Sequential paired electrolysis-enabled synthesis of antifungal-active gem-difluoroalkenes via electrochemical halogen atom transfer
Fuyang
Yue
,
Jiayi
Li
,
Hongjian
Song
 ,
Yuxiu
Liu
,
Yuxiu
Liu
 and
Qingmin
Wang
and
Qingmin
Wang
 *
*
State Key Laboratory of Elemento-Organic Chemistry, Research Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin 300071, People's Republic of China. E-mail: wangqm@nankai.edu.cn
First published on 24th October 2025
Abstract
Fluorine-containing compounds, particularly gem-difluoroalkenes—recognized as bioisosteres of carbonyl groups—hold crucial value in pharmaceuticals, agrochemicals, and functional materials, as they can modulate drug molecules’ lipophilicity, pKa, and bioavailability. Alkyl halides, indispensable building blocks in organic synthesis, serve as alkyl radical precursors; however, generating alkyl radicals from unactivated alkyl halides typically relies on costly photocatalysts, posing a notable challenge. This work presents a practical electrochemical method for the deiodinative gem-difluorovinylation of unactivated primary, secondary, and tertiary alkyl iodides. Operating under mild, transition-metal-free, and sacrificial-anode-free conditions, it integrates electrochemical halogen-atom transfer (e-XAT) with convergent paired electrolysis. The method exhibits broad substrate applicability and excellent compatibility with complex functional groups, enabling late-stage diversification of natural products, biomolecules, and pharmaceuticals. Additionally, glycoside-derived halides were used to synthesize gem-difluoroalkene derivatives; their antifungal activity was evaluated, with several active compounds identified, and preliminary mechanistic studies were conducted.
Green foundation1. This work advances green chemistry via a mild, transition-metal/sacrificial-anode-free electrochemical method, using electricity as a traceless reagent, avoiding costly photocatalysts and toxic waste, and enabling late-stage diversification to cut synthetic steps.2. As the electrochemical means is used, the use of oxidants and metal reagents is avoided. Paired electrolysis prevents the sacrifice of electrodes. 3. The adoption of renewable electricity, and the development of recyclable electrolytes reduce VOCs, carbon footprint, and waste could be investigated to further green the method. |
Introduction
Fluorine-bearing compounds hold significant importance in the realms of pharmaceuticals, agrochemicals, and functional materials. They are capable of modulating the lipophilicity, pKa, conformation, and bioavailability of drug molecules.1 Specifically, gem-difluoroalkenes, often considered as bioisosteres of carbonyl groups, have attracted substantial attention during the drug discovery endeavor.2 For instance, when the carbonyl group in artemisinin is replaced with a difluoroalkene group, the resulting compound exhibits antimalarial activity nearly double that of the original artemisinin2 (as depicted in Fig. 1A). Additionally, the gem-difluoroalkene moiety serves as a valuable precursor. It can be readily converted into monofluoroalkene, difluoromethylene, difluorocyclopropane, and trifluoromethyl groups.3 Given the wide-ranging applications of these compounds, there is an urgent need for effective synthetic methods to obtain highly functionalized gem-difluoroalkenes with diverse structures.Alkyl halides are indispensable building blocks/reagents in organic synthesis and drug discovery, thanks to their structural diversity and easy synthetic access. Recently, they have served as alkyl radical precursors in radical-mediated transformations.4 However, generating alkyl radicals from unactivated alkyl halides is challenging, mainly due to these halides’ extremely low reduction potentials (Ered < −2.0 V vs. SCE). Current alkyl radical generation methods fall into two mechanistically distinct strategies: single-electron transfer (SET) and halogen-atom transfer (XAT). The SET approach is highly reliable in transition-metal catalysis5 and photoredox systems6 (Fig. 1B)—for example, MacMillan recently reported that metallaphotoredox-activated transient silicon radicals facilitate alkyl radical generation from alkyl halides.7 Most recently, Leonori and Juliá developed a method using nucleophilic α-aminoalkyl radicals (easily derived from simple amines in photoredox systems) as XAT agents, which drive homolytic C–halogen bond activation for alkyl radical formation under mild conditions.8 However, all these strategies rely on costly photocatalysts (PC) to generate XAT agents. Thus, simpler, milder, and more efficient approaches to activate unactivated alkyl halides are still in high demand.
As a green and adjustable tool, electrosynthesis has attracted increasing interest. By employing electricity as a traceless redox reagent, it enables precise modulation of electron-transfer processes, offering robust backing for the discovery of novel chemical transformations.9 In an undivided cell, a substrate can undergo sequential oxidation and reduction (or the reverse) at the corresponding electrode. Moreover, direct parallel paired electrolysis that involves a hydrogenation process to form the product (Fig. 1C) is commonly referred to as sequential paired electrolysis.10
Recently, progress has been made in the electrochemical halogen-atom transfer (e-XAT) strategy for the activation of unactivated alkyl iodides.11 Building on this, we propose a convergent paired electrolysis system that integrates e-XAT with selective anodic and cathodic processes to achieve deiodinative gem-difluorovinylation of alkyl halides (Fig. 1D). During the submission of our manuscript, the Zheng group12 reported a study that achieved the cross-coupling of alkyl radicals via the same halogen-atom transfer strategy and convergent paired electrolysis. Similar to their work, our method adopts a transition-metal-free electrochemical strategy, which eliminates the need for both sacrificial anodes and metal catalysts.
In this work, we report a sequential practical electrochemical method for the deiodinative gem-difluorovinylation of unactivated alkyl iodides (primary, secondary, and tertiary) via e-XAT and paired electrolysis under mild, transition-metal-free, and sacrificial-anode-free conditions. This method exhibits a broad substrate scope and excellent compatibility with complex functional groups, and has been further applied to the late-stage diversification of natural products, biomolecules, and pharmaceuticals. We selected glycoside-derived halides as starting materials to synthesize a series of gem-difluoroalkene derivatives. Given the novelty of their structures, we evaluated the antifungal activity of these compounds, identified several derivatives with promising activity, and conducted preliminary studies on their underlying mechanisms.
Results and discussion
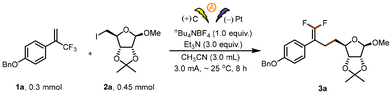
The feasibility study of our hypothesis was performed by selecting 1-(benzyloxy)-4-(3,3,3-trifluoroprop-1-en-2-yl)benzene 1a and (3aS,4S,6R,6aR)-4-(iodomethyl)-6-methoxy-2,2-dimethyl-tetrahydrofuro[3,4-d][1,3]dioxole 2a as model substrates to investigate the reaction (Table 1). After systematic screening of the reaction parameters, we found that in an undivided cell with a carbon plate anode and a Pt plate cathode, defluorinative alkylation of 1a for 8 h at a current of 3.0 mA occurred smoothly in an argon atmosphere at room temperature with tetrabutylammonium tetrafluoroborate as the electrolyte, Et3N (Et3N: Eox = +0.77 V vs. SCE) as the XAT-agent precursor and CH3CN as the solvent. Under these conditions, the desired product 3a was generated in 79% isolated yield (entry 1). Control experiments verified the significance of electricity, the XAT-agent precursor, and the undivided cell for this transformation. Specifically, when electricity was absent (entry 2), the XAT-agent precursor was missing (entry 3), or a divided cell was used instead of an undivided one (entry 4), the reaction either did not occur or was severely affected. Replacing tetrabutylammonium tetrafluoroborate with other electrolytes as the supporting electrolyte under otherwise identical reaction conditions gave lower yields (entries 5–7). Adding a small amount of water to the reaction system did not increase the yield of the reaction (entry 8). Further optimizations were conducted by screening a variety of reaction solvents, such as DMSO, DCM, and HFIP, but they all provided unsatisfactory results (entries 9–11, respectively). Other electrode materials were found to be less effective (entries 12 and 13).| Entry | Deviation from standard conditions | Yieldb (%) |
|---|---|---|
| a Reaction conditions, unless otherwise noted: in an undivided cell equipped with graphite plate electrodes, a solution of 1a (0.3 mmol, 1 equiv.), 2a (0.45 mmol, 1.5 equiv.), Et3N (0.9 mmol, 3.0 equiv.) and n-Bu4NBF4 (0.3 mmol, 1.0 equiv.) in CH3CN (3.0 mL) was electrolyzed at 3.0 mA with stirring for 8 h under argon at room temperature. b The yields were determined by 19F NMR spectroscopy with fluorobenzene as an internal standard, NR = no reaction. c Isolated yield. | ||
| 1 | None | 81 (79c) |
| 2 | No electricity | NR |
| 3 | No Et3N | NR |
| 4 | Divided cell | NR |
| 5 | n-Bu4NClO4 as the electrolyte | 72 |
| 6 | n-Bu4NPF6 as the electrolyte | 62 |
| 7 | Me4NBF4 as the electrolyte | 76 |
| 8 | H2O (0.2 mL) added | 56 |
| 9 | DMSO as the solvent | 37 |
| 10 | DCM as the solvent | 51 |
| 11 | HFIP as the solvent | 11 |
| 12 | RVC(+)–Zn(−) | 30 |
| 13 | RVC(+)–Pt(−) | 80 |
After confirmation of the optimized conditions, we turned our attention to the generality of the protocol (Fig. 2). Initially, α-trifluoromethyl arylalkenes bearing a wide range of functional groups were reacted with (3aS,4S,6R,6aR)-4-(iodomethyl)-6-methoxy-2,2-dimethyl-tetrahydrofuro[3,4-d][1,3]dioxole 2a (Fig. 2). Electron-donating groups linked to the phenyl ring were tolerated well to give good yields of the corresponding gem-difluoroalkenes 3b–3h (58–85%). However, when the para-substituents on the benzene ring were electron-withdrawing groups, the yields of the corresponding products (3i and 3j) were slightly lower compared to those with electron-donating groups. When the para-substituents on the benzene ring were aryl groups, products 3k–3n were obtained in moderate to good yields. The position of electron-donating substituents on the arylalkene has little effect on the yield; products 3o–3q were obtained in 62–80% yields. Disubstituted arylalkenes gave 3r–3y in moderate yields. Substrates containing functionalities useful for further synthetic manipulations, such as a dibenzothiophene (3z, 57%), a naphthalene ring (3aa, 79%) and a dimethylfluorene ring (3ab, 65%), were well tolerated. We further examined the generality of the proposed protocol by screening alkyl iodides and the results are summarized in Fig. 2. We tried many alkyl iodides with spirocyclic structures and some iodinated derivatives of drug molecules, and we found that products 3ac–3al (58–80%) could be obtained in moderate to good yields. Meanwhile, this reaction was also well compatible with some simple alkyl iodides (3am–3ao, 69–85%). In addition, we employed our mild defluorinative alkylation approach to carry out functionalization on certain drug molecules, pesticides, and natural products with complex structures. As a result, the corresponding products (3ap–3as) were acquired in yields ranging from good to excellent. Overall, the outcomes presented in Fig. 2 illustrate the reliability of our method.
To clarify the practicality of these transformations, the gram scale synthesis of product 3a was performed on a 4.0 mmol scale. The reaction proceeded smoothly to provide the desired product in 72% yield (Fig. 3a). Afterwards, control experiments were conducted to gain a more in-depth understanding of the reaction mechanism. When a radical scavenger such as TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl) or 1,1-diphenylethylene was introduced into the model reaction system, the reaction was significantly suppressed (Fig. 3b). Moreover, the captured products 4 and 5 were successfully detected using high-resolution mass spectrometry (HRMS).
Based on literature reports and our experimental observations, we propose the mechanism shown in Fig. 4. Initially, under standard electrochemical conditions, alkyl amine Et3N undergoes anodic oxidation and deprotonation to generate α-amino alkyl radical 6, which possesses strong nucleophilicity (Eox = −1.12 V vs. SCE). Subsequently, α-amino alkyl radical 6 facilitates the formation of the corresponding alkyl radicals 7via halogen-atom transfer with alkyl iodides. The exothermic dissociation of α-iodoamine into iminium iodide provides the thermodynamic energy necessary to drive the process. Next, alkyl radical 7 adds to the α-trifluoromethyl alkene to produce CF3-styrene carbon radical 8. This radical is then reduced at the cathode to form CF3-styrene carbanion intermediate 9, which subsequently undergoes a β-fluoride elimination reaction to afford the target product.
The creation of new agrochemicals hinges on the discovery of novel drug skeletons with unique structures and mechanisms of action. Chitin is an essential component of fungal cell walls, and chitin synthase is crucial for regulating these walls and cell proliferation. This makes it an ideal target for fungicides. Polyoxins are a class of nucleoside antifungal agents produced by Streptomycetes which target chitin synthase. Their core structure consists of uracil and polyoxanone. To expand the structural diversity of fungal chitinase inhibitors, compounds containing difluoroalkyl glycosides were screened for antifungal activity and mechanism of action studies.
Target compounds were screened for bioactivity against eight important pathogenic fungi including Alternaria solani, Botrytis cinerea, Cercospora arachidicola, Fusarium graminearum, Setosphaeria turcica, Pythium aphanidermatum, Rhizoctonia solani and Sclerotinia sclerotiorum at a concentration of 50 µg mL−1 and the results are shown in Table S2. Polyoxin and pyraclostrobin were selected as positive controls. Polyoxin as well as compounds 3 showed moderate inhibitory effects against pathogenic fungi such as C. arachidicola, F. graminearum, P. aphanidermatum, R. solani and S. sclerotiorum. Compounds 3a, 3b, 3d, 3j–3l, 3n, 3o, 3t–3v, 3aa–3ae, and 3ai–3aj showed more than 70.0% inhibition against B. cinerea, which was slightly higher than or comparable to that of polyoxin.
The inhibition rate of compound 3a against B. cinerea was 98.2%, higher than the rate of the commercial fungicide pyraclostrobin (94.3%). Additionally, compounds 3b and 3l exhibited comparable inhibition rates of 73.1% and 72.5% against S. turcica, respectively, to those of polyoxin (72.5%). Furthermore, EC50 assays were conducted on the highly active compounds (Table 2). Compound 3b exhibited moderate antifungal activity against S. turcica, with an EC50 value of 5.84 μg mL−1, which is higher than those of polyoxin (2.25 μg mL−1) and pyrazole (1.77 μg mL−1). In contrast, compound 3a displayed excellent antifungal activity against B. cinerea, with an EC50 value of 2.01 μg mL−1—superior to that of polyoxin (3.02 μg mL−1), but slightly less potent than pyraclostrobin (1.30 μg mL−1). Therefore, compound 3a was selected for mechanism of action studies.
| Fungi | Compd | Regression equation | R | 95% confidence interval (μg mL−1) | EC50 (μg mL−1) |
|---|---|---|---|---|---|
| Botrytis cinerea | 3a | y = 4.790 + 0.691x | 0.9963 | 1.65–2.46 | 2.01 |
| 3b | y = 4.134 + 0.947x | 0.9916 | 7.02–9.61 | 8.21 | |
| 3d | y = 4.077 + 1.163x | 0.9927 | 5.37–7.21 | 6.22 | |
| 3f | y = 4.064 + 1.191x | 0.9815 | 4.82–7.75 | 6.11 | |
| 3d | y = 4.077 + 1.163x | 0.9927 | 5.37–7.21 | 6.22 | |
| 3k | y = 3.137 + 1.336x | 0.9784 | 17.91–34.32 | 24.79 | |
| 3o | y = 3.597 + 1.1574x | 0.9862 | 12.71–20.89 | 16.29 | |
| 3t | y = 4.076 + 0.855x | 0.9915 | 10.09–14.37 | 12.04 | |
| 3u | y = 3.960 + 1.183x | 0.9883 | 6.27–9.15 | 7.57 | |
| 3v | y = 3.885 + 1.247x | 0.9639 | 5.58–11.01 | 7.84 | |
| 3ae | y = 4.351 + 1.286x | 0.9958 | 2.83–3.62 | 3.20 | |
| 3aj | y = 3.501 + 1.262x | 0.9941 | 13.39–17.69 | 15.39 | |
| Polyoxin | y = 3.784 + 2.417x | 0.9592 | 2.14–4.74 | 3.18 | |
| Exserohilum turcicum | 3b | y = 4.186 + 1.063x | 0.9827 | 4.64–7.34 | 5.84 |
| 3l | y = 3.510 + 1.308x | 0.9752 | 10.70–17.74 | 13.77 | |
| Polyoxin | y = 4.481 + 1.474x | 0.9886 | 1.91–2.65 | 2.25 |
The chitin synthase protein of B. cinerea was modelled using AlphaFold2. Molecular docking was then performed using AutoDock and the results were visualised. The molecular docking results showed that both compound 3a and polyoxins bind to the active pocket of chitin synthase, with respective binding energies of −9.6 and −10.8 kcal mol−1. As demonstrated in Fig. 5A and B, compound 3a and polyoxins both form hydrogen bond interactions with ARG1027. Molecular dynamics simulations were performed using GROMACS software to investigate the conformational stability and dynamic behaviour of the ligand–B. cinerea chitinase complex. The RMSD values were calculated using the 50 ns molecular dynamics simulation. Fig. 5C shows that the protein RMSD in the compound 3a complex increases rapidly within the first 5 ns and then stabilises at around 1.0 Å, whereas the protein RMSD in the polyoxin complex remains consistently at around 0.6 Å with minimal fluctuations. This indicates that the polyoxin complex exhibits superior conformational stability. Fig. 5D shows that the ligand RMSD of compound 3a fluctuates between 0.6 and 1.0 Å, indicating high conformational flexibility and less stable binding than polyoxins. While compound 3a forms a relatively stable protein–ligand complex, significant conformational fluctuations during binding may impact its binding affinity and biological activity.
Subsequently, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were employed to examine the morphological and ultrastructural alterations in the B. cinerea mycelium following treatment with compound 3a. SEM images (Fig. 6A and B) revealed that the hyphae in the control group exhibited a smooth surface and intact morphology. By contrast, significant surface damage was evident in the hyphae treated with compound 3a, including shrinkage, wrinkling, and collapse of the hyphal structure. This indicated that the integrity of the cell wall had been compromised. TEM results showed that the control cells exhibited well-defined intracellular structures, including intact cell walls (CW), clear cytoplasmic boundaries, and uniformly distributed organelles (Fig. 6C). In contrast, fungal cells treated with compound 3a displayed thinned cell walls, plasmolysis, cytoplasmic vacuolisation, and organelle disintegration (Fig. 6D). These structural changes suggested that compound 3a disrupted the integrity of the fungal cell wall. Docking results showed that compound 3a interacted with the active site of chitin synthase. These findings indicate that its antifungal activity may be mediated by chitin synthase inhibition, thereby impairing chitin biosynthesis and compromising the cell wall.
Conclusions
This study developed a green electrochemical method for deiodinative gem-difluorovinylation of unactivated alkyl iodides via e-XAT and convergent paired electrolysis, under mild, transition-metal-free, sacrificial-anode-free conditions, solving the problem of activating such alkyl halides without costly photocatalysts. It has a broad substrate scope, enabling late-stage diversification of natural products and drugs, with gram-scale synthesis feasible. Glycoside-derived gem-difluoroalkene derivatives showed good antifungal activity, e.g., 3a against B. cinerea (EC50 = 2.01 μg mL−1) outperforming polyoxin. Mechanistic studies confirmed radical pathways and that 3a inhibited chitin synthase. This work aids gem-difluoroalkene synthesis and new antifungal development.Author contributions
F. Y. conceived the chemistry and designed the experiments under the guidance of Professor Q. W. The experiments and data analysis were conducted by all authors. F. Y. wrote the manuscript. All authors gave approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Date availability
All relevant data are presented in the article and its supplementary information (SI). Supplementary information: general comments, general procedures, optimization details and NMR spectra. See DOI: https://doi.org/10.1039/d5gc05189d.Acknowledgements
We are grateful to the National Natural Science Foundation of China (22271166) and Tianjin Science and Technology Plan Project Technology Innovation Guidance Special Fund (25YDTPJC00600) for generous financial support for our programs.References
- (a) Y. Pan, J. Qiu and R. B. Silverman, J. Med. Chem., 2003, 46, 5292 CrossRef CAS PubMed; (b) P. Jeschke, ChemBioChem, 2004, 5, 570 CrossRef CAS PubMed; (c) Y. Ogawa, E. Tokunaga, O. Kobayashi, K. Hirai and N. Shibata, iScience, 2020, 23, 101467 CrossRef CAS PubMed; (d) Q. Wang, H. Song and Q. Wang, Chin. Chem. Lett., 2022, 33, 626–642 CrossRef CAS; (e) S. Purser, R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC.
- (a) W. R. Moore, G. L. Schatzman, E. T. Jarvi, R. S. Gross and J. R. McCarthy, J. Am. Chem. Soc., 1992, 114, 360 CrossRef CAS; (b) P. Jeschke, Pest Manage. Sci., 2010, 66, 10 CrossRef CAS PubMed; (c) F. Yue, H. Ma, P. Ding, H. Song, Y. Liu and Q. Wang, ACS Cent. Sci., 2023, 9, 2268–2276 CrossRef CAS PubMed.
- (a) J. Wu, S. Zhang, H. Gao, Z. Qi, C. Zhou, W. Ji, Y. Liu, Y. Chen, Q. Li, X. Li and H. Wang, J. Am. Chem. Soc., 2017, 139, 3537 CrossRef CAS PubMed; (b) J. Liu, J. Yang, F. Ferretti, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2019, 58, 4690 CrossRef CAS PubMed; (c) T. Fujita, K. Fuchibe and J. Ichikawa, Angew. Chem., Int. Ed., 2019, 58, 390 CrossRef CAS PubMed; (d) F. Tian, G. Yan and J. Yu, Chem. Commun., 2019, 55, 13486 RSC; (e) F. Yue, J. Liu, H. Ma, Y. Liu, J. Dong and Q. Wang, Org. Lett., 2022, 24, 4019–4023 CrossRef CAS; (f) W. Tang and P. Fan, Org. Lett., 2023, 25, 5756 CrossRef CAS PubMed; (g) F. Yue, M. Li, K. Yang, H. Song, Y. Liu and Q. Wang, Chem. Sci., 2024, 15, 14241–14247 RSC.
- (a) G. C. Fu, ACS Cent. Sci., 2017, 3, 692–700 CrossRef CAS PubMed; (b) D. J. Weix, Acc. Chem. Res., 2015, 48, 1767–1775 CrossRef CAS; (c) M. R. Kwiatkowski and E. J. Alexanian, Acc. Chem. Res., 2019, 52, 1134–1144 CrossRef CAS PubMed; (d) P. Chuentragool, D. Kurandina and V. Gevorgyan, Angew. Chem., Int. Ed., 2019, 58, 11586–11598 CrossRef CAS PubMed; (e) F. Yue, M. Li, F. Yuan, H. Song, Y. Liu and Q. Wang, Chin. Chem. Lett., 2025, 36, 111053 CrossRef CAS.
- (a) H. Chen, X. Jia, Y. Yu, Q. Qian and H. Gong, Angew. Chem., Int. Ed., 2017, 56, 13103–13106 CrossRef CAS PubMed; (b) F. Zhou, J. Zhu, Y. Zhang and S. Zhu, Angew. Chem., Int. Ed., 2018, 57, 4058–4062 CrossRef CAS PubMed; (c) S. Bera, R. Mao and X. Hu, Nat. Chem., 2021, 13, 270–277 CrossRef CAS; (d) D. Qian, S. Bera and X. Hu, J. Am. Chem. Soc., 2021, 143, 1959–1967 CrossRef CAS PubMed; (e) X. Wu, W. Hao, K. Ye, B. Jiang, G. Pombar, Z. Song and S. Lin, J. Am. Chem. Soc., 2018, 140, 14836–14843 CrossRef CAS PubMed; (f) A. Cai, W. Yan, C. Wang and W. Liu, Angew. Chem., Int. Ed., 2021, 60, 27070–27077 CrossRef CAS.
- (a) J. D. Nguyen, E. M. D'Amato, J. M. R. Narayanam and C. R. J. Stephenson, Nat. Chem., 2012, 4, 854–859 CrossRef CAS PubMed; (b) H. Kim and C. Lee, Angew. Chem., Int. Ed., 2012, 51, 12303–12306 CrossRef CAS PubMed.
- (a) P. Zhang, C. C. Le and D. W. C. MacMillan, J. Am. Chem. Soc., 2016, 138, 8084–8087 CrossRef CAS PubMed; (b) D. J. P. Kornfilt and D. W. C. MacMillan, J. Am. Chem. Soc., 2019, 141, 6853–6858 CrossRef CAS; (c) G. H. Lovett, S. Chen, X.-S. Xue, K. N. Houk and D. W. C. MacMillan, J. Am. Chem. Soc., 2019, 141, 20031–20036 CrossRef CAS PubMed; (d) H. A. Sakai, W. Liu, C. C. Le and D. W. C. MacMillan, J. Am. Chem. Soc., 2020, 142, 11691–11697 CrossRef CAS PubMed.
- (a) B. Górski, A.-L. Barthelemy, J. J. Douglas, F. Juliá and D. Leonori, Nat. Catal., 2021, 4, 623–630 CrossRef; (b) Z. Zhang, B. Górski and D. Leonori, J. Am. Chem. Soc., 2022, 144, 1986–1992 CrossRef CAS PubMed.
- (a) R. Francke and R. D. Little, Chem. Soc. Rev., 2014, 43, 2492–2521 RSC; (b) R. Feng, J. A. Smith and K. D. Moeller, Acc. Chem. Res., 2017, 50, 2346–2352 CrossRef CAS PubMed; (c) M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS PubMed; (d) J. C. Siu, N. Fu and S. Lin, Acc. Chem. Res., 2020, 53, 547–560 CrossRef CAS PubMed; (e) H. Wang, X. Gao, Z. Lv, T. Abdelilah and A. Lei, Chem. Rev., 2019, 119, 6769–6787 CrossRef CAS PubMed; (f) P. Xiong and H.-C. Xu, Acc. Chem. Res., 2019, 52, 3339–3350 CrossRef CAS PubMed; (g) L. Ackermann, Acc. Chem. Res., 2020, 53, 84–104 CrossRef CAS PubMed; (h) K.-J. Jiao, Y.-K. Xing, Q.-L. Yang, H. Qiu and T. Mei, Acc. Chem. Res., 2020, 53, 300–310 CrossRef CAS PubMed; (i) X.-Q. Zhou, H.-T. Tang, F.-H. Cui, Y. Liang, S.-H. Li and Y.-M. Pan, Green Chem., 2023, 25, 5024–5029 RSC; (j) W.-J. Wei, X.-Y. Wang, H.-T. Tang, F.-H. Cui, Y.-Q. Wu and Y.-M. Pan, Sci. China: Chem., 2024, 67, 3382–3388 CrossRef CAS.
- (a) B. Wang, X. Zhang, Y. Cao, L. Zou, X. Qi and Q. Lu, Angew. Chem., Int. Ed., 2023, 62, e202218179 CrossRef CAS PubMed; (b) Z. Liu, X. Zhu, J. Guo, C. Ma, Z. Zuo and T. Mei, Sci. Bull., 2024, 69, 1866–1874 CrossRef CAS PubMed; (c) D. Wood and S. Lin, Angew. Chem., Int. Ed., 2023, 62, e202218858 CrossRef CAS PubMed; (d) R. Zhang, L. Li, K. Zhou and N. Fu, Chem. – Eur. J., 2023, 29, e202301034 CrossRef CAS PubMed.
- X. Sun and K. Zheng, Nat. Commun., 2023, 14, 6825 CrossRef CAS PubMed.
- X. Sun and K. Zheng, J. Am. Chem. Soc., 2025, 147, 34767–34776 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |







![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×; and (D) transmission electron micrograph of
000×; and (D) transmission electron micrograph of