Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Interfacial response of Mg–Ca–Si–Zr nanoparticles for transformative orthopedic therapeutics
Priya
Singh
a,
Somesh
Agrawal
b,
Deepak
Khare
 a,
Vinod
Tiwari
b and
Ashutosh Kumar
Dubey
a,
Vinod
Tiwari
b and
Ashutosh Kumar
Dubey
 *a
*a
aDepartment of Ceramic Engineering, Indian Institute of Technology (BHU), Varanasi - 221005, India. E-mail: akdubey.cer@iitbhu.ac.in; Tel: +918726823415
bDepartment of Pharmaceutical Engineering and Technology, Indian Institute of Technology (BHU), Varanasi, 221005, India
First published on 29th May 2025
Abstract
Debris particles, discharged due to degradation and wear, initiate an inflammatory response at the implantation site or lead to aseptic loosening of the prosthesis, ultimately resulting in implant failure over time. The toxicity concern becomes more severe with the release of nano-sized debris particles due to augmented interfacial interactions, even if the bulk counterpart is highly biocompatible. From this perspective, the present study aims to assess the in vivo toxicity, both local and systemic, of Mg1−xCaxSi1−xZrxO3 (x = 0–0.4) [MCSZO-X, X = 0–4] nanoparticles using a rat model. Initially, the in vitro cytotoxicity of varying concentrations (0.25, 2.5, and 25 mg ml−1) of MCSZO-X nanoparticles was evaluated using MG-63 cells. Cell proliferation increases after the early interfacial interactions. Following this, 100 μl of MCSZO nanoparticles (25 mg ml−1) was administered through intra-articular injection into the knee joint of male Wistar rats. Biochemical analyses revealed no pathological changes in the liver and kidney of the injected group of rats. Additionally, the histopathological analyses demonstrated that there is no inflammation resulting from interfacial interactions with injected nanoparticles in various organs such as the liver, heart, kidney and knee. Overall, these findings pave the way for further advancement in bone repair and implant design.
1. Introduction
The wear resistance of implants and the biological response of debris particles are key factors in determining the long-term success of implants.1 Debris particles, released due to degradation, friction, and wear can trigger harmful biological reactions at implantation sites through interfacial interactions, resulting in periprosthetic osteolysis, inflammation, and aseptic loosening.1,2 Specifically, inflammation activates osteoclast cells, leading to an improper balance between osteoclasts and osteoblasts. This imbalance initiates osteolysis, ultimately causing aseptic loosening of the prosthetic implant.1–3 Additionally, the properties of debris particles, including their composition, morphology, volume, and size, play a crucial role in their biological response and profoundly affect the fate of peri-implant cells.4Numerous studies (both, in vitro and in vivo) have demonstrated that nanoparticles consistently raise interfacial concerns because of their specific features such as surface area, morphology, size and concentration.5,6 Wear particles, smaller than 2 μm, can easily enter other organs, penetrate inside the cell through the plasma membrane and induce toxicity, even at the sub-cellular level.7,8 Wang et al.8 revealed that intra-articular injection (with concentrations of 2 and 20 mg ml−1) of TiO2 nanoparticles (38 to 54 nm) into the knees of rats allowed the migration of TiO2 nanoparticles into crucial organs like the heart and liver and resulted in pathological damage to these organs. Additionally, biochemical assessments demonstrated impairments in the renal and hepatic systems of rats.8 Mabrouk et al.9 reported that the performance of the liver is not affected after implantation of pure and BaO (3% and 5%)-doped MgSiO3 nanopowders into the tibia of fractured rats.
Several studies have reported the excellent osteogenic activity and antibacterial efficiency of Mg and Ca silicate-based bioceramics in vitro, which substantiate the potentiality of these materials for bone tissue engineering applications.10–17 Mg1−xCaxSi1−xZrxO3 (x = 0–0.4) has been established as an excellent biomaterial; however, the toxicity of such nanoparticles due to interfacial interactions has not been explored. As a step ahead, this study examined the in vivo toxicity of Mg1−xCaxSi1−xZrxO3 (x = 0–0.4) [MCSZO-X (X = 0–4)] nanoparticles using a rat model.
Initially, the MG-63 cells were exposed to different concentrations of MCSZO-X eluates (0.25, 2.5, and 25 mg ml−1 in normal saline) for 1 and 3 days. Following the in vitro results, the highest concentration (25 mg ml−1) of the MCSZO-X nanoparticle eluates was injected into the knee joints of rats for 7 days. After the designated exposure period, the rats were euthanized. Hematological evaluations were conducted to measure white blood cell (WBC) counts and mean corpuscular volume (MCV). Additionally, biochemical analyses were performed on the serum to assess the overall functional status of the organs in the groups treated with nanoparticles, including evaluations of alkaline phosphatase and creatinine activity. The histopathological evaluations were conducted to identify any potential signs of inflammation in the major organs (kidney, heart and liver) and knee joint.
2. In vivo toxicity assessment of MCSZO-X (X = 0–4) nanoparticles
2.1. Sample preparation and material characterization
The procedure for synthesizing micron-sized MCSZO-X (X = 0–4) powders has been reported in our earlier work.18 The solid-state method was employed to prepare these powders within a compositional range from X = 0 to X = 4. The particle size of the prepared micron-sized powders was reduced via ball milling (Fritsch Pulverisette 5) for 12–14 h at 300 rpm. For this purpose, cylindrical zirconia balls (6 mm diameter, 6 mm height, 0.9 g per ball) were used for grinding. The ratio of the balls to powder was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In a ball mill jar, 50 g (about 56 balls) of zirconia balls were combined with 5 g of MCSZO-X (X = 0–4) powder in 50 ml of ethanol.
1. In a ball mill jar, 50 g (about 56 balls) of zirconia balls were combined with 5 g of MCSZO-X (X = 0–4) powder in 50 ml of ethanol.
2.2. In vivo assessment
2.3. Hematology and biochemical assay
The EDTA (ethylenediaminetetraacetic acid)-coated vials were used to collect the serum samples for hematological analyses, and hematologic toxicity was assessed using an automated hematological analyzer (Cell-Dyn Ruby Hematology Analyzer). Hematological parameters, such as white blood cells (WBC) and mean corpuscular volume (MCV), were estimated. Biochemical testing was performed on the serum, which was obtained after centrifugation (4000 RPM for 10 min) of blood samples, to evaluate the activities of creatinine and alkaline phosphatase (ALP) in rats injected with nanoparticles and compared with the control and saline-treated groups. AUTOSPAN liquid and MKB alkaline phosphatase kits were used for the determination of ALP activity. The entire test was conducted as per the guidelines provided by the manufacturer. Additionally, creatinine levels were also measured using a standard kit (Crystal Chem, IL, USA).2.4. Histopathological analyses
The fixed tissues, including the kidney, liver, and heart, were dehydrated using a series of ethanol solutions. The paraffin blocks were made after fixing them with paraffin wax. Hematoxylin and eosin (H & E) stains were used for staining the tissue blocks after they were divided (10 μm) for histopathological evaluations. The tissues of the fixed knee joint were first dehydrated with ethanol solution, and then decalcification was done with nitric acid solution (10%). The paraffin-embedded joint tissue blocks were prepared for histopathological examination, following a similar procedure to other organs. Nikon Eclipse LV 100 ND fluorescence microscope photographs of stained tissues were obtained.2.5. Statistical analyses
The SPSS software was used to investigate statistically significant differences among various tests using the one-way ANOVA method and Tukey's post hoc tests, at a p ≤ 0.05. For in vivo data analyses, Graph Pad Prism software was used. The body weights of animals were statistically examined through the application of two-way ANOVA, whereas the hematological parameters were assessed via one-way ANOVA (at p > 0.05).3. Results and discussion
3.1. Phase analyses
Fig. 2 represents the XRD patterns of MCSZO-X (X = 0–4) nanoparticles. The XRD pattern confirms the formation of a monoclinic pure MgSiO3 [JCPDS # 35-0610] phase with the P21/c space group. In addition, a few minor peaks were indexed with JCPDS # 34-0189 using the Pmnb space group. However, the peak shifted towards a lower angle with the incorporation of Ca/Zr, increasing the concentration from X = 0 to X = 3, as shown in Fig. 2b. Moreover, the crystallite size was calculated using Scherrer's formula.19,20 The crystallite size decreased from 35 nm to 31 nm as the Ca/Zr increased from X = 0 to X = 3. After that, the crystallite size again increases from 31 nm to 33 nm with an increase in the amount of Ca/Zr, from X = 3 to X = 4.Fig. 2b demonstrates that with increasing concentration from X = 0 to X = 3, the peaks shift towards lower 2θ values from 28.24° to 28.08°. With a further increase in concentration from X = 0 to X = 3, the peak shifts towards the higher angle again from 28.08° to 28.33° (as represented by the enlarged view). The incorporation of larger Ca2+ cations (1.34 Å) at the reduced Mg2+ site (0.72 Å) and Zr4+ cations (0.74 Å) at the diminutive Si4+ site (0.40 Å) leads to an expansion of the lattice in MgSiO3 as the concentrations of Ca and Zr increase from 0 to 0.3, resulting in peak shifts toward lower 2θ values. Nonetheless, as the concentration increases beyond 0.3, the peaks shift to higher angles owing to lattice contraction, stemming from the presence of a greater quantity of Ca2+ at the Mg2+ site.11,21–23
The five polymorphic variants of MgSiO3 adopt either monoclinic or orthorhombic crystal structures.24 As the concentration of Ca and Zr increased from 0 to 0.2, peak positions in the X-ray diffraction patterns shifted to lower 2θ angles, from 22.96° to 22.82°. This shift results from the substitution of larger Ca2+ ions for smaller Mg2+ ions at the A-site and Zr4+ ions for smaller Si4+ ions at the B-site. Conversely, increasing the Ca and Zr concentrations from 0.2 to 0.4 caused the peaks to shift to higher 2θ values, from 22.82° to 23.12°, likely due to lattice contraction in MCSZO-X bioceramics driven by greater Ca2+ substitution at the Mg2+ sites. For compositions with x = 0.3 to x = 0.4, phase analyses revealed minor secondary phases, ZrO2 (JCPDS # 37-1484), CaSiO3 (JCPDS # 43-1460) and CaMgSiO4 (JCPDS # 19-0240). The emergence of an additional secondary phase, Mg2SiO3, poses challenges in synthesizing single-phase MgSiO3. Therefore, with increasing Ca and Zr concentrations from 0.3 to 0.4, further minor phases, including ZrO2, CaSiO3, Ca2MgSiO7, Mg2SiO4, and CaMg(SiO3)2, began to appear.
The FT-IR spectra of MCSZO-X nanoparticles confirmed the incorporation of Ca/Zr in the MgSiO3 structure (Fig. 3). The specific peaks of Si–O at 470, 500, 600, and 1052 cm−1 correspond to bending and stretching vibrations, respectively. The bending vibration observed at around 800 cm−1 in Si–O–Si indicates the formation of MgSiO3.25,26 Moreover, the vibrational peak, representing Si–O within silicate tetrahedra, is observed at 682 cm−1.
Furthermore, the stretching and bending vibrations of Mg–O are associated with the vibrational bands at 517 cm−1 and a peak close to 870 cm−1.27 Additionally, the peaks at 1320 cm−1 and 1127 cm−1 correspond to the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O, respectively.28
O and C–O, respectively.28
3.2. Microstructural analyses
Fig. 4 represents the high-resolution scanning electron microscopic (HRSEM) images of MCSZO-X nanoparticles. The average particle size of MCSZO-X nanoparticles increased from 346 to 452 nm as the amount of Ca/Zr in MCSZO-X was raised from 0 to 4. | ||
| Fig. 4 Scanning electron micrographs of MCSZO-X nanoparticles. (a) X = 0, (b) X = 1, (c) X = 2, (d) X = 3 and (e) X = 4. | ||
3.3. Leaching behavior
Fig. 5 illustrates the amounts of Ca2+, Mg2+, Si4+, and Zr4+ ions, leached from the MCSZO-X nanoparticles after 3, 5, and 7 days of immersion in saline. The leaching of Mg2+ and Si4+ ions from different nanoparticles (M1, M2, M3, M4, and M5) are lower in comparison to pure MCSZO-X (X = 0) nanoparticles as the concentration of Mg2+ and Si4+ decreases with increasing concentrations of Ca2+ and Zr4+ dopants. However, the leaching of Ca2+ and Zr4+ ions increases. In addition, the amount of Ca2+ leached from MCSZO-X nanoparticles also increased with an increase in the immersion time.Bones incorporate ions such as Mg2+, Ca2+, Zr4+, and Si4+, which play vital roles in regulating various metabolic activities, including supporting bone formation and reducing the risk of osteoporosis.10,29–32 Ca plays a vital role and also affects the metabolic functions of osteoblast cells.29,33 Concentrations of Ca2+ ions, above 10 mM, have been shown to exert cytotoxic effects, whereas levels in the 2–4 mM range support osteoblast proliferation and differentiation, thereby, enhancing the osteogenic response.34 Si is essential for bone development and overall bone health.10 Incorporating zirconium into Ca–Si-based bioceramics, like baghdadite, has been reported to enhance the attachment and growth of osteoblast-like cells.35
3.4. Cell viability
The viability of MG-63 cells was evaluated using the MTT assay at different elute concentrations (C1, C2, and C3 for M1, M2, M3, M4 and M5 samples). Fig. 6 demonstrates the proliferation of MG-63 cells on the prepared MCSZO-X nanoparticles after 1 and 3 days of incubation. The viability of osteoblast-like MG-63 cells, cultured on MCSZO-X nanoparticles, was lower than that of the control after 1 day.This is probably due to the physical damage of the cells resulting from early-stage interaction with nanoparticle eluates. Notably, the viability of cells was significantly improved across all concentrations of MCSZO-X nanoparticles after 3 days of culture in comparison to the results obtained after 1 day of incubation. For these samples, the viability was comparable to that of the control group [Fig. 6(b)].
3.5. In vivo studies
The paws of the rats, injected with MCSZO-X nanoparticles (M1, M2, M3, M4 and M5), are similar to those of the saline-treated and control rats, revealing no indication of abnormalities, inflammation, redness, or edema. Likewise, after completion of the experiment, the knee joints of the control, saline and treated rats displayed unchanged morphology.
Furthermore, as aforementioned, no inflammation was detected at the injection site, and no signs of atrophy were noted in the adjacent bone structures, such as the tibia and femur. This result corresponds to the in vitro cytocompatibility of MCSZO-X nanoparticles with varying concentrations of Ca/Zr [Fig. 6].
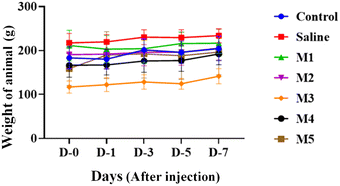
3.5.1.1. Impact of intra-articular injection of MCSZO-X on body weight. The variation in body weight is crucial for assessing whether the injected nanoparticles have adversely affected the function of vital organs.36,37 Consequently, before and after (after 7 days) the injection, the weights of each of the 35 rats were recorded. The control, saline, and MCSZO-X nanoparticle-injected rats did not show any significant weight changes [Fig. 8]. Statistical analyses using two-way ANOVA showed no noticeable variation in body weight.38
 | ||
| Fig. 9 Effect of intra-articular injection of MCSZO-X nanoparticles in rats. (a) WBC count and (b) MCV on day 7 of injection. All results are shown as mean ± standard deviation (n = 5 per group). | ||
The statistical analyses revealed no significant variation in WBC (p > 0.05) among all groups in the MCSZO-X nanoparticle-injected rats in comparison to the saline group [Fig. 9(a)]. Also, there were no notable changes in the MCV (p > 0.05) among all the injected MCSZO-X nanoparticle groups as compared to the saline groups [Fig. 9(b)].
Typically, the hepatic function is assessed by measuring the serum ALP activity level. The breakdown and restoration of liver tissue contribute to alterations in ALP activity.39,40 Additionally, hepatotoxicity raised by chemicals or drugs also increases the ALP activity in blood serum.41,42
As a result, the liver is an essential organ for examining the impact of toxicity induced by nanoparticles. In this study, MCSZO-X nanoparticles were injected into the synovial joint.
ALP, a marker of bone formation, plays a crucial role in determining whether exposure to MCSZO-X nanoparticles leads to any bone abnormalities. The statistical analyses reveal that there were no significant changes in the serum levels (ALP) between the control and MCSZO-X nanoparticle-treated (M1, M2, M3, M4 and M5) groups [Fig. 10(a)]. Consequently, exposure to MCSZO-X nanoparticles did not alter the liver function. Moreover, assessing serum creatinine levels is a typical method for detecting potential adverse effects on renal function caused by the implant or foreign particles.43
Elevated blood creatinine levels indicate reduced kidney filtration capacity.44–47 In this study, the creatinine levels in the blood serum of rats, injected with M1, M2, M3, M4 and M5 nanoparticles, do not show any substantial differences compared to the saline group [Fig. 10(b)]. Also, the MCSZO-X nanoparticles did not cause kidney impairment.
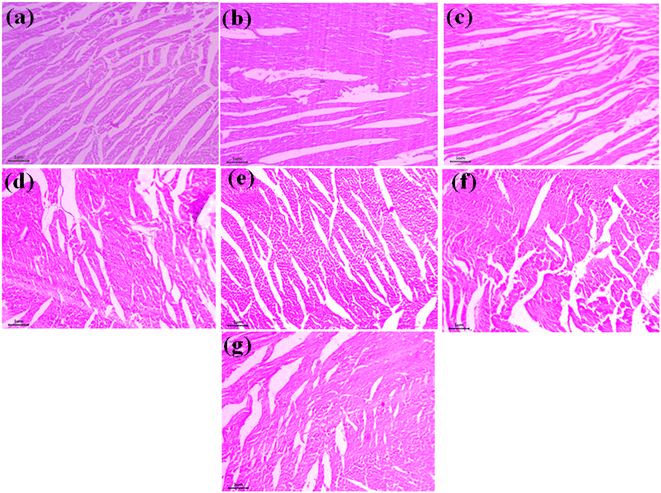
The sections of all the stained organs, in general, reveal a normal appearance without the presence of eluate particles. The MCSZO-X treated groups (M1, M2, M3, M4, and M5) showed no signs of tissue shrinkage, cardiac muscle disorder, vacuolization, and bleeding. The muscle fibers appear straight and organized, similar to those in the control group (Fig. 11). The connective tissues of the hearts in the nanoparticle-treated rat groups show normal architecture. In several studies, it has been observed that exposure to fine concentrated particles can result in irregular beats and, in some cases, cardiac dysfunction.48–52 The injection of TiO2 nanoparticles has been shown to cause swelling of the endothelial cells of the heart after 7 days.8
In this study, the hearts of rats in the M1, M2, M3, M4 and M5 eluate-treated groups did not exhibit enlarged endothelial cells [Fig. 11]. Overall, the cardiac tissues of the rats treated with MCSZO-X (M1, M2, M3, M4 and M5) reveal no histopathological changes. Evidently, the kidney is the main organ of the body that removes foreign nanoparticles.6 As the kidney removes foreign substances from the body by filtering, it is crucial for the kidney to participate in the release of nanoparticles if they reach vital organs. Besides, the histopathology analyses of the kidney tissue are crucial for both identifying the nanoparticles and determining how they may affect the structure and functioning of the kidneys.
Exposure to different nanoparticles, such as ZnO, Au, and TiO2, causes pathological alterations in the kidney, including necrosis, dispersed glomeruli, and tubular dilatation.8,53–55 However, in comparison to the control rat groups, the histopathological images of the kidney sections of the rats injected with MCSZO-X particles (M1, M2, M3, M4 and M5) show unchanged renal tubules within the cortex (absence of any indication of vacuolar degeneration) [Fig. 12].
The kidney sections of rats, treated with the control and MCSZO-X groups, show normal renal cortex and glomerular cells without tubule dilation. Overall, histology of the kidney sections reveals that the intra-articularly injected MCSZO-X nanoparticles (M1, M2, M3, M4 and M5) have no adverse effect on the kidney.
The liver plays a main role in detoxifying the body. This means that the foreign particles can move through the circulatory or lymphatic systems and into the liver.56–58 Cytoplasmic vacuolization disrupts the function of the membrane and can occasionally be a sign of liver injury.59–61 In this study, there is no evidence of any vacuolization in the cytoplasm of hepatocytes in the livers of the rats, treated with MCSZO-X nanoparticles. As a result, histological features of liver cells injected with MCSZO-X particles (M1, M2, M3, M4 and M5) revealed no indications of damage, bleeding, or necrosis near the sinusoids when compared to those of control and saline rats [Fig. 13].
Overall, the histopathological images of the organs, stained with H & E of the M1, M2, M3, M4 and M5 injected nanoparticles reveal a normal appearance comparable to that of the control and saline groups. The major organs of the rats in the nanoparticle-treated groups show no evidence of particle dissemination.
The histological images provide a clear indication of the presence of nanoparticles within the fibroadipose tissue surrounding the synovial joint [Fig. 14(a–e)]. Moreover, the absence of macrophage infiltration is clearly seen [Fig. 14(c–e)], which suggests the biocompatible nature of the prepared MCSZO-X nanoparticles.62,63 Furthermore, the administration of MCSZO-X nanoparticles via intra-articular injection did not lead to any injury to the cartilage or excessive growth of the synovial membrane. The thickening of the synovial membrane occurs due to an increased density of cells resulting from the influx of different cell types.64–66 The histological analyses of the knee joint did not reveal any negative response to the intra-articular administration of MCSZO-X nanoparticles.
The histological analyses of rats, treated with MCSZO-X (M1, M2, M3, M4 and M5) nanoparticles show no signs of inflammation at the implantation site of injection (synovial joint) or in vital organs [Fig. 14]. Moreover, MCSZO-X nanoparticles promote the proliferation of MG-63 cells [Fig. 6]. Overall, MCSZO-X nanoparticles demonstrate in vitro cytocompatibility and in vivo biocompatibility.
In ceramic-based implants, the wear debris particles, such as Al2O3 and ZrO2, are typically submicron in size.67,68 Earlier studies have reported that macrophages engulf submicron-sized particles, which increases the risk of an adverse reaction or inflammation.7,69,70 However, histological images of knee joints treated with MCSZO-X nanoparticles showed no signs of macrophage infiltration (Fig. 14(c–f)), suggesting that MCSZO-X nanoparticles are biocompatible.
4. Conclusion
In vitro investigations indicate that Mg1−xCaxSi1−xZrxO3 nanoparticles promote the proliferation of MG-63 cells up to a concentration of 25 mg ml−1 after initial interfacial interactions. The in vivo assessment revealed that the nanoparticles, injected intraarticularly into the rats, did not migrate to any of the major organs, including the kidney, heart and liver. In addition, the non-toxicity of Mg1−xCaxSi1−xZrxO3 nanoparticles has been established through histological analyses of the knees and vital organs of rats exposed to nanoparticle eluates at concentrations of 0.25, 2.5 and 25 mg ml−1. Also, the absence of nanoparticles within the essential organs suggests that they were not transported to these organs. The histology of the knee tissues of the rats treated with nanoparticles reveals the absence of any indications of inflammation. Furthermore, biochemical parameters (ALP and creatinine) revealed that Mg1−xCaxSi1−xZrxO3 nanoparticles had no toxic effect on the functioning of vital organs.Data availability
The data will be made available upon request.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This research was financially supported by SERB (CRG/2022/002154), Government of India. The authors sincerely thank Dr Bitan Naik from the Department of Pathology, Institute of Medical Sciences (IMS), Banaras Hindu University (BHU), Varanasi, for histopathological imaging.References
- L. Zhang, E.-M. Haddouti, K. Welle, C. Burger, D. C. Wirtz and F. A. Schildberg, et al. The effects of biomaterial implant wear debris on osteoblasts, Front. Cell Dev. Biol., 2020, 8, 352 CrossRef PubMed.
- C. Y. Hu and T.-R. Yoon, Recent updates for biomaterials used in total hip arthroplasty, Biomater. Res., 2018, 22(1), 1–12 CrossRef PubMed.
- I. D. Learmonth, C. Young and C. Rorabeck, The operation of the century: total hip replacement, Lancet, 2007, 370(9597), 1508–1519 CrossRef PubMed.
- A. A. Stratton-Powell, K. M. Pasko, C. L. Brockett and J. L. Tipper, The biologic response to polyetheretherketone (PEEK) wear particles in total joint replacement: a systematic review, Clin. Orthop. Relat. Res., 2016, 474(11), 2394–2404 CrossRef PubMed.
- Z. Chen, H. Meng, G. Xing, C. Chen, Y. Zhao and G. Jia, et al. Acute toxicological effects of copper nanoparticles in vivo, Toxicol. Lett., 2006, 163(2), 109–120 CrossRef CAS PubMed.
- T. Ding, Y. Xue, H. Lu, Z. Huang and J. Sun, Effect of particle size of hydroxyapatite nanoparticles on its biocompatibility, IEEE Trans. NanoBiosci., 2012, 11(4), 336–340 Search PubMed.
- K. J. Margevicius, T. W. Bauer, J. T. McMahon, S. A. Brown and K. Merritt, Isolation and characterization of debris in membranes around total joint prostheses, J. Bone Jt. Surg., Am. Vol., 1994, 76(11), 1664–1675 CrossRef CAS PubMed.
- J.-X. Wang, Y.-B. Fan, Y. Gao, Q.-H. Hu and T.-C. Wang, TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection, Biomaterials, 2009, 30(27), 4590–4600 CrossRef CAS PubMed.
- M. Mabrouk, G. Ibrahim Fouad, H. H. Beherei and D. B. Das, Barium Oxide Doped Magnesium Silicate Nanopowders for Bone Fracture Healing: Preparation, Characterization, Antibacterial and In Vivo Animal Studies, Pharmaceutics, 2022, 14(8), 1582 CrossRef CAS PubMed.
- P. Singh, X. Yu, A. Kumar and A. K. Dubey, Recent advances in silicate-based crystalline bioceramics for orthopedic applications: a review, J. Mater. Sci., 2022, 57(28), 13109–13151 CrossRef CAS.
- P. Singh and A. K. Dubey, Accelerated Osteogenic Response of Electrodynamically Stimulated Mg1–xCaxSi1–xZrxO3 (x = 0–0.4) Bioelectrets, ACS Biomater. Sci. Eng., 2023, 9(11), 6293–6308 CrossRef CAS PubMed.
- P. Singh and A. K. Dubey, Electret-induced antibacterial response of Mg1-xCaxSi1-xZrxO3 (x= 0–0.4) bioceramics, J. Am. Ceram. Soc., 2024, 107(6), 4263–4281 CrossRef CAS.
- S. K. Venkatraman and S. Swamiappan, Review on calcium-and magnesium-based silicates for bone tissue engineering applications, J. Biomed. Mater. Res., Part A, 2020, 108(7), 1546–1562 CrossRef CAS PubMed.
- K. Bavya Devi, S. K. Nandi and M. Roy, Magnesium Silicate Bioceramics for Bone Regeneration: A Review, J. Indian Inst. Sci., 2019, 99(3), 261–288 CrossRef.
- S. Ni and J. Chang, In vitro degradation, bioactivity, and cytocompatibility of calcium silicate, dimagnesium silicate, and tricalcium phosphate bioceramics, J. Biomater. Appl., 2009, 24(2), 139–158 CrossRef CAS PubMed.
- H. Bakhsheshi-Rad, A. Najafinezhad, Z. Hadisi, N. Iqbal, M. Daroonparvar and S. Sharif, et al. Characterization and biological properties of nanostructured clinoenstatite scaffolds for bone tissue engineering applications, Mater. Chem. Phys., 2021, 259, 123969 CrossRef CAS.
- J.-H. Shin, D.-Y. Lee and S.-H. Lee, Comparison of antimicrobial activity of traditional and new developed root sealers against pathogens related root canal, J. Dent. Sci., 2018, 13(1), 54–59 CrossRef PubMed.
- P. Singh and A. K. Dubey, Accelerated Osteogenic Response of Electrodynamically Stimulated Mg1–x Ca x Si1–x Zr x O3 (x= 0–0.4) Bioelectrets, ACS Biomater. Sci. Eng., 2023, 9(11), 6293–6308 CrossRef CAS PubMed.
- B. D. Cullity, Elements of X-ray, Diffraction, Addison-Wesley Publishing, 1956 Search PubMed.
- S. M. Londoño-Restrepo, R. Jeronimo-Cruz, B. M. Millán-Malo, E. M. Rivera-Muñoz and M. E. Rodriguez-García, Effect of the nano crystal size on the X-ray diffraction patterns of biogenic hydroxyapatite from human, bovine, and porcine bones, Sci. Rep., 2019, 9(1), 5915 CrossRef PubMed.
- S. Parthasarathy and V. Parthasarathi, A statistical study on the measurability of Bijvoet differences in crystals with type-I and type-II degree of centrosymmetry, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32(5), 768–771 CrossRef.
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A, 1976, 32(5), 751–767 CrossRef.
- H. Sun, Q. Zhang, H. Yang and J. Zou, (Ca1− xMgx) SiO3: a low-permittivity microwave dielectric ceramic system, Mater. Sci. Eng., B, 2007, 138(1), 46–50 CrossRef CAS.
- J. R. Smyth, Experimental study on the polymorphism of enstatite, Am. Mineral., 1974, 59(3–4), 345–352 CAS.
- C. K. Choi, Comparison between SiOC Thin Film by plasma enhance chemical vapor deposition and SiO2 Thin Film by Fourier Transform Infrared Spectroscopy?, J. Korean Phys. Soc., 2010, 56(4), 1150–1155 CrossRef.
- C. Vancea, M. Mihailescu, A. Negrea, G. Mosoarca, M. Ciopec and N. Duteanu, et al. Batch and fixed-bed column studies on palladium recovery from acidic solution by modified MgSiO3, Int. J. Environ. Res. Public Health, 2020, 17(24), 9500 CrossRef CAS PubMed.
- S. Sagadevan, S. Venilla, A. Marlinda, M. Johan, Y. A. Wahab and R. Zakaria, et al. Effect of synthesis temperature on the morphologies, optical and electrical properties of MgO nanostructures, J. Nanosci. Nanotechnol., 2020, 20(4), 2488–2494 CrossRef CAS PubMed.
- P. Kumar, B. S. Dehiya, A. Sindhu, R. Kumar, C. I. Pruncu and A. Yadav, Fabrication and characterization of silver nanorods incorporated calcium silicate scaffold using polymeric sponge replica technique, Mater. Des., 2020, 195, 109026 CrossRef CAS.
- D. Dufrane, C. Delloye, I. Mckay, P. De Aza, S. De Aza and Y.-J. Schneider, et al. Indirect cytotoxicity evaluation of pseudowollastonite, J. Mater. Sci.: Mater. Med., 2003, 14, 33–38 CrossRef CAS PubMed.
- K. Schwarz, A bound form of silicon in glycosaminoglycans and polyuronides, Proc. Natl. Acad. Sci. U. S. A., 1973, 70(5), 1608–1612 CrossRef CAS PubMed.
- H. Mohammadi, M. Hafezi, N. Nezafati, S. Heasarki, A. Nadernezhad and S. Ghazanfari, et al. Bioinorganics in bioactive calcium silicate ceramics for bone tissue repair: bioactivity and biological properties, J. Ceram. Sci. Technol., 2014, 5(1), 1–12 Search PubMed.
- P. Pravina, D. Sayaji and M. Avinash, Calcium and its role in human body, Int. J. Res. Pharm. Biomed. Sci., 2013, 4(2), 659–668 CAS.
- C. Wu, J. Chang, J. Wang, S. Ni and W. Zhai, Preparation and characteristics of a calcium magnesium silicate (bredigite) bioactive ceramic, Biomaterials, 2005, 26(16), 2925–2931 CrossRef CAS PubMed.
- S. Maeno, Y. Niki, H. Matsumoto, H. Morioka, T. Yatabe and A. Funayama, et al. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture, Biomaterials, 2005, 26(23), 4847–4855 CrossRef CAS PubMed.
- Y. Ramaswamy, C. Wu, A. Van Hummel, V. Combes, G. Grau and H. Zreiqat, The responses of osteoblasts, osteoclasts and endothelial cells to zirconium modified calcium-silicate-based ceramic, Biomaterials, 2008, 29(33), 4392–4402 CrossRef CAS PubMed.
- J. El Hilaly, Z. H. Israili and B. Lyoussi, Acute and chronic toxicological studies of Ajuga iva in experimental animals, J. Ethnopharmacol., 2004, 91(1), 43–50 CrossRef PubMed.
- S. A. Bailey, R. H. Zidell and R. W. Perry, Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint?, Toxicol. Pathol., 2004, 32(4), 448–466 CrossRef PubMed.
- R. Buesen, R. Landsiedel, U. G. Sauer, W. Wohlleben, S. Groeters and V. Strauss, et al. Effects of SiO 2, ZrO 2, and BaSO 4 nanomaterials with or without surface functionalization upon 28-day oral exposure to rats, Arch. Toxicol., 2014, 88, 1881–1906 CrossRef CAS PubMed.
- M. M. Kaplan and A. Righetti, Induction of rat liver alkaline phosphatase: the mechanism of the serum elevation in bile duct obstruction, J. Clin. Invest., 1970, 49(3), 508–516 CrossRef CAS PubMed.
- Z. Gawlik, E. Fiejka, R. Aleksandrowicz and I. Wiśniewska, Activity of alkaline phosphatase in the healing rat liver after hepatectomy, Folia Histochem. Cytochem., 1978, 16(4), 343–349 CAS.
- T. M. Wright and A. M. Vandenberg, Risperidone-and quetiapine-induced cholestasis, Ann. Pharmacother., 2007, 41(9), 1518–1523 CrossRef CAS PubMed.
- A. Singh, T. Bhat and O. Sharma, Clinical biochemistry of hepatotoxicity, J. Clin. Toxicol., 2011, 4, 1–19 Search PubMed.
- P. N. Kidney, Textbook of Biochemistry and Human biology, Prentise Hall India, 1999, pp. 290–296 Search PubMed.
- P. Chan, G. O'hara and A. W. Hayes, Principles and methods for acute and subchronic toxicity, Principles and methods of toxicology, 1982, vol. 12, pp. 17–19 Search PubMed.
- O. Adefemi, A. Elujoba and W. Odesanmi, Evaluation of the toxicity potential of Cassia podocarpa with reference to official Senna, West Afr. J. Pharmacol. Drug Res., 1988, 8, 41–48 Search PubMed.
- R. C. Oh and T. R. Hustead, Causes and evaluation of mildly elevated liver transaminase levels, Am. Fam. Physician, 2011, 84(9), 1003–1008 Search PubMed.
- A. S. Ene-ojo, E. A. Chinedu and F. M. Yakasai, Toxic Effects of Sub-Chronic Administration of Chloroform Extract of Artemisia maciverae Linn on the Kidney of Swiss Albino Rats, 2013.
- K. L. Timonen, E. Vanninen, J. De Hartog, A. Ibald-Mulli, B. Brunekreef and D. R. Gold, et al. Effects of ultrafine and fine particulate and gaseous air pollution on cardiac autonomic control in subjects with coronary artery disease: the ULTRA study, J. Exposure Sci. Environ. Epidemiol., 2006, 16(4), 332–341 CrossRef CAS PubMed.
- D. Q. Rich, W. Zareba, W. Beckett, P. K. Hopke, D. Oakes and M. W. Frampton, et al. Are ambient ultrafine, accumulation mode, and fine particles associated with adverse cardiac responses in patients undergoing cardiac rehabilitation?, Environ. Health Perspect., 2012, 120(8), 1162–1169 CrossRef PubMed.
- A. Peters, R. Hampel, J. Cyrys, S. Breitner, U. Geruschkat and U. Kraus, et al. Elevated particle number concentrations induce immediate changes in heart rate variability: a panel study in individuals with impaired glucose metabolism or diabetes, Part. Fibre Toxicol., 2015, 12(1), 1–11 CrossRef CAS PubMed.
- R. D. Brook, J. R. Brook, B. Urch, R. Vincent, S. Rajagopalan and F. Silverman, Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults, Circulation, 2002, 105(13), 1534–1536 CrossRef CAS PubMed.
- R. B. Hamanaka and G. M. Mutlu, Particulate matter air pollution: effects on the cardiovascular system, Front. Endocrinol., 2018, 9, 680 CrossRef PubMed.
- K. E. Ibrahim, M. G. Al-Mutary, A. O. Bakhiet and H. A. Khan, Histopathology of the liver, kidney, and spleen of mice exposed to gold nanoparticles, Molecules, 2018, 23(8), 1848 CrossRef PubMed.
- G. Yan, Y. Huang, Q. Bu, L. Lv, P. Deng and J. Zhou, et al. Zinc oxide nanoparticles cause nephrotoxicity and kidney metabolism alterations in rats, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2012, 47(4), 577–588 CrossRef CAS PubMed.
- A. Noori, F. Karimi, S. Fatahian and F. Yazdani, Effects of zinc oxide nanoparticles on renal function in mice, Int. J. Biosci., 2014, 5(9), 140–146 CrossRef.
- J. Lipka, M. Semmler-Behnke, R. A. Sperling, A. Wenk, S. Takenaka and C. Schleh, et al. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection, Biomaterials, 2010, 31(25), 6574–6581 CrossRef CAS PubMed.
- M. Husain, D. Wu, A. T. Saber, N. Decan, N. R. Jacobsen and A. Williams, et al. Intratracheally instilled titanium dioxide nanoparticles translocate to heart and liver and activate complement cascade in the heart of C57BL/6 mice, Nanotoxicology, 2015, 9(8), 1013–1022 CrossRef CAS PubMed.
- J. Modrzynska, A. Mortensen, T. Berthing, G. Ravn-Haren, J. Szarek and A. T. Saber, et al. Effect on mouse liver morphology of CeO2, TiO2 and carbon black nanoparticles translocated from lungs or deposited intravenously, Appl. Nanosci., 2021, 2(3), 222–241 Search PubMed.
- M. A. K. Abdelhalim and B. M. Jarrar, Gold nanoparticles administration induced prominent inflammatory, central vein intima disruption, fatty change and Kupffer cells hyperplasia, Lipids Health Dis., 2011, 10, 1–6 CrossRef PubMed.
- M. A. K. Abdelhalim and B. M. Jarrar, Gold nanoparticles induced cloudy swelling to hydropic degeneration, cytoplasmic hyaline vacuolation, polymorphism, binucleation, karyopyknosis, karyolysis, karyorrhexis and necrosis in the liver, Lipids Health Dis., 2011, 10, 1–6 CrossRef PubMed.
- M. A. K. Abdelhalim, Gold nanoparticles administration induces disarray of heart muscle, hemorrhagic, chronic inflammatory cells infiltrated by small lymphocytes, cytoplasmic vacuolization and congested and dilated blood vessels, Lipids Health Dis., 2011, 10(1), 1–9 CrossRef PubMed.
- Z. Sheikh, P. J. Brooks, O. Barzilay, N. Fine and M. Glogauer, Macrophages, foreign body giant cells and their response to implantable biomaterials, Materials, 2015, 8(9), 5671–5701 CrossRef CAS PubMed.
- Z. Xia and J. T. Triffitt, A review on macrophage responses to biomaterials, Biomed. Mater., 2006, 1(1), R1 CrossRef CAS PubMed.
- A. Sergijenko, A. J. Roelofs, A. H. Riemen and C. De Bari, Bone marrow contribution to synovial hyperplasia following joint surface injury, Arthritis Res. Ther., 2016, 18, 1–11 CrossRef PubMed.
- Synovial cellular and molecular markers in rheumatoid arthritis, Seminars in immunopathology, ed. M. Asif Amin, D. A. Fox and J. H. Ruth, Springer, 2017 Search PubMed.
- C. J. Burke, H. Alizai, L. S. Beltran and R. R. Regatte, MRI of synovitis and joint fluid, J. Magn. Reson. Imaging, 2019, 49(6), 1512–1527 CrossRef PubMed.
- S. Lerouge, O. Huk, L. H. Yahia and L. Sedel, Characterization of in vivo wear debris from ceramic—ceramic total hip arthroplasties, J. Biomed. Mater. Res., 1996, 32(4), 627–633 CrossRef CAS PubMed.
- A. Hatton, J. Nevelos, A. Nevelos, R. Banks, J. Fisher and E. Ingham, Alumina–alumina artificial hip joints. Part I: a histological analysis and characterisation of wear debris by laser capture microdissection of tissues retrieved at revision, Biomaterials, 2002, 23(16), 3429–3440 CrossRef CAS PubMed.
- S.-Y. Yang, W. Ren, Y. Park, A. Sieving, S. Hsu and S. Nasser, et al. Diverse cellular and apoptotic responses to variant shapes of UHMWPE particles in a murine model of inflammation, Biomaterials, 2002, 23(17), 3535–3543 CrossRef CAS PubMed.
- G. Thrivikraman, G. Madras and B. Basu, In vitro/in vivo assessment and mechanisms of toxicity of bioceramic materials and its wear particulates, RSC Adv., 2014, 4(25), 12763–12781 RSC.
| This journal is © The Royal Society of Chemistry 2025 |