Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Using a bioderived CO2-responsive polymer as an easily removed pressure sensitive adhesive†
Daniel
Barker
a,
Shannon
Whitty
a,
Tobias
Robert
 c,
Parisa
Bayat
d,
Marc A.
Dubé
c,
Parisa
Bayat
d,
Marc A.
Dubé
 d,
Michael F.
Cunningham
d,
Michael F.
Cunningham
 b,
Guojun
Liu
b,
Guojun
Liu
 *a and
Philip G.
Jessop
*a and
Philip G.
Jessop
 *a
*a
aDepartment of Chemistry, Queen's University, Kingston, ON K7L 3N6, Canada. E-mail: jessop@queensu.ca
bDepartment of Chemical Engineering, Queen's University, Kingston, ON K7L 3N6, Canada
cFraunhofer Institute for Wood Research – Wilhelm-Klauditz-Institut WKI, Riedenkamp 3, 38108 Braunschweig, Germany
dDepartment of Chemical and Biological Engineering, University of Ottawa, Ottawa, Ontario K1N 6N5, Canada
First published on 10th July 2025
Abstract
Pressure sensitive adhesives (PSAs) are used extensively in industry for adhering labels onto substrates. These labels are expected to adhere to the desired substrate during use but be easily removed after serving their purpose. However, strong PSAs can be difficult to remove and can cause significant frustration to consumers and the recycling industry. These conflicting properties of excellent adhesion during use but easy removal at the end-of-life require the material to switch its properties. We have developed a bioderived CO2-responsive adhesive synthesized from a castor oil derivative. The inclusion of CO2-responsive groups allows the bioderived polymer to be readily dissolved into carbonated water. Removal of CO2 and water produces a PSA which displays excellent adhesion comparable to that of commercial cellophane tapes. This new PSA adheres to many common packaging materials such as plastics, metals, and wood. The PSA displays excellent water resistance even after 5 days submerged in neutral water, with no observable loss in adhesive performance. However, exposure to carbonated water causes the PSA to separate easily from the substrate. This bioderived CO2-responsive PSA potentially offers practical advantages in many applications including facilitating the removal of labels from used containers before recycling.
Green foundation1. This work describes a bioderived adhesive that uses carbonated water as solvent for application and removal. The adhesive can be easily removed when the materials to which it is adhered are to be recycled. Easy removal facilitates plastics recycling without contamination from the adhesive.2. This pressure sensitive adhesive addresses functional and environmental problems that are associated with the recycling of products having labels. The adhesive was synthesized using bioderived chemicals, uses carbonated water as a polymerization and removal solvent, and provides the desired adhesive properties when needed. 3. Further optimization of the reaction conditions and introduction of flow chemistry would be useful in limiting reagent use, reducing waste generation, and minimizing energy consumption. Utilizing bioderived amine sources would further reduce the dependence on nonrenewable resources. |
Introduction
Pressure-sensitive adhesives (PSAs) are crucial in the manufacturing and consumer goods sectors. Labels are coated on the back side with PSAs that allow for excellent adhesion to products or packaging but may need to be removed by the customer after purchase or before recycling. However, this does not always happen because some PSAs are difficult to remove, which causes user frustration or can damage the desired item. In addition, adhesive performance plays a significant role in material recycling. In plastic recycling, residual adhesives can alter the properties of polymers once the plastic is melted down. This contamination diminishes the value of the recycled plastic.1,2 The removal of adhesives during the recycling process needs to be facile and inexpensive. Having an adhesive that can switch from strong adhesion during use to no adhesion at the end of life would alleviate consumer frustrations and make the recycling process more efficient and sustainable.Stimuli-responsive materials hold promise in addressing the need for conflicting properties within a single material. Various stimuli, including light, pH, voltage, heat, and ultrasound, have been used to trigger changes in the properties of stimuli-responsive adhesives.3–7 However, using these stimuli for preparing stimuli-responsive PSAs has challenges, particularly in terms of feasibility for consumers to apply the stimulus and large scale use of the stimulus in the recycling industry. Using light as a stimulus appears attractive but has disadvantages, as light-responsive PSAs are usually UV-responsive. The use of UV radiation is potentially harmful to users as significant exposure can lead to cancer.8 The UV radiation must be able to penetrate through any layers between the light source and the adhesive to induce a response. Thermo-responsive adhesives are attractive but can require significant heat exposure, such as 70–80 °C, to induce a rapid response.9,10 Excessive and prolonged heating is costly and could damage the substrate. Voltage as a stimulus is unlikely to be widely used in the recycling industry as the substrate must be conductive and electrically connected to the equipment to induce a response within the adhesive. Ultrasonic debonding would be energy-intensive at scale and, therefore, also unappealing. While stimuli-responsive materials in principle offer a potential solution to conflicting properties within an adhesive, many stimuli are not suitable for solving the recycling problem that adhesives present.
CO2 gas has been shown to induce property changes, like adhesion in certain materials, which are known as CO2-responsive materials.11 CO2 has become widely available to the public with the popularization of at-home carbonated water machines. Industrially, it is a waste gas that is captured, compressed, and sold at low cost.12,13 CO2 as a stimulus has benefits over all the aforementioned stimuli; it is non-hazardous, low cost and does not require the material to be transparent, conductive, or electrically bonded to the equipment to induce a response. No specialized equipment is needed to introduce CO2 into the system. CO2-responsive polymers typically require amine functional groups that have the appropriate basicity to achieve the desired expression of two different properties.14
In the literature, there is precedent for a CO2-responsive adhesive. Richard Weiss and colleagues reported a siloxane polymer containing primary amine side groups.11 When treated with CO2, these side groups formed carbamate salts ([RNH3+][R′HNCO2−], where R and R′ represent different polymer chains), leading to intermolecular electrostatic interactions – ionic crosslinking. However, switching the adhesive back to the CO2-free non-sticky state was not tested.
When CO2 enters water, it forms dissolved CO2 and a small amount of carbonic acid, both of which are acidic. Either of them can then protonate an amine, causing it to become charged, which results in a change in the amine's properties.15 Tertiary or bulky secondary amines are usually selected as the CO2-responsive functionality as these amines do not form carbamate salts. They exclusively form the bicarbonate salt, which can easily be converted back to the neutral amine (Reaction (1)).16 An aqueous polymer solution containing protonated CO2-responsive polymers can be reversed in minutes at ambient temperatures by bubbling with inert gas, or, to accelerate the process, the solution can be heated to >∼60 °C to remove CO2. This switching mechanism can be used to design PSAs that easily dissolve into carbonated water but are poorly water-soluble after CO2 has been removed.
 | (1) |
To our knowledge, there are no reports of a PSA that contains CO2-responsive polymers that use carbonated water as the solvent. This type of CO2-responsive PSA allows the polymer to be synthesized directly in carbonated water as the monomers are CO2-responsive. This CO2-responsive mechanism would eliminate the need for organic solvents during the solution polymerization step and replace them with water, which is significantly safer to handle. Purifying the polymer may also not be needed as the polymerization can go to complete conversion and the polymer is already in the desired application solvent. After the CO2-responsive PSA solution is applied, CO2 and water would evaporate, altering the PSA properties to provide good adhesion and water resistance. At the end of use, carbonated water can again dissolve the PSA for facile removal, although merely softening the polymer coating may be sufficient to allow its removal.
Using CO2-responsive polymers could provide a simple solution for the consumer and recycling industry to separate difficult to remove PSAs from substrates; however, the sustainable design and manufacturing of adhesives also needs to be addressed. Using biological materials to prepare adhesives predates using synthetic polymers. Before the use of synthetic polymers, adhesives were prepared from materials such as animal proteins, starch, and natural rubbers.17–19 These earlier bio-based adhesives were later replaced with synthetic polymers as the synthetic adhesives provided better adhesion strength, and improved water resistance.20 However, with a recent societal shift towards more sustainable and environmentally sourced materials, bio-based adhesives have reemerged with improved water resistance and adhesion. Researchers are modifying bio-based materials like lignin,21 soy meal proteins,22 and triglycerides23 to provide sustainable adhesives with improved performance.
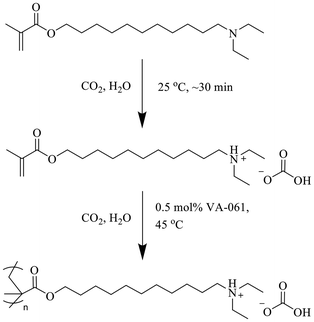
Various biomass feedstocks and bio-based platform chemicals are available to produce bio-based adhesives. Certain material characteristics are sought specifically for PSAs, like excellent tack, shear adhesion, peel strength, and water resistance which are conventionally achieved by using soft petroleum-based hydrophobic polymers like butyl acrylate and 2-ethylhexyl acrylate. To use bio-based materials as an alternative adhesive, the bioderived polymers would ideally mimic petroleum-based adhesives in terms of properties and possibly structure. For example, fatty acids are hydrophobic and exist as oils or waxes. When modified into a polymer, the fatty acid-derived polymers can possess adhesive properties.20 While many bio-based materials can be sourced, we chose to work from fatty acids because their hydrophobicity is similar to that of petroleum-based adhesives. Castor oil is an excellent candidate for sourcing fatty acids that can be readily modified. As a nonedible crop, castor oil consists of triglycerides that contain eight different fatty acids with ricinoleic acid making up about 90% of the composition.24 Pyrolysis of castor oil produces undecenoic acid,25 which can be easily converted into 11-bromoundecanoic acid or 11-bromoundecanol both of which could make suitable starting materials for bioderived adhesives.26,27 The terminal bromide in combination with either the carboxylic acid or alcohol allows for the addition of other functionalities. Polymerizable functional groups and amines can be installed at either end of the molecule to produce a bioderived CO2-responsive monomer.
Herein, a bioderived CO2-responsive PSA is described that can easily dissolve in carbonated water but is resistant to dissolution in neutral water after drying. 11-Bromoundecanol (11-BUDol) was modified using diethylamine and methacryloyl chloride to provide a methacrylate-based monomer with a long alkyl chain, terminated with a CO2-responsive moiety. The monomer was polymerized in carbonated water and the resulting polymer solutions were used without requiring purification. Standard PSA tests in accordance with the Pressure Sensitive Tape Council standards were completed to measure the peel strength, tack, and shear adhesion of the bioderived CO2-responsive PSA. The adhesion properties were compared against commercial PSA tapes. The bioderived CO2-responsive PSA was cast on polypropylene film and adhered to various substrates to observe the adhesion performance. After performance testing the bioderived CO2-responsive PSA was removed easily using carbonated water.
Experimental methods and materials
Materials
Acetonitrile (anhydrous, 99.8%), hexanes (ACS grade), diethylamine (≥99.5%), triethylamine (≥99%), and methacryloyl chloride (97%) were purchased from Millipore Sigma. Toluene (ACS grade) and anhydrous magnesium sulfate were purchased from Fisher Scientific. 11-Bromoundecanol was purchased from TCI America. Sodium carbonate was purchased from VWR International. The thermal initiator 2,2′-azobis[2-(2-imidazolin-2-yl)propane] (VA-061) was purchased from Fujifilm Wako Chemicals and recrystallized from ethanol to remove impurities. Carbon dioxide gas (CO2, 3.0) was purchased from Linde Canada. Scotch® Magic™ tape and Fisherbrand™ labelling tape were purchased from Staples and Fisher Scientific, respectively. Materials were used as received unless otherwise stated.Synthesis of 11-(diethylamino)undecan-1-ol (DEAUol)
To a 250 mL Schlenk flask, under an inert atmosphere, anhydrous acetonitrile (80 mL) was added via a syringe. 11-Bromoundecanol (5 g, 20 mmol), and diethylamine (10 mL, 100 mmol) were then dissolved into the acetonitrile under inert conditions. A condenser was fitted to the flask, and the reaction was refluxed for 24 h. Afterwards, the solvent was evaporated under reduced pressure. A 50 mL solution of saturated sodium carbonate was added to the solution and allowed to stir for 5 min. The product was extracted with 3 × 50 ml of toluene. The organic layer was collected, dried over MgSO4, filtered, and evaporated under reduced pressure. A viscous dark orange oil remained (yield 98%). 1H NMR (499.86 MHz, CDCl3) δ 3.62 (t, J = 6.7 Hz, 2H), 2.52 (q, J = 7.1 Hz, 4H), 2.43–2.34 (m, 2H), 1.55 (p, J = 7.2 Hz, 2H), 1.43 (p, J = 7.2 Hz, 2H), 1.37–1.20 (m, 14H), 1.01 ppm (t, J = 7.2 Hz, 6H). 13C {1H} NMR (125.81 MHz, CDCl3) δ 77.41, 77.16, 76.91, 62.71, 53.02, 46.86, 32.93, 29.69, 29.67, 29.61, 29.53, 27.82, 26.83, 25.89, 11.54 ppm. Calculated M + 1 = 244.26349 g mol−1, observed M + 1 = 244.26408 g mol−1.Synthesis of 11-(diethylamino)undecyl methacrylate (DEAUMA)
To a 100 mL Schlenk flask under an inert atmosphere, anhydrous acetonitrile (30 mL) was added via a syringe, followed by triethylamine (3.26 mL, 23.4 mmol). In a separate flask, under an inert atmosphere, DEAUol (4.75 g, 19.5 mmol) was dissolved in anhydrous acetonitrile (20 mL) and then added to the Schlenk flask via syringe. The solution was chilled in an ice bath for 1 h prior to the dropwise addition of methacryloyl chloride (2.29 mL, 23.4 mol). The addition occurred over 15 min. The mixture was left to react for 24 h, and spontaneously warmed from 0 °C to room temperature (20 °C) over the period of the reaction. After 24 h, the solvent was evaporated under reduced pressure. A 50 mL solution of saturated sodium carbonate was added to the flask and allowed to stir for 5 min. The mixture was poured into a separatory funnel and washed with toluene (3 × 50 ml). The organic fractions were combined, dried over MgSO4, filtered, and evaporated under reduced pressure. The residue was purified by column chromatography using hexanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) triethylamine at v/v = 95/5 as eluent and silica as the stationary phase. The eluate was collected and evaporated under reduced pressure. A clear, slightly yellow oil remained (yield 94%). 1H NMR (499.86 MHz, CDCl3) δ 6.09 (s, 1H), 5.53 (s, 1H), 4.13 (t, J = 6.7 Hz, 2H), 2.51 (q, J = 7.2 Hz, 4H), 2.42–2.35 (m, 2H), 1.93 (s, 3H), 1.70–1.61 (m, 2H), 1.47–1.39 (m, 2H), 1.27 (s, 14H), 1.01 ppm (t, J = 7.1 Hz, 6H). 13C {1H} NMR (125.81 MHz, CDCl3) δ 167.46, 136.59, 125.03, 77.41, 77.16, 76.91, 64.80, 53.09, 46.95, 29.65, 29.61, 29.53, 29.51, 29.26, 28.64, 27.75, 27.11, 26.00, 18.31, 11.75 ppm. Calculated M + 1 = 312.28970 g mol−1, observed M + 1 = 312.29092 g mol−1.
triethylamine at v/v = 95/5 as eluent and silica as the stationary phase. The eluate was collected and evaporated under reduced pressure. A clear, slightly yellow oil remained (yield 94%). 1H NMR (499.86 MHz, CDCl3) δ 6.09 (s, 1H), 5.53 (s, 1H), 4.13 (t, J = 6.7 Hz, 2H), 2.51 (q, J = 7.2 Hz, 4H), 2.42–2.35 (m, 2H), 1.93 (s, 3H), 1.70–1.61 (m, 2H), 1.47–1.39 (m, 2H), 1.27 (s, 14H), 1.01 ppm (t, J = 7.1 Hz, 6H). 13C {1H} NMR (125.81 MHz, CDCl3) δ 167.46, 136.59, 125.03, 77.41, 77.16, 76.91, 64.80, 53.09, 46.95, 29.65, 29.61, 29.53, 29.51, 29.26, 28.64, 27.75, 27.11, 26.00, 18.31, 11.75 ppm. Calculated M + 1 = 312.28970 g mol−1, observed M + 1 = 312.29092 g mol−1.
Polymerization of 11-(diethylamino)undecyl methacrylate (PDEAUMA)
The synthesis of PDEAUMA was achieved by free radical polymerization (FRP) using 2,2′-azobis[2-(2-imidazolin-2-yl)propane] (VA-061) as the initiator. A 15 wt% mixture of DEAUMA monomer in deionized (DI) water was added to a 100 mL cylindrical glass vessel. The mixture was bubbled with CO2 using a 22-gauge stainless steel needle for 30 min to protonate the DEAUMA, thereby making it soluble in carbonated water. VA-061 (0.50 mol%, relative to monomer) was then added to the solution and stirred using a mechanical stirrer for 30 min under continuous bubbling of CO2. The vessel was heated to 45 °C and continuously stirred for 24 h. The system was sealed under an atmosphere of CO2 for the duration of the reaction. After 24 h, the flask was cooled to room temperature and the resulting polymer solution was used for testing with no purification. CO2 was bubbled continuously into the polymer solution during the cooling phase to saturate the solution, preventing the polymer from precipitating out of the solution. The flask was then sealed under an atmosphere of CO2.Material characterization
The purity and composition of each isolated product were determined by performing 1H and 13C NMR spectroscopy using a Bruker NEO-500 MHz spectrometer and deuterated chloroform as the solvent. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) spectroscopy was performed on a Bruker Alpha compact FT-IR spectrometer. Opus v.8.9 software was used to obtain the IR spectrum. Mass spectrometry was performed using a Thermo Scientific Orbitrap Velos Pro Easy-nLC/HESI Hybrid Ion Trap–Orbitrap Mass Spectrometer. Samples were dissolved into methanol at approximately 10 nM and injected directly into the mass spectrometer.Gel permeation chromatography (GPC) was used to obtain the molecular weights and dispersity of PDEAUMA and was compared against calibrated monodisperse polystyrene standards. Samples were dried in a vacuum oven for 24 h, dissolved in tetrahydrofuran (THF) at a concentration of 5 mg mL−1, and filtered using a 0.22 μm Chromspec filter before injection. A Waters 2695 separation module equipped with a Waters 410 differential refractometer used THF as the eluting solvent at a flow rate of 1 mL min−1 through a Waters Styragel HR (4.6 × 300 mm) 4, 3, 1 and 0.5 separation columns. Differential scanning calorimetry (DSC) was performed using a TA Instruments Q100 DSC to determine the polymer dry glass transition temperature (Tg). DSC analysis was performed under a nitrogen atmosphere with a single heating and cooling cycle before Tg determination to remove any thermal history that the polymer had during sample preparation. N2 flow was set to 50 mL min−1. A DSC cycle was performed starting at −70 °C and heating to 150 °C, followed by an isothermal hold for 5 min, then cooling back to −70 °C and heating back to 150 °C. All heating and cooling rates were 10 °C min−1. The PDEAUMA was dried under vacuum for 24 h prior to analysis.
PDEAUMA PSA performance
Pressure Sensitive Tape Council standards (PSTC) including PSTC16, PSTC101, and PSTC107A for tack, 180° peel strength, and shear adhesion, respectively, were followed for sample preparation and testing.28 PDEAUMA 15 wt% solution in carbonated water was cast on 50 μm corona-treated Mylar sheets using a #50 Meyer rod for all samples. The sample was dried under controlled conditions (25 °C and relative humidity (RH) = 50 ± 5%) for 30 min and coated again using a #50 Meyer rod. After the second coating the sample was dried under controlled conditions for 24 h. An Instron 3000 Universal tester and Bluehill 2 Materials Testing Software were used to measure the tack and peel strength as the force per unit width required to remove the PSA from the substrate. Using a lab-built tester, shear adhesion was measured as the time a PSA strip can hold against a constant vertical force. All tests were performed six times, with the average results and standard deviations reported.To test the compatibility of PDEAUMA with various substrates, including glass, polyethylene terephthalate, polypropylene, polystyrene, Teflon, aluminium, matte steel, and wood, PDEAUMA solution in carbonated water was cast on polypropylene film (25.4 mm × 127 mm) using a #50 Meyer rod and dried at ambient conditions to simulate a plastic-backed label. This procedure was performed twice. The label was then applied to the substrate by hand with a 25.4 mm × 76.2 mm area of contact. Adhered substrates were immediately lifted and held for 1 min to observe if the label would separate from the substrate.
Neutral water stability of PDAEUMA
A glass slide (25.4 mm × 76.2 mm) was coated with 15 wt% PDEAUMA solution in carbonated water using a #50 Meyer rod and dried at ambient conditions. This procedure was performed twice in order to increase the thickness. A commercial adhesive, which consisted of a 40 wt% of polyvinyl acetate emulsion (PVA, commercial white glue), was coated onto a separate glass slide using a #50 Meyer rod and dried for 1 h at ambient conditions. This procedure was also performed twice. The coated glass slides were submerged into separate beakers of deionized (DI) water with a pH of approximately 7 and left to soak for 5 days. After 5 days, the coated glass slides were removed from the DI water and left to dry for 10 min at ambient conditions. The dried adhesives were then placed in contact with polypropylene film (25.4 mm × 127 mm) to observe whether their adhesive properties remained.Removal of PDEAUMA using carbonated water
After testing its adhesion, the PDEAUMA coated glass slide was submerged in 500 mL of saturated carbonated water and observed. The carbonated water was stirred at 1000 rpm. To prepare the carbonated water, DI water was sparged with CO2 gas using a gas dispersion tube for 4 h to ensure saturation. The adhesive was wiped with a finger after each minute of exposure to carbonated water until complete removal of the adhesive was achieved.Results and discussion
Synthesis of DEAUMA
Using 11-BUDol as the starting material, a bioderived CO2-responsive monomer was prepared. 11-BUDol was reacted with an excess of diethylamine to ensure the reaction proceeded to completion, and to ensure the HBr generated during the reaction was neutralized. Diethylamine was installed first instead of methacryloyl chloride because it eliminated an undesired side product believed to be the cyclic ether of 11-BUDol that occurred when attempting to install methacryloyl chloride first. DEAUol appeared as a dark orange oil after purification. Following purification, DEAUol was reacted with methacryloyl chloride to produce the monomer DEAUMA (Scheme 1). After purification, a faint yellow oil remained. Both DEAUol and DEAUMA were examined using 1H and 13C NMR spectroscopy to confirm their structures and purity (see ESI†). Mass spectrometry and ATR-IR spectroscopy were also used to confirm the formation of final products.Synthesis of PDEAUMA
To synthesize PDEAUMA, FRP was performed in carbonated water using VA-061 as the thermal initiator (Scheme 2). The tertiary amine groups contained within DEAUMA were protonated by the carbonated water so that the monomer dissolved. VA-061 is a thermal initiator that is soluble in carbonated water. VA-061 contains two imidazoline rings, both of which can become protonated in carbonated water, lowering VA-061's 10 h half-life from 61 °C to 45 °C.29 The lower temperature is advantageous when synthesizing CO2-responsive polymers because as temperature increases, the solubility of CO2 in water decreases, which could cause the monomer and polymer to deprotonate and precipitate, thus potentially hindering the reaction progress. Using 1H NMR spectroscopy, it was determined that the polymerization progressed to >99% conversion in 24 h, which was ideal as this removed the need for a purification step. The polymer solution appeared slightly yellow, translucent, and viscous, similar to syrup. According to GPC analysis, the number and weight average molecular weights were determined to be 19 and 48 kDa respectively, with the dispersity being 2.5. The Tg (glass transition temperature) obtained by DSC analysis was −2.2 °C.PDEAUMA adhesive performance
PSAs used on labels must adhere to a variety of different substrates. To observe the adhesion capabilities of PDEAUMA, it was coated onto a polypropylene film (25.4 mm × 127 mm). After drying, the PDEAUMA coated film was adhered onto glass, various plastics, Teflon, aluminium, matte steel, and wood (Fig. 1). After adhesion had been established, the substrate was lifted off the table by the adhesive tape and held for 1 min. The adhesive tape successfully adhered to all substrates, supported their weight, and did not separate during the duration of testing. PDEAUMA demonstrated excellent adhesion to both smooth and rough surfaces. The PDEAUMA PSA adhered easily to a variety of materials commonly used in packaging and infrastructure industries.An adhesive's tack, peel strength, and shear strength are governed, in part, by molecular weight distribution and the polymer's Tg. A high molecular weight dispersity is beneficial when producing adhesives. Low molecular weight polymers contribute to the tack and peel strength of the adhesive, whereas high molecular weight polymers improve the polymer's shear strength.30 Thus, a broad distribution is expected to exhibit good performance for all three properties. The Tg of an adhesive also affects its tack. Low Tg polymers exhibit better tack, meaning they are stickier than higher Tg polymers. Depending on the application of the PSA, certain properties may be favoured over others. For example, labels require good tack and peel strength as they are bonded directly to substrates and are not under any significant load. However, PSA tapes used to secure items together must have a high shear strength to deal with shear forces applied when under a load.
New bioderived PSAs must have comparable adhesive properties to commercially available adhesives if they are to be commercially viable. PDEAUMA was compared against two commercial PSA tapes, Scotch® Magic™ tape and Fisherbrand™ labeling tape (Table 1, raw data in Fig. S11 through S16†). The adhesive tapes prepared using PDEAUMA demonstrated good adhesion properties without any additives or optimization of the formulation required. The molecular weight distribution obtained by FRP produced an adhesive with good tack and peel strength, ideal for PSAs used in labels. PDEAUMA had higher loop tack and peel strength than Scotch® Magic™ tape. The tack and peel strength of Fisherbrand™ labelling tape did surpass PDEAUMA but this provided a reference for how PDEAUMA compares to other commercial PSA tapes. In the shear adhesion testing PDEAUMA held a 500 g weight for 31 h on average which was lower than that of both the commercial tapes but PDEAUMA was designed as a label PSA, which is not expected to be under significant load. PDEAUMA consistently displayed cohesive failure during the tack, peel strength and shear adhesion testing, as residue remained on both the backing and the substrate after testing. The tack and peel strength results produced by PDEAUMA are comparable to those of some commercial PSA tapes, which is promising for potentially replacing petroleum-based adhesives with bioderived CO2-responsive adhesives.
PDEAUMA water resistance and removal
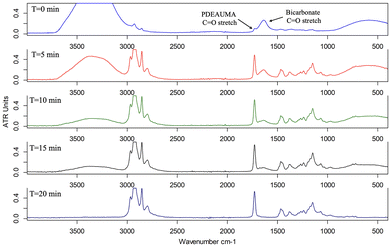
A sought-after feature of most adhesives is their water resistance. A good label PSA should not dissolve or deteriorate if exposed to water during use. Water and bicarbonate species that remain trapped in the adhesive coating after drying pose a concern as this will increase the hydrophilicity of the adhesive. The increased hydrophilicity will lower the adhesive water resistance which is undesirable. To ensure that no water or bicarbonate species remained trapped on the adhesive coating before testing its water resistance, ATR-FTIR was used to analyze PDEAUMA during its drying process. The ATR crystal was coated with the 15 wt% carbonated solution of PDEAUMA using a #50 Meyer rod and then left to dry in ambient conditions. ATR-FTIR spectra were obtained in 5 min increments until no water or bicarbonate peaks remained. The OH stretching of water is observed around 3400 cm−1 and the carbonyl stretching of bicarbonate is observed around 1650 cm−1 (Fig. 2, top). Over 15 min, the water and bicarbonate stretching peak decreased in size. After 20 min there were no observable peaks for water and bicarbonate, indicating the coating was completely dry and no water and bicarbonate species were trapped in the adhesive.After determining that PDEAUMA contains no trapped water or bicarbonate species, the water resistance of the new adhesive was tested. Separate glass slides were coated with either 15 wt% PDEAUMA or 40 wt% PVA solution (commercial white glue), dried and subjected to a water resistance test. The PVA adhesive was used as a visual comparison to demonstrate the water resistance performance of the PDEAUMA adhesive. PVA is water-sensitive, so it was expected to fail when submerged in water. The glass slides were submerged in DI water for 5 days and monitored periodically for any degradation to the coatings (Fig. 3). Within the first minute of testing, the PVA coating immediately whitened, indicating that an interaction with water was occurring. However, for PDEAUMA, the faint whitening of the coatings did not begin until one hour after exposure. After 1 day of exposure, considerable whitening was observed for the PDEAUMA coating. However, no separation or physical disintegration could be observed. The PVA-coated slide had clear signs of coating disintegration, the bottom portion of the adhesive separated from the glass slide and parts of the adhesive were floating in the water. After 5 days, the coated slides were observed again. The PVA adhesive had significant separation and disintegration, but the PDEAUMA-coated slide still showed neither disintegration nor separation. The PDEAUMA-coated slide appeared to be in the same state as it was after 1 day (Fig. 3, lower). The glass slides were removed from the water and dried for 10 min in the air. The damaged PVA coating remained white, indicating irreversible damage. The remaining PVA coating demonstrated no adhesive properties. The PDEAUMA coating whitening subsided as the coating dried over the 10 min. The long alkyl chains of PDEAUMA provide significant hydrophobicity, contributing to the observed water resistance. Whitening did not indicate a performance loss, as the adhesive remained fully attached to the glass surface. The PDEAUMA coating also became tacky again after 10 min of drying; the PDEAUMA-coated glass slide was adhered to a polypropylene film with no issues and could lift the glass slide with no observed performance loss (Fig. 4D).
With PDEAUMA consistently displaying cohesive failure, its ability to be easily removed from backings and valuable substrates is important for recycling these materials. A PDEAUMA-coated glass slide was prepared to represent a discarded substrate with adhesive residue remaining on it. The coated glass slide was placed into carbonated water to cause protonation of the amine groups and debonding of the adhesive from the glass. The coated glass slide was submerged in carbonated water. After 1 min of exposure to carbonated water, swelling of the PDEAUMA coating was observed. A portion of the partially protonated PDEAUMA was removed with a single pass of a finger. After 2 min of exposure, a significant portion of the remaining PDEAUMA was removed and exposed the bare glass. After 3 min of exposure and a single finger wipe, the remaining PDEAUMA was removed, leaving no PDEAUMA on the exposed area (Fig. 5). Faint cloudiness remained around the edges but exhibited no adhesive properties after 30 min of drying at ambient conditions.
The removal experiment was repeated to observe the surface of the adhesive coating more closely using optical microscopy. A new glass slide was coated with 15 wt% PDEAUMA and left to dry in ambient conditions for 24 h. After 24 h, the glass slide was observed using an optical microscope with 20 times magnification to provide better surface detail (Fig. 6A). A portion of the coated glass slide was exposed to 1 mL of carbonated water for 1 min and then wiped once with a finger. This procedure removed a portion of the adhesive (Fig. 6B). Repeating this process twice removed almost all the adhesive in the viewing area (Fig. 6D). As a comparison, a separate part of the coated glass slide, which was not exposed to carbonated water, was wiped three times. Only minor damage was observed to the adhesive coating, indicating that the adhesive coating is strongly adhered to the glass (Fig. 6E). Using carbonated water to protonate PDEAUMA swells and dissolves the polymer, weaking the adhesive bond to the glass, allowing for facile removal compared to abrading the coating with no carbonated water.
The previous experiments represented a label that had been peeled from a substrate, leaving exposed adhesive residue on the desired substrate. However, in certain situations, such as recycling glass or plastic products, the adhesive may not be fully exposed; it remains in contact between the substrate and the label backing. To observe if it was possible to remove the label as well as the adhesive from the substrate simultaneously, paper and polypropylene labels were adhered onto separate glass slides using PDEAUMA (Fig. 7A and D). The labelled glass slides were submerged in 400 mL of saturated carbonated water that was prepared by bubbling CO2 through deionized water for 16 h. The slides were stirred in the carbonated water for 5 min to allow protonation and swelling to occur. PDEAUMA is capable of protonating and swelling enough to be removed in 3 min but given that the presence of the label was expected to hinder the contact between PDEAUMA and carbonated water, more time was given to allow for protonation to occur. After 5 min, the labelled glass slides were removed from the carbonated water and examined. The paper label had considerable damage, with pieces of it separating from the glass slide upon removal from the carbonated water (Fig. 7B). The coated slide was wiped 3 times with a paper towel, successfully cleaning the remaining residue off. Only a small portion of PDEAUMA remained around the edges. However, the polypropylene labelled glass produced undesirable results. The polypropylene is impermeable to water, allowing only the thin edges to be exposed to carbonated water. Protonation and swelling did occur at the edges (Fig. 7E) but the majority of the adhesive remained sticky and resisted removal using the paper towel (Fig. 7F). The experiment was repeated using the polypropylene labelled glass. The exposure time was increased to 1 h. This increased exposure time produced the same result as the prior experiment; protonation and swelling around the edges but no change in properties in the center of the adhesive coating. The impermeable polypropylene, coupled with a low surface area, hindered the mass transfer of carbonated water to PDEAUMA. This prevents most of the PDEAUMA coating from being protonated, which would be necessary for the adhesive to be easily removed. Thus, PDEAUMA adhesive placed between two impermeable materials is not easily removed with carbonated water. However, using porous materials like paper labels allows carbonated water to reach the PDEAUMA layer, causing protonation and swelling which facilitates easy removal of the label and adhesive layer. These results demonstrate that the CO2-responsiveness of the PDEAUMA endows it with all three of the properties that we sought in a PSA: good adhesion performance, water resistance during use, and easy separation from a substrate at the end-of-life.
Conclusions
New sustainable PSAS that perform well during use but are easily removed at the end-of-life will facilitate recycling while minimizing the use of petroleum-based PSAS. Using the fatty alcohol 11-BUDol as the starting material, a bioderived CO2-responsive monomer was synthesized and subsequently polymerized using carbonated water as the solvent. The polymer, PDEAUMA, had tack and peel strengths similar to commercially available Scotch® Magic™ Tape and Fisherbrand™ labelling tape without any additives to improve its adhesive properties. PDEAUMA adhered to many common substrates like glass, various plastics, aluminium, steel, and wood, all of which can be found in the packaging industry. PDEAUMA displayed excellent water resistance over 5 days with no observed degradation. After removal from neutral water and 10 min of drying, PDEAUMA continued to display its adhesive properties with no observable loss in performance. When submerged in carbonated water, PDEAUMA swelled within 1 min and could be partially removed from the substrate with the wipe of a finger. After 3 minutes of total exposure to carbonated water, and three wipes with a finger, PDEAUMA was easily removed from the substrate, demonstrating that PDEAUMA had no adhesion to the substrate. Paper labels adhered to a glass substrate using PDEAUMA can also be removed directly by exposing the entire label to carbonated water. After 5 minutes of exposure, the paper label could be removed and the PDEAUMA residue was wiped off the glass substrate using a paper towel. This bioderived CO2-responsive PSA provides a potential route towards sustainable PSAs that can improve the recyclability of various substrates. While facile removal and the bio-based synthesis are favourable aspects of this PSA, it is difficult at this stage to make an accurate comparison of its environmental harm to that of competing PSAs. In particular, the degradation rate of this polymer in the environment is unknown.Author contributions
D. Barker: conceptualization, methodology, formal analysis, investigation, validation, supervision, writing – original draft, writing – review & editing, visualization. S. Whitty: validation. T. Robert: conceptualization. P. Bayat: validation. Marc A. Dubé; validation, resources. M. F. Cunningham: conceptualization, resources, writing – review & editing, supervision. G. Liu: conceptualization, resources, writing – review & editing, supervision, project administration, funding acquisition. P. G. Jessop: conceptualization, resources, writing – review & editing, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
The authors gratefully acknowledge funding from the Natural Sciences and Engineering Research Council of Canada (NSERC, grant RGPIN-2023-05700). T. R. gratefully acknowledges funding from the German Ministry of Education and Research (FKZ: 031B0772). P. G. J. also thanks the Canada Research Chairs Program for its support. Scotch® Magic™ Tape are registered trademarks of 3M Company. Fisherbrand™ labelling tape is a registered trademark of ThermoFisher Scientific.References
- C. Sato, Recycling and Environmental AspectsHandbook of Adhesion Technology, ed. L. F. M. d. Silva, A. Öchsner and R. D. Adams, Springer Berlin, Heidelberg, 1st edn, 2011, pp. 1505–1526 Search PubMed.
- H. Onusseit, The Influence of Adhesives on Recycling, Resour., Conserv. Recycl., 2006, 46, 168–181, DOI:10.1016/j.resconrec.2005.05.009.
- J. Wang, Z. Dong, J. Chen and S. Chen, Preparation of UV Debonding Acrylate Adhesives by a Postgrafting Reaction, Materials, 2023, 16, 5911, DOI:10.3390/ma16175911.
- P. Karnal, A. Jha, H. Wen, S. Gryska, C. Barrios and J. Frechette, Contribution of Surface Energy to pH-Dependent Underwater Adhesion of an Acrylic Pressure-Sensitive Adhesive, Langmuir, 2019, 35, 5151–5161, DOI:10.1021/acs.langmuir.9b00120.
- M. S. A. Bhuiyan, J. D. Roland, B. Liu, M. Reaume, Z. Zhang, J. D. Kelley and B. P. Lee, In Situ Deactivation of Catechol-Containing Adhesive Using Electrochemistry, J. Am. Chem. Soc., 2020, 142, 4631–4638, DOI:10.1021/jacs.9b11266.
- K. A. Bovaldinova, M. M. Feldstein, N. E. Sherstneva, A. P. Moscalets and A. R. Khokhlov, Thermo-Switchable Pressure-Sensitive Adhesives with Strong Tunable Adhesion Towards Substrate Surfaces of Different Hydrophilicity, Polymer, 2017, 125, 10–20, DOI:10.1016/j.polymer.2017.07.071.
- H. Tachi and K. Suyama, Development of Pressure-Sensitive Adhesives Degradable on Ultrasonic Irradiation, J. Photopolym. Sci. Technol., 2017, 30, 253–257, DOI:10.2494/photopolymer.30.253.
- J. D'Orazio, S. Jarrett, A. Amaro-Ortiz and T. Scott, UV Radiation and the Skin, Int. J. Mol. Sci., 2013, 14, 12222–12248, DOI:10.3390/ijms140612222.
- Z. Wang, L. Guo, H. Xiao, H. Cong and S. Wang, A Reversible Underwater Glue Based on Photo- and Thermo-Responsive Dynamic Covalent Bonds, Mater. Horiz., 2020, 7, 282–288, 10.1039/c9mh01148j.
- P. Lou, X. Li, M. Yao, J. Nie and Y. He, Thermal Debonding Pressure-Sensitive Adhesive Blended with Liquid Crystal Polymers, Prog. Org. Coat., 2023, 185, 107932–107939, DOI:10.1016/j.porgcoat.2023.107932.
- T. Yu, K. Wakuda, D. L. Blair and R. G. Weiss, Reversibly Cross-Linking Amino-Polysiloxanes by Simple Triatomic Molecules. Facile Methods for Tuning Thermal, Rheological, and Adhesive Properties, J. Phys. Chem. C, 2009, 113, 11546–11553, DOI:10.1021/jp900115g.
- R. Pierantozzi, Carbon Dioxide. Kirk-Othmer Encyclopedia of Chemical Technology, 2003. DOI:10.1002/0471238961.0301180216090518.a01.pub2.
- K. Rennert, F. Errickson, B. C. Prest, L. Rennels, R. G. Newell, W. Pizer, C. Kingdon, J. Wingenroth, R. Cooke and B. Parthum, et al., Comprehensive Evidence Implies a Higher Social Cost of CO2, Nature, 2022, 610, 687–692, DOI:10.1038/s41586-022-05224-9.
- A. Darabi, P. G. Jessop and M. F. Cunningham, CO2-Responsive Polymeric Materials: Synthesis, Self-Assembly, and Functional Applications, Chem. Soc. Rev., 2016, 45, 4391–4436, 10.1039/C5CS00873E.
- P. G. Jessop and M. F. Cunningham, CO2-Switchable Materials: Solvents, Surfactants, Solutes and Solids, Royal Society of Chemistry, Cambridge, 2020. 10.1039/9781788012850.
- A. K. Alshamrani, J. R. Vanderveen and P. G. Jessop, A Guide to the Selection of Switchable Functional Groups for CO2-Switchable Compounds, Phys. Chem. Chem. Phys., 2016, 18, 19276–19288, 10.1039/c6cp03302d.
- A. L. Lambuth, Protein Adhesives for Wood, in Handbook of Adhesive Technology, Revised and Expanded, ed. A. Pizzi and K. L. Mittal, Marcel Dekker, 2nd edn, 2003, pp. 435–475 Search PubMed.
- F. A. Keimel, Historical Development of Adhesives and Adhesive Bonding, in Handbook of Adhesive Technology, Revised and Expanded, ed. A. Pizzi and K. L. Mittal, Marcel Dekker, 2nd edn, 2003, pp. 8–21 Search PubMed.
- C. L. Pearson, Animal Glues and Adhesives, in Handbook of Adhesive Technology, Revised and Expanded, ed. A. Pizzi and K. L. Mittal, Marcel Dekker, 2nd edn, 2003, pp. 476–490 Search PubMed.
- M. A. Droesbeke, R. Aksakal, A. Simula, J. M. Asua and F. E. Du Prez, Biobased, Acrylic Pressure-Sensitive Adhesives, Prog. Polym. Sci., 2021, 117, 101396–101407, DOI:10.1016/j.progpolymsci.2021.101396.
- H. Chen, P. Gnanasekar, S. S. Nair, W. Xu, P. Chauhan and N. Yan, Lignin as a Key Component in Lignin-Containing Cellulose Nanofibrils for Enhancing the Performance of Polymeric Diphenylmethane Diisocyanate Wood Adhesives, ACS Sustainable Chem. Eng., 2020, 8, 17165–17176, DOI:10.1021/acssuschemeng.0c05642.
- J. Luo, Y. Zhou, Q. Gao, J. Li and N. Yan, From Wastes to Functions: A New Soybean Meal and Bark-Based Adhesive, ACS Sustainable Chem. Eng., 2020, 8, 10767–10773, DOI:10.1021/acssuschemeng.0c02413.
- Z. Sotoodeh-Nia, A. Hohmann, A. Buss, R. C. Williams and E. W. Cochran, Rheological and physical characterization of pressure sensitive adhesives from bio-derived block copolymers, J. Appl. Polym. Sci., 2018, 135, 46618, DOI:10.1002/app.46618.
- V. R. Patel, G. G. Dumancas, L. C. K. Viswanath, R. Maples and B. J. J. Subong, Castor Oil: Properties, Uses, and Optimization of Processing Parameters in Commercial Production, Lipid Insights, 2016, 9, 1–12, DOI:10.4137/lpi.s40233.
- T. W. Abraham and R. Höfer, Lipid-Based Polymer Building Blocks and Polymers, Polymer Science: A Comprehensive Reference, 2012, vol. 10, pp. 15–58. DOI:10.1016/B978-0-444-53349-4.00253-3.
- B. Berthe, Hydrobromination Method, France, WO2015019028, 2015 Search PubMed.
- A. Horinaka, Preparation of alcohols from carboxylic acids, Japan, JP05271126, 1993 Search PubMed.
- PSTC, Test methods for pressure sensitive adhesive tapes, 2000, pp. 1–133 Search PubMed.
- VA-061, FUJIFILM Wako Chemicals, 2024. https://specchem-wako.fujifilm.com/us/water-soluble-azo-initiators/VA-061.htm (accessed August 28th, 2024) Search PubMed.
- A. Pizzi and K. L. Mittal, Handbook of Adhesive Technology, Revised and Expanded, Marcel Dekker, New York, 2003. DOI:10.1201/9780203912225.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc00630a |
| This journal is © The Royal Society of Chemistry 2025 |