 Open Access Article
Open Access ArticleUrinary excretion kinetics of (poly)phenolic metabolites derived from the consumption of microwaved Piquillo pepper (Capsicum annuum cv. Piquillo)†
Cristina
Del Burgo-Gutiérrez
ab,
Nicole
Tosi
d,
Concepción
Cid
 abc,
Daniele
Del Rio
abc,
Daniele
Del Rio
 de,
Letizia
Bresciani
de,
Letizia
Bresciani
 d,
Iziar A.
Ludwig
d,
Iziar A.
Ludwig
 *abc,
Pedro
Mena
*abc,
Pedro
Mena
 *de and
María-Paz
De Peña
*de and
María-Paz
De Peña
 abc
abc
aDepartment of Nutrition, Food Science and Physiology, School of Pharmacy and Nutrition, University of Navarra, c/Irunlarrea 1, 31008 Pamplona, Spain. E-mail: iludwig@unav.es; Tel: +34 948425600 (Ext. 806652)
bUniversity of Navarra, Center for Nutrition Research, c/Irunlarrea 1, 31008 Pamplona, Spain
cIdiSNA, Navarra Institute for Health Research, c/Irunlarrea 1, 31008 Pamplona, Spain
dHuman Nutrition Unit, Department of Food & Drug, University of Parma, Parma, Italy. E-mail: pedro.mena@unipr.it; Tel: +39 0521 903970
eMicrobiome Research Hub, University of Parma, Parma, Italy
First published on 13th June 2025
Abstract
Consumption of (poly)phenol-containing foods, such as pepper (Capsicum annuum), may have a positive impact on preventing non-communicable diseases. However, native (poly)phenols are extensively transformed, either mediated by the colonic microbiota or as a result of enzymatic phase II reactions. Considering the great interest in these metabolites as biologically active compounds, the present research aimed to evaluate the in vivo metabolism and bioavailability of the phenolic metabolites produced after the consumption of microwaved Piquillo pepper (C. annuum cv. Piquillo). The human intervention study involved 10 healthy volunteers who consumed a portion (90 g) of microwaved Piquillo pepper. Urine was collected before and 24 h after intake at different time intervals. (Poly)phenol metabolites were extracted using μ-SPE and analysed by UHPLC-ESI-QqQ-MS/MS. Twenty urinary metabolites (out of 37 metabolites identified) were exclusively associated with the consumption of microwaved Piquillo pepper, mainly represented by cinnamic and phenylpropanoic acid derivatives (86.2%). Glucuronidation was the main phase II transformation observed after absorption. From the total urine metabolites (17.78 ± 3.20 μmol), the majority were excreted between 4 and 24 hours (11.73 ± 2.80 μmol), suggesting that absorption of (poly)phenols from Piquillo pepper occurs after extensive metabolism in the large intestine. Urinary metabolites showed great interindividual variability in concentration (2.52–30.28 μmol) and metabolite patterns, associated likely with gut microbiota differences. Overall, these metabolites are the ones that could exert health promoting effects at the systemic level, rather than native (poly)phenols. This study paves the way to better understand the benefits of pepper consumption after processing.
Introduction
Peppers (Capsicum annuum) are a rich source of phytochemicals, including (poly)phenolic compounds, which have been extensively studied due to their proposed antioxidant and anti-inflammatory properties.1,2 The Piquillo variety, cultivated in the south of Navarra (Spain) and commercialized under the Protected Designation of Origin (PDO) “Piquillo of Lodosa”, contains a diversity of flavonoids (quercetin and luteolin derivatives) and cinnamic acids, including 4′-hydroxycinnamic acid (p-coumaric acid), 3′,4′-dihydroxycinnamic acid (caffeic acid), 3′-methoxy-4′-hydroxycinnamic acid (ferulic acid) and their glycosylated derivatives.3 Nevertheless, for commercialization purposes, Piquillo pepper is subjected to two distinct industrial thermal processes involving high temperatures: a grilling treatment using direct flame (approximately 700 °C), followed by peeling, and a subsequent canning technique (102 °C). As previously reported,3 these thermal processes affect its (poly)phenolic content and profile. Moreover, although heat treatments generally decrease total (poly)phenolic content,4–6 they might have a positive impact on (poly)phenol bioaccessibility and consequently their bioavailability and bioactivity, by modifying the matrix of treated plant-based foods. Indeed, despite reducing the total (poly)phenolic content, industrial and culinary heat treatments applied to Piquillo pepper may positively affect (poly)phenol bioaccessibility and the formation of (poly)phenol derivatives from gut microbiota catabolism.7Awareness of the relationship between health and diet has been gaining importance over the last few decades, with increasing attention to the consumption of phytochemicals with health-promoting properties. The broadly reported potential effects of (poly)phenols against the onset of several chronic diseases have triggered the need to study their potential mechanisms of action. To do so, understanding the metabolites in circulation represents a fundamental point.8,9
After consumption, some native (poly)phenols are absorbed in the upper gastrointestinal tract after hydrolysis of the sugar unit by the action of lactase phlorizin hydrolase (LPH).9 However, (poly)phenol absorption in the small intestine is low and these compounds reach the colon, where they are extensively catabolized by the gut microbiota, generating a wide array of more potentially absorbable, low molecular weight catabolites.10,11 Once these smaller catabolites are absorbed, they can undergo several phase II metabolic reactions locally and in the liver, before passing into the bloodstream and reaching target cells.8,9 Finally, they are excreted mainly through urine. Main phase II conjugation reactions include the transfer of a methyl group into (poly)phenols’ structure (methylation) mediated by catechol-O-methyltransferase (COMT), glucuronidation by the action of glucuronosyltransferases (UGTs), and sulfation by the action of sulfotransferases (SULTs).9,12,13 (Poly)phenols from plant-based foods do not reach the bloodstream and target cells in their native form generally, but as their phase II conjugated forms and low molecular weight derivatives. Therefore, it is of great interest to identify the (poly)phenol metabolites associated with the health effects attributed to (poly)phenol-containing foods.
In recent years, several studies have evaluated the in vivo metabolism of (poly)phenols and their excretion in urine after the consumption of sources rich in chlorogenic acids such as artichokes14 and coffee,15 berries rich in anthocyanins and flavan-3-ols such as cranberries16,17 and blueberries,18 and flavanone-rich beverages such as orange juice.10,19 Nevertheless, to the best of our knowledge, no studies on the in vivo metabolism and urinary excretion of (poly)phenols derived from the ingestion of Capsicum annuum varieties have been performed. Considering the importance of this vegetable worldwide, this lack of knowledge should be addressed.
Considering the specific (poly)phenolic profile of pepper and the characteristic thermal treatments applied to Piquillo pepper, it is hypothesized that the absorption, distribution, metabolism and excretion (ADME) of their (poly)phenols could differ from those of other plant-based foods. Therefore, the present research aims to evaluate the kinetics of urinary excretion of (poly)phenol metabolites after the consumption of microwaved Piquillo pepper.
Materials and methods
Chemicals and reagents
For HPLC-ESI-MS/MS analysis of Piquillo pepper samples, all solvents were of LC-MS grade. Acetonitrile and formic acid were obtained from Scharlau (Barcelona, Spain) and methanol was purchased from Panreac (Barcelona, Spain). Supplier information of the pure phenolic standards is included in the ESI.†For the analysis of urine samples, OASIS HLB microelution plates (2 mg of sorbent per well, 30 μm) were purchased from Waters Corporation (Eschborn, Germany). All solvents and reagents used for urine analysis were of LC-MS grade. Methanol, acetonitrile, phosphoric acid, acetic acid and ammonium formate were acquired from Sigma-Aldrich (Taufkirchen, Germany) and formic acid was obtained from Fisher Chemical (Thermo Fisher Scientific Inc., San Jose, CA, USA). Ultrapure water from a MilliQ system was obtained from Millipore (Bedford, MA, USA). Manufacturer information of the pure analytical standards for UHPLC-ESI-QqQ-MS/MS analysis of (poly)phenol metabolites in urine samples is given in the ESI.† All phenolics are named following the proposed standardized nomenclature based on their molecular structure.20
Preparation of microwaved Piquillo pepper samples and HPLC-ESI-MS/MS analysis of their (poly)phenolic content
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) and then placed in an ultrasonic bath for 90 minutes and after 10 minutes of centrifugation at 18
50 v/v) and then placed in an ultrasonic bath for 90 minutes and after 10 minutes of centrifugation at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 626g, the supernatant was collected in a 1.5 mL tube. Afterwards, a second extraction of the residue was carried out with 0.25 mL of methanol/acidified water (50
626g, the supernatant was collected in a 1.5 mL tube. Afterwards, a second extraction of the residue was carried out with 0.25 mL of methanol/acidified water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v), followed by 25 minutes of sonication in an ultrasonic bath and 10 minutes of centrifugation at 18
50 v/v), followed by 25 minutes of sonication in an ultrasonic bath and 10 minutes of centrifugation at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 626g. Finally, both aliquots were mixed and filtered with a 0.22 μm PVDF syringe filter for their storage at −18 °C until HPLC-ESI-MS/MS analysis. Each sample was extracted in triplicate.
626g. Finally, both aliquots were mixed and filtered with a 0.22 μm PVDF syringe filter for their storage at −18 °C until HPLC-ESI-MS/MS analysis. Each sample was extracted in triplicate.
A total of 81 (poly)phenols were monitored and identification was carried out by comparing the retention time with available pure phenolic standards and with (poly)phenol databases such as the Human Metabolome Database, PubChem and MassBank of North America. Their chromatographic and spectrometric characteristics are detailed in ESI Table S1.† (Poly)phenol quantification was performed using calibration curves of pure standards, when available. When not available, quantification was carried out with the calibration curves of structurally similar compounds (detailed in ESI Table S1†).3 Chromatograms and spectral data were acquired using Analyst software 1.6.3 (AB SCIEX) and the results were expressed in micromoles (μmol) of (poly)phenol per gram (g) of fresh pepper as mean ± standard deviation (SD).
Human study design
The study protocol was designed in accordance with the guidelines stated in the Declaration of Helsinki and approved by the Ethics Committee of the University of Navarra (reference number: 2021.080 TESIS). Ten healthy participants aged 31.9 ± 7.4 with a body mass index of 22.3 ± 2.9 kg m−2 were enrolled. Study participants declared that they were not pregnant or lactating and had not been diagnosed with any chronic pathology. Moreover, they were not taking any chronic medication or undergoing any antibiotic treatment during the 4 months prior to the study. All volunteers were informed of the details of the protocol and gave their written informed consent prior to their participation. Participants were asked to follow a low-(poly)phenol diet (avoiding fruits, vegetables, legumes, high-fibre foods, and cocoa products as well as coffee, tea, soft drinks, alcoholic beverages, etc.) 48 hours prior to the study, and they were asked to fast for 12 hours prior to the intervention. On the study day, participants were asked to provide a baseline urine sample collected over the previous 10 hours and to consume one serving of microwaved Piquillo pepper (approximately 90 g). After pepper consumption, 24 h urine samples were collected for each participant in 2 L collection containers at different intervals (0–2, 2–4, 4–8, 8–12, and 12–24 h). Urine volume was measured for each sample and aliquots were stored at −80 °C until analysis. During the sampling day, participants were asked to continue the low-(poly)phenol diet and to complete two dietary recalls (one 48 h prior to the study and another one during the sampling day) in order to monitor the adherence to dietary recommendations.Urine sample processing
Urine aliquots were extracted through a microelution solid-phase extraction (μ-SPE) procedure following a validated method.14 Firstly, 100 μL of urine samples were diluted (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) with MilliQ water. Then, 350 μL of each diluted urine was combined with 4% phosphoric acid (1
5) with MilliQ water. Then, 350 μL of each diluted urine was combined with 4% phosphoric acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and an aliquot (600 μL) was loaded onto Oasis 96-well-reversed-phase hydrophilic–lipophilic balanced sorbent plates (μ-SPE HLB plates). Afterwards, the plates were washed with 200 μL of water and then with 200 μL of 0.2% acetic acid and finally, targeted metabolites were eluted three times with 30 μL of methanol with 0.1% formic acid and 10 nM ammonium formate. The extracted samples were taken to a final volume of 130 μL by adding 40 μL of water and stored at −80 °C until UHPLC-ESI-QqQ-MS/MS analysis.
1) and an aliquot (600 μL) was loaded onto Oasis 96-well-reversed-phase hydrophilic–lipophilic balanced sorbent plates (μ-SPE HLB plates). Afterwards, the plates were washed with 200 μL of water and then with 200 μL of 0.2% acetic acid and finally, targeted metabolites were eluted three times with 30 μL of methanol with 0.1% formic acid and 10 nM ammonium formate. The extracted samples were taken to a final volume of 130 μL by adding 40 μL of water and stored at −80 °C until UHPLC-ESI-QqQ-MS/MS analysis.
UHPLC-ESI-QqQ-MS/MS analysis
Metabolite analysis of urine samples was performed on an ultra-high performance liquid chromatograph (UHPLC DIONEX Ultimate 3000) coupled to a TSQ Vantage Triple Quadrupole mass spectrometer (QqQ-MS/MS, Thermo Fisher Scientific Inc.) equipped with a heated-electrospray ionization source (H-ESI-II), following the method previously described by Favari et al.16Chromatographic separation was carried out with a KINETEX EVO C18 column (100 × 2.1 mm, 2.6 μm particle size; Phenomenex, Torrance, CA, USA) working at 40 °C. Mobile phases consisted of 0.01% formic acid in water (A) and 0.01% formic acid in acetonitrile (B). The 12 minute gradient elution started with 5% B, keeping isocratic conditions for 0.5 min, reaching 95% B at 7 min, followed by 1 min at 95% B, and then 4 min at the starting conditions to re-equilibrate the column. The injection volume was 5 μL and the flow rate was set at 0.4 mL min−1. The mass spectrometer operated in negative ionization mode with the temperature maintained at 270 °C for the capillary and at 300 °C for the source. Ultra-high purity argon gas was used for collision-induced dissociation (CID) and the flow was set at 60 units. The auxiliary gas pressure was 10 units and the source voltage was 3 kV.
A total of 120 (poly)phenol metabolites were monitored and metabolite identification was performed by comparing the retention time (Rt), molecular ion mass [M − H]− and MS/MS fragmentation patterns with pure commercial standards, when available, and with data reported in the literature. The chromatographic and spectrometric characteristics of the identified compounds are detailed in ESI Table S2.† Quantification was carried out with calibration curves of available standards or with the most structurally similar compounds when no standards were available (ESI Table S2†). Chromatograms and spectral data were acquired using Xcalibur software 2.1 (Thermo Fisher Scientific Inc.). The results are expressed as μmol of (poly)phenols excreted and indicated as mean ± standard error of the mean (SEM).
Of note, the presence of some (poly)phenol metabolites in basal urine could be due to two main factors: (1) the persistence in circulation of some phenolic metabolites even after following a low-(poly)phenol diet for 48 h, as previously reported in other in vivo studies8,17 and (2) the heterogeneous origin of some low molecular weight phenolics, which can derive from the metabolism of endogenous and exogenous compounds.21,22 Therefore, the amount of (poly)phenol metabolites excreted in urine after the intervention was individually corrected considering the concentration excreted per hour in basal urine (10 h prior to intervention). The basal urine excretion of each metabolite is reported in ESI Table S3.† The kinetics of urinary metabolites excreted during 24 h are expressed as μmol excreted per hour and calculated as the sum of the total compounds excreted during each interval, divided by the total hours of each period.
Statistical analyses were carried out to evaluate the differences in the metabolite excretion in urine at diverse time points using the SPSS v.29.01.00 software package. First the normal distribution of the data was assessed for each (poly)phenolic metabolite. Then, a non-parametric Friedman test was applied followed by the Wilcoxon signed-rank test for multiple comparisons, with significance accepted at p < 0.05.
Results and discussion
(Poly)phenol content of microwaved Piquillo pepper
Piquillo pepper is commonly consumed as a side dish or starter and, therefore, participants consumed an amount equivalent to a typical serving size of 90 g (4–5 pieces) of microwaved Piquillo pepper containing a total of 55.05 ± 1.13 μmol of (poly)phenols. Table 1 shows the ingested amount of each of the 49 identified (poly)phenolic compounds of microwaved Piquillo peppers.| Compounda | μmol per 90 g fresh | |
|---|---|---|
| Non-flavonoids | ||
| Benzene derivatives | ||
| 1 | Benz-1.2-diol | 5.13 ± 0.26 |
| 2 | Benz-1,2,3-triolb | 0.73 ± 0.04 |
| Total benzene derivatives | 5.87 ± 0.28 | |
| Benzoic acids | ||
| 3 | 3-OH-BA | 0.35 ± 0.03 |
| 4 | 4-OH-BA | 0.25 ± 0.02 |
| 5 | 2,5-DiOH-BA | 0.03 ± 0.00 |
| 6 | 3,4-DiOH-BA | 0.91 ± 0.03 |
| 7 | 3-MetOH-BA-4-O-GlucSDb | 8.79 ± 0.50 |
| Total benzoic acids | 10.31 ± 0.47 | |
| Cinnamic acids | ||
| 8 | 4′-OH-CA | 0.23 ± 0.02 |
| 9 | 3′,4′-diOH-CA | 0.06 ± 0.01 |
| 10 | 4′-OH-3′-MetOH-CA | 0.37 ± 0.02 |
| 11 | 3′-OH-4′-MetOH-CA | 0.20 ± 0.01 |
| 12 | 4′-OH-3′,5′-diMetOH-CA | 0.10 ± 0.01 |
| 13 | CA-4′-O-GlucSDb | 13.67 ± 0.23 |
| 14 | 4′-OH-CA-3′-O-GlucSDb | 0.53 ± 0.02 |
| 15 | 3′-MetOH-CA-4′-O-GlucSDb | 5.54 ± 0.18 |
| 16 | 3′,5′-diMetOH-CA-4′-O-GlucSDb | 0.52 ± 0.03 |
| Total cinnamic acids | 21.22 ± 0.15 | |
| Phenylpropanoic acids | ||
| 17 | 3-(3′,4′-diOH-ph)PrA | 0.18 ± 0.01 |
| Total phenylpropanoic acids | 0.18 ± 0.01 | |
| Phenylacetic acids | ||
| 18 | 4′-OH-3′-MetOH-phAcb | 13.98 ± 0.41 |
| Total phenylacetic acids | 13.98 ± 0.41 | |
| Other phenolic acids | ||
| 19 | 4-OH-1,2-BenzPyONb | 0.11 ± 0.01 |
| 20 | 2′-OH-4′MetOH-Ac-phONb | 0.05 ± 0.01 |
| Total other phenolic compounds | 0.17 ± 0.01 | |
| Acyl-quinic acids | ||
| 21 | 5-CQA | 0.15 ± 0.01 |
| 22 | 4-CQA | 0.01 ± 0.00 |
| Total acyl-quinic acids | 0.16 ± 0.01 | |
| Total non-flavonoids | 51.94 ± 1.11 | |
| Compoundb | μmol per 90 g serving | |
|---|---|---|
| Tr = traces.a Full compound names are shown in ESI Table S1.†b Tentatively identified compounds and semiquantified with a structurally similar phenolic standard. | ||
| Flavonols | ||
| 23 | Querc | 0.13 ± 0.06 |
| 24 | IsorhTN | 0.03 ± 0.00 |
| 25 | Querc-3-O-GlucSD | 0.05 ± 0.01 |
| 26 | Querc-3-O-Rha | 0.80 ± 0.01 |
| 27 | IsorhTN-3-O-GlucSD | 0.09 ± 0.01 |
| 28 | Querc-Ace-GlucSDb | Tr |
| 29 | Kmpf-MaO-GlucSDb | 0.03 ± 0.00 |
| 30 | Querc-3-O-Rut | 0.06 ± 0.00 |
| 31 | Querc-3-O-GlucSD-7-O-Rhab | 0.21 ± 0.01 |
| 32 | Querc-3-O-Samb-7-O-Rhab | 0.02 ± 0.00 |
| Total flavonols | 1.41 ± 0.05 | |
| Flavones | ||
| 33 | Lut | 0.05 ± 0.01 |
| 34 | Apig-8-C-GlucSD | 0.02 ± 0.00 |
| 35 | Lut-7-O-GlucSD | 0.03 ± 0.00 |
| 36 | Lut-8-C-GlucSD | 0.25 ± 0.02 |
| 37 | Lut-6-C-GlucSDb | 0.17 ± 0.00 |
| 38 | ChryOL-6-C-GlucSDb | 0.03 ± 0.00 |
| 39 | Apig-Pent-Hexb | 0.13 ± 0.01 |
| 40 | Apig-7-O-(2-O-Ap)GlucSDb | 0.05 ± 0.00 |
| 41 | Lut-6-C-Hex-8-C-Pentb | 0.05 ± 0.01 |
| 42 | Lut-6-C-Pent-8-C-Hexb | 0.03 ± 0.00 |
| 43 | Lut-7-O-(2-O-Ap)GlucSDb | 0.01 ± 0.00 |
| 44 | Apig-6,8-C-diGlucSD | 0.26 ± 0.02 |
| 45 | Lut-6,8-C-diGlucSDb | 0.06 ± 0.00 |
| 46 | Lut-7-O-(2-O-Ap-Ace)GlucSDb | 0.25 ± 0.01 |
| 47 | Lut-7-O-(2-O-Ap-6-O-MaO)GlucSDb | 0.28 ± 0.01 |
| Total flavones | 1.68 ± 0.08 | |
| Flavanones | ||
| 48 | NarGEb | 0.01 ± 0.00 |
| 49 | NarGE-7-O-GlucSD | 0.01 ± 0.00 |
| Total flavanones | 0.02 ± 0.00 | |
| Total flavonoids | 3.12 ± 0.09 | |
| Total phenolic compounds | 55.05 ± 1.13 | |
Considering the (poly)phenols consumed by each participant, non-flavonoids were the most abundant compounds (51.94 ± 1.11 μmol, 94.4%), whereas only 3.12 ± 0.09 μmol corresponded to flavonoids (5.6%) (Table 1). Within the non-flavonoid fraction, four principal subfamilies accounted for nearly all ingested (poly)phenols (93%), including cinnamic acids (21.22 ± 0.15 μmol), phenylacetic acids (13.98 ± 0.41 μmol), benzoic acids (10.31 ± 0.47 μmol), and benzene derivatives (5.87 ± 0.28 μmol) (Table 1). Of those, the major (poly)phenols in microwaved pepper were methylated and/or glucoside derivatives, namely, 3-methoxybenzoic acid-4-O-glucoside (7), cinnamic acid-4′-O-glucoside (13), 3′-methoxycinnamic acid-4′-O-glucoside (15), and 4′-hydroxy-3′-methoxyphenylacetic acid (18). Small amounts of chlorogenic acids, phenylpropanoic acids, and other non-flavonoids were also detected (Table 1). The flavonoid fraction was mainly represented by flavonols (2.5%) and flavones (3%), whereas little amounts of flavanones (<1%) were quantified.
These results differ from those of other pepper varieties that reported flavonoids (luteolin and quercetin derivatives) as the most representative compounds of Capsicum annuum.23–26 Unlike other Capsicum annuum varieties, Piquillo pepper involves two successive industrial treatments before commercialization, namely grilling and canning. As previously reported by Del Burgo-Gutiérrez et al.,3 these industrial treatments, characterised by the use of high temperatures, affect the amount of individual (poly)phenols, especially on the flavonoid fraction, with non-flavonoids becoming the most representative compounds in commercialized canned Piquillo pepper. Moreover, some (poly)phenols were reported to have originated as a result of industrial treatments.3 Therefore, it is important to consider the effect of industrial and/or culinary treatments applied to plant-based foods before consumption, and in particular to Piquillo pepper, before studying the in vivo metabolism of (poly)phenolic compounds.
Urinary profile of (poly)phenol metabolites after consumption of microwaved Piquillo pepper
A total of 37 (poly)phenolic metabolites were detected in urine samples before (basal urine) and after the acute ingestion of microwaved Piquillo pepper (Table S2†). Of those, a total of 17 phenolic metabolites, belonging to different families, such as benzene derivatives, benzaldehydes, hippuric acids, and phenylacetic acids, were present at elevated concentrations in basal urine and, moreover, showed a high variability among volunteers and no clear kinetics in the urine samples during the 24 h post-ingestion. Their presence in basal urine could be associated with (1) their endogenous formation from compounds such as catecholamines, (2) the metabolism of other compounds from the background diet or the protein turnover (i.e, aromatic amino acids), and (3) the previously reported persistence of some phenolic metabolites derived from dietary (poly)phenols, which may remain in circulation in the body and be excreted even beyond 48 hours after ingestion.8,13,14,17,21,22 Therefore, these compounds were classified as (poly)phenol metabolites also derived from other sources and not considered for the study of the bioavailability of Piquillo pepper (poly)phenols. Although these compounds will not be further discussed, their contents are reported in ESI Table S4.†A total of 20 (poly)phenols identified and quantified in urine samples were considered as feasible metabolites derived from the consumption of microwaved Piquillo pepper. These urine metabolites belong to different families including 9 cinnamic acid derivatives (C6–C3 unsaturated), 8 phenylpropanoic acid derivatives (C6–C3), and 3 phenylacetic acid derivatives (C6–C2). These metabolites are in line with the (poly)phenol profile of microwaved Piquillo pepper except for flavonoids, which were not detected in urine samples. This last point might be explained by their low content in the consumed microwaved Piquillo pepper (Table 1). It has been suggested that flavonoid glycosides might be partly absorbed in the small intestine after being hydrolysed into their respective aglycones by the action of LPH and CBG (cytosolic β-glucosidase).9 Nevertheless, the main flavonoids quantified in Piquillo pepper are attached to sugars forming complex structures that might reach the colon intact, where they are subjected to the action of the gut microbiota and are extensively metabolized into smaller catabolites that can be easily absorbed. In particular, it has been suggested that quercetin-3-O-rhamnoside, the main flavonoid present in microwaved piquillo pepper, is not absorbed in the upper GIT and reaches the colon, where it is catabolized into lower molecular weight compounds (i.e. phenylacetic acids) before being absorbed.13
Total urinary recovery of (poly)phenol metabolites
Data on the urine excretion kinetics of each (poly)phenol metabolite at each interval under study (0–2, 2–4, 4–8, 8–12 and 12–24 h) and total daily excretion (24 h) are reported in Table 2. The minimum and maximum for total excretion (24 h) as well as the quotient are also reported in Table 2. The relative contribution (%) of each (poly)phenol class to the total urinary excretion is illustrated in Fig. 1A, while their cumulative 24 h excretion (μmol) is presented in Fig. 1B.| Compounda | n | 0–2 h | 2–4 h | 4–8 h | 8–12 h | 12–24 h | Total excretion (24 h) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| μmol | μmol | μmol | μmol | μmol | Mean ± SEM | Min | Max | Quotient | ||
| Tr = traces.a Full compound names are shown in ESI Table S1.†b Compounds tentatively identified and semiquantified with a structurally similar phenolic standard. Different letters for each row indicate significant differences (p < 0.05) in urinary excretion at different time points. | ||||||||||
| Cinnamic acids | ||||||||||
| OH-CA | 10 | 0.09 ± 0.02b | 0.11 ± 0.02b | 0.15 ± 0.02b | 0.10 ± 0.03ab | 0.04 ± 0.03a | 0.47 ± 0.08 | 0.12 | 0.79 | 6.4 |
| CA-4′-Sulf | 10 | 0.07 ± 0.01b | 0.03 ± 0.01a | 0.05 ± 0.01ab | 0.03 ± 0.01a | 0.01 ± 0.01a | 0.18 ± 0.03 | 0.05 | 0.36 | 6.8 |
| 4′-OH-3′-MetOH-CA-Glyc | 9 | 1.08 ± 0.27b | 0.35 ± 0.11ab | 0.20 ± 0.01a | 0.21 ± 0.11a | 1.07 ± 0.67ab | 2.59 ± 0.75 | 0.32 | 8.02 | 24.8 |
| 4′-OH-CA-3′-Sulfb | 10 | 0.04 ± 0.03b | 0.01 ± 0.01ab | 0.03 ± 0.01ab | 0.04 ± 0.02ab | 0.02 ± 0.02a | 0.13 ± 0.05 | 0.00 | 0.39 | 593.4 |
| 3′-OH-CA-4′-Sulfb | 10 | 0.10 ± 0.02b | 0.05 ± 0.02a | 0.21 ± 0.13ab | 0.19 ± 0.11ab | 0.24 ± 0.12ab | 0.77 ± 0.27 | 0.07 | 1.41 | 19.2 |
| 4′-MetOH-CA-3′-Sulf | 7 | 0.02 ± 0.01a | 0.01 ± 0.01a | 0.02 ± 0.01a | 0.02 ± 0.01a | 0.12 ± 0.05a | 0.19 ± 0.06 | 0.00 | 0.35 | 86.7 |
| 3′-MetOH-CA-4′-Sulf | 10 | 0.41 ± 0.07a | 0.40 ± 0.15a | 0.74 ± 0.20a | 0.46 ± 0.15a | 0.36 ± 0.15a | 2.32 ± 0.49 | 0.07 | 4.39 | 65.8 |
| CA-3′-GlucNDb | 7 | 0.52 ± 0.24a | 0.25 ± 0.09a | 0.44 ± 0.14a | 0.14 ± 0.16a | 0.37 ± 0.19a | 2.15 ± 0.29 | 1.09 | 3.40 | 3.1 |
| 3′-MetOH-CA-4′-GlucND | 9 | 0.80 ± 0.21ab | 0.67 ± 0.17ab | 1.69 ± 0.47b | 1.44 ± 0.59ab | 0.49 ± 0.29a | 5.10 ± 1.40 | 0.13 | 14.33 | 108.6 |
| Total cinnamic acids | 2.83 ± 0.59 | 1.69 ± 0.47 | 3.29 ± 0.64 | 2.77 ± 0.85 | 2.45 ± 0.808 | 12.48 ± 2.23 | 1.09 | 22.78 | 20.9 | |
| Phenylpropanoic acids | ||||||||||
| PhPrA-3′-Sulfb | 9 | 0.04 ± 0.01c | 0.02 ± 0.00bc | 0.08 ± 0.07ab | 0.30 ± 0.30ab | Tra | 0.44 ± 0.37 | 0.04 | 3.41 | 94.7 |
| PhPrA-4′-Sulfb | 8 | 0.11 ± 0.05a | 0.06 ± 0.02a | 0.04 ± 0.02a | 0.05 ± 0.02a | 0.20 ± 0.10a | 0.45 ± 0.12 | 0.02 | 0.96 | 41.8 |
| (4-OH-ph)PrA-3′-Sulf | 10 | 0.13 ± 0.03b | 0.04 ± 0.01ab | 0.07 ± 0.02ab | 0.06 ± 0.03ab | 0.03 ± 0.02a | 0.34 ± 0.06 | 0.04 | 0.66 | 15.0 |
| (3-OH-ph)PrA-4′-Sulfb | 10 | 0.13 ± 0.15b | 0.04 ± 0.02a | 0.05 ± 0.02ab | 0.13 ± 0.04a | 0.24 ± 0.10b | 0.54 ± 0.12 | 0.02 | 1.27 | 56.3 |
| (4′-MetOH-ph)PrA-3′-Sulfb | 8 | 0.07 ± 0.03a | 0.03 ± 0.01a | 0.03 ± 0.02a | 0.03 ± 0.02a | 0.08 ± 0.04a | 0.22 ± 0.10 | 0.06 | 0.82 | 13.6 |
| (3′-MetOH-ph)PrA-4′-Sulf | 4 | 0.12 ± 0.11a | 0.07 ± 0.05a | 0.22 ± 0.26a | 0.83 ± 0.81a | 0.66 ± 0.65a | 1.94 ± 1.89 | 0.01 | 7.61 | 626.3 |
| (4′-MetOH-ph)PrA-3′-GlucND | 9 | 0.27 ± 0.08a | 0.10 ± 0.03a | 0.11 ± 0.04a | 0.12 ± 0.06a | 0.29 ± 0.17a | 0.90 ± 0.24 | 0.03 | 2.47 | 87.3 |
| (3′-MetOH-ph)PrA-4′-GlucNDb | 9 | 0.50 ± 0.16b | 0.17 ± 0.05ab | 0.11 ± 0.05a | 0.06 ± 0.05a | 0.51 ± 0.31ab | 1.35 ± 0.43 | 0.32 | 4.23 | 13.2 |
| Total phenylpropanoic acids | 1.17 ± 0.25 | 0.45 ± 0.10 | 0.55 ± 0.16 | 1.12 ± 0.51 | 1.49 ± 0.59 | 4.62 ± 1.27 | 0.55 | 13.86 | 25.3 | |
| Phenylacetic acids | ||||||||||
| PhA-3′-Sulfb | 9 | 0.01 ± 0.01a | 0.02 ± 0.01a | 0.06 ± 0.02a | 0.10 ± 0.06b | 0.10 ± 0.06ab | 0.29 ± 0.12 | 0.04 | 1.18 | 30.2 |
| 4′-MetOH-PhA-3′-Sulfb | 10 | 0.03 ± 0.01b | 0.02 ± 0.01a | 0.03 ± 0.01ab | 0.02 ± 0.01a | 0.03 ± 0.02a | 0.13 ± 0.03 | 0.00 | 0.33 | 121.9 |
| 3′-MetOH-PhA-4′-Sulfb | 9 | 0.13 ± 0.10b | 0.01 ± 0.00a | 0.01 ± 0.00ab | 0.03 ± 0.01ab | 0.15 ± 0.08b | 0.32 ± 0.14 | 0.02 | 1.20 | 62.1 |
| Total phenylacetic acids | 0.16 ± 0.01 | 0.04 ± 0.02 | 0.10 ± 0.03 | 0.15 ± 0.07 | 0.25 ± 0.11 | 0.68 ± 0.24 | 0.03 | 2.70 | 85.2 | |
| Total metabolites derived from Piquillo pepper | 4.16 ± 0.79 | 2.17 ± 0.52 | 3.90 ± 0.77 | 4.03 ± 1.2 | 4.20 ± 1.31 | 17.78 ± 3.20 | 2.52 | 30.28 | 12.0 | |
On average, 17.78 ± 3.20 μmol of (poly)phenol metabolites were excreted in urine over 24 hours, with cinnamic acid derivatives being the major compounds excreted (12.48 ± 2.23 μmol). High amounts of phenylpropanoic acids were also excreted (4.62 ± 1.27 μmol), whereas much lower levels of phenylacetic acid metabolites were detected (0.68 ± 0.24 μmol). The metabolites excreted corresponded mainly to phase II conjugated compounds, with glucuronidation being the main transformation, with a total excretion of 8.12 ± 1.76 μmol. Sulfation also represented an important (poly)phenol biotransformation, with an excreted amount of sulfate conjugates of 6.81 ± 1.73 μmol. High amounts of methylated metabolites were also excreted after the consumption of microwaved pepper (10.43 ± 2.51 μmol), with the predominance of 3′-O-methylation (9.19 ± 2.30 μmol) over 4′-O-methylation (1.25 ± 0.33 μmol). This could be due to both high COMT-mediated activity and the high amounts of methylated compounds present in microwaved Piquillo pepper (29.54 ± 0.84 μmol) (Table 1).
The total (poly)phenols excreted (17.78 ± 3.20 μmol) for 24 h corresponded to a total urinary recovery of 32.3% (bioavailability value). Nevertheless, some variability was observed among participants, with 2.52 μmol (4.6% recovery) and 30.28 μmol (55% recovery) being the lowest and highest bioavailability values, respectively. The total recovery observed for (poly)phenols of microwaved Piquillo pepper (32.3%) was notably higher in comparison with other raw vegetables such as cranberry juice and wild blueberries, which showed a total recovery of 6.2%17 and 16%,18 respectively. Moreover, in both studies,17,18 hippuric acid derivatives were included and contributed to the majority of the excreted metabolites, whereas for the present research these compounds were excluded due to their probable origin from other sources.8,22 Indeed, the inclusion of these compounds has been reported to cause an overestimation of the recovery after the consumption of sous-vide artichokes, being 40% when hippurates were considered versus 8.9% when excluded.14
In the present study, the total urinary recovery of (poly)phenols from microwaved Piquillo pepper and their metabolites rose on average to 70.6% (18.0–143.8%) when values were not corrected for basal urine excretion, highlighting the importance of excluding compounds potentially arising from other sources, to avoid the overestimation of urinary recovery. Therefore, there is a need to establish the type of compound to be included in (poly)phenol bioavailability studies, as well as those that might be excluded from bioavailability studies when not associated with the dietary challenge. This may also be relevant to better understand the potential bioactivity of (poly)phenols from plant-based foods rather than from other exogenous/endogenous sources.
The high recovery values observed in the present research in comparison with other studies are probably associated with the unique (poly)phenolic profile of microwaved Piquillo pepper compared to other plant-based foods studied. The 32.3% total recovery was in line with the bioavailability expected for dietary sources rich in hydroxycinnamates such as coffee, while it was much higher than for cereals, yerba mate or artichoke, with recovery rates usually <20%.27 This may be explained as a result of the industrial treatment applied to Piquillo pepper for its commercialization, during which cinnamic acids become the most abundant compound class of (poly)phenols.3 Moreover, it is known that the interaction with other components in the food matrix, in particular with fibre, could negatively influence the total urine recovery of (poly)phenols by inhibiting their absorption.28,29 In light of this, the high total recovery observed for (poly)phenols of Piquillo pepper may result from the positive influence of industrial and subsequent culinary treatments applied that enhance the release of (poly)phenols from the food matrix due to cell wall softening, thereby favouring their absorption in the upper gastrointestinal tract. Another underlying reason for the higher recovery observed for Piquillo pepper was the low portion size consumed (90 g) compared to other in vivo studies, which provided participants with larger servings containing greater amounts of (poly)phenols.14,17,18 Though it might sound controversial, consumption of larger amounts of (poly)phenol containing foods might result in an inverse dose–response association, as recently reviewed,22 and/or in a lower urinary recovery, despite a positive dose–response, as previously reported by Feliciano et al.30 Last but not least, a vast array of different metabolites were identified and quantified using appropriate standards in this study, which led to an accurate estimation of the total metabolites excreted, whereas the lack of standards might result in an under- or overestimation of the total excreted amounts of (poly)phenols.27
Urine kinetics
When considering the urine kinetics of (poly)phenol metabolites, compounds that increase within the first hours (0–4 h) are commonly associated with their absorption in the upper gastrointestinal tract, whereas those excreted between 4 and 24 h generally correspond to metabolites reaching the colon and being metabolized by the action of the gut microbiota before absorption.14 The sum of total metabolites excreted in both intervals (0–4 and 4–24 h) as well as the excretion rate (μmol h−1) in each interval are reported in Table 3. As can be observed, the total excretion of (poly)phenol metabolites was higher between 4 and 24 h (11.73 ± 2.80 μmol) compared to the 0–4 h period (6.59 ± 1.20 μmol), which suggests that the majority of excreted compounds are colonic microbial metabolites derived from non-absorbable (poly)phenols. However, the high excretion rate observed in the initial period (0–4 h) (3.29 ± 0.64 μmol h−1) compared to that during the 4–24 h period (2.23 ± 0.54 μmol h−1) also suggested that considerable amounts of (poly)phenols are rapidly absorbed in the upper gastrointestinal tract.| Compounda | n | Total excretion 0–4 h | Total excretion 4–24 h | ||
|---|---|---|---|---|---|
| Sum μmol | μmol h−1 | Sum μmol | μmol h−1 | ||
| a Full compound names are shown in ESI Table S1.† b Compounds tentatively identified and semiquantified with a structurally similar phenolic standard. | |||||
| Cinnamic acids | |||||
| OH-CA | 10 | 0.20 ± 0.04 | 0.10 ± 0.02 | 0.28 ± 0.06 | 0.06 ± 0.01 |
| CA-3′-GlucNDb | 7 | 0.70 ± 0.35 | 0.35 ± 0.17 | 0.91 ± 0.29 | 0.18 ± 0.07 |
| CA-4′-Sulf | 10 | 0.09 ± 0.02 | 0.05 ± 0.01 | 0.09 ± 0.02 | 0.02 ± 0.00 |
| 4′-OH-CA-3′-Sulfb | 10 | 0.05 ± 0.03 | 0.02 ± 0.02 | 0.08 ± 0.05 | 0.02 ± 0.01 |
| 3′-OH-CA-4′-Sulfb | 10 | 0.15 ± 0.03 | 0.07 ± 0.02 | 0.62 ± 0.27 | 0.11 ± 0.06 |
| 3′-MetOH-CA-4′-GlucND | 9 | 1.47 ± 0.29 | 0.82 ± 0.12 | 3.26 ± 1.26 | 0.74 ± 0.31 |
| 4′-MetOH-CA-3′-Sulf | 7 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.11 ± 0.05 | 0.01 ± 0.01 |
| 3′-MetOH-CA-4′-Sulf | 10 | 0.81 ± 0.20 | 0.41 ± 0.10 | 1.51 ± 0.41 | 0.32 ± 0.09 |
| Feruloylglycine | 9 | 1.28 ± 0.34 | 0.71 ± 0.16 | 1.33 ± 0.70 | 0.18 ± 0.08 |
| Total cinnamic acids | 4.77 ± 0.95 | 2.39 ± 0.49 | 8.19 ± 2.00 | 1.64 ± 0.40 | |
| Phenylpropanoic acids | |||||
| PhPrA-3′-Sulfb | 9 | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.34 ± 0.35 | 0.10 ± 0.09 |
| PhPrA-4′-Sulfb | 8 | 0.13 ± 0.06 | 0.08 ± 0.03 | 0.23 ± 0.11 | 0.04 ± 0.01 |
| (4′-OH-ph)PrA-3′-Sulfb | 10 | 0.18 ± 0.03 | 0.09 ± 0.02 | 0.17 ± 0.05 | 0.04 ± 0.01 |
| (3′-OH-ph)PrA-4′-Sulf | 10 | 0.17 ± 0.07 | 0.09 ± 0.03 | 0.42 ± 0.12 | 0.06 ± 0.02 |
| (3′-MetOH-ph)PrA-4′-GlucNDb | 9 | 0.60 ± 0.19 | 0.33 ± 0.09 | 0.61 ± 0.35 | 0.09 ± 0.04 |
| (4′-MetOH-ph)PrA-3′-GlucND | 9 | 0.34 ± 0.10 | 0.19 ± 0.05 | 0.47 ± 0.22 | 0.08 ± 0.03 |
| (4′-MetOH-ph)PrA-3′-Sulfb | 8 | 0.07 ± 0.03 | 0.05 ± 0.02 | 0.11 ± 0.06 | 0.02 ± 0.01 |
| (3′-MetOH-ph)PrA-4′-Sulfb | 4 | 0.08 ± 0.11 | 0.09 ± 0.07 | 0.70 ± 1.10 | 0.26 ± 0.29 |
| Total phenylpropanoic acids | 1.62 ± 0.33 | 0.81 ± 0.16 | 3.05 ± 1.10 | 0.51 ± 0.19 | |
| Phenylacetic acids | |||||
| PhA-3′-Sulfb | 9 | 0.03 ± 0.02 | 0.01 ± 0.01 | 0.24 ± 0.11 | 0.05 ± 0.02 |
| 3′-MetOH-PhA-4′-Sulfb | 9 | 0.12 ± 0.10 | 0.07 ± 0.05 | 0.17 ± 0.08 | 0.02 ± 0.01 |
| 4′-MetOH-PhA-3′-Sulfb | 10 | 0.05 ± 0.01 | 0.02 ± 0.01 | 0.08 ± 0.03 | 0.02 ± 0.00 |
| Total phenylacetic acids | 0.19 ± 0.11 | 0.10 ± 0.06 | 0.49 ± 0.16 | 0.08 ± 0.03 | |
| Total excreted | 6.59 ± 1.20 | 3.29 ± 0.64 | 11.73 ± 2.80 | 2.23 ± 0.54 | |
Considering the twenty metabolites derived from microwaved Piquillo pepper, four cinnamic acid derivatives (3′-methoxycinnamic-4′-glucuronide, 4′-hydroxy-3′-methoxycinnamoylglycine, 3′-methoxycinnamic-4′-sulfate, and cinnamic acid-3′-glucuronide) and two phenylpropanoic acid derivatives (3-(3′methoxyphenyl)propionic-4′-glucuronide and 3-(3′-methoxyphenyl)propionic-4′-sulfate) were the most relevant compounds and accounted for 86.2% of the total metabolites excreted. Fig. 2 illustrates the kinetics of these urinary metabolites excreted during 24 h (μmol per hour). Excretion kinetics over 24 h for minor (poly)phenol metabolites are shown in ESI Fig. S1.†
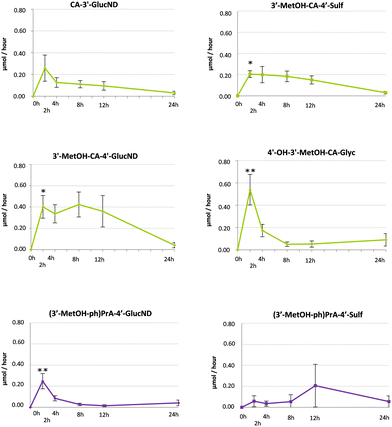 | ||
| Fig. 2 Kinetics of 24 h urinary excretion of the most representative metabolites quantified in urine6 after the consumption of microwaved Piquillo pepper, of which 4 corresponded to cinnamic acid derivatives and 2 compounds were classified as phenylpropanoic acid derivatives. Data are expressed as μmol excreted per hour. Statistically significant differences between consecutive time points were indicated as *p < 0.05 and **p ≤ 0.001. | ||
The excretion rates of all major metabolites except for cinnamic acid-3′-glucuronide (Fig. 2) show a significant increase (p > 0.05) after 2 h and are associated with an early absorption of (poly)phenols, which is in line with the higher excretion rates observed during the first hours (0–4 h) after consumption compared to excretion rates from 4 to 24 h. Interestingly, 3-(3′-methoxyphenyl)propionic-4′-sulfate showed only a slight increase after 2 h, and although a more notable rise was observed from 8 to 12 h, the differences were not significant (p < 0.05) mainly attributed to the inter-individual differences in excretion (Fig. 2). Both the late excretion and the great variability among volunteers point to a colonic origin, probably due to the microbial catabolism of hydroxycinnamates and flavonoids into phenylpropanoic acids via side chain saturation and C-ring fission, respectively.8,13 Similarly, other minor compounds such as 3-(phenyl)propanoic-3′-sulfate and phenylacetic-sulfate showed a major excretion rate in the 8–12 h period (Fig. S1†), putatively associated with the formation of colonic derivatives. Of note, the main metabolite excreted in urine, 3′-methoxycinnamic-4′-glucuronide (Fig. 2), showed high excretion rates between 0 and 2 h and again between 4 and 12 h after consumption, suggesting a biphasic excretion. Other minor metabolites excreted also showed a biphasic excretion, including 3′-hydroxycinnamic-4′-sulfate and 3-(phenyl)propanoic-3′-sulfate (Fig. S1†). The amounts excreted during the first hours might correspond to the absorption of native methoxy-cinnamic acids or other cinnamic acid derivatives, which undergo phase II reactions after absorption, whereas the metabolites quantified after 4 h may derive from the colonic catabolism of the remaining non-absorbed (poly)phenols (flavonoids and non-flavonoids), in line with previous reports.15,27
According to these results, (poly)phenols of microwaved Piquillo pepper seem to be absorbed in two ways: (1) in the upper gastrointestinal tract after hydrolyzation and/or deglycosylation in the brush border by the LHP enzymatic activity or in the cytosol of enterocytes by the action of CBG;8,9 and (2) at the colon level after being metabolized by the gut microbiota, which leads to an overall increase in (poly)phenol bioavailability. The available information on the in vivo urinary excretion and kinetics of (poly)phenols is still limited and, to the best of our knowledge, no research has evaluated the urinary excretion and kinetics of (poly)phenols from Capsicum annuum varieties until now, so comparisons are not possible with other sources rich in hydroxycinnamates. Further pharmacokinetic studies assessing the concentrations of the metabolites in blood may help in designing studies aiming at exploring the effects of pepper-derived metabolites on human health.
Inter-individual variability
Some compounds presented a large quotient and a high SEM (Table 2), showing a relevant inter-individual variability. Several authors have previously reported inter-individual variability in the in vivo metabolism of (poly)phenolic compounds due to intrinsic or extrinsic factors including age, sex, ethnicity, food matrix, gut microbiota composition, dietary patterns, etc.31Fig. 3A and B illustrate the differences among individuals in the cumulative 24 h metabolite excretion of each (poly)phenol metabolite and their relative contribution to the total 24 h metabolite excretion, respectively. The relative contributions of the different phase II metabolites derived from (poly)phenols after absorption are presented in Fig. 3C and D.First, the urinary excretion of (poly)phenol metabolites greatly differed among participants (2.52–30.28 μmol per 24 hours) with a quotient of 12.0 (Table 2 and Fig. 3A). Based on the total 24 h excretion, three groups were identified: participants 6, 7, and 10 showed low excretion (<5 μmol), participants 3, 5, and 9 were classified as medium excretory (approx. 15–20 μmol) and finally, participants 1, 2, 4, and 8 were characterized by high excretion (>25 μmol). Moreover, individual (poly)phenol metabolites did not equally contribute to the total excretion in all participants (Fig. 3B), which was also reflected in the high variability in the excretion quotients of the individual metabolite analysed (Table 2). In particular, (3′-methoxyphenyl)propanoic acid-4′-sulfate (quotient 761.0) and 3′-methoxycinnamic acid-4′-glucuronide (quotient 108.6) were the colonic compounds showing the highest variation among participants, which supports the hypothesis that the gut microbiota are the main factors affecting (poly)phenol metabolism.10,15,30,32
Interestingly, the relative excretion of methylated vs. non-methylated compounds was greater in persons with high and medium excretion in comparison with those with low metabolite excretion (Fig. 3D). The participant showing the highest total excretion (P4) was characterized by a high contribution of methyl derivatives, accounting for 78% (23.60 out of 30.29 μmol), whereas for the lowest excretory participant (P10), methylated compounds accounted for only 34% (0.87 out of 2.57 μmol per 24 h). These differences in the ratio of methylated/non-methylated compounds may suggest that the COMT activity of each participant might be partly associated with a greater total metabolite excretion and therefore recovery and bioavailability.14 However, it should be acknowledged that high amounts of the methylated metabolites found in urine samples might derive directly from the absorption of methylated native compounds in microwaved Piquillo pepper (28% of the total compounds ingested).
For sulfation and glucuronidation, no clear associations were observed with the total amount excreted. Interestingly, no glucuronide metabolites were quantified in the participant with the lowest total excretion (P10) (Fig. 3D). However, glucuronidated metabolites were excreted in higher amounts than sulfated derivatives among the other participants, except for participants 1 and 4 (Fig. 3B). Sulfation occurred mainly at the 3′ position rather than 4′, with no remarkable differences among participants. In contrast, glucuronidation showed again some differences among participants, with 4′ being the predominant position for most participants, especially for high producers, whereas low excretion (P6 and P7) presented relatively higher amounts of 3′-glucuronidated derivatives than 4′ (5.8 and 1.7 folds, respectively), suggesting a possible but not clear association.
Although some associations could be made in the present research based on total urinary excretion, further studies are needed to elucidate the mechanisms underlying the variability observed. Larger intervention studies are warranted to fully understand the metabolic fate of (poly)phenols from Piquillo pepper and establish the effect of inter-individual variability in their bioavailability on the biological efficacy, trying to stratify subjects into metabotypes associated with the consumption of these (poly)phenols.
Conclusion
The absorption, metabolism, and excretion of microwaved Piquillo pepper (poly)phenols after an acute human intervention study were assessed for the first time. A total of 20 metabolites, mainly glucuronide metabolites, were quantified in urine as exclusive Piquillo pepper (poly)phenol metabolites. The total (24 h) urinary excretion of these compounds corresponded to a remarkably high recovery of 32.3% compared with similar in vivo studies of other (poly)phenol containing foods. The great bioavailability observed may be related to the (poly)phenol profile of Piquillo pepper (mainly phenolic acids) and the possible modification of the food matrix as a result of the heat treatments applied, in particular, the industrial treatments (grilling and canning) applied to Piquillo pepper for their commercialization, together with the following culinary process (microwaving). The evaluation of the individual urinary kinetic profiles indicated that most native (poly)phenols reach the colon and undergo extensive catabolism before absorption. Moreover, the gut microbiota may play an important role in the inter-individual variability observed. Studying the bioavailability of vegetables containing (poly)phenols represents a key point to better understand the contribution of these bioactive compounds and their dietary sources to the preventive effects of plant-based diets.Author contributions
Conceptualization: C. C., M.-P. D. P., P. M. and D. D. R.; formal analysis: C. D. B.-G., I. A. L. and P. M.; funding acquisition: C. C., M.-P. D. P., P. M. and D. D. R.; investigation: C. D. B.-G.; methodology: C. D. B.-G., N. T. and L. B.; project administration: C. C., M.-P. D. P., P. M. and D. D. R.; supervision: I. A. L., C. C., M.-P. D. P., P. M. and D. D. R.; writing – original draft preparation: C. D. B.-G.; all the authors contributed to the writing – review and editing of the article and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The datasets generated for this article are available in the Zenodo repository at https://doi.org/10.5281/zenodo.14937406.Acknowledgements
The research leading to these results has received funding from the University of Navarra Research Plan (PIUNA 2018-09) and the “La Caixa” Banking Foundation. I. A. Ludwig is supported by the Gobierno de Navarra (Departamento de Universidad, Innovación y Transformación Digital; grant name and reference: “Talento senior 2021 ANDIA”, 0011-3947-2021-000034). C. Del Burgo-Gutiérrez is grateful to the “Asociación de Amigos de la Universidad de Navarra” for the grant received. The authors thank the volunteers for their participation in the study.References
- G. E. S. Batiha, A. Alqahtani, O. A. Ojo, H. M. Shaheen, L. Wasef, M. Elzeiny, M. Ismail, M. Shalaby, T. Murata, A. Zaragoza-Bastida, N. Rivero-Perez, A. M. Beshbishy, K. I. Kasozi, P. Jeandet and H. F. Hetta, Biological properties, bioactive constituents, and pharmacokinetics of some capsicum spp. and capsaicinoids, Int. J. Mol. Sci., 2020, 21(15), 1–35, DOI:10.3390/ijms21155179.
- N. de Sá Mendes and É.C Branco de Andrade Gonçalves, The role of bioactive components found in peppers, Trends Food Sci. Technol., 2020, 99, 229–243, DOI:10.1016/j.tifs.2020.02.032.
- C. Del Burgo-Gutiérrez, C. Cid, I. A. Ludwig and M. P. de Peña, LC-MS/MS Analysis Elucidates the Different Effects of Industrial and Culinary Processing on Total and Individual (Poly) phenolic Compounds of Piquillo Pepper (Capsicum annuum cv. Piquillo), J. Agric. Food Chem., 2023, 71(15), 6050–6060, DOI:10.1021/acs.jafc.2c07829.
- E. De Santiago, G. Pereira-Caro, J. M. Moreno-Rojas, C. Cid and M. P. De Peña, Digestibility of (Poly)phenols and Antioxidant Activity in Raw and Cooked Cactus Cladodes (Opuntia ficus-indica), J. Agric. Food Chem., 2018, 66(23), 5832–5844, DOI:10.1021/acs.jafc.8b01167.
- I. Juániz, I. A. Ludwig, L. Bresciani, M. Dall'Asta, P. Mena, D. Del Rio, C. Cid and M. P. de Peña, Bioaccessibility of (poly)phenolic compounds of raw and cooked cardoon (Cynara cardunculus L.), after simulated gastrointestinal digestion and fermentation by human colonic microbiota, J. Funct. Foods, 2017, 32, 195–207, DOI:10.1016/j.jff.2017.02.033.
- A. Ribas-Agustí, O. Martín-Belloso, R. Soliva-Fortuny and P. Elez-Martínez, Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods, Crit. Rev. Food Sci. Nutr., 2018, 58(15), 2531–2548, DOI:10.1080/10408398.2017.1331200.
- C. Del Burgo-Gutiérrez, I. A. Ludwig, M. P. de Peña and C. Cid, Industrial and culinary treatments applied to Piquillo pepper (Capsicum annuum cv. Piquillo) impact positively on (poly)phenols’ bioaccessibility and gut microbiota catabolism, Food Funct., 2024, 15, 2443–2458, 10.1039/d3fo04762h.
- D. Del Rio, A. Rodriguez-Mateos, J. P. E. Spencer, M. Tognolini, G. Borges and A. Crozier, Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases, Antioxid. Redox Signaling, 2013, 18(14), 1818–1892, DOI:10.1089/ars.2012.4581.
- A. Rodriguez-Mateos, D. Vauzour, C. G. Krueger, D. Shanmuganayagam, J. Reed, L. Calani, P. Mena, D. Del Rio and A. Crozier, Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update, Arch. Toxicol., 2014, 88(10), 1803–1853, DOI:10.1007/s00204-014-1330-7.
- F. Castello, M. S. Fernández-Pachón, I. Cerrillo, B. Escudero-López, Á. Ortega, A. Rosi, L. Bresciani, D. Del Rio and P. Mena, Absorption, metabolism, and excretion of orange juice (poly)phenols in humans: The effect of a controlled alcoholic fermentation, Arch. Biochem. Biophys., 2020, 695, 108627, DOI:10.1016/j.abb.2020.108627.
- G. Williamson and M. N. Clifford, Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols, Biochem. Pharmacol., 2017, 139, 24–39, DOI:10.1016/j.bcp.2017.03.012.
- M. D'Archivio, C. Filesi, R. Varì, B. Scazzocchio and R. Masella, Bioavailability of the polyphenols: status and controversies, Int. J. Mol. Sci., 2021, 11(4), 1321–1342, DOI:10.3390/ijms11041321.
- S. Yuste, I. A. Ludwig, L. Rubió, M. P. Romero, A. Pedret, R. M. Valls, R. Solà, M. J. Motilva and A. Macià, In vivo biotransformation of (poly)phenols and anthocyanins of red-fleshed apple and identification of intake biomarkers, J. Funct. Foods, 2019, 55, 146–155, DOI:10.1016/j.jff.2019.02.013.
- M. Domínguez-Fernández, P. Young Tie Yang, I. A. Ludwig, M. N. Clifford, C. Cid and A. Rodriguez-Mateos, In vivo study of the bioavailability and metabolic profile of (poly)phenols after sous-vide artichoke consumption, Food Chem., 2022, 367, 130620, DOI:10.1016/j.foodchem.2021.130620.
- P. Mena, L. Bresciani, M. Tassotti, A. Rosi, D. Martini, M. Antonini, A. Cas, R. Bonadonna, F. Brighenti and D. Del Rio, Effect of different patterns of consumption of coffee and a cocoa-based product containing coffee on the nutrikinetics and urinary excretion of phenolic compounds, Am. J. Clin. Nutr., 2021, 114(6), 2107–2118, DOI:10.1093/ajcn/nqab299.
- C. Favari, P. Mena, C. Curti, G. Istas, C. Heiss, D. Del Rio and A. Rodriguez-Mateos, Kinetic profile and urinary excretion of phenylγ-valerolactones upon consumption of cranberry: a dose-response relationship, Food Funct., 2020, 11, 3975–3985, 10.1039/d0fo00806k.
- R. P. Feliciano, A. Boeres, L. Massacessi, G. Istas, M. R. Ventura, C. Nunes Dos Santos, C. Heiss and A. Rodriguez-Mateos, Identification and quantification of novel cranberry-derived plasma and urinary (poly)phenols, Arch. Biochem. Biophys., 2016, 599, 31–41, DOI:10.1016/j.abb.2016.01.014.
- R. P. Feliciano, G. Istas, C. Heiss and A. Rodriguez-Mateos, Plasma and urinary phenolic profiles after acute and repetitive intake of wild blueberry, Molecules, 2016, 21(9), 14–16, DOI:10.3390/molecules21091120.
- G. Pereira-Caro, I. A. Ludwig, T. Polyviou, D. Malkova, A. García, J. M. Moreno-Rojas and A. Crozier, Identification of plasma and urinary metabolites and catabolites derived from orange juice (poly)phenols: Analysis by high-performance liquid chromatography-high-resolution mass spectrometry, J. Agric. Food Chem., 2016, 64(28), 5724–5735, DOI:10.1021/acs.jafc.6b02088.
- C. D. Kay, M. N. Clifford, P. Mena, G. J. McDougall, C. Andres-Lacueva, A. Cassidy, D. Del Rio, N. Kuhnert, C. Manach, G. Pereira-Caro, A. Rodriguez-Mateos, A. Scalbert, F. Tomás-Barberán, G. Williamson, D. S. Wishart and A. Crozier, Recommendations for standardizing nomenclature for dietary (poly)phenol catabolites, Am. J. Clin. Nutr., 2020, 112(4), 1051–1068, DOI:10.1093/ajcn/nqaa204.
- M. N. Clifford, I. A. Ludwig, G. Pereira-Caro, L. Zeraik, G. Borges, T. M. Almutairi, S. Dobani, L. Bresciani, P. Mena, C. R. I. Gill and A. Crozier, Exploring and disentangling the production of potentially bioactive phenolic catabolites from dietary (poly)phenols, phenylalanine, tyrosine and catecholamines, Redox Biol., 2024, 71, 103068, DOI:10.1016/j.redox.2024.103068.
- G. Williamson and M. N. Clifford, A critical examination of human data for the biological activity of phenolic acids and their phase-2 conjugates derived from dietary (poly)phenols, phenylalanine, tyrosine and catecholamines, Crit. Rev. Food Sci. Nutr., 2024, 1–60, DOI:10.1080/10408398.2024.2410874.
- A. P. Cárdenas-Castro, J. J. Rochín-Medina, K. Ramírez, J. Tovar and S. G. Sáyago-Ayerdi, In Vitro Intestinal Bioaccessibility and Colonic Biotransformation of Polyphenols from Mini Bell Peppers (Capsicum annuum L.), Plant Foods Hum. Nutr., 2022, 77(1), 77–82, DOI:10.1007/s11130-022-00948-5.
- I. Juániz, I. A. Ludwig, E. Huarte, G. Pereira-Caro, J. M. Moreno-Rojas, C. Cid and M. P. de Peña, Influence of heat treatment on antioxidant capacity and (poly)phenolic compounds of selected vegetables, Food Chem., 2016, 197, 466–473, DOI:10.1016/j.foodchem.2015.10.139.
- H. Kelebek, O. Sevindik, T. Uzlasir and S. Selli, LC-DAD/ESI MS/MS characterization of fresh and cooked Capia and Aleppo red peppers (Capsicum annuum L.) phenolic profiles, Eur. Food Res. Technol., 2020, 246(10), 1971–1980, DOI:10.1007/s00217-020-03548-2.
- J. G. López-Velázquez, F. Delgado-Vargas, G. López-Ángulo, E. García-Armenta, M. E. López-López, L. E. Ayón-Reyna, D. A. Díaz-Corona and M. O. Vega-García, Phenolic profile associated with chilling tolerance induced by the application of a hot water treatment in bell pepper fruit, J. Food Sci., 2020, 85(7), 2080–2089, DOI:10.1111/1750-3841.15310.
- G. Di Pede, P. Mena, L. Bresciani, M. Achour, R. M. Lamuela-Raventós, R. Estruch, R. Landberg, S. E. Kulling, D. Wishart, A. Rodriguez-Mateos, M. N. Clifford, A. Crozier, C. Manach and D. Del Rio, A Systematic Review and Comprehensive Evaluation of Human Intervention Studies to Unravel the Bioavailability of Hydroxycinnamic Acids, Antioxid. Redox Signaling, 2024, 40(7–9), 510–541, DOI:10.1089/ars.2023.0254.
- E. D. Clarke, M. E. Rollo, C. E. Collins, L. Wood, R. Callister, M. Philo, P. A. Kroon and R. L. Haslam, The Relationship between Dietary Polyphenol Intakes and Urinary Polyphenol Concentrations in Adults Prescribed a High Vegetable and Fruit Diet, Nutrients, 2020, 12(11), 3431, DOI:10.3390/nu12113431.
- Y. Liu, J. Deng, T. Zhao, X. Yang, J. Zhang and H. Yang, Bioavailability and mechanisms of dietary polyphenols affected by non-thermal processing technology in fruits and vegetables, Curr. Res. Food Sci., 2024, 7(8), 100715, DOI:10.1016/j.crfs.2024.100715.
- R. P. Feliciano, C. E. Mills, G. Istas, C. Heiss and A. Rodriguez-Mateos, Absorption, metabolism and excretion of cranberry (poly)phenols in humans: A dose response study and assessment of inter-individual variability, Nutrients, 2017, 9(3), 268, DOI:10.3390/nu9030268.
- C. Favari, J. F. Rinaldi de Alvarenga, L. Sánchez-Martínez, N. Tosi, C. Mignogna, E. Cremonini, C. Manach, L. Bresciani, D. Del Rio and P. Mena, Factors driving the inter-individual variability in the metabolism and bioavailability of (poly)phenolic metabolites: A systematic review of human studies, Redox Biol., 2024, 71, 103095, DOI:10.1016/j.redox.2024.103095.
- N. Tosi, C. Favari, L. Bresciani, E. Flanagan, M. Hornberger, A. Narbad, D. Del Rio, D. Vauzour and P. Mena, Unravelling phenolic metabotypes in the frame of the COMBAT study, a randomized, controlled trial with cranberry supplementation, Food Res. Int., 2023, 172, 113187, DOI:10.1016/j.foodres.2023.113187.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5fo01111f |
| This journal is © The Royal Society of Chemistry 2025 |


