 Open Access Article
Open Access ArticleRed analytical performance index (RAPI) and software: the missing tool for assessing methods in terms of analytical performance†
Paweł Mateusz
Nowak
 a,
Wojciech
Wojnowski
a,
Wojciech
Wojnowski
 bc,
Natalia
Manousi
bc,
Natalia
Manousi
 de,
Victoria
Samanidou
de,
Victoria
Samanidou
 d and
Justyna
Płotka-Wasylka
d and
Justyna
Płotka-Wasylka
 *b
*b
aJagiellonian University in Kraków, Faculty of Chemistry, Department of Analytical Chemistry, Laboratory of Forensic Chemistry, Gronostajowa St. 2, 30-387 Kraków, Poland
bDepartment of Analytical Chemistry, Faculty of Chemistry, Gdańsk University of Technology, 11/12 G. Narutowicza Street, 80-233 Gdańsk, Poland. E-mail: plotkajustyna@gmail.com
cDepartment of Chemistry, University of Oslo, P.O. Box 1033-Blindern, 0315 Oslo, Norway
dLaboratory of Analytical Chemistry, School of Chemistry, Aristotle University of Thessaloniki, GR 54124 Thessaloniki, Greece
eInstitute of Chemical Technologies and Analytics, TU Wien, Getreidemarkt 9/164, 1060 Vienna, Austria
First published on 25th April 2025
Abstract
Although performing validation of an analytical method is a widely accepted standard, assessing and comparing the overall analytical potential covering all validation criteria is not straightforward. To answer the expectations of analytical chemists, we propose a new tool that solves this problem in a simple and user-friendly way. The Red Analytical Performance Index (RAPI) presented in this article is inspired by the Red-Green-Blue assessment model, in which the red colour represents analytical criteria. A simple, open-source software (mostwiedzy.pl/rapi) is used to assess the given method in relation to the ten pre-defined criteria. The performance in particular criteria is scored (0, 2.5, 5.0, 7.5, or 10 points), with the sores mapped to colour intensity and saturation where 0 is white and 10 is dark red. A star-like pictogram is automatically created, and divided into fields related to the particular criteria, with the final, mean quantitative assessment score (0–100) in the middle. It thus shows similarities to the Blue Applicability Grade Index (BAGI) – a recently published “sister” tool dedicated to practical criteria represented by the blue colour. Therefore, RAPI and BAGI can support and supplement known greenness assessment metrics, providing key information about functional characteristics, crucial for the method application. The use of RAPI was demonstrated using examples of various analytical methods, which were assessed in parallel using BAGI and the greenness metrics showing the closest analogy. This provided a comprehensive picture of the varied methods’ characteristics. We believe that RAPI will prove to be an effective and useful support for analytical chemists in methods evaluation and comparison.
Green foundation1. Our work contributes to green chemistry by introducing the Red Analytical Performance Index (RAPI) as a complementary tool to existing greenness assessment metrics. While traditional green chemistry assessment tools focus on environmental impact, RAPI provides a broader evaluation of analytical methods, including functional and validation-related aspects. By integrating RAPI with greenness metrics, analytical chemists can achieve a more holistic view of a method's sustainability and practical applicability, ensuring that environmentally friendly methods are also robust, reliable, and suitable for real-world applications.2. The specific green chemistry achievement of our work lies in the development of a systematic and visual approach to evaluating analytical methods. By using a colour-coded star-like pictogram, RAPI facilitates rapid comparison of analytical performance, including efficiency, sensitivity, and waste generation. In our study, we applied RAPI to various analytical techniques, demonstrating its ability to highlight trade-offs between method robustness and environmental impact. By combining RAPI with green chemistry metrics, we provide a more comprehensive evaluation framework that enables informed decision-making when selecting environmentally friendly analytical procedures. 3. Future research could include using RAPI as a starting point for developing a more complex and holistic assessment system. While RAPI currently focuses on analytical performance, integrating specific green chemistry indicators (e.g., solvent toxicity, energy consumption, waste production) would provide a broader look at the quality of the assessed method. Additionally, further research could apply RAPI to a broader range of analytical techniques, including those in industry settings, to identify the most sustainable and high-performing methods for large-scale applications. Automating the tool with AI-driven optimization could further refine method selection, ensuring that the most environmentally friendly yet analytically sound approaches are prioritized. |
1. Introduction
1.1. Greenness assessment metrics
The concept of Green Analytical Chemistry (GAC) has been known for 25 years,1–3 and has recently become increasingly popular. It has been formalized by the formulation of the “12 Principles of GAC” and the “10 Principles of Green Sample Preparation”.4,5 Although the potential impact of performing a single chemical analysis procedure on the environment and safety may seem small compared to chemical synthesis, the ubiquity of analysis processes in many areas of life (environmental and medical laboratories, food analysis, forensic examinations, quality control, and many others) is undeniable. Moreover, almost every synthesis process requires parallel analytical monitoring and often also the use of analytical techniques to purify the synthesis products. From the point of view of green chemistry, the areas of synthesis and analysis are complementary and equally relevant.Tools dedicated to assessing the greenness of analytical methods are currently of great interest and new metrics are constantly being invented.6–8 Among the most commonly used in the analytical community today are the National Environmental Method Index (NEMI),9 Analytical Eco-Scale,10 Green Analytical Procedure Index (GAPI),11 Analytical GREEnness metric (AGREE),12 Complementary Green Analytical Procedure Index (ComplexGAPI),13 Analytical GREEnness metric for sample preparation (AGREEprep),14 Analytical Method Greenness Score (AMGS),15 Chloroform-oriented Toxicity Estimation Scale (ChlorTox Scale),16 and Sample Preparation Metric of Sustainability (SPMS).17 These metrics enable comparison and selection of the greenest method by using coloured pictograms, quantitative rating systems, or both approaches simultaneously (see Fig. 1). They differ in the level of complexity and the selection of criteria. While useful for identifying methods that seem more environmentally friendly and safe, these tools do not allow for a holistic comparison of methods because they omit the criteria that determine their effectiveness and usefulness.
 | ||
| Fig. 1 Pictograms obtained using the selected assessment tools dedicated to analytical methods: (A) GAPI, (B) ComplexGAPI, (C) AGREE, (D) AGREEprep, (E) RGBfast, (F) BAGI. | ||
1.2. White analytical chemistry and BAGI
An extension of GAC with functional features is the White Analytical Chemistry (WAC) concept introduced in 2021,18 which is currently gaining popularity. WAC refers to the Red-Green-Blue model used for colour coding in electronics, in which white light is obtained by superimposing three primary colours: red, green, and blue. Green is, therefore, one of the three basic attributes in the WAC concept, the other two relate to functional features: red to the validation parameters determining analytical performance, and blue to parameters determining practicality and economy. According to WAC, a whiter method is one that shows a better compromise between all three attributes and is overall better suited to the intended application.18,19 The basic tool for assessing and comparing methods in line with the WAC idea are various versions of the RGB model based on specially prepared Excel sheets.18,20,21 The version called RGBfast21 demonstrates the highest degree of automation of the assessment process and eliminates the need for the user to award points arbitrarily, which increases the objectivity of the results obtained (example assessment outcome is shown in Fig. 1E). An alternative to the RGB model are other approaches that allow assessing methods while taking into account both green and functional criteria, such as HEXAGON and Multi-Criteria Decision Analysis methods (MCDA).22,23 Evaluation of the method in the context of the WAC stipulations is crucial to determine its actual suitability for a specific analytical problem, allowing for maintaining a balance between greenness and functional features.Another approach complementary to greenness assessment tools is the Blue Applicability Grade Index (BAGI).24 It is a model referring to the WAC concept,18 dedicated to the assessment of “blue” criteria, i.e. those determining practicality. The assessment procedure is carried out using open-source software (mostwiedzy.pl/bagi), which, based on a simple automated scoring system of 10 selected criteria, visualizes the method's practicality using a pictogram coloured on a scale of white (bad) – dark blue (good), with the overall assessment result given as the number in the centre of a five-pointed star (scale from 25 to 100, see Fig. 1F). The higher the score, the more practical the method. BAGI has been enthusiastically received in the analytical chemistry community. A question that naturally comes to mind is: is it possible to develop the missing, analogous model dedicated to the “red” criteria of WAC, i.e. those determining analytical performance?
1.3. The aim
The aim of this article is to present a new tool for assessing the “redness” of analytical methods, focusing on the ten basic analytical parameters, called the “Red Analytical Performance Index (RAPI)”. The motivation to develop a new tool was the desire to fill a certain gap in the spectrum of currently available tools. RAPI allows to perform the assessment and comparison of analytical methods in the spirit of WAC in a more comprehensive way. There are many metrics aimed at greenness, BAGI is aimed at blueness, while RAPI is supposed to be their natural complement focused on redness. Although the criteria determining analytical performance are included in the RGB model, their assessment is usually limited to only a few, e.g. three in RGBfast (trueness, precision, LOD),21 or four in RGB12 (scope of application, LOD&LOQ, precision, accuracy).18 By design, RAPI is aligned with general validation guidelines and good laboratory practice, takes into account a number of versatile criteria and allows for obtaining a more holistic picture. In addition, it expresses information in a simple graphical way. Therefore, although RAPI does not directly consider any criteria related to greenness, it promotes green chemistry indirectly by helping to achieve the right balance between greenness and performance.2. RAPI description
The idea of RAPI is similar to that known from BAGI. It employs a simple, open-source Python-based software (https://mostwiedzy.pl/rapi),25 available under the MIT license, that allows the user to make a quick assessment by selecting appropriate options from a drop-down menu. RAPI is primarily dedicated to quantitative analysis methods. The selection of assessment parameters was guided by ICH recommendations for validation,26–29 generally accepted principles and good laboratory practice. Since the number of parameters indicating analytical performance is quite large, we decided to select those that are most expected and universal (apply to all kinds of analytical methods):(1) repeatability (variation in results when measurements are performed by a single analyst using the same equipment over a short timescale),
(2) intermediate precision (variation in results when measurements are made in a single laboratory but under conditions that are more variable than repeatability conditions, e.g. over a longer timescale and/or by different operators),
(3) reproducibility (expected to give the largest variation in results, a measure of the variation obtained in different laboratories, using different equipment, by different operators)27,28 – the expression of these criteria (1–3) is percentage Relative Standard Deviation (RSD%); the use of Analysis of Variance (ANOVA) is recommended for its estimation;27
(4) trueness (expressed in relative error/bias (%), the agreement of the average result of measurements with the true value, measured using Certified Reference Materials (CRMs) or, only if CRMs are unavailable, by adding a known amount of analyte to a sample not previously containing the analyte, or alternatively, by comparing the results with the reference method of verified trueness);
(5) recovery and matrix effect (parameters not required in basic validation but showing additional method's features, recovery expressed quantitatively as the percentage of the added analyte concentration, and the matrix effect expressed qualitatively, depending on the found impact of the matrix on the analytical result);
(6) limit of quantitation (LOQ, expressed for the purpose of RAPI, as a percentage of the mean expected analyte concentration in a given sample type);
(7) working range (expressed as a distance between LOQ and its multiple indicating maximum concentration);
(8) simplified linearity estimation (coefficient of determination obtained for the calibration plot, R2 – although this is not a comprehensive expression of linearity, it was chosen due to its universality);
(9) ruggedness/robustness (expressed as the number of experimental factors that were found not to affect the precision/trueness of the method); and
(10) selectivity (expressed as the number of chemical interferents that were found not to affect the precision/trueness of the method).
Most of them are commonly determined by authors when developing new methods, so a large amount of comparative data can be found in the literature. Some, however, are less frequently tested despite the general recommendations, e.g. reproducibility or ruggedness. We included them to promote a more comprehensive approach and encourage users to enrich their standard validation protocol with missing parameters.
RAPI does not differentiate the importance of individual parameters, the weight of each of them is the same. While in some cases certain criteria may be prominent, and others less influential (e.g. LOQ may seem more important than recovery), there is no solid basis for predicting this in advance and generalizing potential scenarios in the model's structure. The use of RAPI, by definition, is intended to provide complex information regarding the wide spectrum of analytical parameters. Thus, RAPI assessment cannot be the sole basis for determining whether the method is fit-for-purpose.
Each criterion is assessed according to the general scheme shown in Table 1. The scoring scheme is five-level, on a scale of 0 (worst result), 2.5, 5.0, 7.5, and 10 (best result). The value 0 is also given to the method that has not been tested at all for a given parameter, and therefore there is no data to confirm a given method feature. The sum of all scores, in the range of 0–100, is placed in the central part of the pictogram. The better the overall performance of the method, the higher this value, see Fig. 2.
| Number | Criterion name | 10 points | 7.5 points | 5.0 points | 2.5 points | 0 points |
|---|---|---|---|---|---|---|
| 1 | Repeatability | RSD% from <0.5 to <11.3 (depending on concentration), fulfilled on all studied levels | RSD% from <1.0 to <22.7 (depending on concentration), fulfilled on all studied levels | RSD% from <1.5 to <34.0 (depending on concentration), fulfilled on all studied levels | RSD% from <2.0 to <45.3 (depending on concentration), fulfilled on all studied levels | Worse than for 25 points or not tested |
| 2 | Intermediate precision | RSD% from <1.0 to <22.7 (depending on concentration), fulfilled on all studied levels | RSD% from <1.5 to <34.0 (depending on concentration), fulfilled on all studied levels | RSD% from <2.0 to <45.3 (depending on concentration), fulfilled on all studied levels | RSD% from <2.5 to <56.6 (depending on concentration), fulfilled on all studied levels | Worse than for 25 points or not tested |
| 3 | Reproducibility | RSD% from <1.5 to <34.0 (depending on concentration), fulfilled on all studied levels | RSD% from <2.0 to <45.3 (depending on concentration), fulfilled on all studied levels | RSD% from <2.5 to <56.6 (depending on concentration), fulfilled on all studied levels | RSD% from <3.0 to <68.0 (depending on concentration), fulfilled on all studied levels | Worse than for 25 points or not tested |
| 4 | Trueness | As for 75 points, confirmed with CRMs | Error/bias% from <1.0 to <40.0, depending on concentration, fulfilled on all studied levels | Error/bias% from <2.0 to <60.0, depending on concentration, fulfilled on all studied levels | Error/bias% from <3.0 to <80.0, depending on concentration, fulfilled on all studied levels | Worse than for 25 points or not tested |
| 5 | Recovery and matrix effect (ME) | Recovery% from >99.5, <100.5 to >80, <110, depending on concentration, fulfilled on all studied levels; ME is studied and found weak | Recovery% from >99, <101 to >60, <115, depending on concentration, fulfilled on all studied levels; ME is studied and found acceptable | Recovery% from >98, <102 to >40, <120, depending on concentration, fulfilled on all studied levels; ME optionally studied | Recovery% from >97, <103 to >20, <130, depending on concentration, fulfilled on all studied levels; ME optionally studied | Worse than for 25 points or not tested |
| 6 | LOQ (limit of quantification as % of expected mean analyte concentration) | LOQ <1% of mean concentration | LOQ <3% of mean concentration | LOQ <10% of mean concentration | LOQ <25% of mean concentration | Worse than for 25 points or not tested |
| 7 | Working range | Wider than 100 × LOQ | Wider than 30 × LOQ | Wider than 10 × LOQ | Wider than 5 × LOQ | Worse than for 25 points or not tested |
| 8 | Simplified linearity estimation | R 2 > 0.99 | R 2 > 0.97 | R 2 > 0.94 | R 2 > 0.90 | Worse than for 25 points or not tested |
| 9 | Ruggedness/robustness | If demonstrated for at least 5 factors | If demonstrated for at least 3 factors | If demonstrated for at least 2 factors | If demonstrated for at least 1 factor | If not demonstrated |
| 10 | Selectivity | If demonstrated for at least 5 potential interferents | If demonstrated for at least 3 potential interferents | If demonstrated for at least 2 potential interferents | If demonstrated for at least 1 potential interferents | If not demonstrated |
Since there is a confirmed relationship between analyte concentration and parameters such as precision, trueness and recovery, the Horwitz model was adapted and included in the scoring scheme.27–29 In general, regardless of the sample type, it should be expected that the lower the concentration, the higher the RSD and error values should be expected. Accordingly, when assessing a given parameter, the user first indicates the concentration of the analyte, which results in automatic adjustment of the requirements taking into account the adapted Horwitz model.28 Then, the user indicates the value of the parameter, which automatically results in awarding the appropriate score. It is crucial to emphasize that parameters such as precision, trueness and recovery should be tested at multiple concentration levels. Therefore, during the assessment each concentration should be examined individually, and the RAPI pictogram should always indicate the lowest point score as the final value. In other words, if three concentrations were tested and RAPI assigns them e.g. 10, 10 and 7.5 points, the final result should be 7.5.
Another criterion that is assessed “dynamically” is LOQ. Because analytical methods vary greatly in this respect, assessing LOQ requires estimating what the average analyte concentration is expected in the target sample type. LOQ guidelines are expressed as a percentage of this value (Table 1).
The above assumptions allow the assessment guidelines to be adapted to the various specificities of potential analytical methods. They are not perfect, but in our opinion, they are a good compromise between the objectivity of the assessment and the simplicity of using RAPI.
It is also crucial to note that methods can be compared based on the results obtained for the same analyte. To compare methods addressed to several analytes, it is recommended to conduct an independent assessment and comparison for each of them separately. Preferably, the matrix should also be the same, then the comparison can help to select the best method from the set of alternatives. Comparisons assuming different matrices can also be valuable, e.g. to find out how much matrix inconsistency impacts the analytical characteristics. The user should always be aware of the purpose for which RAPI is used.
The star-like shape of the pictogram is similar to that used in BAGI, although it is not identical. The numerical assessment of individual criteria is represented by the lightness of individual fields on a scale of white (0) – dark red (10). The colour scale is perceptually uniform and based on the “reds” sequential colour map developed for the Matplotib library.30 This scale is analogous to BAGI where dark blue is used instead of dark red. The proposed form of graphical representation of results is intended to facilitate interpretation, remembering the characteristics of methods and making decisions. Noteworthy, the numerical scale in BAGI starts with 2.5, not 0. We decided to introduce the additional level in RAPI to increase the exactness of the scoring system for quantitative performance-related criteria (the specificity of blue criteria included in BAGI is more qualitative). In addition, receiving a value of 0 points in the absence of data for a given criterion promotes a comprehensive approach to validation and efforts to obtain additional information about analytical characteristics. In brief, it promotes good analytical practice.
3. Case studies: assessment of the selected procedures using RAPI and complementary tools
RAPI was used to assess the procedures representing varied samples (food, biological, and environmental), analytical instrumentation and sample preparation techniques, as well as different classes of target analytes. In addition, the practicality was assessed using BAGI, whereas greenness was assessed using various variants of GAPI, including original GAPI, ComplexGAPI, Modified GAPI (MoGAPI)31 and Complex Modified GAPI (ComplexMoGAPI).32 The modified GAPI alternatives provide a numerical score for method comparison. The selected methodologies concerned the determination of triazine herbicides, histamine and histidine, parabens as endocrine disruptors, and lead.33–44 For each analyte class, three representative methods were included. The obtained pictograms are shown in Fig. 3, the detailed scores obtained for the particular criteria are shown in the ESI,† and the main assessment results are discussed in the following sections.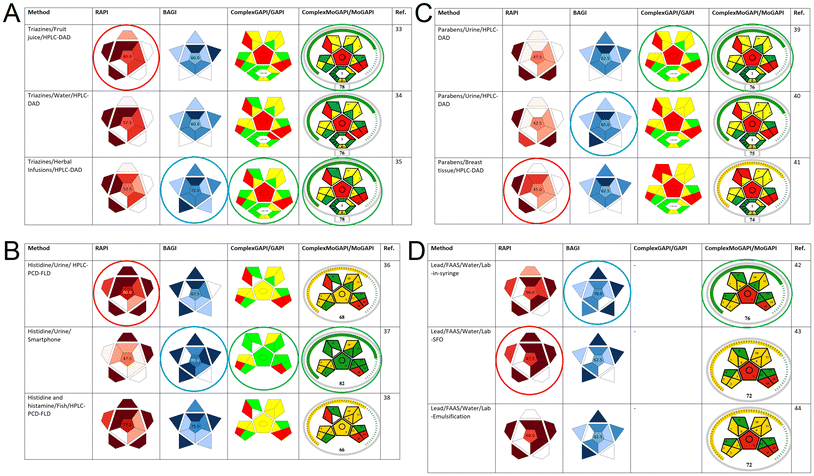 | ||
| Fig. 3 RAPI, BAGI, and GAPI assessment results of different analytical methods. The coloured circles indicate the best results from the set of three alternatives. | ||
3.1. Triazine herbicides
Triazine herbicides comprise a class of pesticides that are widely used to control weeds in different agricultural crops. These pesticides and their degradation products exhibit high toxicity, as well as persistence in water, soil, and crops. RAPI was used to compare the determination of triazines in similar but not the same matrices (it was done intentionally to examine the impact of matrix): in fruit juices after application of fabric phase sorptive extraction (FPSE),33 environmental waters after capsule phase microextraction (CPME),34 and herbal infusions after magnet-integrated fabric phase sorptive extraction (MI-FPSE).35 High-performance liquid chromatography coupled to diode array detection (HPLC-DAD) was used in all cases for the separation and quantification of the target analytes. The obtained RAPI scores were 60.0, 57.5, and 52.5, respectively (Fig. 3A). The robustness, selectivity, and reproducibility of the analytical methods were not examined, thus reducing their overall RAPI score. The superiority of the analytical method for fruit juice analysis can be attributed to its lower LOQ compared to the other two approaches. Moreover, the lower recovery and repeatability values for herbal infusion analysis had a profound negative impact on its RAPI score. However, the method for herbal infusion analysis exhibited higher method practicality (i.e., a BAGI score of 72.5) compared to the analytical methods for environmental water and fruit juice. This is the outcome of the elimination of the evaporation/reconstitution step that was necessary for the former two procedures. The higher practicality was also the result of the utilization of an autosampler to automate the analytical step, the incorporation of many analytes in the analytical scheme, and the higher sample throughput. Finally, the MI-FPSE protocol eliminated the need for additional treatments after the extraction procedure and did not require sample storage, demonstrating its higher compliance with green chemistry. Moreover, as revealed from the additional pictogram of the ComplexMoGAPI, the preparation of the FPSE and MI-FPSE is favourable compared to the CPME media. This is reflected by the scores: 78 for MI-FPSE/FPSE and 76 for CPME protocols. Taking into consideration all results, the determination of triazine herbicides in fruit juices by FPSE was found to be superior in terms of redness, while in herbal infusions by MI-FPSE superior in terms of blueness. The method dedicated to water analysis turned out slightly less green than the other two. Therefore, the strengths of particular methods are different. This confirms the expected impact of matrix and related type of extraction applied on the obtained WAC characteristics.3.2. Histamine and histidine
L-Histidine is an amino acid that is involved in various biological mechanisms in the human body. Histamine is a biogenic amine that can either be formed from L-histidine or come from food intake, and it can cause food poisoning cases at high levels. Thus, the monitoring of these compounds in food and biological samples is of high importance. RAPI was used to assess two analytical methods for histidine determination in urine using high-performance liquid chromatography-post column derivatization-fluorescence detection (HPLC-PCD-FLD)36 and smartphone-based detection,37 as well as an HPLC-PCD-FLD method for the simultaneous determination of histidine and histamine in fish samples.38 The respective scores were 80.0, 47.5, and 77.5 (Fig. 3B). As can be seen, the HPLC-PCD-FLD protocol for urine analysis exhibited the best performance in terms of its figures of merit due to its good trueness, linearity, and precision. The slightly reduced score (77.5) for the HPLC-PCD-FLD protocol for fish analysis follows from the intra-day and inter-day precision. The utilization of smartphone-based detection significantly reduced the RAPI score since it exhibited a narrower linear range, worse trueness and recovery. The practicality assessment using BAGI was generally favourable in each case. As expected, the smartphone-based protocol exhibited multiple advantages since it required only ubiquitous, portable instrumentation instead of advanced analytical instruments, reduced sample requirement, and increased sample throughput. The green character of the proposed protocols was assessed using MoGAPI (instead of ComplexMoGAPI since some input data required for ComplexMoGAPI was unavailable). As expected, the superior green character was observed for the analytical protocol for urine analysis using the smartphone device. This can be attributed to the significantly low requirements for chemicals and the negligible amount of the generated waste. Other benefits of this approach were the reduced energy demands and the complete elimination of the sample preparation step. Overall, the third method addressed to fish samples has not any explicit advantages. The other two methods differ in characteristics. In reality, the lower redness of the smartphone-based method can be problematic. RAPI pictogram attracts attention and informs about potential problems in advance.3.3. Parabens
Parabens are alkyl esters of p-hydroxybenzoic acid that are widely used as preservatives and antimicrobial agents. These chemicals can be easily absorbed into the human body, causing adverse effects on human health by exhibiting endocrine-disrupting action. Three different analytical methods based on FPSE and HPLC-DAD for the determination of parabens in urine and breast tissue samples were evaluated.39–41 Although the protocols were in principle similar, RAPI was able to differentiate their efficiency, producing different scores for all cases. The highest score (47.5) was attained for the protocol for urine analysis (Fig. 3C). A lower score (45.0) was obtained for the breast tissue analysis due to its worse LOQ and the lack of robustness validation. The differences between the two urine analysis protocols were attributed to the different intra-day and inter-day precision, and LOQ. Similar scores of BAGI were achieved, with the slight advantage of the second method addressed to urine samples, showing that these three approaches exhibit quite comparable practical applicability. In terms of their environmental friendliness, all the examined procedures exhibited reduced greenness due to the increased consumption of chemicals and the additional steps (i.e., microextraction, evaporation, reconstitution, etc.). The complex nature of the breast tissue samples further enhanced the complexity of the protocol, reducing its greenness. The highest greenness, according to their ComplexMoGAPI pictograms, was observed for the first method for the determination of parabens in urine due to the lower demand for chemical use compared to the other two protocols. This is an interesting case where each method has its own advantages and dominating “colours”. The indication of the overall whitest one is even more difficult than in the previous cases.3.4. Lead
Lead is one of the most toxic chemical elements that has accumulative properties, and it is considered an environmental priority pollutant due to its adverse effects on human health. The characteristics of three different off-line and on-line analytical methods for lead determination in water samples by flame atomic absorption spectrometry (FAAS) were examined using the RAPI, BAGI, and MoGAPI. The first approach was based on lab-in-syringe liquid-phase microextraction (LIS-LPME),42 the second approach was based on floating organic droplets dispersive liquid–liquid microextraction by a portable microsampling syringe,43 and the third approach was based on emulsification liquid–liquid microextraction.44 In all cases, deep eutectic solvents were used as more environmentally friendly alternatives to conventional organic solvents. The obtained RAPI scores were 60.0, 67.5, and 60.0 (Fig. 3D). The higher RAPI score of the second approach comes from its wider working range and lower LOQ. However, taking into consideration the method's practicality, contradictory results were obtained. In this case, the lab-in-syringe protocol showed the highest practicality (BAGI score of 70.0), in comparison with the other two approaches (BAGI scores of 62.5). This can be attributed to the automation of the analytical method, which enhanced its practicality. Finally, in terms of greenness, the lab-in-syringe protocol showed also higher compliance, as reflected by its MoGAPI score. This results from the lower sample amount and waste generation. All things considered, the method using emulsification is devoid of strong advantages, although the differences are actually quite minor and each method seems to be a good choice in some circumstances. Nevertheless, it has been confirmed that the choice of sample preparation method has a huge impact on the obtained WAC characteristics, in particular, whether the main advantages are red or blue/green criteria.4. Conclusions
RAPI is a new assessment tool, resembling the structure of BAGI, addressing the red attribute of WAC. It enables direct juxtaposition of methods in terms of general analytical performance in numerical and graphical ways. As such, it complements the spectrum of available assessment metrics. We used RAPI in combination with BAGI and various GAPI variants to compare several example analytical methods aimed at different analytes and using different experimental techniques. The results obtained confirm that RAPI allow consideration of varied analytical criteria in a well-balanced manner, and facilitate the comparison of analytical procedures in a fast and effective way. The considered methods turned out to have different advantages and shortcomings, and it was difficult to indicate the overall best ones in the particular cases. Therefore, it is generally desirable to use other assessment systems in parallel, e.g. the new RGBfast model,21 or other greenness metrics, e.g. AGREE,12 AGREEprep,14 ChlorTox Scale,16 AMGS,15 SPMS,17 or others. RAPI can be used in assessing newly developed methods as well as in retrospective literature studies, as analytical parameters are usually well described in the publications. Nevertheless, RAPI is a model based on certain arbitrary assumptions and does not always allow the rigour of the assessment to be adjusted to the specificity of the method. Hence, RAPI should be used as an auxiliary tool, especially in decision-making. In the future, it may be further developed. For instance, it could be adapted to assess a specific group of methods, e.g. based on chromatographic or electrokinetic separation, taking into account additional, more specific assessment criteria.Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its ESI.†Conflicts of interest
The authors declare no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The study was financially supported by the Polish National Science Centre, grant no 2019/35/B/ST4/01022 (recipient P.M.N).References
- P. T. Anastas, Crit. Rev. Anal. Chem., 1999, 29, 167–175 CrossRef CAS.
- M. Koel and M. Kaljurand, Pure Appl. Chem., 2006, 78, 1993–2002 CAS.
- S. Armenta, S. Garrigues and M. de la Guardia, TrAC, Trends Anal. Chem., 2008, 27, 497–511 CrossRef CAS.
- A. Gałuszka, Z. Migaszewski and J. Namieśnik, TrAC, Trends Anal. Chem., 2013, 50, 78–84 CrossRef.
- Á. I. López-Lorente, F. Pena-Pereira, S. Pedersen-Bjergaard, V. G. Zuin, S. A. Ozkan and E. Psillakis, TrAC, Trends Anal. Chem., 2022, 148, 116530 CrossRef.
- M. Sajid and J. Płotka-Wasylka, Talanta, 2022, 238, 123046 CrossRef CAS PubMed.
- J. Martínez, J. F. Cortés and R. Miranda, Processes, 2022, 10, 1274 CrossRef.
- L. Yin, L. Yu, Y. Guo, C. Wang, Y. Ge, X. Zheng, N. Zhang, J. You, Y. Zhang and M. Shi, J. Pharm. Anal., 2024, 101013 CrossRef PubMed.
- L. H. Keith, L. U. Gron and J. L. Young, Chem. Rev., 2007, 107, 2695–2708 CrossRef CAS PubMed.
- A. Gałuszka, Z. M. Migaszewski, P. Konieczka and J. Namieśnik, TrAC, Trends Anal. Chem., 2012, 37, 61–72 CrossRef.
- J. Płotka-Wasylka, Talanta, 2018, 181, 204–209 CrossRef PubMed.
- F. Pena-Pereira, W. Wojnowski and M. Tobiszewski, Anal. Chem., 2020, 92, 10076–10082 CrossRef CAS PubMed.
- J. Płotka-Wasylka and W. Wojnowski, Green Chem., 2021, 23, 8657–8665 RSC.
- W. Wojnowski, M. Tobiszewski, F. Pena-Pereira and E. Psillakis, TrAC, Trends Anal. Chem., 2022, 149, 116553 CrossRef CAS.
- M. B. Hicks, W. Farrell, C. Aurigemma, L. Lehmann, L. Weisel, K. Nadeau, H. Lee, C. Moraff, M. Wong, Y. Huang and P. Ferguson, Green Chem., 2019, 21, 1816–1826 RSC.
- P. M. Nowak, R. Wietecha-Posłuszny, J. Płotka-Wasylka and M. Tobiszewski, Green Anal. Chem., 2023, 5, 100056 CrossRef.
- R. Gonzalez-Martín, A. Gutierrez-Serpa, V. Pino and M. Sajid, J. Chromatogr., A, 2023, 1707, 464291 CrossRef PubMed.
- P. M. Nowak, R. Wietecha-Posłuszny and J. Pawliszyn, TrAC, Trends Anal. Chem., 2021, 138, 116223 CrossRef CAS.
- P. Mateusz Nowak, Green Chem., 2023, 25, 4625–4640 RSC.
- P. M. Nowak and P. Kościelniak, Anal. Chem., 2019, 91, 10343–10352 CrossRef CAS PubMed.
- P. M. Nowak and F. Arduini, Green Anal. Chem., 2024, 10, 100120 CrossRef.
- A. Ballester-Caudet, P. Campíns-Falcó, B. Pérez, R. Sancho, M. Lorente, G. Sastre and C. González, TrAC, Trends Anal. Chem., 2019, 118, 538–547 CrossRef CAS.
- P. M. Nowak, P. Kościelniak, M. Tobiszewski, A. Ballester-Caudet and P. Campíns-Falcó, TrAC, Trends Anal. Chem., 2020, 133, 116065 CrossRef CAS.
- N. Manousi, W. Wojnowski, J. Płotka-Wasylka and V. Samanidou, Green Chem., 2023, 25, 7598–7604 RSC.
- F. Lundh, 1999. An introduction to tkinter. URL: https://www.pythonware.com/library/tkinter/introduction/index.htm.
- P. Konieczka, J. Namieśnik and B. Zygmunt, Ocena i kontrola jakości wyników pomiarów analitycznych, WNT, Warsaw, 2013 Search PubMed.
- Eurachem Guide: The Fitness for Purpose of Analytical Methods – A Laboratory Guide to Method Validation and Related Topics, ed. B. Magnusson and U. Ornemark, 2nd edn, 2014. ISBN 978-91-87461-59-0. Available from https://www.eurachem.org Search PubMed.
- I. Taverniers, M. De Loose and E. V. Bockstaele, TrAC, Trends Anal. Chem., 2004, 23, 535–552 CrossRef CAS.
- W. Horwitz and R. Albert, J. AOAC Int., 1996, 79, 589 CrossRef CAS PubMed.
- J. D. Hunter, Comput. Sci. Eng., 2007, 9, 90–95 Search PubMed.
- F. R. Mansour, J. Płotka-Wasylka and M. Locatelli, Analytica, 2024, 5(3), 451–457 CrossRef.
- F. R. Mansour, K. M. Omer and J. Płotka-Wasylka, Green Anal. Chem., 2024, 10, 100126 CrossRef.
- N. Manousi, V. Alampanos, I. Priovolos, A. Kabir, K. G. Furton, E. Rosenberg, G. A. Zachariadis and V. F. Samanidou, Food Chem., 2022, 373, 131517 CrossRef CAS PubMed.
- N. Manousi, V. Alampanos, I. Priovolos, A. Kabir, K. G. Furton, E. Rosenberg, G. A. Zachariadis and V. F. Samanidou, Talanta, 2021, 234, 122710 CrossRef CAS PubMed.
- N. Manousi, A. Kabir, K. G. Furton, G. A. Zachariadis and E. Rosenberg, Microchem. J., 2022, 179, 107524 CrossRef CAS.
- E. Stampina, A. Tsiasioti, K. Klimatsaki, C. K. Zacharis and P. D. Tzanavaras, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2021, 1173, 122697 CrossRef CAS PubMed.
- M. Tarara, A. Tsiasioti, P. D. Tzanavaras and G. Z. Tsogas, Talanta, 2022, 249, 123685 CrossRef CAS PubMed.
- A. Tsiasioti and P. D. Tzanavaras, Food Chem., 2021, 351, 129351 CrossRef CAS PubMed.
- G. Rigkos, V. Alampanos, A. Kabir, K. G. Furton, Ž. Roje, I. V. Vrček, I. Panderi and V. Samanidou, Biomed. Chromatogr., 2021, 35, e4974 CrossRef CAS PubMed.
- A. Tartaglia, A. Kabir, S. Ulusoy, E. Sperandio, S. Piccolantonio, H. I. Ulusoy, K. G. Furton and M. Locatelli, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2019, 1125, 121707 CrossRef PubMed.
- V. Alampanos, A. Kabir, K. G. Furton, Ž. Roje, I. V. Vrček and V. Samanidou, J. Chromatogr., A, 2020, 1630, 461530 CrossRef CAS PubMed.
- V. Christoforou, N. Manousi, C. K. Zacharis and A. Anthemidis, Sustainable Chem. Pharm., 2024, 39, 101567 CrossRef CAS.
- Naeemullah and M. Tuzen, Talanta, 2019, 196, 71–77 CrossRef CAS PubMed.
- C. Karadaş and D. Kara, Water Supply, 2019, 19, 864–870 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc05298f |
| This journal is © The Royal Society of Chemistry 2025 |

