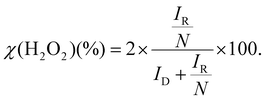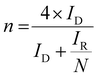Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Self-assembly of semiaromatic poly(amic acid) into flower-like microparticles via one-step precipitation polymerization†
Yuqian
Chen
 a,
Ryohei
Kikuchi
a,
Ryohei
Kikuchi
 b,
Kan
Hatakeyama-Sato
b,
Kan
Hatakeyama-Sato
 a,
Yuta
Nabae
a,
Yuta
Nabae
 *a and
Teruaki
Hayakawa
*a and
Teruaki
Hayakawa
 *a
*a
aDepartment of Materials Science and Engineering, School of Materials and Chemical Technology, Institute of Science Tokyo, 2-12-1 S8-36 Ookayama Meguro-ku, Tokyo 152-8552, Japan. E-mail: nabae.y.aa@m.titech.ac.jp; hayakawa.t.ac@m.titech.ac.jp
bMaterials Analysis Division, Core Facility Center, Research Infrastructure Management Center, Institute of Science Tokyo, 2-12-1 S7-26, Ookayama, Meguro-ku, Tokyo 152-8552, Japan
First published on 25th February 2025
Abstract
Flower-like particles (FLPs) are highly attractive materials owing to their intricate morphologies and high specific surface areas. However, a definitive method for fabricating organic FLPs with unique three-dimensional morphologies has yet to be established. In this paper, we report on a synthetic route for poly(amic acid) (PAA) FLPs using a specially designed semiaromatic PAA consisting of alternate rigid aromatic segments and flexible alkyl segments via one-step precipitation polymerization at room temperature. The particle morphology can be tuned from spherical to flower-like by adjusting the mixed-solvent ratio. Based on small-angle X-ray scattering, wide-angle X-ray diffraction, and polarized optical microscopy analyses, the flower-like morphology is attributed to the microcrystalline structure formed by the folded and stacked alignment of the PAA precursors. Moreover, solubility plays a crucial role in determining the crystallization rate and growth mechanism, thereby leading to variations in the flower-like morphology. Notably, the flower-like morphology is preserved after thermal imidization and carbonization. The as-synthesized carbon flowers demonstrated high catalytic activity and selectivity for the 2-electron electrochemical reduction of oxygen in an acidic electrolyte, which could be attributed to the N-content of 2.72% and the efficient mass transport granted by the open structure of the unique flower-like morphology.
Introduction
Polymeric particles with three-dimensional, higher-order internal and surface morphology, such as Janus,1,2 lamella,3,4 onion-like,5 and golf ball-like,6 have garnered significant interest across a wide range of application areas. The morphology control of these intricate particles often utilizes the bottom-up method, i.e. the self-assembly behavior of the block copolymer precisely prepared through controlled polymerization.2–6 However, polymers synthesized via step-growth polymerization, such as polyimides (PIs) and their precursor poly(amic acid)s (PAAs), hardly form microphase-separated, higher-order structures when combined with other thermodynamically immiscible blocks to form diblock or triblock copolymers. This difficulty arises from the challenges in precisely controlling the molecular weight of PIs.Among various classes of morphologies, flower-like particles (FLPs) exhibit complex, higher-order internal and surface morphologies, offering a large surface area. These characteristics make FLPs promising materials for applications in various fields, such as gas capture,7,8 host materials for targeted drug delivery,9 and catalyst supports.10 So far, there are only a few reports on the successful synthesis of flower-like morphologies in the field of organic chemistry.11–18 For example, Liu et al. reported the FLPs formed from precipitation polymerization of a redox-responsive liquid crystalline monomer.15 Zhang et al. also reported that cellulose stearoyl esters can precipitate into FLPs in non-solvents and the flower-like morphology relates to the polymer molecular weight, concentration of the polymer solution, and the volume ratio of the mixed solvents.16 Chen et al. further demonstrated the facile synthesis of polyacrylonitrile (PAN) FLPs as precursors for carbon flowers.18 Their subsequent investigation revealed that the flower-like morphology originated from orthorhombic PAN crystals.19 However, no universal synthesis route or formation mechanism has been identified for this complex morphology.
While morphology control of PIs is generally challenging,20,21 Sun et al. have reported the higher-order flower-shaped PAA formed by dropping the thermal-treated PAA solution into the poor solvent.22,23 PIs have also been reported to form flower-like morphologies using the solvothermal method with commercially available monomers. This method involves heating the PAA precursor solution to a high temperature under high pressure after solution polymerization. Owing to their high carbon yield and well-preserved morphology after carbonization, PI FLPs have been used as precursors for carbon flowers.7,24–30 However, the solvothermal method has only been successful in producing FLPs in the form of PIs.
In this study, we developed a novel synthetic route to PAA FLPs as the precursor of PI FLPs and carbon flowers. By constructing semiaromatic PAA, the difference in conformational entropy between the aromatic and alkyl segments can induce liquid-crystalline ordering, which opens up the potential for unique self-assembly behaviors.31,32 Unlike in the solvothermal method, the flower-like morphology was achieved concurrently with the progression of precipitation polymerization at room temperature, initially forming PAA FLPs. Precipitation polymerization provides a straightforward bottom-up approach for producing uniform polymer particles. However, the precipitation polymerization of commercially available diamines and dianhydrides has previously only resulted in fine spherical PI microparticles.33–35 Currently, there are no reports of PAA particles bearing complex three-dimensional, higher-order morphologies prepared through one-step precipitation polymerization. Notably, the particle size of the FLPs produced in this study could be tuned by adjusting the mixed solvent ratio. We attribute the formation of the flower-like morphology to the folding and stacking of the semiaromatic PAA synthesized in this study. The flower-like morphology remained well preserved after thermal imidization, with only particle size shrinkage observed following carbonization. The as-synthesized carbon flowers exhibited excellent catalytic activity for the 2-electron pathway oxygen reduction reaction (ORR), benefiting from their intricate hierarchical superstructure and nitrogen content.
Results and discussion
Precipitation polymerization of DA-6/PMDA
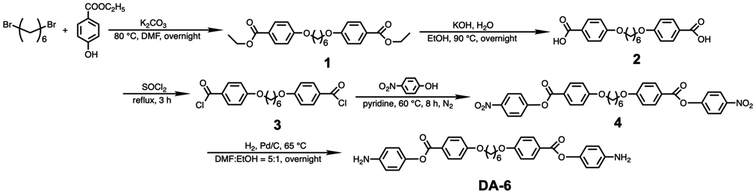
In this study, PAA particles were synthesized through precipitation polymerization involving bis(4-aminophenyl) 4,4′-(hexane-1,6-diylbis(oxy))dibenzoate (DA-6) and pyromellitic dianhydride (PMDA). DA-6 was specifically designed with a flexible six-carbon alkyl segment at its center and rigid aromatic segments at both ends. DA-6 was synthesized according to the procedure depicted in Scheme 1. First, 1 was synthesized via Williamson esterification of 1,6-dibromohexane and ethyl 4-hydroxybenzoate. This intermediate was then cleaved under basic conditions to yield the dicarboxylic acid of 2, which was subsequently reacted with thionyl chloride to form the diacid chloride 3. Next, 3 was esterified with 4-nitrophenol to produce dinitro 4. Finally, 4 was hydrogenated to obtain diamine DA-6. PMDA, a commonly used dianhydride for PI synthesis, was selected to react with DA-6 to introduce rigid segments into semiaromatic PAA (Scheme 2).Precipitation polymerization requires the reaction solvent to be a good solvent for the monomers but a poor solvent for the resulting polymer. As the polymerization reaction progresses, polymeric microspheres form and precipitate out of the homogeneous solution. Acetone is commonly used as the solvent for the precipitation polymerization of PAA particles.33,34 The DA-6 synthesized in this study was insoluble in most common solvents, including ethanol (EtOH), ether, acetone, hexane, tetrahydrofuran (THF), chloroform, dichloromethane (DCM), toluene, ethyl acetate, chlorobenzene, acetonitrile, methyl ethyl ketone, and acetophenone (5 mg mL−1). Meanwhile, it was soluble in 1-methyl-2-pyrrolidone (NMP), N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), N,N-dimethylacetamide (DMAc), and pyridine at room temperature (Table S1†). However, these solvents were not suitable for precipitation polymerization because they also dissolved the resulting PAA. DA-6 is soluble in cyclohexanone and THF upon heating and remains dissolved upon cooling to room temperature. Therefore, precipitation polymerization was conducted using two different mixed solvent systems—cyclohexanone/toluene (cyc/tol) and cyclohexanone/acetone (cyc/ace)—and pure THF, with vigorous stirring at room temperature. In the mixed solvent systems, cyclohexanone served as the good solvent, while toluene and acetone acted as poor solvents. Precipitation polymerization began immediately after the monomer solutions were mixed. Previous studies have shown that solvents significantly influence the morphology of the resulting polymer particles.16,18 Thus, we investigated the effect of the selected solvents on the particle morphology. For the mixed solvents, we varied the volume ratios, while maintaining a constant total solvent volume of 100 mL. The precipitation polymerization of DA-6/PMDA in pure THF resulted in coagulated and irregular multispheres (Fig. S1†).
When a cyc/tol mixed solvent was used, the resulting particles displayed a popcorn-like morphology with irregular sizes and shapes at cyc/tol ratios of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7
2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 3
3, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. In contrast, particles with minute flakes were observed at a cyc/tol ratio of 5
7. In contrast, particles with minute flakes were observed at a cyc/tol ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The effect of monomer concentration was further investigated at a cyc/tol ratio of 7
5. The effect of monomer concentration was further investigated at a cyc/tol ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Increasing the monomer amount to 0.5 mmol and 1 mmol while maintaining the total solvent volume at 100 mL resulted in spherical particles with minute flaky surfaces, which could be considered as an incomplete flower-like morphology (Fig. S2†).
3. Increasing the monomer amount to 0.5 mmol and 1 mmol while maintaining the total solvent volume at 100 mL resulted in spherical particles with minute flaky surfaces, which could be considered as an incomplete flower-like morphology (Fig. S2†).
In contrast, when using cyc/ace as the mixed solvent, distinct FLPs consisting of interleaving nanoflakes were formed at cyc/ace ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 3
8, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 4
7, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, and 5
6, and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (Fig. 1a). However, when the acetone volume ratio was decreased to cyc/ace = 6
5 (Fig. 1a). However, when the acetone volume ratio was decreased to cyc/ace = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the flaky surface disappeared and the morphology transitioned to agglomerated spherical particles. With a further decrease in the acetone volume ratio to cyc/ace = 7
4, the flaky surface disappeared and the morphology transitioned to agglomerated spherical particles. With a further decrease in the acetone volume ratio to cyc/ace = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the independent particle morphology changed to irregular, hairy assemblies (Fig. S3†). The missing mixed volume ratios, such as 1
3, the independent particle morphology changed to irregular, hairy assemblies (Fig. S3†). The missing mixed volume ratios, such as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and 8
9 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, were not investigated because precipitation polymerization could not be established—either the monomer failed to dissolve or the polymer did not precipitate. Monomer concentration is another crucial factor that influences FLP morphology. When the DA-6 monomer concentration was increased to 0.5 mmol while maintaining the total solvent volume at 100 mL with cyc/ace = 4
2, were not investigated because precipitation polymerization could not be established—either the monomer failed to dissolve or the polymer did not precipitate. Monomer concentration is another crucial factor that influences FLP morphology. When the DA-6 monomer concentration was increased to 0.5 mmol while maintaining the total solvent volume at 100 mL with cyc/ace = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 5
6 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the flower-like morphology was observed again (Fig. S3†). The reaction time was maintained at 1 h to optimize yield, while the morphology became blurred when the reaction exceeded 1 h (Fig. S4†).
5, the flower-like morphology was observed again (Fig. S3†). The reaction time was maintained at 1 h to optimize yield, while the morphology became blurred when the reaction exceeded 1 h (Fig. S4†).
 | ||
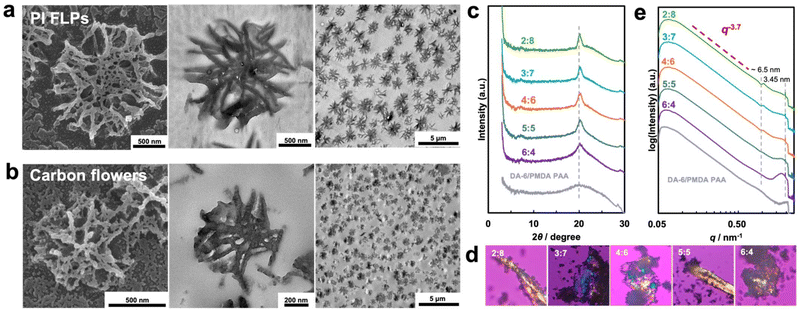
| Fig. 1 Field emission scanning electron microscopy (FE-SEM) images of PAA FLPs (a), PI FLPs (b) and carbon flowers (c) prepared from DA-6/PMDA in cyc/ace with varied mixed solvent ratio. | ||
The inherent viscosity test indicated that PAA particles synthesized via precipitation polymerization in cyc/tol and cyc/ace exhibited relatively low molecular weights compared to PAA prepared through solution polymerization (Table S2†). In a precipitation polymerization, PAA exhibits low reactivity once solid particles precipitate under poor solvent conditions, thereby halting further polymerization and molecular weight growth. In contrast, solution polymerization occurs in a homogeneous system, allowing polymer molecules to interact continuously over an extended reaction time, resulting in a higher molecular weight.
The FLPs prepared in the cyc/ace solvent system exhibited a more developed flower-like morphology. Consequently, we selected these FLPs for subsequent thermal imidization and carbonization. Thermogravimetric analysis (TGA; Fig. S5†) of PAA FLPs revealed the two stages of weight loss corresponding to imidization and carbonization, respectively. The carbonization process began at approximately 336 °C, with a final yield of carbon flowers yield around 20%.
The FLPs demonstrated excellent thermal stability, and their morphology was well maintained after thermal imidization (Fig. 1b). Fourier transform infrared (FT-IR) spectra (Fig. S6a†) displayed absorption peaks corresponding to amide groups at 1661 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) and 1543 cm−1 (C–N–H stretching) in the PAA FLPs, which disappeared after imidization. Additionally, absorption peaks corresponding to imide groups were observed at 1779 cm−1 (C
O stretching) and 1543 cm−1 (C–N–H stretching) in the PAA FLPs, which disappeared after imidization. Additionally, absorption peaks corresponding to imide groups were observed at 1779 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) and 1380 cm−1 (C–N–C stretching) in the PI FLPs, confirming that the imidization process was fully completed.
O stretching) and 1380 cm−1 (C–N–C stretching) in the PI FLPs, confirming that the imidization process was fully completed.
The PAA FLPs were subsequently carbonized under a N2 atmosphere at a ramping rate of 1 °C min−1 to prevent morphological collapse (Fig. 1c). The cross-sectional images of the PI FLPs (Fig. 2a) and carbon flowers (Fig. 2b) prepared in the cyc/ace = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 solvent system reveal an inner hollow morphology, confirming that the FLPs were formed from clusters of interleaving nanoflakes extending from the core to the surface. After carbonization, only size shrinkage was detected, which was attributed to the pyrolysis of the flexible alkyl segments.
8 solvent system reveal an inner hollow morphology, confirming that the FLPs were formed from clusters of interleaving nanoflakes extending from the core to the surface. After carbonization, only size shrinkage was detected, which was attributed to the pyrolysis of the flexible alkyl segments.
Higher-order structure analysis of FLPs
The higher-order structure of the PAA FLPs was analyzed using wide-angle X-ray diffraction (WAXD), polarized optical microscopy (POM), and small-angle X-ray scattering (SAXS) with the DA-6/PMDA in the form of PAA and PI prepared from homogenous solution polymerization serving as a comparison.The WAXD profile of the FLPs (Fig. 2c) presented sharp diffraction peaks at 2θ of approximately 20°, indicating the stacking of aromatic rings and an interchain distance of 0.44 nm for DA-6/PMDA molecules. The POM images of the FLPs prepared at room temperature using different cyc/ace volume ratios consistently exhibited anisotropic bright fields (Fig. 2d). Although differential scanning calorimetry (DSC) thermogram did not indicate any phase transition of PI FLPs between 30 and 300 °C (Fig. S7†), the results of WAXD and POM suggested the presence of a microcrystalline structure in the PAA FLPs.
SAXS profiles of FLPs prepared from cyc/ace solvent system are shown in Fig. 2e. A Bragg diffraction peak was observed at approximately 1.95 nm−1. This result corresponds to a d-spacing of 3.22 nm, consistent with the repeating unit length indicated by the WAXD profile of the DA-6/PMDA PI bulk film at 2θ = 2.56° (Fig. S8a†). The SAXS profiles of the FLPs also exhibited a distinct oscillation signal at approximately 0.97 nm−1, the oscillatory signal corresponds to a d-spacing of approximately 6.5 nm in the Porod region. This scattering region provides information about the primary structure corresponding to the shortest building block, namely, the minimal structural unit of lamellae forming the flower-like morphology. The petal thickness of the FLPs observed from FE-SEM was in the range of 20–40 nm, formed by the multiple stacking of these minimal structural units. In certain areas, a petal layer of approximately 6.5 nm was observed (Fig. S9†). The power-law region to the left of the Porod region exhibited a slope of −3.7, representing the surface fractal dimension of the particle surface.19,34,36 Notably, the signal strength decreased as the flower-like morphology gradually became more blurred, eventually transitioning to completely spherical particles when prepared from cyc/ace = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The incomplete FLPs prepared with cyc/tol = 5
4. The incomplete FLPs prepared with cyc/tol = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 also exhibited a similar signal in this region (Fig. S10†). This unique oscillatory signal suggests the presence of a different higher-order structure than that of the DA-6/PMDA PAA or the PI bulk film prepared from solution polymerization.
5 also exhibited a similar signal in this region (Fig. S10†). This unique oscillatory signal suggests the presence of a different higher-order structure than that of the DA-6/PMDA PAA or the PI bulk film prepared from solution polymerization.
Influence of solubility on FLP morphology
A gradual decrease in the particle size was observed in the PAA FLPs prepared from different cyc/ace volume ratios (Fig. 1a). Specifically, as the solubility of DA-6/PMDA decreased with increasing acetone volume ratio, precipitation formation occurred more rapidly, indicating increased crystalline rate. Under such conditions, the DA-6/PMDA molecules tended to attach randomly to the existing lamellae, forming branched lamellae under vigorous stirring. This resulted in larger particle sizes. The rapid formation of FLPs was confirmed within the first 15 minutes of the reaction (Fig. S4†). In contrast, the polymer chains diffused more effectively in a solvent with higher solubility, leading to relatively lower crystallization rate. Eventually, smaller FLPs with relatively compact layer-by-layer lamellae were formed (Fig. 3a). In comparison, the 4,4′-oxydianiline (ODA)/PMDA particles prepared under the same mixed solvent conditions exhibited smooth surfaces (Fig. S11†). The ODA/PMDA particle size was not influenced by the polymerization solvent, further suggesting that the size variation of the FLPs originated from the semiaromatic molecular structure and crystallization behavior of DA-6/PMDA in cyc/ace. The FLP petals varied slightly from loose to compact with increasing monomer concentration (Fig. S3†), which was also attributed to changes in the crystallization rate. Higher crystallization rate of DA-6/PMDA resulted in the formation of branched lamellae when the solubility of the system was reduced by increasing either the acetone volume ratio or monomer concentration. In contrast, higher solubility in the system promoted layer-by-layer growth, leading to densely packed FLPs (Fig. 3a).Formation mechanism of DA-6/PMDA FLPs
The proposed self-assembly mechanism of PI FLPs prepared via the solvothermal method involves pressure- and temperature-induced crystallization of PI nanosheets.7,12,25,27,28,30 Although an exclusive formation mechanism, synthetic route, solvent, or scaffold that specifically enables the fabrication of other polymeric FLPs is yet to be identified, some studies offer insights into how crystallization may contribute to the development of this fascinating flower-like morphology.11–16,19Typically, the formation of polymer lamellae from solutions or melts is primarily governed by thermodynamic factors. The WAXD profile of DA-6/PMDA PAA prepared from homogenous solution exhibited an amorphous halo (Fig. 2c). In PI films prepared from drop-casting of PAA solution onto a substrate, the polymer chains tend to align in the in-plane parallel direction, which is influenced by shrinkage due to the clamping effect at the film edges during thermal imidization37 and the repeating unit length and interchain distance of the DA-6/PMDA bulk film were investigated using the WAXD profiles (Fig. S8a† and Fig. 3b). In contrast to the bulk films, DA-6/PMDA FLPs exhibited a distinct crystalline behavior, with the petals intersecting to form a fractal morphology. This unique microcrystalline flower-like structure was formed via precipitation polymerization in a poor solvent at room temperature, suggesting that the crystalline process of FLPs in this study was more kinetically driven rather than thermodynamically controlled.
In summary, (1) the WAXD profile and POM images confirm the presence of a microcrystalline structure in the FLPs (Fig. 2c and d). (2) The FLP SAXS profiles display unique oscillatory valleys at approximately 0.97 nm−1 (Fig. 2e), indicating a different chain arrangement compared to the structures of bulk PI films and spherical particles. The corresponding d-spacing of approximately 6.5 nm, which is related to the building block thickness of the flower-like morphology, is approximately twice the length of the repeating unit. (3) Comparative precipitation polymerization of ODA/PMDA in a cyc/ace system yielded only fine spherical particles under conditions that produced FLPs with DA-6/PMDA. This suggests that the unique molecular design of semiaromatic DA-6/PMDA leads to the formation of a flower-like morphology. Based on these observations, we propose that the formation mechanism of the FLPs in this study (Fig. 3c) follows a branching mechanism similar to those of PAN and PI FLPs prepared via solvothermal methods.38 The flexible alkyl segments in DA-6/PMDA allow the PAA chains to fold and stack to form lamellar petals. These lamellae grow on existing petals or branches to create an intersecting, fractal morphology that ultimately assembles into FLPs.
ORR performance of carbon flowers
H2O2 is one of the most important industrial chemicals due to its broad range of applications, including paper and textile manufacturing, wastewater treatment, and more.39,40 Currently, its industrial production primarily relies on the anthraquinone oxidation process, which involves energy-intensive multistep reactions and generates hazardous by-products.41 As an alternative, electrochemical H2O2 production via the 2-electron ORR at a lower potential (0.7 V vs. RHE) can offer a more sustainable and environmentally friendly approach.42Oxygen molecules can also be reduced to H2O via the 4-electron pathway, a process that releases a relatively large free energy (1.23 V vs. RHE) and is widely utilized in fuel cells. However, a 2 + 2 reduction pathway has been identified, originating from the 2-electron ORR.43,44 Therefore, understanding the 2-electron ORR is not only essential for sustainable H2O2 production but also provides deeper insights into the 2 + 2 reduction pathway, which is relevant for practical fuel cell applications.
So far, efforts have been made to improve the catalytic activity and control the catalytic selectivity, such as using the metal-free mesoporous N-doped carbon.45,46 Carbon flowers made from PAN have also been reported to have high selectivity and activity in the production of H2O2 in a KOH electrolyte through the 2-electron ORR.47
To evaluate the 2-electron ORR activity of carbon flowers prepared in this work, rotating ring disk electrode (RRDE) voltammetry was conducted in O2-saturated 0.5 M H2SO4 at a rotation rate of 1600 rpm. Carbon flowers synthesized from cyc/ace = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 with a particle size around 0.8 μm were selected as the representative sample. For comparison, amorphous carbon with the same chemical composition as carbon flowers (Fig. 4a)—carbonized from PAA particles prepared in cyc/tol = 7
8 with a particle size around 0.8 μm were selected as the representative sample. For comparison, amorphous carbon with the same chemical composition as carbon flowers (Fig. 4a)—carbonized from PAA particles prepared in cyc/tol = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. S2†)—as well as commercially available Ketjen Black (KB), were also included.
3 (Fig. S2†)—as well as commercially available Ketjen Black (KB), were also included.
The RRDE voltammogram is shown in Fig. 4b–d. Among all candidates, carbon flowers exhibited the highest 2-electron reduction activity. They also demonstrated the highest half-wave potential of 0.25 V vs. RHE, compared to 0.20 V for amorphous carbon and 0.08 V for KB (Fig. 4c and Table S3†). The superior 2-electron reduction activity of carbon flowers was attributed to their nitrogen content (C: 93.24%, N: 2.72%, H: 0.78%, determined by elemental analysis). Additionally, their unique open flower-like morphology facilitated efficient mass transport of the generated H2O2 and suppressed another 2-electron reduction from H2O2 to H2O,45 contributing to their high H2O2 selectivity of nearly 80% at a wide range of potentials (Fig. 4b and d).
Conclusions
In this study, DA-6/PMDA PAA FLPs were synthesized as precursors through a one-step precipitation polymerization in a cyc/ace mixed solvent. Unlike the conventional solvothermal method, the as-synthesized PAA FLPs were formed directly during polymerization at room temperature. The development of the flower-like morphology was attributed to rapid crystalline formation under poor solubility conditions. The branched growth of the DA-6/PMDA microcrystals into fractal petals was enhanced in solvents with lower solubility, resulting in larger FLPs with a more distinct fractal morphology. The flower-like structure was well preserved after thermal imidization and carbonization, although particle size shrinkage was observed in the carbon flowers. We propose that the flower-like structure arises from folding and stacking of the molecular alignments of DA-6/PMDA. This work offers a facile method of producing carbon flowers from PAA FLPs, which also deepens the understanding of the self-assembly behavior of PAA prepared from polycondensation. The as-synthesized carbon flowers exhibited excellent electrocatalytic activities and selectivity for H2O2 production via 2-electron pathway, attributed to its nitrogen content and unique flower-like morphology. Carbon flowers with hierarchical superstructures are expected to exhibit high activity in various fields, including energy conversion and storage devices, such as electrocatalyst supports, supercapacitors, and gas storage materials.Experimental
Materials
Ethyl 4-hydroxyl benzoate, 1,6-dibromohexane, and 4,4′-oxydianiline (ODA) were purchased from Tokyo Chemical Industry (TCI, Japan). The ODA was recrystallized from EtOH prior to use. PMDA was purchased from Nippon Shokubai and recrystallized from acetic anhydride before use. Cyclohexanone, acetone, toluene, NMP, and THF (stabilizer-free) were purchased from Fujifilm Wako Pure Chemical Industries. All other chemicals were of reagent grade and used as received.Synthesis of DA-6
Precipitation polymerization of DA-6/PMDA
DA-6 is soluble in cyclohexanone and THF upon heating and remains dissolved upon cooling to room temperature. Therefore, THF and cyclohexanone were selected as the candidate solvents for the precipitation polymerization of DA-6 and PMDA. However, the resulting PAA did not precipitate in pure cyclohexanone. Therefore, to identify a suitable solvent, precipitation polymerization was conducted using three solvent systems: pure THF, cyc/tol, and cyc/ace.Precipitation polymerization of DA-6/PMDA in THF
First, 50 mL THF was added to DA-6 (0.25 mmol, 0.1352 g) in a 100 mL round-bottom flask and heated at 70 °C until complete dissolution. The DA-6/THF solution was then cooled to room temperature in the air. Separately, an equimolar ratio of PMDA (0.25 mmol, 0.0545 g) was dissolved in 50 mL THF in a 500 mL round-bottom flask and stirred for 30 min. Once the monomer was well dissolved and dispersed, the DA-6/THF solution was added to the PMDA solution, and the mixture was vigorously stirred at room temperature for 1 h under a N2 atmosphere. The PAA particles were collected using a rotary evaporator and subsequently dried under vacuum at 40 °C overnight to obtain pale yellow PAA particles.Precipitation polymerization in cyc/tol mixed solvent
First, 50 mL cyc/tol mixed solvent (volume ratio, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7
2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 5
3, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 3
5, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) was added to DA-6 (0.25 mmol, 0.1352 g; 0.5 mmol, 0.2704 g; 1 mmol 0.5408 g) in a 100 mL round-bottom flask and heated until complete dissolution. The DA-6 solution was then cooled to room temperature in air. Separately, an equimolar ratio of PMDA (0.25 mmol, 0.0545 g; 0.5 mmol 0.1090 g; 1 mmol 0.2182 g) was dissolved in 50 mL of the same cyc/tol mixed solvent in a 500 mL round-bottom flask and stirred for 30 min at room temperature. Once the monomer was well dissolved and dispersed, the DA-6 solution was added to the PMDA solution. The mixture was then vigorously stirred at room temperature for 1 h under a N2 atmosphere. The PAA particles were collected by centrifugal separation and washed thrice with toluene. After removing the toluene with a rotary evaporator, the powder was dried under vacuum at 40 °C overnight to obtain the pale yellow PAA particles.
7) was added to DA-6 (0.25 mmol, 0.1352 g; 0.5 mmol, 0.2704 g; 1 mmol 0.5408 g) in a 100 mL round-bottom flask and heated until complete dissolution. The DA-6 solution was then cooled to room temperature in air. Separately, an equimolar ratio of PMDA (0.25 mmol, 0.0545 g; 0.5 mmol 0.1090 g; 1 mmol 0.2182 g) was dissolved in 50 mL of the same cyc/tol mixed solvent in a 500 mL round-bottom flask and stirred for 30 min at room temperature. Once the monomer was well dissolved and dispersed, the DA-6 solution was added to the PMDA solution. The mixture was then vigorously stirred at room temperature for 1 h under a N2 atmosphere. The PAA particles were collected by centrifugal separation and washed thrice with toluene. After removing the toluene with a rotary evaporator, the powder was dried under vacuum at 40 °C overnight to obtain the pale yellow PAA particles.
Precipitation polymerization in cyc/ace mixed solvent
DA-6 (0.25 mmol, 0.1352 g) was dissolved in 20 mL cyclohexanone in a 100 mL round-bottom flask and heated until complete dissolution. The DA-6/cyclohexanone solution was then cooled to room temperature in air. Separately, an equimolar ratio of PMDA (0.25 mmol, 0.0545 g) was dissolved in 80 mL acetone in a 500 mL round-bottom flask. Once the monomer was completely dissolved and dispersed, the DA-6 solution was added to the PMDA solution, and the mixture was vigorously stirred at room temperature for 1 h under a N2 atmosphere. PAA particles were collected by centrifugal separation and washed thrice with acetone. After removing the acetone using a rotary evaporator, the powder was dried at 40 °C under vacuum overnight to obtain PAA particles. The same procedure was employed to conduct the precipitation polymerization of DA-6/PMDA using different cyc/ace volume ratios (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 4
7, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 5
6, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 6
5, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 7
4, and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and with a different DA-6 concentration (0.5 mmol).
3) and with a different DA-6 concentration (0.5 mmol).
FT-IR (KBr, v, cm−1): 3450, 3073, 2937, 2865, 1726, 1661, 1607, 1543, 1510, 1412, 1259, 1209, 1199, 1167, 1113, 1074, 1013, 877, 845, 805, 763, 694, 652, 605, 523 cm−1, as shown in Fig. S5a.†1H NMR (400 MHz, DMSO-D6) spectra is shown in Fig. S5b.†
Precipitation polymerization of ODA/PMDA (0.25 mmol) was conducted similarly in the cyc/ace mixed solvent.
Imidization and carbonization
The pale brown powder of PI particles was obtained by heating the DA-6/PMDA PAA particles at 240 °C for 3 h under vacuum. IR (KBr, v, cm−1): 3444, 2933, 2862, 1779, 1727, 1606, 1512, 1468, 1380, 1259, 1210, 1166, 1122, 1073, 1013, 874, 845, 801, 760, 723, 692, 643, 523 cm−1.To convert the PAA particles into carbon particles, the PI particles were carbonized under N2 (50 mL min−1) at a ramping rate of 1 °C min−1 and held at 900 °C for 30 min.
Preparation of DA-6/PMDA bulk film
DA-6/PMDA bulk film were synthesized through a two-step step-growth polymerization between DA-6 and PMDA. DA-6 (497 mg) was added to a 25 mL two-necked flask and dissolved by 1.5 mL NMP under N2 atmosphere. PMDA (200.45 mg) was then added to the flask. After adding another 1.5 mL NMP, the reaction solvent was stirred under Argon atmosphere at room temperature for 24 h. The PI film of DA-6/PMDA was obtained by casting the reaction solvent onto a flat glass schale, ventilated and heated on a hot plate at 80 °C for 1 h before completely imidized at 240 °C for 3 h under vacuum.Characterization
1H NMR spectra were obtained using a JEOL 400 MHz NMR instrument. FT-IR spectra were recorded on a JASCO FT-IR 4100 spectrometer using the KBr pellet method. The inherent viscosities (ηinh) of PAAs with a solid content of 0.5 g dL−1 were measured in N-methyl-2-pyrrolidone (NMP) at 30 °C using an Ostwald viscometer. FE-SEM was conducted using an S-5500 microscope (Hitachi High-Tech Corporation) at an accelerating voltage of 1.0–3.0 kV and the cross-section images were gained from S4700 (Hitachi High-Tech Corporation) operated at 6.0 kV. To obtain cross-sections, samples were embedded in light curable resin (Toagosei LCR-D800) and sectioned using an ultramicrotome. Subsequently, the block faces were plasma etched using a PIB-20 (Vacuum Device) to remove the resin. TEM images were obtained on a H7650 Zero. A (Hitachi High-Tech Corporation) at a 100 kV accelerating voltage. For TEM analysis, samples were embedded in epoxy resin, and then sectioned using an ultramicrotome (Leica Microsystems EM UC7). TGA was performed using an EXSTAR TG/DTA 7300 (Seiko Instruments, Tokyo, Japan) at a heating rate of 1 °C min−1. The optical structure of PAA was observed using a POM (OLYMPUS BX51). SAXS was conducted at the SPring-8 beamline BL40B2 with a wavelength of 1.9 Å and a Pilatus detector. WAXD was performed using a Bruker New D8 DISCOVER instrument. X-rays generated by a Bruker AXS NanoSTAR (output: 50 kV, 100 mA) were converted to monochromatic X-rays using a Göbel mirror, and the sample was irradiated with focused CuKα rays (wavelength: 1.5416 Å). The C, H, and N contents of the carbon flower were determined using a CHN elemental analyzer (J-Science Lab, JM10).Electrochemical study
The catalytic activity of the samples was evaluated using cyclic voltammetry (CV, 10 mV s−1) with a RRDE, sweeping the disk potential between 0 V to 1.0 V vs. RHE. The 3 mg of catalyst powder was ultrasonically dispersed in a mixture of Nafion solution (5 wt%, DEC520, FUJIFILM, 45 μL), ethanol (300 μL), ultrapure water (300 μL) and then was applied to the surface of a glassy carbon electrode. The amount of catalyst loading was 60 μg cm−2. The rotation speed of the RRDE was controlled with an RRDE system (NIKKO KEISOKU, RDE-1) and the electrochemical data were collected with a potentiostat (HZ-700, HAG1232m). CV was first performed in N2-saturated H2SO4 (0.5 M) for cleaning and background, and then in O2-saturated H2SO4. The ORR current was determined by subtracting the N2 current from the O2 current. The potential of the Pt ring electrode was kept at 1.2 V vs. RHE for the detection of H2O2 produced on the disk electrode and the selectivity for the two-electron reduction, χ(H2O2), was calculated using the following equation:The electron transfer number was determined by the ratio of current density for the ring/disk electrode using the following equation:
Author contributions
Y. C. contributed to the investigation, data visualization, and writing the original draft. R. K. was involved in the investigation. K. H. participated in reviewing and editing the manuscript. Y. N. was responsible for conceptualization, funding acquisition, and manuscript review and editing. T. H. contributed to the conceptualization, supervision, and review and editing of the manuscript.Data availability
The data supporting this article have been included as part of the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by JSPS KAKENHI (21K04828) and the New Energy and Industrial Technology Development Organization (NEDO).FE-SEM was performed using equipment shared through the MEXT Project (JPMXS0420900521). CHN elemental analysis was performed under the assistance of Materials Analysis Division, Core Facility Center, Research Infrastructure Management Center. SAXS measurements were carried out at BL40BII at SPring-8 (Proposal Number 2023A1197, 2024B1228) with the assistance of Prof. Tomoyasu Hirai (Osaka Institute of Technology) and Dr Noboru Ohota (JASRI). Y. Chen is supported by a Tokyo Tech Tsubame Scholarship and Scholarships from the Amano Institute of Technology.
References
- J. X. Liu, N. Bizmark, D. M. Scott, R. A. Register, M. P. Haataja, S. S. Datta, C. B. Arnold and R. D. Priestley, JACS Au, 2021, 1, 936–944 CrossRef CAS PubMed.
- C. Li, H. Peng, J. Cai, L. Li, J. Zhang and Y. Mai, Adv. Mater., 2021, 33, 1–10 Search PubMed.
- J. Lee, K. H. Ku, J. Kim, Y. J. Lee, S. G. Jang and B. J. Kim, J. Am. Chem. Soc., 2019, 141, 15348–15355 CrossRef CAS.
- D. Klinger, C. X. Wang, L. A. Connal, D. J. Audus, S. G. Jang, S. Kraemer, K. L. Killops, G. H. Fredrickson, E. J. Kramer and C. J. Hawker, Angew. Chem., Int. Ed., 2014, 53, 7018–7022 CrossRef CAS PubMed.
- D. Varadharajan, H. Turgut, J. Lahann, H. Yabu and G. Delaittre, Adv. Funct. Mater., 2018, 28, 1800846 CrossRef.
- L. Tong, Y. Nabae, T. Hirai, H. Yabu and T. Hayakawa, Macromol. Chem. Phys., 2023, 224, 2200402 CrossRef CAS.
- M. Sharma and M. A. Snyder, Microporous Mesoporous Mater., 2022, 335, 111801 CrossRef CAS.
- H. Sun, Z. Chu, D. Hong, G. Zhang, Y. Xie, L. Li and K. Shi, J. Alloys Compd., 2016, 658, 561–568 CrossRef CAS.
- Z. Wang, H. Yu, K. Ma, Y. Chen, X. Zhang, T. Wang, S. Li, X. Zhu and X. Wang, Bioconjugate Chem., 2018, 29, 2090–2099 CrossRef CAS.
- J. Li, M. Zhang, Z. Song, S. Liu, J. Wang and L. Zhang, Catalysts, 2019, 9, 767 CrossRef CAS.
- T. Nakanishi, K. Ariga, T. Michinobu, K. Yoshida, H. Takahashi, T. Teranishi, H. Möhwald and D. G. Kurth, Small, 2007, 3, 2019–2023 CrossRef CAS PubMed.
- J. Yin, J. Yan, M. He, Y. Song, X. Xu, K. Wu and J. Pei, Chem. – Eur. J., 2010, 16, 7309–7318 CrossRef CAS.
- H. Zhao, X. Guo, S. He, X. Zeng, X. Zhou, C. Zhang, J. Hu, X. Wu, Z. Xing, L. Chu, Y. He and Q. Chen, Nat. Commun., 2014, 5, 3108 CrossRef.
- H. Zhu, T. J. Buchtal and M. Mitsuishi, Appl. Surf. Sci., 2021, 563, 150245 CrossRef CAS.
- X. Liu, M. A. Moradi, T. Bus, M. G. Debije, S. A. F. Bon, J. P. A. Heuts and A. P. H. J. Schenning, Angew. Chem., Int. Ed., 2021, 60, 27026–27030 CrossRef CAS.
- K. Zhang, A. Geissler, X. Chen, S. Rosenfeldt, Y. Yang, S. Förster and F. Müller-Plathe, ACS Macro Lett., 2015, 4, 214–219 CrossRef CAS PubMed.
- Z. Chen, J. Huang, Y. Cui and R. Fu, Carbon, 2022, 190, 395–401 CrossRef CAS.
- S. Chen, D. M. Koshy, Y. Tsao, R. Pfattner, X. Yan, D. Feng and Z. Bao, J. Am. Chem. Soc., 2018, 140, 10297–10304 CrossRef.
- H. Gong, J. Ilavsky, I. Kuzmenko, S. Chen, H. Yan, C. B. Cooper, G. Chen, Y. Chen, J. A. Chiong, Y. Jiang, J. Lai, Y. Zheng, K. H. Stone, L. Huelsenbeck, G. Giri, J. B. H. Tok and Z. Bao, J. Am. Chem. Soc., 2022, 144, 17576–17587 CrossRef CAS.
- C. B. Arrington, D. A. Rau, J. A. Vandenbrande, M. Hegde, C. B. Williams and T. E. Long, ACS Macro Lett., 2021, 10, 412–418 CrossRef CAS.
- M. Lahnsteiner, M. Caldera, H. M. Moura, D. A. Cerrón-Infantes, J. Roeser, T. Konegger, A. Thomas, J. Menche and M. M. Unterlass, J. Mater. Chem. A, 2021, 9, 19754–19769 RSC.
- H. Sun and J. Du, Macromolecules, 2020, 53, 11033–11039 CrossRef CAS.
- H. Sun, X. Li, K. Jin, X. Lai and J. Du, Nanoscale Adv., 2022, 4, 1422–1430 RSC.
- H. Peng, S. Qi, Q. Miao, R. Zhao, Y. Xu, G. Ma and Z. Lei, J. Power Sources, 2021, 482, 228993 CrossRef CAS.
- Y. Wang, Y. Lu, X. Liu, H. Chi, J. Hu, H. Zhao and G. Xiao, J. Energy Storage, 2022, 47, 103656 CrossRef.
- G. Zhao, G. Zou, X. Qiu, S. Li, T. Guo, H. Hou and X. Ji, Electrochim. Acta, 2017, 240, 24–30 CrossRef CAS.
- M. J. Taublaender, M. Reiter and M. M. Unterlass, Macromolecules, 2019, 52, 6318–6329 CrossRef CAS.
- Z. Xu, X. Zhuang, C. Yang, J. Cao, Z. Yao, Y. Tang, J. Jiang, D. Wu and X. Feng, Adv. Mater., 2016, 28, 1981–1987 CrossRef CAS.
- M. Wang, W. J. Bao, J. Wang, K. Wang, J. J. Xu, H. Y. Chen and X. H. Xia, Sci. Rep., 2014, 4, 6606 CrossRef CAS.
- J. Liu, T. Wang and H. Sun, ACS Macro Lett., 2024, 13, 1139–1146 CrossRef CAS.
- J. K. Kallitsis and A. K. Andreopoulou, in Polymer Science: A Comprehensive Reference, ed. K. Matyjaszewski and M. Möller, Elsevier, Amsterdam, 2012, pp. 725–773 Search PubMed.
- B. D. Olsen and R. A. Segalman, Mater. Sci. Eng., R, 2008, 62, 37–66 CrossRef.
- K. Asao, J. Photopolym. Sci. Technol., 2014, 27, 181–185 CrossRef.
- S. Kuretani, Y. Nabae and T. Hayakawa, J. Photopolym. Sci. Technol., 2022, 35, 271–276 CrossRef CAS.
- K. Hatakeyama-Sato, H. Ishikawa, S. Takaishi, Y. Igarashi, Y. Nabae and T. Hayakawa, Polym. J., 2024, 56, 977–986 CrossRef CAS.
- H. Matsuoka, J. Jpn. Oil Chem. Soc., 2000, 49, 1163–1171 CrossRef CAS.
- Y. Nagata, Y. Ohnishi and T. Kajiyama, Polym. J., 1996, 28, 980–985 CrossRef.
- H. Gong, S. Chen, J. B. H. Tok and Z. Bao, Matter, 2023, 6, 2206–2234 CrossRef CAS.
- A. Yu, S. Liu and Y. Yang, Chem. Commun., 2024, 60, 5232–5244 RSC.
- S. Li, J. Ma, F. Xu, L. Wei and D. He, Chem. Eng. J., 2023, 452, 139371 CrossRef CAS.
- J. M. Campos-Martin, G. Blanco-Brieva and J. L. G. Fierro, Angew. Chem., Int. Ed., 2006, 45, 6962–6984 CrossRef CAS.
- K. Otsuka and I. Yamanaka, Electrochim. Acta, 1990, 35, 319–322 CrossRef CAS.
- T. S. Olson, S. Pylypenko, J. E. Fulghum and P. Atanassov, J. Electrochem. Soc., 2010, 157, B54–B63 CrossRef CAS.
- Y. Wu and Y. Nabae, Curr. Opin. Electrochem., 2021, 25, 100633 CrossRef CAS.
- J. Park, Y. Nabae, T. Hayakawa and M. A. Kakimoto, ACS Catal., 2014, 4, 3749–3754 CrossRef CAS.
- Y. Wu, A. Muthukrishnan, S. Nagata and Y. Nabae, J. Phys. Chem. C, 2019, 123, 4590–4596 CrossRef CAS.
- H. Gong, L. Wei, S. Chen, Z. Chen, T. F. Jaramillo and Z. Bao, Nano Res., 2023, 16, 11556–11563 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4lp00327f |
| This journal is © The Royal Society of Chemistry 2025 |