 Open Access Article
Open Access ArticleCO2-Promoted photoredox-catalyzed hydrosulfonylation of alkenes with sulfinates†
Wanhui
Huang
ab,
Ge
Liu
bc,
Fangyuanhang
Yang
 b,
Yuxi
Ren
b,
Yuzhen
Gao
b,
Yuxi
Ren
b,
Yuzhen
Gao
 *abc and
Weiping
Su
*abc and
Weiping
Su
 *abc
*abc
aCollege of Chemistry & Materials Science, Fujian Normal University, Fuzhou 350007, P. R. China
bState Key Laboratory of Structural Chemistry, Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fujian College, University of Chinese Academy of Sciences, Fuzhou, 350002, P. R. China. E-mail: gyz@fjirsm.ac.cn; wpsu@fjirsm.ac.cn
cUniversity of Chinese Academy of Sciences, Beijing, 100049, China
First published on 3rd March 2025
Abstract
Herein, we report a CO2-promoted strategy for the photoredox-catalyzed hydrosulfonylation of alkenes with sulfinates under metal-, acid-, and exogenous reagent-free conditions. This method was compatible with a wide range of functional groups, including complex drug derivatives, making it a versatile and straightforward approach for synthesizing valuable sulfonyl compounds. Notably, CO2, which is an environmentally friendly additive, played an essential role in achieving the products, demonstrating a novel role of CO2 in sulfonylation reactions.
Green foundation1. Carbon dioxide (CO2) is a non-toxic, naturally abundant, stable and low-cost chemical. We demonstrated an approach for the synthesis of highly valuable sulfonyl compounds via CO2-promoted photoredox-catalyzed reactions of alkenes with sodium sulfinates. This method employed low-cost reagents that are readily available and proceeded under metal-, acid- and additional reagent-free conditions. This transformation was significantly influenced by CO2 as no target product was observed when the reaction occurred in the absence of CO2, highlighting the essential role of CO2 in this transformation.2. CO2 is usually used as a versatile C1 synthon for synthesizing valuable chemicals in organic synthesis. Besides, CO2 has proven its remarkable ability to determine the selectivity and reactivity of certain reactions. We presented a “green” concept using CO2 as an essential additive and low-cost reagents that are readily available, and the reaction proceeded under metal-, acid- and additional reagent-free conditions. 3. Unactivated alkenes with a broad range of functional groups (such as hydroxyl, carboxyl, ester, acylamino, ether, silicyl, boryl, terminal halogen, and alkenyl), electron-deficient styrenes, and complex drug derivatives were found to be compatible with the reaction, which will benefit organic chemists to synthesize pharmaceuticals and natural products containing sulfonyl scaffolds and other specific functional groups. |
Organosulfonyl compounds exhibit excellent biological activities and distinct chemical properties, making them extensively utilized in medicine, agriculture, and materials science.1 Compounds with C–sulfonyl bonds are particularly prevalent in drugs and natural products and exhibit antibacterial, anti-inflammatory, antiviral, and anticancer properties (Scheme 1a).1 Consequently, incorporating sulfonyl groups into small molecules is an attractive strategy for drug molecule modifications. Additionally, organosulfonyl compounds can serve as versatile intermediates in organic synthesis to facilitate novel organic transformations.2 Therefore, efficient synthesis of organosulfonyl compounds is of great interest to chemists. Traditional methods for constructing C–sulfonyl bonds, such as sulfonylation with highly reactive nucleophilic reagents3 and Friedel–Crafts-type reactions with sulfonyl halides,4 often require pre-installation of leaving groups, use of strong acids or oxidants and high temperature, which pose challenges related to regioselectivity and functional group compatibility. As a result, developing environmentally friendly and practical methods to synthesize organosulfonyl compounds has become a research hotspot in the fields of organic synthesis and medicinal chemistry.
 | ||
| Scheme 1 (a) Selected bioactive molecules containing sulfone structure. (b) and (c) Photoredox-catalyzed hydrosulfonylation of alkenes. | ||
In organic synthesis, olefin is one of the most abundant and easily accessible feedstock chemicals. Consequently, photo-catalyzed reactions involving olefins and sulfonyl radicals generated from various sulfonyl reagents have emerged as a significant alternative, providing a concise approach for direct sulfonylation to obtain highly valuable sulfonyl compounds.5–11 In 2018, Wu's group achieved photo-induced hydrosulfonylation of alkenes by generating sulfonyl radicals using [DABCO·(SO2)2] (an equivalent SO2 surrogate) with a radical precursor (Scheme 1b, i).7 However, this multicomponent hydrosulfonylation method was only compatible with electron-deficient alkenes. Alternatively, photoredox hydrosulfonylation of alkenes using sulfinates as the radical sources provides a straightforward method to synthesize organosulfonyl compounds (Scheme 1b, ii); however, a noble metal [Ir] and/or excess acid is essential as the catalytic system of this reaction.8 As an important alternative to radical sulfonylation, photoredox-catalyzed hydrosulfonylation of alkenes via hydrogen-atom transfer (HAT)9 (Scheme 1b, iii) or halogen-atom transfer (XAT)10 (Scheme 1b, iv) mechanisms using sulfonyl halides9a–c,10 or sulfonamides9d has also been reported. Notably, an equivalent H-atom donor reagent or XAT reagent is essential for the successful implementation of these transformations. Reactions via an electron donor–acceptor (EDA) pathway without an external photocatalyst have also been demonstrated as efficient protocols for hydrosulfonylation,11 in which a photochemically active EDA complex is generally required. Despite these significant progresses, a general and facile procedure for photoredox radical hydrosulfonylation of alkenes to prepare sulfonyl compounds under metal-, acid- and additional reagent-free conditions is still not achieved.
Carbon dioxide (CO2), which is a non-toxic, naturally abundant, stable and low-cost chemical, can be used as a versatile C1 synthon for synthesizing valuable chemicals in organic synthesis.12,13 Beyond its potential to get converted into value-added products, CO2 has proven its remarkable ability to determine the selectivity and reactivity of certain reactions.14,15 Consequently, in recent years, CO2-promoted reactions are regarded representatives of sustainable approaches and green catalytic chemistry, which is a prosperously developing concept in organic synthesis and has attracted wide attention.
Herein, we demonstrated an approach for the synthesis of highly valuable sulfonyl compounds via a CO2-promoted photoredox-catalyzed reaction of alkenes with sodium sulfinates (Scheme 1c). This method employed low-cost reagents that are readily available and proceeded under metal-, acid- and additional reagent-free conditions. Notably, unactivated alkenes with a broad range of functional groups, electron-deficient styrenes, and complex drug derivatives were compatible with the reaction. As a result, this approach provides a general and straightforward procedure for the synthesis of highly valuable sulfonyl compounds. Notably, consistent with our previous reports,1 this transformation was significantly influenced by CO2 as no target product was observed when the reaction occurred in the absence of CO2, highlighting the essential role of CO2 in sulfonyl radical-involved reactions.
For our investigation, commercially available oct-1-ene (1a) and sodium benzenesulfinate (2a) were chosen as model substrates. As shown in Table 1, the desired sulfonated product 3a was isolated in 95% yield when the reaction was performed under a CO2 atmosphere and catalyzed by 4CzIPN after irradiation using blue LEDs at room temperature (entry 1). Interestingly, the transformation was totally restrained when the reaction was conducted under N2 atmosphere, and most of the substrates remained unreacted, indicating the essential role of CO2 in this reaction (entry 2). Subsequently, control experiments were conducted, and results revealed that both light and photocatalyst (PC) were vital for this transformation (entries 3 and 4), suggesting that the reaction is light-facilitated. A low yield of 3a was afforded when the reaction was carried out in the absence of H2O (entry 5), demonstrating that H2O likely acted as the proton source. Notably, residual crystalline water in substrate 2a might have served as the proton source in this water-free system. Other PCs, such as Ir(ppy)2(dtbbpy)·PF6, Ru(bpy)3Cl2 and Eosin Y, were also tested, and all of them were inferior to 4CzIPN (entries 6–8). After solvent screening, it was found that the choice of solvent played a crucial role in the success of this reaction because the target product 3a could not be obtained or the yield was very low when the reaction was conducted in other solvents, such as CH3CN, DMF, MeOH and DMSO (entries 9–12). This might be attributed to the poor water solubility in other solvents (vs. 1,4-dioxane), limiting efficient protonation, and/or the insufficient redox capability of the excited PCs in other solvents, reducing the single-electron transfer efficiency.
| Entry | Deviation from standard conditions | Yield (%) |
|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol, 1.5 equiv.), 4CzIPN (1 mol%), H2O (5.0 equiv.), 1,4-dioxane (2 mL, 0.1 M), CO2 (1 atm, closed), 5 W × 8 blue LEDs, rt, 16 h. Yield was determined using 1H NMR with CHCl2CHCl2 as the internal standard. b Yield of isolated product shown in parentheses. 4CzIPN: 2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile; ppy: 2-phenylpyridine; dtbbpy: 4,4-di-tert-butyl-2,2′-bipyridine; bpy: 2,2′-bipyridine. | ||
| 1 | None (standard conditions) | 99 (95)b |
| 2 | Under N2 atmosphere | 0 |
| 3 | w/o 4CzIPN | 0 |
| 4 | w/o light | 0 |
| 5 | w/o H2O | 21 |
| 6 | Ir(ppy)2(dtbbpy)·PF6 as PC | 20 |
| 7 | Ru(bpy)3Cl2 as PC | 42 |
| 8 | Eosin Y as PC | 0 |
| 9 | CH3CN as solvent | 13 |
| 10 | DMF as solvent | 0 |
| 11 | MeOH as solvent | 0 |
| 12 | DMSO as solvent | 0 |
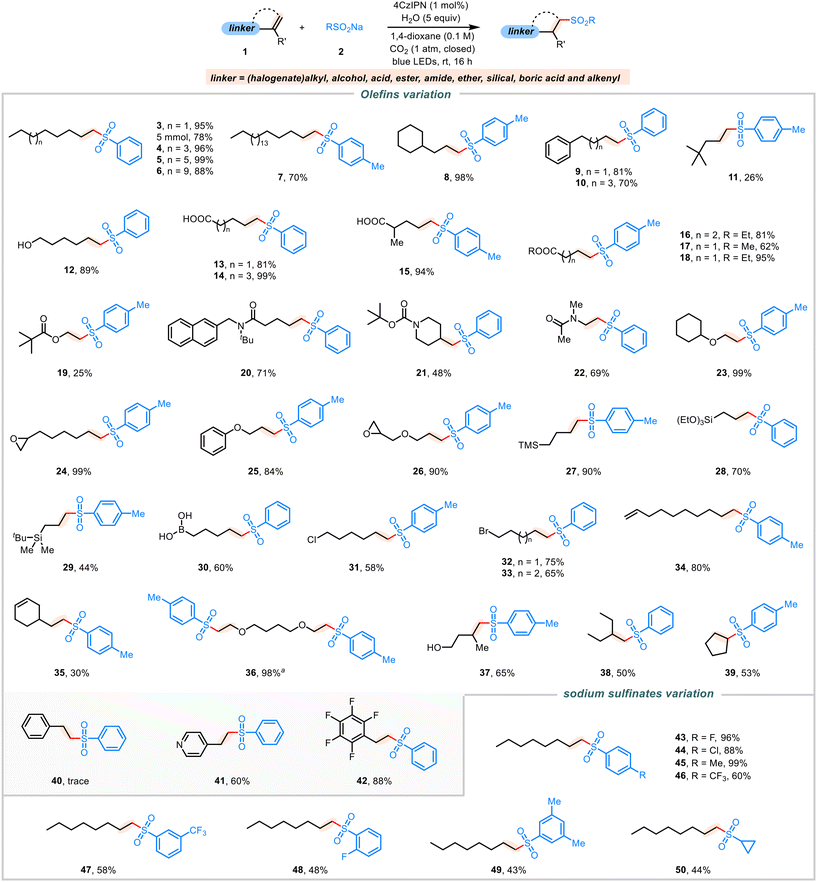
With the optimal reaction conditions in hand, the generality of this transformation was examined. As shown in Scheme 2, a broad range of unactivated alkenes with diverse functional groups were compatible with the reaction. In this transformation, the length of the alkene chain had no significant influence on the yields of products (3–7). All the cyclic (8), phenyl (9, 10) and branched (11) substituents linked to the alkenes were successfully subjected to this reaction, furnishing the desired products in reasonable yields. Active hydrogen did not affect the reaction as all the alkenes containing hydroxyl (12, 37) and carboxyl (13–18) groups efficiently underwent the desired transformation to yield target products in good yields. Furthermore, all the alkenes bearing ester (19), acylamino (20–22) and ether (23–26) groups were proved to be viable substrates to produce corresponding products in moderate to excellent yields. Notably, some sensitive functional groups, such as silicyl (27–29) and boryl (30) remained intact under the standard reaction conditions. Alkenes with terminal chloro (31) or bromo (32, 33) groups also readily accommodated sulfonyl groups in acceptable yields. Substrates with two double bonds were further explored, and the target products (34, 35) were obtained with good selectivity. Notably, the di-sulfonated product 36 was produced in excellent yield when the amount of sulfonate was increased to 2.5 equivalents. β,β-Disubstituted alkenes (37, 38) and an internal alkene (39) were also proved to be suitable substrates. Moreover, alkyl olefins and aryl olefins were examined (40–42). Styrene was incompatible with this reaction (40), but sulfonyl groups were easily introduced in electron-deficient aryl olefins, such as 4-vinylpyridine and penta-fluorovinylbenzene, in good yields (41, 42), indicating that a carbanion intermediate might be involved in this transformation. Subsequently, the scope of sulfinates was also examined. Aryl sulfinates with diverse functional groups (such as fluoro, chloro, methyl and trifluoromethyl) were compatible with this reaction to produce the corresponding products (43–49) in general to good yields. In addition, aliphatic sulfinates, such as sodium cyclopropanesulfinate, readily accommodated sulfonyl groups to deliver the target product 50 in an acceptable yield.
Subsequently, we explored the synthetic potential of this protocol in the late-stage modification of more complex motifs. Both alkenes and sodium sulfinates containing biologically active functional groups were evaluated and proven to be feasible (Scheme 3). Initially, alkenes containing estrone, probenecid, eugenol, cimaterol impurity 3, adamantoic acid, oxaprozin and indomethacin derivatives were examined, and all of them efficiently delivered the desired products (51–57) in moderate to good yields. Furthermore, sulfinates derived from valdecoxib and sildenafil successfully yielded the target products (58 and 59) in acceptable yields.
Several control experiments were performed to investigate the mechanism of this CO2-promoted hydrosulfonylation of unactivated alkenes (Scheme 4). Initially, some radical scavengers, such as 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) or butylated hydroxytoluene (BHT), were added to the reaction mixture under standard reaction conditions, and the reaction was observed to be almost restrained, indicating that a radical pathway might be involved in this reaction (Scheme 4a). Subsequently, ring-opening sulfonated products (62, 63) were isolated in excellent yields when medicinally relevant alkenes, such as nopinene and caryophyllene oxide, were used in this reaction (Scheme 4b). These transformations not only provide an efficient protocol for hydrosulfonylation of medicinally relevant alkenes but also demonstrate that the reaction follows a radical process. The generation of ring-opening product 61 further supported this inference (Scheme 4c). Furthermore, when the reaction was carried out in the presence of D2O under standard reaction conditions, deuterium product d-3 was obtained with a reasonable D/H ratio (Scheme 4d), indicating that a carbanion intermediate might be generated in this reaction. In the methylated reaction system, no carboxylation products were detected during GC-MS and 1H NMR analyses (Scheme 4e), indicating that CO2 did not directly participate as a C1 synthon in this transformation. To further elucidate the role of CO2 in the reaction, additional control experiments were conducted under an N2 atmosphere using alternative acids and buffers (Scheme 4f). When benzoic acid or BF3·Et2O was added, the target product 3 was obtained in acceptable yields (Scheme 4f, entries 1 and 2). To investigate whether CO2 functioned solely via its acidity (via H2CO3 formation, pKa1 ≈ 6.4),16 buffers with comparable pKa values, such as citrate buffer (pKa = 6.4), MES buffer (pKa = 6.1) and phosphate buffer (pKa = 7.2), were employed. Notably, the citrate- and MES-buffer systems did not yield 3 (Scheme 4f, entries 3 and 4), while the phosphate buffer achieved a modest 29% yield (Scheme 4f, entry 5), which was significantly lower than the 95% yield achieved under standard CO2 conditions. These results indicate that CO2 not only generates a weakly acidic microenvironment to facilitate protonation but also potentially modulates the redox properties of the photocatalytic system without being converted into C1-products. Furthermore, light-on–off experiments were carried out to demonstrate that this reaction follows a photoredox-catalyzed radical catalytic pathway instead of a radical chain pathway (details in ESI Fig. 4†).
Based on these mechanistic studies and our previous works, a possible mechanism is proposed (Scheme 4g). Initially, excited PC* is produced upon light irradiation on the PC. Subsequently, a single-electron transfer (SET) process occurred between PC* [Ered1/2 (PC*/PC˙−) = 1.43 V vs. SCE in MeCN for 4CzIPN]17 and phenylsulfinate A (−0.37 V vs. SCE in MeCN for PhSO2Na)18, generating a sulfonyl radical (B) and reduced PC˙− species. Thereafter, the addition of radical B to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond generated a carbon radical intermediate C, which is further reduced by PC˙− to produce the carbanion intermediate D and ground-state PC. Finally, the desired sulfonated products are obtained after protonation from D. It is supposed that CO2 serves a dual role in this transformation, namely, (i) to generate a weakly acidic microenvironment to facilitate protonation and (ii) to potentially modulate the redox properties of the photocatalytic system, ultimately enhancing the reactivity of the sulfonyl radical to ensure efficient electron transfer during the SET process.
C double bond generated a carbon radical intermediate C, which is further reduced by PC˙− to produce the carbanion intermediate D and ground-state PC. Finally, the desired sulfonated products are obtained after protonation from D. It is supposed that CO2 serves a dual role in this transformation, namely, (i) to generate a weakly acidic microenvironment to facilitate protonation and (ii) to potentially modulate the redox properties of the photocatalytic system, ultimately enhancing the reactivity of the sulfonyl radical to ensure efficient electron transfer during the SET process.
Conclusions
In conclusion, we developed a CO2-promoted strategy for photoredox-catalyzed hydrosulfonylation of unactivated alkenes with sulfinates to synthesize highly valuable sulfonyl compounds. A broad range of functional groups, including hydroxyl, carboxyl, ester, acylamino, ether, silicyl, boryl, terminal halogen, and alkenyl, were compatible with this sulfonated transformation, offering a straightforward and clean procedure for the synthesis of sulfonyl products. Notably, the crucial role of CO2 in this transformation highlighted its multifunctional nature via which its acid-promoting and substrate-specific interactions were combined to enable high reactivity under mild conditions. Further CO2-promoted transformations are currently being investigated in our laboratory.Author contributions
Y. Gao conceived and designed the experiments. W. Huang performed the experiments and analysed the data. G. Liu helped analyse the data. F. Yang conducted the X-ray analysis. Y. Gao and W. Su wrote the manuscript, and all authors revised the manuscript.Data availability
All experimental data associated with this study are provided in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 22271285, 21931011), the National Key Research and Development Program of China (2018FYA0704502), the Self-deployment Project Research Program of Haixi Institutes, Chinese Academy of Sciences (CXZX-2022-GH04), and Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China (2021ZZ105).References
- (a) S. Patai, Z. Rappoport and C. J. M. Stirling, The Chemistryof Sulphones and Sulfoxides, Wiley, New York, 1988 Search PubMed; (b) N. A. McGrath, M. Brichacek and J. T. Njardarson, J. Chem. Ed., 2010, 87, 1348 CrossRef CAS; (c) C. L. Percicot, C. R. Schnell, C. Debon and C. Hariton, J. Pharmacol. Toxicol. Methods, 1996, 36, 223 CrossRef CAS PubMed; (d) M. Feng, B. Tang, S. H. Liang and X. Jiang, Curr. Top. Med. Chem., 2016, 16, 1200 CrossRef CAS; (e) F. Zhao, J. Wang, X. Ding, S. J. Shu and H. Liu, Chin. J. Org. Chem., 2016, 36, 490 CrossRef CAS; (f) K. A. Scott and J. T. Njardarson, Top. Curr. Chem., 2018, 376, 5 CrossRef.
- (a) B. M. Trost and C. A. Kalnmals, Chem. – Eur. J., 2019, 25, 11193 CrossRef CAS PubMed; (b) X.-Q. Chu, D. Ge, Y.-Y. Cui, Z.-L. Shen and C.-J. Li, Chem. Rev., 2021, 121, 12548 CrossRef CAS PubMed; (c) M. Nambo, Y. Maekawa and C. M. Crudden, ACS Catal., 2022, 12, 3013 CrossRef CAS.
- (a) B. P. Bandgar, S. V. Bettigeri and J. Phopase, Org. Lett., 2004, 6, 2105 CrossRef CAS PubMed; (b) Y. Fu, W. Zhu, X. Zhao, H. Hügel, Z. Wu, Y. Su, Z. Du, D. Huang and Y. Hu, Org. Biomol. Chem., 2014, 12, 4295 RSC; (c) A. G. Tathe and N. T. Patil, Org. Lett., 2022, 24, 4459 CrossRef CAS PubMed.
- (a) S. Répichet, C. Le Roux, P. Hernandez, J. Dubac and J.-R. Desmurs, J. Org. Chem., 1999, 64, 6479 CrossRef; (b) D. O. Jang, K. S. Moon, D. H. Cho and J.-G. Kim, Tetrahedron Lett., 2006, 47, 6063 CrossRef CAS; (c) N.-W. Liu, S. Liang and G. Manolikakes, Synthesis, 2016, 1939 CAS.
- For selected reviews, see: (a) S. Ye, G. Qiu and J. Wu, Chem. Commun., 2019, 55, 1013 RSC; (b) S. Zhao, K. Chen, L. Zhang, W. Yang and D. Huang, Adv. Synth. Catal., 2020, 362, 3516 CrossRef CAS; (c) D. Zeng, M. Wang, W.-P. Deng and X. Jiang, Org. Chem. Front., 2020, 7, 3956 RSC; (d) D.-Q. Dong, Q.-Q. Han, S.-H. Yang, J.-C. Song, N. Li, Z.-L. Wang and X.-M. Xu, ChemistrySelect, 2020, 5, 13103 CrossRef CAS; (e) D. Joseph, M. A. Idris, J. Chen and S. Lee, ACS Catal., 2021, 11, 4169 CrossRef CAS; (f) S. Liang, K. Hofman, M. Friedrich, J. Keller and G. Manolikakes, ChemSusChem, 2021, 14, 4878 CrossRef CAS.
- Y. Chen, N. McNamara, O. May, T. Pillaiyar, D. C. Blakemore and S. V. Ley, Org. Lett., 2020, 22, 5746 CrossRef CAS PubMed.
- (a) T. Liu, Y. Li, L. Lai, J. Cheng, J. Sun and J. Wu, Org. Lett., 2018, 20, 3605 CrossRef CAS PubMed; (b) X. Wang, M. Yang, W. Xie, X. Fan and J. Wu, Chem. Commun., 2019, 55, 6010 RSC.
- (a) J.-J. Wang and W. Yu, Org. Lett., 2019, 21, 9236 CrossRef CAS PubMed; (b) Y. Zheng, Y. You, Q. Shen, J. Zhang, L. Liu and X.-H. Duan, Org. Chem. Front., 2020, 7, 2069 RSC.
- (a) S. M. Hell, C. F. Meyer, A. Misale, J. B. I. Sap, K. E. Christensen, M. C. Willis, A. A. Trabanco and V. Gouverneur, Angew. Chem., Int. Ed., 2020, 59, 11620–11626 CrossRef CAS PubMed; (b) S. T. Shreiber and G. A. Molander, Org. Lett., 2023, 25, 2084 CrossRef CAS PubMed; (c) C.-M. Li, X.-X. Dong, Z. Wang and B. Zhang, Green Chem., 2023, 25, 4122 RSC; (d) M. J. Tilby, D. F. Dewez, L. R. E. Pantaine, A. Hall, C. Martínez-Lamenca and M. C. Willis, ACS Catal., 2022, 12, 6060 CrossRef CAS PubMed.
- X. Wu and B. Gao, Org. Lett., 2023, 25, 8722 CrossRef CAS.
- (a) M. Kim, E. You, S. Park and S. Hong, Chem. Sci., 2021, 12, 6629 RSC; (b) P. Renzi, E. Azzi, S. Ascensio, S. Parisotto, F. Sordello, F. Pellegrino, G. Ghigo and A. Deagostino, Chem. Sci., 2023, 14, 2721 RSC; (c) Y. Song, C. Li, X. Hu, H. Zhang, Y. Mao, X. Wang, C. Wang, L. Hu and J. Yan, Green Chem., 2024, 26, 6578 RSC; (d) J. D. Lasso, D. J. Castillo-Pazos, J. M. Salgado, C. Ruchlin, L. Lefebvre, D. Farajat, D. F. Perepichka and C.-J. Li, J. Am. Chem. Soc., 2024, 146, 2583 CrossRef CAS PubMed.
- For selected reviews, see: (a) K. Huang, C.-L. Sun and Z.-J. Shi, Chem. Soc. Rev., 2011, 40, 2435 RSC; (b) Y. Yang and J.-W. Lee, Chem. Sci., 2019, 10, 3905 RSC; (c) C. S. Yeung, Angew. Chem., Int. Ed., 2019, 58, 5492 CrossRef CAS PubMed; (d) Z. Fan, Z. Zhang and C. Xi, ChemSusChem, 2020, 13, 6201 CrossRef CAS PubMed; (e) X. He, L.-Q. Qiu, W.-J. Wang, K.-H. Chen and L.-N. He, Green Chem., 2020, 22, 7301 RSC; (f) J. H. Ye, T. Ju, H. Huang, L. L. Liao and D.-G. Yu, Acc. Chem. Res., 2021, 54, 2518 CrossRef CAS PubMed; (g) L. Guo, K. J. Lamb and M. North, Green Chem., 2021, 23, 77 RSC; (h) A. J. Davida and A. Das, Green Chem., 2025, 27, 851 RSC.
- For recent selected examples, see: (a) W. Li, B. Sun, L. Zhang and F. Mo, Green Chem., 2023, 25, 5030 RSC; (b) H. Huang, X. Lin, F. Yang, Y. Ren, Y. Gao and W. Su, Org. Lett., 2024, 26, 11195 CrossRef CAS PubMed; (c) F. Zhang, X.-Y. Wu, P.-P. Gao, H. Zhang, Z. Li, S. Ai and G. Li, Chem. Sci., 2024, 15, 6178 RSC; (d) Y. Liu, G.-H. Xue, Z. He, J.-P. Yue, M. Pan, L. Song, W. Zhang, J.-H. Ye and D.-G. Yu, J. Am. Chem. Soc., 2024, 146, 28350 CAS; (e) Y. Yia and C. Xi, Green Synth. Catal., 2024 DOI:10.1016/j.gresc.2024.09.003.
- For selected reviews, see: (a) P. K. Sahoo, Y. Zhang and S. Das, ACS Catal., 2021, 11, 3414 CrossRef CAS; (b) Z. Zhang and C. Xi, ChemCatChem, 2025, 17, e202401834 CrossRef CAS; (c) W. Schilling and S. Das, Tetrahedron Lett., 2018, 59, 3821 Search PubMed.
- For selected examples, see: (a) G. Pupo, R. Properzi and B. List, Angew. Chem., Int. Ed., 2016, 55, 6099 Search PubMed; (b) J. Ye, I. Kalvet, F. Schoenebeck and T. Rovis, Nat. Chem., 2018, 10, 1037 CrossRef CAS PubMed; (c) D. Riemer, W. Schilling, A. G. Götz, Y. Zhang, S. Gehrke, I. Tkach, O. Hollóczki and S. Das, ACS Catal., 2018, 8, 11679 CrossRef CAS; (d) M. Kapoor, P. Chand-Thakuri and M. C. Young, J. Am. Chem. Soc., 2019, 141, 7980 CrossRef CAS PubMed; (e) Y. Zhao, X. Guo, S. Li, Y. Fan, G.-C. Ji, M. Jiang, Y. Yang and Y.-Y. Jiang, Angew. Chem., Int. Ed., 2022, 61, e202213636 CrossRef CAS PubMed; (f) Z. Fan, S. Chen, S. Zou and C. Xi, ACS Catal., 2022, 12, 2781 CrossRef CAS; (g) S. Chen and C. Xi, Green Chem., 2023, 25, 7978 RSC; (h) Z. Zhang, D. Li and C. Xi, Org. Lett., 2023, 25, 698 CrossRef CAS PubMed; (i) Y. Gao, S. Liu and W. Su, Green Chem., 2023, 25, 7335 RSC; (j) G. Archer, R. Meyrelles, I. Eder, N. Kovács, B. Maryasin, M. Médebielle and J. Merad, Angew. Chem., Int. Ed., 2023, e202315329 Search PubMed; (k) G. Liu, D. Ma, J. Zhang, F. Yang, Y. Gao and W. Su, Nat. Commun., 2024, 15, 10153 CrossRef CAS PubMed; (l) S. Dotzauer, G. B. Hadaf, F. S. Kamounah, A. Kadziola and J.-W. Lee, Catalysts, 2020, 10, 1481 CrossRef CAS.
- A. P. Miller, Lange's Handbook of Chemistry, in Am J Public Health N, 4th edn, 1941, vol. 31, p. 1324 Search PubMed.
- E. Speckmeier, T. G. Fischer and K. Zeitler, J. Am. Chem. Soc., 2018, 140, 15353 CrossRef CAS.
- A. U. Meyer, S. Jäger, D. P. Hari and B. König, Adv. Synth. Catal., 2015, 357, 2050 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2367386 and 2367416. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5gc00057b |
| This journal is © The Royal Society of Chemistry 2025 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sodium 4-methylbenzenesulfinate (0.5 mmol, 2.5 equiv.).
Sodium 4-methylbenzenesulfinate (0.5 mmol, 2.5 equiv.).
