Open Access Article
Open Access Article“On-seawater” accelerated aquacatalysis by edible fatty acids: harnessing the remarkable salting-out effect†
Soo Bok
Kim
 a,
Seok Ju
Hong
a,
Dong Hyeon
Kim
a,
Gang Min
Lee‡
a,
Seok Ju
Hong
a,
Dong Hyeon
Kim
a,
Gang Min
Lee‡
 a,
Yujin
Lim
b,
Sangkyu
Lee
b,
Yongseok
Kwon
a,
Yujin
Lim
b,
Sangkyu
Lee
b,
Yongseok
Kwon
 b and
Han Yong
Bae
b and
Han Yong
Bae
 *a
*a
aDepartment of Chemistry, Sungkyunkwan University, Suwon, 16419, Republic of Korea. E-mail: hybae@skku.edu
bSchool of Pharmacy, Sungkyunkwan University, Suwon 16419, Republic of Korea
First published on 3rd June 2025
Abstract
Medium-chain fatty acids (MCFAs) are widely recognized for their metabolic and therapeutic benefits, yet their potential as catalysts for chemical reactions remains elusive. Herein, we report the development of a sustainable aquacatalytic system utilizing MCFAs, particularly caprylic acid, for Brønsted acid-catalyzed carbonyl allylation under “on-water” conditions. This approach leveraged the salting-out effect induced by NaCl to suppress micelle formation and enhance interfacial catalysis. By employing unrefined seawater and edible sea salt as cost-effective additives, the system enables the efficient allylation of aldehydes without a catalyst and the caprylic acid-catalyzed allylation of ketones. This reaction is scalable to gram-scale synthesis using a chromatography-free purification process, offering a practical and sustainable route for producing active pharmaceutical ingredients. Furthermore, the mild reaction conditions and compatibility with aqueous media facilitated successful on-DNA allylation, underscoring the potential of DNA-encoded library applications in drug discovery. This study highlights the unprecedented utility of MCFAs as renewable catalysts and establishes a versatile and environmentally friendly platform for aquatic organic transformations.
Green foundation1. This study introduces a new sustainable aquacatalytic system utilizing medium-chain fatty acids (MCFAs), especially caprylic acid, for Brønsted acid-catalyzed carbonyl allylation under “on-water” conditions. The system operates efficiently using seawater or edible sea salt, offering a low-cost and environmentally benign alternative to conventional solvent systems.2. The core innovation exploits the salting-out effect to suppress micelle formation and enhance interfacial reactivity, enabling catalyst-free aldehyde allylation and caprylic acid-catalyzed ketone allylation in aqueous media. The reaction conditions are mild, scalable, and compatible with DNA-tagged substrates, opening avenues for pharmaceutical synthesis and drug discovery. 3. Future directions may involve broadening the scope of MCFA-based catalysis across other aqueous transformations, integrating renewable MCFA sources from food or waste streams, and developing entirely water-based catalytic platforms to further enhance the system's green chemistry potential. |
Introduction
Medium-chain fatty acids (MCFAs), which are carboxylic acids with carbon chain lengths of 6–12, are rapidly digested and absorbed by the human body and serve as an efficient energy source.1–4 In addition to their metabolic roles, MCFAs offer a range of health benefits, including weight loss support,5–7 reduction in cardiovascular disease risk,8 enhancement of diet-induced thermogenesis and satiety,9 antimicrobial activity,10 and improved digestive efficiency.11 These unique properties have garnered significant attention in nutritional science and medical research, as they are valuable components in functional foods and therapeutic interventions.3,12–14 In addition to their biological significance, MCFAs exhibit distinctive amphiphilic properties owing to their chemical structure, which features a hydrophilic head and a hydrophobic tail. This structure imparts surfactant-like characteristics, including excellent biocompatibility, biodegradability, low toxicity, and high specificity. These characteristics make MCFAs suitable for applications such as drug delivery systems in both synthetic and biomedical contexts.15 Notably, in aqueous environments, MCFAs can self-assemble into micelles at their critical micelle concentration (CMC), which enables them to perform stably and efficiently under physiological conditions.16,17 Caprylic acid (C8:0), a prominent MCFA, exemplifies the advantages of this class of compounds. The carbon chain enhances its solubility and metabolic efficiency, facilitating its rapid absorption and direct utilization as an energy source in the liver.18 In addition, its potent antimicrobial activity against various pathogens underscores its utility in functional foods and therapeutic applications.10 Furthermore, their ability to form stable micelles highlights their potential as versatile carriers.19,20 However, their role as catalysts in chemical reactions remains entirely elusive (Fig. 1A).Recent studies by our group have revealed that various catalytic transformations can be effectively accelerated under “on-water” conditions,21–25 where reactions occur at the interface between bulk-water and compact-oil phases.26,27 Mechanisms such as the use of Brønsted superacids21 and Brønsted superbases,22,24 and photocatalysis25 were observed, which significantly augmented the catalytic activity at the interface.28 This phenomenon is mainly observed in addition-type reactions (e.g., Michael addition and [2 + 2] cycloaddition), which typically involve a decrease in the volume of the transition state.29 Although multiple factors may contribute, it is hypothesized that the reactions occurring within a water-induced, confined organic cage result in a high-pressure-like effect from the surrounding bulk water.30 In addition, an enriched hydrogen bond donated by water at the interface is a key factor in facilitating this enhancement.31 In particular, we discovered that the salting-out effect induced by high-concentration NaCl solutions suppressed micelle formation and increased the interfacial area, further enhancing the reaction efficiency. We hypothesized that this effect could provide a basis for the efficient functioning of MCFAs as Brønsted acid organocatalysts, particularly below their CMCs (Fig. 1B).
Herein, we report the “on-water” accelerated Brønsted acid-catalyzed aqueous reactions with MCFAs. As a representative model reaction, the allylboration of a general class of carbonyl compounds was demonstrated, exemplifying the scalability of the gram-scale synthesis. A crucial discovery in this study was the specific salting-out effect induced by NaCl, which proved to be essential for the success of the reaction. Remarkably, the reaction was also effectively catalyzed using very low-cost and unrefined additives such as seawater or edible sea salt, enabling the synthesis of high-purity pharmaceutical intermediates (APIs). The concentrated NaCl solution suppressed the self-assembly of MCFAs as surfactants, thereby enhancing the efficiency of the reaction under “on-water” conditions, specifically at the water–oil interface. This finding highlights the pivotal role of the salting-out effect in facilitating interfacial catalysis and offers a simple and sustainable approach for scalable and cost-effective organic transformations (Fig. 1C).
Results and discussion
Condition optimization of catalyst-free allylation of aldehydes
We aimed to develop sustainable allylation reactions of general carbonyl compounds (Table 1). For our model reaction, we selected hydrocinnamaldehyde to initiate investigation with aliphatic aldehydes. Allylboronic acid pinacol ester was selected as the allylating reagent owing to its low toxicity and availability.32 We first explored conventional organic solvents, such as dichloromethane and acetonitrile stirred at 25 °C. However, only very low conversions to product 1 were obtained (23% and 27% for entries 1 and 2, respectively). The neat condition (no solvent) gave a similarly low reactivity (26%, entry 3). Based on our previous findings, we speculate that aqueous conditions with bulk water as the reaction medium may improve the outcome. In particular, allylboronic acid pinacol ester is well known to exhibit reasonable activity in aqueous reaction systems.33,34 To our surprise, “on-water” (water–oil biphasic) conditions were highly effective in this reaction. The use of pure water (10 L mol−1, distilled) significantly improved the results, with >99% conversion in 10 min (entry 6).| Entry | [Reaction medium] | Conv.b (%) | ||||
|---|---|---|---|---|---|---|
| 1 min | 2 min | 3 min | 5 min | 10 min | ||
| a Reactions were conducted using hydrocinnamaldehyde (0.1 mmol, 1.0 equiv.) and allyl-Bpin (0.12 mmol, 1.2 equiv.) in different reaction media (10 L mol−1) at 25 °C. b Conv. (%) was determined by 1H NMR using 1,4-dimethoxybenzene as an internal standard. min = minute. | ||||||
| 1 | CH2Cl2 | 3 | 5 | 8 | 18 | 23 |
| 2 | MeCN | 2 | 3 | 10 | 20 | 27 |
| 3 | Neat | 3 | 5 | 9 | 20 | 26 |
| 4 | TPGS-750-M (2 wt%) | 73 | 75 | 79 | 83 | 87 |
| 5 | D2O | 86 | >99 | >99 | >99 | >99 |
| 6 | H2O | 93 | >99 | >99 | >99 | >99 |
| 7 | H 2 O-NaCl (sat.) | >99 | >99 | >99 | >99 | >99 |
| 8 | H2O-edible salt (sat.) | 95 | >99 | >99 | >99 | >99 |
| 9 | Seawater | 84 | >99 | >99 | >99 | >99 |
| 10 | Seawater-edible salt (sat.) | 96 | >99 | >99 | >99 | >99 |
Typical slowdown effects were observed under on-water conditions. Utilizing a designer surfactant developed by the Lipshutz group (TPGS-750-M, 2 wt% aqueous solution),35 the entire system was emulsified, yielding a conversion of 87% within 10 min (entry 4). Although this outcome was slightly inferior to that achieved with pure water, the result aligned well with established on-water experimental observations.26
To investigate further, D2O—a heavier and more viscous medium compared to H2O—was employed. Similar to the results observed for H2O, complete conversion was achieved within 10 min. However, the initial reaction rate was relatively slower (86% conversion in 1 min, entry 5) compared to the remarkable 93% conversion achieved within 1 min using H2O. This notable difference can be attributed to the higher viscosity of D2O, which likely reduces the effective interfacial surface area between the reactants and the aqueous medium by altering shear forces.31 The reaction likely proceeds through acceleration at the water–oil interface, in line with the “on-water” theory introduced by Sharpless et al. and later supported theoretically by Jung and Marcus.26,31
Next, NaCl was used as a representative salting-out agent.36 This approach yielded exceptional results, achieving >99% conversion within 1 minute (entry 7). These results align with the trends observed in other reported on-water reactions, highlighting accelerated reaction rates due to the effects of aqueous media.26 Based on previous findings that hydrophobic agents enhance the efficiency of addition reactions, we replaced the reagent-grade NaCl (from a chemical supplier) with commercially available edible sea salt (from a grocery market). As a result, 95% conversion was achieved within 1 min, and 99% conversion was observed within 2 min (entry 8). While using bulk water represents an environmentally friendly approach to carbonyl allylation, achieving the initial goal of sustainable conditions and the reliance on distilled water present challenges. The need for repeated distillation in industrial-scale processes leads to an increased consumption of energy and resources.37 From this perspective, seawater, which is one of the most abundant resources on the Earth, captured our attention. The utilization of seawater in chemical processes offers significant advantages in terms of cost, toxicity, and environmental impact.38 Untreated seawater (salinity = 3.5%) sourced directly from Namhae (the southern sea of Korea, Pohang City) was used as the reaction medium (entry 9). The initial reactivity showed a slightly reduced conversion (84% in 1 min) compared to that achieved with distilled water. However, after 2 min, >99% conversion was achieved. We believed that the reactivity observed in seawater could be further enhanced by the addition of edible salt. Remarkably, the modification of this medium to saturated seawater (salinity = 26%) significantly improved the reaction rate, achieving 96% conversion within 1 min and complete conversion (>99% conversion) within 2 min (entry 10). These results highlight the potential for utilizing abundant and sustainable resources to develop sustainable and cost-effective synthetic processes.
Based on the optimized reaction conditions, a preliminary investigation of the substrate scope of aldehydes was conducted (Fig. 2). The reactions were carried out using seawater saturated with edible sea salt as the reaction medium, forming a water–oil biphasic interface. To ensure efficient mixing under these conditions, vigorous stirring (>1000 rpm) was performed.29 Owing to the excellent reactivity of the system, all the reactions were completed within 5 min. The model substrate, hydrocinnamaldehyde, afforded product 1 in 99% isolated yield. To further investigate the reactivity of alkyl aldehydes, reactions were carried out with phenylacetaldehyde and 1-cyclohexenylaldehyde, affording products 2 and 3 in high yields of 95% and 90%, respectively. Cinnamaldehyde, a natural product readily obtained from cinnamon bark, afforded product 4 in 97% yield. Furthermore, the reactions with an aryl aldehyde proceeded smoothly, delivering product 5 in exceptionally high yield (99%). To further expand the substrate scope of aryl aldehydes incorporating electron-withdrawing and donating substituents, varying steric effects were investigated in our reaction system. para-Halogenated substrates (Cl and Br) afforded the corresponding products 6 and 7 in excellent yields (99%). Electron-withdrawing and donating substituents also provided desired products 8–10 in excellent yields (93%–99%). Biphenyl and 2-naphthyl incorporated bulky substrates afforded the desired products 11 and 12 in excellent yields (99%). Moreover, oxygen and sulphur-containing heteroaryl-aldehydes were highly active in the reaction, affording products 13 and 14 in 91% and 99% yields, respectively.
Condition optimization of catalytic allylation of ketones
We then continued to develop an efficient process for the allylation of less reactive ketones as acceptors39 (Table 2). In particular, tetrasubstituted carbon centers in homoallylic alcohols have attracted our attention because of their potential as key intermediates in the synthesis of various active APIs.40,41 Conventional methods such as the Hosomi–Sakurai allylation employing organosilicon reagents such as allyl trimethylsilane in moisture-sensitive metal Lewis acids have been widely investigated.42,43 However, the application of anhydrous conditions to industrial processes poses significant challenges. The avoidance of organic solvents and metals in this catalytic system was expected to have a substantial impact.| Entry | Carboxylic acid or salt (mol%) | Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of acid P of acid |
[reaction medium] | Conv. (%) of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
|---|---|---|---|---|
| a Reactions were conducted using 7 (0.1 mmol, 1.0 equiv.), allyl-Bpin (0.3 mmol, 3.0 equiv.) and a carboxylic acid catalyst (10 mol%) in H2O (10 L mol−1) at 60 °C for 24 h. b Conv. (%) was determined by 1H NMR analysis using 1,4-dimethoxybenzene as an internal standard. c At 25 °C. d At 80 °C. e At 100 °C. f Using allyl-Bpin (5.0 equiv.) at 80 °C. g Isolated yields are given in parentheses. | ||||
| 1c | Without a catalyst | — | H2O | 5 |
| 2 | Without a catalyst | — | H2O | 29 |
| 3 | Formic acid (10) | –0.54 (−0.30) | H2O | 33 |
| 4 | Acetic acid (10) | –0.26 (−0.09) | H2O | 31 |
| 5 | Trifluoroacetic acid (10) | 1.35 (0.21) | H2O | <1 |
| 6 | Benzoic acid (10) | 1.95 (1.65) | H2O | <1 |
| 7 | 4-n-Dodecylbenzoic acid (10) | (6.81) | H2O | <1 |
| 8 | Caproic acid (10) | 1.92 (1.83) | H2O | 40 |
| 9 | Caprylic acid (10) | 3.05 (2.93) | H2O | 53 |
| 10 | Capric acid (10) | 4.09 (4.04) | H2O | 48 |
| 11 | Lauric acid (10) | 5.15 (4.60) | H2O | 45 |
| 12 | Isobutyric acid (10) | 0.94 (0.45) | H2O | 43 |
| 13 | Myristic acid (10) | 6.10 (4.97) | H2O | 32 |
| 14 | Erucic acid (10) | 9.46 (8.10) | H2O | 10 |
| 15d | Caprylic acid (10) | 3.05 (2.93) | H2O | 66 |
| 16e | Caprylic acid (10) | 3.05 (2.93) | H2O | Trace |
| 17d | Caprylic acid (10) | 3.05 (2.93) | H2O-NaCl (sat.) | 79 |
| 18d | Caprylic acid (20) | 3.05 (2.93) | H2O-NaCl (sat.) | 87 |
| 19d | Caprylic acid (30) | 3.05 (2.93) | H2O-NaCl (sat.) | 92 |
| 20f | Caprylic acid (30) | 3.05 (2.93) | H2O | 83 |
| 21f | Sodium caprylate (30) | — | H2O-NaCl (sat.) | 25 |
| 22 | Caprylic acid (30) | 3.05 (2.93) | H 2 O-NaCl (sat.) | >99(99) |
Acetophenone, an aryl-alkyl ketone, was selected as the model substrate, and water was used as the reaction medium. Unlike aldehydes, ketones exhibited significantly lower reactivities under catalyst-free conditions, with negligible formation of product 15 (entry 1). At an elevated temperature of 60 °C, the reaction proceeded with 29% conversion after 24 h of stirring (entry 2). Consequently, we hypothesized that either Brønsted or Lewis acid catalysts would be required and proceeded to screen various candidates.
Inspired by our previous work on multicomponent aquacatalytic allylation, we preferably evaluated strong Brønsted acids.21 However, p-toluenesulfonic acid (PTSA), dodecylbenzenesulfonic acid (DBSA), and bistriflimide led to the complete decomposition of the substrate (∼0%), whereas triflic acid (TfOH) showed limited catalytic activity (38% conversion). Among Lewis acids, tris(pentafluorophenyl)borane (B(C6F5)3), a highly effective catalyst for Mukaiyama aldol reactions in water reported by the Loh group,44 was inactive in this system. Scandium triflate (Sc(OTf)3), commonly utilized by the Kobayashi group,45 demonstrated marginally better activity but suboptimal performance, with a conversion of 54%. These findings highlight the need for novel and efficient catalysts.
Carboxylic acids are notable for their natural abundance, straightforward production, mild acidity, and low toxicity, all of which contribute to their safety and economic viability.46 A diverse range of carboxylic acids was screened as catalysts (10 mol%). Simple highly water-soluble acids, such as formic acid (log ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = −0.54) and acetic acid (log
P = −0.54) and acetic acid (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = −0.26), exhibited no catalytic effect (31–33% conversion), while trifluoroacetic acid (log
P = −0.26), exhibited no catalytic effect (31–33% conversion), while trifluoroacetic acid (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1.35), benzoic acid (log
P = 1.35), benzoic acid (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1.95), and p-dodecylbenzoic acid showed almost no reactivity (<1% conversion, entries 3–7). Considering the aqueous reaction medium, we hypothesized that introducing longer alkyl chains into the carboxylic acid structure might enhance the reactivity. Natural fatty acid screening revealed promising results. Caproic acid (C6:0, log
P = 1.95), and p-dodecylbenzoic acid showed almost no reactivity (<1% conversion, entries 3–7). Considering the aqueous reaction medium, we hypothesized that introducing longer alkyl chains into the carboxylic acid structure might enhance the reactivity. Natural fatty acid screening revealed promising results. Caproic acid (C6:0, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1.92), caprylic acid (C8:0, log
P = 1.92), caprylic acid (C8:0, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 3.05), capric acid (C10:0, log
P = 3.05), capric acid (C10:0, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 4.09) and lauric acid (C8:0, log
P = 4.09) and lauric acid (C8:0, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 5.15), which are key components of MCTs,3 significantly improved the conversion to 40%, 53%, 48% and 45% (entries 8–11). In contrast, longer-chain fatty acids such as myristic acid (C14:0, log
P = 5.15), which are key components of MCTs,3 significantly improved the conversion to 40%, 53%, 48% and 45% (entries 8–11). In contrast, longer-chain fatty acids such as myristic acid (C14:0, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 6.10) and erucic acid (C22:1, log
P = 6.10) and erucic acid (C22:1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 9.46), as well as branched acids such as isobutyric acid (log
P = 9.46), as well as branched acids such as isobutyric acid (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 0.45), showed no further improvement (entries 12–14).
P = 0.45), showed no further improvement (entries 12–14).
Among the tested acids, caprylic acid emerged as the optimal catalyst owing to its lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 3.05). Temperature also played a critical role in the reaction. At 80 °C, the reactivity increased moderately, achieving 66% conversion (entry 15), while at 100 °C, the conversion decreased. This decrease was probably due to the increased solubility of the fatty acid above its CMC,16 which disrupted the biphasic reaction environment for catalysis (entry 16). The salting-out effect of NaCl is particularly crucial in this system, as it further enhances the reactivity. Under optimized conditions, increasing the catalyst loading to 30 mol% led to complete conversion to the desired product 15 (entries 17–20, and 22). The reaction yield dropped dramatically to 25% when sodium caprylate was used (entry 20).
P = 3.05). Temperature also played a critical role in the reaction. At 80 °C, the reactivity increased moderately, achieving 66% conversion (entry 15), while at 100 °C, the conversion decreased. This decrease was probably due to the increased solubility of the fatty acid above its CMC,16 which disrupted the biphasic reaction environment for catalysis (entry 16). The salting-out effect of NaCl is particularly crucial in this system, as it further enhances the reactivity. Under optimized conditions, increasing the catalyst loading to 30 mol% led to complete conversion to the desired product 15 (entries 17–20, and 22). The reaction yield dropped dramatically to 25% when sodium caprylate was used (entry 20).
Substrate scope of allylation of ketones
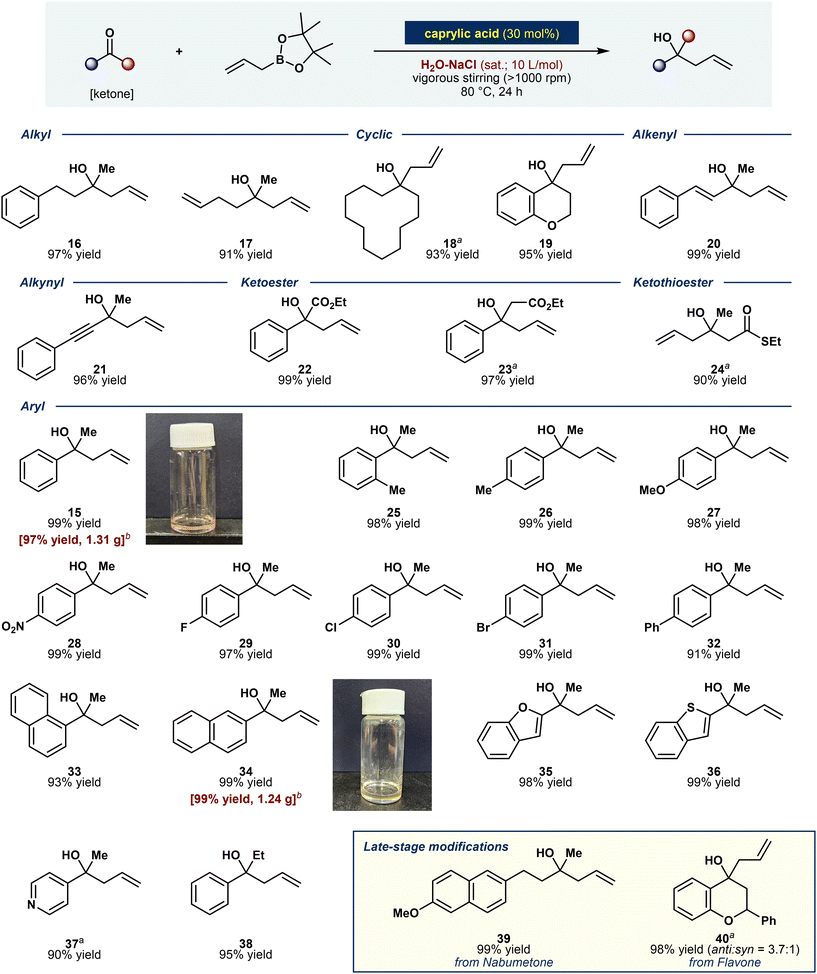
Based on the optimized reaction conditions, the substrate scope for the allylation of various ketones was explored (Fig. 3). Ketones substituted with alkyl–alkyl groups are generally less reactive as acceptors, presenting a significant challenge. However, under our salting-out-enhanced “on-water” conditions, they were successfully converted to compounds 16 and 17 in excellent yields (97% and 91%, respectively). The method was further extended to cyclic ketones, yielding products 18 and 19 with remarkable efficiencies (up to 95% yield). Similarly, alkenyl and alkynyl ketones underwent quantitative transformations into the expected products, 20 and 21, in 99% and 96% yields, respectively. Substrates bearing (thio)ester functional groups, such as β-ketoesters and α-ketothioesters, also exhibited excellent functional group tolerance, yielding compounds 23 and 24 in 97% and 90% yields, respectively. A wide range of (hetero)aryl-alkyl ketones were also converted to the desired products without complications in very high yields (compounds 15–38, up to 99%). Additionally, gram-scale reactions with ketone substrates were successfully conducted, maintaining excellent efficiency (99% yield for compounds 15 and 34).We further demonstrated the utility of this method by performing late-stage modifications on ketone-containing natural products and pharmaceuticals. Nabumetone,47 a nonsteroidal anti-inflammatory drug (NSAID), and flavone,48 a compound commonly found in plant-derived foods, were efficiently transformed into the desired products in high yields (99% yield for compound 39; 98% yield with anti/syn = 3.7/1 for compound 40). This investigation highlights the broad applicability and robustness of the developed aqueous allylation protocol, even with challenging substrates and late-stage functionalization.
Development of chromatography-free purification methods and synthetic applications
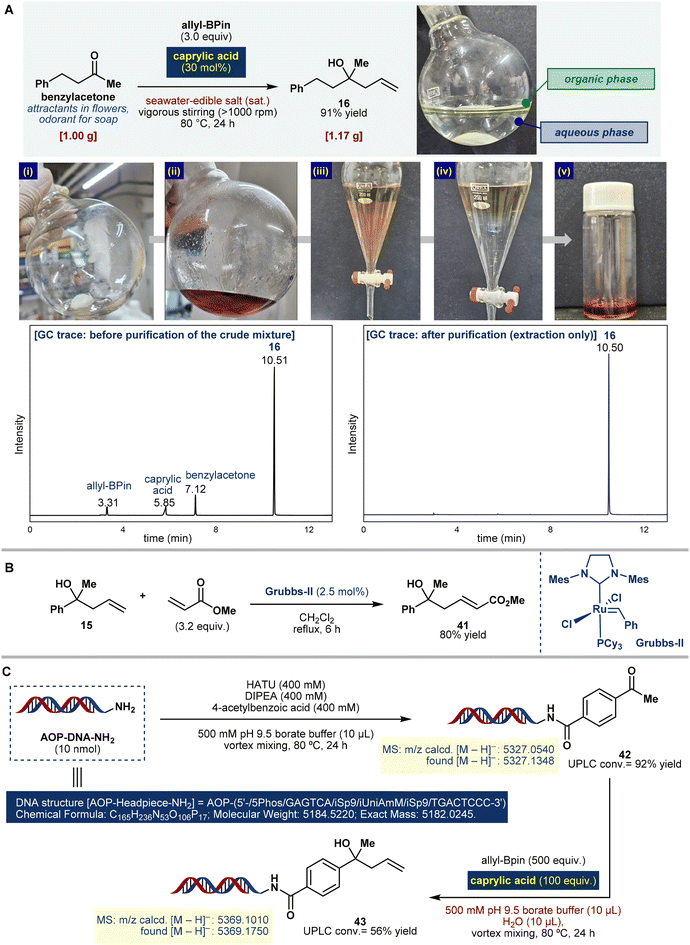
To achieve an efficient and sustainable purification process, it is necessary to develop a purification protocol that does not require column chromatography. A 1.0 gram scale synthesis of benzylacetone was conducted to isolate the product solely through extraction. The reaction was performed using untreated seawater saturated with edible sea salt as a medium (Fig. 4A). Upon completion of the reaction, the crude mixture was subjected to extraction-based purification. The most challenging aspect of the purification process was the removal of the residual allyl-Bpin and caprylic acid. Previous studies have indicated that pinacol boronate esters undergo hydrolysis under acidic conditions.49 Subsequently, 6 N HCl (aq.) was added to the crude mixture, which was then stirred for 24 h. This treatment transformed the pale-yellow crude mixture into a dark red solution, and the resulting unreacted components were simply removed through extraction. Next, the removal of residual caprylic acid, which was used as the catalyst, was investigated. The water solubility of caprylic acid increases with temperature,50 allowing its efficient removal through hot water extraction (see the ESI†). To validate the efficacy of this protocol, gas chromatography (GC) was performed on both crude and purified products. The residual allyl-Bpin, caprylic acid, and minor benzylacetone impurities were removed entirely, yielding a highly pure (>99%) GC trace of product 16. In the case of the aldehyde, the product 1 was also successfully obtained through our chromatography-free protocol (see the ESI†), highlighting its potential for simplifying sustainable and economical processes.To further demonstrate the utility of these reaction products, olefin metathesis was performed using 15 (Fig. 4B). Using the Grubbs-II catalyst, compound 15 was successfully converted to compound 41 with 80% yield. This excellent functional group tolerance underscores the potential of this method for API synthesis.
The compatibility of our catalytic allylation system with water and its transition-metal-free nature suggest that it is particularly practical for biomolecule functionalization applications.51 In this context, the reaction system aligns closely with the biomimetic conditions required for constructing DNA-encoded libraries (DELs), a powerful tool in drug discovery.52 Notably, the allylation of ketones tethered to DNA has not yet been reported, making this a significant opportunity for advancing its biochemical applications. To extend the applicability of this reaction, we investigated its use in on-DNA allylation (Fig. 4C). First, amide coupling was performed by reacting a headpiece-modified DNA molecule53 with 4-acetylbenzoic acid, hexafluorophosphate azabenzotriazole tetramethyl uranium (HATU), N,N-diisopropylethylamine (DIPEA), and pH 9.5 borate buffer at 80 °C for 24 h. Compound 42 was characterized using HPLC and UPLC, achieving a conversion of 92% (HRMS: m/z calcd [M − H]− = 5327.0540; found [M − H]− = 5327.1348). Compound 42 was diluted with double-distilled H2O. Allyl-Bpin (500 equiv.) and caprylic acid (100 equiv.) were added, and the reaction was performed at 80 °C for 24 h. The desired product 43 was successfully obtained with 56% conversion, as confirmed by HPLC and UPLC (MS: m/z calcd [M − H]− = 5369.1010; found [M − H]− = 5369.1750). This study highlights the utility of the developed catalytic system for sustainable and efficient aquatic allylation processes and its broad applicability to advanced biochemical transformations such as DNA-encoded library synthesis.
Mechanistic investigation
Investigating the water-promoted rate acceleration in biphasic reactions involving a catalyst is inherently more complex than that under catalyst-free conditions. The mechanism underlying this enhancement was well categorized by Kobayashi and Kitanosono,54,55 who classified on-water reactions involving catalysts as type III. In our system, elevated temperatures increase the solubility of caprylic acid in water; however, the salting-out effect induced by saturated Na+ and Cl− ions may be expected to suppress micelle formation (illustrated in Fig. 1C). It is hypothesized that the reaction predominantly occurs at the interface between the oil phase (the ketone, allyl boronate, and caprylic acid (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 3.05)) and the ion-saturated aqueous phase. Jung and Marcus proposed that the free –OH groups in bulk water form stronger hydrogen bonds with hydrogen-bond acceptors in the transition state.31 Additionally, based on computational studies, Roke et al. reported that the surface of pure water is more acidic than the bulk,56 suggesting that the acidity of hydrated caprylic acid is enhanced at the oil–water interface. This increased acidity may facilitate strong hydrogen bonding interactions with hydrogen bonding acceptors such as the oxygen atom adjacent to the boron atom in allyl-Bpin.57,58 To further elucidate this phenomenon, density functional theory (DFT) calculations were performed (Fig. 5A). A key aspect of this investigation was to determine whether the catalytic activity of caprylic acid could be attributed to its –COOH group, which activates allyl-Bpin to provide the [intermediate-I]. DFT calculations revealed that the Mulliken charge of the boron atom in allyl-Bpin increased from 1.645 in the absence of a catalyst to 1.961 in the presence of caprylic acid. This suggests that caprylic acid forms a hydrogen-bonded complex with allyl-Bpin, enhancing its Lewis acidity and generating additional reactive organoboron intermediates. For comparison, further calculations were performed to examine the interaction between caprylic acid and the carbonyl oxygen of acetophenone ([intermediate-II]). As a result, the Mulliken charge on the carbonyl carbon of acetophenone increased from 0.126 to 0.246 upon interaction with caprylic acid. This outcome suggests that caprylic acid preferentially interacts with the oxygen atom in allyl-Bpin rather than with the carbonyl oxygen in acetophenone (see the ESI† for details).58
P = 3.05)) and the ion-saturated aqueous phase. Jung and Marcus proposed that the free –OH groups in bulk water form stronger hydrogen bonds with hydrogen-bond acceptors in the transition state.31 Additionally, based on computational studies, Roke et al. reported that the surface of pure water is more acidic than the bulk,56 suggesting that the acidity of hydrated caprylic acid is enhanced at the oil–water interface. This increased acidity may facilitate strong hydrogen bonding interactions with hydrogen bonding acceptors such as the oxygen atom adjacent to the boron atom in allyl-Bpin.57,58 To further elucidate this phenomenon, density functional theory (DFT) calculations were performed (Fig. 5A). A key aspect of this investigation was to determine whether the catalytic activity of caprylic acid could be attributed to its –COOH group, which activates allyl-Bpin to provide the [intermediate-I]. DFT calculations revealed that the Mulliken charge of the boron atom in allyl-Bpin increased from 1.645 in the absence of a catalyst to 1.961 in the presence of caprylic acid. This suggests that caprylic acid forms a hydrogen-bonded complex with allyl-Bpin, enhancing its Lewis acidity and generating additional reactive organoboron intermediates. For comparison, further calculations were performed to examine the interaction between caprylic acid and the carbonyl oxygen of acetophenone ([intermediate-II]). As a result, the Mulliken charge on the carbonyl carbon of acetophenone increased from 0.126 to 0.246 upon interaction with caprylic acid. This outcome suggests that caprylic acid preferentially interacts with the oxygen atom in allyl-Bpin rather than with the carbonyl oxygen in acetophenone (see the ESI† for details).58
To validate whether the reactive intermediate benefited from the on-water effect, the reactivities of various reaction media were compared (Fig. 5B). Reactions were conducted with 10 mol% caprylic acid at an optimized temperature of 80 °C. Bulk water yielded 66% conversion (entry h), whereas organic solvents such as acetone and dichloromethane were completely inactive. Methanol exhibited a limited reactivity (16% conversion; entries a–c). Although the initial rate was slightly slower, D2O gave a result comparable to that of H2O (entry g). The designer surfactant H2O/TPGS (2 wt%) resulted in only 30% yield (entry d), while neat conditions improved the yield to 62% (entry f). These findings indicate that homogeneous reaction systems are unsuitable, with their reaction performance closely linked to the substrate concentration; higher concentrations under neat conditions enhance the reaction. The superior reactivity observed in bulk water is likely derived from the biphasic system, which creates a confined organic cage that exerts a high-pressure-like effect on the reactants.30 This confinement compresses the reactive organic phase, leading to a negative activation volume (ΔV‡ < 0), which is known to accelerate transformations, particularly those involving addition-type reaction mechanisms.29 This hypothesis is consistent with the observed trends: a hydrophobic agent34 like NaCl enhances conversion to 79% (entry e), while an anti-hydrophobic agent59 like LiClO4 reduces it to 52% (entry d). Furthermore, increasing the catalyst loading significantly improves the reactivity, highlighting the critical role of the catalyst (entries j–m).
To ensure that the catalytic system followed a Zimmerman–Traxler-type transition state,60 we replaced allyl-Bpin with trans-crotylboronic acid pinacol ester (compound 44, Fig. 5C). The reaction yielded only compound 45, with a methyl group at the α-position in 95% yield. Notably, a single diastereomer with an anti-configuration was observed, indicating that the nucleophilic attack on the carbonyl carbon occurred exclusively at the terminal sp2-carbon atom. In accordance with our expectations, reactions conducted in organic solvents (MeCN) afforded less than 5% yield, and even under solvent-free conditions, the yield was low (60%). These results support the involvement of the Zimmerman–Traxler transition state.
Conclusions
In summary, we have developed a sustainable aqueous catalytic approach for carbonyl allylation, leveraging unrefined seawater and sea salt to harness the salting-out effect. This method achieved (i) efficient, catalyst-free allylation of aldehydes and (ii) caprylic acid-catalyzed allylation of ketones using a cost-effective, nontoxic fatty acid commonly employed in the food industry, with bulk water as the reaction medium. The process demonstrated scalability, enabling gram-scale synthesis with chromatography-free purification and offering a promising pathway for cost-efficient API production. Additionally, the mild reaction conditions and water compatibility facilitate on-DNA conjugation, potentially transforming the development of DELs, a critical innovation in drug discovery. We believe that this study provides a robust foundation for further advancements in environmentally friendly, water-compatible, and fundamental catalytic systems for challenging applications.Data availability
The data supporting the findings of this study are available within the article and its ESI.† Additional data are available from the corresponding author upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The generous support from the Ministry of Science, ICT, and Future Planning of Korea (RS-2023-00259659 and RS-2023-00219859) and the Ministry of Education (2022R1A6C101A751 and 2022R1A6C102A913) is gratefully acknowledged.References
- L. Huang, L. Gao and C. Chen, Role of Medium-Chain Fatty Acids in Healthy Metabolism: A Clinical Perspective, Trends Endocrinol. Metab., 2021, 32, 351–366 CrossRef CAS PubMed.
- A. C. Bach and V. K. Babayan, Medium-chain triglycerides: an up-date, Am. J. Clin. Nutr., 1982, 36, 950–962 CrossRef CAS PubMed.
- P. G. Roopashree, S. S. Shetty and N. S. Kumari, Effect of Medium Chain Fatty Acid in Human Health and Disease, J. Funct. Foods, 2021, 87, 104724 CrossRef CAS.
- P. Schönfeld and L. Wojtczak, Short- and Medium-Chain Fatty Acids in Energy Metabolism: The Cellular Perspective, J. Lipid Res., 2016, 57, 943–954 CrossRef PubMed.
- A. A. Papamandjaris, D. E. Macdougall and P. J. H. Jones, Medium chain fatty acid metabolism and energy expenditure: Obesity treatment implications, Life Sci., 1998, 62, 1203–1215 CrossRef CAS PubMed.
- C. Vetrani, L. Verde, S. Savastano, A. Colao, G. Muscogiuri and L. Barrea, Supplementation with medium-chain fatty acids increases body weight loss during very low-calorie ketogenic diet: a retrospective analysis in a real-life setting, J. Transl. Med., 2023, 21, 29, DOI:10.1186/s12967-023-03880-7.
- M. P. St-Onge and A. Bosarge, Weight-loss diet that includes consumption of medium-chain triacylglycerol oil leads to a greater rate of weight and fat mass loss than does olive oil, Am. J. Clin. Nutr., 2008, 87, 621–626 CrossRef CAS PubMed.
- C. R. Roe, L. Sweetman, D. S. Roe, F. David and H. Brunengraber, Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride, J. Clin. Invest., 2002, 110, 259–269 CrossRef CAS PubMed.
- M. E. Clegg, M. Golsorkhi and C. J. Henry, Combined medium-chain triglyceride and chilli feeding increases diet-induced thermogenesis in normal-weight humans, Eur. J. Nutr., 2013, 52, 1579–1585 CrossRef CAS PubMed.
- B. Yoon, J. Jackman, E. Valle-González and N. J. Cho, Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications, Int. J. Mol. Sci., 2018, 19, 1114 CrossRef PubMed.
- W. Liu, X. Luo, T. Liu and F. Feng, Study on the digesticharacteristicsics of short-and medium-chain fatty acid structural lipid and its rapid intervention on gut microbes: In vivo and in vitro studies, Food Chem., 2022, 380, 131792 CrossRef CAS PubMed.
- M. Chnadhapuram and Y. R. Sunkireddy, Preparation of palm olein enriched with medium chain fatty acids by lipase acidolysis, Food Chem., 2012, 132, 216–221 CrossRef CAS PubMed.
- K. Nagao and T. Yanagita, Medium-Chain Fatty Acids: Functional Lipids for the Prevention and Treatment of the Metabolic Syndrome, Pharmacol. Res., 2010, 61, 208–212 CrossRef CAS PubMed.
- S. S. Mathiasen, J. M. Kanta, R. P. Frydenberg, A. Lundsgaard, Z. Guo, A. M. Fritzen, B. Kiens, L. Wiking and M. Kleinert, Novel methodology to enrich medium- and short-chain fatty acids in milk fat to improve metabolic health, Food Funct., 2024, 15, 7951–7960 RSC.
- S. Maher, T. W. Leonard, J. Jacobsen and D. J. Brayden, Safety and Efficacy of Sodium Caprate in Promoting Oral Drug Absorption: From in Vitro to the Clinic, Adv. Drug Delivery Rev., 2009, 61, 1427–1449 CrossRef CAS PubMed.
- J. Mehringer, E. Hofmann, D. Touraud, S. Koltzenburg, M. Kellermeier and W. Kunz, Salting-in and Salting-out Effects of Short Amphiphilic Molecules: A Balance between Specific Ion Effects and Hydrophobicity, Phys. Chem. Chem. Phys., 2021, 23, 1381–1391 RSC.
- Y. Zhou, F. Liu, L. Liu, X. Qiu, M. Ye, H. Pan and H. Liu, Effects of length and type of the alkyl chain on the micellization behavior of mixed systems of HS15 with fatty acids, Food Chem., 2022, 397, 133830 CrossRef CAS PubMed.
- K. Traul, A. Driedger, D. Ingle and D. Nakhasi, Review of the Toxicologic Properties of Medium-Chain Triglycerides, Food Chem. Toxicol., 2000, 38, 79–98 CrossRef CAS PubMed.
- M. Ruiz-Rico, C. Fuentes, É. Pérez-Esteve, A. I. Jiménez-Belenguer, A. Quiles, M. D. Marcos, R. Martínez-Máñez and J. M. Barat, Bactericidal Activity of Caprylic Acid Entrapped in Mesoporous Silica Nanoparticles, Food Control, 2015, 56, 77–85 CrossRef CAS.
- K. S. Sung, W. H. Cho, S. H. Cha, Y.-W. Kim, S. H. Choi, H. J. Kim and M. S. Yun, Saturated Fatty Acid Emulsions Open the Blood–Brain Barrier and Promote Drug Delivery in Rat Brains, Pharmaceutics, 2024, 16, 246 CrossRef CAS PubMed.
- P. Goswami, S. Y. Cho, J. H. Park, W. H. Kim, H. J. Kim, M. H. Shin and H. Y. Bae, Efficient Access to General α-Tertiary Amines via Water-Accelerated Organocatalytic Multicomponent Allylation, Nat. Commun., 2022, 13, 2702 CrossRef CAS PubMed.
- S. B. Lee, J. H. Park and H. Y. Bae, Hydrophobic Amplification Enabled High-Turnover Phosphazene Superbase Catalysis, ChemSusChem, 2022, 15, e202200634 CrossRef CAS PubMed.
- J. H. Park, S. B. Lee, B. J. Koo and H. Y. Bae, β-Aminosulfonyl Fluorides via Water-Accelerated N-Heterocyclic Carbene Catalysis, ChemSusChem, 2022, 15, e202201000 CrossRef CAS PubMed.
- J. H. Park, G. A. Gonzalez-Montiel, P. H. Cheong and H. Y. Bae, Alkyl Sulfonyl Fluorides Incorporating Geminal Dithioesters as SuFEx Click Hubs via Water-Accelerated Organosuperbase Catalysis, Org. Lett., 2023, 25, 1056–1060 CrossRef CAS PubMed.
- S. B. Kim, D. H. Kim and H. Y. Bae, “On-Water” Accelerated Dearomative Cycloaddition via Aquaphotocatalysis, Nat. Commun., 2024, 15, 3876 CrossRef CAS PubMed.
- S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, “On water′′: Unique Reactivity of Organic Compounds in Aqueous Suspension, Angew. Chem., Int. Ed., 2005, 44, 3275–3279 CrossRef CAS PubMed.
- A. Chanda and V. V. Fokin, Organic Synthesis “On Water”, Chem. Rev., 2009, 109, 725–748 CrossRef CAS PubMed.
- R. N. Butler and A. G. Coyne, Organic Synthesis Reactions On-Water at the Organic-Liquid Water Interface, Org. Biomol. Chem., 2016, 14, 9945–9960 RSC.
- M. Cortes-Clerget, J. Yu, J. R. A. Kincaid, P. Walde, F. Gallou and B. H. Lipshutz, Water as the reaction medium in organic chemistry: from our worst enemy to our best friend, Chem. Sci., 2021, 12, 4237–4266 RSC.
- Y. Hayashi, W. Tsuboi, M. Shoji and N. Suzuki, Application of High Pressure Induced by Water-Freezing to the Direct Catalytic Asymmetric Three-Component List–Barbas–Mannich Reaction, J. Am. Chem. Soc., 2003, 125, 11208–11209 CrossRef CAS PubMed.
- Y. Jung and R. A. Marcus, On the Theory of Organic Catalysis “on Water”, J. Am. Chem. Soc., 2007, 129, 5492–5502 CrossRef CAS PubMed.
- S. C. Jonnalagadda, P. Suman, A. Patel, G. Jampana and A. Colfer, Allylboration, in Boron Reagents in Synthesis, ACS Symposium Series, 1236; American Chemical Society, Washington, DC, 2016 Search PubMed.
- S. Kobayashi, T. Endo, T. Yoshino, U. Schneider and M. Ueno, Allylation Reactions of Aldehydes with Allylboronates in Aqueous Media: Unique Reactivity and Selectivity that are Only Observed in the Presence of Water, Chem. – Asian J., 2013, 8, 2033–2045 CrossRef CAS PubMed.
- M. Zheng, W. Gao, L. Zhang, K. Yan, Z. Zhang, P. Xie and T.-P. Loh, Allylation of Lactol in Water, Org. Lett., 2024, 26, 10201–10206 CrossRef CAS PubMed.
- B. Lipshutz, S. Ghorai, A. Abela, R. Moser, T. Nishikata, C. Duplais, A. Krasovskiy, R. Gaston and R. Gadwood, Tpgs-750-M: A Second-Generation Amphiphile for Metal-Catalyzed Cross-Couplings in Water at Room Temperature, J. Org. Chem., 2011, 76, 4379–4391 CrossRef CAS PubMed.
- R. Breslow, Hydrophobic Effects on Simple Organic Reactions in Water, Acc. Chem. Res., 1991, 24, 159–164 CrossRef CAS.
- E. A. Aboagye, J. D. Chea and K. M. Yenkie, Systems level roadmap for solvent recovery and reuse in industries, iScience, 2021, 24, 103114 CrossRef CAS PubMed.
- H. Ren, M.-H. Zong, H. Wu and N. Li, Utilization of Seawater for the Biorefinery of Lignocellulosic Biomass: Ionic Liquid Pretreatment, Enzymatic Hydrolysis, and Microbial Lipid Production, ACS Sustainable Chem. Eng., 2016, 4, 5659–5666 CrossRef CAS.
- D. S. Barnett, P. N. Moquist and S. E. Schaus, The Mechanism and an Improved Asymmetric Allylboration of Ketones Catalyzed by Chiral Biphenols, Angew. Chem., Int. Ed., 2009, 48, 8679–8682 CrossRef CAS PubMed.
- C. C. Meyer, K. L. Verboom, M. M. Evarts, W.-O. Jung and M. J. Krische, Allyl Alcohol as an Acrolein Equivalent in Enantioselective C-C Coupling: Total Synthesis of Amphidinolides R, J, and S, J. Am. Chem. Soc., 2023, 145, 8242–8247 CrossRef CAS PubMed.
- V. J. Mayerhofer, M. Lippolis and C. J. Teskey, Dual-Catalysed Inter-molecular Reductive Coupling of Dienes and Ketones, Angew. Chem., Int. Ed., 2024, 63, e202314870 CrossRef CAS PubMed.
- M. Wadamoto and H. Yamamoto, Silver-Catalyzed Asymmetric Sakurai-Hosomi Allylation of Ketones, J. Am. Chem. Soc., 2005, 127, 14556–14557 CrossRef CAS PubMed.
- Z.-Y. Cao, Y. Zhang, C.-B. Ji and J. A. Zhou, Hg(ClO4)2·3H2O Catalyzed Sakurai–Hosomi Allylation of Isatins and Isatin Ketoimines Using Allyltrimethylsilane, Org. Lett., 2011, 13, 6398–6401 CrossRef CAS PubMed.
- Z. Zhang, L. Li, X. Zong, Y. Lv, S. Liu, L. Ji, X. Zhao, Z. Jia and T.-P. Loh, “Water” accelerated B(C6F5)3-catalyzed Mukaiyama-aldol reaction: Outer-sphere activation model, Chin. Chem. Lett., 2024, 110504 Search PubMed.
- S. Kobayashi, Scandium Triflate in Organic Synthesis, Eur. J. Org. Chem., 1999, 15–27 CrossRef CAS.
- P. S. Prabhakar, J. Sahoo, I. A. Alnaser, A. H. Seikh, M. R. Karim and S. Dutta, Aqueous solution of biogenic carboxylic acids as sustainable catalysts and green reaction media for the high-yielding synthesis of Biginelli adducts, Hantzsch esters, and substituted pyridines, RSC Adv., 2024, 14, 39050–39060 RSC.
- F. Cipollone, A. Ganci, M. R. Panara, A. Greco, F. Cuccurullo, C. Patrono and P. Patrignani, Effects of nabumetone on prostanoid biosynthesis in humans, Clin. Pharmacol. Ther., 1995, 58, 335–341 CrossRef CAS PubMed.
- H. J. Heo, D.-O. Kim, S. C. Shin, M. J. Kim, B. G. Kim and D.-H. Shin, Effect of antioxidant flavanone, naringenin, from Citrus junos on neuroprotection, J. Agric. Food Chem., 2004, 52, 1520–1525 CrossRef CAS PubMed.
- S. R. Inglis, E. C. Y. Woon, A. L. Thompson and C. J. Schofield, Observations on the Deprotection of Pinanediol and Pinacol Boronate Esters via Fluorinated Intermediates, J. Org. Chem., 2010, 75, 468–471 CrossRef CAS PubMed.
- F. H. Constable and S. Tegul, Solubility and Heat of Solution of Caprylic Acid in Water, Nature, 1946, 157, 735 CrossRef CAS.
- S. L. Scinto, D. A. Bilodeau, R. Hincapie, W. Lee, S. S. Nguyen, M. Xu, C. W. am Ende, M. G. Finn, K. Lang, Q. Lin, J. P. Pezacki, J. A. Prescher, M. S. Robillard and J. M. Fox, Bioorthogonal chemistry, Nat. Rev. Methods Primers, 2021, 1, 30 CrossRef CAS PubMed.
- A. A. Peterson and D. R. Liu, Small-molecule discovery through DNA-encoded libraries, Nat. Rev. Drug Discovery, 2023, 22, 699–722 CrossRef CAS PubMed.
- S. Eom, T. Kwon, D. Y. Lee, C. H. Park and H. J. Kim, Copper-Mediated Three-Component Reaction for the Synthesis of N-Acylsulfonamide on DNA, Org. Lett., 2022, 24, 4881–4885 CrossRef CAS PubMed.
- T. Kitanosono and S. Kobayashi, Reactions in Water Involving the “On-Water” Mechanism, Chem. – Eur. J., 2020, 26, 9408–9429 CrossRef CAS PubMed.
- T. Kitanosono and S. Kobayashi, Synthetic Organic “Aquachemistry” that Relies on Neither Cosolvents nor Surfactants, ACS Cent. Sci., 2021, 7, 739–747 CrossRef CAS PubMed.
- R. Vácha, S. W. Rick, P. Jungwirth, A. G. F. De beer, H. B. De aguiar, J.-S. Samson and S. Roke, The Orientation and Charge of Water at the Hydrophobic Oil Droplet-Water Interface, J. Am. Chem. Soc., 2011, 133, 10204–10210 CrossRef PubMed.
- J. R. M. Bastidas, A. Chhabra, Y. Feng, T. J. Oleskey, M. R. Smith and R. E. Maleczka, Steric Shielding Effects Induced by Intramolecular C–H⋯O Hydrogen Bonding: Remote Borylation Directed by Bpin Groups, ACS Catal., 2022, 12, 2694–2705 CrossRef PubMed.
- S. Umemiya, S. Osaka, N. Shinagawa, T. Hirata and M. Terada, Chiral Brønsted acid-catalysed enantioselective allylboration of sterically hindered aldehydes enabled by multiple hydrogen bonding interactions, Chem. Sci., 2025, 16, 3865–3871 RSC.
- R. Breslow, Determining the geometries of transition states by use of antihydrophobic additives in water, Acc. Chem. Res., 2004, 37, 471–478 CrossRef CAS PubMed.
- T. Mejuch, N. Gilboa, E. Gayon, H. Wang, K. Houk and I. Marek, Axial preferences in allylation reactions via the Zimmerman–Traxler transition state, Acc. Chem. Res., 2013, 46, 1659–1669 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc01441g |
| ‡ Present address: RIST, Gwangyang, Jeollanam-do, 57801, Republic of Korea. |
| This journal is © The Royal Society of Chemistry 2025 |