 Open Access Article
Open Access ArticleClosed-loop recycling of bio-based disulfide vitrimer via a solvent-and waste-free strategy†
Solène
Guggari
ab,
Fiona
Magliozzi
b,
Samuel
Malburet
 b,
Alain
Graillot
b,
Alain
Graillot
 b,
Mathias
Destarac
a and
Marc
Guerre
b,
Mathias
Destarac
a and
Marc
Guerre
 *a
*a
aLaboratoire SOFTMAT, Université de Toulouse, CNRS UMR 5623, Université Paul Sabatier, 118 route de Narbonne, 31062 Toulouse Cedex 9, France. E-mail: marc.guerre@cnrs.fr
bSPECIFIC POLYMERS, Zac Via Domitia, 150 Avenue des Cocardières, 34160 Castries, France
First published on 19th May 2025
Abstract
This work presents a solvent-free recycling method for epoxy-based vitrimers using cystamine as a reactant and depolymerizing agent. The process depolymerizes the vitrimer network via disulfide exchange, enabling the recovery of amine-terminated oligomers that can be re-crosslinked. Applied to carbon-fiber composites, it offers a scalable, sustainable closed-loop recycling solution.
Green foundation1. This strategy addresses the urgent need for more sustainable solutions in polymer recycling, particularly for high-performance thermoset composites, which are widely used but notoriously difficult to recycle. This study presents a novel closed-loop chemical recycling strategy for disulfide-based epoxy vitrimers using cystamine, a bio-based hardener that acts as both a reactant and a depolymerizing agent.2. A solvent-free process enables the efficient degradation of the vitrimer matrix under mild conditions, producing oligomers that can be directly reused without purification. 3. Efforts to reduce the temperature of depolymerization through the help of catalysis could save energy. |
Introduction
The growing accumulation of plastic waste has emerged as a critical environmental challenge, demanding innovative and scalable recycling strategies that can move us toward a circular materials economy. While mechanical recycling remains the dominant method, it is limited by polymer degradation and contamination, often resulting in downcycled products with inferior properties. This has spurred global interest in chemical and closed-loop recycling approaches, which aim to restore or retain the original performance and purity of polymers.1–3 Thermoplastics and more specifically polyolefins like polyethylene (PE)—which constitute the largest global plastic production—have also seen renewed focus for chemical upcycling and monomer recovery due to their sheer volume and environmental footprint.4,5 Despite recent progress, comprehensive strategies that integrate thermoset and thermoplastic recycling under a unified framework remain scarce.Thermoset composites have become important materials in the construction, transportation, aerospace, military manufacturing, and automotive industries due to their high strength, high modulus and versatile applicability. These materials can effectively reduce the weight of technical components and possess special characteristics that are appealing for high performance applications. Epoxy-based thermoset composites are often the preferred choice among carbon fiber reinforced composites (CFRPs) for high-performance applications owing to their structural stability, high strength, low shrinkage, and resistance to solvents.6–9 However, the irreversible cross-linking of epoxy thermosets limits their recyclability and repairability, leading to environmental concerns as they are often incinerated or discarded at the end of their life.10,11
To address these challenges, various recycling methods have been developed. Mechanical degradation is widely used but can lead to energy-intensive processes and reduced quality of recycled fibers.12–15 Solvolysis, which converts thermoset waste into soluble monomers, has also been explored but often results in downcycling and requires harsh conditions or expensive catalysts.16,17 Consequently, considerable research is now focusing on developing recycling methodologies for epoxy thermosets, with the aim of recovering both monomers and fibers. In this context, dynamic covalent bonds offer a promising solution to the recyclability and healability limitations of conventional epoxy thermosets.18–21 The introduction of dynamic bonds, which are inherently less robust and more prone to cleavage, paves the way for new recycling methods, greatly improving the sustainability and lifespan of these materials. These advancements have sparked renewed interest in the development of more sustainable thermoset systems.
Various dynamic exchanges have been used to chemically recycle dynamic materials. Ester bonds are among the most commonly used due to their susceptibility to hydrolysis or transesterification reactions. For instance, Zhang and co-workers22 demonstrated the degradation of an eugenol-based epoxy vitrimer via ethanol-driven transesterification at 160 °C, catalyzed by zinc acetylacetonate. The decomposed polymers can be reconverted into new thermosetting polymers. However, this process requires high temperatures and releases ethanol. Imine bonds have also been extensively utilized due to their reversible reaction with water. Zhao and co-workers23 prepared an imine-based thermoset from vanillin and 4-aminophenol, which degraded in concentrated hydrochloric acid (HCl) and dimethylformamide mixture (DMF), yielding oligomers with aldehyde or amine end groups. Upon solvent removal, imine bonds re-formed, restoring the thermoset's original properties. Similarly, Weder and co-workers24 applied this concept to vinylogous urethane vitrimers, which can undergo water-induced degradation at moderate temperatures, enabling a nearly quantitative recovery (≈98%). Not only degradable bonds were investigated. Bowman and coworkers25 showed that thiol-thioester networks could be degraded without external reactants by adding acetone, excess tetrathiol monomer, and triethylamine, generating oligomers that could be repolymerized with added diene. These approaches highlight the versatility of dynamic covalent chemistries in enabling material degradation and recyclability. However, many of these recycling methods require harsh conditions, specialized catalysts, or excessive solvent use, which can hamper scalability, increase processing costs, and raise environmental concerns. While the ability to degrade materials with water offers advantages for recycling, it also implies poor stability under typical hydrolytic conditions, making these materials unsuitable for many applications that require long-term performance and durability.
Although disulfide-based vitrimers have been explored for recyclability, existing methods suffer from significant limitations. Tesoro and Sastri26,27 reported degradation of epoxy resins containing disulfide bonds by treatment with triphenylphosphine. However, the degradation products were not reused and the resulting chemical structures were not characterized. More recent recycling methods triggered chemical reduction of the disulfide bond with a reducing agent such as 2-mercaptoethanol or dithiothreitol (DTT) in a suitable solvent leading to a complete dissolution of the network.28–31 DTT enables faster exchange than monothiols due to the stability of the cyclic disulfide formed.32 Alternatively, thiol–disulfide exchange with a disulfide like diphenyldisulfide33 has been used to depolymerize disulfide-containing epoxy vitrimers. However, in all of these strategies, the degraded matrix contains a complex mixture of functional groups, which limits its direct reuse without additional purification or chemical modification. There is thus a clear need to develop more efficient, scalable, and environmentally friendly recycling strategies for disulfide-based vitrimers.
In this work, we present a novel, robust, and versatile recycling method for epoxy vitrimers based on disulfide exchange reactions. Unlike conventional methods that rely on solvents, catalysts, or degrading agents such as DTT, our approach employs a solvent-free process utilizing cystamine, a bio-based synthon that acts as both the degrading and reacting agent. The degradation process leverages disulfide exchanges between the polymer matrix and cystamine, efficiently converting the material into oligomers in bulk without requiring solvent evaporation or purification steps. These oligomers, rich in amine groups, can be directly repurposed as hardeners to re-synthesize the original materials. This recycling concept was further validated by preparing carbon fiber–reinforced composites, where the polymer matrix was successfully removed and reformed.
Results and discussion
Due to the reversible nature of disulfide bonds, disulfide-based vitrimers can be readily degraded using conventional approaches involving organic solvents and an excess of nucleophilic reactants such as thiols. However, this approach poses environmental concerns, as both thiols and most organic solvents efficient at swelling polymer networks are classified as carcinogenic, mutagenic and reprotoxic substances. Additionally, through this process, the depolymerized matrix contains a mixture of degraded oligomers mixed with free thiols and/or hydroxy-functional groups, which cannot be directly reused without purification or reacted via the original chemical process. This limitation hinders the material's recyclability. Therefore, developing inherently recyclable, high-performance vitrimers that can be easily recycled under mild conditions and enable the recovery of all starting materials is a crucial research objective.Therein, we explored a more environmentally friendly recycling method. Specifically, we used cystamine as both a degrading agent and reactive medium (Fig. 1).
We hypothesized that the disulfide groups in the cystamine structure could easily undergo exchanges with the disulfide functionalities in the vitrimer network, resulting in the formation of liquid oligomers with aliphatic primary amine end groups (see Fig. 1). These newly formed amine-containing oligomers are expected to be liquid and reactive with epoxy resins, enabling the reformation of the original material with the same structure and composition. These strategies prevent any loss of matter or the need for additional purification steps or solvent evaporation.
Following the procedure described in Fig. 1, the depolymerization step was carried out using different quantities of cystamine, i.e., 2, 5 or 10 equivalents in weight with respect to the crosslinked materials, respectively corresponding to a recycled material content for hardener mixture calculated as 33 wt%, 17 wt% and 9 wt%. The starting materials were prepared according to an already published procedure using diglycidyl ether of vanillyl alcohol (DGEVA) as epoxy and cystamine as dynamic hardener.34 The crosslinked materials were crushed into powder and immersed in pure cystamine for 24 h at 60 °C. Although 2 equivalents were not enough to completely depolymerize the material, complete degradation of the vitrimer matrix was achieved with 5 and 10 equivalents after around 24 hours. The selection of 5 equivalents of cystamine as the lower limit ensures an optimal balance between effective swelling of the epoxy matrix and a sufficient concentration of disulfide bonds, thereby promoting efficient matrix depolymerization. The resulting viscous liquid, containing oligomers and unreacted cystamine, was used as a cross-linking agent and mixed directly with DGEVA epoxy resin to reform the original vitrimer using a standard curing procedure: 1 hour at 60 °C, followed by 2 hours at 90 °C. The recycled materials obtained were designated as DGEVA/cyst5 (R) and DGEVA/cyst10 (R), corresponding to a final recycled content of approximately 4 wt% and 2 wt%, respectively. To achieve the same physicochemical properties, it is crucial to maintain the initial stoichiometry and carefully control the amount of cystamine added. The ratio of cystamine and the added epoxy must closely match that of the initial material, which in this case is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 epoxy to NH2.
1.2 epoxy to NH2.
The recycled materials, DGEVA/cyst5 (R) and DGEVA/cyst10 (R), were characterized by differential scanning calorimetry (DSC) analysis (Fig. 2). No residual peaks were observed during the first heating cycle, indicating a successful cross-linking process for both materials. As shown in Fig. 2, the DSC thermograms of DGEVA/cyst5 (R) and DGEVA/cyst10 (R) showed comparable glass transition temperatures (Tg), 51 °C and 53 °C for DGEVA/cyst5 (R) and DGEVA/cyst10 (R), respectively. This result shows that reducing the amount of cystamine from 10 to 5 equivalents has no significant impact on the glass transition of the recycled materials. However, a lower limit exists, as using only 2 equivalents of cystamine appears insufficient to fully degrade the matrix.
 | ||
| Fig. 2 DSC thermograms of recycled DGEVA/cyst with 10 (green) and 5 equivalents (blue) of cystamine compared to the original material (orange). | ||
This is likely due to both swelling limitations, which hinder efficient diffusion of the reagent, and concentration effects, where a lower cystamine content reduces the concentration of disulfide groups necessary for effective degradation. To investigate potential structural changes before and after depolymerization, Fourier-transform infrared spectroscopy (FTIR) analysis was performed on both the original and recycled materials (see Fig. 3). No chemical modification was observed, indicating that the epoxy network is effectively restored after the curing cycles.
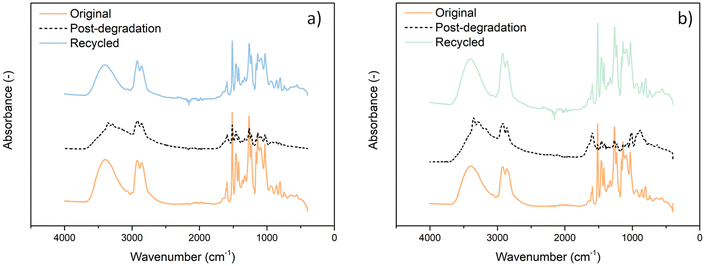 | ||
| Fig. 3 FTIR analysis of post-degraded formulation (dashed line), (a) DGEVA/cyst5 (R, blue) and (b) DGEVA/cyst10 (R, green) compared to the original material (orange). | ||
A complementary study was carried out by Dynamic Mechanical Analysis (DMA) (Fig. 4 and Table S1†). For the two recycled materials, the viscoelastic relaxation temperature α (Tα) corresponds well with the glass transition temperature determined by DSC and are comparable to the original materials. The slightly higher half-height widths of the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak in the recycled materials compared to the original suggest a less homogeneous network structure. This variation may result from differences in polymer chain distribution or crosslink density introduced during the recycling process, potentially leading to a less uniform network structure. In addition, the storage moduli E′ at 25 °C (on the glassy plate) for DGEVA/cyst10 (R) and DGEVA/cyst5 (R) show similar values to the original material (3709 MPa and 3457 MPa, respectively vs. 2980 MPa). The E′ moduli taken on the rubbery plateau (Tα + 30 °C) are also in the same range for DGEVA/cyst and DGEVA/cyst10 (R), while a slight increase is observed for the DGEVA/cyst5 (R) material, which remains within the margin of measurements error.
δ peak in the recycled materials compared to the original suggest a less homogeneous network structure. This variation may result from differences in polymer chain distribution or crosslink density introduced during the recycling process, potentially leading to a less uniform network structure. In addition, the storage moduli E′ at 25 °C (on the glassy plate) for DGEVA/cyst10 (R) and DGEVA/cyst5 (R) show similar values to the original material (3709 MPa and 3457 MPa, respectively vs. 2980 MPa). The E′ moduli taken on the rubbery plateau (Tα + 30 °C) are also in the same range for DGEVA/cyst and DGEVA/cyst10 (R), while a slight increase is observed for the DGEVA/cyst5 (R) material, which remains within the margin of measurements error.
 | ||
Fig. 4 Storage modulus E′ and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of recycled material DGEVA/cyst5 (R) (blue) and DGEVA/cyst10 (R) (green) compared to the original material DGEVA/cyst (orange). δ of recycled material DGEVA/cyst5 (R) (blue) and DGEVA/cyst10 (R) (green) compared to the original material DGEVA/cyst (orange). | ||
The impact of the recycling procedure on vitrimer properties was then evaluated through stress relaxation experiments (Fig. 5a, Table S2 and Fig. S1†).
As shown in Fig. 5a, both recycled networks exhibit complete stress relaxation, albeit at a slower rate than the original material. A direct comparison of relaxation profiles at 140 °C reveals a noticeable deceleration in the exchange dynamics of the recycled networks compared to the original. This observation is confirmed by the relaxation times 〈τ〉, determined using the Kohlrausch–Williams–Watts (KWW) model (see ESI, equation (S1), (S2) and Table S2†).
The relaxation times increase by approximately a factor of 4.5, with 〈τ〉 rising from 74 s for DGEVA/cyst to 341 s and 367 s for DGEVA/cyst10 (R) and DGEVA/cyst5 (R), respectively. This slower relaxation behavior can be attributed to the introduction of heterogeneities and/or structural defects during the recycling process, as observed by DMA, which alters the macromolecular architecture while preserving overall dynamicity. This trend is also reflected in the breadth relaxation factor (β), which decreases from an average value of 0.61 for the original material to 0.55 and 0.47 for DGEVA/cyst10 (R) and DGEVA/cyst5 (R), respectively. Although the thermomechanical properties remain largely unchanged regardless of whether 5 or 10 equivalents of cystamine are used, the β factor suggests that a higher cystamine content (10 equivalents) leads to reduced network heterogeneity. This is likely due to the formation of lower-molecular-weight oligomers during the recycling procedure.
The temperature dependence of relaxation times for both recycled materials follows the Arrhenius equation, consistent with vitrimer behavior (Fig. 5b). The activation energies (Ea) for the recycled materials were determined to be 46 kJ mol−1 for DGEVA/cyst10 (R) and 53 kJ mol−1 for DGEVA/cyst5 (R), respectively, which closely match the activation energy of the original DGEVA/cyst vitrimer (57 kJ mol−1). Overall, these results highlight that the dynamic exchange mechanisms facilitated by cystamine enable an efficient and sustainable recycling process for cystamine-based vitrimer resins.
This new recycling method was then applied to CFRP composites.35,36 Here, the focus is on the recyclability of the resin, setting it apart from CFRP systems in the literature based on dynamic covalent bonds, where recyclability is primarily centered on fiber recovery.
Carbon fiber–reinforced composites were synthesized with DGEVA/cyst resin. Mono- (MO) and multilayered (MU) composites were prepared following the protocol mentioned in the Experimental section and displayed in Fig. 6. Both composites were analyzed by DMA (Fig. S3 and Table S3†).
 | ||
| Fig. 6 Synthesis of carbon fiber composite via a vacuum bag process, followed by depolymerization with cystamine, fiber separation, and subsequent crosslinking of the degraded mixture with epoxy. | ||
The DGEVA/cyst/CF MO and MU composites showed typical mechanical properties for carbon-based composites with modulus E′ of 12.4 GPa and 54.7 GPa, respectively. The MO composite (consisting of epoxy matrix and carbon fibers) was then immersed in 10 weight equivalents of cystamine and stirred at 60 °C for 15 hours. A change in color, a slight increase in viscosity, and a loss of material integrity indicated degradation of the polymer matrix (Fig. 6). Subsequently, the epoxy matrix and carbon fiber were successfully separated manually, and the degraded fraction was crosslinked with DGEVA. Any excess cystamine remaining on the carbon fibers was easily removed by washing with ethanol. All properties were similar to those of the previously reported degraded matrix with similar deviation as observed for non-composite counterpart (Fig. 7). The glass transition temperatures (Tg) were both 53 °C, and the relaxation time of the recycled material at 140 °C was as expected higher than the reference, showing 170 s (vs. 74 s) (Fig. 7, Fig. S2 and Table S4†), but slightly faster than that of the fiber-free recycling strategy (DGEVA/cyst10 (R)). The only significant difference observed was in the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak, which appeared noticeably broader compared to the initial recycling strategy, with, in addition, a lowering of the crosslink density.
δ peak, which appeared noticeably broader compared to the initial recycling strategy, with, in addition, a lowering of the crosslink density.
This broadening and decrease of crosslink density may be attributed to the incomplete removal of cystamine from the carbon fibers, potentially leading to slight deviations in stoichiometry and, consequently, a less uniform network structure. This issue is more challenging to address in fiber-reinforced systems than in non-reinforced polymers. A possible solution would involve implementing an additional washing step to completely eliminate residual cystamine from the fibers, followed by precise recovery and quantification of the removed cystamine to restore the exact stoichiometric balance. While such an approach could theoretically achieve near-perfect stoichiometry, it would introduce an additional processing step requiring a solvent. However, given that cystamine is soluble in ethanol, this step would not necessitate the use of hazardous organic solvents such as DMF. Nevertheless, since the primary objective of this study was to develop a solvent-free recycling methodology, further optimization of this aspect is beyond the current scope.
Conclusions
In this work, we have developed a novel and sustainable chemical recycling strategy for disulfide-based epoxy vitrimer by utilizing a bio-based hardener, cystamine, as a depolymerizing agent. This method enables efficient degradation of the vitrimer matrix under mild conditions while allowing for the direct reuse of the degraded material without additional purification steps. The recovered systems, DGEVA/cyst (R), exhibited thermomechanical properties comparable to the original material, demonstrating successful reprocessability and structural integrity. By leveraging disulfide exchange reactions, we have established a broadly applicable approach for recycling disulfide-containing networks in a simple yet effective manner. This methodology offers a promising route for the depolymerization and recrosslinking of epoxy-based vitrimers, potentially extending to other disulfide vitrimers. The full material recovery, along with its ability to retain functional and mechanical properties, highlights the potential for scalable implementation in sustainable material design. Furthermore, we successfully applied this recycling process to carbon fiber-reinforced polymer (CFRP) composites, demonstrating efficient matrix degradation and matrix recovery. The resulting recycled composites, DGEVA/cyst/CF, maintained high mechanical performance, confirming the feasibility of this closed-loop recycling system. Overall, this study represents a significant advancement in vitrimer recycling by offering an environmentally friendly, efficient, and scalable closed-loop strategy. This breakthrough paves the way for the development of high-performance, inherently recyclable materials, addressing key challenges in polymer sustainability and circular economy initiatives.Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request. All experimental data, including raw measurements, characterization results, and ESI,† will be stored in our institutional database and are accessible for verification purposes upon paper acceptance. Where applicable, additional datasets have been included in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the VIBES project. This project has received funding from the Bio-Based Industries Joint Undertaking (JU) under grant agreement no.101023190. The JU receives support from the European Union's Horizon 2020 research and innovation program and the Bio-Based Industries Consortium. This article reflects only the authors’ view, and the JU is not responsible for any use that may be made of the information it contains. The authors would like to thank Antoine Suty and Hugo Bertrand for their help in the preparation of composites and DMA analyses.References
- J. M. Garcia and M. L. Robertson, Science, 2017, 358, 870–872 CrossRef CAS PubMed.
- L. T. J. Korley, T. H. Epps, B. A. Helms and A. J. Ryan, Science, 2021, 373, 66–69 CrossRef CAS PubMed.
- B. Qin, S. Liu, Z. Huang, L. Zeng, J.-F. Xu and X. Zhang, Nat. Commun., 2022, 13, 7595 CrossRef CAS PubMed.
- S. T. Schwab, M. Baur, T. F. Nelson and S. Mecking, Chem. Rev., 2024, 124, 2327–2351 CrossRef CAS PubMed.
- Y. Ma, X. Jiang, X. Xiang, P. Qu and M. Zhu, Green Chem., 2025, 27, 4040–4093 RSC.
- F.-L. Jin, X. Li and S.-J. Park, J. Ind. Eng. Chem., 2015, 29, 1–11 CrossRef CAS.
- C. May, Epoxy Resins: Chemistry and Technology, Routledge, 2nd edn, 2018 Search PubMed.
- P. Mohan, Polym.-Plast. Technol. Eng., 2013, 52, 107–125 CrossRef CAS.
- H. Dodiuk, Handbook of Thermoset Plastics, William Andrew, 2021 Search PubMed.
- Y. Yang, R. Boom, B. Irion, D.-J. van Heerden, P. Kuiper and H. de Wit, Chem. Eng. Process., 2012, 51, 53–68 CrossRef CAS.
- S. Utekar, V. K. Suriya, N. More and A. Rao, Composites, Part B, 2021, 207, 108596 CrossRef CAS.
- M. M. Haider, S. Nassiri, K. Englund, H. Li and Z. Chen, Transp. Res. Rec., 2021, 2675, 1254–1267 CrossRef.
- W. Post, A. Susa, R. Blaauw, K. Molenveld and R. J. I. Knoop, Polym. Rev., 2020, 60, 359–388 CrossRef CAS.
- P. S. Roy, G. Garnier, F. Allais and K. Saito, ChemSusChem, 2021, 14, 4007–4027 CrossRef CAS PubMed.
- C. Ma, D. Sánchez-Rodríguez and T. Kamo, Polym. Degrad. Stab., 2020, 178, 109199 CrossRef CAS.
- D. Tortorici, Y. Chen, L. Mishnaevsky and S. Laurenzi, Composites, Part A, 2025, 190, 108667 CrossRef CAS.
- W. Ballout, N. Sallem-Idrissi, M. Sclavons, C. Doneux, C. Bailly, T. Pardoen and P. Van Velthem, Sci. Rep., 2022, 12, 5928 CrossRef CAS PubMed.
- W. Denissen, J. M. Winne and F. E. Du Prez, Chem. Sci., 2016, 7, 30–38 RSC.
- N. J. Van Zee and R. Nicolaÿ, Prog. Polym. Sci., 2020, 101233 CrossRef CAS.
- M. Guerre, C. Taplan, J. M. Winne and F. E. D. Prez, Chem. Sci., 2020, 11, 4855–4870 RSC.
- V. Schenk, K. Labastie, M. Destarac, P. Olivier and M. Guerre, Mater. Adv., 2022, 3, 8012–8029 RSC.
- T. Liu, C. Hao, L. Wang, Y. Li, W. Liu, J. Xin and J. Zhang, Macromolecules, 2017, 50, 8588–8597 CrossRef CAS.
- S. Zhao and M. M. Abu-Omar, Macromolecules, 2018, 51, 9816–9824 CrossRef CAS.
- Y. Ma, X. Jiang, Z. Shi, J. A. Berrocal and C. Weder, Angew. Chem., Int. Ed., 2023, 62, e202306188 CrossRef CAS PubMed.
- C. Wang, T. M. Goldman, B. T. Worrell, M. K. McBride, M. D. Alim and C. N. Bowman, Mater. Horiz., 2018, 5, 1042–1046 RSC.
- G. C. Tesoro and V. Sastri, J. Appl. Polym. Sci., 1990, 39, 1425–1437 CrossRef CAS.
- V. R. Sastri and G. C. Tesoro, J. Appl. Polym. Sci., 1990, 39, 1439–1457 CrossRef CAS.
- I. Azcune and I. Odriozola, Eur. Polym. J., 2016, 84, 147–160 CrossRef CAS.
- D. Martinez-Diaz, A. Cortés, A. Jiménez-Suárez and S. G. Prolongo, ACS Appl. Polym. Mater., 2022, 4, 5068–5076 CrossRef CAS.
- B. Li, G. Zhu, Y. Hao and T. Ren, J. Appl. Polym. Sci., 2022, 139, 51589 CrossRef CAS.
- P. Verdugo, D. Santiago, S. De la Flor and À. Serra, ACS Sustainable Chem. Eng., 2024, 12, 5965–5978 CrossRef CAS PubMed.
- W. W. Cleland, Biochemistry, 1964, 3, 480–482 CrossRef CAS PubMed.
- A. Takahashi, T. Ohishi, R. Goseki and H. Otsuka, Polymer, 2016, 82, 319–326 CrossRef CAS.
- S. Guggari, F. Magliozzi, S. Malburet, A. Graillot, M. Destarac and M. Guerre, ACS Sustainable Chem. Eng., 2023, 11, 6021–6031 Search PubMed.
- Y. Yang, R. Boom, B. Irion, D.-J. van Heerden, P. Kuiper and H. de Wit, Chem. Eng. Process., 2012, 51, 53–68 Search PubMed.
- A. Saccani, S. Manzi, I. Lancellotti and L. Lipparini, Constr. Build. Mater., 2019, 204, 296–302 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc01872b |
| This journal is © The Royal Society of Chemistry 2025 |



