Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dried urine spot as a stable, green, and practical microsampling tool in clinical practice for quantification of neopterin and creatinine†
Chaweewan
Suwanvecho
ab,
Lea
Vyleťalová
a,
Nikola
Přívratská
ab,
Pakanan
Laolertworakul
ab,
Dorota
Turoňová
b,
Milan
Vošmik
c,
Lenka Kujovská
Krčmová
*ab and
Frantisek
Svec
a
aThe Department of Analytical Chemistry, Faculty of Pharmacy in Hradec Králové, Charles University, Akademika Heyrovského 1203, 500 05, Hradec Králové, Czech Republic. E-mail: lenka.kujovska@faf.cuni.cz
bThe Department of Clinical Biochemistry and Diagnostics, University Hospital Hradec Králové, Sokolská 581, 500 05, Hradec Králové, Czech Republic
cThe Department of Oncology and Radiotherapy, University Hospital Hradec Králové, Sokolská 581, 500 05, Hradec Králové, Czech Republic
First published on 28th June 2025
Abstract
Dried urine spot (DUS) is demonstrated to be a useful microsampling technique for neopterin and creatinine analysis. The method was verified using samples from 12 healthy volunteers, 12 cancer patients, and 12 patients infected with the SARS-CoV-2 virus and provided results comparable to the routine reference method. DUS samples were prepared using a simple dilute-and-shoot approach with a buffer solution prior to analysis. The developed method requires only 10 μL samples, making it feasible for home sampling when combined with commercially available pre-cut DUS devices with volumetric microfluidics or capillary transfer tubes. The method is environmentally friendly as it minimizes reagent use, requires only 110 μL phosphate buffer and no organic solvents. DUS also requires 250 times less storage space than liquid urine. The technique also improves laboratory work safety by minimizing the use of harmful extraction solvents. Dried samples are non-biohazardous and stable for up to 5 days at 40 °C and for 4 months at room temperature. This allows cost-effective transport via standard mail. The study also evaluated practical considerations for clinical laboratories and home sampling. Volumetric urine sampling is simple using capillary transfer tubes or commercially available volumetric microfluidic devices. When integrated with telemedicine, this microsampling approach can reduce unnecessary hospital visits for patients and improve patient access to clinical testing.
Green foundation1. The study proposes dried urine spot sampling for neopterin and creatinine analysis, reducing urine and buffer volumes tenfold compared to conventional method. It minimizes biohazard waste generation and eliminates toxic reagents, lowering chemical exposure risks for lab staff.2. The method significantly reduces energy consumption throughout the healthcare process by minimizing storage space requirements and eliminating the need for temperature-controlled transport. This enables cost-effective shipping via standard mail or courier services. Moreover, its compatibility with home sampling supports telemedicine initiatives and improves healthcare accessibility, particularly for patients in remote areas. 3. The successful application of the method to real patient sample ensures clinical feasibility potential for broader healthcare implementation. Future research could enhance its greenness by exploring fully biodegradable sampling devices, automating low-energy extraction, and integrating point-of-care testing to simplify workflows and further reduce environmental impact. |
Introduction
Sample collection and preparation are the critical steps in bioanalysis. Conventional sampling techniques often require milliliters of sample, resulting in patient discomfort, especially for invasive collections of samples such as serum, plasma, and cerebrospinal fluid. These methods require multi-step preparation to obtain clear sample for analysis. While still necessary for diagnosis, the conventional approaches are not always practical in certain clinical settings, such as for neonate and elderly. Cost and time concerns arise due to the need for trained personnel, storage, and logistical requirements.1 Liquid samples have several drawbacks during shipping and storage, including the risk of leakage, reliance on cold chain transportation, controlled low-temperature storage, and the need of a large storage capacity.2Currently, the microsampling techniques are increasingly being used to reduce sample volume, enabled by improvements in analytical instrumentation and methodologies.3 These techniques minimize the ratio of extraction solvent to sample volume compared to classical approaches. In line with the principles of green analytical chemistry, microsampling increases the environmental friendliness of the method and reduces the exposure of laboratory personnel to harmful solvents. In addition, microsampling is widely used in clinical research and practice because it is time-saving, cost-effective, and less invasive nature.4
Dried matrix spot techniques, such as dried blood spots were introduced in 1963 for large-scale screening of phenylketonuria.5 However, dried spot techniques are not limited to blood. They are used as an alternative sampling technique for urine, a method called dried urine spot (DUS). It offers non-invasive sampling while simplifying sample shipment and storage. The DUS samples can be stored at room temperature and require minimal storage space.
Human urine can be classified as potentially infectious material if it is visibly contaminated with blood or if originates from individuals with urinary tract infections (categorized as risk group 2 microorganisms).6–8 In contrast, blood samples may contain pathogens with higher infection risk (risk group 3), such as the human immunodeficiency virus, the hepatitis B virus, the hepatitis C virus, and the Mycobacterium tuberculosis.9 The inherently lower biohazard risk of urine, combined with the reduced infectious potential of the dried matrix samples, classifies the DUS sample as non-hazardous material. This allows for shipment via regular mail or courier services.10,11 In-house sampling is possible, eliminating indirect costs associated with hospital visits, which is particularly beneficial for people living in remote areas.12 For quantitative analysis, volumetric microfluidic devices are integrated into dried matrix sampling technique to precisely determine the microscale sample, improving analytical accuracy.13 Nevertheless, variation in small volume sampling and analysis remains a challenging task in this field. The sensitive analytical method is required due to the limited concentration of analyte in defined sample volumes.
Neopterin, a pteridine derivative, is catabolized from guanosine triphosphate (GTP) by GTP cyclohydrolase I, an enzyme induced by interferon-gamma, a Th1 type cytokine. Neopterin serves as a marker of immune system activation and its levels are elevated in the early stages of diseases associated with inflammation.14 Quantification of urinary neopterin concentrations is a widely accepted method for assessing inflammation levels in various pathological conditions, including, trauma,15 cardiovascular disease,16 renal disease,17 rheumatic diseases,18 cancer,19 and infections caused by bacteria, parasites, and viruses.20,21 In addition, it has been used to predict disease severity and prognosis, which has important implications for clinical practice. Urinary neopterin levels are significantly elevated in patients with benign and malignant tumors. Numerous studies have demonstrated the usefulness of relating disease progression, predicting complications, and assessing prognosis and survival with neopterin levels. For example, high urinary neopterin levels are associated with advanced stages of cancer, such as metastasis and recurrence, in breast and colorectal cancers. These levels are elevated in approximately 80% of patients diagnosed with ovarian cancer.14,19,22 Elevated levels have also been observed in gastric, pancreatic, cholangiocarcinoma, and gallbladder cancer, serving as long-term prognostic indicators.23 In hepatocellular carcinoma, neopterin levels correlate with tumor size and survival outcomes.24 As we have previously published, neopterin is one of the biomarkers used to classify SARS-CoV-2 virus (COVID-19) patients based on their risk of death, helping to prioritize intensive care resources.25 After the fourth and seventh days of hospitalization, neopterin concentration decreased significantly due to treatment or the natural disease progression. However, it increased in patients with subsequent post-COVID syndrome.21 Creatinine, a nitrogenous compound produced by the breakdown of creatine in muscles, is excreted through the kidneys and is commonly used to estimate the glomerular filtration rate. In urinary neopterin studies, the neopterin-to-creatinine ratio is used to correct for variability of individuals’ hydration status and urine concentration.26 Therefore, simultaneous measurement of both analytes is necessary for accurate clinical interpretation.
Several approaches to the analysis of neopterin and creatinine in urine have been reported since the 1970s, often using a simple dilute-and-shoot sample preparation technique.27 Although the conventional techniques provide accurate and precise results, they require minimum sample volume of 100 μL.
To the best of our knowledge, no study has reported the methods for the analysis of neopterin and creatinine in DUS that are simple and economical for sample collection and transport. From a holistic perspective, the environmental impact and practicality of analytical method were evaluated via greenness using AGREE28 and BAGI29 software. We demonstrate the application of our approach to real life samples obtained from healthy volunteers, patients infected with the SARS-CoV-2 virus, and cancer patients whose matrices are more complex due to diseases and medications.
Experimental section
Chemicals and reagents
Neopterin (≥97.5%), and creatinine (≥97.5%), were obtained from Sigma Aldrich (Darmstadt, Germany). 6,7-Dimethylpterine was purchased from Cayman Chemical (Michigan, USA). HPLC grade methanol and acetonitrile were acquired from Honeywell (North Carolina, USA). Dibasic potassium phosphate trihydrate (K2HPO4·3H2O) and potassium dihydrogen phosphate (KH2PO4) from Merck (Darmstadt, Germany) were used for buffer preparation. Ultrapure water was produced by the Ultrapure Water System, Goro (Prague, Czech Republic).Standard and buffer preparation
Stock solutions of neopterin (200 μmol L−1), creatinine (500 mmol L−1), and 6,7-dimethylpterin (50 μmol L−1) were prepared by accurately weighing and dissolving these substances in purified water. The internal standard, 6,7-dimethylpterin, was further diluted to 5 μmol L−1 in methanol to allow for rapid evaporation after spiking on the DUS. Stock and diluted internal standard solutions were stored at −20 °C.Working standard solutions were freshly prepared on the day of analysis by dilution with ultrapure water to the desired concentrations using and stored at 4 °C until use.
For the mobile phase, a stock solution of 1 mol L−1 phosphate buffer was prepared by dissolving 57.1 g of dibasic potassium phosphate trihydrate and 102.0 g of potassium dihydrogen phosphate in 1 L of ultrapure water. The working mobile phase (15 mmol L−1, pH 6.45) was prepared freshly on the day of analysis by appropriate dilution.
Chromatographic condition
Chromatographic separations were carried out on a Shimadzu Prominence (Kyoto, Japan) and adapted from our previous study.30 The LC 20 HPLC system was equipped with a SPD-M20A diode array and a RF-10 AXL fluorescence detector. Separation was performed using Chromolith® SpeedRod RP-18e, 50 × 4.6 mm connected to Chromolith® Performance RP-18e, 150 × 3.0 mm with guard column. The column oven was maintained at 25 °C.Gradient elution used 15 mmol L−1 phosphate buffer (pH 6.45) as eluent A and acetonitrile as eluent B at a flow rate of 1.0 mL min−1. The gradient started at 0% eluent B for 1.5 min, increased to 7% eluent B in 0.5 min, held at 7% B for 2.2 min, and returned to the initial conditions at 0% B. The total run time was 8 min, with an injection volume of 1 μL.
The fluorescence detector was programmed at 353 nm excitation and 438 nm emission wavelengths to detect neopterin and the internal standard, while creatinine was detected at 235 nm using the diode array detector.
Clinical sample analysis
To ensure the applicability of the developed method in the clinical practice, its effectiveness was evaluated by determining the levels of neopterin and creatinine levels in healthy volunteers as well as in hospitalized patients infected with COVID and cancer patients. Urine samples were collected from 36 individuals over the age of 18. The healthy volunteers included 4 males and 8 females without any serious medical conditions. There were 12 SARS-CoV-2-infected patients who had been hospitalized due to the disease. The remaining 12 patients had various primary tumors (e.g., distal esophageal cancer, colon cancer, stomach cancer, pancreatic cancer, and rectal cancer) who were undergoing treatment. All of these patients were treated at the University Hospital Hradec Kralove, Czech Republic.All experiments were performed in accordance with the World Medical Association Declaration of Helsinki and the International Ethical Guidelines for Health-related Research Involving Human.31,32 Experiments were approved by the ethics committee at University Hospital Hradec Kralove, Czech Republic (protocol numbers 202007S01P, 202011P04, and 202411P16). Informed consents were obtained from human participants of this study. Urine samples were aliquoted into the microcentrifuge tubes and stored at −80 °C until analysis.
Sample processing
For the reference method, as described by Holeckova et al.,33 100 μL of urine was spiked with the internal standard stock solution (50 μmol L−1), centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g for 45 seconds, diluted 1
100 g for 45 seconds, diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 with eluent A. Finally, 170 μL of the extract was filtered onto a microtitration plate.
10 with eluent A. Finally, 170 μL of the extract was filtered onto a microtitration plate.
For the DUS preparation, 10 μL of urine was spotted on a pre-cut Whatman 903 Protein Saver card filter paper (Cytiva, Marlborough, USA). To simulate home sampling in different locations, the samples were dried at room temperature in the dark and in opened air for 2.5 h. After drying, 5 μL of the diluted internal standard (5 μmol L−1) was applied. The analytes were extracted using 110 μL of eluent A aided with shaking for 5 min. The extract was then filtered into a microtitration plate.
Method validation
The method was validated according to the commonly used bioanalytical validation guideline, the European Medicine Agency (EMA)34 and the US Food and Drug Administration (FDA)35 guidelines. Parameters including selectivity, lower limit of quantitation (LLOQ), limit of detection (LOD), linearity, precision, and accuracy were evaluated. Long term stability and shipping condition simulation were also assessed.Analytical method global assessment
Results and discussion
Optimization of chromatographic conditions and sample preparation
We modified our routine analytical method30 to achieve optimal separation of neopterin, creatinine, and the internal standard. Based on the native fluorescent nature of neopterin and internal standard, they were detected using fluorescence detector at 353 nm excitation and 438 nm emission. Creatinine was monitored using a diode array detector at 235 nm.The stationary phase comprised a Chromolith® SpeedRod RP-18e, 50 × 4.6 mm connected to a Chromolith® Performance RP-18e, 150 × 3.0 mm with guard column. The mobile phase composition and flow rates were optimized. Starting conditions were based on previous literature,27,30 using 15 mmol L−1 phosphate buffer (pH 6.45) with isocratic elution. The flow rate was initially set at 1.0 mL min−1, increased to 3.5 mL min−1 at 3 min, and continued until the end of the analysis at 5 min. The C18 monolith column was selected for its high separation efficiency at a low back pressure. To improve the eluting power for the non-polar internal standard, acetonitrile was added to the mobile phase. The goal of the optimization was to achieve the shortest run time with acceptable separation of the target analytes, suitable for high throughput analysis in clinical research and practice.
We started with the mobile phase composition as described above. Neopterin eluted at 2.5 min, and creatinine at 2.0 min. However, the internal standard was retained on the C18 sorbent until 10.5 min. We tested isocratic elution at a flow rate of 1 mL min−1 using organic solvent, acetonitrile at 3, 5, and 10%. The latter percentage resulted in neopterin and creatinine eluting around 1.6 min. This was close to the dead volume, with coelution to the accompanying interferences in real life samples. Subsequently, a linear gradient of acetonitrile (2.75 to 4 min) was tested to increase the eluting power after neopterin and creatinine were eluted to not change their retention times. The flow rate was changed from 1 to 3.5 min mL−1 at 2.75 min. However, we observed the synchronous noise in the chromatograms when acetonitrile was introduced with changed flow rate. In the gradient elution, we tested different percentages of acetonitrile from 3, to 5, to 10%, while the flow rate was fixed at 1 mL min−1. We observed the best separation of both analytes and internal standard when 5% of acetonitrile was used with 15 min analysis time.
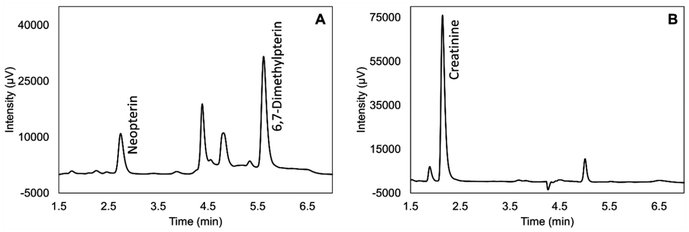
To shorten the run time, we tried to move internal standard peak from 8.5 min close to both target analytes by optimizing the gradient profile. We found out that 7% of acetonitrile with gradient elution as described in the Experimental section showed the best separation with the satisfying run time 8 min. The chromatograms of the standard solution and the real DUS sample are shown in Fig. 1 and 2, respectively.
For sample preparation, we modified our routine procedure by reducing the extraction solvent, 15 mmol L−1 phosphate buffer pH 6.45, to maintain a similar dilution factor. Vortex mixing was performed for 5 min to improve the extraction efficiency. The extracts were then filtered through a 0.2 μm polypropylene filter.
Method validation
| Standard solution | Dried urine spot sample | |||
|---|---|---|---|---|
| Neopterin | Creatinine | Neopterin | Creatinine | |
| RSD: relative standard deviation.a Dried urine spot sample contained neopterin 3000 nmol L−1 and creatinine 12 mmol L−1, standard solution contained neopterin 2300 nmol L−1 and creatinine 8.4 mmol L−1. | ||||
| Retention time (min) | 2.660 | 2.097 | 2.738 | 2.138 |
| Resolution | 8.319 | 2.390 | 1.558 | 1.750 |
| Number of theoretical plates | 2772 | 2856 | 2722 | 2870 |
| Symmetry factor | 1.499 | 1.792 | 1.403 | 1.849 |
| Repeatability of peak area (%RSD) (n = 6)a | 0.48 | 0.34 | 0.51 | 0.32 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nmol L−1, with r of 0.9997 and 1.0000, respectively. Table 2 summarizes the calibration ranges, equations, r, and limits for the target analytes.
000 nmol L−1, with r of 0.9997 and 1.0000, respectively. Table 2 summarizes the calibration ranges, equations, r, and limits for the target analytes.
| Analytes | Neopterin | Creatinine |
|---|---|---|
| r: correlation coefficient, LOD: limit of detection, LLOQ: lower limit of quantitation. | ||
| Calibration range | 55–2750 nmol L−1 | 1.1–220 mmol L−1 |
275–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nmol L−1 000 nmol L−1 |
||
| Regression equation | Y = 0.00011X − 0.00072 |
Y = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 234X + 3167.31 234X + 3167.31 |
| Y = 0.00011X − 0.00164 | ||
| r | 0.9997 | 1.0000 |
| 1.0000 | ||
| LOD | 40 nmol L−1 | 0.25 mmol L−1 |
| LLOQ | 55 nmol L−1 | 1.0 mmol L−1 |
| Neopterin (nmol L−1) | Creatinine (mmol L−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| LLOQ | LowQC | MidQC | HighQC | LLOQ | LowQC | MidQC | HighQC | |
| LLOQ: lower limit of detection, LowQC: low quality control level, midQC: middle quality control level, highQC: high quality control level. | ||||||||
| Concentration from reference method | 52.70 | 826.22 | 3124.70 | 5335.73 | 0.90 | 4.13 | 11.98 | 17.97 |
| Within-run (n = 5) | ||||||||
| Mean concentration | 49.70 | 871.36 | 3260.83 | 5552.95 | 0.93 | 4.26 | 12.59 | 18.51 |
| Precision, %RSD | 1.76 | 1.39 | 2.38 | 1.39 | 0.09 | 0.92 | 1.95 | 1.19 |
| Accuracy, %bias | 5.68 | 0.92 | 0.51 | 0.00 | 3.79 | 1.22 | 2.28 | 0.99 |
| Extraction recovery, % | 94.32 | 100.92 | 100.51 | 100.00 | 103.79 | 101.22 | 102.28 | 100.99 |
| Between-run (n = 3) | ||||||||
| Mean concentration | 49.72 | 847.76 | 3237.74 | 5306.92 | 0.92 | 4.13 | 12.04 | 18.19 |
| Precision, %RSD | 3.00 | 4.78 | 4.49 | 3.91 | 1.38 | 2.80 | 3.63 | 2.24 |
| Accuracy, %bias | 5.65 | 2.61 | 3.62 | 0.54 | 2.51 | 0.11 | 0.55 | 1.23 |
| Extraction recovery, % | 94.35 | 102.61 | 103.62 | 99.46 | 102.51 | 100.11 | 100.55 | 101.23 |
Application
To evaluate the usefulness of the analytical method in clinical research and practice, patient samples with complex matrices reflecting disease progression and the effects of various drugs were tested. No interferences were observed in the retention times of the target analytes. In addition, neopterin and creatinine were quantified in DUS samples obtained from healthy volunteers, COVID-19 patients, and cancer patients (n = 12 per group). In urinary neopterin studies, the neopterin-to-creatinine ratio is used to account for variation in individual hydration status and urine concentration. The results are shown in Table 4.| Analyte | Healthy volunteers (n = 12) | COVID-19 group (n = 12) | Cancer group (n = 12) |
|---|---|---|---|
| Concentration range | |||
| Neopterin (nmol L−1) | 893.20–5139.80 | 546.37–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 922.03 922.03 |
309.79–2973.43 |
| Creatinine (mmol L−1) | 4.54–21.54 | 4.67–22.00 | 1.43–14.09 |
| Ratio | |||
| Neopterin/creatinine ratio (mmol mol−1) | 102.19–374.28 | 100.48–964.94 | 129.94–637.02 |
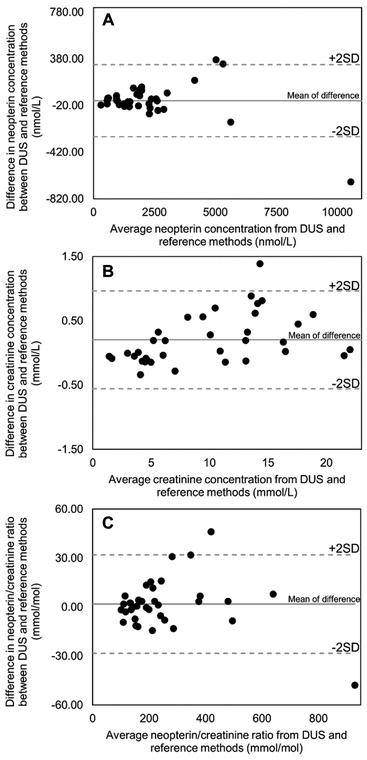
We compared the results obtained from the DUS samples with those obtained by the reference method using Bland–Altman plots as illustrated in Fig. 4. This statistical approach is recommended for evaluating the degree of agreement between two measurement methods in medical laboratories because it focuses on the differences rather than relationships between variables. Correlation analysis, on the other hand, assesses relationships between variables and is not suitable for evaluating method comparability. The differences between the two measurements were plotted against their means. The plots of neopterin, creatinine, and their ratio showed a random distribution of the data points and the majority of data points were within ±2 standard deviation of the mean difference. These results indicate that the DUS method is not different from the reference method, and both methods can be used interchangeably.42 However, a paired t-test was also used to compare the DUS method to those of the reference method. P-Values greater than 0.05 indicated no significant difference (0.42 for neopterin, 0.88 for creatinine, and 0.47 for the neopterin-to-creatinine ratio).
Quantitative analysis requires precise and accurate volume sampling to minimize the analytical bias. Filter paper without volumetric device, such as the Whatman 903 card, requires additional volume metering tools, such as pipette and capillary transfer tube.1 The commercial options with volumetric microfluidic chip such as the HemaXis DB10 (Hemaxis, Switzerland) and Capitainer-B qDBS (Capitainer, Sweden), offer greater convenience for home sampling patients.
Although inaccurate sampling is overcome by the microfluidic chip, the visibility of the dried urine spot is also problematic when the dried spot has to be subpunched as shown in Fig. 5. Therefore, the pre-cut filter paper like Capitainer-B qDBS is more recommended to be easier for cutting after sampling and more convenient for downstream analysis. This commercial option was tested using samples from healthy volunteers, COVID-19, and cancer patients (n = 2 per group), and the results are shown in Table 5. A paired samples t-test revealed no significant differences (p > 0.05) in the concentrations of neopterin, creatinine, or the neopterin/creatinine ratio between the Capitainer-B qDBS method and the reference method.
| Sample | Neopterin concentration (nmol L−1) | Creatinine concentration (mmol L−1) | Neopterin/creatinine ratio (mmol mol−1) | |||
|---|---|---|---|---|---|---|
| Reference method | DUS Capitainer-B qDBS | Reference method | DUS Capitainer-B qDBS | Reference method | DUS Capitainer-B qDBS | |
| 1 | 2386.00 | 2344.08 | 21.50 | 21.32 | 110.98 | 109.95 |
| 2 | 2589.00 | 2568.98 | 16.40 | 16.08 | 157.87 | 159.76 |
| 3 | 2620.18 | 2615.39 | 13.19 | 12.98 | 198.65 | 201.49 |
| 4 | 1836.95 | 1867.40 | 13.04 | 13.37 | 140.90 | 139.67 |
| 5 | 558.08 | 561.76 | 4.16 | 4.34 | 134.02 | 129.44 |
| 6 | 2271.66 | 2294.78 | 3.53 | 3.65 | 643.51 | 628.71 |
| p-Value | 0.892 | 0.902 | 0.332 | |||
DUS sampling is less invasive than DBS and dried plasma spot techniques because it does not require the use of a lancet for sample collection. Additionally, the simpler urine matrix in DUS contains fewer interfering substances such as proteins, lipids, and cells, which simplifies the sample extraction process. These characteristics reduce the need for complex laboratory equipment and decrease solvent consumption. Sample heterogeneity is lower in DUS because urine is not affected by hematocrit. Although volumetric absorptive microsampling (VAMS) has also been used to collect urine,43 the lower viscosity of urine compared to blood may lead to over-adsorption or inconsistent volume uptake, which could affect the accuracy of the analysis. In contrast, when DUS is prepared using a standardized microfluidic compartment to control spot volume, it offers reproducible and homogeneous analyte distribution with minimal variability.
Several challenges may affect the accuracy and reliability of analytical results when implementing volumetric DUS sampling to clinical research and practices. Certain analytes may strongly adsorb to the disc. Special solvents or condition are needed for efficient extraction and reproducible results. These issues can be evaluated through comprehensive method validation. Matrix effect is a common challenge in bioanalysis. However, the filter paper discs also increase matrix complexity, due to fluctuations in urine pH, ionic strength, and other compounds excreted in urine. Drying the discs in open air can degrade analytes leading to inaccurate quantification and making stability studies essential. Lastly, DUS use in clinical setting is limited due to insufficient clinical validation. Fewer biomarkers have been validated using the volumetric DUS than with liquid urine samples. In our study, we addressed these potential limitations through targeted experimental design, which did not significantly impact our results.
In addition to technical considerations, patient compliance and sample collection techniques may differ, especially in home sampling scenarios. For certain populations, such as the elderly or individuals with mobility issues, applying 10 μL of urine to the sampling device can be difficult, especially when using a capillary transfer tube with the Whatman 903 card. Therefore, volumetric DBS devices that are equipped with an overflow mechanism, such as the Capitainer-B qDBS, are recommended due to their ease of use. Moreover, integrating DUS sampling into the clinical workflow may require additional staff training, and regulatory approval. Analytical and clinical validation must be conducted across diverse populations, analytes, and clinical settings. Usability studies and clear instructions are important.
Analytical method global assessment
In addition to considering analytical efficiency through the validation, we evaluated the environmental impact and practicality of the method using AGREE and BAGI software tools. As shown in Fig. 6, the DUS method achieved a slightly higher greenness score than the reference method, mainly due to miniaturization. This method aligns well with the principles of green chemistry in several key areas, including waste reduction, energy efficiency, and the use of safer chemicals. The DUS method requires only 10 μL of urine, which is 10 times less than the reference and previous published method.27,33 This miniaturization significantly reduces waste in terms of both sample volume and overall reagents and consumables use. The DUS method avoids using of organic solvents by employing an 110 μL aqueous buffer for dilution. This eliminates the need for protein precipitation, liquid–liquid extraction, or solid-phase extraction. Consequently, the quantity of hazardous waste and the exposure to harmful chemicals is minimized. The method is energy efficient because it uses a highly sensitive fluorescence detector, eliminating the need for sample preconcentration such as evaporation and derivatization. Our procedure supports high-throughput analysis, allowing multiple samples to be processed in parallel with a simple 5 min vortexing step. This eliminates the need for complex equipment for sample preparation. Furthermore, the energy consumption throughout the entire workflow from sample collection to analysis is significantly reduced compared to liquid urine methods. The DUS samples do not require cold-chain transport and occupy 250 times less storage space than liquid urine samples. They can be stored in smaller, more energy-efficient freezers. Additionally, DUS enhances laboratory safety compared to the reference method by minimizing the risk of spills and contamination. The reference methods require multiple steps such as aliquoting urine from a collection vessel, adding internal standards, and transferring the extraction solvent to liquid urine. These steps can increase the likelihood of accidental spills, which can lead to contamination of work surfaces. The use of a combustible mobile phase and lower sample throughput were the weaknesses. The reference method also shared strengths such as minimal sample preparation steps, no derivatization, low waste generation, multi-analyte capability, and reduction of harmful solvent use. | ||
| Fig. 6 Comparative evaluation of the environmental friendliness (greenness), practicality (blueness), stability, storage space and transportation of dried urine spot method and reference method. 12 principles of green analytical chemistry were evaluated including 1-direct analytical technique, 2-amount of sample, 3-in situ measurement, 4-integration of process, 5-automation and miniaturization, 6-derivatization, 7-waste generation, 8-multianalyte method, 9-energy consumption, 10-reagents from renewable sources, 11-toxic reagents, 12-safety of operator.19 10 attributes of blueness including: 1-type of analysis, 2-the number of analytes that are simultaneously determined, 3-the analytical technique, 4-the number of samples that can be simultaneously treated, 5-the sample preparation, 6-the number of samples that can be analyzed per hour, 7-reagents and materials used, 8-preconcentration, 9-automation level, and 10-amount of sample.20 *The blueness score does not include stability, storage requirements, and transport, which are critical for practical application. | ||
The practicality of the analytical methods was also evaluated. As demonstrated with Fig. 6, DUS and reference methods had equal blueness scores of 80 points in the BAGI tool. The smaller sample volume requirement of the DUS method is a significant advantage, while the reference method is characterized by faster analysis time.
However, beyond the ten attributes evaluated by the BAGI tool, the key parameters such as, stability, storage space, and transportation were not considered to evaluate the applicability of the method. If a sample degrades under certain conditions without proper consideration, it may lead to inaccurate and irreproducible results. In addition, a method that requires a large volume of storage space may be impractical for high-throughput or field application. Biological samples typically require freezer storage, which consumes energy and increases logistical complexity. Transportability is also critical, especially for remote sampling, clinical trials, and global health applications. Liquid samples transport that relies on cold chain logistics increases cost and complexity. We believe these factors should be explicitly considered when evaluating the practical real-world feasibility of an analytical method.
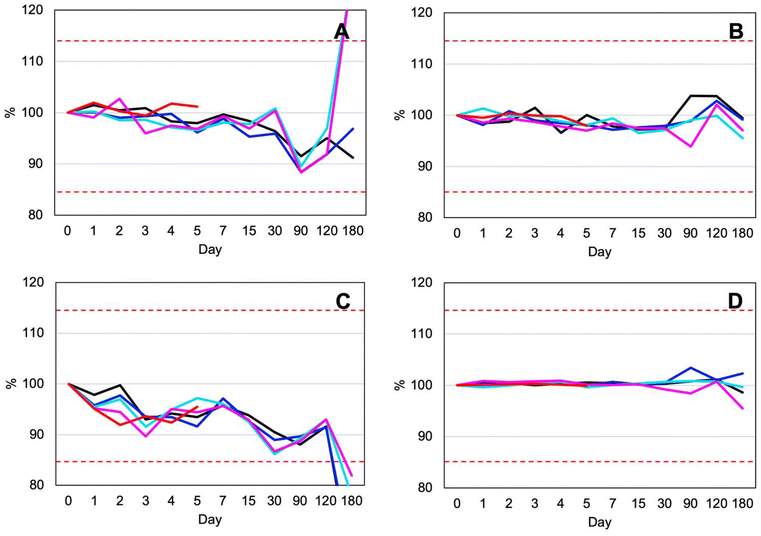
According to recommendations for handling liquid sample and analyte stability,44 shipping samples chilled at 4 °C within 2 weeks using cold chain system is practical in a resource-limited setting due to the lower cost of batch shipping. However, this method requires special packaging, reliable freezer access, and a stable electrical supply with backup power for the freezer. These are challenges that may be difficult to overcome in such environments. Additionally, sample collection usually requires patients to visits to primary care facilities, and longer transportation times further complicate logistics.45 As mentioned in the stability study, DUS samples are more stable than liquid urine samples under all storage conditions, particularly at room temperature and at +40 °C. DUS samples are ideal for use in low-resource settings. They do not require cryostorage and remain stable at room temperature for at least 4 months. This allows for storage at primary care units and shipment to clinical laboratories without temperature control. Due to the non-biohazard nature of the dried matrix spots, DUS sample can also be mailed directly by patients, with no impact from transportation delays.46
Another challenge in routine clinical research is storage space. Biological samples cannot be stored at room temperature due to their limited stability. The freezer, the key piece of equipment, has limited space. For example, liquid urine samples typically require aliquoting into the appropriate volumes for analysis, along with backup aliquots. Batch analysis is commonly used, which increases storage demands even more. There is also the indirect cost of storage equipment and electricity. Typically, we store about 1 mL of urine sample in a 1.5 mL microcentrifuge tube until the day of analysis. Based on the dimensions of the 20 mm width and 41 mm long tube, it each sample requires 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 mm3 of space. While a DUS sample with a diameter of 8 mm requires only 64 mm3 freezer space which is 250-times reduced from storing the liquid urine sample (Fig. 7). DUS overcomes limitation of freezer storage by enabling ambient storage. Long-term stability studies have confirmed the integrity of DUS samples during extended room-temperature storage and transport.
400 mm3 of space. While a DUS sample with a diameter of 8 mm requires only 64 mm3 freezer space which is 250-times reduced from storing the liquid urine sample (Fig. 7). DUS overcomes limitation of freezer storage by enabling ambient storage. Long-term stability studies have confirmed the integrity of DUS samples during extended room-temperature storage and transport.
 | ||
| Fig. 7 Comparison of storage space requirements for a 1.5 mL microcentrifuge tube for liquid urine and a dried urine spot. | ||
Moreover, there are several advantages for patients. Integrating DUS sampling with telemedicine has significant practical potential. Due to their stability and ease of transport, DUS samples can be collected at lower-level healthcare facilities or even in settings with limited resources and then shipped to specialized laboratories for analysis. DUS sampling is ideally suited for monitoring discharged patients or outpatients because it allows them to collect samples at home. The samples can then be mailed to clinical laboratories, where professionals conduct the necessary analyses. Healthcare providers can interpret the results and communicate them back to patients via phone calls or video consultations. This approach improves patient follow-up, reduces the need for hospital visits, and supports continuous care efficiently and conveniently. This flexibility can also lead to higher participation rates and better adherence rates in both clinical research and routine monitoring.
DUS is particularly advantageous for patients in remote areas, where access to clinical laboratories is limited and transporting samples is difficult. It is also beneficial for disabled patients who may have difficulty traveling to healthcare facilities for routine urine collections. Patients who require frequent monitoring, such as those with chronic diseases, organ transplants, or who are on long-term medication, may also find DUS more convenient and less burdensome.
During pandemics, DUS offers key benefits through low-contact sample collection. This reduces the burden on healthcare facilities and minimizes the risk of disease transmission. DUS can also contribute to personalized medicine by enabling the remote monitoring of patient-specific biomarkers in urine. This allows for more frequent sampling without the need for clinic visits and supports real-time adjustments to treatment plans. Importantly, the convenience of at-home sample collection can improve patient compliance with follow-up appointments.
Conclusions
To the best of our knowledge, this is the first study to demonstrate the applicability of DUS sampling for the simultaneous determination of neopterin and creatinine. This alternative microsampling technique was successfully applied for urine collection, requiring only 10 μL of urine, which is 10 times less than the reference method. A simple and robust HPLC-FLD/DAD method was developed and validated. The method demonstrated its potential through the analysis of 36 human DUS samples from healthy volunteers and patients with COVID-19 and cancer, verifying its applicability to different clinical conditions.From a green chemistry perspective, this method minimizes reagent consumption, requiring only 110 μL of phosphate buffer without organic solvents and a simple 5 min vortexing step. The HPLC analysis is still required due to the complexity of the biological matrix. The energy footprint of the sample collection and analysis is significantly reduced by minimizing storage space requirements, allowing 250 times more samples to be stored in the freezer compared to liquid urine. In addition, DUS samples remain stable for up to 5 days at 40 °C and 4 months at room temperature, eliminating the need for temperature-controlled transport and enabling cost-effective shipping via standard mail or courier services. We also evaluated the practicality of the presented method, which should also be considered in the clinical laboratory. Compared to the reference method, its strength lies in its miniaturization.
Volumetric urine sampling can be facilitated with volumetric devices, such as pipettes or capillary transfer tubes. Home-sampling by the patient is possible with commercial options that incorporate volumetric microfluidics. However, subpunching from conventional Whatman 903 Protein Saving Card and HemaXis DB10 is problematic due to the indivisibility of dried spots, making pre-cut DUS devices a more practical alternative. The results of the Capitainer-B qDBS further confirmed its suitability as a home urine sampling tool.
Our method demonstrates significant potential for home sampling. It offers convenience to patients in remote areas and supports telemedicine initiatives. It provides a less burdensome alternative for patients who require frequent monitoring, including those with chronic diseases, organ transplants, on long-term medication, and disabilities. This improves adherence. DUS enables remote sample collection, allowing patients to avoid hospital visits while enabling healthcare providers to obtain results and offer advice via modern telecommunications platforms. DUS may contribute to personalized medicine by facilitating real-time adjustments to treatment plans and improving patient compliance with follow-up care. During pandemics, DUS offers key benefits through low-contact sample collection. This reduces the burden on healthcare facilities and minimizes the risk of disease transmission.
Further studies may incorporated DUS be into the diagnosis or monitoring of other diseases, particularly if relevant urinary markers are identified. Then, sensitive analytical methods should be developed and validated, taking the small sample volume into account. Participants should be involved from the beginning, starting with the sample collection step to assess usability and human factors. Inter-laboratory reproducibility should also be evaluated to support broader implementation. Moreover, future innovations in DUS technique will focus on creating point-of-care devices and integrating DUS with digital tracking systems to improve usability and enable real-time data access. Automating sample processing will enhance efficiency and support broader clinical adoption.
Author contributions
Chaweewan Suwanvecho: methodology, validation, writing – original draft, review and editing, visualization. Lea Vyleťalová: methodology and validation. Nikola Přívratská: validation, data curation. Pakanan Laolertworakul: validation. Dorota Turoňová: validation. Milan Vošmik: resource. Lenka Kujovská Krčmová: conceptualization, data curation, supervision, review and editing. Frantisek Svec: writing, review and editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
The data supporting this article have been included as part of the ESI supplementary data of this publication is openly available at Zenodo https://doi.org/10.5281/zenodo.14903150.† Data collected from human participants, described in Fig. 4, Tables 4 and 5, are not available for confidentiality reasons.Acknowledgements
This study was supported by the Czech Research Health Council (NU21J-02-00021, NU22-01-00151, and NW24-05-00031), Charles University (SVV 260 782), MH CZ—DRO [UHHK, 00179906]. All rights reserved. This research was also co-funded by the European Union under the ATEBIO project (Advanced Techniques for Biomedical Diagnostics, Project ID CZ.02.01.01/00/23_020/0008535).References
- J. D. Freeman, L. M. Rosman, J. D. Ratcliff, P. T. Strickland, D. R. Graham and E. K. Silbergeld, Clin. Chem., 2018, 64, 656–679 CrossRef CAS.
- G. Lenk, S. Sandkvist, A. Pohanka, G. Stemme, O. Beck and N. Roxhed, Bioanalysis, 2015, 7, 2085–2094 CrossRef CAS.
- A. Cafaro, M. Conti, F. Pigliasco, S. Barco, R. Bandettini and G. Cangemi, Biomedicines, 2023, 11, 1962 CrossRef.
- B. U. W. Lei and T. W. Prow, Biomed. Microdevices, 2019, 21, 81 CrossRef.
- R. Gutheir and A. Susi, Pediatrics, 1963, 338–343 CrossRef.
- C. U. Environment Health and, Safety , Feces and Urine-Human Biological Agent Reference Sheets (BARS), https://ehs.cornell.edu/research-safety/biosafety-biosecurity/biological-safety-manuals-and-other-documents/bars-other/feces-and-urine-human, (accessed 18 May, 2025).
- National Aeronautics and Space Administration , JSC Safety & Health Requirements, Part 7 – Health Protection Practices, https://www.nasa.gov/johnson/jsc-safety-health-requirements/#Part7, (accessed 18 May, 2025).
- U.S. Department, of Labor (Occupational Safety and Health Standard) , Bloodborne pathogens, https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030, (accessed 18 May 2025).
- American Biological Safety Association , Risk Group Database, https://my.absa.org/Riskgroups, (accessed 18 May, 2025).
- WHO manual for HIV drug resistance testing using dried blood spot specimens, World Health Organization, Geneva, 3rd edn., 2020 Search PubMed.
- Centers for Disease Control and Prevention , Shipping Guidelines for Dried-Blood Spot Specimens, https://www.cdc.gov/newborn-screening/media/pdfs/2024/05/Bloodspot-Transportation-Guidelines.pdf, (accessed 26 December, 2024).
- T. Meikopoulos, O. Begou, H. Gika and G. Theodoridis, Talanta, 2024, 269, 125489 CrossRef CAS.
- L. Delahaye, H. Veenhof, B. C. P. Koch, J.-W. C. Alffenaar, R. Linden and C. Stove, Ther. Drug Monit., 2021, 43, 310–321 CrossRef.
- R. Sucher, K. Schroecksnadel, G. Weiss, R. Margreiter, D. Fuchs and G. Brandacher, Cancer Lett., 2010, 287, 13–22 CrossRef CAS.
- T. Baydar, O. Yuksel, T. Sahin, K. Dikmen, G. Girgin, H. Sipahi, O. Kurukahvecioglu, H. Bostanci and M. Sare, J. Crit. Care, 2009, 24, 318–321 CrossRef CAS.
- Z. Shao, R. Zhang, K. Shrestha, A. G. Borowski, A. Schuster, A. Thakur, S. L. Hazen and W. H. Tang, Am. J. Cardiol., 2014, 113, 1839–1843 CrossRef CAS.
- H. Y. Lhee, H. Kim, K. J. Joo, S. S. Jung and K. B. Lee, J. Korean Med. Sci., 2006, 21, 678–682 CrossRef CAS.
- A. A. Mangoni and A. Zinellu, Front. Immunol., 2023, 14, 1271383 CrossRef CAS.
- B. Melichar, M. Spisarová, M. Bartoušková, L. K. Krčmová, L. Javorská and H. Študentová, Ann. Transl. Med., 2006, 5, 280 CrossRef.
- M. Eisenhut, J. Biomarkers, 2013, 2013, 196432 Search PubMed.
- L. K. Krcmova, L. Javorska, K. Matousova, P. Smahel, M. Skala, M. Kopecky, C. Suwanvecho, N. Privratska, D. Turonova and B. Melichar, Clin. Chem. Lab. Med., 2024, 62, 1217–1227 CrossRef CAS.
- C. Burton and Y. Ma, Pteridines, 2017, 28, 1–21 CrossRef CAS.
- B. Melichar, D. Solichová, I. Svobodová, L. Urbánek and K. Melicharová, Pteridines, 2006, 17, 20–24 CrossRef CAS.
- H. Kawasaki, H. Watanabe, S. Yamada, K. Watanabe and A. Suyama, Tohoku J. Exp. Med., 1988, 155, 311–318 CrossRef CAS.
- L. K. Krcmova, K. Matousova, L. Javorska, P. Smahel, M. Skala, V. Koblizek, J. Skop, D. Turonova, M. Gancarcikova and B. Melichar, Clin. Chem. Lab. Med., 2023, 61, 2053–2064 CrossRef CAS.
- I. M. Washington and G. Van Hoosier, in The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents, eds. M. A. Suckow, K. A. Stevens and R. P. Wilson, 2012, pp. 57–116 Search PubMed.
- H. Wachter, D. Fuchs, A. Hausen, G. Reibnegger, G. Weiss, E. Werner and G. Werner-Felmayer, Neopterin: biochemistry-methods-clinical application, Walter de Gruyter, 2011 Search PubMed.
- F. Pena-Pereira, W. Wojnowski and M. Tobiszewski, Anal. Chem., 2020, 92, 10076–10082 CrossRef CAS.
- N. Manousi, W. Wojnowski, J. Płotka-Wasylka and V. Samanidou, Green Chem., 2023, 25, 7598–7604 RSC.
- B. Melichar, L. Krcmová, H. Kalabova, I. Svobodova, E. Dragounova, P. Vesely, R. Hyspler, D. Solichova and L. Urbanek, Pteridines, 2006, 17, 145–153 CrossRef CAS.
- Council for International Organizations of Medical Sciences , International Ethical Guidelines for Health-related Research Involving Humans, https://www.ncbi.nlm.nih.gov/books/NBK614410/pdf/Bookshelf_NBK614410.pdf, (accessed 26 June, 2025).
- World Medical Association , WMA Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Participants, https://www.wma.net/policies-post/wma-declaration-of-helsinki/, (accessed 26 June, 2025).
- P. Holeckova, L. Krcmova, H. Kalabova, M. Kasparova, J. Plisek, M. Pala, P. Vitek, D. Solichova, M. Zezulova, H. Studentova and B. Melichar, Int. J. Vitam. Nutr. Res., 2012, 82, 77–84 CrossRef CAS.
- European Medicines Agency , ICH guideline M10 on bioanalytical method validation and study sample analysis Step 5, (accessed 26 December, 2024).
- U.S. Department of Health and, Human Services (Food and Drug Administration) , Bioanalytical Method Validation Guidance for Industry, https://www.fda.gov/media/70858/download, (accessed 26 December, 2024).
- International Conference in Harmonisation (ICH) , ICH Q2(R2) Guideline on validation of analytical procedures, https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q2r2-guideline-validation-analytical-procedures-step-5-revision-1_en.pdf, (accessed 26 December, 2024).
- A. Hausen, D. Fuchs, K. König and H. Wachter, J. Chromatogr., 1982, 227, 61–70 CrossRef CAS.
- R. K. Rakesh Kumar, M. O. Shaikh and C. H. Chuang, Anal. Chim. Acta, 2021, 1183, 338748 CrossRef CAS.
- M. Dvořák, R. Maršala and P. Kubáň, Anal. Chim. Acta, 2023, 1254, 341071 CrossRef.
- M. Newman, D. A. Curran and B. P. Mayfield, J. Clin. Transl. Endocrinol., 2020, 22, 100243 Search PubMed.
- F. Stöth, M. M. Fabritius, W. Weinmann, M. Luginbühl, S. Gaugler and S. König, J. Anal. Toxicol., 2023, 47, 332–337 CrossRef.
- J. M. Bland and D. G. Altman, Lancet, 1986, 8(1), 307–310 CrossRef.
- M. Protti, P. M. Sberna, A. E. Sberna, R. Ferrante, R. Mandrioli and L. Mercolini, J. Pharm. Biomed. Anal., 2021, 204, 114234 CrossRef CAS.
- H. Wachter, D. Fuchs, A. Hausen, G. Reibnegger, G. Weiss, E. R. Werner and G. Werner-Felmayer, Neopterin Biochemistry - Methods - Clinical Application, Walter de Gruyter & Co., Berlin, 1992 Search PubMed.
- M. Mendy, R. T. Lawlor, A. L. van Kappel, P. H. J. Riegman, F. Betsou, O. D. Cohen and M. K. Henderson, Clin. Lab. Med., 2018, 38, 183–207 CrossRef.
- World Health Organization, Guidance on regulations for the transport of infectious substances 2023-2024, World Health Organization, Geneva, 2023 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc00956a |
| This journal is © The Royal Society of Chemistry 2025 |