentrations over a 2 h period (Fig. 5a). The selection of 0.2 to 10 mg L−1 for PFOA solutions was designed to examine adsorption kinetics across a broad concentration range. Regardless of the PFOA concentration, the time-dependent studies demonstrated exceptional PFOA removal, exceeding 98% within 30 minutes using DR4 (Fig. 5b). DR4, with a particle size range of 20–100 μm (Fig. 3a), exhibited rapid adsorption kinetics, achieving greater than 99% PFOA adsorption in less than 60 minutes. This rapid performance may be attributed to its smaller particle size, and thus larger surface area, when compared to commercial anionic exchange resins such as IRA67, IRA400, and IRA910, which typically have particle sizes ranging from 0.3 to 1.2 mm.20,22,31 The kinetics of DR4 at each PFOA concentration were modeled using the linear pseudo-second-order kinetic model (Fig. 5c), and the corresponding rate constants were determined (Fig. 5a). These findings underscore the rapid and efficient adsorption capabilities of DR4, establishing it as an effective anionic exchange resin for the removal of PFOA from water in different concentrations (Fig. 5a, entries I–III). Further, increasing the adsorbent amount improved the adsorption rate (Fig. 5a, entries III–IV).
The capacity of DR4 for PFOA removal from water was investigated. An adsorption isotherm was constructed using 10 mg of resin and PFOA solutions ranging from 1 to 800 mg L−1 (Fig. S15†). The adsorption isotherm exhibited an excellent fit with the Langmuir model, demonstrating remarkable alignment with a correlation coefficient of approximately 0.97 (Fig. S16†). Based on the Langmuir model, the estimated maximum adsorption capacity (Qm) for DR4 was determined to be 3300 mg g−1, representing the highest capacity for PFOA adsorption among reported anionic exchange resins and porous materials (Table S2†). Additionally, the affinity coefficient (KL) was calculated to be 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 L mol−1, indicating a strong affinity between DR4 and PFOA. The Freundlich isotherm, with a lower correlation coefficient of 0.82, did not fit the experimental data as well. The Freundlich constant (KF) for DR4 was determined to be 756.7 (mg g−1) (L mg−1)(1/n), where n was 3.6 (Fig. S17†).
100 L mol−1, indicating a strong affinity between DR4 and PFOA. The Freundlich isotherm, with a lower correlation coefficient of 0.82, did not fit the experimental data as well. The Freundlich constant (KF) for DR4 was determined to be 756.7 (mg g−1) (L mg−1)(1/n), where n was 3.6 (Fig. S17†).
The presence of quaternary ammonium groups with positive charges within the DR4 structure provide electrostatic and ion exchange interactions with PFOA that are likely the primary mechanisms for adsorption.20,22,31 Additionally, factors such as porosity, shape, and particle size of the adsorbents can significantly influence the efficiency of PFOA adsorption.1,11,45 The high capacity and rapid kinetics of DR4 for PFOA removal can be attributed to its synthesis via inverse suspension polymerization. This polymerization method, involving cationic monomers in the presence of a suitable anionic surfactant and porogen, not only increases the number of active monomers on the surface but also enhances the polymer's porosity.40 Consequently, the increased active capturing sites and improved porosity on the DR4 surface enhance the accessibility of active sites for PFOA adsorption, leading to a remarkable improvement in its efficiency compared to other reported anionic exchange resins.20,22,31
3.4 Effect of pH on PFOA adsorption
Since the pH of wastewater can vary widely from acidic to alkaline depending on the source of contamination, the absorption of PFOA was studied at different pH ranges. To illustrate the effect of pH on the adsorption efficiency of DR4, the removal of PFOA was investigated over a broad pH range from 3 to 9 (Fig. 6a). The pH was adjusted using 0.1 M solutions of HCl and NaOH. For each sample, the initial concentration of PFOA was maintained at 0.2 mg L−1, with 10 mg of DR4 and an exposure time of 1 hour. The selected concentration of 0.2 mg L−1 is comparable to the reported maximum PFOA levels in industrial discharge, landfill leachate, wastewater, and contaminated groundwater, ensuring environmental relevance.46,47Changing the pH between 5 and 8 had a minimal impact on the ability of DR4 to remove PFOA added to Milli-Q water. The highest removal efficiency was observed at pH 6, attributed to strong electrostatic interactions between PFOA and DR4. The pKa of PFOA is 2.5,48 indicating that at pH 6 it exists predominantly in its anionic form. This negative charge means that PFOA can effectively interact with the positively charged ammonium functional groups of DR4.20 At pH 9, a decrease of approximately 10% in adsorption efficiency was observed, this is attributed to competition between hydroxide ions and PFOA with the DR4. This competition impedes PFOAs’ anion exchange interactions and reduces the removal efficiency of DR4. Finally, a noticeable decrease in adsorption efficiency was observed at pH 3. Considering the pKa of PFOA, at pH 3, approximately 76% of it remains in its non-ionized form. As a result, its electrostatic attraction to the resin is reduced due to the lower negative charge. Additionally, this decrease in adsorption may also be attributed to the presence of chloride ions from the HCl used to acidify the solution, which could compete with PFOA for adsorption sites.21
3.5 Effect of inorganic salts and natural organic matter on PFOA adsorption
In addition to the pH study, the selectivity of DR4 for the removal of PFOA was examined in the presence of various inorganic salts and natural organic matter. These competitive adsorption studies included common anions found in drinking and groundwater, such as chloride, nitrate, sulfate, and chromate, using NaCl, NaNO3, Na2SO4, and K2CrO4 as their respective sources. Additionally, the impact of natural organic matter (NOM) was represented by humic acid and tannic acid was investigated (Fig. 6b). In each experiment, PFOA, and each inorganic salt or NOM were dissolved in Milli-Q water. For each sample, 10 mg of DR4 was used as the sorbent. The concentration of PFOA was 0.2 mg L−1 and for each salt or NOM the concentration was 5 mg L−1. The total volume for each sample was 20 mL. A control experiment was conducted by adding the same amounts of PFOA and DR4 into Milli-Q water without any additional salts and NOM. Minimal interference from both inorganic and organic pollutants on the PFOA removal efficiency using DR4 was observed (Fig. 6b).To expand the scope of this study, an experiment was designed by introducing PFOA, DR4, and a mixture of NaCl, NaNO3, Na2SO4, K2CrO4, humic acid, and tannic acid into Milli-Q water. The concentration of each salt and NOM was maintained at 5 mg L−1, while the concentration of PFOA was 0.2 mg L− and the amount of DR4 used was 10 mg. The total concentration of inorganic salts and NOM in this experiment was 30 mg L−1, which is 150 times higher than that of PFOA. Compared to the control experiment, no salts or NOM, a reduction of approximately 20% in removal efficiency was observed, attributed to the increased ionic strength of the aqueous solution, which may decrease the interaction between PFOA and the surface of the resin.49
Most studies on adsorbents for PFAS removal from water are done using deionized (DI) or Milli-Q water, often neglecting the impact of dissolved components in tap water. Therefore, PFOA was added to locally sourced tap water from Ithaca, NY and DR4 was used to remove it. The concentration of PFOA was 0.2 mg L−1 and the DR4 amount used was 10 mg. A decrease of around 26% in the efficiency of DR4 in removing PFOA from tap water was observed, this is likely due to the presence of various components in tap water (total hardness 122 mg L−1, total alkanility 104 mg L−1, chloride 52 mg L−1, sulfate 12 μg L−1, and Nitrate 0.26 mg L−1) that may interfere with the PFAS removal process.50
3.6 PFAS removal from aqueous solutions
While PFOA is the most toxic and prevalent PFAS, other PFASs have been found in wastewater and drinking water supplies, therefore we evaluated the ability of DR4 to remove other PFASs at low concentrations. The PFASs used for this experiment were perfluorobutanoic acid (PFBA), perfluorohexanoic acid (PFHA), perfluoro-2-methyl-3-oxahexanoic acid (GenX), perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), and perfluorodecanoic acid (PFDA) (Fig. 6c). The experiments were conducted separately in Milli-Q water (single solute system) with a 1 h exposure period. In each experiment, 10 mg of DR4 was used as the sorbent, with an initial concentration of 20 μg L−1 for each PFAS and a total volume of 100 mL. PFOA, PFOS, and PFDA, characterized by their longer carbon chains, demonstrated significant removal efficiency when treated with DR4. Notably, PFOS exhibited the highest adsorption rate, achieving above 99% removal efficiency after exposure to the resin with only 8 ng L−1 remaining in the solution. The performance of DR4 toward PFOS is likely due to PFOS's high hydrophobicity, and its higher acidity as compared to other PFASs,20 which facilitates better ionization and subsequently enhances the affinity of PFOS for DR4. The removal efficiencies for GenX, PFHA, and PFBA ranged between 86% and 93%, proving that in addition to removing long chain PFASs, DR4 is also effective for the removal of PFASs with shorter carbon chains. PFBA is identified as a leachable, short-chain anionic PFAS, which is difficult to remove due to its shorter carbon chain and higher water solubility. Previous studies with the same initial concentration of PFBA (20 μg L−1) in Milli-Q water have demonstrated PFBA removal efficiencies ranging from 23% to 35%, underscoring the challenge of achieving high removal rates for this compound.30,51 However, this difficulty is notably overcome with DR4, which achieves a removal efficiency of more than 93% for PFBA.To evaluate the impact of co-existing PFASs on adsorption efficiency, a competitive adsorption study was done using a mixture of PFBA, PFHA, GenX, PFOA, PFOS, and PFDA in a multi-solute system (Fig. 6d). The parameters, including the amount of adsorbent, the concentration of each PFAS, the total volume, and the exposure time, were kept consistent with the single solute experiments. Although the total concentration of PFASs in multi-solute solution was 6 times (120 μg L−1) more than that of single solute experiments, there was no change in removal efficiency for PFBA, PFOA, PFOS, and PFDA. Interestingly, for PFHA and especially GenX, the results of the multi-solute experiment showed an improvement in adsorption efficiency compared to the single-solute experiments. The first layer of PFAS adsorption occurs via electrostatic interactions, while the second layer involves hydrophobic interactions between the hydrophobic fluorinated tails of PFAS molecules. After the initial interactions, the surface of DR4 loses some of its electrostatic character as it surrounded by PFAS molecules and becomes more hydrophobic38 due to the hydrophobic fluorocarbon tails that protrude from the PFAS adsorbed onto the resin.35 Thus longer-chain PFAS continue to be attracted to DR4, leading to higher adsorption efficiency compared to short-chain PFAS.52,53 Therefore, hydrophobic interactions between GenX and the resin are enhanced by the increased hydrophobicity of the resins in a solution with a variety of PFAS molecules. These results strongly support the synergistic effect of co-existing PFAS on the adsorption efficiency of DR4.
The average concentration of PFAS in contaminated groundwater is close to 1 μg L−1, therefore we evaluated DR4 for PFAS removal at these concentrations.12 A mixture of PFBA, PFHA, GenX, PFOA, PFOS, and PFDA was added to Milli-Q water at an initial concentration of each PFAS of 200 ng L−1, the amount of DR4 used was 50 mg, the total volume was 20 mL, and the exposure time to the resin was 1 hour. In this solution, the total concentration of PFAS was 1.2 μg L−1, which is close to the average concentration of PFAS in contaminated groundwater.54 All PFAS, except GenX which had a final concentration of 3.2 ng L−1, were not detected in the solution after exposure to the resin. These samples were analyzed by Pace company using LC/MS-MS and the detection limits for each PFAS were: PFBA 8.4 ng L−1; PFHA 1.4 ng L−1; GenX 2.4 ng L−1; PFOA 4.1 ng L−1; PFOS 1.6 ng L−1; and PFDA 2.2 ng L−1.
In a similar study to PFOA in tap water, DR4's efficacy for a competitive adsorption was studied by adding the PFAS to Ithaca, NY tap water. A sample of tap water direct from the tap was analyzed to ensure that none were detected before the study.
A mixture of PFBA, PFHA, GenX, PFOA, PFOS, and PFDA was added to tap water at an initial concentration of each PFAS of 200 ng L−1, the amount of DR4 used was 50 mg, the total volume was 20 mL, and the exposure time to the resin was 1 hour. Despite the dissolved components in tap water impacting the adsorbent, the results indicated that DR4 performed well toward the removal of PFASs from tap water (Fig. 6e). For instance, in addition to PFDA, PFOS was not detected in the tap water after exposure to DR4. The final concentration of PFOA was measured at 75 ng L−1, indicating a significant reduction. Additionally, the concentrations of PFBA, PFHA, and GenX, decreased to 59, 45, and 61 ng L−1, respectively. These findings confirm the practical application of DR4 in removing PFASs from contaminated drinking water.
3.7 Reusability
The reusability of resins for water purification is important for real-use applications. To assess this, DR4 was evaluated after several consecutive adsorption/desorption cycles. Following previously recognized procedures for regenerating anion exchange resins,21,23 desorption was conducted using a mixture of methanol and NaCl solution. The quantities of adsorbed PFOA were measured in each cycle. Results from five cycles indicated that DR4 maintained high efficiency (>99%) for PFOA adsorption, demonstrating excellent structural integrity and resilience through successive adsorption–desorption processes (Fig. 6f).4. Conclusion
In summary, the synthesis and characterization of a new cross-linked anion exchange resin (DR4) by inverse suspension polymerization of APTMAC, a commercially available cationic monomer, in corn oil, cross-linked using BisAAm was reported. To increase the porosity of the resin, PVOH was used as a porogen, and AOT was used as an anionic surfactant to enhance the functionality of ammonium groups on the surface of the resin. Resin DR4 exhibited fast and efficient adsorption capabilities towards various PFAS from contaminated water. The high adsorption capacity of 3300 mg g−1 for PFOA, coupled with rapid adsorption kinetics and high selectivity, is attributed to the cationic surface of DR4, which facilitates electrostatic interactions and anion-exchange ability with PFAS. Furthermore, the high swelling of DR4 enhances the mass transfer of PFASs towards the resin in aqueous solutions, thereby achieving fast adsorption of PFASs from water. Additionally, resin DR4 demonstrated excellent regeneration and reusability, maintaining its performance across multiple cycles of reuse. This underscores its practical application for the removal of PFASs from contaminated water sources.Author contributions
L.K.: conceptualization, formal analysis, data curation, methodology investigation, writing – original draft, writing – review & editing. A.Z.: formal analysis, methodology investigation, data curation, writing – original draft, writing – review and editing. A.A.: project administration, conceptualization, resources, funding acquisition, supervision, writing – review and editing.Data availability
Raw data used to create the visual elements can be found at: https://zenodo.org/records/13984331.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank the Cornell Center for Materials Research (funded by the National Science Foundation under grant DMR-1719875) and the NMR facility at Cornell University (supported by the NSF through MRI grant number CHE-1531632).References
- C. T. Vu and T. Wu, Crit. Rev. Environ. Sci. Technol., 2022, 52, 90–129 CrossRef CAS.
- F. Pérez, M. Nadal, A. Navarro-Ortega, F. Fàbrega, J. L. Domingo, D. Barceló and M. Farré, Environ. Int., 2013, 59, 354–362 CrossRef.
- N. M. Crawford, S. E. Fenton, M. Strynar, E. P. Hines, D. A. Pritchard and A. Z. Steiner, Reprod. Toxicol., 2017, 69, 53–59 CrossRef CAS.
- J. Stone, P. Sutrave, E. Gascoigne, M. B. Givens, R. C. Fry and T. A. Manuck, Am. J. Obstet. Gynecol. MFM, 2021, 3, 100308 CrossRef CAS PubMed.
- L. Cao, Y. Guo, Y. Chen, J. Hong, J. Wu and J. Hangbiao, Chemosphere, 2022, 296, 134083 CrossRef CAS PubMed.
- M.-A. Verner, A. E. Loccisano, N.-H. Morken, M. Yoon, H. Wu, R. McDougall, M. Maisonet, M. Marcus, R. Kishi and C. Miyashita, Environ. Health Perspect., 2015, 123, 1317–1324 CrossRef CAS PubMed.
- Q. Wu, X. Coumoul, P. Grandjean, R. Barouki and K. Audouze, Environ. Int., 2021, 157, 106232 CrossRef CAS PubMed.
- B. Granum, L. S. Haug, E. Namork, S. B. Stølevik, C. Thomsen, I. S. Aaberge, H. van Loveren, M. Løvik and U. C. Nygaard, J. Immunotoxicol., 2013, 10, 373–379 CrossRef CAS.
- J. C. DeWitt, Toxicological effects of perfluoroalkyl and polyfluoroalkyl substances, Springer, 2015 Search PubMed.
- J. L. Domingo and M. Nadal, J. Agric. Food Chem., 2017, 65, 533–543 CrossRef CAS PubMed.
- P. S. Pauletto and T. J. Bandosz, J. Hazard. Mater., 2022, 425, 127810 CrossRef CAS.
- J. Huang, Y. Shi, J. Xu, J. Zheng, F. Zhu, X. Liu and G. Ouyang, Adv. Funct. Mater., 2022, 32, 2203171 CrossRef CAS.
- C. Liu, J. Chu, N. L. Cápiro, J. D. Fortner and K. D. Pennell, J. Hazard. Mater., 2022, 422, 126960 CrossRef CAS.
- X. Liu, C. Zhu, J. Yin, J. Li, Z. Zhang, J. Li, F. Shui, Z. You, Z. Shi and B. Li, Nat. Commun., 2022, 13, 2132 CrossRef CAS PubMed.
- D. Q. Zhang, W. L. Zhang and Y. N. Liang, Sci. Total Environ., 2019, 694, 133606 CrossRef CAS PubMed.
- Y. Liu, T. Li, J. Bao, X. Hu, X. Zhao, L. Shao, C. Li and M. Lu, Appl. Sci., 2022, 12, 1941 CrossRef CAS.
- Z. Du, S. Deng, Y. Bei, Q. Huang, B. Wang, J. Huang and G. Yu, J. Hazard. Mater., 2014, 274, 443–454 CrossRef CAS.
- E. Gagliano, M. Sgroi, P. P. Falciglia, F. G. Vagliasindi and P. Roccaro, Water Res., 2020, 171, 115381 CrossRef CAS PubMed.
- P. McCleaf, S. Englund, A. Östlund, K. Lindegren, K. Wiberg and L. Ahrens, Water Res., 2017, 120, 77–87 CrossRef CAS.
- A. Maimaiti, S. Deng, P. Meng, W. Wang, B. Wang, J. Huang, Y. Wang and G. Yu, Chem. Eng. J., 2018, 348, 494–502 CrossRef CAS.
- Y. Gao, S. Deng, Z. Du, K. Liu and G. Yu, J. Hazard. Mater., 2017, 323, 550–557 CrossRef CAS PubMed.
- W. Wang, X. Mi, Z. Zhou, S. Zhou, C. Li, X. Hu, D. Qi and S. Deng, J. Colloid Interface Sci., 2019, 557, 655–663 CrossRef CAS.
- T. Tamanna, P. J. Mahon, R. K. Hockings, H. Alam, M. Raymond, C. Smith, C. Clarke and A. Yu, Appl. Sci., 2023, 13, 6263 CrossRef CAS.
- D. Abdullatif, A. Khosropour, A. Khojastegi, I. Mosleh, L. Khazdooz, A. Zarei and A. Abbaspourrad, ACS Appl. Polym. Mater., 2022, 5, 412–419 CrossRef.
- A. Yang, C. Ching, M. Easler, D. E. Helbling and W. R. Dichtel, ACS Mater. Lett., 2020, 2, 1240–1245 CrossRef CAS.
- C. Ching, Y. Ling, B. Trang, M. Klemes, L. Xiao, A. Yang, G. Barin, W. R. Dichtel and D. E. Helbling, Water Res., 2022, 209, 117938 CrossRef CAS PubMed.
- W. Ji, L. Xiao, Y. Ling, C. Ching, M. Matsumoto, R. P. Bisbey, D. E. Helbling and W. R. Dichtel, J. Am. Chem. Soc., 2018, 140, 12677–12681 CrossRef CAS PubMed.
- J. Huang, Y. Shi, G. Huang, S. Huang, J. Zheng, J. Xu, F. Zhu and G. Ouyang, Angew. Chem., Int. Ed., 2022, 61, e202206749 CrossRef CAS PubMed.
- A. N. Zeppuhar, D. S. Rollins, D. L. Huber, E. A. Bazan-Bergamino, F. Chen, H. A. Evans and M. K. Taylor, ACS Appl. Mater. Interfaces, 2023, 15, 52622–52630 CAS.
- A. Zarei, A. Khosropour, L. Khazdooz, S. Amirjalayer, A. Khojastegi, A. Zadehnazari, Y. Zhao and A. Abbaspourrad, ACS Appl. Mater. Interfaces, 2024, 16, 9483–9494 CrossRef CAS.
- W. Wang, A. Maimaiti, H. Shi, R. Wu, R. Wang, Z. Li, D. Qi, G. Yu and S. Deng, Chem. Eng. J., 2019, 364, 132–138 CrossRef CAS.
- I. M. Manning, N. G. P. Chew, H. P. Macdonald, K. E. Miller, M. J. Strynar, O. Coronell and F. A. Leibfarth, Angew. Chem., 2022, 134, e202208150 CrossRef.
- X. Tan, J. Zhong, C. Fu, H. Dang, Y. Han, P. Král, J. Guo, Z. Yuan, H. Peng and C. Zhang, Macromolecules, 2021, 54, 3447–3457 CrossRef CAS.
- X. Tan, M. Sawczyk, Y. Chang, Y. Wang, A. Usman, C. Fu, P. Král, H. Peng, C. Zhang and A. K. Whittaker, Macromolecules, 2022, 55, 1077–1087 CrossRef CAS.
- A. Román Santiago, S. Yin, J. Elbert, J. Lee, D. Shukla and X. Su, J. Am. Chem. Soc., 2023, 145, 9508–9519 CrossRef PubMed.
- A. Choudhary and D. Bedrov, ACS Macro Lett., 2022, 11, 1123–1128 CrossRef CAS.
- S. Anjum, M. Arik, A. Patel, N. Abasali, L. Wu and A. Sarkar, ACS Appl. Polym. Mater., 2025, 1187–1193 CrossRef CAS.
- A. Zadehnazari, A. Khosropour, A. Zarei, L. Khazdooz, S. Amirjalayer, F. Auras and A. Abbaspourrad, Small, 2024, 2405176 CrossRef CAS PubMed.
- L. Khazdooz, A. Zarei, G. Meletharayil, R. Kapoor and A. Abbaspourrad, ACS Omega, 2023, 8, 30966–30975 CrossRef CAS.
- M. Enayati, M. Karimi Abdolmaleki and A. Abbaspourrad, ACS Biomater. Sci. Eng., 2020, 6, 2822–2831 CrossRef CAS.
- R. M. Silverstein, F. X. Webster, D. Kiemle and D. L. Bryce, Spectrometric Identification of Organic Compounds, Wiley, 8th Edition, 2014 Search PubMed.
- D. Valade, F. Boschet, S. Roualdès and B. Ameduri, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2043–2058 CrossRef CAS.
- Z. Shi, K. G. Neoh and E. T. Kang, Biomaterials, 2005, 26, 501–508 CrossRef CAS PubMed.
- R. F. Pereira, R. Brito-Pereira, R. Goncalves, M. P. Silva, C. M. Costa, M. M. Silva, V. de Zea Bermudez and S. Lanceros-Méndez, ACS Appl. Mater. Interfaces, 2018, 10, 5385–5394 CrossRef CAS.
- S. Yin and D. Villagrán, Sci. Total Environ., 2022, 831, 154939 CrossRef CAS.
- B. G. Moneta, M. L. Feo, M. Torre, P. Tratzi, S. E. Aita, C. M. Montone, E. Taglioni, S. Mosca, C. Balducci and M. Cerasa, Sci. Total Environ., 2023, 894, 165089 CrossRef CAS PubMed.
- G. R. Johnson, M. L. Brusseau, K. C. Carroll and G. R. Tick.
- Z. Zhang, D. Sarkar, R. Datta and Y. Deng, J. Hazard. Mater. Lett., 2021, 2, 100034 CrossRef CAS.
- W. Wang, Z. Zhou, H. Shao, S. Zhou, G. Yu and S. Deng, Chem. Eng. J., 2021, 412, 127509 CrossRef CAS.
- https://www.cityofithaca.org/DocumentCenter/View/15429 .
- N. Zorigt, A. Zarei, F. Auras, L. Khazdooz, A. Khosropour and A. Abbaspourrad, Small, 2024, 2406805 Search PubMed.
- X. Lei, Q. Lian, X. Zhang, T. K. Karsili, W. Holmes, Y. Chen, M. E. Zappi and D. D. Gang, Environ. Pollut., 2023, 321, 121138 CrossRef CAS PubMed.
- F. Dixit, R. Dutta, B. Barbeau, P. Berube and M. Mohseni, Chemosphere, 2021, 272, 129777 CrossRef CAS.
- D. Mussabek, A. Söderman, T. Imura, K. M. Persson, K. Nakagawa, L. Ahrens and R. Berndtsson, Water, 2022, 15, 137 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed table of synthetic preparations for each resin. Additional figures including FTIR, TGA, SEM of resins and Langmuir and Freundlich isotherms for adsorption of PFAS onto DR4. Comparison table of previously published adsorbents for PFAS with DR4 resin. Raw data used to create the visual elements can be found at: https://zenodo.org/records/13984331. See DOI: https://doi.org/10.1039/d5lp00035a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |





