Open Access Article
Open Access ArticleImproving dietary energy and antioxidative properties benefit early maternal BMI and further manage adverse pregnancy outcomes with better weight gain†
Hang-Yu
Li
 ,
Bing-Jie
Ding
*,
Jia
Wang
,
Xin-Li
Yang
,
Bing-Jie
Ding
*,
Jia
Wang
,
Xin-Li
Yang
 ,
Zhi-Wen
Ge
,
Nan
Wang
,
Ya-Ru
Li
,
Yan-Xia
Bi
,
Cong-Cong
Wang
,
Zheng-Li
Shi
,
Yu-Xia
Wang
,
Yi-Si
Wang
,
Cheng
Li
,
Ze-Bin
Peng
and
Zhong-Xin
Hong
,
Zhi-Wen
Ge
,
Nan
Wang
,
Ya-Ru
Li
,
Yan-Xia
Bi
,
Cong-Cong
Wang
,
Zheng-Li
Shi
,
Yu-Xia
Wang
,
Yi-Si
Wang
,
Cheng
Li
,
Ze-Bin
Peng
and
Zhong-Xin
Hong
 *
*
Department of Clinical Nutrition, Beijing Friendship Hospital, Capital Medical University, Beijing, China. E-mail: hongzhongxin@vip.sina.com; bingjieding@ccmu.edu.cn
First published on 26th February 2025
Abstract
Dietary characteristics affect maternal status in early pregnancy, which is important for later outcomes. However, Chinese dietary guidelines for pregnant women are not specific to obesity, overweight, and underweight. Moreover, since pregnancy is a prolonged process, an intermediate factor is needed to connect early maternal BMI with pregnancy outcomes. In this cohort of 1785 Chinese pregnant women from 2020 to 2022, 37.98% of participants had abnormal BMI in early pregnancy. A lower energy intake from carbohydrates (<50%) but higher intake from protein (>20%) and fat (>30%) resulted in excessive energy consumption, which was a risk factor for maternal obesity (adjusted OR (AOR): 1.49, 95%CI: 1.02–2.17) and overweight (AOR: 1.47, 95%CI: 1.00–2.18). Furthermore, the risk of maternal underweight was increased by a poor antioxidative diet (AOR: 2.80, 95%CI: 1.02–7.66) with a 20.28% lower intake of isoflavones and an imbalanced dietary structure (AOR: 3.95, 95%CI: 1.42–10.95) with less energy from fat (<20%) and unsaturated fatty acids (<3%). Following the timeline from gestation to delivery, early maternal obesity, overweight, and underweight increased the risk of abnormal body weight gain during pregnancy (AOR: 1.91–3.62, 95%CI: 1.20–6.12). Subsequently, abnormal weight gain further provoked adverse pregnancy outcomes, such as gestational diabetes mellitus, hypertensive disorders, cesarean section, and macrosomia (AOR, 1.33–2.58; 95%CI, 1.04–4.17). To minimize these threats, obese/overweight pregnant women in China might have more energy from carbohydrates (>65%) while reducing energy intake from protein (<10%) and fat (<20%). Meanwhile, underweight pregnant women are advised to increase their intake of dietary antioxidants (especially isoflavones) and consume more energy from fat (>30%) and unsaturated fatty acids (>11%). Finally, gestational body weight gain, as a potential intermediate bridge, should receive more attention.
Introduction
Maternal status in early pregnancy is crucial for the long-term quality of life for both pregnant women and neonates.1 Since the increase in total body water during pregnancy makes body mass index (BMI) less reliable,2 maternal BMI in the early stage (around 8 weeks of gestation) has gained more attention.3 In terms of maternal and neonatal health, previous literature has paid more attention to the obese population,4–6 correlating maternal obesity/overweight with various adverse outcomes, such as hypertension, colorectal cancer, and gut dysbiosis.6–8 However, underweight remains a concern in developing regions.9 China, one of the largest developing countries in the world, is undergoing an economic structural transformation. In this recent cohort study from 2020 to 2022 in Beijing, China, we not only focused on pregnant women with higher BMI but also addressed the concerns of those who are underweight.Facing the health threats triggered by abnormal maternal BMI, optimizing dietary structure could be a promising practical strategy.10,11 However, inconsistent results have been reported. Several studies have shown that low-glycemic index foods with higher protein intake might benefit lean mass, weight gain, and pregnancy complications in obese and overweight women.12,13 Whereas other studies have found that protein balance was not related to gestational body weight gain and neonatal adiposity,14 but serum long-chain polyunsaturated fatty acids might be linked to gestational diabetes mellitus.15 For Chinese citizens, the most authoritative and responsible standards to improve food, energy, and nutrient intake are the Dietary Guidelines for Chinese Residents and the Dietary Reference Intakes for China.16–19 However, the current recommendations for pregnant Chinese women are general and do not provide targeted suggestions for maternal obesity, overweight, and underweight.18 We would like to describe maternal dietary characteristics classified by different BMI statuses and hopefully provide several insights for refining the Chinese dietary guidelines for pregnant women. Furthermore, previous inconsistent studies have mainly focused on the amount of food consumption.12–15 We hypothesize that the energy contribution from different macronutrients could be more crucial. Meanwhile, whether other dietary characteristics (such as antioxidative properties) play a role in the process from early maternal BMI to later pregnancy outcomes is worth exploring.
Because the whole pregnancy process has a long period, identifying an anchor point to connect early maternal BMI and later pregnancy outcomes is valuable for clinical practice. Previous evidence implied that gestational body weight gain could be a promising intermediate bridge.20 Since 2009, most studies on gestational body weight gain have been based on recommendations from the American National Academy of Medicine (formerly known as the Institute of Medicine).20–23 However, the recommendations for Americans might not be the best choices for Chinese.24 In 2021, the localized guidelines for gestational body weight gain in China were released,25 which provided us a great opportunity to more reasonably explore the importance of body weight gain among Chinese women during pregnancy. Moreover, previous studies paid more attention to the relationship between the amount of weight gain and adverse pregnancy events.26,27 For example, the excessive amount of body weight gain increased the risk of preeclampsia, while the inadequate amount of that increased the risk of small for gestational age infants in the United States.22 In this study, we comprehensively consider both the total amount of body weight gain before parturition and the average rate of body weight gain per week based on real-world data from China.
In short, the present study assessed early maternal BMI-related dietary characteristics, and targeted dietary recommendations were proposed for pregnant Chinese women who were obese, overweight, and underweight. Additionally, the role of gestational body weight gain as an intermediate bridge to connect abnormal maternal BMI in early gestation and multiple adverse pregnancy events was clarified. Hopefully, our findings could have some significance in managing chronic disease among the pregnant Chinese population.
Materials and methods
Study design, setting, and participants
The present cohort study was conducted at two different campuses of the Beijing Friendship Hospital located in the Xicheng and Tongzhou districts from October 2020 to August 2022, and 1785 participants were included. All procedures were supervised and approved by the Ethics Committee in the Beijing Friendship Hospital, Capital Medical University (No. 2021-P2-128-01), and the Strengthening Reporting of Observational Studies in Epidemiology (STROBE) was followed. The first prenatal visit with gestational file registration around the 8-week gestation was the baseline, and follow-up was processed with subsequent prenatal visits until completing parturition as the endpoint. Inclusion criteria were as follows: (1) age >18, (2) passed the first prenatal examination, and (3) finished dietary survey in a nutrition clinic. Exclusion criteria are as follows: (1) low-quality dietary survey (truncated and incomplete data), (2) multiple pregnancies, (3) not delivering in the investigator's hospital, (4) low-quality data, and (5) unfortunate stillbirth.Exposures and outcomes
Maternal BMI in early pregnancy was the exposure factor (based on self-reported height and weight measurements at baseline). Adverse pregnancy events were outcomes that included three major categories:28 (1) pregnancy complications and comorbidities, such as gestational diabetes mellitus, hypertensive disorders of pregnancy, morning sickness, and thyroid disease; (2) abnormal delivery and its complications, such as delivery mode (cesarean section or natural vaginal delivery), birth injury, fetal distress, the premature rupture of fetal membranes, postpartum hemorrhage, and preterm birth; (3) fetal and neonatal abnormalities, such as meconium-stained amniotic fluid, macrosomia, and low birth weight. More details are presented in ESI.†Gestational body weight gain assessment
Both the total amount and weekly rate of gestational body weight gain were analyzed. The total amount of weight gain was equal to predelivery weight minus baseline weight. The weekly rate of weight gain was equal to the amount of weight gain divided by the gestational weeks. According to the Chinese Nutrition Society guidelines of gestational body weight gain,24,25 for maternal underweight (BMI < 18.5), normal (18.5 ≤ BMI < 24), overweight (24 ≤ BMI < 28), and obesity (BMI ≥ 28), the optimal amounts of weight gain were 11–16 kg, 8–14 kg, 7–11 kg, and 5–9 kg, respectively, and the optimal rates of weight gain were 0.46 (0.37–0.56) kg per week, 0.37 (0.26–0.48) kg per week, 0.30 (0.22–0.37) kg per week, and 0.22 (0.15–0.30) kg per week, respectively.Demographic characteristics and biochemical indexes
Maternal age, gestational registration week (first prenatal visit), delivery week, parity, education level, physical activity, working status/income, and smoking and drinking status were collected and used to address potential bias. Regular blood biochemical indexes were abstracted from the medical records.Dietary survey and calculation of energy and nutrient intake
Based on the Dietary Guidelines for Chinese Residents18 and our previous work,29 a food-frequency questionnaire was used, which contained 67 subtypes of foods involving grains, vegetables, fruits, animal foods, dairy, legumes, nuts, and others. A dietary survey was conducted at gestational registration (first prenatal visit) by nutritionists. Dietary survey data were transformed into the amount of food consumed per day after the quality assessment. According to the China Food Composition Database30 and the Dietary Reference Intakes for China,19 dietary energy and nutrient intake were calculated.Overall dietary characteristics assessment
Pregnant woman-based multidimensional dietary indexes and conceptions were selected to assess dietary status, including dietary quality, antioxidative property, dietary guideline adherence, eating habits, consistency of Dietary Approaches to Stop Hypertension Diet (DASH) principle, anti-inflammatory potential, and dietary diversity. Calculation details of all dietary indexes are presented in ESI Methods.† Only dietary quality and antioxidative property showed significant differences in proportion among maternal BMI groups.Dietary quality was reflected by the Chinese Diet Balance Index for Pregnancy (DBI-P) accompanied by Diet Quality Distance (DQD), High Bound Score (HBS), and Low Bound Score (LBS).31 A lower score of DBI-P with DQD, HBS, and LBS meant better dietary quality. The DBI-P with DQD represented the conditions of an imbalanced diet, which were classified into 4 degrees: high level (>56 points), middle level (39–56 points), low level (20–38 points), and almost no problem (1–19 points). The DBI-P with HBS represented the conditions of excessive dietary intake, which were classified into 5 degrees: high level (>32 points), middle level (23–32 points), low level (12–22 points), almost no problem (1–11 points), and no excessive intake (0 points). The DBI-P with LBS represented the conditions of inadequate dietary intake, which were classified into 5 degrees: high level (>44 points), middle level (31–44 points), low level (16–30 points), almost no problem (1–15 points), and no inadequate intake (0 points). The proportion of dietary quality status among maternal BMI groups was studied and described.
The dietary antioxidative property was reflected by the Dietary Antioxidant Quality Score (DAQS).32 A higher score of DAQS meant a better antioxidative property. The status of dietary antioxidative properties was classified into 4 degrees: very poor quality (0 points), low quality (1–2 points), average quality (3–4 points), and high quality (5–6 points). The proportion of dietary antioxidative properties among maternal BMI groups was studied.
Statistical analysis
Based on SPSS software (version 26.0, IBM, USA), measurement data were described as median [interquartile (IQR)] owing to the lack of distribution normality, and categorical data were described as count (n) and proportion (%). Subsequently, the Kruskal–Wallis test and Chi-square test were used to analyze the differences between maternal BMI groups. The unadjusted odds ratio (UOR) and adjusted OR (AOR) were measured by logistic regression, with demographic characteristics (age, gestational registration week, delivery week, parity, education level, physical activities, working status/income, smoking status, and drinking status) and diabetes mellitus history as covariates. Neonatal delivery mode was further adjusted when abnormal delivery and its complications as well as fetal and neonatal abnormalities were analyzed.33–36 Correlation coefficient (r) was analyzed by Spearman correlation. A P value <0.05 was considered a significant difference.Results
Basic information on pregnant women with abnormal BMI in early pregnancy
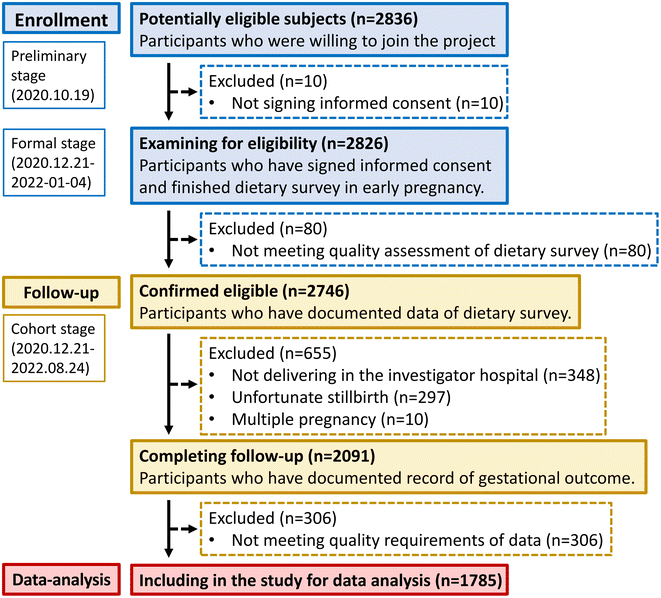
A total of 1785 pregnant women with a median (IQR) age of 31 (29–34) years were involved, and the flowchart is presented in Fig. 1. The median (IQR) weeks of gestational registration and neonatal delivery were 8 (7–9) and 39 (38–40). The majority of participants were primipara, had college and bachelor education, did not regularly exercise, and were still working every day, nonsmoking, and nondrinking (Table 1).| Basic characteristics | Total (n = 1785) | Normal (n = 1107) | Underweight (n = 150) | Overweight (n = 394) | Obesity (n = 134) | P value |
|---|---|---|---|---|---|---|
| Data were presented as median (IQR) or counts with proportion (%). | ||||||
| Age (year) | 31 (29–34) | 31 (29–34) | 30 (28–32) | 32 (30–35) | 33 (30–35) | <0.001 |
| Gestational registration (week) | 8 (7–9) | 8 (7–9) | 8 (79) | 8 (7–9) | 8 (7–9) | 0.062 |
| Delivery week | 39 (38–40) | 39 (39–40) | 39 (39–40) | 39 (38–40) | 39 (38–40) | 0.001 |
| Parity (n, %) | ||||||
| Never | 1291 (72.32%) | 793 (71.64%) | 123 (82.00%) | 285 (72.34%) | 90 (67.16%) | 0.12 |
| One time | 471 (26.39%) | 299 (27.01%) | 27 (18.00%) | 103 (26.14%) | 42 (31.35%) | |
| Two times | 23 (1.29%) | 15 (1.35%) | 0 (0%) | 6 (1.52%) | 2 (1.49%) | |
| Total | 1785 (100%) | 1107 (100%) | 150 (100%) | 394 (100%) | 134 (100%) | |
| Education level (n, %) | ||||||
| Master's degree or above | 382 (21.4%) | 279 (25.2%) | 26 (17.33%) | 66 (16.75%) | 11 (8.21%) | <0.001 |
| College and bachelor | 1165 (65.27%) | 695 (62.78%) | 101 (67.33%) | 265 (67.26%) | 104 (77.61%) | |
| High school or less | 106 (5.94%) | 57 (5.15%) | 10 (6.67%) | 27 (6.85%) | 12 (8.96%) | |
| Unwilling to inform | 132 (7.39%) | 76 (6.87%) | 13 (8.67%) | 36 (9.14%) | 7 (5.22%) | |
| Total | 1785 (100%) | 1107 (100%) | 150 (100%) | 394 (100%) | 134 (100%) | |
| Physical activities (n, %) | ||||||
| Regular exercise | ||||||
| Yes | 285 (15.97%) | 183 (16.53%) | 18 (12.00%) | 57 (14.47%) | 27 (20.15%) | 0.219 |
| No | 1500 (84.03%) | 924 (83.47%) | 132 (88.00%) | 337 (85.53%) | 107 (79.85%) | |
| Total | 1785 (100%) | 1107 (100%) | 150 (100%) | 394 (100%) | 134 (100%) | |
| Walking steps per day | ||||||
| Over 6000 steps | 637 (35.69%) | 389 (35.14%) | 43 (28.67%) | 149 (37.82%) | 56 (41.79%) | 0.283 |
| 3000–6000 steps | 532 (29.8%) | 338 (30.53%) | 45 (30.00%) | 112 (28.43%) | 37 (27.61%) | |
| Less 3000 steps | 616 (34.51%) | 380 (34.33%) | 62 (41.33%) | 133 (33.75%) | 41 (30.6%) | |
| Total | 1785 (100%) | 1107 (100%) | 150 (100%) | 394 (100%) | 134 (100%) | |
| Working status/income (n, %) | ||||||
Not working (<$10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 511 per year) 511 per year) |
310 (17.37%) | 179 (16.17%) | 27 (18.00%) | 76 (19.29%) | 28 (20.9%) | 0.344 |
Working (≥$10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 511 per year) 511 per year) |
1475 (82.63%) | 928 (83.83%) | 123 (82.00%) | 318 (80.71%) | 106 (79.1%) | |
| Total | 1785 (100%) | 1107 (100%) | 150 (100%) | 394 (100%) | 134 (100%) | |
| Smoking status (n, %) | ||||||
| Smoking | 31 (1.74%) | 21 (1.90%) | 1 (0.67%) | 5 (1.27%) | 4 (2.99%) | 0.407 |
| Nonsmoking | 1754 (98.26%) | 1086 (98.10%) | 149 (99.33%) | 389 (98.73%) | 130 (97.01%) | |
| Total | 1785 (100%) | 1107 (100%) | 150 (100%) | 394 (100%) | 134 (100%) | |
| Drinking status (n, %) | ||||||
| Drinking | 199 (11.15%) | 121 (10.93%) | 16 (10.67%) | 52 (13.2%) | 10 (7.46%) | 0.308 |
| Nondrinking | 1586 (88.85%) | 986 (89.07%) | 134 (89.33%) | 342 (86.8%) | 124 (92.54%) | |
| Total | 1785 (100%) | 1107 (100%) | 150 (100%) | 394 (100%) | 134 (100%) | |
The proportions of obese, overweight, underweight, and normal pregnant women were 7.51%, 22.07%, 8.40%, and 62.02%, respectively. Meanwhile, their median (IQR) BMI were 30.5 (29.1–31.8), 25.3 (24.5–26.4), 17.7 (17.3–18.3), and 21.1 (19.9–22.3), respectively. Next, the median (IQR) of predelivery weights among obese, overweight, underweight, and normal groups were 88.25 (83.53–96.00) kg, 78.00 (74.00–83.13) kg, 61.00 (57.53–64.00) kg, and 68.00 (64.00–73.00) kg, respectively. Furthermore, early maternal BMI was positively correlated to predelivery weight (r = 0.751, P < 0.001). Additionally, maternal obesity/overweight had hyperlipidemia with higher levels of glycated hemoglobin, fasting blood glucose, thyroid stimulating hormone, free T3, and creatinine than normal pregnant women. However, maternal underweight showed the opposite trends of serum lipids with lower levels of fasting blood glucose and creatinine (Table 2).
| Biochemical indexes | Normal [as control] | Obesity | P value | Overweight | P value | Underweight | P value |
|---|---|---|---|---|---|---|---|
| Data were presented as median (IQR). Abbreviations: TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OGTT, oral glucose tolerance test; TSH, thyroid stimulating hormone. | |||||||
| Lipid metabolism | |||||||
| TG (mmol L−1) | 0.99 (0.78–1.31) | 1.36 (1.04–1.78) | <0.001 | 1.17 (0.89–1.46) | <0.001 | 0.93 (0.76–1.11) | 0.001 |
| TC (mmol L−1) | 4.36 (3.93–4.88) | 4.68 (4.21–5.44) | 0.01 | 4.57 (4.05–5.05) | <0.001 | 4.21 (3.88–4.73) | 0.008 |
| HDL-C (mmol L−1) | 1.54 (1.35–1.73) | 1.39 (1.19–1.56) | <0.001 | 1.40 (1.26–1.60) | <0.001 | 1.58 (1.43–1.77) | 0.006 |
| LDL-C (mmol L−1) | 2.23 (1.97–2.53) | 2.61 (2.22–3.04) | <0.001 | 2.41 (2.04–2.79) | <0.001 | 2.04 (1.88–2.43) | <0.001 |
| Glucose metabolism | |||||||
| At the time of gestational file registration (first prenatal visit) | |||||||
| Glycated hemoglobin (%) | 5.00 (4.80–5.20) | 5.20 (5.00–5.50) | <0.001 | 5.10 (4.80–5.30) | <0.001 | 5.00 (4.80–5.20) | 0.323 |
| Fasting blood glucose (mmol L−1) | 4.65 (4.44–4.87) | 4.94 (4.67–5.36) | <0.001 | 4.77 (4.51–5.05) | <0.001 | 4.56 (4.39–4.84) | 0.005 |
| At the time of diabetes mellitus screening (within the second trimester) | |||||||
| Fasting blood glucose (mmol L−1) | 4.39 (4.14–4.68) | 4.75 (4.32–5.03) | <0.001 | 4.55 (4.30–4.95) | <0.001 | 4.39 (4.15–4.59) | 0.041 |
| One-hour blood glucose (mmol L−1) | 7.62 (6.48–8.74) | 8.68 (7.02–9.92) | <0.001 | 8.27 (7.07–9.32) | <0.001 | 7.59 (6.55–8.65) | 0.174 |
| Two-hour blood glucose (mmol L−1) | 6.72 (5.92–7.72) | 7.30 (6.14–9.10) | <0.001 | 7.16 (6.34–8.19) | <0.001 | 6.66 (5.50–7.34) | 0.018 |
| OGTT area (mmol L−1 h−1) | 13.11 (11.81–14.75) | 14.61 (12.49–16.58) | <0.001 | 14.12 (12.49–15.64) | <0.001 | 12.65 (11.41–14.42) | 0.082 |
| Thyroid and other metabolic indexes | |||||||
| TSH (μIU mL−1) | 1.11 (0.55–1.87) | 1.45 (0.94–2.21) | <0.001 | 1.34 (0.72–2.02) | 0.061 | 0.97 (0.33–1.56) | 0.098 |
| Free T3 (pg mL−1) | 3.13 (2.88–3.38) | 3.29 (2.97–3.52) | 0.005 | 3.21 (2.98–3.49) | 0.031 | 3.15 (2.89–3.48) | 0.913 |
| Free T4 (ng dL−1) | 0.88 (0.80–0.98) | 0.81 (0.74–0.91) | 0.155 | 0.84 (0.79–0.95) | 0.025 | 0.94 (0.83–1.04) | 0.074 |
| Creatinine (μmol L−1) | 49.40 (45.90–53.60) | 53.00 (49.00–57.18) | <0.001 | 50.40 (45.80–54.80) | 0.005 | 48.00 (44.70–51.10) | 0.002 |
In short, 37.98% of pregnant women had abnormal BMI in early pregnancy with lipid and glucose metabolic disorders, and the positive correlation between early BMI and predelivery weight implied that gestational body weight gain was important.
Characteristics of dietary quality, antioxidative property, food consumption, and energy intake among maternal BMI groups
Based on dietary quality assessment via the DBI-P index, the obese group had a higher proportion of “low level of imbalanced diet” than the normal group (71.64% vs. 60.79%, P < 0.05). The overweight group had a higher proportion of “moderate level of excessive diet” (6.85% vs. 4.16%, P < 0.05) (Table 3). The underweight group had a higher proportion of “high level of imbalanced diet” (5.33% vs. 1.90%, P < 0.05) and “high level of inadequate dietary intake” (10.00% vs. 4.25%, P < 0.05) than the normal group (Table 3). Moreover, the DAQS index suggested that the underweight group had more women with “very poor dietary antioxidative quality” than the normal group (6.00% vs. 1.81%, P < 0.05) (Table 3). No difference had been found in dietary guideline adherence, eating habits, consistency of the DASH principle, anti-inflammatory potential, and dietary diversity (Table S1†).| Overall dietary quality assessment | Normal [as control] | Obesity | P value | Overweight | P value | Underweight | P value |
|---|---|---|---|---|---|---|---|
| Data were presented as counts with proportion (%). Abbreviations: DAQS, dietary antioxidant quality score; DBI-P, Chinese diet balance index for pregnancy; DQD, diet quality distance; HBS, high bound score; LBS, low bound score. | |||||||
| DAQS (n, %) | |||||||
| Very poor quality | 20 (1.81%) | 4 (2.99%) | >0.05 | 9 (2.28%) | >0.05 | 9 (6.00%) | <0.05 |
| Low quality | 58 (5.24%) | 2 (1.49%) | >0.05 | 14 (3.55%) | >0.05 | 7 (4.67%) | >0.05 |
| Average quality | 84 (7.59%) | 6 (4.48%) | >0.05 | 30 (7.61%) | >0.05 | 12 (8.00%) | >0.05 |
| High quality | 945 (85.36%) | 122 (91.04%) | >0.05 | 341 (86.56%) | >0.05 | 122 (81.33%) | >0.05 |
| Total | 1107 (100%) | 134 (100%) | >0.05 | 394 (100%) | >0.05 | 150 (100%) | >0.05 |
| DQD of DBI-P (n, %) | |||||||
| High level of an imbalanced diet (very poor dietary intake) | 21 (1.90%) | 1 (0.75%) | >0.05 | 4 (1.02%) | >0.05 | 8 (5.33%) | <0.05 |
| Moderate level of an imbalanced diet (poor dietary intake) | 263 (23.76%) | 22 (16.42%) | >0.05 | 99 (25.13%) | >0.05 | 43 (28.67%) | >0.05 |
| Low level of an imbalanced diet (imbalanced dietary intake) | 673 (60.79%) | 96 (71.64%) | >0.05 | 252 (63.96%) | >0.05 | 86 (57.33%) | >0.05 |
| Almost no problem (good dietary intake) | 150 (13.55%) | 15 (11.19%) | >0.05 | 39 (9.89%) | >0.05 | 13 (8.67%) | >0.05 |
| Total | 1107 (100%) | 134 (100%) | >0.05 | 394 (100%) | >0.05 | 150 (100%) | >0.05 |
| HBS of DBI-P (n, %) | |||||||
| High level of excessive intake | 5 (0.45%) | 2 (1.49%) | >0.05 | 0 (0.00%) | >0.05 | 1 (0.67%) | >0.05 |
| Moderate level of excessive intake | 46 (4.16%) | 4 (2.99%) | >0.05 | 27 (6.85%) | <0.05 | 7 (4.67%) | >0.05 |
| Low level of excessive intake | 282 (25.47%) | 31 (23.13%) | >0.05 | 112 (28.43%) | >0.05 | 34 (22.67%) | >0.05 |
| Almost no excessive intake | 771 (69.65%) | 97 (72.39%) | >0.05 | 253 (64.21%) | <0.05 | 108 (71.99%) | >0.05 |
| No excessive intake | 3 (0.27%) | 0 (0.00%) | >0.05 | 2 (0.51%) | >0.05 | 0 (0.00%) | >0.05 |
| Total | 1107 (100%) | 134 (100%) | >0.05 | 394 (100%) | >0.05 | 150 (100%) | >0.05 |
| LBS of DBI-P (n, %) | |||||||
| High level of inadequate intake | 47 (4.25%) | 4 (2.99%) | >0.05 | 16 (4.06%) | >0.05 | 15 (10.00%) | <0.05 |
| Moderate level of inadequate intake | 202 (18.25%) | 18 (13.43%) | >0.05 | 69 (17.51%) | >0.05 | 27 (18.00%) | >0.05 |
| Low level of inadequate intake | 482 (43.54%) | 64 (47.76%) | >0.05 | 184 (46.70%) | >0.05 | 69 (46.00%) | >0.05 |
| Almost no inadequate intake | 371 (33.51%) | 47 (35.07%) | >0.05 | 124 (31.47%) | >0.05 | 39 (26.00%) | >0.05 |
| No inadequate intake | 5 (0.45%) | 1 (0.75%) | >0.05 | 1 (0.26%) | >0.05 | 0 (0.00%) | >0.05 |
| Total | 1107 (100%) | 134 (100%) | >0.05 | 394 (100%) | >0.05 | 150 (100%) | >0.05 |
For daily food intake, the obese and overweight groups consumed more animal and plant proteins from unprocessed red meat and other sources. The underweight group consumed less carbohydrate and plant protein from legumes and less animal protein from eggs (Table S2†).
In terms of macronutrients and energy intake, the obese group consumed a higher amount of protein (115.88 vs. 103.41 g day−1, P = 0.011), fat (70.22 vs. 61.12 g day−1, P = 0.035), and total energy (2026.32 vs. 1837.59 kcal day−1, P = 0.014) than the normal group. After analyzing the structure of macronutrient-provided energy, the obese group absorbed more energy derived from protein (463.51 vs. 414.63 kcal day−1, P = 0.011) than the normal group (Table 4). Similarly, the overweight group showed an excessive trend of protein intake (107.13 vs. 103.41 g day−1, P = 0.051) and excessive energy from protein (428.37 vs. 414.63 kcal day−1, P = 0.051) (Table 4). Besides, the underweight group consumed a lower amount of lipids than the normal group, such as cholesterol (413.5 vs. 508.74 mg day−1, P = 0.001), saturated fatty acid (10.28 vs. 12.57 g day−1, P = 0.018), and polyunsaturated fatty acid (5.73 vs. 6.59 g day−1, P = 0.048). Moreover, the underweight group had a trend to absorb less energy derived from protein (360.95 vs. 414.63 kcal day−1, P = 0.065) (Table 4).
| Dietary intake | Normal [as control] | Obesity | P value | Overweight | P value | Underweight | P value |
|---|---|---|---|---|---|---|---|
| Data were presented as median (IQR). Daidzein, glycitein, and genistein are 3 major subtypes of isoflavones. Abbreviations: MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acid. | |||||||
| Macronutrients | |||||||
| Carbohydrate (g day−1) | 225.07 (163.97–319.08) | 244.24 (176.80–376.91) | 0.053 | 236.06 (156.96–352.97) | 0.167 | 221.74 (145.72–324.81) | 0.395 |
| Protein (g day−1) | 103.41 (65.78–151.85) | 115.88 (75.23–181.04) | 0.011 | 107.13 (72.51–173.58) | 0.051 | 89.71 (57.11–148.26) | 0.065 |
| Fat (g day−1) | 61.12 (36.91–98.59) | 70.22 (44.19–114.01) | 0.035 | 65.29 (39.67–102.28) | 0.177 | 52.51 (31.47–89.95) | 0.081 |
| Cholesterol (mg day−1) | 508.75 (331.51–771.28) | 525.41 (394.74–834.64) | 0.062 | 542.24 (348.43–775.69) | 0.288 | 413.50 (223.97–727.56) | 0.001 |
| SFA (g day−1) | 12.57 (8.34–18.48) | 12.68 (8.55–20.65) | 0.343 | 13.14 (8.80–19.41) | 0.165 | 10.82 (6.11–18.17) | 0.018 |
| MUFA (g day−1) | 10.74 (6.78–17.41) | 11.48 (7.30–20.85) | 0.089 | 11.49 (7.55–19.48) | 0.129 | 9.37 (5.27–16.31) | 0.058 |
| PUFA (g day−1) | 6.59 (3.71–10.59) | 6.38 (4.21–11.77) | 0.363 | 6.80 (3.95–11.07) | 0.262 | 5.73 (2.82–9.76) | 0.048 |
| Energy (kcal day−1) | |||||||
| Total energy intake | 1837.59 (1255.99–2629.99) | 2026.32 (1383.32–2836.39) | 0.014 | 1926.97 (1306.66–2794.13) | 0.095 | 1627.14 (1037.45–2686.05) | 0.139 |
| Carbohydrate for energy | 847.70 (612.79–1205.77) | 910.71 (631.65–1426.97) | 0.077 | 892.68 (589.24–1333.13) | 0.193 | 838.15 (557.27–1224.19) | 0.378 |
| Protein for energy | 414.63 (263.32–609.96) | 463.51 (300.94–724.14) | 0.011 | 428.37 (289.88–689.27) | 0.051 | 360.95 (230.24–593.31) | 0.065 |
| Fat for energy | 494.28 (281.72–813.62) | 571.99 (338.32–909.96) | 0.080 | 512.96 (306.58–855.17) | 0.254 | 423.89 (253.52–769.95) | 0.083 |
| Isoflavones (mg day−1) | 1.43 (0.60–3.14) | 1.25 (0.51–2.93) | 0.462 | 1.31 (0.57–3.06) | 0.487 | 1.14 (0.42–2.36) | 0.012 |
| Daidzein (mg day−1) | 2.05 (0.91–4.14) | 1.81 (0.79–3.85) | 0.375 | 1.93 (0.92–3.94) | 0.627 | 1.50 (0.66–3.25) | 0.006 |
| Glycitein (mg day−1) | 0.42 (0.18–0.91) | 0.40 (0.16–0.91) | 0.805 | 0.38 (0.18–1.01) | 0.770 | 0.34 (0.13–0.73) | 0.016 |
| Genistein (mg day−1) | 1.95 (0.72–4.54) | 1.59 (0.58–3.87) | 0.404 | 1.78 (0.63–4.38) | 0.429 | 1.51 (0.43–3.32) | 0.016 |
For micronutrients, the underweight group showed a significant 20.28% lower intake of isoflavones than the normal group (1.14 vs. 1.43 mg day−1, P = 0.012) (Table 4). In fact, all 3 major subtypes of isoflavones showed a decreased intake in the underweight group, including daidzein (1.50 vs. 2.05 mg day−1, P = 0.006), glycitein (0.34 vs. 0.42, P = 0.016), and genistein (1.51 vs. 1.95 mg day−1, P = 0.016) (Table 4). However, the overall intake of vitamins, minerals, and other food components (such as dietary fiber, flavonoids, and anthocyanidins) was adequate among the obese, overweight, and underweight groups (Table S3†).
In short, an early abnormal BMI came with an imbalanced diet. The obese and overweight groups had excessive dietary intake with more energy from protein, so maternal obese and overweight may need to control energy intake derived from protein. Besides, the underweight group had a high level of imbalanced diet with inadequate dietary intake (such as lipids and isoflavones) and less energy from protein. Combining the prevalence of “very poor dietary antioxidative quality” in the underweight group in this study, and the widely known fact that isoflavones possess significant antioxidative property,37,38 more attention should be paid to the isoflavone intake in maternal underweight in China.
Improving dietary energy structure and poor dietary antioxidative property benefited the management of early maternal obesity, overweight and underweight
Next, we assessed the risk of abnormal maternal BMI in early pregnancy induced by inappropriate dietary energy. First, a daily diet with excessive energy intake increased the risk of early maternal obesity (AOR, 1.49; 95%CI, 1.02–2.17) and overweight (AOR, 1.26; 95%CI, 0.99–1.60) (Table 5). Then, according to the Dietary Reference Intakes for China,19 the excessive energy intake among pregnant women could be induced by dietary energy from carbohydrates <50% (AOR, 2.29; 95%CI, 1.86–2.83), protein >20% (AOR, 1.91; 95%CI, 1.52–2.40), and fat >30% (AOR, 2.20; 95%CI, 1.77–2.74) (Table 6). Inversely, energy from fat <20% and unsaturated fatty acids <3% was beneficial in restricting excessive energy intake (AOR, 0.42–0.74; 95%CI, 0.20–0.98) (Table 6).| Risk factors for early abnormal BMI | UOR | P value | AOR | P value |
|---|---|---|---|---|
| The assessment of energy intake was referred to the Dietary Reference Intakes for China, which specified the daily energy requirement of pregnant Chinese women at different ages, gestational stages, and physical activity levels. The assessment of dietary antioxidative status was based on the DAQS score in this study, and the degree of average quality was set as the control. Abbreviations: AOR, adjusted odds ratio; DAQS, dietary antioxidant quality score; UOR, unadjusted odds ratio. | ||||
| Risk from energy intake | ||||
| Excessive energy to obesity | 1.47 (1.03–2.11) | 0.035 | 1.49 (1.02–2.17) | 0.038 |
| Excessive energy to overweight | 1.28 (1.02–1.61) | 0.037 | 1.26 (0.99–1.60) | 0.056 |
| Excessive energy to underweight | 0.87 (0.62–1.24) | 0.442 | 0.87 (0.61–1.25) | 0.463 |
| Inadequate energy to obesity | 0.68 (0.47–0.97) | 0.035 | 0.67 (0.46–0.98) | 0.038 |
| Inadequate energy to overweight | 0.78 (0.62–0.99) | 0.037 | 0.79 (0.63–1.01) | 0.056 |
| Inadequate energy to underweight | 1.15 (0.81–1.63) | 0.442 | 1.14 (0.80–1.64) | 0.463 |
| Risk from the dietary antioxidative status | ||||
| Very poor quality to obesity | 2.80 (0.72–10.86) | 0.137 | 2.28 (0.55–9.46) | 0.256 |
| Very poor quality to overweight | 1.26 (0.52–3.07) | 0.611 | 1.19 (0.48–2.97) | 0.704 |
| Very poor quality to underweight | 3.15 (1.17–8.50) | 0.023 | 2.80 (1.02–7.66) | 0.046 |
| Low quality to obesity | 0.48 (0.09–2.48) | 0.383 | 0.51 (0.10–2.67) | 0.426 |
| Low quality to overweight | 0.68 (0.33–1.39) | 0.284 | 0.69 (0.33–1.43) | 0.312 |
| Low quality to underweight | 0.85 (0.31–2.28) | 0.739 | 0.74 (0.27–2.01) | 0.552 |
| High quality to obesity | 1.81 (0.77–4.23) | 0.172 | 1.71 (0.72–4.07) | 0.222 |
| High quality to overweight | 1.01 (0.65–1.56) | 0.963 | 1.00 (0.64–1.56) | 0.988 |
| High quality to underweight | 0.90 (0.48–1.70) | 0.754 | 0.93 (0.49–1.77) | 0.823 |
| Macronutrient-provided energy | Risk of excessive energy intake | Risk of inadequate energy intake | ||||||
|---|---|---|---|---|---|---|---|---|
| UOR | P value | AOR | P value | UOR | P value | AOR | P value | |
| Abbreviations: AOR, adjusted odds ratio; UFAs, unsaturated fatty acids; UOR, unadjusted odds ratio. | ||||||||
| Carbohydrate for energy | ||||||||
| >65% | 0.74 (0.52–1.06) | 0.098 | 0.76 (0.53–1.10) | 0.145 | 1.35 (0.95–1.93) | 0.098 | 1.31 (0.91–1.88) | 0.145 |
| <50% | 2.26 (1.84–2.78) | <0.001 | 2.29 (1.86–2.83) | <0.001 | 0.44 (0.36–0.54) | <0.001 | 0.44 (0.35–0.54) | <0.001 |
| Protein for energy | ||||||||
| >20% | 1.87 (1.50–2.34) | <0.001 | 1.91 (1.52–2.40) | <0.001 | 0.53 (0.43–0.67) | <0.001 | 0.52 (0.42–0.66) | <0.001 |
| <10% | 1.33 (0.22–8.06) | 0.754 | 1.56 (0.26–9.49) | 0.632 | 0.75 (0.12–4.54) | 0.754 | 0.64 (0.11–3.92) | 0.632 |
| Fat for energy | ||||||||
| >30% | 2.15 (1.74–2.67) | <0.001 | 2.20 (1.77–2.74) | <0.001 | 0.47 (0.38–0.58) | <0.001 | 0.45 (0.37–0.57) | <0.001 |
| <20% | 0.73 (0.55–0.95) | 0.021 | 0.74 (0.56–0.98) | 0.035 | 1.38 (1.05–1.81) | 0.021 | 1.35 (1.02–1.78) | 0.035 |
| UFAs for energy | ||||||||
| >11% | 0.98 (0.80–1.20) | 0.805 | 0.97 (0.78–1.19) | 0.740 | 1.03 (0.84–1.26) | 0.805 | 1.04 (0.84–1.28) | 0.740 |
| <3% | 0.42 (0.20–0.91) | 0.028 | 0.42 (0.20–0.92) | 0.030 | 2.36 (1.10–5.09) | 0.028 | 2.36 (1.09–5.13) | 0.030 |
Besides, the “high level of imbalanced dietary structure” increased the risk of early maternal underweight (AOR, 3.95; 95%CI, 1.42–10.95), and energy intake was also important to maternal underweight. The daily diet with inadequate energy intake could be induced by energy from fat <20% (AOR, 1.35; 95%CI, 1.02–1.78) and unsaturated fatty acids <3% (AOR, 2.36; 95%CI, 1.09–5.13) (Table 6). Inversely, the inadequate energy intake could be controlled by dietary energy from carbohydrate <50% (AOR, 0.44; 95%CI, 0.35–0.54), protein >20% (AOR, 0.52; 95%CI, 0.42–0.66), and fat >30% (AOR, 0.45; 95%CI, 0.37–0.57) (Table 6). More interestingly, we found out that “very poor dietary antioxidative quality” was a significant risk factor for maternal underweight in early pregnancy (AOR, 2.80; 95%CI, 1.02–7.66) (Table 5), which implied that inadequate energy intake and dietary antioxidative property should be concerned for managing underweight among pregnant women in China.
In short, improving the dietary energy structure provided by macronutrients and antioxidative properties contributed by dietary antioxidants (such as isoflavones) were beneficial to the management of maternal BMI in early pregnancy (Fig. 2). To highlight the clinical significance of managing maternal BMI in early pregnancy by optimizing daily diet, next, we explored the connection between early maternal BMI and later pregnancy outcomes.
Abnormal maternal BMI without dietary management in early pregnancy was a risk factor for adverse pregnancy outcomes
In this study, pregnant women suffering from imbalanced diet-related obesity and overweight had a higher proportion of gestational diabetes mellitus than normal pregnant women (47.01% and 36.29% vs. 22.40%, P < 0.05), as did hypertensive disorders of pregnancy (29.01% and 13.96% vs. 5.69%, P < 0.05), cesarean section (61.19% and 52.03% vs. 40.83%, P < 0.05), and preterm birth (9.70% and 8.88% vs. 3.97%), as well as fewer neonates with normal birth weight (88.06% and 89.09% vs. 93.32%, P < 0.05) (Table S4†). Besides, the obese and overweight groups had fewer pregnant women with birth injury (29.10% and 32.74% vs. 41.10%, P < 0.05), which could be attributed to more women undergoing cesarean section and consequently controlling injury from natural vaginal delivery (Table S4†). Other pregnancy events showed no significant difference in proportion among BMI groups (Table S4†).More importantly, maternal obesity increased the risk of gestational diabetes mellitus (AOR, 2.59; 95%CI, 1.76–3.80), hypertensive disorders of pregnancy (AOR, 5.71; 95%CI, 3.49–9.34), and cesarean section (AOR, 1.88; 95%CI, 1.28–2.75). Similarly, maternal overweight also increased the risk of gestational diabetes mellitus (AOR, 1.76; 95%CI, 1.36–2.28), hypertensive disorders of pregnancy (AOR, 2.35; 95%CI, 1.57–3.51), and cesarean section (AOR, 1.40; 95%CI, 1.10–1.78). Although the group of underweight pregnant women showed no significant results in the proportion of adverse pregnancy outcomes, maternal underweight might be disadvantageous to severe morning sickness (AOR, 2.67; 95%CI, 1.00–7.12) (Table 7).
| Adverse pregnancy outcomes | Obesity | Overweight | Underweight | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UOR | P value | AOR | P value | UOR | P value | AOR | P value | UOR | P value | AOR | P value | |
| Abbreviations: AOR, adjusted odds ratio; UOR, unadjusted odds ratio. | ||||||||||||
| Morning sickness | ||||||||||||
| Severe | 0.48 (0.17–1.34) | 0.163 | 0.66 (0.23–1.90) | 0.442 | 0.49 (0.22–1.09) | 0.081 | 0.56 (0.25–1.26) | 0.159 | 2.78 (1.06–7.30) | 0.039 | 2.67 (1.00–7.12) | 0.050 |
| Moderate | 0.58 (0.32–1.04) | 0.069 | 0.74 (0.39–1.38) | 0.338 | 1.23 (0.80–1.89) | 0.344 | 1.36 (0.87–2.12) | 0.173 | 2.14 (0.98–4.70) | 0.057 | 1.93 (0.87–4.28) | 0.104 |
| Mild | 0.60 (0.36–1.02) | 0.057 | 0.80 (0.46–1.39) | 0.421 | 1.03 (0.69–1.53) | 0.905 | 1.16 (0.77–1.76) | 0.471 | 1.79 (0.84–3.79) | 0.131 | 1.61 (0.75–3.43) | 0.222 |
| Gestational diabetes mellitus | 3.07 (2.13–4.44) | <0.001 | 2.59 (1.76–3.80) | <0.001 | 1.97 (1.54–2.53) | <0.001 | 1.76 (1.36–2.28) | <0.001 | 0.60 (0.37–0.96) | 0.032 | 0.64 (0.40–1.03) | 0.067 |
| Hypertensive disorders of pregnancy | 6.80 (4.33–10.68) | <0.001 | 5.71 (3.49–9.34) | <0.001 | 2.69 (1.84–3.94) | <0.001 | 2.35 (1.57–3.51) | <0.001 | 0.34 (0.11–1.09) | 0.070 | 0.37 (0.11–1.19) | 0.094 |
| Thyroid disease | ||||||||||||
| Hypothyroidism | 1.06 (0.65–1.72) | 0.828 | 0.86 (0.51–1.44) | 0.571 | 0.86 (0.62–1.19) | 0.361 | 0.79 (0.56–1.10) | 0.167 | 0.97 (0.60–1.56) | 0.899 | 0.92 (0.57–1.49) | 0.732 |
| Hyperthyroidism | 1.48 (0.43–5.14) | 0.536 | 1.08 (0.29–4.07) | 0.905 | 0.64 (0.22–1.92) | 0.429 | 0.55 (0.18–1.70) | 0.300 | 1.30 (0.38–4.51) | 0.677 | 1.44 (0.41–5.08) | 0.574 |
| Cesarean section | 2.29 (1.58–3.30) | <0.001 | 1.88 (1.28–2.75) | 0.001 | 1.57 (1.25–1.98) | <0.001 | 1.40 (1.10–1.78) | 0.006 | 0.62 (0.43–0.90) | 0.011 | 0.64 (0.44–0.93) | 0.019 |
| Birth injury | 0.59 (0.40–0.87) | 0.008 | 0.96 (0.59–1.57) | 0.883 | 0.70 (0.55–0.90) | 0.004 | 0.85 (0.63–1.15) | 0.299 | 1.07 (0.76–1.51) | 0.715 | 0.76 (0.50–1.13) | 0.176 |
| Preterm birth | 2.60 (1.36–4.96) | 0.004 | 2.21 (0.11–45.18) | 0.606 | 2.36 (1.49–3.73) | <0.001 | 3.40 (0.42–27.67) | 0.252 | 0.49 (0.15–1.61) | 0.241 | 0.59 (0.01–63.62) | 0.824 |
| Fetal distress | 0.96 (0.54–1.72) | 0.890 | 0.74 (0.39–1.41) | 0.358 | 1.25 (0.88–1.77) | 0.208 | 1.02 (0.69–1.50) | 0.936 | 0.98 (0.57–1.71) | 0.949 | 1.11 (0.59–2.06) | 0.753 |
| Premature rupture of fetal membranes | 0.96 (0.62–1.48) | 0.842 | 0.95 (0.60–1.52) | 0.828 | 1.07 (0.82–1.41) | 0.625 | 1.04 (0.78–1.39) | 0.799 | 0.94 (0.62–1.42) | 0.768 | 0.88 (0.58–1.36) | 0.574 |
| Postpartum hemorrhage | 1.55 (0.59–4.10) | 0.376 | 2.25 (0.81–6.24) | 0.119 | 1.04 (0.50–2.17) | 0.913 | 1.00 (0.46–2.17) | 0.996 | 0.82 (0.25–2.72) | 0.741 | 0.61 (0.17–2.13) | 0.436 |
| Meconium-stained amniotic fluid | 0.78 (0.41–1.49) | 0.449 | 0.80 (0.41–1.57) | 0.515 | 0.98 (0.67–1.44) | 0.935 | 1.00 (0.68–1.48) | 0.998 | 1.26 (0.75–2.12) | 0.377 | 1.23 (0.71–2.12) | 0.457 |
| Neonatal birth weight | 1.89 (1.07–3.36) | 0.029 | 1.37 (0.70–2.68) | 0.352 | 1.71 (1.15–2.54) | 0.008 | 1.37 (0.88–2.14) | 0.160 | 0.79 (0.37–1.67) | 0.530 | 0.86 (0.39–1.86) | 0.695 |
| Macrosomia | 1.89 (0.86–4.16) | 0.112 | 1.55 (0.66–3.63) | 0.310 | 1.67 (0.97–2.89) | 0.067 | 1.61 (0.91–2.84) | 0.104 | 1.18 (0.49–2.85) | 0.713 | 1.21 (0.49–2.99) | 0.675 |
| Low birth weight | 1.89 (0.86–4.16) | 0.112 | 0.97 (0.27–3.57) | 0.967 | 1.75 (1.02–3.01) | 0.043 | 0.90 (0.39–2.08) | 0.812 | 0.39 (0.09–1.65) | 0.202 | 0.44 (0.08–2.39) | 0.342 |
In summary, maternal overweight and obesity in early pregnancy showed a direct adverse association with gestational diabetes mellitus (AOR, 1.76–2.59; 95%CI,1.36–3.80), hypertensive disorders of pregnancy (AOR, 2.35–5.71; 95%CI, 1.57–9.34), and cesarean section (AOR, 1.40–1.88; 95%CI, 1.10–2.75), meanwhile, underweight could be related to severe morning sickness (AOR, 2.67; 95%CI, 1.00–7.12) (Fig. 2). Given the long period of pregnancy, the direct association of early maternal BMI with adverse pregnancy events occurring a few months later was rough and incomplete. Therefore, we further explore the role of gestational body weight gain as an intermediate bridge to explain these associations. The total amount of body weight gain before parturition and the average rate of body weight gain per week were considered.
Total amount and weekly rate of gestational body weight gain among different maternal BMI groups
For the total amount of body weight gain, the obese group had a higher proportion of excessive total gain amount than the normal group (43.28% vs. 32.52%), as did the overweight group (51.78% vs. 32.52%). Whereas the underweight group had a lower proportion of excessive total gain amount than the normal group (23.32% vs. 32.52%) (Table S5†). Moreover, the obese group had a higher proportion of inadequate total gain amount than the normal group (24.63% vs. 11.11%). Similar results were found in the overweight (16.75% vs. 11.11%) and underweight groups (20.00% vs. 11.11%) (Table S5†).For the weekly rate of body weight gain, the obese group had a higher proportion of excessive weekly gain rate than the normal group (44.77% vs. 28.91%), as did the overweight group (51.01% vs. 28.91%). Whereas the underweight group had a lower proportion of excessive weekly gain rate than the normal group (20.00% vs. 28.91%) (Table S5†). Furthermore, the obese group had a higher proportion of inadequate weekly gain rate than the normal group (24.63% vs. 13.10%). Additionally, the underweight group had more women with an inadequate weekly gain rate (20.00% vs. 13.10%). However, the overweight group showed no significant result in the proportion of inadequate weekly gain rate compared to the normal group (Table S5†).
In general, the obese and overweight groups had more pregnant women with excessive and inadequate gestational body weight gain. Meanwhile, inadequate weight gain was a notable problem in the underweight group.
Gestational body weight gain could be the intermediate bridge to connect early maternal BMI and adverse pregnancy outcomes
Between early maternal BMI and further gestational body weight gain, obesity increased the risk of excessive total gain amount (AOR, 2.42; 95%CI, 1.58–3.72), inadequate total gain amount (AOR, 3.62; 95%CI, 2.14–6.12), excessive weekly gain rate (AOR, 2.82; 95%CI, 1.83–4.34), and inadequate weekly gain rate (AOR, 3.28; 95%CI, 1.95–5.51). Similarly, overweight increased the risk of excessive total gain amount (AOR, 3.00; 95%CI, 2.30–3.91), inadequate total gain amount (AOR, 2.45; 95%CI, 1.69–3.56), excessive weekly gain rate (AOR, 3.25; 95%CI, 2.49–4.24), and inadequate weekly gain rate (AOR, 2.12; 95%CI, 1.48–3.04). However, underweight only increased the risk of inadequate total gain amount (AOR, 1.91; 95%CI, 1.20–3.07) and inadequate weekly gain rate (AOR, 2.28; 95%CI, 1.48–3.51) (Table 8).| Risk of abnormal weight gain from abnormal maternal BMI | Obesity | Overweight | Underweight | |
|---|---|---|---|---|
| Abbreviations: AOR, adjusted odds ratio; UOR, unadjusted odds ratio. | ||||
| Excessive amount | UOR | 2.34 (1.54–3.54) | 2.85 (2.20–3.69) | 0.71 (0.47–1.08) |
| P value | <0.001 | <0.001 | 0.111 | |
| AOR | 2.42 (1.58–3.72) | 3.00 (2.30–3.91) | 0.67 (0.44–1.02) | |
| P value | <0.001 | <0.001 | 0.061 | |
| Inadequate amount | UOR | 3.89 (2.38–6.38) | 2.70 (1.89–3.85) | 1.79 (1.13–2.83) |
| P value | <0.001 | <0.001 | 0.013 | |
| AOR | 3.62 (2.14–6.12) | 2.45 (1.69–3.56) | 1.91 (1.20–3.07) | |
| P value | <0.001 | <0.001 | 0.007 | |
| Excessive rate | UOR | 2.94 (1.93–4.47) | 3.15 (2.43–4.08) | 0.74 (0.48–1.15) |
| P value | <0.001 | <0.001 | 0.186 | |
| AOR | 2.82 (1.83–4.34) | 3.25 (2.49–4.24) | 0.70 (0.45–1.10) | |
| P value | <0.001 | <0.001 | 0.124 | |
| Inadequate rate | UOR | 3.56 (2.18–5.83) | 2.25 (1.59–3.19) | 2.13 (1.40–3.25) |
| P value | <0.001 | <0.001 | <0.001 | |
| AOR | 3.28 (1.95–5.51) | 2.12 (1.48–3.04) | 2.28 (1.48–3.51) | |
| P value | <0.001 | <0.001 | <0.001 | |
Between gestational body weight gain and later adverse pregnancy outcomes, the excessive total amount of weight gain increased the risk of hypertensive disorders (AOR, 2.08; 95%CI, 1.43–3.03), hypothyroidism (AOR, 1.44; 95%CI, 1.08–1.91), cesarean section (AOR, 1.33; 95%CI, 1.07–1.64), and macrosomia (AOR, 2.49; 95%CI, 1.48–4.17). Meanwhile, the inadequate total amount of weight gain increased the risk of gestational diabetes mellitus (AOR, 2.58; 95%CI, 1.91–3.49) (Table 9). Similarly, the excessive weekly rate of weight gain increased the risk of hypertensive disorders (AOR, 2.37; 95%CI, 1.62–3.47), hypothyroidism (AOR, 1.39; 95%CI, 1.04–1.85), cesarean section (AOR, 1.40; 95%CI, 1.13–1.74), and macrosomia (AOR, 2.16; 95%CI, 1.30–3.60). The inadequate weekly rate of weight gain increased the risk of gestational diabetes mellitus (AOR, 2.29; 95%CI, 1.72–3.06) (Table 9).
| Risk of adverse pregnancy events | Excessive total gain amount | Inadequate total gain amount | Excessive weekly gain rate | Inadequate weekly gain rate | |
|---|---|---|---|---|---|
| Abbreviations: AOR, adjusted odds ratio; UOR, unadjusted odds ratio. | |||||
| Gestational diabetes mellitus | UOR | 0.73 (0.57–0.93) | 2.75 (2.06–3.67) | 0.76 (0.59–0.97) | 2.43 (1.84–3.21) |
| P value | 0.011 | <0.001 | 0.026 | <0.001 | |
| AOR | 0.73 (0.57–0.94) | 2.58 (1.91–3.49) | 0.72 (0.56–0.93) | 2.29 (1.72–3.06) | |
| P value | 0.016 | <0.001 | 0.011 | <0.001 | |
| Hypertensive disorders in pregnancy | UOR | 1.87 (1.31–2.68) | 1.55 (0.95–2.54) | 2.29 (1.60–3.29) | 1.48 (0.90–2.42) |
| P value | 0.001 | 0.079 | <0.001 | 0.119 | |
| AOR | 2.08 (1.43–3.03) | 1.00 (0.58–1.74) | 2.37 (1.62–3.47) | 1.23 (0.72–2.09) | |
| P value | <0.001 | 0.988 | <0.001 | 0.449 | |
| Hypothyroidism | UOR | 1.47 (1.11–1.94) | 1.25 (0.84–1.84) | 1.42 (1.07–1.89) | 1.30 (0.90–1.88) |
| P value | 0.007 | 0.271 | 0.015 | 0.166 | |
| AOR | 1.44 (1.08–1.91) | 1.17 (0.79–1.75) | 1.39 (1.04–1.85) | 1.26 (0.87–1.84) | |
| P value | 0.012 | 0.437 | 0.027 | 0.222 | |
| Cesarean section | UOR | 1.30 (1.06–1.60) | 0.99 (0.74–1.31) | 1.43 (1.17–1.76) | 1.05 (0.80–1.38) |
| P value | 0.011 | 0.936 | 0.001 | 0.732 | |
| AOR | 1.33 (1.07–1.64) | 0.87 (0.65–1.17) | 1.40 (1.13–1.74) | 0.96 (0.72–1.27) | |
| P value | 0.009 | 0.362 | 0.002 | 0.769 | |
| Meconium-stained amniotic fluid | UOR | 0.87 (0.62–1.21) | 0.81 (0.50–1.30) | 0.91 (0.64–1.28) | 1.05 (0.68–1.61) |
| P value | 0.400 | 0.378 | 0.579 | 0.829 | |
| AOR | 0.83 (0.59–1.17) | 0.93 (0.57–1.52) | 0.91 (0.64–1.29) | 1.12 (0.72–1.74) | |
| P value | 0.293 | 0.768 | 0.594 | 0.620 | |
| Macrosomia | UOR | 2.52 (1.53–4.14) | 0.15 (0.02–1.09) | 2.16 (1.33–3.50) | 0.11 (0.02–0.80) |
| P value | <0.001 | 0.060 | 0.002 | 0.029 | |
| AOR | 2.49 (1.48–4.17) | 0.12 (0.02–0.89) | 2.16 (1.30–3.60) | 0.09 (0.01–0.68) | |
| P value | 0.001 | 0.038 | 0.003 | 0.020 | |
In short, following the timeline of gestation to delivery, abnormal maternal BMI in early pregnancy increased the risk of subsequently abnormal gestational body weight gain (AOR, 2.12–3.62; 95%CI, 1.20–6.12). Then, the abnormal weight gain further increased the risk of later adverse pregnancy outcomes, such as gestational diabetes mellitus, hypertensive disorders, hypothyroidism, cesarean section, and macrosomia (AOR, 1.33–2.58; 95%CI, 1.04–4.17). Thus, gestational body weight gain could be the intermediate bridge for connecting early maternal BMI and adverse pregnancy outcomes, so it should be monitored based on Chinese localized standards of total gain amount and weekly gain rate. More importantly, managing maternal BMI in early pregnancy via the improvement of dietary structure (especially aimed at dietary energy and antioxidative property) could prevent these vicious causal associations among pregnant Chinese women from the very beginning (Fig. 2).
Discussion
Owing to distinct ethnic and lifestyles, different institutes and countries published localization standards of BMI for scientific purposes. For example, the ranges of BMI <18.5, 18.5–24.9, 25.0–29.9, and ≥30.0 were considered as underweight, normal weight, overweight, and obesity, respectively, by the World Health Organization and the United Kingdom National Institute for Health and Care Excellence.39 However, the BMI standard for the Chinese was the foundation of the present study, which suggests that <18.5, 18.5–24, 24–28, and ≥28 were classifications of BMI.24,25 Based on the cohort from 2021–2022 in Beijing showed that the prevalence of maternal obesity, overweight, and underweight in early pregnancy were 7.51%, 22.07%, and 8.40%, respectively. The prevalence of abnormal maternal BMI in China was distinct from that in either developing areas (for example, Southern Ethiopia exhibited 41.20% for undernutrition40), or developed countries (for example, the United States exhibited 39.7% for obesity,41 and Japan exhibited 21.7% for underweight42). Thus, pregnant Chinese women had a unique epidemiological distribution of abnormal BMI, so strategies for managing maternal BMI should fit their characteristics.Ideally, the management of pregnant women should be provided by nutritionists and obstetricians in the early stage.41 Previous studies suggested that dietary intervention and physical activity before the second trimester, not oral hypoglycemic agents (such as metformin), might be an optimal strategy.11 Nowadays, inappropriate energy intake among pregnant women is a worldwide problem. The structure of calorigenic nutrients and their food sources might be more important than a simple low-calorie diet.43 In this study, overall maternal dietary characteristics were evaluated by dietary indexes, such as DBI-P and DQAS (which were previously validated in pregnant women in the Guangzhou Yuexiu birth cohort31 and the participants of the Shanghai Women's Health Study32). Meanwhile, detailed features (such as macronutrient and micronutrient intake) were assessed. It turns out that maternal dietary characteristics were different from Western lifestyles or situations in developing areas.40,41 We found out that dietary energy from carbohydrates <50%, protein >20%, and fat >30% were risk factors for excessive energy intake, which further increased the risk of maternal obesity and overweight in early pregnancy. Meanwhile, energy from fat <20% and unsaturated fatty acids <3% increased the risk of inadequate energy intake, which was not good news for maternal underweight. Therefore, the dietary recommendations for pregnant Chinese women should serve general ladies and be more specific to help women who are obese, overweight, and underweight.
Unlike previous studies, which considered that obese women had a hidden hunger for micronutrients,44 in this study, the overall micronutrient intake in the obese and overweight groups was adequate. The underweight group had a 20.28% lower intake of isoflavones with poor dietary antioxidative properties in contrast to the normal group. What is worse, we found that poor dietary antioxidative property was a significant risk factor for maternal underweight in early pregnancy. Isoflavones, as a group of vital phytochemicals in soybeans and their products, have been widely reported to possess antioxidative capacity.45–47 A mechanism study reported that isoflavones could activate the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway to mediate antioxidant responses.37 Additionally, other phytochemicals, including dietary fiber, flavonoids (luteolin, apigenin, quercetin, myricetin, and kaempferol), and anthocyanidins (delphinidin, cyanidin, and peonidin), were adequate among the BMI groups (Table S3†). Besides, in this study, underweight pregnant women had less dietary energy from unsaturated fatty acids, which could be a disadvantage to dietary antioxidative capacity. Unsaturated fatty acids (as essential fatty acids) provide energy for maintaining life and are involved in the antioxidative system.48–50 For example, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) showed antioxidative activity via mitochondrial modulation.48–50 Therefore, to reduce the risk of maternal underweight induced by poor dietary antioxidative property, the lower intake of isoflavones and less energy from unsaturated fatty acids among pregnant Chinese women need to be considered.
To highlight the clinical significance of managing maternal BMI in early pregnancy by optimizing the daily diet, the connection between early maternal BMI and later pregnancy outcomes was further explored. Previous studies reported that abnormal BMI was related to postpartum weight retention in the United Kingdom51 and offspring fat accumulation in Finland.52 We found out that abnormal maternal BMI increased the risk of adverse events in China, such as gestational diabetes mellitus, hypertensive disorders, and cesarean section. Therefore, abnormal BMI in early pregnancy is a serious threat to pregnant Chinese women.
Owing to the long period of the whole pregnancy process, finding an intermediate bridge (such as gestational body weight gain) to explain the direct connection between maternal BMI in early pregnancy and adverse pregnancy outcomes months later seems more reasonable.53 Since 2009, the recommendations of gestational body weight gain from the American National Academy of Medicine (formerly known as the Institute of Medicine) have been globally used to maintain a healthy pregnancy.54–56 In detail, the American standards recommended a total amount of 12.5–18 kg, 11.5–16 kg, 7–11.5 kg, and 5–9 kg body weight gain to underweight, normal, overweight, and obese pregnant women, respectively.56 Corresponding, the optimal average rates of weight gain were 0.51 (0.44–0.58) kg per week, 0.42 (0.35–0.50) kg per week, 0.28 (0.23–0.33) kg per week, and 0.22 (0.17–0.27) kg per week, respectively.56 According to the American standards, data from more than 1 million pregnant women from America, Asia, and Europe showed that 47% of them had excessive gestational body weight gain, while 23% were inadequate.21 However, previous literature in China based on the American version of body weight gain recommendations showed that neither diet intervention nor physical activity benefited the prevention of gestational diabetes mellitus but only restricted gestational body weight gain.57
In 2021, the localized guidelines for gestational body weight gain in China were released.24,25 Based on that, for Chinese maternal underweight, normal, overweight, and obesity, the optimal total amounts of weight gain were 11–16 kg, 8–14 kg, 7–11 kg, and 5–9 kg, respectively; meanwhile, the optimal weekly rates of weight gain were 0.46 (0.37–0.56) kg per week, 0.37 (0.26–0.48) kg per week, 0.30 (0.22–0.37) kg per week, and 0.22 (0.15–0.30) kg per week, respectively.58 According to the localized guidelines in China, 32.53%–51.78% of women in this study had an excessive total amount of weight gain, and 11.11%–24.63% of them were inadequate, meanwhile, the weekly rate of weight gain showed similar results. More importantly, over the time from gestation to delivery, abnormal maternal BMI in early pregnancy increased the risk of abnormal body weight gain, and subsequently, the abnormal body weight gain further increased the risk of adverse pregnancy outcomes. Thus, gestational body weight gain could be an intermediate bridge for connecting early maternal BMI and adverse pregnancy outcomes. Several mechanism studies showed that changes in macronutrient metabolism, oxidative status, immune system, and biome homeostasis might play roles in these serial connections.59,60 Besides, we found an interesting phenomenon that inadequate weight gain, not excess of that, was the risk factor for gestational diabetes mellitus, which might suggest that the guidelines of gestational body weight gain for managing this disease need extra attention.
Finally, based on our findings and the above evidence, we suggested that pregnant Chinese women who were obese or overweight should have more energy from carbohydrates (>65%) and less from protein (<10%) and fat (<20%). However, underweight pregnant women were recommended to increase their intake of dietary antioxidants (especially isoflavones) with more energy from fat (>30%) and unsaturated fatty acids (>11%). In the United States, berries and soluble fiber might be beneficial in ameliorating oxidative stress and metabolic complications during pregnancy,61 while we believe that isoflavone-rich foods (such as soybeans) are more crucial and recommended to underweight pregnant women in China.
Because the present research is still in a primary stage and could only provide exploratory results, in the future, we still need a large population with rigorous statistical analysis (such as rational application of Bonferroni correction) to further verify and confirm the links between protein and obesity, as well as low isoflavones intake and maternal underweight. Previous studies62 suggested that red meat (rich in saturated protein, heme iron, and advanced glycation end products)63 as well as metabolites of animal protein (such as branched-chain and aromatic amino acids)64,65 could be related to obesity and serum insulin and might lead to insulin resistance, β-cell failure, and the development of diabetes mellitus via provoking oxidative stress by upregulating iron load.66 However, more underlying mechanisms among dietary characteristics (such as insufficient isoflavones), maternal BMI, gestational body weight gain, and adverse pregnancy outcomes still need to be revealed. For example, whether dietary protein intake could affect hormonal regulation and thus influence obesity is noteworthy. Moreover, although the correlation between poor antioxidative properties with low isoflavone intake and maternal underweight was found, whether there is a unique metabolic need as well as the molecular mechanism of this correlation is still missing puzzles. Furthermore, trying to normalize dietary energy requirements by body weight in further studies on dietary guidelines among the Chinese population might have unexpected findings. Besides, more pivotal food components and phytochemicals should be identified and applied to improve maternal and neonatal health. For example, in our previous study, natural bioactive components (such as theabrownin from dark tea) significantly reversed obesity and alleviated oxidative stress by gut microbial-mediated serotonin signaling pathways.67,68 Whether adding it to the daily diet could benefit pregnant women is still known.
Conclusions
The prevalences of maternal obesity, overweight, and underweight in early pregnancy were 7.51%, 22.07%, and 8.40% in this study, respectively, which showed distinct differences from the situation in Western countries and other developing areas. Less energy from carbohydrates (<50%) but more from protein (>20%) and fat (>30%) were problems related to maternal obesity and overweight. The poor antioxidative diet with a significant 20.28% lower intake of isoflavones as well as imbalanced dietary structure with less energy from fat (<20%) and unsaturated fatty acids (<3%) were problems in maternal underweight. According to the body weight gain guidelines for pregnant Chinese women, gestational body weight gain was the intermediate bridge to connect early maternal BMI and adverse pregnancy outcomes, so it should be monitored throughout pregnancy in terms of total gain amount and weekly gain rate. To reduce the health burden during pregnancy in China, maternal obesity and overweight should have more energy from carbohydrates (>65%) and less from protein (<10%) and fat (<20%). For maternal underweight, increasing the intake of dietary antioxidants (especially isoflavones) with more energy from fat (>30%) and unsaturated fatty acids (>11%) was recommended.Author contributions
Conceptualization, H.-Y. Li; data curation, H.-Y. Li, B.-J. Ding, J. Wang, X.-L. Yang, Z.-W. Ge, N. Wang, Y.-R. Li, Y.-X. Bi, C.-C. Wang, Z.-L. Shi, Y.-X. Wang, Y.-S. Wang, C. Li, and Z.-B. Peng; formal analysis, H.-Y. Li; funding acquisition, H.-Y. Li, B.-J. Ding, and Z.-X. Hong; investigation, H.-Y. Li, B.-J. Ding, and X.-L. Yang; methodology, H.-Y. Li; project administration, B.-J. Ding and Z.-X. Hong; resources, H.-Y. Li, B.-J. Ding, and Z.-X. Hong; software, H.-Y. Li; supervision, B.-J. Ding and Z.-X. Hong; validation, H.-Y. Li; visualization, H.-Y. Li; writing-original draft, H.-Y. Li; writing-review & editing, H.-Y. Li.Data availability
The raw data files have been uploaded to the online ESI† as an Excel file. However, we declare that the raw data for this research can only be accessed and used as supplementary explanation for this paper. For any other purposes (such as secondary analysis), permission must first be obtained from the corresponding author upon reasonable request, and authorization from both the corresponding author and Beijing Friendship Hospital, Capital Medical University, is required.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to express our gratitude to all participants and their families for their support and for accompanying the pregnant women throughout the pregnancy process. We also appreciate the hard work of all nutritionists, obstetricians, nurses, and schoolteachers. This study is supported by Beijing Friendship Hospital, Capital Medical University (Grant No. YYZZ202345), and the Open Project of Hebei Key Laboratory of Environment and Human Health (Grant No. 202302).References
- E. C. Francis, K. Kechris, T. Jansson, D. Dabelea and W. Perng, Novel metabolic subtypes in pregnant women and risk of early childhood obesity in offspring, JAMA Network Open, 2023, 6, e237030 Search PubMed.
- P. M. Catalano and K. Shankar, Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child, BMJ-Brit. Med. J., 2017, 356, j1 CrossRef PubMed.
- A. Ferrara, M. M. Hedderson, S. D. Brown, S. F. Ehrlich, A.-L. Tsai, J. Feng, M. Galarce, S. Marcovina, P. Catalano and C. P. Quesenberry, A telehealth lifestyle intervention to reduce excess gestational weight gain in pregnant women with overweight or obesity (GLOW): a randomised, parallel-group, controlled trial, Lancet Diabetes Endocrinol., 2020, 8, 490–500 CrossRef.
- A. A. Creanga, P. M. Catalano and B. T. Bateman, Obesity in pregnancy, N. Engl. J. Med., 2022, 387, 248–259 CrossRef CAS PubMed.
- E. Rubini, N. Schenkelaars, M. Rousian, K. D. Sinclair, L. Wekema, M. M. Faas, R. P. M. Steegers-Theunissen and S. Schoenmakers, Maternal obesity during pregnancy leads to derangements in one-carbon metabolism and the gut microbiota: implications for fetal development and offspring wellbeing, Am. J. Obstet. Gynecol., 2022, 227, 392–400 CrossRef CAS PubMed.
- Y. Guo, Z. Wang, L. Chen, L. Tang, S. Wen, Y. Liu and J. Yuan, Diet induced maternal obesity affects offspring gut microbiota and persists into young adulthood, Food Funct., 2018, 9, 4317–4327 RSC.
- M. C. Wang, P. M. Freaney, A. M. Perak, P. Greenland, D. M. Lloyd-Jones, W. A. Grobman and S. S. Khan, Trends in prepregnancy obesity and association with adverse pregnancy outcomes in the United States, 2013 to 2018, J. Am. Heart Assoc., 2021, 10, e020717 CrossRef PubMed.
- C. C. Murphy, P. M. Cirillo, N. Y. Krigbaum, A. G. Singal, M. Lee, T. Zaki, E. Burstein and B. A. Cohn, Maternal obesity, pregnancy weight gain, and birth weight and risk of colorectal cancer, Gut, 2022, 71, 1332–1339 Search PubMed.
- M. F. Young and U. Ramakrishnan, Maternal undernutrition before and during pregnancy and offspring health and development, Ann. Nutr. Metab., 2021, 76, 41–53 CrossRef.
- K. A. M. Okesene-Gafa, M. Li, C. J. D. McKinlay, R. S. Taylor, E. C. Rush, C. R. Wall, J. Wilson, R. Murphy, R. Taylor, J. M. D. Thompson, C. A. Crowther and L. M. E. McCowan, Effect of antenatal dietary interventions in maternal obesity on pregnancy weight-gain and birthweight: Healthy Mums and Babies (HUMBA) randomized trial, Am. J. Obstet. Gynecol., 2019, 221, 152.e1–152.e13 Search PubMed.
- J. Louise, A. J. Poprzeczny, A. R. Deussen, C. Vinter, M. Tanvig, D. M. Jensen, A. Bogaerts, R. Devlieger, F. M. McAuliffe, K. M. Renault, E. Carlsen, N. Geiker, L. Poston, A. Briley, S. Thangaratinam and J. M. Dodd, The effects of dietary and lifestyle interventions among pregnant women with overweight or obesity on early childhood outcomes: an individual participant data meta-analysis from randomised trials, BMC Med., 2021, 19, 128 Search PubMed.
- O. Pellonpera, E. Koivuniemi, T. Vahlberg, K. Mokkala, K. Tertti, T. Ronnemaa and K. Laitinen, Dietary quality influences body composition in overweight and obese pregnant women, Clin. Nutr., 2019, 38, 1613–1619 Search PubMed.
- N. R. W. Geiker, F. Magkos, H. Zingenberg, J. Svare, E. Chabanova, H. S. Thomsen, C. Ritz and A. Astrup, A high-protein low-glycemic index diet attenuates gestational weight gain in pregnant women with obesity: the “An optimized programming of healthy children” (APPROACH) randomized controlled trial, Am. J. Clin. Nutr., 2022, 115, 970–979 CrossRef CAS PubMed.
- M. Kebbe, J. Most, A. D. Altazan and L. M. Redman, No strong evidence of the protein leverage hypothesis in pregnant women with obesity and their infants, Obesity, 2023, 31, 2057–2064 CrossRef CAS PubMed.
- N. Houttu, T. Vahlberg, E. A. Miles, P. C. Calder and K. Laitinen, The impact of fish oil and/or probiotics on serum fatty acids and the interaction with low-grade inflammation in pregnant women with overweight and obesity: secondary analysis of a randomised controlled trial, Br. J. Nutr., 2023, 131, 296–311 CrossRef PubMed.
- J. Lin, F. Yang, M. Lan, Y. Ding and K. Yin, Adhere to the Chinese dietary guidelines associated with better subjective well-being: evidence from a cross-sectional survey and a daily diary investigation, BMC Public Health, 2024, 24, 445 Search PubMed.
- Y. Zhu, Y. Zhang and X. Zhu, The evolution process, characteristics and adjustment of Chinese dietary guidelines: A global perspective, Resour., Conserv. Recycl., 2023, 193, 106964 Search PubMed.
- Chinese Nutrition Society, Dietary guidelines for Chinese residents, People's Medical Publishing House, Beijing, 2022 Search PubMed.
- Chinese Nutrition Society, Dietary reference intakes for China, People's Medical Publishing House, Beijing, 2023 Search PubMed.
- E. A. Nohr, M. Vaeth, J. L. Baker, T. I. A. Sørensen, J. Olsen and K. M. Rasmussen, Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy, Am. J. Clin. Nutr., 2008, 87, 1750–1759 CrossRef CAS PubMed.
- R. F. Goldstein, S. K. Abell, S. Ranasinha, M. Misso, J. A. Boyle, M. H. Black, N. Li, G. Hu, F. Corrado, L. Rode, Y. J. Kim, M. Haugen, W. O. Song, M. H. Kim, A. Bogaerts, R. Devlieger, J. H. Chung and H. J. Teede, Association of gestational weight gain with maternal and infant outcomes, JAMA, J. Am. Med. Assoc., 2017, 317, 2207–2225 CrossRef PubMed.
- J. A. Gavard, Gestational weight gain and maternal and neonatal outcomes in underweight pregnant women: A population-based historical cohort study, Matern. Child Health J., 2017, 21, 1203–1210 CrossRef PubMed.
- C. S. Mogensen, H. Zingenberg, J. Svare, A. Astrup, F. Magkos and N. R. W. Geiker, Gestational weight gain in women with pre-pregnancy overweight or obesity and anthropometry of infants at birth, Front. Pediatr., 2023, 11, 1142920 Search PubMed.
- H. J. Teede, R. Goldstein and C. Harrison, Comparison of Chinese vs. US gestational weight gain guidelines for Chinese women, JAMA Network Open, 2022, 5, e2233256 CrossRef PubMed.
- F. Chen, P. Wang, J. Wang, Z. Liao, X. Zong, Y. Chen, J. Lai, T. Zhang, G. Liu and X. Xie, Analysis and comparison of early childhood nutritional outcomes among offspring of Chinese women under the Chinese 2021 and US 2009 gestational weight gain guidelines, JAMA Network Open, 2022, 5, e2233250 CrossRef.
- A. M. Stuebe, E. Oken and M. W. Gillman, Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain, Am. J. Obstet. Gynecol., 2009, 201, 58.e1–58.e8 Search PubMed.
- C.-X. Zhang, J.-Q. Lai, K.-Y. Liu, N.-H. Yang, G. Zeng, L.-M. Mao, Z.-N. Li, Y. Teng, W. Xia, N. Dai, Z.-X. Wang and Y.-X. Su, Optimal gestational weight gain in Chinese pregnant women by Chinese-specific BMI categories: a multicentre prospective cohort study, Public Health Nutr., 2021, 24, 3210–3220 Search PubMed.
- X. Xie, B. H. Kong and T. Duan, Obstetrics and gynecology, People's Medical Publishing House, Beijing, 2018 Search PubMed.
- C. Li, Y. Li, N. Wang, Z. Ge, J. Wang, B. Ding, Y. Bi, Y. Wang, Y. Wang, Z. Peng, X. Yang, C. Wang and Z. Hong, Comprehensive modulatory effects of whole grain consumption on immune-mediated inflammation in middle-aged and elderly community residents: A real-world randomized controlled trial, Redox Biol., 2024, 76, 103337 CrossRef CAS PubMed.
- National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention, China food composition table, Peking University Medical Press, Beijing, 2018 Search PubMed.
- W. T. Pan, S. Karatela, Q. G. Lu, L. Q. Xie, S. C. Wu, J. Jing and L. Cai, Association of diet quality during pregnancy with maternal glucose metabolism in Chinese women, Br. J. Nutr., 2023, 130, 958–965 CrossRef CAS.
- H. N. Luu, W. Wen, H. Li, Q. Dai, G. Yang, Q. Cai, Y.-B. Xiang, Y.-T. Gao, W. Zheng and X.-O. Shu, Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels?, Antioxid. Redox Signal., 2015, 22, 951–959 Search PubMed.
- D. O. Mook-Kanamori, E. A. P. Steegers, P. H. Eilers, H. Raat, A. Hofman and V. W. V. Jaddoe, Risk factors and outcomes associated with first-trimester fetal growth restriction, JAMA, J. Am. Med. Assoc., 2010, 303, 527–534 CrossRef CAS PubMed.
- S. C. Bath, C. D. Steer, J. Golding, P. Emmett and M. P. Rayman, Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC), Lancet, 2013, 382, 331–337 CrossRef CAS PubMed.
- K. Zhu, J. Wactawski-Wende, P. Mendola, N. I. Parikh, M. J. Lamonte, V. M. Barnabei, R. H. Blair, J. E. Manson, S. Liu, M. Wang, R. A. Wild, A. H. Shadyab, L. Van Horn, E. S. Leblanc, R. Sinkey, P. F. Schnatz, N. Saquib and L. Mu, Adverse pregnancy outcomes and risk of type 2 diabetes in postmenopausal women, Am. J. Obstet. Gynecol., 2024, 230, 93.e1–93.e19 Search PubMed.
- A. J. Gaskins, F. L. Nassan, Y.-H. Chiu, M. Arvizu, P. L. Williams, M. G. Keller, I. Souter, R. Hauser and J. E. Chavarro, Dietary patterns and outcomes of assisted reproduction, Am. J. Obstet. Gynecol., 2019, 220, 567.e1–567.e18 Search PubMed.
- Y. Li and H. Zhang, Soybean isoflavones ameliorate ischemic cardiomyopathy by activating Nrf2-mediated antioxidant responses, Food Funct., 2017, 8, 2935–2944 Search PubMed.
- M.-S. Kim, Y. S. Jung, D. Jang, C. H. Cho, S.-H. Lee, N. S. Han and D.-O. Kim, Antioxidant capacity of 12 major soybean isoflavones and their bioavailability under simulated digestion and in human intestinal Caco-2 cells, Food Chem., 2022, 374, 131493 Search PubMed.
- C. Piernas, M. Patone, N. M. Astbury, M. Gao, A. Sheikh, K. Khunti, M. Shankar-Hari, S. Dixon, C. Coupland, P. Aveyard, J. Hippisley-Cox and S. A. Jebb, Associations of BMI with COVID-19 vaccine uptake, vaccine effectiveness, and risk of severe COVID-19 outcomes after vaccination in England: a population-based cohort study, Lancet Diabetes Endocrinol., 2022, 10, 571–580 Search PubMed.
- S. Zewdie, S. G. Fage, A. K. Tura and F. Weldegebreal, Undernutrition among pregnant women in rural communities in Southern Ethiopia, Int. J. Womens Health, 2021, 13, 73–79 CrossRef.
- P. M. Catalano and G. O. Koutrouvelis, Obesity in pregnancy: ACOG practice bulletin, number 230, Obstet. Gynecol., 2021, 137, E128–E144 CrossRef PubMed.
- R. Shindo, M. Aoki, Y. Yamamoto, T. Misumi, E. Miyagi and S. Aoki, Optimal gestational weight gain for underweight pregnant women in Japan, Sci. Rep., 2019, 9, 18129 Search PubMed.
- S. A. L. Price, P. Sumithran, A. J. Nankervis, M. Permezel, L. A. Prendergast and J. Proietto, Impact of preconception weight loss on fasting glucose and pregnancy outcomes in women with obesity: A randomized trial, Obesity, 2021, 29, 1445–1457 Search PubMed.
- M. Charnley, L. Newson, A. Weeks and J. Abayomi, Pregnant women living with obesity: A cross-sectional observational study of dietary quality and pregnancy outcomes, Nutrients, 2021, 13, 1652 Search PubMed.
- J. Liu, S. K. C. Chang and D. Wiesenborn, Antioxidant properties of soybean isoflavone extract and tofu in vitro and in vivo, J. Agric. Food Chem., 2005, 53, 2333–2340 CrossRef CAS.
- D. M. Balisteiro, C. V. Rombaldi and M. I. Genovese, Protein, isoflavones, trypsin inhibitory and in vitro antioxidant capacities: Comparison among conventionally and organically grown soybeans, Food Res. Int., 2013, 51, 8–14 Search PubMed.
- X. Yu, M. Meenu, B. Xu and H. Yu, Impact of processing technologies on isoflavones, phenolic acids, and antioxidant capacities of soymilk prepared from 15 soybean varieties, Food Chem., 2021, 345, 128612 CrossRef CAS PubMed.
- M. G. Semenova, A. S. Antipova, E. I. Martirosova, S. A. Chebotarev, N. P. Palmina, N. G. Bogdanova, N. I. Krikunova, D. V. Zelikina, M. S. Anokhina and V. V. Kasparov, The relationship between the structure and functionality of essential PUFA delivery systems based on sodium caseinate with phosphatidylcholine liposomes without and with a plant antioxidant: an in vitro and in vivo study, Food Funct., 2022, 13, 2354–2371 Search PubMed.
- G. Li, Y. Li, B. Xiao, D. Cui, Y. Lin, J. Zeng, J. Li, M.-J. Cao and J. Liu, Antioxidant activity of docosahexaenoic acid (DHA) and its regulatory roles in mitochondria, J. Agric. Food Chem., 2021, 69, 1647–1655 CrossRef CAS PubMed.
- B. Xiao, Y. Li, Y. Lin, J. Lin, L. Zhang, D. Wu, J. Zeng, J. Li, J. w. Liu and G. Li, Eicosapentaenoic acid (EPA) exhibits antioxidant activity via mitochondrial modulation, Food Chem., 2022, 373, 131389 CrossRef CAS PubMed.
- S. A. Simpson, E. Coulman, D. Gallagher, K. Jewell, D. Cohen, R. G. Newcombe, C. Huang, J. A. Robles, M. Busse, E. Owen-Jones, D. Duncan, N. Williams, H. Stanton, A. Avery, E. McIntosh and R. Playle, Healthy eating and lifestyle in pregnancy (HELP): A cluster randomised trial to evaluate the effectiveness of a weight management intervention for pregnant women with obesity on weight at 12 months postpartum, Int. J. Obes., 2021, 45, 1728–1739 Search PubMed.
- E. Huvinen, A. K. Tuomaala, P. H. Bergman, J. Meinila, T. Tammelin, J. Kulmala, E. Engberg and S. B. Koivusalo, Ascending growth is associated with offspring adiposity in pregnancies complicated with obesity or gestational diabetes, J. Clin. Endocrinol. Metab., 2021, 106, E1993–E2004 Search PubMed.
- J. M. Petersen, J. A. Hutcheon, L. M. Bodnar, S. E. Parker, K. A. Ahrens and M. M. Werler, Weight gain patterns among pregnancies with obesity and small- and large-for-gestational-age births, Obesity, 2023, 31, 1133–1145 CrossRef CAS PubMed.
- D. S. Feig, L. E. Donovan, R. Corcoy, K. E. Murphy, S. A. Amiel, K. F. Hunt, E. Asztalos, J. F. R. Barrett, J. J. Sanchez, A. de Leiva, M. Hod, L. Jovanovic, E. Keely, R. McManus, E. K. Hutton, C. L. Meek, Z. A. Stewart, T. Wysocki, R. O'Brien, K. Ruedy, C. Kollman, G. Tomlinson and H. R. Murphy, Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial, Lancet, 2017, 390, 2347–2359 Search PubMed.
- J. M. Dodd, J. Louise, A. R. Deussen, R. M. Grivell, G. Dekker, A. J. McPhee and W. Hague, Effect of metformin in addition to dietary and lifestyle advice for pregnant women who are overweight or obese: the GRoW randomised, double-blind, placebo-controlled trial, Lancet Diabetes Endocrinol., 2019, 7, 15–24 CrossRef CAS PubMed.
- K. M. Rasmussen and A. L. Yaktine, Weight gain during pregnancy: Reexamining the guidelines, National Academies Press, Washington, DC, 2009 Search PubMed.
- S. Wu, J. N. Jin, K. L. Hu, Y. Q. Wu and D. Zhang, Prevention of gestational diabetes mellitus and gestational weight gain restriction in overweight/obese pregnant women: A systematic review and network meta-analysis, Nutrients, 2022, 14, 2383 CrossRef PubMed.
- Chinese Nutrition Society, Weight monitoring and evaluation during pregnancy period of Chinese women, Chinese Nutrition Society, 2021 Search PubMed.
- S. Rastogi and D. Rastogi, The epidemiology and mechanisms of lifetime cardiopulmonary morbidities associated with pre-pregnancy obesity and excessive gestational weight gain, Front. Cardiovasc. Med., 2022, 9, 844905 Search PubMed.
- D. Alvarez, Y. Munoz, M. Ortiz, M. Maliqueo, R. Chouinard-Watkins and R. Valenzuela, Impact of maternal obesity on the metabolism and bioavailability of polyunsaturated fatty acids during pregnancy and breastfeeding, Nutrients, 2021, 13, 19 CrossRef CAS PubMed.
- A. Basu, J. Crew, J. L. Ebersole, J. W. Kinney, A. M. Salazar, P. Planinic and J. M. Alexander, Dietary blueberry and soluble fiber improve serum antioxidant and adipokine biomarkers and lipid peroxidation in pregnant women with obesity and at risk for gestational diabetes, Antioxidants, 2021, 10, 1318 CrossRef CAS PubMed.
- T. Y. Luo, H. Y. Chen, H. X. Wei, Y. L. Yang, F. X. Wei and W. Q. Chen, Dietary protein in early pregnancy and gestational diabetes mellitus: a prospective cohort study, Endocrine, 2023, 83, 357–367 CrossRef PubMed.
- W. Bao, Y. Rong, S. Rong and L. Liu, Dietary iron intake, body iron stores, and the risk of type 2 diabetes: a systematic review and metaanalysis, BMC Med., 2012, 10, 119 CrossRef CAS PubMed.
- A. Floegel, N. Stefan, Z. Yu, K. Mühlenbruch, D. Drogan, H.-G. Joost, A. Fritsche, H.-U. Häring, M. Hrabě de Angelis, A. Peters, M. Roden, C. Prehn, R. Wang-Sattler, T. Illig, M. B. Schulze, J. Adamski, H. Boeing and T. Pischon, Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach, Diabetes, 2013, 62, 639–648 Search PubMed.
- T. J. Wang, M. G. Larson, R. S. Vasan, S. Cheng, E. P. Rhee, E. McCabe, G. D. Lewis, C. S. Fox, P. F. Jacques, C. Fernandez, C. J. O'Donnell, S. A. Carr, V. K. Mootha, J. C. Florez, A. Souza, O. Melander, C. B. Clish and R. E. Gerszten, Metabolite profiles and the risk of developing diabetes, Nat. Med., 2011, 17, 448–453 Search PubMed.
- R. C. Cooksey, H. A. Jouihan, R. S. Ajioka, M. W. Hazel, D. L. Jones, J. P. Kushner and D. A. McClain, Oxidative stress, β-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis, Endocrinology, 2004, 145, 5305–5312 Search PubMed.
- H.-Y. Li, S.-Y. Huang, R.-G. Xiong, S.-X. Wu, D.-D. Zhou, A. Saimaiti, M. Luo, H.-L. Zhu and H.-B. Li, Anti-obesity effect of theabrownin from dark tea in C57BL/6J mice fed a high-fat diet by metabolic profiles through gut microbiota using untargeted metabolomics, Foods, 2022, 11, 3000 Search PubMed.
- H.-Y. Li, S.-Y. Huang, D.-D. Zhou, R.-G. Xiong, M. Luo, A. Saimaiti, M.-K. Han, R.-Y. Gan, H.-L. Zhu and H.-B. Li, Theabrownin inhibits obesity and non-alcoholic fatty liver disease in mice via serotonin-related signaling pathways and gut-liver axis, J. Adv. Res., 2023, 52, 59–72 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo06451h |
| This journal is © The Royal Society of Chemistry 2025 |