 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective oxidation of glucose–galactose syrup to gluconic and galactonic acids†
Joseph
Install
 a,
Anže
Zupanc
a,
Anže
Zupanc
 a,
Seonyeong
Kim
a,
Seonyeong
Kim
 b,
Wenjia
Wang
b,
Wenjia
Wang
 b,
Marianna
Kemell
b,
Marianna
Kemell
 a,
George W.
Huber
a,
George W.
Huber
 b and
Timo
Repo
b and
Timo
Repo
 *a
*a
aDepartment of Chemistry, University of Helsinki, A. I. Virtasen aukio 1, Helsinki, Uusimaa, Finland. E-mail: timo.repo@helsinki.fi
bDepartment of Chemical and Biological Engineering, University of Wisconsin-Madison, 1415 Engineering Drive, Madison, WI 53706, USA
First published on 20th May 2025
Abstract
The global food industry, inclusive of dairy, is searching for sustainable upgrading of the large volumes of inedible waste they produce. Presented here is the selective oxidation of Greek yoghurt acid-whey derived glucose–galactose syrup (GGS) to gluconic and galactonic acids. Aldonic acids are high value biproducts with many applications. Our straightforward approach combines efficient catalytic oxidation and simple separation to upgrade a large dairy waste product to higher value products under base free conditions, using an Au/Al2O3 catalyst with 73% and 55% selectivity towards gluconic and galactonic acids, respectively, under conditions of 2.5 bar O2 for 24 h at 80 °C with a low Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GGS ratio of 1
GGS ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000. A selective separation method was developed allowing the separation of the two epimeric acids based solely on their solubilities with high purity.
1000. A selective separation method was developed allowing the separation of the two epimeric acids based solely on their solubilities with high purity.
Green foundation1. An inedible waste stream from the production of Greek yoghurt has been utilised to produce products listed in the top 10 high value bio-products by the Department of Energy. In addition, manipulation of the epimeric relationship of glucose and galactose is performed for the separation of mixed products gluconic and galactonic acids based on solubilities.2. Exploration beyond the conventional lignocellulosic biomass for upgrading of waste to useful products has provided a route to gluconic and galactonic acids simultaneously. Selectivities of 73% and 55% to these aldonic acids using a gold catalyst with an extremely low loading ratio of 1 3. To make the work greener, a non-noble metal catalyst could be used. Further directed scale-up research is required to assess the process feasibility and economics to elevate the research. |
Introduction
Exploitation of unavoidable side-streams and lessening dependence on petroleum derived chemical products are simultaneously achievable by targeted valorisation of biomass waste.1 In addition, reduction of environmental impact of food production is vital in ensuring sustainable maintenance of worldwide food supplies. The dairy industry aims to reduce greenhouse gas emissions, land and water use, which they hold a large share of among the food industry and the wider sectors.2,3 Greek yoghurt production has significantly increased in the last decade, growing from a 1% market share of yogurt products to 40% and 20% in the US and Canada, respectively.3–5 This increase in demand yields large amounts of inedible Greek yoghurt acidic whey (GAW), around 2.1 million tonnes per year,6,7 which is unavoidable due to the yoghurt production process.8 There are limited feasible solutions to upgrading of GAW, which is the most significant by-product of the dairy industry in the US;9 hence it requires a huge waste disposal effort. Therefore, there is an opportunity for bioproducts produced from the waste to provide an additional revenue stream, but more importantly, an environmental benefit for a circular economy.GAW has a high chemical oxygen demand (COD), acidity and mineral content making environmental disposal challenging without further processing.10 Unlike cheese whey, it cannot be used as fertilizer or livestock feed.
Recently a highly effective, multistep process for converting GAW into high purity glucose–galactose syrup (GGS) was developed at the University of Wisconsin–Madison6 as shown in Fig. 1. GGS has a carbohydrate composition and sweetness level similar to sucrose, making it a viable alternative to high-fructose corn syrup (HFCS). Based on our techno-economic analysis, selling GGS at a price comparable to HFCS would make the process economically viable. We have successfully developed a pilot-scale GGS production system at the University of Wisconsin–Madison. Our current focus is to further improve the process economics by upgrading GGS into higher-value products. We identified an opportunity to valorise the GAW-derived product, GGS, as an attractive feedstock for the production of gluconic acid (GA) and galactonic acid (GAL).
 | ||
| Fig. 1 Schematic overview of this work and the developed process for the oxidation of GGS to gluconic acid (GA) and galactonic acid (GAL) and the precipitation of their calcium salts. | ||
GA is an aldonic acid formed via oxidation of the aldehyde group of glucose to a carboxylic acid, and similarly GAL is the monoacid product of galactose oxidation (Fig. 1). GA has applications in a wide array of industries including food additives,11 pharmaceuticals,12 and metal-chelating agents13 and has been found to be an excellent additive for cement which currently constitutes its largest demand by industry (45%).14,15 Owing to these applications the Department of Energy (DoE) named GA in the top 10 bioproduct targets and its market value is predicted to hit $1.9 billion in 2028.16
Fermentation is a common route used for the production of GA from glucose,17,18 with batch processing producing high yields yet hindered by difficulties in separation and operating time and other production roadblocks.19 Thermochemical routes directly from glucose utilising Pt and Au catalysts20–25 are being developed but with applied alkaline conditions the longevity of the catalyst on various supports poses a feasibility problem. Alternative catalysts such as CuO nanoleaves have been reported for these conversions showing promising high selectivity for the oxidation of glucose or cellobiose.26,27
Galactonic acid (GAL), an epimer of gluconic acid, is not as widespread in production nor application, due to the lower availability of galactose compared to glucose. Owing to its similar structure to GA it may have potential applications in similar use cases. For example, the same anti-retardation of the cement effect is observed for GAL as for GA,28 acting by slowing the dehydration of concrete and allowing longer handling times with suitable addition. Although the orientation of the hydroxyl group may not impact its application and value, it impacts the solubility of GAL compared to that of GA, more exaggerated when used as calcium salts.29 A difference in solubility is also observed when comparing aldaric (dicarboxylic) acids derived from glucose and galactose. Glucaric acid from glucose has a high solubility in water; however galactaric acid is insoluble in water and in virtually all common organic solvents.30 This is due to the tighter crystalline packing possible due to its optical symmetry, a trend extended to GA and GAL. The solubility of calcium gluconate is 3.5 g per 100 mL (ref. 31), whereas calcium galactonate has a low solubility in water, even when heat is applied. In this work the solubility is utilised for selective precipitation of the two epimers, aided simply by the low solubility of calcium galactonate.
Here we report a selective, catalytic oxidation of waste-derived GGS into gluconic and galactonic acids using Au catalysts, on various supports, using hydrogen peroxide as a green, more convenient oxidant and molecular O2 for a more economical process. The oxidation does not require the addition of a base, and inexpensive additives are used. Selective precipitation utilising just the solubilities of the aldonic acid calcium salts allows two high-value product streams to be produced as secondary products from GAW.
Experimental
Catalyst preparation
Au/Al2O3 and Au/TiO2 were prepared by deposition–precipitation (DP)32–34 using gamma-phase Al2O3 (Thermo Fisher) and TiO2 (Aeroxide P25, Thermo Fisher) as supports. For the Au precursor, HAuCl4·3H2O (Aldrich >99.5%) was used. The selected support was dried in an oven overnight and a 100 mL solution of HAuCl4·3H2O at 4.2 × 10–3 M was heated to 80 °C and the pH adjusted to 8 using a 1 M solution of NaOH. After maintaining the pH for some time, 1 g of the dried solid support was added, and the resulting slurry was readjusted to pH 8 before stirring for 2 h. After this time the solids were separated by centrifugation, washed thoroughly with H2O and dried overnight. The catalyst material was then calcined in a furnace at 300 °C with a ramp rate of 2 °C min−1 and the target temperature was maintained for 4 h. Other catalysts used in this work, Pt/C (5 wt% & 10 wt%), Pt/Al2O3 (5 wt%) and Pd/C (5 wt%), were sourced commercially from Sigma-Aldrich.Catalyst characterisation
Metal loadings were determined using an Oxford INCA 350 energy-dispersive X-ray microanalysis system (EDS) connected with a Hitachi S-4800 field emission scanning electron microscope (FESEM) used for the energy-dispersive X-ray spectrometry (EDS) measurements. EDS spectra are shown in the ESI (Fig. S1 & S2†). Using the TEM images captured with a TE attachment for the EDS system, the particle size distribution (Fig. 2b) and average particle size were calculated for the prepared catalysts (Table 1). Ultraviolet-Visible (UV-Vis) spectroscopy of solid samples was performed using an Agilent Cary 5000 UV-Vis-NIR spectrophotometer equipped with a Praying Mantis diffuse reflectance accessory. The prepared catalysts were referenced against the relevant support materials. A characteristic absorbance at ca. 540 nm confirmed deposition of Au onto the surface (Fig. 3). | ||
| Fig. 2 (a) TEM image of Au/Al2O3 (scale bar = 300 nm) scale. (b) Particle size distribution of Au NPs on Au/Al2O3. | ||
| Catalyst | Metal loading/wt% | Particle diameter/nm |
|---|---|---|
| Au/Al2O3 | 1 | 6.7 ± 1.1 |
| Au/TiO2 | 3 | 4.9 ± 1.0 |
Product analysis
Product analysis was performed on an Agilent 1200 series HPLC with a diode array detector (DAD) and a refractive index detector (RID) using a Phenomenex Rezex ROA-Organic Acid H+ (8%) column with a 5 mM H2SO4 mobile phase. Product quantities of gluconic, galactonic and glucaric acids were measured at 210 nm in the UV range using external standard curves. Carbohydrate conversions were determined using the RID detector with additional external standard curves. The RID signal for both gluconic and galactonic acids and their respective carbohydrate precursor overlap; however as the carbohydrates are not UV active it was possible to subtract a calculated RID response based on the UV quantifications of gluconic and galactonic acids to quantify the remaining unreacted carbohydrates. Example chromatograms are provided in the ESI (Fig. S3 and S4†). Total organic carbon (TOC) was measured with a Shimadzu© TOC-VCPH Analyzer. Samples were diluted in MQ H2O and degassed, and a 1000 ppm potassium hydrogen phthalate standard was used for the quantification of the organic carbons in the liquid samples. The results are shown in ESI Table S4.†Results and discussion
Catalytic oxidation of GGS using hydrogen peroxide as the oxidant
Building on our previous research using Au NP catalysts to successfully oxidise glucose to gluconic acid under microwave irradiation in H2O without adding a base,22 we began a trial oxidation of GGS simulation feeds with various catalysts using hydrogen peroxide as the oxidant. Only stochiometric amounts of H2O2 are required. Initially microwave (MW) experiments were performed to establish catalyst performance and optimal reaction temperature which can be adapted to conventional heating apparatus. Based on the initial screening it was concluded that Au/Al2O3 at 80 °C provided the highest selectivity for gluconic acid production (Table 2).| Temp/°C | GLU conv./% | GAL conv./% | GA selectivity/% | GALA selectivity/% |
|---|---|---|---|---|
| 40 | 0 | 0 | 0 | 0 |
| 60 | 0 | 0 | 0 | 0 |
| 80 | 43 | 62 | 91 | 16 |
| 100 | 61 | 71 | 85 | 36 |
| 120 | 76 | 81 | 56 | 29 |
| 140 | 95 | 87 | 56 | 51 |
Previous reports of Au, Pt and Pd catalysts for the oxidation of carbohydrates focused on the production of aldaric acids. As these di-acids are not the target in this work we restricted the amount of oxidant to stochiometric amounts to avoid over-oxidation. The importance of oxidant concentration conversion and selectivity of GGS oxidation was assessed by creating a series of H2O2 equivalents. Lower conversion was observed: 72% and 82% for glucose and galactose with 62% and 48% selectivities to the respective aldonic acid using 1 equivalent H2O2 (Fig. 4). This improved to 98% and 94% conversion of glucose and galactose using 4 equivalents with 69% and 57% selectivity, respectively. With the addition of greater equivalents of H2O2 we noticed a slight decrease in selectivity, which is a result of further oxidation to aldaric acids, and small amounts with <10% selectivity to glucaric acid were observed.
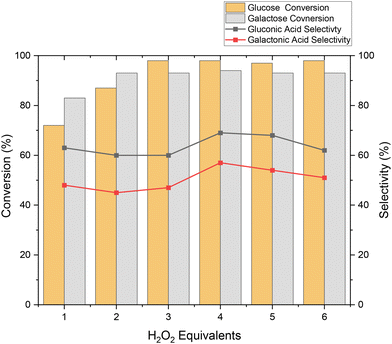 | ||
Fig. 4 Oxidation of GGS with varying H2O2 equivalents. Reaction conditions: 80 °C for 24 h, 10 mL of 2% solution of GGS with 18 mg Au/Al2O3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, GGS 1000, GGS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au). Au). | ||
The dependence on time was assessed. Glucose and galactose conversions in a 3-hour reaction were moderate (and the respective selectivity). A 6-hour reaction was required for >90% conversion (Fig. 5). After 24 hours 98% and 93% conversions of glucose and galactose were achieved with 69% selectivity to gluconic acid and 57% to galactonic acid. Equipped with these results we replaced H2O2 with molecular O2 as the oxidant for more economical conversions. As the stoichiometry of H2O2 can be controlled in an easier way, the insights gained from these oxidations can be applied to those with molecular O2.
 | ||
Fig. 5 GGS oxidation as a function of reaction time. Reaction conditions: 80 °C, 10 mL of 2% solution of GGS with 18 mg catalyst (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, GGS 1000, GGS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au) and 4 equiv. H2O2. Au) and 4 equiv. H2O2. | ||
Catalytic oxidation experimental optimisation using O2 as the oxidant of simulation feeds
Studying O2 as the oxidant for the reaction, we observe selectivity towards the aldonic acids. In a 24 h reaction at 7 bar O2 pressure the Au catalysts (Au/Al2O3 and Au/TiO2) outperformed Pt and Pd catalysts (Fig. 6), even with significantly lower metal loading (1–3 wt% Au and 5–10 wt% Pt and Pd) and the selectivity and conversion were comparable to those of reactions using H2O2. The reactions were carried out without the addition of a base, a benefit of using Au catalysts which does not favour Pt & Pd oxidation as reported in the literature.35Oxidations of glucose with Pt/C are well reported, for the purpose of producing glucaric acid by the oxidation of glucose.20,36,37 Comparably high conversions of glucose and galactose were observed using Pt/C under the applied conditions (Fig. 6), but the selectivity was low, 29% and 21% for gluconic and galactonic acid, respectively. This can be explained by quantifying the glucaric acid in the reaction mixture signifying overoxidation (Fig. S7†), while for galactaric acid it is insoluble and will not be detectable in solution via HPLC analysis. For a 24 h reaction at 5 bar O2 pressure at 80 °C the conversion of glucose and galactose was 79% and 95%, respectively. This translated into 58% and 31% selectivities to gluconic and galactonic acids, with a selectivity to glucaric acid of 32%. This shows easier accessible oxidation of the terminal hydroxyl group with Pt/C supported by studies on the adsorption and reactivity of aldoses on Pt surfaces,38 which for the scope of aldonic acid production is an undesired side product. Au/Al2O3 and Au/TiO2 have lower selectivity towards glucaric acid, reflecting decreased activity of Au for the oxidation of terminal hydroxyl groups.
The conversion at 7 bar (Fig. 6b) for 24 h using Au/Al2O3 was 80% and 88% for glucose and galactose, respectively (52% and 33% selectivities). However, using hydrogen peroxide under the same conditions higher selectivity and conversions were observed for the oxidation products. It was noted that lower pressures favoured higher conversion and acid selectivity. The reaction at 2.5 bar O2 for 24 h at 80 °C gave 98 and 95% conversions of glucose and galactose with 73% and 55% selectivities for gluconic and galactonic acids from the respective monosaccharide (Fig. 6a). This was a marked improvement and highlighted the importance of controlling the O2 pressure during the reaction. The trends in Table 3 for a 6 h reaction showcase the effect of pressure on the GGS conversion and selectivity which emphasises the requirement for appropriate oxygen pressure to reduce catalytic site blocking and destruction of reaction products. This was confirmed using liquid total organic carbon (TOC) analysis. The TOC showed that the pressure applied was positively correlated with total carbon loss (see the ESI for more details†). Catalyst loading is another important aspect to ensure high selectivity and a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000. Au
1000. Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GGS was found to be optimal for adequate conversions and high selectivity, and an increase in the ratio to 1
GGS was found to be optimal for adequate conversions and high selectivity, and an increase in the ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 decreased the conversion. A decrease in the ratio to 1
2000 decreased the conversion. A decrease in the ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 and further to 1
500 and further to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 showed a higher conversion but a decrease in selectivity. This was explained by a positive linear correlation between catalyst
250 showed a higher conversion but a decrease in selectivity. This was explained by a positive linear correlation between catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) feed and total carbon loss (monitored by TOC and detailed in Table S14†).
feed and total carbon loss (monitored by TOC and detailed in Table S14†).
| O2 pressure (bar) | Time (h) | Temp. (°C) | Glucose conversion (%) | Galactose conversion (%) | Selectivity GA (%) | Selectivity GAL (%) |
|---|---|---|---|---|---|---|
| Reaction conditions: 90 mg glucose, 90 mg galactose, 30 mg lactose, 18 mg catalyst, and 10 mL H2O | ||||||
| 1.5 | 6 | 80 | 32% | 39% | 52% | 45% |
| 3 | 6 | 80 | 73% | 75% | 76% | 45% |
| 7 | 6 | 80 | 67% | 73% | 51% | 33% |
| 12 | 6 | 80 | 74% | 79% | 33% | 22% |
Selective separations of gluconic and galactonic salts
As with numerous carbohydrate conversions, the separation of products is a vital and usually a challenging aspect of the process and can account for 20–50% of the total cost of the process.39 Thermo-catalytic treatment of carbohydrates often leads to small organic acidic side products and humin formation. As this oxidation is active at 80 °C, humin formation and side product formation are minimal.40 However, if the intended applications of the gluconic and galactonic acids are pharmaceuticals and food products, these are high purity demanding applications. Therefore, it is desired to have a separation toolkit for further applications. The difference in solubilities in glucose and galactose become more pronounced when oxidised to aldonic and aldaric acids and even more so as calcium salts. The calcium salts calcium gluconate and calcium galactonate are reported to have much lower solubility than the free acids. These are summarised in Table 4 from reported literature values.A simulated mixture of calcium carboxylates obtained from the GGS oxidation, of 5 wt% calcium gluconate and 3 wt% calcium galactonate in H2O, was prepared to test crystallization as a separation approach. Upon cooling to 0 °C, a precipitate formed, and this was confirmed by 1H NMR spectroscopy to be calcium galactonate with high purity (>99%) (Fig. 7a). A solution of calcium gluconate in H2O was left. To precipitate the calcium gluconate, EtOH was added, as the solubility of the salts in EtOH is generally poor. This successfully precipitated calcium gluconate confirmed by 1H NMR with a purity of 83% (Fig. 7b) with the major impurity being calcium galactonate, lending to its trace solubility in H2O.
Applying the successful model precipitation experiments to the obtained oxidation reaction solutions first the solid catalyst was recovered for reuse, then 5 equivalents CaCO3 were added as an inexpensive base and calcium source and the solution was stirred for 2 h. With a solution of calcium gluconate, calcium galactonate and unreacted sugars the selective precipitation method was tried. Initially this was unsuccessful in precipitating calcium galactonate in H2O with both calcium salts co-precipitating once EtOH was added. This can be suggested to be the consequence of strong hydrogen bonding between the components in solution. To promote the calcium galactonate to precipitate out of the solution seed crystallisation of calcium galactonate was tested. A small portion of pure calcium gluconate was added, and the concentrated solution was cooled (detailed in the ESI†). This caused the calcium galactonate in solution to precipitate and the purity was monitored by 1H NMR. After this was separated, we added EtOH to the solution to precipitate calcium gluconate and this was obtained with a purity of 86%.
Conclusions
An efficient approach for the conversion of a dairy waste-derived product GGS from an inedible Greek yogurt side product is outlined. The developed two-step process provides new feedstock streams for the renewable chemical industry. The Au/Al2O3 catalyst prepared selectively oxidized glucose and galactose into their respective aldonic acids, using either H2O2 or O2 as the oxidant with a very low catalyst loading (Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GGS ratio of 1
GGS ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000).
1000).
Gluconic acid, galactonic acid and their respective calcium salts are desired products as they already have a plethora of industrial applications. By manipulating the solubility difference of the produced gluconic and galactonic acids as calcium salts, an effective, simple, and selective precipitation of epimeric products is presented, providing opportunities for high-purity product streams of valuable bioproducts from the mixed monosaccharide GGS. Further studies on the scale-up potential of the oxidation and separation procedures will allow realisation of the utilisation of the large volume of Greek yogurt waste, an important step in removing some of the environmental burden from the dairy industry. To align with a wider theme of a green chemistry future, the applications of sugar derived acids are still being developed and when attention is turned to renewable feedstocks in contrast to petroleum sourced chemicals, we can provide new chemical building blocks with improved functionality as well as helping to secure a greener chemical industry.48
Data availability
The data used for the preparation of this communication are listed in the ESI.†Conflicts of interest
There are no conflicts to declare.References
- C. O. Tuck, Valorization of biomass: Deriving more value from waste (Science (695)), Science, 2012, 338(6107), 604 CAS.
- L. Aleksandrowicz, R. Green, E. J. M. Joy, P. Smith and A. Haines, The impacts of dietary change on greenhouse gas emissions, land use, water use, and health: A systematic review, PLoS One, 2016, 11(11), 1–16, DOI:10.1371/journal.pone.0165797.
- W. Willett, J. Rockström, B. Loken, M. Springmann, T. Lang and S. Vermeulen, et al., Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems, Lancet, 2019, 393(10170), 447–492 CrossRef PubMed.
- C. Houssard, D. Maxime, S. Benoit, Y. Pouliot and M. Margni, Comparative life cycle assessment of five greek yogurt production systems: A perspective beyond the plant boundaries, Sustainability, 2020, 12(21), 1–21 CrossRef.
- D. Rocha-Mendoza, E. Kosmerl, A. Krentz, L. Zhang, S. Badiger and G. Miyagusuku-Cruzado, et al., Invited review: Acid whey trends and health benefits, J. Dairy Sci., 2021, 104(2), 1262–1275 CrossRef CAS PubMed.
- M. J. Lindsay, M. S. Molitor, T. B. Goculdas, J. Zhao, J. R. Featherman and M. Li, et al., Production of glucose-galactose syrup and milk minerals from Greek yogurt acid whey, Green Chem., 2022, 24(21), 8538–8551 RSC.
- W. Wang, O. J. Dziedzic, C. R. Lesnjak, Z. Yu, J. B. Miller and J. R. Featherman, et al., The Kinetics of Aqueous Lactose Hydrolysis with Sulfuric Acid, React. Chem. Eng., 2025 10.1039/D5RE00175G.
- R. Gyawali and S. A. Ibrahim, Effects of hydrocolloids and processing conditions on acid whey production with reference to Greek yogurt, Trends Food Sci. Technol., 2016, 56, 61–76 CrossRef CAS.
- P. Menchik, T. Zuber, A. Zuber and C. I. Moraru, Short communication: Composition of coproduct streams from dairy processing: Acid whey and milk permeate, J. Dairy Sci., 2019, 102(5), 3978–3984, DOI:10.3168/jds.2018-15951.
- M. Nani and K. Krishnaswamy, A natural whitening alternative from upcycled food waste (acid whey) and underutilized grains (millet), Sci. Rep., 2023, 13(1), 1–14, DOI:10.1038/s41598-023-32204-4.
- S. Ramachandran, P. Fontanille, A. Pandey and C. Larroche, Gluconic acid: Properties, applications and microbial production, Food Technol. Biotechnol., 2006, 44(2), 185–195 CAS.
- S. Ramachandran, S. Nair, C. Larroche and A. Pandey, Current Developments in Biotechnology and Bioengineering: Production, Isolation and Purification of Industrial Products, in Gluconic Acid, Elsevier B.V., 2016, pp. 577–599. DOI:10.1016/B978-0-444-63662-1.00026-9.
- K. Fischer and H. P. Bipp, Removal of heavy metals from soil components and soils by natural chelating agents, Part II. Soil extraction by sugar acids, Water, Air, Soil Pollut., 2002, 138(1–4), 271–288 CrossRef CAS.
- S. Ma, W. Li, S. Zhang, D. Ge, J. Yu and X. Shen, Influence of sodium gluconate on the performance and hydration of Portland cement, Constr. Build. Mater., 2015, 91, 138–144, DOI:10.1016/j.conbuildmat.2015.05.068.
- A. M. Cañete-Rodríguez, I. M. Santos-Dueñas, J. E. Jiménez-Hornero, A. Ehrenreich, W. Liebl and I. García-García, Gluconic acid: Properties, production methods and applications—An excellent opportunity for agro-industrial by-products and waste bio-valorization, Process Biochem., 2016, 51(12), 1891–1903 CrossRef.
- Global gluconic acid market is expected to reach USD 1.9 billion by 2028, fiormarkets.com [Internet]. 2021. Available from: https://www.globenewswire.com/news-release/2021/05/04/2221889/0/en/Global-Gluconic-Acid-Market-Is-Expected-to-Reach-USD-1-9-billion-by-2028-Fior-Markets.html#.
- H. Znad, J. Markoš and V. Baleš, Production of gluconic acid from glucose by Aspergillus niger: growth and non-growth conditions, Process Biochem., 2004, 39(11), 1341–1345 CrossRef CAS.
- Y. Jiang, K. Liu, H. Zhang, Y. Wang, Q. Yuan and N. Su, et al., Gluconic Acid Production from Potato Waste by Gluconobacter oxidans Using Sequential Hydrolysis and Fermentation, ACS Sustainable Chem. Eng., 2017, 5(7), 6116–6123 CrossRef CAS.
- P. Pal, R. Kumar and S. Banerjee, Manufacture of gluconic acid: A review towards process intensification for green production, Chem. Eng. Process., 2016, 104, 160–171 CrossRef CAS.
- J. Lee, B. Saha and D. G. Vlachos, Pt catalysts for efficient aerobic oxidation of glucose to glucaric acid in water, Green Chem., 2016, 18(13), 3815–3822 RSC.
- C. Megías-Sayago, J. L. Santos, F. Ammari, M. Chenouf, S. Ivanova and M. A. Centeno, et al., Influence of gold particle size in Au/C catalysts for base-free oxidation of glucose, Catal. Today, 2018, 306(2016), 183–190, DOI:10.1016/j.cattod.2017.01.007.
- S. Rautiainen, P. Lehtinen, M. Vehkamäki, K. Niemelä, M. Kemell and M. Heikkilä, et al., Microwave-assisted base-free oxidation of glucose on gold nanoparticle catalysts, Catal. Commun., 2016, 74, 115–118, DOI:10.1016/j.catcom.2015.11.014.
- X. Liu, Y. Yang, S. Su and D. Yin, Efficient Oxidation of Glucose into Sodium Gluconate Catalyzed by Hydroxyapatite Supported Au Catalyst, Catal. Lett., 2017, 147(2), 383–390 CrossRef CAS.
- M. Besson, F. Lahmer, P. Gallezot, F. Patrick and G. Fleche, Catalytic oxidation of glucose on bismuth-promoted palladium catalysts, J. Catal., 1995, 152, 116–121 CrossRef CAS.
- J. Teržan, A. Sedminek, Ž Lavrič, M. Grilc, M. Huš and B. Likozar, Selective oxidation of biomass-derived carbohydrate monomers, Green Chem., 2023, 25(6), 2220–2240 Search PubMed.
- P. N. Amaniampong, Q. T. Trinh, K. Li, S. H. Mushrif, Y. Hao and Y. Yang, Porous structured CuO-CeO2 nanospheres for the direct oxidation of cellobiose and glucose to gluconic acid, Catal. Today, 2018, 306, 172–182, DOI:10.1016/j.cattod.2017.01.009.
- P. N. Amaniampong, Q. T. Trinh, B. Wang, A. Borgna, Y. Yang and S. H. Mushrif, Biomass Oxidation: Formyl C-H Bond Activation by the Surface Lattice Oxygen of Regenerative CuO Nanoleaves, Angew. Chem., Int. Ed., 2015, 54(31), 8928–8933 CrossRef CAS PubMed.
- W. Hou and J. Bao, Evaluation of cement retarding performance of cellulosic sugar acids, Constr. Build. Mater., 2019, 202, 522–527, DOI:10.1016/j.conbuildmat.2019.01.025.
- X. Hua, X. Zhou and Y. Xu, Directed regulation of whole-cell catalysis for high-quality galactonic acid bio-preparation and characterization by Ca2+, Fuel, 2021, 285(159), 119134, DOI:10.1016/j.fuel.2020.119134.
- J. Install, A. Zupanc, M. Nikunen, J. Jänis and T. Repo, Lactose Utilisation to Furan Carboxylates: A Unique Source for Platform Molecules, Green Chem., 2024, 1–6 Search PubMed.
- World Health Organization. https://chemicalsafety.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=1736. Calcium Gluconate.
- R. Zanella, S. Giorgio, C. R. Henry and C. Louis, Alternative methods for the preparation of gold nanoparticles supported on TiO2, J. Phys. Chem. B, 2002, 106(31), 7634–7642 CrossRef CAS.
- D. L. Nguyen, S. Umbarkar, M. K. Dongare, C. Lancelot, J. S. Girardon and C. Dujardin, et al., Deposition-precipitation versus anionic-exchange Au/Al 2O 3 catalysts: A comparative investigation towards the selective reduction of NO x, Catal. Commun., 2012, 26, 225–230, DOI:10.1016/j.catcom.2012.06.004.
- A. A. Upadhye, I. Ro, X. Zeng, H. J. Kim, I. Tejedor and M. A. Anderson, et al., Plasmon-enhanced reverse water gas shift reaction over oxide supported Au catalysts, Catal. Sci. Technol., 2015, 5(5), 2590–2601 RSC.
- S. Biella, L. Prati and M. Rossi, Selective oxidation of D-glucose on gold catalyst, J. Catal., 2002, 206(2), 242–247 Search PubMed.
- J. M. H. Dirkx and H. S. van der Baan, The oxidation of glucose with platinum on carbon as catalyst, J. Catal., 1981, 67(1), 1–13 Search PubMed.
- M. Comotti, P. C. Della and M. Rossi, Mono- and bimetallic catalysts for glucose oxidation, J. Mol. Catal. A:Chem., 2006, 251(1–2), 89–92 Search PubMed.
- Q. T. Trinh, B. K. Chethana and S. H. Mushrif, Adsorption and Reactivity of Cellulosic Aldoses on Transition Metals, J. Phys. Chem. C, 2015, 119(30), 17137–17145 Search PubMed.
- P. Ibarra-Gonzalez, L. P. Christensen and B. G. Rong, A critical review of separation technologies in lignocellulosic biomass conversion to liquid transportation fuels production processes, Chem. Eng. Commun., 2022, 209(4), 529–554, DOI:10.1080/00986445.2021.1874367.
- I. Van Zandvoort, Y. Wang, C. B. Rasrendra, E. R. H. Van Eck, P. C. A. Bruijnincx and H. J. Heeres, et al., Formation, molecular structure, and morphology of humins in biomass conversion: Influence of feedstock and processing conditions, ChemSusChem, 2013, 6(9), 1745–1758 Search PubMed.
- S. H. Yalkowsky, Y. He and P. Jain, in Handbook of Aqueous Solubility Data, CRC Press, 2nd edn, 2016, p. 308 Search PubMed.
- S. P. Gould, The Final Solubility of d-Galactose in Water, J. Dairy Sci., 1940, 23(3), 227 Search PubMed.
- M. Windholz, S. Budavari and L. Y. F. Stoumtsos, in The Merck Index: An encyclopedia of chemicals and drugs, 9th edn, 1976, p. 575 Search PubMed.
- E. coli, Metabolome database. https://ecmdb.ca/compounds/ECMDB00565. Galactonic acid.
- X. Hua, X. Zhou and Y. Xu, Directed regulation of whole-cell catalysis for high-quality galactonic acid bio-preparation and characterization by Ca2+, Fuel, 2021, 285 Search PubMed.
- E. coli Metabolome database. https://hmdb.ca/metabolites/HMDB0000663. Glucaric acid.
- E. coli Metabolome database. https://hmdb.ca/metabolites/HMDB0000639. Galactaric acid.
- J. B. Zimmerman, P. T. Anastas, H. C. Erythropel and W. Leitner, Designing for a green chemistry future, Science, 2020, 367(6476), 397–400 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc01381j |
| This journal is © The Royal Society of Chemistry 2025 |



