 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cutting-edge development of non-isocyanate polyurethane (NIPU) foams: from sustainable precursors to environmental impact evaluation
Federica
Orabona†
 ab,
Federica
Recupido†
c,
Giuseppe Cesare
Lama
c,
Krzysztof
Polaczek
c,
Francesco
Taddeo
ab,
Federica
Recupido†
c,
Giuseppe Cesare
Lama
c,
Krzysztof
Polaczek
c,
Francesco
Taddeo
 b,
Tapio
Salmi
a,
Martino
Di Serio
b,
Tapio
Salmi
a,
Martino
Di Serio
 b,
Letizia
Verdolotti
b,
Letizia
Verdolotti
 *c and
Vincenzo
Russo
*c and
Vincenzo
Russo
 *ab
*ab
aÅbo Academy University, Laboratory of Industrial Chemistry and Reaction Engineering, Henrikinkatu 2, FI-20500, Turku/Åbo, Finland. E-mail: v.russo@unina.it
bChemical Sciences Department, University of Naples Federico II, Via Cinthia 26, 80126 Naples, Italy
cInstitute for Polymers, Composites, and Biomaterials (IPCB-CNR), Italian National Council, Piazzale E. Fermi 1, 80055, Portici, Italy. E-mail: letizia.verdolotti@cnr.it
First published on 23rd May 2025
Abstract
Polyurethane (PU) foams represent a wide class of polymeric materials, having applications in different sectors ranging from automotive, packaging, and cushioning/bedding to construction. However, their synthesis requires the use of petrol-based components, which are mostly harmful and toxic isocyanates. Considering this, non-isocyanate polyurethane (NIPU) foams have been demonstrated to be promising alternatives to conventional PUs, which are obtained through “isocyanate-free” routes such as the aminolysis of cyclic carbonates with diamines. The building blocks of NIPU foams can be derived from different bio-sources, such as vegetable oils, sugars, and terpenes. Moreover, the final NIPU materials can be fully reprocessed and recycled owing to the presence of suitable functional groups that facilitate dynamic bond exchange. This review aims to comprehensively describe the current state-of-the-art concerning the synthesis and applications of NIPU and hybrid NIPU foams and is divided into three sections: (i) an outline of the synthesis of bio-based NIPU precursors, i.e. cyclic and linear carbonates, diamines and carbamates, from biomass-derived and waste sources such as vegetable oils and CO2 and via environmentally friendly approaches; (ii) analysis of the reprocessability and recyclability of NIPU and composite NIPU foams; and (iii) evaluation of the environmental impacts of NIPU precursors and foams using the life cycle assessment (LCA) technique, preliminary investigations of their techno-economic analysis (TEA), and description of future perspectives.
Green foundation1. The main advances in green chemistry related to the synthesis of sustainable polyurethanes, namely, NIPU (non-isocyanate polyurethane), were discussed.2. This review article focuses on a hot topic as the produced NIPU could be used for a wide range of applications. Moreover, this review is multidisciplinary, linking chemistry, reaction engineering, polymer syntheses and characterization. 3. Our work can inspire the further development of new synthetic routes to produce NIPU, leading to the development of chemical processes to produce sustainable polyurethanes. |
1. Introduction
In the last decades, considerable attention has been paid to polyurethanes (PUs), making them the 6th most used polymers around the world1 and one of the most versatile raw materials for various chemical structures including, foams, elastomers, sealants, and coatings. Among these, PU foams are the largest subgroup, representing more than 60% of the overall PU market.2 They are key materials for modern life, with a broad range of applications in bedding/cushioning (furniture, mattresses, and seats), thermal/acoustic insulation3–5 and shock and water adsorption.6 In 2022, the worldwide production of PU foams was USD 46.80 billion (about 8% of the overall plastic market), which is expected to reach USD 78.16 billion by 20297 (10% of the worldwide plastic market) with a compound annual growth rate (CAGR) of 4.5%.Generally, PUs are obtained via the polyaddition of polyols (containing reactive hydroxyl groups) and di-isocyanates (Scheme 1i) followed by a blowing process, which is triggered by physical or chemical blowing agents (BAs). Physical BAs, such as hydrofluorocarbons (HFCs), hydrocarbons (HCs) and hydrofluoroolefins (HFOs),8,9 are volatile compounds that evaporate to the gas phase owing to the exothermicity of the reaction. Alternatively, chemical BAs generate blowing gas through chemical reactions. For example, water and formic acid can act as chemical BAs, reacting with isocyanate and generating unstable carbamic acid.8 The latter decomposes into amine and CO2, causing blowing10,11 (Scheme 1, ii).2 As a side reaction, urea is formed from the reaction between the isocyanate and amines (Scheme 1, iii).12
Typically, PU precursors are obtained from petrol-based sources. The synthesis of PU foams involves the use of isocyanate products, i.e. methylene-diphenyl di-isocyanate (MDI) and toluene di-isocyanate (TDI), which are obtained using extremely toxic phosgene. Moreover, prolonged exposure to isocyanate products poses significant issues to human health such as dermatitis, eye irritation, and respiratory diseases (asthma).14 Many of them are indeed classified as CMR, i.e., carcinogenic, mutagenic, and reprotoxic.13 In August 2020, the European Union promoted a REACH restriction (ANNEX XVII),15 which limits the use of di-isocyanates (cut-off limit is 0.1%) and introduced mandatory training for employees handling di-isocyanates.
The ongoing challenge in the scientific community is to shift to 100% bio-based raw materials, while maintaining an enhanced functional performance in the final foamed materials. In the past 20 years, both the research community and industry have devoted significant efforts to finding strategies to reduce CO2 emissions and shift from petrol-derived materials to more sustainable sources and technologies. Among them, a common approach involves replacing petrol-based polyols with bio-based polyols employing renewable building blocks derived from either microbial16–18 or lignin-cellulosic biomass19,20 as well as plant oils.21–23 However, although the use of bio-based polyols is a great step forward, the presence of isocyanates enormously affects the transition towards a total green process. Therefore, bio-based isocyanates derived from terpenes, fatty acids, lignin, and amino acids have been developed.24,25 Some of them have already appeared on the market, such as aliphatic isocyanates, i.e. Lupranat® ZERO by BASF,26 Desmodur® QCN 3000 by Covestro,27 and Tolonate X FLO 100 by Vencorex.28 Nevertheless, the toxic phosgene is still partially employed in the manufacturing of the latter products.
The synthesis of non-isocyanate polyurethanes (NIPUs) has been strongly encouraged in the last decade,29–32 despite their initial discovery in 1957 by Dyer and Scott.33 To date, many isocyanate-free alternative pathways have been reported in the literature. Among them, the aminolysis of linear (LCs) and cyclic carbonates (CCs) with diamines34–37 and transurethanization of bis-alkylcarbamates/bis-hydroxyalkylcarbamates with alcohols38 are considered the most promising routes to obtain NIPU foams, as illustrated in Fig. 1.
 | ||
| Fig. 1 Aminolysis and transurethanization as the two most promising eco-friendly routes for the synthesis of NIPU foams. | ||
The aminolysis of dicarbonates with diamines is a solvent-free route resulting in the formation of hydroxy-urethane repeating units and generating the so-called polyhydroxyurethanes (PHUs).34,35 Usually, acid/base catalysts are employed to enhance the reaction rate35 but this process also proceeds under catalyst-free conditions.39 CCs with 5-membered rings (5CCs) are preferably used as monomers for the synthesis of NIPU. They can be obtained through sustainable pathways such as cycloaddition of CO2 to epoxidized vegetable/biomass-derived oils, i.e., sunflower, linseed, and cardanol oils.35,40–42 Recently, terpenes, tannin or lignin derivatives, vanillin, and glycerol43 have been proposed as sustainable building blocks to obtain these precursors. Diamines are usually petrol-based sources; however, they may also be obtained using sustainable building blocks such as fatty acid dimerization.44
The main drawback of the aminolysis pathway is the relatively low reactivity of CCs with diamines, which requires a very long reaction time, limiting the scalability of the process on an industrial level. Thus, to overcome this limitation, new approaches have been developed involving the use of thiols and thiol derivatives.45 More specifically, employing thiols in the formulation of CCs and diamines accelerates the decarboxylation of CCs, also enhancing the aminolysis process. Either linear thiols or cyclic dithiocarbonates (CTCs) can be employed, which exhibit higher reactivity towards amines.45,46 Actually, it has been demonstrated that the reaction time can be reduced from several hours to minutes.45 The backbone of the resulting polymers possesses both urethane and thioether linkages. Therefore, they are defined as non-isocyanate polyhydroxythiourethanes (NIPTU) due to their hybrid nature.45
Another attractive route to obtain NIPUs is the transurethanization (or polycondensation) of carbamates with alcohols. Nowadays, alkylcarbamates can be synthesized via phosgene-free routes, e.g., reactions involving dialkylcarbonates and diamines, making transurethanization safer than the traditional route. In contrast to aminolysis, transurethanization leads to the formation of urethane moieties rather than to hydroxyurethane moieties. Therefore, the resulting chemical structure is closer to that of the classical PUs. However, alcohol is generated as a stoichiometric co-product in the reaction, making downstream separation steps necessary. Thus, to achieve high conversion and degree of polymerization, it is necessary to apply in situ vacuum distillation of the alcohol and use solvents.47 Moreover, organocatalysts such as TBD (triazabicyclodecene) are usually selected and the operating temperatures should exceed 110 °C (ref. 47) for longer reaction times.
Besides the above-mentioned reactions, other isocyanate-free routes have been investigated such as the copolymerization of aziridine with carbon dioxide, the ring-opening polymerization of cyclic carbamates, and the reaction between carbamates and aldehydes (not reviewed herein).31,48 These routes have previously been deeply discussed in similar reviews on this topic, and principally all promising. However, they still involve the use of toxic components i.e., aziridines, aldehydes, and ethyl chloroformate, and have not been studied in the application of PU foams. Therefore, they will not be reviewed herein.
Blowing of NIPU can be performed by either physical or chemical blowing agents,49,50i.e. non-self-blowing or self-blowing routes (in situ CO2 formation), involving the decarboxylation of CCs by either hydrolysis,51 Pearson reaction between CCs and thiols45,52,53 (i.e., S-alkylation) and using amine/CO2 adducts.54 The type and concentration12 of precursors and additives, i.e., epoxy cross-linkers,55 catalysts, and micro-/nano-sized fillers,56 dictate the morphology of the cellular materials (i.e., open/closed cell content, anisotropy, and apparent density) and influence their functional properties. All these parameters can be adjusted to tailor the final characteristics of NIPU foams and make them flexible or rigid materials depending on the final application. Similar to conventional PUs, flexible NIPU foams have open interconnected cell structures with viscoelastic properties, which can be applied in the automotive, cushioning/bedding, and biomedical sectors.14 Rigid NIPU foams have a closed cell morphology, resulting in good insulating materials57 with excellent mechanical characteristics (high stress and impacts). Semi-rigid/semi-flexible foams share the characteristics of both categories and may have potential applications in several sectors, i.e., automotive (e.g. integral skin foams), packaging, and building (spray foams). Fig. 2 displays the morphology, properties, and possible final applications where NIPU foams can replace conventional foams. In comparison with traditional PU materials, NIPU and NIPTU foams have intriguing peculiarities associated with the presence of pending OH or SH functional groups on their polymeric backbone, which play a crucial role in promoting the reprocessability of these materials.32
The recent advances in the synthesis of NIPU foams are described in this review by examining the sustainable routes to obtain the building blocks of the foam precursors. A comprehensive overview on the synthesis of NIPU foams and sustainability aspects such as reprocessability through dynamic chemistry and recycling of these materials, mainly through chemical routes, as well as their environmental assessment, is provided and future developments are illustrated.
2. NIPU precursors
The agricultural waste from agro-industries, crop residues, and livestock is approximately 2 billion tons per year.58 Thus, the valorization of this huge amount of biomass is extremely crucial from the viewpoint of a circular economy. Lignin, cellulose, organic acids, and vegetable oils can be extracted by the decomposition and further treatment of biomass and utilized as a feedstock for the production of added-value products,59 such as biopolymers. Different strategies for the synthesis of natural-source monomers for NIPU foam precursors are discussed in the following sections.2.1 Carbon dioxide
Since the beginning of the 21st century, greenhouse gas (GHG) emissions have shown a continuous upward trend. In particular, carbon dioxide (CO2) emissions from emerging economies have become a dominant contributor, accounting for approximately 74% of the total global emissions in 2023. According to the latest Emissions Database for Global Atmospheric Research (EDGAR) report (2024), the global GHG emissions reached 994 million tonnes of CO2 equivalent 994 (Mt CO2eq) in 2023, an increase of 1.9% compared to the previous year.60 This steady rise highlights the urgent need for effective climate mitigation strategies. In response to regulatory pressure and growing environmental awareness, several major chemical companies have committed to significantly reducing their CO2 emissions, with the long-term goals of achieving net-zero emissions by 2050. These initiatives reflect a broader industrial shift towards sustainability and innovation in carbon management practices, aligned with the objectives of the Paris Agreement.61The most promising solutions are carbon capture and storage (CCS) and carbon capture and utilization (CCU) technologies, which aim to remove CO2 from the atmosphere and either store it securely or convert it into useful products, respectively.42,62 CCS focuses on permanent storage, whereas CCU offers a pathway to transform captured carbon into fuels, chemicals, and materials, effectively closing the carbon loop. Importantly, the utilization of carbon dioxide as a chemical feedstock is not a recent innovation. As early as 1921, the industrial production of urea from CO2 and ammonia was developed, which remains widely used in the fertilizer industry today.42 Additionally, CO2 can be utilized in the dry reforming of methane, a process that converts CO2 and CH4 into synthesis gas (a mixture of H2 and CO), which serves as a precursor for various fuels and chemicals in the energy sector. Another interesting decarbonization pathway is CO2 conversion into cyclic carbonates and carbamates, which are the main precursors of isocyanate-free polyurethanes, as discussed in Section 2.2 and 2.3, respectively.
These examples demonstrate the diverse potential of CO2 as a chemical building block. As technologies mature and economic incentives grow, CCU is expected to play a vital role in industrial decarbonization and the transition to a more circular, low-carbon economy.
2.2 Cyclic and linear carbonates
5CCs are mainly obtained via the cyclo-carbonation of diols (a),70,71 oxidative carboxylation of alkenes (b),72 the reaction of CO2 and halohydrins (c),73 (d) transcarbonation of diols with linear dialkyl carbonates and (e) cycloaddition reaction of CO2 and epoxides,74–77 as illustrated in Scheme 2.
The cyclocarbonation of diols (Scheme 2a) is exceptionally environmentally friendly given that diols can be obtained through the direct treatment of biomass; however, this reaction is hindered by both thermodynamics and kinetics, resulting in a very low yield of cyclic carbonates.78,79 The oxidative carboxylation of olefins (Scheme 2b) allows the one-pot synthesis of cyclic carbonates via the formation of an epoxy intermediate;80 however, to the best of our knowledge, it has never been verified with greener substrates.
The reaction of CO2 and halohydrins (Scheme 2c) can give a relatively high yield of 5CCs,81 but halohydrins are not abundant and halogen acids are inevitably produced as co-products. Another approach is the transcarbonation of diols with short-chained linear carbonates, i.e., dimethyl and diethyl carbonates (DMC and DEC, respectively, Scheme 2d). It is an interesting alternative pathway to synthesize short-chain CCs. In this case, green reactants and mild operating conditions are usually employed.42,48 However, this route has been mainly studied for low molecular weight substrates, and the formation of methanol as a co-product of the reaction makes further separation steps necessary.32,82,83 Among the pathways presented, the cycloaddition of CO2 to epoxides (Scheme 2e) is the most studied. Although it usually needs high temperatures and pressures, it involves the use of CO2 as the reactant and registers 100% atom economy, generating high yields of cyclic carbonates without the formation of any stoichiometric coproducts.84 Considering all the advantages and disadvantages of the reaction routes presented, the carboxylation of diols with dialkyl carbonates (d), and particularly the fixation of carbon dioxide to epoxides (e) are at the moment considered the most promising sustainable pathways because of their feasibility and versatility.85
A broad spectrum of starting bio-based and waste-based materials can be used.86 Namely, 5CCs have been successfully synthesized from vegetable oils,87–89 vanillin,90 lignocellulosic biomass derivatives, e.g. diphenolic acid,91 and terpenes (i.e., limonene).92 In the last few decades, the scientific community has focused on the development of homogeneous as well as heterogeneous catalysts to enhance the reaction rate and the selectivity towards carbonates, while operating under milder temperature and pressure conditions.93 To catalyze epoxy ring opening, organic catalysts such as halide quaternary ammonium salts, e.g., tetrabutylammonium bromide, iodide, and chloride (TBAB, TBAI, and TBAC), are usually utilized, and eventually combined with hydrogen-bond donors (HBD).94,95 HBD are compounds either with OH moieties, metals, or their combination, which act as Lewis acids, making the epoxy ring more prone to nucleophilic attack from Lewis bases. The halide ions, i.e., Cl−, Br− and I−, in TBAX, usually act as Lewis bases, finalizing oxirane ring breakage.95
Regarding the transcarbonation of diols, nucleophilic organocatalysts are usually employed such as cyclic amines, e.g., 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD); however, ionic liquids, molecular sieves, and metal oxides have also been demonstrated to act as selective catalysts for the carbonation of diols to cyclic carbonates.48,83
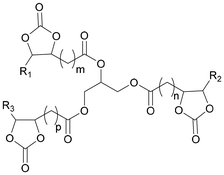
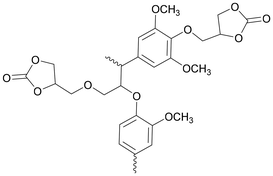
Considerable effort has been made in the application of CCs for the synthesis of NIPU foams.13 Specifically, CCs with a functionality equal to or exciding 2 are preferably applied as monomers for the synthesis of NIPUs with sufficiently high molecular weights. Table 1 presents the double or higher functionalized 5CCs successfully employed as monomers for the synthesis of NIPU foams, together with their molecular structures, raw materials, and synthesis process.
| Chemical properties of the CCs | Reaction conditions | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | Structure | Raw material | CC functionality | T (°C) | P (bar) | Reaction time (h) | Catalyst | Solvent | CCs Yield (%) | |
| Soybean oil cyclic carbonate |
|
Soybean oil | >2 | 100 | 100 | 10 | TBAB + HBD | — | 100 | Grignard et al.103 |
| Poly(ethylene glycol) biscarbonate |

|
PEG | 2 | 80 | 10 | 0.6 | TBAI + HBD | — | 100 | Grignard et al.103 |
| Cyclic carbonate of resorcinol diglycidyl ether (RDGCC) |

|
Resorcinol | 2 | — | 12 | 12 | — | — | 97 | Clark et al.101 |
| Glycerol polyglycidyl carbonate |

|
Glycerol | 3 | 110 | 90 | 24 | TBAI | — | >98 | Blattman et al.50 |
| Sorbitan bis-carbonate |

|
Sorbitol | 2 | 80 | — | 24 | K2CO3 | MetOH | 40 | Clark et al.101 |
| Cyclocarbonated lignin |
|
Lignin | — | 75 | — | 4 | K2CO3 | GC/DMSO | 96–98 | Sternberg and Pilla99 |
| Trimethylol-propane triglycidyl carbonate (TMPTC) |

|
Trimethylol propane | 110 | 90 | 24 | TBAI | — | >8 | Blattman et al.50 | |
| 2.6 | 80 | 15 | 36 | LiBr | DMF | — | Purwanto et al.97 | |||
| Poly(propylene oxide) bis-carbonate (PPOBC) |
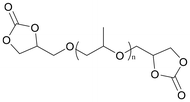
|
PPO | 2 | 80 | 15 | 36 | LiBr | DMF | 76 | Purwanto et al.,97 |
| Ethoxylated TMPTC (EO-TMPGC) |

|
Trimethylol propane | 3 | 140 | 10 | 96 | TBAB | — | 100 | Blattman et al.50 |
| Cardolite® NC-514 cyclic carbonate |

|
Cashew nutshell liquid | 2 | 80 | — | 80 | TBAI | DMF | 100 | Purwanto et al.,45 |
| Linseed oil cyclic carbonate |

|
Linseed oil | >2 | 120 | 60 | 12 | TBAB | — | 79 | Wang et al.89 |
| TC6 |

|
Trimethylol propane allyl ether | 4 | 60 | 1 | 16 | AIBN | 1,4-Dioxane | 87 | Coste et al.,107 |
| SR GreenPoxy 33 cyclic carbonate |

|
SR GreenPoxy (mixture of DGEBA and 1,4-butanediol diglycidyl ether) | >2 | 105 | 1 | 12 | TBAB | — | 85 | Chaib et al.,55 |
| Polypropylene oxide bis-thiocarbonate (PPOTC) |

|
PPO | 2 | 60 | 1 | 96 | LiBr | EtOAc | 85 | Coste et al.,107 |
Bis-cyclic carbonates, i.e., containing two cyclic carbonate functionalities, are the most common CCs, which have been synthesized using several alternative renewable sources. One of the first approaches was to introduce renewable epoxies in fossil-based epoxies to increase the bio-based content of the final product but maintaining most of the properties of the traditional products. For instance, a commercial mixture of 1,4-butanediol diglycidyl ether (bio-based) and the diglycidyl ether of bisphenol A, i.e., DGEBA (fossil-based) named SR GreenPoxy 33 (Sicomin epoxy systems), was successfully converted to the respective 5CCs using atmospheric CO2 at 105 °C and promoted by TBAB.55
Cashew nutshell liquid (CNSL) has been recently explored as a raw material for the synthesis of bis-cyclic carbonate precursors. CNSL is an interesting biowaste material given that it is rich in phenolic derivatives, i.e. anacardic acid and cardol, which can be converted into valuable cardanol.96 After further treatment, cardanol can be functionalized into many compounds. The Cardolite company produces a cardanol-based diepoxy named Cardolite® NC-514, which has been employed as a starting material in the synthesis of cyclic carbonates by the group of Torkelson.45,97 The complete conversion of cardanol diepoxy into the respective bis-cyclic carbonate was registered after 80 h of reaction at 80 °C and in the presence of dimethylformamide (DMF) as a solvent.98 The final product was utilized for the synthesis of NIPU foams after the separation of the solvent.
Another interesting source of NIPU foam precursors is lignin and its derivatives. Lignin is a widely abundant biopolymer with a complex chemical structure and rich in phenolic hydroxy functional groups. Kraft lignin, which is a type of lignin obtained from the waste of the pulp and paper industries, has been converted into cyclocarbonated lignin in a two-step process involving oxyalkylation with glycerol carbonate (GC), followed by transesterification with dimethylcarbonate (DMC) in DMSO (dimethyl sulfoxide).99 As a result, the lignin-based bis-cyclic carbonate was found to have exceptionally high reactivity towards diamines. However, the yield of oxy-alkylated lignin in the first reaction was below 10% due to side reactions. Unfortunately, lignin is difficult to handle due to its very low solubility and brittleness, high-temperature sensitivity, and heterogeneity. Therefore, future research on this process is necessary to overcome these limits.
Besides lignin, its phenolic derivatives can be employed in the synthesis of 5CCs. For instance, a very interesting product of lignin depolymerization, i.e., resorcinol, can be coupled with CO2 at 12 bar after being functionalized with epichlorohydrin.100 The resulting 5-membered bis-cyclocarbonate can be used as a monomer for the synthesis of NIPHU foams. The synthesis of bis-cyclic carbonates from sugars has also been reported. For instance, sorbitol is a very promising bio-based building block for the synthesis of fossil-free polymeric materials. Sorbitol-based CCs101 have been prepared starting from sorbitol and dimethyl carbonate, and successfully employed as co-monomers of diamines in the preparation of flexible NIPU foams.
Multifunctional cyclic carbonates, with functionalities higher than 2, are particularly interesting, given that they enable the creation of highly crosslinked, isocyanate-free polyurethane foams. Compared to bifunctional monomers, they donate higher cross-linking density to the final material given that the other cyclocarbonate moieties act as cross-linkers between the polymer chains. An example of multifunctional cyclic carbonates is trimethylolpropane triglycidyl carbonate (TMPTC), which is obtained from trimethylolpropane by functionalization with epichlorohydrin, followed by coupling with CO2. TMPTC has three cyclic carbonate functionalities and can be either used in copolymerization with diamines51 or further ethoxylated into a TMPTC derivative (EO-TMPGC). Blattman et al.50 selected a blend of these carbonates in the synthesis of NIPU foams to tailor the structure and properties of the final material. According to the authors, blending TMPTC with EO-TMPGC resulted in higher flexibility of the polymeric matrix owing to the longer ether chains of EO-TMPGC. The possibility to blend different cyclic carbonates has also been explored by other research groups. Cornille et al. first synthesized TMPGC and a polypropylene oxide biscarbonate (PPOBC) by coupling CO2 to the respective tri- and di-glycidyl ethers.102 They prepared different blends of TMPGC and PPOBC, investigating the effect of the CC formulations on the characteristics of the final material in the aminolysis process. More recently, Bourguignon et al.53 synthesized a new glycerol-based trifunctional 5CC from CO2 and bio-based glycerol triglycidylether. As a result, employing this 5CC in the synthesis of NIPU foams rather than TMPTC endowed the final material with a higher bio-based content (up to 90%).53
Cyclic carbonates with a functionality exceeding 3 can be obtained from epoxidized vegetable oils. The possibility of employing vegetable oils as a source of NIPU foam precursors has indeed been explored by some research groups. For instance, the carbonation of linseed oil epoxy was carried out under solvent-free conditions at 60 bar and 120 °C in the presence of TBAB, resulting in highly functionalized monomers for the synthesis of NIPU foams.89 Grignard et al. produced soybean oil cyclic carbonates by coupling CO2 with epoxidized soybean oil (ESBO) at 100 bar and 100 °C and employing a bifunctional catalytic system of TBAB with 1,3-bis(hydroxyhexafluoroisopropyl)benzene as a hydrogen bond donor.
The carbonation of ESBO was compared with that of a bifunctional epoxy compound, i.e., poly(ethylene glycol) diglycidyl ether. As a result, the reactivity of the compound containing terminal epoxy groups was higher given that shorter reaction times were observed under milder operating conditions, i.e., at a 10-times lower pressure. A similar trend of the reactivity was translated to the carbonation products in the aminolysis process to obtain NIPU foams.103 Hence, multifunctional cyclic carbonates are valuable monomers in the synthesis of highly cross-linked NIPU foams; however, the carbonation rate of these compounds is lower compared to bifunctional carbonates because the internal epoxy groups are more sterically hindered than the terminal groups.
As demonstrated by the above-mentioned studies, replacing toxic monomers with bio-based cyclic carbonates as monomers for NIPU foams is not only feasible but already shown to be possible. The diversity of renewable/waste sources that can be employed in the synthesis of CCs is a great advantage given that it does not limit the production of CCs to a single feedstock. Moreover, CCs can be obtained in most cases under solvent-free conditions in very high yields, and thus no additional separation steps are required.
To date, very few techno-economic assessments have been conducted exclusively focusing on low molecular weight cyclic carbonates, such as ethylene carbonate (EC) and propylene carbonate (PC).104 The main challenges identified include the need for high pressures, the cost and availability of the starting materials. However, the price of the final CCs can become more competitive in the future, especially considering the savings on CO2 taxes that the plant can achieve by utilizing CO2 as a feedstock, as pointed out by Mishra and Peter.105 The Life Cycle Assessment (LCA) of ethylene carbonate production from coupling CO2 to epoxide was demonstrated to be more environmentally friendly compared to the traditional routes. Moreover, employing CO2 as a raw material is economically beneficial given that it is widely available and inexpensive. The main disadvantages are the relatively high pressures and temperatures employed, which increase the overall process cost.106 Also, Pescarmona95 emphasized that employing CO2 in the synthesis of cyclic carbonates cannot counterbalance anthropogenic carbon dioxide emissions alone because of the large gap between the latter and the actual annual production of 5CCs. Another important point is that all the cyclic carbonates presented were obtained employing discontinuous processes, which are generally not the most convenient on an industrial scale. To date, the continuous approaches, e.g., microreactors and tubular reactors, have been mainly applied for the carbonation of low-molecular-weight monofunctional epoxides, e.g. ethylene oxide, 1,2-epoxyoctane, and styrene oxide92 but not other more complex bio-based systems.85
Nevertheless, different phosgene-free routes have been proposed as promising alternatives in the last decade. The possibility of coupling CO2 with 1,3-diols is undoubtedly the most attractive in terms of sustainability (Scheme 3a). Generally, this reaction is carried out in the presence of a solvent, i.e., chloroform and acetonitrile, at relatively low temperatures and pressures, catalyzed by mild and strong bases, and eventually combined with a dehydrating agent when the base is moisture sensitive.71 The first example of the base-catalyzed cyclocarbonation of diols is the work by McGuire et al.71 In particular, 6-, 7- and 8-membered CCs with one carbonate functionality were synthesized from different diols and CO2 at mild temperatures and pressures. High conversions of diols were achieved, whereas the yields of the resulting 6, 7, and 8 CCs were 71%, 61%, and 43%, respectively, due to side reactions.
 | ||
| Scheme 3 Main routes for the synthesis of 6,7 and 8 CCs: (a) cyclocarbonation of diols and (b) reaction of diols with chloroformate. | ||
Another possibility is to combine diols with ethyl chloroformate (Scheme 3b). For instance, bis-6CCs were prepared starting from castor oil-derived methyl 10-undecenoate through a four-step process.109 In particular, in the second step, undecenoate 1,3-diol is produced and reacts with ethyl chloroformate to obtain the respective 6CCs. To increase the CC functionality, dimerization of the 6CCs was carried out; as a result, the undecenoate bis-6-membered CC was obtained. Finally, the authors compared the aminolysis kinetics of the 6CCs and the equivalent 5-membered CCs. Again, the former monomer was more prone to react with amines. However, the synthesis of these monomers involves many steps and the use of solvents i.e., DMF, tetrahydrofuran (THF), and pentane. Moreover, the conversion of the reactants and the yields of the main products of each step are below unity, and thus separation steps between the process steps are required.
Despite the positive results, the high ring strain and instability of the larger CC rings promote side reactions, impairing the selectivity for the aminolysis main product. Compared to their 5-membered counterparts, high yields of larger CC rings are indeed less likely achieved, which is the main drawback of their eventual upscaling.69 Thus, their application in the synthesis of NIPU is still limited. To the best of our knowledge, only one research article has been published regarding the synthesis of NIPU foams with 6-membered CCs from phosgene-free sources,110 while no scientific report employing 7 or 8 CCs to produce NIPU foams was recorded, suggesting the infancy of research in this field.
Similar to traditional CCs, CTCs can be synthesized using diols and the harmful (thio)phosgene.46 Alternatively, CTCs can be obtained by the reaction between either diols or epoxides (Scheme 4b) with carbon disulfide (CS2), i.e., a gaseous by-product from the fiber industry.111
 | ||
| Scheme 4 Main phosgene-free routes for the synthesis of cyclic thiocarbonates (CTCs): cycloaddition of carbon disulfide to (a) diols and (b) epoxides. | ||
Given that the reactivity of substrates with CS2 is higher than CO2, milder reaction conditions, i.e., room temperature and short reaction times, are usually sufficient to get high yields of CTCs. Depending on the starting material, catalyst, solvent, and operating conditions, cyclic mono- or di-thiocarbonates can be synthesized (Scheme 4). In case (a) (Scheme 4), one sulfur atom is present in the cyclic structure in place of oxygen, whereas in case (b), two sulfur atoms replace two oxygen atoms, respectively. Under specific reaction conditions, e.g., high temperature and in the presence of protic and highly polar solvents, cyclic trithiocarbonates and episulfide can be produced as by-products.112 The choice of the oxirane substrate influences the selectivity of the main products. In fact, the reaction of glycidyl phenyl ether with CS2 in THF at room temperature and catalyzed by the alkali metal halide LiBr yields 97% of the corresponding five-membered cyclic dithiocarbonate in 4.5 h of reaction. However, when employing different epoxides such as vicinal disubstituted epoxides, the yield of the cyclic dithiocarbonates is lower, and longer reaction times are needed.113
Recently, Coste et al. synthesized a thiocarbonate with a double functionality by reacting poly(propylene oxide) diglycidyl ether and carbon disulfide for 96 h at 60 °C and in the presence of LiBr as a homogeneous catalyst. Under these conditions, the thiocarbonation reaction resulted in the formation of a cyclic di-thiocarbonate with a yield of up to 85%.46,107
However, despite the highly interesting properties of CTCs as possible alternatives to CCs, academic research has mainly been focused on the latter subject, as proven by the huge gap in the number of publications on CTCs compared to that of CCs. In fact, in the period 1990–2024, only 1141 articles were published on cyclic thio- and dithio-carbonates, whereas 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 854 articles were published on traditional cyclic carbonates (Scopus, Keywords: cyclic dithiocarbonates OR cyclic thiocarbonates, cyclic carbonates, October 2024).
854 articles were published on traditional cyclic carbonates (Scopus, Keywords: cyclic dithiocarbonates OR cyclic thiocarbonates, cyclic carbonates, October 2024).
Among the dialkyl carbonates, dimethyl carbonate (DMC) is the most utilized for this purpose, given that it is the most prone to nucleophilic substitution.116 The carboxymethylation reaction is usually carried out at a relatively mild temperature and using an organic solvent. Depending on the operating conditions and catalyst used, a mixture of linear and cyclic carbonates might be obtained.80 Besides alcohols, tannins, lignin, and sugars have recently appeared as alternative sources of hydroxyl groups in the transcarbonation with DMC.117
For instance, the carboxymethylation of glucose with DMC was reported by Xi et al.,118 who conducted the reaction in an aqueous environment at 50 °C for 40 min. As a result, a bio-based carbonate monomer was obtained, and then further reacted with diamines to generate urethane bonds for the synthesis of NIPU foams.115 Under similar synthetic conditions, the transcarbonation of tannins with DMC was carried out, demonstrating the feasibility of this reaction with different renewable sources. The tannin-based carbonate was utilized for the synthesis of NIPU foams.119
However, the lack of work utilizing the transcarbonation pathway to produce monomers for the synthesis of NIPU foams is surprising given that this route is relatively easy compared to that for obtaining cyclic carbonates. In fact, it proceeds at high reaction rates at low temperatures (below 90 °C) and it does not require any cyclization step.114 Conversely, it is a relatively new approach given that the first article on this topic only appeared five years ago.117 Moreover, the polycondensation of linear carbonates with diamines produces alcohols as co-products that need to be separated compared to the polyaddition of cyclic carbonates with diamines, which yields only the isocyanate-free polyurethane unit.48 Thus, considering all the reasons discussed, more research on the topic is expected to be carried out in the following years.
2.3 Carbamates
The catalyzed polycondensation of di-carbamates with either polyols or diamines is an alternative approach for the synthesis of NIPU materials.120 In the case of isocyanates, carbamates have been traditionally synthesized from phosgene; however, more sustainable routes have been recently identified. For instance, Unverferth et al.47 developed a novel method to synthesize dicarbamates from castor oil in five reaction steps. However, although this is an interesting sustainable approach, the high number of reactions and separation steps required may limit its feasibility on a large scale. Thus, the most promising phosgene-free processes for the synthesis of carbamates are illustrated in Scheme 6, i.e., the reaction of urea or urea derivatives with alcohol (a), aminolysis of dialkyl carbonates (b) and carboxylation of amines with CO2 and an alcohol (c).Phosgene-free pathways (a) and (b) in Scheme 6 to obtain carbamates are well-known, where the former is usually preferred to the latter from an economical point of view, given that the cost of urea is certainly lower than that of carbonates. However, urea is chemically more stable, and therefore less reactive than carbonates. Moreover, to the best of our knowledge, the alcoholysis of urea and urea derivatives has not been utilized to date for the production of monomers dedicated to the application of NIPU foams.
Pathway (b) is regarded as environmentally friendly given that it involves dialkyl carbonates such as DMC, which are considered green and safe chemicals, and diamines obtained, for example, through fermentation processes (Paragraph 2.3). Moreover, the reaction conditions are usually relatively mild, and the reaction rates quite rapid when employing a specific catalyst.121–123 A recent publication applied this pathway for the synthesis of carbamates by reacting DMC and hexamethylene diamine for 1 h at 70 °C. The resulting carbamate was reacted with D-xylose as a bio-sourced alcohol to yield NIPU foams.124 Similarly, dicarbamates were synthesized from DMC and Priamine 1074 for 5 h at 80 °C employing TBD as the catalyst. The same authors utilized the biscarbamate monomers further in the synthesis of NIPU foams through the transurethanization approach.124
Particularly, the carboxylation of amines with CO2 and an alcohol (c) is attractive from both an environmental and technological viewpoint.125 This pathway is intriguing given that it involves the utilization of CO2.126 However, the formation of water as a co-product is a thermodynamic barrier in the reaction; therefore, dehydrating agents usually need to be employed as well as harsh temperature and pressure conditions, e.g., up to 120 °C and 50 bar, respectively.127,128 Thus, to mitigate these issues, different approaches have been investigated. For instance, replacing alcohols with regenerable metal alkoxides was demonstrated not only to remove the need for dehydrating agents but also reduce the operating CO2 pressure to 1 bar.127 In the work by Zheng et al.,128 aromatic carbamates were obtained under milder reaction conditions (60 °C and 10 bar) employing protonic ionic liquids both as catalysts and substrates and CH2Br2 as a solvent/dehydrating agent. The authors obtained a high amine conversion (97%) and selectivity to the desired carbamate product (99%), demonstrating the multiple recyclability of the catalyst as well. A similar approach that does not require the use of alcohols is the preparation of dicarbamates as amine–CO2 adducts. Recently, Choong et al.54 synthesized different adducts via the reaction between triethylenetetramine and CO2 catalyzed by organic bases, i.e., DBN. The resulting adducts were successfully utilized as co-monomers in the synthesis of NIPU foams. Another class of isocyanate-free carbamate monomers is the bis-carbonylimidazolides (BCI). They can be obtained from the reaction between a diol, e.g., 1,4-butanediol, and carbonyldiimidazole in a relatively few steps and with a final yield exceeding 90%. BCI monomers can be particularly beneficial in the application of NIPU foams given that they spontaneously release CO2 at 140 °C, providing an in situ blowing effect. In fact, the reaction between BCI monomers and triamine resulted in the formation of flexible as well as rigid NIPU foams.129 Despite the few examples available, the use of carbamates as monomers for the synthesis of NIPU foams through the transurethanization approach is highly interesting. Through this process, traditional urethane linkages are obtained, rather than the hydroxyurethane linkages obtained in the polyaddition of CCs with diamines. For this reason, the interest in this pathway may encourage the scientific community to conduct further research on the synthesis of sustainable carbamates and their use in the formulation of foamed polyurethane materials.
2.4 Diamines
Amines with a double primary amine moiety are usually utilized as co-monomers in the reaction with cyclic carbonates. The choice of a specific diamine is particularly crucial given that it can influence the kinetics of the polyaddition reaction with cyclic carbonates as well as the properties of the final material. Depending on the final application of the NIPU foams, either linear or cycloaliphatic diamines can be employed, while aromatic diamines are usually utilized for NIPU coatings and adhesives. For instance, linear aliphatic diamines can be more suitable to synthesize soft and flexible NIPU foams. However, the longer the carbon chain, the lower the reactivity of the diamine. Alternatively, cycloaliphatic diamines can provide rigidity, but also brittleness to the material.110Diamines are traditionally produced from fossil-based substrates, e.g. propylene and butadiene. Owing to the recent cutting-edge advances in bio-engineering, diamines nowadays can be synthesized in more sustainable ways.130 A recent review by Wang et al.131 described all the strategies for the bio-synthesis of both aliphatic and aromatic diamines in detail. However, aliphatic diamines are mainly selected for the synthesis of NIPU foams. In particular, the diamines discussed hereafter together with their structure, bio-source, and production process are presented in Table 2.
| Substrate | Structure | Renewable building block | Process | Ref. |
|---|---|---|---|---|
| 1,3-Diaminopropane (1,3-DAP) |

|
Glucose | Fermentation of sugars with bacteria | Wang et al.127 |
| 1,4-Diaminobutane (1,4-DAB) or putrescine |

|
Glucose | Fermentation of sugars with bacteria | Wang et al.127 |
| Succinic acid | Chemical conversion of succinic acid | Sen Choong et al.54 | ||
| 1,5-Diaminopentane (1,5-DAP) or cadaverine |

|
Glucose, xylose | Fermentation of sugars with bacteria | Wang et al.,127 Dros et al.,137 Meyer et al.140 |
| N 1-(3-Aminopropyl)butane-1,4-diamine or spermidine |
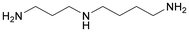
|
Glucose | Fermentation of sugars with bacteria | Dros et al.137 |
| 1,6-Diaminohexane |
|
Starch | Chemical conversion of hydroxymethylfurfural to 1,6-butandiol, followed by high-pressure amination | Dros et al.,137 Radzik et al.134 |
| Biomass | Hydrogenation of bio-adiponitrile | Lee et al.136 | ||
| L-Lysine |

|
L-Lysine | Fermentation of sugars and ammonia with bacteria | Meyer et al.,140 Booysen et al.141 |
| 1,10-Diaminodecane |

|
Castor oil | Chemical conversion of castor oil | Sen Choong et al.54 |
| Isophorone diamine (IPDA) |

|
Bio-based acetone | Condensation of acetone followed by hydrocyanation and amination | /crosslinkers.evonik.com/en/144 |
| Cycloaliphatic diamines, priamine™ |

|
Fatty acids | Chemical conversion of fatty acids | https://www.cargill.com/bioindustrial/priamine 143 |
Bio-based diamines with 3–5 carbon atoms in their backbone can be biotechnologically produced through the fermentation of biomass-derived sugars, e.g. glucose and xylose. In particular, 1,3-diaminopropane, putrescine (1,4-diaminobutane, DAB), cadaverine (1,4-diaminopentane, DAP), and spermidine (N1-(3-aminopropyl)butane-1,4-diamine) can be obtained via the metabolic pathway of different bacteria such as E. coli and C. glutamicum,131,132 either naturally or synthetically with the aid of specific enzymes. Alternatively, bio-based 1,4-butandiamine can be obtained using biomass-derived succinic acid,133 while cadaverine can be produced from the decarboxylation of L-lysine catalyzed by organocatalysts.43,101
Sustainable pathways for the production of renewable longer-chain diamines have been reported in the literature. For instance, 1,10-decanediamine has been prepared by the chemical conversion of castor oil in a three-step process. In particular, castor oil-derived sebacic acid was first reacted with ammonia, followed by dehydration and catalytic hydrogenation steps.134 Hexamethylenediamine (HMDA), also known as 1,4-hexanediamine,50,135 has also been successfully synthesized from renewable feedstock such as starch.50 In particular, starch-derived 5-hydroxymehylfurfural (HMF)136 can be converted into HMDA through three different catalytic processes. Two possible routes are hydrogenation to 2,5-bis(hydroxymethyl)tetrahydrofuran (THFDM), followed by either amination and hydro-deoxygenation or hydrogenolysis and amination. Another possibility is the oxidation of HMF followed by reductive amination and hydrodeoxygenation.137
Moreover, in 2013, the American company Rennovia developed new technology for the synthesis of renewable adipic acid-based HMDA.138 Other vegetable oil-derived diamines are produced by Croda under the commercial name of Priamine™. Specifically, they are a class of cycloaliphatic diamines derived from fatty acid dimers121 and some of them, i.e., Priamine™ 1073 and Priamine™ 1074, have been successfully employed in the synthesis of NIPU foams.49,138
Another trend in the field of bio-monomers for aminolysis reactions is the utilization of amino acids. Employing amino acids as amines can be particularly beneficial from both an environmental and economical point of view given that they are natural and inexpensive feedstock, which can be produced by the fermentation of sugars.139,140 To date, L-lysine, L-glutamine, L-arginine, and L-asparagine have already been explored as natural amines in the reaction with cyclic carbonates to produce NIPU foams.141 The experiments employing L-glutamine, L-arginine, and L-asparagine did not result in the production of NIPU foams, given that heterogeneous mixtures of the amine and the cyclic carbonate were obtained, whereas the reactions with L-lysine successfully produced NIPU foams. According to the authors, the successful results with L-lysine can be explained by its higher solubility in the reaction mixture compared to the other amino acids, owing to its particular structure carrying a positive charge. Among the amino acids studied, L-lysine appears to be the most promising for the synthesis of isocyanate-free foams owing to the above-mentioned reasons, but also because for future scale-up, it is already available on the market in large amounts as a nutrition supplement.141
Cycloaliphatic diamines are interesting building blocks in the cycloaddition reaction with cyclic carbonates. In particular, they can offer more rigidity to the final material owing to their structure compared to linear diamines. Among the bio-based cycloaliphatic diamines, isophorone diamines (IPDA) are quite promising given that isophorone can be synthesized from bio-based acetone. In particular, the group of Caillol have already coinvestigated the potential use of isophorone diamine (IPDA) in the reaction with six-membered cyclic carbonates to produce rigid NIPU foams.110
Considering the wide variety of bio-based diamines presented, it is clear that both the academic and industrial communities have made many attempts to develop natural alternatives to the traditional fossil-based diamines. In fact, some renewable diamines, i.e., Priamine™, have already reached the market level, while others are expected to reach it in the near future.142,143 For instance, Cathay Biotechnology142 has recently scaled up the biosynthesis of cadaverine from natural sources, while the bio-process for the production of 1,4-diaminobutane is expected to be developed on the industrial scale in the next few years. This reflects the increasing interest in the utilization of bio-based diamines. Their industrial production is expected to reach a very high market demand in the near future given that they will be employed not only in the synthesis of NIPU but also in the production of bio-based polyamides.143
3. Synthesis of NIPU foams
In this section, we provide a general overview on the preparation of NIPU foams. As mentioned in the Introduction, it has been reviewed that aminolysis between CCs and diamines represents the most investigated polymerization route to obtain NIPUs in general and NIPU foams.13,31,32,39,48 The presence of CCs allows several OH pendant groups to be achieved in the resulting polyhydroxyurethane chain, which are crucial in the reprocessability of the material (see Section 5).The selection of different CC and diamine structures and functionality, as well as the ratio of CC/NH2 groups determine the different degrees of crosslinking, and thus the mechanical, thermal, chemical/physical, and functional properties of the foamed materials. To prepare porous materials, either physical or chemical blowing agents are needed. However, the aminolysis process is slower than the conventional PU-based synthesis route, and thus quite short reaction times are needed to ensure gas trapping within polyurethane matrices and get porous structures.34 Therefore, currently, this is the main limitation in the technology shift. In this sense, NIPU foam-blowing routes can be classified into two macro-categories, non-self and self-blowing routes, as will be discussed in the next sections.
3.1. Non self-blowing routes
Non-self-blowing processes require the use of physical or chemical BAs to allow the formation of gases and let the blowing process progress.13 In the case of physical BAs, to accurately select physical BAs, it is crucial to verify their physical and chemical characteristics, such as boiling point and heat of vaporization from the viewpoint of economy and process safety.2 In detail, lower boiling temperature BAs enable controllable foaming reactions, resulting in a lower energy input and avoiding critical degradation temperatures for foams. The thermal conductivity (λ) of the physical BAs in the gas phase is crucial given that it plays a significant role in determining the final conductivity of the resulting NIPU foams. Another relevant aspect is the Global Warming Potential (GWP) factor, an estimation of the ozone depletion according to the Montreal and Kyoto Protocols.2,145 The physical BAs used in synthesis of non-self-blowing NIPU foams are summarized in Table 3.| Type of BA | Structure | Boiling temperature (°C) | Heat of vaporization (kJ mol−1) | Molar mass (g mol−1) | λ (mW mK−1) at 25 °C | GWP (−) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| HFC-2345fa is the commercial name of 1,1,1,3,3-pentafluoropropane, HFC-365mfc is the commercial name of 1,1,1,3,3-pentafluorobutane and Solkane 365/227 is the commercial name of the mixture of 1,1,1,3,3-pentafluorobutane (93 wt%) and 1,1,1,2,3,3,3-heptafluoropropane (7 wt%). | ||||||||
| HCs | Pentane |

|
36.1 | 27.6 | 72.1 | 16.4 | 11 | Figvosky et al.146–150 |
| Cyclo pentane |

|
49.1 | 28.7 | 70.1 | 18.76 | 11 | Figvosky et al.149,150 Marrucho et al.151 | |

|
Iso-pentane |

|
28.2 | 24.7 | 72.15 | 11.2 | 11 | Figvosky et al.,149,150 Peyrton & Avérous2 |
| HFCs | HFC-2345fa |

|
15.3 | 26.0 | 134.1 | 12.5 | 990 | Figvosky et al.,147 Peyrton & Avérous2 |
| HFC-365mfc |

|
40.2 | 26.0 | 148.1 | 11.6 | 910 | Figvosky et al.148–150 | |
| Solkane 365/277 |

|
30 | — | 149.6 | 10.1 | 725 | Lauth et al.,152 Blattman et al.50 | |
| Supercritical CO2 | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
−78.6 | 6.8 | 44 | — | 1 | Grignard et al.103 | |
Preliminary investigations on NIPU foams focused on the use of low-boiling physical BAs. Figovsky and co-authors patented in 2004146 the synthesis of acrylic NIPU foams through the radical polymerization of acrylate, whereas the blowing reaction could be carried out by using low-boiling HCs such as pentane or cyclopentane.146–149 Subsequently, non-isocyanate sprayable materials were also patented by the same research group in 2015.150 Specifically, two different mixtures called amino-reactive components, i.e. a mixture of epoxy and cyclocarbonate functional groups, and amino-containing groups, i.e. primary amine, were properly mixed in a reaction chamber and the resulting system was conveyed through an intermediate chamber, where the process parameters can be controlled, and then sprayed onto a clean surface through a nozzle. For the blowing reaction, the authors proposed several physical BAs such as HCs or both saturated and unsaturated HFCs, i.e., HFC-365mfc or HFC-234fa. As a result, the sprayed foams, i.e., with open/closed cell characteristics, were found to be tack-free within 60 s, with a thermal transmission exceeding 0.5 W m−2 K−1.
The use of sustainable precursors in the synthesis of NIPU foams was proposed by Blattmann et al.,50 where bio-based open cell NIPU foams with tailored properties were synthesized at room temperature. A suitable blend of rigid carbonated trimethylolpropane glycidyl ether polyglycidyl-ethers (TMPGE, 60 wt%) and flexible ethoxylated TMP (EO-TMPGC, 40 wt%) was employed given that they possessed both rigid and flexible features, providing adequate pot life and gelation times. HMDA and commercial ozone-free-HFC Solkane 365/227 were selected as the curing agent and BA, whereas DABCO 33 was selected as the gelling catalyst (1 wt%). This route represents an easy one-pot process to obtain flexible NIPU foams within a relatively short time (20–30 min) at room temperature. As a result, foams with open cells ranging from 132 μm to 184 μm and apparent density in the range 83–219 kg m−3, were obtained. The smallest pores and density as well as the highest height were achieved at the shortest mixing time, corresponding to a lower viscosity of the reaction mixture.
Based on this technology, a patent was released by the French company Faurecia Interieur Industrie for the production of NIPU foams for the automotive sector.152 However, the resulting foams possess a high apparent density, corresponding to poor foaming conditions, which limits their use. Conclusively, non-self-blowing routes have produced flexible and semiflexible NIPU foamed materials with an apparent density of 400 kg m−3 in most cases, making them applicable in the automotive sector as integral foams, i.e. for car seats and steering wheels. However, some environmental and toxicity issues should be noted such as the use of physical BA with high GWP and relatively high boiling temperatures, which can lead to high foaming temperatures, and thus compromise their stability.
Closed cell structures with a low density (30–100 kg m−3, rigid foams) and rather low thermal conductivity (λ = 50 mW m−1 K−1) were for the first time prepared using supercritical CO2 (sc-CO2) as a BA at high temperature and high pressure.103 In particular, sc-CO2 has been widely recognized as an inert and green physical blowing agent, enabling the fine-tuning of rigid microcellular structures.153–155 Foams were synthesized using a single-step procedure employing a bio-based amino-telechelic oligoamide and cyclic carbonates obtained from epoxidation of soybean oil at 80 °C for 3 h, and by impregnating sc-CO2 within polymer matrices at different times and pressures. The blowing reaction was carried out by pressurizing CO2 at a temperature close to the melting point of the polymer matrix, while expansion took place due to rapid decompression.103 It was demonstrated that long impregnation times promoted higher CO2 solubility within the polymeric matrix, and thus the formation of narrow and small closed cell morphologies (in the range of 4–22 μm), which was ascribed to high melting temperature (Tm) of the polymer matrix, corresponding to enhanced cell nucleation and the melt strength during foaming. Alternatively, higher pressure (300 bar) induced a more homogenous cell size distribution (range 2–5 μm), translating to low thermal conductivity, which was found comparable to that of conventional thermal insulating materials such as glass wool and wood.156 Recently, Mao et al.157 suggested an sc-CO2-assisted-foaming process by employing a bisphenol A diglycidyl ether (EOBPA)-based cyclic carbonate oligomer and bio-based diamine (HMDA). sc-CO2 was utilized under lower pressure conditions than in ref. 103 (150 bar) and 80 °C for 6 h. The partially bio-based NIPU foams exhibited rigid structures with an average size of 9–22 μm, corresponding to the maximum compressive strength between 120–150 kPa and accomplishing overall compressive behavior close to that of conventional PU foams. Although the use of green physical BA is promising, it should be noted that the use of supercritical CO2 requires specific pressurized conditions, and thus high-cost devices, which may limit the scaling-up of this process.
Besides the use of physical BAs, another valid approach involves exploiting the thermal decomposition of inorganic salts, such as sodium bicarbonate (NaHCO3), to generate CO2 and water (Scheme 7). This is a green and human-safe approach as well as a widely consolidated route to synthesize foams from thermoplastic materials.3 Usually, this decomposition occurs at around 145 °C–150 °C, which matches the chemistry of NIPU foams, as reported by the recent patent by Zeller and Carter158 and the study by Amezúa-Arranz et al.159 In the latter work, the authors studied the effect of the particle size distribution and concentration of NaHCO3, given that both have an impact on the foam reactivity. The best results were obtained using 13 mm particles, attaining a good comprise between the polymerization and blowing reactions, whereas the narrowest pore size distribution was achieved using the highest SC concentration (1.5%), corresponding to relatively low-density foamed systems.
Table 4 summarized the most significant non-self-blowing routes together with their potential environmental impacts and applications.
| Cyclic carbonates | Diamines | Physical BAs | Reaction T and time | Foam morphology | Properties | Applications | Environmental/toxicity/process drawbacks aspects | Ref. |
|---|---|---|---|---|---|---|---|---|
| CCs | Primary diamines | HFC, pentane, cyclopentane | 80 °C, 3 h, 80 °C, 4 h, 120 °C, 2 h | Open cells | ρ in the range of 200–600 kg m−3, tear strength in the range of 3–3.5 MPa, elongation strain >50% | Seating | Use of high GWP-BAs, not sustainable building blocks for precursors are not included. Relatively high-boiling temperature-BAs | Figvosky et al.149,150 |
| TMPGC/EO-TMPGC | HMDA | Solkane 365/227 | 80 °C for 2–6 min, curing at 80 °C for 14 h | Open cells | ρ = 80–220 kg m−3, cell size = 130–180 μm, hysteresis = 13%, hardness = 3 kPa | Automotive | Low VOC emission, formaldehyde-BA, poor foaming (high-density) | Blattman et al.,50 Lauth et al.152 |
| PEG-bisCC, Soybean oil CCs | 1,2-Diaminoethane | Sc-CO2 | 120 °C for 3 h or 24 h | Closed cells | ρ = 110–180 kg m−3 | Thermal insulation | Ecofriendly process/Solvent free | Grignard et al.103 |
| λ = 50–60 mW mK−1 | High cost of supercritical CO2 equipment | |||||||
| High pressure and timing reaction. | ||||||||
| Higher λ and high density compared to that of conventional PUs | ||||||||
| ECBPA-based CC | C36 Alkylenediamine | Sc-CO2 | 80 °C for 6 h, at 150 bar | Closed cells | ρ in the range of 32 and 215 kg m−3 with pore sizes of 10–20 μm, compressive strength in the range of 49.5 and 123.4 kPa | Not indicated | Ecofriendly process/solvent free | Mao et al.157 |
| HDMA | High cost of supercritical CO2 equipment, high-density foams (poor foaming) | |||||||
| EDA | ||||||||
| Tris-CCs | HMDA | NaHCO3 | 150 °C for 6 min | Open cells | ρ = 220–500 kg m3 | Not indicated | Ecofriendly process, high temperatures required | Amezúa-Arranz et al.159 |
| Cell size ranging from 150 to 390 μm |
3.2. Self-blowing processes
Self-blowing, also named self-foaming, involves in situ gas formation with the aid of chemical reactions. According to the definition given in the review by Detrembleur and co-authors,160 self-blowing routes involve the incorporation of chemical blowing agents covalently bound to a polymeric matrix, promoting its expansion by the production of gaseous compounds. Based on their mechanism, self-blowing routes are classified into thermolysis and condensation-based routes160 (Fig. 3).In the first case, blowing is induced by the thermal degradation of chemical BAs anchored to the polymeric matrix, leading to the formation of volatile compounds, such as CO2, water, and alcohol. Condensation routes are endogenous systems, where blowing takes place simultaneously with curing, involving condensation reactions between the functional groups of the precursors and producing expanded materials. Usually, small molecules are employed such as water, thiols, and alcohols. In the synthesis of NIPU foams, two approaches have been selected. In the case of thermolysis, foam formation is driven by the thermal decarboxylation of carbamates, either directly or facilitated by the presence of carbonates or carboxylic acids. Condensation routes involve the reaction of siloxane-based BAs with diamines and the reaction of cyclic carbonates with either thiols or water, i.e. S-alkylation and hydrolysis, respectively.
The most significant chemical BAs employed in self-blowing routes are illustrated in Fig. 4.
In the following sections, the foaming routes are described together with the foam properties. The examined NIPU foams are grouped based on their morphological characteristics, i.e., open-cell and closed-cell structures, and the most relevant morphological, thermal, chemical, physical, and mechanical properties are reviewed.
 | ||
| Scheme 8 Blowing reaction between PHMS (MH 15) and diamine. Reproduced from ref. 49 with permission from Elsevier Ltd., Copyright (2015). License Number 6034151206250. | ||
The effect of the two types of petrol-based cyclic carbonates, i.e. poly(propylene oxide) bis-carbonate and trimethylolpropane tris-carbonate (TMP-Tri-C5) and conventional diamines (Jeffamine EDR 148 and Priamine 1073), on the final characteristics of flexible foams was examined. Five different formulations were obtained depending on the functionalities of the selected cyclic carbonates as well as their chemical structures. Therefore, foamed materials with various morphological characteristics, ranging from semi-flexible to flexible materials, with coarse and interconnected pores (1000–1500 μm-average size), as well as different apparent densities (190–300 kg m−3), were produced (Fig. 5).
 | ||
| Fig. 5 SEM images of developed NIPU foams using PHMS as the blowing agent. Reproduced from ref. 49 with permission from Elsevier Ltd. Copyright (2015). License Number 6034151206250. | ||
The proposed route to formulate NIPU foams required long reaction times and high-temperature conditions, followed by similar curing times, making it practically unfeasible for upscaling purposes; the aminolysis route at room temperature was implemented by the same authors in a later publication,161 although with extremely long reaction times of about three days. Another relevant work was published by Coste et al.,110 where tetrafunctional 6CC from petrol-based sources was selected as precursors, together with diamines with different reactivities (IPDA, cadaverine, Priamine®, and MXDA). This selection enabled the aminolysis process to be carried out at lower operating temperatures (50 °C for 4 h) in the absence of catalysts. As a result, the chemical structures of the diamines implied that different gel times corresponded to different chemical and physical characteristics of the foams. Specifically, diamines with higher reactivity and no steric hindrance allowed gel times (200–400 s) closer to that of conventional PU foams to be achieved. Nonetheless, also in this case, the use of petrol-based precursors was still implicated.
Sustainable rigid NIPU foams based on lignin-derived-cyclic carbonates, obtained through the above-mentioned blowing route, were synthesized via a blowing reaction between diamines and PHMS for the first time by Sternberg & Pilla in 2020.99,162 As mentioned in Section 2.1.1, this work is at the vanguard in the preparation of sustainable NIPU foams from industrial Kraft lignin, overcoming its limitations by proposing a green route that enabled to develop both cyclic carbonate precursors and curing agents. In this case, blowing was carried out using PMHS at 1.5% and 3.0% volume fraction of the total reaction mixture, followed by curing at 150 °C for 4 h. As a result, the rigid foamed materials showed comparable gelation times in the range of 3 min with respect to the conventional PU foams, owing to the combination of high aromaticity provided by the lignin-based precursors and the elasticity of the aliphatic fatty acids-diamines. The obtained rigid foams exhibited porosities in the range of 0.7–0.8% and compressive strengths exceeding 100 kPa under the investigated conditions.
Using a similar foaming route, Li et al.163 suggested the synthesis of lignin-based NIPU foams for wound dressing applications. The addition of silver nanoparticles at different concentrations in the polymeric matrix allowed remarkable antimicrobial properties to be achieved against common pathogens such as Staphylococcus aureus and Escherichia coli (up to 95%).
Conclusively, the use of PHMS as a chemical BA may be associated with potential flammability issues owing to the release of H2. Moreover, the employed operating conditions are still not comparable with that of conventional PU foams as well as the use of precursors from non-sustainable sources, making this foaming route not completely captivating from an environmental point of view. Concerning the foam properties, both open and closed-cell structures presented high apparent densities in the range of 200–400 kg m−3 with performances lower than that of their PU counterparts (Table 5). Additionally, it can be concluded that mainly open-cell foams have been synthesized through this foaming route, which limits their applicability.
| Cyclic carbonates | Diamines | Process conditions | Morphology | Properties | Applications | Ref. |
|---|---|---|---|---|---|---|
| PPO-Bis-C5/TMP-Tri-C5 | Priamine 1073/Jeffamine® EDR-148 | 80 °C, 24 h, curing at 80 °C for 14 h or 25 °C for 3 days | Open cells | ρ in the range of 100–200 kg m−3 | Integral foams for automotive (seating, steering wheel) | Cornille et al.49,161 |
| Thermal stability >300 °C | ||||||
| Lignin-based CC | Priamine 1074 | 80 °C for 4 h, 150 °C for 4 h, 80 °C for 2 h, curing at 150 °C for 12 h | Open/closed cells | ρ in the range of 100–300 kg m−3 | Insulation, packaging | Sternberg & Pilla99,162 |
| E = 1.1.–1.7 MPa, σ (10%) = 105–180 kPa, hysteresis loss = 19–37% | ||||||
| Lignin-based CC | Petrol-based diamine (@China Evolution Technology Co) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CC/NH2, silver nanoparticles at 6.5%, 8% and 10%, curing at 150 °C for 12 h 2 CC/NH2, silver nanoparticles at 6.5%, 8% and 10%, curing at 150 °C for 12 h |
Open cells | Compressive strength at 50% in the range of 28–200 kPa | Wound dressing | Li et al.163 |
| Thermal stability > 350 °C, 99% cell reduction for S. aureus and E. coli within 8 h | ||||||
| 6-Membered CC (TC6) | MDXA, triethylamine, isophoronediamine, cadaverine | 50 °C, overnight. Curing at 120 °C for 2 h | Open/closed cells | ρ in the range of 150–300 kg m−3, cell size = 210–1000 μm, σ (50%) = 140–250 kPa | Insulation, packaging | Coste et al.110 |
3.2.2.1 Thermally and carboxylic acid-driven decarboxylation. Decarboxylation of bis-cyclic carbonates was introduced in 2018 by North and co-workers101 (Scheme 9). The process involved of the formation of bis-carbonates, which can react with diamines, to get PHUs via a solvent-free polymerization, whereas no chemical BAs are needed and in situ CO2 is generated. The examined work represents an important contribution to NIPU research, given that foamed materials with high renewable content (≥90%) were obtained utilizing bio-based building blocks, i.e., sorbitol and L-lysine for the synthesis of CCs and diamines (e.g., cadaverine), through a solvent-free and thermally driven-process. The synthesis was carried out at 100 °C to get rigid-foamed materials, whereas CO2 is well entrapped in the polymeric matrix. A similar approach was selected by Anitha et al.100 to prepare flexible NIPU foams at room temperature for 18 h by selecting resorcinol diglycidylether-based cyclic carbonates and amine-terminated oligomer phenylhydroxylamine (AOPHA), whereas the process was catalyzed by TBD. However, this special route requires harsh conditions and unique chemical compounds (i.e., sorbitol-based), which makes it rather unpractical in many cases.
 | ||
| Scheme 9 Thermally driven decarboxylation of bis-cyclic carbonates derived from sorbitol. Reproduced from ref. 100 with permission from Elsevier Ltd., Copyright (2022). License Number 6034160465054. | ||
Other decarboxylation routes have been reported, whereas acid chemical BAs, i.e., citric,57,117,118,164,165 aconitic, malic, and maleic166 and formic acids57 (Fig. 3), have been involved. Glutaraldehyde is indicated as a curing agent, which easily reacts with amine groups.
In the publications by the group of Pizzi,57,117,118,164 bio-based dimethyl carbonates from mimosa tannin were synthesized and reacted with diamines to get an NIPU resin (defined as tannin-based non-isocyanate polyurethane foams, TNIPU). Interestingly, closed/open-cell systems were obtained with relevant specific compressive strength (higher than 1 kPa kg−1 m−3). Moreover, the flame retardant properties were tested, resulting in comparable features of the conventional PU foams with a limiting oxygen index (LOI) of 27.5%.164 However, it must be emphasized that the use of glutaraldehyde implies environmental and health issues.32
Table 6 summarizes the foaming routes based on decarboxylation and the respective NIPU foams together with their properties and potential applications.
| Carbonate-based precursors | Diamines | Decarboxylation agents | Process conditions | Morphology | Properties | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Bis-carbonate-sorbitol derived-CC | HMDA Cadaverine | Temperature | 100 °C for 20 h | Closed cell | Thermal stability >250 °C Tg about 6 °C | Not indicated | Clark et al.101 |
| Dimethyl carbonate from mimosa tannin | HMDA | NaHCO3/maleic acid | 25 °C for 5 h and curing at 103 °C for 4 h | Closed cell | Thermal stability > 300 °C | Not indicated | Xi et al.117,118 |
| Dimethyl carbonate from tannin | HMDA | Formic acid | 83 °C for 10 h | Closed cell | Maximum compressive strength = 0.8 MPa, LOI = 24.45% | Fire proofing | Zhao et al.57 |
3.2.2.2 S-Alkylation. S-Alkylation (or thiolization, Scheme 10a) offers a greener alternative condensation route to produce hybrid NIPU foams, known as NIPTU, utilizing thiols (–SH) as blowing agents.
 | ||
| Scheme 10 (a) S-Alkylation of CCs with thiols.167 (B) S-Alkylation of CCs with latent thiols and aminolysis of CCs. Reproduced from ref. 52, with permission from the American Chemical Society, Copyright (2022). | ||
The thiol compounds mentioned in the literature are shown in Fig. 3. To date, mainly aliphatic ester- and ether-based compounds have been employed as blowing agents, i.e., 2,2′-(ethylenedioxy)diethanethiol. However, aromatic-based thiols have recently gained attention. They are alternatives to aliphatic thiols owing to their higher basicity, corresponding to higher reactivity and lower gelling times. The group of Detrembleur introduced S-alkylation in 2020167 through the nucleophilic addition of a thiol-(ether (ethylenedioxy)diethanethiol) to diacyl-carbonates (Pearson's reaction) to obtain hydroxy thioether and CO2 (Scheme 10a).
The authors investigated the regioselectivity of the aminolysis/S-alkylation, examining different organo-based catalysts, such as DBU, TBD, DABCO. It was found that, although aminolysis can be carried out without the use of a catalyst at 80 °C, S-alkylation requires suitable organo-based catalysts. Specifically, in the presence of DBU, the resulting foamed materials presented high-density-flexible-like structures (200–400 kg m−3), whereas the dimensional stability was achieved owing to the combination of adequate polymerization and expansion. Specifically, strategies were adopted to enhance foaming and reduce the bubble coalescence, such as higher operation temperature (up to 120 °C), pre-polymerization under ambient conditions or by employing nucleating agents and surface stabilizers, by tailoring microcellular characteristics.167
Subsequently, S-alkylation has been widely recognized as a valuable self-blowing route to prepare NIPTU foams with different characteristics,45,52,97,168 where sustainable precursors, for instance, from the epoxidation of vegetable oils such as CNSL, linseed oil and dimeric acid were employed, achieving a sustainability index of ≥80%.89 The process was carried out under high-temperature conditions between 80 °C–120 °C, although the alkylation process was optimized, resulting in shorter gelling times.
Purwanto et al.52,168 examined the effect of the thiol concentration and type on the final characteristics of the foamed materials. In the first work,52 employing the same reaction conditions and thiol compound, as reported by Monie et al., investigated the S-alkylation route through a rheologically guided approach, whereas under the investigated conditions (120 °C), the gelling times were drastically reduced from several hours to 20 min. A structure–property relationship was proposed for the first time according to the well-established Gibson–Ashby model,2,11,45,169 correlating the mechanical performances with the cellular struts and the apparent density. Tunable characteristics (rise height, foam density, and cellular parameters) were achieved by varying the thiol concentration. In a subsequent work,168 the authors focused their attention on the use of aliphatic thiols with different functionalities (from 2 to 4, S2, S3, and S4) reacting with bio-based CC obtained from the epoxidation of a dimeric acid called GS-120 to modulate the final characteristics of the foamed materials. In all these cases, open-cell foams were obtained (Fig. 6a), with a nice narrow size distribution in the range of 0.2–0.3 mm. The effect of the chemical structure of the thiol was crucial to obtain different cross-link structures, resulting in different compressive properties; at increased thiol functionalities, the compressive and cyclic compressive properties increased, being three or four times higher than that of functionality equal to 2 (Fig. 6b).168
 | ||
| Fig. 6 (a) Digital and SEM images of NIPTU foams with different thiols. (b) Compressive and cyclic compressive stress vs strain curves of the selected foams. Reproduced from ref. 168, with the permission from Elsevier Ltd, Copyright (2024). License number 6034161388565. | ||
However, it is noteworthy that the use of thiols may be associated with the release of volatile organic compounds (VOCs) or some leaching and toxicity issues, and therefore as already described in Section 2.2.2, an interesting way to overcome these issues is to “mask” thiols. This process involves incorporating them into a polymeric matrix, by either employing dithiocarbonates or using thiolactones, enabling the release of thiol groups during aminolysis.52,107 The first approach was introduced by Coste, Negrell & Caillol in 2022;107 a one-pot foaming route was successfully established at 90 °C for 24 h, employing DBU as the catalyst and TMPC and Jeffamine EDR-148 as precursors. The resulting foams presented open-cell structures stabilized by the aid of surfactants and nanometric fillers and two glass transition temperatures in the range of −30 °C to −20 °C (associated with thiocarbonates) and another between 10–20 °C related to the carbonate. In the work by Monie et al.,52N-acetylhomocysteine thiolactone (NAHcT), a thiol-ester derived from bio-based sources (homocysteine), was utilized. This compound initiates a cascade reaction involving sequential steps, as follows: (1) aminolysis of thiol dicarbonates; (2) formation of thiol intermediates; and (3) aminolysis of CC with diamines (Scheme 10b). Also, in this case, aminolysis of NAHTC was found to be much more rapid than the traditional aminolysis process, attaining complete CC conversion within 50 min in the absence of catalysts. Also, in this case, high-density and flexible foams were obtained with comparable properties to that reported by Coste et al.107
Recently, the same research group introduced aromatic thiols (i.e., thiophenol and bis(4-mercaptophenyl) sulfide) as blowing agents (BAs) for NIPTU foams,170 allowing foam production to be carried out under ambient conditions. A combination of aromatic thiols and epoxide additives was proposed. Specifically, in the first case, the higher basicity of aromatic thiols (i.e., higher nucleophilicity) than aliphatic thiols promotes an acid–base reaction with diamines. Alternatively, the presence of epoxides, namely trimethylpropane triglycidyl ether (TMPE), was essential, given that the aminolysis of epoxy is a well-established method in epoxy resin manufacturing and is known to be exothermic.171,172 As a result, the foaming times were found to be drastically shorter than that obtained for the conventional s-alkylation routes using aliphatic thiols, i.e., between 1 to 10 min. Based on different formulations, i.e., ratio of NH2/CC/epoxides/SH, tunable foam characteristics were attained from open-cells and open/closed to closed-cell morphologies with the characteristics (i.e., Tg from −17 °C to 50 °C) effectively comparable to that of conventional PUs. Recently, the group of Verdejo55 proposed a similar approach to prepare NIPU foams with a high sustainability index employing bio-based epoxy monomers. These recent trends present a new opportunity to scale up the route for industrial-level NIPTU foam production, leveraging existing PU infrastructures to create more sustainable materials. For example, this process would be potentially applicable in cases where flexible foams are grown free-rising at room temperature such as mattresses, cushioning and seatings.
3.2.2.3 Hydrolysis. An attractive and greener method to synthesize PHU foams was reported by Detrembleur and co-workers in 2022.51 This method involved using water as a chemical BA, aiming to mimic the reaction conditions of conventional PU foams; thus, combining aminolysis with CC hydrolysis (Scheme 11). Generally, the hydrolysis of CCs requires a large amount of water under a strong alkaline environment.173 Nevertheless, the authors addressed this limitation by synthesizing water-self-blown foams with partial hydrolysis, leading to the formation of diols. The latter ones were not involved in further crosslinking and were available as pendant groups. This aspect was crucial to ensure the recycling and reprocessability of the foam via compression molding and re-utilizing it as polymeric films (see Section 4).
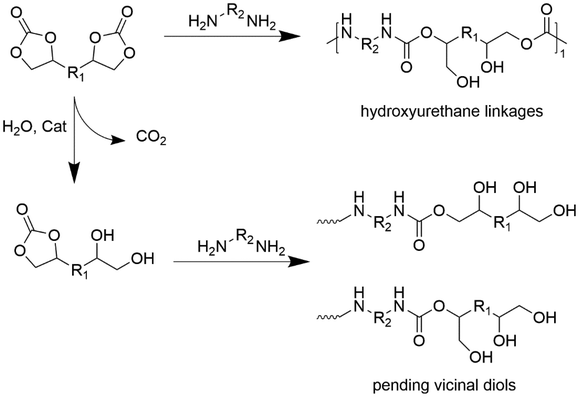 | ||
| Scheme 11 Hydrolysis mechanism to obtain self-blowing NIPU foams. Reproduced from ref. 51, with permission from John Wiley & Sons, Copyright (2022). License number 6034200603992. | ||
Regarding the chemistry of the process, the authors prepared different materials starting from conventional tris(cyclic carbonate) and by screening different amines (both aromatic and aliphatic) as well as temperature and reaction times (80 °C and 100 °C at 3 h and 5 h) and water contents.
Under the selected conditions, it was found that aminolysis yield exceeded 90% within 30 min. Foams with flexible and rigid morphologies were obtained, whereas the addition of nanoclays, e.g. hydrotalcite at 12 wt%, was also fundamental for homogenizing the resulting cellular structures (Fig. 7a). Overall, self-blown foams showed thermal and mechanical behaviors, approaching that of PU materials including compressive strength of 40% in the range of 0.1–0.20 MPa (Fig. 7b, top images). The implementation of the selected technology on a larger scale was demonstrated for the first time; in particular, reaction injection molding (RIM) was selected to fabricate NIPU foams in a closed preheated mold of defined shapes (Fig. 7b, bottom images). A solvent-free route was proposed through preheating at 100 °C and subsequent injection of the precursor mixtures into the preheated mold. The final NIPU foam characteristics were found to be comparable with that of the foams obtained at a lower scale. This represents the first real approach to scale-up this route on a real case, i.e. RIM technology.51
 | ||
| Fig. 7 (a) Morphological investigation of the obtained foams with and without reinforcing filler (hydrotalcite). (b) Comparison between classic foaming process (left) and fast foaming process (right): density, gel content, and thermal properties(top images). Upscaling of NIPU foam production up to 650 g in a closed mold, and SEM images of the scaled-up formulation (bottom images). Reproduced from ref. 51, with permission from John Wiley & Sons, Copyright (2022). License number 6034200603992. | ||
However, it should be noted that this approach, although promising, is only suitable for established PU technologies, requiring high temperatures, such as RIM. Therefore, in this case, epoxy-based click reactions were also suggested by the same authors to prepare NIPU foams under ambient conditions53 through cascade exothermic reactions, as follows: (1) aminolysis of CC, (2) hydrolysis of CC and (3) aminolysis of the epoxy monomer. More specifically, the authors demonstrated that partial replacement of TMPC with the equivalent epoxy monomer (TMPE) resulted in rapid foaming (in 3 min), having high foaming reactivity. Several hybrid foams with open/closed cell structures with a density less than 500 kg m−3 having different thermomechanical properties according to the employed epoxy concentrations were obtained; at lower concentrations, Tg was about 21 °C, similar to other NIPU foams, while, at higher concentrations, Tg was about 35–40 °C, closer to that of epoxy monomers. Interestingly, the foams were also synthesized by selecting bio-based precursors such as glycerol-based CC and epoxy (GTC and GTE) and bio-based diamines (HMDA) and natural fillers, whose properties were found to be comparable to that of conventional PU foams (apparent density lower than 300 kg m−3 and GC higher than 90%), achieving high levels of sustainability (90%). Conclusively, the combination of CC hydrolysis with a click reaction presents the most promising approach for synthesizing a diverse range of NIPU-foamed materials to meet the high demand in the PU market. This process is solvent-free, requiring minimal water amounts and avoiding the release of low-molecular-weight organic compounds. Furthermore, it offers versatility in the bio-based precursor selection, enabling the production of various foamed materials.
To avoid drastic operating conditions, a noteworthy strategy is to synthesize CO2 adducts from carbamates54 (Scheme 12) with a dual purpose, i.e., to act as both the blowing agent and the co-monomer. In particular, the authors started from triethylenetetramine (TETA), which reacts with CO2 for 15 min under ambient conditions in the presence of organic catalysts, to obtain amine–CO2 adducts. This latter reacted with CCs and diamines under milder conditions (i.e., 50–60 °C), resulting in CO2 desorption and leading to CO2-blown NIPU foams. The authors investigated the effect of different precursor concentrations as well as temperatures ranging from 50 °C to 100 °C on CO2 desorption, confirming the variations in foaming rates. Closed and open cell structures were attained, where the best chemical physical and chemical properties, i.e., the apparent density of 200 kg m−3, Tg = 16 °C cell size distribution < 600 μm, and GC > 90%, were linked to the use of bis-AEE as diamine.
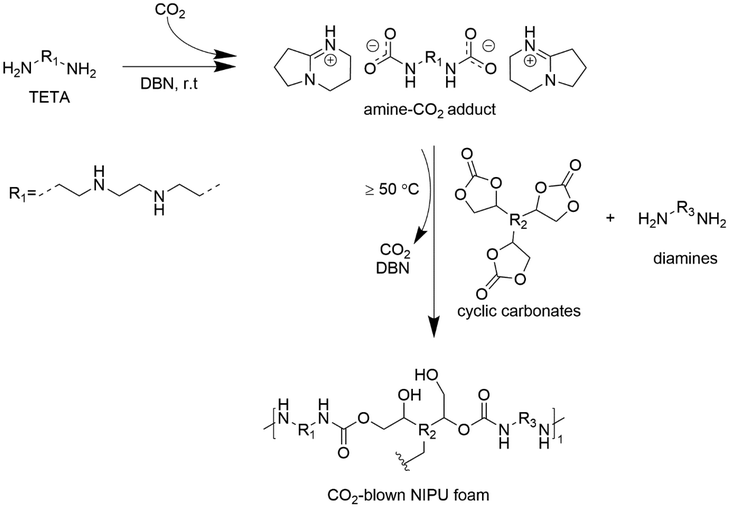 | ||
| Scheme 12 Mechanism for the synthesis of CO2-blown NIPU foams starting from CO2-adduct products. Reproduced from ref. 54, with the permission from the American Chemical Society, Copyright (2023). | ||
Overall, the decarboxylation of carbamates has unused potential and it should be further explored to develop process conditions that more closely align with that of conventional polyurethane materials. Furthermore, it seems to be less energetically favored compared to CC decarboxylation routes, whereas solvent-free and one-pot reactions as well as a broader variety of precursors can be employed.
4 Composite NIPU foams
As reported in Section 3.2, the use of fillers (both from synthetic and natural origins, i.e., inorganic systems such as silica and clay nano or microparticles) have been employed to improve the cell nucleation and cell size distribution of the resulting NIPU foams.51–53,167,168 However, in compliance with the 3R principle of the circular economy plan, reuse, reduce, recycle, presented by the European Commission in 2015,174 a potential solution is to use natural or waste materials from agricultural or industrial sources as fillers. This is an established practice for conventional PU materials, whereas both organic and inorganic fillers have been widely used, such as lignocellulose,11,19,175,176 proteins from agricultural sources such as walnut shells, rice husk, potato, and chitin2,177–179 or animal waste from industrial production, e.g. eggshells, chicken feathers, and leather waste.180,181 It has been demonstrated that these fillers, once intercalated within PU matrices, enable remarkable mechanical and functional performances within specific concentration ranges, as well as reduce the environmental impact of the material. Therefore, the same concept can be easily extended to NIPU foams.In this regard, a relevant contribution was recently reported by Trojanowska et al.56 Different types of bio-fillers, such as keratin, cellulose, gelatin, zein, chitosan, and sodium lignosulfonate, were employed at different concentrations, ranging from 10 wt% to 30 wt%. More specifically, the authors focused their attention on the use of proteins with different amino acid compositions, e.g. cysteine, alanine, lysine, tryptophan, and prolamine, being sources of thiol groups. This allowed the S-alkylation of 5CCs to be performed without the aid of an external chemical BA (Fig. 8i). Surprisingly, operating at 100 °C for 5 h, the amount of thiol was not sufficient for S-alkylation; conversely, the evidence of foaming could be explained merely by the presence of the water content within the solid fillers, which was sufficient to promote the hydrolysis of 5CCs (Scheme 11). This represents a step forward in the production of self-blowing composite NIPU foams through the simple valorization of waste from the agricultural and industrial sectors, without the aid of further reagents. For a specific filler, the concentration effect is crucial to impart different characteristics to the foamed materials, i.e., apparent density (between 200–400 kg m−3) and GC (80%–90%), whereas the lowest apparent density and the highest GC were found between 5 wt% and 20 wt%. Moreover, at a given concentration level (10 wt%), the effect of this type of filler was noticed in terms of morphological and mechanical properties (Fig. 8ii). After the proteins were selected, rigid and semi-rigid foams were obtained with compressive specific strengths (26.3 kPa kg−1 m3), whereas the use of polysaccharides provided flexible foams with much weaker mechanical properties (0.7–6 kPa kg−1 m3). The final properties of the foam were influenced by the dispersion of the type of fillers (i.e., proteins or polysaccharides) within the PHU matrices, leading to distinct interactions and macromolecular rearrangements. This study opens the possibility to use biomass as a reinforcing/reactive component in PHU formulations, tuning the foam characteristics according to the filler selection. Further research is needed either to have full comprehension of the interaction between the bio-filler and the PHU matrices and explore the potential of different waste-derived fillers, for example, with different granulometric characteristics and using other natural and waste sources, imparting specific functional characteristics, i.e., thermal insulation. Attention should be shifted towards optimizing the synthesis route and preparing composite materials with comparable features to that of their composite PU counterparts.
 | ||
| Fig. 8 (i) Synthesis of bio-based composite NIPU foams using waste fillers. (a) Description of bio-based fillers. (b) Reaction mechanism to get hybrid NIPU foams. (c) Example of a typical liquid formulation and its foaming with some representative foams. (ii) A and (B) Digital and SEM images of foams using different biofillers at 10 wt%. (C) Compressive stress vs strain of the selected systems. Reproduced from ref. 56, with the permission from The Royal Chemical Society, Copyright (2024). | ||
5. Recycling and reprocessability of NIPU foams
The typical recycling of conventional PU foam systems primarily relies on mechanical methods, such as shredding, tearing, and grinding the foam and incorporating the resulting granules or powder as a filler in newly manufactured products. Chemical and enzymatic methods are also used, involving the depolymerization and cleavage of urethane bonds, followed by conversion into feedstocks such as polyol and isocyanate precursors.182 Subsequently, these feedstocks can be reintegrated to produce new-generation materials with advanced functional properties (i.e. upcycling), in line with the principles of the circular economy paradigm.183 However, recycling pathways for isocyanate-free polyurethanes including foams have been poorly explored. The only reported evidence of chemical recycling of NIPU foams via hydrolysis was recently documented by Sternrberg & Pilla.162 The authors investigated the recycling of lignin-based NIPU foam in an alkaline solution (0.1 M and 0.2 M KOH) and ethylene glycol (EG) under high pressure in an autoclave. Following the reaction, solid lignin was separated from the liquid mixture containing diamines and surfactant, which was then solubilized in dichloromethane (DCM). Upon precipitation of the mixture at pH 2, lignin powder was produced, which can be reused in the formulation of new-generation foamed materials. The authors compared the recycling yield of the hydrolysis process to that of the glycolysis route (using EG as a solvent) at various reaction times (2 h, 3 h, and 6 h). The highest lignin recovery of 93% was achieved with EG in 0.2 M KOH solution after 6 h. It was observed that the properties of the second-generation materials differed slightly from that of the virgin materials, with increased density and a lower compressive modulus.In the case of conventional PU foams, which are thermoset materials, recycling and reprocessing are limited by their cross-linked structure. Consequently, the development of new recycling methods and reprocessing routes has become an important and rapidly growing field of research. To achieve this, dynamic covalent polymeric networks (DCPNs), also referred to as covalent adaptive networks (CANs),184–187 have been in development, and in recent years, have been proposed for the recycling of conventional PU foams186 and PHU,187–189 NIPU,189–192 NIPU and NIPTU foams.45,167–169 CANs or DCNPs are stable under typical use conditions, but when exposed to specific external stimuli, such as temperature, UV light, pH, and solvents, their covalent bonds can rearrange. The incorporation of CANs allows the reprocessing of cross-linked materials, given that reversible bond breaking enables the material to flow upon heating. This significantly enhances their reprocessability and recyclability, while also potentially providing properties such as shape memory and self-healing.
In particular, dynamic bonds and their associated reactions include ester, carbamate, urea, imine, disulfide, acetal, siloxane and silyl ester bonds, as well Diels–Alder reactions.189,193,194 Reactions between carbamate or urethane groups and hydroxyl groups, essential for the reprocessing and recycling of NIPU and PHU, are termed transcarbamoylation or transurethanization. Urethane exchange specifically refers to reactions that do not involve interactions with hydroxyl groups. Transcarbamoylation can occur via two mechanisms, dissociative (an elimination–addition mechanism involving bond breaking and reformation of alcohol and isocyanate moieties) or associative (an addition–elimination displacement mechanism).195 Associative transesterification reactions and dissociative reversible cyclic carbonate aminolysis can also participate in rearranging the PHU structure during reprocessing (Scheme 13).191
 | ||
| Scheme 13 Reversible cyclic carbonate aminolysis, transesterification and transcarbamoylation reactions.188 | ||
Although conventional PU macromolecule reprocessing has been deemed inefficient and slow, requiring the use of appropriate catalysts, such as tin-based catalysts (dibutyltin dilaurate, DBTDL)184 or 4-(dimethylamino)pyridine (DMAP), PHU networks are superior in their reprocessing ability due to the presence of primary and secondary hydroxyl groups, which can readily partake in carbamate exchange reactions (transcarbamoylation and transurethanisation) under catalyst-free conditions.52,55,194–197 The occurrence of reversible dissociative ring-opening/ring-closing of cyclic carbonate by amine, together with associative transcarbamoylation during PHU reprocessing (T = 140 °C, P ∼ 10 MPa for 2 h), was discovered by Chen et al.191 in 2017 and confirmed in subsequent studies.194 The occurrence of transesterification reactions was also confirmed by Hu et al.,188 who reprocessed dynamic PHU networks synthesized from bio-derived carbonated soybean oil (CSBO) and sorbitol ether carbonate (SEC) with either a synthetic diamine or a bio-based diamine using compression molding at T = 110 °C, P ∼ 11 MPa for 30 min. It was confirmed that transesterification reactions occur under these conditions but are relatively slow. Additionally, it was demonstrated that SEC-based PHU exhibited significantly worse reprocessability compared to CSBO-based PHU due to the higher functionality of groups responsible for cross-linking with short chains between adjacent groups, which led to steric effects and limited mobility of functional groups, both during synthesis and reprocessing. Purwanto et al.97 reported studies on the reprocessability of flexible NIPU foams obtained from the reaction of cyclic carbonates, amines, and thiols. The NIPU foams were reprocessed three times by compression molding at T = 160 °C, P ∼ 16 MPa for 3 h to produce recycled films. DBU used in the foam synthesis process also acted as a catalyst during reprocessing. It was observed that during reprocessing, the unreacted cyclic carbonate was consumed, leading to a post-curing effect and an increase in the glass transition temperature of the film compared to the original NIPU foam. In subsequent work, the same authors reported the synthesis and reprocessing of NIPU foams based on CNSL-derived cyclic carbonates. Foam scraps were subjected to compression molding at T = 140 °C, P ∼ 16 MPa for 2 h to produce recycled films.45 Similarly, Detrembleur and coworkers53 reported the reprocessability of water-blown self-blowing NIPU foams owing to the presence of pending diols groups. Compression moulding was conducted at 160 °C at 2 tons per cm2 pressure for 2 h. Recently, Purwanto et al.97 reprocessed bio-based NIPU foams into bulk films using compression molding at T = 120 °C, P ∼ 10 MPa for 1 h. It was determined that the rearrangement of the PHU network was primarily driven by transcarbamoylation reactions, with contributions from transesterification and reversible cyclic carbonate aminolysis. However, it was found that at temperatures below 150–160 °C, transesterification is largely inactive or significantly suppressed, and only at temperatures above 180 °C the active rearrangement of ester bonds occurred, allowing increased mobility in the PHU network to rearrange. Moreover, the presence of hydroxyl groups in the NIPU material backbone resulted in an increase its hydrophilicity, which may promote hydrolysis, and thus lead to a decrease in the mechanical performances. Monie et al.167 explored the reprocessing of cross-linked network PHU foams into films or structural coatings through heat treatment. The obtained films presented integral structures (no cracks or holes were present), while preserving the crosslinked structures of the original foams. A second life to NIPU materials was then provided as coatings (combined with nylon films) for wear applications, closing the loop of the circular economy. Conclusively, in a recent study, Chen et al.197 developed a series of NIPTU foams through catalyst-free synthesis. The highly dynamic disulfide crosslinks in these foams allow reprocessing by compression molding at temperatures between 140 °C and 180 °C. NIPTU foams were also reprocessed using extrusion and injection molding to reform the material and produce films. The self-healing properties of NIPTU foams were also validated. The reprocessing route is schematically shown in Fig. 9.
 | ||
| Fig. 9 Bio-based, catalyst-free non-isocyanate polythiourethane (NIPTU) foams characterized by dynamic chemical structure, fast reprocessability, extrudability and re-foamability. Reproduced from ref. 197, with permission from Elsevier Ltd., Copyright (2024). License number 6034210145754. | ||
Overall, the presence of dynamic covalent bonds in polymer networks facilitates the reprocessability and potential reuse of the materials. However, several challenges must still be addressed.198 For instance, CANs are mainly derived from petrol-based systems, although few emerging alternatives based on sustainable aromatic building blocks such as lignin, CNSL, tannins and others199 have recently been reported in the literature. Another drawback is that incorporating CANs into the polymer matrix can reduce the mechanical performance of the material, making it more susceptible to deformations such as creep,200,201 a time-dependent deformation under constant stress, particularly at elevated temperatures below the reprocessing threshold. In contrast, conventional thermosets, characterized by permanent covalent cross-links, exhibit intrinsic resistance to creep.200,201 In the study by Hu et al.,202 reprocessable PHU networks reinforced with polyhedral oligomeric silsesquioxanes (POSS) were examined for their creep resistance at elevated temperatures. The results indicated that these dynamic PHU networks exhibited minimal creep, comparable to permanently cross-linked materials at 80–90 °C (approximately 50–60 °C below the reprocessing temperature) under a constant 3.0 kPa shear stress. Indeed, the addition of POSS significantly enhanced the thermal stability of the material without affecting its creep behavior.
Despite their considerable potential, the challenges associated with CANs, make the technological transfer from laboratory to industrial applications impractical from both an economic and environmental perspective. The complexity of their production process, reliance on petroleum-based systems, the potential reduction in mechanical performance, and difficulties in monitoring the reactivity of CANs during reprocessing all contribute to this challenge. As a result, the overall costs and technological hurdles prevent the scaling-up of these materials to industrial levels in the near future.
6. Environmental assessment of precursors and NIPU foams
The evaluation of the environmental impact and potential hazards of products and technologies is critical. Indeed, the mere use of sustainable chemical building blocks and renewable processes might not always guarantee higher levels of sustainability as well as reduced environmental impact of the large-scale production processes.11 In this regard, Life Cycle Assessment (LCA) is a standardized and globally recognized methodology adopted by several companies and academic research units to estimate the environmental impacts according to well-defined parameters known as impact categories (ICs), such as global warming potential, GWP11,203 and eco and human toxicological indicators, such as freshwater aquatic ecotoxicity, FAETP, and human toxicity, HTTP, throughout its entire process, from the production of precursors to waste management.204LCA analysis is regulated by two standards, ISO 14040 and ISO 14044, and involves a “cradle to grave” approach, which is devoted to investigating the life cycles of products and materials comprising the following stages: (i) extraction of raw materials, (ii) processing, (iii) transportation, (iv) usage, (v) waste disposal and (vi) recycling and re-use of waste materials as new raw materials.205 All these points are investigated throughout the LCA main process, which is divided into four different phases. Briefly, the first phase is the definition of goal and scope, where the scope of the analysis is determined, including the product or process under study, the extent of the analysis, and the specific IC from the EN 15804 standard that will guide the assessment. The second phase involves the life cycle inventory (LCI), which entails collecting data on all the inputs such as raw materials and energy and outputs, including emissions to air, land, or water, related to the specific system to be analyzed. The third phase is the life cycle impact assessment (LCIA), where the collected data are used to quantify the environmental impacts of the product or the process. The impact is measured using indicators with corresponding equivalent units, such as carbon dioxide equivalents (CO2-eq) for climate change, allowing an easier comparison of the impacts between different substances. Finally, the fourth phase is the interpretation of the data, where the results are analyzed to draw the conclusions. This step includes comparing the environmental performance of the products to similar products, identifying the opportunities for reducing the impact, and exploring the pathways to enhance the efficiency. To perform this comprehensive analysis regarding a single topic, sophisticated software is necessary, where most common ones are SimaPro™![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 206 Gabi™,207 Ecochain Mobius™, OneClick LCA™,208 OpenLCA™
206 Gabi™,207 Ecochain Mobius™, OneClick LCA™,208 OpenLCA™![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and Umberto.209
11 and Umberto.209
Owing to the environmental issues related to PU production, the development of NIPU foams as more sustainable alternatives to PU foams can be of pivotal interest in the field. Thus, a sustainability assessment of this new class of materials is noteworthy. However, the number of publications on the detailed LCA assessment of NIPU foams as well as regarding their economic feasibility is still extremely limited due to the partial data sets comprehending the raw materials, processes, and services involved in the production of NIPU foams. In addition, the sensitivity concerning the environmental issue represented by the use of di-isocyanates and translated into the studies of NIPU production was initiated approximately twenty years ago.210 From a wider perspective, the introduction of NIPU-based materials on the market may have several environmental benefits, such as the total absence of solvents and the use of greener building blocks.211
6.1. Environmental assessment of precursors
Preliminary studies reported the environmental assessment of NIPU precursors, specifically about 5CCs. LCA analysis on CCs was mostly performed on that synthesized from captured CO2, being proposed as a sustainable CC synthesis route, with a consistent reduction in the GWP parameter and the usage of fossil oil.212 EC was selected as a model precursor given that available data sets in the inventory for LCA are available, even if collected by processes conducted at a low Technology Readiness Level (TRL). As reported by Jahanbani,213 the synthesis of EC involves the reaction between ethylene oxide and CO2 at 120 °C and 16 bar. CO2 was extracted from waste gas streams derived from other chemical processes, whereas the production of ethylene oxide was mainly based on bioethanol from wood waste, together with an energy supply derived from renewable sources. The LCA was evaluated for GWP and human health as ICs. It was found that the best reduction of these two categories was achieved by involving the epoxide obtained from bio-ethylene oxide, and CO2 captured with systems in which renewable energy was employed. On the contrary, the LCA evaluation of the synthesis of other NIPU precursors, i.e. carbamates or diamines, has not been reported in the open literature to date.In the doctoral dissertation of C. M. Laprise,214 an LCA was performed focusing on the epoxidation process, which is crucial for synthesizing epoxidized fatty oils (EFO) from natural oils such as methyl oleate (MO), oleic acid (OA), and fish oil (FO). This process is pivotal given that the resulting epoxides are used to prepare cyclic carbonates by reacting with CO2, which react with amines to create NIPUs. It is evident that the LCA analysis was performed in conjunction with the four epoxidation processes explored, enabling a greener route to be selected for the production of the NIPU. Besides GWP, eight more ICs were selected including acidification potential (AP), ozone depletion potential (OD), smog formation potential (SFP), human toxicity by ingestion (INGTP) and inhalation (INHTP) potentials, persistence (PER), bioaccumulation (BIOACC), and abiotic resource depletion potential (ADP).
The analysis found that the method using 3-chloroperoxybenzoic acid (m-CPBA, Route 1) had the highest GWP at 800 units, mostly due to the heavy use of unpleasant toxic solvents, e.g. dichloromethane (CH2Cl2), which contributed to a high human toxicity potential and smog formation. This method was deemed the least “green”. The second and third routes considered the use of sulfuric acid (H2SO4) and choline chloride–oxalic acid (ChCl–OxA), respectively, together with the use of H2O2. These two methods resulted in much greener processes compared to Route 1, but they still had a significant impact, especially due to the use of corrosive acids and the high inhalation and ingestion indices due to the use of hydrogen peroxide, yielding a GWP of 192 and 133, respectively. However, in the fourth method, H2O2 was maintained and used with formic acid (HCOOH), which made Route 4 the greenest option, with the lowest global warming potential of 54 units, with no acidification, and with low toxicity levels. This route had a high efficiency and minimal environmental impact, making it the most promising for sustainable epoxidation. Additionally, using dimethyl carbonate (DMC), obtained in an eco-friendly way215 as a solvent further improved the green profile by replacing CH2Cl2.
6.2. Environmental assessment of NIPU materials
Liang and co-workers216 conducted an accurate LCA and techno-economic analysis of the production and reprocessing of NIPU and NIPTU foamed materials using data from patents, the open literature, and their experiments. Focusing on LCA, they selected three ICs, namely greenhouse gas (GHG) emissions, use of fossil energy, and water consumption, to conduct the analysis on PHU, NIPTU, and the reprocessed NIPUs. These data were compared to the same metrics with conventional PU. The study considered bulk materials, not foams, but provided useful information about the nature and the weight of each NIPU precursor on the final LCA analysis. This study did not include concerns regarding the use of blowing agents, which would have shown a substantial increase in GHG emissions and the use of fossil energy, even if cyclopentane alone was included. However, they analyzed four different NIPU production pathways, as follows: (1) biomass-based PHU, (2) second-generation PHU via depolymerization, (3) biomass-based NIPTU, and (4) second-generation NIPTU via depolymerization (Fig. 10). As a result, the water and fossil energy use were significantly higher for both the virgin PHU and NIPTU production than for their traditional counterpart, while the GHG emissions were lower for PHU production and narrowly higher for NIPTU. Concerning the reprocessing of both PHU and NIPTU, the water usage was significantly higher with respect to the traditional PU foams and virgin PHU and NIPTU, use of fossil energy, and GHG emissions decreased to a lower amount, depending on whether PHU or NIPTU were involved. The value of the LCA analysis lies in its ability to identify the key material input parameters that have the greatest impact on the selected ICs. This insight enables the authors to provide clear guidance on optimizing the production process to align with the desired sustainability goals of the final products. Given that XDA was selected and fossil-based methanol was replaced with bio-methanol, one-third of the fossil energy use was saved, decreasing with respect to the traditional PU foam production. Moreover, a 16% reduction in fossil energy use could be achieved in the NIPTU production if xylene was recycled. The same authors conducted for the first time a TEA assessment of the resulting NIPU foams. Understanding the economic viability of NIPU foams is essential for assessing their potential impact on the current market. Thus, a sensitivity analysis was carried out to identify cost items, such as taxes, operating expenses, fixed costs, utilities, and raw material costs. The authors reported that the minimum selling price (MSP) of NIPU, PHU, and second-generation PHU foams could be lower than or comparable to that of conventional petroleum-based flexible foams, which serve as a cost benchmark in the range of 3.5–4.5 USD per kg. However, achieving cost competitiveness largely depends on the careful selection of raw materials, such as diamines and solvents used in foam reprocessing (e.g., THF), which significantly influence the material costs. Furthermore, optimizing the design of pilot or industrial-scale production facilities is recommended to align operating costs with that of existing polyurethane (PU) manufacturing lines. Nevertheless, as a main result of this preliminary study, the authors found that from an economic point of view, the best option was to reprocess NIPU foams, in agreement with the circular economy perspectives. | ||
| Fig. 10 Impact categories of different NIPU foams: (a) fossil energy consumption, (b) GHG emissions, and (c) water consumption impacts of NIPU production and reprocessing. Impacts for PHU and NIPTU production are compared with the production of PU flexible and rigid foams. Orange bars are representative of PU/NIPU production, and blue bars are representative of NIPU reprocessing. Reproduced from ref. 216 with permission from the American Chemical Society, Copyright (2024). Licensed under CC-BY-NC-ND 4.0. | ||
Concerning NIPU-based foams, the evaluation of the environmental profile of lignin-based NIPU foams has been reported by Sternberg & Pilla.162 The main topic was the evaluation of the energy consumption and GWP of both virgin foam production (first generation) and chemical recycling process (second generation). The authors compared several ICs of both generations of NIPU foams. In the production of first-generation NIPU foam, the recycling process was performed with different NIPU: solvent ratios of 10% and 20%. The GWP of the recycled NIPU was three times higher than that of the first-generation NIPU, given that low loading ratios (10% NIPU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent) were used. However, by increasing the loading ratio to 20%, the GWP index of the recycled NIPU was reduced, making it only 47% higher than its first-generation counterpart. If industrial process steam was used instead of electricity, a 17.6% reduction in GWP was registered, bringing the GWP of the recycled NIPU much closer to the first-generation value. If process steam was used for the total energy demand, it might have decreased by around 26%. Additionally, using the appropriate amount of ethylene glycol (EG) in the recycling process could potentially decrease the environmental impacts even further, i.e. a 5% EG mixture in the solvent could decrease the GWP of recycled NIPU below the first-generation value.159 Nevertheless, given that the LCA was based on hypothetical scaled-up laboratory procedures, it indicated that with further optimization on a factual industrial scale, the chemical recycling process can achieve even lower environmental impacts than indicated by the initial laboratory results.
solvent) were used. However, by increasing the loading ratio to 20%, the GWP index of the recycled NIPU was reduced, making it only 47% higher than its first-generation counterpart. If industrial process steam was used instead of electricity, a 17.6% reduction in GWP was registered, bringing the GWP of the recycled NIPU much closer to the first-generation value. If process steam was used for the total energy demand, it might have decreased by around 26%. Additionally, using the appropriate amount of ethylene glycol (EG) in the recycling process could potentially decrease the environmental impacts even further, i.e. a 5% EG mixture in the solvent could decrease the GWP of recycled NIPU below the first-generation value.159 Nevertheless, given that the LCA was based on hypothetical scaled-up laboratory procedures, it indicated that with further optimization on a factual industrial scale, the chemical recycling process can achieve even lower environmental impacts than indicated by the initial laboratory results.
7. Conclusions and future perspectives
Over the last decade, non-isocyanate polyurethane (NIPU) foams have gained considerable attention as alternatives to isocyanate-based PU foams owing to the chief environmental concerns associated with isocyanate compounds. These materials provide interesting features compared to the conventional PU systems, such as a unique structure characterized by an abundance of hydroxyl groups in the polymer backbone, which makes them fully recyclable and reprocessable. NIPU foams can be synthesized by two main environmentally friendly routes, i.e. transurethanization of carbamates with alcohols, and aminolysis of linear or cyclic carbonates with diamines. The building blocks of NIPU foams can be produced via different reactions and from various renewable and waste sources, such as vegetable oils, lignin, terpenes, and sugars.In this review article, a comprehensive analysis on the synthesis of NIPU foams is provided, from the synthesis of the bio-based precursors to the environmental (Life Cycle Assessment, LCA) and techno-economic (TEA) assessment of the production processes and final materials. Particular attention was dedicated to the blowing routes, which can be roughly divided into two main categories, non-self-blowing (where physical BAs are employed) and self-blowing routes (consisting of endogenous reactions such as decarboxylation of 5 CCs via S-alkylation, hydrolysis or via suitable chemical BAs). The chemical, physical, mechanical, and thermal characteristics together with the potential applications of the resulting foams were discussed herein. To improve the exothermicity, and thus let the synthesis be conducted at room conditions and at relatively short reaction times (5–30 min), an intriguing and promising way based on epoxy reactions has been set up by the recent literature. Although LCA and TEA analyses have been poorly investigated in the NIPU foam field, preliminary efforts proved the potential economic feasibility of the current routes for NIPU foams as well as the reduced environmental impacts. This gives hope to boost their market in the next few years, leading to potential replacement of the conventional PU materials in several applications. Nevertheless, future investigations are required to examine these routes as well as to exploit different bio-based precursors as well as solid fillers to enhance the final performances of the resulting materials. Compared to isocyanate-based PU foams, NIPU foams have been indicated as fully reprocessable materials owing to the presence of OH or SH functional groups, which can promptly take part in carbamate exchange reactions, either with the aid of specific catalysts or under catalyst-free conditions. As a result, the materials can undergo self-healing or even complete reshaping, providing a valuable solution for the end-life management of thermosetting PU-based systems. Conclusively, efforts should be made in the near future to address the hydrophilicity of these materials through the use of dedicated additives or fillers, and thus by accurately optimizing foam formulations.
Possible future research should address the upcycling of NIPU foams, by reintegrating NIPU foam scraps as building blocks for new-generation materials. Contextually, the evaluation of the environmental impact of NIPU precursors and foams through LCA and TEA should be investigated more extensively. Detailed insights into each stage of the life cycle of foam, from the selection of the building blocks to the end-of-life phases, should be provided, highlighting potential environmental impacts at each step.
Abbreviations
| ADP | Abiotic depletion potential |
| AIBN | Azobisisobutyronitrile |
| AOPHA | Amine-terminated oligomer phenylhydroxylamine |
| AP | Acidification potential |
| BA | Blowing agent |
| BCI | Bis-carbonyl imidazoline |
| BIOACC | Bioaccumulation |
| Bis AEE | 1,2-Bis(aminoethoxy)ethane |
| Br− | Bromide ion |
| CAGR | Compound annual growth rate |
| CANs | Covalent adaptable networks |
| CC | Cyclic carbonate |
| C. glutamicum | Corynebacterium glutamicum |
| CH2Br2 | Dibromomethane |
| CH2Cl2 | Dichloromethane |
| ChCl–OxA | Chloride–oxalic acid |
| Cl− | Chloride ion |
| CLSO | Carbonated linseed oil |
| CMR | Carcinogenic, mutagenic and reprotoxic |
| CNSL | Cashew nutshell liquid |
| CO2 | Carbon dioxide |
| CO2-eq | Carbon dioxide equivalent |
| CSBO | Carbonated soybean oil |
| CS2 | Carbon disulfide |
| CTC | Cyclic dithiocarbonate |
| DA | Dimeric acid |
| DAB | 1,4-Diaminobutane |
| DABCO | Diaminobicyclooctane |
| DAP | 1,5-Diaminopentane |
| DBN | 1,5-Diazabicyclo(4.3.0)non-5-ene |
| DBTDL | Dibutyltin dilaurate |
| DBU | 1,5-Diazabiciclo(5.4.0)undec-7-ene |
| DCM | Dichloromethane |
| DCPN | Dynamic covalent polymeric network |
| DEC | Diethyl carbonate |
| DMAP | (Dimethylamino)pyridine |
| DMC | Dimethyl carbonate |
| DMF | Dimethyl formamide |
| DMSO | Dimethyl sulfoxide |
| E. coli | Escherichia coli |
| EC | Ethylene carbonate |
| EDT | Ethane-1,2-dithiol |
| EFO | Epoxidized fatty oil |
| EG | Ethylene glycol |
| EOBPA | Bisphenol A diglycidyl-ether |
| EO-TMPTC | Ethoxylated trimethylolpropane triglycidyl carbonate |
| EO-TMPGC | Ethoxylated TMPTC derivatives |
| ESBO | Epoxidized soybean oil |
| EtOAc | Ethyl acetate |
| FAETP | Fresh water aquatic ecotoxicity |
| FBC | Furan bis(cyclic carbonate) |
| FO | Fish oil |
| GC | Glycerol carbonate |
| GHG | Greenhouse gas |
| GTC | Glycerol tricyclic carbonate |
| GTE | Glycerol triglycidylether |
| GWP | Global warming potential |
| HBD | Hydrogen-bond donors |
| HC | Hydrocarbon |
| HCl | Chloride acid |
| HCOOH | Formic acid |
| HFC | Hydrofluorocarbon |
| HFO | Hydrofluorolefine |
| HMDA | Hexamethylendiamine |
| HMF | Hydroxymethylfurfural |
| HTTP | Human toxicity |
| HX | Halide acid |
| H2 | Hydrogen |
| H2O | Distilled water |
| H2O2 | Hydrogen peroxide |
| H2S | Hydrogen sulfide |
| I− | Iodide ion |
| IC | Impact category |
| INGTP | Ingestion toxicity potential |
| INHTP | Inhalation toxicity potential |
| IPDA | Isophorone diamine |
| ISO | International standard organization |
| K2CO3 | Potassium carbonate |
| KOH | Potassium hydroxide |
| LC | Linear carbonate |
| LCA | Life cycle assessment |
| LCI | Life cycle inventory |
| LCIA | Life cycle impact assessment |
| LiBr | Lithium bromide |
| LOI | Limiting oxygen index |
| m-CPBA | 3-Chloroperoxybenzoic acid |
| MDI | Methylene-diphenyl di-isocyanate |
| MDXA | N-Methyldiethanolamine |
| MetOH | Methanol |
| MO | Methyl oleate |
| MSP | Minimum selling price |
| NaHCO3 | Sodium bi-carbonate |
| NAHcT | N-Acetylhomocysteine thiolactone |
| NCO | Isocyanate functional group |
| NH2 | Amino group |
| NIPU | Non-isocyanate polyurethane |
| NITPU | Non-isocyanate polythiourethane |
| OA | Oleic acid |
| OD | Ozone depletion potential |
| OH | Hydroxyl group |
| O2 | Oxygen |
| PC | Propylene carbonate |
| PDMS | Polydimethylsiloxane |
| PEG | Polyethylene glycol |
| PER | Persistence |
| PHU | Polyhydroxy urethane |
| pMC | Percent modern carbon |
| PMHS | Polymethyl-hydrogen siloxane |
| POSS | Polyhedral oligomeric silsesquioxanes |
| PPO-Bis-C5 | Polypropylene oxide bis-carbonate |
| PPOBC | Poly(propylene oxide) bis-carbonate |
| PU | Polyurethane |
| RDGCC | Resorcinol diglycidyl ether cyclic carbonates |
| REACH | Regulation on registration, evaluation, authorisation and restriction of chemicals |
| RIM | Reaction injection molding |
| ROH | Alcohol |
| RT | Room temperature |
| S. aureus | Staphylococcus aureus |
| SC | Sodium carbonate |
| Sc-CO2 | Supercritical carbon dioxide |
| SEC | Sorbitol ether carbonate |
| SEM | Scanning electron microscopy |
| SFP | Smog formation potential |
| T | Temperature |
| TADE | 3,3,4,4-Tetraaminodiphenyl ether |
| TBAB | Tetrabutylammonium bromide |
| TBAC | Tetrabutylammonium chloride |
| TBAI | Tetrabutylammonium iodide |
| TBD | Triazabicyclodecene |
| T d,5% | Degradation temperature at 5% weight loss variation |
| TDI | Toluene di-isocyanate |
| TEA | Techno-economic analysis |
| TETA | Triethylenetetramine |
| TEGDT | 3,6-Dioxa-1,8-octanedithiol |
| THF | Tetrahydrofuran |
| THFDM | 2,5-bis(hydroxymethyl)tetrahydrofuran |
| T g | Glass transition temperature |
| T m | Melting temperature |
| TMP | Tri-methyl propane |
| TMPE | Trimethylolpropane triglycidyl ether |
| TMPTC | Trimethylol propane triglycidyl carbonate |
| TMP-Tri-C5 | Trimethylolpropane tris-carbonate |
| TNIPU | Tannin based-non isocyanate polyurethane foams |
| TRL | Technology readiness level |
| VOC | Volatile organic compounds |
| USD | United States Dollar |
| XDA | m-Xylylenediamine |
| Y(NO3)3·6H2O | Lanthanide salt |
| 1,3-BAC | 1,3-Bis(aminomethyl)cyclohexane |
| 5CC | 5-Membered ring cyclic carbonate |
| 6 CC | 6-Membered ring cyclic carbonate |
| 7CC | 7-Membered ring cyclic carbonate |
| 8CC | 8-Membered ring cyclic carbonate |
Greek symbols
| ρ | Apparent density |
| λ | Thermal conductivity |
| σ (40%) | Compressive strength at 40% deformation |
| σ* | Specific compressive strength |
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was carried out within the Made in Italy–Circular and Sustainable Extended Partnership (MICS) and received funding from the European Union Next-Generation EU (Piano Nazionale di Ripresa e Resilienza (PNRR) –missione 4 componente 2, investimento 1.3 –D.D. 1551.11-10-2022 PE00000004) and the Horizon EU project INTEGRANO-G.A. 101138414 (title: Multidimensional Integrated 623 Quantitative Approach To Assess Safety And Sustainability Of Nanomaterials In Real Case Life Cycle Scenarios Using Nanospecific Impact Categories). This manuscript reflects only the authors’ views and opinions, and neither the European Union nor the European Commission can be considered responsible for them. The work is part of the activities financed by the Academy of Finland through the Academy Professor grants 319002, 320115, 345053 (Tapio Salmi, Federica Orabona). Economic support from Åbo Akademi University Graduate School (Federica Orabona) is gratefully acknowledged. Walter Ahlström Foundation is gratefully acknowledged for the financial support (Federica Orabona). Marco Fiume (IPCB-CNR) is gratefully acknowledged for designing and developing the graphical abstract.References
- D. Simón, A. M. Borreguero, A. de Lucas and J. F. Rodríguez, Waste Manag., 2018, 76, 147–171 CrossRef PubMed.
- J. Peyrton and L. Avérous, Mater. Sci. Eng., R, 2021, 145, 100608 Search PubMed.
- S. A. Madbouly, Curr. Opin. Green Sustainable Chem., 2023, 42, 100835 CrossRef CAS.
- A. Yadav, F. M. De Souza, T. Dawsey and R. K. Gupta, Ind. Eng. Chem. Res., 2022, 61, 15046–15065 CrossRef CAS.
- W. Liu, Y. C. Chang, C. Hao, H. Liu, J. Zhang, D. Mielewski and A. Kiziltas, ACS Appl. Polym. Mater., 2022, 4, 5056–5067 CrossRef CAS.
- A. Pascarella, F. Recupido, G. C. Lama, L. Sorrentino, A. Campanile, B. Liguori, M. Berthet, G. Rollo, M. Lavorgna and L. Verdolotti, Adv. Eng. Mater., 2024, 26(7), 2301888 CrossRef CAS.
- Future Market Insights , Polyurethane Foam Market, https://www.marketsandmarkets.com (access on 27 th May 2024).
- G. Coste, C. Negrell and S. Caillol, Eur. Polym. J., 2020, 140, 110029 CrossRef CAS.
- S. Bobbo, G. Di Nicola, C. Zilio, J. S. Brown and L. Fedele, Int. J. Refrig., 2018, 90, 181–201 CrossRef CAS.
- M. Kirpluks, D. Kalnbunde, H. Benes and U. Cabulis, Ind. Crops Prod., 2018, 122, 627–636 CrossRef CAS.
- F. Recupido, G. C. Lama, M. Ammendola, F. D. L. Bossa, A. Minigher, P. Campaner, A. G. Morena, T. Tzanov, M. Ornelas, A. Barros, F. Gomes, V. Bouça, R. Malgueiro, M. Sanchez, E. Martinez, L. Sorrentino, L. Boggioni, M. Perucca, S. Anegalla, R. Marzella, P. Moimare and L. Verdolotti, Polymer, 2023, 267, 126574 CrossRef.
- S. L. D. Randall and S. Lee, The Polyurethanes Book, Wiley, Michigan, USA, 1st edn, 2002 Search PubMed.
- S. El Khezraji, H. Ben Youcef, L. Belachemi, M. A. Lopez Manchado, R. Verdejo and M. Lahcini, Polymers, 2023, 15(2), 254 CrossRef CAS.
- S. F. Melo, A. Nondonfaz, A. Aqil, A. Pierrard, A. Hulin, C. Delierneux, B. Ditkowski, M. Gustin, M. Legrand, B. M. E. Tullemans, S. L. N. Brouns, A. Nchimi, R. Carrus, A. Dejosé, J. W. M. Heemskerk, M. J. E. Kuijpers, J. Ritter, U. Steinseifer, J. C. Clauser, C. Jérôme, P. Lancellotti and C. Oury, Biomater. Sci., 2024, 12, 2149–2164 RSC.
- European Commission , REACH Regulation, 2020, https://www.environment.ec.europa.eu/topics/chemicals/reach-regulation_en (accessed 15 th September 2023).
- H. Sardon, D. Mecerreyes, A. Basterretxea, L. Avérous and C. Jehanno, ACS Sustainable Chem. Eng., 2021, 9, 10664–10677 CrossRef CAS.
- P. Furtwengler and L. Avérous, Polym. Chem., 2018, 9, 4258–4287 RSC.
- U. Cabulis and A. Ivdre, Curr. Opin. Green Sustainable Chem., 2023, 44, 100866 CrossRef CAS.
- F. Recupido, G. C. Lama, M. Lavorgna, G. G. Buonocore, R. Marzella and L. Verdolotti, Food Packag. Shelf Life, 2023, 40, 101175 CrossRef CAS.
- A. Duval, D. Vidal, A. Sarbu, W. Renè and L. Avérous, Mater. Chem. Today, 2022, 24, 100793 CrossRef CAS.
- A. Fridrihsone, F. Romagnoli, V. Kirsanovs and U. Cabulis, J. Cleaner Prod., 2020, 266, 121403 CrossRef CAS.
- M. Kurańska, K. Polaczek, M. Auguścik-Królikowska, A. Prociak and J. Ryszkowska, Polymer, 2020, 190, 122164 CrossRef.
- A. E. Coman, J. Peyrton, G. Hubca, A. Sarbu, A. R. Gabor, C. A. Nicolae, T. V. Iordache and L. Avérous, Eur. Polym. J., 2021, 149, 110363 CrossRef CAS.
- J. Niesiobędzka and J. Datta, Green Chem., 2023, 25, 2482–2504 RSC.
- B. Quienne, F. Cuminet, J. Pinaud, M. Semsarilar, D. Cot, V. Ladmiral and S. Caillol, ACS Sustainable Chem. Eng., 2022, 10, 7041–7049 CrossRef CAS.
- Lupranat® ZERO , https://chemicals.basf.com (accessed on 18 th February 2024).
- Desmodur® QCN 3000 , https://solutions.covestro.com (accessed on 18 th February 2024).
- Vencorex® 100 , https://www.vencorex.com (accessed on 18 th February 2024).
- J. Guan, Y. Song, X. Yin, M. Zou, Y. Zhao, X. Thao and Q. Zheng, Ind. Eng. Chem. Res., 2011, 50(11), 6517–6527 CrossRef CAS.
- M. S. Kathalewar, P. B. Joshi, A. S. Sabnis and V. C. Malshe, RSC Adv., 2013, 3, 4110 RSC.
- A. Gomez-Lopez, F. Elizalde, I. Calvo and H. Sardon, Chem. Commun., 2021, 57, 12254–12265 RSC.
- A. Delavarde, G. Savin, P. Derkenne, M. Boursier, R. Morales-Cerrada, B. Nottelet, J. Pinaud and S. Caillol, Prog. Polym. Sci., 2024, 151, 101805 CrossRef CAS.
- E. Dyer and H. Scott, J. Am. Chem. Soc., 1957, 79(3), 672–675 CrossRef CAS.
- A. Cornille, R. Auvergne, O. Figovsky, B. Boutevin and S. Caillol, Eur. Polym. J., 2017, 87, 535–552 CrossRef CAS.
- C. Carré, Y. Ecochard, S. Caillol and L. Avérous, ChemSustChem, 2019, 12, 3410–3430 CrossRef.
- L. Maisonneuve, O. Lamarzelle, E. Rix, E. Grau and H. Cramail, Chem. Rev., 2015, 115, 12407–12439 CrossRef CAS.
- N. Yadav, F. Seidi, D. Crespy and V. D'Elia, ChemSusChem, 2019, 12, 724–754 CrossRef CAS PubMed.
- A. Mouren and L. Avérous, Chem. Soc. Rev., 2023, 52, 277–317 RSC.
- C. Carré, L. Bonnet and L. Avérous, RSC Adv., 2015, 5, 100390–100400 RSC.
- M. Rayung, N. A. Ghani and N. Hasanudin, RSC Adv., 2024, 14, 9273–9299 RSC.
- C. Claver, M. Bin Yeamin, M. Reguero and A. M. Masdeu-Bultó, Green Chem., 2020, 22, 7665–7706 RSC.
- L. Guo, K. J. Lamb and M. North, Green Chem., 2021, 23, 77–118 RSC.
- M. Ghasemlou, F. Daver, E. P. Ivanova and B. Adhikari, Eur. Polym. J., 2019, 118, 668–684 CrossRef CAS.
- K. Błażek, P. Kasprzyk and J. Datta, Polymer, 2020, 205, 122768 CrossRef.
- N. S. Purwanto, Y. Chen and J. M. Torkelson, ACS Appl. Polym. Mater., 2023, 5, 6651–6661 CrossRef CAS.
- H. Tomita, F. Sanda and T. Endo, Macromolecules, 2001, 34, 727–733 CrossRef CAS.
- M. Unverferth, O. Kreye, A. Prohammer and M. A. R. Meier, Macromol. Rapid Commun., 2013, 34, 1569–1574 CrossRef CAS.
- F. Mundo, S. Caillol, V. Ladmiral and M. A. R. Meier, ACS Sustainable Chem. Eng., 2024, 12, 6452–6466 CrossRef CAS.
- A. Cornille, S. Dworakowska, D. Bogdal, B. Boutevin and S. Caillol, Eur. Polym. J., 2015, 66, 129–138 CrossRef CAS.
- H. Blattmann, M. Lauth and R. Mülhaupt, Macromol. Mater. Eng., 2016, 301, 944–952 CrossRef CAS.
- M. Bourguignon, B. Grignard and C. Detrembleur, Angew. Chem., Int. Ed., 2022, 61(51), e202213422 CrossRef CAS.
- F. Monie, B. Grignard and C. Detrembleur, ACS Macro Lett., 2022, 11, 236–242 CrossRef CAS PubMed.
- M. Bourguignon, B. Grignard and C. Detrembleur, J. Am. Chem. Soc., 2024, 146(1), 988–1000 CrossRef CAS PubMed.
- P. Sen Choong, Y. L. E. Hui and C. C. Lim, ACS Macro Lett., 2023, 12, 1094–1099 CrossRef.
- M. Chaib, S. El Khezraji, S. Thakur, H. Ben Youcef, M. Lahcini and R. Verdejo, React. Funct. Polym., 2024, 200, 105924 CrossRef CAS.
- D. Trojanowska, F. Monie, G. Perotto, A. Athanassiou, B. Grignard, E. Grau, T. Vidil, H. Cramail and C. Detrembleur, Green Chem., 2024, 26, 8383–8394 RSC.
- Y. Zhao, Q. Zhang, H. Lei, X. Zhou, G. Du, A. Pizzi and X. Xi, Int. J. Biol. Macromol., 2024, 258, 128994 CrossRef CAS.
- R. Singh, R. Das, S. Sangwan, B. Rohatgi, R. Khanam, S. K. P. G. Peera, S. Das, Y. A. Lyngdoh, S. Langyan, A. Shukla, M. Shrivastava and S. Misra, Environ. Sustainability, 2021, 4, 619–636 CrossRef CAS.
- B. Koul, M. Yakoob and M. P. Shah, Environ. Res., 2022, 206, 112285 CrossRef CAS.
- M. Crippa, D. Guizzardi, F. Pagani, M. Banja, M. Muntean, E. Schaaf, F. Monforti-Ferrario, W. Becker, R. Quadrelli, A. Risquez Martin, P. Taghavi-Moharamli, J. Köykkä, G. Grassi, S. Rossi, J. Melo, D. Oom, A. Branco, J. San-Miguel, G. Manca, E. Pisoni, E. Vignati and F. Pekar, Publications Office of the European Union 2024, JRC138862, https://publications.jrc.ec.europa.eu/repository/handle/JRC138862 (accessed on 16 th May 2025).
- The Paris Agreement ( https://unfccc.int/process-and-meetings/the-paris-agreement, accessed on 16 th May 2025).
- P. Zhou and M. Wang, Ecol. Econ., 2016, 125, 47–59 CrossRef.
- T. Cogliano, R. Turco, M. Di Serio, T. Salmi, R. Tesser and V. Russo, Ind. Eng. Chem. Res., 2024, 63(26), 11231–11262 CrossRef CAS.
- M. Usman, A. Rehman, F. Saleem, A. Abbas, V. C. Eze and A. Harvey, RSC Adv., 2023, 13, 22717–22743 RSC.
- R. Villa, R. Porcar, S. Nieto, A. Donaire, E. Garcia-Verdugo, S. V. Luis and P. Lozano, Green Chem., 2021, 23, 4191–4200 RSC.
- M. Alves, B. Grignard, S. Gennen, R. Mereau, C. Detrembleur, C. Jerome and T. Tassaing, Catal. Sci. Technol., 2015, 5, 4636–4643 RSC.
- S. J. Groszos and E. K. Drechsel, United States Patent Office Method of preparing a Polyurethane, US Patent, 2802022, 1957 Search PubMed.
- E. J. C. Lopes, A. P. C. Ribeiro and L. M. D. R. S. Martins, Catalysts, 2020, 10(5), 479 CrossRef CAS.
- T. Werner and N. Tenhumberg, J. CO2 Util., 2014, 7, 39–45 CrossRef CAS.
- M. Tamura, M. Honda, Y. Nakagawa and K. Tomishige, J. Chem. Technol. Biotechnol., 2014, 89, 19–33 CrossRef CAS.
- T. M. McGuire, E. M. López-Vidal, G. L. Gregory and A. Buchard, J. CO2 Util., 2018, 27, 283–288 CrossRef CAS.
- J. Sun, L. Liang, J. Sun, Y. Jiang, K. Lin, X. Xu and R. Wang, Catal. Surv. Asia, 2011, 15, 49–54 CrossRef CAS.
- S. G. Khokarale and J. P. Mikkola, RSC Adv., 2019, 9, 34023–34031 RSC.
- R. Turnaturi, C. Zagni, V. Patamia, V. Barbera, G. Floresta and A. Rescifina, Green Chem., 2023, 25, 9574–9602 RSC.
- T. Theerathanagorn, T. Kessaratikoon, H. U. Rehman, V. D'Elia and D. Crespy, Chin. J. Chem., 2024, 42, 652–685 CrossRef CAS.
- J. Steinbauer, C. Kubis, R. Ludwig and T. Werner, ACS Sustainable Chem. Eng., 2018, 6, 10778–10788 CrossRef CAS.
- V. D'Elia and A. W. Kleij, Green Chem. Eng., 2022, 3, 210–227 CrossRef.
- W. Y. Pérez-Sena, X. Cai, N. Kebir, L. Vernières-Hassimi, C. Serra, T. Salmi and S. Leveneur, Chem. Eng. J., 2018, 346, 271–280 CrossRef.
- A. F. Guzmán Agudelo, W. Y. Pérez-Sena, N. Kebir, T. Salmi, L. A. Ríos and S. Leveneur, Chem. Eng. Sci., 2020, 228, 115954 CrossRef.
- L. Wang, S. Que, Z. Ding and E. Vessally, RSC Adv., 2020, 10, 9103–9115 RSC.
- T. Hirose, S. Shimizu, S. Qu, H. Shitara, K. Kodama and L. Wang, RSC Adv., 2016, 6, 69040–69044 RSC.
- M. M. Mazurek-Budzyńska, G. Rokicki, M. Drzewicz, P. A. Guńka and J. Zachara, Eur. Polym. J., 2016, 84, 799–811 CrossRef.
- M. Selva, A. Caretto, M. Noè and A. Perosa, Org. Biomol. Chem., 2014, 12, 4143–4155 RSC.
- A. Rehman, F. Saleem, F. Javed, A. Ikhlaq, S. W. Ahmad and A. Harvey, J. Environ. Chem. Eng., 2021, 9(2), 105113 CrossRef CAS.
- M. Aresta, A. Dibenedetto and A. Angelini, Chem. Rev., 2014, 114(3), 1709–1742 CrossRef CAS.
- F. De La Cruz-Martínez, M. Martínez De Sarasa Buchaca, J. Martínez, J. Fernández-Baeza, L. F. Sánchez-Barba, A. Rodríguez-Diéguez, J. A. Castro-Osma and A. Lara-Sánchez, ACS Sustainable Chem. Eng., 2019, 7, 20126–20138 CrossRef.
- W. Y. Perez-Sena, K. Eränen, N. Kumar, L. Estel, S. Leveneur and T. Salmi, J. CO2 Util., 2022, 57, 101879 CrossRef CAS.
- J. Martínez, F. De La Cruz-Martínez, M. M. De Sarasa Buchaca, M. P. Caballero, R. M. Ojeda-Amador, M. D. Salvador, G. Fregapane, J. Tejeda, J. A. Castro-Osma and A. Lara-Sánchez, J. Environ. Chem. Eng., 2021, 9(4), 105464 CrossRef.
- T. Wang, H. Deng, H. Zeng, J. Shen, F. Xie and C. Zhang, ACS Sustainable Resour. Manage., 2024, 1, 462–470 CrossRef CAS.
- M. Fache, E. Darroman, V. Besse, R. Auvergne, S. Caillol and B. Boutevin, Green Chem., 2014, 16, 1987–1998 RSC.
- Z. Ma, C. Li, H. Fan, J. Wan, Y. Luo and B. G. Li, Ind. Eng. Chem. Res., 2017, 56, 14089–14100 CrossRef CAS.
- A. Rehman, A. M. L. Fernández, M. F. M. G. Resul and A. Harvey, J. CO2 Util., 2019, 29, 126–133 CrossRef CAS.
- V. Schimpf, B. S. Ritter, P. Weis, K. Parison and R. Mülhaupt, Macromolecules, 2017, 50, 944–955 CrossRef CAS.
- M. Bähr, A. Bitto and R. Mülhaupt, Green Chem., 2012, 14, 1447–1454 RSC.
- P. Pescarmona, Curr. Opin. Green Sustainable Chem., 2021, 29, 100457 CrossRef CAS.
- S. K. Kyei, W. I. Eke, R. D. Nagre, I. Mensah and O. Akaranta, Clean. Waste Syst., 2023, 6, 100116 CrossRef.
- N. S. Purwanto, Y. Chen, T. Wang and J. M. Torkelson, Polymer, 2023, 272, 125858 CrossRef CAS.
- F. Jaillet, E. Darroman, B. Boutevin and S. Caillol, OCL:Oilseeds Fats, Crops Lipids, 2016, 23(5), D511 CrossRef.
- J. Sternberg and S. Pilla, Green Chem., 2020, 22, 6922–6935 RSC.
- S. Anitha, G. Unnikrishnan and K. S. Santhosh Kumar, Mater. Lett.:X, 2022, 14, 100142 CAS.
- J. H. Clark, T. J. Farmer, I. D. V. Ingram, Y. Lie and M. North, Eur. J. Org. Chem., 2018, 4265–4271 CrossRef CAS.
- A. Cornille, C. Guillet, S. Benyahya, C. Negrell, B. Boutevin and S. Caillol, Eur. Polym. J., 2016, 84, 873–888 CrossRef CAS.
- B. Grignard, J. M. Thomassin, S. Gennen, L. Poussard, L. Bonnaud, J. M. Raquez, P. Dubois, M. P. Tran, C. B. Park, C. Jerome and C. Detrembleur, Green Chem., 2016, 18, 2206–2215 RSC.
- W. Lin, Z. Xuen, E. D. Chandrakumar, A. Chun Minh Loy, J. Vongsvivut and S. Bhattacharya, ACS Sustainable Chem. Eng., 2024, 12(51), 18320–18334 CrossRef.
- V. Mishra and S. C. Peter, Chem. Catal., 2024, 4, 100796 CrossRef CAS.
- V. Rodin, L. Zeilerbauer, J. Lindorfer, C. Paulik and D. Finger, Front. Sustainable, 2022, 3, 799389 CrossRef.
- G. Coste, C. Negrell and S. Caillol, Macromol. Rapid Commun., 2022, 43, 13 CrossRef PubMed.
- H. Tomita, F. Sanda and T. Endo, J. Polym. Sci., Part A:Polym. Chem., 2001, 39, 4091–4100 CrossRef CAS.
- L. Maisonneuve, A. L. Wirotius, C. Alfos, E. Grau and H. Cramail, Polym. Chem., 2014, 5, 6142–6147 RSC.
- G. Coste, D. Berne, V. Ladmiral, C. Negrell and S. Caillol, Eur. Polym. J., 2022, 176, 111392 CrossRef CAS.
- C. Díez-Poza, L. Álvarez-Miguel, M. E. G. Moquera and C. J. Whiteoak, Org. Biomol. Chem., 2023, 21, 3733–3755 RSC.
- W. Choi, M. Nakajima, F. Sanda and T. Endo, Macromol. Chem. Phys., 1998, 199(9), 1909–1915 CrossRef CAS.
- N. Kihara, Y. Nakawaki and T. Endo, J. Org. Chem., 1995, 60(2), 473–475 CrossRef CAS.
- M. Włoch and J. Datta, Polyur Pol, Blends Interpetreting Pol Net, 2017, ch. 7, pp. 169–202 Search PubMed.
- A. Farzan, S. Borandeh, H. Baniasadi and J. Seppälä, eXPRESS Polym. Lett., 2024, 18(1), 88–101 CrossRef CAS.
- P. Kongpanna, V. Pavarajarn, R. Gani and S. Assabumrungrat, Chem. Eng. Res. Des., 2015, 93, 496–510 CrossRef CAS.
- X. Xi, A. Pizzi, C. Gerardin, H. Lei, X. Chen and S. Amirou, Polymers, 2019, 11(11), 1802 CrossRef CAS PubMed.
- X. Xi, A. Pizzi, C. Gerardin and G. Du, J. Renewable Mater., 2019, 7, 3 Search PubMed.
- X. Chen, X. Xi, A. Pizzi, E. Fredon, X. Zhou, J. Li, C. Gerardin and G. Du, Polymers, 2020, 12(4), 750 CrossRef CAS.
- K. Błażek and J. Datta, Crit. Rev. Environ. Sci. Technol., 2019, 49, 173–211 CrossRef.
- Y. Ono, Catal. Today, 1997, 35(1), 2 Search PubMed.
- H. Q. Li, Y. Cao, X. T. Li, L. G. Wang, F. J. Li and G. Y. Zhu, Ind. Eng. Chem. Res., 2014, 53(2), 626–634 CrossRef CAS.
- P. Singh and R. Kaur, J. Polym. Environ., 2023, 31, 743–753 CrossRef CAS.
- V. Valette, N. Kébir, F. B. Tiavarison, F. Burel and L. Lecamp, React. Funct. Polym., 2022, 181, 105416 CrossRef CAS.
- R. H. Heyn, I. Jacobs and R. C. Carr, Adv. Org. Chem., 2014, 66, 83–115 CAS.
- K. Takeuchi, K. Matsumoto, N. Fukaya, K. Osakada, K. Sato and J. C. Choi, Dalton Trans., 2022, 51, 15631–15643 RSC.
- L. Wang, C. Qi, W. Xiong and H. Jiang, Chin. J. Catal., 2022, 43, 1598–1617 CrossRef CAS.
- L. Zheng, G. Yang, J. Liu, X. Hu and Z. Zhang, Chem. Eng. J., 2021, 425, 131452 CrossRef CAS.
- J. I. Sintas, J. D. Wolfgang and T. E. Long, Polym. Chem., 2023, 14, 1497–1506 RSC.
- V. Froidevaux, C. Negrell, S. Caillol, J. P. Pascault and B. Boutevin, Chem. Rev., 2016, 116, 14181–14224 CrossRef CAS.
- X. Wang, S. Gao, J. Wang, S. Xu, H. Li, K. Chen and P. Ouyang, Chin. J. Chem. Eng., 2021, 30, 4–13 CrossRef CAS.
- S. Y. Lee, J. Nielsen, G. Stephanopoulos, C. Wittmann and J. C. Liao, Industrial Biotechnology: Microorganisms, John Wiley & Sons, Incorporated, 2017, ch. 6 and 7 Search PubMed.
- P. F. H. Harmsen, M. M. Hackmann and H. L. Bos, Biofuels, Bioprod. Biorefin., 2014, 8, 306–324 CrossRef CAS.
- R. Radzik, A. Leszczyńska and K. Pielichowski, Polym. Bull., 2020, 77, 501–528 CrossRef.
- L. Wang, G. Li and Y. Deng, Appl. Environ. Microbiol., 2020, 86, 1–15 Search PubMed.
- J. Lee, Y. Lee, S. Kim, E. E. Kwon and K. Y. A. Lin, Korean J. Chem. Eng., 2021, 38, 1079–1086 CrossRef CAS.
- A. B. Dros, O. Larue, A. Reimond, F. De Campo and M. Pera-Titus, Green Chem., 2015, 17, 4760–4772 RSC.
- Rennovia , Rennovia Develops Process To Produce 100% Bio-Based Nylon, (2023), https://sustainablebrands.com/read/chemistry-materials-packaging/rennovia-develops-process-to-produce00-bio-based-nylon (accessed on 17 th November 2023).
- T. A. Ewing, N. Nouse, M. van Lint, J. van Haveren, J. Hugenholtz and D. S. van Es, Green Chem., 2022, 24, 6373–6405 RSC.
- H. Meyer, W. Minas and D. Schmidhalter, in Industrial Biotechnology, Wiley, 2017, ch. 1, pp. 1–53 Search PubMed.
- J. Booysen, S. Marx, L. C. Muller, U. Vermeulen and A. Grobler, Synthesis of Novel Non-Isocyanate Polyhydroxyurethane from L-Lysine and Its Application, International Institute of Engineers, 2015. DOI:10.15242/iie.e1115053.
- CATHAY Industrial Biotech , Pentamethylene diamine, (2023), https://www.cathaybiotech.com/en/(accessed on 17 th November 2023).
- Cargill , Priamine™, (2023), https://www.cargill.com/bioindustrial/priamine (accessed on 17 th November 2023).
- EVONIK , Evonik launches first renewable isophorone-based products, (2022) https://crosslinkers.evonik.com/en/-169696.html (accessed on 17 th November 2023).
- R. W. Johnson, Int. J. Refrig., 2004, 27(7), 794–799 CrossRef CAS.
- O. Figovsky, L. Shapovalov and O. Axenov, Surf. Coat. Int., Part B, 2005, 87(2), 83–90 CrossRef.
- O. Figovsky, R. Potashnikov, A. Leykin, L. Shapovalov and S. Sivokon, Canadian Patent, CA2840738A1, 2014 Search PubMed.
- O. Figovsky and L. Shapovalov, Unites States Patent, US 7232877B2, 2007 Search PubMed.
- O. Figovsky, R. Potashnikov, A. Leykin, L. Shapovalov and S. Sivokon, Unites States Patent, US US20150024138A, 2015 Search PubMed.
- O. Figovsky, A. Leykin and L. Shapovalov, Altern. Energy Ecol. (ISJAEE)., 2016, 3(4), 95–108 CrossRef.
- I. M. Marrucho, F. Santos, N. S. Oliviera and R. Dohrn, J. Cell. Plast., 2005, 41, 3 Search PubMed.
- M. Lauth, R. Mülhaupt and H. Blattmann, Unites States Patent, US20170218124A1, 2017 Search PubMed.
- Z. Yang, D. Hu, T. Liu, Z. Xu and L. Zhao, J. Supercrit. Fluids, 2019, 153, 104601 CrossRef CAS.
- L. Verdolotti, M. Oliviero, M. Lavorgna, V. Iozzino, D. Larobina and S. Iannace, J. Cell. Plast., 2015, 51, 75–87 CrossRef.
- P. Belmonte, J. M. García-Vargas, J. F. Rodriguez, S. Medorvarschi, I. Garrido, M. T. García and M. J. Ramos, J. CO2 Util., 2023, 71, 102475 CrossRef CAS.
- A. M. Papadopoulos, Energy Build., 2005, 37, 77–86 CrossRef.
- H. I. Mao, C. W. Chen, H. C. Yan and S. P. Rwei, J. Appl. Polym. Sci., 2022, 139, 35 Search PubMed.
- M. A. Zeller and P. Carter, International Patent no. WO2021/067976A1, 2021.
- C. Amezúa-Arranz, M. Santiago-Calvo and M. Á. Rodríguez-Pérez, Eur. Polym. J., 2023, 197, 112366 CrossRef.
- F. Monie, T. Vidil, B. Grignard, H. Cramail and C. Detrembleur, Mater. Sci. Eng., R, 2021, 145, 100628 CrossRef.
- A. Cornille, C. Guillet, S. Benyahya, C. Negrell, B. Boutevin and S. Caillol, Eur. Polym. J., 2016, 84, 873–888 CrossRef CAS.
- J. Sternberg and S. Pilla, Nat. Sustainable, 2023, 6, 316–324 CrossRef.
- J. Li, X. Xu, X. Ma, M. Cui, X. Wang, J. Chen, J. Zhou and J. Chen, ACS Appl. Bio. Mater., 2024, 7, 1301–1310 CrossRef CAS.
- X. Chen, J. Li, X. Xi, A. Pizzi, X. Zhou, E. Fredon, G. Du and C. Gerardin, Polym. Degrad. Stab., 2020, 175, 109121 CrossRef CAS.
- T. Dong, E. Dherssa, M. Wiatrowski, A. Patres Pereira, A. Zeller, L. M. L. Laurens and P. T. Pienkos, ACS Sustainable Chem. Eng., 2021, 9, 12858–12869 CrossRef CAS.
- D. L. Smith, D. Rodriguez-Melendez, S. M. Cotton, Y. Quan, Q. Wang and J. C. Grunlan, Polymers, 2022, 14(22), 5019 CrossRef CAS PubMed.
- F. Monie, B. Grignard, J. M. Thomassin, R. Mereau, T. Tassaing, C. Jerome and C. Detrembleur, Angew. Chem., Int. Ed., 2020, 59, 17033–17041 CrossRef CAS PubMed.
- N. S. Purwanto, Y. Chen and J. M. Torkelson, Eur. Polym. J., 2024, 206, 112775 CrossRef CAS.
- F. Rahimidehgolan and W. J. Altenhof, Composites, Part B, 2023, 253, 110513 CrossRef CAS.
- M. Bourguignon, B. Grignard and C. Detrembleur, Polym. Chem., 2025, 16, 192–203 RSC.
- Z. Ran, X. Liu, X. Jiang, Y. Wu and H. Liao, Thermochim. Acta, 2020, 692, 178735 CrossRef CAS.
- J. A. Marsella and W. E. Sterner, J. Polym. Sci., Part A:Polym. Chem., 2000, 38, 5 CrossRef.
- M. Bourguignon, J. M. Thomassin, B. Grignard, C. Jerome and C. Detrembleur, ACS Sustainable Chem. Eng., 2019, 7, 12601–12610 CAS.
- European Commission. Closing the Loop An EU Action Plan forthe Circular Economy, Brussels; 2015, https://www.eea.europa.eu/policy-documents/com-2015-0614-final (accessed on 11 th October 2024).
- S. Silvano, P. Moimare, L. Gryshchuk, E. Barak-Kulbak, F. Recupido, G. C. Lama, L. Boggioni and L. Verdolotti, Int. J. Biol. Macromol., 2024, 278, 135282 CrossRef CAS.
- O. H. Santos, M. Coelho da Silva, V. R. Silva, W. N. Mussel and M. I. Yoshida, J. Hazard. Mater., 2017, 324, 406–413 CrossRef CAS PubMed.
- S. Członka, M. F. Bertino and K. Strzelec, Polym. Test., 2018, 68, 135–145 CrossRef.
- F. De Luca Bossa, C. Santillo, L. Verdolotti, P. Campaner, A. Minigher, L. Boggioni, S. Losio, F. Coccia, S. Iannace and G. C. Lama, Materials, 2020, 13(1), 211 CrossRef.
- S. M. Husainie, X. Deng, M. Abu Ghalia, J. Robinson and H. E. Naguib, Mater. Today Commun., 2021, 27, 102187 CrossRef CAS.
- S. Członka, M. F. Bertino, K. Strzelec, A. Strąkowska and M. Masłowski, Polym. Test., 2018, 69, 225–237 CrossRef.
- S. Członka, N. Sienkiewicz, A. Strąkowska and K. Strzelec, Polym. Test., 2018, 72, 32–45 CrossRef.
- G. Rossignolo, G. Malucelli and A. Lorenzetti, Green Chem., 2023, 26, 1132–1152 RSC.
- F. Recupido, G. C. Lama, S. Steffen, C. Dreyer, H. Seidlitz, V. Russo, M. Lavorgna, F. De Luca Bossa, S. Silvano, L. Boggioni and L. Verdolotti, Ecotoxicol. Environ. Saf., 2024, 269, 115758 CrossRef CAS PubMed.
- S. A. Madbouly, Curr. Opin. Green Sustainable Chem., 2023, 42, 100835 CrossRef CAS.
- X. L. Zhao, P. X. Tian, Y. D. Li and J. B. Zeng, Green Chem., 2022, 24, 4363–4387 RSC.
- S. Kamarulzaman, Z. M. Png, E. Q. Lim, I. Z. S. Lim, Z. Li and S. S. Goh, Chem, 2023, 9, 2771–2816 CAS.
- D. J. Fortman, J. P. Brutman, C. J. Cramer, M. A. Hillmyer and W. R. Dichtel, J. Am. Chem. Soc., 2015, 137, 14019–14022 CrossRef CAS.
- S. Hu, X. Chen and J. M. Torkelson, ACS Sustainable Chem. Eng., 2019, 7, 10025–10034 CrossRef CAS.
- Y. Chen, M. Laporte and J. M. Torkelson, J. Polym. Res., 2024, 31, 1–12 CrossRef.
- X. Chen, L. Li, T. Wei, D. C. Venerus and J. M. Torkelson, ACS Appl. Mater. Interfaces, 2019, 11, 2398–2407 CrossRef CAS PubMed.
- X. Chen, L. Li, K. Jin and J. M. Torkelson, Polym. Chem., 2017, 8, 6349–6355 Search PubMed.
- A. Hernández, H. A. Houck, F. Elizalde, M. Guerre, H. Sardon and F. E. Du Prez, Eur. Polym. J., 2022, 168, 111000 Search PubMed.
- Y. Chen, N. Mielke, N. S. Purwanto, B. Chen, C. D. Malliakas and J. M. Torkelson, Macromolecules, 2024, 57, 490–502 CrossRef CAS.
- Y. Chen, N. S. Purwanto, B. Chen, T. Wang, S. Kim, Y. W. Huang, W. R. Dichtel and J. M. Torkelson, Chem. Eng. J., 2024, 496, 154035 Search PubMed.
- C. Bakkali-Hassani, D. Berne, V. Ladmiral and S. Caillol, Macromolecules, 2022, 55, 7974–7991 Search PubMed.
- X. Chen, L. Li, T. Wei and J. M. Torkelson, Macromol. Chem. Phys., 2019, 220, 1–9 Search PubMed.
- Y. Chen, N. S. Purwanto, B. Chen, T. Wang, S. Kim, Y. W. Huang, W. R. Dichtel and J. M. Torkelson, Chem. Eng. J., 2024, 496, 154035 CrossRef CAS.
- S. Kotanen, M. Poikelispää, A. Efimov, T. Harjunalanen, C. Mills, T. Laaksonen and E. Sarlin, Int. J. Adhes. Adhes., 2021, 110, 102950 CrossRef CAS.
- L. Sougrati, A. Duval and L. Avérous, Mater. Sci. Eng., R, 2024, 161, 100882 CrossRef.
- Y. You, M. R. Fu, M. Z. Rong and M. Q. Zhang, Mater. Chem. Today, 2022, 23, 100687 CrossRef CAS.
- Y. Gu, Y. Liu and M. Chen, Chem, 2021, 7(8), 1990–1992 CAS.
- S. Hu, X. Chen, M. A. Bin Rusayyis, N. S. Purwanto and J. M. Torkelson, Polymer, 2022, 252, 124971 CrossRef CAS.
- M. Mudersbach, M. Jürgens, M. Pohler, S. Spierling, V. Venkatachalam, H. J. Endres and L. Barner, Macromol. Rapid Commun., 2023, 2300466 Search PubMed.
- R. Silva, A. Barros-Timmons and P. Quinteiro, J. Cleaner Prod., 2023, 430, 139697 CrossRef CAS.
- D. Pan, X. Yu and Y. Zhou, J. Cleaner Prod., 2023, 416, 137921 CrossRef CAS.
- A. Ana, S. M. Ioannidou, N. Giannakis, G. Feijoo, M. T. Moreira and A. Koutinas, Ind. Crops Prod., 2023, 205, 117504 CrossRef.
- C. C. Höhne, A. Salles, P. Brantsch, T. Reichert and R. Hanich-Spahn, J. Phys.: Conf. Ser., 2023, 2526, 012053 CrossRef.
- E. Suttie, C. Hill, G. Sandin, A. Kutnar, C. Ganne-Chédeville, F. Lowres and A. C. Dias, Performance Bio-based Build. Mat, 2017, ch. 9, pp. 547–591 Search PubMed.
- H. E. Wray, S. Luzzi, P. D'Arrigo and G. Griffini, ACS Sustainable Chem. Eng., 2023, 11(21), 8065–8074 CrossRef CAS.
- M. D. Shalati, J. H. McBee, W. J. DeGooyer, F. Thys, L. Van der Ven and R. T. M. Leijzer, Prog. Org. Coat., 2003, 48, 236–250 CrossRef CAS.
- J. Datta and M. Włoch, Polym. Bull., 2016, 73, 1459–1496 CrossRef CAS.
- G. Garcia-Garcia, M. C. Fernandez, K. Armstrong, S. Woolass and P. Styring, ChemSusChem, 2021, 14, 995–1015 CrossRef CAS PubMed.
- M. Jahanbani, Master In Env. Sci, University of Quebec, Canada, 2022 Search PubMed.
- C. M. Laprise, Masters thesis, Memorial University of Newfoundland, St. John's, Canada, 2019.
- B. J. Davies, M. Šarić, M. C. Figueiredo, N. C. Schjødt, S. Dahl, P. G. Moses, M. Escudero-Escribano, M. Arenz and J. Rossmeisl, ACS Catal., 2018, 51(11), 859–866 Search PubMed.
- C. Liang, Y. Jadidi, Y. Chen, U. Gracida-Alvarez, J. M. Torkelson, T. R. Hawkins and J. B. Bunn, ACS Sustainable Chem. Eng., 2024, 12(32), 12161–12170 CrossRef CAS PubMed.
Footnote |
| † These authors equally contributed to this review. |
| This journal is © The Royal Society of Chemistry 2025 |














