Open Access Article
Open Access ArticleMagnetic pillar induced Poiseuille-like flow in microfluidic channels with viscous and viscoelastic fluids†
Charles Paul
Moore
 *ab,
Stefan
Rouach
b,
Marine
Le Goas
b,
Sandra
Lerouge
b,
Nicolas
Tsapis
c,
Jérôme
Fresnais
*ab,
Stefan
Rouach
b,
Marine
Le Goas
b,
Sandra
Lerouge
b,
Nicolas
Tsapis
c,
Jérôme
Fresnais
 a and
Jean-François
Berret
a and
Jean-François
Berret
 *b
*b
aCNRS, Laboratoire de Physicochimie des Electrolytes et Nanosystèmes Interfaciaux, PHENIX, UMR8234, Sorbonne Université, F-75252 Paris Cedex 05, France
bUniversité Paris Cité, CNRS, Matière et systèmes complexes, 75013 Paris, France. E-mail: paul.moore@u-paris.fr; jean-francois.berret@u-paris.fr
cUniversité Paris-Saclay, CNRS, Institut Galien Paris-Saclay, 91400 Orsay, France
First published on 2nd May 2025
Abstract
Mucociliary clearance in mammals serves as the primary defense mechanism for removing particulate matter deposited in the pulmonary airways. Dysfunctions in this process are linked to serious respiratory diseases and can hinder effective drug delivery to the lungs. Microfluidic systems have emerged as a promising alternative for replicating lung functions in non-cellular physiological environments, offering a more controllable approach compared to in vivo and in vitro assays. Here we present a microfluidic platform featuring a closed-loop circular microchannel, integrating a thousand 75 μm-high magnetic pillars arranged in a square array. Made of polydimethylsiloxane and loaded with iron microparticles, the pillars are studied using scanning electron microscopy and magnetometry; their internal structure and bending response to a magnetic field are quantitatively analyzed. Using a combination of experimental data and finite element simulations, we found that the magnetic torque induced by permanent magnets dominates over magnetic force, generating fluid flow in the microchannel. Under the application of a rotating field, the time-dependent deflection of the pillars closely mimics the behavior of lung cilia, exhibiting alternating effective and recovery strokes. The velocity profiles of viscous and viscoelastic fluids are examined, and shown to display Poiseuille-type flow. By varying the viscosity of the fluids across four orders of magnitude, we identified a transition in propulsion regimes between viscous and elastic-driven flows. This active microfluidic platform offers a promising approach for modeling mucociliary clearance in drug delivery applications.
1 Introduction
Humans inhale approximately 10 m3 of air daily, carrying particulate matter, much of which deposits in the pulmonary airways.1 To counteract this, the respiratory system relies on mucociliary clearance (MCC) as a key defense mechanism in the tracheobronchial region to mitigate the effects of inhaled particles and pathogens. The MCC integrates a 5–10 μm thick mucus layer with the coordinated beating of cilia, which propel mucus from the respiratory tract and direct it into the digestive system for clearance.2–4 Cilia are versatile structures found across many living systems, aiding for lubrication or enabling motility in organisms like protozoa and some aquatic invertebrates.5–9 In lung diseases such as ciliary dyskinesia and cystic fibrosis however, disruptions in cilia coordination or changes in mucus rheology can impair respiratory function. These issues, compounded by alterations in mucus composition, can hinder effective drug delivery to the airways.1,3,10–12 In the context of local drug delivery to the lungs, advancing therapeutic strategies remains a critical challenge in medical engineering and personalized medicine.13,14To overcome the limitations of in vivo studies in the formulation of lung-specific drugs, in vitro experiments on reconstituted human bronchial epithelium were conducted, allowing for in situ study of changes in mucus rheology and the observation of cilia efficiency and coordination.15–18 These approaches also enable the study of mucus flow and the evaluation of drug diffusion into the mucus layer.1 However, cultures maintained at the air–liquid interface can occasionally produce non-physiological flow patterns, which may complicate the interpretation of ciliary transport in the context of native bronchial function. Still, when both ciliary density and mucus viscosity reach physiologically relevant levels, the system is capable of generating efficient, long-range unidirectional mucus flow, a key component of effective mucociliary clearance.16 Despite its limitations, the 2D reconstructed human bronchial epithelium remains a valuable model for studying the interface between air, mucus, and lung cells.
Complementary to in vivo and in vitro approaches, synthetic ciliated substrate alternatives have been developed in parallel using established microfluidic and soft lithography methods19–23 and more recently through laser printing techniques,24 allowing the precise fabrication of biomimetic structures. Pulmonary cilia are 7 μm long and 200 nm in diameter, with a surface density of 200–300 cilia per cell.25 These cilia exhibit a progressive, coordinated and asymmetric movement, called a metachronal wave, and beat at a frequency of 10–20 Hz, leading in humans to mucus clearance at a speed of 40–90 μm s−1.4,26 To date, research in this field has focused not on replicating ciliated substrates with exact physiological characteristics of density, aspect ratio, or size, but on developing responsive substrates that can mimic MCC functions.27,28 To achieve this, researchers took inspiration from elastomeric pillar arrays, developed about 20 years ago, designed for measuring micrometer-scale forces or crafting superhydrophobic substrates.29–31 These ciliated substrates, typically composed of PDMS or poly(acrylamide) were however not suitable for remote actuation.32,33 For controlled deflection, active particles—often magnetic—are incorporated into the elastomer, enabling pillar orientation on demand.19–21,23,28,34–41 Magnetic particles commonly incorporated into elastomers include iron (Fe0) or iron oxide particles (maghemite γ-Fe2O3, magnetite Fe3O4), with sizes ranging from a few nanometers to several micrometers.35 Notably, 0.5 μm iron carbonyl microparticles (hereafter noted FeMPs) offer an excellent balance of high magnetization, chemical stability, and cost-effectiveness.42
Among the various devices recently developed for microfluidic applications, a series of high-performance microchips has been designed to efficiently propel viscous liquids in confined environments. Over the past decade, significant advancements have been achieved, including the miniaturization of cilia19,35,43,44 and pillars,28,39,41 increased flow rates,20 programmable pillar movements,38–41 and the generation of metachronal waves reminiscent of lung ciliated cells.20–22,38,39 Yet, limited attention has been given to studying velocity profiles in closed microfluidic chips or exploring the rheological properties of the fluids in motion. The above-mentioned studies commonly rely on the local application of magnetic fields, generated by either electro- or permanent magnets, which produce fields between 10 and 100 mT at the pillar level. Such magnetic fields are inherently non-homogeneous, with their amplitude and orientation varying spatially. This spatial variation induces both magnetic forces and torques on the system,35,45 which can influence chip performance. To date, the relative contributions of these effects have been inadequately examined.27,42,43,46 The present study seeks to address these gaps by investigating key factors such as the nature of induced flows, the influence of fluid viscoelasticity, and the dynamics of magnet-pillar interactions via physics simulations.
Here, we fabricated a circular closed-loop microchannel using soft lithography, incorporating an array with 1070 magnetic pillars arranged on a square grid and defining the actuation zone.20 These pillars loaded with iron carbonyl particles are characterized in terms of composition and density, and their mechanical response to a magnetic field is analyzed quantitatively. Using finite element simulations, combined with experimental data, we model the impact of the magnetic field on pillar bending and find that the magnetic torque dominates over the magnetic force. The pillars are activated by the field created by rotating permanent magnets, producing fluid flow in the microchannel. Velocity fields studied for viscous and viscoelastic fluids over more than 4 orders of magnitudes in viscosity show Poiseuille-type profiles in the channel, as well as a propulsion regime transition between viscous and elastic driven flows.
2 Results and discussion
2.1 Pillar characterization
 | ||
| Fig. 1 a) Scanning electron microscopy (SEM) image of a PDMS pillar array. Inset: close-up view of a single pillar highlighting its geometric dimensions (dPillar = 20 μm, lPillar = 75 μm). A small bulge, resulting from mold construction, is visible 25 μm from the base. b) Schematic of the fabrication process for magnetic pillars: a slurry of ethanol and FeMPs is poured into the counter-mold; ethanol is then evaporated, and PDMS elastomer is added over the particles and cured. c) SEM image of a magnetic pillar array, showing FeMPs as white dots concentrated in the upper part of the pillars; insets: SEM image and iron EDX mapping of a pillar bent toward the substrate. d) Mass absorption coefficient, εm, of pure iron, FeMPs, and magnetic pillars dissolved in high-concentration hydrochloric acid. The absorbance signal reflects the formation of FeCl4− (tetrachloroferrate) ions, allowing for iron quantification.51 e) Magnetization curves comparing FeMP powder and magnetic pillars. | ||
Iron quantification was performed using two methods: a colorimetric assay via UV-visible spectroscopy and vibrating sample magnetometry (VSM). The first method involved measuring the mass absorption coefficient εm of iron in FeMPs and in magnetic pillars dissolved in 37% hydrochloric acid (HCl), comparing these values to the εm of iron. For this reference, iron data were obtained from 10 nm maghemite (γ-Fe2O3) nanoparticles dissolved in HCl, and then normalized by iron content.51,52 In Fig. 1d, the mass absorption coefficient of FeMPs is shown to be reduced by 32% compared to pure iron, suggesting the microparticles contain in average 68% elemental iron (Fe0), with the remainder likely due to carbonyl groups or additives. The curve in blue in Fig. 1d represents εm for 4070 magnetic pillars dissolved in concentrated HCl. Comparing this with the FeMP reference reveals an average volume fraction ϕFeMP of 22% in the pillars. Values for hFeMP and ϕFeMP are key parameters for subsequent pillar bending simulations.
Further iron quantification was performed using VSM. Magnetization curves M(H) for both FeMPs powder samples and pillar arrays were measured as a function of the magnetic field (ESI† S3). For the powder, the data was normalized by the effective volume of FeMPs in the sample. On the other hand, the raw M(H) pillar data was normalized by the total pillar volume within the investigated array. As shown in Fig. 1e, both systems display similar behavior: a linear increase at low fields, followed by a saturation plateau around 106 A m−1, with a lower saturation value for the pillars. The absence of hysteresis in the field cycles confirms superparamagnetic behavior. Analysis of the linear region suggests a magnetic particle size estimate of 3 nm, slightly smaller than the 9 nm-crystallite size measured by wide-angle X-ray scattering (ESI† S4). The FeMP saturation value ms = 1.90 × 106 A m−1 is also in agreement with previous determination.42 Comparing VSM data from Fig. 1e, the pillars reach a saturation magnetization of 20.5% relative to the FeMPs. This result aligns well with the colorimetric findings which indicated a value of 22%. Finally, this fabrication method achieves iron pillar concentrations that are twice higher than those obtained by directly mixing FeMPs with PDMS prior to molding,42 with the added benefit of localizing iron in the upper part of the pillars.40
2.2 Magnetic bending
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μm above the pillars during approach. Fig. 2b shows the magnetic field B(y) and the magnetic field gradient dB(y)/dy as a function of the magnet distance to the microchip center. The maximum magnetic field, approximately 0.4 T, was recorded directly beneath the prism tip, corresponding to a gradient of 250 T m−1. Notably, at distances greater than 10 mm from the magnet tip, the field gradient nears zero. As the magnet moves closer to the array, the pillars bend due to the concomitant influence of magnetic force and torque. Fig. 2c displays a chronophotograph of three rows of pillars in the array at varying distances from the magnet tip, ranging from 12 mm to 0 mm, clearly illustrating that the deflection δPillar(y) increases as the distance y decreases (ESI,† Movie S1). To quantify the deflection behavior, pillar bending was recorded by optical microscopy over 3 fields of view containing a total of 87 pillars in a sequence of images over time, then converted into distance data δPillar(y). Fig. 2d presents these deflections as a shaded surface across all 87 pillars. The deflection pattern is consistent for all collected data, showing an inflection point around y = 3 mm and reaching a saturation as the magnet approaches directly above the pillars. Maximum deflection values for this array range from 35 to 45 μm. Moderate variability in pillar responses is observed, likely due to differences in FeMP distribution or pillar elasticity. The curve in red at the center of the shaded region represents the average deflection, calculated as a 10-point moving average. Below 1 mm, the deflection plateaus, which could be attributed to various factors, including the geometry of the magnetic field that increasingly aligns or pulls pillars upward toward the magnet as it nears. The following section presents finite element simulations of pillar bending, analyzing the effects of induced magnetic forces and torques.
μm above the pillars during approach. Fig. 2b shows the magnetic field B(y) and the magnetic field gradient dB(y)/dy as a function of the magnet distance to the microchip center. The maximum magnetic field, approximately 0.4 T, was recorded directly beneath the prism tip, corresponding to a gradient of 250 T m−1. Notably, at distances greater than 10 mm from the magnet tip, the field gradient nears zero. As the magnet moves closer to the array, the pillars bend due to the concomitant influence of magnetic force and torque. Fig. 2c displays a chronophotograph of three rows of pillars in the array at varying distances from the magnet tip, ranging from 12 mm to 0 mm, clearly illustrating that the deflection δPillar(y) increases as the distance y decreases (ESI,† Movie S1). To quantify the deflection behavior, pillar bending was recorded by optical microscopy over 3 fields of view containing a total of 87 pillars in a sequence of images over time, then converted into distance data δPillar(y). Fig. 2d presents these deflections as a shaded surface across all 87 pillars. The deflection pattern is consistent for all collected data, showing an inflection point around y = 3 mm and reaching a saturation as the magnet approaches directly above the pillars. Maximum deflection values for this array range from 35 to 45 μm. Moderate variability in pillar responses is observed, likely due to differences in FeMP distribution or pillar elasticity. The curve in red at the center of the shaded region represents the average deflection, calculated as a 10-point moving average. Below 1 mm, the deflection plateaus, which could be attributed to various factors, including the geometry of the magnetic field that increasingly aligns or pulls pillars upward toward the magnet as it nears. The following section presents finite element simulations of pillar bending, analyzing the effects of induced magnetic forces and torques.
 | ||
| Fig. 2 a) Schematic representation of the device used to study pillar bending featuring phase contrast optical microscopy for visualization. b) Magnetic field B(y) and magnetic field gradient dB(y)/dy generated by two 12 mm-cubic N48 neodymium magnets 200 μm above the pillar tips as a function of the distance y. c) Chronophotograph showing the bending of pillars as the magnet moves closer to the chip center. The deflection, δPillar(y), is marked in the image. d) Variation of deflection δPillar(y) as a function of the distance between pillars and magnet. The area in gray represents the response of 87 individual pillars, whereas the curve in red is the 10-point moving average (see also Movie S1 in ESI†). | ||
 | ||
| Fig. 3 a) Simulated 3D-mapping of the magnetic field lines created by the magnetic pen and iron prism displayed in Fig. 2a. The field mapping was validated by comparing it with the field shown in Fig. 2b. b) The bending of a single pillar at distances y = 10, 2, 1 and 0 mm (from left to right panels) from the magnet displayed in Fig. 3a. The pillar is modeled as a 75 μm-long, slightly tapered beam fixed at its base and free at its end. c) Simulated deflection δPillar(y) as a function of the distance from the tip for different values of hFeMP/lPillar, where hFeMP designates the height of the FeMP-loaded region and lPillar the total pillar length (75 μm). In this simulation, ϕFeMP = 22%, and E = 0.75 MPa. The curve in blue labelled “0.50 (f)” is obtained for force alone, while the curves in shades of red include force and magnetic torque. The area in gray represents the response of 87 individual pillars. d) Same as in Fig. 3c) simulating the effect of the pillar Young modulus E. e) Simulated deflection δPillar as a function of the magnetic field B for different values of the FeMP volume fraction ϕFeMP. | ||
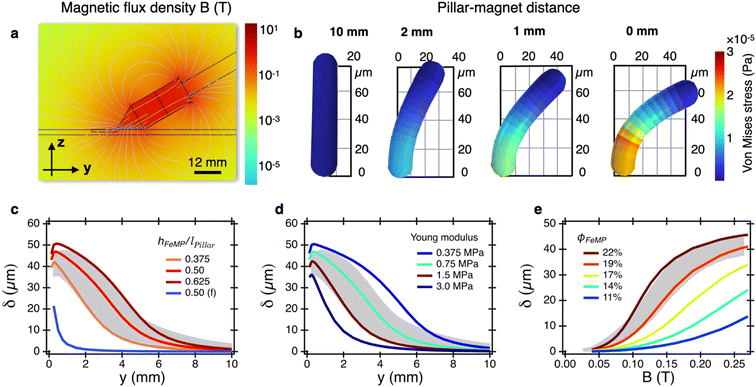
Pillar deflection was simulated separately using the COMSOL beam simulation environment.53 A single pillar, modeled as a slightly tapered beam 75 μm long and 20 μm in diameter, fixed at its base and free at its end, was designed to match SEM measurements. The beam was subjected to a magnetic force as well as a magnetic torque perpendicular to the bending plane. The bending of a simulated pillar for a magnet located at 10, 2, 1 and 0 mm is shown in Fig. 3b. As the magnet gets closer, bending increases. The simulations provide the von Mises stress distribution along the beam, visualized by the color scale. It is found that the stress is highest at around 20 μm above its base. The von Mises stress values indicate that the pillar is only slightly outside the region of linear behavior for typical PDMS.54 This occurs when the pillar is just below the prism extremity.
Fig. 3c presents a direct comparison of pillar bending driven by magnetic force (in blue) versus combined magnetic force and torque (in shades of red) effects. In this simulation, the FeMP volume fraction is set to its experimental value, ϕFeMP = 22%, and the pillar Young modulus E is fixed at 0.75 MPa. This value is consistent with those reported in the literature using beam bending methods.55 The simulation indicates that magnetic force alone underestimates the experimentally observed deflection (shaded region), highlighting that both torque and force contributions are essential for accurate interpretation. This represents a key finding of this work: magnetic force alone cannot account for the deflection of PDMS pillars under current conditions.
Simulations were also conducted across various hFeMP/lPillar values, with optimal agreement closely matching the experimental data in Fig. 1c. At short distances, deflection saturation occurs due to the magnetic field predominantly vertical orientation, which aligns the pillar vertically as well. As a second parameter, we varied the PDMS material properties to simulate the effect of elasticity on pillar bending (Fig. 3d), conducting simulations at hFeMP/lPillar = 0.5 and ϕFeMP = 22%. We found that increasing E led to a decrease in deflection δPillar. The relationship between E and δPillar is however nonlinear, with the most pronounced effect occurring at intermediate distances (1–5 mm). Compared to experimental data, the stiffest and softest beams fell outside the 95% confidence interval, with the best fit observed for E = 0.75 MPa.
In a final simulation, we examine the effect of the magnetic field on pillar deflection (Fig. 3e), keeping the parameters E, hFeMP and ϕFeMP as before. Moreover, the magnetic field strength was modeled based on the experimental data shown in Fig. 2b. As B increases, the simulated pillar deflection also rises, consistent with the quadratic dependence of torque on field amplitude. Fig. 3e further explores the impact of varying ϕFeMP on δPillar: reducing the FeMP fraction below the experimental value results in a decrease in deflection, with only ϕFeMP ≥ 19% falling within the 95% confidence interval of experimental data. In summary to this part, finite element simulations show strong agreement with experimental results for various parameters, including hFeMP/lPillar, E, and ϕFeMP, validating the simulation scheme adopted here and underscoring the dominant role of magnetic torque in pillar deflection.
2.3 Closed-loop microchannel with pillar-driven flow
Fig. 4f presents the tip displacement of the pillar xPillar(t) along the x-axis over two full magnet rotations, with rotation angles π, 2π, 3π and 4π indicated at the top. The time points corresponding to Fig. 4c–e are also marked. The displacement profile exhibits asymmetric square-wave patterns, characterized by a gradual rise followed by a sharp drop. This asymmetry is even more evident in Fig. 4g, which shows the x-component of the tip velocity dxPillar(t)/dt. For much of the cycle, the velocity remains in the 100 μm s−1 range, punctuated by spikes reaching velocities up to 20 mm s−1 as the pillar gets back to its equilibrium position. This time-dependent deflection closely resembles the behavior of lung cilia, with alternating effective and recovery strokes. The effective stroke is marked by sharp drops in xPillar(t) at t = 0.175, 0.35, 0.525 and 0.70 s. Finally, Fig. 4f and g reveal distinct profiles between the 0/π and π/2π periods, attributed to slight misalignments in magnet positioning during the latter half of each rotation. To provide a more complete description of pillar motion, we point out that the pillars exhibit slight displacements in the lateral y-direction during the recovery stroke, indicating limited out-of-plane motion. ESI† S5 presents a comparison of the displacements, xPillar(t) and yPillar(t) over several periods of magnet rotation along with the corresponding velocity profiles. Notably, the peak velocity along the y-axis reaches approximately 3% of the maximum value observed along the x-axis (0.6 versus 20 mm s−1). In Fig. 4h, we examine the velocity spikes associated with the effective stroke and its propagation along a row of pillars, shown here for a sequence of five pillars. The pillars, spaced 212 μm apart in the chosen configuration, exhibit a noticeable time shift of the velocity spikes, indicating a phase difference between their motions. This observation suggests the occurrence of a progressive wave in the pillar beating along the x-axis.56 By plotting the temporal phase shift against the pillar positions (inset), we obtain a linear relationship, where the slope corresponds to the inverse phase velocity Vϕ = 48 mm s−1, in good agreement with the magnet rotation speed. The progressive wave is also characterized by a wave vector k = 0.73 mm−1 and a wavelength λ = 8.6 mm, which can serve as metrics for evaluating the efficiency of pillared arrays or facilitating comparisons between devices.56 To conclude this section, we have shown that in a closed-loop microfluidic chip with magnetic pillars standing on the bottom, a progressive wave in pillar beating is induced, exhibiting characteristics similar to the ciliary beat observed in certain organisms and organs.
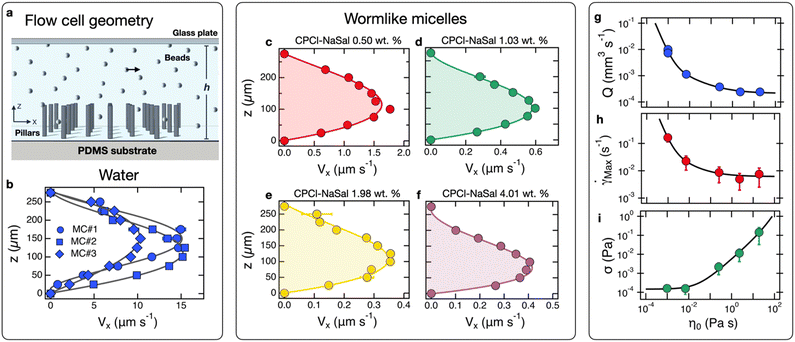
Fig. 5b presents the flow profile of water at 21 °C at the center of the observation zone. The experiment was performed using three different microchips, noted MC#1, MC#2 and MC#3 to evaluate the performance variability arising from fabrication. In all three cases, the maximum velocity VMax reaches values on the order of 10–15 μm s−1, corresponding to Reynolds numbers Re ∼10−3. The velocity maxima are located around 125–150 μm, i.e. roughly at the microchannel midplane. Regarding VMax, we confirmed previous findings20,39 that the maximum flow velocity is proportional to the magnet angular frequency ω, and found VMax(ω) = 0.84ω, where VMax is expressed in μm s−1. The flow profile in Fig. 5b resembles Poiseuille flow in a Hele–Shaw channel but deviates slightly from the theoretical expression57Vx(z) ∼ z(h − z). Finally, it is worth noting that this flow differs from that found by Shields et al., for which the maximum was located at the pillar tips, and decreased above.19 Secondly, we studied velocity profiles in cetylpyridinium chloride (CPCl) and sodium salicylate (NaSal) viscoelastic wormlike micellar solutions (Fig. 5c–f). In the semi-dilute concentration regime (cCPCl–NaSal > 0.3 wt%) these micelles form an entangled network58 akin to that observed in polymer solutions or in the mucin glycoprotein hydrogel.3,59 For CPCl–NaSal concentrations between 0.5 and 4 wt%, the static viscosity η0 increases from 0.007 to 20 Pa s and the elastic moduli G0 from 0.2 to 20 Pa (Table 1), covering the typical viscosity and elasticity ranges of pulmonary mucus.60–62 In particular, CPCl–NaSal solutions at 1.03 and 1.98 wt% yield moduli comparable to those of human mucus.62
| C CPCl–NaSal | η 0 | G 0 | τ | |
|---|---|---|---|---|
| wt% | Pa s | Pa | s | |
| Water | 0 | 0.00098 | n.d. | n.d. |
| CPCl–NaSal | 0.50 | 0.007 | 0.17 | 0.04 |
| CPCl–NaSal | 1.03 | 0.25 | 1.5 | 0.17 |
| CPCl–NaSal | 1.98 | 2.3 | 7 | 0.34 |
| CPCl–NaSal | 4.01 | 19.1 | 29 | 0.71 |
The velocity profiles obtained with microchannel MC#2 exhibit a Poiseuille-like flow, similar to that of water, with velocity maxima slightly off-center. As surfactant concentration—and consequently viscosity—increases, maximum velocities decrease sharply. To quantify this effect, we have plotted in Fig. 5g–i the flow rate Q, the maximum shear rate ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) Max and shear stress σMax = η0
Max and shear stress σMax = η0![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) Max generated by pillar actuation as a function of η0, respectively. For both Q and
Max generated by pillar actuation as a function of η0, respectively. For both Q and ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) Max, a sharp decrease is observed at low viscosity, followed by a plateau for η0 > 1 Pa s. Conversely, σMax is found to be stable at low viscosity and then increases linearly with η0. The derived
Max, a sharp decrease is observed at low viscosity, followed by a plateau for η0 > 1 Pa s. Conversely, σMax is found to be stable at low viscosity and then increases linearly with η0. The derived ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) Max for wormlike micelles are notably small, on the order of 10−2 s−1, leading to a Weissenberg number Wi =
Max for wormlike micelles are notably small, on the order of 10−2 s−1, leading to a Weissenberg number Wi = ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) Maxτ ≪ 1, where τ is the micellar network relaxation time (Table 1).58,63,64 In rheology, particularly for viscoelastic liquids, the inequality Wi ≪ 1 unequivocally indicates that the flow occurs in the Newtonian regime, where viscosity versus shear rate remains constant.64 This suggests that, on average, the flow of surfactant solutions remains that of a Newtonian fluid across the chip height.
Maxτ ≪ 1, where τ is the micellar network relaxation time (Table 1).58,63,64 In rheology, particularly for viscoelastic liquids, the inequality Wi ≪ 1 unequivocally indicates that the flow occurs in the Newtonian regime, where viscosity versus shear rate remains constant.64 This suggests that, on average, the flow of surfactant solutions remains that of a Newtonian fluid across the chip height.
We also observed that pillar beating remains unaffected by fluid viscosity; for viscosities ranging from 0.001 to 20 Pa s, the displacement xPillar(t) and velocity dxPillar(t)/dt profiles are consistent with those shown in Fig. 4f and g. Locally however, particularly near the pillars, velocities can reach up to 20 mm s−1 during their effective stroke, corresponding to angular frequencies of ωPillar ∼ 270 rad s−1. Under these conditions, the product ωPillarτ is much larger than one, demonstrating that during this short period, surfactant solutions behave as a purely elastic material. We propose that the transition in Q(η0), ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) Max(η0) and σMax(η0) observed between the low and high viscosity regions arise from the elasticity of the micellar network. These findings will need to be validated using data from mucus gel samples.
Max(η0) and σMax(η0) observed between the low and high viscosity regions arise from the elasticity of the micellar network. These findings will need to be validated using data from mucus gel samples.
3 Conclusion
This study presents the development and characterization of magnetically actuated flexible PDMS pillar arrays as a tool for microfluidic flow generation and analysis. Fabricated using soft lithography, the pillars were embedded with iron carbonyl microparticles, allowing for precise magnetic responsiveness. Structural characterization, including SEM and EDX analysis, revealed uniform FeMP distribution, predominantly localized in the upper half of the pillars, a key factor in achieving controlled bending behavior. Quantitative magnetic and mechanical analyses demonstrated the critical contributions of both magnetic force and torque in pillar deflection, as confirmed through experiments and finite element simulations. The integration of these pillars into a closed-loop microchannel enabled effective flow actuation in low Reynolds number conditions, mimicking biological ciliary motion. Through systematic flow measurements, the study demonstrated consistent Poiseuille-like flow profiles in Newtonian fluids and explored the impact of increasing fluid viscosity and viscoelasticity, achieving stable actuation across a wide viscosity range. Key findings include the scaling of maximum shear stress with fluid viscosity and the emergence of plateau behaviors in flow rate and shear rate, indicating a regime transition between viscous and viscoelastic flows. The observed flow patterns, combined with the controllability and versatility of this platform, highlight its potential for mimicking pulmonary mucus clearance. Future work may focus on refining pillar designs, expanding flow applications, and integrating this platform with real-time sensing technologies for more advanced biological and industrial applications.4 Materials and methods
4.1 Materials
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 base-to-crosslinker ratio). The PDMS counter-mold was baked at 150 °C for 60 min to ensure complete crosslinking.
1 base-to-crosslinker ratio). The PDMS counter-mold was baked at 150 °C for 60 min to ensure complete crosslinking.
Magnetic pillars were fabricated first by filling the pillar wells with FeMPs.42 The design consisted of a 1 × 1 cm square containing an array of pillars with diameters of 20 μm, arranged on square pitches of 66 or 150 μm, and reaching a height of 75 μm. A 50 wt% solution of FeMP in ethanol was pipetted over the counter-mold at a coverage of 2 μL per square millimeter of the pillar array. This FeMP slurry was then rubbed into the mounter-mold surface containing pillar wells. The excess ethanol was removed by placing the counter-mold in a vacuum chamber, leaving only dry FeMPs. The excess FeMP on the counter-mold surface was removed using microfiber damp with ethanol. The filling process described above was repeated 5 times. The subsequent steps for fabricating the array follow the same procedure outlined earlier for filling the counter-mold with PDMS.
For the microchannel design, the first layer consisted of both the microchannel walls as well as a 4 × 6 mm pillar array with circles measuring 20 μm in diameter, spaced 150 μm apart on a square lattice (Fig. 4a). The first layer was laminated with a 25 μm photoresist layer, followed by a 50 μm layer before UV exposure (leading to lPillar = 75 μm), while the second layer consisted solely of the walls. Two additional 100 μm photoresist layers were laminated and exposed to UV solely on the microchannel walls, resulting in a total height of 275 μm. Care was taken to align the two layers of the design using the maskless lithography system. The pillar array is positioned 7.5 mm from the center of the channel loop, opposite to a pillar free observation area and linked by a fluidic canal. To actuate the magnetic pillars, two neodymium magnets were positioned beneath the microchannel, as illustrated in Fig. 4b. These magnets were mounted on a custom stand connected to an electric motor, which enabled controlled rotation in either direction at frequencies ν ranging from 0 to 2.8 Hz. Their alignment ensured that the pillars alternated between a strong and weak magnetic field during rotation. The entire setup was mounted on an upright optical microscope with a 10× objective to facilitate real-time observation of pillar movement. Flow profiles measurements in water were conducted using three different microchips, labeled MC#1, MC#2, and MC#3, to assess performance variability due to fabrication.
4.2 Methods
| FMag = (M·∇)B | (1) |
 | (2) |
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Conceptualization: JF, JFB, NT, SL – methodology: CPM, JF, JFB, MLG, SR – software: CPM – formal analysis: CPM, JF, JFB, MLG, SR – investigation: CPM, JF, JFB, MLG, SR – supervision: JF, JFB, SL – writing – original draft: CPM, JF, JFB – writing – review and editing: JFB – funding acquisition: JF, JFB, NT, SL.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the ImagoSeine facility (Jacques Monod Institute, Paris, France), and the France BioImaging infrastructure supported by the French National Research Agency (ANR-10-INSB-04, “Investments for the future”). We also thank the ITODYS SEM facility (Université Paris Cité, CNRS UMR 7086, Paris, France) and Dr Sarra Derouich for her help with the SEM and EDX analyses. David Montero from the IPM SEM facility (Sorbonne Université, FR2482, Paris, France) is also acknowledged for help with the secondary SEM analysis. We thank David Hrabovsky from the MPBT platform (Sorbonne University, FR 2769, Paris, France) for VSM measurements. We would like to acknowledge the work of Frederik Gélébart from the PHENIX laboratory (Sorbonne Université, UMR 8232, Paris France) for designing and assembling the motorized magnetic axis which was affixed to our microscope. This research was supported in by the Agence Nationale de la Recherche under the contract ANR-17-CE09-0017 (AlveolusMimics) and ANR-21-CE19-0058-1 (MucOnChip).References
- B. C. Huck, X. Murgia, S. Frisch, M. Hittinger, A. Hidalgo, B. Loretz and C.-M. Lehr, Adv. Drug Delivery Rev., 2022, 183, 114141 CrossRef CAS PubMed.
- L. E. Wallace, M. Liu, F. J. M. Van Kuppeveld, E. De Vries and C. A. M. De Haan, Trends Microbiol., 2021, 29, 983–992 CrossRef CAS PubMed.
- R. Bansil and B. S. Turner, Adv. Drug Delivery Rev., 2018, 124, 3–15 CrossRef CAS PubMed.
- X. M. Bustamante-Marin and L. E. Ostrowski, Cold Spring Harbor Perspect. Biol., 2017, 9, a028241 CrossRef PubMed.
- S. A. Halbert, P. Y. Tam and R. J. Blandau, Science, 1976, 191, 1052–1053 CrossRef CAS PubMed.
- K. Inaba, Mol. Hum. Reprod., 2011, 17, 524–538 CrossRef CAS PubMed.
- R. Jeanneret, V. Kantsler and M. Polin, Nat. Commun., 2016, 7, 12518 CrossRef CAS PubMed.
- H. Riisgård and P. Larsen, Mar. Ecol.: Prog. Ser., 2010, 418, 255–293 CrossRef.
- A. Sironen, A. Shoemark, M. Patel, M. R. Loebinger and H. M. Mitchison, Cell. Mol. Life Sci., 2020, 77, 2029–2048 CrossRef CAS PubMed.
- J. Y. Lock, T. L. Carlson and R. L. Carrier, Adv. Drug Delivery Rev., 2018, 124, 34–49 CrossRef CAS PubMed.
- G. A. Duncan, J. Jung, J. Hanes and J. S. Suk, Mol. Ther., 2016, 24, 2043–2053 CrossRef CAS PubMed.
- J. V. Fahy and B. F. Dickey, N. Engl. J. Med., 2010, 363, 2233–2247 CrossRef CAS PubMed.
- L. J. Kricka, Clin. Chim. Acta, 2001, 307, 219–223 CrossRef CAS PubMed.
- B. Mohelnikova-Duchonova, World J. Gastroenterol., 2014, 20, 10316 CrossRef CAS PubMed.
- M.-K. Khelloufi, E. Loiseau, M. Jaeger, N. Molinari, P. Chanez, D. Gras and A. Viallat, Sci. Rep., 2018, 8, 2447 CrossRef PubMed.
- S. Gsell, E. Loiseau, U. D'Ortona, A. Viallat and J. Favier, Sci. Rep., 2020, 10, 8405 CrossRef CAS PubMed.
- G. Lacroix, W. Koch, D. Ritter, A. C. Gutleb, S. T. Larsen, T. Loret, F. Zanetti, S. Constant, S. Chortarea, B. Rothen-Rutishauser, P. S. Hiemstra, E. Frejafon, P. Hubert, L. Gribaldo, P. Kearns, J.-M. Aublant, S. Diabate, C. Weiss, A. de Groot and I. Kooter, Appl. In Vitro Toxicol., 2018, 4, 91–106 CrossRef PubMed.
- K. B. Sivars, U. Sivars, E. Hornberg, H. Zhang, L. Brändén, R. Bonfante, S. Huang, S. Constant, I. Robinson, C. J. Betts and P. M. Åberg, Toxicol. Sci., 2018, 162, 301–308 CrossRef CAS PubMed.
- A. R. Shields, B. L. Fiser, B. A. Evans, M. R. Falvo, S. Washburn and R. Superfine, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 15670–15675 CrossRef CAS PubMed.
- S. Zhang, Z. Cui, Y. Wang and J. M. J. den Toonder, Lab Chip, 2020, 20, 3569–3581 RSC.
- H. Gu, Q. Boehler, H. Cui, E. Secchi, G. Savorana, C. De Marco, S. Gervasoni, Q. Peyron, T.-Y. Huang, S. Pane, A. M. Hirt, D. Ahmed and B. J. Nelson, Nat. Commun., 2020, 11, 2637 CrossRef CAS PubMed.
- F. Tsumori, R. Marume, A. Saijou, K. Kudo, T. Osada and H. Miura, Jpn. J. Appl. Phys., 2016, 55, 06GP19 CrossRef.
- B. Zhou, W. Xu, A. A. Syed, Y. Chau, L. Chen, B. Chew, O. Yassine, X. Wu, Y. Gao, J. Zhang, X. Xiao, J. Kosel, X.-X. Zhang, Z. Yao and W. Wen, Lab Chip, 2015, 15, 2125–2132 RSC.
- S. Zhang, X. Hu, M. Li, U. Bozuyuk, R. Zhang, E. Suadiye, J. Han, F. Wang, P. Onck and M. Sitti, Sci. Adv., 2023, 9, eadf9462 CrossRef CAS PubMed.
- A. E. Tilley, M. S. Walters, R. Shaykhiev and R. G. Crystal, Annu. Rev. Physiol., 2015, 77, 379–406 CrossRef CAS PubMed.
- W. M. Foster, E. Langenback and E. H. Bergofsky, J. Appl. Physiol., 1980, 48, 965–971 CrossRef CAS PubMed.
- T. u. Islam, Y. Wang, I. Aggarwal, Z. W. Cui, H. E. Amirabadi, H. Garg, R. Kooi, B. B. Venkataramanachar, T. S. Wang, S. Z. Zhang, P. R. Onck and J. M. J. den Toonder, Lab Chip, 2022, 22, 1650–1679 RSC.
- Z. Luo, X. A. Zhang, B. A. Evans and C.-H. Chang, ACS Appl. Mater. Interfaces, 2020, 12, 11135–11143 CrossRef CAS PubMed.
- O. du Roure, A. Saez, A. Buguin, R. H. Austin, P. Chavrier, P. Silberzan and B. Ladoux, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 2390–2395 CrossRef CAS PubMed.
- J. Bico, É. Reyssat and B. Roman, Annu. Rev. Fluid Mech., 2018, 50, 629–659 CrossRef.
- A. Lafuma and D. Quere, Nat. Mater., 2003, 2, 457–460 CrossRef CAS PubMed.
- H. Becker and L. E. Locascio, Talanta, 2002, 56, 267–287 CrossRef CAS PubMed.
- J. le Digabel, N. Biais, J. Fresnais, J.-F. Berret, P. Hersen and B. Ladoux, Lab Chip, 2011, 11, 2630–2636 RSC.
- T. u. Islam, Y. Bellouard and J. M. J. den Toonder, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2104930118 CrossRef CAS PubMed.
- B. A. Evans, A. R. Shields, R. L. Carroll, S. Washburn, M. R. Falvo and R. Superfine, Nano Lett., 2007, 7, 1428–1434 CrossRef CAS PubMed.
- B. L. Fiser, A. R. Shields, M. R. Falvo and R. Superfine, J. Micromech. Microeng., 2015, 25, 025004 CrossRef CAS PubMed.
- R. M. Judith, B. Lanham, M. R. Falvo and R. Superfine, PLoS One, 2018, 13, e0200345 CrossRef PubMed.
- T. Wang, T. ul Islam, E. Steur, T. Homan, I. Aggarwal, P. R. Onck, J. M. J. den Toonder and Y. Wang, Lab Chip, 2024, 24, 1573–1585 RSC.
- Z. Cui, Y. Wang, S. Zhang, T. Wang and J. M. J. d. Toonder, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2304519120 CrossRef CAS PubMed.
- Z. Wang, K. Wang, D. Liang, L. Yan, K. Ni, H. Huang, B. Li, Z. Guo, J. Wang, X. Ma, X. Tang and L.-Q. Chen, Adv. Mater., 2020, 32, 2001879 CrossRef CAS PubMed.
- W. Jiang, L. Wang, B. Chen and H. Liu, J. Micromech. Microeng., 2023, 33, 015003 CrossRef CAS.
- B. Bolteau, F. Gelebart, J. Teisseire, E. Barthel and J. Fresnais, ACS Appl. Mater. Interfaces, 2023, 15, 35674–35683 CrossRef CAS PubMed.
- M. R. Clary, S. N. Cantu, J. A.-C. Liu, E. E. Evans and J. B. Tracy, Adv. Mater. Technol., 2024, 9, 2302243 CrossRef CAS.
- A. Grein-Iankovski, A. Graillot, M. Radiom, W. Loh and J.-F. Berret, J. Phys. Chem. C, 2020, 124, 26068–26075 CrossRef CAS.
- S. Zhang, Y. Wang, R. Lavrijsen, P. R. Onck and J. M. J. den Toonder, Sens. Actuators B: Chem., 2018, 263, 614–624 CrossRef CAS.
- M. Vilfan, A. Potocnik, B. Kavcic, N. Osterman, I. Poberaj, A. Vilfan and D. Babic, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 1844–1847 CrossRef CAS PubMed.
- D. Qin, Y. Xia and G. M. Whitesides, Nat. Protoc., 2010, 5, 491–502 CrossRef CAS PubMed.
- B. Bolteau, K. Dos Santos, F. Gelebart, J. Gomes, J. Teisseire, E. Barthel and J. Fresnais, Langmuir, 2019, 35, 9133–9138 CrossRef CAS PubMed.
- A. Saberi, S. Zhang, C. van den Bersselaar, H. Kandail, J. M. J. den Toonder and N. A. Kurniawan, Soft Matter, 2019, 15, 1435–1443 RSC.
- X. M. Yang and Z. W. Zhong, J. Appl. Polym. Sci., 2021, 138, 50460 CrossRef CAS.
- A. Galimard, M. Safi, N. Ould-Moussa, D. Montero, H. Conjeaud and J. F. Berret, Small, 2012, 8, 2036–2044 CrossRef CAS PubMed.
- V. Torrisi, A. Graillot, L. Vitorazi, Q. Crouzet, G. Marletta, C. Loubat and J.-F. Berret, Biomacromolecules, 2014, 15, 3171–3179 CrossRef CAS PubMed.
- COMSOL Multiphysics® v. 6.2. COMSOL AB, Stockholm, Sweden, 2019 Search PubMed.
- T. K. Kim, J. K. Kim and O. C. Jeong, Microelectron. Eng., 2011, 88, 1982–1985 CrossRef CAS.
- H. Varner and T. Cohen, Soft Matter, 2024, 20, 9174–9183 RSC.
- M. Bottier, M. Peña Fernández, G. Pelle, D. Isabey, B. Louis, J. B. Grotberg and M. Filoche, PLoS Comput. Biol., 2017, 13, e1005552 CrossRef PubMed.
- E. Guyon, J.-P. Hulin, L. Petit and C. D. Mitescu, Physical Hydrodynamics, Oxford University Press, 2015 Search PubMed.
- J.-F. Berret, Molecular Gels: Materials with Self-Assembled Fibrillar Networks, 2006, pp. 667–720 Search PubMed.
- J. Carpenter, Y. Wang, R. Gupta, Y. Li, P. Haridass, D. B. Subramani, B. Reidel, L. Morton, C. Ridley, W. K. O'Neal, M.-P. Buisine, C. Ehre, D. J. Thornton and M. Kesimer, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2104490118 CrossRef CAS PubMed.
- S. K. Lai, Y.-Y. Wang, D. Wirtz and J. Hanes, Adv. Drug Delivery Rev., 2009, 61, 86–100 CrossRef CAS PubMed.
- C. E. Wagner, B. S. Turner, M. Rubinstein, G. H. McKinley and K. Ribbeck, Biomacromolecules, 2017, 18, 3654–3664 CrossRef CAS PubMed.
- M. Radiom, R. Hénault, S. Mani, A. G. Iankovski, X. Norel and J.-F. Berret, Soft Matter, 2021, 17, 7585–7595 RSC.
- J. F. Berret, J. Appell and G. Porte, Langmuir, 1993, 9, 2851–2854 CrossRef CAS.
- R. G. Larson, The Structure and Rheology of Complex Fluids, Oxford University Press, New York, 1998 Search PubMed.
- H. Rehage and H. Hoffmann, J. Phys. Chem., 1988, 92, 4712–4719 CrossRef CAS.
- J.-F. Berret and A. Graillot, Langmuir, 2022, 38, 5323–5338 CrossRef CAS PubMed.
- S. P. Timoshenko and S. Woinowsky-Krieger, Theory of Plates and Shells, New York, 2nd edn, 1959 Search PubMed.
- A. D. Shine and R. C. Armstrong, Rheol. Acta, 1987, 26, 152–161 CrossRef CAS.
- M. Sato and Y. Ishii, J. Appl. Phys., 1989, 66, 983–985 CrossRef.
- F. Mousseau and J.-F. Berret, Soft Matter, 2018, 14, 5764–5774 RSC.
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, Nat. Methods, 2012, 9, 671–675 CrossRef CAS PubMed.
- J. Y. Tinevez, N. Perry, J. Schindelin, G. M. Hoopes, G. D. Reynolds, E. Laplantine, S. Y. Bednarek, S. L. Shorte and K. W. Eliceiri, Methods, 2017, 115, 80–90 CrossRef CAS PubMed.
- M. Dessard, J.-B. Manneville and J.-F. Berret, Nanoscale Adv., 2024, 6, 1727–1738 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Size distribution of the iron carbonyl microparticles (S1) – elemental maps and fractions of carbon, silicon, iron and oxygen obtained from energy-dispersive X-ray (EDX) analysis (S2) – iron carbonyl microparticle (FeMPs) powder magnetization curve (S3) – wide-angle X-ray scattering (WAXS) on FeMP iron microparticles (S4) – comparison of pillar motion along the x- and y-direction (S5) – bead motion shows pulsatile pattern as a function of time (S6) – image of magnetic micropillar arrays in binocular microscopy (S7) – magnetic field measurements under translational motion: experiments and simulations (S8) – detailed simulation procedure (S9). See DOI: https://doi.org/10.1039/d5lc00035a |
| This journal is © The Royal Society of Chemistry 2025 |