Open Access Article
Open Access ArticleFull conversion of grass biomass into sustainable functional antimicrobial bioplastics†
José David
Estrada-Sotomayor
 a,
Łukasz
Łopusiewicz
bc,
Erlantz
Lizundia
a,
Łukasz
Łopusiewicz
bc,
Erlantz
Lizundia
 de,
Sebastian
Guenther
c and
Danila
Merino
de,
Sebastian
Guenther
c and
Danila
Merino
 *af
*af
aBasque Center for Macromolecular Design and Engineering (POLYMAT), University of the Basque Country (UPV/EHU), Avenida de Tolosa 72, 20018, Donostia-San Sebastian, Spain. E-mail: danila.merino@ehu.eus
bSchool of Medical & Health Sciences, University of Economics and Human Sciences in Warsaw, 59 Okopowa Str., Warszawa, 01-043, Poland
cInstitute of Pharmacy, Department Pharmaceutical Biology, Greifswald University, Friedrich-Ludwig-Jahn-Str. 17, 17489 Greifswald, Germany
dLife Cycle Thinking Group, Department of Graphic Design and Engineering Projects, Faculty of Engineering in Bilbao. University of the Basque Country (UPV/EHU), Bilbao, 48013, Spain
eBCMaterials, Basque Center for Materials, Applications and Nanostructures, UPV/EHU Science Park, Leioa, 48940, Spain
fIkerbasque, Basque Foundation for Science, 48009 Bilbao, Spain
First published on 27th March 2025
Abstract
The environmental impact of non-degradable single-use plastics poses a significant challenge to current sustainability efforts. To foster a sustainable circular economy, this study introduces grass biomass as a renewable resource for the production of innovative bioplastics. The research involves the direct conversion of grass waste into composite bioplastics through alkaline hydrolysis, offering a transformative approach to plastic manufacturing. The hydrolysis process was optimized by varying treatment times and alkaline concentrations, with the ideal conditions identified as 1 M NH3 and 24 hours of treatment. Subsequently, the incorporation of ε-polylysine (PL) enhanced the mechanical properties of the bioplastics by acting as a plasticizer. Mechanical testing revealed that samples containing 10% and 20% PL exhibited comparable rigidity, with a Young's modulus of approximately 700 MPa and a tensile strength of 10 MPa. Moreover, the addition of PL, up to 20%, significantly improved the water resistance of the bioplastics, evidenced by decreased moisture content and water solubility. Additionally, the bioplastics demonstrated effective antimicrobial activity against Escherichia coli and Staphylococcus aureus, as well as significant antioxidant activity. Life cycle assessment (LCA) and life cycle costing (LCAA) results demonstrate the potential environmental benefits of manufacturing grass biomass into plastic films, with a significant reduction in greenhouse gases, cumulative energy demand (CED), and cost when compared to benchmark packaging plastics. These promising properties indicate that these biomaterials could be effectively utilized in real-world applications, with potential application as sustainable biobased packaging materials.
Green foundation1. Our work advances green chemistry by introducing a solvent-minimized process to transform grass biomass into functional bioplastics. This approach eliminates the need for intensive chemical extractions, reduces waste, and enhances material performance through a single-step hydrolysis method.2. Our bioplastics achieve a cradle-to-gate carbon footprint of 1.69 kg CO2 per kg, significantly lower than PLA (3.40 kg CO2 per kg) and polyethylene terephthalate (PET) (3.62 kg CO2 per kg). The process utilizes mild alkaline hydrolysis instead of organic solvents, cutting down hazardous waste. The resulting materials are fully bio-based, biodegradable, antimicrobial, and antioxidant, reducing the need for synthetic additives and preservatives. 3. Future research could focus on recycling ammonia from the hydrolysis process to further reduce environmental impact. Additionally, scaling up production with renewable energy sources and optimizing biopolymer interactions could enhance both the sustainability and functionality of these materials. |
1. Introduction
With over 400 million metric tonnes produced globally in 2023, plastics have become essential in everyday life.1,2 Their high tunability in terms of mechanical, barrier or thermal properties makes them especially valuable in the packaging industry, where they dominate due to their versatility.3 Lightweight polymers such as polyethylene (PE), polyethylene terephthalate (PET), and polycarbonate (PC) are commonly used in packaging for their excellent mechanical strength, barrier properties, and resistance to chemical and environmental degradation.4,5 However, the same durability and resistance to degradation that make these materials so valuable have also become significant environmental issues, especially in the context of single-use plastics.6In Europe, nearly one in four plastic items ends up in landfills, contributing to the accumulation of post-consumer plastic waste.7 This waste degrades soil quality,2 generates micro- and nanoplastics,8,9 and poses threats to human health10 and agricultural productivity.11 Plastic waste in landfills also risks leaching into water bodies, harming aquatic ecosystems,2 causing bioaccumulation of hazardous substances,12 and releasing toxic additives into the oceans.12 These escalating environmental challenges underscore the urgent need for sustainable alternatives. One proposed solution is a transition to a circular economy.
A key aspect of this shift is the development of bioplastics as alternatives to conventional plastics.13 Among potential raw materials, lignocellulosic biomass stands out as particularly promising. An estimated 1.3 billion tonnes of this biomass, rich in cellulose (40–60 wt%), hemicellulose (20–40 wt%), and lignin (10–24 wt%), are generated annually around the globe. Furthermore, much of this biomass is available as waste from various industries. However, current studies on bioplastics derived from lignocellulosic biomass tend to focus on extracting macromolecules such as cellulose and lignin by using organic solvents and energy-intensive processes that compromise long-term sustainability.14 These approaches also isolate specific components, leaving residues and underutilizing the full potential of the biomass.
Recent research has explored hydrolysis-based processes as a greener alternative for converting biomass such as spinach stems, citrus peels, and carrot pomace into bioplastic films with desirable properties.15–19 This process involves partial hydrolysis of lignin, pectin and hemicellulose, disrupting plant cell walls and releasing cellulose nano- and microfibers, which are regenerated into a composite material. In these composites, cellulose nanofibrils are embedded within an amorphous polymer matrix.15 While these biomass-derived bioplastics exhibit good mechanical properties and thermal stability, film formation success depends on the raw material composition. For instance, peanut shells, despite forming continuous films, resulted in highly brittle materials, likely do to their low hemicellulose content.19 Additionally, these bioplastics often degrade rapidly due to their high hydrophilicity and susceptibility to microbial attack, highlighting challenges that require further optimization.
Furthermore, life cycle assessment (LCA) and life cycle costing (LCC) results are valuable to confirm that the attributes of environmental and economic sustainability, which include the use of naturally derived materials and straightforward processing with low energy and chemical requirements, are translated in materials with low environmental impact.20,21 Furthermore, LCA enables the determination of the environmental hotspots during the product's lifecycle, allowing the eco-design the material to enhance sustainability.22
In this study, Brachiaria decumbens grass was selected as the starting biomass, given its abundance as a non-edible resource and its global availability. Though grass is primarily used for biofuel production,23,24 its potential for bioplastic development remains underexplored. To enhance the performance of these bioplastics, ε-polylysine (PL), a biopolymer known for its antimicrobial properties, was incorporated into the formulation. Recent advancements have improved the production efficiency of PL, making it more cost-effective.25 As a homopolymer of amino acids, PL consists of 25–35 lysine residues connected by peptide bonds between the ε-amino and α-carboxylic groups,26 offering water solubility and biodegradability, which makes it compatible with the alkaline media used in grass-derived bioplastic preparation.19
However, due to its low molecular weight, PL alone cannot produce high-quality films.27 To overcome this limitation, crosslinking PL with reducing sugars through Schiff base formation has been investigated.28 This strategy aims to enhance the mechanical properties, flexibility, and insolubility of the bioplastics.27,28 Since hydrolysis of lignocellulosic biomass produces reducing sugars, exploring their potential for crosslinking with PL to develop high-quality, ductile films with antimicrobial properties is of significant interest.
The objective of this study is to develop a novel bioplastic from underutilized Brachiaria decumbens grass biomass as a sustainable alternative to conventional plastics in packaging applications. We have optimized the conditions for alkaline hydrolysis of grass to produce bioplastics with enhanced mechanical properties. Additionally, PL was incorporated to impart antimicrobial properties. The bioplastics were characterized in terms of their physicochemical, morphological, optical, mechanical, and barrier properties. Furthermore, their antibacterial efficacy was tested against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus), two common pathogens affecting both human health and food safety. The antioxidant properties of the materials were also evaluated to assess potential applicability in food contact materials, including cutlery, trays, and fast-food packaging.
2. Materials and methods
2.1 Materials
PL of 95% purity was purchased from BLDpharm. Ammonium hydroxide of ∼30–33% NH3 in H2O was purchased from Fluka. NaClO2 purity of 80% was purchased from Sigma Aldrich. The grass biomass was manually collected from a public park in San Sebastian, Spain.2.2 Grass biomass pre-treatment
Cut grass biomass was rinsed with water to remove any possible impurities and was dried in an oven at 60 °C until friable consistency. The dried leaves were blended in an AMZCHEF “professional blender” at high intensity for two cycles. The obtained powder was later sieved in an Endecotts™ MIN200 (London, UK) sieve to obtain particle sizes of <53 μm. The resulting powder was stored at room temperature in a PE bag to avoid humidification and placed away from the sun to prevent any degradation.2.3 Optimization of the hydrolysis process
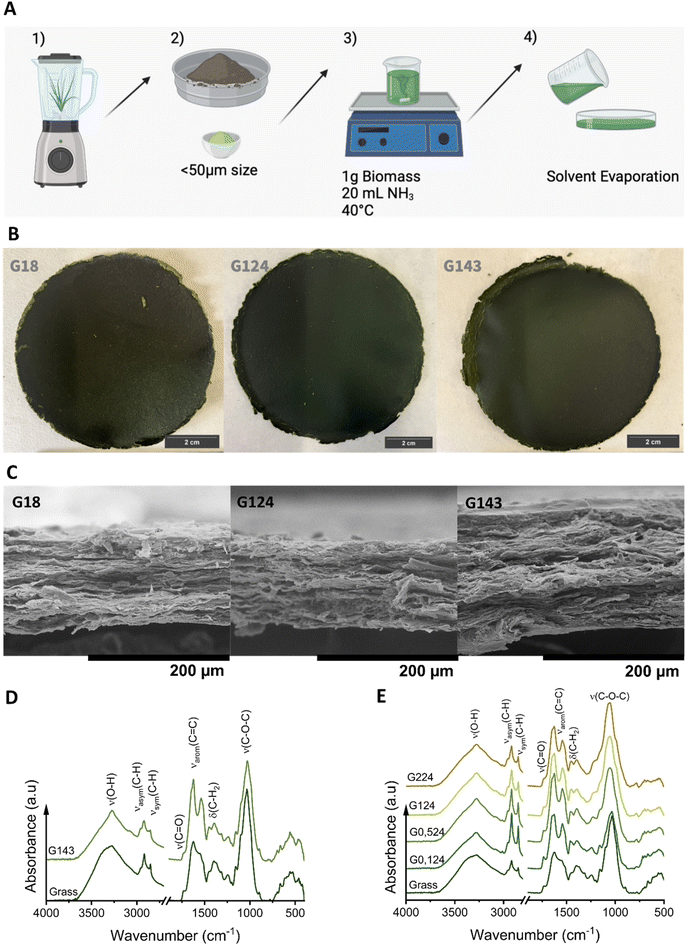
Alkaline hydrolysis was selected as the treatment for the biomass, since cellulose micro- and nano-fibrillation has been reported at this condition.19 The hydrolysis consisted of the treatment of 1 g of biomass with 20 mL of ammonium hydroxide at 40 °C for various durations. To determine the optimal hydrolysis conditions, different concentrations of ammonium hydroxide were tested: 0.1, 0.5, 1, and 2 M, along with varying treatment times: 8, 24, and 43 h. Table 1 provides a summary of samples’ name according to the hydrolysis conditions tested. Following the hydrolysis treatment, the solutions were cast onto polytetrafluoroethylene (PTFE) Petri dishes, and the solvent was left to evaporate, forming bioplastic films. A scheme of the procedure to obtain the hydrolysed grass films can be seen in Fig. 1A.| Sample name | Grass (g) | PL (g) | NH3 (mL) | NH3 concentration (M) | Hydrolysis time (h) |
|---|---|---|---|---|---|
| G0.18 | 1 | — | 20 | 0.1 | 8 |
| G0.58 | 1 | — | 20 | 0.5 | 8 |
| G18 | 1 | — | 20 | 1 | 8 |
| G28 | 1 | — | 20 | 2 | 8 |
| G0.124 | 1 | — | 20 | 0.1 | 24 |
| G0.524 | 1 | — | 20 | 0.5 | 24 |
| G124 | 1 | — | 20 | 1 | 24 |
| G224 | 1 | — | 20 | 2 | 24 |
| G0.143 | 1 | — | 20 | 0.1 | 43 |
| G0.543 | 1 | — | 20 | 0.5 | 43 |
| G143 | 1 | — | 20 | 1 | 43 |
| G243 | 1 | — | 20 | 2 | 43 |
| GPL10 | 0.9 | 0.1 | 20 | 1 | 24 |
| GPL20 | 0.8 | 0.2 | 20 | 1 | 24 |
| GPL30 | 0.7 | 0.3 | 20 | 1 | 24 |
2.4 Bioplastics preparation
Once the optimal hydrolysis conditions were established, bioplastics were prepared by incorporating PL at concentrations of 10, 20, and 30 wt% to act as an antimicrobial agent. These bioplastics were designated as GPL10, GPL20, and GPL30, respectively, as shown in Table 1. PL was added to the formulation before the introduction of ammonium hydroxide.2.5 Characterization of bioplastics
To quantify the holocellulose (HoC) content, that is, the content of hemicellulose and cellulose biopolymers a modified version of Wise's method for non-woody biomasses was utilized.29 In this procedure, 2 g of biomass were added to an Erlenmeyer flask with 150 mL of deionized H2O and 1.08 mL of glacial acetic acid. The mixture was heated to 96 °C and stirred for 90 minutes. After heating, the solution was cooled in a refrigerator for 1 hour. Subsequently, the mixture was centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 minutes, the supernatant was discarded, and the pellets were placed in an oven at 110 °C overnight to remove excess water. The percentage of HoC was calculated with the following equation:
000g for 10 minutes, the supernatant was discarded, and the pellets were placed in an oven at 110 °C overnight to remove excess water. The percentage of HoC was calculated with the following equation:
 | (1) |
Hemicellulose (HC) was removed from holocellulose by diluted acid hydrolysis using hydrochloric acid.30 The extracted holocellulose was treated for 2 h under reflux with 45 mL of HCl at 1 M. Once cooled it was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min, the supernatant was discarded, and the solid was cleaned with warm water (approx. 50 °C) and centrifuged again at the same conditions. The falcons were placed in an oven at 110 °C overnight and the following equations were used to determine cellulose and hemicellulose content in biomass:
000g for 10 min, the supernatant was discarded, and the solid was cleaned with warm water (approx. 50 °C) and centrifuged again at the same conditions. The falcons were placed in an oven at 110 °C overnight and the following equations were used to determine cellulose and hemicellulose content in biomass:
 | (2) |
 | (3) |
At last, the acid-insoluble lignin (AL) was determined by the method proposed by Ioelovich.30 According to this method, 0.3 g of biomass was hydrolysed at room temperature using 72 wt% H2SO4 for 2 h. After this period, the acid was diluted with 45 mL of distilled water and heated at boiling temperature under reflux for 2 h. After reflux, the solution was refrigerated for 30 min, and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. The precipitate was washed with distilled water and placed in an oven at 110 °C until constant weight. The following equation was used to determine the lignin percentage in biomass:
000g for 10 min. The precipitate was washed with distilled water and placed in an oven at 110 °C until constant weight. The following equation was used to determine the lignin percentage in biomass:
 | (4) |
To prepare the DNS working reagent, 10 g of DNS was dissolved in 200 mL of distilled water with continuous stirring. Next, 16 g of NaOH was dissolved in 150 mL of distilled water and slowly added to DNS solution. This mixture was incubated at 50 °C with constant stirring until a clear solution was obtained. Subsequently, 403 g of sodium potassium tartrate tetrahydrate was added to the DNS/NaOH mixture, which was filtered through filter paper. The final volume of the mixture was adjusted to 1000 mL with distilled water.
For the assay, 1 mL of each extract was mixed with 1 mL of 0.05 M acetate buffer (pH 4.8), followed by the addition of 3 mL of DNS working reagent. Then, the mixture was vigorously shaken and incubated in boiling water for 5 minutes, afterward cooled to room temperature. The samples were transferred to a 96-well microplate, and absorbance was measured at 540 nm using a BMG Labtech Clariostar Plus microplate reader (Ortenberg, Germany). A glucose standard prepared in acetate buffer was used to generate the calibration curve.
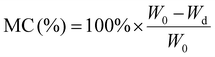 | (5) |
 | (6) |
 | (7) |
Antimicrobial properties were also studied according to the ASTM E 2180-01 based on methodology described in previous study.33 As the first step of the experiments, E. coli and S. aureus, cultures originated from 24 h growth (coming from stock cultures) were prepared. The concentrations of the cultures were standardized to 1.5 × 108 CFU mL−1. The concentration of each culture was measured using Amersham Biosciences Ultraspec 10 Cell Density Meter (Slough, UK). The agar slurry was prepared by dissolving 0.9 g of NaCl and 0.3 g of agar–agar in 100 mL of deionized water and autoclaved for 15 min at 121 °C and equilibrated at 45 °C (one agar slurry was prepared for each strain). Then, 1 mL of the culture (separately) was placed into the 100 mL of agar slurry. The final concentration of each culture was 1.5 × 107 CFU mL−1 in molten agar slurry. The square samples of each film were introduced (separately) into the sterile Petri dishes with a diameter of 55 mm. Inoculated agar slurry (1.0 mL) was pipetted onto each square sample. The samples were incubated 24 h at 30 °C with relative humidity at 90%. After incubation the samples were aseptically removed from the Petri dishes and introduced into the 9 mL of sterile physiological saline (0.9% NaCl). The samples were then vortexed 1 min. The dispersion facilitated the complete release of the agar slurry from the samples. Then serial dilutions of the initial inoculum were performed. Each dilution was spread into the LB Agar (Carl Roth Gmbh) and incubated at 30 °C for 24 h. The results were presented as an average value with standard deviations.
ABTS radical scavenging activity was determined by mixing 30 μL of film extracts with 1500 μL of ABTS radical solution (produced by mixing 7 mM ABTS with 2.45 mM potassium persulfate) and incubating in the dark for 6 min. Then, the absorbance at 734 nm was measured using BMG Labtech Clariostar plus multiplate reader (Ortenberg, Germany). Trolox solutions were used to prepare the calibration curve. The results were expressed as μM Trolox equivalents (TE)/100 mg of film sample.
- Polylactic acid production, granulate|polylactic acid, granulate|Cutoff, U – GLO & extrusion, plastic film|extrusion, plastic film|Cutoff, U – RER.
- Polyethylene terephthalate production, granulate, bottle grade|polyethylene terephthalate, granulate, bottle grade|Cutoff, U – RER & extrusion, plastic film|extrusion, plastic film|Cutoff, U – RER.
- Packaging film production, low density polyethylene|packaging film, low density polyethylene|Cutoff, U – RER.
Besides, the OpenLCA 2.4 software and the ecoinvent v3.11 database has been utilized to determine the energy-related properties and cost aspects of conventional plastic materials. The cumulative energy demand (CED), cumulative exergy demand (CExD) and economic cost (based on a life cycle costing, LCC) of producing 1 kg of plastic film were calculated and compared with common plastic materials. For the grass bioplastic, the CED, CExD, and the economic cost are based on the material utilization for grass bioplastic, excluding capital expenses and the energy consumption to avoid biasing the results by high-energy consumption that is not representative of the full-scale process. However, this analysis can provide a useful overview on the potential cost of the developed material.
3. Results and discussion
3.1 Optimization of hydrolytic conditions during bioplastics preparation from grass
The content of the cellulose, hemicellulose, and lignin in the raw grass biomass was included in Table 2. Note that the sum of the reported values does not equal 100%., the rest corresponds to extractives and minerals (∼11 wt%) whose composition has not been determined. The results indicate cellulose as the majoritarian component, followed by hemicellulose, in agreement with the values reported in the literature for other grass species.30,35Grass-derived bioplastics obtained through alkaline hydrolysis (Fig. 1B and Fig. S1 in the ESI†) exhibited a uniform appearance, with a distinctive dark green colour and a paper-like texture. The films displayed a balanced combination of roughness and rigidity, which gradually decreased with increasing hydrolysis time, resulting in a smoother surface.
SEM micrographs of the bioplastics’ cross-section (Fig. 1C and Fig. S2†) revealed the absence of large individual grass particles, confirming successful film formation after hydrolysis. The films exhibit a layered structure with visible fibrillar and sheet-like formations. Notably, as hydrolysis time increases, the layers become progressively more defined and compact, indicating structural rearrangement. This suggests that longer hydrolysis times enhance the cohesive structure and overall integrity of the films.
Fig. 1D shows the FTIR spectra of grass before and after hydrolysis, with G143 as a representative sample, highlighting the chemical changes induced by the process. Several characteristic peaks are observed in the grass prior to hydrolysis. The stretching vibration corresponding to hydroxyl groups, ν(O–H), appears at 3350 cm−1, while the asymmetric, νasym(C–H), and symmetric, νsym(C–H), stretching vibrations, primarily associated with the methylene groups of the carbohydrate chain, are located at 2920 and 2850 cm−1, respectively.15 The band that appears in the range 1736–1729 cm−1 corresponds to the stretching of the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), primarily due to the hemicellulose and lignin, typically related to ester groups.36 The peaks between 1650 and 1500 cm−1 are mainly attributed to the aromatic stretching of C
O), primarily due to the hemicellulose and lignin, typically related to ester groups.36 The peaks between 1650 and 1500 cm−1 are mainly attributed to the aromatic stretching of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, indicating the presence of lignin, and count also with the contribution of N–H bending of amines and amides in proteins and adsorbed water molecules. The region from 1460 cm−1 to 1316 cm−1, shows multiple overlapping signals, including C–H in-plane bending, CH2 asymmetric and symmetric bending, and O–H bending.37 A minor band at 1250 cm−1 corresponds to C–O aryl groups present in lignin. Lastly, the strong signals at 1150 cm−1 and 1030 cm−1 correspond to C–O–C asymmetric stretching and C–O–C ring skeletal vibrations, due to the presence of hemicellulose and cellulose.15,37
C bonds, indicating the presence of lignin, and count also with the contribution of N–H bending of amines and amides in proteins and adsorbed water molecules. The region from 1460 cm−1 to 1316 cm−1, shows multiple overlapping signals, including C–H in-plane bending, CH2 asymmetric and symmetric bending, and O–H bending.37 A minor band at 1250 cm−1 corresponds to C–O aryl groups present in lignin. Lastly, the strong signals at 1150 cm−1 and 1030 cm−1 correspond to C–O–C asymmetric stretching and C–O–C ring skeletal vibrations, due to the presence of hemicellulose and cellulose.15,37
Four notable changes are observed after hydrolysis. First, the band corresponding to the hydroxyl groups increases, as confirmed by the ratio of intensities between O–H band and C–H2 band. Second, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band at 1730 cm−1 shows a reduction in intensity, indicating that the alkaline hydrolysis produces the partial deacetylation of the hemicelluloses.38 Third, the intensity of the C–O–C band at 1030 cm−1 also decreases, suggesting the breakdown of glycosidic bonds. Finally, the peaks centred at 1650 cm−1, 1500 cm−1 and 1250 cm−1 due to the presence of aromatic compounds also increase intensity suggesting the possible formation of pseudo lignin.39–41
O band at 1730 cm−1 shows a reduction in intensity, indicating that the alkaline hydrolysis produces the partial deacetylation of the hemicelluloses.38 Third, the intensity of the C–O–C band at 1030 cm−1 also decreases, suggesting the breakdown of glycosidic bonds. Finally, the peaks centred at 1650 cm−1, 1500 cm−1 and 1250 cm−1 due to the presence of aromatic compounds also increase intensity suggesting the possible formation of pseudo lignin.39–41
Fig. 1E illustrates the effect of NH3 concentration at a fixed hydrolysis time (24 h) on the final chemical properties of the bioplastics. As NH3 concentration increases, the intensity of the C–O–C linkage bands decreases, a trend also observed in films prepared at different times (Fig. S3†). This reduction is consistent with literature, indicating that the amorphous hemicellulose undergoes hydrolysis by breaking the C–O–C backbone and generating reducing sugars.42 Using the C–H bond as a reference, the intensity of the O–H groups increases with the increase of NH3 concentration. The ratio of intensities between these groups starts at 0.65 for NH3 at 0.1 M, 24 h and increases up to 1.02 for NH3 at 1M over the same period. This suggests that higher NH3 concentrations lead to more extensive hydrolysis of the amorphous components.
To further investigate the impact of hydrolysis on the physicochemical properties of the developed bioplastics, we performed a thermogravimetric analysis. Fig. 2A illustrates the TGA curves showing the effect of time on grass hydrolysis at 1 M NH3, while Fig. 2B presents the derivative curves for the same samples. Additional TGA curves for other films can be found in Fig. S4 in the ESI.† As shown in Fig. 2A and B, both raw grass and its derived bioplastics exhibit two main weight loss events. The first, around 100 °C, is attributed to the evaporation of water from the samples which corresponded to an approximately 5% weight loss for all the samples. The second weight loss, corresponds to the overlapped thermal degradation of the three primary components: cellulose (220 °C–360 °C),37 hemicellulose (200–280 °C),37 and lignin (290–700 °C).37 Additionally, Fig. 2A indicates that G143 exhibits the lowest thermal stability, starting to degrade at lower temperatures compared to the grass and other samples. Moreover, the shoulder associated with hemicellulose degradation diminishes after hydrolysis, suggesting that hemicelluloses are particularly affected by the process, while cellulose remains relatively unaffected, at least for grass hydrolysis conducted at 8 and 24 hours. In contrast, grass bioplastics obtained after 43 hours of hydrolysis show also a decrease in the cellulose peak intensity, indicating partial hydrolysis of both hemicellulose and cellulose.
Regarding lignin, an increase in the intensity of the shoulder associated with its thermal degradation, centered at 400 °C, is observed after hydrolysis. This increase can be attributed to the condensation of sugars formed during hydrolysis, resulting in the formation of a pseudo-lignin.41,43,44 This observation is further supported by the increase in aromatic groups detected in the FTIR analysis after hydrolysis.41
Lastly, to determine the optimal hydrolytic conditions for preparing bioplastics from grass biomass, we analysed the mechanical properties of the bioplastics, with key results presented in Fig. 2C and D.
Both reaction time and NH3 concentration significantly impact the final mechanical properties of the bioplastics. The Young's modulus, which reflects the films’ stiffness, remains low after a short hydrolysis time of 8 hours, indicating that the bioplastics have not yet developed sufficient structural integrity. This suggests that extended hydrolysis times are necessary to enhance the mechanical properties, regardless of the NH3 concentration.
In terms of tensile strength, the films subjected to 8 hours of hydrolysis consistently display the lowest values, with no significant differences observed across varying NH3 concentrations. However, hydrolysis times of 24 and 43 hours reduced the variation in both tensile strength and Young's modulus among the bioplastics. This indicates that longer hydrolysis not only improves the overall mechanical properties but also results in more uniform materials.
Maintaining a fixed hydrolysis time while increasing NH3 concentration enhances both tensile strength and Young's modulus. This improvement arises from the increased hydrolysis of amorphous regions, which disrupts cell walls more effectively and facilitates the formation of a biocomposite material reinforced by cellulose fibrils. However, mechanical properties begin to decline beyond a critical NH3 concentration. Excessive hydrolysis can lead to the degradation of amorphous components, resulting in a lower quality biocomposite. These results are in line with the results obtained by TGA. Notably, this critical NH3 concentration varies with hydrolysis time: 1 M for 24 hours and 0.5 M for 43 hours.
Based on the mechanical test results, hydrolysis conditions of 1 M NH3 for 24 hours were selected, as they produced rigid films with a Young's modulus of 1998 MPa ± 310 MPa and a tensile strength of 19 MPa ± 2 MPa. These conditions align with those reported in the literature for the alkaline hydrolysis of other plant biomass.24
3.2 Optimizing conditions for preparation of antimicrobial bioplastics from grass biomass
PL was incorporated to optimize the functional properties of bioplastics from grass biomass. The bioplastics incorporating different PL concentrations exhibited a homogenous and smooth appearance, with no noticeable differences across varying PL content (Fig. 3A). However, significant differences were observed in the visual appearance of the films. The addition of PL resulted in a smoother surface with a subtle glow, enhancing the visual appeal. Moreover, the PL-containing samples lost their original paper-like rigidity and become ductile.The SEM micrographs of the cross-sections (Fig. 3B) reveal significant structural changes with varying PL content. As previously discussed, the cross-section of G124 (0% PL) (shown in both Fig. 1B and 3B for comparison) is rough and fibrous, and presents loosely packed layers. Upon the addition of PL, the cross-sections become progressively more compact and denser. This structural densification suggests a strong interaction between the grass components and PL, leading to enhanced cohesion within the bioplastic matrix.45
To investigate the chemical interactions between grass polymer components and PL, the FTIR spectra of pure PL, G124 (0% PL), and GPL30 are compared in Fig. 3C. Spectra of other PL compositions are provided in Fig. S5 in ESI.†
The FTIR spectra of pure PL polymer shows a band at 3200 cm−1 corresponding to N–H stretching. A prominent signal at 3060 cm−1 accompanied by another at 1600cm−1 to 1500 cm−1 are attributed to the NH3+ side chain groups.46,47 This is followed by distinct peaks at 2940 and 2860 cm−1, which correspond to the asymmetric and symmetric stretching of C-H2 bonds, respectively. The amide I band at 1640 cm−1 is characteristic of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching,46,48 while the amide II band at 1530 cm−1 is due to the in-plane deformation of the NH group.46 Lastly, the band at 1245 cm−1 corresponds to C–N stretching in the amide group.48
O stretching,46,48 while the amide II band at 1530 cm−1 is due to the in-plane deformation of the NH group.46 Lastly, the band at 1245 cm−1 corresponds to C–N stretching in the amide group.48
When comparing the FTIR spectra of pure PL, G124 (0% PL), and GPL30 (30%PL), no new peaks are observed in the GPL30 sample. This indicates that no covalent crosslinking occurs between the functional groups of the hydrolysed biomass and PL. However, shifts to lower wavenumbers are noted for bands corresponding to functional groups involved in hydrogen bonding, such as amide I and the C–N stretching. These shifts suggest that the interaction between PL and biomass is mainly due to hydrogen bonds.49
The formation of a Schiff base between the pending amino group of PL and the carbonyl groups of reducing sugars (produced during hydrolysis) was expected, which would have been indicated by the appearance of an imine peak around 1660 cm−1 in the FTIR spectra.50 However, the absence of this peak suggests that PL did not react with the reducing sugars.51 Schiff base formation would also have reduced the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band intensity due to the consumption of carbonyl groups.50 Therefore, the reducing sugar content was measured in raw grass and bioplastics with varying PL content (0%, 10%, 20%, and 30% by weight) (Fig. 3D).
O band intensity due to the consumption of carbonyl groups.50 Therefore, the reducing sugar content was measured in raw grass and bioplastics with varying PL content (0%, 10%, 20%, and 30% by weight) (Fig. 3D).
When comparing the reducing sugars content of raw grass with that of the bioplastics containing 0% PL, no significant increase in reducing sugars content was observed, which is unexpected since hydrolysis of polysaccharides yields reducing sugars.42 However, as indicated by the earlier FTIR and TGA results, it is likely that the reducing sugars are reacting to form pseudo-lignin immediately after production, thereby preventing their interaction with the amino groups in PL. Additionally, an increase in PL content in the bioplastic formulations corresponds with a decrease in reducing sugar levels, likely due to the dilution effect of PL, which does not contribute to the final product's reducing sugars content.
The DTGA curves for selected samples are illustrated in Fig. 4A, while the complete DTGA curves for all samples can be found in ESI Fig. S6.† For PL, thermal degradation occurs in two distinct steps. The first degradation temperature, observed at 86 °C, primarily results from water evaporation, as PL is hygroscopic and rapidly absorbs moisture. Subsequently, thermal degradation is observed at two temperatures of maximum degradation rate: 297 °C and 444 °C. The first temperature is associated with transamidation reactions that reduce the molecular weight of the sample, leading to the formation of volatile compounds, while the second temperature is linked to the carbonization of the remaining components.45
Interestingly, the bioplastic films containing PL exhibit multiple degradation temperatures that do not correspond to those of the individual raw materials (PL and G124). These degradation steps present temperatures of maximum degradation rates at 190 °C and 210 °C, indicating the formation of specific interactions or complexes between PL and the polymers in the biomass, as well as with low molecular weight components present in the biomass.50,52 A comparison with the literature and the FTIR spectra suggests that this stabilization may be attributed to hydrogen bonding between PL and the hydrolyzed grass components.45,53
To further investigate these interactions, DSC analysis was conducted to assess changes in the glass transition temperature (Tg) relative to PL content in the films. The DSC curves (Fig. 4B) reveal a single Tg within the studied temperature range. The addition of PL slightly decreases the Tg of the bioplastics, acting as a plasticizer. However, even with high concentrations of PL, the Tg only decreases by 2.7 °C compared to the film without PL. This minimal decrease in the glass transition temperature may be attributed to the hydrogen bonding that restricts the mobility of the polymer chains,54 in addition to the higher molecular weight of PL compared to other common plasticizers, such as glycerol.
Subsequently, we analyzed the mechanical properties of the bioplastics, with the results summarized in Table 3. The data indicate that PL effectively acted as a plasticizer within the bioplastic matrix, as confirmed by the DSC analysis.49 Notably, the addition of PL significantly reduced the Young's modulus from approximately 2000 MPa to around 750 MPa with 20% PL incorporation. Furthermore, the inclusion of PL enhanced the ductility of the material, as evidenced by the increase in strain at break, which rose from 1.1% to 1.6% (Table 3). However, the tensile strength of the grass bioplastics decreased with the addition of PL, dropping from 21 MPa at 0% PL to 10 MPa.
| Material | Young's modulus (MPa) | Tensile strength (MPa) | Strain at break (%) | Cumulative energy demand (CED) | Cumulative exergy demand (CExD) | Cost of production (EUR per kg) | Ref. |
|---|---|---|---|---|---|---|---|
| G124 | 2055 ± 126 | 21 ± 1 | 1.1 ± 0.1 | — | — | — | This work |
| GPL10 | 846 ± 91 | 12 ± 3 | 1.8 ± 0.4 | 25.4 MJ | 26.4 MJ | — | This work |
| GPL20 | 735 ± 119 | 10 ± 1 | 1.6 ± 0.2 | 34.1 (1.41 EUR per kg with no ε-polylysine) | This work | ||
| GPL30 | 583 ± 88 | 10 ± 1 | 2.75 ± 0.3 | This work | |||
| LDPE | 100–300 | 9–15 | 300–500 | 99.7 MJ | 115.9 MJ | 2.97 | 55 and 56 |
| HDPE | 800–1600 | 10–60 | 150–400 | 57 and 58 | |||
| Poly-lactic acid (PLA) | 8600 | 120 | 30 | 77.5 MJ | 99.7 MJ | 1.48 | 64 |
| Thermoplastic starch | 400–1000 | 0.9–22 | 3–60 | 67.8 MJ | 78.8 MJ | 0.45 | 59 |
| Lime peel | 441 ± 43 | 14 ± 2 | 4.6 ± 1.2 | — | — | — | 61 |
| Avocado peel | 342 ± 46 | 18 ± 3 | 16.8 ± 6.1 | — | — | — | 62 |
| Potato peel | 432 ± 86 | 13 ± 2 | 18.4 ± 4.3 | — | — | — | 63 |
When comparing the properties of the synthesized bioplastics with those of conventional packaging materials (Table 3), it is evident that the Younǵs modulus and tensile strength (TS) of these bioplastics are comparable to those of commonly used packaging materials such as low-density polyethylene (LDPE),55,56 high-density polyethylene (HDPE),57,58 and thermoplastic starch.59 However, the ductility of the PL-grass films is noticeably lower than that of these materials, which could limit their applicability in scenarios where ductility is crucial. Considering their high elastic modulus and tensile strength, these bioplastics may be well-suited for rigid packaging applications, such as trays or covers, as illustrated in the graphical abstract.60
In comparison to lime peel,61 avocado peel62 and potato peel63 derived bioplastics the rigidity of the PL-grass biofilms is nearly doubled, while the tensile strength falls within a similar range. However, the previous bioplastics have demonstrated superior ductility.
In addition, Table 3 presents the sustainability assessment of the grass-derived bioplastics, focusing on their cumulative energy demand (CED), cumulative exergy demand (CExD), and economic cost based on life cycle costing (LCC). The results underscore the potential advantages of these bioplastics over conventional plastics. The CED and CExD of the bioplastic containing 20% PL (GPL20) were determined at 25.4 MJ and 26.4 MJ, respectively, which are substantially lower than those of widely used LDPE (99.7 MJ, 115.9 MJ) and PLA (77.5 MJ, 99.7 MJ), indicating a reduced environmental footprint in terms of energy consumption. However, the economic analysis highlights that the production cost of GPL20 remains relatively high, primarily due to the inclusion of PL. Notably, when excluding PL from the cost calculation, the price (1.41 EUR per kg) becomes competitive with PLA (1.48 EUR per kg). Future advancements in PL production and cost optimization could further improve the economic feasibility of these bioplastics. Given their lower energy demands and the potential for future cost reductions, these materials show promise as sustainable alternatives for rigid packaging applications, aligning with circular economy principles in the packaging industry.
Regarding interactions with water, the addition of PL yields notable changes. As shown in Fig. 5A, the moisture content of the samples is significantly reduced with the incorporation of PL. This effect may be attributed to the formation of hydrogen bonds, where the hydroxyl groups in the grass interact with PL, thereby limiting moisture absorption and enhancing the structural integrity of the bioplastic.65
As illustrated in Fig. 5B, the solubility of the samples initially decreases by 50% with the addition of up to 20 wt% of PL. However, as the PL content increases beyond this point, the solubility subsequently rises, with PL alone exhibiting 100% solubility in water. Notably, the GPL10 and GPL20 samples demonstrate the best water stability, showing significant resistance to solubility compared to the sample without PL (G124).
The water vapor permeability results are summarized in Fig. 5C. The addition of PL to the bioplastic films increases the WVP; however, no statistically significant differences were observed with respect to varying PL content. The values obtained are comparable to those reported in the literature for other bioplastics, like mango peel,66 wheat starch67 and potato starch films,68 with WVP values of 8.8 × 10−9, 2.8 × 10−9 and 1.5 × 10−9 g Pa−1 s−1 m−1, respectively.
Finally, the results of the water contact angle analysis support the role of hydrogen bonding as a stabilizing force in the PL-grass films. As illustrated in Fig. 5D, the incorporation of PL renders the films slightly more hydrophobic. This change arises from the hydrogen bonding between the polar groups of the components, which reduces the likelihood of water molecules interacting with the film.69
Finally, the antimicrobial properties of bioplastics were studied against both Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria. The results obtained using the ASTM E 2180-01 method, shown in Fig. 6A, demonstrate that the growth of both bacteria is inhibited in the presence of PL. For E. coli, no bacterial growth was observed after the addition of 20% PL, while for S. aureus, growth inhibition occurred only at 30% PL.
To confirm the diffusion of PL, an agar diffusion test was performed with the film against S. aureus (Fig. S7†) and E. coli (Fig. S8†). The results indicate that bioplastics acquire antimicrobial properties with the addition of PL.25,26 A clear increase in the inhibition halo is observed as PL content increases, while the film containing 0% PL (G124) does not generate an inhibition halo. In contrast, PL control discs show a distinct inhibition halo, confirming its role as an antimicrobial agent.
The inhibition mechanisms of PL against Gram-positive and Gram-negative bacteria have been extensively reported. PL inhibits bacterial growth by affecting the permeability of their cell walls and membranes.25 For E. coli, it has been shown that PL interacts with the phospholipid groups in the cell membrane, causing distortion and damage.25 In the case of S. aureus, PL induces structural changes in the peptidoglycans of the cell wall, increasing permeability.70
Regarding the antioxidant properties of the film an interesting behaviour can be observed in Fig. 6B. The antioxidants that quench and scavenge the radicals of ABTS are compounds capable of donating hydrogen or electrons, additionally the antioxidant compounds are responsible of stabilizing the new radical generated within the structure. Phenols have shown to produce this antioxidant behaviour, that are strongly affected by the degree of substitution of the aromatic ring.71 This explains the different behaviour observed for the bioplastic films. Firstly, when no PL is present in the films, the antioxidant properties are solely dependent on the hydrolysis products of the grass. The alkaline hydrolysis of the grass produces phenols, through lignin hydrolysis, and reducing sugars, through hemicelluloses hydrolysis.42 Both compounds can stabilize and scavenge the radicals of ABTS effectively reaching a scavenging percentage of up to 80%. However, a different behaviour is observed after the incorporation of PL. With the addition of PL the availability of this antioxidants is reduced due to a diluting effect generated by the PL. With the addition of PL, the hydrogen bonds between PL and hydrolysis products decreases significantly the scavenging capacity (25%). This is due to the inability of the highly antioxidant compounds of hydrolysis to donate hydrogen atoms to stabilize the radicals as they are imprisoned by the interaction with PL. At last, the increase in PL to higher percentages also lead to an increase in scavenging percentage that can be attributed to the increase of amino groups in lysine, which concentration increases as more PL is present in the film. This amino groups have been reported to have a low antioxidant activity72.
Life cycle assessment (LCA) was applied to grass biomass bioplastic development to explore its potential for sustainable material development. The disaggregated climate change potential for a 100 year time horizon (expressed in CO2 equivalents) of the GPL20 film for a cradle-to-gate system boundary is provided in Fig. 7. The production of the GPL20 film has a footprint of 3.29 kg CO2 equiv. kg−1, where steps 2, and specially steps 5 and 6, bear the largest share. Spherically, the step 2 (drying) has a contribution of 0.46 kg CO2 equiv. kg−1, while steps 5 and 6 add 1.78 and 1.01 kg CO2 equiv. kg−1, respectively. The largest contributors to greenhouse gas emissions during steps 5 are the use of ammonia (0.89 kg CO2 equiv. kg−1), the electricity consumption (0.63 kg CO2 equiv. kg−1) and the incorporation of ε-polylysine (0.25 kg CO2 equiv. kg−1). It is important to note that the climate change potential of the GPL20 films remains below the footprint showed by benchmark plastic films such as PLA (3.45 kg CO2 equiv. kg−1), PET (3.62 kg CO2 equiv. kg−1), LDPE (3.40 kg CO2 equiv. kg−1). Similarly, the grass bioplastics show a lower carbon footprint than the 4.4–4.8 kg CO2 equiv. kg−1 reported for pomegranate peel extract-infused carboxymethyl cellulose films (that have antioxidant and antibacterial properties),73 while it remains at the lower end of the range of 1.82–6.00 kg CO2 equiv. kg−1 showed by alveolus trays produced by thermoforming of vegetable waste.74 More importantly, if the biogenic carbon content is accounted as sequestered carbon according to the PAS 2050 carbon accounting protocol (i.e., long-term applications),75,76 a total climate change potential of 1.69 kg CO2 equiv. kg−1 is achieved. The reduced carbon footprint over petroleum-derived plastic films matches literature and highlights the potential of bio-based products to deliver materials with reduced greenhouse gas emissions.77
Additional environmental impact categories in the light of the EF v3.1 impact assessment methodology are provided in Fig. S9.† The results show a remarkable variability depending on the category under consideration. With values of 36 MJ kg−1, 1.08 × 10−4 kg Sb eq. kg−1, and 0.25 kBq U235 eq. kg−1, significant benefits are observed in categories such as energy resources (non-renewable), material resources (metals and minerals) and ionizing radiation (human health), respectively. These benefits are explained by the biobased character of the grass bioplastics, which avoids the need to extract non-renewable resources. On the contrary, the acidification and water use derived from grass biomass bioplastic manufacturing results one-to-two orders of magnitude larger than that observed for conventional packaging plastics. 99% of the acidification originates from the NH3 gas released during solvent evaporation (step 6),78 while 78% of water uses are related to the significant electricity consumption during steps 5 and 6. Therefore, implementing an ammonia recovery process and enhanced process efficiency upon upscaling can certainly reduce the environmental impacts and increase the sustainability of the grass bioplastic. In the light of LCA results, the developed materials show promising attributes for sustainable packaging solutions.
4. Conclusions
Bioplastics were successfully prepared from grass biomass, which underwent alkaline hydrolysis with NH3. The resulting films exhibited competitive mechanical properties against petroleum-derived plastics, achieving a Young's modulus of 2200 MPa and a tensile strength of 22 MPa. However, these films demonstrated low ductility, with a strain at break of less than 1%. The mechanical properties were influenced by both the hydrolysis time and the concentration of the NH3 solution used. Optimal conditions were established at 24 hours of hydrolysis with 1 M NH3, yielding the best mechanical performance. TGA and FTIR analyses confirmed that the hydrolysis process preferentially targeted hemicellulose and lignin polymers. Although these techniques provide useful insights into compositional changes, direct polymer molecular weight measurements would better elucidate chain scission and reorganization during hydrolysis, which represents a promising avenue for future research.To enhance the properties of the bioplastics and incorporate antimicrobial functionality, grass biomass was combined with the antimicrobial PL peptide. The primary interaction between PL and hydrolyzed grass was identified as hydrogen bonding, as evidenced by FTIR spectra. Notably, there was no chemical crosslinking between PL and the hydrolysis products. Instead, the reducing sugars are believed to form pseudo-lignin rather than crosslinking with PL, as indicated by TGA, RSC, and FTIR results.
The inclusion of PL in the hydrolysis mixture produced of more flexible materials due to its plasticizing effect, resulting in reduced Young's modulus and tensile strength while slightly enhancing ductility. This effect was further confirmed by a decrease in the glass transition temperature (Tg) with increasing PL content, and the films’ cross-sectional morphology became smoother compared to bioplastics without PL.
In terms of water interactions, all bioplastics maintained a moisture content below 10%. The addition of up to 20 wt% PL resulted in a significant reduction in water solubility. These observations were attributed to hydrogen bonding interactions between the polar groups of the hydrolyzed biomass and PL, inhibiting water absorption.
Moreover, the incorporation of PL into the bioplastics resulted in antimicrobial films effective against E. coli and S. aureus. The GPL20 films demonstrated complete inhibition of E. coli growth and a 99% reduction in S. aureus growth compared to films lacking PL. Furthermore, antioxidant properties were observed, which can be beneficial for extending food shelf life.
Life cycle assessment highlights the potential of grass biomass for sustainable material development, outperforming benchmark packaging biobased or fossil-based alternatives. The ease of processing in combination with the biogenic carbon storage results in films with a cradle-to-gate climate change potential of 1.69 kg CO2 eq. kg−1, while PLA, PET and LDPE films carbon footprint values of 3.40–3.62 kg CO2 eq. kg−1.
Given their antimicrobial properties, favourable mechanical characteristics, and interactions with water, GPL20 films represent a promising material for further investigation into sustainable food packaging applications, particularly for rigid or single-use packaging solutions. However, it is important to note that this study was conducted on a laboratory scale (3 g batches) sufficient for prototyping, such as fabricating a sample tray. Future work should focus on scaling up production by optimizing drying techniques, improving biomass dispersion, and evaluating large-scale manufacturability to facilitate industrial adoption.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
J. D. E. S. would like to thank the members of the Sustainable Biocomposite Materials (SusBioComp) group for their support and advice during the seminar sessions. Gratitude is also extended to the members of Sardon's Lab for their assistance during their time in the laboratory. Additionally, thanks are given to Jorge Olmedo for conducting the TGA analysis. E. L. is grateful for the financial support from the University of the Basque Country (Convocatoria de ayudas a grupos de investigación GIU21/010). D. M. gratefully acknowledges financial support from the Basque Science Foundation for Science (Ikerbasque), POLYMAT, the University of the Basque Country (UPV/EHU), and the European Union's Horizon Europe research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 101103474. Additionally, D. M. thanks the support from the María de Maeztu Excellence Unit (CEX2023-001303-M), funded by MCIN/AEI/10.13039/501100011033.References
- Plastics Europe, 2023.
- Y. Chen, A. K. Awasthi, F. Wei, Q. Tan and J. Li, Sci. Total Environ., 2021, 752, 141772 CrossRef CAS PubMed
.
-
S. Alavi, S. Thomas, K. P. Sandeep, N. Kalarikkal, J. Varghese and S. Yaragalla, Polymers for Packaging Applications, CRC Press, 2014 Search PubMed
.
-
B. Tajeddin and M. Arabkhedri, in Polymer Science and Innovative Applications, ed. M. A. A. AlMaadeed, D. Ponnamma and M. A. Carignano, Elsevier, 2020, pp. 525–543 Search PubMed
.
- J. Tan, S. K. Tiwari and S. Ramakrishna, Mater. Circ. Econ., 2021, 3, 7 CrossRef
.
- K. Babaremu, O. P. Oladijo and E. Akinlabi, Adv. Ind. Eng. Polym. Res., 2023, 6, 333–340 CAS
.
- Plastics Europe, 2022.
- A. Kumar, S. Mishra, R. Pandey, Z. G. Yu, M. Kumar, K. S. Khoo, T. K. Thakur and P. L. Show, TrAC Trends Anal. Chem., 2023, 158, 116869 CAS
.
- Z. Li, Y. Yang, X. Chen, Y. He, N. Bolan, J. Rinklebe, S. S. Lam, W. Peng and C. Sonne, Chemosphere, 2023, 313, 137637 CAS
.
- E. C. Emenike, C. J. Okorie, T. Ojeyemi, A. Egbemhenghe, K. O. Iwuozor, O. D. Saliu, H. K. Okoro and A. G. Adeniyi, Heliyon, 2023, 9, e20440 Search PubMed
.
- L. He, Z. Li, Q. Jia and Z. Xu, Science, 2023, 379, 547–547 CAS
.
- Z. Qaiser, M. Aqeel, W. Sarfraz, Z. Fatima Rizvi, A. Noman, S. Naeem and N. Khalid, Case Stud. Chem. Environ. Eng., 2023, 8, 100536 CAS
.
- D. G. Bucknall, Philos. Trans. R. Soc., A, 2020, 378, 20190268 CAS
.
- K. N. Onwukamike, S. Grelier, E. Grau, H. Cramail and M. A. R. Meier, ACS Sustainable Chem. Eng., 2019, 7, 1826–1840 CAS
.
- A. I. Quilez-Molina, U. Chandra Paul, D. Merino and A. Athanassiou, ACS Sustainable Chem. Eng., 2022, 10, 15402–15413 CAS
.
- A. N. M. A. Haque, R. Remadevi, X. Wang and M. Naebe, Mater. Chem. Phys., 2020, 239, 122009 Search PubMed
.
- J. S. Yaradoddi, N. R. Banapurmath, S. V. Ganachari, M. E. M. Soudagar, A. M. Sajjan, S. Kamat, M. A. Mujtaba, A. S. Shettar, A. E. Anqi, M. R. Safaei, A. Elfasakhany, M. I. Haque Siddiqui and M. A. Ali, J. Mater. Res. Technol., 2022, 17, 3186–3197 CAS
.
- D. Merino, R. Simonutti, G. Perotto and A. Athanassiou, Green Chem., 2021, 23, 5956–5971 RSC
.
- D. Merino and A. Athanassiou, Chem. Eng. J., 2023, 454, 140171 CrossRef CAS
.
- L. Chen, T. Bi, E. Lizundia, A. Liu, L. Qi, Y. Ma, J. Huang, Z. Lu, L. Yu, H. Deng and C. Chen, Innovation, 2024, 5, 100655 CAS
.
- Z. Hao, W. Y. Hamad and P. Yaseneva, Chem. Eng. J., 2023, 147160 CAS
.
- R.
L. de Lapuente Díaz de Otazu, O. Akizu-Gardoki, B. de Ulibarri, M. Iturriondobeitia, R. Minguez and E. Lizundia, Sustain. Prod. Consum., 2021, 29, 718–729 Search PubMed
.
- M. F. de Souza, Ç. Akyol, B. Willems, A. Huizinga, S. van Calker, M. Van Dael, A. De Meyer, R. Guisson, E. Michels and E. Meers, Waste Manage., 2024, 182, 1–10 Search PubMed
.
- J. C. García, A. Alfaro, J. M. Loaiza, S. Lozano-Calvo and F. López, Biomass Convers. Biorefin., 2024, 14, 8307–8320 Search PubMed
.
- L. Wang, C. Zhang, J. Zhang, Z. Rao, X. Xu, Z. Mao and X. Chen, Front. Bioeng. Biotechnol., 2021, 9, 748976 CrossRef PubMed
.
- S. Chen, S. Huang, Y. Li and C. Zhou, Front. Chem., 2021, 9, 659304 CrossRef CAS
.
- K. Ushimaru, Y. Hamano, T. Morita and T. Fukuoka, ACS Omega, 2019, 4, 9756–9762 CAS
.
- K. Ushimaru, T. Morita and T. Fukuoka, ACS Omega, 2020, 5, 22793–22799 CAS
.
- A. Álvarez, S. Cachero, C. González-Sánchez, J. Montejo-Bernardo, C. Pizarro and J. L. Bueno, Carbohydr. Polym., 2018, 189, 250–256 Search PubMed
.
- M. Ioelovich, Bioresources, 2015, 10(1), 1879–1914 Search PubMed
.
- Ł. Łopusiewicz, N. Śmietana, D. Paradowska and E. Drozłowska, Microorganisms, 2022, 10, 300 Search PubMed
.
- S. Macieja, B. Środa, B. Zielińska, S. Roy, A. Bartkowiak and Ł. Łopusiewicz, Int. J. Mol. Sci., 2022, 23, 15560 CAS
.
- Ł. Łopusiewicz, P. Kwiatkowski, E. Drozłowska, P. Trocer, M. Kostek, M. Śliwiński, M. Polak-Śliwińska, E. Kowalczyk and M. Sienkiewicz, Polymers, 2021, 13, 499 Search PubMed
.
- A. Laurent, B. P. Weidema, J. Bare, X. Liao, D. Maia de Souza, M. Pizzol, S. Sala, H. Schreiber, N. Thonemann and F. Verones, J. Ind. Ecol., 2020, 24, 986–1003 Search PubMed
.
- C. Chan, D. Martin, E. Gauthier, P. Jensen, B. Laycock and S. Pratt, Polymers, 2022, 14, 3704 CAS
.
- V. E. Ottah, A. L. Ezugwu, T. C. Ezike and F. C. Chilaka, Heliyon, 2022, 8, e09714 CAS
.
- G. S. Cano-Díaz, A. Rosas-Aburto, E. Vivaldo-Lima, L. Flores-Santos, M. A. Vega-Hernández, M. G. Hernández-Luna and A. Martinez, Ind. Eng. Chem. Res., 2021, 60, 3502–3515 CrossRef
.
- W. Peng, L. Wang, M. A. K. O. T. O. Ohkoshi and M. Zhang, Cellul. Chem. Technol., 2015, 49(9–10), 756–764 Search PubMed
.
- D. M. D. Carvalho and J. L. Colodette, BioResources, 2017, 12, 6907–6923 Search PubMed
.
- R. Kumar, F. Hu, P. Sannigrahi, S. Jung, A. J. Ragauskas and C. E. Wyman, Biotechnol. Bioeng., 2013, 110, 737–753 CrossRef CAS
.
- S. D. Shinde, X. Meng, R. Kumar and A. J. Ragauskas, Green Chem., 2018, 20, 2192–2205 CAS
.
- S. Park, S. J. Kim, K. C. Oh, L. Cho, Y. K. Jeon and D. H. Kim, Energy, 2023, 283, 128548 CrossRef
.
- M. Henrikki Sipponen, V. Pihlajaniemi, S. Sipponen, O. Pastinen and S. Laakso, RSC Adv., 2014, 4, 23177–23184 RSC
.
- P. Sannigrahi, D. H. Kim, S. Jung and A. Ragauskas, Energy Environ. Sci., 2011, 4, 1306–1310 RSC
.
- S. Razani and A. Dadkhah Tehrani, Polym. Bull., 2024, 81, 4893–4909 CrossRef CAS
.
- M. Rozenberg and G. Shoham, Biophys. Chem., 2007, 125, 166–171 CrossRef CAS PubMed
.
- D. Sebben and P. Pendleton, Spectrochim. Acta, Part A, 2014, 132, 706–712 Search PubMed
.
- G. Zhu, N. Guo, X. Yan, J. Dong, X. Chen and H. Lu, Coatings, 2023, 13, 1832 CAS
.
- Z. Yu, G. Rao, Y. Wei, J. Yu, S. Wu and Y. Fang, Int. J. Biol. Macromol., 2019, 141, 545–552 CAS
.
- X. He, Y. Li, L. Zhang, R. Du, Y. Dai and Z. Tan, Cellulose, 2021, 28, 2833–2847 CAS
.
- S. C. Shukla, A. Singh, A. K. Pandey and A. Mishra, Biochem. Eng. J., 2012, 65, 70–81 CAS
.
- W. Liao, X. Liu, Q. Zhao, Z. Lu, A. Feng and X. Sun, Int. J. Biol. Macromol., 2023, 253, 127231 CAS
.
- J. Hu, W. Jiao, Q. Chen, B. Liu and M. Fu, Food Sci. Nutr., 2023, 11, 5188–5198 CAS
.
- R. G. M. van der Sman, J. Phys. Chem. B, 2013, 117, 16303–16313 Search PubMed
.
-
G. Wypych, in Handbook of Polymers (Second Edition), ed. G. Wypych, ChemTec Publishing, 2016, pp. 178–184 Search PubMed
.
- X. Zhao, K. Cornish and Y. Vodovotz, Environ. Sci. Technol., 2020, 54, 4712–4732 Search PubMed
.
-
G. Wypych, in Handbook of Polymers (Second Edition), ed. G. Wypych, ChemTec Publishing, 2016, pp. 156–163 Search PubMed
.
- M. Zhang, G. M. Biesold, W. Choi, J. Yu, Y. Deng, C. Silvestre and Z. Lin, Mater. Today, 2022, 53, 134–161 Search PubMed
.
- Y. Zhang, C. Rempel and Q. Liu, Crit. Rev. Food Sci. Nutr., 2014, 54, 1353–1370 CAS
.
- V. Dwibedi, G. Kaur, N. George, P. Rana, Y. Ge and T. Sun, Food Packag. Shelf Life, 2024, 46, 101385 CrossRef CAS
.
- P. Rodsamran and R. Sothornvit, Food Hydrocolloids, 2019, 97, 105173 Search PubMed
.
- D. Merino, L. Bertolacci, U. C. Paul, R. Simonutti and A. Athanassiou, ACS Appl. Mater. Interfaces, 2021, 13, 38688–38699 CrossRef CAS
.
- D. Merino, U. C. Paul and A. Athanassiou, Food Packag. Shelf Life, 2021, 29, 100707 CAS
.
- N. G. Khouri, J. O. Bahú, C. Blanco-Llamero, P. Severino, V. O. C. Concha and E. B. Souto, J. Mol. Struct., 2024, 1309, 138243 CAS
.
-
G. Wypych, Handbook of Material Weathering, Elsevier, 2018 Search PubMed
.
- C. Torres-León, A. A. Vicente, M. L. Flores-López, R. Rojas, L. Serna-Cock, O. B. Alvarez-Pérez and C. N. Aguilar, LWT, 2018, 97, 624–631 Search PubMed
.
- G. P. Bruni, J. P. de Oliveira, L. M. Fonseca, F. T. da Silva, A. R. G. Dias and E. da Rosa Zavareze, Starch, 2020, 72, 1900051 CAS
.
- A. Rejak, A. Wójtowicz, T. Oniszczuk, D. Niemczuk and M. Nowacka, Teka Komisji Motoryzacji i Energetyki Rolnictwa, 2014, 14(3), 89–94 Search PubMed
.
- Q. Sun, Molecules, 2022, 27, 7009 CAS
.
- Z. Tan, Y. Shi, B. Xing, Y. Hou, J. Cui and S. Jia, Bioresour. Bioprocess., 2019, 6, 11 Search PubMed
.
- P. R. Salgado, M. E. López-Caballero, M. C. Gómez-Guillén, A. N. Mauri and M. P. Montero, Food Hydrocolloids, 2012, 29, 374–381 CrossRef CAS
.
-
K. Chie, Effect of glycation on ε-polylysine with dextran using maillard reaction on its antimicrobial activity, antioxidant activity, and stability, Doctoral dissertation, California State Polytechnic University, Pomona, 2022 Search PubMed
.
- H. Baniasadi, Z. Fathi, E. Lizundia, C. D. Cruz, R. Abidnejad, M. Fazeli, P. Tammela, E. Kontturi, J. Lipponen and J. Niskanen, Food Hydrocolloids., 2025, 158, 110525 CrossRef CAS
.
- M. Gallo, G. Arrighi, L. Moreschi, A. Del Borghi, A. Athanassiou and G. Perotto, ACS Sustainable Chem. Eng., 2022, 10, 13936–13944 CAS
.
- S. Wang, W. Wang and H. Yang, Int. J. Environ. Res. Public Health, 2018, 15(10), 2060, DOI:10.3390/ijerph15102060
.
- R. Garcia and F. Freire, J. Cleaner Prod., 2014, 66, 199–209 CAS
.
- E. A. R. Zuiderveen, K. J. J. Kuipers, C. Caldeira, S. V. Hanssen, M. K. van der Hulst, M. M. J. de Jonge, A. Vlysidis, R. van Zelm, S. Sala and M. A. J. Huijbregts, Nat. Commun., 2023, 14, 8521 CrossRef CAS
.
- K. E. Wyer, D. B. Kelleghan, V. Blanes-Vidal, G. Schauberger and T. P. Curran, J. Environ. Manage., 2022, 323, 116285 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc00643k |
| This journal is © The Royal Society of Chemistry 2025 |