 Open Access Article
Open Access Article3D printable lignin-caprolactone material†
Banchamlak Bemerw
Kassaun
ab,
Luyao
Wang
b,
Oskar
Backman
b,
Chunlin
Xu
 *b and
Pedram
Fatehi
*b and
Pedram
Fatehi
 *ab
*ab
aDepartment of Chemical Engineering, Lakehead University, 955 Oliver Road, Thunder Bay, ON P7B 5E1, Canada. E-mail: pfatehi@lakeheadu.ca
bLaboratory of Natural Materials Technology, Faculty of Science and Engineering, Åbo Akademi University, Henrikinkatu 2, Turku, FI-20500, Finland. E-mail: chunlin.xu@abo.fi
First published on 19th February 2025
Abstract
The use of lignin in three-dimensional (3D) printing materials has been considered a viable strategy to generate sustainable 3D printing objects. However, complex molecular structures, high viscosity, and charring of lignin impair its 3D printability. This study investigated the synthesis of lignin-caprolactone polymer and its fused deposition modeling (FDM)-3D printing performance. Lignin-caprolactone polymerization was carried out with ethanol-soluble fractionated birch alkali lignin (LE) and caprolactone (CL). Results showed that ethanol fractionation reduced lignin's molecular weight from 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 to 3827 g mol−1 and increased its hydroxyl group concentration. The melt temperature, viscosity, and polymerization degree were considered in the Box-Behnken surface approach to obtain lignin-caprolactone with the best results. Compared to unfractionated lignin caprolactone (LPO), fractionated lignin-caprolactone polymer (LEPO) had a 10.9% higher grafting ratio, 69.43% rise in melt temperature (Tm), and 85.71% increase in glass transition temperature. The melt rheological investigation showed that LEPO's lower viscosity (160.9 Pa s) and shear-thinning behavior than those of LPO made it more suitable for the 3D printing application. The 15 °C delay in G′ and G′′ crossover points of LEPO compared to LPO improved 3D printing adhesion layers. Furthermore, LEPO exhibited superior mechanical characteristics and a greater water contact angle (92°) than LPO. The reduction in molecular weight distribution of lignin (due to ethanol fractionation) prior to copolymerization facilitated the production of a 3D-printable polymer containing 75% lignin. By tailoring the melt and viscosity parameters of the lignin-caprolactone copolymer, the lignin-copolymer exhibited improved 3D printing performance, which offers advantages over lignin composite 3D printing.
870 to 3827 g mol−1 and increased its hydroxyl group concentration. The melt temperature, viscosity, and polymerization degree were considered in the Box-Behnken surface approach to obtain lignin-caprolactone with the best results. Compared to unfractionated lignin caprolactone (LPO), fractionated lignin-caprolactone polymer (LEPO) had a 10.9% higher grafting ratio, 69.43% rise in melt temperature (Tm), and 85.71% increase in glass transition temperature. The melt rheological investigation showed that LEPO's lower viscosity (160.9 Pa s) and shear-thinning behavior than those of LPO made it more suitable for the 3D printing application. The 15 °C delay in G′ and G′′ crossover points of LEPO compared to LPO improved 3D printing adhesion layers. Furthermore, LEPO exhibited superior mechanical characteristics and a greater water contact angle (92°) than LPO. The reduction in molecular weight distribution of lignin (due to ethanol fractionation) prior to copolymerization facilitated the production of a 3D-printable polymer containing 75% lignin. By tailoring the melt and viscosity parameters of the lignin-caprolactone copolymer, the lignin-copolymer exhibited improved 3D printing performance, which offers advantages over lignin composite 3D printing.
Green foundation1. This work introduces a simple molecular weight upgrading and a solvent-free polymerization reaction (i.e., a green process) to fabricate a sustainable polymer (lignin-caprolactone, LCP, polymer) with outstanding characteristics that are suitable for 3D printing.2. This work illustrates that, by fractionating lignin and controlling its molecular weight, it is possible to generate LCP that contains a large proportion of lignin derivative (>75 wt%) and has excellent thermal and rheological properties, which can be readily used for 3D printing excluding the necessity of other chemicals in the 3 D printing formulation. 3. In future work, other lignin fractionation processes may be explored to mimic the molecular weight of lignin so that a larger proportion of lignin can be used in 3D printing material fabrication. |
1. Introduction
Fused deposition modeling (FDM) is a widely used extrusion-based technique for fabricating 3D printing materials.1,2 Generally, rheological properties, thermal conductivity, and shear rate influence polymeric materials’ printability via FDM.3 Currently, acrylonitrile butadiene styrene, polyethylene terephthalate, polycarbonate, polyether ether ketone, polypropylene, polylactic acid, and nylon are commonly used in FDM-3D printing.4,5 Despite their high efficiency, they are environmentally unfriendly and expensive.2 Thus, the incentive for generating sustainable 3D printing material is high.The use of various biomass materials in 3D printing, such as cellulose and hemicellulose, has gained attention due to their renewable nature and potential to reduce environmental impacts.6,7 These materials have been utilized to produce biodegradable composites for fabricating 3D printed components, which provide advantages for sustainable manufacturing.8 Nevertheless, the usability of cellulose and hemicellulose in 3D printing in the FDM technique is limited owing to their low-temperature stability, moisture sensitivity, weak mechanical properties, and challenges in extrusion caused by their fibrous nature.9 Thanks to its high thermal stability, moisture resistance, higher mechanical properties, antioxidant, antibacterial, and biodegradable properties, lignin, i.e., a plentiful and sustainable biopolymer, has gained attention for fabricating sustainable 3D printing materials.10,11
Lignin has traditionally been underutilized and dominantly burnt as an energy source.12 Its rich aromatic structure makes it suitable for creating valuable products, such as bio-adhesives, bioplastics, and carbon fibers.13,14 Nevertheless, the complex structure of lignin, its undesired thermal properties (e.g., its ability to char at high temperatures), and rheological properties (e.g., shear thickening behavior, which causes increased resistance to flow or deformation) have hindered its application in 3D printing applications.15–17 For this reason, the application of lignin in 3D printing via FDM is limited to lignin being blended at a low quantity (e.g., 2–40%) with other polymers, such as acrylonitrile-butadiene-styrene (ABS), polylactic acid (PLA) and polyamide (PA).17–20
The source and extraction process of lignin also influences its applicability in FDM 3D printing. Alkali hardwood lignin is especially beneficial compared to the softwood kraft lignin because of its less structural variability, which mitigates the risk of nozzle blockage.21,22 For example, Nguyen et al. mixed hardwood lignin and thermoplastic nylon 12 and reported improved rigidity and decreased melting viscosity of lignin for use as a 3D printing material.17 In another study, an organosolv hardwood lignin exhibited high compatibility with polylactic acid (PLA), and its 15 wt% lignin-loaded composition exhibited outstanding printability via the FDM process.15 While incorporating lignin into polymer blends offers advantages, the exceptional properties (e.g., mechanical stability, UV protection, and antioxidant) of lignin in the blend are constrained by the percentage of lignin (40 wt% max) in the matrix.23,24 Additionally, the blending process frequently results in phase separation, further restricting the ultimate characteristics of the printed material. One way to widen the use of lignin in a 3D printable formulation is to crosslink lignin and other thermoplastic polymers to improve their thermal and rheological properties. The performance of polymerized lignin in 3D printing applications has yet to be explored. The present work examined polymerizing lignin with caprolactone (CL) to generate sustainable 3D printing material as the first objective.
Polycaprolactone (PCL) is a biodegradable polyester with a low melting point of approximately 60 °C and a glass transition temperature of roughly −60 °C.25,26 PCL is a favorable thermoplastic polymer for producing FDM scaffolds for tissue engineering applications due to its biocompatibility, biodegradability, low melting temperature, low glass transition temperature, and high thermal stability.27,28 Generally, carbon nanotubes, pristine graphene, and tricalcium phosphate are included in the PCL matrix to improve the mechanical properties and structural integrity of PCL materials.29–31 However, these fillers would limit their biodegradability and biocompatibility.32 In the past, caprolactone (CL), the monomer for PCL, was polymerized with kraft lignin to produce thermoplastic materials by compression molding with PCL.33 However, the report did not elaborate on the reaction fundamentals and the molding characteristics of the lignin-caprolactone polymer. As the characteristics of such polymers would significantly impact their 3D printing affinity, the second objective of this study was to understand the polymerization reaction system to achieve a sustainable polymer with the best printing performance.
In this study, the lignin was fractionated by ethanol to reduce its molecular weight before polymerizing with CL.34,35 Ethanol fractionation is chosen for upgrading lignin properties (e.g., molecular weight distribution) due to its simplicity, less severe process conditions, and the specific solubility of lignin in ethanol, as opposed to other techniques, such as lignin depolymerization and acid catalytic upgrading.36–40 This study presents a ring-opening polymerization of birch alkali lignin (L) and CL to produce a 3D printable polymer with exceptional flow characteristics. The properties of L were improved using ethanol fractionation, and the ethanol soluble fractionated lignin (LE) resulted in a lower molecular weight than L, which was then used to fabricate lignin-caprolactone polymer with best properties (LEPO) following the Box-Behnken (BBD) response surface method (RSM). The printability of the selected sample was assessed by running melt rheology. Then, the 3D printing methods of FDM were employed to study the 3D printing performance of the generated sustainable material. The mechanical strength, surface properties, and appearance of 3D-printed structures were evaluated comprehensively. This work demonstrated a promising approach to utilizing polymerized lignin for a microstructurally well-organized 3D printed material fabrication.
2. Method and materials
2.1. Materials
Birch alkali lignin (BL) was supplied by CH-Bioforce Oy (Espoo, Finland), ethanol (96%), hexane (99%), ε-caprolactone (CL), dibutyltin dilaurate (DBTDL, 95%), methanol (95%), dimethyl sulfoxide (DMSO-d6), chloroform (CDCl3), anhydrous pyridine, endo-N-hydroxy-5-norbornene-2,3-dicarboximide, chromium(III) acetylacetonate (Cr(acac)3), 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (Cl-TMDP), dimethyl sulfoxide-d6 (DMSO-d6), chloroform-d containing 0.03%, tetramethylsilane (TMS), and polycaprolactone (PCL, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) were purchased from Sigma Aldrich.
000 g mol−1) were purchased from Sigma Aldrich.
2.2. Ethanol fractionation of birch alkali lignin
Birch alkali lignin (L) was fractionated using ethanol. The amount of solvent and lignin was set to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt/wt (70 g of ethanol for 10 g of lignin). After stirring for 2 h, the insoluble and soluble parts of lignin were separated using a vacuum filter. The ethanol-soluble part of lignin (LE) was taken and treated by a rotary evaporator to separate lignin from ethanol. Subsequently, samples were collected, washed with hexane (20 mL) to remove the extractives, and dried in a 60 °C oven for 48 hours. Eqn (S1) (ESI)† was used to measure the yield of the lignin fractionation process.
1 wt/wt (70 g of ethanol for 10 g of lignin). After stirring for 2 h, the insoluble and soluble parts of lignin were separated using a vacuum filter. The ethanol-soluble part of lignin (LE) was taken and treated by a rotary evaporator to separate lignin from ethanol. Subsequently, samples were collected, washed with hexane (20 mL) to remove the extractives, and dried in a 60 °C oven for 48 hours. Eqn (S1) (ESI)† was used to measure the yield of the lignin fractionation process.
2.3. Experimental design by box-Behnken (BBD)- response surface method (RSM) for lignin-caprolactone (LEP) polymerization
The experiments for the polymerization of lignin and caprolactone (LEP) were designed using response surface methodology (RSM) based on a box-Behnken design (BBD). The BBD-RSM approach was used to evaluate the impact of three independent factors on the LEP polymer's viscosity and melt temperature. To accomplish this goal, the influence of three experimental factors (the ratio of caprolactone monomer to the hydroxyl group of lignin (CL/OH), the concentration of catalyst, and the reaction duration) was assessed. These factors were evaluated at three levels: low, medium, and high, represented by coded values of −1, 0, and +1, respectively, in Table S1.† To achieve this objective, 17 experiments were devised using the Box-Behnken Design (BBD) methodology. All second-order regression coefficients were determined by the statistical analysis of the findings using the ANOVA technique. Afterward, the best reaction conditions were determined by merging the acquired outcomes and graphing an equation for each response variable. The statistical analysis of multivariable equations, derivation of coefficients for a second-order regression model, and the examination of the impacts of factors on variables were conducted using design expert statistical software (Version 12, State-Ease Inc, USA). All BBD-RSM equations are listed in eqn (S2)–(S5).†2.4. Polymerization of lignin and caprolactone
Lignin and caprolactone polymerization generates lignin caprolactone polymer (LEP) without the need for a solvent.33 Caprolactone was used as a monomer for ring-opening polymerization (ROP), lignin was used as a micro initiator, and DBTDL was used as a catalyst. The LE was mixed with CL based on the CL/OH ratio of lignin (as listed in Table S1†) at 50 °C under nitrogen and stirred for 30 min. DBTDL (based on the wt% ratio of lignin listed in Table S1†) was added slowly to the mixture, which was heated to 150 °C under magnetic stirring (250 rpm). ROP is a form of chain-growth polymerization in which the terminal end of a polymer chain acts as a reactive center where further cyclic monomers (CL) can react by opening its ring structure and forming a longer polymer chain. The –COO– group is formed via the opening of the CL ring and reacts with the OH group of lignin to form an ester linkage, leaving –OH at the end of the CL chain, which subsequently reacts with CL monomers to grow a PCL (Fig. S1b†). When the reaction was complete (for the durations listed in Table S1†), the product was cooled to room temperature, and the mixture was washed several times with cool methanol to remove impurities. The overall schematics of the reaction are described in Fig. S1a.† The reaction conditions for the samples detailed in the main documents, LEP2, LEP5, and LEP6, are as follows: CL/OH ratios of 2.6, 1.73, and 0.83 mmol g−1, respectively; a reaction time of 420 minutes for all; and catalyst concentrations of 0.5, 0.75, and 1 wt% based on lignin content, respectively (Table S2†). The optimized conditions were a CL/OH ratio (mmol g−1) of 1.1, a reaction time of 420 min, and a catalyst concentration of 1 wt% based on lignin amount (Table S2†), which generated the sample labeled as LEP. A control sample with unfractionated lignin (L) was produced under the same conditions as LEPO and labeled as LPO.2.5. Lignin filament extrusion
Samples prepared under the optimization conditions were used to generate filaments. The lignin caprolactone polymer filament was generated using ethanol-fractionated lignin copolymer under optimized conditions (LEP), and the control sample was generated by unfractionated lignin under the same conditions and labeled as LP. The LEP and LP were extruded using a compounding twin extrusion machine (Xplore MC15HT). The extrusion was conducted at 100 °C for LPO and 80 °C for LEPO at a speed of 5 rpm to generate filaments with a 1.5–1.7 mm diameter.2.6. 3D printing of LPO and LEPO polymers
The printability of LPO and LEPO was assessed by the fused deposition modeling (FDM) method using extrusion-based 3D printing (Printer One, BRINTER Ltd, Finland) equipped with a 0.55 mm nozzle diameter. The samples were prepared as a pallet by chopping the prepared filaments and fed to a granular tool printhead with the temperature set at 100 °C for LPO and 80 °C for LEPO. The print bed was prepared by coating a tin PCL layer for better grip throughout the study. An eight-layered honeycomb structure was printed at a speed of 8 mm s−1, pressure of 200 mbar, layer height of 0.2 mm, and shell thickness of 0.5 mm.2.7. Characterization of lignin and lignin-caprolactone polymers
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) that contained relaxation reagent of chromium(III) acetylacetone (Cr(acsac)3, 1.3 μmol) and internal standard of endo-N-hydroxy-5-norbornene-2-3-dicarboximide (12 μmol). The ratio of internal standard to lignin was set to 0.6 mmol g−1. The hydroxyl groups were phosphorylated with 0.1 mL of 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane for 30 min before 31P-NMR measurement. The concentration of Cr(acac)3 was 0.002 M to ensure the complete relation of the phosphorus nuclei before applying the radiofrequency pulse. Spectrum acquisition parameters were a 10 s pulse delay, 2.0 s acquisition time, and 64 scans. The hydroxyl group was used to calculate the grafting percentage of CL on the hydroxyl group of lignin according to eqn (S6).†1H-NMR and HSQC NMR analyses were performed on L, LE, LEP, LPO, and LEPO samples. The 1H-NMR setup was set to 16 scans, 3.28 s acquisition, 1 s relaxation, and 90° pulse at room temperature. L and LE samples were prepared by dissolving the vacuum oven-dried (40 °C) samples in 80 mg mL−1 DMSO, while LEP and LPO were prepared by dissolving 80 mg samples in 0.75 mL of CDCl3 (0.03 v/v% of TMS). The HSQC NMR spectra were acquired using the HSQCEDETGPSISP2.3 pulse sequence with a relaxation delay of 2.0 s and an acquisition time of 0.15 s, recording 256 increments of 80 scans per increment. The results were assessed using Top Spin 4.02 software. The 1H-NMR spectra of the LEPO and LPO were used to quantify the degree of polymerization (n) in the PCL chain segments, following eqn (S7).† The major linkages of L and LE were quantified according to eqn (S8) and (S9).†
1, v/v) that contained relaxation reagent of chromium(III) acetylacetone (Cr(acsac)3, 1.3 μmol) and internal standard of endo-N-hydroxy-5-norbornene-2-3-dicarboximide (12 μmol). The ratio of internal standard to lignin was set to 0.6 mmol g−1. The hydroxyl groups were phosphorylated with 0.1 mL of 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane for 30 min before 31P-NMR measurement. The concentration of Cr(acac)3 was 0.002 M to ensure the complete relation of the phosphorus nuclei before applying the radiofrequency pulse. Spectrum acquisition parameters were a 10 s pulse delay, 2.0 s acquisition time, and 64 scans. The hydroxyl group was used to calculate the grafting percentage of CL on the hydroxyl group of lignin according to eqn (S6).†1H-NMR and HSQC NMR analyses were performed on L, LE, LEP, LPO, and LEPO samples. The 1H-NMR setup was set to 16 scans, 3.28 s acquisition, 1 s relaxation, and 90° pulse at room temperature. L and LE samples were prepared by dissolving the vacuum oven-dried (40 °C) samples in 80 mg mL−1 DMSO, while LEP and LPO were prepared by dissolving 80 mg samples in 0.75 mL of CDCl3 (0.03 v/v% of TMS). The HSQC NMR spectra were acquired using the HSQCEDETGPSISP2.3 pulse sequence with a relaxation delay of 2.0 s and an acquisition time of 0.15 s, recording 256 increments of 80 scans per increment. The results were assessed using Top Spin 4.02 software. The 1H-NMR spectra of the LEPO and LPO were used to quantify the degree of polymerization (n) in the PCL chain segments, following eqn (S7).† The major linkages of L and LE were quantified according to eqn (S8) and (S9).†
3. Results and discussion
3.1. Ethanol fractionation of birch alkali lignin
The chemical structure of lignin substantially impacts the characteristics of the material incorporating it.43 Therefore, the chemical structure, molecular weight, and thermal properties of birch alkali lignin, unfractionated (L) and ethanol fractionated (LE), were assessed and reported in Fig. 1. The molar mass distributions of L and LE are illustrated in Fig. 1a. The molecular weight (Mw) and polydispersity (ĐM) values are displayed in Table 1. The LE exhibited a significantly lower Mw and ĐM than L. The reduced Mw and ĐM result from ethanol fractionation, which solubilized part of lignin fragments.35,44,45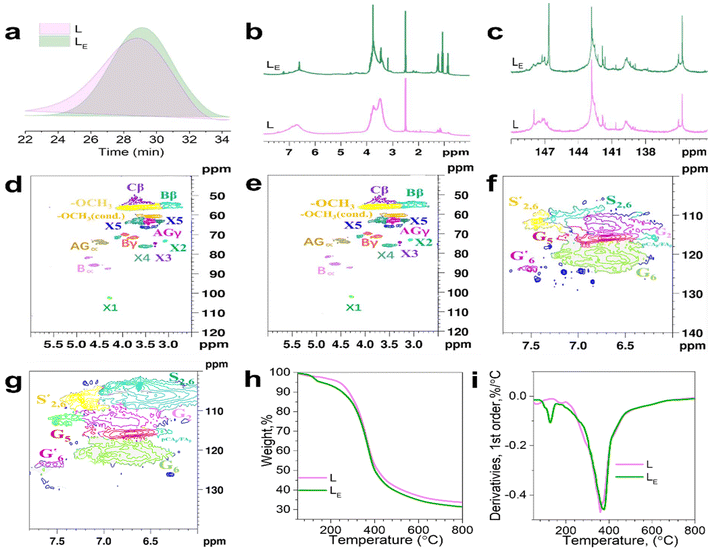 | ||
| Fig. 1 Molar mass distribution (a), 1H NMR (b), 31P NMR (c), HSQC spectra of oxygenated aliphatic linkages (d and e), aromatic linkages (f and g) of L and LE, and TGA (h) and DTG (i) of L and LE. | ||
| Sample | Yield (wt%) | Grafting (%) | Molar mass characteristics | Lignin hydroxyl group amount (mmol g−1) | The concentration of interunit linkages (% of C9 units) | Thermal characteristics (°C) | DP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mw (g mol−1) | Đ M | Total phenolic-OH | Aliphatic-OH | Carboxylic-OH | β-O-4 | β–β | β-5 | T o | T 50% | DTGmax | T g | T m | ||||
| L | 100 | — | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 870 |
2.98 | 2.61 | 1.38 | 0.59 | 229.4 | 74.1 | 41.8 | 239 | 416 | 360 | 120 | — | — |
| LE | 54.2 | — | 3827 | 1.00 | 2.77 | 1.44 | 0.64 | 64.3 | 27.8 | 64.3 | 123 | 398 | 380 | 111.5 | — | — |
| LEP2 | — | 48.6 | 141![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
— | 1.25 | 0.87 | 0.36 | — | — | — | 257.8 | 394.2 | 400 | −20 | 50 | 7.02 |
| LEP5 | — | 33.6 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 340 |
— | 1.71 | 1.04 | 0.47 | — | — | — | 257.8 | 404.8 | 395 | −22 | 52 | 5.91 |
| LEP6 | — | 25.6 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 880 |
— | 2.51 | 1.00 | 0.40 | — | — | — | 266.9 | 398.4 | 395 | −20 | 53 | 4.60 |
| LEPO | — | 26.6 | 402![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
— | 2.13 | 0.95 | 0.56 | — | — | — | 292.2 | 396.8 | 396.7 | −26 | 49 | 4.97 |
| LPO | — | 15.7 | 728![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
— | 2.17 | 0.97 | 0.69 | — | — | — | 287.0 | 391.7 | 396.6 | −14 | 47 | 5.16 |
The 1H NMR spectra of L and LE polymers are presented in Fig. 1b. The spectra revealed that aromatic protons appeared in the region of 6.0–7.5 ppm, methoxy proton at 3.75 ppm, aliphatic proton at 0.85–2.2 ppm, and DSMO solvent at 2.5 ppm.39 LE showed stronger aliphatic signals that belonged to ethanol residues. The quantity of aliphatic, guaiacyl, C5-substituted, and carboxylic hydroxyl groups in L and LE was determined by quantitative 31P NMR analysis (Fig. 1c), and the hydroxyl group concentration of L and LE are listed in Table 1. Phenolic hydroxyl groups substantially rose compared to aliphatic and carboxylic hydroxyl groups for LE compared to L. The C5-substituted hydroxyl groups exhibited the most significant rise from the constituents of phenolic hydroxyl groups. In addition, the interunit linkages and substructures of L and LE were analyzed by HSQC NMR to understand the changes in lignin structure further. The spectra are presented in Fig. 1(d–g), and the δC/δH ppm are presented in Table S3.† Quantitative HSQC was used to evaluate the inter-unit linkages in L and LE using the guaiacol (G2) and syringyl (S2,6) signals as an internal standard described according to eqn (S8) and (S9),† and values are listed in Table 1.46 The oxygenated aliphatic region (δC/δH 40–120/2.5–6.0) of L and LE showed signals presented in Fig. 1(d and e). The dominant interunit linkages in L and LE were the diacylglycerol-β-aryl ether link (β-O-4′) followed by pinoresinol (β–β′) and minor amounts of phenylcoumaran (β-5′). Relative to L, the LE interunit linkages showed a 165% reduction, which was prominent for β-O-4 (Table 1). This reduction could result from the breakage of β-O-4 linkages due to the ethanol fractionation.47 This is further supported by the increased phenolic hydroxyl group content from 31P NMR analysis (Fig. 1c). Furthermore, the aromatic linkages (δC/δH 100–140/6.0–8) were analyzed, and the results are presented in Fig. 1(f and g). The results showed a vigorous intensity of the S units in LE compared to L. The increase in the S units is consistent with the result of P-NMR, where the phenolic hydroxyl group showed an increase.
The thermal properties of L and LE were assessed using TGA and DSC to understand the thermal alteration of lignin after ethanol fractionation. The TGA and DTG curves are presented in Fig. 1(h and i). The results indicated that the LE had a lower To (onset temperature) and T50% (the temperature at which 50% of the weight was lost) than L (Table 1). However, the DTGmax of LE was 20 °C higher than L. The lower molecular weight of LE than L could be the reason for the lower To and T50%. The increased phenolic hydroxyl group of LE contributed to the higher DTGmax due to the higher frequency of intermolecular hydrogen bond interactions of phenolic hydroxyl groups.48 The glass transition (Tg) of L and LE were analyzed using DSC, and the results are presented in Table 1. In general, the Tg of LE was lower than L, which is attributed to the reduced molecular weight, narrower molecular weight distribution (Table 1), and change in the hydroxyl group of LE, all of which would increase the chain mobility and thus reduce Tg.
3.2. Lignin and caprolactone polymerization via ring opening polymerization (ROP)
The fractionated lignin (LE) and caprolactone (CL) polymerization were designed using BBD-RMS. Lignin-(OH) served as a macroinitiator with Sn(Oct)2 catalyst and CL as a monomer.29 ROP is a polymerization process where the reactive center of a polymer chain is the terminal end. This reactive center allows for the opening of cyclic monomers (CL). It forms a more extended polymer chain (Fig. S1b†).17,29 The impact of reaction conditions on the response variables (viscosity, melt temperature, and degree of polymerization) is discussed in the ESI with details in Fig. S5, S6 and Tables S5–S7.† This document places particular emphasis on the selected polymers (LEP2, LEP5, and LEP6) and the optimized polymer (LEPO) and control polymer (LPO).The increase in the grafting percentage in LEPO (compared with LPO) is attributed to the increased hydroxyl groups of LE due to ethanol fractionation (Table 1 and Fig. 1c), creating more initiation sites for the CL ring opening and subsequent grafting. Previous research indicated that the CL monomers would mainly react with the aliphatic hydroxyl groups of kraft lignin, and this reactivity would be dependent on the CL/OH ratios.49 However, the reactivity of phenolic hydroxyl was higher when the ratio of CL/OH was higher.50 Similarly, LEP2 had the highest grafting percentage and substitution of phenolic hydroxyl groups (54%), followed by LEP5 with phenolic hydroxyl groups (38%) between the three samples. This behavior could be due to the higher CL concentration in LEP2 than in LEP5, which would provide an advantage in reacting with lignin's hydroxyl sites. LEP6 had the lowest grafting percentage and the highest substitution of the hydroxyl group (30%).
This is due to the higher molecular weight of the polymers after lignin-caprolactone polymerization, which resulted in the grafting of PCL chains to the lignin backbone, improving its thermal stability (Table 1 or Fig. 4a and b). Similarly, the Tm of LEP polymers was analyzed (Fig. 4c) and reported in Table 1. It is crucial to note that the Tm was recorded during the initial heating cycle of the DSC analysis due to the disappearance of the Tm in the last heating cycle. The disappearance of the Tm could result from the lower concentration of CL/OH (0.8–2.6 mmol), leading to a short PCL chain length (DP from Table 1) in the lignin molecular structure. Also, the shorter chain of PCL in lignin could experience restricted molecular mobility due to the random and rigid structure of lignin.52 All the samples exhibited lower Tg (obtained from the second heating cycle of DSC) than L and LE (Table S4†). The decreased Tg in the polymers may be attributed to increased free volume, allowing molecular movement due to the grafting of PCL on the lignin backbone.52 Also, the difference in Tg and Tm between LEP2, LEP5, and LEP6 is insignificant due to the greater lignin concentration in all the samples and the short chain length of PCL, as shown in Table 1.
LPO and LEPO were selected for further rheological analysis and 3D printing due to lower viscosity with a more shear-thinning behavior than the other samples, making them suitable candidates for FDM 3D printing. Temperature ramp and frequency sweep analysis provide information on molecular entanglement, molecular relaxation, layer stability, and guidelines of layer adhesion for FDM 3D printability.59 The printability of LEP and LPO was assessed using a temperature ramp experiment. The complex viscosity, storage (G′), and loss modulus (G′′) decreased with reducing temperature for both polymers (Fig. 5a and b). The complex viscosity, G′, and G′′, of LPO were higher than those of LEPO, which might be related to the higher Mw of LPO (Table 1). Furthermore, the point at which G′ and G′′ intersect (i.e., cross-over point) appears at a lower temperature (55 °C) for LPO than for LEPO (40 °C). This provides an insight into the material's behavior during printing, implying that LPO behaved more like a solid at a faster rate than LEPO with the temperature decreasing. The loss modulus (G′′) and storage modulus (G′) of a polymer imply the layer adhesion and quality of 3D printable materials.60 Strong interfacial bonding between layers is crucial for strong printing. An increment in the storage modulus often correlates with improved stiffness and better layer adhesion. Conversely, decreasing loss modulus may enhance printability by reducing energy dissipation during extrusion.61 In this regard, LEPO had a much more printable behavior than LPO. In addition, the ratio between G′ and G′′ (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) of LEPO was lower than that of LPO and closer to 1 (Fig. 5c). This indicates that LEPO had more balanced viscous and elastic properties, i.e., an ideal balance for FDM printing where a balanced flow during extrusion and shape retention after printing would be necessary.62 In addition, the complex viscosity from the frequency sweep analysis in Fig. 5d exhibited that both polymers had similar flow behaviour, indicating that they could flow at their melting temperature in the printing nozzle in the extrusion step. However, the significantly higher viscosity of LPO could cause filament buckling during nozzle extrusion, which is typical for materials with a higher viscosity.63 The final mechanical properties of the printed parts are directly dependent on the interlayer adhesion between the layers deposited subsequently. The adhesion between layers is determined by the diffusion of polymer chains across the interface and the reorganization of macromolecules to their initial state.64 This process is directly influenced by the molten material's elastic or viscous properties and the polymer's relaxation time.64,65 The frequency sweep analysis for both polymers shows G′′ > G′ indicating an interlayer adhesion (Fig. 5e and f). In comparison, the LPO had a higher G′ and G′′ than LEPO, indicating that LPO possessed more solid-like properties than LEPO. The filaments were extruded using a filament extrusion machine, and the appearance of LEPO and LPO is displayed in Fig. 5g and Fig. S7a.†
δ) of LEPO was lower than that of LPO and closer to 1 (Fig. 5c). This indicates that LEPO had more balanced viscous and elastic properties, i.e., an ideal balance for FDM printing where a balanced flow during extrusion and shape retention after printing would be necessary.62 In addition, the complex viscosity from the frequency sweep analysis in Fig. 5d exhibited that both polymers had similar flow behaviour, indicating that they could flow at their melting temperature in the printing nozzle in the extrusion step. However, the significantly higher viscosity of LPO could cause filament buckling during nozzle extrusion, which is typical for materials with a higher viscosity.63 The final mechanical properties of the printed parts are directly dependent on the interlayer adhesion between the layers deposited subsequently. The adhesion between layers is determined by the diffusion of polymer chains across the interface and the reorganization of macromolecules to their initial state.64 This process is directly influenced by the molten material's elastic or viscous properties and the polymer's relaxation time.64,65 The frequency sweep analysis for both polymers shows G′′ > G′ indicating an interlayer adhesion (Fig. 5e and f). In comparison, the LPO had a higher G′ and G′′ than LEPO, indicating that LPO possessed more solid-like properties than LEPO. The filaments were extruded using a filament extrusion machine, and the appearance of LEPO and LPO is displayed in Fig. 5g and Fig. S7a.†
Overall, using several characterization methods clearly showed that ethanol fractionation had a noticeable effect on the physicochemical properties of the lignin-caprolactone polymer, as stated in the overall performance of LPO and LEPO. The molecular weight, molecular weight distribution, and hydroxyl group content were altered due to ethanol separation, resulting in a lower molecular weight and increased hydroxyl groups. This led to LE having a more significant advantage than L in interacting with CL, as seen by a higher grafting in the hydroxyl groups (Fig. S2†). LEPO had a lower viscosity and better rheological, mechanical, and hydrophobic properties than LPO because it had a lower molecular weight (Table 1), which may be attributed to improved free volume for molecular chain mobility and conformational rearrangement. Table 2 shows that earlier publications have identified interfacial incompatibility between lignin and other polymers in the system. This incompatibility has restricted lignin's presence in the final products.
| Materials | Solvent | Filament preparation method | Lignin amount, wt% | Other components con. % | Viscosity, Pa S | Advantages | Shortcomings | Ref. |
|---|---|---|---|---|---|---|---|---|
| Lignin-caprolactone and acrylonitrile-butadiene-styrene | Solvent-free | Blending | 5 | ABS, 90 PCL, 5 | 2130 | Lignin-PCL improved the processability of the composite | - Stability of composite material not reported | 66 |
| - Lower concentration of lignin | ||||||||
| - Still used | ||||||||
| Lignin, polycaprolactone, and curcumin | Solvent-free | Blending | 10 | Curcumin, 5 PCL, 85 | NR | Lignin improved the antibacterial and antimicrobial properties | - Mechanical properties not reported | 67 |
| - Lower concentration of lignin | ||||||||
| Enzymatic lignin, polycaprolactone and polyurethane | Solvent-free | Blending | 20 | PCL, 30 | 100 | Lignin enhanced the rigidity of the composite | Interface incompatibility between lignin and other components | 68 |
| PU, 55 | ||||||||
| Polylactic acid, polycaprolactone, and lignin | Solvent-free | Blending | 2 | PCL, 24 | NR | Lignin improved the thermal recovery and mechanical properties | Lower concentration of lignin | 69 |
| PLA, 65 | ||||||||
| Lignin and caprolactone | Solvent-free | Copolymerization | 75 | Caprolactone, 25 | 160.9 | - Higher concentration of lignin | Application of 3D printed material needs investigation | This work |
| - Ease of use | ||||||||
| - Superior mechanical properties |
3.3. 3D printability of lignin-caprolactone polymer
The 3D printability of LPO and LEPO was assessed using FDM, and images of the 3D printed parts are displayed in Fig. 6. The pallets for both polymers were rolled, loaded, and printed using a 3D printer; however, as seen from the printed parts, a visible gap between layers indicates low interlayer diffusion and adhesion between deposited layers in the LPO samples (Fig. 6a′ and b′). Conversely, LEPO showed no gaps between printed layers, indicating better interlayer adhesion and filling. This behavior could be attributed to the very high viscosity (Fig. 5a and d) and molecular weight of LPO (Table 1), inducing thermal crosslinking, which might lead to reducing molecular mobility and interfacial diffusion, thus reducing adhesion between deposited layers.20Furthermore, the intersection point observed during the melt rheology investigation (Fig. 5b) of LPO occurred at higher temperatures (55 °C), indicating that the sample might lose its ability to melt more quickly when exposed to lower temperatures. This could hinder the bonding of layers, as seen in Fig. 6a′ and b′. The higher 3D printability of LEPO than LPO might be ascribed to improved layer adhesion originating from the lowered molecular weight and viscosity of LEPO due to LE's lower molecular weight achieved by ethanol fractionation (Table 1). Therefore, we can conclude that the ethanol fractionation of alkali lignin improved the 3D printability of lignin-caprolactone polymers.
3.4. Implications
In the past, lignin polymers were used in 3D printing in polymer blends with other polymers, such as acrylonitrile-butadiene-styrene, polycaprolactone, cummin, polycaprolactone/polyurethane, and polylactic acid following the FDM-3D printing technique, as indicated in Table 2. The addition of lignin-caprolactone polymer (15 wt%) to acrylonitrile-butadiene-styrene led to a decrease in viscosity (2130 Pa s) compared to the formulations without lignin-caprolactone polymer (4270 Pa s) under similar temperatures and shear rates.66 In another study, incorporating lignin-caprolactone polymer (40 wt%) into polycaprolactone and curcumin improved the blend's thermal stability even though the rheological properties were not reported.67Table 2 shows that earlier publications have identified interfacial incompatibility between lignin and other polymers in the system. This incompatibility has restricted lignin's presence in the final products. Utilizing a homogeneous copolymer would facilitate the manufacturing process. The current study is unique compared to the existing literature since the lignin-caprolactone polymer was used solitarily for 3D printing after mimicking the characteristics of lignin polymer via solvent fractionation (ethanol). The total concentration of lignin present in the lignin-caprolactone matrix was 75 wt% (i.e., a truly sustainable material), based on the calculation in (eqn (S1)†), with much lower viscosity (160.9 Pa s) and ease of printing. Considering the ethanol fractionation, the overall use of lignin in the produced 3D printing material would be 40.12 wt% of the original pristine lignin. The results imply that almost 60% of pristine lignin is still available for other uses and can be incorporated into other value-added applications if collected after filtration. This study also conducted an analysis of variance (ANOVA) on the responses (viscosity, melt temperature, and degree of polymerization) of lignin-caprolactone polymer, considering three variables: CL/OH ratio, reaction time, and catalyst concentration in Tables S5–S7.† The results indicate that all reaction parameters significantly affect the response variables, with p-values less than 0.05. Additionally, suitable models for predicting the response variables are presented, according to eqn (S10)–(S12).† This work provides an excellent insight into the use of lignin polymer for FDM-3D printing, which would reduce the use of inorganic and synthetic polymers in 3D printed materials, tackling the problem of sustainability. Future research may explore the substitutions of hexane with a more environmentally friendly solvent utilized for the extraction of extractives from post-ethanol fractionated lignin, as well as the DBTDL catalyst with environmentally friendly catalysts.4. Conclusion
This work aimed to reduce the molecular weight and molecular weight distribution of birch alkali lignin (L) via ethanol fraction before polymerization of the fractionated lignin (LE) with caprolactone for FDM-3D printing. LE showed a higher hydroxyl group content, lower molecular weight, and lower poly dispersibility than L. The increase in hydroxyl groups created more reactive sites on the lignin structure for the caprolactone grafting. The copolymerization of LE and caprolactone was optimized by considering the melt temperature (Tm), degree of polymerization (DP), and viscosity as the primary factors of its printability. The results confirmed that the optimized sample (LEPO), produced by a CL/OH ratio of 1.15 mmol g−1, a reaction duration of 420 minutes, and a catalyst concentration of 1 wt% showed better printability with a melt temperature of 48 °C, shear-thinning behavior, low viscosity (160.9 Pa s) and better thermal stability with an onset temperature 292.2 °C. The melt rheology results demonstrated that LEPO exhibited superior interlayer adhesion during printing and mechanical capabilities to unfractionated lignin-caprolactone (LPO) polymer. LE's decreased molecular weight and narrower molecular weight distribution contributed to LEPO's reduced viscosity. Furthermore, the occurrence of the cross-over points between G′ and G′′ appearing 15 °C higher than LPO provided LEPO with a favorable condition for improved layer formation and enhanced bonding between layers. LEPO's water contact angle value was higher than those of LPO by 30°, which was consistent with the higher grafting percentage of CL (11% higher) to the hydroxyl groups of LEPO than LPO. Based on this study's findings, the reduced variability in the lignin structure after ethanol fractionation of lignin is a viable approach for producing lignin-caprolactone polymer for 3D printable materials, where 75% of the fractionated lignin (representing 40.12% unfractionated pristine alkali birch lignin) was part of the lignin-caprolactone polymer. More extensive research is required to explore the usefulness of this 3D-printed lignin-caprolactone polymer in areas such as bio-medical applications.Author contributions
Banchamlak Bemerw Kassaun: writing the original draft, conceptualization, methodology, and investigation. Luyao Wang: methodology, investigation. Oskar Backman: methodology, investigation. Chunlin Xu: writing-review & editing, project administration, methodology, investigation, conceptualization; Pedram Fatehi: writing-review and editing, project administration, methodology, investigation, conceptualization.Data availability
The data supporting this article have been included as part of the ESI.† No software or codes were used in this article, and extra data can be obtained upon request from the corresponding author.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
The authors acknowledge the Globalink MITACS national research organization, Northern Ontario Heritage Fund Corporation, NSERC, Business Finland (project 43674/31/2020), and part of the research used Research Council of Finland Research Infrastructure “Printed Intelligence Infrastructure kkjure” (PII-FIRI).References
- T. Yao, J. Ye, Z. Deng, K. Zhang, Y. Ma and H. Ouyang, Composites, Part B, 2020, 188, 107894 CrossRef CAS.
- S. Garzon-Hernandez, A. Arias and D. Garcia-Gonzalez, Composites, Part B, 2020, 201, 108373 CrossRef CAS.
- Z. Fu, S. Naghieh, C. Xu, C. Wang, W. Sun and X. Chen, Biofabrication, 2021, 13, 033001 CrossRef CAS PubMed.
- N. R. Madhu, H. Erfani, S. Jadoun, M. Amir, Y. Thiagarajan and N. P. S. Chauhan, Int. J. Adv. Manuf. Technol., 2022, 122, 2125–2138 CrossRef PubMed.
- L. Shao, Y.-C. Chang, C. Hao, M.-E. Fei, B. Zhao, B. J. Bliss and J. Zhang, Green Chem., 2022, 24, 8716–8724 RSC.
- Y. Bozkurt and E. Karayel, J. Mater. Res. Technol., 2021, 14, 1430–1450 CrossRef CAS.
- G. Chyr and J. M. DeSimone, Green Chem., 2023, 25, 453–466 RSC.
- S. Shariatnia, A. Veldanda, S. Obeidat, D. Jarrahbashi and A. Asadi, Composites, Part B, 2019, 177, 107291 CrossRef CAS.
- Ö. Yapar, in Additive Manufacturing in Multidisciplinary Cooperation and Production, Springer, 2023, pp. 79–102. DOI:10.1007/978-3-031-37671-9_8.
- D. S. Bajwa, G. Pourhashem, A. H. Ullah and S. G. Bajwa, Ind. Crops Prod., 2019, 139, 111526 CrossRef CAS.
- S. Wasti, E. Triggs, R. Farag, M. Auad, S. Adhikari, D. Bajwa, M. Li and A. J. Ragauskas, Composites, Part B, 2021, 205, 108483 CrossRef CAS.
- A. E. Kazzaz, Z. H. Feizi and P. Fatehi, Green Chem., 2019, 21, 5714–5752 RSC.
- O. Yu and K. H. Kim, Appl. Sci., 2020, 10, 4626 CrossRef CAS.
- M. Fazeli, S. Mukherjee, H. Baniasadi, R. Abidnejad, M. Mujtaba, J. Lipponen, J. Seppälä and O. J. Rojas, Green Chem., 2024, 26, 593–630 RSC.
- V. Mimini, E. Sykacek, S. N. A. Syed Hashim, J. Holzweber, H. Hettegger, K. Fackler, A. Potthast, N. Mundigler and T. Rosenau, J. Wood Chem. Technol., 2019, 39, 14–30 CrossRef CAS.
- X. Zhang, M. Morits, C. Jonkergouw, A. Ora, J. J. Valle-Delgado, M. Farooq, R. Ajdary, S. Huan, M. Linder and O. Rojas, Biomacromolecules, 2020, 21, 1875–1885 CrossRef CAS PubMed.
- N. A. Nguyen, S. H. Barnes, C. C. Bowland, K. M. Meek, K. C. Littrell, J. K. Keum and A. K. Naskar, Sci. Adv., 2018, 4, eaat4967 CrossRef CAS PubMed.
- J. Domínguez-Robles, N. K. Martin, M. L. Fong, S. A. Stewart, N. J. Irwin, M. I. Rial-Hermida, R. F. Donnelly and E. Larrañeta, Pharmaceutics, 2019, 11, 165 CrossRef PubMed.
- S. Wasti, E. Triggs, R. Farag, M. Auad, S. Adhikari, D. Bajwa, M. Li and A. J. Ragauskas, Composites, Part B, 2021, 205, 108483 CrossRef CAS.
- N. A. Nguyen, C. C. Bowland and A. K. Naskar, Appl. Mater. Today, 2018, 12, 138–152 CrossRef.
- P. Schlee, O. Hosseinaei, C. A. O'Keefe, M. J. Mostazo-López, D. Cazorla-Amorós, S. Herou, P. Tomani, C. P. Grey and M.-M. Titirici, J. Mater. Chem., 2020, 8, 23543–23554 RSC.
- L. S. Ebers, A. Arya, C. C. Bowland, W. G. Glasser, S. C. Chmely, A. K. Naskar and M. P. Laborie, Biopolymers, 2021, 112, e23431 CrossRef CAS PubMed.
- V. V. Adithyamol, S. Gupta and B. Kandasdubramanian, Biomed. Mater. Devices, 2024, 1–13, DOI:10.1007/s44174-024-00193-1.
- H. Ye, Y. He, H. Li, T. You and F. Xu, Polymers, 2023, 15, 2806 CrossRef CAS PubMed.
- M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484–3504 RSC.
- R. M. Mohamed and K. Yusoh, Adv. Mater. Res., 2016, 1134, 249–255 Search PubMed.
- M. Bartnikowski, T. R. Dargaville, S. Ivanovski and D. W. Hutmacher, Prog. Polym. Sci., 2019, 96, 1–20 CrossRef CAS.
- V. Guarino, G. Gentile, L. Sorrentino and L. Ambrosio, EPST, 2002, pp. 1–36. DOI:10.1002/0471440264.pst658.
- C. M. B. Ho, A. Mishra, P. T. P. Lin, S. H. Ng, W. Y. Yeong, Y. J. Kim and Y. J. Yoon, Macromol. Biosci., 2017, 17, 1600250 CrossRef PubMed.
- W. Wang, G. F. Caetano, W.-H. Chiang, A. L. Braz, J. J. Blaker, M. A. C. Frade and P. J. Bártolo, Int. J. Bioprint., 2016, 2, 95–104 CrossRef CAS.
- K. Liu, J. Sun, Q. Zhu, X. Jin, Z. Zhang, Z. Zhao, G. Chen, C. Wang, H. Jiang and P. Zhang, Ceram. Int., 2022, 48, 24032–24043 CrossRef CAS.
- Z. Muwaffak, A. Goyanes, V. Clark, A. W. Basit, S. T. Hilton and S. Gaisford, Int. J. Pharm., 2017, 527, 161–170 CrossRef CAS PubMed.
- I.-K. Park, H. Sun, S.-H. Kim, Y. Kim, G. E. Kim, Y. Lee, T. Kim, H. R. Choi, J. Suhr and J.-D. Nam, Sci. Rep., 2019, 9, 7033 CrossRef PubMed.
- K. L. Kadam, C. Y. Chin and L. W. Brown, Environ. Prog. Sustainable Energy, 2009, 28, 89–99 CrossRef CAS.
- H. Sadeghifar and A. Ragauskas, ACS Sustainable Chem. Eng., 2020, 8, 8086–8101 CrossRef CAS.
- Y. Wang, L. Wei, Q. Hou, Z. Mo, X. Liu and W. Li, Fermentation, 2023, 9, 386 CrossRef CAS.
- C. A. E. Costa, F. M. Casimiro, C. Vega-Aguilar and A. E. Rodrigues, ChemEngineering, 2023, 7, 42 CrossRef CAS.
- R. Liu, A. Smeds, L. Wang, A. Pranovich, J. Hemming, S. Willfor, H. Zhang and C. Xu, ACS Sustainable Chem. Eng., 2021, 9, 13862–13873 CrossRef CAS.
- L. Wang, L. Lagerquist, Y. Zhang, R. Koppolu, T. Tirri, I. Sulaeva, S. V. Schoultz, L. Vähäsalo, A. Pranovich and T. Rosenau, ACS Sustainable Chem. Eng., 2020, 8, 13517–13526 CrossRef CAS.
- C. Gao, M. Li, C. Zhu, Y. Hu, T. Shen, M. Li, X. Ji, G. Lyu and W. Zhuang, Composites, Part B, 2021, 205, 108530 CrossRef CAS.
- G. Zinovyev, I. Sulaeva, S. Podzimek, D. Rössner, I. Kilpeläinen, I. Sumerskii, T. Rosenau and A. Potthast, ChemSusChem, 2018, 11, 3259–3268 CrossRef CAS PubMed.
- X. Meng, C. Crestini, H. Ben, N. Hao, Y. Pu, A. J. Ragauskas and D. S. Argyropoulos, Nat. Protoc., 2019, 14, 2627–2647 CrossRef CAS PubMed.
- D. Diment, O. Tkachenko, P. Schlee, N. Kohlhuber, A. Potthast, T. M. Budnyak, D. Rigo and M. Balakshin, Biomacromolecules, 2023, 25, 200–212 CrossRef PubMed.
- M. Gigli and C. Crestini, Green Chem., 2020, 22, 4722–4746 RSC.
- R. Liu, A. Smeds, T. Tirri, H. Zhang, S. Willfor and C. Xu, ACS Sustainable Chem. Eng., 2022, 10, 14588–14599 CrossRef CAS.
- M. Sette, R. Wechselberger and C. Crestini, Chem. – Eur. J., 2011, 17, 9529–9535 CrossRef CAS PubMed.
- T. Pang, G. Wang, H. Sun, W. Sui and C. Si, Ind. Crops Prod., 2021, 165, 113442 CrossRef CAS.
- J.-H. Choi, S.-M. Cho, J.-C. Kim, S.-W. Park, Y.-M. Cho, B. Koo, H. W. Kwak and I.-G. Choi, ACS Omega, 2021, 6, 1534–1546 CrossRef CAS PubMed.
- N. A. Nguyen, K. M. Meek, C. C. Bowland and A. K. Naskar, Data Brief, 2019, 22, 392–399 CrossRef PubMed.
- M. C. Najarro, M. Nikolic, J. Iruthayaraj and I. Johannsen, ACS Appl. Polym. Mater., 2020, 2, 5767–5778 CrossRef CAS.
- E. A. Capanema, M. Y. Balakshin and J. F. Kadla, J. Agric. Food Chem., 2004, 52, 1850–1860 CrossRef CAS PubMed.
- T. Hatakeyama, S. Yamashita and H. Hatakeyama, J. Therm. Anal. Calorim., 2021, 143, 203–211 CrossRef CAS.
- J. F. Vega, J. Otegui, J. Ramos and J. Martínez-Salazar, Rheol. Acta, 2012, 51, 81–87 CrossRef CAS.
- S. Middleman, J. Appl. Polym. Sci., 1967, 11, 417–424 CrossRef CAS.
- S. J. Kozmiski, Effect of Molecular Weight Distribution on Viscosity-shear Rate and Die Swell Behavior in Homologous Polystyrene Blends, The Pennsylvania State University, 1984 Search PubMed.
- S. Wickramasinghe, T. Do and P. Tran, Polymers, 2020, 12, 1529 CrossRef CAS PubMed.
- Y. Tlegenov, W. F. Lu and G. S. Hong, Prog. Addit. Manuf., 2019, 4, 211–223 CrossRef.
- H. Zhang, L. Zhang, H. Zhang, J. Wu, X. An and D. Yang, Int. J. Adv. Manuf. Technol., 2021, 117, 3549–3562 CrossRef.
- M. V. Candal, I. Calafel, N. Aranburu, M. Fernández, G. Gerrica-Echevarria, A. Santamaría and A. J. Müller, Addit. Manuf., 2020, 36, 101408 CAS.
- A. Patti, S. Acierno, G. Cicala and D. Acierno, ChemEngineering, 2022, 7, 1 CrossRef.
- S. Thumsorn, W. Prasong, T. Kurose, A. Ishigami, Y. Kobayashi and H. Ito, Polymers, 2022, 14, 2721 CrossRef CAS PubMed.
- R. Arrigo and A. Frache, Polymers, 2022, 14, 1754 CrossRef CAS PubMed.
- K. Ilyés, N. K. Kovács, A. Balogh, E. Borbás, B. Farkas, T. Casian, G. Marosi, I. Tomuţă and Z. K. Nagy, Eur. J. Pharm. Sci., 2019, 129, 110–123 CrossRef PubMed.
- A. Das, J. A. Riet, M. J. Bortner and C. McIlroy, Phys. Fluids, 2022, 34, 123108 CrossRef CAS.
- Y. Weng, M. Li, D. Zhang, M. J. Tan and S. Qian, Cem. Concr. Res., 2021, 143, 106386 CrossRef CAS.
- S.-H. Kim, K. Choi, H. R. Choi, T. Kim, J. Suhr, K. J. Kim, H. J. Choi and J.-D. Nam, ACS Omega, 2019, 4, 10036–10043 CrossRef CAS PubMed.
- J. Domínguez-Robles, E. Cuartas-Gómez, S. Dynes, E. Utomo, Q. K. Anjani, U. Detamornrat, R. F. Donnelly, N. Moreno-Castellanos and E. Larrañeta, Sustainable Mater. Technol., 2023, 35, e00581 CrossRef.
- F. Suo, X. Bai, Y. Liu, M. Xu, T. Gu, L. Cao, X. Lv, X. Zhang and Y. Yao, Int. J. Biol. Macromol., 2024, 132943, DOI:10.1016/j.ijbiomac.2024.132943.
- B. Liu, X. Gu, X. Chen, Y. Yang, J. Zou, H. Yin and Y. Cai, J. Appl. Polym. Sci., 2024, 141, e55252 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc06179a |
| This journal is © The Royal Society of Chemistry 2025 |





