Co-pyrolysis of biomass and plastic waste into carbon materials with environmental applications: a critical review†
Jiaqi
Deng‡
a,
Baojun
Yi‡
 *ab,
Ondřej
Mašek
*ab,
Ondřej
Mašek
 c,
Xiangzhou
Yuan
c,
Xiangzhou
Yuan
 d,
Sung Yeon
Hwang
e,
Hwai Chyuan
Ong
f,
Zewen
Hua
a and
Yong Sik
Ok
d,
Sung Yeon
Hwang
e,
Hwai Chyuan
Ong
f,
Zewen
Hua
a and
Yong Sik
Ok
 *b
*b
aCollege of Engineering, Huazhong Agricultural University, No. 1, Shizishan Street, Hongshan District, Wuhan, 430070, PR China. E-mail: bjyi@mail.hzau.edu.cn
bKorea Biochar Research Center, Division of Environmental Science and Ecological Engineering, Korea University, Seoul 02841, Republic of Korea. E-mail: yongsikok@korea.ac.kr
cUK Biochar Research Centre (UKBRC), School of GeoSciences, University of Edinburgh, King's Buildings, Edinburgh, EH9 3JN, UK
dMinistry of Education of Key Laboratory of Energy Thermal Conversion and Control, School of Energy and Environment, Southeast University, Nanjing 210096, China
eDepartment of Plant & Environmental New Resources and Graduate School of Biotechnology, Kyung Hee University, Gyeonggi-Do 17104, Republic of Korea
fDepartment of Engineering, School of Engineering and Technology, Sunway University, Jalan Universiti, Bandar Sunway, 47500 Petaling Jaya, Selangor, Malaysia
First published on 13th March 2025
Abstract
The urgent need for global energy transformation and environmental protection, combined with widespread plastic contamination, has stimulated research into the co-pyrolysis of biomass and plastic waste. This approach challenges traditional resource utilization methods and opens new pathways for sustainable energy generation and waste management. The reaction processes and mechanisms of both biomass pyrolysis and various plastic wastes are comprehensively examined. The influences of co-processing parameters on the composition, content, and product characteristics are analysed, providing a solid theoretical foundation for the large-scale production and application of co-pyrolysis. Besides the potential problems related to plastic co-pyrolysis, the enhancement of carbon materials’ properties by plastic waste in co-pyrolysis is also explored, which is significant for the functionalization of carbon materials. The reaction mechanism, reaction process, reaction conditions, and products generated from the co-pyrolysis of biomass and plastic waste are discussed. In the co-pyrolysis of biomass (especially lignocellulosic biomass) and plastic waste, PET, PU, and PVC are more conducive to the production of carbon materials, while PP, PE, and PS are more favourable for the generation of bio-oils. The interaction between the hydroxyl radicals provided by biomass and the hydrogen radicals provided by plastic waste enhances the reaction. Slow co-pyrolysis at 500 °C, a feedstock ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (biomass to plastic waste), and a suitable catalyst (such as zeolites) are more beneficial for carbon materials in terms of the yield, porosity, and production rate. Co-pyrolysis carbon materials possess superior hydrophobicity and adsorption properties compared to conventional carbon materials and can be enriched in elements often absent from the original carbon materials, such as nitrogen. These potential advantages of co-pyrolyzing plastics with biomass open new prospects beyond waste management, such as enhanced material development for a range of environmental and agricultural applications.
1 (biomass to plastic waste), and a suitable catalyst (such as zeolites) are more beneficial for carbon materials in terms of the yield, porosity, and production rate. Co-pyrolysis carbon materials possess superior hydrophobicity and adsorption properties compared to conventional carbon materials and can be enriched in elements often absent from the original carbon materials, such as nitrogen. These potential advantages of co-pyrolyzing plastics with biomass open new prospects beyond waste management, such as enhanced material development for a range of environmental and agricultural applications.
Green foundation1. Proposed a novel strategy for co-pyrolyzing biomass with plastics to produce advanced carbon materials, systematically evaluating their reaction mechanisms, process dynamics, and environmental benefits, providing insights into sustainable material design.2. Developed efficient co-pyrolysis methods that highlighted the potential of carbon materials to replace costlier or less environmentally friendly alternatives. By demonstrating versatile applications, this research significantly contributes to advancing green and sustainable development. 3. Identified promising future directions for carbon materials derived from biomass and plastic co-pyrolysis in areas such as energy storage, agricultural applications, and broader sustainability. Emphasized the impact of plastic type on carbon material properties and explored optimized applications of modified carbon materials, driving innovations in green chemistry and sustainable technologies. |
Introduction
Solid waste, in typical linear economic concepts, pertains to any material that is discarded or abandoned as a result of human activities and represents a prevalent source of environmental pollution.1 Plastic waste has drawn significant attention due to the increasing risk associated with microplastics.2 Over the past years, large quantities of solid waste have inevitably been produced with approximately 1.5–1.7 billion tons per year of solid waste being generated worldwide.3 Simultaneously, the rapid exhaustion of natural resources and conventional fossil fuels, along with growing energy security concerns and environmental challenges, are increasingly drawing attention to sustainable energy sources.4 Thus, the conversion of biomass and appropriate solid waste fractions into electricity, heat, chemicals, and materials is a highly promising area, considering sustainability and environmental impact.Biomass is derived from a diverse range of sources, such as agricultural residues, energy crops, industrial production residues, livestock, sewage, municipal waste, and so on.5 Given the immense quantity of biomass produced globally each year, biomass holds vast potential as a source for alternative energy transition. Biomass possesses the advantages of abundance, renewability, and the potential to be CO2 neutral. Biomass is the fourth most significant energy source and currently constitutes approximately 14% of annual global energy consumption.6 The utilization of biomass with lower energy density to obtain more energy-dense products that can directly substitute fossil fuel products is increasingly significant.
Physical–chemical, bio-chemical, and thermo-chemical processes are all competent at converting biomass into biofuels.1 Thermo-chemical conversion refers to a set of processes that involve the application of heat and chemical reactions to transform a substance into different forms or products, and it is the predominant technology for converting biomass.4 This typically encompasses pyrolysis, gasification, and combustion.7 Among these, pyrolysis is attracting considerable attention due to its flexibility in terms of product outputs and suitability for operation across different scales.8 The thermal degradation of organic molecules converts various forms of biomass into fuels, chemicals, and solid carbon products.9 The flexibility of the feedstock and the diversity of possible products make this technology an important contributor to the transition from high carbon intensity linear economy models to a net-zero circular economy.
Plastic waste is a type of polymeric solid waste that encompasses items such as plastic bags, bottles, packaging, and plastic components from consumer goods that are no longer required or have reached the end of their service life. The production of industrial plastics started to expand around 1950. Current global production of plastic waste is approximately 200 million tons per annum.10 Plastic waste has revolutionized our society, offering a wide array of materials at relatively low cost and having extensive applications in industry and all aspects of life. Nevertheless, the issue of plastic waste management has also emerged. Plastic waste management pertains to the systematic approach and set of strategies utilized to handle, control, and address plastic waste throughout its life cycle. The global accumulation of plastic waste without appropriate management will surpass the planet's capacity in the future.11 Currently, conventional methods for managing plastic waste encompass landfilling, stockpiling, incineration, and recycling.5 Over time, unregulated landfilling of plastic waste leads to the formation of macro-, meso-, micro-, and nanoparticles that accumulate in the environment.12 This plastic waste is so small (from mm to nm) that it can affect the environment and human health in ways that we are only just beginning to understand.13 In addition to landfill, the incineration of plastic waste has several negative environmental impacts related to the release of numerous hazardous substances that can pose a threat to human health.14 These traditional methods of disposing of plastic waste are a serious menace to the planet.15 Therefore, there is an urgent necessity to reduce plastic waste generation and to explore alternative approaches of transforming plastic waste into higher value-added products, safeguarding the environment and human health.
Plastic waste has been extensively studied for many years, with a particular emphasis on the process of thermochemical recovery of plastic waste from low-grade to petrochemical feedstock. Generating energy from recycled plastic waste helps to reduce the reliance on fossil fuels by minimizing waste and addressing energy shortages. Thermochemical treatment methods such as gasification and pyrolysis can offer more efficient value-added products compared to biotechnology.16 In particular, gasification of plastic waste has the potential to reduce its quantity and increase energy recovery.17 However, the single gasification of plastic waste frequently encounters operational challenges due to issues such as inconsistent feedstock quality, complex chemical reactions, and high energy consumption.18 The utilization of plastic waste as a pyrolysis feedstock, particularly for polyolefins, has been a subject of discussion in recent years.19 This is because of its capacity to produce a series of relatively clean fractions suitable for reuse. In addition, unlike biomass, polyolefins have a low oxygen content, enabling higher bio-oil yields and high calorific value syngas.19 However, the pyrolysis of plastic waste alone has disadvantages such as plugging, inhibition of fluidization, generation of contaminants, etc.20 The co-pyrolysis of biomass and plastic waste resolves these issues and the interaction between them during the process improves the quality of the final product.21
Plastic waste has a relatively high H/C ratio. Incorporating plastic waste into the biomass pyrolysis process can improve the H/C ratio of the feedstock, thereby providing a greater amount of hydrogen for the pyrolysis process.22 As a result, the co-pyrolysis of plastic and biomass is currently regarded as a feasible and effective approach for enhancing product quality. Due to the low oxygen and high hydrogen content of plastic waste, their co-pyrolysis with biomass can provide H radicals and promote hydroxyl radical–hydrogen radical interactions.23 Thus, the combining of plastic waste and biomass for co-pyrolysis could potentially provide a means of generating renewable energy and reducing environmental pollution.
Co-pyrolysis of different raw materials in an inert environment enables the exploitation of specific interactions between the feedstocks to produce high-performance products. In general, co-pyrolysis typically involves two or more feedstocks being pyrolyzed under homogeneous mixing circumstances.3 Due to the efficiency and operational simplicity of co-pyrolysis in generating valuable products, co-pyrolysis approaches have garnered considerable focus in research undertakings,24 and they can offer a more economically beneficial solution for waste management and the production of electricity, heat, and materials, presenting the potential for rapid commercial utilization.25
Co-pyrolysis of biomass and plastic waste is a promising technological method and represents a straightforward, effective, and alternative solution (Fig. 1). With an escalating amount of waste being utilized as a raw material, co-pyrolysis can considerably reduce waste that would otherwise be bound for landfill. The pyrolysis products can also strengthen a nation's energy security, while concurrently maintaining environmental and ecosystem safety.26 Consequently, co-pyrolysis can be applied as efficient and economically viable recycling technology to reduce waste.27 The plastic waste typically used in pyrolysis includes high-density polyethylene (HDPE), low-density polyethylene (LDPE), polypropylene (PP), polystyrene (PS), polyethylene terephthalate (PET), polyacarbonates (PCs), and polyvinyl chloride (PVC).3
 | ||
| Fig. 1 Conversion and application of co-pyrolysis of biomass and plastic waste.28 | ||
Due to their significance, the reaction mechanisms and interactions between biomass and plastic waste during co-pyrolysis are presented in this paper. Firstly, an in-depth examination of the composition, structure, and elemental composition of biomass and plastic waste was carried out. Detailed pyrolysis reaction processes and mechanisms of these two feedstocks were explored, which provided a solid foundation for comprehending their interactions during co-pyrolysis. Furthermore, this work elucidates the reaction mechanisms and interactions of biomass and different plastic waste under co-pyrolytic conditions, focusing on how plastic waste influences the co-pyrolysis products, and the significance of appropriate plastic selection for a specific target product. In addition, the impacts of process parameters on the composition, content, and properties of the co-pyrolysis products are analyzed, and these analyses offer theoretical support for their application in large-scale production. The enhancement and modification of carbon material properties by plastic waste during co-pyrolysis is also deliberated; this is of great significance for the development of novel functional carbon materials and applications. Finally, this paper reviews the key literature on the co-pyrolysis of biomass and plastic waste from recent years, highlighting research progress and current challenges, with the aim of guiding future developments in the field.
Literature statistics on the co-pyrolysis of biomass and plastic waste
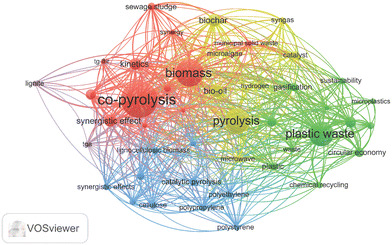
A bibliometric analysis of the co-pyrolysis of biomass and plastic waste was performed to identify reliable recommendations for future research (Fig. 2). Data on research relevant to the co-pyrolysis of biomass and plastic waste were retrieved from the Web of Science Core databases (accessed until 19 April, 2024). We employed the search terms “co-pyrolysis” or “biomass” or “plastic waste”, and the time period of 2014 to 2023. After cursory examination and elimination of duplicate data, 4000 articles were selected for bibliometric analysis, and the data were visualized using VOSviewer software and bibliometric software to identify 5399 keywords with a minimum threshold of 30 occurrences.Keyword analysis reveals that in the last decade, concomitant with the escalating attention devoted to renewable energy and environmental protection, the domains of biomass, plastic waste, co-pyrolysis, pyrolysis, and biochar have emerged as hotspots and trends in research. The techno-economic viability of these approaches underpins their research prominence. Biomass and plastic waste co-pyrolysis, for instance, offers the potential to combine the advantages of both feedstocks. The products obtained from this process often include valuable gases, liquids, and solids. Gaseous products such as hydrogen and methane offer potential for clean energy production. Liquid products, including bio-oils, can be refined and utilized as alternative fuels. Solid biochar has applications in soil improvement and carbon sequestration. These research hotspots and trends reflect the current urgent need for sustainable development and environmental protection. It is necessary to intensify research and explore more innovative and effective technological routes to promote the development of relevant fields, achieve the coordinated development of the economy, society, and the environment, and fully exploit the potential of biomass, plastic waste, co-pyrolysis, and pyrolysis for a more sustainable future.
Countries’ cooperation and co-citation analyses indicate that China, the United States, India, and Australia are the predominant countries in terms of cooperative published research. The upward trend in the number of publications on co-pyrolysis demonstrates the growing global significance of the field (Fig. 3).
 | ||
| Fig. 3 Number of published papers during 2014–2024. (a) Co-pyrolysis; (b) Co-pyrolysis biochar (source: Web of Science, until 20 January, 2025). | ||
A number of reviews have been published on biomass and plastic waste co-pyrolysis, and this section discusses the most influential reviews within this field, as presented in Table 1. A significant portion of the focus has been placed on investigating the effects of process parameters on co-pyrolysis and value-added effects on the bio-oil products, while largely neglecting the reaction mechanisms during co-pyrolysis as well as the effects of plastic waste on the enhancement of carbon materials.
| Title | Characteristics of the review | Publication details | Citations |
|---|---|---|---|
| Co-pyrolysis of waste plastic and solid biomass for synergistic production of biofuels and chemicals – a review | This review is to discuss the pyrolysis mechanisms in the co-pyrolysis of plastics and biomass for oil production and to present different catalytic co-pyrolysis reactors as well as several catalytic treatments. | Progress in Energy and Combustion Science (2021)28 | 272 |
| Co-pyrolysis of biomass and plastic wastes: a review on reactants synergy, catalyst impact, process parameter, hydrocarbon fuel potential, COVID-19 | This review focuses on the synergistic effects of co-pyrolysis and reviews several catalysts that affect the yield and composition of bio-oils, as well as discussing the process parameters for co-pyrolysis of biomass and plastics. | Journal of Environmental Chemical Engineering (2021)29 | 48 |
| Co-pyrolysis of biomass and plastic waste as a thermochemical conversion technology for high-grade biofuel production: recent progress and future directions elsewhere worldwide | This review focuses on the advantages of the co-pyrolysis process, the yield of the co-pyrolysis products, and the synergistic effects of the co-pyrolysis process, aiming to show that co-pyrolysis of biomass and plastic wastes is more beneficial than pyrolysis of biomass alone. | Energy Conversion and Management (2018)26 | 361 |
| Valorization of municipal wastes using co-pyrolysis for green energy production, energy security, and environmental sustainability: a review | This review summarises the latest technological advances and applications for the recovery of value-added products from municipal waste through co-pyrolysis, highlights the characteristics of the liquid fuels produced by co-pyrolysis, and concludes that co-pyrolysis is a viable and sustainable method of recovering biofuels from municipal waste as a green source of energy and for energy security. | Chemical Engineering Journal, (2021)30 | 106 |
| A critical review on co-gasification and co-pyrolysis for gas production | This review discusses the co-pyrolysis and co-gasification of plastics and biomass for hydrogen production and analyses the advantages of co-pyrolysis over pyrolysis alone and the improvement of the product. | Renewable and Sustainable Energy Reviews (2022)31 | 49 |
| Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review | This review summarises the pyrolysis mechanism of lignocellulosic biomass and describes in detail the intermediate changes that occur during pyrolysis of lignocellulosic biomass. It aims to illustrate the pyrolysis properties of lignocellulosic biomass. | Progress in Energy and Combustion Science (2017)32 | 1667 |
| Recent advances in catalytic co-pyrolysis of biomass and plastic waste for the production of petroleum-like hydrocarbons | This review focuses on recent advances in catalytic co-pyrolysis and indicates that catalytic co-pyrolysis can effectively improve the quality of bio-oil. The role of different catalysts is also discussed. | Bioresource Technology (2020)33 | 196 |
| A review on co-pyrolysis of biomass: an optional technique to obtain a high-grade pyrolysis oil | This review describes the co-pyrolysis process in terms of process mechanism, feedstock, co-pyrolysis phenomena, by-product characteristics and economic assessment, and points out that the properties of pyrolysis oils can be improved by co-pyrolysis technology. | Energy Conversion and Management (2014)27 | 597 |
| Review on synergistic effects during co-pyrolysis of biomass and plastic waste: significance of operating conditions and interaction mechanism | The aim of this review is to consider the influence of various factors such as plastic type, biomass type, mixing ratio, reactor type, heating rate, reaction temperature and catalyst on the synergistic effect. In addition, plausible interaction mechanisms related to synergistic effects during synergistic pyrolysis are presented. | Biomass and Bioenergy (2022)34 | 61 |
| A review of pyrolysis technologies and feedstock: a blending approach for plastic and biomass towards optimum carbon materials yield | This review discusses biochar production technologies, pyrolysis technology mechanisms, types of pyrolysis and reactor types. The effects of process parameters such as temperature, heating rate, reactor bed height and type, residence time, pressure, feedstock type and feedstock mixing ratio on biochar yield and properties are reviewed. | Renewable and Sustainable Energy Reviews (2022)35 | 115 |
| Thermochemical conversion of plastic waste to fuels: a review | This review summarises advances in the catalytic thermochemical conversion of various types of plastic waste, including pyrolysis, gasification, and hydrothermal processes. Current challenges and future prospects for catalytic thermochemical conversion of plastic waste are also discussed. | Environmental Chemistry Letters (2021)36 | 156 |
| A unified view on catalytic conversion of biomass and plastic waste | This review focuses on catalyst design in the catalytic conversion of biomass and waste plastics and explores the catalytic conversion of biomass and plastic wastes from a unified perspective. | Nature Reviews Chemistry (2022)37 | 179 |
This review aims to provide a holistic assessment of the co-pyrolysis topic in its entirety, commencing from the characteristics of the feedstock, analyzing the similarities and differences between biomass and plastic waste that affect the pyrolysis process, thereby enabling an understanding of the specific reactions and interactions between the different components when they are co-pyrolyzed, and to analyze the specific impacts of plastic waste on carbon materials in conjunction with the variations of diverse process parameters, and subsequently to achieve the leap from the technology to practical applications.
Property comparisons between biomass and plastic waste
Comparison of biomass and plastic waste structures
Plastic waste and biomass, as two different pyrolysis feedstocks, are similar in structure but have significant differences. Plastic waste is typically derived from petrochemicals, whereas biomass is derived from plants or other forms of organic matter. Biomass is composed of hydrocarbon bonds and aromatic epoxides, as well as small amounts of inorganic substances. Plastic waste, on the other hand, consists mainly of hydrocarbons, including linear and aromatic polymers.29 It is clear that the overall molecular structures of plastic waste and biomass are different. Plastic waste is composed of synthetic polymers whose molecular chains can be linear, branched or cross-linked, whereas biomass is composed of naturally occurring polymers that typically have more complex biological structures and functions. Nevertheless, a careful comparative study of biomass and plastic waste reveals compositional and structural similarities at both the macroscopic and microscopic levels. A structural comparison between a repeating unit of wood and a plastic structure is shown in Fig. 4. Firstly, it is clear that both contain long-chain polymers, and both have linkages between the polymer chains. These linkages increase the resistance of the feedstock to chemical degradation and change the nature of the products.38 Secondly, biomass and plastic waste predominantly consist of the same elements, mainly C, H, O, and N. Consequently, biomass and plastic waste share similar chemical bonds, including C–C, C–O, and C–N. These similarities have comparable physical properties, such as hydrophobicity. They may also exhibit similar behavior during thermochemical conversion, and biomass and plastic waste are frequently converted using the same thermochemical conversion strategy.37 The main disparity lies in the fundamental nature of plastic waste and biomass. Plastic waste typically originates from the polymerization of multiple monomers, creating a relatively uniform and consistent structure. In contrast, biomass exhibits a significant degree of compositional variation. It encompasses a wide array of components such as cellulose, hemicellulose, lignin, and various extractives. The intricate composition of biomass implies that its conversion and utilization require more sophisticated and customized approaches compared to the relatively straightforward treatment of plastic waste, which is dominated by a specific polymeric structure. | ||
| Fig. 4 Comparison of typical plastic waste and biomass structures.10,27,37 | ||
Pyrolysis properties of biomass
Pyrolysis, also known as thermal decomposition, refers to the decomposition of biomass into carbon materials, bio-oils and pyrolysis gas. This is generally done over a temperature range of 250–900 °C.9 Biomass pyrolysis involves a variety of endothermic and exothermic processes in which a series of reactions occur, including dehydration, depolymerisation, isomerisation, aromatisation, decarboxylation, and carbonization.39 The primary reactions include bond cleavage, which produces carbon materials, a depolymerisation process for bio-oil synthesis, and gaseous products resulting from the cleavage step.28 The primary constituents of biomass typically consist of cellulose (between 32% and 45%), hemicellulose (between 19% and 25%), and lignin (between 14% and 26%).40 Hemicellulose occupies the interstitial space between micro- and macro-primary fibers within the cellulose matrix, while lignin fulfills a structural role by enveloping cellulose and hemicellulose.41The monomeric composition of cellulose is (C6H10O5)n linked by β-1,4-glycosidic linkages of pyrene-D-glucose units.32 Cellulose has a crystalline structure formed by hydrogen bonds.6 Consequently, cellulose pyrolysis serves as the basis of biomass conversion. Hemicellulose is an amorphous short-chain linear polysaccharide with an average composition of (C5H8O4)n.42 Lignin is a mononuclear aromatic polymer and is typically found in the form of lignocellulosic complexes.28 There are three types of phenylpropane unit that form the isomeric structure of lignin, namely the p-hydroxyphenyl unit (H), the guaiacyl unit (G), and the syringyl unit (S).42 Lignin in wood decomposes at relatively high temperatures compared to cellulose and hemicellulose and has strongly hydrophobic properties.9 Thermal analyses indicate that hemicellulose decomposes at 250 °C to 350 °C, cellulose pyrolysis occurs at 325 °C to 400 °C, and the pyrolysis temperature range for lignin is generally considered to be 300 °C to 500 °C.43 Typically, lignin is pyrolysed at higher temperatures and cellulose and hemicellulose promote the deoxygenation of polymers within the lignin structure.44
The characteristics of biomass pyrolysis depend on the content of the components.45 The cellulose content determines the liquid product yield, the hemicellulose content determines the gaseous product yield, and the lignin content determines the biochar yield.46 Among other things, pyrolysis at relatively slow heating rates and temperatures below 450 °C will produce higher yields of carbon materials. Conversely, higher temperatures reduce carbon material production and enhance the formation of other liquids.47
Due to the high degree of polymerisation of the cellulose chains, depolymerisation is the most critical reaction of cellulose pyrolysis. The mechanisms for cellulose depolymerisation include homolytic, heterogeneous and synergistic cleavage.42 In cleavage, the β-1,4-glycosidic linkage is fragmented to give rise to two radicals. Besides, hydrolysis is thought to be the cause of the cleavage of the C–O bond, as a significant amount of water molecules can be produced during the pyrolysis process.48
The pyrolysis of lignin is much more complicated than that of hemicellulose and cellulose. During pyrolysis, the degradation of lignin begins with the cleavage of relatively weak bonds at relatively low temperatures. These weak bonds are less stable and have lower bond dissociation energies, making them more prone to breakage under mild thermal conditions. As the temperature rises during the pyrolysis process, degradation proceeds with the cleavage of stronger bonds. These stronger bonds require higher energy input in the form of increased temperature to undergo cleavage.28 Small molecule aldehydes, toluene, styrene and guaiacyl hydroxyls are the predominant products in the initial stage of lignin degradation, while p-hydroxyphenol is the further degradation product.49
Generally, the pyrolysis products of biomass are acidic and have a relatively low calorific value.50 Furthermore, the composition of the feedstock is also a factor influencing the properties of carbon materials. Biomass rich in lignin has been developed into macro-porous carbon materials, while biomass rich in cellulose has predominantly been developed into micro-porous carbon materials.51 Micro-pores typically result in a high specific surface area and superior absorptive capacity.52
Pyrolysis properties of plastic waste
Plastic waste is composed of macromolecular substances polymerised from monomers by addition polymerisation or polycondensation.53 This article focuses on commonly used plastic waste such as PET, PU, PVC, HDPE, PP, PS, and PCs.The range of 350 °C to 700 °C represents typical temperatures for the pyrolysis of plastic waste. Specifically, PP and PS show single-stage degradation with initial temperatures of 240 °C and 330 °C, respectively, and maximum degradation temperatures of 425 °C and 470 °C, respectively. PE has a two-stage degradation pattern. The first stage occurs at 270–400 °C, while the second stage of degradation starts at 400 °C and reaches maximum conversion at 480 °C.54 The pyrolysis conversion mechanisms of plastic waste are shown in Fig. 5.
 | ||
| Fig. 5 Diagram of the pyrolysis mechanism for plastic waste pyrolysis.10,55,56 | ||
Thermal degradation of plastic waste typically involves random fracture and chain end fracture mechanisms, or they may act simultaneously, either through the generation of free radicals or the formation of long carbon chains. Among these, the free radical mechanism is more often used to explain the degradation of plastic waste.25
Secondly, the thermal decomposition of PP is a stochastic cleavage along the main chain, as shown in Fig. 5b. Each random scission gives rise to a primary radical and a secondary radical, which are successively converted into alkanes, alkenes, and alkadienes in a manner similar to that of PE.4 In addition, the predominant products of PP pyrolysis are formed by the decomposition of secondary radicals, with only a small amount of product being derived from primary radicals. Another programme was developed to generate olefinic products and another secondary radical through a back-loading reaction and a β-cracking reaction. The aim was the formation of olefins and alkadienes. The newly formed radical follows similar sequential procedures for decomposition or, alternatively, β-scission can give rise to a terminally unsaturated polymer, which can undergo an atom abstraction reaction for β-scission, thereby resulting in the formation of another radical and many olefinic diene products.10
Co-pyrolysis of biomass and plastic waste
There is a vast variety of plastic waste, and variations in its chemical composition and structure lead to distinct differences in the products of pyrolysis. While exploring the co-pyrolysis of plastic waste and biomass, researchers have discovered that there are substantial differences in the main products obtained during the pyrolysis of various types of plastic. Based on this phenomenon, it is significant to categorize different plastic waste into groups according to the predominant products for a more systematic and in-depth study and elaboration.In the co-pyrolysis process of carbon-producing plastic waste, the products are more inclined to generate carbon materials of certain value. These carbon materials have extensive applications in fields such as energy storage and adsorbents. In contrast, oil-producing plastic waste mainly produces liquid oil products during pyrolysis, which can be transformed into fuels or chemical raw materials after further processing and refinement. This classification study approach helps in understanding the pyrolysis characteristics and product distribution patterns of each plastic waste more precisely, thereby providing a theoretical basis for optimizing the pyrolysis process and enhancing the quality and yield of the products. The co-pyrolysis reaction mechanism of various types of plastic waste combined with biomass is shown in Fig. 6.
 | ||
| Fig. 6 Interaction mechanism for plastic waste and biomass co-pyrolysis.3,28,64,65 | ||
Co-pyrolysis for carbon materials
In addition, there is not only a synergistic carbon formation effect between PET and biomass, but this effect may also reduce the activation energy of PET. A study of the co-pyrolysis of sardine waste with PET showed an increase in the volatile content and a decrease in the activation energy compared to the pyrolysis carried out in isolation.69 The result of reaction between PET and macadamia nut shells shows that the volatiles produced by PET could influence the charcoal extracted from the nut shells to form a charcoal-like structure.70 In addition, the interaction between PET and biomass serves to stabilise the free radicals of charcoal, increasing the concentration of free radicals and allowing PET to degrade at relatively lower temperatures.71 Co-pyrolysis of municipal solid waste and PET has also been examined; the interaction between the two becomes more prominent when a greater amount of biomass is involved in the co-pyrolysis process.72
The energy required for the co-pyrolysis of biomass and PET mixtures may increase with increasing plastic blends, because PET plastic requires more energy for pyrolysis than wood.12 The pyrolysis of biomass starts before that of polyester plastics, so the volatiles in the biomass can further contribute to the pyrolysis of polyester plastics.73 PET usually has an aromatic configuration and a high activation energy. The synergistic effect of this biomass pyrolysis on plastics significantly reduces the activation energy.74 Furthermore, PET has a relatively low effective H/C ratio and is rich in aromatic structures, thus facilitating the production of carbon materials during pyrolysis. The specific surface area of char produced by adding PET is higher than that of char produced from biomass alone, and the promoting effect gradually increases with increasing temperature and the temperature increase results in an increase of the aromaticity and hydrophobicity of carbon materials.75 In addition, the polyester plastics significantly increased the adsorption capacity of the carbon materials during co-pyrolysis with biomass.76 Therefore, the co-pyrolysis of polyester plastics and biomass can be exploited for the production of high performance adsorbent carbon materials.75
In conclusion, there is an interaction between the co-pyrolysis of PET and biomass. Biomass starts to degrade at a relatively low temperature compared to PET and produces OH radicals, which then interact with the H radicals of PET to facilitate the degradation process of PET, allowing it to be broken down into small molecules more quickly than in the case of pyrolysis alone. This also increases the yield of levoglucan and promotes the dehydration of levoglucosan to levoglucan saponin. Additionally, the co-pyrolysis of PET with biomass is highly beneficial for the yield of carbon materials. Compared to biomass pyrolysis alone, co-pyrolysis with PET not only enhances the yield of carbon materials but also modifies the physical properties of carbon materials. This means that the co-pyrolysed carbon materials have a higher specific surface area and cation exchange capacity, which increases the adsorption capacity of the carbon materials. The adsorption capacity of carbon materials is thereby improved. The interaction between PET and biomass also stabilizes the free radicals within char, allowing it to be degraded at lower temperatures.
Co-pyrolysis for bio-oil
The co-pyrolysis of PP, PE which includes HDPE and LDPE, and PS together with biomass appears to be more favorable for increased bio-oil generation.80 The polyolefin plastic inhibits polymerization and cross-linking reactions between the biomass components through encapsulation, thereby suppressing carbon material formation and enhancing bio-oil production.81 Though all polyolefin plastic waste significantly enhances the hydrocarbon content of bio-oils and decreases oxygenated compounds, the difference lies in the fact that PE and PP facilitate the formation of aliphatic hydrocarbons, while PS promotes the formation of aromatic hydrocarbons.82 Free radical interactions during co-pyrolysis can lead to improved bio-oil yield and stability.83 Generally, PP, PE, and PS provide in situ hydrogen during co-pyrolysis with biomass as a means to increase the ratio of H to C in the pyrolysis products, thereby resulting in enhanced bio-oil yields and an increased calorific value of the bio-oils.29In the case of PP, as the temperature progressively ascends, cellulose and the like start to undergo a pyrolysis procedure, and the long bonds of PP begin to fracture, and the two come into contact and interact.84 As the temperature progressively attained a level above 500–800 °C, the interaction between cellulose and PP gave rise to an augmentation in bio-oil yield. In this process, the extraction reaction of hydroxyl, hydrogen, and methyl groups is promoted, which consequently results in an increase in the yield of bio-oils.85 Furthermore, the cooperative impacts of the co-pyrolysis of PP and biomass typically contribute to the diminution of activation energy in the reaction procedure. The synergistic effect is also manifested in the conveyance of hydrogen from the plastic, which leads to the bio-oils obtained from the mixture of wood and PP generally being hydrogen-rich.28 A study of the pyrolysis of tobacco and PP revealed a significant reduction in activation energy compared to the pyrolysis of tobacco alone.86 The co-pyrolysis of lignin and PP was subjected to an in-depth investigation; the synergistic effect of this process resulted in higher yields of liquid bio-oils and lower yields of char.34 This indicates that plastic waste and biomass demonstrate favorable synergies for enhancing the yields of hydrocarbons and the feed conversion rates.
The interaction between PS and cellulose facilitates superior bio-oil yields and characteristics, while simultaneously reducing the yields of gas and carbon.82 The reaction process is similar to that of PP. Nonetheless, PS is capable of promoting the generation of a greater amount of bio-oils during the co-pyrolysis process, due to its analogous pyrolysis temperature to that of biomass and secondary reactions of pyrolyzed substances.87 The co-pyrolysis of grapeseed and PS within a fixed-bed reactor disclosed that the incorporation of PS led to a higher thermal energy value of bio-oils in contrast to biomass pyrolysis alone.28 Similar to PP, PS also diminishes the activation energy. The addition of PS enhanced the reactivity of walnut shells and peach pits, which significantly increased the extent of pyrolysis degradation process, together with the initial and final temperatures.88 Particularly, there are greater yields of toluene, ethylbenzene, and naphthalene in the catalyzed rapid pyrolysis of PS in combination with cellulose.89 Furthermore, the utilization of PS for char production has relatively minor advantages. At lower pyrolysis temperatures, PS is preferentially deposited onto the surface of the biomass, which facilitates adhesion for the formation of carbon materials.90 However, with the increase of temperature, PS will gradually volatilize and be converted into liquid bio-oil and syngas.91 In conclusion, depending on specific requirements, the addition of PS to biomass can significantly produce more bio-oils with higher calorific values. However, the presence of PS favors the production of carbon materials when co-pyrolysis is carried out with certain specific biomasses and the specific characteristics of the carbon materials are related to the pyrolysis temperature.
PE can be divided into HDPE and LDPE, and like PP and PS, PE is conducive to bio-oil production, with LDPE being more effective than HDPE.92 The interaction between PE and cellulose during the process of mixed pyrolysis has a tendency to increase the non-condensable gases, restrain the generation of char, and enhance bio-oils owing to the re-polymerization of C–C bonds and the coupling reactions of lignin.93 It is widely believed that polyethylene plays a role in supplying H radicals to biomass during co-pyrolysis. In particular, the addition of HDPE to biomass co-pyrolysis reduces the formation of catalysts and oxygenated compounds due to the low fixed carbon content of HDPE.94 Furthermore, in contrast to HDPE, LDPE possesses more branches and less powerful intermolecular forces. LDPE is a polyolefin with high molecular weight that undergoes incomplete pyrolysis and experiences chain breaks, thereby giving rise to the formation of larger molecules.95 Owing to the decomposition of LDPE and biomass via free radicals, LDPE is capable of inhibiting the generation of coke in the course of biomass pyrolysis.96 By virtue of the relatively low thermal stability of biomass, it undergoes decomposition prior to LDPE, thereby generating primary radicals. With the temperature rising, LDPE starts to disintegrate into hydrocarbons that are rich in H radicals, and the H radicals originating from LDPE facilitate the secondary decomposition of biomass to yield volatiles.97 These volatile constituents prevent LDPE from enveloping the biomass through softening at elevated temperatures, thereby suppressing the formation of coke during biomass pyrolysis.98 Concurrently hydrogen transfer resulting from chain breakage of HDPE promotes the decomposition of cellulose, and the oxygenates within cellulose facilitate the breaking and cracking of HDPE.99 Therefore, in the course of co-pyrolysis of biomass with HDPE, HDPE typically exhibits a favorable impact on the bio-oils.100 LDPE also contributes to decreasing the activation energy.101 The hydrogen atoms transported from HDPE can facilitate the production of hydrocarbons through strengthening the cleavage and deoxidation reactions.102 The pyrolysis of a mixture of pinewood and HDPE in a two-column reactor resulted in an increase of pyrolyzed bio-oils by a factor of the HDPE added compared to that of pyrolyzed bio-oils alone.94 The co-pyrolysis of wastepaper and HDPE exhibited synergistic effects on the properties of bio-oils. This highlights the roles of hydrogen replenishment and the deoxygenation of HDPE. The co-pyrolysis of discarded newspapers and HDPE generated a greater number of bio-oils and a lower amount of gas compared to the theoretical values.103
In addition, when PE and biomass are co-pyrolyzed, the pyrolysis temperature of both is significantly reduced, and co-pyrolysis accelerates the decomposition rate of the two substances.104 The co-pyrolysis of HDPE and potato materials was examined, and the primary thermal degradation occurred in the range of 178 °C to 378 °C, while the decomposition of HDPE alone mainly occurred at approximately 400 °C.105
In conclusion, when the intended product is bio-oils, PP, PE, and PS can be selected for co-pyrolysis with biomass. During the reaction, they will supply H radicals and lower the activation energy, thereby facilitating the reaction. PP and PS undergo analogous degradation processes, and both degrade prior to biomass. Conversely, PE is degraded later than biomass. In particular, the initial pyrolysis temperature of PE during co-pyrolysis is significantly lower than that of PE pyrolysis alone, which is conducive to accelerating the reaction rate of pyrolysis.
Co-pyrolysis mechanism
A more comprehensive analysis of the reaction mechanism between plastic waste and biomass is essential for process improvement.106 The co-pyrolysis of biomass and plastic waste is divided into slow and fast pyrolysis processes.107 The main product of slow pyrolysis is carbon materials, and the main product of fast pyrolysis is pyrolyzed bio-oils.100 The co-pyrolysis products from biomass and various plastic wastes are qualitatively and quantitatively superior to biomass pyrolysis products.108The synergistic mechanism of catalytic co-pyrolysis between plastic waste and biomass at elevated temperatures is capable of uncovering highly complex chemical pathways. Among a variety of consecutive or parallel reaction pathways, a considerable number of free radicals are involved as reaction intermediates in the catalytic replicative cleavage.99 Cellulose undergoes processes such as dehydration, decarbonisation, and decarboxylation, and thermal degradation produces furan chemicals. Hemicellulose is depolymerised to form furan compounds. Lignin is primarily depolymerised to produce phenolic chemicals.109 Plastic waste is pyrolysed by random scissions and chain-end cracking. Chain-end cracking produces olefins and hydrogen. Random scissions produce high molecular weight waxy intermediates, which undergo catalytic cracking and amphoteric ionisation to produce light olefins.110 These two mechanisms can operate simultaneously, producing both free radicals and long carbon chains.111 In addition, free radical fragments can be converted into straight-chain hydrocarbons through hydrogen transfer processes.112
During co-pyrolysis, olefins from plastics react with furan compounds from biomass to form aromatic hydrocarbons.111 Free radicals generated during the co-pyrolysis of biomass also contribute to the degradation of plastic polymers.25 Among them, lignin is the main provider of reactive radicals during rapid pyrolysis of biomass. Therefore during co-pyrolysis, lignin may have a significant influence on the degradation of plastic waste.113 In addition, the plastic that gradually dissolves during the pyrolysis process also covers the biomass pellets, facilitating energy transfer during the reaction.38
The rate of intramolecular hydrogen bond transfer that determines the type of products depends on the location of free radicals, which have different reaction pathways.108 Because biomass is a strong hydrogen acceptor and plastic waste has a relatively high hydrogen content, plastic waste typically provides hydrogen to biomass.114 Hydrogen extracted from plastic waste can inhibit cross-linking and polymerisation reactions.94 This suggests that hydrogen transfer during co-pyrolysis contributes to the stabilization of biomass primary products. Furthermore, hydrogen produced by most plastic waste enhances the deoxygenation of biomass and reduces the activation energy of the reaction.29 The reaction process involves interactions between biomass and plastic. Additionally, multiple degradation pathways also promote the generation of free radicals, allowing multiple degradation processes to occur at relatively lower temperatures.71 Hydrogen take up by reactive radicals, oxygenates, and polyethylene can accelerate the cleavage of polymer chains and their derivatives.115 Overall, most of the free radicals produced by the pyrolysis of plastics bind to oxygen radicals in the biomass, enhancing the deoxygenation of biomass and accelerating the rate of the reaction, allowing it to decompose at temperatures lower than the original pyrolysis temperature. It also inhibits some of the intermediate reactions and prevents the intermediate products from volatilizing under the effect of elevated temperatures; this contributes to the stabilization of the primary products produced.
Kinetic and thermogravimetric analyses (TGA) also support the above conclusion.26 At the start of co-pyrolysis, the biomass initially decomposes at relatively low temperatures, producing OH radicals and high molecular weight organic matter. Different plastic wastes decompose at different times, but all produce H radicals, which interact with the primary products produced by the biomass.28 With increasing temperature, the large organic molecules are broken down into smaller molecules.25 The co-pyrolysis process tends to occur faster and the onset temperature of co-pyrolysis varies due to the activation energy generated by specific interactions between different plastic wastes and biomass. It has been shown that single pyrolysis of PP starts at 400 °C. When tobacco stalks are co-pyrolysed with PP, pyrolysis starts at 450 °C. The activation energy for the co-pyrolysis of tobacco stems and PVC was reduced by about 50% in the first stage of pyrolysis compared to PVC pyrolysis alone.116 TGA showed that the activation energy of co-pyrolysis was usually lower than the pyrolysis of the plastic alone, showing a synergistic effect. TGA of rubber seed husk and HDPE showed that the activation energy of their co-pyrolysis was lower than that of HDPE alone.117 TGA of PCs and pine showed an overlap in their activation energy distributions. This overlap led to an interaction between the two, resulting in an enhanced degree of co-pyrolysis. The activation energy of PCs in the presence of pinewood was reduced compared to PC decomposition alone.118 TGA of microalgae, wood and PP showed that the addition of PP reduced the pyrolysis temperatures of wood and microalgae and that the co-pyrolysis of microalgae Chlorella vulgaris, wood and PP exhibited a synergistic effect between 300 °C and 400 °C.119 In summary, the generally accepted mechanism for the co-pyrolysis of biomass and plastic wastes can be summarized in two steps: (1) Pyrolysis of biomass generates free radicals that inhibit inter- and intramolecular hydrogen transfer in plastic waste to produce aliphatic hydrocarbons and reduced olefins; and (2) Hydrogen transferred from plastic waste reacts with biomass radicals to produce stable primary products. In addition, the entire reaction pathway during catalytic co-pyrolysis primarily proceeds via the cleavage of abundant –O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –C
O, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–O–C moieties in biomass.120 In conclusion, the interactions during co-pyrolysis are a key factor improving the yield and properties of pyrolysis products.
O, and C–O–C moieties in biomass.120 In conclusion, the interactions during co-pyrolysis are a key factor improving the yield and properties of pyrolysis products.
The hydrocarbon pool mechanism and the Diels–Alder mechanism are the main mechanisms of the reaction. The hydrocarbon pool mechanism is an indirect mechanism in methanol-to-hydrocarbon (MTH) reactions that explains the formation of the initial C–C bond. The hydrocarbon pool mechanism postulates that a series of unsaturated hydrocarbons are initially formed and these compounds form a “hydrocarbon pool” on the catalyst surface. The compounds in this pool are then subjected to a series of reactions involving methylation, cracking and recombination to produce low carbon olefin products.121 The Diels–Alder reaction, also known as the bis-alkene addition reaction, is a cycloaddition reaction in which a conjugated diene reacts with a substituted alkene (commonly referred to as a dienophile surrogate) to give rise to a substituted cyclohexene.122 The Diels–Alder reaction and hydrocarbon pooling mechanisms are primarily responsible for the conversion of phenols into aromatics via dehydration, cracking and oligomerisation.94 In addition, furans and light olefins give rise to aromatic compounds via the Diels–Alder reaction, followed by dehydration reactions to potentiate the aromatic hydrocarbons.123 In particular, the removal of hydrogen by oxygenated compounds in the biomass enables the conversion of plastic waste into olefins and alkanes.124 Pyrolysis experiments on biomass with PE showed that the Diels–Alder reaction between cellulose- and xylan-derived furans and polyethylene-derived light olefins was facilitated, increasing the yield of aromatic hydrocarbons.111
Co-pyrolysis with chlorinated plastics
The chlorine radicals generated during the degradation of PVC can initiate condensation reactions, cyclisation and aromatisation. At the same time, PVC can increase the reactivity of biomass and accelerate the reaction rate.28 It has been found that the addition of PVC contributes to the decomposition of pinewood in the lower temperature range, due to the fact that dehydrochlorination of PVC leads to accelerated decomposition of pinewood by hydrochloric acid.23 In addition, increased coke production from the co-pyrolysis of pine and PVC can also be attributed to the fact that PVC produces hydrochloric acid at relatively low temperatures (from 230 °C to 300 °C) and facilitates the dehydration of cellulose into aldehyde compounds, resulting in an increase in carbon production due to the reduction of hydrogen and oxygen atoms in cellulose.128 Studies on the co-pyrolytic interactions between PVC and poplar or rice have shown that co-pyrolysis is more pronounced between PVC and rice and between PVC and poplar than between rice and poplar. Hydrochloric acid produced by PVC was able to participate in the pyrolysis of rice and formed organic chlorides between 350 °C and 500 °C.129 PVC is capable of modifying the pyrolysis performance of lignocellulosic biomass with respect to aspects such as the reactive property. The co-pyrolysis reactivity of cherry seed/PVC is two orders of magnitude higher than that of cherry seed alone at the same heating rate. This increase in reactivity is thought to be due to the incorporation of highly electronegative chloride ions into the chemical structure of PVC.130 The co-pyrolysis of cellulose and PVC was conducted in polar and non-protonic solvents. The fragmentation of vinyl chloride in tetrahydrofuran at the early reaction stage plays a catalytic role in enhancing the depolymerization process of cellulose and accelerating the conversion process. The co-solvents constituted by tetrahydrofuran and water can further enhance the conversion and liquid yield.131
The distribution of halogens among the pyrolysis products varies depending on the type of pyrolyzed plastic and the process conditions. The thermal degradation of PVC can be roughly partitioned into two steps that partially overlap: (1) at approximately 360 °C, dechlorination occurs, where the majority (approximately 99%) of chlorine departs in the form of hydrochloric acid; and (2) the residual polyolefinic structure above 360 °C undergoes intramolecular cyclization and cross-linking, thereby giving rise to the main aromatic compounds.133 This constitutes a crucial attribute of PVC, which can be utilized in a dehalogenation process referred to as progressive pyrolysis. Existing studies utilize several potential mechanisms for dehalogenation during pyrolysis: (a) The generation of hydrochloric and hydrobromic acids through a free radical process, which subsequently exit the system, and this is the predominant pathway of stepwise pyrolysis; (b) β-H elimination on Lewis acid sites through direct elimination or via two-stage dissociative adsorption reactions by means of interactions between the metal oxides and the organic halogens; (c) Intense neutralization reactions between hydrobromic acid or hydrochloric acid and intense neutralization reactions between the metal substances, respectively forming bromides and chlorides.134
Nevertheless, not all halogen atoms can be eliminated during pyrolysis through the natural release route. Hence, it is of great significance to identify a suitable dehalogenation approach. Considerable efforts have been dedicated to seeking an adsorbent/catalyst that can further decrease the halogen content. Among the most effective dehalogenating agents hitherto discovered are CaCO3, Ca(OH)2, Na2CO3, Fe3O4, and Fe.135 Furthermore, halogen removal can be carried out in different ways, depending on the suitability of the different reactions, and dehalogenation can be conducted before, during, or after pyrolysis. For more effective dehalogenation and decreased cost, it is expedient to incorporate dehalogenation, typically by means of performing pyrolysis vapour condensation in the presence of a catalyst or solid adsorbent, respectively, to allow the pyrolysis vapour to pass through iron oxide–carbon or calcium carbonate composites, thereby achieving a higher degree of dehalogenation.134 Alternatively, to circumvent inhibitory interactions, classification and dechlorination can be implemented prior to pyrolysis and preheated to approximately 380 °C, such that the majority of chlorine within PVC is liberated as hydrochloric acid and adsorbed by a solid adsorbent.9
The complete debromination of dodecylbenzene ethane (DecaBDE) within PS was accomplished through wet grinding in a stirred ball mill for 24 h, by utilizing sodium hydroxide in ethylene glycol as a dehalogenation reagent within a temperature range of 150–190 °C.132 Consequently, PP incorporating DecaBDE can be subjected to debromination with inexpensive and abundantly available solid reagents (namely, Fe and silica), which is typically carried out at the pretreatment stage. Additionally, the hydrothermal method is an efficacious approach for the dechlorination of PVC, and high dechlorination efficiencies can be attained.136 The employment of hydrothermal dechlorination circumvents the liberation of HCl during the catalytic cracking process, which may even give rise to catalyst poisoning, and it possesses advantages over the traditional pyrolysis dechlorination process. However, the disadvantages of high-pressure systems, mix of heteroatoms, and secondary contamination should not be ignored.137
Currently, mechanical force chemical (MC) debromination technology is used as an expected non-combustion treatment means for debromination. MC refers to the utilization of multiple modalities of mechanical force (such as collision, compression, shear, and friction) with the aim of inducing a chemical reaction. Destruction through MC does not require heating or off-gas handling, and persistent organic pollutants (POPs) are decomposed into carbon, carbon dioxide, water, and inorganic halogenated compounds, thus preventing the generation of unintended pollutants like PBDD/Fs. Nevertheless, MC still has certain deficiencies such as the requirement for large equipment, low reaction rate, and strict reaction conditions.138
Furthermore, dehalogenation methods can be categorized as physical and chemical dehalogenation, each possessing distinct characteristics. Physical dehalogenation depends on physical processes or apparatus to accomplish dehalogenation. Sub-/supercritical fluids exert a remarkable dehalogenating and carbonizing effect on halogenated plastic waste and form distinctive carbon structures. Sub-/supercritical carbon dioxide has emerged as the most prevalent green medium for the simultaneous detoxification and conversion of polymer wastes into carbon-containing materials due to the simplicity of phase separation and relatively milder operating conditions in comparison with other sub-/supercritical solvents.139 Chemical dehalogenation refers to the substitution or decomposition of halogens into substances of lower toxicity via the reaction of chemical reagents with halogen-containing organic substances. This approach typically encompasses glycolic acid dehalogenation and alkali-catalyzed decomposition. In situ methods are not sufficiently efficient at dehalogenation and may even lead to an increase in the chlorine content. Superior results are frequently achieved by employing alkali absorbents and metal oxides (such as CaO, Ca(OH)2, CaCO3, and Fe3O4), which immobilize Cl in the form of metal chlorides.133 Research into the dehalogenation of plastic waste has made some progress, but there are still a number of key issues to be resolved, such as improving dehalogenation efficiency, minimising energy consumption and reducing environmental impact. Future research will continue to focus on these issues and strive to find more optimal solutions.
Co-pyrolysis with other plastic waste
PCs represent a category of thermoplastic polymers that incorporate carbonate groups within their chemical architectures.99 Similar to other plastic waste, PCs facilitate co-pyrolysis and diminish the activation energy. In particular, PCs typically exhibit a propensity for greater gas production and lower yields of tar and carbon.140 This phenomenon implies that the interactions existing between PCs and biomass, encompassing gas-phase volatile interactions as well as volatile–solid interactions, enhance the overall conversion of the solid feedstock into gas. Additionally, the pyrolysis of PCs has a tendency to generate a greater amount of phenol via deoxygenation.118 The decomposition of PCs is predominantly executed via the chain scission mechanism, and the primary reactions involve an intramolecular exchange reaction and hydrolysis, which give rise to terminal hydroxyl oligomers and carbon dioxide within the temperature range from 400 to 500 °C.141 Blending pine with PCs augments this particular pathway by means of forming stable phenolic intermediates in conjunction with the lignin fraction, thereby facilitating the decomposition and conversion of pine into low molecular weight aromatics in the form of volatile substances, which lead to a reduction in the formation of charcoal by approximately 10%.118 Co-pyrolysis experiments conducted on pine and PCs demonstrated that molten PCs constituted a physical obstruction that impeded liberation of compounds and heat transfer. Additionally, the carbon materials played the role of a catalyst in accelerating the decomposition of PCs, which yielded inert char.142By comparing the experimental outcomes of the co-pyrolysis of mixtures of pine and PCs with the weighted average of pyrolysis of the individual feedstocks, the magnitude of the interactive effect of their co-pyrolysis on gas production can be ascertained. Compared with the theoretical values of single pyrolysis, the total yields of H2, CO, and others were respectively enhanced by 33%, 36%, and 19%, thereby indicating positive synergies between pine and PC.65 Conversely, there is a certain impediment to the formation of CnHm. The variance in the synergistic behavior of the diverse gas components might be ascribed to the fact that the interaction between the intermediates of pine and PC during co-pyrolysis leads to the generation of a greater quantity of oxygenated substances, while resulting in fewer hydrocarbons.143 The pyrolysis of PC and pinewood results in the reduction of phenolics due to the alkali metal-catalyzed deoxidation of biomass and cleavage of phenolic intermediates.140 Moreover, the replicative cleavage of PCs and lignin undergoes pyrolysis conversion, which transforms the monophenols from PCs into bisphenols.144
Certain researchers have postulated that the pyrolysis of biomass takes place prior to that of polyester plastic waste, and the volatiles within the biomass further contribute to the pyrolysis of polyester plastic waste.145 PCs absorb water from the reaction. The result is a reduction in the water content of the bio-oils and the production of highly valuable hydrolysates such as aromatic acids.132 Moreover, the addition of lignin during the pyrolysis of PCs can influence the decomposition of PCs into phenolic compounds by facilitating the liberation of CO during co-pyrolysis, while concomitantly suppressing the liberation of aromatic compounds.141
Influence of co-pyrolysis process parameters
Influence of pyrolysis process parameters
The yield and composition of products depend not only on the type of plastic being co-pyrolyzed, but also on the process parameters, encompassing the heating rate, particle size, reaction temperature, pressure, and catalyst.3 Furthermore, these process parameters exert an influence on the synergistic effects among the feedstock mixtures within the co-pyrolysis process, and on the various reaction mechanisms.26 Selecting the optimal reaction conditions for the desired product constitutes the key to improving production efficiency.146Temperature is a very important co-pyrolysis parameter that is critical for both the yield and the physicochemical properties of the carbon material, such as stability, specific surface area, pore structure and functional groups of the carbon material.147 Concerning the main constituents of biomass (cellulose, hemicellulose, and lignin), the temperature degradation ranges are respectively 325–400 °C, 250–350 °C, and 300–500 °C.43 For plastic waste, the spectrum of degradation temperatures typically either overlaps with or is higher than that of biomass, and it varies for different sorts of polymers.54 It was shown that the yield of biochar showed a decreasing trend with the gradual increase of pyrolysis temperature. In contrast, the yield of bio-oils gradually increased.148 A study showed that biomass gave the highest yield of carbon materials when pyrolysed at 350 °C, and that higher pyrolysis temperatures on this basis resulted in an increase in the surface area, an increase in pore size, and an increase in pH and carbon content.149 However, when biomass is co-pyrolysed with plastics, the optimal temperature shows a slight variation depending on the composition of diverse raw materials.150
The mixing ratio of feedstocks is likewise a crucial factor influencing the yield and composition of co-pyrolysis, and a large number of studies have focused on understanding the influence of blending biomass with various plastic wastes on the yield and productivity. Some investigations have demonstrated that the char yield ascends with an increasing blending ratio of PET.28 The interaction between biomass and PET is more prominently manifested during co-pyrolysis when the biomass fraction assumes a predominant position (higher carbon production synergies for 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 blends as opposed to 50
30 blends as opposed to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 or 30
50 or 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 blends).72 It has also been evidenced that blends of biomass and PET in a ratio of 3
70 blends).72 It has also been evidenced that blends of biomass and PET in a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 yield more pronounced synergistic effects compared to blends in ratios of 1
1 yield more pronounced synergistic effects compared to blends in ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 5
1 or 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.69 Consequently, the feedstock mixing ratio is a notable variable affecting the carbon materials.151 Additionally, a higher proportion of plasticity in the feedstock leads to the melting of the plastic, which results in an increase of the carbon material yield.99 The blending ratio between biomass and plastic waste exerted an influence on the product distribution. It was also noted that diverse feedstock blends yielded bio-oils of differing qualities.151 Furthermore, the co-pyrolysis of lignin and PP for the generation of bio-oils encompassing naphthenic and aromatic hydrocarbons was examined. The blends of biomass and PET in a ratio of 70
1.69 Consequently, the feedstock mixing ratio is a notable variable affecting the carbon materials.151 Additionally, a higher proportion of plasticity in the feedstock leads to the melting of the plastic, which results in an increase of the carbon material yield.99 The blending ratio between biomass and plastic waste exerted an influence on the product distribution. It was also noted that diverse feedstock blends yielded bio-oils of differing qualities.151 Furthermore, the co-pyrolysis of lignin and PP for the generation of bio-oils encompassing naphthenic and aromatic hydrocarbons was examined. The blends of biomass and PET in a ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 were more conducive to the output of bio-oils as compared to blends with ratios of 50
30 were more conducive to the output of bio-oils as compared to blends with ratios of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 or 30
50 or 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70.152 The synergistic effect of the process leads to a greater yield of bio-oils while simultaneously resulting in a reduced yield of carbon materials. Hence, when the intended products are carbon materials, the percentage of biomass can be augmented, whereas when the generation of bio-oils is the objective, a rise in the percentage of plastic is necessary.
70.152 The synergistic effect of the process leads to a greater yield of bio-oils while simultaneously resulting in a reduced yield of carbon materials. Hence, when the intended products are carbon materials, the percentage of biomass can be augmented, whereas when the generation of bio-oils is the objective, a rise in the percentage of plastic is necessary.
The heating rate constitutes a crucial parameter for ascertaining the composition of product. Differences in heating rates during the reaction process have an effect on the compositional percentage of pyrolysis products. Faster heating rates accelerate the cracking of the reacting feedstock, leading to more fragmentation of the feedstock, which increases oil and gas production. A slower heating rate triggers a secondary cracking phase, which results in more deposition of the reactive feedstock, favouring the production of carbon materials.153 For carbon materials, changes in heating rate affect the surface morphology of the carbon material. As the heating rate increases, the rate of evaporation of volatiles increases, leading to a decrease in the surface area and porosity of the carbon material.154 It was found that faster heating rates led to a decrease in the oxygen content within the carbon material.155 In addition, it was found that the heating rate had a greater effect on the yield and surface morphology of the carbon material, and less effect on the composition and other properties of the carbon material.156
Catalysts frequently prove advantageous in facilitating the attainment of the desired products, and hence it is of great significance to possess an in-depth comprehension of the properties of catalysts and to choose an appropriate catalyst. The incorporation of a catalyst promotes the cracking of pyrolysis vapors and enhances the selectivity for desired compounds via the dehydration, decarbonization, and deoxygenation of deoxygenated compounds.67 Furthermore, the catalyst assists in steering of the reaction towards specific products through the interaction of its structure with the pyrolysis reactants and the resulting product.157 During the process of pyrolysis, the catalyst expedites a series of reactions, thereby enhancing product selectivity and quality.158 The efficacy of a catalyst is contingent upon its acidic nature, redox characteristics, and porosity. The modulation of the acidity of the catalyst in accordance with the density, strength, and type of catalyst is of crucial importance in the design of the catalyst, as these elements exert specific influences on activity, product selectivity, and reaction pathways.49 There are many types of catalysts used to perform catalytic pyrolysis, such as homogeneous catalysts, acid mesoporous materials, non-acid mesoporous solids, fluid catalytic cracking (FCC) catalysts, zeolites and metal oxides. The most commonly used catalysts are zeolites, siliceous alumina, MCM-41 and FCC.159 Common catalysts used in the preparation of carbon materials are nickel oxides, magnesium oxides, alumina, ZMS-5 and Al-MCM-41.159 It has been proved that most of these inorganic salt type catalysts can be used to increase the production of carbon materials while reducing the production of oil and gas.35 It has been shown that ZSM-5 is more conducive to changing the acid or alkali content of the biomass, leading to an increase in biochar production, than using sodium carbonate and alumina as catalysts.160 Most studies have shown that most catalysts possess the ability to increase biochar yield during co-pyrolysis.161
The size of the feedstock particles also has an impact on the products of co-pyrolysis.162 Larger particles (exceeding 0.5 mm) are prone to show poorer heat transfer to the inner surface of the raw material particles, resulting in decreased volatile yields due to the larger temperature difference among particles. Nevertheless, particle size (>1 mm) has no significant effect on bio-oil yield, suggesting that endothermicity or mass transfer have negligible effects on the process. It has also been reported that larger particle sizes result in less heat transfer to the interior of the feedstock.162
In conclusion, diverse process parameters tend to alter the product orientation of co-pyrolysis, and they should be judiciously selected in order to attain the most efficacious reaction process and optimal quality of product composition. Temperature is critical for the yield and physicochemical properties of carbon materials. As the pyrolysis temperature gradually increases, the yield of carbon materials tends to decrease, and generally speaking, the highest yield of carbon materials is found at 350 °C. The mixing ratio of the raw materials also plays an important role in the formation of the product. The synergistic effect of the two in producing carbon materials is more pronounced when biomass is more predominant. In addition, an increase in plasticised plastics also leads to an increase in the production of carbon materials. Changes in the heating rate also affect the properties of the carbon material, with slower heating rates favouring increased yields of carbon material. Typically, the presence of a catalyst tends to favour the production of carbon materials.
Plastic waste affects the co-pyrolyzed carbon material properties
The production of carbon materials and their use have important social and economic benefits. The process of producing carbon materials is important for mitigating climate change, improving soil structure, remediating contaminated water bodies and providing energy.35 Co-pyrolyzed carbon materials are a product of feedstock interactions during the pyrolysis process. The majority of co-pyrolysis processes not only enhance the yield of carbon materials but also modify the original properties and composition of the co-pyrolyzed carbon materials.163 Plastic waste and biomass are no exception when co-pyrolysing. In the co-pyrolysis of plastic waste and biomass to produce carbon materials, the presence of plastics changes the properties of the carbon materials, while different types of plastics have different effects on the properties of the carbon materials.164 Typically, carbon materials produced from the co-pyrolysis of plastic waste and biomass have various advantages, such as high specific surface area, good pore structure and abundant functional groups.165Specific elements contained within plastic waste can to some extent modify the carbon materials.166 A typical example is PVC. Certain researchers have ascertained that during the co-pyrolysis of PVC and biomass, PVC typically contributes to increasing the carbon material yield. Synergistic effects on char and oil properties were explored in experiments on the co-pyrolysis of pinewood (PW) and PVC, which showed that the co-pyrolysis of PW–PVC blends produced more char (15.5–27.9%) and less oil (7.2–14.4%) than the expected values, and that the PW–PVC interactions caused the pyrolysis onset temperature of PW to decrease significantly.128 It also illustrates that the interaction of PVC with biomass favours the lowering of the H/C atomic ratio of charcoal, thus improving the chemical stability of charcoal.167 In addition, the presence of PVC facilitates the synthesis of high-performance porous carbon materials. In co-pyrolysis experiments with PVC and lignin and rice husk, it was found that the presence of PVC favoured the enhancement of the specific surface area of the carbon material products, resulting in higher microporosity, which had a significant effect on the adsorption capacity of the carbon materials.168 This facilitates the synthesis of high-performance porous carbon materials.
In addition, the incorporation of nitrogen-containing plastic wastes in co-pyrolysis facilitates the increase in carbon material yield and nitrogen content of the carbon material.169 Co-pyrolysis of PU and biomass may be used as a typical example. In the co-pyrolysis of PU and biomass, nitrogen present in the PU interacts with the biomass, resulting in a higher nitrogen content in the resulting carbon material.170 This facilitates the enhancement of the surface activity of carbon materials and the formation of abundant functional groups, while also improving their chemical stability.171 In addition, the addition of nitrogen provides carbon materials with superior chemical properties, which helps to expand their application areas, making them capable of removing heavy metals, etc.171
PET also improves the yield of carbon materials. In the co-pyrolysis of PET and Paulownia wood, it was found that the yield of carbon material increased with the proportion of PET, and as the co-mixing ratio of PET increased, more of the PET product could react with the charcoal in Paulownia wood, resulting in higher charcoal yields in the co-pyrolysis process.66 Slow pyrolysis of PET and solid biomass mixtures also showed that PET–solid biomass blends increased the yield of pyrolysis products, oil and char, while decreasing the gas yield, compared to the pyrolysis rates of the individual components.68 Moreover, the presence of PET facilitates the stabilisation of free radicals in the carbon material, allowing it to start forming at lower temperatures.79 Mixed co-pyrolysis of PET with macadamia nut shells showed that carbon in the macadamia nut shells trapped volatiles in PET, resulting in the formation of a charcoal-like structure, which gave a synergistic carbon yield and accelerated the reaction.70 This also favours a faster reaction to produce more carbon material. In addition, the presence of PET significantly enhances the adsorption properties of carbon materials, which is very favourable for the preparation of high-performance adsorbent carbon material products.75
In summary, the presence of plastic waste has a significant effect on the performance of carbon materials compared to single-component pyrolysis of biomass. The most significant of these is the increased yield of carbon material. The ability of the plastic waste to form a covering film over the surface of the biomass during the pyrolysis process has led to an increase in the yield of carbon materials. Moreover, the intermediates produced during the pyrolysis of plastic wastes can interact with the carbon material, which is conducive to changing some of the properties of the carbon material. When plastic waste contains specific elements, it is often possible for the carbon material to capture these specific elements. As a result, this means co-pyrolytic carbon materials tend to have high performances. The specific surface area, pore structure, and functional groups of co-pyrolysed carbon materials are often superior to those produced by the pyrolysis of a single material. This makes co-pyrolyzed biochar more advantageous in the agricultural sector, the environmental sector and the energy sector. It can be used to improve soil structure, remediate polluted water bodies and provide energy.
Prospects and challenges
In recent years, the global focus has been intensely centered on the co-pyrolysis of biomass and plastic waste as feasible technology, dealing with the problems of waste handling and disposal while concomitantly contributing to the mitigation of CO2 emissions. It is an important process for converting waste into energy. In comparison with biomass pyrolysis in isolation, co-pyrolysis exhibits synergistic effects in terms of char and bio-oil production and resolves issues such as toxicity resulting from the mono-pyrolysis of plastic waste as well as the relatively low calorific value of biomass pyrolysis. Nevertheless, the specific reactions between plastic waste and biomass in the co-pyrolysis process remain indistinct, and further investigations into their properties and conversion approaches are required.This review illustrates the pyrolysis products obtained from the co-processing of solid wastes, such as plastic waste and biomass, and promotes sustainable solutions for the efficient management of waste and energy generation. In particular, the carbon material products obtained from the co-pyrolysis are of great importance for sustainable development. Co-pyrolytic carbon materials play an important role in many areas. In soil improvement, they have the ability to increase soil fertility due to their richness in various elements, and they also improve the structure of the soil, which helps the growth of plants.30 In the treatment of environmental pollution, the co-pyrolyzed carbon material has a good adsorption capacity by virtue of its porous nature, and it can adsorb heavy metal ions, harmful gases, etc., which is conducive to improved water and air quality.26 The clarification of the interaction between the two feedstocks, which is a crucial factor in differentiating co-pyrolysis from traditional pyrolysis, will facilitate the better design of systems for co-pyrolysis, resulting in higher yields of carbon materials with physicochemical properties that are more appropriately aligned to the desired requirements, while also enhancing the quality of other by-products during the process. Evidently, the mechanism of interaction is primarily the transfer of free radicals. Nevertheless, it is difficult to interpret the extent of interaction using data from diverse experiments. Currently, most of the relevant studies are still restricted to the laboratory and very few are performed on a pilot scale. To realize sustainable development, large-scale investigations on biomass–plastic co-pyrolysis are needed to provide more generalized data.
The interaction between plastic waste and biomass in the co-pyrolysis process constitutes an intriguing matter, as, if appropriately manipulated, it potentially enhances the efficiency of the plastic waste recycling procedure while increasing the value of organic waste that would otherwise be destined for landfill. Co-pyrolysis technology has the economic benefit of considerably reducing the volume of waste, thereby diminishing the number of landfill sites required, economizing on waste disposal costs, and addressing certain environmental concerns affiliated with landfill waste disposal. Hence, the development of co-pyrolysis technologies for plastic waste and biomass is of fundamental importance. Pyrolysis not only offers an effective modality for waste management and carbon utilization but also minimizes non-sustainable routes such as landfill and incineration while capitalizing on them.
Nevertheless, it also encounters several challenges, including feedstock variability, instability and deactivation of catalysts, as well as the complexity of synergistic mechanisms. In this section, the review offers insights into future research orientations based on the aforementioned summarized content. Additionally, subsequent observations regarding future technological progressions and products are made in accordance with our own comprehension: (1) Conduct research on pretreatment technologies, particularly lignocellulosic biomass, with the aim of enhancing the interaction between biomass and the intermediates generated through the co-pyrolysis of plastic waste, and to increase the yield of the requisite carbon materials. (2) Research on plastic waste pyrolysis holds greater application prospects for the utilization of pure plastics that are difficult to obtain from actual plastic waste. (3) Conduct mechanistic studies based on synergistic effects of biomass and plastic waste to provide guidance on pretreatment processes, feedstock mixing ratios, catalysts and reactor design. (4) Expanding the application range of biomass and plastic waste co-pyrolysis and other functional products to improve commercial performance. (5) Increase real-field pilot large-scale studies to address the problems and challenges that may arise in practical applications. (6) There are no extensive experimental studies on the hydrodynamic aspects, and more attention should be given to research in this direction to improve the yield of practical production and to solve problems in the operation process. (7) Focus on its commercial viability and work towards attaining the UN's sustainable development goals, especially in terms of economic viability in the storage and transportation of raw materials. (8) Conduct life cycle and environmental impact assessments, and analyze specific energy sources, options, and other parameters in accordance with the applicable national circumstances to demonstrate the advanced and practical status of this technology. (9) Address the issue of emissions and waste generated during the plastic pyrolysis process and reduce the recycling period.
Based on the above, we have outlined a development pathway for co-pyrolysis of biomass and plastic waste, as shown in Fig. 7. Firstly, co-pyrolysis facilitates the restructuring of industrial systems, driving the development of the upstream industries related to resource recycling and the downstream industries in chemical production. Secondly, by advancing basic research on co-pyrolysis, we can accelerate its commercialization, ultimately leading to the establishment of a global community collaborating on resources, technology, economics and environmental sustainability. Furthermore, the selection of future technologies will be contingent upon numerous factors, including the accessibility of plastic waste and biomass stockpiles, along with sustainability, financial expenditure, and process efficiency. It also requires several initiatives and technical support to make the technology more attractive. Concurrently, governmental organizations should also develop more sustainable disposal policies.
Conclusions
An overview of the overall reaction process, interaction mechanisms and contributing effects to the products produced by the co-pyrolysis of biomass and plastic waste is presented. There are diverse interactions for different plastic waste and biomass co-pyrolysis processes, which not only boost the yield and caloric content but also improve the physicochemical properties of the products. Plastic waste such as PET, PU, and PVC is favorable for promoting the output of carbon materials, while PP, PE, and PS are more conducive to bio-oil production. The product ratios are influenced by factors such as feedstock composition, structure, ratio, and pyrolysis conditions. Comparisons are also made between the similarities and differences in the natures of the feedstocks and individual pyrolysis. The paper summarizes the impacts of pyrolysis reaction temperature zones and operating parameters on the efficacy and yield of co-pyrolysis, encompassing the optimal temperature, feedstock mixing ratio, and heating rate, as well as the improvement by catalysts on the performance of carbon materials. PP and PS tend to decompose prior to the biomass, yielding small molecules that are deposited on the surface of the biomass, while LDPE decomposes after the biomass, generating H-radicals that facilitate the secondary decomposition of the biomass, which are beneficial for preventing volatiles from covering the biomass. Typically, 500 °C is regarded as the optimal temperature for co-pyrolysis. When the biomass is more preponderant, it is favorable for the output of carbon materials. Additionally, relatively faster heating rates are frequently employed to generate bio-oils, while slow pyrolysis is often the primary approach for producing carbon materials. Furthermore, the performance of carbon materials can be enhanced by utilizing different catalysts. In particular, this paper comprehensively summarizes a number of common dehalogenation approaches that can be employed to effectively eliminate halogenated elements from plastic waste, depending on the specific time period for removal.Data availability
Data supporting this review are included in the article's ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Key Research and Development Program of Hubei Province (2023BBB001), the National Key Research and Development Program of China (2021YFD1600503), and Support Enterprise Technology Innovation and Development Projects of Hubei Province (2021BAB115). Baojun Yi acknowledges support from the China Scholarship Council (202206765005) and Korea University for hosting his visiting research. This work was supported by the Technology Innovation Program (Project No. 00432915, Development of biodegradable polymer and their applications using high-performance enzyme activation technologies for acceleration of biodegradability) funded by the Ministry of Trade, Industry & Energy (MOTIE, korea). This work was supported by Korea University Grant. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1A6A1A10045235).References
- S. A. Raja, Z. R. Kennedy, B. C. Pillai and C. L. R. Lee, Energy, 2010, 35, 2819–2823 CrossRef CAS.
- C. Ravichandran and G. Venkatesan, Environ. Monit. Assess., 2021, 67–103 Search PubMed.
- W.-H. Chen, C. Naveen, P. K. Ghodke, A. K. Sharma and P. Bobde, Fuel, 2023, 345, 128177 CrossRef CAS.
- M. Singh, S. A. Salaudeen, B. H. Gilroyed, S. M. Al-Salem and A. Dutta, Biomass Convers. Biorefin., 2023, 13, 8747–8771 CrossRef.
- A. Antelava, N. Jablonska, A. Constantinou, G. Manos, S. A. Salaudeen, A. Dutta and S. M. Al-Salem, Energy Fuels, 2021, 35, 3558–3571 CrossRef CAS.
- K. B. Ansari, B. Kamal, S. Beg, M. A. Wakeel Khan, M. S. Khan, M. K. Al Mesfer and M. Danish, Renewable Sustainable Energy Rev., 2021, 150, 111454 CrossRef CAS.
- R. Mishra, H. C. Ong and C.-W. Lin, Energy Convers. Manage., 2023, 284, 116983 Search PubMed.
- M. Haghighat, N. Majidian, A. Hallajisani and M. Samipourgiri, Sustainable Energy Technol. Assess., 2020, 42, 100870 CrossRef.
- A. I. Osman, M. Farghali, I. Ihara, A. M. Elgarahy, A. Ayyad, N. Mehta, K. H. Ng, E. M. Abd El-Monaem, A. S. Eltaweil, M. Hosny, S. M. Hamed, S. Fawzy, P.-S. Yap and D. W. Rooney, Environ. Chem. Lett., 2023, 21, 1419–1476 CrossRef CAS.
- H. Li, H. A. Aguirre-Villegas, R. D. Allen, X. Bai, C. H. Benson, G. T. Beckham, S. L. Bradshaw, J. L. Brown, R. C. Brown, V. S. Cecon, J. B. Curley, G. W. Curtzwiler, S. Dong, S. Gaddameedi, J. E. García, I. Hermans, M. S. Kim, J. Ma, L. O. Mark, M. Mavrikakis, O. O. Olafasakin, T. A. Osswald, K. G. Papanikolaou, H. Radhakrishnan, M. A. Sanchez Castillo, K. L. Sánchez-Rivera, K. N. Tumu, R. C. Van Lehn, K. L. Vorst, M. M. Wright, J. Wu, V. M. Zavala, P. Zhou and G. W. Huber, Green Chem., 2022, 24, 8899–9002 RSC.
- X. Yuan, X. Wang, B. Sarkar and Y. S. Ok, Nat. Rev. Earth Environ., 2021, 2, 659–660 CrossRef CAS PubMed.
- P. Samanta, S. Dey, D. Kundu, D. Dutta, R. Jambulkar, R. Mishra, A. R. Ghosh and S. Kumar, Trends Environ. Anal. Chem., 2022, 36, e00181 CrossRef CAS.
- Y. Chae and Y.-J. An, Environ. Pollut., 2018, 240, 387–395 CrossRef CAS PubMed.
- P. C. Ojha, S. S. Satpathy, A. K. Ojha, L. B. Sukla and D. Pradhan, Sci. Total Environ., 2022, 832, 155072 CrossRef CAS PubMed.
- M. Ghayebzadeh, H. Taghipour and H. Aslani, Sci. Total Environ., 2020, 733, 138942 CrossRef CAS PubMed.
- C. Li, X. Yuan, Z. Sun, M. Suvarna, X. Hu, X. Wang and Y. S. Ok, Bioresour. Technol., 2022, 346, 126582 CrossRef CAS PubMed.
- R.-X. Yang, K.-H. Chuang and M.-Y. Wey, Energy Fuels, 2018, 32, 5462–5470 CrossRef CAS.
- A. Fazil, S. Kumar and S. M. Mahajani, Energy, 2022, 243, 123055 Search PubMed.
- C. Abdy, Y. Zhang, J. Wang, Y. Yang, I. Artamendi and B. Allen, Resour., Conserv. Recycl., 2022, 180, 106213 CrossRef CAS.
- A. V. Bridgwater, Biomass Bioenergy, 2012, 38, 68–94 Search PubMed.
- S.-Y. Oh and Y.-D. Seo, Bioresour. Technol., 2016, 218, 77–83 CrossRef CAS PubMed.
- J. A. Rodriguez, J. F. Lustosa Filho, L. C. A. Melo, I. R. de Assis and T. S. de Oliveira, J. Anal. Appl. Pyrolysis, 2021, 155, 105036 CrossRef CAS.
- H. Fan, J. Gu, S. Hu, H. Yuan and Y. Chen, J. Energy Inst., 2019, 92, 1926–1935 CrossRef CAS.
- W. Chen, S. Shi, J. Zhang, M. Chen and X. Zhou, Energy Convers. Manage., 2016, 112, 41–48 CrossRef CAS.
- N. Sophonrat and W. Yang, Energy Procedia, 2017, 142, 315–320 CrossRef CAS.
- B. B. Uzoejinwa, X. He, S. Wang, A. El-Fatah Abomohra, Y. Hu and Q. Wang, Energy Convers. Manage., 2018, 163, 468–492 CrossRef CAS.
- F. Abnisa and W. M. A. Wan Daud, Energy Convers. Manage., 2014, 87, 71–85 CrossRef CAS.
- Z. Wang, K. G. Burra, T. Lei and A. K. Gupta, Prog. Energy Combust. Sci., 2021, 84, 100899 CrossRef.
- K. B. Ansari, S. Z. Hassan, R. Bhoi and E. Ahmad, J. Environ. Chem. Eng., 2021, 9, 106436 CrossRef CAS.
- W. A. Wan Mahari, E. Azwar, S. Y. Foong, A. Ahmed, W. Peng, M. Tabatabaei, M. Aghbashlo, Y.-K. Park, C. Sonne and S. S. Lam, Chem. Eng. J., 2021, 421, 129749 CrossRef CAS.
- S. Mariyam, M. Shahbaz, T. Al-Ansari, H. R. Mackey and G. McKay, Renewable Sustainable Energy Rev., 2022, 161, 112349 CrossRef CAS.
- S. Wang, G. Dai, H. Yang and Z. Luo, Prog. Energy Combust. Sci., 2017, 62, 33–86 Search PubMed.
- H. W. Ryu, D. H. Kim, J. Jae, S. S. Lam, E. D. Park and Y.-K. Park, Bioresour. Technol., 2020, 310, 123473 CrossRef CAS PubMed.
- S. B. Engamba Esso, Z. Xiong, W. Chaiwat, M. F. Kamara, X. Longfei, J. Xu, J. Ebako, L. Jiang, S. Su, S. Hu, Y. Wang and J. Xiang, Biomass Bioenergy, 2022, 159, 106415 CrossRef CAS.
- A. Al-Rumaihi, M. Shahbaz, G. McKay, H. Mackey and T. Al-Ansari, Renewable Sustainable Energy Rev., 2022, 167, 112715 CrossRef CAS.
- S. Nanda and F. Berruti, Environ. Chem. Lett., 2021, 19, 123–148 CrossRef CAS.
- K. Lee, Y. Jing, Y. Wang and N. Yan, Nat. Rev. Chem., 2022, 6, 635–652 CrossRef PubMed.
- W. Cai, X. Wang, Z. Zhu, R. Kumar, P. Nana Amaniampong, J. Zhao and Z.-T. Hu, Fuel, 2023, 353, 129210 CrossRef CAS.
- A. K. Sharma, P. Ghodke, P. K. Sharma, S. Manna, A. Pugazhendhi, L. Matsakas and A. Patel, Biomass Convers. Biorefin., 2022, 14, 5261–5274 CrossRef.
- M. S. Mettler, D. G. Vlachos and P. J. Dauenhauer, Energy Environ. Sci., 2012, 5, 7797–7809 RSC.
- P. Kostetskyy and L. J. Broadbelt, Energy Fuels, 2020, 34, 15195–15216 CrossRef CAS.
- B. Hu, B. Zhang, W.-l. Xie, X.-y. Jiang, J. Liu and Q. Lu, Energy Fuels, 2020, 34, 10384–10440 Search PubMed.
- P. K. Ghodke, A. K. Sharma, J. K. Pandey, W.-H. Chen, A. Patel and V. Ashokkumar, J. Environ. Manage., 2021, 298, 113450 CrossRef CAS PubMed.
- A. Nanduri, S. S. Kulkarni and P. L. Mills, Renewable Sustainable Energy Rev., 2021, 148, 111262 Search PubMed.
- E. Önal, B. B. Uzun and A. E. Pütün, Energy Convers. Manage., 2014, 78, 704–710 CrossRef.
- R. Kumar, V. Strezov, H. Weldekidan, J. He, S. Singh, T. Kan and B. Dastjerdi, Renewable Sustainable Energy Rev., 2020, 123, 109763 Search PubMed.
- B. Samal, K. R. Vanapalli, B. K. Dubey, J. Bhattacharya, S. Chandra and I. Medha, Sci. Total Environ., 2021, 794, 148723 CrossRef CAS PubMed.
- N. Almagro-Herrera, S. Lozano-Calvo, A. Palma, J. C. García and M. J. Díaz, Fuel, 2024, 367, 131501 Search PubMed.
- H. Ben and A. J. Ragauskas, Energy Fuels, 2011, 25, 2322–2332 CrossRef CAS.
- W. Cai, R. Liu, Y. He, M. Chai and J. Cai, Fuel Process. Technol., 2018, 171, 308–317 CrossRef CAS.
- H. Li, X. Dong, E. B. da Silva, L. M. de Oliveira, Y. Chen and L. Q. Ma, Chemosphere, 2017, 178, 466–478 Search PubMed.
- N. A. Qambrani, M. M. Rahman, S. Won, S. Shim and C. Ra, Renewable Sustainable Energy Rev., 2017, 79, 255–273 Search PubMed.
- N. M. L. Hansen and D. Plackett, Biomacromolecules, 2008, 9, 1493–1505 Search PubMed.
- M. X. J. Wee, B. L. F. Chin, A. Saptoro, C. L. Yiin, J. J. Chew, J. Sunarso, S. Yusup and A. Sharma, Front. Chem. Sci. Eng., 2023, 17, 1141–1161 Search PubMed.
- X. Zhang, H. Lei, S. Chen and J. Wu, Green Chem., 2016, 18, 4145–4169 RSC.
- Y. Matsuzawa, M. Ayabe, J. Nishino, N. Kubota and M. Motegi, Fuel, 2004, 83, 1675–1687 Search PubMed.
- S. Singh, T. Patil, S. P. Tekade, M. B. Gawande and A. N. Sawarkar, Sci. Total Environ., 2021, 783, 147004 CrossRef CAS PubMed.
- D. Zhao, X. Wang, J. B. Miller and G. W. Huber, ChemSusChem, 2020, 13, 1764–1774 CrossRef CAS PubMed.
- A. Guyot, Polym. Degrad. Stab., 1986, 15, 219–235 CrossRef CAS.
- W. H. Starnes, Prog. Polym. Sci., 2002, 27, 2133–2170 CrossRef CAS.
- B. Gautam, M.-R. Huang, C.-C. Lin, C.-C. Chang and J.-T. Chen, Polym. Degrad. Stab., 2023, 217, 110528 CrossRef CAS.
- R. K. Jha, B. J. Neyhouse, M. S. Young, D. E. Fagnani and A. J. McNeil, Chem. Sci., 2024, 15, 5802–5813 Search PubMed.
- A. Rahimi and J. M. García, Nat. Rev. Chem., 2017, 1, 0046 CrossRef.
- S. Fakudze and J. Chen, Chem. Eng. J., 2023, 457, 141004 CrossRef CAS.
- B. Xing, Y. Hu, D. Xu, B. Li, Y. Li, W. Guan, R. Li and S. Wang, Energy Fuels, 2023, 37, 4769–4790 Search PubMed.
- L. Chen, S. Wang, H. Meng, Z. Wu and J. Zhao, Appl. Therm. Eng., 2017, 111, 834–846 CrossRef CAS.
- A. C. Dyer, M. A. Nahil and P. T. Williams, J. Energy Inst., 2021, 97, 27–36 Search PubMed.
- Ö. Çepelioğullar and A. E. Pütün, J. Anal. Appl. Pyrolysis, 2014, 110, 363–374 CrossRef.
- R. K. Mishra, A. Sahoo and K. Mohanty, Bioresour. Technol., 2019, 289, 121608 CrossRef CAS PubMed.
- K.-H. Ko, A. Rawal and V. Sahajwalla, Energy Convers. Manage., 2014, 86, 154–164 Search PubMed.
- K.-H. Ko, V. Sahajwalla and A. Rawal, Bioresour. Technol., 2014, 170, 248–255 CrossRef CAS PubMed.
- E. Ansah, L. Wang and A. Shahbazi, Waste Manage., 2016, 56, 196–206 CrossRef CAS PubMed.
- Y. Keramatian, C. Li, X. Hu and M. Gholizadeh, Biomass Convers. Biorefin., 2023, 13, 16099–16113 Search PubMed.
- S. Kumagai, M. Yamamoto, Y. Takahashi, T. Kameda, Y. Saito and T. Yoshioka, Energy Fuels, 2019, 33, 6837–6841 Search PubMed.
- C. Li, Y. Sun, Q. Li, L. Zhang, S. Zhang, H. Wang, G. Hu and X. Hu, Renewable Energy, 2022, 189, 139–151 CrossRef CAS.
- X. Cui, J. Zhang, M. Pan, Q. Lin, M. B. Khan, X. Yang, Z. He, B. Yan and G. Chen, J. Hazard. Mater., 2021, 416, 125793 Search PubMed.
- K. Kositkanawuth, A. Bhatt, M. Sattler and B. Dennis, Energy Fuels, 2017, 31, 5088–5096 Search PubMed.
- H. Stančin, M. Šafář, J. Růžičková, H. Mikulčić, H. Raclavská, X. Wang and N. Duić, Renewable Energy, 2022, 196, 1218–1228 Search PubMed.
- T. N. L. T. Ngo and K.-Y. Chiang, Sustainable Environ. Res., 2021, 31, 23–31 CrossRef CAS.
- G. Özsin and A. E. Pütün, J. Cleaner Prod., 2018, 205, 1127–1138 Search PubMed.
- H. Yuan, H. Fan, R. Shan, M. He, J. Gu and Y. Chen, Energy Convers. Manage., 2018, 157, 517–526 Search PubMed.
- H. Stančin, M. Šafář, J. Růžičková, H. Mikulčić, H. Raclavská, X. Wang and N. Duić, Process Saf. Environ. Prot., 2021, 145, 1–11 CrossRef.
- J. D. Martínez, A. Veses, A. M. Mastral, R. Murillo, M. V. Navarro, N. Puy, A. Artigues, J. Bartrolí and T. García, Fuel Process. Technol., 2014, 119, 263–271 CrossRef.
- A. Ashraf, G. Liu, M. Arif, B. Yousaf, P. Akhtar, A. Rashid, H. Gulzaman, R. Safeer, M. S. Rashid and M. I. S. Haider, Process Saf. Environ. Prot., 2024, 184, 766–781 Search PubMed.
- D. K. Ojha and R. Vinu, RSC Adv., 2015, 5, 66861–66870 Search PubMed.
- H. Hassan, J. K. Lim and B. H. Hameed, Bioresour. Technol., 2016, 221, 645–655 Search PubMed.
- M. Brebu, S. Ucar, C. Vasile and J. Yanik, Fuel, 2010, 89, 1911–1918 Search PubMed.
- P. Rutkowski and A. Kubacki, Energy Convers. Manage., 2006, 47, 716–731 Search PubMed.
- L. Zhu, W. Cai, J. Li, D. Chen and Z. Ma, Energy, 2024, 296, 131241 Search PubMed.
- B. Samal, K. R. Vanapalli, B. K. Dubey, J. Bhattacharya, S. Chandra and I. Medha, Energy, 2021, 232, 121050 Search PubMed.
- R. Kumar Mishra and K. Mohanty, Carbon Resour. Convers., 2020, 3, 145–155 Search PubMed.
- M. Asmadi, H. Kawamoto and S. Saka, J. Anal. Appl. Pyrolysis, 2011, 92, 417–425 Search PubMed.
- D. K. Shen, S. Gu, K. H. Luo, S. R. Wang and M. X. Fang, Bioresour. Technol., 2010, 101, 6136–6146 CrossRef CAS PubMed.
- M. H. Rahman, P. R. Bhoi, A. Saha, V. Patil and S. Adhikari, Energy, 2021, 225, 120231 Search PubMed.
- Y. Zheng, L. Tao, X. Yang, Y. Huang, C. Liu and Z. Zheng, J. Anal. Appl. Pyrolysis, 2018, 133, 185–197 Search PubMed.
- D. Han, L. Sun, L. Chen, S. Yang, T. Li, X. Xie, M. Xu, W. Tang, B. Zhao, H. Si and D. Hua, J. Fuel Chem. Technol., 2024, 52, 481–495 Search PubMed.
- C. Jia, S. Xie, W. Zhang, N. N. Intan, J. Sampath, J. Pfaendtner and H. Lin, Chem. Catal., 2021, 1, 437–455 CrossRef CAS.
- K. Pyra, K. A. Tarach, A. Śrębowata, I. Melián-Cabrera and K. Góra-Marek, Appl. Catal., B, 2020, 277, 119070 Search PubMed.
- A. H. Zulkafli, H. Hassan, M. A. Ahmad, A. T. Mohd Din and S. M. Wasli, Arabian J. Chem., 2023, 16, 104389 CrossRef CAS.
- F. Paradela, F. Pinto, I. Gulyurtlu, I. Cabrita and N. Lapa, Clean Technol. Environ. Policy, 2009, 11, 115–122 CrossRef CAS.
- Z. Xiang, J. Liang, H. M. Morgan, Y. Liu, H. Mao and Q. Bu, Bioresour. Technol., 2018, 247, 804–811 CrossRef CAS PubMed.
- T. He, S. Zhong, C. Liu, A. Shujaa and B. Zhang, Waste Manage., 2021, 128, 189–199 Search PubMed.
- W. Chen, S. Shi, M. Chen and X. Zhou, Waste Manage., 2017, 67, 155–162 Search PubMed.
- E. Önal, B. B. Uzun and A. E. Pütün, Fuel Process. Technol., 2012, 104, 365–370 CrossRef.
- S. Xiong, J. Zhuo, H. Zhou, R. Pang and Q. Yao, J. Anal. Appl. Pyrolysis, 2015, 112, 66–73 Search PubMed.
- C. Dorado, C. A. Mullen and A. A. Boateng, Appl. Catal., B, 2015, 162, 338–345 Search PubMed.
- W.-H. Chen, C.-W. Wang, H. C. Ong, P. L. Show and T.-H. Hsieh, Fuel, 2019, 258, 116168 CrossRef CAS.
- S. E. Levine and L. J. Broadbelt, Polym. Degrad. Stab., 2009, 94, 810–822 Search PubMed.
- X. Zhang, K. Rajagopalan, H. Lei, R. Ruan and B. K. Sharma, Sustainable Energy Fuels, 2017, 1, 1664–1699 Search PubMed.
- X. Wei, P. Liu, S. Huang, Y. Wu and S. Wu, Fuel, 2024, 363, 131015 Search PubMed.
- X. Zhang, H. Lei, L. Zhu, M. Qian, X. Zhu, J. Wu and S. Chen, Appl. Energy, 2016, 173, 418–430 Search PubMed.
- A. R. Aghamiri and P. Lahijani, Biomass Bioenergy, 2024, 183, 107120 Search PubMed.
- P. F. Britt, A. C. Buchanan, K. B. Thomas and S.-K. Lee, J. Anal. Appl. Pyrolysis, 1995, 33, 1–19 CrossRef.
- X. Li, J. Li, G. Zhou, Y. Feng, Y. Wang, G. Yu, S. Deng, J. Huang and B. Wang, Appl. Catal., A, 2014, 481, 173–182 Search PubMed.
- A. K. Panda, R. K. Singh and D. K. Mishra, Renewable Sustainable Energy Rev., 2010, 14, 233–248 CrossRef CAS.
- R. Chen, J. Zhang, L. Lun, Q. Li and Y. Zhang, Bioresour. Technol., 2019, 292, 121970 Search PubMed.
- Q. H. Ng, B. L. F. Chin, S. Yusup, A. C. M. Loy and K. Y. Y. Chong, Appl. Therm. Eng., 2018, 138, 336–345 CrossRef CAS.
- K. G. Burra and A. K. Gupta, Appl. Energy, 2018, 220, 408–418 CrossRef CAS.
- K. Azizi, M. Keshavarz Moraveji and H. Abedini Najafabadi, Bioresour. Technol., 2017, 243, 481–491 Search PubMed.
- R. Xu, C. Yan, Q. Liu, E. Liu, H. Zhang, X. Zhang, X. Yuan, L. Han, H. Lei, R. Ruan and X. Zhang, Fuel Process. Technol., 2022, 227, 107127 Search PubMed.
- M. Huang, W. Cai, L. Zhu, J. Li and Z. Ma, Ind. Crops Prod., 2024, 211, 118273 Search PubMed.
- S. Chen and Y. H. Hu, Sci. Total Environ., 2023, 905, 167251 CrossRef CAS PubMed.
- Y. Liu, K. Chandra Akula, K. Phani Raj Dandamudi, Y. Liu, M. Xu, A. Sanchez, D. Zhu and S. Deng, Chem. Eng. J., 2022, 446, 137238 Search PubMed.
- M. S. Abbas-Abadi, K. M. Van Geem, M. Fathi, H. Bazgir and M. Ghadiri, Energy, 2021, 232, 121085 Search PubMed.
- Y. Tang, Q. Huang, K. Sun, Y. Chi and J. Yan, Bioresour. Technol., 2018, 249, 16–23 CrossRef CAS PubMed.
- J. Shen, Y. Wu, G. Lan, Y. Xia, B. Yan, Y. Li, Y. Zhang, Y. Yu, C. Fu, A. Xu, J. Zhou, A. Zhu and D. Chen, J. Environ. Chem. Eng., 2023, 11, 110406 CrossRef CAS.
- X. Li, S. Guo, D. Shen, J. Shentu, L. Lv, S. Qi, M. Zhu and Y. Long, Waste Manage., 2024, 175, 22–29 Search PubMed.
- P. Lu, Q. Huang, A. C. Bourtsalas, Y. Chi and J. Yan, Fuel, 2018, 230, 359–367 CrossRef CAS.
- H. Zhou, A. Meng, Y. Long, Q. Li and Y. Zhang, J. Anal. Appl. Pyrolysis, 2014, 108, 19–25 CrossRef CAS.
- G. Özsin and A. E. Pütün, Energy Convers. Manage., 2019, 182, 143–153 Search PubMed.
- J. Braden and X. Bai, Waste Manage., 2018, 78, 894–902 Search PubMed.
- G. Cagnetta, K. Zhang, Q. Zhang, J. Huang and G. Yu, Waste Manage., 2018, 75, 181–186 Search PubMed.
- J. Hubáček, J. Lederer, P. Kuráň, P. Koutník, Z. Gholami, M. Zbuzek and M. Bačiak, Fuel Process. Technol., 2022, 231, 107226 Search PubMed.
- J. López, L. Amodio, M. del Mar Alonso-Doncel, J. Cueto, H. Hernando, M. Mazur, J. Čejka, P. Pizarro and D. P. Serrano, J. Environ. Chem. Eng., 2024, 12, 111790 CrossRef.
- D. R. Rout, H. M. Jena, O. Baigenzhenov and A. Hosseini-Bandegharaei, Sci. Total Environ., 2023, 863, 160871 CrossRef CAS PubMed.
- Y. Wang, K. Wu, Q. Liu and H. Zhang, Process Saf. Environ. Prot., 2021, 149, 105–114 Search PubMed.
- N. Dong, H. Hui, S. Li and L. Du, J. Anal. Appl. Pyrolysis, 2023, 169, 105817 CrossRef CAS.
- S. Lu, X. Guo, H. He, Y. Peng, J. Ding, J. Ding, H. Zhu, Q. Muhammad, I. Rytöluoto and A. Tenhunen, J. Environ. Chem. Eng., 2023, 11, 109916 CrossRef CAS.
- C.-C. Zhang, N.-m. Zhu, F.-S. Zhang, X.-H. Yue and M. Wang, Chem. Eng. J., 2022, 431, 134140 CrossRef CAS.
- X. Liu, K. R. G. Burra, Z. Wang, J. Li, D. Che and A. K. Gupta, Appl. Energy, 2021, 289, 116662 CrossRef CAS.
- W. Jin, D. Shen, Q. Liu and R. Xiao, Polym. Degrad. Stab., 2016, 133, 65–74 Search PubMed.
- J. Zhang, W. Ji, Y. Yuan, W. Nan and W. Yuan, Energy, 2024, 292, 130548 CrossRef CAS.
- X. Liu, K. R. G. Burra, Z. Wang, J. Li, D. Che and A. K. Gupta, J. Energy Resour. Technol., 2021, 143, 052107 Search PubMed.
- M. Brebu and I. Spiridon, Polym. Degrad. Stab., 2012, 97, 2104–2109 Search PubMed.
- M. Wang, C. Zhou, C. Li, W. Zhu, J. Shi and G. Liu, Therm. Sci. Eng. Prog., 2023, 45, 102144 Search PubMed.
- M. Van de Velden, J. Baeyens, A. Brems, B. Janssens and R. Dewil, Renewable Energy, 2010, 35, 232–242 CrossRef CAS.
- V. Dhyani and T. Bhaskar, Renewable Energy, 2018, 129, 695–716 Search PubMed.
- H. S. Choi, Y. S. Choi and H. C. Park, Renewable Energy, 2012, 42, 131–135 CrossRef CAS.
- A. Shaaban, S.-M. Se, M. F. Dimin, J. M. Juoi, M. H. Mohd Husin and N. M. M. Mitan, J. Anal. Appl. Pyrolysis, 2014, 107, 31–39 CrossRef CAS.
- R. Pogaku, B. S. Hardinge, H. Vuthaluru and H. A. Amir, Biofuels, 2016, 7, 647–660 CrossRef CAS.
- D. Phakedi, A. U. Ude and P. O. Oladijo, J. Anal. Appl. Pyrolysis, 2021, 155, 105077 Search PubMed.
- S. Ryu, H. W. Lee, Y.-M. Kim, J. Jae, S.-C. Jung, J.-M. Ha and Y.-K. Park, Appl. Surf. Sci., 2020, 511, 145521 CrossRef.
- J. Ahmad, F. Patuzzi, U. Rashid, M. Shahabz, C. Ngamcharussrivichai and M. Baratieri, Environ. Technol. Innovation, 2021, 21, 101310 Search PubMed.
- D. Angın and S. Şensöz, Int. J. Phytorem., 2014, 16, 684–693 CrossRef PubMed.
- J. Akhtar and N. Saidina Amin, Renewable Sustainable Energy Rev., 2012, 16, 5101–5109 Search PubMed.
- D. Chen, Y. Li, K. Cen, M. Luo, H. Li and B. Lu, Bioresour. Technol., 2016, 218, 780–788 Search PubMed.
- M. V. Rocha, A. J. Vinuesa, L. B. Pierella and M. S. Renzini, Therm. Sci. Eng. Prog., 2020, 19, 100654 CrossRef.
- D. V. Suriapparao, R. Gautam and L. Rao Jeeru, Bioresour. Technol., 2022, 357, 127357 CrossRef CAS PubMed.
- R. Miandad, M. A. Barakat, A. S. Aburiazaiza, M. Rehan and A. S. Nizami, Process Saf. Environ. Prot., 2016, 102, 822–838 Search PubMed.
- K. Smets, A. Roukaerts, J. Czech, G. Reggers, S. Schreurs, R. Carleer and J. Yperman, Biomass Bioenergy, 2013, 57, 180–190 Search PubMed.
- S. D. Stefanidis, K. G. Kalogiannis, E. F. Iliopoulou, C. M. Michailof, P. A. Pilavachi and A. A. Lappas, J. Anal. Appl. Pyrolysis, 2014, 105, 143–150 CrossRef CAS.
- H. Yang, R. Yan, T. Chin, D. T. Liang, H. Chen and C. Zheng, Energy Fuels, 2004, 18, 1814–1821 Search PubMed.
- Y. Li, H. Yu, L. Liu and H. Yu, J. Hazard. Mater., 2021, 420, 126655 Search PubMed.
- D. Chen, L. Yin, H. Wang and P. He, Waste Manage., 2014, 34, 2466–2486 CrossRef CAS PubMed.
- M. J. Ahmed and B. H. Hameed, J. Cleaner Prod., 2020, 265, 121762 Search PubMed.
- S.-Y. Oh and T.-C. Seo, RSC Adv., 2019, 9, 28284–28290 RSC.
- M. K. Rafiq, S. D. Joseph, F. Li, Y. Bai, Z. Shang, A. Rawal, J. M. Hook, P. R. Munroe, S. Donne, S. Taherymoosavi, D. R. G. Mitchell, B. Pace, M. Mohammed, J. Horvat, C. E. Marjo, A. Wagner, Y. Wang, J. Ye and R.-J. Long, Sci. Total Environ., 2017, 607–608, 184–194 CrossRef CAS PubMed.
- N. Zhang and Y. Shen, Bioresour. Technol., 2019, 284, 325–332 CrossRef CAS PubMed.
- H. Stančin, H. Mikulčić, N. Manić, D. Stojiljiković, M. Vujanović, X. Wang and N. Duić, Energy, 2021, 237, 121592 CrossRef.
- Z. Tang, W. Chen, Y. Chen, H. Yang and H. Chen, Bioresour. Technol., 2019, 274, 145–152 Search PubMed.
- J. Meng, M. Tao, L. Wang, X. Liu and J. Xu, Sci. Total Environ., 2018, 633, 300–307 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc04842c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |