 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The hydrogen economy fairytale†
Tycho
Ehrhardt
 and
Gadi
Rothenberg
and
Gadi
Rothenberg
 *
*
Van‘t Hoff Institute for Molecular Sciences, University of Amsterdam, Science Park 904, 1098 XH, Amsterdam, The Netherlands. E-mail: g.rothenberg@uva.nl
First published on 27th May 2025
Abstract
We present a quantitative and realistic analysis of the current situation of hydrogen production worldwide. Subsequently, we calculate the thresholds needed for applying so-called “green hydrogen” as an energy carrier on a scale that would make a sizeable change in the world energy market. Using a simple back-of-the-envelope calculation, we show that green hydrogen cannot account for even 10% of the world energy demand by 2050. Considering also the time and investment required for building a worldwide green hydrogen infrastructure, we conclude that the hydrogen economy narrative, while elegant and desirable, has no basis in reality in the 21st century.
Green foundation1. We discuss the feasibility of the hydrogen economy becoming a reality in the 21st century, based on simple economic and chemical calculations.2. Many people are interested in the hydrogen economy. Some even think, mistakenly, that it is on our doorstep. Our realistic analysis shows that this is not the case. 3. Most likely, the future of the field of world energy demand in the coming decades will be fossil-carbon based, with an increasing role for wind and solar power. However, because of the growing demand for energy, especially by 2.5 billion people in Africa and Southeast Asia, the overall energy demand in the world will increase such that all energy sources will have to be utilised. Green hydrogen will not become a main energy carrier in this century. This might not be what people would like to hear, but it is the truth. Knowing the facts will help people steer towards a better future. |
Introduction
Anthropogenic emission of CO2 is one of mankind's most pressing problems.1 It causes weather extremes, accelerates sea level rising, and damages ecosystems.2 These emissions are caused by our burning of fossil fuels. In 2023, 80% of the global primary energy supply came from coal, oil, and natural gas, with over 37 billion tonnes of CO2 emitted.3,4 The total emissions of greenhouse gases (GHGs) topped 53 billion tons of CO2-equivalents.5Yet fossil fuels are not just energy sources, but also energy carriers. Any plan for phasing them out requires an alternative carrier. Hydrogen was already proposed as an alternative over a century ago by Haldane.6 The term “Hydrogen Economy” was coined in 1972 by Bockris, who envisaged hydrogen production based on atomic power, and using it directly as a fuel for producing electricity in fuel cells and as a reductant and source of hydrogen for the chemical industry.7 Since then, the Hydrogen Economy concept has gained popularity, thanks to a narrative based on five simple facts:
• Hydrogen is the most abundant element in the universe;
• It is easily produced from water by electrolysis;
• When burned, in a combustion engine or in a fuel cell, it produces only energy and water vapor;
• Its gravimetric heating value is higher than that of natural gas, gasoline or diesel;
• The energy for making hydrogen can come from sunlight or wind (free and renewable energy sources).
This narrative carries a powerful message: Given enough wind turbines and/or photovoltaic cells, the world economy can run on hydrogen, with no fossil fuels and zero carbon emissions.
The problem is that this is only a good story. Hydrogen is the most abundant element in the universe, but there is no free hydrogen available on Earth. You can easily produce it from water by electrolysis (and many kids do so in school experiments), but large-scale electrolysis is simply too expensive. Its gravimetric energy density is very high, but unfortunately hydrogen is a gas, and its volumetric energy density is very low (see Table 1). And while it is true that given enough wind turbines and/or photovoltaic cells the world could run on hydrogen, the question is how much time and money would it cost to build these turbines and what is the alternative cost?
| Fuela | Energy density | |
|---|---|---|
| Gravimetric (W h kg−1) | Volumetric (W h L−1) | |
| a At 298 K and 1 bar, unless noted otherwise. b Compressed to 350 bar. c Compressed to 700 bar. d Liquified at 20 K and 1 bar. | ||
| Hydrogen | 33.360 | 3 |
| Hydrogenb | 33.360 | 981 |
| Hydrogenc | 33.360 | 1659 |
| Hydrogend | 33.360 | 2805 |
| Natural gas | 14.900 | 10 |
| Gasoline | 12.900 | 9500 |
| Diesel | 12.620 | 9611 |
In this paper we compare the chemistry, thermodynamics and energy costs for producing hydrogen from fossil fuels and renewable resources. We analyze the current situation of hydrogen production worldwide, examining the practical possibilities for making green hydrogen (defined as hydrogen made by water electrolysis using renewable energy) on a large scale. Subsequently, we compare the hydrogen production options in the US, Europe and China and examine the ambitious goals for renewable hydrogen, set by the US department of energy (1 US$ per kg H2 in 2031), and the European Union (2.2 $ per kg H2 by 2030).10,11 Based on this data, we predict the likelihood for large-scale green hydrogen production in the coming decades.
Results and discussion
Comparison scales and nomenclature
A key difficulty in the discussion of the energy transition in general and hydrogen in particular is that different studies use different units. Energy and power are mixed, and units are hard to compare (MJ, kWh, toe and BTU are a few common examples). Moreover, many studies present relative values, rather than absolute ones. Even when such reports are written in good faith, this practice can be misleading. For clarity, we use here only three units: Watt (W) for measuring the rate of power, Watt-hour (Wh) for measuring energy, and US$ per kg (herein: $ per kg) for measuring cost per amount. All other units are converted into these. A conversion table between all the major units used is included in the ESI.† Further, we present absolute numbers, rather than percentages (e.g., “in 2023 there were 28.677 on-shore wind turbines operating in Germany, with a total capacity of 61.010 MW, compared to 53.912 MW in 2019”, rather than “on-shore wind turbine capacity in Germany increased by 13% from 2019 to 2023”).The current situation
In 2023, 97 million tons of hydrogen were produced worldwide. Of these, 93.1 million tons were produced using fossil-carbon resources, mostly natural gas and coal. To put this in perspective, the energy needed for producing this much hydrogen is roughly equivalent to the annual energy consumption of Germany.12,13 These 97 million tons are equivalent to 3.201.000 GW h or 3.201 TW h of energy. Again, this may sound like a lot, but it is <2% of the world energy consumption, which was 183.000 TW h in 2023.13 Of this total, less than 1 million tons were low-emission hydrogen (in combination with carbon capture and storage, CCS) and only 100.000 tons were green hydrogen (Fig. 1). Nearly half of the hydrogen produced today is used in petroleum refining. Another third is used for making ammonia.12In a hydrogen economy, hydrogen would be used as an energy carrier. Not all processes would run on hydrogen – many sectors could be electrified directly. The ones that cannot are steel production (5% of the world energy demand),14,15 shipping (3%, expected to double by 2050),16 aviation (3%), and seasonal energy storage (10–15%).17,18 Hydrogen would therefore have to account for at least 20% of the world energy demand, or 36.000 TW h in 2023 terms (giant circle in Fig. 1). Note that for mobile applications, the low volumetric energy density is a problem. This can be solved by compression, liquefaction or by converting it into chemicals that can release hydrogen on-demand.19 However, this increases both infrastructure and operational costs.
Hydrogen production methods
Hydrogen can be produced from fossil resources such as oil, coal, and natural gas. Natural gas, which is mostly methane, has the highest hydrogen content.20 It is also an abundant resource: In 2022, proven global reserves topped 180 trillion m3, enough to meet current production rates at least until 2070.13 Methane is the most common feedstock for making hydrogen, accounting for 62% of global production, and 95% of the hydrogen produced in the US.12,21Hydrogen is also produced from coal, via energy-intensive gasification with steam. Coal gasification accounts for 20% of global hydrogen production.12 China, which has large coal reserves but relatively little oil and gas, is by far the biggest player in this field, using it for almost two-thirds of its hydrogen.12,22 Worldwide coal reserves are even more abundant than natural gas, and are estimated to last well over 100 years at the current usage rate.13
Although biomass is a sustainable feedstock for producing hydrogen, it has two critical drawbacks. First, it contains only 6 wt% hydrogen.23 Second, it contains roughly 50% carbon,24 much of which is bound to oxygen atoms. This lowers the utility of biomass gasification compared to coal. Similarly, biological and photochemical hydrogen production methods are excluded, as these low-TRL methods are not applicable on any scale >50 ktpa.25,26
Steam methane reforming (SMR)
This process accounts for nearly half of the global supply of hydrogen today.27 It converts natural gas and steam into syngas (eqn (1)). The CO can be further reacted with water to give H2 and CO2 in the water gas shift reaction (WGSR, eqn (2)).28 The combined process gives four equivalents of hydrogen per equivalent of methane (eqn (3)), and emits (on paper) 5.5 kg of CO2 per kg H2.| CH4 + H2O ⇌ 3H2 + CO ΔH = +206 kJ mol−1 | (1) |
| CO + H2O ⇌ CO2 + H2 ΔH = –41.2 kJ mol−1 | (2) |
| CH4 + 2H2O ⇌ CO2 + 4H2 ΔH = +164.8 kJ mol−1 | (3) |
Autothermal reforming (ATR)
The intrinsic energy need of SMR requires external energy, which is provided by burning methane. Another option is the partial oxidation (POX) of methane (eqn (4)). This exothermic reaction can provide energy to keep the reforming process running, while also producing two equivalents of hydrogen.36| CH4 + ½O2 → 2H2 + CO ΔH = –22.6 kJ mol−1 | (4) |
POX typically runs at high temperatures, 1200–1500 °C.37 A catalytic alternative using nickel-based catalysts can reduce the temperature to 800–900 °C, but the catalysts suffer from coking and sintering.37,38 These catalysts can also catalyse SMR, so hydrogen can be produced simultaneously from both processes in a single reactor, by introducing oxygen in the stream. Just like in SMR, POX can be followed by the WGSR (eqn (2)).
Combining SMR and POX into a thermally neutral process is called autothermal reforming (ATR). Since both POX and WGSR are exothermic, they can offset the energy requirement of the SMR process. Theoretically, an energy-neutral ATR can produce 3.3 equivalents of hydrogen per mole of methane (eqn (5)), with a corresponding CO2 production of 6.6 kg per kg H2.
| CH4 + 1.30H2O + 0.35O2 → 3.30H2 + CO2 ΔH = 0 kJ mol−1 | (5) |
ATR requires a careful monitoring of the oxygen to carbon ratio, to limit the combustion of methane to CO2. The process requires a stream of oxygen gas, which is produced with an air separation plant, at an added energy cost.39 Hydrogen is then isolated from the gas stream using pressure swing absorption, leading to a 99.9% pure hydrogen stream, the total process emits 8.4 kg of CO2 per kg of hydrogen.35
Coal gasification (CG)
This process has two steps. First, the coal is reacted with water to give syngas (eqn (6)). Then, to produce more hydrogen, this reaction is followed by WGSR (eqn (2)), giving a total conversion to CO2 and hydrogen (eqn (7)).40| C + H2O → H2 + CO | (6) |
| C + 2H2O → 2H2 + CO2 | (7) |
CG is generally performed above 900 °C, and any quality of coal can be used.41 The process is well established (the Wrinkler gasification process was commercialized already in 192642). Of the fossil-carbon based methods, CG emits the most CO2 at 11 kg per kg of hydrogen produced. Actual emissions, including heating and operating energy, are ca. 20.8 kg CO2 per kg H2.43
Natural gas decomposition (NGD)
Thermal decomposition of methane yields solid carbon and hydrogen, avoiding CO2 emission (eqn (8)). The reaction is endothermic, requiring temperatures above 1200 °C. Catalytic decomposition can lower this to 450–750 °C.44 The catalysts are nickel- or iron-based, commonly promoted with cobalt.45 But just like with SMR and ATR, these catalysts deactivate by sintering and coking. At lower pressure, the equilibrium favors hydrogen production. To decrease the methane partial pressure nitrogen is co-fed into the reactor (this is more efficient than operating at sub-atmospheric pressures).46| CH4 → 2H2 + C | (8) |
NGD produces 3 kg of carbon per kg H2. The carbon byproduct can be stored permanently, avoiding any CO2 emissions. It can also be sold, offsetting some of the production cost of the process, but the worldwide demand for such carbon is only 15 million tons.47 The emissions of process heating using natural gas are 1.5 kg CO2 per kg H2.48
Electrolysis
Electrochemical water splitting (eqn (9)) was already used commercially in the 1890s to generate hydrogen for airships.49 Water electrolysis involves two half-reactions, the cathodic hydrogen evolution reaction (HER) and the anodic oxygen evolution reaction (OER). It is done in an electrochemical cell, comprising an anode, a cathode, a separator or membrane, and a (liquid or solid) electrolyte that facilitates charge transfer.50–52 For scale-up purposes, the electrochemical cells are assembled in a so-called stack.| 2H2O → 2H2 + O2 | (9) |
Stoichiometrically, making 1 kg of hydrogen requires 9 kg of water. Deionised water is used to optimize electrode lifetimes.53 The energy needed for electrolysis is supplied by electricity, and the efficiency is described by the electrical power input per kilogram of hydrogen (Wh kg−1 H2).54
Alkaline water electrolysis (AWE)
This is the most mature water splitting technology.55 It relies on the transport of OH− ions (generally supplied by dissolving KOH in water) for charge transfer between the electrodes. The two half-reactions are catalyzed by a nickel cathode and a cobalt oxide anode, respectively (eqn (10) and (11)).50 A diaphragm that facilitates the transport of hydroxide ions separates the two half-cells. This diaphragm minimizes the distance between electrodes, while preventing hydrogen crossover. Most AWE systems use Zirfon® diaphragms, a polysulfone material containing ZrO2 particles.56Cathode:
| 2H2O + 2e− → H2 + 4OH− | (10) |
Anode:
| 4OH− → 2H2O + O2 + 4e− | (11) |
AWE electrolyzers can generate hydrogen of >99.99% purity at industrial scale. A typical electrolyzer produces hydrogen with a 76% efficiency, corresponding to 52 kWh kg−1 H2.57
Proton exchange membrane (PEM) electrolysis
This system was developed by General Electric in the 1960s to overcome current density limits with AWE systems.51 Unlike AWE, it uses an acidic medium, which is highly corrosive, requiring noble metal electrodes.58 The HER is catalyzed by platinum, where protons are reduced and adsorbed on the surface, combining to form hydrogen (eqn (12)).51 The OER (eqn (13)) is done using an iridium oxide (IrO2) catalyst.59Cathode:
| 2H+ + 2e− → H2 | (12) |
Anode:
| 2H2O → O2 + 4H+ + 4e− | (13) |
The proton transfer membrane is usually made of Nafion®, a stable copolymer51 comprising a tetrafluoroethylene backbone, with perfluorovinylether side-chains terminated with sulfonate groups.60 Nafion membranes give less crossover than AWE diaphragms, leading to higher H2 purities (>99.999%). PEM can give a higher current density compared to AWE (1.5 A cm−2 and 0.5 A cm−2, respectively61), but the bottleneck is the need for iridium. There is simply not enough iridium available for large-scale PEM electrolysis. Developments in iridium recycling are ongoing, but meeting iridium demand for a large-scale PEM industry requires a reduction of the catalyst loading from 700 kg per GW to 50 kg per GW. This will not happen in the foreseeable future.59,62
Solid oxide electrolysis cells (SOEC)
In this method, electrolysis is performed at higher temperatures (700–900 °C) converting steam to hydrogen.63 The oxide anion (O2−) is used as the charge carrier, transported through a ceramic electrolyte (usually yttria substituted zirconia, YSZ).52 The cathode is typically a ceramic compound of the electrolyte with nickel (Ni-YSZ), which converts steam to hydrogen (eqn (14)).64 At the anode, where the O2− ion is oxidized (eqn (15)), a strontium-doped lanthanum material is generally used.52Cathode:
| H2O + 2e− → H2 + O2− | (14) |
Anode:
| 2O2− → O2 + 4e− | (15) |
The higher operating temperature of SOECs improves process efficiency. Energy consumption is significantly lower than AWE or PEM systems. The electrolysis stacks take the majority of the energy but are near 100% efficient, although additional energy is needed for heating. To improve efficiency, SOECs can be coupled with other industrial processes, utilizing waste heat streams. Industrial production utility of hydrogen using SOECs is roughly 45 kWh kg−1 H2 for a water feedstock, going as low as 40 kWh kg−1 H2 when steam or waste heat is supplied from other industrial processes.65,66
Comparing economic viability
We use a simple model to calculate the production costs (in $ per kg H2, see details and calculation examples in the ESI†) based on the prices of natural gas, coal, and electricity (the cost of water was fixed at 0.33 $ per m3 and has a negligible effect67,68). The natural gas cost estimates in North America, Asia and Europe were done using the prices at the Henry Hub (Henry), the Japan/Korea marker (JKM), and the Title Transfer Facility (TTF) Dutch gas trading platform, respectively. For comparing fossil-carbon-based methods, we fixed the industrial price of electricity at the global average of 12.7 $ per MWh (September 2023).69Fig. 2 shows the cost of hydrogen in different regions depending on the cost of natural gas, as well as the average price of natural gas in 2023. For SMR and ATR we also show the combination with carbon capture and storage (CCS). As carbon-based hydrogen is produced through mature technologies, we assume that the future production costs will depend mainly on the price of fossil fuels. | ||
| Fig. 2 The cost of hydrogen in US dollars, depending on natural gas price. With the 2023 average natural gas price at the Henry hub (Henry, 2.7 $ per GJ) the Japan Korea Marker (JKM, 14 $ per GJ) and the Title Transfer Facility (TTF, 13 $ per GJ).70 Figure based on data from ESI.† | ||
Looking at Fig. 2, we see that at lower natural gas prices, SMR is most cost effective, but as natural gas prices increase above about 8 $ per GJ, ATR becomes the more attractive option. This comes from the trade-off of higher capital cost with higher thermal efficiency, leading to a decreased natural gas consumption compared to SMR.71
Fig. 3 shows the analogous case for coal. We see that producing hydrogen by coal gasification is cost-effective at current coal prices in Europe (Antwerp–Rotterdam–Amsterdam, ARA), the US, and South China.72 Note that making hydrogen via coal gasification is cheaper than using natural gas in both Europe (TTF, ARA) and China (JKM, South China).
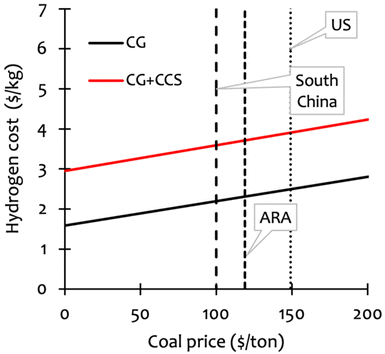 | ||
| Fig. 3 The cost of hydrogen produced through coal gasification in $ per kg H2, depending on the coal price per metric tonne, with the coal price marked in the US (149 $ per mt), Europe (ARA, 119 $ per mt) and China (South China, 100 $ per mt).72 Figure based on data from ESI.† | ||
The effect of carbon taxes
Carbon taxation strongly influences the final production costs, and can increase the economic viability of green hydrogen. Currently, there is no carbon tax in the US or in China. However, several European countries implement a carbon tax, ranging from $2/ per t CO2 in Estonia up to $120 per t CO2 in Switzerland.73Fig. 4 shows the European case where low-carbon hydrogen (using carbon capture and storage) becomes competitive at a carbon tax of approximately 60 $ per t. From that point onward, ATR with CCS becomes the most cost-effective method of producing low-carbon hydrogen from fossil-carbon in Europe. This is one reason why it is the preferred low-carbon hydrogen production method in the port of Rotterdam, where the current carbon tax is $80 per t CO2, and expected to increase to $140 per t CO2 in 2030.74,75 Taking the 2023 average prices for coal and natural gas, we see that CO2 taxes would need to surpass 80 $ per t for ATR-CCS to become the most attractive method in the US. For China CG-CCS becomes the financially preferred method at a tax rate of 70 $ per t CO2 eq. Carbon taxes are sensitive to political changes. For example, they were repealed by new governments in Australia and locally in Alberta, Canada.76,77 The resulting uncertainty drives investors away from large-scale green hydrogen projects.78 | ||
| Fig. 4 The effect of carbon taxation on the cost of hydrogen, based on natural gas prices in Europe (13 $ per GJ), coal prices (119 $ per t), and levelised cost of electricity (LCOE, 0.130 $ per kWh).3,70,71 The percentages with CCS represent the fraction captured. Figure based on data from ESI.† | ||
The viability of green hydrogen
The cost of hydrogen produced through water electrolysis depends on fixed costs and on the cost of electricity. The former depends on the capital expenditure (CAPEX) as well as operating costs and plant lifetime. Fixed costs are expected to decrease as technology improves and production scales up.79,80Fig. 5 shows the current hydrogen production cost, depending on the price of electricity and assuming production at full capacity. We see that hydrogen from AWE is cheaper at lower electricity costs, but is overtaken by SOEC with waste heat at higher energy prices, as that needs significantly less energy. However, electrolysis is more expensive compared to carbon-based methods, when coupled with grid electricity. This could be addressed by using other sources of energy, directly coupled with the electrolysis systems. In the US, EU, and China, the levelized cost of energy (LCOE) is lowest for onshore wind power, with respectively 0.04, 0.06, and 0.045 $ per kWh.3 But even at this price, green hydrogen cannot compete with fossil-carbon-based hydrogen in China and the US. | ||
| Fig. 5 Calculated cost of hydrogen produced through different electrolysis technologies, depending on the regional levelised cost of electricity (LCOE). Data based on current costs of technology, and assuming production at full capacity. See ESI for calculation details.† | ||
Another issue is the intermittent availability of Wind and solar power for hydrogen production. This requires compensation through higher production capacity. For example, if solar or wind power is available 20% of the time, a five-fold increase in electrolyzer capacity is needed. Of the available options, wind turbines offer an optimum between energy cost and availability (geothermal energy is continuous, but comes at triple the cost of wind power81).
A typical modern wind turbine is about 100 meters high, generates 2 MW, costs about $2.5 million and weighs about 200 tons. Large offshore turbines can generate up to 15 MW, cost about $13 million and weigh 500 tons.82 Operational capacity depends on the wind available, but the average across wind farms is about 65%. With 8640 hours in a year, a 2 MW wind turbine generates typically 2 × 8640 × 0.65 = 11.232 MW h or 11.2 GW h every year.
An ideal electrolyzer would require 39.4 kilowatt hours (kWh) of electricity to produce a kilogram of hydrogen. Typical real-life values are ca. 50 kWh kg−1 H2. Based on this, meeting the 2023 world demand of 97 million tons via electrolysis would require 4.850 TW h, or 434.000 wind turbines. The installed world wind turbine capacity in 2023 was 5.879 TW h.83 So in theory, if nearly all the wind power worldwide would be used for making hydrogen via electrolysis, it could cover the 97 million tons. But this is only in theory. In real life, <100 TW h of renewable energy was used for making hydrogen in 2023.12
Even the theoretical capacity for making green hydrogen is still a far cry from the transition to the hydrogen economy. A 20% share for hydrogen as an energy carrier would require 36.000 TW h. This would mean a six-fold increase of the number of wind turbines worldwide, to 2.5 million turbines. At the current rate of installation, this will take 50 years (in 2023, a record of 100 GW were installed, which equates to 50.000 turbines of 2 MW).83 But since the average lifetime of a turbine is 20 years, the installation capacity will have to double every 20 years to increase the numbers of turbines thereafter. Reaching the 20% hydrogen threshold by 2050, or indeed even by 2060, is unrealistic. In fact, considering that in 2023 green hydrogen accounted for only 0.05% of the world energy demand, reaching even a 10% share by 2050 is unrealistic.
Other regions
The Middle East and North Africa (MENA) have abundant solar possibilities. The problem is that they are also rich in oil and gas. Low fossil fuel prices limit the development of renewables. Furthermore, improving living standards are increasing energy consumption in MENA by 3% annually.84 Renewable energy will be used to fulfill increasing demand, before attempts are made to phase out fossil-carbon based energy. Similarly, Australia has significant renewable energy potential. It is projected to produce up to 3 million tpa of green hydrogen by 2040, and could export this hydrogen to resource-strained trading partners such as South Korea and Japan. But this is only 3% of today's hydrogen market.85The outlook for hydrogen in the coming decades
Today, fossil-carbon based hydrogen is the most cost-effective. In the US, low natural gas prices make SMR the most attractive option. In Europe, the geopolitical situation has strongly influenced natural gas prices.86 As a result, CG is followed by ATR in producing the lowest-cost hydrogen. However, if CO2 emissions are taxed at 60 $ per t, ATR-CCS becomes cheaper. In China, the difference between coal and natural gas prices is even larger, making CG the cheapest method. Green hydrogen is not competitive.By 2030, US natural gas prices at the Henry Hub are expected to rise to 5.13 $ per GJ.87 SMR would still be the most cost-effective production method, at just under 1.5 $ per kg H2. The European price of green hydrogen is expected to be on-par with today's ATR-CCS production methods, when emissions are taxed at >60 $ per t CO2 eq. However, when the current geopolitical tensions ease, the European and Asian natural gas prices are expected to move towards about 7.60 $ per GJ.88 This means that in Europe, SMR would be the cheapest method of producing hydrogen, but if CO2 taxes would exceed 70 $ per t, ATR-CCS will be the most cost-effective. In China, lower natural gas prices result in similar costs for SMR and CG (1.85 and 1.79 $ per kg H2, respectively). A carbon tax of 70 $ per t would make hydrogen via ATR-CCS the cheapest. Green hydrogen would remain uncompetitive.
By 2040, US natural gas prices are expected to increase to 6.22 $ per GJ.87 If European and Asian gas prices follow a similar trend from 2030 onwards, natural gas would cost about 9.21 $ per GJ. Carbon-based hydrogen would still remain the cheapest option.
Conclusions
Current hydrogen production is based on demand from the petroleum refining and ammonia sectors, not as an energy carrier. Decarbonizing industrial sectors or transportation requires large-scale investment and long-term worldwide government support, neither of which are available.The hydrogen economy narrative glosses over the barriers for building the required infrastructure. It assumes a joint global commitment and a surplus of cheap renewable energy. In reality, world energy demand will only increase in the coming decades. India, Africa and South America are home to over 40% of the world's population, yet account for only 13% of its energy demand. To reach the same quality of life as in Europe or North America, their energy footprint would have to increase five-fold. This means that the world will continue using all available energy, irrespective of its source.
The ambitious green hydrogen goals of the US Government and the European Union are unrealistic. With no clear and convincing business case, there is no incentive for large-scale investment in water electrolysis. The low cost and abundance of fossil fuels, and the absence of a unified worldwide carbon tax policy rule out any chance that green hydrogen would account for even 10% of the world energy demand by 2050. Even if there were a clear incentive by then, building a worldwide green hydrogen infrastructure would take decades. Fairytales are nice, but in reality there will be no hydrogen economy in the 21st century.
Methods
The methods and models used for calculating the hydrogen production costs, as well as example calculations and a full set of parameters and their sources are included in the ESI.†Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interests to declare.References
- G Rothenberg, A realistic look at CO2 emissions, climate change and the role of sustainable chemistry., Sust. Chem. Clim. Action, 2023, 2, 100012 Search PubMed.
- H. Lee and J. Romero, Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Intergovernmental Panel On Climate Change (IPCC), Geneva, 2023 Search PubMed.
- “IEA, World Energy Outlook 2024,” can be found under https://www.iea.org/reports/world-energy-outlook-2024, 2024. Accessed 13-2-2025.
- “IEA, CO2 Emissions in 2023,” can be found under https://www.iea.org/reports/co2-emissions-in-2023, 2024. Accessed 13-2-2025.
- European Commission, in Joint Research Centre, IEA, GHG Emissions of All World Countries, Publications Office, LU, 2024 Search PubMed.
- J. B. S. Haldane, DAEDALUS or Science and the Future, in A Paper Read to the Heretics, Cambridge, 4th February 1923, Kegan Paul, Trench, Trubner & Co., Ltd, London, 1923 Search PubMed.
- J. O. Bockris, Science, 1972, 176, 1323–1323 CrossRef CAS PubMed.
- B. C. Tashie-Lewis and S. G. Nnabuife, Chem. Eng. J. Adv., 2021, 8, 100172 CrossRef CAS.
- J. O. Abe, A. P. I. Popoola, E. Ajenifuja and O. M. Popoola, Int. J. Hydrogen Energy, 2019, 44, 15072–15086 CrossRef CAS.
- “Participation of Ursula von der Leyen, President of the European Commission, in the European Hydrogen Week,” can be found under https://audiovisual.ec.europa.eu/en/photo/P-052483~2F00-09, 2021. Accessed 7-1-2025.
- U.S. Departement of Energy, “Hydrogen shot,” can be found under https://www.energy.gov/topics/hydrogen-shot, 2021. Accessed 7-1-2025.
- IEA, “Global Hydrogen Review 2024,” can be found under https://www.iea.org/reports/global-hydrogen-review-2024, 2024. Accessed 8-1-2025.
- “Energy Institute Statistical Review of World Energy 2024,” can be found under https://www.energyinst.org/statistical-review, 2024. Accessed 8-1-2025.
- W. Liu, H. Zuo, J. Wang, Q. Xue, B. Ren and F. Yang, Int. J. Hydrogen Energy, 2021, 46, 10548–10569 CrossRef CAS.
- R. R. Wang, Y. Q. Zhao, A. Babich, D. Senk and X. Y. Fan, J. Cleaner Prod., 2021, 329, 129797 CrossRef CAS.
- Z. Wan, A. El Makhloufi, Y. Chen and J. Tang, Mar. Pollut. Bull., 2018, 126, 428–435 CrossRef CAS PubMed.
- A. M. Oliveira, R. R. Beswick and Y. Yan, Curr. Opin. Chem. Eng., 2021, 33, 100701 CrossRef.
- B. Stolz, M. Held, G. Georges and K. Boulouchos, Nat. Energy, 2022, 7, 203–212 CrossRef.
- E. Rivard, M. Trudeau and K. Zaghib, Materials, 2019, 12, 1973 CrossRef CAS PubMed.
- S. Faramawy, T. Zaki and A. A.-E. Sakr, J. Nat. Gas Sci. Eng., 2016, 34, 34–54 CrossRef CAS.
- T. L. LeValley, A. R. Richard and M. Fan, Int. J. Hydrogen Energy, 2014, 39, 16983–17000 CrossRef CAS.
- IEA, “Opportunities for Hydrogen Production with CCUS in China,” can be found under https://www.iea.org/reports/opportunities-for-hydrogen-production-with-ccus-in-china, 2022. Accessed 13-2-2025.
- M. Buffi, M. Prussi and N. Scarlat, Biomass Bioenergy, 2022, 165, 106556 CrossRef CAS.
- C. L. Williams, R. M. Emerson and J. S. Tumuluru, in Biomass Vol. Estim. Valorization Energy, InTech, 2017 Search PubMed.
- M. González Martínez, M. Elsaddik and A. Nzihou, Int. J. Hydrogen Energy, 2023, 48, 22113–22131 CrossRef.
- V. B.-Y. Oh, S.-F. Ng and W.-J. Ong, J. Cleaner Prod., 2022, 379, 134673 CrossRef CAS.
- S. Masoudi Soltani, A. Lahiri, H. Bahzad, P. Clough, M. Gorbounov and Y. Yan, Carbon Capture Sci. Technol., 2021, 1, 100003 CrossRef CAS.
- C. Ratnasamy and J. P. Wagner, Catal. Rev., 2009, 51, 325–440 CrossRef CAS.
- L. Barelli, G. Bidini, F. Gallorini and S. Servili, Energy, 2008, 33, 554–570 CrossRef CAS.
- R. Kothari, D. Buddhi and R. L. Sawhney, Renewable Sustainable Energy Rev., 2008, 12, 553–563 CrossRef CAS.
- S. Z. Abbas, V. Dupont and T. Mahmud, Int. J. Hydrogen Energy, 2017, 42, 2889–2903 CrossRef CAS.
- P. Smirniotis and K. Gunugunuri, Water Gas Shift Reaction: Research Developments and Applications, Elsevier, 2015 Search PubMed.
- W. Shi, H. Yang, Y. Shen, Q. Fu, D. Zhang and B. Fu, Int. J. Hydrogen Energy, 2018, 43, 19057–19074 CrossRef CAS.
- A. Golmakani, S. Fatemi and J. Tamnanloo, Sep. Purif. Technol., 2017, 176, 73–91 CrossRef CAS.
- P. Sun, B. Young, A. Elgowainy, Z. Lu, M. Wang, B. Morelli and T. Hawkins, Environ. Sci. Technol., 2019, 53, 7103–7113 CrossRef CAS PubMed.
- R. Carapellucci and L. Giordano, J. Power Sources, 2020, 469, 228391 CrossRef CAS.
- A. H. Elbadawi, L. Ge, Z. Li, S. Liu, S. Wang and Z. Zhu, Catal. Rev., 2021, 63, 1–67 CrossRef CAS.
- M.-C. Zhan, W.-D. Wang, T.-F. Tian and C.-S. Chen, Energy Fuels, 2010, 24, 764–771 CrossRef CAS.
- National Research Council and National Academy of Engineering, The Hydrogen Economy: Opportunities, Costs, Barriers, and R&D Needs, National Academies Press, Washington, D.C., 2004 Search PubMed.
- E. Shoko, B. McLellan, A. L. Dicks and J. C. D. da Costa, Int. J. Coal Geol., 2006, 65, 213–222 CrossRef CAS.
- A. Midilli, H. Kucuk, M. E. Topal, U. Akbulut and I. Dincer, Int. J. Hydrogen Energy, 2021, 46, 25385–25412 CrossRef CAS.
- D. A. Bell, B. F. Towler and M. Fan, Coal Gasification and Its Applications, Elsevier, 2011 Search PubMed.
- “IEA, Comparison of the emissions intensity of different hydrogen production routes,” can be found under https://www.iea.org/data-and-statistics/charts/comparison-of-the-emissions-intensity-of-different-hydrogen-production-routes-2021, 2021. Accessed 14-1-2025.
- L. Alves, V. Pereira, T. Lagarteira and A. Mendes, Renewable Sustainable Energy Rev., 2021, 137, 110465 CrossRef CAS.
- N. Sánchez-Bastardo, R. Schlögl and H. Ruland, Ind. Eng. Chem. Res., 2021, 60, 11855–11881 CrossRef.
- M. Younessi-Sinaki, E. A. Matida and F. Hamdullahpur, Int. J. Hydrogen Energy, 2009, 34, 3710–3716 CrossRef CAS.
- F. Rosner, T. Bhagde, D. S. Slaughter, V. Zorba and J. Stokes-Draut, J. Cleaner Prod., 2024, 436, 140224 CrossRef CAS.
- B. Parkinson, J. W. Matthews, T. B. McConnaughy, D. C. Upham and E. W. McFarland, Chem. Eng. Technol., 2017, 40, 1022–1030 CrossRef CAS.
- T. Smolinka, H. Bergmann, J. Garche and M. Kusnezoff, in Electrochem. Power Sources Fundam. Syst. Appl, Elsevier, 2022, pp. 83–164 Search PubMed.
- M. El-Shafie, Results Eng., 2023, 20, 101426 CrossRef CAS.
- M. Carmo, D. L. Fritz, J. Mergel and D. Stolten, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef CAS.
- A. A. AlZahrani and I. Dincer, Energy, 2022, 243, 123042 CrossRef CAS.
- J. A. Turner, Science, 2004, 305, 972–974 CrossRef CAS PubMed.
- The Hydrogen Economy, National Academies Press, Washington, D.C., 2004 Search PubMed.
- D. Jang, H.-S. Cho, S. Lee, M. Park, S. Kim, H. Park and S. Kang, J. Cleaner Prod., 2023, 424, 138862 CrossRef CAS.
- I. Genné, S. Kuypers and R. Leysen, J. Membr. Sci., 1996, 113, 343–350 CrossRef.
- “Green hydrogen systems, HYPROVIDE X-series,” can be found under https://www.greenhydrogensystems.com/, 2024. Accessed 13-1-2025.
- P. Millet, N. Mbemba, S. A. Grigoriev, V. N. Fateev, A. Aukauloo and C. Etiévant, Int. J. Hydrogen Energy, 2011, 36, 4134–4142 CrossRef CAS.
- P. Shirvanian and F. van Berkel, Electrochem. Commun., 2020, 114, 106704 CrossRef CAS.
- K. A. Mauritz and R. B. Moore, Chem. Rev., 2004, 104, 4535–4586 CrossRef CAS PubMed.
- H. Ito, T. Maeda, A. Nakano and H. Takenaka, Int. J. Hydrogen Energy, 2011, 36, 10527–10540 CrossRef CAS.
- C. Minke, M. Suermann, B. Bensmann and R. Hanke-Rauschenbach, Int. J. Hydrogen Energy, 2021, 46, 23581–23590 CrossRef CAS.
- G. Min, S. Choi and J. Hong, Appl. Energy, 2022, 328, 120145 CrossRef CAS.
- B. Singh, S. Ghosh, S. Aich, B. Roy and J. Power, Sources, 2017, 339, 103–135 CrossRef CAS.
- A. Chmielewski, J. Kupecki, L. Szablowski, K. Fijalkowski, K. Bogdzinski, O. Kulik and T. Adamczewski, in Currently Available and Future Methods of Energy Storage, WWF, Poland, 2020 Search PubMed.
- G. Jiménez-Martín, X. Judez, M. Aguado and I. Garbayo, Int. J. Hydrogen Energy, 2024, S0360319924054466, DOI:10.1016/j.ijhydene.2024.12.239.
- W. Kuckshinrichs, T. Ketelaer and J. C. Koj, Front. Energy Res., 2017, 5, 1 Search PubMed.
- X. Liu, S. Shanbhag, T. V. Bartholomew, J. F. Whitacre and M. S. Mauter, ACS EST Eng., 2021, 1, 261–273 CrossRef CAS.
- “Electricity prices,” can be found under https://www.globalpetrolprices.com/electricity_prices/, 2023. Accessed 13-2-2025.
- “IEA, Gas Market Report, Q1-2024,” can be found under https://www.iea.org/reports/gas-market-report-q1-2024, 2024. Accessed 13-2-2025.
- A. O. Oni, K. Anaya, T. Giwa, G. Di Lullo and A. Kumar, Energy Convers. Manage., 2022, 254, 115245 CrossRef CAS.
- “EIA, Quarterly Coal Report October–December 2023,” can be found under https://www.eia.gov/coal/production/quarterly/, 2024. Accessed 13-2-2025.
- A. Mengden, “Carbon taxes in Europe,” can be found under https://taxfoundation.org/data/all/eu/carbon-taxes-in-europe-2023/, 2023. Accessed 13-2-2025.
- “TNO, Blue hydrogen as accelerator and pioneer for energy transition in the industry,” can be found under https://repository.tno.nl/SingleDoc?find=UID%2065c255a9-1006-4992-8dbf-21c411e64663, 2019. Accessed 13-2-2025.
- “Minimum fee CO2 emissions to increase for industrial companies,” can be found under https://business.gov.nl/amendment/co2-emissions-fee-increase/, 2024. Accessed 3-2-2025.
- M. Hammerle, R. Best and P. Crosby, Energy Econ., 2021, 101, 105420 CrossRef.
- M. Mildenberger, E. Lachapelle, K. Harrison and I. Stadelmann-Steffen, Nat. Clim. Change, 2022, 12, 141–147 CrossRef.
- Z. Y. Dong, J. Yang, L. Yu, R. Daiyan and R. Amal, Int. J. Hydrogen Energy, 2022, 47, 728–734 CrossRef CAS.
- B. Yang, R. Zhang, Z. Shao and C. Zhang, Int. J. Hydrogen Energy, 2023, 48, 13767–13779 CrossRef CAS.
- H. Z. Böhm Andreas Goers, S. Tichler and P. Robert Kroon, in Innovative Large-Scale Energy Storage Technologies and Power-to-Gas Concepts after Optimization, Report on Experience Curves and Economies of Scale, 2018 Search PubMed.
- C. Clauser and M. Ewert, Renewable Sustainable Energy Rev., 2018, 82, 3683–3693 CrossRef.
- “Manufacturers and turbines,” can be found under https://www.thewindpower.net/turbines_manufacturers_en.php, 2025. Accessed 13-2-2025.
- “International statistics,” can be found under https://www.wind-energie.de/english/statistics/, 2025. Accessed 13-2-2025.
- F. Razi and I. Dincer, Renewable Sustainable Energy Rev., 2022, 168, 112763 CrossRef CAS.
- L. Andeobu, S. Wibowo and S. Grandhi, Int. J. Hydrogen Energy, 2024, 87, 1207–1223 CrossRef CAS.
- C. W. Su, M. Qin, H.-L. Chang and A.-M. Ţăran, Resour. Policy, 2023, 81, 103381 CrossRef.
- “Natural Gas Forecast & Price Predictions April 2024 and Beyond,” can be found under https://capex.com/eu/overview/natural-gas-price-prediction, 2024. Accessed 13-2-2025.
- M. Fulwood, in A New Global Gas Order? Part 1, The Oxford Institute For Energy Studies, Oxford, 2023 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc00946d |
| This journal is © The Royal Society of Chemistry 2025 |

