Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Time-of-day-dependent effects of a green tea extract on postprandial glycemia and insulinemia in healthy adults: a randomized, controlled, double-blind, cross-over intervention†
Noha
Sulaimani
 abc,
Erika J.
Rosbotham
ad,
Rebekah
Warnock
ad,
Louise
Polzella
a,
Rebecca
Judowski
a,
Luca
Nicolotti
ef,
Michael J.
Houghton
abc,
Erika J.
Rosbotham
ad,
Rebekah
Warnock
ad,
Louise
Polzella
a,
Rebecca
Judowski
a,
Luca
Nicolotti
ef,
Michael J.
Houghton
 ab,
Gary
Williamson
ab,
Gary
Williamson
 ab and
Maxine P.
Bonham
ab and
Maxine P.
Bonham
 *a
*a
aDepartment of Nutrition, Dietetics and Food, Monash University, Notting Hill, 3168, Australia. E-mail: Maxine.bonham@monash.edu
bVictorian Heart Institute, Monash University, Clayton, 3168, Australia
cDepartment of Food and Nutrition, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
dNutrition Innovation Centre for Food and Health (NICHE), School of Biomedical Sciences, Ulster University, Coleraine, BT52 1SA, UK
eThe Australian Wine Research Institute, Adelaide, SA 5064, Australia
fMetabolomics Australia, The Australian Wine Research Institute, Adelaide, SA 5064, Australia
First published on 28th April 2025
Abstract
Glucose homeostasis is intricately associated with circadian rhythms, and disrupting these rhythms, due to mistimed eating, can increase the risk of metabolic dysfunction. Although green tea (poly)phenols are recognized for their potential to lower postprandial glycemia, time-of-day effects of green tea extract incorporated into a starch-based meal remain unexplored. We hypothesized that combining green tea extract with a starch-rich meal could lower postprandial glucose and insulin in both the morning and evening. A four-arm randomized, double-blind, controlled, cross-over intervention was conducted on fourteen healthy adults. Fasted volunteers attended twice in the morning (08:00) and twice in the evening (18:00), consuming either a control (white rice) or test meal (green tea extract-enriched white rice). Blood glucose and insulin concentrations were measured at several time points over 180 minutes, and incremental areas under the curve (iAUC) and peak blood concentrations were calculated. Postprandial glucose levels were higher in the evening compared to the morning, while insulin levels were lower in the evening, regardless of the intervention. The green tea extract meal did not significantly alter the glucose and insulin iAUC compared to the control meal during the morning and evening. Although green tea extract did not affect postprandial glucose concentrations, it significantly decreased peak insulin levels (629 ± 313 pmol L−1, P = 0.04) 30 minutes after the morning meal. Green tea (poly)phenols delay postprandial insulin in the morning but not in the evening, revealing a time-of-day dependent effect on insulin sensitivity.
1. Introduction
Glucose homeostasis is regulated by circadian rhythms, the internal biological clock that consists of a central brain clock and peripheral tissue clocks. Behavioral factors, such as mistimed food intake, can disrupt circadian rhythms, mainly impacting peripheral clocks across various tissues including the liver, pancreas, muscles, and adipose tissues. This disruption can adversely influence glucose metabolism, potentially leading to an increased risk of developing type 2 diabetes.1,2 Evening carbohydrate ingestion, compared to morning intake, results in an extended and elevated postprandial glucose response in healthy adults.3 In addition, two recent meta-analyses of acute studies in healthy individuals substantiate that extending eating time beyond 18:00 is linked to augmented postprandial glucose spikes compared to earlier in the day, irrespective of meal composition.4,5 However, findings for insulin are less consistent, with some studies indicating higher postprandial insulin area under the curve (AUC) or incremental area under the curve (iAUC) in the evening,5 but others reporting no significant difference in postprandial insulin AUC between morning and evening.4 These variations have been attributed to circadian differences in insulin sensitivity,6,7 liver responsiveness,1,2,6 and pancreatic β-cell activity.8,9 Thus, it is crucial to consider dietary approaches aimed at modulating postprandial glycemia, where mistimed food intake can exacerbate these variations in physiological processes by disrupting circadian rhythms, ultimately leading to dysregulation of glucose metabolism.10,11Naturally occurring bioactive compounds in plants, such as (poly)phenols and their subclasses, including flavonoids like catechins, have been shown to modulate postprandial blood glucose excursions. As such, the incorporation of (poly)phenols into meals, especially at times of relative insulin resistance (i.e. at night) may have ramifications for managing blood glucose. Intervention studies in healthy humans have reported that (poly)phenols, as extracts or (poly)phenol-rich foods, can attenuate postprandial hyperglycemia.12–17 Green tea, high in catechins such as (−)-epigallocatechin gallate (≥50% of total catechins) and (−)-epicatechin gallate (∼33%),18 has demonstrated a postprandial glucose-lowering effect in some studies on healthy individuals.18–20 This effect is ascribed to its inhibitory action on the activity of key enzymes, α-glucosidase21 and α-amylase,18,22,23 involved in carbohydrate digestion, as well as on glucose transporters24 and on starch hydrolysis via starch-polyphenol complex formation.22
Whilst some studies have examined the effects of (poly)phenols in general on metabolic health, the possible time-of-day influence of (poly)phenol ingestion and its subsequent impact on postprandial glycemia are yet to be extensively explored. To date, two human trials have investigated the time effect of a green tea beverage, revealing a notable decrease in postprandial blood glucose levels in the evening compared to the morning,25,26 but, to our knowledge, no previous studies have explored the role of green tea incorporated into food on time-of-day effects on postprandial glycemia or insulin. It has been reported that consumption of green tea in combination with starchy food reduces both glucose and insulin levels in a dose-dependent manner in healthy individuals,18 as EGCG slows down starch hydrolysis via complexation with starch, not via direct inhibition of α-amylase.22 We, therefore, tested the hypothesis that green tea extract incorporated into a starch-rich food could lower postprandial glucose and insulin in both the morning and evening and designated the change in glucose and insulin iAUC from time 0–180 minutes as the primary outcome measure.
2. Methods
2.1. Study design
This study was a double-blind, controlled, randomized cross-over intervention carried out on a total of 14 healthy volunteers in the morning and evening. The study protocol was approved by the Monash University Human Research Ethics Committee (ethics approval number 18721) and registered with the Australian New Zealand Clinical Trials Registry (registration number ACTRN12619000789167) and can be accessed at https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=377433&isReview=true. This study was conducted between June and November 2019 and recruitment was stopped when the sample size was reached. Each participant provided written informed consent before the commencement of the study.2.2. Subjects
Participants were recruited via a snowball sampling method, utilizing flyers and online advertising. Male and female volunteers between the ages of 18 and 65, with a body mass index (BMI) ranging from 18.5 to <28 kg m−2, fasting blood glucose <5.5 mmol L−1 and normal blood pressure, were eligible for inclusion. Exclusion criteria were as follows: taking medication for blood glucose control or other natural health products known to affect (poly)phenol metabolism, bioavailability, or action on target pathways (such as fish oil), having any gastrointestinal problems that could interfere with (poly)phenol absorption and intestinal functions, having any serious health conditions (e.g., thyroid or liver dysfunction or recent major surgery), possessing an implanted cardiac defibrillator, being pregnant or breastfeeding, consuming more than four alcoholic drinks per day, or shift workers.2.3. Randomization and blinding
Using computer-generated randomization, participants were allocated in a random sequence to either the control in the morning, green tea extract-rich meal (GTE) in the morning, control in the evening, or GTE in the evening. The researcher, participants and data analysts were blinded to the intervention being conducted in each session, labeling intervention groups with letter codes (A, B, C, or D) to conceal their identity. To maintain blinding for both participants and the researcher, both meals were colored using synthetic black food coloring. Participant identification during analysis of blood samples was also concealed from the researchers using a three-digit code.2.4. Test meal
All meals consisted of a carbohydrate load in the form of jasmine rice (64 g raw weight), containing 50 g available carbohydrates as estimated using FoodWorks 10 (Xyris Software, Australia Pty Ltd, Spring Hill, Queensland, Australia). Rice was cooked in 400 mL of water and 6 g of chicken stock, with 6 g of Nature's Way ® Matcha Green Tea Powder (containing ∼1.06 g of (poly)phenols) added to the test meal. The control meal was identical to the test meal except for the absence of green tea extract. Both meals were supplemented with 2.5 mL of Queen Black Food Color Gel to ensure blinding of researchers and participants.2.5. Procedure
Each participant attended four sessions at the Monash University Be Active Sleep Eat (BASE) facility in Melbourne, Australia, two of which were morning visits and two were evening visits, with the participants receiving either the control or test meal in a randomized pattern, as described above, on a total of four occasions. A one-week minimum washout period was implemented between each treatment.2.5.2.1. Morning sessions. Participants were given a standardized commercially-available meal for dinner (energy content: 1530–1670 kJ) to consume between 19:00–21:00 the night before each testing day and then were asked to fast overnight (≥9 h), except for water. Participants were also instructed to abstain from engaging in vigorous exercise and consuming (poly)phenol-rich foods and beverages for 24 hours preceding each testing session, with a list of suggested foods provided before the start of the study.
On the morning of each testing session, at 08:00, a fasting blood sample was collected, via finger prick, to assess fasting blood glucose and insulin. Anthropometric measurements (weight, height, weight circumference, and fat mass percentage) were also collected. Thirty minutes after baseline sampling, participants were provided with the meal and asked to consume it within 15 minutes. Blood was sampled through finger prick at pre-determined intervals to measure levels of postprandial blood glucose (15, 30, 45, 60, 90, 120, 150 and 180 minutes) and insulin (30, 60, 90, 120 and 180 minutes) (Fig. 1).
2.5.2.2. Evening sessions. Evening sessions followed the same protocol as the morning sessions, except for consuming the same standardized meal between 08:00–09:00, followed by a fasting period until 18:00, when a fasting blood sample and anthropometric measurements were collected. Subsequent to test meal consumption, blood samples were collected at the same pre-specified postprandial intervals over the next three hours (Fig. 1).
2.6. Analysis of (poly)phenols in test meal
The green tea extract-containing meal given to participants was analyzed by liquid chromatography-mass spectrometry (LC-orbitrap-MS), as previously described,30 and high-performance liquid chromatography with photodiode-array detection (HPLC-DAD).2.7. Blood glucose and insulin measures
Finger pricks were performed using a Unistick® 3 Extra single-use lancets (Owen Mumford Ltd, Oxfordshire, United Kingdom) and capillary glucose concentrations were assessed with the HemoCue Glucose 201 RT System (Radiometer Pacific Pty Ltd, Mount Waverley, Victoria, Australia), following standard procedures. Plasma insulin concentrations were measured by Human Insulin ELISA kit (#EZHI-14K, Millipore, Merck Life Science, Bayswater, Victoria, Australia), according to the manufacturer's instructions.2.8. Anthropometric data and blood pressure
Height, weight and body composition data were collected after removing shoes and socks. Height was measured using Harpenden Stadiometer (Holtain Ltd, Crymych, UK). Weight and body composition (% fat mass, % fat free mass) were determined using the SECA mBCA 515 medical body composition analyzer (SECA, Hamburg, Germany). Waist circumference measurements were taken directly over bare skin, aligned with the naval. Resting blood pressure was measured with a portable sphygmomanometer (Welch Allyn, Skaneateles Falls, New York, USA), with two measurements taken and averaged for analysis.2.9. Dietary assessment and physical activity
Participants’ 24-hour dietary recalls (four per individual) were analyzed using FoodWorks 10 software (Xyris Software (Australia) Pty Ltd, Spring Hill, Queensland, Australia), with a standardized set of assumptions developed and applied where detailed information was unavailable across all recalls. Average daily energy, carbohydrate, protein, and fat intakes were calculated, with average (poly)phenol intake estimated from the FFQ data. The IPAQ score was calculated and participants were designated as having low, medium or high activity levels.2.10. Intolerance symptoms
To evaluate the incidence and intensity of side effects, an intolerance symptoms questionnaire was completed by participants 24 hours following test meal consumption. They were asked to report whether they had experienced any adverse reactions, such as headache, anxiety, tiredness/exhaustion, lack of energy, tendency to become rapidly exhausted, changes in appetite (either increased or decreased), hiccups, nausea, vomiting, indigestion, stomach or abdominal pain, constipation, diarrhea, gas, abdominal bloating, cardiac palpitations, balance disorders, reduced capacity to concentrate, feeling cold, muscle or joint pain, numbness, burning or itching sensations, and dark or depressing thoughts.2.11. Statistical analysis
Given the lack of prior equivalent data on the effects of an evening (poly)phenol treatment, power analysis was derived from outcomes of a previous study assessing the impact of green tea consumption in the morning on postprandial glycemia.31 The sample size was determined to observe a change in the primary variable, 40 units in the postprandial blood glucose iAUC between the treatment and control, based on iAUC of 178 ± 47 mmol L−1. At a two-sided power of 80% and a significance level of 0.05, a minimum of 14 participants was required to complete each arm of the study, with participants acting as their controls.Postprandial blood glucose and insulin responses were estimated using the incremental areas under curves (iAUC) over 180 minutes, and peak blood concentrations recorded. Using SPSS 29.0 software (IBM® SPSS® Statistics Corp, Chicago, IL, USA), the iAUC was calculated applying the trapezoidal method, excluding baseline values, and is expressed as mmol L−1 min−1 for glucose and pmol L−1 min−1 for insulin. Data are presented as mean ± standard deviation (SD). Normality of data distribution was tested by the Shapiro–Wilk test and visual inspection of residuals plots. Where results were non-normally distributed, data transformation was conducted using the natural logarithm. A two-factor repeated measures analysis of variance (ANOVA), with Tukey's post-hoc comparisons tests, was carried out to investigate the interaction between the two meals (control and test meal) and time of day on blood glucose and insulin responses across the post-meal period (0–180 min). Differences in postprandial intervals, iAUC and peak blood concentration among the four groups were considered significant at P < 0.05, with 95% confidence intervals (CI). Statistical analyses and visualization of figures were performed using GraphPad Prism 9.3.1 software (GraphPad Software, Boston, MA, USA).
3. Results
3.1. Participants
Fourteen subjects completed the 4-way intervention (Fig. 2), with baseline characteristics shown in Table 1. All participants were within a healthy weight range, with a normal fasting blood glucose concentration (<5.5 mmol L−1), and normal blood pressure readings. No adverse reactions after ingestion of the test meal were reported, as indicated by responses to an intolerance symptoms questionnaire.| Characteristics | Mean (SD) |
|---|---|
| a Values reported as median (IQR); IQR: interquartile range; SD: standard deviation; M: male; F: female. b n = 13 due to unreported data from one participant. Average (poly)phenol intake was estimated from FFQ data based on the Phenol-Explorer 3.6 database on (poly)phenol content in foods28 and the USDA Database for the Flavonoid Content of Selected Foods, Release 3.1.29 Physical activity level was measured via the International Physical Activity Questionnaire (IPAQ); BMI: body mass index. | |
| Age (years) | 19.5 (2.25)a |
| BMI (kg m−2) | 22.7 (3.2) |
| Waist circumference (cm) | 75.7 (9.9) |
| Fat mass (%) | 27.2 (8.2)a |
| Fat free mass (%) | 72.8 (8.2)a |
| Fasting blood glucose (mmol L−1) | 4.3 (1.0)a |
| Systolic blood pressure (mmHg) | 117.9 (14.0) |
| Diastolic blood pressure (mmHg) | 74.3 (8.0) |
| Estimated (poly)phenol intake (mg day−1) | 901 mg (403) |
| n (frequency) | |
| Sex | M: 5 (35.7%); F: 9 (64.3%) |
| Physical activity levelb | Moderate: 6 (46%); high: 7 (54%) |
3.2. Postprandial plasma glucose response
There were significant main effects of time of day on postprandial glucose levels (Fig. 3). As shown in Fig. 3A, there were significantly higher glucose concentrations at 60 min (P < 0.01), 90 min (P < 0.001), and 120 min (P < 0.01) during the evening session compared to the morning session, irrespective of the meal consumed. The postprandial glucose iAUC was approximately two-fold higher in the evening versus the morning (P < 0.01) for both meals (Fig. 3B). However, no significant differences were observed in peak plasma glucose concentration between time of the day (P = 0.07) (Fig. 3C). There were no main effects of treatment or time of day × treatment interactions observed in the mean postprandial blood glucose concentrations, glucose iAUC or glucose peak between the treatment groups (Fig. 3A, B and C).3.3. Postprandial plasma insulin response
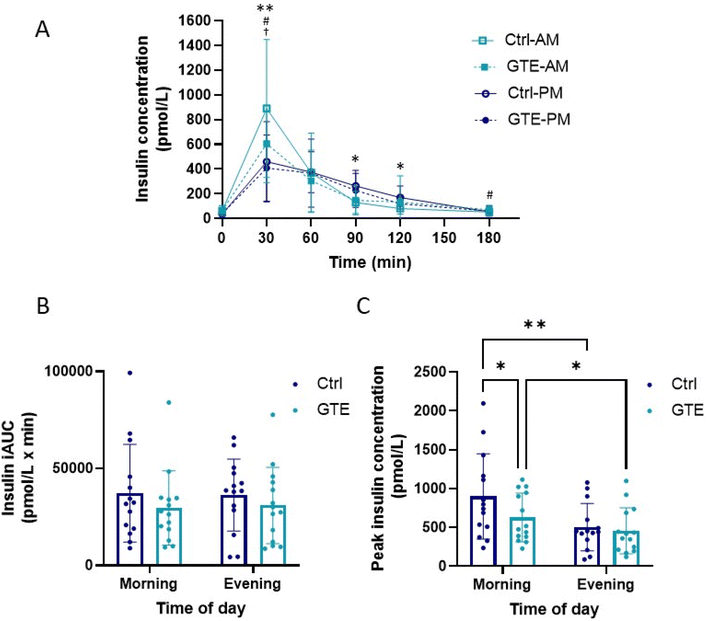
Postprandial plasma insulin concentrations are presented in Fig. 4. Two-factor repeated measures ANOVA revealed significant main effects of time of day (P < 0.001) and interaction of time of day × intervention (P < 0.001), but not of the intervention (P = 0.18), on mean postprandial blood insulin concentrations (Fig. 4A). In terms of main effects of time of day, postprandial insulin concentrations were lower in the evening than in the morning, specifically at 30 min, in both the control group (P = 0.002) and GTE group (P = 0.01). Of note, there was a 32% reduction in mean plasma insulin concentration at 30 min after consuming GTE in the morning compared to the control (P = 0.04), indicating the interaction of time of day × intervention. There was a main effect of intervention (P = 0.004) on insulin iAUC (Fig. 4B), but no specific significant differences detected by the post-hoc comparisons tests. Significant main effects of time of day (P = 0.0002), intervention (P = 0.014) and time of day × treatment interaction (P = 0.031) were observed for the insulin peak (Fig. 4C). Notably, the insulin peak declined in the evening compared to the morning by 44% in the control group (P = 0.0002) and ∼30% in the GTE group (P = 0.01). The GTE considerably reduced peak insulin in the morning by 30% compared to the control (P = 0.04), but not in the evening (P = 0.8).3.4. (Poly)phenol composition of the test meal
Table 2 shows the (poly)phenol constituents of the test meal analyzed using LC-orbitrap-MS. Epicatechin, (−)-epigallocatechin gallate, (−)-catechin gallate, catechin, and rutin are the predominant (poly)phenols in the green tea extract-enriched rice, demonstrating that the test meal retained (poly)phenols during the meal preparation process. This retention was further confirmed by quantifying EGCG, the most abundant polyphenol, in the test meal using HPLC, which indicated that the meal contained 127.9 mg EGCG per portion of rice consumed (0.552 mg EGCG per gram of eaten rice).| Tentative compounds identified by Liquid Chromotography | Formula | MW | t R (min) | Weight normalized relative peak area (divided by 1000) |
|---|---|---|---|---|
| The bold typeface represents (poly)phenols identified in green tea as evidenced by literature.28,56–59 Many of the other compounds have been described in tea as well, including the hydroxycinnamic acids.a Kaempferol 3-neohesperidoside may be analogous to kaempferol-3-O-rutinoside due to their identical chemical formula with a slight difference in molecular weight (594.16 and 594.52, respectively). tR: retention time in minutes; MW: molecular weight. | ||||
| Epicatechin | C15 H14 O6 | 290.08 | 17.28 | 923![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 156 156 |
| Epigallocatechin gallate | C22 H18 O11 | 458.09 | 23.65 | 648![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 997 997 |
| 24.38 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 947 947 |
|||
| (−)-Catechin gallate | C22 H18 O10 | 442.09 | 24.22 | 271![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 648 648 |
| 24.82 | 169![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 271 271 |
|||
| Catechin | C15 H14 O6 | 290.08 | 9.41 | 264![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 442 442 |
| Rutin | C27 H30 O16 | 610.15 | 25 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 843 843 |
| 3-p-Coumaroylquinic acid | C16 H18 O8 | 338.10 | 14.86 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 299 299 |
| 18.95 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 372 372 |
|||
| 11.57 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 876 876 |
|||
| trans-5-O-(4-Coumaroyl)-D-quinic acid | C16 H18 O8 | 338.10 | 6.05 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 148 148 |
| (−)-Epiafzelechin 3-O-gallate | C22 H18 O9 | 426.10 | 25.12 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 133 133 |
| 6-O-Galloyl-Î2-D-glucose | C13 H16 O10 | 332.07 | 1.36 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 691 691 |
| 2.41 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 186 186 |
|||
| Kaempferol 3-neohesperidoside | C27 H30 O15 | 594.16 | 22.99 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 522 522 |
| Isovitexin 2′′-O-arabinoside | 24.14 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 037 037 |
||
| 24.59 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 911 911 |
|||
| Quercetin-3β-D-glucoside | C21 H20 O12 | 464.10 | 25.07 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 594 594 |
| 24.91 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 405 405 |
|||
| Myricetin 3-O-β-D-galactopyranoside | C21 H20 O13 | 480.09 | 24.26 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 003 003 |
| Leucodelphinidin | C15 H14 O8 | 322.07 | 6.46 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 037 037 |
| Theogallin | C14 H16 O10 | 344.07 | 2.29 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 746 746 |
| Neochlorogenic acid | C16 H18 O9 | 354.10 | 12.08 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 225 225 |
| (+)-Gallocatechin | C15 H14 O7 | 306.07 | 17.98 | 9712 |
| Gentisic acid | C7 H6 O4 | 154.03 | 4.59 | 9235 |
4. Discussion
We conducted a randomized, double-blind, controlled, cross-over trial to test the hypothesis that (poly)phenol-rich green tea extract (GTE) combined with a starchy meal could attenuate postprandial glycemia and insulinemia in healthy individuals in both the morning and evening. Postprandial glucose response was increased in the evening compared to the morning, independently of the intervention, but there was no effect on glucose response by the GTE at either time of day. In contrast to glucose, insulin was decreased in the evening compared to the morning, regardless of the GTE, while the GTE delayed insulin secretion in response to the starch-based meal in the morning.The augmented postprandial glucose response observed here in the evening, irrespective of the intervention, concurs with similar studies that have examined the impact of meal timing on postprandial glycemia in healthy individuals,32,33 and supports meta-analyses showing time-of-day differences in postprandial glucose response.4,5 The insulin concentration was substantially lower in the evening 30 minutes after meal intake, aligning with other research.25,34 These responses are attributed to a close interconnection between physiological processes, metabolic homeostasis and circadian rhythms. Insulin secretion and insulin sensitivity exhibit diurnal variations, being profoundly lower in the evening than in the morning.1,2,6 In both rats9 and humans,8 insulin secretion is regulated by the pancreatic circadian clock, with Circadian Locomotor Output Cycles Kaput (CLOCK) and Brain and Muscle ARNT-Like 1 (BMAL1) playing a crucial role in activating genes related to insulin production and secretion, and therefore maintaining glucose metabolism. Similarly, human muscle tissue displays greater insulin sensitivity in the morning as opposed to the evening,35 indicating diurnal variations influenced by the molecular clock in the muscle. BMAL1 regulates insulin-dependent translocation of glucose transporter GLUT4 to the cell surface.36 Further, CLOCK and BMAL1 regulate insulin sensitivity by modulating insulin-stimulated phosphorylation of proteins in the insulin signaling pathway, including insulin receptor substrate-1 (IRS-1), protein kinase B (AKT) and glycogen synthase kinase-3β (GSK-3β), as well as expression of sirtuin1 (Sirt1). Sirt1 is a key enzyme responsible for the deacetylation of several proteins involved in the insulin signaling pathway, thereby impacting the circadian clock and metabolic processes in muscle cells.37
The addition of green tea (poly)phenols to a rice dish did not impact glucose homeostasis either in the morning or the evening, compared to rice alone. In contrast, Takahashi et al.25,26 reported a significant attenuation of postprandial glucose in the evening, but not in the morning, after consumption of a green tea drink together with a mixed meal in healthy subjects, and a comparable effect was observed with a single dose of (poly)phenol-rich mulberry leaf extract consumed with a mixed meal.34 Conversely, there was no effect on postprandial glucose by a brown seaweed extract when consumed before bread in the morning or evening.38,39 A limited number of studies have compared the effect of (poly)phenols on postprandial glucose response in the morning to that in the evening, with most reporting data on the effect of (poly)phenols on postprandial glucose only in the morning after fasting. Some studies have observed an effect of a (poly)phenol-rich food or extract in the morning, but many do not.40–44 For example, green tea did not impact postprandial glycemia when consumed with a meal,40 nor when incorporated into bread.41 Despite previous studies showing mixed results regarding the effects of (poly)phenols on postprandial glycemia, this discrepancy can be explained in part by inter-individual variations in the bioavailability and metabolism of (poly)phenols, the combined effects of various compounds in the food matrix on the biological outcomes of (poly)phenols, the concentration and composition of (poly)phenol consumed,45,46 or the diverse mechanisms of (poly)phenol action.
Our findings suggest that the lack of effect by the GTE on glucose response is conceivably due to no influence on carbohydrate digestion18 or glucose absorption.24,47 Despite this, diurnal variations in the expression of glucose transporters may influence metabolic responses to meals consumed at different times of the day. For example, expression of rat intestinal transporters sodium-dependent glucose co-transporter-1 (SGLT1), glucose transporter 2 and 5 (GLUT2 and GLUT5) exhibited circadian oscillations.48–50 However, this seems unrelated to our observed results, as postprandial glucose responses remained unaffected following the GTE meal. Indeed, the human pancreatic α-amylase, essential for carbohydrate digestion, does not follow a rhythmic pattern throughout the day, suggesting that this enzyme is not under direct circadian regulation.51
An important finding in the present study is that consuming green tea extract added to a starch-based meal in the morning resulted in a pronounced and significant reduction in the postprandial insulin peak (30 min). Despite this, limited short-term human intervention studies have shown no time-of-day-dependent effects of (poly)phenols on postprandial insulinemia.25,26,34,38,39 The reduction in insulin observed in our study was not observed previously with the acute ingestion of a green tea beverage25,26 or mulberry leaf extract with a mixed meal,34 or brown seaweed extract with bread.38,39 However, in trials run in the morning in healthy individuals, insulin concentrations were blunted after consuming a green tea extract added to bread,41 a strawberry beverage with a high-carbohydrate moderate-fat meal,42 berries with white bread,43 or baobab fruit extract-enriched bread,41 with no discernible effects on glucose concentrations.
In the current study, the observed effect on insulinemia may be explained by the circadian rhythmicity of insulin sensitivity, with cells being more responsive to insulin in the morning than in the evening. The absence of an effect by the (poly)phenol-rich GTE on postprandial insulinemia in the evening is likely due to the lower baseline insulin response in the evening rather than an effect on circadian processes by the (poly)phenols, particularly since this was a single-dose postprandial study that did not implicate the mechanism of GTE (poly)phenols on circadian genes. However, the green tea polyphenols, namely (−)-epicatechin, (−)-epigallocatechin and (−)-epigallocatechin gallate, are rapidly absorbed and reach circulation within 30 minutes post-consumption,52 allowing them to exert immediate effects on insulin sensitivity. Consecutive ingestion of a green tea beverage increased insulin in the evening while decreasing glucose in healthy individuals, suggesting its potential efficacy in improving reduced insulin sensitivity in the evening.26 Despite the notable scarcity of publications concerning the impact of (poly)phenols on circadian-metabolic responses in humans, effects by green tea (poly)phenols on circadian processes have been demonstrated in cultured cells.53 In cultured rat muscle cells, EGCG facilitated GLUT4 translocation to the cell membrane by insulin-induced phosphorylation of AKT, subsequently enhancing glucose uptake.54 Further, EGCG modulated insulin signaling in human HepG2 cells, metabolically stressed with high glucose, by reducing serine (ser 307) phosphorylation and activating tyrosine phosphorylation of IRS-1 via Adenosine Monophosphate-activated Protein Kinase (AMPK) activation, and thereby enhancing hepatic insulin sensitivity.55 These data indicate that habitual (poly)phenol consumption has the potential to improve insulin sensitivity through circadian genes, opening up a new avenue for exploring their time-of-day effects on disturbed glucose metabolism caused by mistimed eating.
This study is the first to investigate the efficacy of a green tea extract incorporated into a starch-based meal consumed in the morning and evening, demonstrating its potent impact on lowering postprandial insulin levels exclusively in the morning. This finding suggests that combining GTE into morning meals could be a promising strategy for enhancing insulin sensitivity and improving metabolic health. Such an approach could benefit both the general population and individuals with metabolic disorders, potentially reducing the risk of developing type 2 diabetes and cardiovascular diseases. The consistently controlled fasting duration (≥9 h) for both morning and evening trials allowed for precise assessment of the time-of-day effect on meal consumption. A limitation of the study is that healthy participants were recruited, and the effect was not examined on individuals with insulin resistance, obesity or diabetes, nor on populations experiencing mistimed eating and circadian disruption, such as shift workers. Plasma incretins such as glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1), were not measured, which could provide deeper insights into how green tea (poly)phenols interact with glucose absorption rates and explain the observed differences in insulin concentrations at different times of day.
5. Conclusion
Green tea extract incorporated into rice delayed postprandial insulin in the morning, but not in the evening, with no effect on postprandial glucose. While postprandial glucose was higher in the evening compared to the morning, insulin was lower in the evening, regardless of the intervention. Considering the protective effects of (poly)phenol-rich foods or extracts against the risk of type 2 diabetes, further long-term intervention and mechanistic studies are essential to disentangle how (poly)phenols, whether consumed independently or integrated with meals at different times of the day, can improve metabolic health. Additionally, future research is needed to determine whether circadian interactions occur primarily at the level of digestion, glucose transporters (e.g., by comparing equal amounts of different carbohydrate sources) or insulin sensitivity.Abbreviations
| AKT | Protein kinase B |
| BMAL1 | Brain and muscle ARNT-like 1 |
| CLOCK | Circadian locomotor output cycles kaput |
| EGCG | (−)-Epigallocatechin gallate |
| GTE | Green tea extract-rich meal |
| GLUT2/4/5 | Glucose transporter 2/4/5 |
| GSK-3β | Glycogen synthase kinase-3β |
| iAUC | Incremental area under the curve |
| IRS-1 | Insulin receptor substrate-1 |
| Sirt1 | Sirtuin1 |
Author contributions
Noha Sulaimani: investigation, formal analysis, writing – original draft, writing – review & editing, visualization. Erika J Rosbotham: methodology, investigation, writing – review & editing. Rebekah Warnock: methodology, investigation, writing – review & editing. Louise Polzella: investigation, writing – review & editing. Rebecca Judowski: investigation, writing – review & editing. Luca Nicolotti: methodology, investigation, writing – review & editing. Michael J. Houghton: methodology, formal analysis, writing – review & editing, supervision. Gary Williamson: resources, writing – review & editing, supervision, project administration, funding acquisition. Maxine P. Bonham: conceptualization, methodology, resources, writing – review & editing, supervision, project administration, funding acquisition.Data availability
Data from the human study cannot be made available due to ethical confidentiality requirements.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The work was funded by Monash University, by a King Abdulaziz University scholarship to NS. Funding for the insulin measurements was generously provided by the Andrea Joy Logan Trust. We would like to acknowledge the participants. We thank Dr Helen Tsimiklis for generously providing access to the centrifugal evaporator at Monash University and Dr Lavaraj Devkota for conducting HPLC analysis. This project used NCRIS-enabled ‘Metabolomics Australia’ infrastructure. Metabolomics South Australia is funded through Bioplatforms Australia Pty Ltd (BPA), and investment from the South Australian State Government and The Australian Wine Research Institute.References
- E. M. Speksnijder, P. H. Bisschop, S. E. Siegelaar, D. J. Stenvers and A. Kalsbeek, Circadian desynchrony and glucose metabolism, J. Pineal Res., 2024, 76, e12956 CrossRef CAS PubMed.
- D. J. Stenvers, F. Scheer, P. Schrauwen, S. E. la Fleur and A. Kalsbeek, Circadian clocks and insulin resistance, Nat. Rev. Endocrinol., 2019, 15, 75–89 CrossRef PubMed.
- G. K. W. Leung, C. E. Huggins and M. P. Bonham, Effect of meal timing on postprandial glucose responses to a low glycemic index meal: A crossover trial in healthy volunteers, Clin. Nutr., 2019, 38, 465–471 CrossRef CAS PubMed.
- R. S. de Almeida, L. P. Marot, C. O. C. Latorraca, R. Oliveira and C. A. Crispim, Is evening carbohydrate intake in healthy individuals associated with higher postprandial glycemia and insulinemia when compared to morning intake? A systematic review and meta-analysis of randomized crossover studies, J. Am. Nutr. Assoc., 2023, 42, 349–360 CAS.
- G. K. W. Leung, C. E. Huggins, R. S. Ware and M. P. Bonham, Time of day difference in postprandial glucose and insulin responses: Systematic review and meta-analysis of acute postprandial studies, Chronobiol. Int., 2020, 37, 311–326 CrossRef CAS.
- A. Saad, C. Dalla Man, D. K. Nandy, J. A. Levine, A. E. Bharucha, R. A. Rizza, R. Basu, R. E. Carter, C. Cobelli, Y. C. Kudva and A. Basu, Diurnal pattern to insulin secretion and insulin action in healthy individuals, Diabetes, 2012, 61, 2691–2700 CrossRef CAS PubMed.
- T. Gibson and R. J. Jarrett, Diurnal variation in insulin sensitivity, Lancet, 1972, 2, 947–948 CrossRef CAS PubMed.
- C. Saini, V. Petrenko, P. Pulimeno, L. Giovannoni, T. Berney, M. Hebrok, C. Howald, E. T. Dermitzakis and C. Dibner, A functional circadian clock is required for proper insulin secretion by human pancreatic islet cells, Diabetes, Obes. Metab., 2016, 18, 355–365 CrossRef CAS PubMed.
- B. Marcheva, K. M. Ramsey, E. D. Buhr, Y. Kobayashi, H. Su, C. H. Ko, G. Ivanova, C. Omura, S. Mo, M. H. Vitaterna, J. P. Lopez, L. H. Philipson, C. A. Bradfield, S. D. Crosby, L. JeBailey, X. Wang, J. S. Takahashi and J. Bass, Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes, Nature, 2010, 466, 627–631 CrossRef CAS.
- R. Davis, C. Murgia, A. L. Dordevic, M. P. Bonham and C. E. Huggins, Diurnal variation in gene expression of human peripheral blood mononuclear cells after eating a standard meal compared with a high protein meal: A cross-over study, Clin. Nutr., 2021, 40, 4349–4359 CrossRef CAS PubMed.
- S. M. T. Wehrens, S. Christou, C. Isherwood, B. Middleton, M. A. Gibbs, S. N. Archer, D. J. Skene and J. D. Johnston, Meal timing regulates the human circadian system, Curr. Biol., 2017, 27, 1768–1775 CrossRef CAS PubMed.
- S. A. Coe, M. Clegg, M. Armengol and L. Ryan, The polyphenol-rich baobab fruit (Adansonia digitata L.) reduces starch digestion and glycemic response in humans, Nutr. Res., 2013, 33, 888–896 CrossRef CAS.
- R. Törrönen, E. Sarkkinen, T. Niskanen, N. Tapola, K. Kilpi and L. Niskanen, Postprandial glucose, insulin and glucagon-like peptide 1 responses to sucrose ingested with berries in healthy subjects, Br. J. Nutr., 2012, 107, 1445–1451 CrossRef.
- J. Hlebowicz, G. Darwiche, O. Björgell and L. O. Almér, Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects, Am. J. Clin. Nutr., 2007, 85, 1552–1556 CrossRef CAS.
- R. Törrönen, M. Kolehmainen, E. Sarkkinen, H. Mykkänen and L. Niskanen, Postprandial glucose, insulin, and free fatty acid responses to sucrose consumed with blackcurrants and lingonberries in healthy women, Am. J. Clin. Nutr., 2012, 96, 527–533 CrossRef PubMed.
- J. A. Bryans, P. A. Judd and P. R. Ellis, The effect of consuming instant black tea on postprandial plasma glucose and insulin concentrations in healthy humans, J. Am. Coll. Nutr., 2007, 26, 471–477 CrossRef CAS PubMed.
- E. Makarova, P. Górnaś, I. Konrade, D. Tirzite, H. Cirule, A. Gulbe, I. Pugajeva, D. Seglina and M. Dambrova, Acute anti-hyperglycaemic effects of an unripe apple preparation containing phlorizin in healthy volunteers: a preliminary study, J. Sci. Food Agric., 2015, 95, 560–568 CrossRef CAS.
- H. Nyambe-Silavwe and G. Williamson, Polyphenol- and fibre-rich dried fruits with green tea attenuate starch-derived postprandial blood glucose and insulin: A randomised, controlled, single-blind, cross-over intervention, Br. J. Nutr., 2016, 116, 443–450 CrossRef CAS.
- H. Tsuneki, M. Ishizuka, M. Terasawa, J. B. Wu, T. Sasaoka and I. Kimura, Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans, BMC Pharmacol., 2004, 4, 18 CrossRef.
- M. Takahashi, M. Miyashita, K. Suzuki, S. R. Bae, H. K. Kim, T. Wakisaka, Y. Matsui, M. Takeshita and K. Yasunaga, Acute ingestion of catechin-rich green tea improves postprandial glucose status and increases serum thioredoxin concentrations in postmenopausal women, Br. J. Nutr., 2014, 112, 1542–1550 CrossRef CAS.
- E. Barber, M. J. Houghton and G. Williamson, Flavonoids as human intestinal α-glucosidase inhibitors, Foods, 2021, 10, 1939 CrossRef CAS.
- R. Visvanathan, M. J. Houghton and G. Williamson, Maltoheptaoside hydrolysis with chromatographic detection and starch hydrolysis with reducing sugar analysis: Comparison of assays allows assessment of the roles of direct α-amylase inhibition and starch complexation, Food Chem., 2021, 343, 128423 CrossRef CAS.
- R. Visvanathan, M. J. Houghton, E. Barber and G. Williamson, Structure-function relationships in (poly)phenol-enzyme binding: Direct inhibition of human salivary and pancreatic α-amylases, Food Res. Int., 2024, 188, 114504 CrossRef CAS.
- Y. Kobayashi, M. Suzuki, H. Satsu, S. Arai, Y. Hara, K. Suzuki, Y. Miyamoto and M. Shimizu, Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism, J. Agric. Food Chem., 2000, 48, 5618–5623 CrossRef CAS PubMed.
- M. Takahashi, M. Ozaki, M. Miyashita, M. Fukazawa, T. Nakaoka, T. Wakisaka, Y. Matsui, M. Hibi, N. Osaki and S. Shibata, Effects of timing of acute catechin-rich green tea ingestion on postprandial glucose metabolism in healthy men, J. Nutr. Biochem., 2019, 73, 108221 CrossRef CAS.
- M. Takahashi, M. Ozaki, M. Tsubosaka, H. K. Kim, H. Sasaki, Y. Matsui, M. Hibi, N. Osaki, M. Miyashita and S. Shibata, Effects of timing of acute and consecutive catechin ingestion on postprandial glucose metabolism in mice and humans, Nutrients, 2020, 12, 565 CrossRef CAS.
- C. L. Craig, A. L. Marshall, M. Sjöström, A. E. Bauman, M. L. Booth, B. E. Ainsworth, M. Pratt, U. Ekelund, A. Yngve, J. F. Sallis and P. Oja, International physical activity questionnaire: 12-country reliability and validity, Med. Sci. Sports Exercise, 2003, 35, 1381–1395 CrossRef.
- V. Neveu, J. Perez-Jiménez, F. Vos, V. Crespy, L. du Chaffaut, L. Mennen, C. Knox, R. Eisner, J. Cruz, D. Wishart and A. Scalbert, Phenol-Explorer: an online comprehensive database on polyphenol contents in foods, Database, 2010, 2010, bap024 CrossRef CAS PubMed.
- U.S. Department of Agriculture, USDA Database for the Flavonoid Content of Selected Foods, Release 3.1, https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/methods-and-application-of-food-composition-laboratory/mafcl-site-pages/flavonoids/, September 2019.
- M. Farazi, M. J. Houghton, L. Nicolotti, M. Murray, B. R. Cardoso and G. Williamson, Inhibition of human starch digesting enzymes and intestinal glucose transport by walnut polyphenols, Food Res. Int., 2024, 189, 114572 CrossRef CAS PubMed.
- L. Chepulis, H. Al-Aubaidy and R. Page, Effects of selected antioxidant food extracts on postprandial glucose responses in healthy individuals, Funct. Foods Health Dis., 2016, 6, 493–505 CrossRef CAS.
- M. Gibbs, D. Harrington, S. Starkey, P. Williams and S. Hampton, Diurnal postprandial responses to low and high glycaemic index mixed meals, Clin. Nutr., 2014, 33, 889–894 CrossRef CAS PubMed.
- M. Takahashi, M. Ozaki, M. I. Kang, H. Sasaki, M. Fukazawa, T. Iwakami, P. J. Lim, H. K. Kim, S. Aoyama and S. Shibata, Effects of meal timing on postprandial glucose metabolism and blood metabolites in healthy adults, Nutrients, 2018, 10, 1763 CrossRef PubMed.
- M. Takahashi, Y. Mineshita, J. Yamagami, C. Wang, K. Fujihira, Y. Tahara, H. K. Kim, T. Nakaoka and S. Shibata, Effects of the timing of acute mulberry leaf extract intake on postprandial glucose metabolism in healthy adults: A randomised, placebo-controlled, double-blind study, Eur. J. Clin. Nutr., 2023, 77, 468–473 CrossRef CAS PubMed.
- A. Verrillo, A. De Teresa, C. Martino, G. Di Chiara, M. Pinto, L. Verrillo, F. Torello and A. Gattoni, Differential roles of splanchnic and peripheral tissues in determining diurnal fluctuation of glucose tolerance, Am. J. Physiol., 1989, 257, E459–E465 CAS.
- K. A. Dyar, S. Ciciliot, L. E. Wright, R. S. Biensø, G. M. Tagliazucchi, V. R. Patel, M. Forcato, M. I. Peña-Paz, A. Gudiksen, F. Solagna, M. Albiero, I. Moretti, K. L. Eckel-Mahan, P. Baldi, P. Sassone-Corsi, R. Rizzuto, S. Bicciato, H. Pilegaard, B. Blaauw and S. Schiaffino, Erratum to “Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock” [Mol Metab 3 (2014) 29–41], Mol. Metab., 2014, 3, 857 CrossRef CAS PubMed.
- J. Liu, B. Zhou, M. Yan, R. Huang, Y. Wang, Z. He, Y. Yang, C. Dai, Y. Wang, F. Zhang and Q. Zhai, CLOCK and BMAL1 regulate muscle insulin sensitivity via SIRT1 in male mice, Endocrinology, 2016, 157, 2259–2269 CrossRef CAS.
- M. Murray, A. L. Dordevic, L. Ryan and M. P. Bonham, A single-dose of a polyphenol-rich fucus vesiculosus extract is insufficient to blunt the elevated postprandial blood glucose responses exhibited by healthy adults in the evening: A randomised crossover trial, Antioxidants, 2019, 8, 49 CrossRef CAS PubMed.
- M. Murray, A. L. Dordevic, L. Ryan and M. P. Bonham, The impact of a single dose of a polyphenol-rich seaweed extract on postprandial glycaemic control in healthy adults: A randomised cross-over trial, Nutrients, 2018, 10, 270 CrossRef PubMed.
- J. Josic, A. T. Olsson, J. Wickeberg, S. Lindstedt and J. Hlebowicz, Does green tea affect postprandial glucose, insulin and satiety in healthy subjects: A randomized controlled trial, Nutr. J., 2010, 9, 63 CrossRef PubMed.
- S. Coe and L. Ryan, White bread enriched with polyphenol extracts shows no effect on glycemic response or satiety, yet may increase postprandial insulin economy in healthy participants, Nutr. Res., 2016, 36, 193–200 CrossRef CAS PubMed.
- I. Edirisinghe, K. Banaszewski, J. Cappozzo, K. Sandhya, C. L. Ellis, R. Tadapaneni, C. T. Kappagoda and B. M. Burton-Freeman, Strawberry anthocyanin and its association with postprandial inflammation and insulin, Br. J. Nutr., 2011, 106, 913–922 CrossRef CAS PubMed.
- R. Törrönen, M. Kolehmainen, E. Sarkkinen, K. Poutanen, H. Mykkänen and L. Niskanen, Berries reduce postprandial insulin responses to wheat and rye breads in healthy women, J. Nutr., 2013, 143, 430–436 CrossRef PubMed.
- M. E. Clegg, M. Pratt, C. M. Meade and C. J. Henry, The addition of raspberries and blueberries to a starch-based food does not alter the glycaemic response, Br. J. Nutr., 2011, 106, 335–338 CrossRef CAS PubMed.
- P. Mena and D. Del Rio, Gold standards for realistic (poly)phenol research, J. Agric. Food Chem., 2018, 66, 8221–8223 CrossRef CAS PubMed.
- C. Manach, D. Milenkovic, T. Van de Wiele, A. Rodriguez-Mateos, B. de Roos, M. T. Garcia-Conesa, R. Landberg, E. R. Gibney, M. Heinonen, F. Tomás-Barberán and C. Morand, Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction, Mol. Nutr. Food Res., 2017, 61, 1600557 CrossRef PubMed.
- D. Ni, Z. Ai, D. Munoz-Sandoval, R. Suresh, P. R. Ellis, C. Yuqiong, P. A. Sharp, P. J. Butterworth, Z. Yu and C. P. Corpe, Inhibition of the facilitative sugar transporters (GLUTs) by tea extracts and catechins, FASEB J., 2020, 34, 9995–10010 CrossRef CAS PubMed.
- A. Tavakkolizadeh, A. Ramsanahie, L. L. Levitsky, M. J. Zinner, E. E. Whang, S. W. Ashley and D. B. Rhoads, Differential role of vagus nerve in maintaining diurnal gene expression rhythms in the proximal small intestine, J. Surg. Res., 2005, 129, 73–78 CrossRef CAS PubMed.
- S. G. Houghton, A. E. Zarroug, J. A. Duenes, M. E. Fernandez-Zapico and M. G. Sarr, The diurnal periodicity of hexose transporter mRNA and protein levels in the rat jejunum: Role of vagal innervation, Surgery, 2006, 139, 542–549 CrossRef PubMed.
- C. P. Corpe and C. F. Burant, Hexose transporter expression in rat small intestine: Effect of diet on diurnal variations, Am. J. Physiol., 1996, 271, G211–G216 CAS.
- A. Rivera-Coll, X. Fuentes-Arderiu and A. Díez-Noguera, Circadian rhythms of serum concentrations of 12 enzymes of clinical interest, Chronobiol. Int., 1993, 10, 190–200 CrossRef CAS PubMed.
- M. Renouf, K. Redeuil, K. Longet, C. Marmet, F. Dionisi, M. Kussmann, G. Williamson and K. Nagy, Plasma pharmacokinetics of catechin metabolite 4′-O-Me-EGC in healthy humans, Eur. J. Nutr., 2011, 50, 575–580 CrossRef CAS PubMed.
- N. Sulaimani, M. J. Houghton, M. P. Bonham and G. Williamson, Effects of (poly)phenols on circadian clock gene-mediated metabolic homeostasis in cultured mammalian cells: A scoping review, Adv. Nutr., 2024, 15, 100232 CrossRef CAS PubMed.
- L. Xu, W. Li, Z. Chen, Q. Guo, C. Wang, R. K. Santhanam and H. Chen, Inhibitory effect of epigallocatechin-3-O-gallate on α-glucosidase and its hypoglycemic effect via targeting PI3K/AKT signaling pathway in L6 skeletal muscle cells, Int. J. Biol. Macromol., 2019, 125, 605–611 CrossRef CAS PubMed.
- C. L. Lin and J. K. Lin, Epigallocatechin gallate (EGCG) attenuates high glucose-induced insulin signaling blockade in human hepG2 hepatoma cells, Mol. Nutr. Food Res., 2008, 52, 930–939 CrossRef CAS PubMed.
- N. López-Gutiérrez, R. Romero-González, P. Plaza-Bolaños, J. L. Martínez Vidal and A. Garrido Frenich, Identification and quantification of phytochemicals in nutraceutical products from green tea by UHPLC–Orbitrap-MS, Food Chem., 2015, 173, 607–618 CrossRef PubMed.
- X.-H. Zhang, Q. Zhou, Z. Liu, X.-D. Qing, J.-J. Zheng, S.-T. Mu and P.-H. Liu, Comparison of three second-order multivariate calibration methods for the rapid identification and quantitative analysis of tea polyphenols in Chinese teas using high-performance liquid chromatography, J. Chromatogr. A, 2020, 1618, 460905 CrossRef CAS PubMed.
- B. Hu, L. Wang, B. Zhou, X. Zhang, Y. Sun, H. Ye, L. Zhao, Q. Hu, G. Wang and X. Zeng, Efficient procedure for isolating methylated catechins from green tea and effective simultaneous analysis of ten catechins, three purine alkaloids, and gallic acid in tea by high-performance liquid chromatography with diode array detection, J. Chromatogr. A, 2009, 1216, 3223–3231 CrossRef CAS PubMed.
- L. S. Lee, S. H. Kim, Y. B. Kim and Y. C. Kim, Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity, Molecules, 2014, 19, 9173–9186 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo04843a |
| This journal is © The Royal Society of Chemistry 2025 |