Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Green epoxidation of unactivated alkenes via the catalytic activation of hydrogen peroxide by 4-hydroxybenzaldehyde
Efthymios T.
Poursaitidis
 a,
Christiana
Mantzourani
a,
Christiana
Mantzourani
 a,
Ierasia
Triandafillidi
a,
Ierasia
Triandafillidi
 a,
Maroula G.
Kokotou
a,
Maroula G.
Kokotou
 b and
Christoforos G.
Kokotos
b and
Christoforos G.
Kokotos
 *a
*a
aLaboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis, Athens 15771, Greece. E-mail: ckokotos@chem.uoa.gr
bLaboratory of Chemistry, Department of Food Science and Human Nutrition, Agricultural University of Athens, Iera Odos 75, Athens 11855, Greece
First published on 11th August 2025
Abstract
In the quest for green, inexpensive and sustainable methods for the epoxidation of unactivated alkenes, for both academic and industrial applications, the interest in effective organocatalytic activators of hydrogen peroxide is gaining increased attention. In this work, we report the innovative use of a commercially available aldehyde, 4-hydroxybenzaldehyde, as an effective activator of H2O2 in a substoichiometric amount (20 mol%) for the selective epoxidation of alkenes. A plethora of alkenes were selectively epoxidised in very good to high yields. Thorough mechanistic studies revealed a novel, complex epoxidation mechanism involving a Payne/Dakin tandem pathway. 4-Hydroxybenzaldehyde seems to be mainly converted to hydroquinone/benzoquinone, which have been uncovered as efficient activators of hydrogen peroxide for the epoxidation of alkenes. This so far unidentified oxidative pathway may find new potential applications for oxidative transformations.
Green foundation1. In the quest for green, inexpensive and sustainable methods for the epoxidation of unactivated alkenes, for both academic and industrial applications, the interest in effective organocatalytic activators of hydrogen peroxide is gaining increased attention. Herein, the use of a commercially available organocatalyst (4-hydroxybenzaldehyde) is described, bypassing the need for metal-based catalyst activators of hydrogen peroxide. Additionally, the reaction is performed in a solution of methanol-aqueous buffer, bypassing the need for toxic and non-green organic solvents.2. The use of a commercially available and cheap organocatalyst as an activator of hydrogen peroxide is described. Thus, the use of metals or stoichiometric amounts of aldehydes is not needed. Furthermore, the identification of a commercially available aldehyde is crucial as the previous catalyst of choice required a laborious synthesis. Additionally, in most cases, the purification of products is achieved by extraction and column chromatography is not required. 3. The reaction mechanism is also studied thoroughly. These results can be the foundation for further reaction development on heteroatom oxidation and/or epoxidation-cyclization reactions. Moreover, in the future, the identification of a more active hydroquinone–quinone system could lead to a lower catalyst loading or be the initial point for the development of an asymmetric version. |
Introduction
Olefin epoxidation is among the most important transformations in organic synthesis as epoxides constitute highly diverse intermediates, finding numerous applications.1 Since its discovery, the epoxidation reaction has been approached via various angles, the most common being the use of peracids as the epoxidation agent, first established in 1909 by Prileschajew,2 with m-CPBA emerging as an oxidant of choice, although it was required in stoichiometric amounts. A landmark in chemistry was the pioneering studies by Sharpless and Katsuki, who managed to achieve the first asymmetric epoxidation of double bonds, specifically on allylic alcohols, with high stereoselectivity, using a titanium complex with a tartrate ligand as the catalyst and TBHP as the oxidant, changing modern understanding on how to implement chemical processes.3 Organocatalysis, a field that has gained increasing attention over the last twenty-five years and significantly altered modern organic synthesis, has provided additional environmentally friendly protocols for the epoxidation reaction, avoiding the use of expensive metal catalysts.4 Even though the first epoxidation employing solely metal-free compounds can be attributed to Prileschajew,2 it was not until many years later that ground-breaking organocatalytic epoxidation reactions began to appear.4 The studies by Adam,5 Curci,5,6 Mello,6 Yang,7 Denmark8 and Shi9 introduced dioxiranes from ketones as potent oxidising agents, either used directly or generated in situ from activated ketones, in combination with oxone as the oxidant. Apart from ketones, researchers have explored the use of other carbonyl-containing moieties as potential mediators in oxidation reactions. The first application of aldehydes in the epoxidation of olefins dates back to 1991 by Mukaiyama et al.,10 where a nickel complex was used as the catalyst along with molecular oxygen as the oxidant. However, an over-stoichiometric amount of isobutyraldehyde had to be employed as the reducing agent. Later, different metal complexes and/or different aldehydes were also employed (Scheme 1A).11 Following Mukaiyama, other researchers invested in a variety of complexes that were able to promote the reaction under similar conditions.12 Mechanistic studies by Lassila,13 Nam14 and Nolte15 concluded that the reactivity of aldehydes as co-reagents is based on their autooxidation pathway with O2, which generates either peracids or acyl peroxy radicals. These, in the presence of metal complexes, can generate oxo-metallic species. Thus, in order to obtain high epoxidation yields, an over-stoichiometric amount of aldehyde was often needed to outcompete the inevitable oxidation pathway towards the carboxylic acid, which is the terminal product of the catalytic cycle of the reaction. In a recent study, the use of beta-zeolites as mediators managed to reduce the amount of isobutyraldehyde to 1.5 equivalents.12j | ||
| Scheme 1 Epoxidation of unactivated olefins employing aldehydes: (A)–(C) Previous work and (D) this work. | ||
Not long after Mukaiyama's work, Ishii's group proved that the presence of a metal catalyst is not a requisite for the activation of aldehydes.16 By removing the metal and increasing the reaction temperature to 40 °C, high yields were achieved in relatively short reaction times. In the same vein, other researchers have used 2-ethylhexanal as the solvent,17 while others applied radical initiators.18 Recently, 50 mol% of butyraldehyde was employed for the selective epoxidation of the volatile β-ionone in water.19 In addition, other metal-free approaches using aldehydes have been reported in the literature. Perfluorocarbons were used by Pozzi as an inert reaction medium that dissolves molecular oxygen from the atmosphere to promote the reaction,20 while Yang's group focused on the in situ generation of oxaziridines as the active oxidants.21 Notably, asymmetric epoxidation has also been achieved by Bez and Zhao,22 who proposed a dioxirane intermediate, derived from a chiral aldehyde, as the active oxygen-transfer reagent. Furthermore, processes have been developed utilizing N-hydroxyphthalimide as the catalyst, along with acetaldehyde.23 All of these methods certainly have their own merits; however, in most cases, they all suffer from the necessity of using stoichiometric or over-stoichiometric amounts of the aldehyde, where some substrates are not viable or cannot be explored further under these reaction conditions (Scheme 1B).
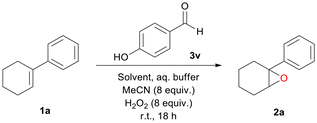
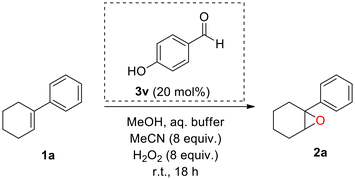
Nowadays, the need for sustainability has greatly increased; thus, green and inexpensive methods are imperative. Hydrogen peroxide is considered a green oxidant that is less commonly used in these reactions, due to its poor oxidation power on its own, as it requires activation by a catalyst to form a reactive intermediate.24 In this context, our group has established the use of commercially available 2,2,2-trifluoroacetophenone as a potent organocatalyst for the epoxidation of various olefins.25 The efficiency of this method was later expanded to other oxidative transformations as well.26 Recently, we disclosed the first catalytic use of an atropoisomeric aldehyde as an organocatalyst for the epoxidation reaction (Scheme 1C).27 Although a powerful method, it proved not to be enantioselective, while the copious synthesis of the catalyst limits its application further. Herein, we sought to explore the possibility of simple, commercially available aldehydes serving as potential organocatalysts in substoichiometric amounts. Not only do we report our research on the catalytic scope of various simple benzaldehydes for the epoxidation of 1-phenyl-1-cyclohexene (1a) using hydrogen peroxide as a green oxidant but also further describe the application of this protocol in different substrates. To our knowledge, this is the first use of a commercially available aldehyde as an H2O2 activator employed in substoichiometric amounts (20 mol%) for the epoxidation of unactivated alkenes. Apart from its diverse substrate scope, the simple and straightforward reaction conditions and its environmental friendliness make this process really appealing. Moreover, a thorough study of the reaction mechanism uncovered a previously unidentified mechanistic pathway, leading to a new mechanism of action. This hidden and previously underdeveloped activation pathway may uncover new oxidation processes with unrecognised potential.
Results and discussion
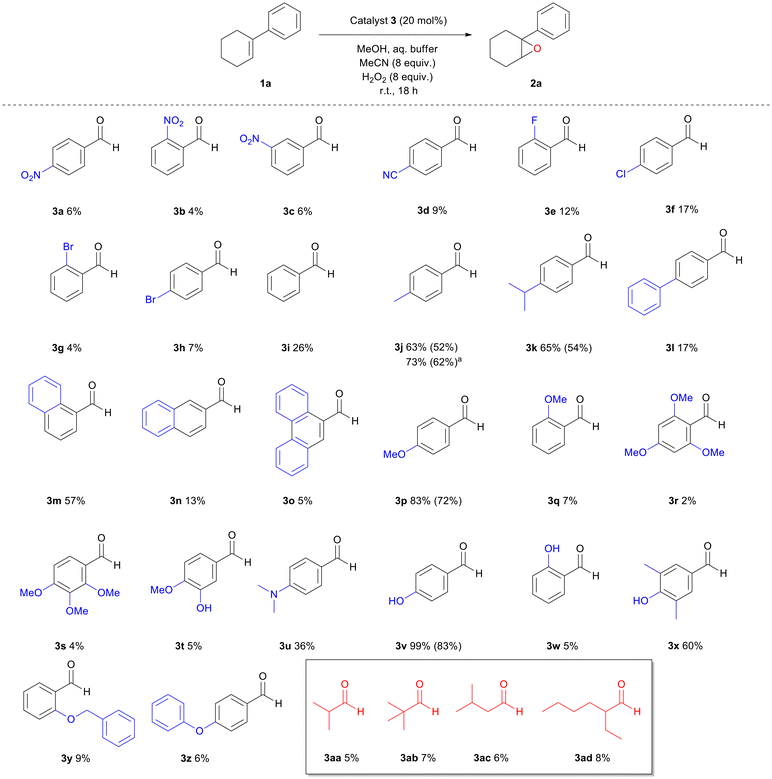
We began our studies by testing a variety of substituted benzaldehydes as the organocatalysts for the oxidation of 1-phenyl-1-cyclohexene (1a), a common substrate used to test electrophilic oxidation processes (Scheme 2). The reactions were carried out with 20 mol% catalyst loading of each aldehyde in methanol, along with an aqueous buffer solution, 8 equiv. of acetonitrile and hydrogen peroxide (30% w/w), mimicking our previous reaction conditions.27 All nitrobenzaldehydes (3a–c), regardless of the position of the nitro group, along with 4-cyanobenzaldehyde (3d), afforded a low conversion to the corresponding epoxide. In the case of halogenated benzaldehydes 3e–h, conversions were slightly higher, as 4-chlorobenzaldehyde (3e) afforded the best results among them, while 2-bromobenzaldehyde (3g) afforded the lowest conversion. When benzaldehyde (3i) was employed, the conversion of the reaction increased up to 26%. Substitution with a methyl or an isopropyl group at the para position of the aromatic ring significantly increased the yield (3j or 3k) up to 65%. Increasing the amounts of MeCN and H2O2 enhanced the outcome in the case of 3j. The use of benzaldehydes 3l, 3n or 3o indicated that the presence of an extended aromatic conjugation is not a good option, with the exception of 1-naphthylaldehyde (3m), which was almost as good as 4-methyl or 4-isopropyl benzaldehyde (3j or 3k). Having concluded that mild activation of the ring is promising for the reaction, we next tested the effect of strong activating groups. To our delight, strong activation seemed to facilitate the reaction (3p and 3v), with 4-hydroxybenzaldehyde (3v) affording an almost quantitative conversion of the alkene (99%). However, the position of the activating group is also important as ortho-substitution does not seem to work (3qvs.3p or 3wvs.3v). Adding more than one activating group to the aromatic scaffold was also not beneficial (3r–3t). In the literature, aliphatic aldehydes are often employed in Mukaiyama-type epoxidation reactions. None of 3aa–3ad were able to promote the epoxidation reaction. From screening 30 commercially available aldehydes, we identified that five aldehydes, in substoichiometric amounts, led to higher than 50% yield. All were para-substituted benzaldehydes, while 4-hydroxybenzaldehyde stood out by providing almost quantitative conversion. Thus, to the best of our knowledge, these represent the first successful examples of commercially available aldehydes that are capable of promoting an epoxidation reaction. Among the aromatic aldehydes tested, we concluded that 4-hydroxybenzaldehyde (3v) is the best organocatalyst/H2O2 activator for the aldehyde-catalysed epoxidation reaction.Other than methanol, a variety of organic solvents were employed (Table 1); however, none of them led to sufficient conversions (Table 1, entries 6–11), except from water-miscible solvents, such as MeCN (Table 1, entry 12) or other alcohols, such as EtOH (Table 1, entry 4) or t-BuOH (Table 1, entry 5). By performing the control experiments in all three alcohols (in the absence of the organocatalyst), methanol led to the lowest background reaction (Table 1, entries 13–15). Considering that 4-hydroxybenzaldehyde (3v) led to an excellent result in methanol, while simultaneously methanol is, among these three, the simplest and cheapest alcohol, making this protocol more cost-friendly, we decided to perform our protocol in methanol. Low catalyst loading did not seem to benefit the reaction (Table 1, entries 2 and 3).
| Entry | 3v (mol%) | Solvent | Conversiona (%) |
|---|---|---|---|
| a Conversion determined by 1H-NMR, yield of 2a after isolation by column chromatography is given in parentheses. b Total volume of MeCN was 0.57 mL. | |||
| 1 | 20 | MeOH | 99 (83) |
| 2 | 10 | MeOH | 88 |
| 3 | 5 | MeOH | 70 |
| 4 | 20 | EtOH | 99 (83) |
| 5 | 20 | t-BuOH | 100 (83) |
| 6 | 20 | EtOAc | 19 |
| 7 | 20 | CHCl3 | 17 |
| 8 | 20 | CH2Cl2 | 21 |
| 9 | 20 | Et2O | 28 |
| 10 | 20 | 2-Me-THF | 29 |
| 11 | 20 | Toluene | 9 |
| 12b | 20 | MeCN | 100 (78) |
| 13 | — | MeOH | 5 |
| 14 | — | EtOH | 9 |
| 15 | — | t-BuOH | 30 |
With the optimum conditions in hand, we then sought to explore the substrate scope of the aldehyde-catalysed epoxidation with a plethora of diverse alkenes (Scheme 3). For the majority of trisubstituted olefins examined (2a–2i), 8 equiv. of H2O2/MeCN were sufficient to obtain the corresponding products in good to high yields. 1-Phenylcycloheptene (1e) required a two-fold increase in the oxidant, in order to reach a sufficient yield. The labile acetal group was well tolerated, as highlighted in the case of 2h. α-Pinene (1i) was selectively converted to α-pinene oxide 2i, while no ring opening occurred. (2-(Cyclohex-1-en-1-yl)ethyl)benzene (1c) proved to be the most difficult substrate to epoxidise, among the trisubstituted alkenes, needing up to 30 equiv. to reach a mediocre yield. Next, various disubstituted alkenes were studied. cis-Cyclic alkenes led to high to quantitative yields (2j–m). In the case of cyclohexene oxide (2j), the reduced isolated yield was attributable to its high volatility. Increasing the amount of oxidant to 30 equiv. allowed cis-1,4-dichloro-2-butene (1n) to afford epoxide 2n in 63% yield after isolation by column chromatography. Such substrates failed to be epoxidised using our previous protocols.27 Disubstituted trans-olefins were well tolerated, as shown by the epoxidation of trans-β-methylstyrene and allylic alcohols in high yields (2o–q). In the case of 1,1-disubstituted olefins, α-methylstyrene (1r) was easily epoxidised to 2r; however, other substrates with bulkier groups required more equivalents of the oxidant (2s, 64% yield). 4-Methylene-1-tosylpiperidine (1t) appeared to be insoluble in the reaction mixture, leading to a low conversion (25%). To counteract this issue, a mixture of ethyl acetate and methanol was used as the solvent. This enhanced the reaction outcome, leading to 51% of 2t. Further increasing the oxidant to 30 equiv. led to an even better result (78%). Furthermore, 8 equiv. of oxidant were sufficient for styrene (1u), 4-fluoro-styrene (1v) or 4-methyl-styrene (1y) to reach quantitative conversions. Notably, styrene on a larger scale (1.04 g, 10.00 mmol) required 30 equiv. of H2O2 to reach reaction completion, affording 2u in 75% yield. 4-Chlorostyrene (1w) also afforded 2w in high yield, although 4-bromo-styrene (1x) and 4-tert-butyl-styrene (1z) required 30 equiv. of oxidant. Leaving 1z stirring for 42 hours, the yield of 2z was slightly better. Other terminal olefins (1aa–1ac) required 30 equiv. to yield medium to good results. N-Allylbenzamide (1ac), being highly nucleophilic, required only 4 h to afford the corresponding epoxide (2ac) in a mediocre yield. Finally, some naturally occurring alkenes were tested. Both double bonds of geraniol and limonene were epoxidised, yielding only the corresponding diepoxides in both cases, with 8 equiv. of oxidant. Reducing the oxidant to 2 equiv. with limonene afforded both mono-epoxide 2af and diepoxide 2ag, but at the cost of reduced yield in both products. On the contrary, utilising 2 equiv. of oxidant with geraniol (2ae), geraniol diepoxide still prevailed; however, a small amount of the 6,7-mono-epoxide was detected and isolated. A natural steroid, cholesterol, exhibiting low solubility in methanol, was unable to participate in the reaction successfully. By substituting methanol with t-BuOH,25 along with an additional amount of chloroform to facilitate the solubility of the substrate, the epoxidation of cholesterol was achieved. There are some limitations to this epoxidation protocol, namely, substrates with long aliphatic chains or electron-poor double bonds are not well tolerated, affording the corresponding products in zero or low yields (see SI).28 Notably, in many cases where quantitative transformation of the substrate occurred (2d, 2g, 2h, 2j–2m, 2o–2r, 2u, 2v, 2y, 2ae, and 2ag), the desired epoxides could be obtained by simple aqueous extraction, with no further need for purification. This is an appealing attribute for industries, as it prevents further waste production for purification methods. Furthermore, from the known literature, a main disadvantage in these kinds of reactions is the production of unwanted oxidation side products, such as allylic oxidation of cyclic alkenes23,24 or oxidative cleavage in the case of styrene and its analogues. None of these side products were observed when applying the present protocol.
The mechanism of the reaction was the next topic that drew our attention. As mentioned in the introduction, in metal-free Mukaiyama epoxidation, the main reactive intermediates are usually peracids or acyl peroxy radicals, which lead to the need for stoichiometric amounts of the aldehyde.13–15 In combination with the fact that aliphatic aldehydes are usually employed in such conditions, we can safely assume that the developed protocol does not follow a Mukaiyama-type mechanism. In our previous work, however, we demonstrated the formation of a dioxirane, arising from the aldehyde (catalyst), as the active oxidant.27 To identify the potential active species, we initially conducted some control experiments (Table 2). Removing the components of the reaction one by one resulted in no product formation (Table 2, entries 3, 4 and 6), verifying that H2O2 is indeed the oxidant; however, on its own, it cannot promote the reaction, and all the other reagents are equally important. Interestingly, in the absence of an organic solvent, 25% of the epoxide was formed, leading us to the conclusion that, even though methanol is a necessary medium to the aqueous phase, the catalyst possibly forms a water-soluble salt under these conditions (Table 2, entry 5). Altering the pH of the buffer solution proved critical for the reaction outcome (Table 2, entries 7–10). The importance of the pH and the necessity of MeCN in the reaction mixture led us to suspect that the intermediate of Payne29 is involved. The lack of oxygen from air significantly reduced the yield (Table 2, entry 11), and the low yield observed upon addition of TEMPO to the reaction mixture suggests that a radical pathway is also present (Table 2, entry 12). Lastly, in order to rule out the possibility of a peracid moiety as the active intermediate in the reaction, 4-hydroxybenzoic acid was employed, instead of the aldehyde, as the catalyst, and a low yield was observed (Table 2, entry 13). These results indicate that a pathway similar to our previous method27 may exist that leads to the formation of the corresponding dioxirane from 3v as the active oxidant species. However, if this were the only viable pathway, we should not observe such enormous fluctuations in yield when 3x or any other substituted benzaldehyde at the para position was employed instead of 3v.
| Entry | Deviation from standard conditions | Conversiona (%) |
|---|---|---|
| a Conversion determined by 1H-NMR, yield of 2a after isolation by column chromatography in parentheses. | ||
| 1 | None | 99 (83) |
| 2 | 1 h reaction time | 12 |
| 3 | No H2O2 | 0 |
| 4 | No MeCN | 0 |
| 5 | No MeOH | 25 |
| 6 | No aq. buffer | 0 |
| 7 | Water instead of aq. buffer | 0 |
| 8 | aq. buffer pH = 9 | 23 |
| 9 | aq. buffer pH = 10 | 56 |
| 10 | aq. buffer pH = 14 | 41 |
| 11 | No O2 (under Ar) | 24 |
| 12 | TEMPO (1 equiv.) | 13 |
| 13 |

|
17 |
Furthermore, according to the literature, under similar reaction conditions, Dakin oxidation also occurs.29c,30 If that was the case, 4-hydroxybenzaldehyde (3v) would decompose to hydroquinone (4) and subsequently oxidize to 1,4-benzoquinone (5). To test whether this was the case and if 4 or 5 can promote oxidation reactions, we tested these commercially available oxidation by-products of 3v as potential catalysts in our protocol. Interestingly, both 4 and 5 promoted the epoxidation of 1a (Scheme 4). In the literature, only one study reports the use of phenol in an epoxidation reaction; however, not as a catalyst but as the solvent.31 This strengthens the notion that the reaction mechanism followed herein is unprecedented, concerning the catalytic activation of H2O2 by phenols and/or quinones. To shed light on the reaction mechanism, HRMS experiments were conducted for the epoxidation reaction of 1a with 3v, 4 or 5 as the catalyst.28 Direct infusion-high resolution mass spectrometry (DI-HRMS) is a powerful analytical platform that can be employed for the discovery of fleeting key intermediates in organic reactions.32 By providing high-resolution exact mass information, DI-HRMS has previously been implemented in mechanistic studies for the identification of organic species that cannot be isolated.32 In this study, along with the expected intermediates that might participate in the reaction mechanism based on our previous study,27 new intermediates were detected, implying an entirely different mechanism for the epoxidation of olefins.
 | ||
| Scheme 4 Epoxidation of 1-phenyl-1-cyclohexene: (A) by hydroquinone (4), (B) by 1,4-benzoquinone (5) and (C) by 4-hydroxybenzaldehyde-3,5-d2 (3v–D) as the catalysts. | ||
Furthermore, we performed NMR monitoring experiments, which verified that Dakin oxidation of 3v indeed occurs.28 We performed the monitoring of the reaction with the omission of the olefin and after 5 min of exposure of 3v under the standard reaction conditions, hydroquinone (4) was the major compound identified by NMR.28 After 25 min of exposure, except from hydroquinone (4), benzoquinone (5) and the epoxide of maleic acid were identified, while after 18 h of exposure, degradation of hydroquinone was observed, detecting only the epoxide of maleic acid.28 According to the HRMS data recorded in negative ionization mode28 and the recorded NMR data,28 we propose that 4-hydroxybenzaldehyde (3v) is converted to hydroquinone (4) by a tandem Payne/Dakin reaction,29c as depicted in Scheme 5. However, a similar mechanism to the atropoisomeric aldehyde may occur as a minor pathway.27,28 As depicted in Scheme 5, Dakin oxidation can proceed via two pathways. The most common is via the formation of perhydrate I, which leads to formate IV (blue arrows). Another pathway reported in the literature is a tandem Payne/Dakin reaction, in which aldehyde 3v reacts with peroxycarboximidic acid II (Payne's intermediate)26–28 to form intermediate III, which collapses to afford formate IV and acetamide. Hydrolysis of formate IV ultimately leads to the production of hydroquinone (4), which continues its journey in the next part of the mechanistic scenario depicted in Scheme 6.
 | ||
| Scheme 5 Oxidation of 4-hydroxybenzaldehyde 3v to hydroquinone (4) via Dakin oxidation (blue arrows) or via Payne/Dakin oxidation (green arrows). | ||
Hydroquinone (4) is known to react with oxygen under alkaline conditions to afford 1,4-benzoquinone (5) (Scheme 6A).33 Based on our DI-HRMS studies,28 we propose that 1,4-benzoquinone (5) can react with Payne's intermediate II to generate intermediate V, which collapses releasing acetamide and dioxirane VI as a possible oxidant (Scheme 6A).28 Ions corresponding to the exact masses of intermediates V and VI were identified.28 However, since 5 is highly electrophilic, it can be directly attacked by hydrogen peroxide to form perhydrates VII or VIII (Scheme 6B). Perhydrates are also known for their reactivity towards epoxidation.34 Even though in Scheme 6B, we depict VIII as the species performing the epoxidation reaction, we cannot exclude the possibility that the epoxidation occurs by VII. In addition, through an intermolecular step (blue arrows), VII leads to VII′, which can then collapse via a retro [4 + 2] mechanism to 4 and eventually return to benzoquinone 5. Ions corresponding to the exact masses of intermediates VII and VIII were also identified.28
Furthermore, under these reaction conditions, 1,4-benzoquinone (5) [or hydroquinone (4) directly] is known to react with H2O2 or O2, leading to 2,5-dihydrobenzoquinone.35 We suspect that the formation of 2,5-dihydrobenzoquinone proceeds via a free radical semiquinone formation pathway, based on early studies under similar reaction conditions.35b,c The presence of 2,5-dihydrobenzoquinone was verified not only by HRMS,28 but also visually, as a pink colour was observed after the addition of H2O2, which can be attributed to its salt formation.35a,36 Moreover, the colour slowly dissipated, and after approximately 1 h, the reaction mixture became colourless; this, however, does not signify reaction completion (Table 2, entry 2). The discoloration of 2,5-dihydrobenzoquinone is known in the literature and extensively studied by Hosoya and Rosenau.37 Evidence via HRMS suggested that 2,5-dihydrobenzoquinone can also participate in the activation of hydrogen peroxide and perform oxidations. However, based on our NMR studies, we were not able to verify such structures; only the final decomposition products of 2,5-dihydrobenzoquinone (maleic acid, the epoxide of maleic acid and acetic acid) could be identified.28 This is in accordance with the literature.37c In light of this, we examined the catalytic activity of these compounds, which, as expected, did not promote the epoxidation reaction.28 At that point, we concluded that epoxidation occurs before the terminal degradation of the catalyst, which correlates well with the slightly enhanced outcome of 2z observed after prolonged reaction time (Scheme 3).
To further explore the reaction mechanism, we continued mechanistic experiments examining the catalytic activity of both 4 or 5 under an argon atmosphere and in the presence of TEMPO.28 In all cases, low yields were obtained. Since a recent study showed that the aerobic oxidation of 4 to 5 under alkaline conditions proceeds via an ionic pathway rather than a radical one,33 from the above-mentioned experiments, we deduced that the presence of O2 is vital for the oxidation of hydroquinone to benzoquinone.
Lastly, we believe that the pH is of critical importance in this case. Buffer solutions of different pH values possibly favours catalyst degradation, rather than the epoxidation path.37b With the data in hand, it seems that pH = 12 is the optimal value for Payne's intermediate generation and catalyst degradation. We believe that at low pH (e.g., pH = 9 and 10), catalyst decomposition is faster than the epoxidation pathway, while at high pH (e.g., pH = 14), degradation is slower, and the formation of Payne's intermediate is not favored.37b
One way to verify the integrity of the observed intermediates by HRMS experiments, is to introduce a deuterated version of the catalyst. To this end, we synthesised 4-hydroxybenzaldehyde-3,5-d2 (3v–D) from 3v. While inferior in terms of epoxidation yield (Scheme 4C), some of the deuterated counterparts of the proposed intermediates were indeed observed by DI-HRMS.28 Last but not least, in order to ensure that the active oxidant is not of radical nature, we employed alkene 1ai as a radical clock (Scheme 7).27,38
Indeed, the only compounds observed in the crude 1H-NMR were unreacted alkene 1ai and the corresponding epoxide 2ai,28 without any by-products derived from the ring opening of the cyclopropane moiety (Scheme 7).
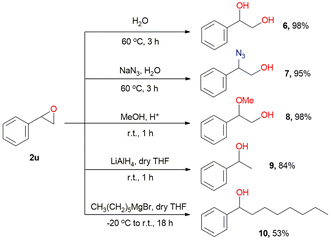
To demonstrate the practical utility of this approach, we performed ring-opening reactions of styrene oxide 2u with various nucleophiles, affording the corresponding products 6–10 in moderate to high yields (Scheme 8).
Considering all the data, we suggest that inexpensive, commercially available benzaldehydes are able to promote the epoxidation reaction in catalytic amounts in the presence of H2O2 and air. Benzaldehydes susceptible to Dakin oxidation, with 4-hydroxybenzaldehyde being the optimum among them, are revealed as exceptional epoxidation catalysts. The position of the functional group on the aryl moiety is also critical, since ortho-substituted electron-rich benzaldehydes produce catechols, which are not known for any oxidative properties (since we employed catechol as a catalyst and the reaction did not proceed, see SI).28 Lastly, meta-substitution leads to the corresponding benzoic acids, which are not viable for epoxidation under these reaction conditions. This hidden oxidation pathway is reported for the first time and has never been identified or proposed in the past, at least to our knowledge.
Conclusions
In conclusion, screening a large set of commercially available aldehydes revealed that para-substituted benzaldehydes can activate hydrogen peroxide to perform the epoxidation of alkenes. 4-Hydroxybenzaldehyde was identified as a simple and cheap activator of hydrogen peroxide towards epoxidation through an unprecedented reactivity. Mechanistic experiments uncovered a complex scenario that leads to epoxidation. This study reveals that the main mechanistic pathway of activation of hydrogen peroxide by 4-hydroxybenzaldehyde occurs via intermediates that formed after Dakin oxidation, namely, hydroquinone or benzoquinone. Such compounds are also reported for the first time as hydrogen peroxide activators, and their use in oxidation reactions in substoichiometric amounts is reported and can account for most of the reaction yield. The increased yield of 4-hydrobenzaldehyde vs. hydroquinone itself is possibly attributed to the gradual release of hydroquinone in the reaction mixture, which partially delays the decomposition pathway. According to the literature, these reaction conditions possibly lead to terminal degradation of the aldehyde; however, this occurs only after it activates H2O2 for the epoxidation of olefins. A variety of olefins were easily epoxidised with high selectivity and good to high yields, and in some cases, the products could be retrieved via extractions, avoiding further purification. This work provides new mechanistic insights that could possibly lead to new asymmetric epoxidation protocols.Author contributions
C. G. K. and I. T. conceived the study and designed the experiments. E. T. P. performed the experiments and analysed the data. C. M. and M. G. K designed the experiments for the HRMS studies and analysed the data. E. T. P. wrote the first draft of the manuscript, and C. G. K. performed the final editing of the manuscript. The manuscript was written through contributions from all the authors.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article (optimisation studies experimental procedures and data, mechanistic studies and DI-HRMS studies) have been included as part of the SI. See DOI: https://doi.org/10.1039/d5gc02537k.Acknowledgements
The authors gratefully acknowledge the Hellenic Foundation for Research and Innovation (HFRI) for the financial support through a grant, which is financed by 1st Call for H.F.R.I. Re-search Projects to Support Faculty Members & Researchers and the procurement of high-cost research equipment grant (grant number 655).References
- For selected reviews, see: (a) O. A. Wong and Y. Shi, Chem. Rev., 2008, 108, 3958–3987 CrossRef CAS PubMed; (b) Y. Zhu, Q. Wang, R. G. Cornwall and Y. Shi, Chem. Rev., 2014, 114, 8199–8256 CrossRef CAS PubMed; For books, see: (c) A. K. Yudin, Aziridines and Epoxides in Organic Synthesis, Wiley-VCH, Weinheim, 2006 CrossRef; (d) G. Centi, S. Perathoner and S. Abate, Modern Heterogeneous Oxidation Catalysis: Design, Reactions and Characterization, Wiley-VCH, Weinheim, 2009.
- N. Prileschajew, Berichte, 1909, 42, 4811–4815 CrossRef CAS.
- T. Katsuki and K. B. Sharpless, J. Am. Chem. Soc., 1980, 102, 5974–5976 CrossRef CAS.
- For a review, see: R. L. Davis, J. Stiller, T. Naicker, H. Jiang and K. A. Jorgensen, Angew. Chem., Int. Ed., 2014, 53, 7406–7426 CrossRef CAS PubMed.
- For selected examples, see: (a) W. Adam, R. Curci and J. O. Edwards, Acc. Chem. Res., 1989, 22, 205–211 CrossRef CAS; (b) W. Adam, P. B. Rao, H.-G. Degen, A. Levai, T. Patonay and C. R. Saha-Möller, J. Org. Chem., 2002, 67, 259–264 CrossRef CAS PubMed.
- For a selected example, see: R. Mello, M. Fiorentino, O. Sciacovelli and R. Curci, J. Org. Chem., 1988, 53, 3890–3891 CrossRef CAS.
- For selected examples, see: (a) D. Yang, Y.-C. Yip, M.-W. Tang, M.-K. Wong, J.-H. Zheng and K.-K. Cheng, J. Am. Chem. Soc., 1996, 118, 491–492 CrossRef CAS; (b) D. Yang, M.-K. Wong, Y.-C. Yip, X.-C. Wang, M.-W. Tang, J.-H. Zheng and K.-K. Cheng, J. Am. Chem. Soc., 1998, 120, 5943–5952 CrossRef CAS.
- For selected examples, see: (a) S. E. Denmark, Z. Wu, C. M. Crudden and H. Matsuhashi, J. Org. Chem., 1997, 62, 8288–8289 CrossRef CAS PubMed; (b) S. E. Denmark and H. Matsuhashi, J. Org. Chem., 2002, 67, 3479–3486 CrossRef CAS PubMed.
- For selected examples, see: (a) Y. Tu, Z.-X. Wang and Y. Shi, J. Am. Chem. Soc., 1996, 118, 9806–9807 CrossRef CAS; (b) Z.-X. Wang, Y. Tu, M. Frohn, J.-R. Zhang and Y. Shi, J. Am. Chem. Soc., 1997, 119, 11224–11235 CrossRef CAS; (c) C. P. Burke and Y. Shi, J. Org. Chem., 2007, 72, 4093–4097 CrossRef CAS PubMed; (d) N. Nieto, I. J. Munslow, H. Fernadez-Perez and A. Vidal-Ferran, Synlett, 2008, 2856–2858 CAS.
- (a) T. Yamada, T. Takai, O. Rhode and T. Mukaiyama, Chem. Lett., 1991, 20, 1–4 CrossRef; (b) T. Yamada, T. Takai, O. Rhode and T. Mukaiyama, Bull. Chem. Soc. Jpn., 1991, 64, 2109–2117 CrossRef CAS.
- For examples of the Mukaiyama epoxidation-type reaction, see: (a) T. Takai, E. Hata, T. Yamada and T. Mukaiyama, Bull. Chem. Soc. Jpn., 1991, 64, 2513–2518 CrossRef CAS; (b) T. Yamada, K. Imagawa and T. Mukaiyama, Chem. Lett., 1992, 21, 2104–2112 Search PubMed; (c) T. Yamada, K. Imagawa, T. Nagata and T. Mukaiyama, Chem. Lett., 1992, 21, 2231–2234 CrossRef; (d) T. Mukaiyama, T. Yamada, T. Nagata and K. Imagawa, Chem. Lett., 1993, 22, 327–330 CrossRef; (e) K. Imagawa, T. Nagata, T. Yamada and T. Mukaiyama, Chem. Lett., 1994, 23, 527–530 CrossRef; (f) T. Nagata, K. Imagawa, T. Yamada and T. Mukaiyama, Chem. Lett., 1994, 23, 1259–1262 CrossRef; (g) T. Nagata, K. Imagawa, T. Yamada and T. Mukaiyama, Bull. Chem. Soc. Jpn., 1995, 68, 1455–1465 CrossRef CAS.
- For selected examples, see: (a) D. D. Díaz, S. S. Gupta, J. Kuzelka, M. Cymborowski, M. Sabat and M. G. Finn, Eur. J. Inorg. Chem., 2006, 4489–4493 CrossRef; (b) D. Shabashov and M. P. Doyle, Tetrahedron, 2013, 69, 10009–10013 CrossRef CAS; (c) L. Hadian-Dehkordi and H. Hosseini-Monfared, Green Chem., 2016, 18, 497–507 RSC; (d) X. He, L. Chen, X. Zhou and H. Ji, Catal. Commun., 2016, 83, 78–81 CrossRef CAS; (e) G. Yang, H. Du, J. Liu, Z. Zhou, X. Hu and Z. Zhang, Green Chem., 2017, 19, 675–681 RSC; (f) K. Berijani and A. Morsali, J. Catal., 2019, 378, 28–35 CrossRef CAS; (g) X. Zhou, Q. Wang, W. Xiong, L. Wang, R. Ye, G. Xiang, C. Qi and J. Hu, Synlett, 2020, 1789–1794 CAS; (h) K. Berijani and A. Morsali, Inorg. Chem., 2021, 60, 206–218 CrossRef CAS PubMed; (i) C. Li, N. Pu, K. Huang, C. Xia, X. Peng, M. Lin, B. Zhu and X. Shu, Green Chem., 2022, 24, 6200–6214 RSC; (j) Z. Zhou, K. Zhang, P. He, H. Chang, M. Zhang, T. Yan, X. Zhang, Y. Li and Z. Cao, Angew. Chem., Int. Ed., 2024, e202419900 Search PubMed.
- K. R. Lassila, F. J. Waller, S. E. Werkheiser and A. L. Wressell, Tetrahedron Lett., 1994, 35, 8077–8080 CrossRef CAS.
- W. Nam, H. J. Kim, S. H. Kim, R. Y. N. Ho and J. S. Valentine, Inorg. Chem., 1996, 35, 1045–1049 CrossRef CAS PubMed.
- (a) B. B. Wentzel, P. A. Gosling, M. C. Feiters and R. J. M. Nolte, J. Chem. Soc., Dalton Trans., 1998, 13, 2241–2246 RSC; (b) B. B. Wentzel, P. L. Alsters, M. C. Feiters and R. J. M. Nolte, J. Org. Chem., 2004, 69, 3453–3464 CrossRef CAS PubMed.
- K. Kaneda, S. Haruna, T. Imanaka, M. Hamamoto, Y. Nishiyama and Y. Ishii, Tetrahedron Lett., 1992, 33, 6827–6830 CrossRef CAS.
- C. Lehtinen and G. Brunow, Org. Process Res. Dev., 1999, 3, 101–103 CrossRef CAS.
- (a) A. Köckritz, M. Blumenstein and A. Martin, Eur. J. Lipid Sci. Technol., 2008, 110, 581–586 CrossRef; (b) M. Hong, J. Min and S. Wang, ChemistrySelect, 2018, 3, 4818–4821 CrossRef CAS.
- X. Zhang, Z. Wei and L. Yu, React. Chem. Eng., 2023, 8, 1700–1704 RSC.
- G. Pozzi, F. Montanari and M. T. Rispens, Synth. Commun., 1997, 27, 447–452 CrossRef CAS.
- (a) D. Yang, C. Zhang and X.-C. Wang, J. Am. Chem. Soc., 2000, 122, 4039–4043 CrossRef CAS; (b) M.-K. Wong, L.-M. Ho, Y.-S. Zheng, C.-Y. Ho and D. Yang, Org. Lett., 2001, 3, 2587–2590 CrossRef CAS PubMed.
- G. Bez and C.-G. Zhao, Tetrahedron Lett., 2003, 44, 7403–7406 CrossRef CAS.
- (a) F. Minisci, C. Gambarotti, M. Pierini, O. Porta, C. Punta, F. Recupero, M. Lucarini and V. Mugnaini, Tetrahedron Lett., 2006, 47, 1421–1424 CrossRef CAS; (b) R. Spaccini, L. Liguori, C. Punta and H. R. Bjørsvik, ChemSusChem, 2012, 5, 261–265 CrossRef CAS PubMed.
- (a) I. Triandafillidi, D. I. Tzaras and C. G. Kokotos, ChemCatChem, 2018, 10, 2521–2535 CrossRef CAS; (b) E. T. Poursaitidis, P. L. Gkizis, I. Triandafillidi and C. G. Kokotos, Chem. Sci., 2024, 15, 1177–1203 RSC.
- D. Limnios and C. G. Kokotos, J. Org. Chem., 2014, 79, 4270–4276 CrossRef CAS PubMed.
- (a) D. Limnios and C. G. Kokotos, ACS Catal., 2013, 3, 2239–2243 CrossRef CAS; (b) D. Limnios and C. G. Kokotos, Chem. – Eur. J., 2014, 20, 559–563 CrossRef CAS PubMed; (c) E. Voutyritsa, A. Theodorou, M. G. Kokotou and C. G. Kokotos, Green Chem., 2017, 19, 1291–1298 RSC; (d) I. Triandafillidi and C. G. Kokotos, Org. Lett., 2017, 19, 106–109 CrossRef CAS PubMed; (e) I. Triandafillidi, M. Raftopoulou, A. Savvidou and C. G. Kokotos, ChemCatChem, 2017, 9, 4120–4124 CrossRef CAS; (f) A. Theodorou and C. G. Kokotos, Adv. Synth. Catal., 2017, 359, 1577–1581 CrossRef CAS; (g) A. Theodorou and C. G. Kokotos, Green Chem., 2017, 19, 670–674 RSC; (h) A. Tsoukaki, E. Skolia, I. Triandafillidi and C. G. Kokotos, Eur. J. Org. Chem., 2022, e202200463 CrossRef CAS; (i) A. Theodorou, I. Triandafillidi and C. G. Kokotos, Adv. Synth. Catal., 2018, 360, 951–957 CrossRef CAS.
- I. Triandafillidi, M. G. Kokotou, D. Lotter, C. Sparr and C. G. Kokotos, Chem. Sci., 2021, 12, 10191–10196 RSC.
- For full optimization and mechanistic studies, see the SI.
- (a) G. B. Payne and P. H. Williams, J. Org. Chem., 1961, 26, 651–659 CrossRef CAS; (b) L. A. Arias, S. Adkins, C. J. Nagel and R. D. Bach, J. Org. Chem., 1983, 48, 888–890 CrossRef CAS; (c) S. Ye, J. J. Hu and D. Yang, Angew. Chem., Int. Ed., 2018, 57, 10173–10177 CrossRef CAS PubMed.
- H. D. Dakin, Am. Chem. J., 1909, 42, 477–498 Search PubMed.
- J. Wahlen, D. E. De Vos and P. A. Jacobs, Org. Lett., 2003, 5, 1777–1780 CrossRef CAS PubMed.
- For selected studies using DI-HRMS to study reaction mechanisms, see: (a) F. Bachle, J. Duschmale, C. Ebner, A. Pfaltz and H. Wennemers, Angew. Chem., Int. Ed., 2013, 52, 12619–12623 CrossRef PubMed; (b) F. Bachle, I. Fleischer and A. Pfaltz, Adv. Synth. Catal., 2015, 357, 2247–2254 CrossRef; (c) P. G. Iseneger, F. Bachle and A. Pfaltz, Chem. – Eur. J., 2016, 22, 17595 CrossRef PubMed; (d) N. Kaplaneris, C. Spyropoulos, M. G. Kokotou and C. G. Kokotos, Org. Lett., 2016, 18, 5800–5803 CrossRef CAS PubMed; (e) I. Triandafillidi, M. G. Kokotou and C. G. Kokotos, Org. Lett., 2018, 20, 36–39 CrossRef CAS PubMed; (f) C. S. Batsika, C. Koutsilieris, G. S. Koutoulogenis, M. G. Kokotou, C. G. Kokotos and G. Kokotos, Green Chem., 2022, 24, 6224–6231 RSC; (g) O. G. Mountanea, C. Mantzourani, M. G. Kokotou, C. G. Kokotos and G. Kokotos, Eur. J. Org. Chem., 2023, e202300046 CrossRef CAS; (h) O. G. Mountanea, D. Psathopoulou, C. Mantzourani, M. G. Kokotou, E. A. Routsi, D. Tzeli, C. G. Kokotos and G. Kokotos, Chem. – Eur. J., 2023, 29, e202300556 CrossRef CAS PubMed; (i) E. T. Poursaitidis, F. Trigka, C. Mantzourani, M. G. Kokotou, I. Triandafillidi and C. G. Kokotos, Eur. J. Org. Chem., 2024, e202400082 CrossRef CAS.
- J. Luo, J. Yao and H. Li, Green Chem., 2022, 24, 3218–3224 RSC.
- (a) R. P. Heggs and B. Ganem, J. Am. Chem. Soc., 1979, 101, 2484–2486 CrossRef CAS; (b) P. A. Ganeshpure and W. Adam, Synthesis, 1996, 179–188 CrossRef CAS; (c) W. Adam, H.-G. Degen and C. R. Saha-Möller, J. Org. Chem., 1999, 64, 1274–1277 CrossRef CAS.
- (a) R. G. Jones and H. A. Shonle, J. Am. Chem. Soc., 1945, 67, 1034–1035 CrossRef CAS; (b) J. A. Pedersen, J. Chem. Soc., Perkin Trans. 2, 1973, 424–431 RSC; (c) A. Brunmark, J. Biolumin. Chemilumin., 1989, 4, 219–225 CrossRef CAS PubMed.
- R. Sůra and M. Antalík, Spectrochim. Acta, Part A, 2022, 271, 120863 CrossRef PubMed.
- (a) T. Hosoya and T. Rosenau, J. Org. Chem., 2013, 78, 3176–3182 CrossRef CAS PubMed; (b) T. Hosoya and T. Rosenau, J. Org. Chem., 2013, 78, 11194–11203 CrossRef CAS PubMed; (c) N. S. Zwirchmayr, T. Hosoya, U. Henniges, L. Gille, M. Bacher, P. Furtmüller and T. Rosenau, J. Org. Chem., 2017, 82, 11558–11565 CrossRef CAS PubMed; (d) M. Bacher, T. Hosoya, N. S. Zwirchmayr, S. Nomura, L. Gille, T. Dietz, T. Erata, A. Potthast, T. Vuorinen and T. Rosenau, Cellulose, 2018, 25, 3197–3203 Search PubMed.
- For a radical ring opening example of cyclopropane, see: (a) Q.-Q. Zhao, X.-S. Zhou, S.-H. Xu, Y.-L. Wu, W.-J. Xiao and J.-R. Chen, Org. Lett., 2020, 22, 2470–2475 CrossRef CAS PubMedFor radical clock examples, see: (b) Y. Li, D. E. Wise, J. K. Mitchell and M. Parasram, J. Org. Chem., 2023, 88, 717–721 CrossRef CAS PubMed; (c) T. Kösel, G. Schulz, G. Dräger and A. Kirschning, Angew. Chem., Int. Ed., 2020, 59, 12376–12380 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 equiv. of MeCN/H2O2 were used. Yield is determined by 1H-NMR, and the yield of the isolated product after column chromatography is given in parentheses.
16 equiv. of MeCN/H2O2 were used. Yield is determined by 1H-NMR, and the yield of the isolated product after column chromatography is given in parentheses.