 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Investigating the hypoglycaemic potential of processed apple and acarbose combination in vitro, ex vivo, and in vivo: the role of quercetin-3-glucoside in steering α-glucosidase inhibition
Umberto
Lanza
 a,
Marilisa
Alongi
a,
Marilisa
Alongi
 *a,
Barbara
Frossi
b,
Carlo
Pucillo
b,
Monica
Anese
a and
Maria Cristina
Nicoli
a
*a,
Barbara
Frossi
b,
Carlo
Pucillo
b,
Monica
Anese
a and
Maria Cristina
Nicoli
a
aDepartment of Agricultural, Food, Environmental and Animal Sciences, University of Udine, Udine, Italy. E-mail: marilisa.alongi@uniud.it
bDepartment of Medicine, University of Udine, Udine, Italy
First published on 3rd February 2025
Abstract
This study investigated the interaction between apple juice (AJ) and acarbose (A) in modulating glycaemic responses, with the aim of validating in vivo results previously observed in vitro. When administered to rats, AJ alone reduced the glycemic curve, but the combination of AJ with increasing doses of A resulted in higher glycemic responses, suggesting an antagonistic interaction in α-glucosidase inhibition. To explore this mechanism, quercetin-3-glucoside (Q-3-G), a major phenolic compound in AJ, was tested for α-glucosidase inhibition in vitro. Q-3-G and A together showed reduced inhibitory efficacy compared to either compound alone, consistent with in vivo findings. Ex vivo studies in Caco-2 cells further supported this antagonism. Sucrose hydrolysis experiments showed that low concentrations of Q-3-G increased residual sucrose when combined with moderate concentrations of A, but higher concentrations of Q-3-G favoured sucrose hydrolysis regardless of A levels. The results highlight the antagonistic interaction between Q-3-G and A in inhibiting α-glucosidase and emphasise the need to combine in vitro, ex vivo and in vivo studies to evaluate food-drug interactions. This comprehensive approach is essential before advocating the use of functional foods alongside pharmacological therapies.
Introduction
Type 2 diabetes mellitus (T2DM) is one of the major global health burdens, affecting a large number of people worldwide, and greatly increasing the risk of a variety of complications, including cardiovascular disease, neuropathy, and kidney damage.1 T2DM is characterised by insulin resistance along with impaired glucose metabolism, leading to chronic hyperglycaemia, which requires effective management strategies to maintain glycaemic control and prevent long-term health problems. One of the critical points of control in T2DM patients is the postprandial glucose level. There is a rise in blood sugar following a meal; therefore, modulation of postprandial hyperglycaemia is important to reduce the overall burden of diabetes.2Among the various strategies that have been used to control postprandial glucose levels, one is the inhibition of carbohydrate-digesting enzymes, in particular α-glucosidase. This enzyme breaks down complex carbohydrates into simple sugars, which can be absorbed by the intestinal brush border membrane of the intestine. Current pharmaceutical interventions make use of acarbose, a well-documented α-glucosidase inhibitor, which has been used to reduce postprandial glucose spikes.3 However, long-term use of acarbose can have several unwanted gastrointestinal side effects, such as bloating, cramping, and flatulence, which can affect a patient's compliance with prescribed treatment regimens.4
In recent years, there has been a growing interest in the potential of bioactive compounds in plant food to help reduce the incidence of T2DM. In particular, polyphenols have shown an antihyperglycemic effect. In fact, polyphenols may exert antidiabetic effects via their ability to inhibit human amylin (hA) aggregation and to modulate oxidative stress, inflammation, and other pathways that are β-cell-protective or insulin-sensitizing.5 Among phenolic compounds, the most promising are the flavonoid glycosides, such as quercetin-3-glucoside, which have gained particular interest due to their documented activity against α-glucosidase.6,7 Due to their antidiabetic potential, phenolic compounds are interesting candidates for therapeutic application either alone or as adjuvants in the treatment of diabetes.8,9 Combining polyphenolic compounds with traditional pharmacological agents presents a new avenue in the treatment of T2DM that may have a dual impact by enhancing therapeutic efficacy and reducing drug doses and related adverse effects.
Most plant foods are consumed as derivatives rather than in their native form. The technological interventions used to obtain these derivatives can induce complex changes in the composition, especially with regard to the bioactive compounds. Therefore, processing can greatly affect the possible antidiabetic potential.10
Among plant-based foods, apples are the most consumed fruit worldwide with apple juice being the most consumed derivative.11 The production of apple juice involves several technological interventions, including pasteurisation, which have a significant impact on phenolic content.12 and thus on potential health benefits.
In this context, a previous study showed how apple juice and acarbose were able to synergistically inhibit α-glucosidase.13 in a specific concentration range. However, the results in this study were obtained with an in vitro approach, which alone cannot be considered reliable to conclude on the efficacy of apple juice and acarbose combination for T2DM management. Conversely, further studies using more complex in vitro assays as well as in vivo trials would be required to prove the intervention. Furthermore, apple juice is a highly complex food matrix since it contains many bioactive compounds.13 These include certain compounds that are known for possessing α-glucosidase inhibitory activity.7 Due to the complexity of the matrix considered it was not possible to determine which of these compounds contributed the most to the observed effect.
In light of these considerations, the present study aimed to investigate the potential hypoglycaemic effect of combining apple juice with acarbose, focusing on the role of quercetin-3-glucoside (Q-3-G), one of the most bioaccessible phenolic compounds in apple juice able to effectively inhibit α-glucosidase.9,14 First, the effects of apple juice and acarbose on the glycaemic response were assessed in vivo using rat models to confirm the results previously obtained in vitro.13 Subsequently, to elucidate the results obtained in vivo, the α-glucosidase inhibitory activity of Q-3-G, acarbose, and their combination was evaluated both in vitro and in an ex vivo cellular assay.
Materials and methods
Chemicals and materials
Quercetin-3-glucoside, acarbose, sucrose, Dulbecco's Modified Eagle Medium (DMEM) high glucose, Fetal Bovine Serum (FBS), Dimethyl Sulfoxide (DMSO), nitrophenyl-α-D-glucopyranoside, α-glucosidase (from Saccharomyces cerevisiae) were purchased from Sigma Aldrich, Milano, Italy. DMEM w/o glucose was purchased from Gibco, USA. ThinCert® polyester insert plates were purchased from Greiner Bio-One International GmbH. K-SUFRG enzymatic kit was purchased from Megazyme International (Bray, Wicklow County, Ireland).Commercial apple juice (Mela limpida 100%, Skipper-Zuegg, Verona, Italy) was purchased on the local market. Apple juice contained 57.3 mg mL−1 fructose, 26.3 mg mL−1 glucose and 12.6 mg mL−1 sucrose.15
In vivo study of apple juice-acarbose interaction
The experimental setup was designed based on a previous study.15 Young adult (six-week-old) male Wistar rats (Rattus norvegicus, n = 20) weighing 242 ± 12 g were obtained from Envigo RMS Srl. They were housed in wire-bottomed cages in a room with controlled temperature (25° C) and lighting (12 h light/dark cycle) and had free access to water and to a commercial diet (Envigo RMS Srl) for 1 week. All procedures were carried out according to the guidelines enforced in Italy (D. lgs 116/1992) and in compliance with the guide of the National Research Council (National Research Council, 2011), upon approval by the Italian Committee for Bioethics (no. 196/2019-PR del 6/3/2019, D. lgs 26/2014).After 15 h fasting, apple juice combined with different dosages of acarbose was orally administered to rats. In particular, aliquots of 3.6 mL juice were administered based on the available carbohydrate content. Considering apple juice sugar concentration, this was equal to 1.58 g per kgbw, which is the concentration recommended to test the glycaemic response in rat models.16 Acarbose dosage corresponded to 32.28 mg per kgbw and was chosen based on the typical human daily dose of acarbose (300 mg per 60 kgbw) and according to the conversion factor reported by Nair et al.17 Similar to what was reported by Zhang et al.,18 two additional dosages were selected, corresponding respectively to 6.46 and 3.23 mg kg−1.
Blood samples were collected from tail veins at 0 (prior to the administration), 15, 30, 45, 60, 90 and 120 min (after the administration) to assay plasma glucose concentration employing Accu-Chek® glucometer (Roche Diabetes Care Italy S.p.A., Monza, Italy).16 Results were expressed as mean ± SEM in mmol L−1 and plotted against time.
α-Glucosidase in vitro inhibitory activity
Quercetin-3-glucoside and acarbose α-glucosidase inhibitory activity were assessed spectrophotometrically with a microplate reader (Synergy H1, BioTek, Vermont, USA) following the method of Singh et al.19 with some modifications. Q-3-G was solubilised in DMSO to obtain a 30 mg mL−1 stock solution. Acarbose was solubilised in potassium phosphate buffer to obtain a 6 mg mL−1 stock solution. α-Glucosidase from Saccharomyces cerevisiae was solubilized in potassium phosphate buffer 0.1 M, pH 7 to obtain a 1 U mL−1 stock solution. The assay was carried out in a 96-well polyester microplate. Acarbose (from 20 to 1200 μg mL−1, corresponding to a range of 1 to 70 μL of stock solution) and Q-3-G (from 1 to 65 μg mL−1, corresponding to a range of 1 to 6 μL of stock solution) were added with 10 μL of α-glucosidase and the volume was made up to 270 μL with potassium phosphate buffer 1 M, pH 7. The plate was incubated at 37 °C for 10 minutes. After incubation, 30 μL of 100 mM nitrophenyl-α-D-glucopyranoside substrate solution was added and the absorbance at 420 nm was recorded every 30 s for 15 minutes. The evolution of the absorbance was plotted against time and the kinetic constant was calculated with a linear regression model for both control (without the inhibitors) and quercetin-3-glucoside or acarbose assay wells. The % of inhibition was calculated using eqn (1) where ks and kc were the kinetic constants calculated in the presence and the absence of the inhibitors, respectively. Results were reported as % of inhibition against inhibitor concentration (means ± standard deviation of three different biological replicates). | (1) |
The method proposed by Chou20 was used, with some modifications, to investigate quercetin and acarbose interaction towards α-glucosidase inhibition. Combined systems of quercetin-3-glucoside and acarbose with a proportionally increasing concentration of both Q-3-G and acarbose were obtained according to eqn (2):
| Fmn × [(Dm)q + (Dm)a] = (Cn)q,a | (2) |
where Fmn represents a multiplicative factor, (Dm)q and (Dm)a are the doses of quercetin-3-glucoside and acarbose able to produce a 30% α-glucosidase inhibition (i.e., IC30) and (Cn)q,a is the total concentration of quercetin-3-glucoside and acarbose in the combined system. Six combined systems were obtained by substituting six different multiplicative factors (i.e., 0.25; 0.5; 1.5; 2; 2.5; 3.5) in eqn (2). The combined systems were tested for their ability to inhibit α-glucosidase and the inhibition percentage was plotted against the concentration. The sum of quercetin-3-glucoside and acarbose doses (Dx)q,a corresponding to an effect x was thus determined, and the relevant single doses of quercetin-3-glucoside and acarbose were calculated by eqn (3) and (4), respectively:
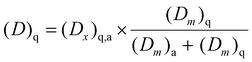 | (3) |
 | (4) |
The Combination Index (CI) was finally calculated by eqn (5):
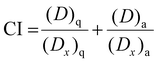 | (5) |
Caco-2 cell cultures
Caco-2 cells (ATCC HTB-37) from passages 30 to 40 were cultured and maintained in DMEM containing 10% v/v FBS, non-essential amino acids (1% v/v), 100 U mL−1 penicillin and 100 μg mL−1 streptomycin in 75 cm2 plastic flasks. Cells were seeded in 0.4 μm ThinCert® polyester insert plates. The cell density was 2.6 × 105 cells per cm2. The cells were grown and differentiated for 21 days on ThinCert® inserts under a humidified atmosphere of air, 5% CO2 and at 37 °C before being used for experiments. Culture media in the upper and lower culture chambers was replaced every 2–3 days. After 21 days, Caco-2 cells form a tight monolayer resembling human intestinal epithelium expressing brush border digestive enzymes with α-glucosidase activity.21α-Glucosidase inhibitory activity assessment in Caco-2 cells
After differentiation, Caco-2 cells grown on ThinCert® inserts were starved for 2 hours in DMEM w/o glucose + 10% FBS. Then, DMEM w/o glucose, supplemented with 110 mM sucrose as a glucose source, was added to the upper chamber of the insert in the presence of different concentrations of Q-3-G and acarbose. Acarbose and Q-3-G were first dissolved in DMSO to obtain 2 mM and 0.8 mM stock solutions, respectively. These stock solutions were then added to the culture media to reach final concentrations of 0.5, 5, 25, and 50 μM for acarbose, and 2 and 25 μM for Q-3-G, maintaining a final volume of 4 mL per condition. The volumes added for each condition were as follows: for acarbose (0.5–50 μM), 1 μL (0.5 μM), 10 μL (5 μM), 50 μL (25 μM), and 100 μL (50 μM) of the 2 mM stock were added; for Q-3-G (2–25 μM), 10 μL (2 μM), 50 μL (10 μM), 125 μL (25 μM) of the 0.8 mM stock were added. The final DMSO concentration in the culture media never exceeded 0.5% v/v. Of these prepared solutions, 100 μL were added to the culture inserts. Over 1 or 2 hours, 10 μL samples of the culture media in the upper chamber were collected at different times, and sucrose concentration was assayed by using the K-SUFRG enzymatic kit to assess possible differences in the amount of hydrolysed sucrose in the differently treated cells. The results are expressed as the percentage of sucrose remaining in the culture media at each time point relative to the initial sucrose concentration at time t0.Statistical analysis
Results are expressed as averages of at least three measurements carried out on two replicated samples and are reported as means ± standard deviation. Inhibition curve fitting was performed using R (version 3.2.3, The R Foundation for Statistical Computing, Vienna, Austria) by employing nlstools package.22Results and discussion
Effect of apple juice and acarbose combination on the glycaemic response in vivo
A previous study from our laboratory13 investigated the antihyperglycemic properties of apple juice. More specifically, apple juice inhibits α-glucosidase activity in vitro. The study also examined the combined effect of apple juice with the well-known antihyperglycemic drug acarbose, discovering both a synergistic and an antagonistic behaviour dependent on the combination of concentrations. In particular, the apple juice-acarbose combined system exhibited an effect up to 40% α-glucosidase inhibition, whereas higher concentrations led to an antagonistic behaviour.While these results are promising from the perspective of reducing drug dosage and thus the related undesired side effects, their validation through in vivo experiments is crucial.
To test this, we looked at how well apple juice and acarbose worked together in real life in our study. Specifically, we administered rats different doses of acarbose combined with apple juice by mouth and monitored their blood sugar for up to 120 minutes after they received the samples (Fig. 1). Rats fed with apple juice showed a reduced glycaemic response compared to those fed with glucose. Specifically, glucose caused a glycaemic peak after 45 min from ingestion, while apple juice did not cause any glycaemic peak. A higher glycaemic curve was previously observed by feeding healthy rats with a sugar solution mimicking apple juice composition, instead of apple juice.15 This effect was attributed to apple juice bioactive compounds, such as phenolic ones.
Although the same amount of available carbohydrates was administered to rats, the differences in the glycaemic profile may be attributed to: (i) apple juice did not contain only glucose, that is the most readily absorbable sugar, but also fructose and sucrose as the main sugars representing the available carbohydrate fraction;13 (ii) apple juice contains phenolic compounds able to inhibit α-glucosidase, which is one of the key enzymes responsible for carbohydrate hydrolysis.7 When apple juice was combined with the lowest dose of acarbose considered in the present study (3.23 mg kg−1), the glycaemic response was slightly higher compared to what was observed for apple juice alone. Higher doses of acarbose, namely 6.46 and 32.28 mg kg−1, further increased the glycaemic response. This trend suggests the possible existence of an antagonistic relationship between acarbose and apple juice in modulating the glycaemic response.
To date, no in vivo studies regarding the potential combined effect of acarbose and apple juice have been conducted.23
Nevertheless, apple juice is a highly complex matrix, containing a huge number of bioactive compounds that may affect the glycaemic response.
Effect of quercetin and acarbose combination on α-glucosidase in vitro inhibition
To shed light on the results acquired in vivo (Fig. 1), a top-down approach was followed.Specifically, we focussed on a single phenolic compound, namely quercetin-3-glucoside (Q-3-G). Q-3-G was selected based on its high bioaccessibility in apple derivatives15,24 and considering its α-glucosidase inhibitory activity.7
An in vitro experiment was thus carried out to test the interactive behaviour between acarbose and Q-3-G in inhibiting α-glucosidase. The results relevant to α-glucosidase inhibitory activity of acarbose (A), quercetin-3-glucoside (Q-3-G) and their combined system (A + Q-3-G) are shown in Fig. 2.
Fig. 2 also shows the inhibitory capacity of Q-3-G as a function of its concentration, which assumed a bell-shaped curve. It must be noticed that the maximum inhibition carried out by Q-3-G barely reached 45% at 30 μg mL−1. Nevertheless, in the inhibition range from 0 to 45%, Q-3-G was more effective in inhibiting α-glucosidase compared to acarbose. However, when Q-3-G concentrations increased above 40 μg mL−1, the opposite effect was obtained.
The combination of acarbose and Q-3-G resulted in a decreased efficacy of α-glucosidase inhibition compared to Q-3-G and acarbose alone (Fig. 2), supporting the hypothesis of an antagonistic behaviour between the two compounds and in line with in vivo observations (Fig. 1). Interestingly, the shape of the inhibition curve of the combined system is similar to the one obtained with Q-3-G alone, suggesting a “leading” role of Q-3-G in determining the observed effect. The inhibition range observed for Q-3-G was in agreement with the results of Barber et al.7 up to a concentration of 30 μg mL−1. Above such a value, these authors observed a further increase in the inhibition, while our results showed a progressive decrease. However, it must be pointed out that Barber et al. (2021)7 considered the quercetin aglycone. Other authors reported on the reduced efficacy of Q-3-G compared to the aglycone.25 Such a reduction was attributed to the glycosylation of the hydroxyl group, namely the substitution of the hydroxyl moiety with a glycoside.26 In particular, glycosylation increased the molecular size, polarity, and non-planar structure of the compound, resulting in a higher steric hindrance that probably weakened the binding capacity to α-glucosidase and therefore reduced the inhibition efficacy.25
Nevertheless, it must be pointed out that quercetin is typically found in plant-based food, and thus in apple and its derivatives, in its glycosylated form rather than as an aglycone and thus it is expected to be mainly consumed in this form.27
Since in a previous study a synergistic behaviour was observed between acarbose and apple juice,13 we further investigated this behaviour following the same approach,20 but considering a simplified model system. In particular, we used Q-3-G, selected as one of the most bioaccessible compounds possessing α-glucosidase inhibitory capacity, instead of apple juice and explored its possible interactions with acarbose. Briefly, this approach requires to obtain the IC50 (half-maximal inhibitory concentration) of the compounds under investigation and to use these values to produce combined systems at increasing concentration based on multiplicative factors. To this purpose, the experimental data relevant to acarbose and Q-3-G (Fig. 2) were fitted to obtain the equations (Table 1) able to describe the inhibitory capacity as a function of concentration.
| Equation | A | Q-3-G | Q-3-G + A |
|---|---|---|---|
| Parameter | y = a + bx + cx1.5 + d√x | y = ax3 + b√x + cx | a + bx + cx1.5 + dx2 + e√x |
| a | −1.1057 | −0.0001 | 0.0601 |
| b | −0.2186 | 13.2907 | −1.3787 |
| c | 0.0022 | −0.8679 | 0.2740 |
| d | 7.2019 | n.d. | −0.0207 |
| e | n.d. | n.d. | 6.4640 |
Since Q-3-G was not able to induce α-glucosidase inhibition higher than 50%, instead of determining the IC50, as required by Chou,20 the IC30, i.e., the dose necessary to obtain 30% of inhibition, was determined for acarbose and Q-3-G, and corresponded to 25.53 μg mL−1 and 6.86 μg mL−1, respectively. Therefore, the IC30 was used instead of the IC50 to produce the combined systems that were tested against α-glucosidase (Fig. 2).
To gain more insights regarding the possible antagonistic behaviour towards α-glucosidase inhibition between acarbose and Q-3-G, we calculated the combination index (CI) from the inhibition data of the combined system,20 and results are shown in Fig. 3. The CI provides an indication of the interaction between different compounds in the overall enzyme inhibition range. In particular, CI > 1 indicates antagonistic effect, CI < 1 means synergic effect and CI = 1 stands for additive effect.
According to CI values, antagonism towards α-glucosidase inhibition between acarbose and Q-3-G prevailed (Fig. 3), in line with our hypothesis.
These results partially agree with what observed in our previous study,13 in which however antagonism between acarbose and apple juice only occurred for α-glucosidase inhibition values above 40%.
Effect of quercetin and acarbose combination on sucrose hydrolysis in a cell model
To better understand the impact of acarbose and Q-3-G on α-glucosidase inhibition, and thus on sugar absorption, we employed a cellular model (i.e., Caco-2 cells) to resemble in vitro the intestinal brush border membrane. Caco-2 cells were first incubated in DMEM without glucose and then treated with 110 mM sucrose, alone or with different concentrations of acarbose (0.5, 5, 25, 50 μM), Q-3-G (2, 25 μM), and their combinations. The sucrose concentration was measured over time to account for the amount of sucrose non-hydrolysed to glucose by α-glucosidase. Fig. 4 shows the concentration of sucrose, expressed as a percentage of the initial sucrose concentration at t0 in the supernatant of cells treated with acarbose (Fig. 4a), and Q-3-G (Fig. 4b). The highest was the residual sucrose, the most effective was the tested compound in restraining sucrose hydrolysis and thus inhibiting α-glucosidase. As expected, the highest concentration of acarbose (50 μM) was the most efficient in inhibiting sucrose hydrolysis (Fig. 4a). Conversely, lower concentrations of the drug proved to be inefficient in reducing sucrose hydrolysis. These results are in line with what was reported by other authors on the efficacy of acarbose.28 However, these studies investigated the ability of acarbose to inhibit the α-glucosidase in Caco-2 cell extracts, whereas, to the best of our knowledge, no direct measurements of the remaining non-hydrolysed sucrose on the apical side of intact cells are available in the literature. As reported in Fig. 4b, Q-3-G was able to reduce sucrose hydrolysis both at low (2 μM) and high (25 μM) concentrations, compared to cells only fed with sucrose and not treated with any inhibitor (CTRL), confirming the efficacy of Q-3-G in restraining sucrose hydrolysis. Overall, these results show that acarbose and Q-3-G led to a similar range of residual sucrose, indicating a similar effect on sucrose hydrolysis inhibition. This means that the efficacy of Q-3-G is in the same range as that of acarbose, a well-known α-glucosidase inhibitor used in type 2 diabetes treatment.To further substantiate the evidence collected in vitro about the antagonistic interaction between acarbose and Q-3-G in inhibiting α-glucosidase (Fig. 2), Caco-2 cells were treated with their combination at different concentrations. Fig. 5 shows the residual sucrose concentration as a function of acarbose concentration for the lowest (2 μM, Fig. 5a) and the highest (25 μM, Fig. 5b) Q-3-G concentration tested, after 1 and 2 h from the beginning of the experiment.
Overall, residual sucrose concentration ranged between 25 and 80% depending on the concentration of Q-3-G, acarbose and time.
In particular, considering the lowest Q-3-G concentration (Fig. 5a), it can be noticed that residual sucrose after 1 h increased from around 60 to 80% by increasing acarbose from 0.5 to 25 μM. A further increase in acarbose concentration to 50 μM however caused a drop to <50% in the residual sucrose. After 2 h from the beginning of the experiment, a lower residual sucrose was measured for all acarbose concentrations compared to what observed after 1 h. Also in this case, the residual sucrose increased with acarbose concentration up to 25 μM (∼65%), followed by a significant drop (∼25%) with 50 μM acarbose. These results indicate that even if acarbose alone was effective only at the highest concentration (Fig. 4a), its combination with Q-3-G dramatically changed the scenario.
When the highest Q-3-G concentration (Fig. 5b) was combined with acarbose, a different trend in residual sucrose as a function of acarbose concentration was observed both after 1 and 2 h. When comparing 2 μM (Fig. 5a) and 25 μM (Fig. 5b) Q-3-G, the difference in residual sucrose became more pronounced at higher acarbose concentrations. Specifically, when 25 μM acarbose was combined with 25 μM Q-3-G, residual sucrose decreased. In contrast, when 50 μM acarbose was combined with 25 μM Q-3-G, residual sucrose increased.
Overall, these results further support the hypothesis of an antagonistic relationship between the two compounds, which seems to be exacerbated when the system under investigation is “crowded”.
Conclusions
This study looked at how apple juice interacts with acarbose, focusing on quercetin-3-glucoside (Q-3-G) as the main bioactive constituent, and considered possible effects of their combination in the context of α-glucosidase activity inhibition and glycaemic response modulation.The in vivo study we performed in rat models showed that apple juice might work against acarbose. When apple juice was administered on its own, it caused a lower blood sugar response than glucose. When apple juice was administered with increasing doses of acarbose, it led to a higher glycaemic response, which backs up the hypothesis of antagonism. It is also worth mentioning that apple juice is a complex food matrix, containing a whole range of different bioactive compounds, that makes tricky the result interpretation. Based on these assumptions, we considered a simplified model, focussing on quercetin-3-glucoside (Q-3-G), which is among the most bioaccessible phenolic compounds in apple juice able to effectively inhibit α-glucosidase. We found that acarbose and Q-3-G, when tested separately, could inhibit α-glucosidase activity to some extent. However, when they were combined, their inhibitory activity was significantly reduced, which supports the antagonism hypothesis. This was backed up by the combination index (CI) calculation, which showed that the interaction of acarbose and Q-3-G was mainly antagonistic within the studied concentration range. Cell-based assays on Caco-2 cultures showed that at higher Q-3-G concentrations, its combination with acarbose led to less inhibition of sucrose hydrolysis than each compound alone, confirming once more that acarbose and Q-3-G did not work well together. This trend was even more noticeable when the system was more “crowded”, which suggests that the interaction between the two compounds might depend on concentration.
All things considered, these results suggest that, even though Q-3-G shows promise as an α-glucosidase inhibitor, combining it with acarbose might not be the best approach, given the antagonistic effects observed.
Based on these considerations, there is a need for more research on how this happens and on comparing the effects of quercetin in different forms (i.e., glycosylated versus aglycone) to understand this topic better. Moreover, the complex composition of food like apple juice requires to look closely at how different compounds interact when combining dietary supplements and pharmaceutical therapies for managing type 2 diabetes. As more and more people are looking at functional foods as a way to help treat different illnesses, including diabetes, it is really important to understand how drugs and the natural ingredients in these foods could affect each other. Our study showed that acarbose and quercetin, which were thought to work well together, actually have an opposing effect. This finding shows that we need to take a more comprehensive approach, like the one we have outlined in this study. This approach integrates in vitro investigations that are validated through ex vivo and in vivo studies. This approach is key to accurately assessing how functional foods interact and what that means for using them in a therapeutic setting.
Author contributions
Umberto Lanza: investigation, data curation, formal analysis, visualization writing – original draft; Marilisa Alongi: conceptualization, methodology, data curation, formal analysis, writing – original draft; Barbara Frossi: methodology, supervision; Carlo Pucillo: resources; Monica Anese: conceptualization, resources; Maria Cristina Nicoli: conceptualization, supervision, resources; all authors equally contributed to writing – review & editing.Data availability
The authors confirm that the data supporting the findings of this study are available within the article.Conflicts of interest
The authors declare that there are no competing interests.References
- R. A. DeFronzo, E. Ferrannini, L. Groop, R. R. Henry, W. H. Herman, J. J. Holst, F. B. Hu, C. R. Kahn, I. Raz, G. I. Shulman, D. C. Simonson, M. A. Testa and R. Weiss, Type 2 diabetes mellitus, Nat. Rev. Dis. Primers, 2015, 1, 1–23 CrossRef PubMed.
- R. A. Defronzo, From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus, Diabetes, 2009, 58, 773–795 CrossRef CAS PubMed.
- J. L. Chiasson, R. G. Josse, R. Gomis, M. Hanefeld, A. Karasik and M. Laakso, Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial, Lancet, 2002, 359, 2072–2077 CrossRef CAS PubMed.
- T. M. S. Wolever, R. Radmard, J.-L. Chiasson, J. A. Hunt, R. G. Josse, C. Palmason, N. W. Rodger, S. A. Ross, E. A. Ryan and M. H. Tan, One-year Acarbose Treatment Raises Fasting Serum Acetate in Diabetic Patients, Diabetic Med., 1995, 12, 164–172 CrossRef CAS PubMed.
- T. Nie and G. J. S. Cooper, Mechanisms Underlying the Antidiabetic Activities of Polyphenolic Compounds: A Review, Front. Pharmacol., 2021, 12, 798329 CrossRef CAS PubMed.
- G. Williamson, Possible effects of dietary polyphenols on sugar absorption and digestion, Mol. Nutr. Food Res., 2013, 57, 48–57 CrossRef CAS PubMed.
- E. Barber, M. J. Houghton and G. Williamson, Flavonoids as Human Intestinal α-Glucosidase Inhibitors, Foods, 2021, 10, 1939 CrossRef CAS PubMed.
- O. Kwon, P. Eck, S. Chen, C. P. Corpe, J. Lee, M. Kruhlak and M. Levine, Inhibition of the intestinal glucose transporter GLUT2 by flavonoids, FASEB J., 2007, 21, 366–377 CrossRef CAS PubMed.
- R. Dhanya, Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy, Biomed. Pharmacother., 2022, 146, 112560 CrossRef CAS PubMed.
- L. Arfaoui, Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability, Molecules, 2021, 26, 2959 CrossRef CAS PubMed.
- U.S. Department of Agriculture, FoodData Central, 2024, https://fdc.nal.usda.gov/.
- C. Schulze, A. Bangert, G. Kottra, K. E. Geillinger, B. Schwanck, H. Vollert, W. Blaschek and H. Daniel, Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans, Mol. Nutr. Food Res., 2014, 58, 1795–1808 CrossRef CAS PubMed.
- M. Alongi, G. Verardo, A. Gorassini and M. Anese, Effect of pasteurization on in vitro α-glucosidase inhibitory activity of apple juice, LWT, 2018, 98, 366–371 CrossRef CAS.
- Y. Q. Li, F. C. Zhou, F. Gao, J. S. Bian and F. Shan, Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase, J. Agric. Food Chem., 2009, 57, 11463–11468 CrossRef CAS PubMed.
- M. Alongi, G. Verardo, A. Gorassini, S. Sillani, C. Degrassi and M. Anese, Reformulation and food combination as strategies to modulate glycaemia: the case of apple pomace containing biscuits administered with apple juice to healthy rats, Int. J. Food Sci. Nutr., 2021, 72, 174–183 CrossRef CAS PubMed.
- D. P. Belobrajdic, J. Wei and A. R. Bird, A rat model for determining the postprandial response to foods, J. Sci. Food Agric., 2017, 97, 1529–1532 CrossRef CAS PubMed.
- A. B. Nair and S. Jacob, A simple practice guide for dose conversion between animals and human, J. Basic Clin. Pharm., 2016, 7, 27 CrossRef PubMed.
- Q. Zhang, X. Xiao, M. Li, W. Li, M. Yu, H. Zhang, Z. Wang and H. Xiang, Acarbose Reduces Blood Glucose by Activating miR-10a-5p and miR-664 in Diabetic Rats, PLoS One, 2013, 8, e79697 CrossRef PubMed.
- K. Singh, A. Kafka, B. H. Kang, R. Goundra, Y. I. Kwon and E. Apostolidis, In vitro evaluation and determination of responsible fraction of coffee beans and dried sugar beet leaves for alpha-glucosidase inhibition, Int. J. Appl. Res. Nat. Prod., 2014, 7, 15–20 Search PubMed.
- T. C. Chou, Drug combination studies and their synergy quantification using the chou-talalay method, Cancer Res., 2010, 70, 440–446 CrossRef CAS PubMed.
- L. Tor, The impact of food bioactives on health: in vitro and ex vivo models, ed. K. Verhoeckx, P. Cotter and I. López Expósito, Springer International Publishing, Cham, 1st edn, 2015, vol. 10, pp. 103–111 Search PubMed.
- F. Baty, C. Ritz, S. Charles, M. Brutsche, J. P. Flandrois and M. L. Delignette-Muller, A Toolbox for Nonlinear Regression in R: The Package nlstools, J. Food Biochem., 2015, 66, 1–21 Search PubMed.
- J. J. Uuh Narvaez and M. R. Segura Campos, Combination therapy of bioactive compounds with acarbose: A proposal to control hyperglycemia in type 2 diabetes, J. Food Biochem., 2022, 46, 1–14 CrossRef PubMed.
- M. Alongi, G. Verardo, A. Gorassini, M. A. Lemos, G. Hungerford, G. Cortella and M. Anese, Phenolic content and potential bioactivity of apple juice as affected by thermal and ultrasound pasteurization, Food Funct., 2019, 10, 7366–7377 RSC.
- L. Han, H. Wang, J. Cao, Y. Li, X. Jin, C. He and M. Wang, Inhibition mechanism of α-glucosidase inhibitors screened from Tartary buckwheat and synergistic effect with acarbose, Food Chem., 2023, 420, 136102 CrossRef CAS PubMed.
- L. Flores-Bocanegra, A. Pérez-Vásquez, M. Torres-Piedra, R. Bye, E. Linares and R. Mata, α-Glucosidase Inhibitors from Vauquelinia corymbosa, Molecules, 2015, 20, 15330–15342 CrossRef CAS PubMed.
- J. Lee and A. E. Mitchell, Pharmacokinetics of quercetin absorption from apples and onions in healthy humans, J. Agric. Food Chem., 2012, 60, 3874–3881 CrossRef CAS PubMed.
- A. Pyner, H. Nyambe-Silavwe and G. Williamson, Inhibition of human and rat sucrase and maltase activities to assess antiglycemic potential: Optimization of the assay using acarbose and polyphenols, J. Agric. Food Chem., 2017, 65, 8643–8651 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |





